Abstract
To investigate the changes and significance of IL-37 in patients with sepsis.
A total of 50 patients with sepsis between September 2016 and October 2017 at the intensive care unit (ICU) of the First Affiliated Hospital of Shihezi University School of Medicine were selected as the sepsis group, 30 age and sex-matched healthy controls were selected as the control group. The levels of IL-37 in serum were measured by enzyme-linked immunosorbent assay (ELISA) on day 1 and day 7 of the sepsis patients.
The levels of serum IL-37 in the sepsis group on day 1 [(39.13 ± 34.35)pg/mL] were significantly higher than that in the control group [(23.75 ± 2.52)pg/mL] with significant difference (P <.05). The levels of IL-37 in the sepsis group after treatment [(30.57 ± 11.01)pg/mL] on day 7 were obviously lower than that before treatment without statisticaly difference (P >.05). A correlation analysis showed that the levels of serum IL-37 and IL-1β were positively correlated.
The level of IL-37 observed in sepsis was found to correlate with the severity of the inflammatory reaction. IL-37 could be an important cytokine in the control of sepsis by suppressing the production of pro-inflammatory cytokines.
Keywords: IL-1β, interleukin-37, PCT, sepsis
1. Introduction
Sepsis is defined as a life-threatening organ dysfunction that is caused by a dysregulated host response to infection.[1] Sepsis and septic shock are major healthcare problems, affecting millions of people around the world each year, and killing as many as 1 in 4 (and often more).[2] Sepsis is the primary cause of death from infection, especially if not recognized and treated promptly. Its recognition mandates urgent attention.
Interleukin-37 (IL-37), a new member of interleukin-1 (IL-1) family cytokine, is recently identified as a natural inhibitor of innate immunity. IL-37 plays a protective role in many diseases, but there is less research on sepsis.[3] It is an important anti-inflammatory cytokine, which may be involved in the regulation of the pathogenesis of sepsis. This study aimed to measure serum levels of IL-37 in sepsis patients and to investigate its role in sepsis. IL-37 may provide a novel research target for the pathogenesis and therapy of sepsis.
2. Materials and methods
2.1. Study
Prospective observational study
2.2. Patients
This study was conducted between September 2016 and October 2017 at the intensive care unit (ICU) of the First Affiliated Hospital of Shihezi University School of Medicine, China. A total of 50 patients with sepsis were selected as the experimental group, which were diagnosed according to the latest 2016 diagnostic criteria for sepsis. For patients with infection or suspected infection in ICU, they would be diagnosed with sepsis when their sequential organ failure assessment (SOFA) scores ≥2. These patients included 36 patients with pulmonary infection, 3 patients with acute urinary tract infection, 2 patients with acute infectious diarrhea, 4 patients with biliary tract infection, 2 patients with acute tracheobronchitis, 1 patient with liver abscess and 2 patients with skin and soft tissue infection. 30 age and sex-matched healthy controls were selected as the control group. The levels of IL-37 in serum were measured by enzyme-linked immunosorbent assay (ELISA) on day 1 and day 7 of patients with sepsis. Written informed consent was obtained from each patient. The study approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University School of Medicine.
2.3. Laboratory measurement
Blood samples were immediately centrifuged for 10 minutes at 3500r/min, and serum was collected and stored in polypropylene vials at − 80°C until assayed. Concentrations of serum IL-37, IL-1β, and IL-10 were measured by an ELISA, following the manufacturer's instructions.
2.4. Statistical analysis
All of the data were given as the mean ± SD. When comparing only 2 groups, Student t test was used. For comparisons involving 3 groups, 1-way analysis of variance (ANOVA) followed by Neuman–Keuls post hoc test was used. Spearman's correlation was used to calculate the correlations between serum IL-37 concentrations and the other parameters. In all of the tests, a value of P <.05 was considered to be statistically significant. The SPSS statistical software (version 23) was used for all calculations.
3. Results
3.1. Baseline characteristics
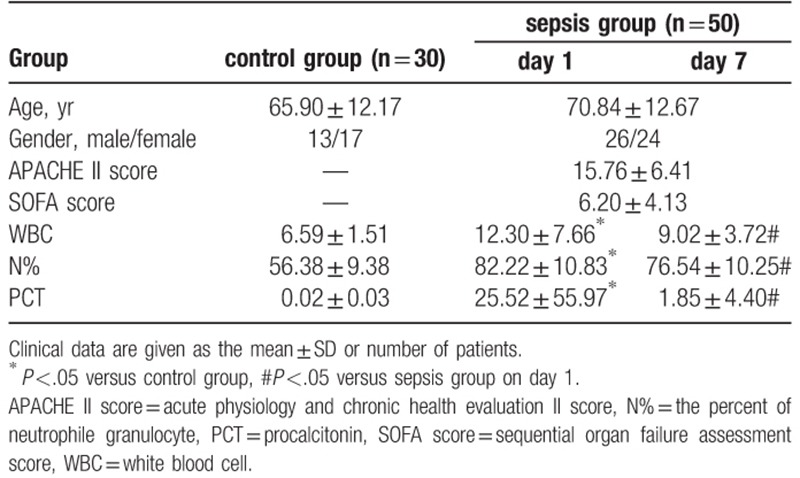
The baseline characteristics of patients are presented in Table 1. We observed significantly increased white blood cell (WBC) counts, the percent of neutrophile granulocyte (N%), and procalcitonin (PCT) levels in the sepsis group compared with those in the control group. Furthermore, the WBC count, N%, and PCT levels decreased significantly in the sepsis group on day 7 after standard treatment.
Table 1.
Baseline characteristics.
3.2. Levels of serum IL-37, IL-1β, and IL-10 in the 2 groups
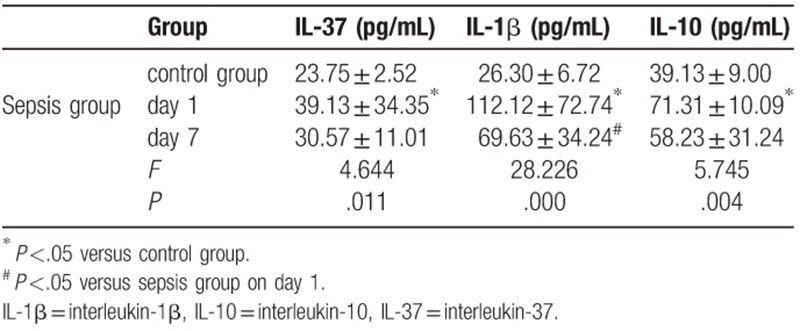
As shown in Table 2, sepsis patients showed significantly increased serum levels of IL-37, IL-1β, IL-10 on day 1 compared with those in the control group. Sepsis patients showed significantly decreased IL-1β levels on day 7 compared with the levels observed on day 1. There were no significant differences in serum IL-37 and IL-10 levels between day 1 (39.13 ± 34.35 and 71.31 ± 10.09) and day 7 (30.57 ± 11.01 and 58.23 ± 31.24) in the sepsis group (Table 2).
Table 2.
Serum IL-37, IL-1β and IL-10 concentrations levels in each group.
3.3. Spearman's correlation analysis
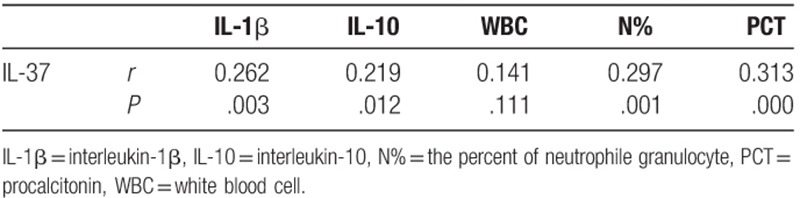
We assessed whether serum IL-37 concentrations were associated with levels of IL-1β, IL-10, WBC counts, N%, and PCT. A correlation analysis indicated that serum levels of IL-37 and IL-1β, IL-10, N% and PCT were positively correlated. There was no significant correlation between serum IL-37 level and WBC count (Table 3).
Table 3.
Correlation analysis of IL-37 and IL-1β, IL-10, WBC, N%, PCT.
4. Discussion
In sepsis, the immune response that is induced by an invading pathogen fails to return to homeostasis. This culminates in a pathological syndrome that is characterized by sustained excessive inflammation and immune suppression. The condition of sepsis patients develops rapidly. Although there are good treatment and monitoring measures, the morbidity and mortality of sepsis are still high, which is a prominent problem faced in the global medical field.[4] Early recognition is essential to reduce sepsis-associated mortality. It was believed that sepsis was a result of tissue and organ damage due to excessive inflammation. However, an increasing number of studies have found that the pathophysiological process of sepsis is very complicated and includes various aspects of inflammation, immunity and coagulation dysfunction, as well as various changes in cell function, metabolism, and microcirculation.
Our results indicated that serum IL-37 levels increased significantly in sepsis patients compared with the healthy control group. After administering standardized anti-infective and other symptomatic treatment for 7 days, serum IL-37 levels decreased significantly in sepsis patients. However, sepsis patients still showed higher serum IL-37 concentrations than patients in the healthy control group. In addition, we found that IL-37 and the pro-inflammatory factor IL-1β showed the same trend. On correlation analysis, a positive correlation was observed between the 2. IL-37, formerly termed IL-1 family member 7 (IL-1 family 7, IL-1F7), was originally a cytokine identified in 2000.[5] IL-37 has various immunomodulatory effects. Its main role is to suppress the inflammatory response. It has been reported that IL-37 is detected in lymph nodes, liver, subcutaneous fat, placenta, colon, lung, kidney, testis, thymus, and uterus and acts as an anti-inflammatory cytokine. IL-37 strongly inhibits lipopolysaccharide (LPS)-induced expression of IL-1α, IL-1β, tumor necrosis factor (TNF), IL-23, IL-17, IL-18, interferon-γ (IFN-γ), and CC chemokines including monocyte chemoattractant protein (MCP)-5/CCL12. It reduces levels of CXC chemokines such as IL-8, BCA-1/CXCL13, and macrophage inflammatory protein (MIP)-2/CXCL2 in neutrophils. IL-37 is a natural inhibitor of immune responses and down-regulates IL-1-induced cJun and reduces expression of the mitogen-activated protein (MAP) kinase p38α, signal transducer and activator of transcription (STAT) transcription factors, p53, and pro-inflammatory signals; this affects cellular differentiation and proliferation.[6,7]
Our findings showed that the body produces a large number of pro-inflammatory factors such as TNF-α and IL-1β in the early stages of sepsis, which initiate inflammatory reactions and cascade amplification. Anti-inflammatory cytokines have the effect of antagonizing inflammatory mediators and inhibiting the development of inflammation, mainly including IL-10, transforming growth factor-β (TGF-β) and so on. When inflammatory reactions occur in sepsis patients, negative feedback increases the concentration of anti-inflammatory factors, this inhibits generation and release of pro-inflammatory mediators, and this, in turn, inhibits monocytes/macrophages-associated inflammation. In sepsis patients, imbalance of pro-inflammatory cytokines and anti-inflammatory factors is often observed. If these levels are not controlled, inflammatory cells cannot be activated effectively, and sufficient levels of anti-inflammatory cytokines are not produced. The body then develops immune dysfunction easily and thus sepsis progression is aggravated. IL-37 has been identified as a natural suppressor of innate inflammatory responses, whereas IL-1β is one of the initiation factors of the inflammatory response. Our results also showed positive correlation between serum IL-37 and IL-1β levels on correlation analysis, suggesting the critical role of IL-37 in sepsis.
Moreover, the latest international guidelines for management of sepsis and septic shock suggest that measurement of PCT levels can be used to shorten the antimicrobial therapy duration in sepsis patients.[2] Our results revealed that IL-37 and PCT levels were positively correlated. However, elevated PCT levels are also observed in response to non-infectious factors, such as severe trauma, extensive surgery, and burns, because the body releases large amounts of damage-associated molecular patterns under these circumstances. As IL-37 is an anti-inflammatory cytokine, its levels better reflect the severity of the inflammatory response. Thus, early intervention can be provided to sepsis patients based on the increase in IL-37 levels.
However, the present study is limited by the small number of samples. We also failed to stratify sepsis patients according to severity and cytological analysis. It has been reported that IL-37 is detected in many target organs such as lymph nodes, liver, lung, and kidney, but we only did serological analysis. Further research is needed for follow-up work. IL-37 is a natural inhibitor of innate immunity. It plays a regulatory role in many inflammatory reactions and immune-related diseases.[8–10] In the inflammation process, IL-37 exerts anti-inflammatory effects by entering the nucleus and binding to Smad3 to form a complex that regulates gene transcription.[11] It is believed that it can provide new ideas and targets for the treatment of sepsis with the continuous research on IL-37.
5. Conclusion
IL-37 levels observed in sepsis were found to correlate with severity of the inflammatory reaction. IL-37 could be playing an important role in sepsis control by suppressing the production of pro-inflammatory cytokines.
Author contributions
Yong-Chun Wang, Guo-Peng Weng, and Qing-Hong Cheng conceived the study and designed the experiments. Yong-Chun Wang, Guo-Peng Weng, Jian-Ping Liu, and Lei Li contributed to data extraction, performed the analysis, and interpreted the results. Yong-Chun Wang wrote the first draft. Yong-Chun Wang and Guo-Peng Weng contributed to the critical revision of the article. All authors read and approved the final manuscript.
Conceptualization: Yong-Chun Wang, Guo-Peng Weng, Qing-Hong Cheng.
Data curation: Yong-Chun Wang, Guo-Peng Weng, Jian-Ping Liu, Lei Li.
Formal analysis: Yong-Chun Wang, Guo-Peng Weng, Jian-Ping Liu, Lei Li.
Investigation: Yong-Chun Wang, Guo-Peng Weng, Jian-Ping Liu, Lei Li.
Project administration: Qing-Hong Cheng.
Writing – original draft: Yong-Chun Wang.
Writing – review & editing: Yong-Chun Wang, Guo-Peng Weng, Jian-Ping Liu, Lei Li, Qing-Hong Cheng.
Qing-Hong Cheng orcid: 0000-0002-1089-0057.
Footnotes
Abbreviations: ELISA = enzyme-linked immunosorbent assay, ICU = intensive care unit, IL = interleukin, N% = the percent of neutrophile granulocyte, PCT = procalcitonin, TNF = tumor necrosis factor, WBC = white blood cell.
The study approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University School of Medicine, China. Approval Number is 2016-115-01.
This work was supported by the Science and Technology Plan Projects of the Xinjiang Production and Construction Corps in China (2016AD003).
Written informed consent was obtained from each patient.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Please contact author for data requests.
References
- [1].Singer M, Deutschman C, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- [3].Zhang Y, Xiong X, Dermatology DO. Research progress on IL-37 and the correlation with diseases. Basic Clin Med 2015;35:549–53. [Google Scholar]
- [4].Martin-Loeches I, Garnacho-Montero J, Nseir S. Focus on infection and sepsis 2017. Intensive Care Med 2017;43:1–3. [DOI] [PubMed] [Google Scholar]
- [5].Boraschi D, Lucchesi D, Hainzl S, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw 2011;22:127–47. [DOI] [PubMed] [Google Scholar]
- [6].Palomo J, Dietrich D, Martin P, et al. The interleukin (IL)-1 cytokine family—balance between agonists and antagonists in inflammatory diseases. Cytokine 2015;76:25–37. [DOI] [PubMed] [Google Scholar]
- [7].Garlanda C, Dinarello C, Mantovani A. The interleukin-1 family: back to the future. Immunity 2013;39:1003–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu K, Min X, Lin Y, et al. Increased IL-37 concentrations in patients with arterial calcification. Clin Chim Acta 2016;461:19–24. [DOI] [PubMed] [Google Scholar]
- [9].Ji Q, Zeng Q, Huang Y, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm 2014;2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Charrad R, Berraïes A, Hamdi B, et al. Anti-inflammatory activity of IL-37 in asthmatic children: correlation with inflammatory cytokines TNF-(, IL-(, IL-6 and IL-17A. Immunobiology 2016;221:182–7. [DOI] [PubMed] [Google Scholar]
- [11].Nold MF, Noldpetry CA, Zepp JA, et al. Interleukin 37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010;11:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


