ABSTRACT
Background
Huntington's disease (HD) is characterized by chorea, balance and gait impairments, and cognitive deficits, which increase fall risk. Dual task (DT) and environmentally challenging paradigms reflect balance related to everyday life. Furthermore, the impact of cognitive deficits on balance dysfunction and falls in HD is unknown.
Objective
To determine the impact of DT interference, sensory feedback, and cognitive performance on balance and falls in HD.
Methods
Seventeen participants with HD (55 ± 9.7 years) and 17 age‐matched controls (56.5 ± 9.3 years) underwent quantitative balance testing with APDM inertial sensors. Postural sway was assessed during conditions of manipulated stance, vision, proprioception, and cognitive demand. The DT was a concurrent verbal fluency task. Neuropsychological assessments testing multiple cognitive domains were also administered.
Results
HD participants exhibited significantly greater total sway area, jerk, and variability under single‐task (ST) and DT conditions compared to controls (P = 0.0002 – < 0.0001). They also demonstrated greater DT interference with vision removed for total sway area (P = 0.01) and variability (P = 0.02). Significantly worse postural control was observed in HD with vision removed and reduced proprioception (P = 0.001 – 0.01). Decreased visuospatial performance correlated with greater total sway and jerk (P = 0.01; 0.009). No balance parameters correlated with retrospective falls in HD.
Conclusions
HD participants have worse postural control under DT, limited proprioception/vision, and greater DT interference with a narrowed base and no visual input. These findings may have implications for designing motor and cognitive strategies to improve balance in HD.
Keywords: cognition, balance; Huntington's disease; inertial sensors; postural sway
Introduction
Huntington's disease (HD) is a progressive, autosomal dominant, neurodegenerative disease caused by an expanded CAG repeat (> 40) in the gene for the huntingtin protein (HTT). Motor system involvement in HD typically begins with incoordination and progresses to chorea, rigidity, and akinesia. Neuronal death in the striatal division of the basal ganglia1 causes chorea, the hallmark motor deficit in HD,2 resulting in gait and balance dysfunction, falls, and morbidity.3, 4 The basal ganglia also helps integrate proprioceptive, visual, and vestibular signals critical for maintaining balance.5 Balance impairments in HD are thus highlighted by difficulty utilizing sensory cues to maintain postural control.6 The striatum also plays a role in cognition via networks with the prefrontal cortex.7 Cognitively, HD patients have difficulty holding, shifting, and dividing their attention and struggle when responding to multiple stimuli simultaneously,8, 9 which may further exacerbate motor deficits.
Dual‐task (DT) cognitive‐motor paradigms are used to evaluate difficulty‐dividing attention between multiple tasks, movement automaticity, and the effects of cognitive interference on motor tasks.10, 11 As HD progresses, automaticity changes, such that previously automatic tasks, including walking or balancing require increased cognitive resources.12, 13, 14 Ultimately, this progressive neurodegeneration increases fall risk in HD.8, 13 The loss of automaticity in HD is also seen with fine motor skills.15 However, a prior DT study utilizing a circle‐tracing task did not find significant cognitive interference on the speed of this task in HD.16
Patients with HD show the greatest cognitive deficits in the domains of executive function, processing speed, attention, visuospatial ability, and short‐term memory.14, 17, 18 In other movement disorders, such as Parkinson's disease (PD) and multiple sclerosis (MS), cognitive deficits negatively impact balance and gait, leading to falls and progressive disability.19, 20 Furthermore, current understanding of postural control suggests it is an active process, requiring attentional resources.21 Previous studies found HD participants demonstrate decreased gait speed, cadence, and stride length while dual‐tasking.13, 22 However, the extent to which dual‐tasking and cognitive deficits exacerbate balance dysfunction in HD is unknown.
Previously, difficulties in DT gait performance were associated with increased falls in PD23 and HD.22 We hypothesize that DT balance assessments and knowledge of how cognition impacts balance will provide important information about fall risk in HD. DT training programs have shown success in enhancing gait, balance, and cognitive processing while reducing fall risk in the elderly and in PD.24, 25, 26 Therefore, this study could lead to treatment interventions targeted at motor and cognitive impairments in HD. The goals of this study were to: (1) determine the impact of altered sensory input, stance, and DT cognitive interference on postural control in HD; (2) identify which cognitive deficits might be associated with balance deficits and falls in HD; and (3) examine whether challenging balance conditions, including the DT, are associated with a retrospective history of falls in HD participants.
Methods
Study Participants
HD participants were recruited from the Rush University Medical Center (RUMC) Movement Disorders HD clinic; age‐ and sex‐matched healthy controls were recruited from the RUMC community or friends of the HD participants. Inclusion criteria were, (1) clinical diagnosis of HD by a movement disorders/HD expert (JGG) for HD participants,27, 28 (2) > 21 years of age, (3) ability to stand unsupported for ≥30 seconds, (4) ability to ambulate without an assistive device, and (5) the ability to follow protocol‐specific directions as confirmed by a family member and/or caregiver. Participants diagnosed with juvenile HD, those who had lower limb orthopedic surgery within the past year, or those who had any additional neurological or musculoskeletal disorders negatively affecting balance were excluded from the study. The exclusion criteria for controls were the same, but also excluded individuals with cognitive impairment. Participants were classified as having a choreatic, hypokinetic‐rigid, or mixed phenotype as previously described.29 All participants provided informed consent in accordance with the RUMC Institutional Regulatory Board.
Postural Sway Assessments
Quantitative balance analysis under single task (ST) and dual task (DT) conditions was performed using the well‐validated, reliable inertial sensor instrumented SWAY (i‐SWAY) system with balance metrics generated by Mobility Lab Software.30, 31 An Opal wearable inertial sensor was placed at the lumbar spine (L5), the approximate center of mass location.31 Participants performed i‐SWAY trials under increasingly difficult ST and DT conditions. Participants were asked to stand still for 30 seconds, barefoot, hands at their sides, and heel‐to‐heel distance set at 25 cm for those whose height was <165 cm, and 30.5 cm for those >165 cm, in accordance with the Neurocom Smart Balance Master system protocol, another quantitative, validated balance measurement system.32 The main outcome variables selected for analyses were: (1) 95% ellipse sway area (m2/s4), (2) root mean square (RMS) sway (m/s2), and (3) jerk (m2/sec5).30 These variables were selected a priori out of 33 APDM‐generated variables because these have been found to be sensitive measures of balance dysfunction in other movement disorders30, 31, 33 and have good‐to‐excellent reliability.31 A detailed description of selected sway variables are included in Supporting Table 1. Postural sway was assessed under various conditions of stance (feet apart/together), support surface (firm/on foam; foam pad was a Balance‐pad Elite), and visual input (eyes open/closed), as well as with or without DT. The extent of DT interference, or the dual‐task cost (DTC) in balance performance was defined as DTC (%) = (DT‐ST/ST)*100, as previously described.34 The ST conditions were based on the Modified Clinical Test of Sensory Integration in Balance (CTSIB‐M), which is used clinically to determine aberrant sensory‐motor integration.35 The DT consisted of a simultaneous verbal fluency task (the Controlled Oral Word Association test, COWAT36), each with different letters for firm surface conditions. In the DT conditions, no instructions were given on which task to prioritize. The trials were conducted in a non‐random order with increasing difficulty. Participants were carefully monitored during all trials for safety by the study investigator (NLP), standing directly next to the participant during the entire testing protocol.
Neuropsychological, Balance, and Clinical Rating Scale Assessments
Cognitive function was assessed with the following tests: (1) Montreal Cognitive Assessment (MoCA; global cognition)37; (2) Digit Span forwards, backwards, and sequencing (WAIS‐IV) (attention and working memory)38; (3) Symbol Digit Modalities Test (SDMT; information processing speed)39; (4) Consortium to Establish a Registry for Alzheimer's disease (CERAD word list memory with delayed recall; memory)40; (5) Judgment of Line Orientation (JLO; visuospatial perception)41; and (6) animal naming (verbal fluency).42 This cognitive battery was chosen because it spans multiple cognitive domains known to be deficient in HD. The Unified Huntington's disease Rating Scale motor section was administered by a movement disorder/HD neurologist (JGG) and provided a total motor score (UHDRS‐TMS).27 Participants were asked to self‐report the number of falls they had in the past 12 months. They were also administered the Berg Balance Scale (BBS)43 and the Activities‐Specific Balance Confidence Scale (ABC)44 to obtain functional performance‐based balance information and determine participant's perception of their balance impairment.
Statistical Analyses
Clinical characteristics were compared between HD participants and healthy controls using two‐tailed Student t‐tests for parametric and normally distributed measures, or the Mann‐Whitney U test for variables that were not continuous or did not have normal distributions. Differences in i‐SWAY variables under ST and DT conditions and the DTC for each of the primary outcome variables between HD participants and healthy controls were examined with the same statistical tests. Bonferroni corrections were applied to account for multiple conditions/outcomes on the i‐SWAY (adjusted P value ≤0.0017). A two‐way mixed ANOVA with Bonferroni corrections was performed with the within‐patients factor being the four conditions of the CTSIB‐M: (1) feet apart/eyes open/firm (AOF), (2) feet apart/eyes closed/firm (ACF), (3) feet apart/eyes open/foam (AOFo), and (4) feet apart/eyes closed/foam (ACFo) and a between‐patients factor of group (controls versus HD).
Correlations between i‐SWAY measures and cognitive test scores, UHDRS‐TMS, ABC, BBS, and retrospective falls were examined in the HD group using Spearman's rho. The statistical significance for these comparisons was set at P = 0.05 given the exploratory nature of this work, the large number of variables tested, and correlations performed to reduce overlooking potential significant relationships due to Type II errors.
Results
Participant Characteristics
Seventeen individuals with HD and 17 age‐matched controls participated in the study. Demographic and clinical features of the participant groups are in Table 1. UHDRS‐TMS ranged from seven to 39 with seven participants in the seven to 20 group, and nine in the 21 to 40 group. Eight HD participants were mixed phenotype, four choreatic and four hypokinetic‐rigid, with one participant not having a UHDRS‐TMS recorded. HD participants scored significantly worse than controls on measures of global cognition (MoCA, P = 0.0009), response inhibition (stroop, P = 0.007), processing speed (SDMT, P < 0.0001), verbal fluency (COWAT, P < 0.0001), visuospatial abilities (JLO, P = 0.0083), and working memory (digit span, P = 0.0087). Unexpectedly, performance on memory‐delayed recall (CERAD word list) was not significantly different between HD participants and controls. HD participants reported having significantly lower balance confidence on the ABC (P = 0.0001), performed worse on the BBS (P < 0.0001), and had a higher number of falls within the past year (P = 0.0007) compared to controls.
Table 1.
Participant characteristics
| Health controls | Huntington's disease | |
|---|---|---|
| (n = 17) | (n = 17) | |
| Age (years) | 56.47 ± 9.30 (37‐69) | 55 ± 9.66 (36‐67) |
| Sex | 8 Females, 9 Males | 7 Females, 10 Males |
| BMI (kg/m) | 26.29 ± 5.22 (20.8‐37.8) | 24.68 ± 3.79 (17.80‐31.00) |
| Years of education | 16.59 ± 2.82 | 15.59 ± 2.67 |
| UHDRS‐TMS | ‐‐‐‐ | 21.86 ± 9.86 (7‐39) |
| Trunk chorea (subscore) | ‐‐‐‐ | 0.69 ± 0.79 (0‐2) |
| Phenotype | ‐‐‐‐ | 4 choreatic, 4 hypokinetic/rigid, 8 mixed |
| Disease duration (years) | ‐‐‐‐ | 5 ± 2.8 (3‐13) |
| ABC | 95.38 ± 5.05 (83.7‐100) | 81.20 ± 13.2 (50.31 – 100) *** |
| BBS (0‐56) | 55.88 ± .33 (55‐56) | 51.18 ± 3.15 (44‐56) **** |
| One‐year retrospective Falls (#) | 0.176 ± 0.529 (0‐2) | 2.29 ± 2.69 (0‐10) *** |
| MoCA | 26.47 ± 2.79 (20‐30) | 22.70 ± 3.46 (12‐28) *** |
| SDMT | 99.34 ± 13.42 (80.4‐131.1) | 70.89 ± 20.74 (45.5‐105.9) **** |
| Stroop‐CW | 45.5 ± 8.36 (35‐59) | 37.19 ± 7.89 (25‐52) ** |
| CERAD‐recall | 6.35 ± 1.69 (4‐10) | 5.59 ± 2.24 (2‐10) |
| JLO | 12.35 ± 1.87 (8‐15) | 10.06 ± 2.79 (5‐14) ** |
| Digit span total | 11.12 ± 2.47 (5‐14) | 8.23 ± 3.45 (1‐15) ** |
| Animal naming (#) | 37.41 ± 8.44 (20‐51) | 21.76 ± 9.73 (8‐53) |
All values are mean ± SD with range in brackets unless indicated otherwise.
Abbreviations: ABC, Activity Specific Balance Confidence scale; BBS, Berg Balance Scale; BMI, Body Mass Index; CW, Stroop, Color‐Word; CERAD, Consortium to Establish a Registry for Alzheimer's disease; JLO, Judgment of Line Orientation; MoCA, Montreal Cognitive Assessment; SDMT, Symbol Digit Modalities Test; UHDRS‐TMS, Unified Huntington's Disease Rating Scale‐total motor score.
Standardized Digit Span values were compared between Huntington's disease patients and controls. Note that the SDMT, Stroop‐CW, CERAD‐recall, and digit span were scaled to the patient's age and years of education. Significant differences are bolded.
Self‐reported in last year, 1 year fall history.
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
Postural Sway Assessments
Single and Dual‐Task Results
Because of the wide range of UHDRS‐TMS scores in the HD group, we performed a sub‐analysis examining potential differences in postural sway scores between participants with lower TMS scores (7–20) versus those with higher scores (21–40). There were no statistical differences in any balance parameters between these two subgroups; therefore, all data were combined for subsequent analysis. HD participants demonstrated greater total sway, jerk, and RMS sway under all i‐SWAY conditions compared to controls, including both ST (Table 2) and DT conditions (Table 3; P = 0.0002 to <0.0001). HD participants also had significantly greater DTC for total sway area (P = 0.01) and RMS sway (P = 0.02) with feet together on a firm surface and eyes closed (TCF; Fig. 1).
Table 2.
Balance comparisons between controls and HD participants on the single task CTSIB‐M i‐SWAY conditions and narrowed stance condition
| CTSIB‐M | ||
|---|---|---|
| Condition 1: Feet apart–eyes open–firm surface | ||
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.01 ± 0.009 | 0.31 ± 0.28 *** |
| Total jerk (m2/s5) | 0.56 ± 0.36 | 19.78 ± 20.33 *** |
| RMS sway (m/s2) | 0.06 ± 0.02 | 0.19 ± 0.08 *** |
| Condition 2: Feet apart–eyes closed–firm surface | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.02 ± 0.01 | 0.31 ± 0.30 *** |
| Total jerk (m2/s5) | 1.42 ± 1.55 | 20.69 ± 30.25 *** |
| RMS sway (m/s2) | 0.07 ± 0.02 | 0.19 ± 0.10 *** |
| Condition 3: Feet apart–eyes open–foam surface | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.05 ± 0.04 | 0.62 ± 0.42 *** |
| Total jerk (m2/s5) | 1.43 ± 1.30 | 31.60 ± 25.81 *** |
| RMS sway (m/s2) | 0.08 ± 0.03 | 0.26 ± 0.09 *** |
| Condition 4: Feet apart–eyes closed–foam surface | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.12 ± 0.06 | 1.05 ± 0.93 *** |
| Total jerk (m2/s5) | 4.26 ± 3.16 | 56.15 ± 45.65 *** |
| RMS sway (m/s2) | 0.13 ± 0.03 | 0.34 ± 0.12 *** |
| Narrowed base of support | ||
|---|---|---|
| Feet together–eyes open–firm surface | ||
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.06 ± 0.02 | 0.48 ± 0.42 *** |
| Total jerk (m2/s5) | 1.38 ± 0.77 | 20.55 ± 13.77 *** |
| RMS sway (m/s2) | 0.08 ± 0.01 | 0.24 ± 0.11 *** |
| Feet together–eyes closed–firm surface | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.10 ± 0.06 | 0.91 ± 0.78 *** |
| Total jerk (m2/s5) | 3.16 ± 3.16 | 45.10 ± 40.62 *** |
| RMS sway (m/s2) | 0.10 ± 0.03 | 0.30 ± 0.14 *** |
Data reported as mean ± SD. 95% Ellipse sway area refers to the area of an ellipse covering 95% of the points in both the coronal and sagittal planes, putting more weight on regions more frequently visited. Root mean square (RMS) is the extent of postural sway calculated as RMS of the sway angle in both the AP and ML directions. Total jerk is method to quantify the amount of active postural corrections. Significant differences are bolded.
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
Table 3.
Balance comparisons between controls and HD participants on the dual task (DT) i‐SWAY conditions
| Condition 1: Feet apart–eyes open–firm surface–DT “C” | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.05 ± 0.07 | 1.38 ± 1.96 *** |
| Total jerk (m2/s5) | 4.97 ± 7.09 | 100.59 ± 133.61 *** |
| RMS sway (m/s2) | 0.09 ± 0.04 | 0.37 ± 0.24 *** |
| Condition 2: Feet apart–eyes closed–firm surface–DT “L” | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.04 ± 0.03 | 1.11 ± 1.75 *** |
| Total jerk (m2/s5) | 2.39 ± 2.05 | 79.96 ± 98.22 *** |
| RMS sway (m/s2) | 0.08 ± 0.03 | 0.34 ± 0.23 *** |
| Condition 3: Feet together–eyes open–firm surface–DT “A” | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.11 ± 0.09 | 1.60 ± 2.06 *** |
| Total jerk (m2/s5) | 4.57 ± 4.36 | 112.25 ± 148.97 *** |
| RMS sway (m/s2) | 0.12 ± 0.05 | 0.40 ± 0.25 *** |
| Feet together–eyes closed–firm surface–DT “S” | ||
|---|---|---|
| Variable | Controls | HD |
| 95% Ellipse sway area (m2/s4) | 0.09 ± 0.04 | 1.67 ± 1.83 *** |
| Total jerk (m2/s5) | 3.41 ± 1.86 | 95.68 ± 99.82 *** |
| RMS sway (m/s2) | 0.11 ± 0.02 | 0.41 ± 0.22 *** |
Mean differences between controls and HD patients’ sway characteristics under dual task (DT) (controlled oral word association task [COWAT] letters C, L, A, S) reported as mean ± SD. Significant differences are bolded.
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
Figure 1.
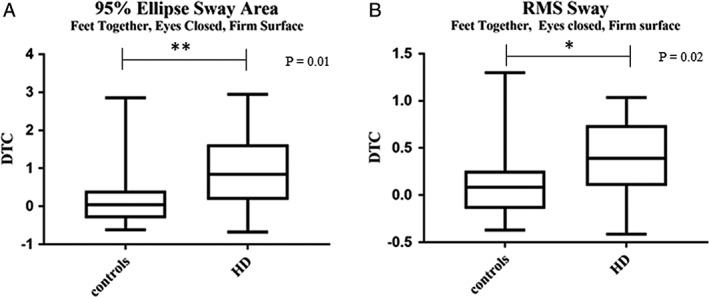
Dual‐task costs (DTC) on the instrumented sway (i‐SWAY) for (A) total sway area and (B) root mean square (RMS) sway between controls and HD patients. DTC defined as (DT‐ST)/ST scores x 100. Data expressed as mean ± SD. *P < 0.05, **P ≤ 0.01.
Clinical Test of Sensory Integration and Balance‐Modified (CTSIB‐M) Results
There was a significant interaction effect between group and CTSIB‐M conditions (P = 0.0009); therefore, the within‐group comparisons were done separately for each group for all three postural sway parameters.
Between Group Comparisons
The HD group exhibited significantly worse total sway and sway variability in all four CTSIB‐M conditions and worse total jerk on three of the four conditions compared to controls (Fig. 2; Table 2). Individuals with HD demonstrated significantly more sway than controls in the AOF (P = 0.002), ACF (P = 0.004), AOFo (P = 0.0001), and ACFo conditions (P = 0.003); significantly more jerk than controls in the AOF (P = 0.005), ACF (P = 0.07), AOFo (P = 0.0008), and ACFo conditions (P = 0.001); significantly more sway variability than controls in the AOF (P < 0.0001), ACF (P = 0.0004), AOFo (P < 0.0001), and ACFo conditions (P < 0.0001).
Figure 2.
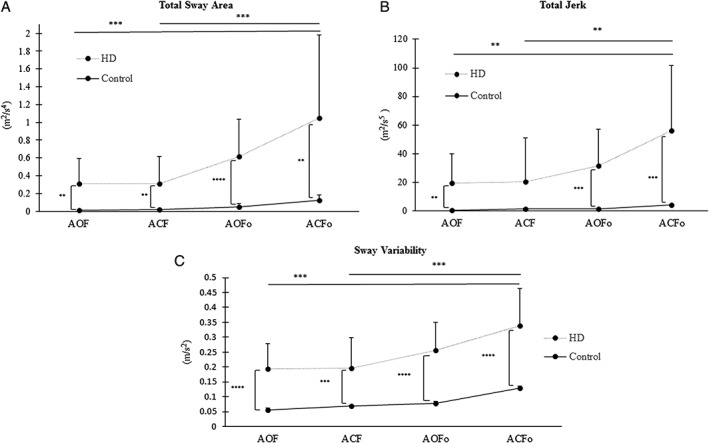
Within and between group comparisons of (A) total sway area, (B) total jerk, and (C) RMS sway values of the HD and control groups under the four conditions of the (CTSIB‐M) modified clinical test of sensory integration and balance; (AOF) feet apart, eyes open, firm surface feet apart; (ACF) eyes closed, firm surface; (AOFo) feet apart, eyes open, foam surface; and (ACFo) feet apart, eyes closed, foam surface. All values are expressed as mean + SD. **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Within‐Group Comparisons
Individuals with HD demonstrated greater total sway (P = 0.001), jerk (P = 0.01), and sway variability (P = 0.001) during the ACFo surface condition compared to ACF condition (Fig. 2). Additionally, HD participants exhibited greater total sway (P = 0.001), jerk (P = 0.01), and sway variability (P = 0.001) with ACFo, compared to AOF.
Correlations Between Cognition, i‐SWAY, UHDRS, and Falls in HD (Table 4; Fig. 2)
Table 4.
Correlations of cognitive/UHDRS‐TMS and iSway parameters
| MoCA | SDMT | Stroop | Digit span | CERAD‐recall | Animal naming | JLO | UHDRS‐TMS | |
|---|---|---|---|---|---|---|---|---|
| Condition 1: Feet apart, eyes open, firm surface | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.258 | ‐0.382 | ‐0.366 | ‐0.073 | ‐0.018 | ‐0.31 | ‐0.32 | 0.528 * |
| Total jerk (m2/s5) | ‐0.174 | ‐0.132 | ‐0.293 | 0.105 | 0.089 | ‐0.15 | ‐0.328 | 0.251 |
| RMS sway (m/s2) | ‐0.248 | ‐0.208 | ‐0.464 | 0.032 | ‐0.001 | ‐0.275 | ‐0.298 | 0.321 |
| Feet apart, eyes closed, firm surface | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.136 | ‐0.064 | ‐0.135 | 0.099 | 0.089 | ‐0.08 | ‐0.343 | 0.189 |
| Total jerk (m2/s5) | ‐0.14 | 0.128 | ‐0.133 | 0.262 | 0.166 | ‐0.086 | ‐0.497 * | 0.049 |
| RMS sway (m/s2) | ‐0.113 | 0.044 | ‐0.281 | 0.162 | 0.089 | ‐0.086 | ‐0.344 | 0.027 |
| Feet together, eyes open, firm surface | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.262 | ‐0.439 | ‐0.349 | ‐0.266 | ‐0.075 | ‐0.364 | ‐0.134 | 0.679 ** |
| Total jerk (m2/s5) | ‐0.224 | ‐0.196 | ‐0.037 | ‐0.006 | 0.063 | ‐0.22 | ‐0.209 | 0.478 |
| RMS sway (m/s2) | ‐0.248 | ‐0.392 | ‐0.319 | ‐0.326 | ‐0.131 | ‐0.456 | ‐0.094 | 0.557* |
| Feet together, eyes closed, firm surface | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.41 | ‐0.574 * | ‐0.343 | ‐0.309 | ‐0.142 | ‐0.448 | ‐.033 | 0.644 ** |
| Total jerk (m2/s5) | ‐0.348 | ‐0.468 | ‐0.133 | ‐0.187 | 0.033 | ‐0.255 | ‐0.43 | 0.525 * |
| RMS sway (m/s2) | ‐0.340 | ‐0.554 * | ‐0.355 | ‐0.318 | ‐0.122 | ‐0.43 | ‐0.308 | 0.662 ** |
| Feet apart, eyes open, foam surface | ||||||||
| 95% Ellipse sway area (m2/s4) | 0.032 | ‐0.314 | ‐0.419 | ‐0.046 | 0.121 | ‐0.084 | ‐0.216 | 0.479 |
| Total jerk (m2/s5) | 0.113 | 0.039 | ‐0.174 | 0.306 | 0.280 | 0.073 | ‐0.264 | 0.062 |
| RMS sway (m/s2) | 0.11 | ‐0.203 | ‐0.468 | 0.025 | 0.219 | ‐0.066 | ‐0.245 | 0.36 |
| Feet apart, eyes closed, foam surface | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.230 | ‐0.059 | ‐0.038 | ‐0.069 | ‐0.058 | 0.081 | ‐0.06 | 0.264 |
| Total jerk (m2/s5) | ‐0.108 | 0.181 | ‐0.034 | 0.248 | 0.023 | 0.213 | 0.009 | ‐0.134 |
| RMS sway (m/s2) | ‐0.183 | ‐0.042 | ‐0.116 | ‐0.108 | 0.001 | 0.07 | ‐0.134 | 0.214 |
| Feet apart, eyes open, dual‐task “C” | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.176 | ‐0.262 | ‐0.302 | ‐0.058 | 0.065 | ‐0.304 | ‐0.617 ** | 0.296 |
| Total jerk (m2/s5) | ‐0.14 | ‐0.066 | ‐0.155 | 0.062 | 0.089 | ‐0.23 | ‐0.549 * | 0.133 |
| RMS sway (m/s2) | ‐0.126 | ‐0.228 | ‐0.387 | ‐0.064 | 0.102 | ‐0.269 | ‐0.581 * | 0.23 |
| Feet apart, eyes closed, dual‐task “L” | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.143 | ‐0.150 | ‐0.234 | 0.111 | 0.152 | ‐0.166 | ‐0.569 * | 0.186 |
| Total jerk (m2/s5) | ‐0.216 | ‐0.064 | ‐0.138 | 0.159 | 0.131 | ‐0.118 | ‐0.624 ** | 0.035 |
| RMS sway (m/s2) | ‐0.049 | ‐0.076 | ‐0.205 | 0.178 | 0.253 | ‐0.015 | ‐0.544 * | 0.102 |
| Feet together, eyes open, dual‐task “A” | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.142 | ‐0.164 | ‐0.059 | ‐0.028 | 0.157 | ‐0.122 | ‐0.527 * | 0.292 |
| Total jerk (m2/s5) | ‐0.044 | ‐0.047 | ‐0.202 | 0.188 | 0.253 | ‐0.15 | ‐0.507 * | 0.075 |
| RMS sway (m/s2) | ‐0.132 | ‐0.137 | ‐0.038 | ‐0.042 | 0.116 | ‐0.139 | ‐0.528 * | 0.165 |
| Feet together, eyes closed, dual‐task “S” | ||||||||
| 95% Ellipse sway area (m2/s4) | ‐0.245 | ‐0.267 | ‐0.127 | ‐0.112 | ‐0.084 | ‐0.175 | ‐0.557 * | 0.395 |
| Total jerk (m2/s5) | ‐0.248 | ‐0.216 | ‐0.149 | 0.037 | 0.615 | ‐0.114 | ‐0.634 ** | 0.254 |
| RMS sway (m/s2) | ‐0.163 | ‐0.275 | ‐0.140 | ‐0.109 | ‐0.045 | ‐0.196 | ‐0.551 * | 0.399 |
Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer's disease; CW, Stroop, Color‐Word; JLO, Judgment of Line Orientation; MoCA, Montreal Cognitive Assessment; SDMT, Symbol Digit Modalities Test; TMS, Total Motor Score; UHDRS, Unified Huntington's disease Rating Scale.
Digit span values were correlated with HD patients’ iSWAY performance under varying sensory conditions. Significant differences are bolded. All values are Spearman's rho (r).
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
Visuospatial function was significantly associated with certain i‐SWAY variables under ST and DT conditions in HD participants (Fig. 3). Lower JLO scores were correlated with (1) greater total sway, under AOF, DT (r = ‐0.617; P = 0.0097), and (2) greater total jerk ACF under both ST (r = ‐0.551, P = 0.0443) and DT (r = ‐0.624; P = 0.0087). Additionally, impaired visuospatial processing was associated with greater DTC under feet together, eyes open, firm (TOF), resulting in increased total sway (r = ‐0.593, P = 0.014), jerk (r = ‐0.552, P = 0.023) and TCF conditions, resulting in increased total sway (r = ‐0.492, P = 0.047) and sway variability (r = ‐0.489, P = 0.048). Lower SDMT scores correlated with greater total sway (r = ‐0.574, P = 0.018) and greater RMS sway (r = ‐0.554, P = 0.023) under TCF, ST. UHDRS‐TMS correlated with greater total sway under TOF (r = 0.679; P = 0.0048) and TCF (r = 0.644; P = 0.006) ST conditions, as well as greater RMS sway (r = 0.662; P = 0.006) with TCF, ST condition. UHDRS‐TMS did not correlate with any balance variables under DT conditions. The number of falls self‐reported in the previous year did not correlate with any cognitive test scores or balance parameters under either ST or DT conditions.
Figure 3.
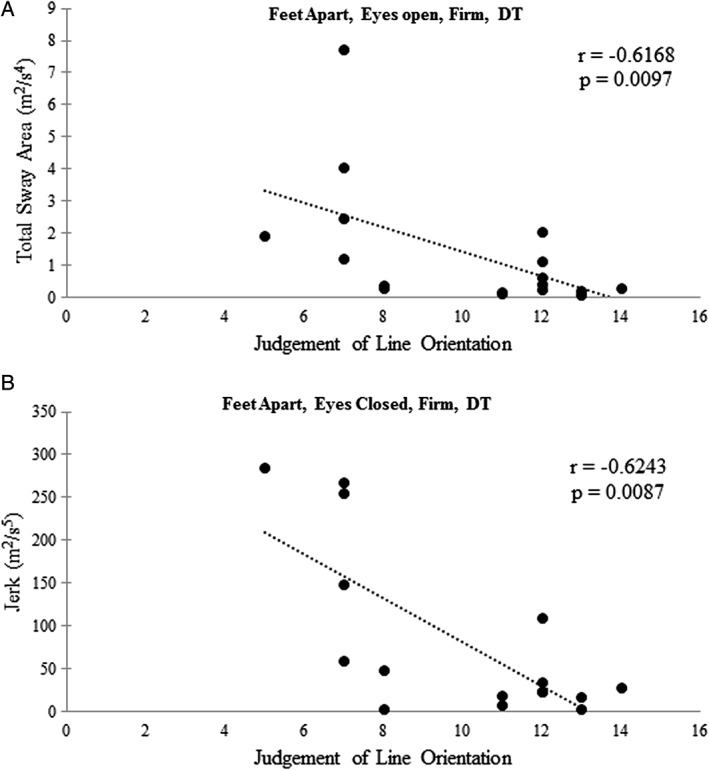
Spearman's correlations (rho) between judgement of line orientation (JLO) scores and (A) total sway area and (B) total jerk under dual task (DT) conditions in HD patients.
Discussion
Postural stability was once thought to be under the control of a few balance centers in the central nervous system.45 However, this view evolved to characterize balance as a complex motor skill controlled by a variety of sensorimotor and cognitive processes and their respective neural pathways.45, 46, 47 Our study found that individuals with HD, compared to controls, have significant cognitive interference when visual input was eliminated and base of support was narrowed, resulting in impaired postural control. Furthermore, the increased postural instability under DT was associated with impaired visuospatial processing. To our knowledge, this is the first study to investigate key characteristics of postural control in HD using inertial sensors, and we found several postural sway domains compromised with reduced visual and proprioceptive input and during cognitive‐motor DT. Our findings are in line with previous work demonstrating that vision is important for stabilizing balance by continually updating the nervous system on body position within a changing environment.48 In the present study, removing vision and narrowing the base of support challenged the neuromotor control of balance, especially under DT, perhaps due to cognitive interference. More specifically, dual tasking produced a “jerkier,” more variable postural sway in HD, which could lead to increased fall risk. These findings suggest that performing a verbal fluency task while balancing significantly interferes with the neural resources necessary to maintain postural control, suggesting competition for common neural networks that are deficient in HD, a theory previously proposed in PD21, 49 and older adults.21, 50
Our findings are related to prior gait studies in HD where performing a cognitive‐motor DT resulted in decreased stride length, cadence,13 and gait speed,13, 22 with increased gait speed DTC.22 Our findings are also consistent with the elevated DTC for combined cognitive and balance tasks in individuals with other neurodegenerative diseases, including MS and PD.51, 52
Under ST conditions, individuals with HD exhibited greater total sway, jerkiness, and variability compared to controls. Our CTSIB‐M findings highlight balance difficulties in HD when proprioception is reduced and when predominantly relying on vestibular information to maintain balance. These results are consistent with previous studies showing that HD participants demonstrate increased sway when proprioceptive and visual cues were altered.6, 53, 54 However, our study is unique in that we characterized balance deficits in three specific domains that measure different aspects of postural stability.31
Our finding that impaired visuospatial perception in HD significantly correlated with a greater, jerkier sway path under DT, suggests that HD participants may depend heavily on their visuospatial system, especially during DT to maintain balance. Visuospatial skills are important for gait and postural control55, 56 and are modulated by the posterior parietal and occipital cortices, areas of volume loss in HD.57, 58 The ability to identify and manipulate where an object is in space involves activation of the parietal lobes, primary motor and premotor cortices, and the basal ganglia.57 Prefrontal cortical degeneration in HD59 would likely contribute to these deficits, given that this area mediates the ability to perform a cognitive motor DT involving executive function.60 While our observations make sense for the eyes open condition, the correlations obtained with the eyes closed condition are not as clear. It is possible that even when HD participants have reduced visuospatial skills, they are likely to use whatever visuospatial capacity they have during balance control, such that eliminating any visual cues caused greater balance impairments. This scenario was made even more challenging by the verbal fluency DT. Future neurophysiological studies employing techniques such as functional near‐infrared spectroscopy (fNIRS)61 while performing balance tasks might help elucidate neural mechanisms for postural control deficits in HD. fNIRS was able to detect changes in prefrontal cortical activation during DT gait paradigms in PD.62 Therefore, a DT fNIRS study could provide a better understanding of prefrontal cortical activation patterns when cognitive loads are imposed on postural control in HD.
We found a reduction in information processing speed was correlated with impaired postural control under the ST conditions of reduced base of support and removed vision, suggesting inadequate cortical processing did not allow HD participants to quickly adapt to these conditions. Lower processing speed has been found to be associated with worse postural stability and increased falls in MS,63 worse turning in PD,64 and slower gait speed in the elderly,65 further highlighting the importance of this cognitive domain in the neural control of balance.66 We did not find significant correlations between the domains of attention, executive function, memory, or global cognition and postural instability. In the past, deficits in executive function were found to compromise a person's dual‐tasking ability, negatively affecting gait and balance in both HD and PD.67, 68 We attribute our lack of significant correlations in the present study to our relatively small sample size.
Contrary to our expectations, the number of self‐reported falls in the past year did not correlate with balance variables under any of the conditions. Retrospective self‐report questionnaires, however, rely on the participants’ self‐awareness and long‐term memory and are vulnerable to under‐reporting. Future studies with prospective fall assessments, caregiver corroboration, or an activity‐monitoring device might provide a more accurate fall report.
The strengths of this study are (1) the use of a sensitive, reliable inertial sensor system to measure balance control in HD under conditions reflecting everyday situations, including cognitive DT; (2) the use of an extensive neuropsychological testing battery that captures multiple cognitive domains and their potential correlations with postural sway and fall risk; and (3) examination of falls in HD, which to date has been understudied. Although this study highlights important negative consequences of DT cognitive interference and altered sensory input on postural control, there are limitations to address in future research. Subsequent studies would benefit from a larger sample size to strengthen potential associations between cognitive domains and balance impairments and stratify HD groups into various levels of motor and cognitive function. A more thorough investigation into visual‐cognitive deficits by utilizing an extensive visual cognition test battery would be beneficial in providing insight into the relationship between visual and balance deficits in HD. Furthermore, incorporating eye‐tracking technology into future balance and gait studies would address the impact of saccadic dysfunction, an early symptom of HD,69 on postural control and fall risk.
In conclusion, HD participants exhibit the most detrimental effects of cognitive interference on postural control with a reduced base of support and vision eliminated. In addition, impaired visuospatial perception and processing speed was associated with worse postural control under DT and ST, respectively. These findings also identified potential future therapeutic strategies to improve balance and reduce fall risk in HD. For example, DT cognitive motor training paradigms,70 virtual reality based rehabilitation,71 and cognitive remediation therapies72 have been shown to improve balance and turning and reduce falls in neurodegenerative disorders.73 Future investigations on the impact of these therapeutic approaches in HD are warranted.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
N.P.: 1A, 1B, 1C, 2A, 2B, 2C, 3A
J.G.: 1A, 1B, 1C, 2C; 3B
B.O.: 2C
B.B.: 1A, 3B
J.O.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: This study was approved by the Rush University Medical Center institutional review board (IRB); ID# 16050204. Informed consent was obtained from all study participants prior to testing. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: Nicollette Purcell reports no disclosures or conflicts of interests related to this manuscript. Jennifer G. Goldman reports no conflicts of interests related to this manuscript. Bichun Ouyang reports no conflicts of interest related to this manuscript. Bryan Bernard reports no disclosures or conflicts of interests related to this manuscript. Joan A. O'Keefe reports no conflicts of interests related to this manuscript.
Financial Disclosures for the previous 12 months: Nicollette Purcell: none. Jennifer Goldman has received grant/research support from National Institutes of Health, Michael J. Fox Foundation, Parkinson Foundation, CHDI, Rush University, Acadia, and Biotie/Accorda (site‐PI), consulting fees from Acadia, Aptinyx, and honoraria from the International Parkinson and Movement Disorder Society, and American Academy of Neurology. Bryan Bernard: none. Joan A. O'Keefe receives research support from the NIH (K01 HD088762).
Supporting information
Supporting Table 1. iSWAY Variable Description
Expanded descriptive definitions of the three selected APDM™ iSWAY variables.
Acknowledgments
We would like to thank our HD and control participants for their participation in this study, as well as the Rush University Movement Disorders Huntington's Disease Society of America Center of Excellence clinical and research team for their assistance in recruitment, especially Kim Janko, Sarah Chen, and Courtney Timms.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Aylward EH, Codori AM, Rosenblatt A, et al. Rate of caudate atrophy in presymptomatic and symptomatic stages of huntington's disease. Mov Disord 2000;15(3):552–560. [DOI] [PubMed] [Google Scholar]
- 2. Bates GP, Dorsey R, Gusella JF, et al. Huntington disease. Nat Rev Dis Primers 2015;23;1:1–21. [DOI] [PubMed] [Google Scholar]
- 3. Rao AK, Muratori L, Louis ED, Moskowitz CB, Marder KS. Spectrum of gait impairments in presymptomatic and symptomatic huntington's disease. Mov Disord 2008;23(8):1100–1107. [DOI] [PubMed] [Google Scholar]
- 4. Medina LD, Pirogovsky E, Salomonczyk D, et al. Postural limits of stability in premanifest and manifest huntington's disease. J Huntingtons Dis 2013;2(2):177–184. [DOI] [PubMed] [Google Scholar]
- 5. Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol 2008;255:4:19–29. [DOI] [PubMed] [Google Scholar]
- 6. Tian J, Herdman SJ, Zee DS, Folstein SE. Postural stability in patients with huntington's disease. Neurology 1992;42(6):1232–1238. [DOI] [PubMed] [Google Scholar]
- 7. Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci 2001;1(2):137–160. [DOI] [PubMed] [Google Scholar]
- 8. Maurage P, Heeren A, Lahaye M, et al. Attentional impairments in huntington's disease: a specific deficit for the executive conflict. Neuropsychology 2017;31(4):424–436. [DOI] [PubMed] [Google Scholar]
- 9. Aron AR, Watkins L, Sahakian BJ, Monsell S, Barker RA, Robbins TW. Task‐set switching deficits in early‐stage huntington's disease: Implications for basal ganglia function. J Cogn Neurosci 2003;15(5):629–642. [DOI] [PubMed] [Google Scholar]
- 10. Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age‐related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture 2007;26(1):113–119. [DOI] [PubMed] [Google Scholar]
- 11. Toulotte C, Thevenon A, Watelain E, Fabre C. Identification of healthy elderly fallers and non‐fallers by gait analysis under dual‐task conditions. Clin Rehabil 2006;20(3):269–276. [DOI] [PubMed] [Google Scholar]
- 12. Thompson JC, Poliakoff E, Sollom AC, Howard E, Craufurd D, Snowden JS. Automaticity and attention in huntington's disease: when two hands are not better than one. Neuropsychologia 2010;48(1):171–178. [DOI] [PubMed] [Google Scholar]
- 13. Delval A, Krystkowiak P, Delliaux M, et al. Role of attentional resources on gait performance in huntington's disease. Mov Disord 2008;23(5):684–689. [DOI] [PubMed] [Google Scholar]
- 14. Paulsen JS. Cognitive impairment in huntington disease: diagnosis and treatment. Curr Neurol Neurosci Rep 2011;11(5):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson JC, Poliakoff E, Sollom AC, Howard E, Craufurd D, Snowden JS. Automaticity and attention in huntington's disease: when two hands are not better than one. Neuropsychologia 2010;48(1):171–178. [DOI] [PubMed] [Google Scholar]
- 16. Vaportzis E, Georgiou‐Karistianis N, Churchyard A, Stout JC. Effects of task difficulty during dual‐task circle tracing in huntington's disease. J Neurol 2015;262(2):268–276. [DOI] [PubMed] [Google Scholar]
- 17. Teixeira AL, de Souza LC, Rocha NP, Furr‐Stimming E, Lauterbach EC. Revisiting the neuropsychiatry of huntington's disease. Dement Neuropsychol 2016;10(4):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaportzis E, Georgiou‐Karistianis N, Churchyard A, Stout JC. Dual task performance may be a better measure of cognitive processing in huntington's disease than traditional attention tests. J Huntingtons Dis 2015;4(2):119–130. [DOI] [PubMed] [Google Scholar]
- 19. Amboni M, Barone P, Iuppariello L, et al. Gait patterns in parkinsonian patients with or without mild cognitive impairment. Mov Disord 2012;27(12):1536–1543. [DOI] [PubMed] [Google Scholar]
- 20. Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: an investigation of cognitive‐motor dual tasking in multiple sclerosis. Mult Scler 2009;15(10):1215–1227. [DOI] [PubMed] [Google Scholar]
- 21. Woollacott M, Shumway‐Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 2002;16(1):1–14. [DOI] [PubMed] [Google Scholar]
- 22. Fritz NE, Hamana K, Kelson M, Rosser A, Busse M, Quinn L. Motor‐cognitive dual‐task deficits in individuals with early–mid stage huntington disease. Gait Posture 2016;49:283–289. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs JV, Nutt JG, Carlson‐Kuhta P, Allen R, Horak FB. Dual tasking during postural stepping responses increases falls but not freezing in people with parkinson's disease. Parkinsonism Relat Disord 2014;20(7):779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dorfman M, Herman T, Brozgol M, et al. Dual‐task training on a treadmill to improve gait and cognitive function in elderly idiopathic fallers. J Neurol Phys Ther 2014;38(4):246–253. [DOI] [PubMed] [Google Scholar]
- 25. Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with parkinson's disease? J Gerontol A Biol Sci Med Sci 2011;66(2):234–240. [DOI] [PubMed] [Google Scholar]
- 26. Fritz NE, Cheek FM, Nichols‐Larsen DS. Motor‐cognitive dual‐task training in persons with neurologic disorders: a systematic review. J Neurol Phys Ther 2015;39(3):142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huntington SG. Unified huntington's disease rating scale: reliability and consistency. Mov Disord 1996;11(2):136–142. [DOI] [PubMed] [Google Scholar]
- 28. Reilmann R, Leavitt BR, Ross CA. Diagnostic criteria for huntington's disease based on natural history. Mov Disord 2014;29(11):1335–1341. [DOI] [PubMed] [Google Scholar]
- 29. Hart EP, Marinus J, Burgunder JM, et al. Better global and cognitive functioning in choreatic versus hypokinetic‐rigid huntington's disease. Mov Disord 2013;28(8):1142–1145. [DOI] [PubMed] [Google Scholar]
- 30. Mancini M, Horak FB, Zampieri C, Carlson‐Kuhta P, Nutt JG, Chiari L. Trunk accelerometry reveals postural instability in untreated parkinson's disease. Parkinsonism Relat Disord 2011;17(7):557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mancini M, Salarian A, Carlson‐Kuhta P, et al. ISway: a sensitive, valid, and reliable measure of postural control. J Neuroeng Rehabil 2012;16:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Natus MI. Objective quatification of balance and mobility. Neurocom International Inc. 2007:12. [Google Scholar]
- 33. Dewey DC, Miocinovic S, Bernstein I, et al. Automated gait and balance parameters diagnose and correlate with severity in parkinson disease. J Neurol Sci 2014;345(1‐2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwenk M, Zieschang T, Oster P, Hauer K. Dual‐task performances can be improved in patients with dementia: a randomized controlled trial. Neurology 2010;74(24):1961–1968. [DOI] [PubMed] [Google Scholar]
- 35. Shumway‐Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther 1986;66(10):1548–1550. [DOI] [PubMed] [Google Scholar]
- 36. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14(2):167–177. [PubMed] [Google Scholar]
- 37. Freitas S, Simoes MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and alzheimer disease. Alzheimer Dis Assoc Disord 2013;27(1):37–43. [DOI] [PubMed] [Google Scholar]
- 38. Wechsler D. Wechsler adult intelligence scale WAIS‐IV; technical and interpretive manual. London: Pearson; 2008. [Google Scholar]
- 39. Smith A. The symbol‐digit modalities test: a neuropsychologic test of learning and other cerebral disorders. Seattle: Special Child Publications; 1968. p 83. [Google Scholar]
- 40. Seo EH, Lee DY, Lee JH, et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry 2010;18(9):801–809. [DOI] [PubMed] [Google Scholar]
- 41. Gullett JM, Price CC, Nguyen P, Okun MS, Bauer RM, Bowers D. Reliability of three benton judgment of line orientation short forms in idiopathic parkinson's disease. Clin Neuropsychol 2013;27(7):1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kertesz A. Western aphasia battery. San Antonio: The Psychological Corporation; 1982. [Google Scholar]
- 43. Berg K, Wood‐Dauphinee S, Williams JI. The balance scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med 1995;27(1):27–36. [PubMed] [Google Scholar]
- 44. Powell LE, Myers AM. The activities‐specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1995;50A(1):M28–34. [DOI] [PubMed] [Google Scholar]
- 45. Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 2006;35 Suppl 2:ii7–ii11. [DOI] [PubMed] [Google Scholar]
- 46. Horak FB, Macpherson J. Exercise regulation and integration of multiple systems In: Handbook of Physiology. New York: Oxford University Press; 1996. [Google Scholar]
- 47. Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture 2001. Dec;14(3):203–210. [DOI] [PubMed] [Google Scholar]
- 48. Lord SR. Visual risk factors for falls in older people. Age Ageing 2006. Sep;35 Suppl 2:ii42–45. [DOI] [PubMed] [Google Scholar]
- 49. Camicioli R, Oken BS, Sexton G, Kaye JA, Nutt JG. Verbal fluency task affects gait in parkinson's disease with motor freezing. J Geriatr Psychiatry Neurol 1998;11(4):181–185. [DOI] [PubMed] [Google Scholar]
- 50. Shumway‐Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci 2000;55(1):M10–16. [DOI] [PubMed] [Google Scholar]
- 51. Holmes JD, Jenkins ME, Johnson AM, Adams SG, Spaulding SJ. Dual‐task interference: the effects of verbal cognitive tasks on upright postural stability in parkinson's disease. Parkinsons Dis 2010;2010:696492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Butchard‐MacDonald E, Paul L, Evans JJ. Balancing the demands of two tasks: an investigation of cognitive‐motor dual‐tasking in relapsing remitting multiple sclerosis. J Int Neuropsychol Soc 2018;24(3):247–258. [DOI] [PubMed] [Google Scholar]
- 53. Panzera R, Salomonczyk D, Pirogovosky E, et al. Postural deficits in huntington's disease when performing motor skills involved in daily living. Gait Posture 2011;33(3):457–461. [DOI] [PubMed] [Google Scholar]
- 54. Salomonczyk D, Panzera R, Pirogovosky E, et al. Impaired postural stability as a marker of premanifest huntington's disease. Mov Disord 2010. Oct 30;25(14):2428–2433. [DOI] [PubMed] [Google Scholar]
- 55. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord 2017. Jan;10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morris R, Lord S, Bunce J, Burn D, Rochester L. Gait and cognition: mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci Biobehav Rev 2016;64:326–345. [DOI] [PubMed] [Google Scholar]
- 57. Labuschagne I, Cassidy AM, Scahill RI, et al. Visuospatial processing deficits linked to posterior brain regions in premanifest and early stage huntington's disease. J Int Neuropsychol Soc 2016;22(6):595–608. [DOI] [PubMed] [Google Scholar]
- 58. Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of huntington's disease in the longitudinal TRACK‐HD study: cross‐sectional analysis of baseline data. Lancet Neurol 2009;8(9):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dominguez DJF, Poudel G, Stout JC, et al. Longitudinal changes in the fronto‐striatal network are associated with executive dysfunction and behavioral dysregulation in huntington's disease: 30 months IMAGE‐HD data. Cortex 2017;92:139–149. [DOI] [PubMed] [Google Scholar]
- 60. Strobach T, Antonenko D, Abbarin M, Escher M, Floel A, Schubert T. Modulation of dual‐task control with right prefrontal transcranial direct current stimulation (tDCS). Exp Brain Res 2018;236(1):227–241. [DOI] [PubMed] [Google Scholar]
- 61. Fishburn FA, Norr ME, Medvedev AV, Vaidya CJ. Sensitivity of fNIRS to cognitive state and load. Front Hum Neurosci 2014;8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thumm PC, Maidan I, Brozgol M, et al. Treadmill walking reduces pre‐frontal activation in patients with parkinson's disease. Gait Posture 2018;62:384–387. [DOI] [PubMed] [Google Scholar]
- 63. Wajda DA, Motl RW, Sosnoff JJ. Correlates of dual task cost of standing balance in individuals with multiple sclerosis. Gait Posture 2014;40(3):352–356. [DOI] [PubMed] [Google Scholar]
- 64. Pal G, O'Keefe J, Robertson‐Dick E, Bernard B, Anderson S, Hall D. Global cognitive function and processing speed are associated with gait and balance dysfunction in parkinson's disease. J Neuroeng Rehabil 2016;13(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the mayo clinic study of aging. J Gerontol A Biol Sci Med Sci 2013;68(8):929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sosnoff JJ, Balantrapu S, Pilutti LA, Sandroff BM, Morrison S, Motl RW. Cognitive processing speed is related to fall frequency in older adults with multiple sclerosis. Arch Phys Med Rehabil 2013;94(8):1567–1572. [DOI] [PubMed] [Google Scholar]
- 67. Kloos AD, Kegelmeyer DA, Fritz NE, Daley AM, Young GS, Kostyk SK. Cognitive dysfunction contributes to mobility impairments in huntington's disease. J Huntingtons Dis 2017;6(4):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dirnberger G, Jahanshahi M. Executive dysfunction in parkinson's disease: a review. J Neuropsychol 2013;7(2):193–224. [DOI] [PubMed] [Google Scholar]
- 69. Blekher T, Johnson SA, Marshall J, et al. Saccades in presymptomatic and early stages of huntington disease. Neurology 2006;67(3):394–399. [DOI] [PubMed] [Google Scholar]
- 70. Fernandes A, Rocha N, Santos R, Tavares JM. Effects of dual‐task training on balance and executive functions in parkinson's disease: a pilot study. Somatosens Mot Res 2015;32(2):122–127. [DOI] [PubMed] [Google Scholar]
- 71. Peruzzi A, Zarbo IR, Cereatti A, Della Croce U, Mirelman A. An innovative training program based on virtual reality and treadmill: effects on gait of persons with multiple sclerosis. Disabil Rehabil 2017;39(15):1557–1563. [DOI] [PubMed] [Google Scholar]
- 72. Milman U, Atias H, Weiss A, Mirelman A, Hausdorff JM. Can cognitive remediation improve mobility in patients with parkinson's disease? Findings from a 12 week pilot study. J Parkinsons Dis 2014;4(1):37–44. [DOI] [PubMed] [Google Scholar]
- 73. Paul SS, Dibble LE, Peterson DS. Motor learning in people with parkinson's disease: implications for fall prevention across the disease spectrum. Gait Posture 2018;61:311–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table 1. iSWAY Variable Description
Expanded descriptive definitions of the three selected APDM™ iSWAY variables.


