Abstract
Background and Objectives
To document racial/ethnic and nativity differences by gender in cognitive life expectancies among older adults in the United States.
Research Design and Methods
Sullivan-based life tables were used to estimate cognitively normal, cognitively impaired/no dementia (CIND), and dementia life expectancies by gender for White, Black, U.S.-born Hispanic, and foreign-born Hispanic adults 50 years and older in the Health and Retirement Study.
Results
Among women, the number of years spent living with dementia for Whites, Blacks, U.S.-born Hispanics, and foreign-born Hispanics was 1.6, 3.9, 4.7, and 6.0 years, respectively. For men, Whites lived 1.1 years with dementia compared to 3.1 years for Blacks, 3.0 years for U.S.-born Hispanics and 3.2 years for foreign-born Hispanics. Similar patterns were observed for race/ethnic and nativity differences in CIND life expectancies. Blacks and Hispanics spend a larger fraction of their remaining years with CIND and dementia relative to Whites, regardless of gender. Foreign-born Hispanic men and women and Black men are particularly disadvantaged in the proportion of years spent after age 50 with CIND and/or dementia.
Discussion and Implications
Disparities in cognitive life expectancies indicate that intervention strategies should target the specific needs of minority and immigrant older adults with dementia. Given that education is a strong predictor of cognitive health, improving access to the social and economic resources that delay dementia onset is key to improving the well-being of diverse older adults.
Keywords: Cognitive impairment, Dementia, Life expectancy, Race/ethnicity, Nativity
Cognitive impairment and dementia are major health issues confronting older adults in the United States. An estimated 13.9% of adults aged 71 and older have dementia (Plassman et al., 2007) and an additional 22% have cognitive impairment that is not severe enough to warrant a diagnosis of dementia (Plassman et al., 2008). There is some evidence that the prevalence and incidence of dementia in high-income countries is declining (Larson, Yaffe, & Langa, 2013). However, the number of older adults living with dementia may increase in the coming decades unless the prevalence of potentially modifiable dementia risk factors can be reduced (Norton, Matthews, Barnes, Yaffe, & Brayne, 2014). In the United States, the aging of the baby boomers will contribute to an increase in the number of older adults living with Alzheimer’s disease and related dementias (Hebert, Weuve, Scherr, & Evans, 2013).
Research on health inequalities indicates that compared to older non-Hispanic Whites (hereafter, “Whites”), older African Americans (hereafter, “Blacks”) have a greater risk for dementia (Barnes & Bennett, 2014). Evidence of differences in dementia risk between Hispanics and Whites is less clear with some studies reporting Hispanics to be at an increased risk (Tang et al., 2001) and others reporting no significant differences in dementia risk (Mayeda, Glymour, Quesenberry, & Whitmer, 2016). Disparities in dementia risk are likely due in part to a cumulative effect of social, demographic, and health factors over the life course, including low literacy (Sisco et al., 2015), poor childhood living conditions (Zhang, Hayward, & Yu, 2016), and high prevalence of chronic health conditions (Noble, Manly, Schupf, Tang, & Luchsinger, 2012).
Recent research on dementia is also interested in investigating differences at various stages of disease onset and development. Cognitive impairment/no dementia (CIND) is an intermediate state between normal cognition and dementia in which an older adult has self-reported concerns about memory or exhibits cognitive functioning that is lower than expected for a person’s age and education (Fisher et al., 2011). CIND is similar to mild cognitive impairment (MCI) and may represent the prodromal stage of dementia. The average conversion rate of CIND to dementia is approximately 10% (Peters et al., 2013). Racial and ethnic differences for CIND and MCI are unclear with some (Manly et al., 2008) but not all studies (Manly et al., 2005; Plassman et al., 2011) reporting that the prevalence and incidence of CIND or MCI is higher for older Black and Hispanic adults compared to Whites.
Prior research has documented racial/ethnic disparities in dementia risk; however, less research has examined potential differences in the number of years in later life with CIND or dementia. Also, few studies have been based on nationally representative samples including representation from the largest racial/ethnic groups in the United States, in particular Hispanics. Furthermore, the heterogeneity of the U.S. Hispanic population is often overlooked in research on disparities in CIND and dementia. More than 60% of the Hispanic population in the United States is of Mexican origin (U.S. Census Bureau, 2011), the majority of which is U.S. born (Gonzalez-Barrera & Lopez, 2013). Foreign-born Hispanics, particularly those who migrated as young adults, tend to have higher cognition, slower cognitive decline, and spend more years cognitive impairment-free compared to U.S.-born Hispanics (Garcia et al., 2017; Hill, Angel, & Balistreri, 2012; Hill, Angel, Balistreri, & Herrera, 2012; Weden et al., 2017). The importance of nativity for health disparities research has been attributed in part to the healthy immigrant hypothesis (Bostean, 2013). In addition, research shows that Hispanics have significantly longer life expectancy than Whites and Blacks (Markides & Eschbach, 2005), particularly the foreign born (Lariscy, Hummer, & Hayward, 2015). The high risk for dementia coupled with extended longevity means that Hispanics may spend more years in late life with dementia.
The present analysis uses a life course framework to examine the proportion of the life span after age 50 spent living with CIND and dementia for Whites, Blacks, and U.S.-born and foreign-born Hispanic adults in the United States. The life course can refer to complex interrelationship between events and transitions across important life domains (e.g., education, employment, health, family) that occur as a person ages. As a research framework, the life course is well suited to examine how life events and experiences that occur early in life contribute to health outcomes (Dannefer, 2003) and cognitive function (Lyu & Burr, 2016) in old age. Education is an important component of the life course because it is often attained early in life and is closely related to other dimensions of the life course, such as occupational status and health. Furthermore, an individual’s level of educational attainment can be influenced by family factors (e.g., parents’ education, value placed on attaining education), a person’s environment (e.g., access to/quality of education), and an individual’s ability to learn new information. Prior research shows that foreign-born Hispanics complete fewer years of education, on average than U.S.-born Hispanics (Gonzalez et al., 2009; Weden et al., 2017). Furthermore, foreign-born Hispanics frequently migrate to the United States as young (age 18–34) or middle-aged (35–49) adults (Gubernskaya, 2015). Consequently, foreign-born Hispanics who migrated to the United States were likely educated in Mexico or their country of origin. Considerable improvements have been made to the quality of education in Latin American countries, including Mexico (Palafox, Prawda, & Velez, 1994), however, educational opportunities in rural regions of Mexico and other Latin American countries are still limited (Gertler, Patrinos, & Rubio-Codina, 2012).
Greater educational attainment has been consistently associated with lower risk of cognitive impairment and dementia (Caamaño-Isorna, Corral, Montes-Martínez, & Takkouche, 2006; Cagney & Lauderdale, 2002; Downer, Garcia, Saenz, Markides, & Wong, 2017; Weden et al., 2017). The apparent benefit of formal education to cognitive functioning has been largely attributed to older adults with higher education having greater cognitive reserve (Meng & D’Arcy, 2012). Cognitive reserve can be defined as the brain’s ability to maximize cognitive performance through using more efficient neural networks (Stern, 2002). Thus, older adults with higher reserve are able to sustain greater damage to the brain than older adults with less reserve before cognitive deficits are observed. Older adults with higher education may also be better able to compensate for damage to the brain and maintain normal cognitive functioning by using alternative neural networks not typically used when completing a cognitive task (Stern, 2002). This is supported by evidence from neuroimaging studies that indicate older adults with high cognitive function engage more brain regions when performing a cognitive task compared to adults who are cognitively impaired (Cabeza, Anderson, Locantore, & McIntosh, 2002; Rosen et al., 2002).
This study builds on previous research that points to race/ethnicity and nativity as important factors in shaping cognitive functioning in later life by examining differences in the number of years after age 50 with normal cognition, CIND, and dementia. Determining if older Blacks and Hispanics spend a greater amount of their later years with CIND and dementia will provide important insight into the burden of these conditions in minority and immigrant populations. We use data from the Health and Retirement Study (1998–2012) to estimate cognitively normal, CIND, and dementia life expectancies among Whites, Blacks, U.S.-born Hispanics, and foreign-born Hispanics. This examination takes into account the demographic heterogeneity of the older adult population and is especially timely given the rapid population aging that U.S. minority and immigrant groups are experiencing. We also describe educational achievement across racial/ethnic groups because previous research has revealed that low educational attainment and poor health behaviors are associated with an increase in the number of years spent living with cognitive impairment or dementia (Lievre, Alley, & Crimmins, 2008). The substantial differences between Whites, Blacks, and Hispanics in educational attainment, health conditions, and health behaviors may contribute to racial/ethnic and nativity disparities in cognitively normal life expectancy. Thus, we hypothesize that older Black and Hispanic adults will spend a greater number of years after age 50 with CIND or dementia compared to Whites.
Methods
Data
This study uses data from the Health and Retirement Study (HRS) and the public-use National Health Interview Survey Linked Mortality Files (NHIS-LMF). The HRS is used to obtain CIND/dementia prevalence in a large nationally representative noninstitutionalized population aged 50 years and older in the United States. The analysis is based on data from 1998 to 2012 of the RAND HRS Version O Data File (RAND, 2016). Our estimates of mortality are obtained from the public-use NHIS-LMF for 1997–2009. The NHIS-LMF provides mortality follow-up from the National Death Index through December 31, 2011. We use the NHIS-LMF to estimate mortality due to the small number of deaths among Hispanics in the HRS. [The HRS has 306 deaths for all U.S.-born Hispanics and 330 deaths for all foreign-born Hispanics. The NHIS-LMF contains 1,248 deaths for foreign-born Hispanic males aged 50 years and older, 962 deaths for U.S.-born Hispanic males, 1,055 deaths for foreign-born Hispanic females, and 831 deaths for U.S.-born Hispanic females.] Previous research shows the quality of mortality linkages for older Hispanics in the NHIS-LMF to be highly accurate (Lariscy et al., 2015). Furthermore, life expectancies based on this data (Hayward, Hummer, Chiu, Gonzalez-Gonzalez, & Wong, 2014) closely match the U.S. life tables published by the National Center for Health Statistics (Arias, 2010). We use pooled waves across survey years of the HRS to estimate differences by race/ethnicity and nativity for cognitive life expectancies (cognitively normal, CIND, and dementia) and death for adults who are 50 years and older. The HRS follows up with individuals who become institutionalized; however, our life table results are representative of the civilian, noninstitutionalized population. That is, we censor the observations of respondents who have entered nursing homes at follow-up. The final analytic sample includes 32,406 unique individuals that contribute 146,593 age-specific observations.
Measures
Cognitive functioning of HRS participants who are able to complete a direct interview is assessed by a modified version of the Telephone Interview for Cognitive Status (TICS-M; Brandt, Spencer, & Folstein, 1988). We used a 27-point scale that includes the following items: immediate and delayed word recall, serial seven subtraction, and counting backwards (Crimmins, Kim, Langa, & Weir, 2011). A score of 12 or greater was defined as normal cognitive function; a score of 7–11 as CIND, and a score of 6 or less as dementia (Crimmins et al., 2011; Langa et al., 2010). Following previous research (Crimmins et al., 2011), we combine three proxy interview questions to categorize cognitive status for participants who were unable to complete a direct interview: proxy-reported memory (score 0 [excellent]–4 [poor]); number of limitations in instrumental activities of daily living (managing money, taking medication, preparing meals, use a telephone, and grocery shopping; score 0–5); and an interviewer assessment of difficulty completing the interview due to cognitive limitation (score 0–2). Proxies with a score of 6 or greater are categorized as dementia, a score of 3–5 as CIND, and a score of 2 or less as normal cognitive function.
Sociodemographic variables used in the analysis include race/ethnicity, nativity, sex, age, and years of education. Race/ethnicity and nativity are self-reported in the HRS and NHIS-LMF. We include Whites, Blacks, and Hispanics. Hispanics are further divided by nativity. Due to small sample size, we omit Asian-Americans and other racial/ethnic groups. Sex corresponds to whether the respondent identifies as female or male. To assess how cognitive status varies by age, we include seven age categories that allow for reasonable cell sizes: 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85 years and older. Finally, educational level is measured by years of formal schooling.
Cognitive Life Expectancies
The construct of healthy life expectancy refers to the number of years an individual can expect to live in good health or absent of a particular health condition (Dubois & Hebert, 2006), in this case CIND or dementia. The construct is particularly appealing to compare across population groups with different age distributions (Jagger, Cox, Le Roy, & EHEMU, 2006) because healthy life expectancy takes into account age-specific prevalence of health conditions and age-specific mortality risks. Thus, the interpretation is the number of years that individuals in the group can expect to spend in each health state, assuming that they live the rest of their lives under the observed disease and mortality risks of the current cohorts in the group.
We integrated information on age-specific CIND and dementia prevalence from the HRS with age-specific mortality information from the NHIS-LMF to calculate Sullivan life table models of cognitively normal, CIND, and dementia life expectancy for each sex and race/ethnic/nativity group (Sullivan, 1971). The prevalence is estimated with multinomial logistic regression models and sampling weights provided by HRS. Weights apply to those residing in the community; for those living in a nursing home, weights are assigned a zero value (RAND, 2016). We estimate mortality rates with Gompertz hazard models and sampling weights from the NHIS-LMF. The mortality rates are used to calculate total life expectancy. This method divides total life expectancy at age 50 into the different health states based on the age-specific prevalence of each cognitive state (cognitively normal, CIND, and dementia). The cognitive life expectancies calculated by this method are the number of remaining years (at age 50) a population can expect to live in each state (Jagger et al., 2006). A weighted bootstrapping technique was used to obtain standard errors by resampling our analysis sample, which allowed us to estimate sampling variability for the life table functions and life expectancies. Based on the 300 bootstrap samples for each group, confidence intervals were obtained for the life expectancies.
Results
Table 1 presents sociodemographic characteristics and age-specific prevalence rates of CIND and dementia for the study sample. Overall, respondents with normal cognition are younger and more educated than individuals with CIND and dementia regardless of race/ethnicity, nativity, or sex. The prevalence of CIND and dementia varies largely by race/ethnicity and nativity, with Black, U.S.-born Hispanics, and foreign-born Hispanics at a severe disadvantage relative to Whites. Furthermore, the results for 5-year age groups suggest that minority and foreign-born Hispanic men and women not only exhibit higher prevalence rates of CIND and dementia than Whites at age 50–54, but their rates increase rapidly with advancing age relative to older White adults.
Table 1.
Sociodemographic Characteristics and Age-Specific Prevalence of Cognitive Health by Race/Ethnicity/Nativity and Sex
| Whites | Blacks | U.S. Hispanics | FB Hispanics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dementia | CIND | Normal | Dementia | CIND | Normal | Dementia | CIND | Normal | Dementia | CIND | Normal | |
| Panel A: Females | ||||||||||||
| N | 3,709 | 7,692 | 51,106 | 1,810 | 3,706 | 8,776 | 403 | 924 | 2,186 | 529 | 1,464 | 2,970 |
| Mean age | 80.2 | 74.7 | 65.3 | 75.7 | 67.5 | 62.3 | 76.2 | 67.2 | 62.2 | 73.0 | 66.4 | 62.0 |
| Mean education | 10.8 | 11.6 | 13.3 | 8.9 | 10.7 | 12.9 | 6.6 | 8.7 | 11.5 | 5.0 | 6.1 | 9.1 |
| Age group | ||||||||||||
| 50–54 | 0.5 | 3.6 | 95.9 | 2.3 | 19.8 | 78.0 | 2.0 | 20.7 | 77.3 | 3.9 | 22.2 | 73.9 |
| 55–59 | 0.6 | 5.0 | 94.5 | 3.7 | 20.4 | 75.8 | 2.5 | 18.4 | 79.2 | 4.4 | 23.1 | 72.5 |
| 60–64 | 0.9 | 5.7 | 93.4 | 4.7 | 21.0 | 74.4 | 5.9 | 20.4 | 73.8 | 5.9 | 28.1 | 66.0 |
| 65–69 | 1.4 | 7.8 | 90.8 | 7.5 | 27.8 | 64.7 | 5.3 | 26.0 | 68.8 | 8.0 | 33.5 | 58.5 |
| 70–74 | 2.7 | 11.9 | 85.4 | 12.2 | 33.6 | 54.3 | 11.2 | 34.0 | 54.8 | 12.9 | 33.1 | 54.0 |
| 75–79 | 5.5 | 17.2 | 77.4 | 21.7 | 36.6 | 41.7 | 17.4 | 30.9 | 51.8 | 18.4 | 43.3 | 38.3 |
| 80–84 | 9.2 | 23.8 | 67.0 | 30.6 | 39.4 | 30.0 | 30.1 | 42.3 | 27.6 | 27.7 | 39.5 | 32.8 |
| ≥85 | 19.0 | 33.0 | 48.0 | 47.1 | 35.1 | 17.9 | 50.9 | 33.4 | 15.7 | 45.0 | 36.7 | 18.3 |
| Panel B: Males | ||||||||||||
| N | 2,206 | 6,835 | 37,689 | 1,126 | 2,495 | 4,890 | 253 | 666 | 1,677 | 293 | 965 | 2,223 |
| Mean age | 75.7 | 71.1 | 64.1 | 71.9 | 65.9 | 61.5 | 72.2 | 66.1 | 61.5 | 72.0 | 65.2 | 61.6 |
| Mean education | 10.5 | 11.5 | 13.8 | 8.0 | 10.5 | 12.7 | 7.4 | 9.4 | 11.8 | 4.8 | 6.1 | 9.5 |
| Age group | ||||||||||||
| 50–54 | 0.9 | 5.7 | 93.4 | 3.0 | 20.7 | 76.3 | 3.5 | 18.6 | 77.9 | 4.7 | 20.6 | 74.7 |
| 55–59 | 0.9 | 7.1 | 92.0 | 4.0 | 22.5 | 73.5 | 2.9 | 17.5 | 79.7 | 3.5 | 21.7 | 74.8 |
| 60–64 | 1.2 | 8.8 | 90.1 | 7.3 | 26.6 | 66.1 | 3.1 | 17.5 | 79.4 | 3.3 | 29.3 | 67.4 |
| 65–69 | 2.1 | 10.8 | 87.1 | 9.2 | 31.4 | 59.4 | 5.9 | 26.7 | 67.4 | 4.3 | 27.9 | 67.9 |
| 70–74 | 3.4 | 15.3 | 81.3 | 14.9 | 35.3 | 49.8 | 7.8 | 30.8 | 61.4 | 6.2 | 28.9 | 64.9 |
| 75–79 | 5.6 | 21.0 | 73.4 | 24.2 | 36.3 | 39.6 | 16.7 | 38.5 | 44.8 | 15.1 | 33.1 | 51.8 |
| 80–84 | 8.9 | 26.2 | 64.9 | 31.6 | 41.5 | 27.0 | 36.1 | 43.1 | 20.9 | 22.0 | 43.2 | 34.8 |
| ≥85 | 16.4 | 33.8 | 49.8 | 43.0 | 41.1 | 15.9 | 46.3 | 34.3 | 19.4 | 44.7 | 35.6 | 19.8 |
Note: CIND = cognitive impairment/no dementia; FB = foreign born. 32,406 unique individuals contribute 146,593 age-specific observations.
Source: HRS 1998–2012.
Cognitively Normal Expectancies
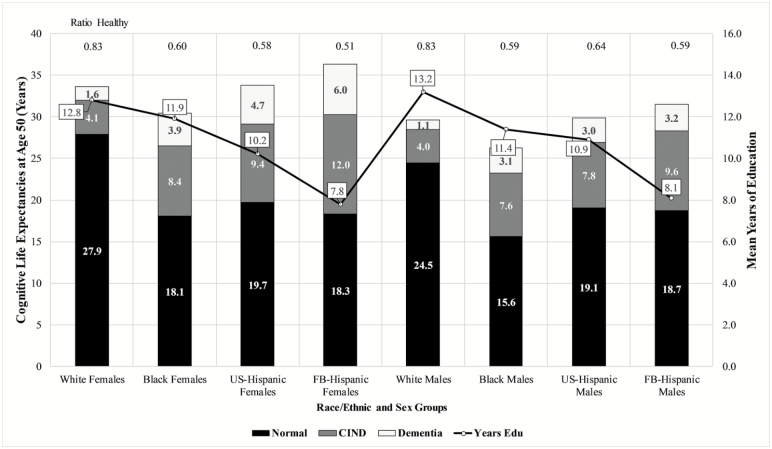
Table 2 shows life expectancies at age 50 and average years of education by race/ethnicity and sex (see Figure 1). In general, females exhibited longer life expectancy than males for all race and ethnic groups. Foreign-born Hispanics (36.3 years for women and 31.6 years for men) had longer total life expectancies, and U.S.-born Hispanics (33.7 years for women and 29.9 years for men) had comparable total life expectancies relative to Whites (33.6 years for women and 29.6 years for men). Conversely, Black women (30.4 years) and men (26.3 years) had lower total life expectancies compared to Whites. Note that these estimates for Black and White men and women at age 50 are slightly higher than life expectancies reported for the same period by Arias (2010) using vital statistics data. This may be due in part to the exclusion of institutionalized adults in the NHIS. In addition, Arias (2010) did not examine life expectancies among Hispanics by nativity; however, her estimates for Hispanics aged 50 and older were 34.9 years for Hispanic women and 31.2 years for Hispanic men, which closely approximate our results discussed earlier. In terms of education, White men and women attained the highest levels of education (13.2 and 12.8 years), whereas foreign-born Hispanic men and women were the most disadvantaged in terms of educational achievement (8.1 and 7.8 years, respectively).
Table 2.
Cognitive Life Expectancies at Age 50 by Race/Ethnicity, Nativity, and Sex
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| White | Black | U.S. Hispanic | FB Hispanic | White | Black | U.S. Hispanic | F.B. Hispanic | |
| Years (SE) | Years (SE) | Years (SE) | Years (SE) | Years (SE) | Years (SE) | Years (SE) | Years (SE) | |
| Total life expectancy | 33.6 (0.08) | 30.4 (0.20)*** | 33.7 (0.45) | 36.3 (0.36)*** | 29.6 (0.07) | 26.3 (0.21)*** | 29.9 (0.42) | 31.6 (0.37)*** |
| Normal cognition | 27.9 (0.09) | 18.1 (0.26)*** | 19.7 (0.51)*** | 18.3 (0.50)*** | 24.5 (0.10) | 15.6 (0.52)*** | 19.1 (0.52)*** | 18.7 (0.48)*** |
| CIND | 4.1 (0.06) | 8.4 (0.22)*** | 9.4 (0.46)*** | 12.0 (0.46)*** | 4.0 (0.07) | 7.6 (0.44)*** | 7.8 (0.44)*** | 9.6 (0.43)*** |
| Dementia | 1.6 (0.04) | 3.9 (0.18)*** | 4.7 (0.36)*** | 6.0 (0.43)*** | 1.1 (0.04) | 3.1 (0.36)*** | 3.0 (0.36)*** | 3.2 (0.33)*** |
| Ratio cognitively healthy | 0.83 (.002) | 0.60 (0.001)*** | 0.58 (0.02)*** | 0.51 (0.01)*** | 0.83 (0.003) | 0.59 (0.01)*** | 0.64 (0.02)*** | 0.59 (0.01)*** |
| Education | 12.8 (2.5) | 11.9 (3.0) | 10.2 (3.9) | 7.8 (4.8) | 13.2 (2.9) | 11.4 (3.5) | 10.9 (3.9) | 8.1 (4.8) |
| Total exposure | 118,200 | 27,224 | 6,656 | 9,368 | 91,256 | 16,492 | 5,036 | 6,798 |
Note: CIND = cognitive impairment/no dementia.
Source: HRS 1998–2012 and NHIS-LMF (1997–2009).
*p ≤ .05. **p ≤ .01. ***p ≤ .001.
Figure 1.
Cognitive-related life expectancies and years of education at age 50 by race/ethnicity, nativity, and sex. Source: HRS 1998–2012 and NHIS-LMF (1997–2009). CIND = cognitive impairment no dementia; FB = foreign born; US = U.S. born.
Among women, cognitively normal life expectancy at age 50 is 27.9 years for Whites, 18.1 years for Blacks, 19.7 years for U.S.-born Hispanics, and 18.3 years for foreign-born Hispanics. The results indicate that minority and foreign-born women are expected to spend a significantly lower proportion of their remaining years after age 50 in a cognitively normal state compared to White women. Foreign-born Hispanic women in particular are at a severe disadvantage relative to other groups in the ratio of years (0.51) lived in late-life cognitively normal.
CIND and Dementia Life Expectancies
Compared to White women, who are expected to spend only 1.6 years with dementia, foreign-born Hispanic women are expected to spend 6.0 years living with dementia. The longer total life expectancies at age 50 for foreign-born Hispanic females (36.3 years) compared to White females (33.6 years) and higher prevalence rates of CIND and dementia contribute to the fewer number of years spent in a cognitively normal state.
For men, a similar pattern emerges. Whites are expected to spend more years cognitively normal (24.5 years), compared to Blacks (15.6 years), U.S.-born Hispanics (19.1 years), and foreign-born Hispanics (18.7 years). Similar to women, all minority and foreign-born men are disadvantaged compared to Whites in the proportion of years after age 50 spent cognitively normal. However, older foreign-born Hispanic adults are the most disadvantaged group in regard to years lived with CIND and dementia. Although older White men are expected to spend approximately 4 years with CIND and 1 year with dementia, older foreign-born Hispanic males are expected to spend 9.6 years with CIND and 3.2 years dementia. As with foreign-born Hispanic women, the additional years spent with cognitive impairment among foreign-born Hispanic men can be attributed to both higher prevalence rates of CIND and dementia and greater longevity compared to White men.
Discussion
The older adult population is rapidly aging and becoming more racially and ethnically diverse. Hispanic and Black older adults are at a higher risk for dementia and cognitive impairment than non-Hispanic White older adults; yet, prior to this study, there was a lack of information on disparities in the number of years in late life spent with CIND or dementia. The current analysis documented that Hispanics and Blacks at age 50 are estimated to spend a greater number of years with CIND and dementia and, consequently, a lower proportion of their remaining lifespan with normal cognition. Our estimates reveal that relative to Whites at age 50, the number of years expected to spend with dementia is approximately three times more for Blacks, 3.5 times more for U.S.-born Hispanics, and 4.5 times more for foreign-born Hispanics.
Several factors may contribute to the observed racial/ethnic and nativity differences in cognitive life expectancies. First, Hispanics, particularly those born outside of the United States, have a longer total life expectancy than Whites (Cantu, Hayward, Hummer, & Chiu, 2013). Longer cognitive life expectancies with CIND and dementia among foreign-born Hispanic men and women observed in the present study may reflect the overall survival advantage of this population. Second, the mean age for respondents with CIND and dementia was much older for White men and women than other groups. In addition, White men and women exhibited a lower prevalence of CIND and dementia at all age groups. The combination of extended longevity and higher risk for dementia implies that Hispanics in particular should expect a heavier burden of CIND and dementia. Furthermore, survival rates decrease with older age of dementia diagnosis (Brookmeyer, Corrada, Curriero, & Kawas, 2002; Mayeda et al., 2017), which may contribute to the shorter life expectancy with dementia observed for Whites.
Second, differences in the age distribution and prevalence of CIND and dementia are partly attributed to differences in education across racial/ethnic and immigrant groups. The relationship between lower educational achievement and higher risk for dementia is well established (Caamaño-Isorna et al., 2006; Stern et al., 1994). The protective role of education may be due to related factors such as occupation over the life span and literacy in developing cognitive reserve as well as access to financial and social resources that protect against the risk of dementia. Future research is needed to explore in more detail the pathways through which different racial/ethnic/nativity and gender groups benefit from educational achievement to reduce the risk for cognitive impairment in old age.
Finally, the longer life expectancy with CIND and dementia for U.S.-born and foreign-born Hispanics may be due to differences in how dementia symptoms manifest and progress in these populations. For example, using clinical samples, O’Bryant and colleagues (2007, 2013) have reported that older Mexican Americans are diagnosed with dementia at younger ages and have more severe dementia symptoms than Whites. In addition, Black adults diagnosed with dementia tend to experience slower rates of cognitive decline than Whites (Barnes et al., 2005). Blacks and Hispanics have also been found to have lower mortality than Whites following a diagnosis of dementia (Helzner et al., 2008). Furthermore, previous research shows that older adults who initially have high cognitive reserve can exhibit rapid cognitive decline once the level of cognitive reserve is no longer sufficient to compensate for the increasing burden of dementia pathology in the brain (Stern, Albert, Tang, & Tsai, 1999). Taken together, these findings indicate that dementia type, severity, and progression may differ by racial/ethnic and nativity. We suggest this as a key area for future research.
Evaluating potential differences in cognitive impairment–free life expectancy by race/ethnicity and nativity is key to developing interventions that appropriately address the needs of older adults and their families. Black and Hispanic older adults are more reliant on family for support than White older adults (Rote & Moon, 2016). The longer life expectancy with CIND and dementia for minority older adults may result in family members providing care for a longer period of time. This makes it necessary to develop and implement culturally sensitive interventions that are competent in meeting the needs of minority older adults and their family caregivers.
This analysis has important limitations. The cutoffs used to define CIND and dementia in the present analysis were created by the HRS to match the prevalence of CIND and dementia in the Aging, Demographic, and Memory Study (ADAMS), which is a substudy of the HRS. The ADAMS was conducted primarily with the non-Hispanic White population, and these cutoffs have been used to study race and ethnic disparities in cognitive impairment in the HRS (Langa, Kabeto, & Weir, 2010). However, these cutoffs may not be appropriate for defining CIND and dementia in Black and Hispanic populations given the considerable racial/ethnic and nativity differences in education. In addition, not accounting for education may have led to some older adults with low education to be incorrectly classified as having CIND or dementia, and result in an overestimation of the prevalence of CIND and dementia in populations with low educational attainment.
A second potential limitation is the cognitive scores of participants who received the TICS over multiple observation waves may have been influenced by practice effects. Not accounting for practice effects in an analysis may result in incorrect estimates of change in cognitive function (Goldberg, Harvey, Wesnes, Snyder, & Schneider, 2015). Practice effects appear to have the greatest impact immediately after the first test exposure, and the magnitude of the practice effect decreases over time (Vivot et al., 2016). We assessed the possible impact of practice effects by dropping the first observation for each participant and repeating the analysis. The results of the sensitivity analysis were consistent with the initial findings (results available on request).
Despite the high prevalence of dementia in minority and immigrant populations, Blacks and Hispanics are more likely than Whites to lack knowledge about important risk factors for dementia and to have a fatalistic mindset toward developing dementia (Roberts, McLaughlin, & Connell, 2014). Public health programs are needed to educate and increase awareness about dementia in U.S. minority and immigrant populations to prevent or delay the onset of cognitive impairment. In addition, considerable efforts need to be made to develop culturally responsive programs and services that address the needs of older minority and immigrant adults with dementia and their family caregivers. This is especially salient, as previous research has also shown that these minority and immigrant populations of old adults, are expected to spend a larger fraction of their remaining years with physical disabilities compared to Whites (Cantu et al., 2013; Hayward et al., 2014). The combined impact of disparities in physical and cognitive limitations in old age implies that the burden of caregiving is expected to be especially prevalent in minority and immigrant populations.
Funding
We gratefully acknowledge financial support for this research provided by the National Institute on Aging (5T32AG270, T32AG000037, P30AG043073, P30AG043097, and R01 AG10939-22).
Conflict of Interest
None reported.
References
- Arias E. (2010). United States life tables by Hispanic origin. Vital and Health Statistics, 2, 1–33. [PubMed] [Google Scholar]
- Barnes L. L., & Bennett D. A (2014). Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff (Millwood), 33, 580–586. doi:10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L., Wilson R. S., Li Y., Aggarwal N. T., Gilley D. W., McCann J. J., & Evans D. A (2005). Racial differences in the progression of cognitive decline in Alzheimer disease. Am J Geriatr Psychiatry, 13, 959–967. doi:10.1176/appi.ajgp.13.11.959 [DOI] [PubMed] [Google Scholar]
- Bostean G. (2013). Does selective migration explain the Hispanic paradox? A comparative analysis of Mexicans in the U.S. and Mexico. J Immigr Minor Health, 15, 624–635. doi:10.1007/s10903-012-9646-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J., Spencer M., & Folstein M (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1, 111–118. [Google Scholar]
- Brookmeyer R., Corrada M. M., Curriero F. C., & Kawas C (2002). Survival following a diagnosis of Alzheimer disease. Arch Neurol, 59, 1764–1767. [DOI] [PubMed] [Google Scholar]
- Caamaño-Isorna F., Corral M., Montes-Martínez A., & Takkouche B (2006). Education and dementia: A meta-analytic study. Neuroepidemiology, 26, 226–232. doi:10.1159/000093378 [DOI] [PubMed] [Google Scholar]
- Cabeza R., Anderson N. D., Locantore J. K., & McIntosh A. R (2002). Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage, 17, 1394–1402. [DOI] [PubMed] [Google Scholar]
- Cagney K. A., & Lauderdale D. S (2002). Education, wealth, and cognitive function in later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 57, P163–P172. [DOI] [PubMed] [Google Scholar]
- Cantu P. A., Hayward M. D., Hummer R. A., & Chiu C. T (2013). New estimates of racial/ethnic differences in life expectancy with chronic morbidity and functional loss: Evidence from the National Health Interview Survey. J Cross Cult Gerontol, 28, 283–297. doi:10.1007/s10823-013-9206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Kim J. K., Langa K. M., & Weir D. R (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), i162–i171. doi:10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer D. (2003). Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 58, S327–S337. [DOI] [PubMed] [Google Scholar]
- Downer B., Garcia M. A., Saenz J., Markides K. S., & Wong R (2017). The role of education in the relationship between age of migration to the United States and risk of cognitive impairment among older Mexican Americans. Res Aging, 0164027517701447 [Epub ahead of print]. doi:10.1177/0164027517701447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., & Hebert R (2006). Cognitive-impairment-free life expectancy for Canadian seniors. Dement Geriatr Cogn Disord, 22, 327–333. doi:10.1159/000095593 [DOI] [PubMed] [Google Scholar]
- Fisher G. G., Franks M. M., Plassman B. L., Brown S. L., Potter G. G., Llewellyn D., … Langa K. M (2011). Caring for individuals with dementia and cognitive impairment, not dementia: Findings from the aging, demographics, and memory study. J Am Geriatr Soc, 59, 488–494. doi:10.1111/j.1532-5415.2010.03304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. A., Saenz J. L., Downer B., Chiu C. T., Rote S., & Wong R (2017). Age of migration differentials in life expectancy with cognitive impairment: 20-year findings from the Hispanic-EPESE. The Gerontologist, gnx062. doi:10.1093/geront/gnx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler P. J., Patrinos H. A., & Rubio-Codina M (2012). Empowering parents to improve education: Evidence from rural Mexico. Journal of Development Economics, 99, 68–79. [Google Scholar]
- Goldberg T. E., Harvey P. D., Wesnes K. A., Snyder P. J., & Schneider L. S (2015). Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement (Amst), 1, 103–111. doi:10.1016/j.dadm.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H. M., Ceballos M., Tarraf W., West B. T., Bowen M. E., & Vega W. A (2009). The health of older Mexican Americans in the long run. Am J Public Health, 99, 1879–1885. doi:10.2105/AJPH.2008.133744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Barrera A., & Lopez M. H (2013). A demographic portrait of Mexican-origin Hispanics in the United States. Washington, DC: Pew Research Hispanic Center; Retrieved from http://www.pewhispanic.org/files/2013/05/2013-04_Demographic-Portrait-of-Mexicans-in-the-US.pdf [Google Scholar]
- Gubernskaya Z. (2015). Age at migration and self-rated health trajectories after age 50: Understanding the older immigrant health paradox. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 279–290. doi:10.1093/geronb/gbu049 [DOI] [PubMed] [Google Scholar]
- Hayward M. D., Hummer R. A., Chiu C. T., Gonzalez-Gonzalez C., & Wong R (2014). Does the Hispanic paradox in U.S. adult mortality extend to disability?Popul Res Policy Rev, 33, 81–96. doi:10.1007/s11113-013-9312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert L. E., Weuve J., Scherr P. A., & Evans D. A (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology, 80, 1778–1783. doi:10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner E. P., Scarmeas N., Cosentino S., Tang M. X., Schupf N., & Stern Y (2008). Survival in Alzheimer disease: A multiethnic, population-based study of incident cases. Neurology, 71, 1489–1495. doi:10.1212/01.wnl.0000334278.11022.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. D., Angel J., & Balistreri K. S (2012). Does the “healthy immigrant effect” extend to cognitive aging? In J. Angel F. Torres-Gil, & K. Markides (Eds.), Aging, health, and longevity in the Mexican-origin population (pp. 19–33). New York: Springer Publishing Company. [Google Scholar]
- Hill T. D., Angel J. L., Balistreri K. S., & Herrera A. P (2012). Immigrant status and cognitive functioning in late-life: An examination of gender variations in the healthy immigrant effect. Soc Sci Med, 75, 2076–2084. doi:10.1016/j.socscimed.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger C., Cox B., Le Roy S.,& EHEMU. (2006). Health expectancy calculation by the Sullivan method. Retrieved from http://reves.site.ined.fr/en/resources/computation_online/sullivan/ [Google Scholar]
- Langa K. M., Kabeto M., & Weir D (2010). Report on race and cognitive impairment using HRS, 2010 Alzheimer’s Disease Facts and Figures. Retrieved from http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf. [Google Scholar]
- Lariscy J. T., Hummer R. A., & Hayward M. D (2015). Hispanic older adult mortality in the United States: New estimates and an assessment of factors shaping the Hispanic paradox. Demography, 52, 1–14. doi:10.1007/s13524-014-0357-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. B., Yaffe K., & Langa K. M (2013). New insights into the dementia epidemic. N Engl J Med, 369, 2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievre A., Alley D., & Crimmins E. M (2008). Educational differentials in life expectancy with cognitive impairment among the elderly in the United States. J Aging Health, 20, 456–477. doi:10.1177/0898264308315857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J., & Burr J. A (2016). Socioeconomic status across the life course and cognitive function among older adults: An examination of the latency, pathways, and accumulation hypotheses. J Aging Health, 28, 40–67. doi:10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- Manly J. J., Bell-McGinty S., Tang M. X., Schupf N., Stern Y., & Mayeux R (2005). Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Archives of Neurology, 62, 1739–1746. doi:10.1001/archneur.62.11.1739 [DOI] [PubMed] [Google Scholar]
- Manly J. J., Tang M. X., Schupf N., Stern Y., Vonsattel J. P., & Mayeux R (2008). Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol, 63, 494–506. doi:10.1002/ana.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markides K. S., & Eschbach K (2005). Aging, migration, and mortality: Current status of research on the Hispanic paradox. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, 68–75. [DOI] [PubMed] [Google Scholar]
- Mayeda E. R., Glymour M. M., Quesenberry C. P., Johnson J. K., Perez-Stable E. J., & Whitmer R. A (2017). Survival after dementia diagnosis in five racial/ethnic groups. Alzheimers Dement, 13, 761–769. doi:10.1016/j.jalz.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda E. R., Glymour M. M., Quesenberry C. P., & Whitmer R. A (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement, 12, 216–224. doi:10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., & D’Arcy C (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS ONE, 7, e38268. doi:10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J. M., Manly J. J., Schupf N., Tang M. X., & Luchsinger J. A (2012). Type 2 diabetes and ethnic disparities in cognitive impairment. Ethn Dis, 22, 38–44. [PMC free article] [PubMed] [Google Scholar]
- Norton S., Matthews F. E., Barnes D. E., Yaffe K., & Brayne C (2014). Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol, 13, 788–794. [DOI] [PubMed] [Google Scholar]
- O’Bryant S. E., Humphreys J. D., Schiffer R. B., & Sutker P. B (2007). Presentation of Mexican Americans to a memory disorder clinic. Journal of Psychopathology and Behavioral Assessment, 29, 137–140. [Google Scholar]
- O’Bryant S. E., Johnson L., Balldin V., Edwards M., Barber R., Williams B., … Hall J (2013). Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis, 33, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palafox J. C., Prawda J., & Velez E (1994). Primary school quality in Mexico. Comparative Education Review, 38, 167–180. [Google Scholar]
- Peters M. E., Rosenberg P. B., Steinberg M., Norton M. C., Welsh-Bohmer K. A., Hayden K. M., … Cache County I (2013). Neuropsychiatric symptoms as risk factors for progression from CIND to dementia: The Cache County Study. Am J Geriatr Psychiatry, 21, 1116–1124. doi:10.1097/JGP.0b013e318267014b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B., … Wallace R. B (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29, 125–132. doi:10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B., … Wallace R. B (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B. L., Langa K. M., McCammon R. J., Fisher G. G., Potter G. G., Burke J. R., … Wallace R. B (2011). Incidence of dementia and cognitive impairment, not dementia in the United States. Annals of Neurology, 70, 418–426. doi:10.1002/ana.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND (2016). RAND HRS data, Version O. Santa Monica, CA: RAND Center for the Study of Aging.
- Roberts J. S., McLaughlin S. J., & Connell C. M (2014). Public beliefs and knowledge about risk and protective factors for Alzheimer’s disease. Alzheimers Dement, 10, S381–S389. doi:10.1016/j.jalz.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A. C., Prull M. W., O’Hara R., Race E. A., Desmond J. E., Glover G. H., … Gabrieli J. D (2002). Variable effects of aging on frontal lobe contributions to memory. Neuroreport, 13, 2425–2428. doi:10.1097/01.wnr.0000048001.96487.05 [DOI] [PubMed] [Google Scholar]
- Rote S. M., & Moon H (2016). Racial/ethnic differences in caregiving frequency: Does immigrant status matter?The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, gbw106. doi:10.1093/geronb/gbw106 [DOI] [PubMed] [Google Scholar]
- Sisco S., Gross A. L., Shih R. A., Sachs B. C., Glymour M. M., Bangen K. J., … Manly J. J (2015). The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 557–567. doi:10.1093/geronb/gbt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460. [PubMed] [Google Scholar]
- Stern Y., Albert S., Tang M. X., & Tsai W. Y (1999). Rate of memory decline in AD is related to education and occupation: Cognitive reserve?Neurology, 53, 1942–1947. [DOI] [PubMed] [Google Scholar]
- Stern Y., Gurland B., Tatemichi T. K., Tang M. X., Wilder D., & Mayeux R (1994). Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA: The Journal of the American Medical Association, 271, 1004–1010. [PubMed] [Google Scholar]
- Sullivan D. F. (1971). A single index of mortality and morbidity. HSMHA Health Rep, 86, 347–354. [PMC free article] [PubMed] [Google Scholar]
- Tang M. X., Cross P., Andrews H., Jacobs D. M., Small S., Bell K., … Mayeux R (2001). Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 56, 49–56. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau (2011). Table 9: Resident population by race, Hispanic origin, and age: 2000 and 2009. Washington, DC: U.S. Census Bureau; Retrieved from http://www.census.gov/prod/2011pubs/11statab/pop.pdf [Google Scholar]
- Vivot A., Power M. C., Glymour M. M., Mayeda E. R., Benitez A., Spiro A. 3rd, … Gross A. L (2016). Jump, hop, or skip: Modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol, 183, 302–314. doi:10.1093/aje/kwv212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weden M. M., Miles J. N., Friedman E., Escarce J. J., Peterson C., Langa K. M., & Shih R. A (2017). The Hispanic paradox: Race/ethnicity and nativity, immigrant enclave residence and cognitive impairment among older US adults. J Am Geriatr Soc, 65, 1085–1091. doi:10.1111/jgs.14806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Hayward M. D., & Yu Y. L (2016). Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav, 57, 184–199. doi:10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]