Abstract
Vaccine effectiveness (VE) was evaluated on a farrow-to-finish farm experiencing mortality due to Streptococcus suis. Direct, indirect, total, and overall vaccine effectiveness were analyzed by vaccinating only 75% of pigs in each litter. Cox’s regression and logistic regression revealed total and overall VE to be 27% and 21%, respectively.
Résumé
Études sur le terrain évaluant l’efficacité directe, indirecte, totale et globale du vaccin autogène pour Streptococcus suis chez les porcelets sevrés. L’efficacité vaccinale (EV) a été évaluée dans une ferme de naissage-engraissage aux prises avec des mortalités causées par Streptococcus suis. L’efficacité vaccinale directe, indirecte, totale et globale ont été analysées en vaccinant seulement 75 % des porcs dans chaque portée. La régression de Cox et la régression logistique ont révélé que l’EV totale et l’EV globale étaient de 27 % et 21 %, respectivement.
(Traduit par Isabelle Vallières)
Introduction
Streptococcus suis is considered to be one of the most important pathogens affecting nursery pigs (1,2). There are currently 35 serotypes of S. suis identified based on the capsular polysacharide (cps), in addition to untypable strains that are un-encapsulated or do not contain a cps moiety (3). In most cases, pigs that are colonized with S. suis remain healthy; however, on occasion systemic infection and clinical disease can occur (4,5).
Control of S. suis disease by vaccination is challenging because of several factors. Although the bacteria are present in the upper respiratory tract of almost all pigs, clinical disease only occurs sporadically. The multiple serotypes of S. suis all have their own set of virulence factors, and the pathogenesis of S. suis is still not well understood (6). Overall, vaccination against S. suis is not a common practice on North American swine farms, and there are no commercial vaccines licensed in Canada for the control of S. suis (6). If vaccines are used on a farm, they are typically serotype-specific autogenous bacterins, based on the serotype isolated from meningeal swabs, and delivered to individual pigs or sows via intramuscular (IM) injection (7). The overall effectiveness of autogenous vaccines against S. suis has not been determined (2). The overarching aim of this study was to determine the effectiveness of vaccination with an autogenous vaccine in preventing mortality due to S. suis in nursery pigs.
A 300-sow farrow-to-finish herd reported high nursery pig mortality during a 6-month period from October 2011 to March 2012, with many of the pigs exhibiting neurological clinical signs consistent with a diagnosis of meningitis. In addition, some pigs in good body condition were found dead without previous signs of illness. Most pig deaths occurred between 6 and 9 wk of age. Postmortem examination and microbiological culture of 12 clinical cases confirmed meningitis due to S. suis serotype 2 as the cause of mortality.
The farm practiced weekly weaning with lactation length varying between 21 and 30 d. The facility had 6 farrowing rooms with 14 farrowing crates per room and operated on an all-in/all-out basis per room. There were 8 nursery rooms, which varied in size and pen conformation. Two rooms contained raised decks with 8 pens measuring 1.22 m × 2.44 m and typically holding 12 pigs per pen. Two other rooms of similar size contained slightly larger pens at floor level (1.52 m × 2.44 m) with on average 13 pigs per pen. There were also 4 larger rooms containing 4 floor-level pens (2.7 m × 4.5 m) holding about 35 pigs each. The nursery rooms were also operated using an all-in/all-out flow. The herd was closed with breeding stock being replaced from within the herd. Further biosecurity measures included shower-in/shower-out, locked doors, and on-site composting of dead stock.
All pigs at weaning received Mycoplasma hyopneumoniae-Haemophilus parasuis combination bacterin (Suvaxyn MH/HPS; Zoetis, Kirkland, Quebec), and a porcine circovirus vaccine (Ingelvac Circoflex; Boehringer Ingelheim, Burlington, Ontario). The M. hyopneumoniae-H. parasuis vaccination was boosted after 3 wk. The starter diet fed for approximately the first week after weaning contained 220 mg/kg of lincomycin (Lincomix 44; Bio Agri Mix, Mitchell, Ontario).
Antimicrobial susceptibility testing revealed the S. suis isolates were resistant to penicillin and so individual pigs with clinical signs were treated with ceftiofur (Excenel RTU, 50 mg ceftiofur per mL; Zoetis), 5 mg/kg body weight (BW), IM. Mortality continued to be high because of the difficulty of identifying sick pigs early enough for antibiotic treatment to be effective. It was decided to approach the problem by instituting an autogenous vaccination program. However, considering previous literature on vaccination for S. suis that had little success (1–3), it was decided to maintain a proportion of control pigs and focus on multiple measures of vaccine effectiveness. We used pigs as a target for vaccination over sows as vaccination at the pig-level was routine practice on the farm and we wanted to maintain farm protocol.
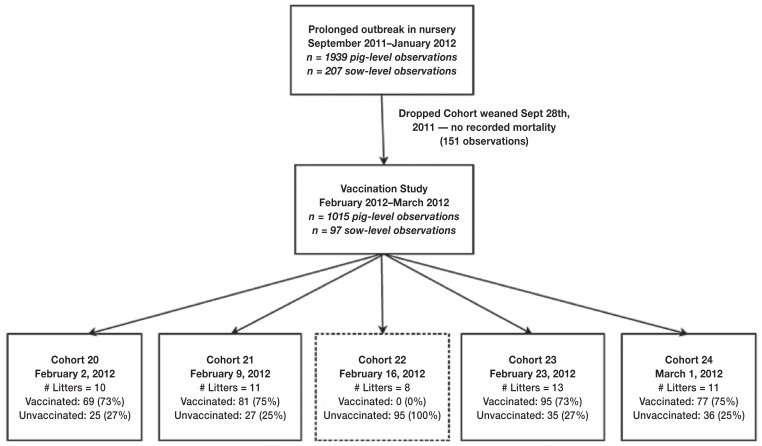
This study included data from 24 weekly cohorts of weanling pigs that entered the nursery phase between October 2011 and March 2012. In 4 cohorts, a randomized pig-level study, blocked by litter, was conducted between February 2nd and March 1st, 2012 (Figure 1). For the 4 cohorts of the vaccine study, meningeal swabs were tested to confirm S. suis, by culture and matrix-assisted laser desorption/ionization-time of flight (MADLI-TOF) method. All laboratory analysis was conducted at the Animal Health Laboratory at the University of Guelph. Direct effectiveness was evaluated based only on mortality due to clinical signs consistent with S. suis infection. The remaining 20 cohorts were evaluated using mortality due to any cause based on available data, in order to estimate indirect, total, and overall vaccine effectiveness.
Figure 1.
Design of the vaccine study conducted February 2, 2012 to March 1, 2012 during a prolonged outbreak of S. suis disease. All piglets from each weaning cohort were involved in the vaccination study, the pigs to be vaccinated were randomly selected from each litter and vaccinated 1 day before entry into the nursery and again 3 weeks later. All piglets were monitored for mortality during the nursery period.
For the vaccine study, approximately 75% of pigs from each litter were randomly selected using systematic random sampling and identified by ear tag. The selected pigs received 2 mL of the autogenous S. suis serotype 2 vaccine IM, a day before entry into the nursery and a 2-mL booster 3 wk after entering the nursery. The other 25% of pigs were left unvaccinated.
The S. suis vaccine used in the study was made at Gallant Custom Laboratory, Cambridge, Ontario, using one S. suis serotype 2 isolate. The vaccine isolate was recovered from a meningeal swab sample collected from a previous clinical case of S. suis on the same farm during an outbreak of disease due to S. suis serotype 2.
The data on all-cause mortality in 20 unvaccinated cohorts during the 6-month study period recorded by the farm manager were used. These records also included information on the date of weaning, date of death, sow and litter information including parity, number of pigs born alive, number of pigs weaned, and pre-weaning mortality. Sows were excluded if information about the weaning date or the number of pigs weaned was missing. The vaccination study data set included sow and litter information, date of weaning, weight of piglet at weaning, date of death, cause of death, gender, vaccination status, and cohort. The time at risk was defined as 9 wk, or the approximate time a pig spent in the nursery room, as well as immediately following the nursery phase. Mortalities that occurred after the period of risk were excluded from analysis.
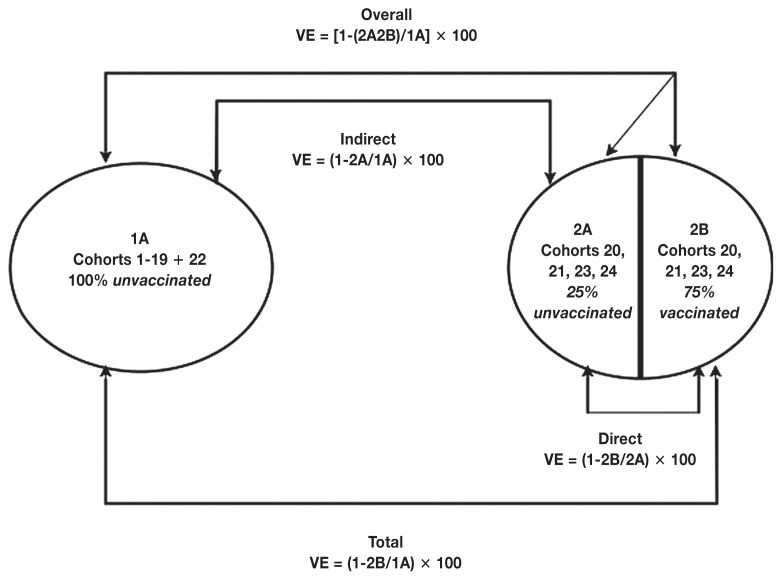
Indirect vaccine effectiveness was considered at the group-level effects by comparing mortality in the unvaccinated pigs exposed to the vaccinated group to the mortality of non-vaccinated pigs in groups with no exposure to vaccinated pigs. However, the total vaccine effectiveness was based on survival of pigs that did receive the vaccine in the population vaccinated. Overall vaccine effectiveness was based on the complete herd-level mortality. The different measures of vaccine effectiveness were evaluated as described by Halloran et al (8) (Figure 2).
Figure 2.
Vaccine effectiveness (VE) as described by Halloran et al (8) where “1” is before the introduction of the vaccine into the population of piglets and “2” is after the introduction of the vaccine into the population. The full calculations for VE are listed and can also be interpreted as 1-RR (relative risk) for each measure of VE.
Vaccine effectiveness was calculated as follows and an illustration of all measures of VE and their derivation can be found in Figure 2. Direct effects were calculated by:
| (Equation 1) |
Indirect vaccine effects are the herd level effects in the pigs that did not receive a vaccine after widespread vaccination. Indirect vaccine effects were calculated by:
| (Equation 2) |
Total vaccine effects are slightly different from indirect effects, as it focuses on the survival of pigs that did receive the vaccine in the population vaccinated. Total effects were calculated by:
| (Equation 3) |
Overall vaccine effectiveness focuses on the complete herd-level mortality. It is described by Halloran et al (7) as the weighted average of indirect effects compared to the weighted average of total effects. Overall effects were calculated by:
| (Equation 4) |
The relative risks described were calculated using 2 models in Stata 14 (StataCorp, College Station, Texas, USA). A Cox’s-proportional hazard model was used to test the direct vaccine effects with morality due to S. suis as the outcome of interest. A generalized linear model, with a binomial distribution and log link, was used to evaluate indirect, total, and overall vaccine effectiveness on all-cause mortality. The hazard ratio (HR) from the Cox’s regression model evaluating direct VE was used as a substitute for relative risk (RR) during percentage VE calculations.
Finally, the residuals and influence statistics for the total and overall VE were evaluated by running binomial logistic regression models on the same data.
In total, 2977 pigs were included in the study over a 6-month period. There was a post-weaning mortality of 358 (12%). The mean littermate post-weaning mortality was 14.4%. Of the 435 pigs included in the vaccination study, 39 (9%) died, of which 35 (90%) deaths were due to clinical signs of S. suis disease. Post-weaning littermate mortality due to S. suis infection in the 4 vaccination cohorts showed high variability with the mean litter-level mortality of 14.0%. The HR for vaccination status was not altered by any of the potential confounding variables, and there was no significant interaction between “vaccination” and “cohort.”
The final model results for all measures of effectiveness, along with their calculated VEs, are shown in Table 1. Direct VE was 1.6% but did not appear to have significant effects on mortality due to S. suis (P > 0.05). The final Cox’s hazard regression model was adjusted for the effect of clustering within litters.
Table 1.
Estimations of vaccine effectiveness (VE) of an autogenous bacterin against Streptococcus suis in pigs during the nursery phase.
| Measure | Relative risk | 95% CI | P-value | VE (%) | 95% CI |
|---|---|---|---|---|---|
| Direct VE | 0.98a | 0.46, 2.12 | 0.982 | 1.6 | < 0, 54 |
| Indirect VE | 0.92 | 0.61, 1.39 | 0.705 | 7.6 | < 0, 49 |
| Total VE | 0.72 | 0.52, 1.01 | 0.058 | 27.6 | < 0, 48 |
| Overall VE | 0.79 | 0.60, 1.03 | 0.082 | 21.3 | < 0, 40 |
HR (hazard ratio) from Cox’s regression used as a substitute for RR (relative risk).
CI — confidence interval.
Data analysis revealed cohorts 9 and 20 to be potential outliers. Removal of cohort 9 resulted in minor changes to all levels of VE. Removal of cohort 20, the first vaccination cohort, increased all measures of VE (Table 2). Most notably, total and overall VE became statistically significant (P < 0.05). The total VE increased from 27% to 54% and overall VE increased from 23% to 50%.
Table 2.
Estimations of vaccine effectiveness (VE) of an autogenous bacterin against Streptococcus suis in pigs during the nursery phase after removal of cohort 20, an influential observation determined through deviance residual analysis.
| Measure | Relative risk | 95% CI | P-value | VE (%) | 95% CI |
|---|---|---|---|---|---|
| Direct VE | 0.93a | 0.30, 2.92 | 0.902 | 7.0 | < 0, 70 |
| Indirect VE | 0.75 | 0.45, 1.35 | 0.277 | 24.4 | < 0, 55 |
| Total VE | 0.45 | 0.28, 0.75 | 0.002 | 54.2 | 25, 72 |
| Overall VE | 0.50 | 0.40, 0.82 | 0.002 | 50.0 | 18, 60 |
HR (hazard ratio) from Cox’s regression used as a substitute of RR (relative risk).
CI — confidence interval.
Removal of both influential observations (cohorts 9 and 20) resulted in total and overall VE becoming statistically significant (P < 0.05). The indirect VE increased from 7.6% to 31.3% but remained statistically nonsignificant (P > 0.05). Herd immunity, in a vaccinated population is based on 3 main factors: the characteristics of pathogen transmission, the ability of the vaccine to offer protection against a pathogen, and the vaccination protocol or coverage in a population (8). Vaccination against infectious diseases can result in a reduction in the transmission probability and a reduction in the duration of infectiousness, subsequently altering the population dynamics involved with infectious agents (8). Most of the population-level studies for vaccinations have been conducted on human populations. To the best of our knowledge, this is the first study which used indirect analysis of vaccine effectiveness for S. suis in swine herds. This study proposed the possibility of existence of the total and overall vaccine effects, indicating alternative methods of studying vaccination effectiveness beyond direct effects. It is a common approach that vaccines are evaluated under controlled experimental conditions using randomization, which is a gold standard for evaluation of direct vaccine effectiveness as well as for assessment of vaccine safety. Specifically, with respect to S. suis, there is a lack of research surrounding population-level vaccine effectiveness in commercial settings.
In this study, vaccine effectiveness differed considerably based on the level of analysis, both in terms of the magnitude of effect and its associated statistical significance. The direct effect of vaccination was not statistically significant; however, the calculated total and overall VE showed potential protective effects.
The magnitude of VE measures increased when the 2 outlier cohorts were excluded. These were outliers based on unusual mortality levels for the observed outbreak. One outlier (Cohort 9) had no mortality, which was very low compared to the average 14.4% per litter experienced over the duration of the outbreak. Another outlier (Cohort 20), the first vaccination cohort, had very high mortality reaching > 30%, compared to the average 14.0% mortality per litter experienced during the vaccination trial. These variations may be due to the presence of other serotypes on the farm and the exponential increase of S. suis cases due to direct transmission that occurs during a severe outbreak of S. suis disease (6). These variations can also be attributed to the sporadic nature of this disease, which illustrates the limitation of having a study with a short duration. However, given the available information, exclusion of the cohorts demonstrates for most cohorts involved in the study, that there was evidence for protection from disease due to S. suis at the population level following vaccination.
The major concern with the validity of our results at the group level is that vaccination was attempted in 4 out of 5 consecutive cohorts. It is possible that mortality due to S. suis showed some cyclical variation and was therefore confounded by the short duration of the study. Streptococcus suis has the potential to cause severe to moderate outbreaks sporadically in nursery pigs and therefore there is a possibility that the high and low mortalities experienced could be attributed to the sporadic nature of the disease (9). In future studies, this should be improved by including a follow-up period after vaccination and restricting the outcome analysis to mortality strictly due to S. suis.
Under a commercial setting, the overall vaccine effectiveness is commonly evaluated by comparison of completely vaccinated and completely non-vaccinated cohorts. While the benefit of such an approach is that the overall VE can be considered, the drawback is that the results could be influenced by potential confounding factors, not associated with VE. These confounders could include the virulence of the strain affecting the cohort having the potential to change over time as multiple strains can infect a herd and even a single pig; therefore, a single vaccine for serotype 2 may not have been effective for the entire study period. Additionally, as we did not evaluate the sow immunity against S. suis or test and serotype every isolate, it is possible that the vaccine effectiveness was confounded by these factors (10).
This retrospective study is not unbiased and future study is needed to have a better randomization of entire groups with respect to their vaccination status. However, taking into consideration all the limitations of retrospective analysis, this study demonstrates the potential for an alternate method for analyzing vaccine effectiveness in a commercial swineherd. Specifically, this study highlighted moderate to high total and overall vaccine effectiveness against S. suis at the population level on a farm experiencing high mortality. Future studies should be aimed at developing a vaccination protocol on-farm that can evaluate these direct and indirect effects without the restrictions that accompanied this retrospective analysis.
Acknowledgments
Funding for this research project was received from Ontario Pork and the University of Guelph-Ontario Ministry of Agriculture, Food, and Rural Affairs Research Partnership, and from the Natural Sciences and Engineering Research Council (NSERC). CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent — An update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3:1–45. doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottschalk M. Streptococcosis. In: Zimmerman JJ, Karicker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012. pp. 841–855. [Google Scholar]
- 3.Gottschalk M, Lacouture S, Bonifait L, Roy D, Fittipaldi N, Grenier D. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Quebec, Canada. Vet Microbiol. 2013;162:819–825. doi: 10.1016/j.vetmic.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Devriese LA, Ceyssens K, Hommez J, Kilpper-Bälz R, Schleifer KH. Characteristics of different Streptococcus suis ecovars and description of a simplified identification method. Vet Microbiol. 1991;26:141–150. doi: 10.1016/0378-1135(91)90050-p. [DOI] [PubMed] [Google Scholar]
- 5.MacInnes JI, Desrosiers R. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can J Vet Res. 1999;63:83–89. [PMC free article] [PubMed] [Google Scholar]
- 6.Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012;7:259–279. doi: 10.2217/fmb.11.149. [DOI] [PubMed] [Google Scholar]
- 7.Baums CG, Bru C, Kock C, Beineke A, Waldmann K, Valentin-Weigand P. Immunogenicity of an autogenous Streptococcus suis bacterin in pre-parturient sows and their piglets in relation to protection after weaning. Clin Vaccine Immunol. 2010;10:1589–1597. doi: 10.1128/CVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halloran ME, Struchiner CJ, Longini M. Study designs for evaluating diffferent efficacy and effectiveness aspects of vaccines. Am J Epidemiol. 2008;4:29–36. doi: 10.1093/oxfordjournals.aje.a009196. [DOI] [PubMed] [Google Scholar]
- 9.Staats JJ, Feder I, Okwumabua O, Chengappa MM. Review Article Streptococcus suis: Past and present. Vet Research Commun. 1997;21:381–407. doi: 10.1023/a:1005870317757. [DOI] [PubMed] [Google Scholar]
- 10.Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]