ABSTRACT
Background
It has been suggested that sensory impairments contribute significantly to the motor deficits secondary to impaired sensorimotor integration in Parkinson's disease. Speech and swallowing are likely to become disordered in PD, and there is evidence that impaired upper airway sensation also contributes to these disorders.
Objectives
The goal of this study was to investigate the relationship between perception of general respiratory sensation, speech, and swallowing in PD.
Methods
Thirteen people with PD and 14 age‐equivalent controls volunteered to participate. Randomized blocks of inspiratory resistive loads were delivered, and participants gauged the magnitude of the loads using a modified Borg scale. The magnitude estimates were then compared to results of speech and swallowing evaluations using multivariate analysis of variance and a stepwise linear regression model.
Results
There was a significant overall interaction between the participant group (PD versus control) and respiratory load (F [10, 300] = 2.138; P = .022). A significant regression equation containing a predictor speech variable respiratory rating was found (F [1,22] = 6.946), P = .023), with a moderate effect size of R2 = .387.
Conclusions
People with PD have blunted perception of respiratory resistive loads when compared with age‐equivalent healthy adults. Results also suggest that blunted ME of resistive loads could contribute to changes in respiratory drive for speech (i.e., loudness).
Keywords: dysarthria, dysphagia, Parkinson's disease, respiratory sensation
Introduction
Parkinson's disease (PD) is a chronic and progressive disease characterized primarily by motor signs, including resting tremor, rigidity, and postural reflex impairment. In addition to these classic motor signs, people with PD exhibit deficits in multiple sensory modalities, including proprioception, kinesthesia, mechanosensation, and olfaction.1, 2, 3, 4 It has been suggested that sensory impairments contribute significantly to the motor deficits secondary to impaired sensorimotor integration within basal ganglia structures. Somatosensory deficits are directly associated with a decline in motor functions such as posture and gait, and much of the literature supports the hypothesis that there may be a sensory origin to PD motor signs.3
In addition to gait and postural changes, people with PD often present with disordered speech (dysarthria), including hypophonia and disordered swallowing (dysphagia).5 A unifying theme between these functions is the integral role of the respiratory system and upper airway for executing these behaviors. The production of phonation and speech are dependent upon both respiratory and laryngeal subsystems working in a coordinated and synergistic fashion. The respiratory system provides tracheal pressure that is requisite to initiating and sustaining vocal fold vibration, which is highly dependent on the lung volume at speech initiation.6 Also, expiratory airflow allows for the development of intraoral pressures necessary for accurate production of pressure consonants during speech.7, 8 Concerning swallowing, in order to protect the airway from aspirate material, the ventilatory respiratory pattern is inhibited during the swallow (referred to as the swallow pause). This swallow‐breathing pause usually ends with the resumption of the expiratory phase of the respiratory cycle, in particular for single swallows of thin liquids, which are presumed to be protective.9
Another unifying aspect of speech and swallowing is that they both depend on sensory system input to guide motor output. For example, motor aspects of swallowing are adjusted based on the sensation of bolus characteristics such as volume and viscosity. The amplitude of movement for speech articulators, including the vocal folds, depends on proprioceptive and kinesthetic information from the oral cavity and larynx. Similar to gait and postural deficits, it has been suggested that sensory perception deficits may underlie disorders of speech and swallowing in PD.4, 10, 11
Given the known sensory deficits in PD, and the integral role of the respiratory system for execution of speech and swallowing, it is reasonable to hypothesize that altered respiratory sensation may occur in PD, which may contribute to the development of dysarthria and dysphagia. Respiratory sensation can be studied by applying respiratory resistive loads that increase the work of breathing, then asking participants to estimate the magnitude of the load presented.12 Because it requires little training and the scales used are typically very easy for patients to understand, magnitude estimation (ME) is a technique that is well suited for studying sensory perception in PD.13 This methodology has been used on many different patient populations and is sensitive to disease state, either in a heightened manner (i.e., anxiety)14 or in a blunted manner (i.e., children with life‐threatening asthma).15
The goal of this study was to compare magnitude estimation (ME) of respiratory resistive loads in people with PD compared to age‐matched healthy adults (controls). It was hypothesized that the PD group would exhibit blunted ME of respiratory resistive loads compared to the controls. A secondary aim was to determine the relationship between the ME of respiratory resistive loads and clinical speech and swallowing metrics in PD. It was hypothesized that the blunted ME would be linearly related to severity of speech and swallowing metrics, providing support to our overall hypothesis, that blunted respiratory sensation contributes to the development of speech and swallowing disorders in PD patients.
Methods
The University of Florida Institutional Review Board approved this study, and all participants provided written and verbal informed consent to participate. The study was a prospective experimental study with two participant groups. There were 13 adults with PD (PD group) diagnosed by a fellowship‐trained movement disorders neurologist, according to strict UK brain bank criteria. Also, there were 14 age‐equivalent adults without a history of PD (control group). Participants were recruited from the University of Florida Center for Movement Disorders and Neurorestoration (UF CMDNR; PD group) and spouses or caregivers that accompanied patients to appointments at the center (control group). Exclusion criteria were: (1) neurologic disease other than PD (PD group); (2) any neurologic disease (control group); history of cancer in the head, neck, or lungs; (4) currently smoking, or smoking within the previous five years; (6) history of breathing disorders or diseases (e.g., COPD, lung cancer); (7) history of severe cognitive deficits (dementia); and (8) severe neuropsychological disorder (i.e., severe depression: 31 or greater on the BDI II).
Baseline Depression and Apathy
There is a significant impact of emotion on respiratory perceptual ratings.12 Baseline depression and apathy scores were measured using two validated scales, the Beck Depression Index ‐ II (BDI II) and Marin Apathy Index (MAI).
Baseline Pulmonary Function Tests
In order to ensure that differences in respiratory sensation were not related to group differences in pulmonary function, we performed pulmonary function tests on all study participants. The forced expired volume in the first second (FEV1) of a forced vital capacity (FVC) exhalation was measured using a digital spirometer (Spirovision 3 + m, Futuremed). The FEV1, FVC, and ratio of FEV1/FVC were expressed as a percent‐predicted value.
Inspiratory Resistive Load Presentation
Participants were seated facing away from the experimental apparatus. A facemask was placed securely over the face and connected to a non‐rebreathing valve (Hans Rudolph, 2700 series), in line with a differential pressure transducer. The inspiratory port of the valve was connected to the resistive loading manifold, which consisted of five differential resistors ranging from 2.5 to 40 cmH2O of resistive pressure, separated by stoppered ports. There was also a “no‐load” condition where no resistive pressure was applied. Removal of the stoppered ports applied the load (Fig. 1). The pressure transducer measured mouth pressure and airflow, which was digitized and recorded to a desktop computer using LabChart software.
Figure 1.
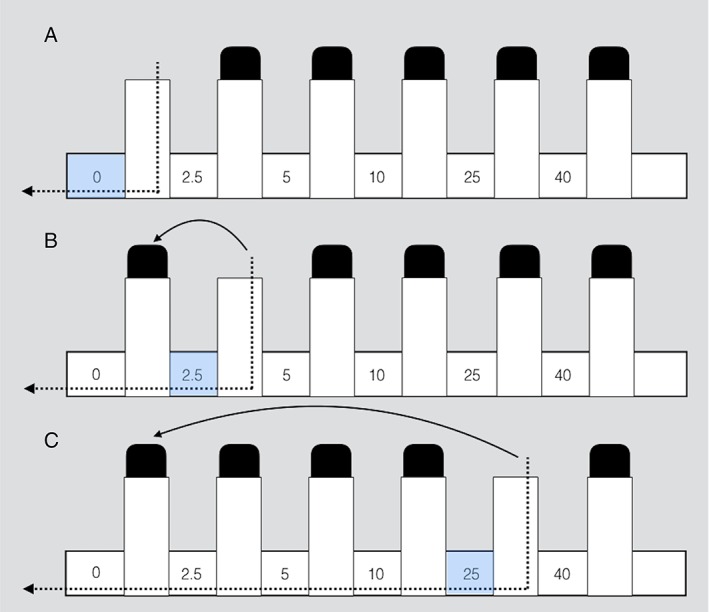
Schematic of the inspiratory load application method. Each of the 3 configurations (A, B, and C) represent a different applied load, with no‐load (0) in part A, 2.5cmH2O load in part B, and 25 cmH2O load in Part C.
Participants were familiarized with the loads in a practice session before the experimental protocol, and the load extremes (i.e., no‐load and maximum load) were presented as anchors for a modified Borg Scale, which was used as the measure of magnitude estimation. During the experiment, the resistive loads were applied in a randomized block design, with each loaded breath separated by at least three unloaded breaths. Two blocks were completed with each load presented between three and five times within each block. Therefore, there were a total of 15 to 25 loaded breaths (3–5 loads by five presentations) per block. Following each loaded breath (including the no‐load condition), participants provided an estimate of the perceived difficulty of inhaling against the load using a modified Borg scale (Supporting Table 1). The ME and load resistance were then plotted on a log‐log linear scale, generating a sensitivity slope (ME slope), that represents sensitivity to increases in resistive load.16
Speech and Swallowing Functions
For PD participants, the most recent speech and swallowing evaluation performed in our clinic were reviewed. These measures of speech and swallowing were selected from the active clinical protocol at the UF CMDNR. This protocol was developed according to the recommended speech evaluation tasks for dysarthria as described by Duffy (Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 2 nd Edition, 2005). Four speech‐language clinicians, with between four and nine years of experience, completed speech evaluations. Evaluations were performed independently, and any difficult or questionable evaluations were discussed at a monthly consensus meeting. Speech evaluations consisted of several tasks that target seven speech subsystem domains, defined in Supporting Table 2. The outcome data consisted of maximum phonation duration on the vowel “ah” (in seconds), and ratings across the seven speech domains. The severity ratings were made on a Likert scale ranging from zero (no dysfunction) to seven (anarthric; Supporting Table 3). The motor speech diagnosis (dysarthria type) was also recorded.
Swallowing evaluations were completed under videofluoroscopy with patients viewed in the lateral plane. The evaluation included multiple swallows of thin liquid barium, pudding‐thick barium, and a cracker or cookie coated with barium. For this study, we recorded the worst observed penetration‐aspiration score (PAS) during the study. The PAS is a validated scale documenting penetration or aspiration of bolus material (Supporting Table 4).17 The PAS served as our metric of swallowing safety.
Data Analysis
Three statistical models were used to analyze the data. First, in order to determine if participant characteristics including age and pulmonary function (FVC, FEV1/FVC), depression, apathy, and ME sensitivity slope differed between the PD and control groups, we utilized a one‐way analysis of variance (ANOVA) with independent variable group (PD vs control) and dependent variables ME slope, age, FCV, and FEV1/FVC, BDI, and MAI. Next, in order to determine whether the ME of each respiratory resistive load differed between the PD and control groups, we used a multivariate analysis of variance (MANOVA) with independent variables resistive load applied (2.5, 5, 10, 25, and 40 cm H20) and group (PD vs control) and dependent variables, including the magnitude estimation (ME) and airflow rate (L/s) during the applied inspiratory load.
In order to address the second aim that focused on the relationship between speech and swallowing metrics and respiratory sensation, we used a stepwise linear regression model with dependent variable ME slope, and predictor variables maximum phonation duration, severity ratings across the seven speech domains, and PAS. Our alpha level was set at P < .05 for all statistical models used.
Results
Participant characteristics, including sex; age; predicted FEV1, FVC, and FEV1/FVC %; depression (BDI); apathy (MAI); and ME slope are shown in Table 1. Results of the one‐way ANOVA showed a significant difference between the PD and control group for ME slope (F [1,25] = 17.327; P < .000; Fig. 2); however, there were no significant group differences between other variables in the model.
Table 1.
Participant demographic information
| Participant group | Sex | Age (years); (standard deviation) | Hoehn & Yahr Stage | Disease duration (years); (standard deviation) | Former smoker? | FEV1 (% predicted); (standard deviation) | FVC (% predicted); (standard deviation) | FEV1/FVC (% predicted); (standard deviation) | BDI Median score (range) | MAI Median score (range) | ME slope (standard deviation) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
PD
(n = 14) |
6 Female 8 Male |
69.14 (5.86) | 1 ‐ 1 2 ‐ 5 2.5 ‐ 1 3 ‐ 4 4 ‐ 3 |
7.07 (4.95) | n = 2 | 86.50 (12.62) | 86.14 (12.81) | 99.07 (4.51) | 5.5 (1 ‐ 12) | 14 (1 ‐ 20) | 0.18 (.07)** |
|
Control
(n = 13) |
7 Female 6 Male |
64.85 (9.70) | N/A | N/A | n = 4 | 90.62 (17.50) | 88.70 (15.83) | 99.54 (12.10) | 4 (1 ‐ 6) | 8 (1 ‐ 18) | 0.28 (.06)** |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BDI, Beck depression inventory II; MAI, Marin apathy scale; ME is magnitude estimation.
Indicates P < .001.
Figure 2.
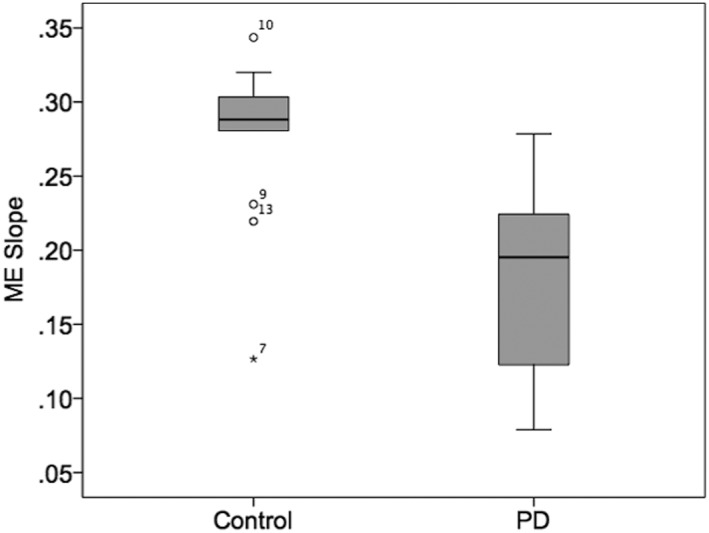
Statistically significant differences in magnitude estimation (ME) slope between the control group (control) and Parkinson's disease group (PD); bars represent standard error.
Results of the MANOVA revealed a significant overall interaction between participant group (PD vs control) and respiratory load (F [10, 300] = 2.138; P = .022). Tests of between‐subjects tests showed the interaction effect was only significant for ME (F [5] = 3.399; P = .006; Fig 3, panel A). While the interaction effect was not significant for airflow (F [5] = .209; P = .958), there were significant main effects for group (F [1] = 4.887; P = .029) and load (F [5] = 9.569; P < .000) on airflow (Fig 3, panel B).
Figure 3.
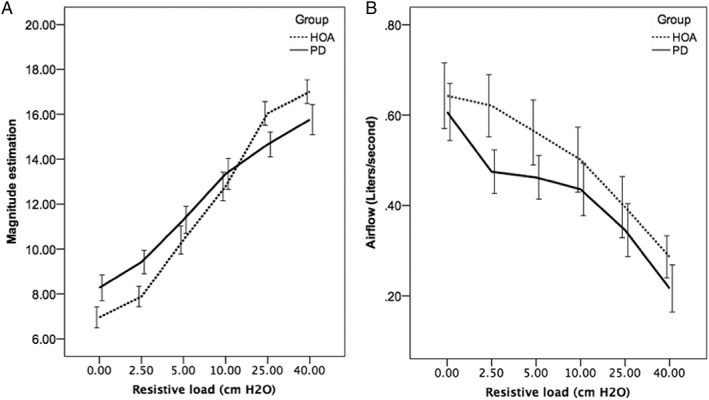
Results for the magnitude estimation (panel A) and airflow (panel B) across the six resistive loads. Bars represent standard error. Healthy older adults (HOA) are the control group. PD is Parkinson's disease group.
Multiple linear regression was calculated to determine whether there was a relationship between ME slope, maximum phonation duration, the seven speech domains, and swallowing safety (PAS). A significant regression equation containing the predictor variable respiratory rating was found (F [1,22] = 6.946; P = .023), with an R2 of .387. The predicted ME slope was equal to .280 ‐.031(respiratory rating), showing the ME slope decreased by .031 for every 1‐unit increase in severity rating of the respiratory domain (Fig. 4).
Figure 4.
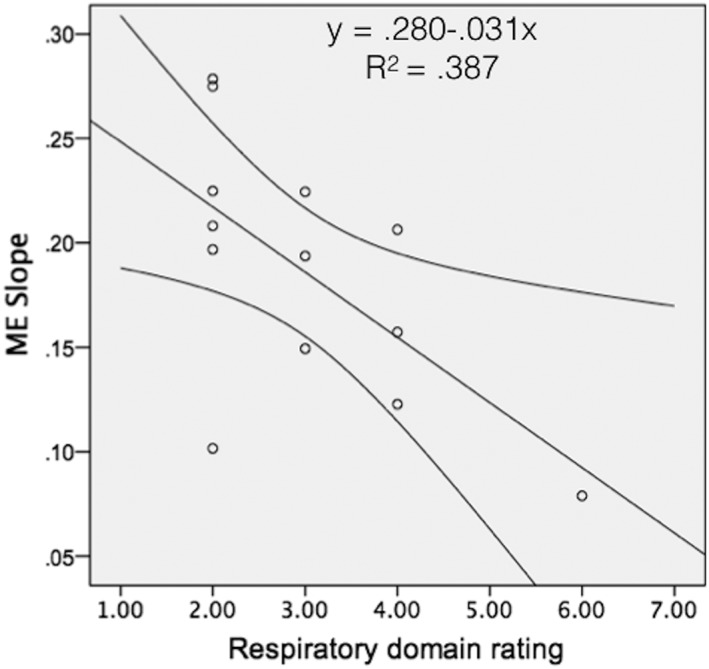
Linear regression model illustrating the slope of the line of best fit for magnitude estimation (ME) slope (y‐axis) and respiratory domain of the speech evaluation (x‐axis).
Discussion
The goal of the current study was to determine whether respiratory sensation was blunted in PD as compared to age‐equivalent healthy control participants. We also sought to investigate whether there was a relationship between respiratory load ME and the functions of speech and swallowing in PD patients. It was hypothesized that the PD group would exhibit blunted ME of respiratory resistive loads compared to the controls. Further, it was hypothesized that the blunted ME would be linearly related to severity of speech and swallowing metrics, providing support to our overall hypothesis: that blunted respiratory sensation contributes to the development of speech and swallowing disorders in patients with PD.
As can be appreciated in Fig. 3, panel A, there were significant differences between the PD and control groups’ ME at the low (0, 2.5, and 5 cm H20) and high resistive loads (25 and 40 cm H20), leading to an overall blunting of the ME slope for the PD group (Fig. 2). These findings support reports of changes in airway sensation in PD patients. Hammer and colleagues found decreased laryngeal sensitivity to air‐puffs targeting the arytenoids.4 Similarly, PD patients demonstrated a blunted urge to cough to various concentrations of capsaicin compared to controls, which was blunted further in PD patients with dysphagia.18 Results of the current study expand our understanding of airway sensation in PD, indicating that in addition to reduced sensation related to the larynx and cough, there is also reduced perception of general airway somatosensation evoked with resistive loads. This understanding is important, because the ability to perceive and process sensory information related to airway function is necessary to produce overlaid somatosensory respiratory functions, such as speech and swallowing.19, 20, 21, 22 The neuropathology of such changes or deficits could exist in multiple locations, either in the periphery or central nervous system. Mu and Sanders found aggregates of alpha‐synuclein protein in the sensory nerve terminals of people with PD premortem, suggesting that possibly the initial sensory stimulus is inadequately transduced to sensory afferents.23 Another possibility is that there is over‐gating of sensory information by the thalamus, whereby the thalamus inhibits throughput of sensory signals to the primary sensory cortex.24 Indeed, changes in sensory processing of both discriminative and affective components of a sensory stimulus could contribute to these findings, as the neuropathology of PD is known to extend beyond the basal ganglia.25, 26 With the current methodology, it is not possible to pinpoint which, if any, of these possibilities are correct. However, there are studies underway utilizing methods that can begin to address this important question.
In addition to blunted ME slopes, the PD participants demonstrated reduced inspiratory airflow rates at all loads applied (Fig. 3, panel B). Given the relationship between airflow, resistance, and pressure (pressure = resistance x airflow), this would indicate the PD group had reduced mouth pressure during the applied resistances when compared to the control group. Thus, it is possible that the ME differences between the PD and control groups were directly related to perceptual differences stemming from different mouth pressures. This hypothesis can reasonably explain the reduced ME ratings at the higher 25 and 40 cm H20 loads; however, it cannot explain increased ME ratings at the lower 0, 2.5, and 5 cm H20 loads. That is, if the differences were explained based on differences in mouth pressure, the expected direction would be reduced rather than increased ME ratings at the low inspiratory loads. Thus, it is unlikely differences in airflow and mouth pressure thoroughly explain the overall blunted ME slope in PD participants.
There is evidence that altered or abnormal processing of sensory information affects the generation and modulation of limb movement27, 28 as well as voice and speech production4, 21, 22 in PD. There have been several studies that show exaggerated pitch compensation in response to perturbed fundamental frequency (F0) in people with PD, as compared to control participants.20, 21, 22 These research groups concluded that abnormal sensorimotor integration of F0 could relate to an abnormal weighting of auditory feedback in the setting of reduced laryngeal somatosensory feedback, leading to a greater degree of pitch compensation in PD. Results of the current study showed a moderate effect size (R2 = .387) for the relationship between the severity rating of the respiratory domain and the ME slope, indicating that other factors other than just sensory perception of respiratory stimuli may influence respiratory drive for speech in PD. Specifically, the ME slope decreased by approximately .031 for every 1‐point increase in respiratory domain severity. Thus, it may be that reduced somatosensory information from the respiratory system contributes to the reduced respiratory support for speech that commonly occurs in PD. These patients may also experience reduced laryngeal somatosensation,4 and thus, two potentially faulty feedback mechanisms (respiratory and laryngeal) may co‐occur, leading to reduced maximum loudness and general monoloudness that is seen in patients with PD. This hypothesis cannot be sufficiently addressed with the current methodology, and the effect size was only moderate, so future studies should be designed to explore combined measures of laryngeal and respiratory sensation, and detailed motor speech outcomes.
The study's results failed to find a significant relationship between the measure of swallowing safety, PAS, and the ME slope. This finding may reflect differences in sensorimotor integration between swallowing and speech, or the lack of specificity of the PAS metric. Regarding the former possibility, while the sensory receptors in the oral cavity, oropharynx, and hypopharynx are primarily important for initiating the cascade of events that lead to adequate airway protection during swallowing, the sensors that transduce information regarding respiration and inspiratory loads (joint receptors and airway stretch receptors) are more important for attaining the requisite lung volume and subglottal pressure necessary for speech. Thus, ME of respiratory resistive loads would not necessarily reflect somatosensory changes elsewhere in the upper airway that could result in changes to swallowing safety. Also, the PAS is a functional measure of swallowing safety and not one of swallowing physiology. It may be that more detailed measures of swallowing physiology would reveal a relationship to respiratory sensation. Thus, it would be prudent in future studies to look at more specific swallowing timing and bolus flow measures, or the respiratory pattern surrounding the swallow, in order to better understand the potential relationship between respiratory sensation and swallowing physiology.
Conclusions
Results of this study support the hypothesis that people with PD have blunted perception of respiratory resistive loads as compared with age‐equivalent healthy adults. Results also suggest that blunted ME of resistive loads could contribute to changes in respiratory drive for speech (i.e., loudness); however, future studies are needed to adequately understand this relationship. A large‐scale study is currently underway that utilizes these methods in order to glean a comprehensive understanding of those mechanisms, which may contribute to dysarthria and dysphagia in PD.
Authors Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
K.H.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
M.T.: 2C, 3B
A.B.: 1B, 1C, 2C, 3C
Disclosures
Ethical Compliance Statement: The institutional review board (IRB) of the University of Florida reviewed and approved the study's protocol. All participants provided written and verbal informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the authors report conflicts of interest regarding the current manuscript.
Financial Disclosures for the previous 12 months: Karen Hegland is employed by the University of Florida. She received an honorarium from the Parkinson's Foundation in October 2017. She receives research funding from the National Institutes of Health: NICHD, NINDS, NIDCD, OD (grants). Michelle Troche is employed by Columbia University, Teacher's College. She receives grant funding from the Michael J. Fox Foundation. Alexandra Brandimore is employed by the University of South Florida.
Supporting information
Supporting Table 1. Modified Borg Scale for rating magnitude of the inspiratory load.
Supporting Table 2. Speech tasks administered during the clinical evaluation, and the associated speech domain that is assessed with each task.
Supporting Table 3. Rating scale for assessing each speech domain, along with the interpretation of each rating.
Supporting Table 4. Penetration‐aspiration scale.16
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Quinn, NP . Parkinson's disease: clinical features. Baillieres Clin Neurol 1997;6(1):1–13. [PubMed] [Google Scholar]
- 2. Quinn, NP , Rossor MN, and Marsden CD. Olfactory threshold in Parkinson's disease. J Neurol Neurosurg Psychiatry 1987;50(1):88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konczak J, Corcos DM, Horak F. Proprioception and motor control in Parkinson's disease. J Mot Behav 2009;41(6):543–552. [DOI] [PubMed] [Google Scholar]
- 4. Hammer MJ, Murphy CA, Abrams TM. Airway somatosensory deficits and dysphagia in Parkinson's disease. J Parkinsons Dis 2013;3(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg 2008;16(3):205–210. [DOI] [PubMed] [Google Scholar]
- 6. Van Den Berg J. Myoelastic‐aerodynamic theory of voice production. J Speech Hear Res 1958;1(3):227–244. [DOI] [PubMed] [Google Scholar]
- 7. Gilbert HR. Oral airflow during stop consonant production. Folia Phoniatr (Basel) 1973;25(4):288–301. [DOI] [PubMed] [Google Scholar]
- 8. Stathopoulos ET, Weismer G. Oral airflow and air pressure during speech production: a comparative study of children, youths and adults. Folia Phoniatr (Basel) 1985;37(3‐4)152–159. [DOI] [PubMed] [Google Scholar]
- 9. Martin‐Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg 2005;131(9):762–770. [DOI] [PubMed] [Google Scholar]
- 10. Kwan LC, Whitehill TL. Perception of speech by individuals with Parkinson's disease: a review. Parkinsons Dis 2011;2011:389767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ho AK, Bradshaw JL, Iansek R, Alfredson R. Speech volume regulation in Parkinson's disease: effects of implicit cues and explicit instructions. Neuropsychologia 1999;37(13):1453–1460. [DOI] [PubMed] [Google Scholar]
- 12. Tsai HW, Chan PY, von Leupoldt A, Davenport PW. The impact of emotion on the perception of graded magnitudes of respiratory resistive loads. Biol Psychol 2013;93(1):220–224. [DOI] [PubMed] [Google Scholar]
- 13. Clark JP, Adams SG, Dykstra AD, Moodie S, Jog M. Loudness perception and speech intensity control in Parkinson's disease. J Commun Disord 2014;51:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Kifle Y, Seng V, Davenport PW. Magnitude estimation of inspiratory resistive loads in children with life‐threatening asthma. Am J Respir Crit Care Med 1997;156(5):1530–1535. [DOI] [PubMed] [Google Scholar]
- 15. Tiller J, Pain M, Biddle N. Anxiety disorder and perception of inspiratory resistive loads. Chest 1987;91(4):547–551. [DOI] [PubMed] [Google Scholar]
- 16. Davenport PW, Kifle Y. Inspiratory resistive load detection in children with life‐threatening asthma. Pediatr Pulmonol 2001;32(1):44–48. [DOI] [PubMed] [Google Scholar]
- 17. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration‐aspiration scale. Dysphagia 1996;11(2):93–98. [DOI] [PubMed] [Google Scholar]
- 18. Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW.Decreased cough sensitivity and aspiration in Parkinson's disease. Chest 2014;146(5):1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson's disease. J Neurol 2010;257(10):1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mollaei F, Shiller DM, Baum SR, Gracco VL. Sensorimotor control of vocal pitch and formant frequencies in Parkinson's disease. Brain Res 2016;1646:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mollaei F, Shiller DM, Gracco VL. Sensorimotor adaptation of speech in Parkinson's disease. Mov Disord 2013;28(12):1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen X, Zhu X, Wang EQ. Sensorimotor control of vocal pitch production in Parkinson's disease. Brain Res 2013;1527:99–107. [DOI] [PubMed] [Google Scholar]
- 23. Mu L, Sobotka S, Chen J, et al. Alpha‐synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol 2013;72(2):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pitts T, Hegland KW, Sapienza CM, Bolser DC, Davenport PW. Alterations in oropharyngeal sensory evoked potentials (PSEP) with Parkinson's disease. Respir Physiol Neurobiol 2016;229:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol 2000;247:2:II3–10. [DOI] [PubMed] [Google Scholar]
- 26. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease‐related pathology. Cell Tissue Res 2004;318(1):121–134. [DOI] [PubMed] [Google Scholar]
- 27. Byblow WD, Summers JJ, Lewis GN, Thomas J. Bimanual coordination in Parkinson's disease: deficits in movement frequency, amplitude, and pattern switching. Mov Disord 2002;17(1):20–29. [DOI] [PubMed] [Google Scholar]
- 28. Lewis GN, Byblow WD. Altered sensorimotor integration in Parkinson's disease. Brain. 2002;125(Pt 9):2089–2099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table 1. Modified Borg Scale for rating magnitude of the inspiratory load.
Supporting Table 2. Speech tasks administered during the clinical evaluation, and the associated speech domain that is assessed with each task.
Supporting Table 3. Rating scale for assessing each speech domain, along with the interpretation of each rating.
Supporting Table 4. Penetration‐aspiration scale.16


