Abstract
The lipolytic effects of growth hormone (GH) have been known for half a century and play an important physiological role for substrate metabolism during fasting. In addition, sustained GH-induced lipolysis is causally linked to insulin resistance. However, the underlying molecular mechanisms remain elusive. In the present study, we obtained experimental data in human subjects and used human adipose-derived stromal vascular cells (hADSCs) as a model system to elucidate GH-triggered molecular signaling that stimulates adipose tissue lipolysis and insulin resistance in human adipocytes. We discovered that GH downregulates the expression of fat-specific protein (FSP27), a negative regulator of lipolysis, by impairing the transcriptional ability of the master transcriptional regulator, peroxisome proliferator-activated receptor-γ (PPARγ) via MEK/ERK activation. Ultimately, GH treatment promotes phosphorylation of PPARγ at Ser273 and causes its translocation from nucleus to the cytosol. Surprisingly, FSP27 overexpression inhibited PPARγ Ser273 phosphorylation and promoted its nuclear retention. GH antagonist treatment had similar effects. Our study identifies a novel signaling mechanism by which GH transcriptionally induces lipolysis via the MEK/ERK pathway that acts along PPARγ-FSP27 in human adipose tissue.
Keywords: ATGL, CIDEC, diabetes, fat metabolism, growth hormone, insulin resistance, lipase, lipid droplets, obesity
INTRODUCTION
The rise in circulating free fatty acids (FFA) was initially identified as the most sensitive response to growth hormone (GH) more than half a century ago (30). The subsequent advent of accurate immunoassays revealed an endogenous GH pattern in serum (9) that mirrored that of FFA with suppressed postprandial levels and elevated fasting levels (33). The physiological implications were provided in studies with GH and insulin administration using the human forearm by observing that the lipolytic effect of GH was accompanied by increased fatty acid (FA) uptake and oxidation in skeletal muscle at the expense of glucose (31, 43).
More recent experimental studies in human subjects demonstrate that the lipolytic effect of GH is blocked by acipimox, an antilipolytic agent that acts via a unique receptor in adipocytes to suppress the hormone-sensitive lipase (HSL) (25). The suppression of lipolysis by acipimox also abrogates the antagonistic effects of GH on insulin-stimulated muscle glucose uptake (25), thus corroborating previous observations (43).
The clinical significance of these effects has been tested in adult patients with GH deficiency (GHDA) during fasting (27) as well as during a hypoglycemic clamp (17) showing that GH plays a crucial role for lipid mobilization and utilization when glucose availability is limited. It is also well documented that prolonged GH replacement in GHDA results in a gradual reduction in fat mass toward normal levels (18). On the other hand, sustained and unregulated GH excess may cause glucose intolerance due to the insulin antagonistic effects as seen in patients with active acromegaly (23). Notwithstanding these important effects of GH, the underlying molecular mechanisms have never been investigated in human models.
The cell death inducing DFFA like effector (CIDE) proteins associate with lipid droplets and regulate FA homeostasis in adipocytes (28, 29). A CIDE protein family member, fat specific protein 27 (FSP27), regulates lipid droplet dynamics and lipolysis in adipocytes through suppression of the catalytic capacity and transcription of adipose tissue glycerol lipase (ATGL), the rate-limiting enzyme in lipolysis (10, 15, 28, 39). Consistent with these studies, FSP27 levels are positively associated with insulin sensitivity in obese humans (19, 29), and loss of function mutation of FSP27 in humans leads to increased lipolysis (34). In addition, adipose-specific disruption of FSP27 causes insulin resistance in high-fat-fed mice (41).
In the present study, we discovered that GH-induced lipolysis in human subjects is tightly associated with acute reduction in FSP27 in subcutaneous adipose tissue, which is mediated via activation of a MEK/ERK signaling pathway that suppresses peroxisome proliferator-activated receptor-γ (PPARγ) transcriptional activity. Additionally, we show that enforced expression of FSP27 inhibits phosphorylation of PPARγ at Ser273, thus stabilizing PPARγ in the nucleus and suppressing GH-induced lipolysis in human adipocytes. Taken together, our results provide a coherent molecular mechanism whereby GH stimulates lipolysis in human adipose tissue.
MATERIALS AND METHODS
Studies in human subjects.
The human study was conducted as outlined in Fig. 1A. Eight healthy young men (body mass index 22.5 ± 1.5 kg/m2) in the age range 19–23 yr were studied in a single blind randomized crossover design. The subjects reported to the laboratory at 0800. On four different study days separated by at least one week, each participant was subjected to a saline infusion for 270 min following an overnight (12-h) fast (“Control”), a GH infusion for 270 min (30 ng·kg−1·min−1, Genotropin; Pfizer) following an overnight fast (“GH”), a saline infusion following a 36-h fast (“Fasting”), and a GH infusion following a 36-h fast (“GH + Fasting”). Adipose tissue biopsies were taken at time (t) = 120 min in the basal period state and t = 270 min during a hyperinsulinemic-euglycemic clamp. All participants gave oral and written informed consent to participate. The study protocol was approved by The Regional Committee on Health Research Ethics and The Danish Data Protection Agency and was conducted in accordance with the Helsinki Declaration. The research protocol was registered at clinicaltrials.gov (NCT01209429). Data from this study focusing on insulin sensitivity in skeletal muscle have been previously published (24).
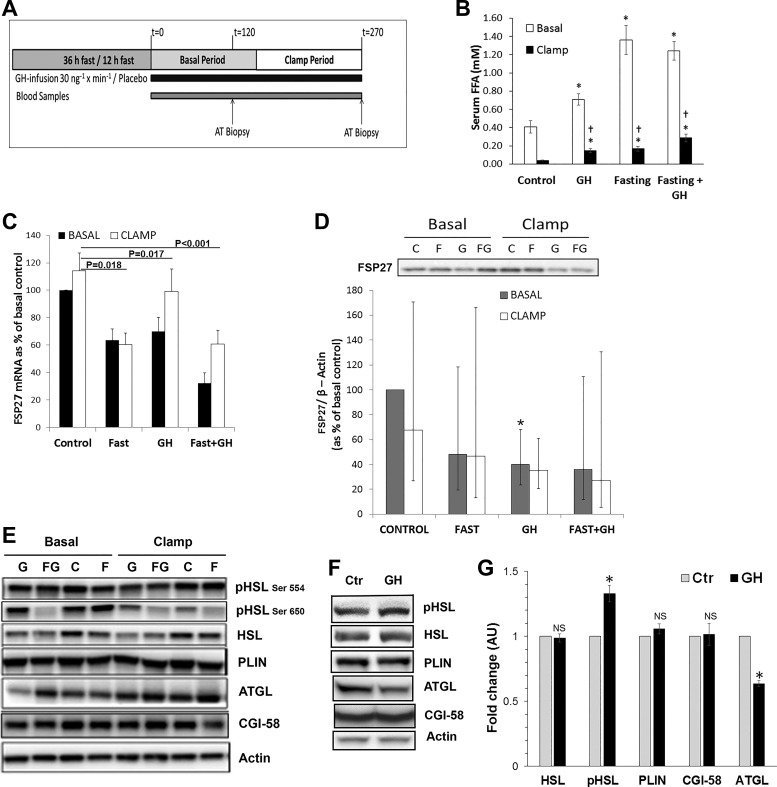
Fig. 1.
Growth hormone (GH) acutely induces lipolysis and reduces fat-specific protein (FSP27) expression in adipose tissue of human subjects. A: schematic representation of the human subject study design. B: serum free fatty acid (FFA) concentrations of plasma collected under both basal and clamped conditions from patients treated on four separate occasions with either saline or GH infusion following either an overnight or 36-h fasting period. C: mRNA expression of FSP27 in adipose tissue of GH-infused human subjects under basal and clamped conditions. D: FSP27 protein expression and quantification in adipose tissue of GH-infused human subjects under basal and clamped conditions. E: expression of lipid droplet-associated proteins in sc adipose tissue of GH-infused human subjects under basal and clamped conditions. F: protein expression of lipid droplet-associated proteins upon GH stimulation in cultured differentiated human adipocytes. In Western blot, actin was used as a loading control. Total hormone-sensitive lipase (HSL) protein was used as a loading control for phosphorylated HSL. G: quantification of lipid droplet-associated proteins upon GH stimulation in cultured differentiated human adipocytes. C, control (Ctr); F, fasting; G, growth hormone; FG, fasting + growth hormone; NS, not significant. *P < 0.05. †Effects of insulin under clamped conditions.
Circulating hormones and metabolites.
Plasma glucose was measured in duplicates immediately after collection on an YSI 2300 Stat Plus (YSI). GH was analyzed using time-resolved fluoroimmunoassay (AutoDELFIA, PerkinElmer, Turku, Finland), and free fatty acids (FFA) were analyzed with a commercial kit (Wako Chemicals, Neuss, Germany).
Cell culture.
Human adipocyte stem cells (hADSCs) from subcutaneous fat were procured from the Boston Nutrition Obesity Research Center adipocyte core. Cells were seeded at a density of 5,000 cells·ml−1·well−1 in a 12-well plate, in growth medium that was made using 13.5 g of α-MEM powder (GIBCO) with 10% FBS (GIBCO), 100 U/ml of penicillin and streptomycin (GIBCO), and 25 mM sodium bicarbonate (Fisher Scientific) reconstituted in double-distilled water (ddH2O) to 1 liter, pH 7.2–7.3. This growth medium was changed every 2 days, until cells reached 90% confluence. To allow the cells to differentiate, the growth medium was then replaced by complete differentiation medium, which consisted of: 13.5 g of Dulbecco’s modified Eagle’s medium powder-F-12 (GIBCO), 100 U/ml of penicillin and streptomycin (GIBCO), 15 mM HEPES (Sigma), 25 mM sodium bicarbonate (Fisher Scientific), 33 μM biotin (Sigma), 17 μM pantothenate (Sigma), 0.5 mM IBMX (Sigma), 10 mg/l transferrin (Sigma), 1 μM rosiglitazone (BioMol), 100 nM dexamethasone (Sigma), 100 nM human insulin (recombinant), and 2 nM triiodo-l-thyronine (T3) (Sigma), reconstituted in ddH2O to 1 liter, pH 7.4. The day the cells were first given complete differentiation media is designated as day 0. The media was changed every 2 days until the preadipocytes were fully differentiated into mature human white adipocytes that were filled with lipid droplets. Following differentiation, the cells were maintained in maintenance medium until fully differentiated, with the media being changed every 2 days. Maintenance medium was made of the similar constituents as complete differentiation media, except without rosiglitazone, IBMX, T3, and transferrin. Additionally, the concentrations of human insulin and dexamethasone used were 10 nm each.
Adenovirus transduction.
FSP27-cyan fluorescent protein (CFP) and control CFP adenoviruses were generated at the Adenoviral Vector Core Facility at Tufts Medical Center. Virus was added at multiplicity of infection of 100 to the human adipocytes as described previously (11). Cells were incubated for another 24–48 h to allow for protein expression before they were treated with growth hormone.
Lipolysis.
Cultured and fully differentiated primary human adipocytes were treated with GH and washed two times with PBS. Cells were incubated with Krebs-Ringer-bicarbonate HEPES buffer supplemented with 4% bovine serum albumin with or without GH. Krebs-Ringer bicarbonate buffer was collected after 30 min to assay for glycerol as a measure of lipolysis using a triglyceride determination kit (Sigma).
Protein analysis.
Western Blot analyses were used to assess total and phosphorylated (p) levels of relevant proteins in adipose tissue biopsies. Antibodies for pHSLSer554, pHSLSer650, and HSL were purchased from Cell Signaling Technology (Beverly, MA). Antibodies for CIDEA, ATGL, and CGI-58 were purchased from Abcam (Cambridge, UK). The antibody for perilipin (PLIN) 1 was purchased from ABR Affinity Bioreagents (Golden, CO), and the antibody for CIDEC/FSP27 was purchased from Novus Biologicals (Littleton, CO). Anti-rabbit IgG horseradish peroxidase (Santa Cruz Biotechnology, Dallas, TX) was used as a secondary antibody.
Frozen adipose tissue biopsies (~100 mg) were homogenized in a buffer containing 50 mM HEPES, 20 mM NaF, 2 mM NaOV, 5 mM EDTA, 5 mM N-acetylmethionine, 10 µM trichostatin A, protease inhibitor cocktail (HALT; Thermo Specific, Waltham, MA), and 5% SDS in a Precellys 24 homogenizer (Bertin Technologies, Montigny-Le-Bretonneux, France) for 2 × 30 s at 6,000 revolutions/min (rpm). Samples were subsequently thermomixed (Eppendorf, Hamburg, Germany) at 37°C and 900 rpm for 1 h. The lipid fraction was isolated by centrifugation at 14,000 g for 20 min at room temperature, and the infranatant was collected and stored at −80°C until used for Western blot analysis. Protein aliquots were separated by gel electrophoresis using StainFree 4–15% CriterionXT gels (Bio-Rad), electrotransferred to a polyvinylidene fluoride membrane, blocked for 2 h in Tris-buffered saline-Tween (TBS-T) containing 1% bovine serum albumin, and incubated in primary antibody for one to three nights. Following primary incubation, the membranes were incubated in secondary antibody for 2 h at room temperature, and protein levels were determined using chemiluminescent technology (Bio-Rad). Protein signals were quantified using Image Laboratory (version 4.0.1; Bio-Rad). Proteins were expressed as a ratio to the content of β-actin except for FSP27, which was also expressed as a ratio to the total content of protein using the Stain Free method (13). HSL phosphorylations are expressed as a ratio of HSL protein.
Protein extracts from differentiated adipocytes were prepared using lysis buffer supplemented with protease and phosphatase inhibitors. Approximately 25–30 μg of protein were loaded on 7.5% PAGE for separation. Proteins were transferred to PVDF membranes at 50 V and 4°C for 2 h. Membranes were blocked for 1 h in 5% nonfat milk or 4% BSA in Tris-buffered saline-Tween at room temperature. Blots were incubated with the indicated primary antibody overnight at 4°C, and secondary antibody [horseradish peroxidase-coupled goat anti-rabbit (catalog no. sc-2030, 1:10,000; Santa Cruz Biotechnology) or mouse anti-rabbit IgG (catalog no. L27A9, 1:6,000; Cell Signaling Technology)] for 1 h at room temperature. Super Signal West Dura extended-duration substrate (catalog no. 34075; Thermo Scientific) was used for detection.
Lipid droplet staining.
Cells were washed with PBS, fixed in 4% formaldehyde for 20 min, and quenched with 0.1 M glycine. Cells were then incubated with 0.5 µg/ml of Nile Red or HCS LipidTOX-Deep Red stain for 30 min. Cells were then washed with PBS, and the cover slips were mounted on glass slides with VectaShield (Vector Laboratories) mounting media.
Morphometric analysis on lipid droplets.
The morphometric analysis on lipid droplets was performed on confocal images by using Metamorph version 7.1. After subtracting the background, the cells were outlined and threshold for Nile red intensity. Each droplet was identified as a discrete object using object classifiers in the metamorph program. Only the droplets that were identified individually by the program were chosen for analysis. Pixel-to-micrometer ratio was assigned, and the program measured the radius and volume (equivalent sphere volume) of the Nile red-stained lipid droplets in cubic micrometers. In each condition, 10–25 cells were measured for total volume of droplets per cell.
Immunostaining and confocal imaging.
For determination of PPARγ localization, human adipocytes were cultured and differentiated on cover slips. Immunostaining was performed using anti-PPARγ polyclonal antibody (1:200 dilution; Cell Signaling) followed by labeling with the florescent secondary antibody. Labeled cells were washed three times with PBS and mounted on slides in mounting media with DAPI. Confocal microscopy was performed using a Nikon A1Rsi confocal microscope with a ×60 oil immersion objective. Images were processed using Nikon software.
RNA Isolation and Quantitative PCR.
The expression of mRNA was analyzed using real-time reverse transcriptase PCR (RT-PCR). TriZol (GIBCO BRL, Life Technologies, Roskilde, Denmark) was used to extract total RNA from adipose tissue samples, and RNA was quantified by measuring absorbance at 260 and 280 nm with a ratio ≥1.8. Ribosomal RNAs 18S and 28S on agarose gel were inspected visually to check RNA integrity.
As described by the manufacturer (Verso cDNA kit; VWR, Herlev, Denmark), cDNA was prepared using random hexomer primers and then adding the specific primers in form of a PCR master mix. SYBR-green real-time RT-PCR assay (KAPA SYBR Fast Universal kit; Ken-En-Tec, Taastrup, Denmark) in a 384-well format in a LightCycler from Roche (Roche Applied Science, Mannheim, Germany) was used to quantify genes. Increases in fluorescence were measured in real time, and cDNA was amplified in separate tubes. All samples were amplified in duplicate. Relative gene expression and threshold cycle were calculated as described in User Bulletin No- 2, 1997 from Perkin Elmer (Perkin Elmer Cetus, Norwalk, CT) using the formula: k·2−ΔΔCT, were k is a constant, set to 1. β2-Microglobulin was used as an internal control (no change of expression during the interventions). Negative controls were made using a similar set-up using no reverse transcriptase and no PCR products.
For in vitro experiments using human adipocytes, cDNA was synthesized according to the manufacturer’s instructions using random primers and reverse transcriptase (catalog no. K1622; Thermofisher). Quantitative real-time PCR was performed using SYBR Green and the Bio-Rad CFX Connect Real-Time System.
Statistics.
Normality was tested with the Shapiro-Wilk test and by using the normal probability plot on raw data. Non-normal distributed data were transformed. Differences between study days were tested using a two-way repeated-measurement analysis of variance (ANOVA). When significant main effects or interactions were found, the Student-Newman-Keuls test was used for post hoc testing. Statistical significance was assumed for P < 0.05. Ln-transformed data are presented as geometric means ± 95% confidence intervals (CI), and nontransformed data are presented as arithmetic means ± SE unless otherwise stated.
RESULTS
Growth hormone-induced adipose tissue lipolysis primarily involves suppression of antilipolytic signals: Human in vivo studies.
Administration of GH stimulates lipolysis after a time lag of 2–3 h (22); therefore, subcutaneous adipose tissue biopsies were taken at t = 120 min in the basal state and t = 270 min during a hyperinsulinemic-euglycemic clamp (Fig. 1A). Consistent with our hypothesis, serum FFA levels were significantly elevated in GH, Fasting, and GH + Fasting conditions compared with basal and clamped Control (Fig. 1B). Cytokine-inducible SH2-containing protein (CISH) mRNA expression, used as a positive control, was upregulated after GH (P < 0.001), Fasting (P = 0.012), and GH + Fasting (P < 0.001) (data not shown). GH alone had no significant impact on ATGL mRNA expression, whereas Fasting and GH + Fasting downregulated ATGL mRNA expression. HSL mRNA expression did not significantly change in response to either GH or Fasting but was reduced by GH + Fasting (P = 0.03) (Table 1). HSL phosphorylation at the Ser650 residue, which activates HSL, was significantly increased after GH + Fasting (P < 0.05) and Fasting (P < 0.001) despite a reduction in total HSL levels. As shown in Table 1, this stimulatory effect remained after normalization of pHSL Ser650 to the total levels of HSL protein (P < 0.05). GH antagonized the inhibitory effect of insulin on HSL Ser650 phosphorylation during Control (35% reduction, P = 0.014) and Fasting (50%, P < 0.001) (Fig. 1E and Table 1). Similarly, we observed an increase in pHSL and decrease in ATGL protein expression in our in vitro model using human adipocytes (Fig. 1F).
Table 1.
Geometric mean (95% confidence intervals) mRNA expression of HSL, CGI-58, and ATGL and protein expression of total HSL, pHSL Ser650, pHSL Ser554, and ATGL
| Control |
Fasting |
GH |
GH + Fasting |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| mRNA | ||||||||
| HSL | ||||||||
| Basal | 0.40 | 0.27:0.58 | 0.28 | 0.19:0.42 | 0.29 | 0.19:0.43 | 0.15* | 0.07:0.30 |
| Clamp | 0.34 | 0.22:0.51 | 0.23 | 0.17:0.33 | 0.22 | 0.14:0.35 | 0.16 | 0.11:0.24 |
| CGI-58 | ||||||||
| Basal | 0.03 | 0.02:0.05 | 0.07*** | 0.05:0.10 | 0.04 | 0.03:0.06 | 0.04* | 0.03:0.06 |
| Clamp | 0.03 | 0.02:0.05 | 0.07 | 0.05:0.10 | 0.05 | 0.03:0.07 | 0.07** | 0.05:0.09 |
| ATGL | ||||||||
| Basal | 0.06 | 0.05:0.07 | 0.04* | 0.03:0.05 | 0.05 | 0.04:0.07 | 0.03** | 0.02:0.04 |
| Clamp | 0.06 | 0.04:0.06 | 0.04 | 0.04:0.05 | 0.05 | 0.04:0.07 | 0.04 | 0.03:0.05 |
| Protein | ||||||||
| HSL | ||||||||
| Basal | 1.04 | 0.75:1.45 | 0.71* | 0.45:1.10 | 0.81 | 0.58:1.15 | 0.55 | 0.28:1.08 |
| Clamp | 0.93 | 0.54:1.58 | 0.56 | 0.28:1.12 | 0.66 | 0.29:1.49 | 0.52 | 0.31:0.89 |
| pHSL Ser650 | ||||||||
| Basal | 0.66 | 0.45:0.99 | 1.43*** | 0.95:2.16 | 0.74 | 0.46:1.17 | 1.11* | 0.72:1.17 |
| Clamp | 0.44† | 0.31:0.62 | 0.70† | 0.44:1.12 | 0.69 | 0.39:1.21 | 0.93 | 0.67:1.28 |
| pHSL Ser554 | ||||||||
| Basal | 0.80 | 0.60:1.07 | 0.89 | 0.62:1.27 | 0.85 | 0.60:1.20 | 0.91 | 0.63:1.32 |
| Clamp | 0.83 | 0.60:1.15 | 0.94 | 0.59:1.51 | 0.84 | 0.61:1.15 | 0.96 | 0.66:1.38 |
| ATGL | ||||||||
| Basal | 1.26 | 0.85:1.89 | 1.31 | 0.92:1.87 | 1.11 | 0.89:1.38 | 1.53 | 1.10:2.35 |
| Clamp | 1.34 | 1.02:1.75 | 1.48 | 0.82:2.67 | 1.45 | 0.85:2.49 | 1.59 | 0.96:2.61 |
Data on phosphorylated hormone-sensitive lipase (pHSL) are expressed relative to total HSL. GH, growth hormone; ATGL, adipose tissue glycerol lipase. The following symbols indicate post hoc statistics:
P < 0.05,
P < 0.01,
P < 0.001 vs. control, and
P < 0.05 vs. basal.
Previous studies have shown that FSP27 suppresses lipolysis by storing triglycerides in lipid droplets (10, 15, 28, 29). We therefore tested if GH negatively regulates expression of FSP27 and other lipid droplet-associated proteins to promote lipolysis. FSP27 mRNA expression was significantly reduced by GH, Fasting, and GH + Fasting under our experimental conditions in human subjects. (Fig. 1C). Moreover, FSP27 protein levels were also reduced by ~60% after GH infusion (P = 0.033) (Fig. 1D). We detected no significant change in PLIN and CGI-58 protein levels after GH exposure either in human adipose tissue biopsies or in human adipocytes. ATGL protein levels, however, decreased significantly upon GH treatment in human adipocytes but remained unchanged in human subjects (Fig. 1, E and F).
GH-induced lipolysis is FSP27 mediated in human adipocytes.
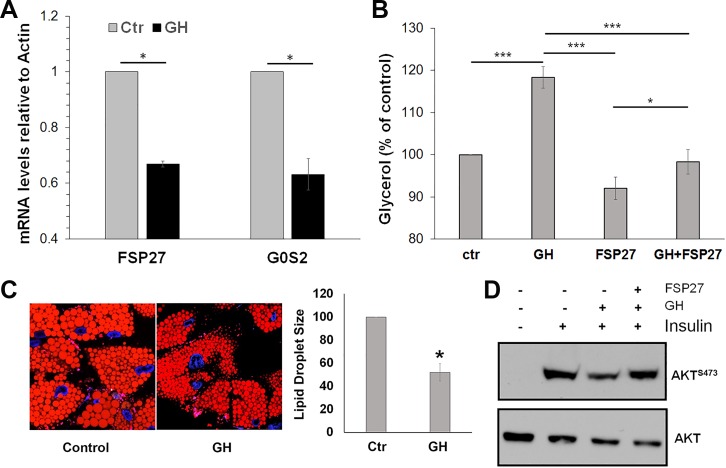
To further investigate that GH-induced lipolysis is dependent on FSP27, we used hADSC isolated from human subcutaneous adipose tissue in our subsequent studies. Previous studies have shown that hADSCs maintain their genetic characteristics in culture even after differentiation and therefore serve as an excellent model to study human adipose tissue metabolic functions (20). GH treatment of these adipocytes (250 ng/ml; 2 h) reduced FSP27 mRNA expression and the expression of G0S2 mRNA, another lipid droplet-associated protein that regulates adipose lipolysis (42) (Fig. 2A). Glycerol release, as a measure of lipolysis, was increased by 20% (Fig. 2B), mimicking the results in human subjects. In addition, GH treatment led to lipid droplet fragmentation in adipocytes (Fig. 2C). These results supported our in vivo finding that GH-induced lipolysis involves suppression of FSP27.
Fig. 2.
Fat-specific protein (FSP27) protects against growth hormone (GH)-induced lipolysis and improves insulin signaling in human adipocytes. A: qPCR analysis of FSP27 and G0S2 mRNA from differentiated human adipocytes treated with 250 ng/ml GH for 2 h. Data are shown as mean ± SE of 3 independent experiments. B: lipolysis (glycerol release) from human adipocytes treated with GH for 2 h in the presence of control adenovirus (expressing CFP only) or adenonovirus expressing FSP27. C: Nile red staining showing lipid droplet size in control and GH-stimulated differentiated human adipocytes. Lipid droplets show significant reduction in size upon GH treatment. D: Western blot showing impaired AKT signaling (pAKT Ser473) and total AKT in primary human adipocytes. Ctr, control. Data are shown as means ± SE of 3 independent experiments. *P < 0.05 and ***P < 0.001.
Consistent with the role of FFAs in reducing insulin signaling (3), we found that GH treatment decreased insulin-stimulated Akt phosphorylation at Ser473 in the adipocytes (Fig. 2D). Our previous studies have shown that FSP27 knockdown in adipocytes derived from hADSCs increases lipolysis and impairs insulin-stimulated insulin signaling, whereas FSP27 overexpression protects against FFA-induced insulin resistance (10). Consistent with these studies, we found that adenovirus-mediated FSP27 overexpression abrogated GH-induced stimulation of lipolysis and reduction in insulin-mediated AKT phosphorylation at Ser473 (Fig. 2, B and D). These results show that GH induces insulin resistance in adipocytes by regulating lipolysis via FSP27.
FSP27 inhibits MAPK-mediated PPARγ Ser273 phosphorylation.
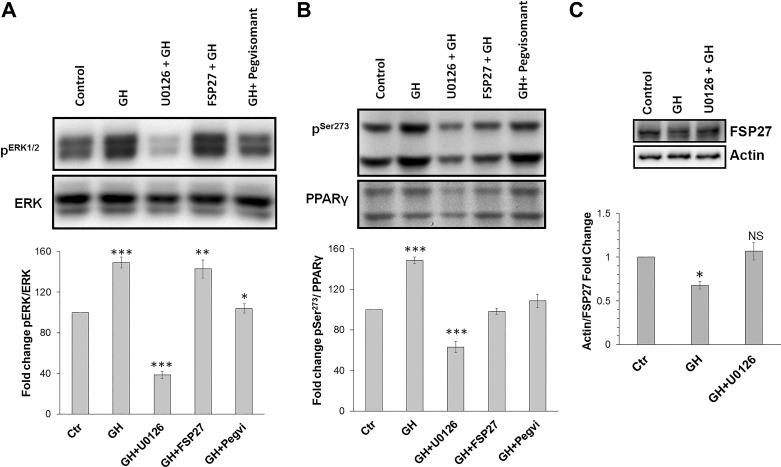
Previous studies have demonstrated that MAPK pathway activation decreases PPARγ transcriptional activity via MEK/ERK activation (5), and MEK/ERK is known to be activated by GH in the mouse 3T3–442A cells (14). ERK1/2, a downstream mediator of MAPK signaling, can phosphorylate PPARγ Ser273, and many studies have linked PPARγ Ser273 phosphorylation with the development of insulin resistance (2, 6, 7, 21). Because FSP27 is regulated by PPARγ in adipocytes (26, 29), we hypothesized that GH-induced activation of the MEK/ERK pathway downregulates FSP27 transcription via Ser273 phosphorylation and subsequent nuclear destabilization of PPARγ. Treatment of adipocytes with GH (250 ng/ml) for 2 h increased ERK1/2 activation, which was inhibited by a small molecule inhibitor of MEK, U-0126 (Fig. 3A). There was no effect of FSP27 overexpression on ERK1/2 phosphorylation (Fig. 3A). We next tested if the GH antagonist, pegvisomant, could block GH-induced ERK1/2 phosphorylation. As shown in Fig. 3A, pegvisomant completely inhibited GH-mediated ERK1/2 phosphorylation, showing a direct role of GH in MEK/ERK activation. Next, we found that GH treatment increased PPARγ Ser273 phosphorylation, which was reduced by U-0126 (Fig. 3B). Unexpectedly, FSP27 overexpression inhibited GH-stimulated increase in PPARγ Ser273 phosphorylation similar to that seen with pegvisomant treatment (Fig. 3B). We next tested if GH downregulates FSP27 protein expression. Interestingly, GH treatment of differentiated human adipocytes resulted in downregulation of FSP27 protein expression, and use of MEK inhibitor U-0126 blocked this effect, suggesting that GH-mediated decrease in FSP27 expression is mediated via the MEK/ERK pathway (Fig. 3C). Because FSP27 overexpression had no effect on ERK1/2 phosphorylation, which is upstream of PPAR Ser273 phosphorylation, it suggested that FSP27 might specifically protect PPARγ against inhibitory ERK and or Cdk5-dependent Ser273 phosphorylation, a root cause for pathogenesis of insulin resistance (2).
Fig. 3.
Fat-specific protein (FSP27) and pegvisomant protect against peroxisome proliferator-activated receptor-γ (PPARγ) Ser273phosphorylation. A: Western blot and quantification of ERK1/2 phosphorylation. B: Western blot and quantification of PPARγ Ser273 phosphorylation. C: Western blot and quantification showing that FSP27 protein expression decreases upon growth hormone (GH) treatment, and small molecule inhibitor of MEK, U-0126, can block MEK/ERK-mediated downregulation of FSP27 expression. Ctr, control; NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001. Data are shown as mean ± SE of 3 independent experiments.
FSP27 overexpression promotes nuclear retention of PPARγ.
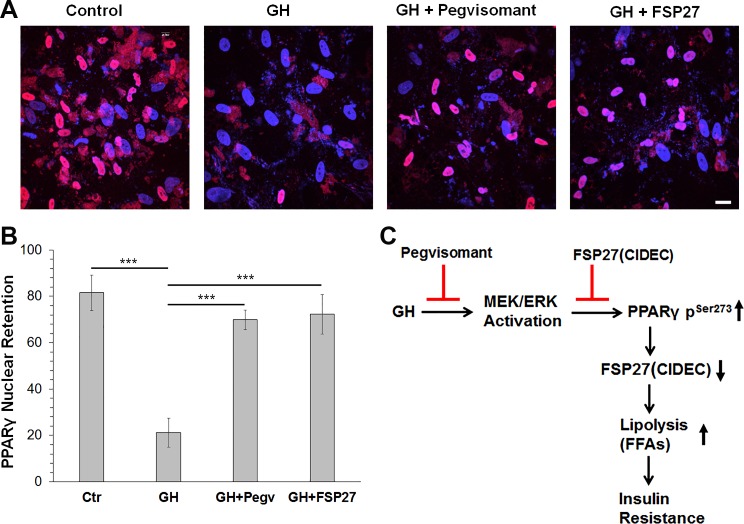
Phosphorylation of PPARγ at Ser273 leads to its relocalization from the nucleus to the cytoplasm and subsequent degradation (7, 40). We therefore studied the role of FSP27 overexpression in PPARγ localization upon GH treatment in human adipocytes. Interestingly, FSP27 overexpression retained PPARγ in the nucleus (Fig. 4, A and B). Similarly, pegvisomant also stabilized PPARγ in the nucleus. Our results suggest that FSP27 protects PPARγ from kinases like MEK/ERK or Cdk5 that have been known to phosphorylate PPARγ at Ser273 phosphorylation that lead to its degradation and, therefore, downregulation of its targets, including FSP27 and G0S2.
Fig. 4.
Fat-specific protein (FSP27) overexpression retains peroxisome proliferator-activated receptor-γ (PPARγ) in the nucleus. A: confocal images showing PPARγ distribution in human adipocytes. Red, PPARγ; blue (DAPI), nucleus. FSP27-overexpressed adipocytes were treated with 250 ng/ml growth hormone (GH) or with GH + pegvisomant for 14 h before immunostaining with PPARγ antibodies. B: quantitation of number of nuclei with PPARγ (pink) or without PPARγ (blue) in a given field. Ctr, control. C: model outlining GH-mediated development of insulin resistance based on our results in human adipocytes and subjects. Data are shown as means ± SE of 3 independent fields with ~25 cells/field. ***P < 0.001.
DISCUSSION
Our study provides a novel mechanism whereby GH stimulates human adipose tissue lipolysis by downregulating or destabilizing FSP27 and PPARγ, both of which are critical regulators of adipose tissue homeostasis. Mechanistically, we show that GH suppresses expression of FSP27, a lipid droplet protein that inhibits lipolysis, via an MEK/ERK pathway. FSP27 expression is stimulated by PPARγ, and we showed that GH-induced MEK/ERK activation led to phosphorylation of PPARγ at Ser273. Phosphorylation of PPARγ at Ser273 promotes export of PPARγ from the nucleus and thereby suppresses its transcriptional activity. FSP27 and pegvisomant, in turn, protect against GH-induced lipolysis, block MEK/ERK-mediated phosphorylation of PPARγ at Ser273, maintain PPARγ nuclear localization, and improve insulin signaling. Pegvisomant mediated protection against GH action on MEK/ERK activation, and PPARγ-Ser273 phosphorylation (Fig. 4) showed that GH acts directly through its receptors to activate PPARγ degradation via the MEK/ERK pathway.
The observation that GH exposure and fasting in human subjects induce lipolysis via HSL activation in adipose tissue underpins the pivotal role of HSL (1, 8, 12) and is compatible with ours’ and others’ previous observation that GH-induced lipolysis is abrogated by suppression of HSL (25, 36, 37). Our previous studies show that FSP27 interacts with the rate-limiting lipase ATGL to impair its lipolytic capacity in human adipocytes (10) and also recruits Egr1 to inhibit ATGL transcription (39). In our human subjects, we did not record consistent effects of GH alone on either CGI-58 or PLIN1. CGI-58 is an activator of ATGL, and PLIN1 is a lipid droplet-associated protein that acts as a scaffold to protect lipid droplets in adipocytes (4). Both of these effects are compatible with fasting-induced activation of lipolysis but do not seem to depend on GH. The antilipolytic effect of the hyperinsulinemic-euglycemic clamp in the control experiment was diminished by GH. Our mechanistic in vitro data suggest that the suppressive effects of GH on FSP27 could, at least in part, cause this effect. FSP27 overexpression protected against GH-induced lipolysis in cultured human adipocytes, which is perhaps due to its effect on ATGL (10). Whether FSP27 also regulates HSL activation remains to be investigated.
Our data show that ATGL gene expression in adipose tissue of human subjects reduced upon fasting alone and in combination with GH under basal conditions; however, ATGL expression was maintained under clamped conditions, consistent with the findings that insulin acutely regulates expression of PPARγ in human adipose tissue (32). Similar results were obtained in cultured human adipocytes. On the other hand, the expression of CGI-58 did not change in these conditions. Perhaps an interplay between FSP27, G0S2, and CGI-58 to regulate ATGL-mediated lipolysis has an overall effect of increased lipolysis. It is also possible that, in the absence of FSP27 and G0S2, ATGL is readily available to CGI-58 to induce lipolysis under basal conditions. Furthermore, lipid droplet fragmentation might have an additional effect on increased lipolysis due to an increased surface area of lipid droplets.
Proinflammatory cytokines and obesity increase PPARγ Ser273 phosphorylation by stimulating Cdk5, which is both necessary and sufficient to phosphorylate PPARγ at Ser273 in cultured adipocytes (2, 7). However, metabolic analysis of adipose-specific deletion of Cdk5 knockout mouse shows increased levels of PPARγ Ser273 phosphorylation, impaired insulin, and glucose tolerance, strongly suggestive of a compensatory mechanism (2). A previous study has proposed that Cdk5 negatively regulates MEK, and therefore Cdk5 deletion may render MEK/ERK constitutively active and lead to PPARγ phosphorylation at Ser273 (38). Interestingly, a recent study has provided evidence for the hypothesis that the ERK-CDK5 axis controls the diabetogenic actions of PPARγ and that ERK inactivation eliminates inhibitory phosphorylation of PPARγ at Ser273 (2). Similarly, GH increased PPARγ phosphorylation at Ser273 in a MEK/ERK-dependent manner. Of special interest was the observation that FSP27 overexpression blocked PPARγ Ser273 phosphorylation, suggesting that FSP27 protects PPARγ Ser273 from inhibitory phosphorylation by ERK/Cdk5. FSP27 did not block MEK/ERK pathway activation in response to GH signaling; however, pegvisomant blocked the MEK/ERK pathway and protected PPARγ Ser273 phosphorylation. Additionally, GH-mediated MEK activation caused nuclear export of PPARγ, whereas FSP27 overexpression retained PPARγ in the nucleus.
We observed that GH treatment increased lipolysis and suppressed insulin signaling in adipocytes in vitro, which was reversed by FSP27 overexpression, consistent with previous studies showing that FSP27 protects against insulin resistance in adipose tissue. As previously mentioned, suppression of lipolysis with acipimox abrogates GH-induced insulin resistance in skeletal muscle (25, 35–37), which also supports a causal link between elevated fatty acid levels and insulin resistance. However, in vivo studies in human subjects do not support that GH-induced insulin resistance involves suppression of insulin signaling (16). It also remains to be investigated if inhibitors of lipolysis other than acipimox exhibit similar effects.
In summary, based on our mechanistic studies, we propose that GH-induced lipolysis depends on suppression of FSP27 expression via a PPARγ-dependent pathway. Because lipolysis is both critical for survival during fasting and causally linked to insulin resistance, our findings may identify therapeutic targets for both weight control and diabetes.
GRANTS
This work was supported by start-up funds from the Ohio University College of Osteopathic Medicine (V. Puri), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-101711 (V. Puri), the Osteopathic Heritage Foundation’s Vision 2020 (V. Puri), the state of Ohio's Eminent Scholar Program that includes a gift from Milton and Lawrence Goll (J. J. Kopchick), a MERCK-CCI award (2018; J. O. L. Jørgensen), and a research grant from The Lundbeck Foundation (J. O. L. Jørgensen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.K., J.O.L.J., and V.P. conceived and designed study; V.M.S., E.T.V., N.J., P.K.-T., B.N., T.S.N., M.H.V., N.M., and R.S. performed experiments; V.M.S., E.T.V., N.J., R.S., J.J.K., J.O.L.J., and V.P. analyzed data; V.M.S., E.T.V., N.J., R.S., K.Y.L., J.J.K., J.O.L.J., and V.P. interpreted results of experiments; V.M.S., E.T.V., and N.J. prepared figures; V.M.S., E.T.V., N.J., J.J.K., J.O.L.J., and V.P. drafted manuscript; V.M.S., E.T.V., N.J., P.K.-T., B.N., T.S.N., M.H.V., N.M., R.S., K.Y.L., J.J.K., J.O.L.J., and V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Andreas Buch Møller and Helle Zibrandtsen are acknowledged for technical support.
Correspondence may also be addressed to J. O. L. Jørgensen (email: joj@clin.au.dk) and J. J. Kopchick (e-mail: kopchick@ohio.edu).
REFERENCES
- 1.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273: 215–221, 1998. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 2.Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik-Bogoslavski D, Shulman GI, Gygi SP, Spiegelman BM. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 517: 391–395, 2015. doi: 10.1038/nature13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10, 1997. doi: 10.2337/diab.46.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48: 2547–2559, 2007. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol 27: 803–817, 2007. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med 312: 1473–1479, 1985. doi: 10.1056/NEJM198506063122302. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 466: 451–456, 2010. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garton AJ, Campbell DG, Cohen P, Yeaman SJ. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett 229: 68–72, 1988. doi: 10.1016/0014-5793(88)80799-3. [DOI] [PubMed] [Google Scholar]
- 9.Glick SM, Roth J, Yalow RS, Berson SA. Immunoassay of human growth hormone in plasma. Nature 199: 784–787, 1963. doi: 10.1038/199784a0. [DOI] [PubMed] [Google Scholar]
- 10.Grahn TH, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, Smas CM, Zechner R, Lass A, Puri V. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem 289: 12029–12039, 2014. doi: 10.1074/jbc.M113.539890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grahn TH, Zhang Y, Lee MJ, Sommer AG, Mostoslavsky G, Fried SK, Greenberg AS, Puri V. FSP27 and PLIN1 interaction promotes the formation of large lipid droplets in human adipocytes. Biochem Biophys Res Commun 432: 296–301, 2013. doi: 10.1016/j.bbrc.2013.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266: 11341–11346, 1991. [PubMed] [Google Scholar]
- 13.Gürtler A, Kunz N, Gomolka M, Hornhardt S, Friedl AA, McDonald K, Kohn JE, Posch A. Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem 433: 105–111, 2013. doi: 10.1016/j.ab.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 273: 31327–31336, 1998. doi: 10.1074/jbc.273.47.31327. [DOI] [PubMed] [Google Scholar]
- 15.Jambunathan S, Yin J, Khan W, Tamori Y, Puri V. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One 6: e28614, 2011. doi: 10.1371/journal.pone.0028614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessen N, Djurhuus CB, Jørgensen JO, Jensen LS, Møller N, Lund S, Schmitz O. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab 288: E194–E199, 2005. doi: 10.1152/ajpendo.00149.2004. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen JO, Møller J, Alberti KG, Schmitz O, Christiansen JS, Orskov H, Moller N. Marked effects of sustained low growth hormone (GH) levels on day-to-day fuel metabolism: studies in GH-deficient patients and healthy untreated subjects. J Clin Endocrinol Metab 77: 1589–1596, 1993. doi: 10.1210/jcem.77.6.8263146. [DOI] [PubMed] [Google Scholar]
- 18.Jørgensen JO, Vahl N, Hansen TB, Thuesen L, Hagen C, Christiansen JS. Growth hormone versus placebo treatment for one year in growth hormone deficient adults: increase in exercise capacity and normalization of body composition. Clin Endocrinol (Oxf) 45: 681–688, 1996. doi: 10.1046/j.1365-2265.1996.8720883.x. [DOI] [PubMed] [Google Scholar]
- 19.Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, Chiang SH, Nielsen AR, Fischer CP, Pedersen BK, MacDougald OA. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem 283: 14355–14365, 2008. doi: 10.1074/jbc.M708323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MJ, Pickering RT, Puri V. Prolonged efficiency of siRNA-mediated gene silencing in primary cultures of human preadipocytes and adipocytes. Obesity (Silver Spring) 22: 1064–1069, 2014. doi: 10.1002/oby.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, Bandyopadhyay G, Scadeng M, Ofrecio JM, Nalbandian S, Olefsky JM. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell 147: 815–826, 2011. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Møller N, Jørgensen JO, Alberti KG, Flyvbjerg A, Schmitz O. Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. J Clin Endocrinol Metab 70: 1179–1186, 1990. doi: 10.1210/jcem-70-4-1179. [DOI] [PubMed] [Google Scholar]
- 23.Møller N, Schmitz O, Jøorgensen JO, Astrup J, Bak JF, Christensen SE, Alberti KG, Weeke J. Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab 74: 1012–1019, 1992. doi: 10.1210/jcem.74.5.1569148. [DOI] [PubMed] [Google Scholar]
- 24.Nellemann B, Vendelbo MH, Nielsen TS, Bak AM, Høgild M, Pedersen SB, Biensø RS, Pilegaard H, Møller N, Jessen N, Jørgensen JO. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol (Oxf) 210: 392–402, 2014. doi: 10.1111/apha.12183. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S, Møller N, Christiansen JS, Jørgensen JO. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50: 2301–2308, 2001. doi: 10.2337/diabetes.50.10.2301. [DOI] [PubMed] [Google Scholar]
- 26.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118: 2808–2821, 2008. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nørrelund H, Djurhuus C, Jørgensen JO, Nielsen S, Nair KS, Schmitz O, Christiansen JS, Møller N. Effects of GH on urea, glucose and lipid metabolism, and insulin sensitivity during fasting in GH-deficient patients. Am J Physiol Endocrinol Metab 285: E737–E743, 2003. doi: 10.1152/ajpendo.00092.2003. [DOI] [PubMed] [Google Scholar]
- 28.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem 282: 34213–34218, 2007. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 29.Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA 105: 7833–7838, 2008. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raben MS. Growth hormone. 1. Physiologic aspects. N Engl J Med 266: 31–35, 1962. doi: 10.1056/NEJM196201042660109. [DOI] [PubMed] [Google Scholar]
- 31.Rabinowitz D, Zierler KL. A metabolic regulating device based on the actions of human growth hormone and of insulin, singly and together, on the human forearm. Nature 199: 913–915, 1963. doi: 10.1038/199913a0. [DOI] [PubMed] [Google Scholar]
- 32.Rieusset J, Andreelli F, Auboeuf D, Roques M, Vallier P, Riou JP, Auwerx J, Laville M, Vidal H. Insulin acutely regulates the expression of the peroxisome proliferator-activated receptor-gamma in human adipocytes. Diabetes 48: 699–705, 1999. doi: 10.2337/diabetes.48.4.699. [DOI] [PubMed] [Google Scholar]
- 33.Roth J, Glick SM, Yalow RS, Bersonsa. Hypoglycemia: a potent stimulus to secretion of growth hormone. Science 140: 987–988, 1963. doi: 10.1126/science.140.3570.987. [DOI] [PubMed] [Google Scholar]
- 34.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magré J, Raymond-Barker P, Murgatroyd PR, Chawla A, Skepper JN, Chatterjee VK, Suliman S, Patch AM, Agarwal AK, Garg A, Barroso I, Cinti S, Czech MP, Argente J, O’Rahilly S, Savage DB; LD Screening Consortium . Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med 1: 280–287, 2009. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgin B, Marcovecchio ML, Williams RM, Jackson SJ, Bluck LJ, Humphreys SM, Acerini CL, Dunger DB. Effects of growth hormone and free fatty acids on insulin sensitivity in patients with type 1 diabetes. J Clin Endocrinol Metab 94: 3297–3305, 2009. doi: 10.1210/jc.2009-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC. Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol 149: 511–519, 2003. doi: 10.1530/eje.0.1490511. [DOI] [PubMed] [Google Scholar]
- 37.Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC. Inhibition of the rise in FFA by Acipimox partially prevents GH-induced insulin resistance in GH-deficient adults. J Clin Endocrinol Metab 86: 5813–5818, 2001. doi: 10.1210/jcem.86.12.8096. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem 277: 528–534, 2002. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]
- 39.Singh M, Kaur R, Lee MJ, Pickering RT, Sharma VM, Puri V, Kandror KV. Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J Biol Chem 289: 14481–14487, 2014. doi: 10.1074/jbc.C114.563080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan X, Cao Z, Li M, Xu E, Wang J, Xiao Y. TNF-α downregulates CIDEC via MEK/ERK pathway in human adipocytes. Obesity (Silver Spring) 24: 1070–1080, 2016. doi: 10.1002/oby.21436. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka N, Takahashi S, Matsubara T, Jiang C, Sakamoto W, Chanturiya T, Teng R, Gavrilova O, Gonzalez FJ. Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J Biol Chem 290: 3092–3105, 2015. doi: 10.1074/jbc.M114.605980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Lu X, Lombès M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab 11: 194–205, 2010. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zierler KL, Rabinowitz D. roles of insulin and growth hormone, based on studies of forearm metabolism in man. Medicine (Baltimore) 42: 385–402, 1963. doi: 10.1097/00005792-196311000-00002. [DOI] [PubMed] [Google Scholar]