Abstract
Rationale:
Rhabdomyosarcoma (RMS) has known as a highly malignant soft tissue sarcoma, representing 5% to 10% of all solid tumors in childhood. Alveolar rhabdomyosarcoma (ARMS) of the retrorectal-presacral space is an extremely rare lesion for adult, no study has been reported in the English literature.
Patient concerns:
A 51-year-old male presented with abdominal pain for 1 month, significantly worse when having a bowel movement.
Diagnosis:
Computed tomography (CT) and magnetic resonance imaging (MRI) of the pelvis showed a solid-cystic, enhancing lesion of dimension located in retrorectal-presacral space. The surgical specimen was reported as ARMS after pathological evaluation.
Interventions:
The tumor was complete surgical resection, and after surgery, the patient was treated with combination chemotherapy.
Outcomes:
At 23 months follow up, the patient was asymptomatic with no evidence of metastases or local recurrence.
Lessons:
Improvements in imaging in addition to early surgical intervention and chemotherapy treatment are crucial to improve survival chances against RMS.
Keywords: alveolar, computed tomography, magnetic resonance imaging, retrorectal-presacral space, rhabdomyosarcoma
1. Introduction
Rhabdomyosarcoma (RMS), a rare malignancy originating from striated muscle tissue or primitive mesenchymal tissue, is typically observed in childhood and adolescence. Only a very small portion of RMS cases have been reported to develop in the adult population older than 20 years.[1] Adult RMS accounts for only 1% to 3% of all malignant soft tissue tumors.[2] The head and neck are the most frequent sites of origin, followed by the genitourinary tract, extremities, trunk, retroperitoneum, and uncommon regions.[3–8] One of the least common sites for RMS is the retrorectal-presacral space. We present the case of a 51-year-old male with primary alveolar rhabdomyosarcoma (ARMS) of the retrorectal-presacral space, and to our knowledge, it is the first reported case of an ARMS developed in this location. Rarity of this disease and unusual presentation prompted us to report this case.
2. Ethical review
2.1. Ethical approval
This article does not contain any studies with human or animals performed by any of the authors.
2.2. Informed consent
Informed consent was obtained from the patient for publication of this case report and accompanying images.
2.3. Case description
A 51-year-old male presented with abdominal pain for 1 month, significantly worse when having a bowel movement. Therefore, he was admitted to our hospital. There were neither important risk factors such as family history nor an important history of medical/surgical treatment. Physical examination revealed an abdominal mass below the level of the umbilicus. No fever or history of weight loss was reported by the patient. Laboratory data, including blood test, chemistry, and tumor markers, were within normal limits.
Computed tomography (CT) of the abdomen showed a sharply defined solid-cystic mass (87×93×98 mm in size) with irregular density in retrorectal-presacral space, pushing and compressing the adjacent rectum (Fig. 1). Magnetic resonance imaging (MRI) of the pelvis demonstrated a sharply defined solid-cystic mass containing irregular intensities. T1-weighted images revealed isointense and low-signal intensity, T2-weighted images revealed multiple cystic lesions of varying sizes of high-signal intensity, the solid components, and septums display intense but inhomogeneous enhancement on contrast-enhanced T1-weighted imaging (Fig. 2).
Figure 1.
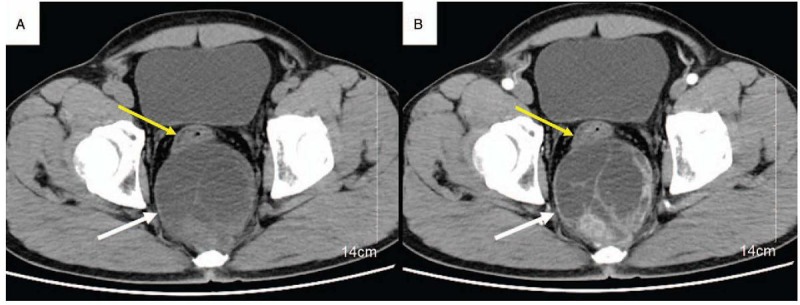
Axial non-contrast (A) and post-contrast CT (B) showed a sharply defined solid-cystic mass (white arrow), with irregular density in retrorectal-presacral space, pushing, and compressing the adjacent rectum (yellow arrow), the solid components and septums display intense but inhomogeneous enhancement on contrast-enhanced.
Figure 2.
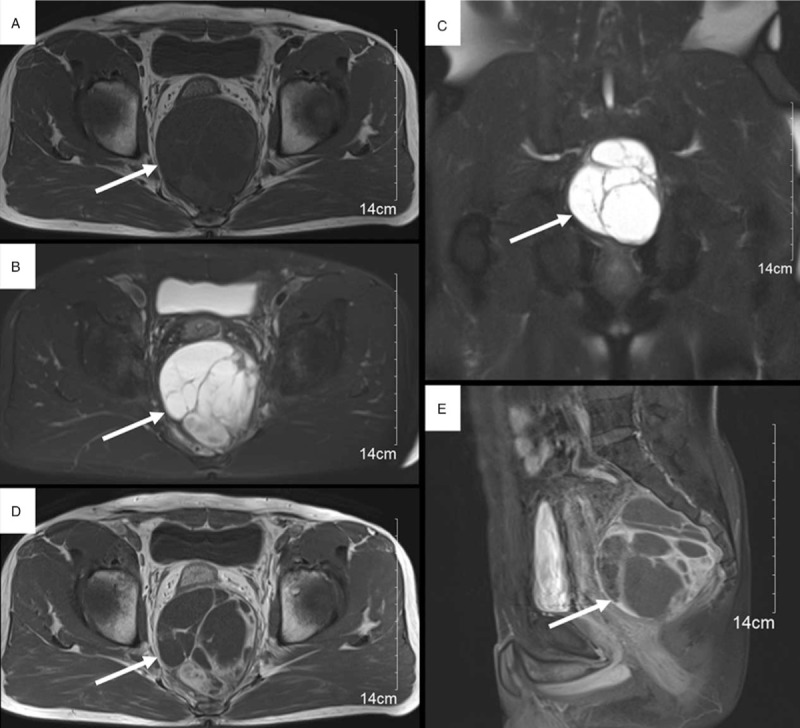
MRI (A—E) demonstrated a sharply defined solid-cystic mass (arrow) containing irregular intensities. Axial T1-weighted image (A) revealed isointense and low-signal intensity. Axial (B) and coronal (C) T2-weighted images revealed multiple cystic lesions of varying sizes of high-signal intensity, the solid components, and septums display intense but inhomogeneous enhancement on contrast-enhanced axial (D) and sagittal (E) T1-weighted images. MRI = magnetic resonance imaging.
Then, the patient underwent tumor resection. Surgery revealed a mass (95x100 mm), with soft texture, which was located at the retrorectal-presacral space. The surgical specimen was reported as ARMS after pathological evaluation. Microscopically, neoplastic cells had large, pleomorphic nuclei with considerable pleomorphism. Bizarre multinucleated giant cells with abundant eosinophilic cytoplasms were also present (Fig. 3A). Immunohistochemical evaluations revealed positive staining for CD99, vimentin, CD34, and Desmin (Fig. 3B). The staging of this patient was stage-I according to the American Intergroup Rhabdomyosarcoma Study (IRS). After surgery, the patient was treated with combination chemotherapy. Vinorelbine, ifosfamide, and actinomycin D were administered intravenously. At 23 months follow up, the patient was asymptomatic with no evidence of metastases or local recurrence. He underwent a metastatic work-up that included a thoracic and abdominal CT scan with and without contrast enhancement and a total body bone scan. These results were negative for tumor.
Figure 3.
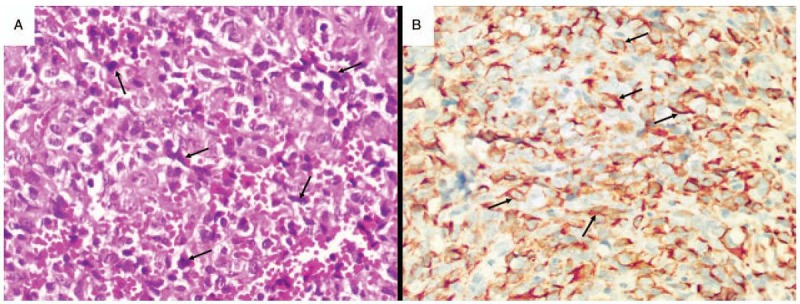
(A, B) Histopathological and immunohistochemical imaging of the case: (A) Most of the tumour cells (arrows) are spindled and arranged in interlacing fascicles. The nuclei are large and pleomorphic, and mitotic figures are easily found (400x). (B) Immunohistochemical positive staining for desmin (arrows).
3. Discussion
RMS is the most common sarcoma in the pediatric population, and it is very uncommon in adults,[2] it is thought to arise from immature mesenchymal cells that are committed to skeletal muscle lineage, but these tumors are also known to arise in tissues in which striated muscle is not normally found. RMS is classified into 4 major subtypes, embryonal RMS (ERMS), alveolar RMS (ARMS), pleomorphic RMS (PRMS) and spindle cell/sclerosing RMS (SRMS).[9] ARMS is most commonly seen in older children and young adults.[10]
RMS can occur at various sites in the body, such as the head, neck, trunk, and extremities, including sites lacking normal striated muscle cells. The most common primary site in adults is the extremities, and the head and neck parameningeal region is the most common site in children.[11] Available data on the extremely infrequent localisations of adulthood, such as larynx,[12] renal pelvis,[13] subcutaneous tissue,[8] uterine,[14] retroperitoneal space,[7] and so on, are insufficient and scarce, mostly presented as case reports or single institution experiences with small numbers of patients. In fact, our patient is the first single-case report of ARMS arising from the retrorectal-presacral space in an adult male, within our thorough investigation.
Imaging findings of RMS are nonspecific.[6] The tumors may appear isointense and low signal intensity to surrounding skeletal muscle on T1-weighted MRI, high and heterogeneous signal on T2-weighted images and contrast-enhanced to variable degrees on T1-weighted sequences.[15,16] Alveolar subtypes, in particular, may show areas of hemorrhage and necrosis as well.[17] But, in the case presented here, CT of the abdomen showed a sharply defined solid-cystic mass with irregular density in retrorectal-presacral space. MRI of the pelvis demonstrated a well-defined solid-cystic enhancing mass. T1-weighted images revealed isointense and low-signal intensity, T2-weighted images revealed multiple cystic lesions of varying sizes of high-signal intensity, the solid components, and septums display intense but inhomogeneous enhancement on contrast-enhanced T1-weighted imaging. On imaging, the multiple cystic lesions of tumor on T2-weighted imaging with high-signal intensity is thought to be due to the necrosis.
There are multimodality treatment options for adult RMS, including surgery, radiation therapy for control of residual bulk or microscopic tumor and systemic combination chemotherapy for primary cytoreduction and eradication of gross and micrometastases.[18] Complete surgical resection is the mainstay of curative therapy to ablate the disease for early tumor stage in the absence of distant metastases. All patients with RMS must receive combination chemotherapy as soon as diagnosis is made or after resection. It improves survival significantly. RMS generally has a dismal prognosis, which has been described in most reports. The best prognostic factors are stage I with small size, less than 5 cm, absence of nodal involvement, and young age, less than 10 years.[19] The 51-year-old male patient in our case underwent tumor resection and received combination chemotherapy and achieved a satisfactory outcome, and so far he is being followed up.
4. Conclusions
In conclusion, this case has reminded us that ARMS could occur in uncommon site and older male. All radiologists should be aware its imaging features. Improvements in imaging in addition to early surgical intervention and chemotherapy treatment are crucial to improve survival chances. An accumulation of such cases is needed for further evaluation and research.
Author contributions
Conceptualization: Liang-Ji Lu, Xiu-Juan Sun.
Formal analysis: Xiu-Liang Zhu.
Investigation: Xiu-Liang Zhu, Wei-Wei Su, Xiu-Juan Sun.
Methodology: Li-Ding Yao.
Supervision: Xiu-Juan Sun.
Validation: Jin-Long Tang.
Writing – original draft: Xiu-Liang Zhu.
Footnotes
Abbreviations: ARMS = alveolar rhabdomyosarcoma, CT = computed tomography, MRI = magnetic resonance imaging, RMS = rhabdomyosarcoma.
L-LJ and S-XJ contributed equally to this work.
The authors have no conflicts of interest to declare.
References
- [1].Liu W, Jiang L, Jin Y, et al. Alveolar rhabdomyosarcoma of the sphenoid sinus mimicking optic neuritis presenting with intermittent visual loss in an adult. Onco Targets Ther 2016;14:6333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dumont SN, Araujo DM, Munsell MF, et al. Management and outcome of 239 adolescent and adult rhabdomyosarcoma patients. Cancer Med 2013;2:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hosseini MS, Ashrafganjoei T, Sourati A, et al. Rhabdomyosarcoma of cervix: a case report. Iran J Cancer Prev 2016;9:e4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kobayashi K, Matsumoto F, Kodaira M, et al. Signifcance of delayed primary excision in localized nonmetastatic adult head and neck rhabdomyosarcoma. Cancer Med 2016;5:2708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schildhaus HU, Lokka S, Fenner W, et al. Spindle cell embryonal rhabdomyosarcoma of the prostate in an adult patient—case report and review of clinicopathological features. Diagn Pathol 2016;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bressner JA, McCarthy EF, Fayad LM, et al. Primary rhabdomyosarcoma of the distal femoral diaphysis: a case report and review of the literature. Skeletal Radiol 2016;45:1391–5. [DOI] [PubMed] [Google Scholar]
- [7].Yadav SK, Sinha DK, Ahmed A, et al. Primary intra-abdominal rhabdomyosarcoma in an adult: an unusual presentation and review of literature. Indian J Surg Oncol 2015;6:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Watanabe M, Ansai SI, Iwakiri I, et al. Case of pleomorphic rhabdomyosarcoma arising on subcutaneous tissue in an adult patient: Review of the published works of 13 cases arising on cutaneous or subcutaneous tissue. J Dermatol 2017;44:59–63. [DOI] [PubMed] [Google Scholar]
- [9].IARC PressLyon, Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumours pathology and genetics of tumours of soft tissue and bone. 2007. [Google Scholar]
- [10].Parham DM, Ellison DA. Rhabdomyosarcomas in adults and children: an update. Arch Pathol Lab Med 2006;130:1454–65. [DOI] [PubMed] [Google Scholar]
- [11].Sultan I, Qaddoumi I, Yaser S, et al. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol 2009;27:3391–7. [DOI] [PubMed] [Google Scholar]
- [12].Chiramel GK, Chacko BR, Thomas R, et al. A rare and unusual occurrence of rhabdomyosarcoma arising from the larynx. Indian J Cancer 2015;52:125–6. [DOI] [PubMed] [Google Scholar]
- [13].Tsai WC, Lee SS, Cheng MF, et al. Botryoid-type pleomorphic rhabdomyosarcoma of the renal pelvis in an adult. Urol Int 2006;77:89–91. [DOI] [PubMed] [Google Scholar]
- [14].Yamada S, Harada Y, Noguchi H, et al. Embryonal rhabdomyosarcoma arising from the uterine corpus in a postmenopausal female: a surgical case challenging the genuine diagnosis on a cytology specimen. Diagn Pathol 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Song L, Chong V, et al. Multiparametric MRI findings of sinonasal rhabdomyosarcoma in adults with comparison to carcinoma. J Magn Reson Imaging 2017;45:998–1004. [DOI] [PubMed] [Google Scholar]
- [16].Yu X, Yang Y, Zhang B, et al. Misdiagnosis of primary pleomorphic rhabdomyosarcoma of the right thigh in a young adult: A case report. Oncol Lett 2016;12:1921–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seynaeve PC, De Visschere PJL, Mortelmans LL. De Schepper AM, et al. Tumors of muscular origin. Imaging of soft tissue tumors. 3rd ed.Germany: Springer; 2006. 293–310. [Google Scholar]
- [18].Elsebaie MAT, Amgad M, Elkashash A, et al. Management of low and intermediate risk adult rhabdomyosarcoma: a pooled survival analysis of 553 patients. Sci Rep 2018;8:11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leaphart CD, Rodeberg D. Pediatric surgical oncology: management of rhabdomyosarcoma. Surg Oncol 2007;16:73–185. [DOI] [PubMed] [Google Scholar]


