Abstract
Differences in the timing of exoskeleton melanization and sclerotization are evident when comparing eusocial and solitary bees. This cuticular maturation heterochrony may be associated with life style, considering that eusocial bees remain protected inside the nest for many days after emergence, while the solitary bees immediately start outside activities. To address this issue, we characterized gene expression using large-scale RNA sequencing (RNA-seq), and quantified cuticular hydrocarbon (CHC) through gas chromatography-mass spectrometry in comparative studies of the integument (cuticle plus its underlying epidermis) of two eusocial and a solitary bee species. In addition, we used transmission electron microscopy (TEM) for studying the developing cuticle of these and other three bee species also differing in life style. We found 13,200, 55,209 and 30,161 transcript types in the integument of the eusocial Apis mellifera and Frieseomelitta varia, and the solitary Centris analis, respectively. In general, structural cuticle proteins and chitin-related genes were upregulated in pharate-adults and newly-emerged bees whereas transcripts for odorant binding proteins, cytochrome P450 and antioxidant proteins were overrepresented in foragers. Consistent with our hypothesis, a distance correlation analysis based on the differentially expressed genes suggested delayed cuticle maturation in A. mellifera in comparison to the solitary bee. However, this was not confirmed in the comparison with F. varia. The expression profiles of 27 of 119 genes displaying functional attributes related to cuticle formation/differentiation were positively correlated between A. mellifera and F. varia, and negatively or non-correlated with C. analis, suggesting roles in cuticular maturation heterochrony. However, we also found transcript profiles positively correlated between each one of the eusocial species and C. analis. Gene co-expression networks greatly differed between the bee species, but we identified common gene interactions exclusively between the eusocial species. Except for F. varia, the TEM analysis is consistent with cuticle development timing adapted to the social or solitary life style. In support to our hypothesis, the absolute quantities of n-alkanes and unsaturated CHCs were significantly higher in foragers than in the earlier developmental phases of the eusocial bees, but did not discriminate newly-emerged from foragers in C. analis. By highlighting differences in integument gene expression, cuticle ultrastructure, and CHC profiles between eusocial and solitary bees, our data provided insights into the process of heterochronic cuticle maturation associated to the way of life.
Introduction
The exoskeleton (cuticle) enables insects to exploit a multitude of ecological habitats, and is central to their evolutionary success and worldwide expansion. It is necessary for muscles attachment, and for protection against predators, injuries, and pathogens [1]. In addition, its thickness is positively correlated with the resistance to some types of insecticides [2]. The exoskeleton is periodically shed and a new, larger one is formed, this characterizing the successive molting episodes that allow for insect growth and development. Its composition is defined by the secretion of products synthesized by the epidermis as well as by the uptake of molecules from other sources, for instances, hemolymph [3]. These products are used for cuticle renewal at each molting episode coordinated by changes in the titer of 20-hydroxyecdysone (20E), the active product of ecdysone hydroxylation. The Ashburner model postulated to explain 20E-induced chromosomal puffs in the larval salivary glands of D. melanogaster have ultimately led to the knowledge of molecular elements regulating molting and metamorphosis [4]. When 20E binds to the heterodimeric receptor consisting of EcR (Ecdysone receptor) and Usp (Ultraspiracle) proteins, its trigger a transcription factor regulatory cascade. Upstream elements of this cascade respond to the high 20E titer that also induces apolysis and initiates molting, whereas most downstream elements are only induced by the subsequent decrease in 20E titer. Binding sites for several of the transcription factors in this cascade were identified in many cuticular protein genes [5], suggesting that they, and other genes involved in cuticle remodeling [6, 7] are indirectly regulated by 20E.
The exoskeleton comprises an inner procuticle formed by layers of endocuticle and exocuticle, an outer epicuticle and the superficial envelope. The procuticle consists of a variety of proteins and chitin, a polymer of the glucose-derived N-acetylglucosamine. Chitin is a major compound in the insect exoskeleton [8]. Key enzymes in the chitin biosynthetic pathway are the highly conserved chitin synthases (ChS) that catalyze the transformation of UDP-N-acetylglucosamine to chitin. Chitin-modifying enzymes, specifically chitin deacetylases (Cdas), catalyze the conversion of chitin to chitosan, a polymer of β-1,4-linked d-glucosamine residues. Mutations in Cda genes are lethal to insect embryos, suggesting that these enzymes play critical roles during development, including the molting process [9]. Molting involves digestion of the actual cuticle, a process mediated by chitin-degrading enzymes, chitinases (Cht), which accumulate in the molting fluid [10]. The epicuticle does not contain chitin, but contains proteins and lipids and is rich in quinones, which are oxidized derivatives of aromatic compounds [11]. Together with chitin, the structural cuticular proteins constitute the bulk of insect cuticle. Based on defining sequence domains, they have been classified into twelve families [12]. Proteins in the CPR family, with the largest number of members, contain the R&R Consensus [13, 14]. Some other structural cuticular proteins pertain to the Tweedle (Twdl) class [15], or were classified as Cuticular Proteins of Low Complexity–Proline-rich (CPLCP), Cuticular Proteins with Forty-four amino acid residues (CPF), Cuticular proteins analogous to peritrophins (Cpap), Glycine-Rich cuticular Proteins (GRP), and apidermins (Apd), among other classes. Some cuticular proteins, however, do not fill the features for inclusion in the pre-established classes. The main components of the envelope are the cuticular hydrocarbons (CHC) [16] that play roles in chemical communication (unsaturated CHC) [17, 18] and, together with other lipids, act as a barrier against insect desiccation by preventing water loss (mainly n-alkanes) [17, 19]. Key enzymes in CHC biosynthetic pathways occurring in the epidermis-associated oenocytes are the desaturases and elongases [20–22]. We previously determined gene expression profiles of six desaturases and ten elongases in the developing integument of A. mellifera, and correlated them with n-alkanes, methyl-alkanes, dimethyl-alkanes, alkenes and alkadienes quantification profiles [23]. Besides highlighting the CHC composition underlying envelope formation, these data provided clues to predict the function of these genes in CHC biosynthetic pathways.
In addition to chitin, cuticular proteins, CHCs, and other compounds, melanin pigments are crucial for the exoskeleton formation in insects. The chemical reactions in the core of the melanin biosynthetic pathway are evolutionary conserved. This pathway comprises the conversion of tyrosine into 3,4-dihydroxyphenylalanine (dopa) by the action of tyrosine hydroxylase (TH). Dopa is converted to dopamine, the primary precursor of insect melanin, via a decarboxylation reaction catalyzed by dopa decarboxylase (Ddc). Dopa or dopamine is further oxidized to dopaquinone or dopaminequinone, and finally these pigment precursors are converted into dopa-melanin or dopamine-melanin through reactions catalyzed by dopachrome conversion enzyme, a product of the yellow gene, and laccase2 (Lac2). Alternatively, dopamine is acetylated to N-acetyl-dopamine (NADA), and in conjugation with α-alanine originates N-β-alanyldopamine (NBAD). Both catechols are precursors for production of colorless and yellowish sclerotins [24, 25]. Thus, melanization occurs concomitantly to sclerotization through a shared biosynthetic pathway. Both processes are fundamental for the exoskeleton development [26], and are developmentally regulated by 20E [27, 28].
Among bees, we can distinguish the solitary and eusocial species. In the solitary species, every female constructs its own nest where it lay eggs, but does not provide care for the ecloded larvae. In contrast, the social organization is grounded on the division of labor between fertile queens and more or less sterile, or completely sterile, workers that are engaged in nest construction and maintenance, besides caring for the queen’s offspring [29, 30]. The search for genomic signatures of eusociality evolution in bees has grown since the publication of the A. mellifera genome [31] and gained force with the recent release of two Bombus species genomes [32] and the study of Kapheim et al. [33] comparing the genomes of ten bee species.
In this context, we draw our attention to the fact that bee species greatly vary in the grade of cuticle melanization/sclerotization at the emergence time (adult ecdysis). In a previous study on the morphology of the developing adult cuticle [34], we observed that in eusocial bees, but not in the solitary ones, the process of cuticle melanization/sclerotization leading to cuticle maturation is extended to the adult stage. After emergence, workers from eusocial species (including the primitively eusocial bees from Bombini) spend some days performing inside nest activities, and during this period they stay protected in a safe and provisioned environment [35] where the hygienic behavior provides a certain level of immunity [36]. In contrast, the newly emerged solitary bees immediately leave the nest. Therefore, they need a fully mature cuticle to protect them in the external environment. This shift in the timing of cuticle maturation seems a case of heterochrony, which is defined as a change in the timing of development of a tissue or anatomical part relative to an ancestor, or between taxa [37].
Here, we used the integument (cuticle and its subjacent epidermis) in an approach based on large-scale RNA sequencing (RNA-seq), transmission electron microscopy (TEM) and gas chromatography-mass spectrometry (GC/MS) to describe cuticle maturation in two eusocial bee species, Apis mellifera (Apini) and Frieseomelitta varia (Meliponini), and a solitary bee species, Centris analis (Centridini), the solitary lifestyle being considered the ancestral condition for bees [38]. TEM was also used for studying the ultrastructure of the cuticle of the primitively eusocial bee, Bombus brasiliensis (Bombini), the facultatively eusocial Euglossa cordata (Euglossini), and the solitary bee, Tetrapedia diversipes. The phylogenetic relationships among the studied bee species considering their social complexity levels is shown in S1 Fig. The combined methodological tools allowed us to compare the integuments at the morphological and molecular levels, besides highlighting differences that could be related to the heterochronic process of cuticle maturation. Among the genes expressed in the integument, we focused on those involved in the melanization/sclerotization pathway, related to chitin, genes encoding structural cuticular proteins, regulators of cuticle renewal and tanning, desaturase and elongase genes potentially involved in CHC biosynthesis, circadian clock genes that could determine the rhythm of cuticle layers deposition [39, 40], and genes encoding pigments other than melanin. Our study included bees at three different developmental phases: pharate-adults at the Pbm phase (classified according to Michelette and Soares [41]), where the adult cuticle in process of pigmentation is apparent underneath the disintegrating pupal cuticle [42], newly-emerged (newly-ecdysed) bees, and foragers. During this time interval, the remarkable developmental events are the intensification of pigmentation/sclerotization of the adult cuticle (cuticle maturation) that can extend through early adulthood, the imaginal molting culminating in the ecdysis, and the emergence of the adults from the brood cells.
The comparison of integument transcriptomes of three bee species at these developmental points gave us back the discovery of distinct genetic signatures, and highlighted differences in gene set expression profiles. The use of TEM and CHC analysis complemented these data by adding new information on cuticle ultrastructure and chemical profiles of its superficial layer, the envelope.
Results
Differential gene expression in the integument of A. mellifera, F. varia and C. analis during adult cuticle formation/maturation
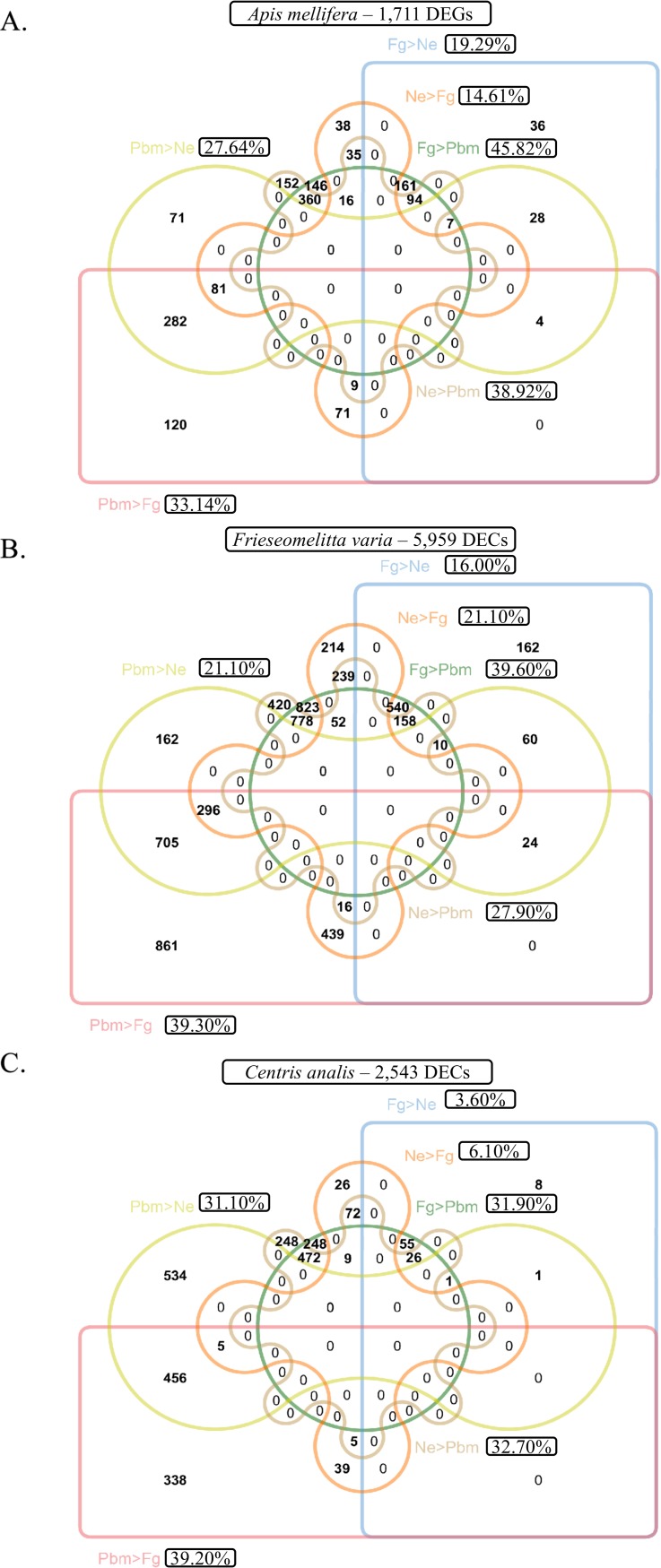
We identified the expression of 13,200 genes in the developing integument of A. mellifera, and 55,209 and 30,161 contigs in the developing integument of F. varia and C. analis, respectively (S1 File). The data obtained from the three biological samples of each developmental phase, Pbm (pharate adult), Ne (newly-emerged) and Fg (forager) of each bee species, in a total of 27 transcriptomes, were used in Pearson correlation analysis in order to check reproducibility. A hierarchical clustering on pairwise correlation is shown in S2 Fig. In general, the samples of the same developmental phase (biological triplicates) joined together, indicating that they are more similar to each other than to samples of the other developmental phases. As expected, for the three bee species, the least correlated samples were those originated from the Pbm and Fg integuments. When filtering these data sets for the genes (DEGs) or contigs (DECs) differentially expressed between the developmental phases, we found 1,711 DEGs for A. mellifera, 5,959 DECs for F. varia and 2,543 DECs for C. analis, representing 12.96%, 10.8%, and 8.4% of the identified genes, respectively. Fig 1 shows the number and percentages of genes that were upregulated in the comparisons between the developmental phases of the three bee species. These percentages of upregulated genes significantly differed between the developmental phases of the bee species (z test, p≤0.001 for all comparisons excluding one where p = 0.017), except for one of the comparisons: F. varia and C. analis showed similar proportions (z test, p = 0.950) of genes upregulated in the Pbm phase in detriment of the Fg fase (Fig 1B: Pbm>Fg = 39.3%; Fig 1C: Pbm>Fg = 39.2%). In addition, it was evident that C. analis showed the lowest percentages of genes upregulated in the comparisons between the Ne and Fg phases (Fig 1C: Ne>Fg = 6.1% and Fg>Ne = 3.6%), comparatively suggesting a higher stability of the adult cuticle from the emergence to the forager phase.
Fig 1.
Venn diagrams constructed with the genes and contigs differentially expressed in the integument of the developmental phases of (A) A. mellifera, (B) F. varia, and (C) C. analis. The number of genes upregulated in each pairwise comparison is indicated. Pbm: pharate adults; Ne: newly-emerged bees; Fg: foragers.
S2 File specifies the genes upregulated between the developmental phases and bee species. Among the DEGs and DECs, it was clear that those encoding structural cuticular proteins, such as those in the CPR, Twdl, and Cpap families, and also chitin-related genes with roles in chitin metabolism, modification and degradation, were upregulated in the Pbm and/or Ne phases of the three bee species here studied. A series of sequences containing the chitin-binding peritrophin A domain were similarly overrepresented in the integument of the Pbm and/or Ne phases of F. varia and C. analis, thus being candidates to participate as structural proteins or enzymes in adult cuticle development. In contrast, genes encoding odorant-binding proteins (OBPs) that bind to pheromones thus serving as insect chemoreceptors, as well as genes encoding a variety of CYPs (cytochrome P450), and antioxidant proteins like glutathione-S-transferase (GST), glutathione peroxidase (GTPx), thioredoxin peroxidase (TPX), and superoxide dismutase (SOD), were more expressed in the mature integument of foragers of the three bee species. Therefore, genes related to discrimination of odors and chemical communication, like those encoding OBPs, which are essential for feeding, mating, toxic substances recognition [43], and kin recognition [44], were upregulated in foragers. Similarly, genes with roles in the metabolism of xenobiotics, like the CYP genes [45] and genes related to the antioxidant systems that respond to dietary and exogenous oxidants [46], which seem important for foragers in their interactions with the environment, were also upregulated in foragers. Consistent with the higher levels of juvenile hormone (JH) in foragers [47], transcripts for genes related to JH activity, specifically Krüppel homolog 1 (Kr-h1), and JH-esterase (jhe), were found in higher levels in the Fg integument of F. varia and C. analis than in the younger phases; transcripts for a JH-inducible (JHI-1) protein were overrepresented in the Fg and Ne integuments of A. mellifera in comparison to the Pbm integument. Curiously, and perhaps linked to the reproductive activity, the Fg integument of C. analis showed a higher expression of the ecdysone receptor (EcR) and seven-up, an orphan nuclear receptor belonging to the steroid receptor gene superfamily [48]. However, seven-up was also overexpressed in the Fg integument of the sterile F. varia. Defense response genes (defensin, apidaecin) were also highly expressed in the integument of A. mellifera foragers suggesting that they potentially respond more efficiently to infectious agents than the younger bees (S2 File). Such developmental differences in gene expression in the integument highlighted genes related to adult cuticle formation and also genes related to the functionality of the mature integument.
To make more comprehensive the RNA-seq analysis of the integument, we searched the Gene Ontology (GO) functional terms for all A. mellifera DEGs and all F. varia and C. analis DECs. The GO annotations for Molecular Function, Cellular Component and Biological Process categories are described in S3 File. We then extracted from this analysis the functional terms more evidently related to cuticle development (Fig 2). Structural molecule activity, chitin-binding, and chitin metabolic process were categories overrepresented in the younger phases, i.e., the Pbm and Ne phases of the three bee species. Structural constituent of cuticle, structural constituent of chitin-based cuticle, and other cuticular components-related GO categories also included genes more expressed in the Pbm and Ne integuments of both, or one of the eusocial species. Functional categories related to the epidermis, which is the tissue responsible for secreting the cuticle, specifically epithelium development, epithelial cell differentiation/development, cell adhesion, cell junction organization/assembly, among other categories, were also more represented in the younger Pbm and Ne bees, but only of the eusocial species. For the three bee species, the DEGs and DECs more related to the functionality of the integument of newly-emerged (Ne) and forager bees (Fg) (here named older phases for simplification), were included in the following overrepresented GO terms: fatty acid biosynthetic process, lipid biosynthetic process, organic acid biosynthetic process, and carboxylic acid biosynthetic process. These terms and others overrepresented in the older Ne and Fg phases of F. varia and C. analis, i.e., very-long-chain fatty acid metabolic process, and fatty acid metabolic process, could be tentatively related to CHC biosynthetic pathways. For F. varia and/or C. analis, functional terms related to pigmentation pathways (pigmentation, pigment metabolic process, pigment biosynthetic process, pigmentation during development, and terms related to eye pigments), were also significantly more represented in the Ne and Fg phases. These GO results (Fig 2) evidenced the similarities and differences in terms of cuticle-related functional attributes between the developmental phases and bee species. Some functional categories were shared by the three bee species, and a larger number of categories were shared by the two eusocial species than by one of them and C. analis.
Fig 2. Gene Ontology (GO) functional terms attributed to integument genes during adult cuticle development and maturation.
The functional terms more represented in the Pbm and Ne phases than in the Fg phase are indicated as Younger>Fg, and those more represented in the Ne and Fg phases than in the Pbm phase are reported as Older>Pbm. The green box includes GO terms related to the cuticle-producing tissue, the epidermis. Purple box: GO terms associated to structural components of the cuticle. Black box: GO terms potentially associated to CHC biosynthetic pathways. Yellow box: GO terms related to pigments and pigmentation.
Distance correlation analysis based on the RNA-seq data is partially consistent with the earlier cuticle maturation in the solitary C. analis in comparison to the eusocial A. mellifera
We used the DEGs and DECs in a distance correlation analysis in order to measure the clustering potential of the studied developmental phases of each bee species (Fig 3). This strategy allowed us to know for each of the bee species how near, or distant from each other are the Pbm, Ne and Fg developmental phases in terms of gene expression levels/patterns in the integument. Assuming that the cuticle of solitary bee species is sufficiently mature at the emergence, the hypothesis approached here was that the integument samples of the Ne and Fg phases of C. analis would cluster together, and separately from the Pbm samples. In contrast, in the eusocial species, the Pbm and Ne samples would group together, with the Fg samples forming a more distant group. The results of the distance correlation analysis using all the C. analis DECs and A. mellifera DEGs were only partially consistent with this hypothesis. As expected, our analyses showed that the A. mellifera foragers were joined together in a statistically supported group (AU = 100; BT = 99), and separated from the Pbm and Ne phases. The Pbm and Ne phases formed another cluster supported by AU values of 92, therefore a little below the 95% (AU = 95) cutoff value, and BT = 67. This analysis shows that consistently with our hypothesis, the A. mellifera foragers indeed form a separate group, distant from the Pbm and Ne phases. However, the lack of statistical support for the cluster formed by the Pbm and Ne phases highlighted that the integument of these developmental phases also show a certain grade of differential gene expression. The distance correlation analysis using C. analis DECs joined the Pbm phase integuments in a statistically supported cluster (AU = 99; BT = 100), separated from the Ne and Fg phases, as expected. However, the cluster grouping the Ne and Fg phases was not statistically supported (AU = 29; BT = 48), thus highlighting differences in integument gene expression between these phases of the solitary bee. In contrast with our hypothesis, the distance correlation analysis grouped the Ne and Fg phases of F. varia in a statistically supported cluster (AU = 99; BT = 99), separated from the Pbm phase (AU = 100; BT = 100) in spite of the very distinct cuticle melanization patterns and hardness that they exhibit.
Fig 3. Distance correlation analysis between developmental phases based on the expression of DEGs and DECs.
(A) A. mellifera; (B) F. varia; (C) C. analis. Green values (BP): bootstrap support. Red values (AU): cluster support. Arrows point to significant clusters (AU > 95%). Branch edges are shown in gray. Pbm = pharate adults. Ne = newly emerged bees. Fg = foragers.
Gene expression profiles in the integument of the eusocial (A. mellifera and F. varia) and solitary (C. analis) bee species
Heatmaps representing the expression profiles of classes of cuticle-related genes through the Pbm, Ne and Fg developmental phases were constructed and clearly showed differences between the bee species (S3 Fig).
We found in the RNA-seq libraries seven genes involved in the biosynthesis of melanin and sclerotizing compounds (see a representation of the melanin/sclerotin biosynthetic pathway in Shamim et al. [24]. The genes with roles in the melanization/sclerotization pathway, except for dopamine N-acetyltransferase (Dat), were more expressed in the younger phases (Pbm and/or Ne) of A. mellifera. Similarly, these genes, including Dat, were more expressed in the younger phases of F. varia. In contrast, in C. analis, the majority of the genes in this class, tan, ddc, lac2, yellow-y, did not significantly change their expression levels, ebony was highly expressed in the Ne and Fg phases, and the expression profile of Dat also differed from both eusocial species. TH was the only gene in this class showing a significantly higher expression level in the very same developmental phase (Ne) of the three bee species.
Searching for genes related to pigmentation pathways other than the melanin biosynthetic pathway in the integument RNA-seq libraries, such as those genes involved in pterin, ommochromes, and heme formation, we found 17 genes in A. mellifera, and 18 genes in F. varia and also in C. analis, including cardinal, scarlet, brown, vermillion, light, sepia, and henna (this one involved in both biopterin formation, and tyrosine formation for the melanization process), thus indicating that their products are necessary in the adult cuticle. We also observed that a higher proportion (66.7%) of these genes displayed higher expression levels in the adults (Ne, Fg, or both phases) of F. varia in comparison to A. mellifera (29.4%) and C. analis (27.8%) (S3 Fig).
Concerning genes encoding chitin-related proteins, we found 17, 16 and 33 of these genes in A. mellifera, F. varia and C. analis, respectively. The four Cda genes [Cda4, Cda5, vermiform (verm), and serpentine (serp)] found in the eusocial species, and five (Cda4-like, Cda-like-1, Cda-like-2, verm, serp) of the six Cda genes found in C. analis showed the higher expression in the Pbm, Ne, or both developmental phases, the other C. analis Cda gene (Cda5-like) showed no significant expression levels variation throughout the developmental phases. One of the two ChS genes found in A. mellifera (krotzkopf verkehrt, acronym kkv) and F. varia (ChS6), and two of the four ChS genes found in C. analis (ChS-kkv-like-1, ChS6-like1) were also more expressed in the Pbm and/or Ne phases whereas the other ChS genes of the three bee species did not show significant expression level variation. Six of the eleven Cht-like genes of A. mellifera (Cht-like2, Cht-2 like, chitooligosaccharidolytic-domain-like, Cht5, chitotriosidase, Cht3), and four of the ten Cht-like genes of F. varia (Cht-like1, Cht-like, chitooligosaccharidolytic-domain-like, Cht2-like2) were also more expressed in the Pbm and/or Ne phases, the remaining showing no significant variation in expression levels, except for the catalytically inactive chitinase-like encoding gene, Idgf-4, which is significantly more expressed in A. mellifera foragers. In contrast, only a small number (Cht-like12, Cht-like4, Cht-like10, Cht-like1) of the 22 Cht-like genes of C. analis were more expressed in these phases (Pbm and/or Ne), the remaining showing no significant changes in expression levels, except for Idgf-4, which is more expressed in Ne and Fg phases (S3 Fig).
The majority of the CPR genes (encoding cuticle proteins containing the RR1 or RR2 Consensus types) in the eusocial species showed significant variation in expression levels through the studied developmental phases. The proportions of RR1 and RR2 genes showing variable expression in the heatmaps illustrating S3 Fig corresponded to 94.1% and 80.9% in A. mellifera, and 66.7% and 70.6% in F. varia, respectively. In contrast, lower proportions of RR1 and RR2 genes in C. analis, corresponding to 40% and 35% respectively, showed significant variation in transcript levels. For the three bee species, most of the genes showing changing transcript levels were more expressed in the Pbm or both Pbm/Ne phases. Interestingly, a few CPR genes were significantly more expressed in the Ne phase [AmCPR19, AmCPR27, AmSgAbd1-like (an RR-endocuticle structural glycoprotein also identified in Schistocerca gregaria), FvUnCPR-1], or in both Ne and Fg phases (CaSgAbd1-like and AmUnCPR-RR2-5), and only a CPR gene, the RR1 motif AmCPR13 gene, showed a higher expression exclusively in foragers. Similarly, a higher proportion of the non-RR cuticular protein genes showed significant transcript levels variation in A. mellifera and F. varia, 90.6% and 72.2% respectively, in comparison to C. analis (64.5%). These genes were also mostly more expressed in the Pbm or Pbm/Ne phases of the three bee species. However, like some CPR genes, there were non-RR genes displaying the highest expression in adults (Ne, Fg or both phases), specifically, Apd genes in F. varia (FvApd-1) and C. analis (CaApd-1 and CaApd-2), and Cpap genes in C. analis (CaUnCpap-3, CaUnCpap-4, CaUnCpap-9, CaCpap3-e) (S3 Fig).
For the three studied bee species, a higher proportion of genes encoding elongases (Elo-genes) and desaturases (Desat-genes) putatively involved in CHC biosynthesis were more expressed in adults (Ne, Fg, or both phases) than in the Pbm phase. However, in C. analis, a higher proportion (66.7%) of these genes increased significantly their expression levels from the Pbm to the Ne phase in comparison to F. varia (26.7%) and A. mellifera (39.1%) (S3 Fig).
A higher proportion of the regulatory genes was significantly more expressed in the Pbm phase of A. mellifera (50%) and F. varia (28.6%) than in C. analis (4.5%) in which the majority of the genes (72.7%) did not show significant difference in expression levels between the developmental phases. Some regulatory genes had a higher expression in adults (Ne, Fg or both phases) of A. mellifera [Ammirr (mirror), AmUsp, AmCCAP (Crustacean Cardioactive Peptide), AmKr-h1 and Amhairy], F. varia (FvKr-h1), and C. analis (CaKr-h1, Camirr, CaE75, CaEcR and Cahairy). Two of the regulatory genes in A. mellifera, AmE75 and AmMblk (Mushroom body large type Kenyon cell specific protein-1 or E93-like), which were highly expressed in the younger Pbm phase, were also highly expressed in the older Fg phase (S3 Fig).
Four among the seven circadian rhythm genes of A. mellifera [Clk (Clock), Cry (Cryptochrome), Per (Period) and Tim2 (Timeless2)] showed the highest expression in the Pbm phase. This is in contrast to the majority of the circadian rhythm genes in F. varia [Clk, Per, Pdp1 (Par domain protein I), Vri (vrille), Tim2] and C. analis (Per, Vri, Cry, Clk, Tim2), which did not significantly change their expression levels. The genes Vri, Cyc (cycle), and Pdp1 in A. mellifera, Cyc in F. varia, and Cyc and Pdp1 in C. analis showed the highest expression in adults (Ne, Fg or both phases) (S3 Fig). It is noteworthy that the expression of these genes could be oscillating in the integument during the time interval of approximately 8h needed for collection and sampling the bees. Therefore, this should be considered when analyzing their expression profiles.
In summary, the following main differences between the social and solitary bee species were highlighted in the heatmaps (S3 Fig): (a) A higher proportion of genes involved in the melanization/sclerotization pathway, cuticle formation (RR1, RR2, and non-RR genes), and regulation (regulatory genes) showed significant transcript levels variation through the studied developmental phases of A. mellifera and F. varia in comparison with C. analis. Most of these genes were more expressed in the Pbm or Pbm/Ne phases. In C. analis, the higher proportion of genes displaying no differences in expression levels through the studied phases, were possibly highly expressed earlier, before the Pbm phase, for faster cuticle formation and maturation, but this assumption requires further investigations; (b) The number of chitin-related genes, higher in C. analis, and not their expression patterns, distinguished this species from the eusocial A. mellifera and F. varia; (c) A higher proportion of desaturase and elongase genes putatively involved in CHC biosynthesis showed significantly increased expression levels at the emergence (Ne phase) of C. analis in comparison to the eusocial ones, which is consistent with an accelerated process of cuticle maturation in the solitary bee.
Importantly, all the gene classes here studied included representatives showing increased or high expression levels in the mature integument of foragers indicating that the mature cuticle is a dynamic structure requiring structural and regulatory elements for its maintenance.
Correlation among expression profiles of genes candidates to play roles in cuticle formation/maturation in the eusocial (A. mellifera and F. varia) and solitary (C. analis) bees
We used Pearson’s correlation in order to measure the strength of the linear association between the expression profiles of 119 genes related to cuticle development and maturation (shown in S3 Fig), which shared potential orthology relationships between the bee species. A fraction of these ortholog genes showed non-significantly correlated transcript levels fluctuation among the bee species, thus highlighting peculiarities in cuticle development for each species. However, 76 orthologs (S1 Table and S4 Fig) displayed expression profiles significantly correlated at least between two of the three bee species. Importantly, the expression profiles of 21 among these 76 genes were positively correlated between the eusocial species, and negatively or non-correlated with the solitary bee (r≥0.6 and p≤0.1). In addition, other six genes, whose transcripts were not identified in C. analis, showed expression profiles positively correlated between the eusocial species. Therefore, these 27 genes are possibly contributing to differences in the processes of cuticle development and maturation in the eusocial bees versus the solitary bee. Thus, the expression profiles of genes related to the melanization/sclerotization pathway (ebony, tan) and chitin [Idgf4-like, Cda5, chitooligosacchariodolytic-domain-like], genes encoding cuticular structural proteins containing the RR1 or RR2 domains (CPR14, CPR17, CPR18, CPR23, CPR25, CPR26), or lacking these domains (Apd-3, Apd-like), and also genes in CHC pathways (Desat-GB40659, Elo-GB54401, Elo-GB54302, Elo-GB45596, Elo-GB46038), regulators of cuticle development [Ethr (Ecdysis triggering hormone receptor), E74, Hr4 (Hormone receptor 4), Hr38 (Hormone receptor 38), FTZ-F1 (Fushi tarazu-factor 1), rickets, Ptx-1 (bicoid-related Paired-type homeobox gene D), circadian rhythm genes (Tim2) and a gene in the non-melanin pigmentation pathways, ALAS (δ-aminolevulinic acid synthase)], suggest roles in the differential cuticle development in the solitary versus eusocial bees.
Among the above cited 76 orthologs, we also found genes whose expression profiles were positively correlated between the solitary and eusocial bees. Thus, the following 23 genes shared expression profiles positively correlated between A. mellifera and C. analis: yellow-y (melanization /sclerotization pathway), Cda4 and ChS-kkv-like1 (chitin-related), SgAbd2-like and 97Ea-like (CPR-RR1 class), CPR10 (RR2 class), Twdl(Grp), Cpap3-a, Cpap3-b and Cpap3-c (non-RR class), Desat-GB48195, Desat-GB45034, Desat-GB42217, Elo-GB51249 and Elo-GB54404 (CHC pathways), Usp, Crustacean Cardioactive Peptide Receptor—CCAPR, mirr and hairy (regulatory genes), Cyc (circadian rhythm), vermillion, sepia and pinta-like (other pigmentation biosynthetic pathways than melanin). Similarly, the following 12 genes shared expression profiles positively correlated between F. varia and C. analis: Cda4 and ChS6 (chitin-related), Cpap3-c (non-RR class), Elo-GB54404 (CHC pathways), Bursicon—Bursβ, CCAP and E75 (regulatory genes), Cyc (circadian rhythm), vermillion-like, cardinal-like, light and scarlet (other pigmentation biosynthetic pathways than melanin).
Co-expression networks reconstructed with genes related to cuticle development and maturation, and common interactions between the networks of the eusocial A. mellifera and F. varia bees
The genes related to cuticle formation and maturation in A. mellifera, F. varia, and C. analis were separately used for co-expression networks reconstruction (S5–S7 Figs). The gene co-expression networks for the eusocial species, A. mellifera and F. varia, showed common interactions among regulatory elements [FTZ-F1, E74, Hr4, Hr46 (Hormone receptor 46)], genes encoding structural cuticular proteins (CPR14, CPR17, CPR23, CPR24, CPR25, Apd-3 and Apd-like), and encoding the elongase Elo-GB54302, Cdas [verm, serp, Cda5], and Lac2 (Fig 4). However, by intersecting the gene co-expression networks of the eusocial A. mellifera and the solitary C. analis, we found only one common interaction comprising the genes yellow-y and Cpap3-a. Similarly, only the interactions between CPR16 and Eh-like (Eclosion hormone), and tan/Elo-GB45596 were highlighted as being common to the eusocial F. varia and the solitary C. analis after superimposing their respective gene co-expression networks.
Fig 4. Overlapping interactions in the gene co-expression networks reconstructed with A. mellifera (S5 Fig) and F. varia (S6 Fig) genes related to cuticle formation and maturation.
Ultrastructure and thickness of the developing adult cuticle shows conspicuous differences among the eusocial, primitively eusocial, facultatively eusocial, and solitary bee models
The morphology of the developing adult cuticle is shown for the eusocial A. mellifera and F. varia bees, for the primitively eusocial Bombus brasiliensis, for the facultatively social Euglossa cordata, and for two solitary bees, C. analis and T. diversipes (Fig 5). For A. mellifera, there were no noticeable modifications in cuticle ultrastructure from the pharate-adult phase (Pbm) to 48h after emergence. Up to this time, only the exocuticle was deposited. At 72h, endocuticle layers became apparent in the micrographs (Fig 5A). Cuticle ultrastructure was very similar in 96h-aged A. mellifera bees and foragers (Fig 5A). We then measured the thickness of the cuticle in seven time points of A. mellifera development (Fig 5A’). As the cuticle measurements in the groups of bees aging 0h to 96h post-emergence, and in the group of foragers, did not show a normal distribution (Shapiro-Wilk normality test, p = 0.0074) we used the Kruskal-Wallis test associated with the post hoc Conover-Iman test and Bonferroni correction to compare the sample collection data. Foragers have a significantly thicker cuticle in comparison to the earlier developmental phases, i.e., the Pbm phase, and bees at 0h, 24h and 48h after emergence (Fig 5A’). At 72h and 96h post-emergence, cuticle measurements values did not significantly differ from foragers. Differently, the cuticle of the eusocial F. varia showed very little variation in morphology (Fig 5B), and no significant variation in thickness (Fig 5B’) from the Pbm phase to the forager time. For the solitary species, C. analis, we observed remarkable differences in cuticle ultrastructure (Fig 5C) and thickness (Fig 5C’) between the Pbm and Ne phases, whereas the cuticles of the Ne and Fg phases were very similar. Pore canals are abundant in the Pbm cuticle of C. analis. At the Ne and Fg phases, the C. analis cuticle can be described as a succession of lamellae, the most superficial ones, i.e., those first deposited, became thicker and reached a higher degree of differentiation (Fig 5C). Like C. analis, the cuticle of B. brasiliensis (Fig 5D and 5D’), E. cordata (Fig 5E and 5E’), and T. diversipes (Fig 5F and 5F’), did not show noticeable ultrastructural changes, or statistically significant thickness differences, from the emergence (Ne phase) to the forager time (Fg phase).
Fig 5. Ultrastructure and thickness of the developing and mature adult cuticle of bees differing in ways of life.
(A) A. mellifera (eusocial), (B) F. varia (eusocial), (C) C. analis (solitary), (D) B. brasiliensis (primitively eusocial), (E) E. cordata (facultatively eusocial), and (F) T. diversipes (solitary). Developmental phases are indicated: Pbm (pharate-adult); Ne (newly-emerged, 0h); 24h, 48h, 72h, and 96h after adult emergence; Fg (forager). The cuticle/epidermis junction was used to align the cuticle images. The number of cuticle samples measured (N) is indicated for the Pbm, Ne and Fg phases of each bee species. (A’- F’) Cuticle thickness measurements (μm) for the corresponding bee species (see S3 Table). Different lowercase letters indicate significant statistical difference between the developmental phases of each species. The scales bars are set to 1 μm.
S3 Table summarizes the means and standard deviations of cuticle thickness measurements (μm) in the developmental phases of the studied bee species. It was evident that while cuticle thickness remains unaltered from the Pbm to the Ne phases of the eusocial species (A. mellifera and F. varia), it triples in C. analis. When comparing cuticle thickness of the Ne and Fg phases of the six studied species, we did not find significant differences, except for A. mellifera, which showed an increase in cuticle thickness of more than two times. The measurements in S3 Table also highlighted that F. varia has the thinner cuticle.
Together, these data indicate that the process of cuticle deposition in the solitary species (C. analis and T. diversipes), primitively eusocial species (B. brasiliensis), and facultatively eusocial species (E. cordata) is completed or almost completed at the time of adult emergence. In contrast, in A. mellifera, the endocuticle is deposited only after the emergence. Surprisingly, the cuticle of the eusocial F. varia species does not undergo significant variation in ultrastructure and thickness from the Pbm to the Fg phases, although a great increase in pigmentation and sclerotization is clearly noticed in in vivo observations.
Cuticular n-alkanes mark the earlier cuticle maturation in the solitary C. analis compared to the eusocial A. mellifera and F. varia bee species
The CHC composition of the superficial cuticle layer, the envelope, was determined for A. mellifera, F. varia and C. analis as another strategy potentially able to uncover differences that could be associated to the cuticle maturation heterochrony. The proportion of CHCs in the chromatograms, the significance level of each peak and the contribution of these peaks for discriminating the developmental phases of the eusocial and solitary species are shown in S4 File. The Euclidean distance clustering analysis applied to the total CHC quantification data clearly discriminated the Fg phase from the earlier Pbm and Ne phases in the eusocial bees, A. mellifera and F. varia, as well as in the solitary C. analis (S8 Fig). Total CHC quantification data grouped together the Pbm and Ne samples of A. mellifera (AU = 100; BT = 100), F. varia (AU = 100; BT = 100), and C. analis (AU = 96; BT = 88). For F. varia, the group including Ne samples showed AU = 95 and BT = 87, which is a moderate to high BT value usually associated with Bayesian posterior probabilities ≥ 95% [49]. A similar result was obtained for the F. varia Pbm samples (AU = 94; BT = 87). For the two eusocial species, the Fg samples grouped with maximal AU (100) and BT (100) values. For C. analis, however, these values were not significant (AU = 78; BT = 57) (S8 Fig).
When we analyzed separately the CHC classes, n-alkanes discriminated the F. varia foragers (Fg) as a separate group (AU = 97; BT = 76), and the Ne and Pbm phases were clustered together (AU = 98; BT = 77). A similar result was obtained for the A. mellifera foragers (Fg) (AU = 94; BT = 84) that were separated from the Pbm/Ne cluster (AU = 85; BT = 77), although with AU values lower than the 95% cutoff. However, the n-alkanes did not significantly distinguish the developmental phases of C. analis (S8 Fig).
The unsaturated CHCs data from A. mellifera did not give us back a strong support for distinguishing the developmental phases. Although all the Ne samples and the majority of the Pbm samples have been grouped with a high AU value (99%), the BT = 1 value was low. Three of the A. mellifera foragers (Fg) escaped from the main cluster formed by twelve foragers (AU = 96; BT = 23). In contrast, the unsaturated CHCs discriminated each of the developmental phases of F. varia. The groups of Pbm samples (AU = 99; BT = 93) and Ne samples (AU = 99; BT = 94) were maintained together in a larger cluster (AU = 99; BT = 98), and separately from the group of Fg samples (AU = 99; BT = 97). This CHC class clustered together the Pbm and Ne samples of C. analis (AU = 96; BT = 80). The Fg samples of C. analis were separated into two clusters with AU cutoff values close to 95%. The main Fg cluster is supported by AU = 94 and BT = 70. The other Fg cluster (AU = 93 and BT = 83) is closer to Ne and Pbm phases (S8 Fig).
Branched CHCs from A. mellifera clearly clustered the Fg samples (AU = 97; BT = 94). The Ne and Pbm phases were joined together in a single well-supported group (AU = 100; BT = 100). In F. varia, separation of Fg from the earlier phases was not clear: three of the fifteen Fg samples joined to the group encompassing the Pbm and Ne samples, this group being supported by 98% AU, but showing a low BT value (BT = 3). The F. varia forager samples were also clustered with low BT values. In the solitary C. analis, the branched CHCs clustered six of the seven Fg samples into a single group (AU = 97; BT = 72), and all the Ne samples plus two of the four Pbm samples were clustered together in another group supported by AU = 99, but presenting a low BT value (BT = 39) (S8 Fig).
These data on the Euclidean distance based on the relative quantification of CHCs was contrasted with the results on the absolute quantification of CHCs (CHC μg per bee) (Table 1 and S4 File). Table 1 shows that Fg bees of the eusocial species have significantly higher quantities of n-alkanes than the Ne and Pbm bees, which is not true for C. analis. Therefore, for the n-alkanes, the results of absolute quantification (Table 1) are consistent and reinforce the distance correlation analysis based on the relative quantification data (S8 Fig). In addition, absolute quantification of unsaturated CHCs also distinguished the foragers from the earlier developmental phases in A. mellifera, but not in C. analis. For A. mellifera, the absolute quantifications of unsaturated CHCs (Table 1) matched the results of relative quantifications (S8 Fig), and confirmed our previous data [23]. For C. analis, the increase in absolute quantities of unsaturated CHCs in the Ne phase was statistically significant (Table 1), suggesting that as earlier as in this phase, the C. analis cuticle reaches maturity. However, this was not confirmed by the distance correlation analysis based on the relative quantifications data. For F. varia, the mass of unsaturated compounds could not be quantified due to their very low quantities.
Table 1. Absolute quantification of n-alkanes and unsaturated CHCs in the cuticle of eusocial and solitary bee species.
Developmental phases are indicated: Pbm (pharate-adults), Ne (newly emeged bees), Fg (foragers). Means and standard deviations (STD) of 3 samples (N = 3) per developmental phase. Different lowercase letters in the Sig (statistical significance) column indicate difference between the developmental phases of each species.
| N-alkanes | ||
| A. mellifera | ||
| Developmental phases | Mean ± STD | Sig. |
| Pbm | 9.35965938 ± 2.89275421 | a |
| Ne | 7.36272669 ± 1.44535498 | a |
| Fg | 18.2360314 ± 4.35877417 | b |
| F. varia | ||
| Developmental phases | Mean ± STD | Sig. |
| Pbm | 1.63324436 ± 0.15390427 | a |
| Ne | 3.40142407 ± 1.35387231 | a |
| Fg | 9.28337077 ± 3.03839358 | b |
| C. analis | ||
| Developmental phases | Mean ± STD | Sig. |
| Pbm | 6.56549413 ± 1.62457012 | a |
| Ne | 14.8349947 ± 0.32610609 | b |
| Fg | 13.3273848 ± 5.07830924 | ab |
| Unsaturated | ||
| Apis mellifera | ||
| Developmental phases | Mean ± STD | Sig. |
| Pbm | 0.60042039 ± 0.17210242 | a |
| Ne | 0.92421769 ± 0.09047864 | a |
| Fg | 6.5543118 ± 2.38207067 | b |
| C. analis | ||
| Developmental phases | Mean ± STD | Sig. |
| Pbm | 9.24954719 ± 2.48578756 | a |
| Ne | 19.242 ± 2.40516304 | ab |
| Fg | 28.3380901 ± 11.855003 | b |
In summary, the Euclidean distance analysis based on the relative quantifications of n-alkanes, as well as the absolute quantifications of n-alkanes and unsaturated CHCs, were consistent with the hypothesis of interdependence between cuticle maturation timing and the eusocial/solitary ways of life. These analyses distinguished the foragers from the younger bees, but only in A. mellifera and F. varia, this being interpreted as the cuticle achieving its complete maturation tardily in the eusocial species, whereas the solitary bee emerges with an already mature cuticle.
Discussion
The RNA-seq analysis revealed the set of genes expressed in the integument of three bee species, and also the changes in gene expression as the adult cuticle is deposited and differentiates in a mature and fully functional cuticle. For A. mellifera, for which we have the sequenced genome, the genes expressed in the integument represented 95.07% of the genes in the released genome assembly version 4.5. Similar proportions will likely be found for F. varia and C. analis in the near future, after the sequencing of their respective genomes. Selected genes with potential roles in cuticle formation and maturation were characterized in terms of differential expression profiles. Co-expression networks were reconstructed. In parallel, we examined the ultrastructure of the developing adult cuticle of bee species. Furthermore, the CHC composition of the envelope, the less known cuticle layer, was also characterized. Our data expanded the knowledge on the insect integument. It is our expectation that the obtained data provide a valuable resource for future studies on exoskeleton formation and maturation in insects.
Expression profiles of cuticle-related genes may significantly differ during adult cuticle formation/maturation, and among bee species
In general, genes involved in adult cuticle formation in A. mellifera show higher expression soon after the ecdysteroid titer peak that signalizes pupal cuticle apolysis and the beginning of the pharate-adult stage [50, 51]. Consistently, the majority of the integument genes showing expression levels variation in the three bee species, and identified as playing roles in cuticle melanization/sclerotization, cuticle structure (RR1, RR2, and non-RR genes), and regulation of the molting events (regulatory genes), displayed a higher expression in pharate-adults (Pbm phase), sometimes extending their higher expression up to the emergence time (Ne phase). However, we found genes, including those related to melanization/sclerotization, other pigmentation pathways, chitin, and structural cuticle proteins, which showed the highest expression later, at emergence (Ne phase), and even in foragers (Fg phase), suggesting that their products are incorporated into the mature cuticle. Moreover, all transcripts identified in higher quantities during cuticle formation in pharate-adults were also identified in the newly emerged and forager bees, although in lower quantities. Their products may be involved in adult cuticle maintenance. Our gene expression findings indicate that the structure of the mature cuticle entails a dynamism, which has been up to now mainly characterized in studies on CHC composition of its most superficial layer, the envelope ([23], this work).
Among the genes identified in the RNA-seq analysis of the integument, we focused on classes of genes playing roles in cuticle formation and maturation, such as those below discriminated.
Genes related to cuticle pigmentation and sclerotization
The expression patterns of the first gene in the pigmentation/sclerotization biosynthetic pathway, TH, were positively correlated between A. mellifera, F. varia and C. analis, and apparently, TH does not contribute to the differential timing of cuticle pigmentation among them. Lower levels of TH transcripts were verified for the forager bees of the three bee species, which is consistent with the reported reduction in TH transcripts levels in T. castaneum [52, 53] and Diacamma sp [54] following the emergence. However, the expression patterns of ebony and tan, whose protein products act in a reversible reaction between dopamine and NBAD sclerotin [55], were positively correlated exclusively between the eusocial species, thus differentiating these species from the solitary one. The expression profiles of the remaining genes in the melanization/sclerotization pathway, including the Lac2 gene previously characterized in A. mellifera [56], did not show such correlation patterns. Interestingly, Dat showed significantly increased expression in the mature cuticle of A. mellifera foragers, which is an uncommon pattern for genes in the melanization/sclerotization pathway.
We also observed that in general, the genes involved in the biosynthesis of other pigments except melanin displayed a higher expression levels in adults (Ne, Fg, or both phases) of F. varia, which may be tentatively interpreted as these genes playing roles in the process of post-ecdysial cuticle pigmentation in this bee species. Two of these genes, cardinal and scarlet, are both necessary for ommochromes formation in B. mori [57], and are associated to the formation of red and brown pigments [58]. The expression profiles of light, which is required for pigment granules formation [59], were positively correlated in F. varia and C. analis, and might be related to the brownish and reddish color pattern typical of the cuticle of these two species. The expression profiles of the gene encoding ALAS, which catalyzes the first enzymatic step in heme biosynthesis, were positively correlated exclusively between the eusocial species, F. varia and A. mellifera. ALAS might be involved in detoxification, as suggested for D. melanogaster [60, 61], and in prevent dehydration [62]. Interestingly, in contrast to the eusocial bees, the expression of ALAS is higher in the Pbm phase of C. analis, which may suggests that mechanisms of protection against cuticle dehydration develop anticipatedly in the solitary species.
Chitin-related genes
In insects, Cht, Cda, and ChS genes have been described as highly expressed during cuticle renewal at the pharate-adult development [8, 63–68]. This was also observed in the bee species here studied. The more evident exception was the Idgf4 (Imaginal disc growth factor 4)-like gene. We included Idgf4-like in the group of chitin-related genes because its predicted product shares a high level of sequence similarity with chitinases, although members of the Idgf protein family lack chitinase activity due to an amino acid residue substitution in the catalytic site. Idgf proteins and chitinases have been considered members of a same protein family (glycosylhydrolase family 18) [69, 70]. Idgf proteins may have evolved from an ancestral chitinase but acquired a new function that does not require chitinase catalytic activity [71]. A function as growth factors in imaginal disc proliferation and differentiation has been suggested [72]. The non-enzymatic Idgf4 product is also required for cuticle formation, possibly acting as a structural protein in the protection of the newly synthesized cuticle matrix against degradation during molting [70]. Idgf proteins do not contain the typical chitin-binding domain (ChBD). However, they show the (β/α)8 TIM barrel structure, suggesting that it is important for binding carbohydrate substrates, most likely chitin or other carbohydrates containing N-acetylglucosamine [69, 70]. The expression of Idgf4 gene increases in newly-emerged C. analis, and like reported for T. castaneum [69], this may be important for the transition to the adult stage. In A. mellifera and F. varia, the expression of Idgf4-like is high in foragers, supporting roles in the mature adult cuticle.
Concerning the Cda genes, in Drosophila, they have a strict relationship with the mechanical properties of the exoskeleton [73], and this might be true for the Cda genes expressed in the integument of the bee species. The other class of chitin-related genes encodes ChS enzymes, which catalyze the last step in the chitin biosynthetic pathway and have been implied in the synthesis of epidermal cuticle in T. castaneum [74]. A ChS gene, CS-1, also called kkv, is required for procuticle formation, stabilization of the epicuticle, and attachment of the cuticle to the epidermis in D. melanogaster [75]. We found a kkv gene in A. mellifera (Amkkv) and three potential orthologs in C. analis (CaChS-kkv-like 1, CaChS-kkv-like 2, CaChS-kkv-like 3); this gene was not identified in the F. varia integument transcriptome.
Genes encoding structural cuticular proteins
The large number of different cuticular protein genes found in insect genomes suggested that their products display redundant and complementary functions [76]. A variable number of genes encode the different classes of structural cuticular proteins in the three bee species and other hymenopterans (S2 Table). Thirty-two CPR genes had been previously identified in A. mellifera [12]. We detected other six CPR genes in our RNA-seq analysis of the A. mellifera integument, and also 32 and 35 CPR genes in the integument of F. varia and C. analis, respectively. In addition to have roles as structural proteins in the horizontally arrayed cuticular laminae, the function of some CPR proteins in T. castaneum was associated to the formation and organization of the pore canals vertically extended across the cuticle [77, 78]. This finding and the variety of CPR genes identified up to now suggest that distinct and additional functions are yet to be discovered for members of the CPR protein class.
Like the class of CPR proteins, Twdl proteins are structural cuticular components that effectively bind chitin, as demonstrated in Bombyx mori [79]. Two Twdl genes were previously characterized in the thoracic integument of A. mellifera [50], and now in the abdominal integument, thus indicating that Twdl proteins participate of both rigid (thoracic) and more flexible (abdominal) cuticles. Like A. mellifera, C. analis has two Twdl genes, but we identified only one in F. varia.
Two CPLCP-encoding genes as reported in Willis [12] were herein confirmed in A. mellifera. Genes in this family were identified in insect genomes in general and are very enriched in mosquito genomes [80]. Based on sequence homology, we could not identify CPLCP transcripts in the F. varia and C. analis abdominal integument.
CPF proteins were associated to the outer cuticle layers of A. gambiae and, apparently, do not bind chitin [81]. Three CPF genes were previously reported for A. mellifera [12] and one of them, AmCPF1, was validated in the thoracic integument through microarray analysis [51]. Here we found CPF1 and CPF2 transcripts in the abdominal integument of A. mellifera, and in addition, transcripts for two other CPF proteins, AmUnCPF1 and AmUnCPF2. We also identified one CPF gene in F. varia and one in C. analis.
Apd genes seem exclusive of hymenopterans and three of these genes were previously identified in A. mellifera [82]. Their transcript levels in the thoracic integument were higher in pharate-adults compared to earlier developmental phases [51]. Here, we detected one more Apd gene in A. mellifera, AmApd-like, and three Apd genes in F. varia as well as in C. analis.
Cpap proteins are essential for the correct formation of the cuticular exoskeleton and elytra in T. castaneum [83]. In our RNA-seq analysis, we identified transcripts of three Cpap1 genes (encoding Cpap proteins containing one chitin-binding domain) in A. mellifera and two Cpap1 genes in F. varia, and also verified that the C. analis integument is very enriched in Cpap1 transcripts (n = 12), and also in Cpap transcripts (n = 11) that we could not classify as encoding Cpap1 or Cpap3 (containing three chitin-binding domains). The number of Cpap3 genes (5 genes) in A. mellifera [12] is here confirmed, and two and seven Cpap3 genes were found in the F. varia and C. analis integument transcriptomes, respectively. It is important to observe that the genes originally named as Am-C and Am-D by Soares et al. [51] were here renamed as AmCpap3-c and AmCpap3-d.
The genes, dumpy (dp), knk (knickkopf) and Rtv (Retroactive) have also been identified as encoding cuticular proteins. In D. melanogaster, dp play roles in cuticle formation [12]. We detected transcripts for dp in the abdominal integument of A. mellifera, but not in the integument of the other two bee species. The genes knk and Rtv are both involved in chitin filament assembly and chitin lamellogenesis, which are essential for procuticle organization and integument differentiation in Drosophila [84]. In T. castaneum, Rtv activity is essential for localization of the Knk protein, facilitating its transport to the cuticle [85, 86]. The co-expression of Rtv and knk in A. mellifera, as shown in the reconstructed co-expression network, supports interaction of their respective products, as verified in T. castaneum. We also found knk transcripts in C. analis integument transcriptome, but not in F. varia. Rtv transcripts were not detected in the integument of these two bee species.
Genes encoding desaturases and elongases potentially involved in CHC biosynthesis
CHC biosynthesis occurs in the epidermis-associated oenocytes [20] through biosynthetic pathways where desaturase and elongase enzymes have essential roles. Previously, we characterized the gene expression profiles of six desaturases and ten elongases in the developing integument of A. mellifera [23]. Our RNA-seq data confirmed these findings, besides identifying three more desaturase genes and other four genes encoding elongases potentially involved in CHC biosynthesis for deposition in the cuticular envelope. For A. mellifera, F. varia and C. analis, a higher proportion of the differentially expressed desaturase and elongase genes showed increased expression in the adults (Ne and/or Fg phases), and only for the eusocial species there were genes more expressed in the pharate-adults (Pbm phase). Among the desaturase and elongase genes, we highlight the expression profiles of Desat-GB40659, Elo-GB54401, Elo-GB54302, Elo-GB45596 and Elo-GB46038 orthologs, all showing positive correlation exclusively between the eusocial species.
Genes of the ecdysone signaling cascade regulating cuticle formation and ecdysis in the integument
We detected in the integument the expression of genes that are part of the signaling cascade underlying insect molting and ecdysis, such as EcR, Usp, E74, E75, FTZ-F1, CCAP, CCAPR, Eth (Ecdysis triggering hormone), Ethr, and Eh [87]. Importantly, transcripts for these regulators were also detected in greater or lesser levels after ecdysis, in the integument of adult bees. Usp, which together with EcR forms the nuclear receptor complex that binds 20E and regulates the expression of a cascade of ecdysone-responsive genes, showed the higher expression in A. mellifera foragers. This is here tentatively related to the elevated JH titer at this phase of A. mellifera worker life [43] once Usp also has been proposed as a mediator of JH action [88].
CCAP, hairy, mirr, and Kr-h1 in A. mellifera, CCAP, Kr-h1, and Met (Methoprene-tolerant) in F. varia, and Kr-h1, E75, EcR, hairy, and mirr in C. analis showed increased expression levels at the Ne and/or Fg phases. The roles of theses genes in adult bees, evidently dissociated from the molting events and metamorphosis, are yet to be determined. Kr-h1 is a direct JH-response gene. Met, the JH receptor, has roles in the crosstalk of JH and 20E signaling pathways, which are critical in the regulation of insect metamorphosis [89]. Since Met, and also hairy, mediate the action of JH on gene regulation [90], they certainly are needed in adult bees where JH has important physiological roles. The mirr gene encodes a homeodomain transcription factor with roles in Drosophila oogenesis [91]. To our knowledge, its role in the integument has not yet been studied.
Some of the identified regulatory genes have been described as playing roles in cuticular melanization, as an example, the Abdominal B (Abd-B) Hox gene, which regulates yellow in the pigmentation/sclerotization pathway in Drosophila [92]. Hairy, which is a pair-rule gene in Drosophila embryos [93], may be involved in the polarity of abdominal segment melanization. The heterodimeric neuropeptide bursicon, composed by the gene products Bursα and Bursβ, is responsible for the regulation of the laccase2-encoding gene, and is crucial for the melanization/sclerotization of the newly formed cuticle [94, 95]. Bursicon interacts with the target tissue through its receptor, the product of the rickets gene, whose transcripts were also identified in our RNA-seq analysis of the integument of the three bee species.
Searching for clues linking cuticle maturation heterochrony to eusocial or solitary life styles in the RNA-seq analysis
Our RNA-seq analyses were used to discover active genes in the integument of three bee species and, in addition, we looked for differences in gene expression profiles that could be linked to the heterochronic cuticle maturation dependent on the social/solitary ways of life. The following main findings highlighted differences in integument gene expression distinguishing the eusocial A. mellifera and F. varia from the solitary C. analis: (a) In contrast to the eusocial species, a smaller proportion of the genes differentially expressed in the integument was upregulated in C. analis foragers in comparison to the newly emerged bees, and vice-versa, which is consistent with the cuticle of the solitary bee reaching maturity already at the emergence time; (b) The GO analysis including all the integument genes displaying orthology relationship with Drosophila genes highlighted functional categories that were mainly shared by both eusocial species in detriment of the solitary C. analis; (c) The Euclidean distance analysis based on the set of genes differentially expressed in the integument of A. mellifera and C. analis is partially consistent with the earlier cuticle maturation in the solitary bee. For F. varia, however, the results of this analysis are in contrast to our hypothesis; (d) In contrast to the eusocial species, most of the genes for melanization/sclerotization, genes encoding RR1, RR2, or non-RR structural proteins, and also regulatory genes, did not show significant expression level variations in C. analis. Such differential fluctuation in transcript levels during development may have possibly contributed to the molecular heterochrony of cuticle maturation associated with bee life style. In addition, consistent with the comparatively earlier cuticle maturation process in the solitary bee, we found a higher proportion of CHC biosynthesis-related genes (desaturase and elongase genes) with significantly increased expression levels at the emergence (Ne phase) of C. analis in comparison to the eusocial bees. (e) Correlation analysis showed that a fraction of cuticle-related genes displayed congruent expression profiles between the eusocial species, but not with the solitary one, these genes possibly contributing to the heterochronic process of cuticle maturation; (f) By superimposing the integument gene co-expression networks constructed for the three bee species, we found common interactions for the eusocial species, which were not seem when we compared these species with the solitary one. The combined co-expression networks of the social species highlighted regulatory genes (FTZ-F1, E74, Hr4, Hr46) in addition of structural genes, and a gene involved in pigmentation pathway. These regulatory genes are all susceptible to ecdysone (in Drosophila) and involved in processes triggered by this hormone, such as cuticle formation leading to ecdysis. Further studies should be undertaken to confirm these network interactions as well as whether these genes regulate the heterochrony of the cuticle maturation process.
Taken together, the comparative approach of the RNA-seq data highlighted suitable gene expression signatures related to adult cuticle formation and maturation in the bee species, in addition of revealing differences in gene expression that may possibly be involved in cuticle maturation heterochrony. Yet, this process may have entailed changes in the expression profiles of regulators of molting and metamorphosis.
Abdominal adult cuticle deposition timing and its ultrastructure exhibit marked differences between the bee species
Cuticle ultrastructure and thickness did not significantly vary between the pharate adults (Pbm phase), newly-emerged (Ne) and foragers (Fg) of F. varia, as evidenced by TEM analysis. This was an unexpected result, considering that at the emergence time, F. varia workers visibly show an immature cuticle, i.e., incompletely pigmented and sclerotized. Therefore, the evident intensification of cuticular pigmentation and sclerotization in F. varia in the subsequent days after the emergence, which is necessary for flight and task performances outside the nest, do not imply in changes in abdominal cuticle thickness. It is possible, however, that thickness measurements taken from cuticular regions other than the abdominal, could evidence a different result, considering that regions of the insect body may diverge in the number of cuticle layers [96] and, consequently, in cuticle thickness.
In contrast, in A. mellifera, cuticle deposition is extended through the initial adult stage. Only in the honeybee we could identify post-ecdysially-deposited cuticle layers. Both, the pre- and post-ecdysially-deposited cuticle layers, or laminae, form the procuticle, which corresponds to the largest portion of the cuticle in insects in general. The term exocuticle has been used synonymously with pre-ecdysial cuticle, whereas those layers deposited post-ecdysially form the endocuticle. However, there is some divergence concerning these concepts [97]. In beetles, for example, up to three endocuticle layers are already present in specific areas of the body surface at the time of the adult ecdysis [96]. In Sarcophaga bullata flies, deposition of endocuticle occurs before the adult ecdysis [98].
As expected, the solitary bees, C. analis and T. diversipes, and even the primitively eusocial B. brasiliensis and the facultatively eusocial E. cordata, showed a fully deposited cuticle as soon as they emerge, and newly-emerged and forager bees in each of these species displayed similar cuticle ultrastructure, pigmentation and sclerotization. The rapid cuticle maturation in E. cordata is consistent with its nesting biology and social structure. E. cordata nests are founded by a single female that build up until ten brood cells. The offspring will leave the nest immediately after the emergence for founding new nests. However, daughters may return to the maternal nest, thus resulting in a facultatively social organization with a dominant female (the mother) and its subordinate daughters. There are also nests formed by sisters’ females or even by unrelated females, the oldest one showing dominance over the youngest. The dominant female produces all the offspring and rarely leaves the nest, whereas the subordinates assume the tasks of nest provisioning and maintenance, and they also lay trophic eggs that are eaten by the dominant [99–101]. Such female associations may have preceded the highly eusocial way of life [102]. Therefore, in E. cordata, as well as in the truly solitary bees, C. analis and T. diversipes, rapid cuticle maturation is the condition for the immediate exit from the nest after emergence.
This situation is somewhat diverse for the primitively eusocial Bombus. In B. brasiliensis, as demonstrated here, the final adult cuticle ultrastructure and thickness are achieved at the emergence. This would allow the workers start foraging soon, as reported for B. atratus workers that may leave the nest as soon as at the emergence day (0 day). However, workers of this species may start foraging later, at the age of 10–20 days [103], thus similar to the eusocial bees. Moreover, younger workers in the genus Bombus have, in general, incompletely pigmented cuticle and hairs, denoting that cuticle maturity was not yet completely achieved. Such characteristics that seem intermediary to the eusocial and solitary condition may be inherent to the primitively eusocial species, but this requires further investigation. Studies correlating the grade of cuticle pigmentation with the age of starting foraging among primitively eusocial bee species should clarify this issue.
Our TEM analysis and thickness measurements showed that in the same abdominal segment, cuticle ultrastructure greatly differs between the bee species, not only in the number of the adjacently arranged chitin/protein sheets (laminae), but also in the morphology of the most superficial layers. Except for F. varia, these results are consistent with a cuticle development timing adapted to the life style, as observed for the highly eusocial A. mellifera, the facultatively eusocial E. cordata, the primitively eusocial B. brasiliensis, and the solitary C. analis and T. diversipes bees.
Considering that the timing of cuticle deposition is peculiar to bee species, and that cuticle deposition rhythm in Drosophila is regulated by a peripheral circadian oscillator in the epidermal cells, which requires the expression of the clock genes Per, Tim2, Cyc, and Clk [39], and also that a Cry clock gene regulates the rhythm of cuticle deposition in the bean bug Riptortus pedestris [40], we compared the expression of seven circadian rhythm genes (Per, Tim2, Cyc, Clk, Cry, Vri and Pdp1) in the developing integument of A. mellifera, F. varia and C. analis.
Consistent with the differences in the timing of cuticle deposition, the expression profiles of Clk in A. mellifera, F. varia, and C. analis were negatively or non-correlated. Similarly, the expression profiles of Cry in A. mellifera and C. analis were negatively correlated (Cry was not identified in F. varia), as well as the expression of Per. It is likely that AmPer has roles in adult cuticle organization. Interaction of AmPer and other genes involved in cuticle formation was specifically observed in A. mellifera, whose sequenced genome gives more support for gene co-expression network reconstruction. In A. mellifera, Per was co-expressed with the knk gene, which in T. castaneum was associated with stabilization of the cuticular laminae [104]. Both genes were co-expressed with structural cuticular protein genes such as AmCpap3-a, AmTwdl(Grp), AmUnCPR-RR2-2, AmCPR26, Am49Ah-like and AmSgAbd2-like, and also with Amyellow-y, a gene in the yellow family, involved in cuticle pigmentation [105]. The expression profiles of another clock gene, Tim2, were positively correlated between the eusocial species, with a marked decrease in expression levels at the emergence, suggesting roles in the final step of adult cuticle formation in these bees. The Pdp1 gene encodes a basic leucine zipper transcription factor and is expressed at high levels in the epidermis and other tissues of Drosophila embryos. Pdp1 is an essential clock gene linked to the circadian rhythm. It is a regulator of Clk and other clock genes, such as Tim, Per, and Pdf (Pigment dispersing factor), a neuropeptide controlling circadian behavioral rhythms [106, 107]. Pdp1 seems an important gene in the C. analis integument since it is connected with nine structural cuticle protein genes, three chitin-related genes, and two desaturase encoding genes in the co-expression network. However, it is significantly more expressed after the emergence, when the cuticle of the solitary bee is already formed, thus virtually excluding a role in cuticle laminae deposition rhythm. Differently from C. analis, Pdp1 was not co-expressed in the networks reconstructed with the A. mellifera and F. varia genes involved in cuticle formation and maturation. Some of the cuticular genes were also co-expressed with Cyc in the integument of A. mellifera and C. analis.
Cuticular n-alkanes as markers of cuticle maturity in bees
N-alkanes are structural lipids in the insect cuticle [17, 108], where they compose the envelope [109]. The absolute quantities of n-alkanes were significantly higher in the foragers than in the earlier developmental phases of the eusocial A. mellifera and F. varia species. The n-alkanes detected in higher proportions in A. mellifera foragers than in the newly-emerged were C23, C24, C25, C26, C27, C29, C31, and C33, the C25 and C27 n-alkanes presenting the highest proportions. The analysis of the individual CHC peaks obtained from F. varia also showed higher proportions of C27 and C29, besides a higher proportion of C22, in the foragers. All these n-alkanes, except C27 and C33, were also proportionally increased in foragers than in newly-emerged bees of the eusocial Melipona marginata [110]. These data are consistent with previous reports on higher levels of n-alkanes in A. mellifera foragers [111] and in foragers of an ant species, Pogonomyrmex barbatus [112]. In contrast, the proportions and absolute quantities of n-alkanes did not differentiate foragers from the newly-emerged in C. analis. Furthermore, the absolute quantities of unsaturated CHCs also did not differentiate C. analis foragers from the newly-emerged, but significantly distinguished these developmental phases of A. mellifera. Together, these findings may be interpreted as the solitary bee displaying an accelerated process of cuticle maturation in comparison to the eusocial ones. N-alkanes may be markers of cuticle structure maturation. Long-chain alkanes are thought to increase cuticle waterproofing [112–113], suggesting that this essential ability for the performance of extra-nidal activities was acquired earlier in the development of C. analis. At the adult emergence, the solitary bee already has the chemical profile needed for a prompt interaction with the environment outside the nest. Consistently, the levels of n-alkanes also did not significantly differ between young and old females of the solitary leafcutter bee species, Megachile rotundata [114].
Conclusions
Using RNA-seq analysis of the integument of two eusocial bee species, A. mellifera and F. varia, and a solitary bee, C. analis, we identified genes involved in cuticle (exoskeleton) formation and maturation. The expression profiles of these genes were determined at three developmental time points corresponding to adult cuticle deposition/differentiation at the pharate-adult stage, newly-ecdysed cuticle, and fully developed cuticle of forager bees. TEM analysis of the cuticle at these time points, including other bee species, and CHC profiles determination were performed in addition to the transcriptome analysis. Together, these experimental approaches provided novel data on integument developmet. We also searched for clues in integument gene expression, structure, and CHC profiles that could be consistent with the premise that eusociality might have entailed heterochronic changes in cuticle development, resulting in faster cuticle maturation in the solitary bee, thus allowing flight and forager activities immediately after emergence, and in slow cuticle maturation in the eusocial bees, which benefit from the protected nest environment for a period of time after the emergence. The RNA-seq analysis highlighted differences in gene expression, GO functions, and gene co-expression networks that are consistent with our hypothesis. The results obtained with cuticle ultrastructure analysis in the solitary, primitively eusocial, and facultatively eusocial species in comparison to the eusocial A. mellifera, as well as cuticular n-alkanes absolute quantifications in A. mellifera, F. varia, and C. analis, also revealed interesting differences, which are consistent with cuticle maturation heterochrony. The distance correlation analysis based on RNA-seq data only partially supports our hypothesis. For F. varia, the results of this analysis, as well as the micrographs obtained from cuticle ultrastructure analysis, are in contrast to our hypothesis. This study expands our understanding on the molecular biology and structure of the developing integument, besides highlighting differences in the process of cuticle maturation related to the eusocial/solitary behaviors.
Materials and methods
Sample collection
We collected workers of A. mellifera (Africanized) and F. varia from colonies maintained in the Experimental Apiary of the Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil. Trap-nests to collect samples of the solitary species C. analis and T. diversipes were made [115] and placed in the Experimental Apiary area. Additional bee species (B. brasiliensis, E. cordata and T. diversipes) were obtained from donations (see acknowledgments section). Samples of A. mellifera, F. varia, and C. analis for the RNA-seq analysis were collected in a time interval of approximately 8 hours, between 8:00 a.m. and 4:00 p.m.
We used females in three developmental phases: pharate adults (Pbm phase), newly emerged adults (Ne) and foragers (Fg). Pharate adults of A. mellifera, F. varia, and C. analis were collected from their respective brood cells. They were identified according a criterium previously established for A. mellifera, based on an intermediary degree of thoracic cuticle pigmentation [41]. The newly emerged bees (0h) of the eusocial (A. mellifera, and F. varia), and solitary (C. analis and T. diversipes) species were collected immediately after they emerged from their brood cells or nests (0h). By marking the thorax of newly emerged A. mellifera workers with a small spot of water-based, non-toxic paint (Uni-Posca paint markers), and returning them to the colony, we could identify and collect them after 24h, 48h, 72h, and 96h. The facultatively eusocial E. cordata bees were obtained through donation. The newly emerged were 0 to 24h-old, as established during behavior observation studies under controlled conditions. E. cordata foragers were the dominant in the nest, besides performing foraging activities. A criterium based on body color (setal pigmentation) was used to identify newly emerged and foragers of B. brasiliensis (primitively eusocial). The cuticle of the newly emerged is not fully pigmented and is covered by unpigmented setae. Foragers are fully pigmented. This criterium traditionally used for Bombus was recently described in detail [116]. Carrying pollen bees from the solitary and social species, and building-nest females from the solitary species, were identified as foragers. B. brasiliensis, E. cordata and T. diversipes specimens were exclusively used for cuticle morphology studies through TEM. In this case, we used the Ne and Fg phases.
RNA extraction and sequencing
For each developmental phase (Pbm, Ne and Fg) of A. mellifera and F. varia, we prepared three independent samples, each made with five abdominal integuments. For the corresponding developmental phases of C. analis, we prepared three independent samples, each containing three abdominal integuments. The RNA extractions were made using TRIzol reagent (Invitrogen) following manufacturer's instruction. The extracted RNAs (2 μg/per sample) were sent to a facility (Laboratório Central de Tecnologias de Alto Desempenho em Ciências da Vida, Universidade Estadual de Campinas, Campinas, Brazil) to access sample quality through a 2100 Bioanalyzer and for library preparation (TruSeq RNA—Illumina) and RNA sequencing in an Illumina HiSeq 2500 equipment (paired-end reads, 2 x 100 bp read length). We obtained an average of 30 million reads per sample, with 90% of the bases showing quality scores > Q30. The RNA-seq data is deposited at the National Center for Biotechnology Information (NCBI) database under the BioProject ID PRJNA490324.
Adapters trimming and quality check
We used the software Scythe v. 0.991 (https://github.com/vsbuffalo/scythe) for trimming 3' standard Illumina adapter sequence. We followed a Cutadapt v. 1.4.1 [117] trimming at 5' ends of the reads, and we filtered reads with Phred quality > 20. The trimmed sequences were filtered using the software PRINSEQ-lite v. 0.20.3 [118] and sequence quality was evaluated through the software FastQC v. 0.11.2 [119].
Transcriptome assembly and gene expression
We aligned the high quality reads from A. mellifera against its genome v. 4.5 using the software TopHat v. 2.0.9 [120]. The A. mellifera aligned sequences were quantified and the developmental phases compared using the software Cufflinks v. 2.1.1 [121]. The extension Cuffmerge integrated the reads to the mapping results and the tool Cuffdiff checked the expression levels for each sample and the significance of comparisons. CuffmeRbund R package v. 2.8.2 allowed us to access all this information [122].
For F. varia and C. analis, we used the software Trinity (trinityrnaseq_r2014717) [123, 124]. The N50 contig length (smallest contig length for which the sum of fewest contigs corresponds to 50% or more of the assembly [125]) of all transcripts of F. varia was 2372 and of C. analis was 2440. Orthology search was performed through the software InParanoid 8 [126]. We only accepted those transcripts with higher similarity with A. mellifera than to Drosophila melanogaster. Statistical evaluation of these data was done with the R software v. 3.1.2, using the packages R DESeq2 v. 1.6.3 [127] and edgeR v. 3.8.6 [128]. We considered as differentially expressed between developmental phases, those contigs with significant results for both R packages.
All heat maps were designed using the function heatmap.2 from gplots R package [129]. For all groups of genes, we measured the clustering potential of the samples for each phase and species. For this approach, we used the R package pvclust v. 1.3.2 [130] based on correlation distances, with a complete linkage method, and 10,000 bootstrap replication. We used unbiased p values (AU) and bootstrap values as measurements of clusters' significance. Clusters showing AU > 95% were considered statistically significant [130].
Molecular and functional characterization of differentially expressed genes
For the analysis of gene expression in A. mellifera, we filtered the differentially expressed genes using the following thresholds: q-value < 0.05; Log 2 Fold Change ≤ -1 or ≥ 1 and Fragments Per Kilobase of transcript per Million mapped reads (FPKM) ≥ 5. In the case of the other two bee species, F. varia and C. analis, we used the parameters cited in the previous section. For the Gene Ontology (GO) enrichment analysis we used the A. mellifera gene IDs to look for D. melanogaster ortologues in Fly Base, through the support of the online softwares g:Profiler (http://biit.cs.ut.ee/gprofiler/gorth.cgi), and g:Orth function [131, 132]. The same was done for F. varia and C. analis but using A. mellifera ortologues. We filtered the Drosophila IDs to avoid ID repetition and used them to generate an input list for the software DAVID v. 6.7 (http://david.abcc.ncifcrf.gov) [133, 134], used to perform the Gene Ontology analysis. The annotated functions belonged to Biological Process (BioP), Cellular Components (CC) and Molecular Function (MF) categories. Structural cuticular protein encoding genes were classified in accordance with the software CutProtFam-Pred (http://aias.biol.uoa.gr/CutProtFam-Pred/home.php) [135]. Venn diagrams were plotted with the online version of the software jvenn [136] (<http://bioinfo.genotoul.fr/jvenn/example.html>).
Transcription factor binding sites search with TRANSFAC
Transcription factors whose binding sites could be enriched in specific groups of A. mellifera gene models were searched using TRANSFAC [137] against insects database. For this enrichment analysis, we searched the 5' UTR regions covering -3,000 bases relative to the transcription start sites of the genes related to sclerotization/melanization processes, chitin, CHC biosynthetic pathways, regulation of cuticle formation and maturation, circadian rhythm, and non-melanization pigmentation pathways (see gene IDs S1 File). We excluded the genes with 5' UTR < 500 bases. We used the A. mellifera genes in this analysis once it is the only among the species here studied with an available reference genome. For the transcription factor FTZ-F1 binding sites, we used the TRANSFAC database (DROME$FTZF1_01, DROME$FTZF1_02, and DROME$FTZF1_03 from D. melanogaster, and BOMMO$CPR92_01, and BOMMO$CPR92_02 from B. mori) to generate a positional matrix. Here, we only highlighted those transcription factor binding sites (TFBS), which could be relevant for insect cuticle formation and maturation (see Discussion section).
Gene co-expression networks
The networks were plotted based on the correlation of gene expression in the integument of the analyzed bee species. We used the software Cytoscape v. 3.3.0 [138]–for Linux, and its plugin ExpressionCorrelation App v. 1.1.0. We only accepted correlations ≥ +0.95 or ≤ -0.95 and p ≤ 0.05.
Transmission electron microscopy (TEM)
We dissected the integument from the right anterior region of the third abdominal tergite of the studied bee species. The ultrastructure of the integument was compared between species and between the developmental phases (Pbm, Ne and Fg) using 11 A. mellifera integument pieces (4 from Pbm, 3 from Ne, and 4 from Fg phases), 9 integuments from F. varia (3 Pbm, 3 Ne, 3 Fg), 11 integuments from C. analis (4 Pbm, 4 Ne, 3 Fg), 4 integuments from B. brasiliensis (2 Ne, 2 Fg), 6 integuments from E. cordata (3 Ne, 3 Fg), and 6 integuments from T. diversipes (3 Ne, and 3 Fg). The Ne and Fg phases of B. brasiliensis were recognized based on the grade of body pigmentation criterion (less intense in the Ne bees) and for the Fg phase we also examined the wings in the search for erosion signals that could indicate intense foraging activity. The integument samples were fixed in 5% glutaraldehyde in cacodylate buffer 0.1 M, pH 7.2, during 2 h under shaking, washed 3X in the cacodylate buffer, fixed in osmium tetroxide 1% diluted in phosphate buffer 0.1M, pH 7.2, dehydrated in acetone and propylene oxide and embedded in resin. We used uranyl acetate for enhancing image contrast. The ultrathin sections were examined in a Jeol-Jem-100cx-II Electron Microscope and the software ImageJ v. 10.2 [139] was used to measure integument thickness. Measurements were compared among the developmental phases and species using Analysis of Variance (ANOVA) associated with the Tukey's Honestly Significance Difference (Tukey's HSD) post hoc test in R software v. 3.1.2, except for the A. mellifera and B. brasiliensis data. As for A. mellifera the data did not present a normal distribution (Shapiro-Wilk normality test) we used the Kruskal-Wallis test associated with the post hoc Conover-Iman test and Bonferroni correction, and for B. brasiliensis we used the Student's t-test [140].
Cuticular hydrocarbon profiles
The quantification of CHCs was based on their peak area in each chromatogram. For the analysis of relative peak area, we collected 15 bees per developmental phase for each of the highly eusocial species, while for C. analis we obtained four Pbm-staged bees, seven Ne and seven Fg bees. The F. varia hind legs were removed to avoid resin contamination. Except for this species, we bathed each sample in 1.5 ml of n-hexane 95% (Mallinckrodt Chemicals) for 1 min and 30 s to extract the CHCs. Due to the small size of F. varia species, we used 500 μl of n-hexane to extract the CHCs [141]. The extracts were dried under N2 flow and resuspended in 160 μl of n-hexane (100 μl for F. varia extracts) before running the analysis. CHC identification was made in a Gas Chromatograph / Mass Spectrometer (GC-MS) system (Shimadzu GCMS model QP2010), equipped with a 30 m DB-5MS column using helium as the carrier gas (1 ml/min), through electronic ionization (EI) mode. CHC relative quantification and normalization of the peak areas were performed following Falcon et al. [23] description. We also compared each developmental phase considering the classes of CHC (n-alkanes, unsaturated, and branched alkanes), repeating the normalization process for each case.
In order to verify differences between the developmental phases and between the bee species, we performed a clustering approach as described in the section Transcriptome assembly and gene expression, but using the Euclidean distance instead of the correlation distance. We also verified the compounds that better explained the detected differences. With this purpose, we performed a Principal Component Analysis (PCA) using the R software. The variation of each CHC peak area between the developmental phases was accessed through a Tukey's HSD test in R software.
Additionally, we calculated the absolute quantities of n-alkanes and unsaturated CHC per bee for the species A. mellifera, F. varia, and C. analis. An analytical curve [142] was built to establish the correlation between the quantities of the used standards and the CHCs. It is described by the equation: y = ax +b, where y is the known amount of the standard, x is the quantity of the unknown CHC, a is the peak area of the standard, and b is the area of the intercepted background. We prepared the curve based on the n-alkanes standards C23, C25, and C32 (Alltech Corporation) and using 1.25, 2.5, 5, 10, 15 and 20 μg/ml of each alkane. To the standard solutions and to each sample, we added 100 μl of the internal standard α-colestane (6.25 μg/μl) (Sigma-Aldrich) under the previously cited chromatography conditions. The values of the correlation curve (R) between CHCs and standards were ≥ 0.99. After curve preparation, CHCs were quantified in three independent samples, each prepared with individual bees, for each developmental phase and species. Due to the reduced body size of F. varia, we used pools of three bees for each one of the three independent samples per developmental phase and the obtained CHC values were corrected accordingly. To calculate the concentrations of compounds up to C24, we used the curve of C23; for compounds from C25 up to C29, we used the C25 curve; and for compounds larger than C29 up to C35 we used the curve of C32. We followed an ANOVA associated with the post hoc Tukey's HSD test to compare the absolute quantity of each CHC (n-alkanes and unsaturated) between developmental phases. The absolute quantities of the F. varia unsaturated compounds were not calculated once their quantities are very low.
Ethics statement
The bee samples that we collected are in agreement with Brazilian laws. A license is not required for exogenous species collection. Native bees were sampled under the register: Ministério do Meio Ambiente—MMA, Instituto Chico Mendes de Conservação da Biodiversidade–ICMBio, Sistema de Autorização e Informação em Biodiversidade–SISBIO, license number: 41883–1; authentication number: 71367685; and license number: 41883–2; authentication number: 39944978.
Supporting information
Dark blue: sociality independent of its complexity. Light blue: eusociality. Red: primitively social. Green: facultatively social. Made in LibreOf@ice Draw; based in Kapheim et al. [33].
(TIF)
Hierarchical cluster analysis with Pearson correlation based on the RNA-seq data obtained from the integument triplicates of pharate adults (Pbm), newly-emerged (Ne) and foragers (Fg) of the three bee species: (A) A. mellifera, (B) F. varia and (C) C. analis. The numbers 1, 2 and 3 following the Pbm, Ne, and Fg abbreviations indicate the independent samples of each developmental phase.
(TIF)
Representative heatmaps of gene expression profiles through the Pbm (pharate-adult), Ne (newly-emerged), and Fg (forager) developmental phases of (A) A. mellifera, (B) F. varia, and (C) C. analis. Genes were grouped according to their potential function in adult cuticle formation and maturation. Different lowercase letters on the heatmaps means statistically significant difference (see Materials and Methods) in the expression levels between the developmental phases of each bee species.
(TIF)
Expression profiles of ebony, tan, Idgf4-like, Cda5, chitooligosaccharidolytic-domain-like, CPR14, CPR17, CPR25, CPR26, Apd-like, Elo-GB54302, Elo-GB54401, Elo-GB45596, Ethr, E74, Hr4, Hr38, FTZ-F1, rickets, Tim2, and ALAS were positively correlated between the eusocial bee species, and negatively or non-correlated with the solitary bee. Expression profiles of CPR18, CPR23, Apd-3, Desat-GB40659, Elo-GB46038, and Ptx-1 were positively correlated between the eusocial species, the basal line in the graphic representations indicating undetected orthologs in C. analis. Pbm: pharate adults, Ne: newly emerged, and Fg: foragers. Color key at the bottom of figure.
(TIF)
The genes are indicated in the nodes, and the edges represent significant correlation among genes.
(TIF)
The genes are indicated in the nodes, and the edges represent significant correlation among genes.
(TIF)
The genes are indicated in the nodes, and the edges represent significant correlation among genes.
(TIF)
Red boxes indicate significant clusters with 95% of confidence. Arrows indicate significantly supported clusters. AU clusters’ support (red values); BP bootstrap support (green values); Branches’ edges (gray values). Pharate-adults (Pbm), newly emerged (Ne), and forager (Fg) bees. The number after each developmental phase indicates the individual bee in the analysis.
(TIF)
Comparisons of gene expression levels through the pharate-adult (Pbm), newly-emerged (Ne) and forager (Fg) developmental phases of A. mellifera, F. varia, and C. analis. Blue: significant positive correlation. Red: significant negative correlation. (-): undetectable gene expression.
(XLS)
(XLS)
Apis mellifera (A. mel.), Frieseomelitta varia (F. var.), Centris. analis (C. ana.), Bombus brasiliensis (B. bra.), Euglossa cordata (E. cor.), and Tetrapedia diversipes (T. div.). Developmental phases: Pbm (pharate-adult); Ne (newly-emerged, 0h); 24h, 48h, 72h, and 96h after adult emergence; Fg (forager).
(DOC)
For F. varia and C. analis, which unlike A. mellifera do not have the genome sequenced, we have included functional information obtained from the de novo characterization of their respective transcriptomes, reconstructed from the RNA-Seq data.
(XLS)
Pharate-adult (Pbm), newly-emerged (Ne) and forager (Fg) developmental phases. The bee species and developmental phases compared are specified in the inferior margin of each table in this File. For example: Amel_Pbm>Ne means the list of A. mellifera genes upregulated in Pbm in comparison to Ne.
(XLS)
The bee species and developmental phases compared are specified in the inferior margin of each table in this File. For example: Amel_Pbm_Ne>Fg_ID means the bees’ gene IDs and Fly Base gene IDs from higher expressed genes at younger developmental phases; and Amel_Ne_Fg>Pbm_GO means the Gene Ontology of the higher expressed genes at older developmental phases based on their fly orthologues IDs.
(XLS)
Pharate-adult (Pbm), newly-emerged (Ne) and forager (Fg) developmental phases.
(XLS)
Acknowledgments
We would like to thank Sidnei Mateus, Solange Augusto, Gabriela Freiria and Anete Pedro Lourenço by the bee specimens provided.
Data Availability
The RNA-seq data is deposited at the National Center for Biotechnology Information (NCBI) database under the BioProject ID PRJNA490324.
Funding Statement
This work was supported by São Paulo Research Foundation (FAPESP) grants to MMGB (2014/13136-0), ZLPS (2011/03171-5), and NPL (2014/50265-3). Fellowships to TF (FAPESP 2012/24284-5 and PNPD Programa Nacional de Pós-Doutorado CAPES/HCPA 88887.160608/2017-00). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hopkins TL, Kramer KJ. Insect cuticle sclerotization. Annul Rev Entomol. 1992;37: 273–302. [Google Scholar]
- 2.Wood OR, Hanrahan S, Coetzee M, Koekemoer LL, Brooke BD. Cuticle thikening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit Vectors. 2010;3: 67 10.1186/1756-3305-3-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csikós G, Molnár K, Borhegyi NH, Talián GC, Sass M. Insect cuticle, an in vivo model of protein trafficking. J Cell Sci. 1999;112: 2113–2124. [DOI] [PubMed] [Google Scholar]
- 4.Hill RJ,Billas IML, Bonneton F,Graham LD, Lawrence MC. Ecdysone receptors: from the Ashburner model to structural biology. Annu Rev Entomol. 2013;58: 251–271. 10.1146/annurev-ento-120811-153610 [DOI] [PubMed] [Google Scholar]
- 5.Ali MS, Iwanaga M., Kawasaki H. Ecdysone-responsive transcriptional regulation determines the temporal expression of cuticular protein genes in wing discs of Bombyx mori. Gene 2013;512: 337–347. 10.1016/j.gene.2012.09.126 [DOI] [PubMed] [Google Scholar]
- 6.Hiruma K, Riddiford LM. The coordination of the sequential appearance of MHR4 and dopa decarboxylase during the decline of the ecdysteroid titer at the end of the molt. Mol Cell Endocrinol. 2007;276: 71–79. 10.1016/j.mce.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Gu J, Huang L-X, Gong Y-J, Zheng S-C, Liu L, Huang L-H, et al. De novo characterization of transcriptome and gene expression dynamics in epidermis during the larval-pupal metamorphosis of common cutworm. Insect Biochem Mol Biol. 2013;43: 794–808. 10.1016/j.ibmb.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 8.Kramer JK, Dziadik-Turner C, Koga D. Chitin metabolism in insects In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. Oxford: Pergamon Press; 1985. pp. 75–115. [Google Scholar]
- 9.Dixit R, Arakane Y, Specht CA, Richard C, Kramer KJ, Beeman RW, et al. Domain organization and phylogenetic analysis of proteins from the chitin deacetylase gene family of Tribolium castaneum and three other species of insects. Insect Biochem Mol Biol. 2008;38: 440–451. 10.1016/j.ibmb.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 10.Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol. 2003;206: 4393–4412. [DOI] [PubMed] [Google Scholar]
- 11.Locke M, Krishnan N. The distribution of phenoloxidases and polyphenols during cuticle formation. Tissue Cell. 1971;3: 103–126. [DOI] [PubMed] [Google Scholar]
- 12.Willis JH. Structural cuticular proteins from arthropods: annotation, nomenclature and sequence characteristics in the genomic era. Insect Biochem Mol Biol. 2010;40: 189–204. 10.1016/j.ibmb.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebers JE, Riddiford LM. Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J Mol Biol. 1988;203: 411–423. [DOI] [PubMed] [Google Scholar]
- 14.Andersen SO. Amino acid sequence studies on endocuticular proteins from the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol. 1998;28: 421–434. [DOI] [PubMed] [Google Scholar]
- 15.Guan X, Middlebrooks BW, Alexander S, Wasserman SA. Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc Natl Acad Sci. 2006;103: 16794–16799. 10.1073/pnas.0607616103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepburn HR. Structure of integument In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. Oxford: Pergamon Press; 1985. pp. 1–58. [Google Scholar]
- 17.Gibbs AG. Lipid melting and cuticular permeability: new insights into an old problem. J Insect Physiol. 2002;48: 391–400. [DOI] [PubMed] [Google Scholar]
- 18.Howard RW, Blomquist GJ. Ecological, behavioral and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50: 371–393. 10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- 19.Edney EB. Water balance in desert arthropods. Science. 1967;156: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 20.Piek T. Synthesis of wax in the honeybee (Apis mellifera L.). J Insect Physiol.1964;10: 563–572. [Google Scholar]
- 21.Blomquist GJ, Dillwith JW. Cuticular lipids In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Oxford: Pergamon Press; 1985. vol. 3, pp. 117–154. [Google Scholar]
- 22.Blomquist GJ, Jurenka R, Schal C, Tittiger C. Pheromone production: biochemistry and molecular biology In: Gilbert LI, editor. Insect Endocrinology. New York: Elsevier; 2012. pp. 523–567. [Google Scholar]
- 23.Falcon T, Ferreira-Caliman MJ, Nunes FMF, Tanaka ED, Nascimento FS, Bitondi MMG. Exoskeleton formation in Apis mellifera: cuticular hydrocarbons profiles and expression of desaturase and elongase genes during pupal and adult development. Insect Biochem Mol Biol. 2014;50: 68–81. 10.1016/j.ibmb.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 24.Shamim G, Ranjan SK, Pandey DM, Ramani R. Biochemistry and biosynthesis of insect pigments. Eur J Entomol. 2014;111: 149–164. [Google Scholar]
- 25.Solano F. Melanins: skin pigments and much more—types, structural models, biological functions, and formation routes. New J Sci. 2014: 1–28. Article ID 498276. 10.1155/2014/498276. [DOI] [Google Scholar]
- 26.Andersen SO. Insect cuticular sclerotization: a review. Insect Biochem Mol Biol. 2010;40: 166–178. 10.1016/j.ibmb.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 27.Hiruma K, Riddiford LM, Hopkins TL, Morgan TD. Roles of dopa decarboxylase and phenoloxidase in the melanization of the tobacco hornworm and their control by 20-hydroxyecdysone. J Comp Physiol B. 1985;155: 659–669. [DOI] [PubMed] [Google Scholar]
- 28.Zufelato MS, Bitondi MMG, Simões ZLP, Hartfelder K. The juvenile hormone analog pyriproxyfen affects ecdysteroid-dependent cuticle melanization and shifts the pupal ecdysteroid peak in the honey bee (Apis mellifera). Arthropod Struct Dev. 2000;19: 111–119. [DOI] [PubMed] [Google Scholar]
- 29.Michener CD. The social behavior of the bees. Massachusetts: Harvard University Press; 1974. [Google Scholar]
- 30.Wilson EO. Sociobiology. The New Synthesis. Cambridge: Harvard University Press; 1975. [Google Scholar]
- 31.Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443: 931–949. 10.1038/nature05260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadd BM, Barribeau SM, Bloch G, de Graaf DC, Dearden P, Elsik CG, et al. The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol. 2015;16: 76 10.1186/s13059-015-0623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapheim KM, Pan H, Li C, Salzberg SL, Puiu D, Magoc T, et al. Genomic signatures of evolutionary transitions from solitary to group living. Science. 2015;348: 1139–1143. 10.1126/science.aaa4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias-Neto M, Nascimento ALO, Bonetti AM, Nascimento FS, Mateus S, Garófalo CA, Bitondi MMG. Heterochrony of cuticular differentiation in eusocial corbiculate bees. Apidologie. 2013;45: 397–408. [Google Scholar]
- 35.Hansell MH. The ecological impact of animal nests and burrows. Funct Ecol. 1993;7: 5–12. [Google Scholar]
- 36.Evans JD, Spivak M. Socialized medicine: individual and communal disease barriers in honey bees. J Invertebr Pathol. 2010;103: S62–S72. 10.1016/j.jip.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 37.West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci. 2005;102: 6543–6549. 10.1073/pnas.0501844102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson EO, Hölldobler B. Eusociality: origin and consequences. Proc Natl Acad Sci. 2005;205: 13367–13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito C, Goto SG, Shiga S, Tomioka K, Numata H. Peripheral circadian clock for the cuticle deposition rhythm in Drosophila melanogaster. Proc Natl Acad Sci. 2008;105: 8446–8451. 10.1073/pnas.0800145105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeno T, Katagiri C, Numata H, Goto SG. Causal involvement of mammalian-type cryptochrome in the circadian cuticle deposition rhythm in the bean bug Riptortus pedestris. Insect Mol Biol. 2011;20: 409–415. 10.1111/j.1365-2583.2011.01075.x [DOI] [PubMed] [Google Scholar]
- 41.Michelette ERF, Soares AEE. Characterization of preimaginal developmental stages in Africanized honey bee workers (Apis mellifera L). Apidologie. 1993;24: 431–440. [Google Scholar]
- 42.Elias-Neto M, Soares MPM, Bitondi MMG. Changes in integument structure during the imaginal molt of the honey bee. Apidologie 2009;40: 29–39. [Google Scholar]
- 43.Zhou J-J. Odorant-binding proteins in insects In Litwack G, editor. Vitamins & Hormones. Burlington: Academic Press; 2010;83: pp. 241–272. [DOI] [PubMed] [Google Scholar]
- 44.Forêt S, Maleszka R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res. 2006;16: 1404–1413. 10.1101/gr.5075706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott JG, Wen Z.Cytochromes P450 of insects: the tip of the iceberg. Pest Manag Sci. 2001;57: 958–967. 10.1002/ps.354 [DOI] [PubMed] [Google Scholar]
- 46.Mittapalli O, Neal JJ, Shukle RH. Antioxidant defense response in a galling insect. Proc Natl Acad Sci. 2007;104: 1889–1894 10.1073/pnas.0604722104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang ZY, Robinson GE, Tobe SS, Yagi KJ, Strambi C, Strambi A, et al. Hormonal regulation of behavioural development in the honey bee is based on changes in the rate of juvenile hormone biosynthesis. J Insect Physiol. 1991;37: 733–741. [Google Scholar]
- 48.Mlodzik M, Hiromi Y, Weber U, Goodman CS, Rubin GM. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990;60: 21l–224. [DOI] [PubMed] [Google Scholar]
- 49.Zander RH. Minimal values for reliability of bootstrap and jackknife proportions, decay index, and Bayesian posterior probability. PhyloInformatics. 2004;2: 1–13. [Google Scholar]
- 50.Soares MPM, Silva-Torres FA, Elias-Neto M, Nunes FMF, Simões ZLP, Bitondi MMG. Ecdysteroid-dependent expression of the Tweedle and Peroxidase genes during adult cuticle formation in the honey bee, Apis mellifera. PloS One. 2011;6: e20513 10.1371/journal.pone.0020513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soares MPM, Barchuk AR, Simões ACQ, Cristino AS, Freitas FCP, Canhos LL, et al. Genes involved in thoracic exoskeleton formation during the pupal-to-adult molt in a social insect model, Apis mellifera. BMC Genomics. 2013;14: 576 10.1186/1471-2164-14-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arakane Y, Lomakin J, Beeman RW, Muthukrishnan S, Gehrke SH, Kanost MR. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J Biol Chem 2009. a;284: 16584–16594. 10.1074/jbc.M901629200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorman MJ, Arakane Y. Tyrosine hydroxylase is required for cuticle sclerotization and pigmentation in Tribolium castaneum. Insect Biochem Mol Biol. 2010;40: 267–273. 10.1016/j.ibmb.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyazaki S, Okada Y, Miyakawa H, Tokuda G, Cornette R, Koshikawa S, et al. Sexually dimorphic body color is regulated by sex-specific expression of Yellow gene in ponerine ant, Diacamma sp. PLoS One. 2014;9: e92875 10.1371/journal.pone.0092875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright TR F. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv Genet. 1987; 24: 127–222. [PubMed] [Google Scholar]
- 56.Elias-Neto M, Soares MPM, Simões ZLP, Hartfelder K, Bitondi MMG. Developmental characterization, function and regulation of a Laccase2 encoding gene in the honeybee, Apis mellifera (Hymenoptera, Apinae). Insect Biochem Mol Biol. 2010;40: 241–251. 10.1016/j.ibmb.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 57.Osanai-Futahashi M, Tatematsu KI, Futahashi R, Narukawa J, Takasu Y, Kayukawa T, et al. Positional cloning of a Bombyx pink-eyed white egg locus reveals the major role of cardinal in ommochrome synthesis. Heredity. 2016;116: 135–145. 10.1038/hdy.2015.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugumaran M. Complexities of cuticular pigmentation in insects. Pigment Cell Melanoma Res. 2009;22: 523–525. 10.1111/j.1755-148X.2009.00608.x [DOI] [PubMed] [Google Scholar]
- 59.Lloyd V, Ramaswami M, Krämer H. Not just pretty eyes: Drosophila eye-colour mutations and lysosomal delivery. Trends Cell Biol. 1998;8: 257–259. [DOI] [PubMed] [Google Scholar]
- 60.Hamza I, Dailey HA. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta. 2012;1823: 1617–1632. 10.1016/j.bbamcr.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stubenhaus BM, Dustin JP, Neverett ER, Beaudry MS, Nadeau LE, Burk-McCoy E, et al. Light-induced depigmentation in planarians models the pathophysiology of acute porphyrias. eLife. 2016;5: e14175 10.7554/eLife.14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaik KS, Meyer F, Vázquez AV, Flötenmeyer M, Cerdán ME, Moussian B. δ-Aminolevulinate synthase is required for apical transcellular barrier formation in the skin of the Drosophila larva. Eur J Cell Biol. 2012;91: 204–215. 10.1016/j.ejcb.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 63.Xi Y, Pan PL, Ye YX, Yu B, Zhang CX. Chitin deacetylase family genes in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol Biol. 2014;23: 695–705. 10.1111/imb.12113 [DOI] [PubMed] [Google Scholar]
- 64.Arakane Y, Hogenkamp DG, Zhu YC, Kramer KJ, Specht CA, Beeman RW, et al. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem Mol Biol. 2004;34: 291–304. 10.1016/j.ibmb.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 65.Arakane Y, Dixit R, Begum K, Park Y, Specht CA, Merzendorfer H, et al. Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem Mol Biol. 2009. b;39: 355–365. 10.1016/j.ibmb.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 66.Tellam RL, Vuocolo T, Johnson SE, Jarmey J, Pearson RD. Insect chitin synthase: cDNA sequence, gene organization and expression. Eur J Biochem. 2000;267: 6025–6042. [DOI] [PubMed] [Google Scholar]
- 67.Gagou ME, Kapsetaki M, Turberg A, Kafetzopoulos D. Stage-specific expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila metamorphosis. Insect Biochem Mol Biol. 2002;32: 141–146. [DOI] [PubMed] [Google Scholar]
- 68.Ampasala DR, Zheng S, Zhang D, Ladd T, Doucet D, Krell PJ, et al. An epidermis-specific chitin synthase cDNA in Choristoneura fumiferana: cloning, characterization, developmental and hormonal-regulated expression. Arch Insect Biochem Physiol. 2011;76: 83–96. 10.1002/arch.20404 [DOI] [PubMed] [Google Scholar]
- 69.Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc Natl Acad Sci. 2008;105: 6650–6655. 10.1073/pnas.0800739105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pesch Y-Y, Riedel D, Patil KR, Loch G, Behr M. Chitinases and Imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci. Rep. 2016;6: 18340 10.1038/srep18340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development. 1999;126: 211–219. [DOI] [PubMed] [Google Scholar]
- 72.Hipfner DR, Cohen SM. New growth factors for imaginal discs. BioEssays. 1999;21: 718–720. [DOI] [PubMed] [Google Scholar]
- 73.Luschnig S, Bätz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16: 186–194. 10.1016/j.cub.2005.11.072 [DOI] [PubMed] [Google Scholar]
- 74.Arakane Y, Muthukrishnan S, Kramer KJ, Specht CA, Tomoyasu Y, Lorezen MD, et al. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol. 2005;14: 53–463. [DOI] [PubMed] [Google Scholar]
- 75.Moussian B, Schwarz H, Bartoszewski S, Nüsslein-Volhard C. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol. 2005;264: 117–130. 10.1002/jmor.10324 [DOI] [PubMed] [Google Scholar]
- 76.Pan PL, Ye YX,Lou YH, Lu JB, Cheng C, Shen Y, et al. A comprehensive omics analysis and functional survey of cuticular proteins in the brown planthopper. Proc Natl Acad Sci. 2018;115: 5175–5180. 10.1073/pnas.1716951115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noh MY, Kramer KJ, Muthukrishnam S, Kanost MR, Beeman RW, Arakane A. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem Mol Biol. 2014;53: 22–29. 10.1016/j.ibmb.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 78.Noh MY, Muthukrishnam S, Kramer KJ, Arakane Y. Tribolium castaneum RR-1 cuticular protein TcCPR4 is required for formation of pore canals in rigid cuticle. PLoS Genet. 2015;11: e1004963 10.1371/journal.pgen.1004963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang L, Liang J, Zhan Z, Xiang Z, He N. Identification of the chitin-binding proteins from the larval proteins of silkworm, Bombyx mori. Insect Biochem Mol Biol. 2010;40: 228–234. 10.1016/j.ibmb.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 80.Cornman RS, Willis JH. Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol Biol. 2009;18: 607–622. 10.1111/j.1365-2583.2009.00902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Togawa T, Dunn WA, Emmons AC, Willis J. CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem Mol Biol. 2007;37: 675–688. 10.1016/j.ibmb.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 82.Kucharski R, Maleszka J, Maleszka R. Novel cuticular proteins revealed by the honey bee genome. Insect Biochem Mol Biol. 2007;37: 128–134. 10.1016/j.ibmb.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 83.Jasrapuria S, Specht CA, Kramer KJ, Beeman RW, Muthukrishnan S. Gene families of cuticular proteins analogous to peritrophins (CPAPs) in Tribolium castaneum have diverse functions. PLoS One 2012;7: e49844 10.1371/journal.pone.0049844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moussian B, Taång E, Tonning A, Helms S, Schwarz H, Nüsslein-Volhard C, et al. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development. 2006;133: 163–171. 10.1242/dev.02177 [DOI] [PubMed] [Google Scholar]
- 85.Chaudhari SS, Arakane Y, Specht CA, Moussian B, Boyle DL, Park Y, et al. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc Natl Acad Sci. 2011;108: 17028–17033. 10.1073/pnas.1112288108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaudhari SS, Arakane Y, Specht CA, Moussian B, Kramer KJ, Muthukrishnan S. et al. Retroactive maintains cuticle integrity by promoting trafficking of Knickkopf into the procuticle of Tribolium castaneum. PLoS Genet. 2013;9: e1003268 10.1371/journal.pgen.1003268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zitnan D, Adams ME. Neuroendocrine regulation of ecdysis In Gilbert LI, editor. Insect Endocrinology. San Diego: Elsevier Academic Press; 2012. pp. 253–309. [Google Scholar]
- 88.Jones G, Sharp PA. Ultraspiracle: an invertebrate nuclear receptor for juvenile hormones. Proc Natl Acad Sci. 1997;94: 13499–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jindra M, Uhlirova M, Charles J-P, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genetics. 2015;11: e1005394 10.1371/journal.pgen.1005394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saha TT, Shin SW, Dou W, Roy S, Zhao B, Hou Y, et al. Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proc Natl Acad Sci. 2016;7: E735–E743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao D, Woolner S, Bownes M. The Mirror transcription factor links signalling pathways in Drosophila oogenesis. Dev Genes Evol. 2000;210: 449–457 10.1007/s004270050332 [DOI] [PubMed] [Google Scholar]
- 92.Jeong S, Rokas A, Carroll SB. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell. 2006;125: 1387–1399. 10.1016/j.cell.2006.04.043 [DOI] [PubMed] [Google Scholar]
- 93.Carroll SB, Laughon A, Thalley BS. Expression, function, and regulation of the hairy segmentation protein in the Drosophila embryo. Genes Dev. 1988;2, 883–890. [DOI] [PubMed] [Google Scholar]
- 94.Song Q. Bursicon, a neuropeptide hormone that controls cuticle tanning and wing expansion In: Gilbert LI, editor. Insect Endocrinology. New York: Academic Press; 2012. pp. 93–105. [Google Scholar]
- 95.Costa CP, Elias-Neto M, Falcon T, Dallacqua RP, Martins JR, Bitondi MMG. RNAi-mediated functional analysis of bursicon genes related to adult cuticle formation and tanning in the honeybee, Apis mellifera. PLoS One. 2016;11: e0167421 10.1371/journal.pone.0167421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zelazny B, Neville AC. Endocuticle layer formation controlled by non-cyrcadian clocks in beetles. Insect Physiol. 1972;18: 1967–1979. [Google Scholar]
- 97.Andersen SO, Hojrup P, Roepstorff P. Insect cuticular proteins. Insect Biochem Mol Biol. 1995;25: 153–176. [DOI] [PubMed] [Google Scholar]
- 98.Whitten J. Coordinated development in the fly foot: sequential cuticle secretion. J Morphol. 1969;127: 73–104. [Google Scholar]
- 99.Garófalo CA. Social structure of Euglossa cordata nests (Hymenoptera: Apidae: Euglossini). Entomol Gen. 1985;11: 77–83. [Google Scholar]
- 100.Augusto SC, Garófalo CA. Comportamento das fêmeas nas associações formadas em ninhos de Euglossa cordata (Hymenoptera; Apidae; Euglossini) In: Encontro sobre Abelhas, Ribeirão Preto, SP, Brazil; 1994. pp. 171–181. [Google Scholar]
- 101.Freiria GA, Garófalo CA, Del Lama MA. The primitively social behavior of Euglossa cordata (Hymenoptera, Apidae, Euglossini): a view from the perspective of kin selection theory and models of reproductive skew. Apidologie. 2017;48: 523–532. [Google Scholar]
- 102.Cardinal S, Danforth BN. The antiquity and evolutionary history of social behavior in bees. PloS One. 2011;6: e21086 10.1371/journal.pone.0021086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silva-Matos EV, Garófalo CA. Worker life tables, survivorship, and longevity in colonies of Bombus (Fervidobombus) atratus (Hymenoptera: Apidae). Rev Biol Tropical. 2000;48: 657–664. [PubMed] [Google Scholar]
- 104.Chaudhari SS, Moussian B, Specht CA, Arakane Y, Kramer KJ, Beeman RW, et al. Functional specialization among members of Knickkopf family of proteins in insect cuticle organization. PLoS Genet. 2014;10: e1004537 10.1371/journal.pgen.1004537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hinaux H, Bachem K, Battistara M, Rossi M, Xin Y, Jaenichen R, et al. Revisiting the developmental and cellular role of the pigmentation gene yellow in Drosophila using a tagged allele. Dev Biol. 2018;438: 111–123 10.1016/j.ydbio.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 106.Reddy KL, Rovani MK, Wohlwill A, Katzen A, Storti RV. The Drosophila Par domain protein I gene, Pdp1, is a regulator of larval growth, mitosis and endoreplication. Dev Biol. 2006;289: 100–114. 10.1016/j.ydbio.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 107.Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, et al. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112: 329–341. [DOI] [PubMed] [Google Scholar]
- 108.Gibbs A, Pomonis JG. Physical properties of insect cuticular hydrocarbons: the effects of chain length, methyl-branchingand unsaturation. Comp Biochem Physiol. 1995;112B: 243–249. [Google Scholar]
- 109.Lockey KH. Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol Part B: Comp Biochem. 1988;89: 595–645. [Google Scholar]
- 110.Ferreira-Caliman MJ, Nascimento FS, Turatti IC, Lopes NP, Zucchi R. The cuticular hydrocarbons profiles in the stingless bee Melipona marginata reflect task-related differences. J Insect Physiol. 2010;56: 800–804. 10.1016/j.jinsphys.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 111.Kather R, Drijfhout FP, Martin SJ. Task group differences in cuticular lipids in the honey bee Apis mellifera. J Chem Ecol. 2011;37: 205–212. 10.1007/s10886-011-9909-4 [DOI] [PubMed] [Google Scholar]
- 112.Wagner D, Brown MJF, Broun P, Cuevas W, Moses LE, Chao DL, et al. Task-related differences in the cuticular hydrocarbon composition of the harvester ants, Pogonomyrmex barbatus. J Chem Ecol. 1998;24: 2021–2037. [Google Scholar]
- 113.Gibbs AG. Water-proofing properties of cuticular lipids. Amer Zool.1998;38: 471–482. [Google Scholar]
- 114.Paulmier I, Bagnères AG, Afonso CMM, Dusticier G, Rivière G, Clément JL. Alkenes as sexual pheromone in the alfalfa leaf-cutter bee Megachile rotundata. J Chem Ecol. 1999;25: 471–490. [Google Scholar]
- 115.Jesus BMV, Garófalo CA. Nesting behaviour of Centris (Heterocentris) analis (Fabricius) in southeastern Brazil (Hymenoptera, Apidae, Centridini). Apidologie. 2000;31: 503–515. [Google Scholar]
- 116.Tian L, Hines HM. Morphological characterization and staging of bumble bee pupae. PeerJ. 2018; 6:e6089 10.7717/peerj.6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17: 10–12. [Google Scholar]
- 118.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27: 863–864. 10.1093/bioinformatics/btr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc.
- 120.Trapnell C, Patcher L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25: 1105–1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and abundance estimation from RNA-Seq reveals thousands of new transcripts and switching among isoforms. Nature Biotechnol. 2010;28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protoc. 2012;7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nature Biotechnol. 2011;15: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protoc. 2013;8: 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller JR, Koren S, Sutton G. Assembly algorithms for the next-generation sequencing data. Genomics. 2010;95: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sonnhammer ELL, Östlund G. InParanoid 8: ortology analysis between 273 proteomes, mostly eukaryotic. Nucleic Acids Res. 2014;43: D234–D239. 10.1093/nar/gku1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15: 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robinson MD, Smyth GK. Small sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics. 2008;9: 321–332. 10.1093/biostatistics/kxm030 [DOI] [PubMed] [Google Scholar]
- 129.Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T, et al. gplots: various R programming tools for plotting data. R package version 2.16.0. 2015. Available from: http://CRAN.R-project.org/package=gplots
- 130.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22: 1540–1542. 10.1093/bioinformatics/btl117 [DOI] [PubMed] [Google Scholar]
- 131.Reimand J, Kull M, Peterson H, Hansen J, Vilo J. g:Profiler—a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007;35: W193–W200. 10.1093/nar/gkm226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reimand J, Arak T, Vilo J. g:Profiler—a web server for functional interpretation of gene lists (2011 update). Nucleic Acids Res. 2011;39: W307–W315. 10.1093/nar/gkr378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protocols. 2009. a;4: 44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 134.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009. b;37: 1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ioannidou ZS, Theodoropoulou MC, Papandreou NC, Willis JH, Hamodrakas SJ. CutProtFam-Pred: detection and classification of putative structural cuticular proteins from sequence alone, based on profile hidden Markov models. Insect Biochem Mol Biol. 2014;52: 51–59. 10.1016/j.ibmb.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15 10.1186/1471-2105-15-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, et al. TRANSFAC: and integrated system for gene expression regulation. Nucleic Acids Res. 2000;28: 316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13: 2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abramoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Internat. 2004;11: 36–42. [Google Scholar]
- 140.Fay DS, Gerow K. A biologist's guide to statistical thinking and analysis. In: Hobert O, editor. Wormbook; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nunes TM, Nascimento FS, Turatti IC, Lopes NP, Zucchi R. Nestmate recognition in a stingless bee: does the similarity of chemical cues determine guard acceptance? Anim Behav. 2008;75: 1165–1171. [Google Scholar]
- 142.Analytical Methods Committee. Internal quality control of analytical data. Analyst 1995;120: 29–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dark blue: sociality independent of its complexity. Light blue: eusociality. Red: primitively social. Green: facultatively social. Made in LibreOf@ice Draw; based in Kapheim et al. [33].
(TIF)
Hierarchical cluster analysis with Pearson correlation based on the RNA-seq data obtained from the integument triplicates of pharate adults (Pbm), newly-emerged (Ne) and foragers (Fg) of the three bee species: (A) A. mellifera, (B) F. varia and (C) C. analis. The numbers 1, 2 and 3 following the Pbm, Ne, and Fg abbreviations indicate the independent samples of each developmental phase.
(TIF)
Representative heatmaps of gene expression profiles through the Pbm (pharate-adult), Ne (newly-emerged), and Fg (forager) developmental phases of (A) A. mellifera, (B) F. varia, and (C) C. analis. Genes were grouped according to their potential function in adult cuticle formation and maturation. Different lowercase letters on the heatmaps means statistically significant difference (see Materials and Methods) in the expression levels between the developmental phases of each bee species.
(TIF)
Expression profiles of ebony, tan, Idgf4-like, Cda5, chitooligosaccharidolytic-domain-like, CPR14, CPR17, CPR25, CPR26, Apd-like, Elo-GB54302, Elo-GB54401, Elo-GB45596, Ethr, E74, Hr4, Hr38, FTZ-F1, rickets, Tim2, and ALAS were positively correlated between the eusocial bee species, and negatively or non-correlated with the solitary bee. Expression profiles of CPR18, CPR23, Apd-3, Desat-GB40659, Elo-GB46038, and Ptx-1 were positively correlated between the eusocial species, the basal line in the graphic representations indicating undetected orthologs in C. analis. Pbm: pharate adults, Ne: newly emerged, and Fg: foragers. Color key at the bottom of figure.
(TIF)
The genes are indicated in the nodes, and the edges represent significant correlation among genes.
(TIF)
The genes are indicated in the nodes, and the edges represent significant correlation among genes.
(TIF)
The genes are indicated in the nodes, and the edges represent significant correlation among genes.
(TIF)
Red boxes indicate significant clusters with 95% of confidence. Arrows indicate significantly supported clusters. AU clusters’ support (red values); BP bootstrap support (green values); Branches’ edges (gray values). Pharate-adults (Pbm), newly emerged (Ne), and forager (Fg) bees. The number after each developmental phase indicates the individual bee in the analysis.
(TIF)
Comparisons of gene expression levels through the pharate-adult (Pbm), newly-emerged (Ne) and forager (Fg) developmental phases of A. mellifera, F. varia, and C. analis. Blue: significant positive correlation. Red: significant negative correlation. (-): undetectable gene expression.
(XLS)
(XLS)
Apis mellifera (A. mel.), Frieseomelitta varia (F. var.), Centris. analis (C. ana.), Bombus brasiliensis (B. bra.), Euglossa cordata (E. cor.), and Tetrapedia diversipes (T. div.). Developmental phases: Pbm (pharate-adult); Ne (newly-emerged, 0h); 24h, 48h, 72h, and 96h after adult emergence; Fg (forager).
(DOC)
For F. varia and C. analis, which unlike A. mellifera do not have the genome sequenced, we have included functional information obtained from the de novo characterization of their respective transcriptomes, reconstructed from the RNA-Seq data.
(XLS)
Pharate-adult (Pbm), newly-emerged (Ne) and forager (Fg) developmental phases. The bee species and developmental phases compared are specified in the inferior margin of each table in this File. For example: Amel_Pbm>Ne means the list of A. mellifera genes upregulated in Pbm in comparison to Ne.
(XLS)
The bee species and developmental phases compared are specified in the inferior margin of each table in this File. For example: Amel_Pbm_Ne>Fg_ID means the bees’ gene IDs and Fly Base gene IDs from higher expressed genes at younger developmental phases; and Amel_Ne_Fg>Pbm_GO means the Gene Ontology of the higher expressed genes at older developmental phases based on their fly orthologues IDs.
(XLS)
Pharate-adult (Pbm), newly-emerged (Ne) and forager (Fg) developmental phases.
(XLS)
Data Availability Statement
The RNA-seq data is deposited at the National Center for Biotechnology Information (NCBI) database under the BioProject ID PRJNA490324.