ABSTRACT
Background
Postural abnormalities in Parkinson's disease (PD) patients and unimpaired elderly are not well differentiated. Factors related to postural abnormality associated with PD are controversial.
Objective
We assessed differences in postural change between PD patients and unimpaired elderly and elucidated factors related to abnormal posture in PD patients.
Methods
We measured the dropped head angle (DHA), anterior flexion angle (AFA), and lateral flexion angle (LFA) of the thoracolumbar spine of an unprecedented 1,117 PD patients and 2,732 general population participants (GPPs) using digital photographs. Two statistical analyses were used for elucidating factors related to these angles.
Results
In GPPs, age was correlated with DHA, AFA, and LFA. DHAs, AFAs, and LFAs of PD patients and age‐matched GPPs were 21.70° ± 14.40° and 13.13° ± 10.79°, 5.98° ± 12.67,°and − 3.82° ± 4.04°, and 0.86° ± 4.25° and 1.33° ± 2.16°, respectively. In PD patients, factors related to DHA were age, male sex, and H & Y stage during ON time. Factors related to AFA were age, duration of disease, H & Y stage during ON and OFF times, pain, vertebral disease, and bending to the right. A factor related to LFA was AFA.
Conclusions
DHA and AFA of GGPs correlated with age and were larger in PD patients than those with in GPPs. Some PD patients showed angles far beyond the normal distribution. Thus, factors associated with disease aggravation affected postural abnormality in PD patients.
Keywords: Parkinson's disease, postural abnormality, general population, related factor, digital camera photograph
Parkinson's disease (PD) patients often show a stooped and bent posture. In addition to the “common” stooped and bent posture, some patients show remarkable anteflexion (camptocormia), lateral leaning (Pisa syndrome), and dropped head. The frequency of camptocormia is 6.9%,1 Pisa syndrome is 8.8%,2 and dropped head is 1.5% to 6.3%.3, 4, 5 However, the exact frequency of these postures is unclear owing to differences in target patients and methods for measuring and judging postural abnormalities. Physically unimpaired people in the general population also develop bent posture with age.6 The study by Oeda et al.7 is the only previous study in which the postures of PD patients and general population participants (GPPs) were digitally imaged and compared. However, they examined comparatively few cases and did not measure the lateral flexion angle (LFA) of the thoracolumbar spine. We measured the dropped head angle (DHA), anterior flexion angle (AFA) of the thoracolumbar spine, and LFA from digital photographs and compared them with those of 1,117 PD patients and age‐matched GPPs. To clarify related factors, we investigated the background and treatment of PD patients. As for therapeutic agents, we focused on dopamine agonists because abnormal postures related to these agents have been reported previously.8, 10
Patients and Methods
PD Patients and GPPs
A total of 1,117 PD patients (540 males, 577 females; average age: 70.4 years) in 15 medical institutions of Tokyo metropolitan district and 2,732 medical checkup GPPs (1,199 males, 1,533 females; average age: 64.9 years) in five city areas of Japan (Shimotsuke City, Ayakawa City, Kami‐ichi Town, Karatsu City, and Wara Town) were registered. This medical checkup service is performed once a year for independent businessmen and housewives by the government. The registration period was from April 2012 to March 2014. The clinical diagnosis of all PD patients was defined as per the UK Brain Bank Criteria.11
Measurement Procedure
We took digital photographs from the back and side of PD patients and GPPs. We identified the (1) external acoustic foramen, (2) acromion, and (3) greater trochanter in the printed photographs. Furthermore, we measured the DHA, AFA, and LFA with a straight line that linked those points and the perpendicular line by hand (Fig. 1).
Figure 1.
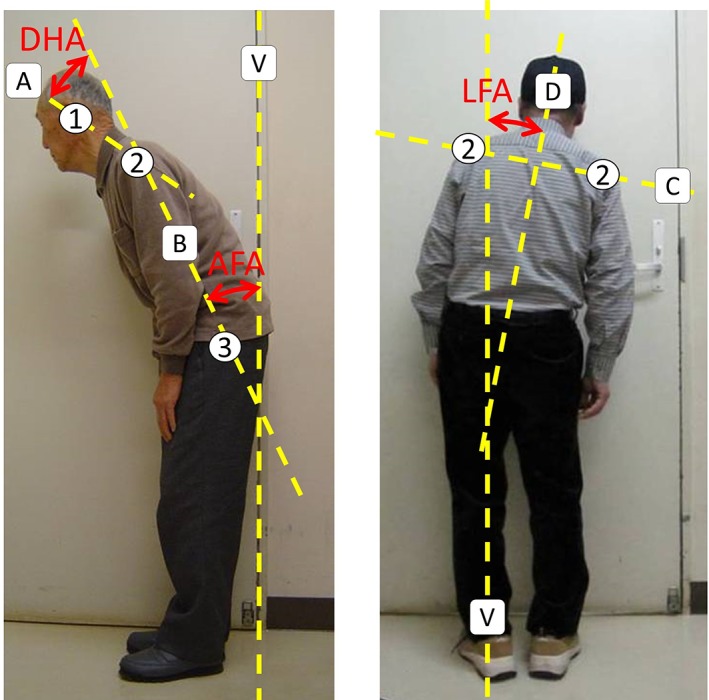
Measurement procedure. 1: external acoustic foramen; 2: acromion; and 3: greater trochanter. A straight line A: between the external acoustic foramen and acromion; A straight line B: between the acromion and greater trochanter. The DHA is made by the straight lines A and B. The AFA is made by straight line B and a vertical line V. A straight line C: between both acromions shown in the back photographs and a straight line D crossing line C at a right angle. The LFA is made by straight line D and vertical line V. Plus sign indicates right direction and minus sign indicates left direction.
PD patients assume an almost anteflexion posture when walking, but can adopt a vertical posture if they stop and make the effort. We photographed postures for these two situations (i.e., standing as straight as possible and relaxing while walking). Because there was correlation among these two postures, we used photographs taken in a relaxed state.
For PD patients, we investigated age, sex, onset side, type of oral medicine and dosage amount, physical work strength required in the occupation, presence of pain, rehabilitation to correct abnormal posture, vertebral disease, operation for PD, H & Y stage during ON and OFF times, and duration of PD. The work strength of the occupation was classified as first (slight, such as office and sewing workers), second (middle, such as salespersons and housewives), and third (severe, such as agricultural and industry workers) degrees. For GPPs, we only collected data on age and sex. We analyzed all GPPs and matched them by age with each PD patient by generating a random number to match against the same number assigned to PD patients for each age. This study was approved by the independent ethics committee of the Jichi Medical University.
Statistical Analysis
We analyzed the H & Y stage and physical work strength of the occupation classification under conditions of both pain and rehabilitation to correct abnormal posture to obtain two values representing the degree classification and analyzed the duration of PD and medication dosage as numerical values.
The levodopa‐equivalent dose (LED) was assumed to have been as follows: pergolide, 1,000 μg; cabergoline, 2 mg; talipexole, 1.6 mg; pramipexole, 2 mg; ropinirole, 9 mg; and l‐dopa/dopa decarboxylase inhibitor (DCI), 100‐mg equivalency. Selegiline was assumed to have been given as one daily output × 30% of levodopa/DCI and entacapone one daily output × 20% of l‐dopa/DCI, regardless of a daily dose.12
Two types of statistical analysis were conducted. First, Spearman's single‐correlation analysis was used for correlations between a measurement angle and each factor as a pair. Statistical significance was set to P < 0.05 for coefficients of correlation |r| > 0.06 and to P < 0.001 for |r| > 0.10. Second, multiple regression analysis (step‐wise method), which was a multivariate analysis, was used. Factors with high levels of significance were included in the final model without excluding basic factors, such as age or sex. To assess differences between PD patients and GPPs, we conducted the Mann‐Whitney U test when there was a variance in the distribution between the groups and Student's t test when there was no variation. The level of statistical significance was set to P < 0.05. StatFlex software (Version 6; Artech Co., Ltd., Tokyo, Japan) was used for all statistical analyses.
Results
DHA
DHA was significantly larger in PD patients (mean ± standard deviation [SD]: 21.70° ± 14.40°] than in age‐matched GPPs (13.13° ± 10.79°; Fig. 2A). DHA was 25.39° ± 13.68° in male PD patients, 18.25° ± 14.23° in female PD patients, 15.48° ± 10.54° in male GPPs, and 10.94° ± 10.57° in female GPPs. In both groups, DHAs were significantly larger in males than in females (Fig. 3A,D).
Figure 2.
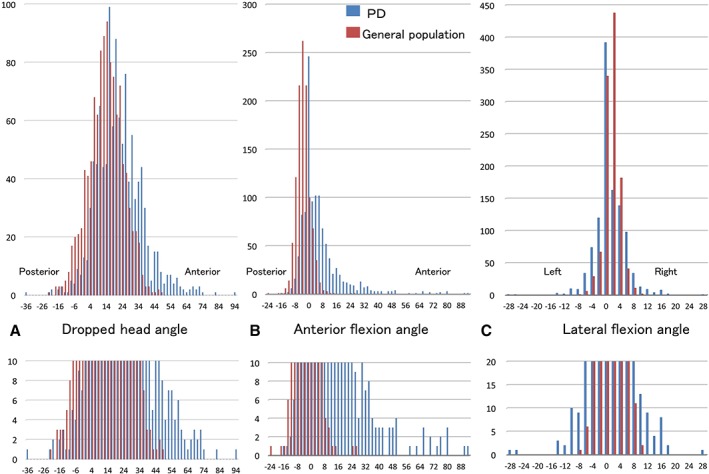
Comparison between the measured angles in the age‐matched general population participants and the angles in the PD patients. Upper section: total distribution. Lower section: extended indication to watch small distribution (n = 0–10).
Figure 3.
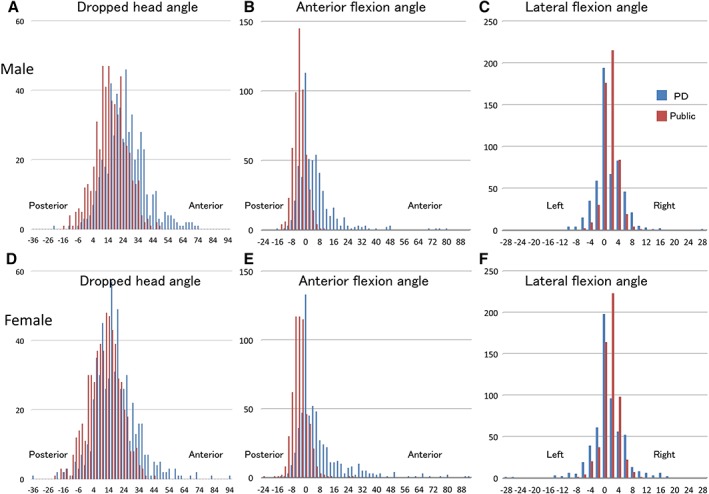
Comparison between the measured angles in the age‐matched general population participants and the angles in the PD patients (540 males and 577 females). Upper section: males. Lower section: females.
In GPPs, coefficients of correlation between DHA and age were 0.0904 (P = 0.0017) in total, 0.0944 (P = 0.832) in males, and 0.0929 (P = 0.211) in females.
Characteristically, distribution of DHAs was significantly wider in PD patients than in GPPs; 16.2% of PD patients had DHAs >34.7° (the mean + 2 SDs of DHA in GPPs). Moreover, 4.0% of PD patients had DHAs >48.9° (the maximum DHA in GPPs). The maximum DHA in PD patients was 94.2° (Fig. 2A, lower panel).
In the Spearman single‐correlation analysis of DHA‐related factors in PD patients, factors that exhibited equilateral correlation and P < 0.001 were male sex; duration of PD; H & Y stage during ON and OFF times; l‐dopa/DCI dosage; l‐dopa/DCI + adjuvant, such as selegiline or entacapone LED; total LED; and work strength of the occupation. Factors that exhibited equilateral correlation and P < 0.05 were age, operation for PD, and presence of pain.
In the multiple regression analysis, the factor of male sex showed equilateral correlation and P < 0.001. Factors that showed equilateral correlation and P < 0.005 were age and H & Y stage during ON time. AFA showed an inverse correlation and P < 0.001.
Factors of age, male sex, and H & Y stage during ON time showed significant correlation in both analyses (Table 1).
Table 1.
Relationship between the measured angles and PD patient background
| DHA | AFA | LFA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCA | MRA | α = −1.1519 ± 3.72984 | SCA | MRA | α = −15.68 ± 2.89 | SCA | MRA | α = 2.10 ± 1.16 | |||||||
| r | P Value | β | std β | P Value | r | P Value | β | std β | P Value | r | P Value | β | std β | P Value | |
| Age | 0.092 | 0.002** | 0.13 ± 0.053 | 0.063 | 0.013* | 0.196 | <0.0001*** | 0.084 ± 0.041 | 0.061 | 0.043* | −0.0282 | 0.3442 | −0.031 ± 0.016 | −0.053 | 0.057 |
| Sex (male) | 0.273 | <0.0001*** | 6.29 ± 0.86 | 0.230 | <0.0001*** | −0.035 | 0.236 | −0.96 ± 0.69 | −0.047 | 0.160 | 0.0101 | 0.7345 | 0.15 ± 0.27 | 0.013 | 0.575 |
| Duration | 0.111 | 0.000*** | 0.05 ± 0.099 | 0.012 | 0.610 | 0.242 | <0.0001*** | 0.47 ± 0.76 | 0.180 | <0.001*** | 0.072 | 0.0162* | −0.017 ± 0.031 | −0.022 | 0.578 |
| H & Y (ON) | 0.168 | <0.0001*** | 2.82 ± 0.95 | 0.088 | 0.003** | 0.448 | <0.0001*** | 3.45 ± 0.74 | 0.140 | <0.001*** | 0.0633 | 0.0362* | −0.17 ± 0.30 | −0.015 | 0.570 |
| H & Y (OFF) | 0.141 | <0.0001*** | 0.18 ± 0.87 | 0.002 | 0.840 | 0.426 | <0.0001*** | 1.66 ± 0.68 | 0.073 | 0.015* | 0.0965 | 0.0014** | 0.37 ± 0.27 | 0.037 | 0.168 |
| PD operation | 0.052 | 0.029* | 3.34 ± 2.03 | 0.055 | 0.100 | 0.018 | 0.547 | −3.34 ± 1.60 | −0.037 | 0.037* | 0.0287 | 0.3369 | 0.65 ± 0.63 | 0.023 | 0.304 |
| l‐dopa + assist LED | 0.156 | <0.0001*** | 0.0041 ± 0.0023 | 0.067 | 0.068 | 0.249 | <0.0001*** | ‐0.0016 ± 0.0018 | −0.023 | 0.360 | 0.0804 | 0.007** | 0.00026 ± 0.00070 | 0.019 | 0.712 |
| Agonist LED | ‐0.0088 | 0.768 | ‐0.0006 ± 0.0079 | ‐0.0098 | 0.940 | 0.053 | 0.075 | ‐0.013 ± 0.0062 | −0.066 | 0.031* | 0.0593 | 0.0468* | 0.001 ± 0.0024 | 0.025 | 0.672 |
| Total LED | 0.138 | <0.0001*** | Excludeda | 0.248 | <0.0001*** | Excludeda | 0.0891 | 0.0028** | Excludeda | ||||||
| Job | 0.137 | <0.0001*** | 1.17 ± 0.61 | 0.048 | 0.056 | 0.029 | 0.333 | 0.85 ± 0.48 | 0.044 | 0.078 | −0.0108 | 0.7181 | −0.10 ± 0.19 | −0.0076 | 0.600 |
| Pain | 0.064 | 0.032* | 0.62 ± 1.00 | 0.016 | 0.540 | 0.317 | <0.0001*** | 3.90 ± 0.78 | 0.160 | <0.001*** | 0.0324 | 0.2775 | 0.021 ± 0.31 | 0.0089 | 0.947 |
| Spinal lesion | 0.026 | 0.383 | 0.61 ± 1.13 | 0.013 | 0.590 | 0.197 | <0.0001*** | 2.43 ± 0.89 | 0.080 | 0.006** | −0.0165 | 0.581 | −0.23 ± 0.35 | −0.026 | 0.508 |
| Rehabilitation | 0.015 | 0.614 | 0.59 ± 1.15 | 0.007 | 0.610 | 0.084 | 0.005* | −0.78 ± 0.90 | ‐0.031 | 0.390 | −0.0431 | 0.1486 | −0.83 ± 0.36 | −0.063 | 0.019* |
| Dropped head angle | — | — | — | — | — | −0.0354 | 0.236 | −0.097 ± 0.024 | ‐0.12 | <0.001*** | 0.0469 | 0.1157 | 0.017 ± 0.0095 | 0.045 | 0.069 |
| Anterior flexion angle | ‐0.0354 | 0.236 | ‐0.16 ± 0.039 | ‐0.12 | <0.0001*** | — | — | — | — | 0.1441 | <0.0001*** | 0.034 ± 0.012 | 0.087 | 0.005** | |
| Lateral flexion angle | 0.047 | 0.116 | 0.18 ± 0.098 | 0.045 | 0.069 | 0.144 | <0.0001*** | 0.22 ± 0.077 | 0.087 | 0.005** | — | — | — | — | |
In the rank‐difference Spearman correlation analysis, the coefficient of correlation |r| value is low for P values with many patients. The items with P < 0.05 are displayed in bold.
In the multiple regression analysis, the l‐dopa + assist drug LED and agonist LED were excluded by multicollinearity leftacteristics. The items with P < 0.05 are displayed in bold.
Excluded by multicollinearity.
P < 0.05;
P < 0.01;
P < 0.001.
Abbreviation: stdβ, standard partial regression coefficient.
AFA
Larger AFA indicates stronger anteflexion. AFA was anteflexion in PD patients (5.98° ± 12.67°) and dorsoflexion in age‐matched GPPs (−3.82° ± 3.04°; Fig. 2B). AFA was 4.86° ± 10.71° in PD males, 7.02° ± 14.19° in PD females, −3.93° ± 3.58° in male GPPs, and − 3.72° ± 4.45° in female GPPs. In PD patients, AFAs were significantly larger in females than in males, whereas in GPPs there was no significant difference between the sexes (Fig. 3B,E).
In the analysis of GPPs, coefficients of correlation between AFAs and age were 0.110 (P < 0.001) in total, 0.0386 (P = 1.000) in males, and 0.107 (P = 0.022) in females.
Similar to the distribution of DHAs, the distribution of AFAs was wider in PD patients than in GPPs. The 39.5% of PD patients had AFAs >4.28° (the mean + 2 SDs of AFA in GPPs; Fig. 2B, lower panel).
In the Spearman single‐correlation analysis of AFA‐related factors in PD patients, factors that showed equilateral correlation and P < 0.001 were age, duration of PD, H & Y stage during ON and OFF times, l‐dopa/DCI + adjuvants LED, total LED, spinal disease, and LFA to the right. Factors of the presence of rehabilitation showed an inverse correlation and P < 0.01.
In the multiple regression analysis, factors of duration of PD, H & Y stage during ON time, and pain showed equilateral correlation and P < 0.001. Factors of spinal disease and LFA to the right showed equilateral correlation and P < 0.01. Factors of age and H & Y stage during OFF time showed equilateral correlation and P < 0.05. The factor of DHA showed an inverse correlation and P < 0.001. Factors of dopamine agonist LED and the presence of operation for PD showed an inverse correlation and P < 0.05.
Factors of age, duration of PD, H & Y stage during ON and OFF times, pain, spinal disease, and LFA to the right showed significant correlations in both analyses (Table 1).
LFA
There was no dominant side of onset of the disease; 410 cases had right‐sided onset, 366 had left‐sided onset, and 341 had bilateral onset or lack of information. The 238 (58.0%) cases in the right‐sided onset group bended to the right side, and the 126 (34.4%) cases in the left‐sided onset group bended to the left side. Only in the cases that the onset side is clear, there was no statistical relation between onset side and bending side; however, there was a tendency of bending to right‐side in both onset side groups.
The LFA tended to incline to the right more in age‐matched GPPs (1.33° ± 2.16°) than in the PD patients (0.86° ± 4.25°; P < 0.001; Fig. 2C). In GPPs, LFA was 1.38° ± 2.00° in males and 1.28° ± 2.30° in females. In PD patients, LFA was 0.98° ± 3.84° in males and 0.75° ± 4.61° in females. In both groups, there was no significance difference in LFAs between the sexes (Fig. 3C,F).
In the analysis of GPPs, positive correlation of “left‐side inclination” was observed between LFA and age either in the total analysis or in males and females.
Similar to the distributions of DHA and AFA, that of LFA was wider in PD patients than in GPPs. Twelve (8.68%) PD patients had LFA > −2.98°/+5.65° (the mean − 2/+2 SDs of LFAs of GPPs; Fig. 2C, lower panel).
In the Spearman single‐correlation analysis of LFA‐related factors in PD patients, the factor of AFA showed equilateral (right‐sided) correlation and P < 0.001. Factors that showed equilateral correlation and P < 0.01 were H & Y stage during OFF time; l‐dopa/DCI + adjuvants LED; and total LED. Factors that showed equilateral correlation and P < 0.05 were H & Y stage during ON time, duration of PD, and dopamine agonist LED.
In the multiple regression analysis, the factor of AFA showed equilateral correlation and P < 0.05. Factors of presence of rehabilitation showed inverse (left‐sided) correlation and P < 0.05.
Only AFA showed correlation in both analyses (Table 1).
Finally, as supplementary analysis, in the single correlative analysis involving all 2,732 GPPs, age was correlated with DHA (r = 0.0848), AFA (r = 0.1134), and LFA (r = 0.0780; P < 0.0001).
Discussion
In this study, we measured DHA, AFA, and LFA in 1,117 PD patients and in 2,732 medical checkup GPPs and conducted an age‐matched comparison of these angles between the two groups.
We confirmed that DHA and AFA were larger in PD patients than in age‐matched GPPs. Notably, AFA was dorsoflexion in GPPs but anteflexion in PD patients. Drzal‐Grabiec et al. reported that the angle of inclination of the trunk in healthy individuals aged >60 years declines backward (dorsoflexion) compared to that in young individuals.6 DHA was larger in males in both PD patients and GPPs. Because AFA is larger in females, the head may turn upward to compensate. In both PD patients and GPPs, DHAs of both sexes were inversely correlated with AFA (P < 0.01).
The hallmark of angles in PD patients was the very wide distribution. Many PD patients showed angles larger than the average + 2 SDs of GPPs for all three angles; 16.2% for DHA; 39.5% for AFA; 8.68% on the right side in LFA; and 12.0% on the left side in LFA. These results demonstrate that some PD patients exhibited extremely abnormal posture, which was rarely observed in GPPs.
Some researchers use an arbitrary number of at least 45° of AFA for defining camptocormia.1 In this study, PD patients with an AFA of ≥45° accounted for 2.24%, which was relatively less compared with that observed in previous studies (6.9% by Tiple et al.,1 4.1% by Seki et al.,13 and 6.5% by Song et al.14 Bonanni et al.15 defined Pisa syndrome as an LFA of >15°, whereas Tinazzi et al.2 defined it as an LFA of >10°. In this study, PD patients with an LFA of ≥15° accounted for 1.25% and ≥10° accounted for 4.65%, which was relatively less compared with 8.8% in the study by Tinazzi et al.2 These discrepancies may be a result of differences in patient backgrounds and measuring methods. Some researchers did not measure,1, 4, 16 some measured by hand,7, 13 or by wall goniometer,2, 14, 15 some measured using a computer software,17 and some used a mechanical computer‐assisted, hand‐held device for measuring the angle of vertebral bones. 18, 19
Regarding the onset side of PD symptoms, the lateral flexion side might not depend on the onset side. Absolute values of angles leaning to the right and left were offset because we assigned the plus sign to the right side and minus sign to the left side for the statistical analysis.
Some previous studies have reported factors influencing postural abnormalities in PD as Table 2. Djaldetti et al.20 first reported a relationship between anterior waist bending and male sex, duration of PD, and H & Y stage. Bloch et al.21 found that the UPDRS Part III scores of patients with waist bending were significantly high, especially on the walk item. Tiple et al.1 noted that patients with waist bending were older, had higher l‐dopa dosages and durations, higher H & Y stage, higher rigidity, and akinesia scores of the UPDRS than those without waist bending. Seki et al.,13 Song et al.,14 and Ameghino et al.16 reported H & Y stage and UPDRS Part III score as common factors for camptocormia. Moreover, Ameghino et al.16 indicated the relationship between postural abnormality and exposure to dopamine agonists or amantadine. Kashihara et al.22 found that DHAs, AFAs, and LFAs of PD patients correlated with H & Y stage and that AFA and LFA were influenced by age, female sex, deterioration of Mini‐Mental State Examination (MMSE), degree of lumbago, and duration of disease. Oeda et al.7 found DHA was influenced by H & Y stage and a history of postural abnormalities induced by dopamine agonists, and AFA was influenced by age, duration of disease, H & Y stage, UPDRS Part III score, dosage of l‐dopa, LED of dopamine agonists, vertebral disease, and mental symptoms. Zinazzi et al.2 reported age, body mass index, and falls as factors related to lateral flexion other than those of severity of PD. Benninger et al.18 found no correlation between the angle of incline and age, sex, disease duration, total UPDRS score, and presence of low back pain. Khlebtovsky et al.19 found that the spinal angle was associated with age, older age at disease onset, disease duration, UPDRS motor and posture scores, and presence of back pain.
Table 2.
Postural abnormality and related factors in PD
| Author | Year | N | Postural Abnormality | Method | Related Factors |
|---|---|---|---|---|---|
| Djaldetti et al.20 | 1999 | 8 | Camptocormia | Unmeasured | Male sex, duration of PD, H & Y stage |
| Bloch et al.21 | 2006 | 63 | Camptocormia | Unmeasured | UPDRS Part III scores |
| Tipple et al.1 | 2009 | 275 | Camptocormia | Unmeasured (partially goniometer) | Age, duration of PD, dosage of l‐dopa, duration of l‐dopa treatment, H & Y stage, rigidity and bradykinesia item score of UPDRS PPart III, dementia, vertebral surgery |
| Seki et al.13 | 2011 | 531 | Camptocormia | Measured (photograph) | Age, H & Y stage, UPDRS Part III scores, total LED, dosage of l‐dopa, urinary incontinence, severe constipation |
| Kashihara et al.22 | 2012 | 365 | Dropped head | Unmeasured | H & Y stage |
| Camptocormia | H & Y stage, age, female sex, deterioration of MMSE score, duration of PD, lumber pain | ||||
| Lateral flexion | H & Y stage, age, female sex, deterioration of MMSE score, duration of PD, lumber pain | ||||
| Song et al.14 | 2013 | 705 | Camptocormia | Measured (goniometer) | Duration of PD, H & Y stage, UPDRS Part III scores, motor fluctuation, dyskinesia, MMSE |
| Oeda et al.7 | 2013 | 216 | Dropped head | Measured (photograph) | H & Y stage, history of postural abnormalities induced by dopamine agonists |
| Camptocormia | Age, duration of PD, H & Y stage, UPDRS Part III score, dosage of l‐dopa, LED of dopamine agonists, vertebral disease, mental symptoms | ||||
| Zinazzi et al.2 | 2015 | 1,631 | Lateral flexion | Measured (goniometer) | Age, body mass index, duration of PD, H & Y stage, UPDRS scores, LED, ongoing pharmacological therapy, falls, veering gait |
| Khlebtovsky et al.19 | 2017 | 190 | Camptocormia | Measured (spinal mouse) | Age, older age at disease onset, duration pf PD, UPDRS motor and posture scores, back pain |
| Ameghino et al.16 | 2018 | 63 | Dropped head | Unmeasured | Urinary incontinence, exposure to pribedil and quetiapine |
| Camptocormia | Age, history of orthopedic disorder, duration of PD, H & Y stage, UPDRS Part III scores, exposure to dopamine agonists and amantadine | ||||
| Lateral flexion | H & Y stage, less depression, exposure to amantadine | ||||
| Present study | 2018 | 1,117 | Dropped head | Measured (photograph) | Age, male sex, H & Y stage during ON time |
| Camptocormia | Duration of PD, H & Y stage during ON and OFF times, spinal disease, LFA to the right | ||||
| Lateral flexion | Anterior flexion angle |
In the present study, DHA and AFA of PD patients were associated with factors related to disease progression, including H & Y stage during ON and OFF times, and duration of PD. DHA and AFA were related to the dosage of l‐dopa/DCI, LED of l‐dopa/DCI + adjuvant, and total LED. However, we regarded these as confounding factors because quantities of medicines taken increased with disease progression. Recently, postural abnormalities attributed to dopamine agonists have been reported.8, 9, 10 Therefore, we confirmed the correlation between each angle and the dosage of LED of dopamine agonists. Consequently, LED of dopamine agonists showed an inverse correlation with AFA. We speculated that the use of dopamine agonists in patients with extremely abnormal posture was discontinued when it was widely recognized in Japan that these abnormalities could be caused by dopamine agonists.
We found that LFA inclined to the right side and was related to the medicines taken, H & Y stage, and duration of disease. Rehabilitation to correct abnormal posture demonstrated inverse (left‐sided) correlation. Paolucci et al.23 also reported the efficacy of perceptive training for treating balance impairment in PD patients.
Benninger et al.18 and Djaldetti et al.20 reported that there was no significant correlation between postural abnormality and pain. Conversely, Khlebtovsky et al.19 and Kashihara et al.22 noted a correlation between postural abnormality and lumbago. In the present study, DHA and AFA were correlated with pain. As described in several previous reports,1, 7, 16, 18 we found that a past history of vertebral operations or diseases can promote the anteflexion of the trunk and that a past history of vertebral disease was a significant factor related to AFA. In addition, AFA was larger and DHA was smaller for people with occupations that required higher physical strength.
Conclusion
We measured DHA, AFA, and LFA of PD patients and GPPs and statistically confirmed that DHA and AFA of PD patients were larger than those of age‐matched GPPs. In GPPs, DHA, AFA, and LFA showed normal distribution pattern. In PD patients, these angles also showed an almost normal distribution, whereas in some patients, these angles were showed skewed distribution.
DHA, AFA, and LFA correlated with age in GPPs.
The relationship between postural abnormality and patient background or treatment was elucidated using two statistical analyses. Factors related to DHA were age, male sex, and H & Y stage during ON time. Factors related to AFA were age, duration of disease, H & Y stage during ON and OFF times, pain, vertebral disease, and lateral flexion angle to the left side. The only factor related to LFA was AFA. DHA, and AFA were associated with the dosage of l‐dopa/DCI, LED of l‐dopa/DCI + adjuvant, and total LED. LED of dopamine agonists showed an inverse correlation with AFA. We speculated that types and dosage of a therapeutic drug did not essentially influence the angles because the dosage of drugs increased as the severity of disease increased.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
Ken‐ichi Fujimoto: 1A, 1B, 1C, 2A, 2C, 3B
Yoshihito Ando: 1C, 2A, 2B, 3A
Yasuyuki Okuma: 1C, 2C, 3B
The other authors: 1C, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work in consistent with those guidelines. The authors confirm that the approval of an institutional review board and patient consent were not required for this work.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Acknowledgments
We thank the JMS cohort II study group; Representative: Shizukiyo Ishikawa (Jichi Medical University, Department of Public Health), Staff: Shimotsuke City in Tochigi, Ayakawa City in Kagawa, Kami‐ichi Town in Toyama, Karatsu City in Saga, and Wara Town in Gifu.
We thank Enago (https://www.enago.jp/) for the English language review.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Tiple D, Fabbrini G, Colosimo C, et al. Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry 2009;80:145–148. [DOI] [PubMed] [Google Scholar]
- 2. Tinazzi M, Fasano A, Geroin C, et al. Pisa syndrome in Parkinson disease: an observational multicenter Italian study. Neurology 2015;85:1769–1779. [DOI] [PubMed] [Google Scholar]
- 3. Askmark H, Eeg‐Olofsson K, Johansson A, Nilsson P, Olsson Y, Aquilonius S. Parkinsonism and neck extensor myopathy: a new syndrome or coincidental findings? Arch Neurol 2001;58:232–237. [DOI] [PubMed] [Google Scholar]
- 4. Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson's disease. Mov Disord 2006;21:1213–1216. [DOI] [PubMed] [Google Scholar]
- 5. Fujimoto K. Dropped head in Parkinson's disease. J Neurol 2006;253(Suppl 7):VII21–VII26. [DOI] [PubMed] [Google Scholar]
- 6. Drzal‐Grabiec J, Rykala J, Podgorska J, Snela S. Changes in body posture of women and men over 60 years of age. Ortop Traumatol Rehabil 2012;14:467–475. [DOI] [PubMed] [Google Scholar]
- 7. Oeda T, Umemura A, Tomita S, Hayashi R, Kohsaka M, Sawada H. Clinical factors associated with abnormal postures in Parkinson's disease. PLoS One 2013;8:e73547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uzawa A, Mori M, Kojima S, et al. Dopamine agonist‐induced antecollis in Parkinson's disease. Mov Disord 2009;24:2408–2411. [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Jeon BS, Kim SH, Han SH. Reversible antecollis associated with pramipexole in a patient with Parkinson's disease. J Clin Neurosci 2012;19:903–904. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki M, Hirai T, Ito Y, et al. Pramipexole‐induced antecollis in Parkinson's disease. J Neurol Sci 2008;264:195–197. [DOI] [PubMed] [Google Scholar]
- 11. Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson's disease. Ann Neurol 1992;32(Suppl):S125–S127. [DOI] [PubMed] [Google Scholar]
- 12. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 13. Seki M, Takahashi K, Koto A, et al. Camptocormia in Japanese patients with Parkinson's disease: a multicenter study. Mov Disord 2011;26:2567–2571. [DOI] [PubMed] [Google Scholar]
- 14. Song W, Guo X, Chen K, et al. Camptocormia in Chinese patients with Parkinson's disease. J Neurol Sci 2014;337:173–175. [DOI] [PubMed] [Google Scholar]
- 15. Bonanni L, Thomas A, Varanese S, Scorrano V, Onofrj M. Botulinum toxin treatment of lateral axial dystonia in Parkinsonism. Mov Disord 2007;22:2097–2103. [DOI] [PubMed] [Google Scholar]
- 16. Ameghino L, Bruno V, Merello M. Postural disorders and antiparkinsonian treatments in Parkinson disease: an exploratory case‐control study. Clin Neuropharmacol 2018;41:123–128. [DOI] [PubMed] [Google Scholar]
- 17. Margraf NG, Wolke R, Granert O, et al. Consensus for the measurement of the camptocormia angle in the standing patient. Parkinsonism Relat Disord 2018;52:1–5. [DOI] [PubMed] [Google Scholar]
- 18. Benninger F, Khlebtovsky A, Roditi Y, et al. Beneficial effect of levodopa therapy on stooped posture in Parkinson's disease. Gait Posture 2015;42:263–268. [DOI] [PubMed] [Google Scholar]
- 19. Khlebtovsky A, Djaldetti R, Rodity Y, et al. Progression of postural changes in Parkinson's disease: quantitative assessment. J Neurol 2017;264:675–683. [DOI] [PubMed] [Google Scholar]
- 20. Djaldetti R, Mosberg‐Galili R, Sroka H, Merims D, Melamed E. Camptocormia (bent spine) in patients with Parkinson's disease—characterization and possible pathogenesis of an unusual phenomenon. Mov Disord 1999;14:443–447. [DOI] [PubMed] [Google Scholar]
- 21. Bloch F, Houeto JL, Tezenas du Montcel S, et al. Parkinson's disease with camptocormia. J Neurol Neurosurg Psychiatry 2006;77:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kashihara K, Imamura T. Clinical correlates of anterior and lateral flexion of the thoracolumbar spine and dropped head in patients with Parkinson's disease. Parkinsonism Relat Disord 2012;18:290–293. [DOI] [PubMed] [Google Scholar]
- 23. Paolucci T, Morone G, Fusco A, et al. Effects of perceptive rehabilitation on balance control in patients with Parkinson's disease. NeuroRehabilitation 2014;34:113–120. [DOI] [PubMed] [Google Scholar]


