Abstract
The amyloid precursor protein (APP) is a type I transmembrane glycoprotein widely studied for its role as the source of β-amyloid peptide, accumulation of which is causal in at least some cases of Alzheimer’s disease (AD). APP is expressed ubiquitously and is involved in diverse biological processes. Growing bodies of evidence indicate connections between AD and somatic metabolic disorders related to type 2 diabetes, and App−/− mice show alterations in glycemic regulation. We find that App−/− mice have higher levels of insulin-degrading enzyme (IDE) mRNA, protein, and activity compared with wild-type controls. This regulation of IDE by APP was widespread across numerous tissues, including liver, skeletal muscle, and brain as well as cell types within neural tissue, including neurons, astrocytes, and microglia. RNA interference-mediated knockdown of APP in the SIM-A9 microglia cell line elevated IDE levels. Fasting levels of blood insulin were lower in App−/− than App+/+ mice, but the former showed a larger increase in response to glucose. These low basal levels may enhance peripheral insulin sensitivity, as App−/− mice failed to develop impairment of glucose tolerance on a high-fat, high-sucrose (“Western”) diet. Insulin levels and insulin signaling were also lower in the App−/− brain; synaptosomes prepared from App−/− hippocampus showed diminished insulin receptor phosphorylation compared with App+/+ mice when stimulated ex vivo. These findings represent a new molecular link connecting APP to metabolic homeostasis and demonstrate a novel role for APP as an upstream regulator of IDE in vivo.
Keywords: Alzheimer’s disease, amyloid precursor protein, insulin, insulin-degrading enzyme, microglia
INTRODUCTION
Alzheimer’s disease (AD) is characterized in part by the robust accumulation of extracellular plaque aggregates of the amyloid β-peptide (Aβ) in the central nervous system (CNS), and multiple lines of evidence indicate that Aβ is a causal factor in AD (23, 76). While familial AD appears to arise from elevated production of longer (42- or 43-amino acid) forms of Aβ, sporadic cases of AD may be more dependent upon compromised clearance of the peptide, which comprises both efflux from the brain into the vasculature and proteolysis. Leading candidates for proteolytic destruction of Aβ include the metalloproteases neprilysin and insulin-degrading peptide (IDE) (5). IDE is a zinc metalloprotease discovered for its ability to hydrolyze insulin, but it has numerous other substrates; in addition to Aβ, it is active against pancreatic amylin (43). All insulin-sensitive tissues degrade insulin, and IDE is established to play an important role in regulating insulin signaling and an essential role in insulin catabolism (15).
Aβ is generated by enzymatic cleavage of the much larger amyloid precursor protein (APP). The genes encoding APP are widely conserved among other species, suggesting the protein performs important or advantageous biological functions (81). APP is part of a three-member family which includes amyloid precursor-like protein (APLP)-1 and -2 (58). While expression of APLP1 is restricted to the CNS, both APP and APLP2 are expressed ubiquitously and can be detected in a variety of tissues (48, 77); only APP contains the Aβ sequence. APP and APLPs have diverse and critical roles in development and physiology, both inside and outside of the CNS (8), highlighted by the neonatal lethality of App/Aplp2 double knockouts (30). Deletion of the App gene alone confers a more benign phenotype, including reduced animal body weight and organ size, changes in grip strength, and broad changes in metabolism (57, 70, 91).
Mounting evidence indicates connections between AD and perturbations in insulin/glucose regulation related to type 2 diabetes mellitus (27, 32, 50, 56, 79, 80). APP may be involved in these links, as illustrated by several lines of evidence. For instance, genetic ablation of APP perturbs basal glycemic levels (62). A recent report demonstrates numerous metabolic differences in App−/− mice, including the finding that circulating levels of insulin are lower in App−/− mice (12). In tissue from obese human subjects, increased expression of APP in adipocytes correlates with the degree of insulin resistance, hyperinsulinemia, and plasma Aβ1–40 levels (46, 47). In wild-type C57BL/6 mice, high-fat diet feeding increases the expression of APP in both hippocampus and adipose tissue (71).
Among its diverse actions, APP contributes to cell adhesion and other aspects of the extracellular matrix that appear to involve complex relationships with proteases (84). In addition to its processing by the α-, β-, γ-, and ε-secretases, APP itself modulates protease activity. The splice variants expressed in nonneuronal cells contain a Kunitz-type serine protease inhibitor domain (9), and other protease inhibitor domains are universal to all splice variants (29, 33). In our recent work, we found that IDE levels are higher in pancreatic tissue extracts and primary pancreatic islet cultures derived from App−/− compared with App+/+ mice (41). In this study, we have expanded upon our initial observation of increased IDE in the App−/− pancreas by demonstrating that regulation of IDE by APP occurs in other tissues, including the brain, highlighting implications for metabolism of Aβ.
MATERIALS AND METHODS
Animals
The APP knockout mice (App−/−) strain B6.129S7-Apptm1Dbo/J (https://www.jax.org/strain/004133) and wild-type (App+/+) C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). APP is knocked out in App−/− mice by deletion of the promoter region and exon 1 of the App gene (91). Both male and female mice were utilized in this study. Mice were provided with food and water ad libitum and housed in a 12:12-h light-dark cycle. Where indicated, normal mouse chow was replaced with a “Western diet” (ENVIGO TD.88137): 42% kcal from fat, 34% sucrose by weight. Western diet TD.88137 has the following composition by weight: 17.3% protein, 48.5% carbohydrate, and 21.2% fat with a calorie content of 4.5 kcal/g. Two control diets were used (ENVIGO TD.08485 and LabDiet JL Rat and Mouse/Auto 6F FK67), having 17–19% protein, 55–61% carbohydrate, and 5.2–7.3% fat by weight. Control diets had a composition of 13–16% kcal from fat and 3.45–3.60 kcal/g. Such studies began with experimentally naive male mice that were 8 wk of age [first glucose tolerance test (GTT)], and diets deviated at 9 wk of age. Animals were euthanized by CO2 asphyxiation and cardiac exsanguination, and blood was cleared by perfusion with phosphate-buffered saline. Animal use was approved by the University of North Dakota Institutional Animal Care and Use Committee and the Central Arkansas Veterans Healthcare System Animal Care and Use Committee. This study conforms to the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals.
Antibodies
Antibodies against full-length APP (ab32136), IDE (ab32216) were purchased from Abcam (Cambridge, MA) (10, 42). Antibodies p-AKT Thr308 (13038) and AKT (C67E7) were purchased from Cell Signaling Technology (Danvers, MA). The polyclonal antibody against IDE utilized for immunohistochemistry was purchased from Biolegend (product no. 840301, San Diego, CA) and was formerly available from Covance as product no. PRB-282C (87). Anti-GAPDH antibody (6C5) and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Technologies (Santa Cruz, CA). Antibodies used in WES capillary Western blots included β-tubulin (2146S, Cell Signaling), insulin receptor (3025S, Cell Signaling), and p-IGF1R-IRβ (3024S, Cell Signaling).
RNA Extraction and RT-qPCR
Total RNA from hippocampus tissue from App+/+ and App−/−mice was isolated using TRIzol Reagent (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Briefly, the tissue samples preserved in Allprotect Tissue Reagent (Qiagen, Valencia, CA) were washed once with PBS and homogenized in TRIzol using a Bullet Blender Storm 24 tissue homogenizer (Next Advance, Averill Park, NY) with 5-mm stainless steel beads (Qiagen). Extracted RNA samples were quantified using a NanoDrop spectrophotometer (ThermoFisher Scientific). For each sample, cDNA was generated from 1 µg of total RNA using iScript Reverse Transcription Supermix (Bio-Rad Laboratories, Hercules, CA) per the manufacturer’s specifications. A previously described primer set for Ide (forward: 5′-CTGTGCCCCTTGTTTGATGC-3′; reverse: 5′-GTTCCCCGTAGCCTTTTCCA-3′) (42) was purchased from Millipore Sigma (St. Louis, MO). qPCR was performed in triplicate using iTaq Universal SYBR Green Supermix and the CFX96 Touch Real-Time PCR Detection System with CFX Manager 3.0 software (Bio-Rad). Ribosomal protein 18S (PrimePCR SYBR Green Assay Rsp18, Bio-Rad) was used as a reference gene, and Rsp18 Cq values were used to normalize respective Ide Cq values (ΔCq). Relative Ide mRNA expression was determined as 2−ΔΔCq and is shown as means ± SE for each mouse strain.
Cell Lines and Cell Culture
Microglia cell line.
The SIM-A9 mouse microglial cell line was previously developed and characterized in our laboratory (59) and is available from the American Type Culture Collection (ATCC CRL-3265). The cells were grown in DMEM/F12 (product no. 12400024, ThermoFisher Scientific) supplemented with 10% FBS and penicillin, neomycin, and streptomycin.
Mouse neuron primary culture.
Neurons were cultured from App+/+and App−/− brains on embryonic day 16 (4, 18, 35, 66). Embryos were harvested and brains were placed into sterile dissection media (0.5 mM EDTA, 100 µM EGTA, 5.5 mM glucose, in PBS). Meninges were removed, and cortices were harvested from both hemispheres, minced in dissection media, and treated with 0.25% trypsin. Tissue was digested for 20 min at 37°C. Digestion was terminated by adding 10 ml DMEM/F12 containing 10% FBS. The cells were then allowed to settle in the bottom of the pipette tip and then added to 10 ml of Neurobasal media supplemented with B27 and l-glutamine, penicillin, streptomycin, and neomycin, then triturated ~20 times. Cells were cultured in six-well plates coated overnight with poly-l-lysine. Neuron cultures were grown at 37°C in 5% CO2 (53).
Mouse primary microglia and astrocyte culture.
Microglia and astrocyte cultures were established from 1-day-old App+/+ and App−/− neonatal pups as previously described (53). Meninges were removed from cerebrum, and cortical tissue was harvested in dissection media (0.5 mM EDTA, 100 µM EGTA, 5.5 mM glucose, in PBS) on ice. Cortices were then digested in trypsin for 15 min at 37°C. Digestion was terminated using DMEM/F12 media containing 10% FBS. Cortical tissue from each mouse was cultured separately in a T75 flask with 20 ml of DMEM/F12 supplemented with 10% FBS, penicillin, streptomycin, and neomycin. Media was supplemented on day 2 and replaced after 1 wk. After 2 wk of culture, microglia were separated from astrocytes by shaking flasks at 200 rpm for 45–60 min. The microglial suspension was collected and plated for microglial culture. Astrocytes adherent to the flasks were removed with trypsin and cultured on six-well plates for experiments.
GTT and Insulin Tolerance Test
Beginning at 7 wk of age, blood was collected from male mice and blood glucose ([Glc]b) was measured using a glucometer (AlphaTRAK II, Abbott Laboratories). For the GTT, mice were fasted 4 h (with access to water); [Glc]b was determined before intraperitoneal injection of Glc (2 mg/g) and at 15, 30, 60, and 120 min thereafter. Some GTT results are expressed as incremental area under the curve; the difference between initial [Glc]b and subsequent [Glc]b values (TN – T0) for each mouse was used for this calculation to remove differences in basal [Glc]b. For the insulin tolerance test (ITT), [Glc]b was determined in nonfasted mice before intraperitoneal injection of insulin (0.75 U/g) and at 15, 30, 60, and 120 min thereafter. GTT and ITT were performed on the same day of the week in alternating weeks. Some ITT results are expressed as incremental area over the curve; the difference between initial [Glc]b and subsequent [Glc]b values (T0 – TN) for each mouse was used for this calculation to remove differences in basal [Glc]b. For experiments in aged animals, mice were fasted 5 h (with access to water); [Glc]b was determined before intraperitoneal injection of Glc (2 mg/g) and at 15, 30, 60, and 120 min thereafter.
Insulin ELISA
Blood (25–30 µl) was collected at the 0- and 30-min time points during the GTT performed at week 6 of the Western diet feeding and centrifuged for 10 min at 2,000 g and 4°C to extract serum. Insulin concentrations were measured from 10-µl aliquots of serum using an Ultra-Sensitive Mouse Insulin ELISA Kit (Crystal Chem) according to the manufacturer’s protocol.
RNA Interference Knockdown of APP
RNA interference (RNAi)-mediated knockdown of the mouse APP gene in SIM-A9 microglia was achieved using Dharmacon (Lafayette, CO) Accell SMARTpool small interference RNA (siRNA; product no. E-043246-00-0050), with nontargeting RNA (product no. D-001910-01-50)-treated cells used as controls. siRNA was utilized by combining the Accell siRNA with Accell delivery media per the manufacturer’s instructions, and cells were treated with 1 µM RNA for 6 days. After 3 days of treatment (72 h), siRNA-containing media was supplemented 1:1 with normal DMEM/F12 with 10% FBS. SIM-A9 cell protein was harvested on day 7 for Western blot analyses.
Western Blot Analyses
Hippocampus, gastrocnemius, liver tissues, and primary cell cultures were lysed in RIPA buffer containing protease inhibitor (P8340, Sigma, St. Louis, MO) on ice. Protein concentrations were determined using the Bradford method (3). Five to ten micrograms of protein was resolved by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h in Tris-buffered saline containing Tween 20 (TBST) and 5% BSA solution, and then they were incubated overnight in 5% BSA-TBST solution containing the primary antibody. The next day, primary antibodies were washed off the membrane with TBST, and HRP-conjugated secondary antibodies were applied to membranes in 5% BSA-TBST for 2 h. Luminol chemiluminescence was utilized for visualization of proteins with the Aplegen Omega Lum G Gel Documentation System (Gel, San Francisco, CA). Western blot analysis was performed using Adobe Photoshop CS6 (Adobe Systems, San Jose, CA). To quantify Western blot bands (i.e., IDE), a box was traced around the protein band of interest. The mean pixel intensity of the area of the box was then measured using the histogram. The box was then moved to additional bands until all bands of interest were quantified. The measured values of bands of interest were then normalized to the mean intensity of the loading control protein for each individual sample. Finally, all normalized protein values were divided by the mean of the App+/+ condition to obtain a mean of 1.0 for App+/+ samples.
IDE Activity Assays
IDE activity assays were purchased from AnaSpec (catalog no. AS-72231, Fremont, CA). The assay utilizes a FRET substrate which emits increased fluorescence when cleaved by IDE. This increased fluorescence is due to liberation of 5-carboxyfluorescein (5-FAM) from a quenching molecule also on the IDE substrate. Hippocampus, liver, and gastrocnemius were collected on ice from App+/+ and App−/− mice. Our preliminary experiments utilizing this assay suggested that IDE enzyme activity is negatively affected by sonication. Tissue was homogenized on ice in assay buffer using a Powermasher II (Nippi, Japan). Fifty microliters of individual brain homogenate was loaded into a well of a 96-well plate containing 50 µl of substrate solution. The fluorescence intensity was measured on a Biotek Plate reader using an excitation of 485 nm and emission of 528 nm in 5-min increments. Fluorescent units measured were converted to concentrations of 5-FAM. The protein concentration of each tissue homogenate was determined using the Bradford method, and 5-FAM concentrations were normalized to the total protein in each sample. Total IDE activity in each sample was calculated using the formula ([final concentration] − [initial concentration]/time × volume) × dilution factor described by Kurauti et al. (42) and normalized as described above.
Immunohistochemistry
Left hemispheres of mouse brains were fixed in 4% paraformaldehyde/PBS for 48 h and embedded in a 15% gelatin matrix as described previously (60). The brains were cryosectioned into 40-µm serial sections by a sliding microtome (Leica SM 2000R, Leica Biosystems, Buffalo Grove, IL). The free-floating sections were incubated in the anti-IDE antibody diluted 1:200 overnight at 4°C in PBS containing 1% Triton X-100, 3% BSA, and 2% horse serum. Visualization of the antigen was carried out using a VECTASTAIN Elite ABC-HRP Kit with VIP as the chromogen (Vector Laboratories, Burlingame, CA). The brain sections were mounted onto subbed glass slides and dehydrated through an ethanol gradient before coverslipping using Permount mounting medium (ThermoFisher).
APP Plasmids and Transfections
pCAX APP 751 and pCAX APP 695 were a gift from Drs. Dennis Selkoe and Tracy Young-Pearse and are available from Addgene as plasmids 30138 and 30137 (88). Plasmids were prepared using a Qiagen Endofree Plasmid Giga Kit (product no. 12391). Primary astrocyte cultures were transfected using Lipofectamine 3000 (ThermoFisher Scientific) as per the manufacturer’s instructions.
APP Peptides
Bacterial recombinant sAPPα695 was purchased from Sigma-Aldrich (product no. S9564). Other sAPP peptides utilized in this study were expressed in an eukaryotic system. HEK293 cells stably transfected with a vector encoding human APP7511–668, APP6951–612, were grown to subconfluence in T175 flasks and changed to a serum-free medium supplemented with 0.5 mM l-glutamine, 50 µM ethanolamine, and 10 nM sodium selenite. After 4 days, the conditioned medium was loaded onto a 15-ml DE52 column to concentrate the acidic proteins. The column was eluted with phosphate buffer (pH 7.4) containing 0.75 M NaCl, 0.5 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (PMSF); 1-ml fractions were collected. The fractions containing sAPPα (determined by immunodetection on dot blots) were pooled and dialyzed 1 h at 4°C vs. 2 liters of PBS (pH 7.4) containing 1 mM EDTA, 1 mM PMSF; the dialysate was loaded onto a 5-ml Supelco Hi-Trap heparin column (GE Healthcare). The heparin column was eluted with an FPLC pump-controlled salt gradient, starting with 20 mM phosphate buffer (pH 7.4) containing 150 mM NaCl, 1 mM EDTA, and 1 mM PMSF and finishing with the same buffer containing 1 M NaCl; 0.5-ml fractions were collected. The sAPPα-containing fractions were pooled and dialyzed 1 h at 4·C vs. 2 liters of 20 mM Tris∙HCl, pH 8, containing 20 mM NaCl, 1 mM EDTA, and 1 mM PMSF; the dialysate was loaded onto a Bio-Scale Q5 anion-exchange column (Bio-Rad). The Q5 column was eluted with an FPLC pump-controlled salt gradient, starting with a 20 mM Tris∙HCl buffer (pH 8) containing 20 mM NaCl, 1 mM EDTA, and 1 mM PMSF and finishing with the same buffer containing 1 M NaCl; 0.5-ml fractions were collected. The sAPPα-containing fractions were further analyzed by SDS-PAGE with silver staining.
Recombinant bacterial-produced APP fragments were applied at a concentration of 20 nM in serum-free DMEM/F12, while eukaryotic-produced fragments were applied at a concentration of 200 nM. Fragments were applied for a total of 72 h and refreshed at 24 and 48 h.
Secretase Inhibitor Treatment
Secretase inhibitors were applied to SIM-A9 cells in DMEM/F12 supplemented with 1% FBS, penicillin, streptomycin, and neomycin for 1 wk at a concentration of 1 µM. Fresh inhibitors and media were applied daily to the cells. Neither overt toxicity nor increased LDH activity in cell culture media was observed. LDH activity assays were purchased from Cayman Chemical (product no. 601170) and were performed per the manufacturer’s instructions. The α-secretase inhibitor GI254023X was purchased from Sigma-Aldrich. The recently described β-secretase inhibitor Verubescestat was purchased from Selleckchem (Houston, TX). The widely used γ-secretase inhibitor DAPT was purchased from Sigma-Aldrich. The dose of 1 µM is well above the IC50 value of Verubescestat, reported at 3.4 nM for murine BACE1. The reported IC50 for DAPT is 0.02 µM. The reported IC50 for GI254023X inhibition of ADAM10 is 5.3 nM (14, 31, 37, 49).
Hippocampus Synaptosome Preparation and Stimulation
Synaptosomal insulin responsiveness was evaluated by ex vivo stimulation of isolated synaptosomal preparations as previously described (20). Briefly, frozen App+/+ and App−/− mouse hippocampi were homogenized using SynPER reagent (ThermoScientific) with 1% protease inhibitor cocktail and phosphatase inhibitor cocktail, and homogenates were centrifuged at 1,230 g for 10 min at 4·C. The supernatant was collected and centrifuged once more at 15,000 g for 20 min at 4°C. The pellet was resuspended in a physiological buffer, HEPES-buffered Krebs-like (HBK) buffer (143 mM NaCl, 4.7 mM KCl, 1.3 mM MgSO4, 1.2 mM CaCl2, 20 mM HEPES, 0.1 mM NaH2PO4, and 10 mM d-glucose, pH 7.4), and aliquoted into tubes of equal protein for unstimulated and insulin-stimulated samples. All tubes received 8 mM ATP, and insulin stimulation was performed with 10 or 200 nM of diluted U-100 insulin. All tubes were incubated at 37°C for 15 min. Samples were pelleted at 10,000 g for 10 min at 4°C and resuspended in RIPA buffer (75 mM NaCl, 25 mM Na2PO4, 1 mM EDTA, 0.5% NP-40, and 0.5% Triton X-100) plus 1% protease inhibitor cocktail and phosphatase inhibitor cocktail. The bicinchoninic acid assay method was used to prepare samples of equal protein concentration for WES capillary Western blot technology (ProteinSimple, San Jose, CA). Data are reported as area under the peak for the specified proteins.
Statistical Analysis
Statistical analysis was performed using SigmaPlot 12.0 software. Values were averaged ± SE, and statistical significance was determined via Student’s t-test or one-way ANOVA as appropriate. In the case of statistical significance, the Tukey-Kramer post hoc test or Holm-Sidak multiple pairwise comparisons were used where applicable. The difference in the mean relative Ide mRNA expression values was statistically analyzed by performing an unpaired t-test with the Welch correction factor using GraphPad Prism 7.03 software (GraphPad Software, La Jolla, CA). P values <0.05 were considered statistically significant.
RESULTS
IDE Protein and mRNA Were Increased in App−/− Tissues
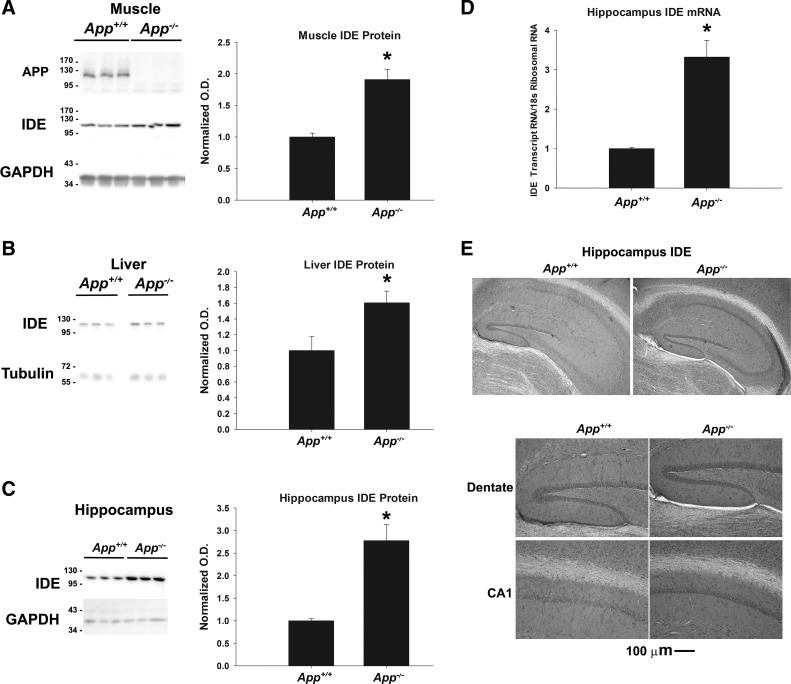
We began examining the potential role of APP in regulating IDE in skeletal muscle from two-month-old female App+/+ C57BL/6 and App−/− mice via Western blot analysis of homogenates. As expected, APP was not detectable in gastrocnemius tissue collected from App−/− mice but was detectable in App+/+ controls (Fig. 1A). Interestingly, IDE protein levels were found to be significantly higher in App−/− muscle (n = 6, P < 0.001) compared with the controls. Next, we examined liver tissue collected from App+/+ and App−/− animals (Fig. 1B). Similar to the skeletal muscle, we observed higher IDE protein levels in liver tissue extracts from App−/− mice compared with App+/+ controls (n = 7, P = 0.023). These data, along with our previous observations of elevated IDE protein in pancreatic extracts from App−/− mice (41), suggested that APP negatively regulates IDE levels in numerous peripheral tissues.
Fig. 1.
Insulin-degrading enzyme (IDE) was increased in App−/− tissues. A–C: IDE protein was measured in liver (n = 7), gastrocnemius (n = 6), and hippocampal tissue (n = 6–7) by Western blotting and normalized to GAPDH as a loading control. Normalized optical density is graphed as mean values ± SE. D: Ide mRNA levels were measured in hippocampus extracts (n = 4–5) and normalized to ribosomal 18S RNA as a loading control. Statistical significance (*P < 0.05) was determined by Student’s t-test. E: immunohistochemistry for IDE protein was performed on App+/+ and App−/− brain tissue sections (n = 4–6). Representative images at ×10 magnification are shown.
Due to the potential role of IDE in Aβ clearance, we next sought to examine whether ablation of the APP gene altered IDE protein levels in the brain. To identify which cell types may be most abundantly expressing IDE, we performed immunohistochemistry to detect IDE in App+/+ and App−/− brain tissue. IDE was diffusely detectable throughout the brain, including within the white matter. Although neurons within the hippocampus were stained to a greater extent, IDE immunostaining did not specifically label a single cell type in the CNS. This is in agreement with previous research demonstrating the expression of IDE in multiple cell types of the brain, including neurons, astrocytes, and microglia (see http://www.brainrnaseq.org/) (90). Because of this high expression, as well as the critical roles in normal memory and AD pathology played by the hippocampus, we assessed IDE protein levels in this region from App+/+ and App−/− brains by Western blotting (n = 6–7) (Fig. 1C). IDE protein levels were found to be almost threefold higher in App−/− hippocampus tissues compared with App+/+ controls (P < 0.001). This suggested that APP regulation of IDE extends to the CNS and is not limited to peripheral organs. We then examined mRNA levels to examine whether APP may be regulating IDE at the transcriptional or posttranslational levels. When relative abundance of Ide transcripts were determined using RT-qPCR (n = 4/5), we observed a significantly higher level of Ide mRNA in App−/− hippocampus tissue compared with App+/+ controls (P = 0.002) (Fig. 1D). Similar to protein levels of IDE, we observed a difference in Ide mRNA expression in App−/− hippocampus tissue of greater than threefold compared with App+/+ controls. Collectively, these data suggested that APP acts as a negative regulator of IDE levels and that ablation of APP increases IDE at the levels of transcription and protein. Furthermore, our immunohistochemistry suggests that IDE is produced throughout the brain in many cell types, and IDE expression is particularly increased in hippocampal neurons in App−/− mouse brain.
IDE Expression Was Dependent on APP Levels in Cultured Neurons, Astrocytes, and Microglial Cells
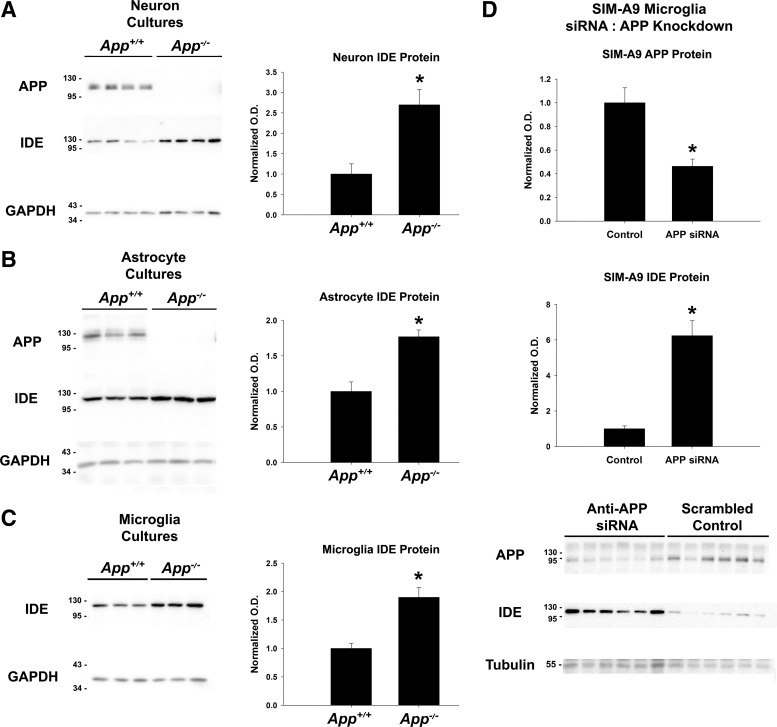
Following our observation that mRNA and protein levels for IDE were robustly increased in the hippocampus tissue of App−/− mice, we next sought to determine which cell type(s) found in the brain are responsible for the difference in IDE content. (Fig. 2A). As expected, APP was abundant in App+/+ cultures of cortical neurons and not detectable in App−/− cultures. Consistent with our observations in homogenized hippocampus, total IDE protein levels were significantly higher in App−/− neuronal cultures compared with App+/+ controls (n = 4, P < 0.010). We also established cultures of astrocytes and microglia from App+/+ and App−/− mice to determine whether APP regulates IDE protein in these glial cell types in a manner similar to neurons (n = 3/condition). The APP detected in cultures of astrocytes and microglia had lower mobility, predicted from the differential expression of KPI-containing splice variants in these various cell types. IDE protein levels were found to be significantly higher in both astrocyte (Fig. 2B; P = 0.009) and microglia (Fig. 2C, P = 0.009) cultures relative to App+/+ controls. These data suggested that APP regulates IDE in both neurons and glia and is not restricted to a specific isoform of APP expressed in limited cell types. Furthermore, this suggests that multiple cell types are responsible for increased total IDE levels found in App−/− hippocampus extracts.
Fig. 2.
IDE was increased in App−/− cell cultures. A–C: primary cell cultures of neurons (n = 4), astrocytes (n = 3), and microglia from App+/+, App−/− and the APP/PS1 mouse model of Alzheimer’s disease (AD) were grown and assessed for IDE protein content by Western blotting with GAPDH as a loading control. Significance (*P < 0.05) was determined by Student’s t-test. D: cultures of SIM-A9 mouse microglia cells (n = 6/condition) were treated with either scrambled RNA or anti-APP small interference (si) RNA for 1 wk. After treatment, total cellular IDE content was assessed by Western blotting with α-tubulin as a loading control. Significance (*P < 0.05) was determined by Student’s t-test. Normalized optical density is graphed as mean values ± SE.
Given that we observed increased IDE in App−/− microglia and that microglial IDE may be particularly important for degrading Aβ, we next tested the hypothesis that knockdown of APP with siRNA in the SIM-A9 microglial cell line will increase IDE levels in vitro (Fig. 2D). Knockdown of APP protein was performed over 1 wk with either an anti-App siRNA pool or a scrambled RNA control (n = 6/condition). As expected, treatment with anti-App siRNA significantly reduced APP as measured by Western blotting (P = 0.003). IDE protein levels were robustly and significantly increased in microglia (P < 0.001) treated with anti-App siRNA, suggesting that knockdown of APP in vitro is sufficient to increase IDE protein levels in cultured cells. This also suggests that APP has a direct role in regulating IDE and that the increased levels of IDE observed in the App−/− mice is not a consequence of overall differences in the physiology of the animals.
IDE Activity Was Increased in App−/− Tissues
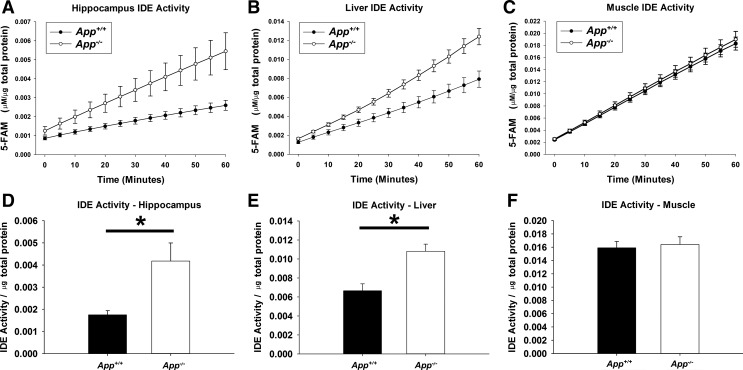
To confirm a potential impact on substrates of the differential levels of IDE protein, we also compared the enzymatic activity of IDE in App+/+ and App−/− mouse tissues. To measure IDE activity, we utilized a commercially available IDE activity assay. This assay uses a FRET peptide IDE substrate that emits increased 5-FAM fluorescence when hydrolyzed by the IDE enzyme. Hippocampus, gastrocnemius, and liver tissue was collected from 2-mo-old App+/+ and App−/− mice (n = 6/condition) and homogenized. Each tissue homogenate was then mixed with the IDE substrate, and fluorescence was measured over time (Fig. 3). The IDE activity of each tissue extract was calculated and normalized to the total protein content of the sample. Significantly higher IDE activity was observed in App−/− hippocampus (P = 0.016) and liver extracts (P = 0.003), indicating that the higher IDE protein likely leads to differences in the stability of IDE substrates in these tissues. Unlike hippocampus and liver tissue, no significant differences in IDE activity were observed between App+/+ and App−/− gastrocnemius tissue extracts (P = 0.746).
Fig. 3.
IDE activity was increased in App−/− tissues. App+/+ and App−/− hippocampus (A), liver (B), and skeletal muscle (C) (n = 6/tissue) were harvested on ice and rapidly assayed for IDE activity over the course of 1 h. The IDE activity assay uses a FRET peptide which is liberated from a quencher and exhibits increased fluorescence when cleaved by IDE. Relative fluorescent units are converted to concentrations of carboxyfluorescein (FAM) via standard curve. The data were normalized to the amount of total protein collected from each tissue. IDE activity was calculated from hippocampus (D), liver (E), and skeletal muscle (F), and significance (*P < 0.05) was determined by Student’s t-test. Data are graphed as means ± SE.
Insulin Levels and Insulin Signaling Were Altered in App−/− Hippocampus Tissue and Synaptosomes
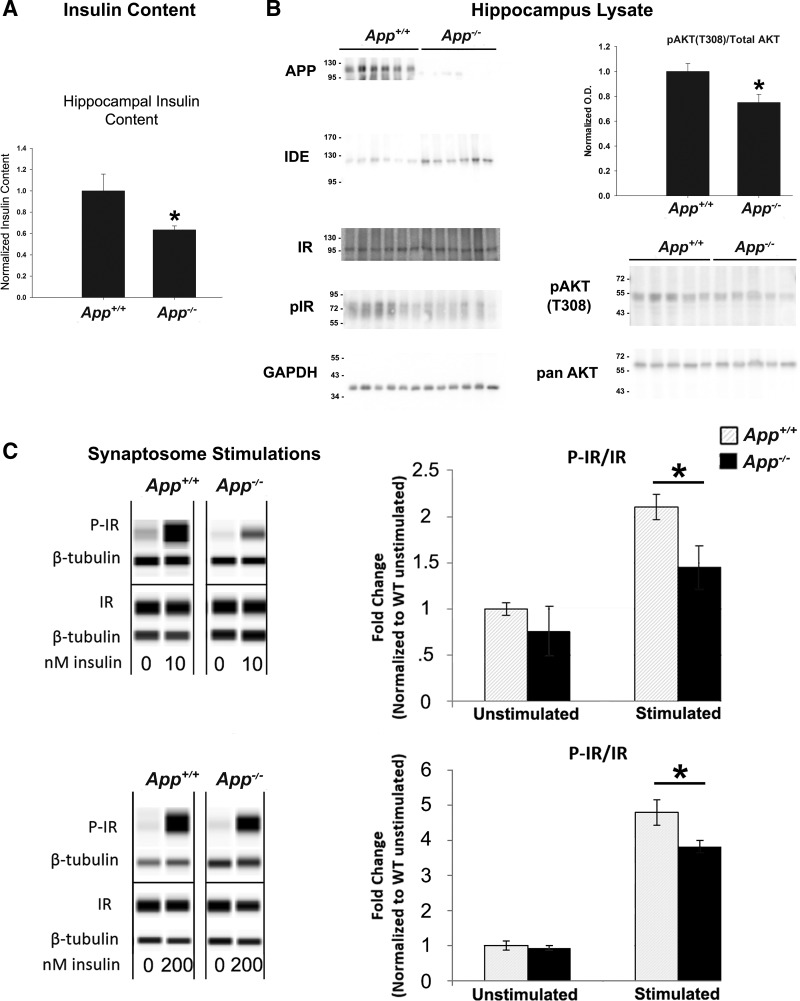
We next sought to probe for consequences of increased IDE protein and enzyme activity on insulin levels and insulin-signaling markers in hippocampus tissue. We measured the insulin levels of 2-mo-old App+/+ and App−/− hippocampus extracts by ELISA (n = 11/13). Hippocampus extracts from App−/− mice had significantly lower hippocampal insulin levels compared with App+/+ animals (P = 0.032), suggesting that total insulin levels in the brain are reduced in these animals (Fig. 4A). We next examined hippocampus extracts from App+/+ and App−/− mice for changes in insulin-signaling markers. While we were unable to clearly detect phosphorylated insulin receptor in these lysates (Fig. 4B), we also examined the T308 residue of AKT, as it is well established to be phosphorylated in response to insulin signaling (1). We observed significantly lower (P = 0.025) levels of pAKT T308 in App−/− hippocampal lysates compared with App+/+ controls, suggesting that App−/− animals have diminished insulin signaling in brain tissue.
Fig. 4.
Insulin signaling and total insulin levels were altered in the App−/− brain. A: total hippocampal insulin levels were measured in RIPA extractions of App+/+ and App−/− hippocampus tissue by ELISA (n = 13). B: insulin-signaling cascade markers were assessed by Western blotting in 2-mo-old App+/+ and App−/− hippocampal tissue (n = 5–6/condition). C: hippocampal synaptosomes from 2-mo-old App+/+ and App−/− animals were prepared and stimulated with 10 (n = 5/5) or 200 nM insulin (n = 6/4). Phosphorylated insulin receptor (pIR), total IR, and β-tubulin protein levels were measured using the WES system. Data were collected using the area under the peak for the specified proteins, and analysis was performed using a Student’s t-test with significance (*P < 0.05). Data are graphed as means ± SE.
To further examine insulin signaling in the brains of these animals, we prepared synaptosomes from App+/+ and App−/− brain tissues and stimulated them acutely with insulin ex vivo. Stimulated synaptosome lysates were then analyzed for protein and phosphorylated protein content by WES capillary Western blotting. We have previously utilized this method to examine insulin signaling in the brains of other transgenic mice (72), and the details of this method have been expanded upon in recent literature (20). App+/+ and App−/− synaptosomes were stimulated with either 10 or 200 nM insulin (n = 4–6/condition). Interestingly, App−/− synaptosomes showed diminished phosphorylation of the insulin receptor compared with App+/+ synaptosomes when stimulated with either 10 (P = 0.0315) or 200 nM (P = 0.0265) insulin. (Fig. 4C). No differences in the ratio of phosphorylated insulin receptor to total insulin receptor were observed in the unstimulated synaptosomes. These data suggest that App−/− animals have impaired insulin signaling in the brain at the level of the insulin receptor.
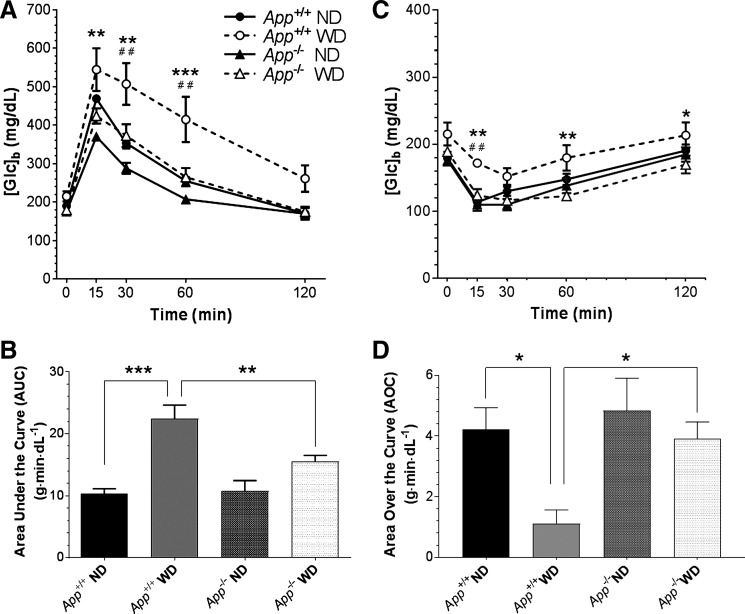
App−/− Mice Had Low Basal Levels of Circulating Insulin and Were Protected from Impairment of Glucose Tolerance by a Western Diet
While our data suggest App−/− animals have changes in brain insulin signaling, their higher levels of IDE in peripheral tissues suggested they might exhibit somatic effects as well. Paradigms of diet-induced obesity have been documented to elevate markers of metabolic syndrome, such as impaired glucose tolerance secondary to insulin resistance. We fed App+/+ and App−/− littermates a Western diet (high fat and high sucrose) for 13 wk. Consistent with previous studies, App+/+ mice on the Western diet showed impaired glucose tolerance in GTTs. At week 0 (7 wk of age), before the introduction of the Western diet, App+/+ and App−/− mice showed nearly identical responses to glucose challenge (not shown). By week 8 (7 wk of diet manipulation; 15 wk of age), App+/+ mice showed blood glucose concentrations ([Glc]b) that were higher on the Western diet compared with normal diet controls (Fig. 5A). Conversely, the [Glc]b in App−/− mice fed the Western diet was not different from normal diet controls. To ascertain that the differences in GTT involved differences in glucose excursion, independent of differences in initial [Glc]b, the GTT profiles were integrated as area under the curve using at each time point [Glc]b values from which the initial [Glc]b had been subtracted individually for each animal. The mean of these values over weeks 14–20 (13–19 wk on diet) showed an overall impairment of glucose tolerance in App+/+ mice fed the Western diet, but App−/− mice failed to reach this state on either diet (Fig. 5B). In addition, the Western diet raised fasting [Glc]b in App+/+ but not App−/− mice.
Fig. 5.
App−/− mice maintain glucose tolerance on a Western diet (WD). App+/+ or App−/− mice were maintained on a normal diet (ND) or WD. A: glucose tolerance test (GTT) at 7 wk on diet (**P < 0.01, ***P < 0.001, App+/+ WD vs. App−/− WD at the indicated time points; ##P < 0.01, App+/+ WD vs. App+/+ ND). B: mean of GTTs performed during weeks 13–19 on diet, expressed as area under the curve, zeroed to the initial [GTT]b for each mouse (***P < 0.001, **P < 0.01). C: [Glc]b in response to insulin at 8 wk on diet (*P < 0.05, **P < 0.01, App+/+ WD vs. App−/− WD at the indicated time points; ##P < 0.01, App+/+ WD vs. App+/+ ND). D: mean of insulin tolerance tests (ITTs) performed during weeks 12–18 on diet, expressed as area over the curve, capped at the initial [Glc]b for each mouse (*P < 0.05).
An ITT was used to evaluate insulin sensitivity in weeks alternating with the GTT. At week 1 (7 wk of age; before dietary manipulation), App+/+ and App−/− mice had similar insulin sensitivity, with App−/− mice trending toward a more robust response (data not shown). By week 9 (16 wk of age; 8 wk on the Western diet), there was a significant difference in the Western diet-fed mice as a function of genotype (Fig. 5C). To ascertain that the difference between App+/+ and App−/− mice reflected differences in insulin resistance and was independent of differences in initial [Glc]b, the area of the deflection from the initial [Glc]b over time was integrated. In contrast to the GTT calculations, ITTs were integrated as area over the curve. For this calculation, the [Glc]b at each time point was subtracted from the initial [Glc]b, in effect setting the ceiling for the curve at the initial [Glc]b for each animal. The mean of these values over weeks 13–19 (12–18 wk on diet) showed insulin resistance in App+/+ mice fed the Western diet, but App−/− mice were protected from this outcome (Fig. 5D).
The above results indicate that App−/− mice retained greater insulin sensitivity than their App+/+ counterparts on a Western diet. We measured blood insulin levels in a fasted state and 30 min after a glucose injection. While the App+/+ mice fed the Western diet clearly exhibited hyperinsulinemia, App−/− mice maintained normal basal insulin levels on this diet (Table 1). In fact, App−/− mice on normal and Western diets had lower fasting insulin levels than their App+/+ counterparts, potentially implicating a role for elevated IDE in regulating circulating insulin levels. Due to the low basal levels of insulin observed in the fasting state of App−/− mice, the change in insulin levels in response to glucose was much greater in these animals.
Table 1.
Insulin levels at 6 wk on a Western diet
| Mouse Group |
[Insulin], ng/ml |
|||
|---|---|---|---|---|
| Genotype | Dieta | T0 | T30b | %Δc |
| App+/+ | ND | 0.355 ± 0.0823 | 0.680 ± 0.0930 | 111.7 ± 78.04 |
| WD | 1.990 ± 0.107 | 2.077 ± 0.0947 | 7.07 ± 33.34 | |
| App−/− | ND | 0.111 ± 0.0354 | 0.466 ± 0.0386†† | 699.7 ± 292.0* |
| WD | 0.289 ± 0.0567* | 0.627 ± 0.0591† | 160.0 ± 55.18 | |
Values are means ± SE.
ND, normal diet; WD, Western diet;
30 min after challenge with glucose (2 g/kg body wt);
group mean of the percent changes calculated for each mouse.
P ≤ 0.05,
P ≤ 0.01 vs. T0.
P ≤ 0.05 vs. App+/+ counterpart.
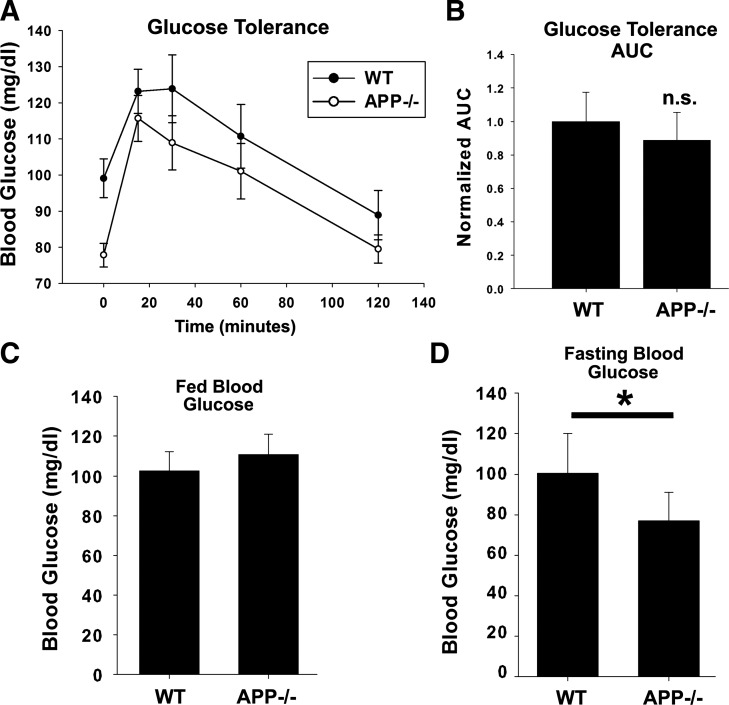
Aged App−/− Mice Have Normal Glucose Tolerance but Are Hypoglycemic When Fasted
Aging is a well-established risk factor for both diabetes and Alzheimer’s disease (36, 65). While, as stated above, we found no differences in glucose tolerance in young App−/− animals on a normal chow diet compared with App+/+ controls, we also tested 12-mo-old cohorts of both App+/+ and App−/− mice (n = 11/13) on normal chow to examine the potential consequences that may result from the increased levels of IDE while aging (Fig. 6). Animals were fasted for 5 h on the day of the experiment and then administered 2 g/kg body weight glucose by intraperitoneal injection. Blood glucose was monitored just before injection and at 15, 30, 60, and 120 min after injection. Data were graphed as blood glucose vs. time, and the area under the curve was assessed. No significant difference was observed in overall glucose tolerance (P = 0.12) between App+/+ and App−/− mice. However, we did observe a robust and significant reduction in the fasting blood glucose levels of App−/− mice (P = 0.002) compared with App+/+ controls. Blood glucose was also measured in free fed App+/+ and App−/− mice (n = 5/condition) and was not significantly different (P = 0.221). These data suggest aged App−/− mice on normal chow remain glucose tolerant but are hypoglycemic when fasted, potentially indicating that increased IDE levels at older ages may have consequences on normal glucose homeostasis.
Fig. 6.
Aged App−/− animals are hypoglycemic when fasted. A: twelve-month-old App+/+ and App−/− mice (n = 11–13) were fasted and subjected to GTT with intraperitoneal injections of glucose. B: area under the curve (AUC) was measured for each animal’s blood glucose over time, and App+/+ and App−/− animals were compared by Student’s t-test. Blood glucose was measured and graphed in free fed (C; n = 5) and fasted (D; n = 11–13) App+/+ and App−/− mice. Significance (*P < 0.05) was determined by Student’s t-test. Data are graphed as means ± SE.
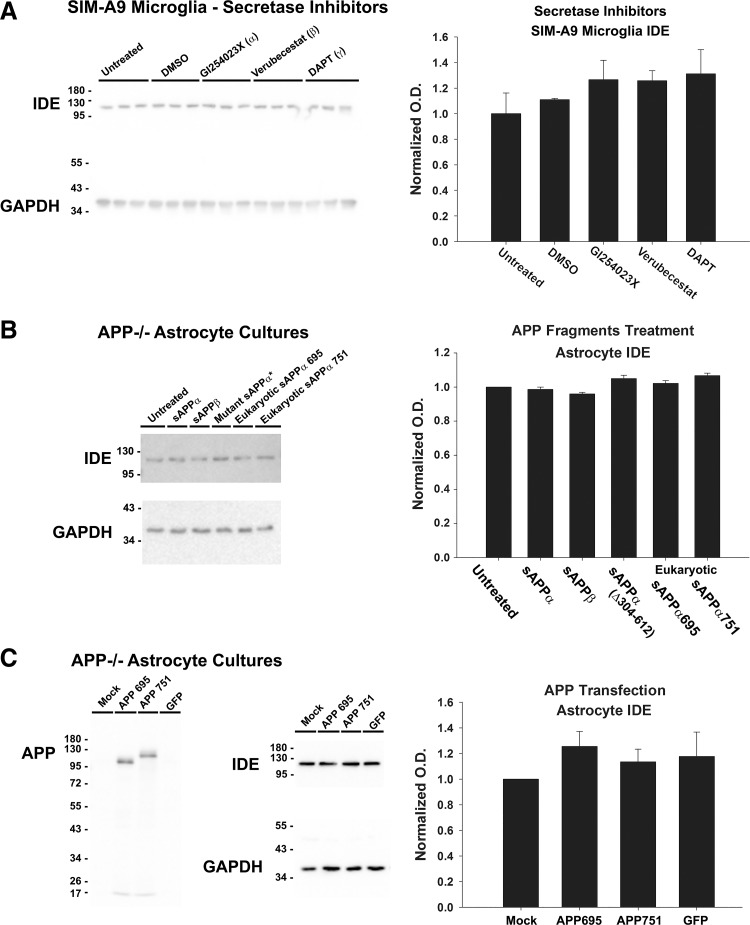
Overexpression of APP or Treatment with Secretase Inhibitors Did Not Alter IDE Levels
APP is cleaved by a variety of enzymes termed secretases, which result in the production of various APP fragments. To examine whether IDE regulation occurs in an APP fragment-specific manner and whether selective inhibition of secretase isotypes differentially alters levels of IDE, SIM-A9 microglia cells were treated for 7 days in DMEM with 1% serum in the presence or absence of GI254023X (α-secretase inhibitor), Verubecestat (β-secretase inhibitor), or DAPT (the γ-secretase inhibitor) (Fig. 7A). SIM-A9 cells were chosen for this experiment as they are amenable to culturing in low-serum conditions and had shown an increase in IDE protein levels when APP was knocked down with siRNA (Fig. 2D). No overt toxicity or change in LDH activity in the media was observed from this treatment (data not shown), indicating the secretase inhibitors did not cause robust cell death. No significant difference was found in the IDE protein content of SIM-A9 cells treated with inhibitors compared with untreated or vehicle controls (n = 3/treatment, P = 0.45) (Fig. 7A).
Fig. 7.
APP fragments, APP overexpression, and secretase inhibitors did not alter IDE in App−/− cells. A: SIM-A9 microglia (n = 3/condition) were treated with secretase inhibitors, DMSO vehicle, or left untreated for 1 wk, and IDE protein content was measured. Significance (P < 0.05) was determined by 1-way ANOVA. B: App−/− primary astrocyte cultures (n = 4/condition) were treated with bacterial recombinant sAPPα, sAPPβ, mutant N-terminal sAPPα, or eukaryotic-derived sAPPα695 and 751. IDE protein was measured and significance (P < 0.05) was determined by 1-way ANOVA. C: App−/− astrocyte primary cultures (n = 6/condition) were transfected with APP695, APP751, or green fluorescent protein (GFP) plasmids or mock transfected. IDE protein was measured and significance (P < 0.05) was determined by 1-way ANOVA. Data are graphed as means ± SE.
Next, to further examine whether a specific APP metabolite may be capable of changing IDE levels, we tested whether recombinant sAPP fragments would reduce protein levels of IDE in App−/− astrocyte primary cultures. sAPP is thought to exert effects on cells by inducing signaling through a variety of receptors (25). Cells were treated for 3 days with 20 nM prokaryotically expressed recombinant sAPPα, sAPPβ, or mutant sAPPα lacking N-terminal amino acids 304–612; eukaryotically expressed sAPPα695 or sAPPα751 were also tested at 200 nM. These sAPP fragments did not alter IDE levels in cultures, indicating that the full-length APP protein or another APP fragment is required for differences in IDE levels observed in App−/− tissues (Fig. 7B).
Finally, we tested whether overexpression of the APP695 isoform or the APP751 isoform in App−/− astrocytes was sufficient to reduce levels of IDE. Primary astrocyte cultures were either mock transfected or transfected with plasmids coding for APP695, APP751, or green fluorescent protein. Cellular protein was collected after 5 days, and IDE was measured by Western blotting. Surprisingly, overexpression of APP in App−/− astrocytes did not significantly change IDE levels (P = 0.56) (Fig. 7C).
DISCUSSION
Glucose hypometabolism in the brain is observed in advanced AD, in animal models of AD, and in Down syndrome patients (44, 64, 67, 86). AD patients exhibit type 2 diabetes or impaired glucose tolerance at twice the rate of age-matched controls (85). A plethora of evidence shows an association between type 2 diabetes and cognitive decline, but recent studies have challenged the hypothesis that AD is promoted by type 2 diabetes, as the latter is better correlated with vascular dementia (33, 34, 40, 54). Moreover, diabetes has not been found to exacerbate Aβ deposition in all studies (68, 75). The converse, however, may be true, as indicated by the perturbation of peripheral insulin/glucose regulation in AD models (51, 52, 61, 63). Most of these models involve overexpression of APP, and evidence suggests the precursor participates in metabolic homeostasis. For example, App−/− knockout mice, in addition to having lower body weight, show reduced weight gain compared with App+/+ controls when placed on a high-fat diet (70). In muscle tissue, sAPPα potentiates glucose uptake (26). Our data demonstrate that ablation of the APP gene in mice or prolonged knockdown of APP in cell culture results in higher levels of IDE compared with App+/+ controls. Higher levels of IDE protein were observed in muscle, liver, and hippocampal tissue extracts from App−/− animals. In agreement with this, primary cell cultures of neurons, astrocytes, and microglia from App−/− mice all had elevated IDE levels compared with App+/+ cultures. Furthermore, Ide mRNA levels were significantly increased in hippocampal tissue from App−/− animals compared with controls, suggesting that increased IDE protein was due to increased transcription. The effect seen in vivo did not appear to result from indirect effects of developmental abnormalities because knockdown of APP in cultured cells was sufficient to acutely elevate protein levels of IDE.
We observed robust and significantly higher IDE activity in both hippocampus and liver extracts from App−/− animals compared with App+/+ controls. IDE activity was not statistically different in skeletal muscle tissue extracts despite increased total IDE proteins levels. This could be due to the nature of the activity assay, which uses cleavage of a FRET peptide as an indicator of IDE activity. Muscle tissue may contain other enzymes capable of cleaving the FRET substrate. Interestingly, muscle tissue lysates had additional, lower-molecular-weight IDE bands on Western blots compared with other tissues, suggesting there may be alternative forms of IDE in muscle or that the enzyme is subject to an unusually higher rate of proteolysis in this tissue.
Although the brain is traditionally viewed as an insulin-insensitive organ, recent literature demonstrates insulin and insulin signaling are involved in the normal physiology of the brain (17, 22, 39). Moreover, impaired insulin signaling may play a role in AD (74, 80). Indeed, intranasal insulin is under investigation as a therapeutic intervention for AD (7). We observed significantly lower hippocampal insulin levels in App−/− animals, suggesting that higher IDE activity is tantamount to greater insulin catabolism. This concept is in general agreement with previous research showing IDE−/− mice have impaired tissue insulin degradation (16).
Our data also indicate that increased IDE protein and activity alter brain insulin signaling. While we did not clearly detect phosphorylated insulin receptor in our hippocampus homogenates, possibly due to the transient nature of insulin receptor phosphorylation, we did observe modest but significantly reduced levels of pAKT(T308) in the App−/− hippocampus. This suggested that insulin signaling may be impaired in App−/− brain tissues, perhaps due to lower levels of insulin itself. However, we also observed diminished phosphorylation of the insulin receptor following acute stimulation of App−/− synaptosomes compared with App+/+ controls. This suggests that differences in signaling in App−/− brains may occur via receptor dysfunction as well.
To examine physiological consequences of increased tissue levels of IDE in App−/− mice, we assessed insulin in serum. Six weeks of a Western diet produced hyperinsulinemia in App+/+ mice but not App−/− mice. Pharmacological inhibition of IDE in vivo has been shown to alter insulin levels and impair glucose tolerance (13). Consistent with this finding, elevated IDE in the App−/− mice correlated with protection against insulin resistance and glucose intolerance. It is possible that suppression of circulating insulin permits the maintenance of greater insulin sensitivity in App−/− mice, which may manifest in the lower serum insulin levels observed in App−/− mice. Indeed, this in agreement with a recent report showing reduced pancreatic β cell mass in App−/− mice challenged with a high-fat diet (12). It should be noted that while levels of serum insulin were reduced in fasted App−/− mice, their pancreatic response to glucose showed a greater dynamic response, achieving levels similar to those in App+/+ mice. This exaggerated responsiveness appears to be consistent with the phenotype of isolated pancreatic islets in a previous report (82). In addition to impacts on insulin, IDE may alter peripheral glucose regulation through its degradation of glucagon (78). In agreement with our study, recent work by Czeczor et al. (12) observed lower plasma levels of insulin in fasted App−/− mice fed a high-fat diet for 14 wk. However, they observed that a high-fat diet caused glucose intolerance while insulin tolerance remained normal in the App−/− mice. The incongruity is perhaps due to the large difference in sucrose composition in the diets, which was 34% by mass in our Western diet study and 20% in the study by Czeczor et al.
It is interesting to note that while App−/− mice retained insulin sensitivity in the periphery when placed on a western diet, they had impaired insulin signaling in the brain. The reason for this is unclear. However, insulin sensitivity in tissues is affected by numerous factors, including phosphorylation status of the insulin receptor substrate 1 (IRS-1), downstream of the insulin receptor (11, 55). Surprisingly, hippocampal synaptosomes from App−/− animals demonstrated acute insulin resistance at the level of the insulin receptor suggesting a complex response to elevated IDE in the brain. Since the brain is exposed to much lower levels of insulin than peripheral organs it is not surprising that elevated IDE activity in the brain may have differential consequences on tissue insulin signaling compared with the periphery. Furthermore, it is known that the adult brain expresses a different isoform of the insulin receptor which has a greater affinity for both insulin and IGF-II compared with the insulin receptor predominantly found in peripheral tissues (2, 21, 24). This difference in central and peripheral insulin receptor isoforms may further account for the apparent differential effects of increased IDE in the brain compared with periphery.
To further examine the potential functional consequences of increased tissue levels of IDE in aging animals, we performed glucose tolerance testing in 12-mo-old male and female wild-type and App−/− animals. Pharmacological inhibition of the IDE in vivo has been shown to alter insulin levels and affect glucose tolerance (13). While we did not observe a significant difference in overall glucose tolerance between wild-type and App−/− animals, we were surprised to observe that when aged App−/− animals were fasted, they became hypoglycemic. This is in contrast to our prior analysis of glucose levels in 2-mo App−/− animals, which did not reveal this phenomenon despite increased levels of pancreatic IDE (41). This finding, however, is in general agreement with work by Needham et al. (62), which demonstrated lower plasma glucose in both App−/− and APLP2−/− mice. Other groups have shown that aged App−/− animals have impaired spatial learning with age (57, 69). It can be speculated that aging may reveal phenotypes present in App−/− mice not observed at earlier ages. Indeed, it has been shown that aged App−/− mice, but not young App−/− mice, show fewer dendritic spines and changes in spine morphology in addition to impaired LTP (45, 83). The compromised metabolic homeostasis we observed in aged animals may reveal the potentially deleterious consequences of increased IDE in tissues, despite normal overall glucose tolerance. IDE has been implicated in the degradation of the glucose-elevating hormone glucagon, which may account in part for the hypoglycemia we observed in fasting conditions (78).
The mechanism by which ablation of APP results in increased IDE transcription remains to be elucidated. The APP intracellular domain can interact with other proteins such as Fe65, Dab1, and the histone acetyltransferase Tip60 to transduce intracellular signals (6, 38). One of these accessory proteins is NEDD8, which activates ubiquitin-proteasome degradation of substrates in the Cullin pathways (73). However, little is known about degradation of IDE via the ubiquitin-proteasome system, and our analysis of mRNA suggests a pretranslational site of action. Treatment of the SIM-A9 microglia cell line with secretase inhibitors did not significantly change IDE levels, suggesting that APP processing is not necessary for the impact on IDE. Previous research has indicated that APP may be able to affect transcription independently of γ-secretase activity (28). Regardless, a reliance on holo-APP would not be inconsistent with schemes in which both the intact precursor and one of its fragments are involved. It has been suggested that sAPPα requires the APP holoprotein for its normal signaling functions (89). A recent report has shown that viral overexpression of sAPPα in the brain increases IDE and restores memory deficits in a mouse model for AD (19). Perhaps the increased IDE observed in the App−/− mice results from loss of normal sAPPα-APP signaling. In our study, in vitro treatment of App−/− astrocytes with recombinant sAPPα or sAPPβ did not alter IDE protein levels, nor did treatment of the SIM-A9 microglia cell line with α- or β-secretase inhibitors. It was also somewhat surprising that overexpression of human APP695 or APP751 was not sufficient to change IDE levels in App−/− astrocyte cultures. This may be due to abnormal processing or trafficking of the exogenous, overexpressed APP. Regardless, long-term siRNA knockdown of APP in vitro was sufficient to robustly change IDE levels, supporting our observations in the App−/− mice. It is also possible that changes in IDE occur indirectly from loss of APP instead of APP being directly involved in IDE transcription.
In conclusion, we present new evidence that APP suppresses the levels of IDE mRNA, protein, and enzymatic activity, both in the brain and in peripheral organs. These findings represent a new link connecting APP to metabolic homeostasis and expand on the already substantial connections between AD and metabolism. Furthermore, these findings may have relevance for situations, empirical or otherwise, in which holo-APP is overexpressed, wherein greater restriction of IDE levels may alter Aβ accumulation.
GRANTS
S.W.B. and R.D.H. were supported by NIH Grant P01AG012411. J.A.K., G.D.M., K.L.P., and C.K.C. were supported by NIH Grants R01AGO48993 and RO1AGO42819 and NIH/NIGMS Grant P20GM113123.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.K, W.F.F, R.D.H, G.T., S.W.B., and C.K.C. conceived and designed research; J.A.K., W.F.F., N.A.S., G.D.M., K.L.P., and R.D.H. performed experiments; J.A.K., W.F.F., N.A.S., G.D.M., K.L.P., K.N.-C., R.D.H., G.T., S.W.B., and C.K.C. analyzed data; J.A.K., W.F.F., N.A.S., G.D.M., K.L.P., K.N.-C., R.D.H., G.T., S.W.B., and C.K.C. interpreted results of experiments; J.A.K., W.F.F., N.A.S., and R.D.H. prepared figures; J.A.K. drafted manuscript; J.A.K., W.F.F., N.A.S., G.D.M., K.L.P., K.N.-C., R.D.H., G.T., S.W.B., and C.K.C. edited and revised manuscript; J.A.K. and C.K.C. approved final version of manuscript.
REFERENCES
- 1.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996. doi: 10.1002/j.1460-2075.1996.tb01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30: 586–623, 2009. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 35: 567–576, 1993. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 5.Bush AI, Pettingell WH Jr, de Paradis M, Tanzi RE, Wasco W. The amyloid beta-protein precursor and its mammalian homologues. Evidence for a zinc-modulated heparin-binding superfamily. J Biol Chem 269: 26618–26621, 1994. [PubMed] [Google Scholar]
- 6.Cao X, Südhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293: 115–120, 2001. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 7.Chapman CD, Schioth HB, Grillo CA, Benedict C. Intranasal insulin in Alzheimer’s disease: food for thought. Neuropharmacology 136: 196–201, 2018. doi: 10.1016/j.neuropharm.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Neve R, Zheng H, Griffin W, Barger S, Mrak R. Cycle on wheels: is APP key to the AppBp1 pathway? Austin Alzheimers Parkinsons Dis 1: id1008, 2014. [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke JR, Lyra E Silva NM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, Katashima CK, Razolli D, Carvalho BM, Frazão R, Silveira MA, Ribeiro FC, Bomfim TR, Neves FS, Klein WL, Medeiros R, LaFerla FM, Carvalheira JB, Saad MJ, Munoz DP, Velloso LA, Ferreira ST, De Felice FG. Alzheimer-associated Aβ oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Mol Med 7: 190–210, 2015. doi: 10.15252/emmm.201404183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colvin BA, Rogers VA, Kulas JA, Ridgway EA, Amtashar FS, Combs CK, Nichols MR. The conformational epitope for a new Aβ42 protofibril-selective antibody partially overlaps with the peptide N-terminal region. J Neurochem 143: 736–749, 2017. doi: 10.1111/jnc.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab 11: 84–92, 2010. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czeczor JK, Genders AJ, Aston-Mourney K, Connor T, Hall LG, Hasebe K, Ellis M, De Jong KA, Henstridge DC, Meikle PJ, Febbraio MA, Walder K, McGee SL. APP deficiency results in resistance to obesity but impairs glucose tolerance upon high fat feeding. J Endocrinol 237: 311–322, 2018. doi: 10.1530/JOE-18-0051. [DOI] [PubMed] [Google Scholar]
- 13.Deprez-Poulain R, Hennuyer N, Bosc D, Liang WG, Enée E, Marechal X, Charton J, Totobenazara J, Berte G, Jahklal J, Verdelet T, Dumont J, Dassonneville S, Woitrain E, Gauriot M, Paquet C, Duplan I, Hermant P, Cantrelle FX, Sevin E, Culot M, Landry V, Herledan A, Piveteau C, Lippens G, Leroux F, Tang WJ, van Endert P, Staels B, Deprez B. Catalytic site inhibition of insulin-degrading enzyme by a small molecule induces glucose intolerance in mice. Nat Commun 6: 8250, 2015. doi: 10.1038/ncomms9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem 76: 173–181, 2001. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 15.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 19: 608–624, 1998. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 16.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA 100: 4162–4167, 2003. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez AM, Hernandez-Garzón E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, Santi A, Trueba-Saiz A, García-Guerra L, Pose-Utrilla J, Fielitz J, Olson EN, Fernandez de la Rosa R, Garcia Garcia L, Pozo MA, Iglesias T, Araque A, Soya H, Perea G, Martin ED, Torres Aleman I. Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes 66: 64–74, 2017. doi: 10.2337/db16-0861. [DOI] [PubMed] [Google Scholar]
- 18.Floden AM, Li S, Combs CK. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J Neurosci 25: 2566–2575, 2005. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fol R, Braudeau J, Ludewig S, Abel T, Weyer SW, Roederer JP, Brod F, Audrain M, Bemelmans AP, Buchholz CJ, Korte M, Cartier N, Müller UC. Viral gene transfer of APPsα rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol 131: 247–266, 2016. doi: 10.1007/s00401-015-1498-9. [DOI] [PubMed] [Google Scholar]
- 20.Franklin W, Taglialatela G. A method to determine insulin responsiveness in synaptosomes isolated from frozen brain tissue. J Neurosci Methods 261: 128–134, 2016. doi: 10.1016/j.jneumeth.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 19: 3278–3288, 1999. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166: 867–880, 2016. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890, 1984. doi: 10.1016/S0006-291X(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 24.Gray SM, Barrett EJ. Insulin transport into the brain. Am J Physiol Cell Physiol 315: C125–C136, 2018. doi: 10.1152/ajpcell.00240.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habib A, Sawmiller D, Tan J. Restoring soluble amyloid precursor protein α functions as a potential treatment for Alzheimer’s disease. J Neurosci Res 95: 973–991, 2017. doi: 10.1002/jnr.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton DL, Findlay JA, Montagut G, Meakin PJ, Bestow D, Jalicy SM, Ashford ML. Altered amyloid precursor protein processing regulates glucose uptake and oxidation in cultured rodent myotubes. Diabetologia 57: 1684–1692, 2014. doi: 10.1007/s00125-014-3269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris RA, Tindale L, Lone A, Singh O, Macauley SL, Stanley M, Holtzman DM, Bartha R, Cumming RC. Aerobic glycolysis in the frontal cortex correlates with memory performance in wild-type mice but not the APP/PS1 mouse model of cerebral amyloidosis. J Neurosci 36: 1871–1878, 2016. doi: 10.1523/JNEUROSCI.3131-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hass MR, Yankner BA. A gamma-secretase-independent mechanism of signal transduction by the amyloid precursor protein. J Biol Chem 280: 36895–36904, 2005. doi: 10.1074/jbc.M502861200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassing LB, Johansson B, Nilsson SE, Berg S, Pedersen NL, Gatz M, McClearn G. Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer’s disease: a population-based study of the oldest old. Int Psychogeriatr 14: 239–248, 2002. doi: 10.1017/S104161020200844X. [DOI] [PubMed] [Google Scholar]
- 30.Heber S, Herms J, Gajic V, Hainfellner J, Aguzzi A, Rülicke T, Kretzschmar H, von Koch C, Sisodia S, Tremml P, Lipp HP, Wolfer DP, Müller U. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J Neurosci 20: 7951–7963, 2000. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoettecke N, Ludwig A, Foro S, Schmidt B. Improved synthesis of ADAM10 inhibitor GI254023X. Neurodegener Dis 7: 232–238, 2010. doi: 10.1159/000267865. [DOI] [PubMed] [Google Scholar]
- 32.Hoyer S. Abnormalities of glucose metabolism in Alzheimer’s disease. Ann N Y Acad Sci 640: 53–58, 1991. doi: 10.1111/j.1749-6632.1991.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 33.Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 102: 1186–1195, 2003. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen KT, Iverfeldt K. Amyloid precursor protein and its homologues: a family of proteolysis-dependent receptors. Cell Mol Life Sci 66: 2299–2318, 2009. doi: 10.1007/s00018-009-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J Neurochem 100: 1407–1420, 2007. doi: 10.1111/j.1471-4159.2006.04330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyani RR, Golden SH, Cefalu WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care 40: 440–443, 2017. doi: 10.2337/dci17-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, Egan M, Ereshefsky L, Hodgson RA, Hyde LA, Jhee S, Kleijn HJ, Kuvelkar R, Li W, Mattson BA, Mei H, Palcza J, Scott JD, Tanen M, Troyer MD, Tseng JL, Stone JA, Parker EM, Forman MS. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med 8: 363ra150, 2016. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- 38.Kimberly WT, Zheng JB, Guénette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem 276: 40288–40292, 2001. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- 39.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63: 2232–2243, 2014. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohjima M, Sun Y, Chan L. Increased food intake leads to obesity and insulin resistance in the tg2576 Alzheimer’s disease mouse model. Endocrinology 151: 1532–1540, 2010. doi: 10.1210/en.2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulas JA, Puig KL, Combs CK. Amyloid precursor protein in pancreatic islets. J Endocrinol 235: 49–67, 2017. doi: 10.1530/JOE-17-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurauti MA, Costa-Júnior JM, Ferreira SM, Santos GJ, Sponton CHG, Carneiro EM, Telles GD, Chacon-Mikahil MPT, Cavaglieri CR, Rezende LF, Boschero AC. Interleukin-6 increases the expression and activity of insulin-degrading enzyme. Sci Rep 7: 46750, 2017. doi: 10.1038/srep46750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurochkin IV, Guarnera E, Berezovsky IN. Insulin-degrading enzyme in the fight against Alzheimer’s disease. Trends Pharmacol Sci 39: 49–58, 2018. doi: 10.1016/j.tips.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Lao PJ, Handen BL, Betthauser TJ, Mihaila I, Hartley SL, Cohen AD, Tudorascu DL, Bulova PD, Lopresti BJ, Tumuluru RV, Murali D, Mathis CA, Barnhart TE, Stone CK, Price JC, Devenny DA, Johnson SC, Klunk WE, Christian BT. Alzheimer-like pattern of hypometabolism emerges with elevated amyloid-β burden in Down syndrome. J Alzheimers Dis 61: 631–644, 2018. doi: 10.3233/JAD-170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KJ, Moussa CE, Lee Y, Sung Y, Howell BW, Turner RS, Pak DT, Hoe HS. Beta amyloid-independent role of amyloid precursor protein in generation and maintenance of dendritic spines. Neuroscience 169: 344–356, 2010. doi: 10.1016/j.neuroscience.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YH, Martin JM, Maple RL, Tharp WG, Pratley RE. Plasma amyloid-beta peptide levels correlate with adipocyte amyloid precursor protein gene expression in obese individuals. Neuroendocrinology 90: 383–390, 2009. doi: 10.1159/000235555. [DOI] [PubMed] [Google Scholar]
- 47.Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity (Silver Spring) 16: 1493–1500, 2008. doi: 10.1038/oby.2008.267. [DOI] [PubMed] [Google Scholar]
- 48.Lorent K, Overbergh L, Moechars D, De Strooper B, Van Leuven F, Van den Berghe H. Expression in mouse embryos and in adult mouse brain of three members of the amyloid precursor protein family, of the alpha-2-macroglobulin receptor/low density lipoprotein receptor-related protein and of its ligands apolipoprotein E, lipoprotein lipase, alpha-2-macroglobulin and the 40,000 molecular weight receptor-associated protein. Neuroscience 65: 1009–1025, 1995. doi: 10.1016/0306-4522(94)00555-J. [DOI] [PubMed] [Google Scholar]
- 49.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen 8: 161–171, 2005. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 50.Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, Mahan TE, Sutphen CL, Holtzman DM. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest 125: 2463–2467, 2015. doi: 10.1172/JCI79742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macklin L, Griffith CM, Cai Y, Rose GM, Yan XX, Patrylo PR. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp Gerontol 88: 9–18, 2017. doi: 10.1016/j.exger.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Maianti JP, McFedries A, Foda ZH, Kleiner RE, Du XQ, Leissring MA, Tang WJ, Charron MJ, Seeliger MA, Saghatelian A, Liu DR. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature 511: 94–98, 2014. doi: 10.1038/nature13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manocha GD, Floden AM, Rausch K, Kulas JA, McGregor BA, Rojanathammanee L, Puig KR, Puig KL, Karki S, Nichols MR, Darland DC, Porter JE, Combs CK. APP regulates microglial phenotype in a mouse model of Alzheimer’s disease. J Neurosci 36: 8471–8486, 2016. doi: 10.1523/JNEUROSCI.4654-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matioli MNPDS, Suemoto CK, Rodriguez RD, Farias DS, da Silva MM, Leite REP, Ferretti-Rebustini REL, Pasqualucci CA, Filho JW, Grinberg LT, Nitrini R. Association between diabetes and causes of dementia: evidence from a clinicopathological study. Dement Neuropsychol 11: 406–412, 2017. doi: 10.1590/1980-57642016dn11-040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morino K, Neschen S, Bilz S, Sono S, Tsirigotis D, Reznick RM, Moore I, Nagai Y, Samuel V, Sebastian D, White M, Philbrick W, Shulman GI. Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes 57: 2644–2651, 2008. doi: 10.2337/db06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, Tsui W, Ginestroni A, Bessi V, Fayyazz M, Caffarra P, Pupi A. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 47: 1778–1786, 2006. [PubMed] [Google Scholar]
- 57.Müller U, Cristina N, Li ZW, Wolfer DP, Lipp HP, Rülicke T, Brandner S, Aguzzi A, Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell 79: 755–765, 1994. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 58.Müller UC, Zheng H. Physiological functions of APP family proteins. Cold Spring Harb Perspect Med 2: a006288, 2012. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagamoto-Combs K, Kulas J, Combs CK. A novel cell line from spontaneously immortalized murine microglia. J Neurosci Methods 233: 187–198, 2014. doi: 10.1016/j.jneumeth.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagamoto-Combs K, Manocha GD, Puig K, Combs CK. An improved approach to align and embed multiple brain samples in a gelatin-based matrix for simultaneous histological processing. J Neurosci Methods 261: 155–160, 2016. doi: 10.1016/j.jneumeth.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J Neurochem 120, Suppl 1: 167–185, 2012. doi: 10.1111/j.1471-4159.2011.07510.x. [DOI] [PubMed] [Google Scholar]
- 62.Needham BE, Wlodek ME, Ciccotosto GD, Fam BC, Masters CL, Proietto J, Andrikopoulos S, Cappai R. Identification of the Alzheimer’s disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J Pathol 215: 155–163, 2008. doi: 10.1002/path.2343. [DOI] [PubMed] [Google Scholar]
- 63.Niwano H, Embury PB, Greenberg BD, Ratnoff OD. Inhibitory action of amyloid precursor protein against human Hageman factor (factor XII). J Lab Clin Med 125: 251–256, 1995. [PubMed] [Google Scholar]
- 64.Oh H, Madison C, Baker S, Rabinovici G, Jagust W. Dynamic relationships between age, amyloid-β deposition, and glucose metabolism link to the regional vulnerability to Alzheimer’s disease. Brain 139: 2275–2289, 2016. doi: 10.1093/brain/aww108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Oliveira FF, de Almeida SS, Chen ES, Smith MC, Naffah-Mazzacoratti MDG, Bertolucci PHF. Lifetime risk factors for functional and cognitive outcomes in patients with Alzheimer’s disease. J Alzheimers Dis 65: 1283–1299, 2018. doi: 10.3233/JAD-180303. [DOI] [PubMed] [Google Scholar]
- 66.Pacifici M, Peruzzi F. Isolation and culture of rat embryonic neural cells: a quick protocol. J Vis Exp (63): e3965, 2012. doi: 10.3791/3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parent MJ, Zimmer ER, Shin M, Kang MS, Fonov VS, Mathieu A, Aliaga A, Kostikov A, Do Carmo S, Dea D, Poirier J, Soucy JP, Gauthier S, Cuello AC, Rosa-Neto P. Multimodal imaging in rat model recapitulates Alzheimer’s disease biomarkers abnormalities. J Neurosci 37: 12263–12271, 2017. doi: 10.1523/JNEUROSCI.1346-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peila R, Rodriguez BL, Launer LJ; Honolulu-Asia Aging Study . Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 51: 1256–1262, 2002. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 69.Phinney AL, Calhoun ME, Wolfer DP, Lipp HP, Zheng H, Jucker M. No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience 90: 1207–1216, 1999. doi: 10.1016/S0306-4522(98)00645-9. [DOI] [PubMed] [Google Scholar]
- 70.Puig KL, Brose SA, Zhou X, Sens MA, Combs GF, Jensen MD, Golovko MY, Combs CK. Amyloid precursor protein modulates macrophage phenotype and diet-dependent weight gain. Sci Rep 7: 43725, 2017. doi: 10.1038/srep43725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One 7: e30378, 2012. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puig KL, Kulas JA, Franklin W, Rakoczy SG, Taglialatela G, Brown-Borg HM, Combs CK. The Ames dwarf mutation attenuates Alzheimer’s disease phenotype of APP/PS1 mice. Neurobiol Aging 40: 22–40, 2016. doi: 10.1016/j.neurobiolaging.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, Portet F, Dartigues JF, Alpérovitch A, Barberger-Gateau P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes Care 32: 169–174, 2009. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ribe EM, Lovestone S. Insulin signalling in Alzheimer’s disease and diabetes: from epidemiology to molecular links. J Intern Med 280: 430–442, 2016. doi: 10.1111/joim.12534. [DOI] [PubMed] [Google Scholar]
- 75.Roberts RO, Knopman DS, Cha RH, Mielke MM, Pankratz VS, Boeve BF, Kantarci K, Geda YE, Jack CR Jr, Petersen RC, Lowe VJ. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med 55: 759–764, 2014. doi: 10.2967/jnumed.113.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8: 595–608, 2016. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA 85: 7341–7345, 1988. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature 443: 870–874, 2006. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 7: 63–80, 2005. doi: 10.3233/JAD-2005-7107. [DOI] [PubMed] [Google Scholar]
- 80.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122: 1316–1338, 2012. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tharp WG, Sarkar IN. Origins of amyloid-β. BMC Genomics 14: 290, 2013. doi: 10.1186/1471-2164-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tu Z, Keller MP, Zhang C, Rabaglia ME, Greenawalt DM, Yang X, Wang IM, Dai H, Bruss MD, Lum PY, Zhou YP, Kemp DM, Kendziorski C, Yandell BS, Attie AD, Schadt EE, Zhu J. Integrative analysis of a cross-loci regulation network identifies App as a gene regulating insulin secretion from pancreatic islets. PLoS Genet 8: e1003107, 2012. doi: 10.1371/journal.pgen.1003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tyan SH, Shih AY, Walsh JJ, Maruyama H, Sarsoza F, Ku L, Eggert S, Hof PR, Koo EH, Dickstein DL. Amyloid precursor protein (APP) regulates synaptic structure and function. Mol Cell Neurosci 51: 43–52, 2012. doi: 10.1016/j.mcn.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Nostrand WE, Farrow JS, Wagner SL, Bhasin R, Goldgaber D, Cotman CW, Cunningham DD. The predominant form of the amyloid beta-protein precursor in human brain is protease nexin 2. Proc Natl Acad Sci USA 88: 10302–10306, 1991. doi: 10.1073/pnas.88.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wijesekara N, Goncalves da Silva RA, De Felice FG, Fraser PE. Impaired peripheral glucose homeostasis and Alzheimer’s disease. Neuropharmacology 136: 172–181, 2018. doi: 10.1016/j.neuropharm.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 86.Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KA, Hooper NM, Vardy ER, Wu D, Unwin RD, Faull RL, Dowsey AW, Cooper GJ. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Sci Rep 6: 27524, 2016. doi: 10.1038/srep27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yfanti C, Mengele K, Gkazepis A, Weirich G, Giersig C, Kuo WL, Tang WJ, Rosner M, Schmitt M. Expression of metalloprotease insulin-degrading enzyme insulysin in normal and malignant human tissues. Int J Mol Med 22: 421–431, 2008. doi: 10.3892/ijmm_00000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 27: 14459–14469, 2007. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev 3: 15, 2008. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–11947, 2014. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisodia SS, Chen HY, Van der Ploeg LH. Beta-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81: 525–531, 1995. doi: 10.1016/0092-8674(95)90073-X. [DOI] [PubMed] [Google Scholar]