Abstract
Major depressive disorder (MDD) is the most prevalent comorbid mental disorder among people with substance use disorders. The MDD can be both primary and substance-induced and its accurate diagnosis represents a challenge for clinical practice and treatment response. Recent studies reported alterations in the circulating expression of inflammatory mediators in patients with psychiatric disorders, including those related to substance use. The aim of the study was to explore TNF-α, IL-1β, CXCL12, CCL2, CCL11 (eotaxin-1) and CX3CL1 (fractalkine) as potential biomarkers to identify comorbid MDD and to distinguish primary MDD from substance-induced MDD in patients with substance disorders. Patients diagnosed with cocaine (CUD, n = 64) or alcohol (AUD, n = 65) use disorders with/without MDD were recruited from outpatient treatment programs [CUD/non-MDD (n = 31); CUD/primary MDD (n = 18); CUD/cocaine-induced MDD (N = 15); AUD/non-MDD (n = 27); AUD/primary MDD (n = 16) and AUD/alcohol-induced MDD (n = 22)]. Sixty-two healthy subjects were also recruited as control group. Substance and mental disorders were assessed according to “Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision” (DSM-IV-TR) and a blood sample was collected for determinations in the plasma. The cocaine group showed lower TNF-α (p<0.05) and CCL11 (p<0.05), and higher IL-1β (p<0.01) concentrations than the control group. In contrast, the alcohol group showed higher IL-1β (p<0.01) and lower CXCL12 (p<0.01) concentrations than the control group. Regarding MDD, we only observed alterations in the cocaine group. Thus, CUD/MDD patients showed lower IL-1β (p<0.05), CXCL12 (p<0.05) and CCL11 (p<0.05), and higher CXC3CL1 (p<0.05) concentrations than CUD/non-MDD patients. Moreover, while CUD/primary MDD patients showed higher CCL11 (p<0.01) concentrations than both CUD/non-MDD and CUD/cocaine-induced MDD patients, CUD/cocaine-induced MDD patients showed lower CXCL12 (p<0.05) concentrations than CUD/non-MDD patients. Finally, a logistic regression model in the cocaine group identified CXCL12, CCL11 and sex to distinguish primary MDD from cocaine-induced MDD providing a high discriminatory power. The present data suggest an association between changes in inflammatory mediators and the diagnosis of primary and substance-induced MDD, namely in CUD patients.
Introduction
Major depressive disorder (MDD) is the most prevalent comorbid mental condition in subjects with substance use disorders (SUD) and closely related to their poor prognosis. As expected, due to their marked clinical severity, these dual diagnosed patients also present considerable psychosocial acuteness and make greater use of health resources, including emergency services and psychiatric admissions [1–3]. The identification of MDD in substance users, however, is often complicated because of its inherent characteristics, that is to say, the acute and chronic effects related to substance consumption/withdrawal can mimic depression. Currently, diagnosis is syndromic and established with clinical criteria using “Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision” (DSM-IV-TR), “DSM 5th Edition” (DSM-5) [4–5] or “International Classification of Diseases and Related Health Problems 10th Edition” (ICD-10) [6], the availability of specific biomarkers to facilitate the diagnosis of MDD in SUD are therefore required [7]. Among others, modulation of inflammatory mediators such as cytokines and chemokines has been recognized as a possible target. A growing body of literature has established that cytokines can play a critical role in the pathogenesis of both MDD [8–9] and SUD [10–13]. Preclinical and clinical studies in MDD and SUD have reported alterations in the immune system and dysregulation of the hypothalamic-pituitary-adrenal axis (HPA) [14–15]. These changes result in a chronic inflammatory response and a disruption of brain integrity and homeostasis as consequence of neuroinflammation [16]. In the brain, glial activation results in the release of cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), inflammatory mediators that influence the central immune system, modulate neural activity, and regulate the HPA axis [14, 17–18]. Chemokines, small chemoattractant proteins, also act as modulators in neuronal transmission and participate in the communication between glia and neurons [8, 19].
An increasing quantity of research has focused on the leading role of inflammatory mediators in the pathophysiology of a variety of psychiatric problems including bipolar [20] and post-traumatic stress disorders [21], schizophrenia [22], and MDD [23]. With respect to MDD, patients show increased serum levels of TNF-α and it has been reported that elevated serum levels of CCL11 are associated with suicidal ideations in such patients [24]. Moreover, there are studies suggesting that inflammatory markers have the potential to predict antidepressant treatment outcomes [25–26]. Regarding SUD, preclinical models of cocaine exposure have described that stromal derived factor-1/SDF-1 (CXCL12) participates in the modulation of cocaine-induced behavioral effects such as locomotion and stereotypes [27]. Previous studies in abstinent subjects with CUD found a positive correlation between plasma levels of IL-1β, fractalkine (CX3CL1), and CXCL12 with a number of DSM-IV-TR criteria for CUD, and with increased prevalence of comorbid psychiatric disorders relative to those users with no other psychiatric diagnosis [11]. With respect to alcohol, in preclinical models it has been reported to disrupt the cytokine profile during neuronal differentiation and influence adult neurogenesis, thus providing potential mechanisms to understand alcohol effects on cerebral development [28]. Other clinical studies have indicated that alcohol dependence is characterized by enhanced glial IL-1β in the cerebral cortex [29] and elevated chemokine monocyte chemotactic protein 1/MCP-1 (CCL2) in different brain regions [10]. Furthermore, a recent study in patients diagnosed with alcohol use disorders (AUD) showed lower plasma levels of CXCL12 and CX3CL1 than in controls. Additionally, AUD patients with comorbid depressive and/or anxiety disorders have been reported to have alterations in the plasma levels of the chemokine eotaxin-1 (CCL11) [30].
Because the peripheral alterations of inflammatory molecules may modulate and/or reflect neuroinflammatory events in the brain, certain cytokines and chemokines could be reliable candidates to detect MDD in SUD patients. Changes in peripheral inflammatory mediators might thus contribute to improving diagnosis, treatment strategy, and medical approach for these psychiatric patients [7, 31]. The aim of the present study is to explore whether relevant proinflammatory cytokines such as TNF-α, and IL-1β, and chemokines such as CXCL12, CCL2, CCL11, and CX3CL1 could be potential biomarkers for the detection of co-occurrence of MDD in patients with CUD and/or AUD, and to distinguish primary MDD from substance-induced MDD.
Materials and methods
Subjects
The present cross-sectional study was conducted in white Caucasian population, and included SUD [cocaine (CUD) or alcohol (AUD)] patients diagnosed with/without comorbid MDD, and healthy control subjects. Patients were recruited at the addiction treatment facilities of the Instituto de Neuropsiquiatría y Adicciones-Parc Salut Mar (Barcelona, Spain), Hospital Universitario 12 de Octubre (Madrid, Spain), and Centro Provincial de Drogodependencias (Málaga, Spain). Control participants were included from data bases of healthy subjects willing to participate in medical research projects at the Unidad de Farmacología del IMIM-Hospital del Mar Research Institute (Barcelona, Spain), Hospital Universitario 12 de Octubre (Madrid, Spain), and Hospital Regional Universitario de Málaga (Málaga, Spain).
Cocaine (n = 64) and alcohol (n = 65) groups were classified into six different subgroups: CUD with no MDD (CUD/non-MDD, n = 31), CUD with primary MDD (CUD/primary MDD, n = 18); CUD with cocaine-induced MDD (CUD/cocaine-induced MDD, n = 15), AUD with no MDD (AUD/non-MDD, n = 27), AUD with primary MDD (AUD/primary MDD, n = 16); AUD with alcohol-induced MDD (AUD/alcohol-induced MDD, n = 22).
All the subjects met the inclusion criteria, which were: both genders, age ≥ 18 years old up to 65 years of age and at least 4 weeks of abstinence in the case of subjects with SUD. Those patients who were diagnosed with MDD had to be in remission and “Hamilton Depression Rating Scale” (HDRS) scores had to be lower than 6. We considered 4 weeks of abstinence (SUD) and remission phase (MDD) because patients have to be as pathophysiologically stable as possible for clinical assessments and biochemical determinations. The exclusion criteria included: cognitive or language limitations that precluded evaluations and pregnant or breastfeeding women. SUD patients suffering from any psychiatric disorder in Axis I (DSM-IV-TR) [4] other than MDD and/or CUD/AUD (except from nicotine use disorder) were excluded. In the control group, participants with psychiatric disorders in Axis I (DSM-IV-TR), SUD (except nicotine use disorder) or family history of MDD were also excluded. Other excluding factors for all participants were: use of anti-inflammatory drugs or MAOI’s, personal history of cancer; major surgery within 6 months prior to the study; long-term inflammatory disease; any chronic illnesses that could interfere in the study such as cardiovascular, respiratory, renal, hepatic, endocrine, gastro-intestinal, hematological, neurological diseases; and infectious diseases (including HIV, hepatitis B, and hepatitis C). Clinical assessments and blood/urine analyses were performed to assure these participation criteria.
Ethics statements
Written informed consents were obtained from each participant after a complete description of the study. All the participants had the opportunity to discuss any questions or issues. The study and protocols for recruitment were approved by the Ethics Committee of the Hospital Regional Universitario de Málaga (CP14/00173, CP14/00212 and PI13/02261) and by the Research Ethical Committee from the CEIC-Parc de Salut Mar (2012/4903/I; 2012/4751/I; 2009/3494/I) in accordance with the Ethical Principles for Medical Research Involving Human Subjects adopted in the Declaration of Helsinki by the World Medical Association (64th WMA General Assembly, Fortaleza, Brazil, October 2013), Recommendation No. R (97) 5 of the Committee of Ministers to Member States on the Protection of Medical Data (1997), and Spanish data protection act (Ley Orgánica 15/1999 de Protección de Datos, LOPD). All collected data were given code numbers in order to maintain privacy and confidentiality.
Clinical assessments
Substance and non-substance use disorders were diagnosed according to the DSM-IV-TR criteria [4] using the Spanish version of the “Psychiatric Research Interview for Substance and Mental Disorders” (PRISM) [4, 32]. PRISM is a semi-structured interview that has demonstrated good psychometric properties in terms of test-retest reliability, inter-rater reliability, and validity for primary MDD and substance-induced MDD, with kappa ranging from 0.66 and 0.75 [32–33]. All the interviews were performed by trained and experienced psychologists.
Collection of plasma samples
Blood samples were obtained in the morning after fasting for 8–12 h by experienced nurses. Participants were comfortably seated in a chair for 5 min before the blood draw, and were asked whether they had allergies, phobias or a history of vaso-vagal response during previous injections or blood draws. The participants could be placed in the supine position by adjusting the chair (backrest) if necessary. After the blood draw, participants were invited for breakfast prior to the psychiatric interviews.
Venous blood was extracted into 10 mL K2 EDTA tubes (BD, Franklin Lakes, NJ, USA) and immediately processed to obtain plasma. Blood samples were centrifuged at 2,200 x g for 15 min (4°C) and individually assayed to detect infectious diseases by 3 commercial rapid tests for HIV, hepatitis B, and hepatitis C (Strasbourg, Cedex, France). Finally, plasma samples were individually characterized, registered, and stored at -80°C until further analyses.
Multiplex immunoassays
Proinflammatory cytokines and chemokines were chosen based on previous studies regarding relevant inflammatory mediators in psychiatric disorders and SUD using a Luminex Platform for quantification [11, 13, 34]. A Bio-Plex Suspension Array System 200 (Bio-Rad Laboratories, Hercules, CA, USA) was used to quantify cytokine and chemokine concentrations in plasma with a ProcartaPlex Immunoassay panel and an appropriate Plasma Standard Diluent Kit (Invitrogen, ThermoFisher Scientific, Waltham, MA, USA). A human cytokine/chemokine 6-plex panel was used to simultaneously detect the following analytes: TNF-α, IL-1β, CXCL12; CCL2, CCL11, and CX3CL1. The measurements of these inflammatory mediators in plasma were performed following the manufacturer´s instructions [11]. Raw data (mean fluorescence intensity) were analyzed using the Bio-Plex Manager Software 4.1 (Bio-Rad laboratories, Hercules, CA, USA). Plasma concentrations were expressed as pg/mL. According to manufacturer’s specifications (ProcartaPlex Human Cytokine/Chemokine Simplex Kit), assay sensitivity and standard curve range for each analyte were as follows: 0.4 pg/mL and 8.54–35,000 pg/mL for TNF-α (catalog #: EPX01A-10223-901); 0.2 pg/mL and 2.44–10,000 pg/mL for IL-1β (catalog #: EPX01A-10224-901); 20.5 pg/mL and 17.1–70,000 pg/mL for CXCL12 (catalog #: EPX01A-12138-901); unknown and 37.5–23,300 pg/mL for CCL2 (catalog #: EPX01B-10281-901); 1.4 pg/mL and 0.61–2,500 pg/mL for CCL11 (catalog #: EPX01B-12120-901); and 0.5 pg/mL and 2.08–8,500 pg/mL for CX3CL1 (catalog #: EPX01A-12121-901). Inter-assay and intra-assay (samples in duplicate) coefficients of variation (%CV) were as follows: 7.1% and 6.5% for TNF-α; <5% and 8.9% for IL-1β; 7.8% and 9.0% for CCL11; 7.2% and 6.4% for CX3CL1; <5% and 9.8% for CCL2; and 5% and 10.7% for CXCL12.
Statistical analysis
All data in the tables are expressed as number and percentage of subjects [n (%)] or mean and standard deviation [mean (SD)]. The significance of differences in categorical and normal continuous variables was determined using Fisher’s exact test (chi-square test) and Student’s t-test, respectively.
One-way analysis of covariance (ANCOVA) was performed to assess the effects of independent factors (i.e., grouping variables such as types of SUD, diagnosis of MDD, and types of MDD) on the plasma concentrations of cytokines and chemokines (dependent variables), controlling for additional independent variables and covariates [e.g., sex, age, body mass index (BMI), and antidepressant medication]. The post hoc tests for multiple comparisons were performed using Sidak’s correction test. Prior to performing one-way ANCOVA, logarithm (10)-transformation for dependent variables was used to ensure statistical assumptions for positive skewed distributions.
Binary logistic models were employed to distinguish between primary MDD and substance-induced MDD for SUD patients using full models that included cytokine and chemokine concentrations, sex, age, and BMI, and adjusted models (variables were chosen using a backward stepwise approach). Receiver operating characteristic (ROC) analysis was performed to determine the accuracy of these models comparing the area under the curve (AUC) and representative cut-off scores for the distinction between types of MDD, which included estimations of sensitivity and specificity.
Statistical analysis was carried out with the GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA, USA), and IBM SPSS Statistical version 22 (IBM, Armonk, NY, USA). A p-value<0.05 was considered statistically significant.
Results
Sociodemographic and clinical characteristics of the sample
Table 1 shows the sociodemographic characteristics and depression-related variables of 191 participants in this cross-sectional study who were divided into 3 groups: cocaine, alcohol and control groups.
Table 1. Baseline sociodemographic and clinical characteristics of the study sample.
| VARIABLES | CONTROL GROUP (n = 62) | COCAINE GROUP (CUD) (n = 64) | ALCOHOL GROUP (AUD) (n = 65) | |||||
|---|---|---|---|---|---|---|---|---|
| Non-MDD (n = 31) | Primary MDD (n = 18) | Cocaine-Induced MDD (n = 15) | Non-MDD (n = 27) | Primary MDD (n = 16) | Alcohol-Induced MDD (n = 22) | |||
| SOCIODEMOGRAPHIC VARIABLES | ||||||||
| Age [Mean (SD)] | Years | 34.82 (6.23) | 36.52 (6.47) | 40.89 (10.11) | 35.67 (9.34) | 46.78 (6.71) aaa | 47.31 (6.03) aaa | 46.64 (8.77) aaa |
| BMI [Mean (SD)] | Kg/m2 | 24.89 (3.49) | 24.64 (3.05) | 26.69 (5.75) | 26.16 (4.23) | 25.63 (3.87) | 27.68 (4.58) a | 25.23 (3.57) |
| Sex [n (%)] | Women | 29 (46.8) | 6 (19.4) a | 5 (27.8) | 4 (26.7) | 7 (25.9) | 5 (31.3) | 10 (45.5) |
| Men | 33 (53.2) | 25 (80.6) | 13 (72.2) | 11 (73.3) | 20 (74.1) | 11 (68.8) | 12 (54.5) | |
| Marital Status [n (%)] | Single | 28 (45.2) | 12 (38.7) | 6 (33.3) aa | 11 (73.3) | 9 (33.3) | 3 (18.8) | 8 (36.4) |
| Cohabiting | 24 (38.7) | 11 (35.5) | 3 (16.7) | 1 (6.7) | 13 (48.1) | 7 (43.8) | 9 (40.9) | |
| Separated | 6 (9.7) | 8 (25.8) | 9 (50.0) | 3 (20.0) | 5 (18.5) | 5 (31.3) | 4 (18.2) | |
| Widow/er | 4 (6.5) | - | - | - | - | 1 (6.3) | 1 (4.5) | |
| Education [n (%)] | Elementary | 1 (1.6) | 16 (51.6) aaa | 10 (55.6) aaa | 7 (46.7) aaa | 10 (37.0) aaa | 4 (25.0) aaa | 6 (27.3) aaa |
| Secondary | 22 (35.5) | 11 (35.5) | 4 (22.2) | 4 (26.7) | 14 (51.9) | 8 (50.0) | 7 (31.8) | |
| University | 39 (62.9) | 4 (12.9) | 4 (22.2) | 4 (26.7) | 3 (11.1) | 4 (25.0) | 9 (40.9) | |
| Occupation [n (%)] | Employed | 41 (66.1) | 15 (48.4) | 10 (55.6) | 4 (26.7) aa | 12 (44.4) | 6 (37.5) | 9 (40.9) |
| Unemployed | 21 (33.9) | 16 (51.6) | 8 (44.4) | 11 (73.3) | 15 (55.6) | 10 (62.5) | 13 (59.1) | |
| DEPRESSION-RELATED VARIABLES | ||||||||
| Age of onset [Mean (SD)] | Years | - | - | 33.33 (11.30) | 31.40 (8.63) | - | 42.31 (7.67) | 39.29 (9.21) |
| Number of episodes [Mean (SD)] | - | - | 2.44 (1.89) | 4.20 (6.47) | - | 3.54 (5.13) | 4.00 (5.66) | |
| Age at last episode [Mean (SD)] | Years | - | - | 38.61 (10.25) | 31.50 (5.17) c | - | 47.23 (6.07) | 46.64 (8.77) |
| Remission [Mean (SD)] | Months | - | - | 15.00 (19.88) | 29.56 (35.64) | - | 6.38 (4.05) | 14.24 (18.24) |
| Current antidepressant treatment [n (%)] | - | - | 10 (55.6) | 4 (26.7) | 5 (18.5) | 11 (68.8) bb | 9 (40.9) | |
| Family history of MDD [n (%)] | - | 5 (16.1) | 9 (50.0) | 4 (26.7) | 15 (55.6) | 9 (56.3) | 12 (54.5) | |
(a) p<0.05
(aa) p<0.01 and
(aaa) p<0.001 denote significant differences versus control group
(bb) p<0.01 denotes significant differences versus non-MDD subgroup
(c) p<0.05 denotes significant differences versus primary MDD subgroup
Abbreviations: AUD = alcohol use disorders; BMI = body mass index; CUD = cocaine use disorders MDD = major depressive disorders
The mean age of the cocaine group was 37.6 years, and 23.4% were women. The most common marital status was single, mainly in cocaine-induced MDD patients, with elementary education (51.6%). As compared with the control group, we observed significant differences in the percentage of women (CUD/non-MDD patients were 19% women; p<0.05), marital status (CUD/primary MDD patients were mainly separated; p<0.01), education (p<0.001), and occupation (CUD/cocaine-induced MDD patients were mainly unemployed; p<0.01). In the alcohol group, the mean age was 46.9 years, and 33.8% were women. In contrast, cohabiting was the most common marital status, and education at secondary or higher level reached 69.2%. The comparison with the control group revealed significant differences in the mean age (p<0.001), BMI (AUD/primary MDD had higher BMI than controls; p<0.05), and education (p<0.001).
Regarding MDD-related variables in the cocaine group, the mean age of onset was 32.5 years and 3.2 episodes. However, there were significant differences in the age of the last episode when primary and cocaine-induced patients were compared (p<0.05). In the alcohol group, the mean age of onset was 40.6 years and 3.81 episodes. No differences were found in the age of the last episode in AUD patients. Finally, although remission and current treatment with antidepressants were apparently different as compared both primary and substance-induced MDD subgroups in the cocaine and alcohol groups, statistical analysis found no significant differences.
Inflammatory mediators in relation to substance use disorders
The impact of SUD on the plasma concentrations of proinflammatory cytokines and chemokines was investigated using a one-way ANCOVA [cocaine group (CUD/non-MDD patients), alcohol group (AUD/non-MDD patients), and control group], and controlling for sex, age, and BMI. Plasma concentrations and statistical analysis of inflammatory mediators according to SUD are described in Table 2.
Table 2. Plasma concentrations of inflammatory mediators in participants grouped according to the type of substance use disorder.
| VARIABLE | CONTROL GROUP (n = 62) | COCAINE GROUP (CUD) Non-MDD (n = 31) | ALCOHOL GROUP (AUD) Non-MDD (n = 27) | ANCOVA (Statistics)(1) | ||
|---|---|---|---|---|---|---|
| F-value | df | p-value | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | ||||
| TNF-α (pg/mL) | 11.32±4.91 | 8.00±5.11 a | 10.27±5.97 | 4.540 | 2, 113 | 0.013 |
| IL-1β (pg/mL) | 0.724±0.76 | 1.346±0.87 aa | 1.764±1.13 aa | 7.891 | 2,103 | 0.001 |
| CXCL12 (pg/mL) | 287.2±44.53 | 287.9±60.34 | 240.1±77.69 aa, b | 5.662 | 2,114 | 0.005 |
| CCL2 (pg/mL) | 35.29±16.37 | 38.70±9.303 | 39.01±16.28 | 2.198 | 2, 114 | 0.116 |
| CCL11 (pg/mL) | 148.5±61.24 | 126.5±54.00 a | 128.8±37.10 | 3.737 | 2, 114 | 0.027 |
| CX3CL1 (pg/mL) | 2.606±1.37 | 1.987±1.67 | 1.839±1.09 | 1.886 | 2, 113 | 0.156 |
(1) Statistical analysis was conducted on the logarithmic transformed values to ensure statistical assumptions. Data were analyzed by ANCOVA controlling for age, sex, and BMI
(a) p<0.05 and
(aa) p<0.01 denote significant differences versus control group after post hoc comparisons
(b) p<0.05 denotes significant differences versus cocaine non-MDD group after post hoc comparisons
Missing participants: TNF-α (n = 1, control); IL-1β (n = 4, control; n = 7, CUD); CX3CL1 (n = 1, CUD)
Abbreviations: ANCOVA = analysis of covariance; AUD = alcohol use disorders; CUD = cocaine use disorders; df = degree of freedom; Non-MDD = non-major depressive disorders
One-way ANCOVA revealed a main effect of SUD on TNF-α (p<0.05) concentrations. While the cocaine group showed significantly lower TNF-α concentrations than the control (p<0.05), there were no differences in TNF-α concentrations between the alcohol group and the control. In the case of IL-1β, there was also a main effect of SUD (p<0.01) and both cocaine and alcohol groups reported higher IL-1β concentrations than the control (p<0.01). Regarding chemokine concentrations, the statistical analysis revealed a main effect of SUD on CXCL12 (p<0.01) and CCL11 (p<0.05) concentrations. The multiple comparisons test showed significantly lower CXCL12 concentrations in the alcohol group than in the cocaine (p<0.05) and control (p<0.01) groups, but also significantly lower CCL11 (p<0.05) in the cocaine group than in the control (p<0.05). In contrast, no main effects of SUD were detected on plasma concentrations of CCL2 and CX3CL1.
Inflammatory mediators in relation to comorbid major depressive disorders
We investigated whether comorbid MDD was associated with alterations in the plasma concentrations of these inflammatory mediators in the two groups of SUD patients. A one-way ANCOVA was performed for the cocaine (CUD/non-MDD and CUD/MDD patients) and alcohol groups (AUD/non-MDD and AUD/MDD patients) controlling for sex, age, BMI, and antidepressant medication. Plasma concentrations and statistical analysis of inflammatory mediators according to MDD in the cocaine and alcohol groups are described in Table 3.
Table 3. Plasma concentrations of inflammatory mediators in the cocaine and alcohol groups according to comorbid depression.
| VARIABLE | COCAINE GROUP (CUD) (n = 64) | ANCOVA (Statistics) (1) | ALCOHOL GROUP (AUD) (n = 65) | ANCOVA (Statistics) (1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-MDD (n = 31) | MDD (n = 33) | F-value | df | p-value | Non-MDD (n = 27) | MDD (n = 38) | F-value | df | p-value | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| TNF-α (pg/mL) | 8.00±5.11 | 9.80±5.58 | 0.992 | 1,57 | 0.319 | 10.27±5.97 | 9.67±5.03 | 0.077 | 1,59 | 0.782 |
| IL-1β (pg/mL) | 1.346±0.868 | 0.699±0.327 | 4.211 | 1,46 | 0.046 | 1.764±1.129 | 1.646±1.137 | 0.105 | 1,59 | 0.747 |
| CXCL12 (pg/mL) | 287.9±60.34 | 252.5±41.04 | 5.222 | 1,58 | 0.026 | 240.1±77.69 | 241.8±110.3 | 0.053 | 1,59 | 0.819 |
| CCL2 (pg/mL) | 38.70±9.303 | 43.36±12.89 | 0.853 | 1,58 | 0.360 | 39.01±16.28 | 34.89±16.14 | 1.568 | 1,59 | 0.215 |
| CCL11 (pg/mL) | 126.5±54.00 | 171.10±98.14 | 4.778 | 1,58 | 0.033 | 128.8±37.10 | 111.9±35.3 | 2.899 | 1,59 | 0.094 |
| CX3CL1 (pg/mL) | 1.987±1.666 | 2.342±1.252 | 5.807 | 1,55 | 0.019 | 1.839±1.090 | 2.212±1.298 | 1.742 | 1,59 | 0.192 |
(1) Statistical analysis was conducted on the logarithmic transformed values to ensure statistical assumptions. Data were analyzed by ANCOVA controlling for age, sex, BMI, and antidepressant medication
Missing participants: TNF-α (n = 1, CUD/MDD); IL-1β (n = 7, CUD/non-MDD; n = 5, CUD/MDD); CX3CL1 (n = 1, CUD/non-MDD; n = 2, CUD/MDD)
Abbreviations: ANCOVA = analysis of covariance; AUD = alcohol use disorders; CUD = cocaine use disorders; df = degree of freedom; MDD = major depressive disorder
One-way ANCOVA showed a main effect of MDD on the plasma concentrations of IL-1β (p<0.05), CXCL12 (p<0.05), CCL11 (p<0.05), and CX3CL1 (p<0.05) in the cocaine group, but no significant effects on any of these inflammatory mediators in the alcohol one. CUD/MDD patients showed significantly lower IL-1β and CXCL12 concentrations than CUD/non-MDD patients, and significantly higher CCL11 and CX3CL1 concentrations than CUD/non-MDD patients.
Inflammatory mediators in relation to primary and substance-induced major depressive disorders
SUD patients who were diagnosed with MDD were divided into primary and substance-induced MDD according to DSM-IV-TR criteria. We investigated the impact of the type of comorbid MDD in CUD and AUD patients using one-way ANCOVA for the cocaine (CUD/non-MDD, CUD/primary MDD, and CUD/cocaine-induced MDD patients) and alcohol groups (AUD/non-MDD, AUD/primary MDD, and AUD/cocaine-induced MDD patients), controlling for sex, age, BMI, and antidepressant medication. Plasma concentrations and statistical analysis of inflammatory mediators according to type of MDD in the cocaine and alcohol groups are described in Table 4.
Table 4. Plasma concentrations of inflammatory mediators in the cocaine and alcohol groups according to the type of depression.
| VARIABLE | COCAINE GROUP (CUD) (n = 64) | ANCOVA (Statistics) (1) | ALCOHOL GROUP (AUD) (n = 65) | ANCOVA (Statistics) (1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-MDD (n = 31) | Primary MDD (n = 18) | Cocaine-Induced-MDD (n = 15) | F-value | df | p-value | Non-MDD (n = 27) | Primary MDD (n = 16) | Alcohol-Induced-MDD (n = 22) | F-value | df | p-value | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| TNF-α (pg/mL) | 8.00±5.11 | 11.63±6.38 | 7.45±3.25 | 0.751 | 2,56 | 0.477 | 10.27±5.97 | 9.62±5.17 | 9.70±5.05 | 0.102 | 2,58 | 0.875 |
| IL-1β (pg/mL) | 1.346±0.868 | 0.660±0.389 | 0.760±0.202 | 2.411 | 2,45 | 0.101 | 1.764±1.129 | 1.343±0.692 | 1.867±1.347 | 0.217 | 2,58 | 0.806 |
| CXCL12 (pg/mL) | 287.9±60.34 | 265.5±42.56 | 236.9±34.26 a | 4.453 | 2,57 | 0.016 | 240.1±77.69 | 248.7±66.69 | 236.8±134.8 | 0.415 | 2,58 | 0.662 |
| CCL2 (pg/mL) | 38.70±9.303 | 45.40±14.15 | 40.91±11.16 | 0.501 | 2,57 | 0.609 | 39.01±16.28 | 30.96±13.71 | 37.74±17.45 | 1.444 | 2,58 | 0.244 |
| CCL11 (pg/mL) | 126.5±54.00 | 211.7±111.4 aa | 124.3±49.9 bb | 6.445 | 2,57 | 0.003 | 128.8±37.10 | 115.4±39.4 | 109.3±32.8 | 1.401 | 2,58 | 0.255 |
| CX3CL1 (pg/mL) | 1.987±1.666 | 2.272±1.195 | 2.439±1.371 | 0.134 | 2,54 | 0.875 | 1.839±1.090 | 2.116±1.387 | 2.283±1.259 | 0.864 | 2,58 | 0.427 |
(1) Statistical analysis was conducted on the logarithmic transformed values to ensure statistical assumptions. Data were analyzed by ANCOVA controlling for age, sex, BMI, and antidepressant medication.
(a) p<0.05 and
(aa) p<0.01 denote significant differences versus cocaine non-MDD group after post hoc comparisons
(bb) p<0.01 denotes significant differences versus CUD primary MDD group after post hoc comparisons
Missing participants: TNF-α (n = 1, CUD/cocaine-induced MDD); IL-1β (n = 7, CUD/non-MDD; n = 1, CUD/primary MDD; n = 4, CUD/cocaine-induced MDD); CX3CL1 (n = 1, CUD/non-MDD; n = 2, CUD/cocaine-induced MDD)
Abbreviations: ANCOVA = analysis of covariance; AUD = alcohol use disorders; CUD = cocaine use disorders; df = degree of freedom; MDD = major depressive disorders
In a similar manner to previous results with comorbid MDD diagnosis, one-way ANCOVA revealed a main effect of the type of MDD on the plasma concentrations of CXCL12 (p<0.05) and CCL11 (p<0.01) in the cocaine group, but not in the alcohol one. The multiple comparisons test showed that CXCL12 concentrations were significantly lower in CUD/cocaine-induced MDD patients than in CUD/non-MDD patients (p<0.05). The post hoc test also revealed that CCL11 concentrations were significantly higher in CUD/primary MDD patients than in CUD/non-MDD (p<0.01) and CUD/cocaine-induced MDD (p<0.01) patients.
Inflammatory mediators in cocaine use disorders as potential biomarkers of comorbid major depressive disorders
Logistic regression models were generated to evaluate whether these cytokine and chemokine concentrations could serve as explanatory variables in order to discriminate between primary MDD and substance induced-MDD in the cocaine group. The regression analyses included additional variables (age, sex, and BMI) using different approaches and the resulting models are represented in Table 5.
Table 5. Binary logistic regression models for predicting cocaine-induced depression through plasma concentrations of inflammatory mediators, sex, age, and BMI.
| MODEL | VARIABLE | B | SEM | W | df | p value | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
|
LOGISTIC REGRESSION FULL MODEL 1 Method: Enter |
TNF-α | -0.204 | 0.144 | 2.005 | 1 | 0.157 | 0.816 | 0.616 | 1.081 |
| IL-1β | 2.809 | 2.493 | 1.270 | 1 | 0.260 | 16.597 | 0.125 | 2198 | |
| CXCL12 | -0.021 | 0.017 | 1.401 | 1 | 0.237 | 0.980 | 0.947 | 1.014 | |
| CCL2 | 0.070 | 0.073 | 0.921 | 1 | 0.337 | 1.073 | 0.930 | 1.238 | |
| CCL11 | -0.017 | 0.012 | 1.890 | 1 | 0.169 | 0.983 | 0.959 | 1.007 | |
| CX3CL1 | 0.308 | 0.602 | 0.262 | 1 | 0.609 | 1.361 | 0.418 | 4.426 | |
| Age | -0.019 | 0.106 | 0.033 | 1 | 0.856 | 0.981 | 0.797 | 1.208 | |
| Sex | 2.788 | 1.503 | 3.443 | 1 | 0.064 | 16.25 | 0.855 | 308.8 | |
| BMI | -0.084 | 0.170 | 0.243 | 1 | 0.622 | 0.920 | 0.659 | 1.283 | |
| Constant | 5.369 | 5.991 | 0.803 | 1 | 0.370 | 214.7 | - | - | |
|
LOGISTIC REGRESSION MODEL 2 Method: Backward Stepwise |
CXCL12 | -0.022 | 0.013 | 2.829 | 1 | 0.093 | 0.978 | 0.954 | 1.004 |
| CCL11 | -0.015 | 0.007 | 4.567 | 1 | 0.033 | 0.985 | 0.971 | 0.999 | |
| Sex | 2.037 | 0.976 | 4.354 | 1 | 0.037 | 7.669 | 1.132 | 51.973 | |
| Constant | 6.610 | 3.506 | 3.555 | 1 | 0.059 | 742.329 | |||
Abbreviations: B = coefficient; SEM = standard error; W = Wald test; df = degrees of Freedom; CI = confidence interval
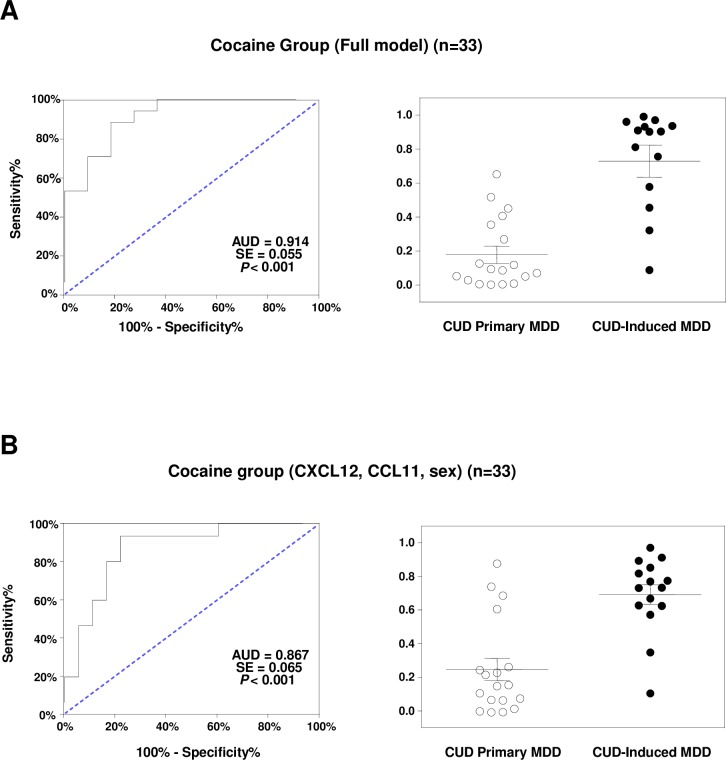
The full model included plasma concentrations of TNF-α, IL-1β, CXCL12, CCL2, CCL11, and CX3CL1, and age, sex and BMI as independent variables in the logistic regression analysis in the cocaine group. As depicted in Fig 1A, the ROC curve analysis showed an AUC = 0.914 (p<0.001), which indicates a high discriminative power. A representative cut-off value was 0.452 [sensitivity 88.24 (95%CI = 63.56–98.54) and specificity 81.82 (95% CI = 48.22–97.72)]. Regarding the scatter plot of the predictive probabilities for the type of MDD, their means were significantly different between primary MDD and cocaine-induced MDD patients (U = 16.00, p<0.001).
Fig 1. ROC analysis and scatter dots for two logistic models to distinguish primary MDD from cocaine-induced MDD in the cocaine group.
(A) ROC analysis of a full model: TNF-α, IL-1β, CXCL12, CCL2, CCL11 CX3CL1, sex, age and BMI; and a scatter plot for the predictive probabilities. (B) ROC analysis of an adjusted model: CXCL12, CCL11 and sex; and a scatter plot for the predictive probabilities. Lines on the scatter plots are means and SD.
To identify the most discriminative variables, a backward stepwise approach was used to generate a logistic regression model in the cocaine group. This model was restricted to 3 explanatory variables in the equation: CXCL12 and CCL11 concentrations and sex. As depicted in Fig 1B, the ROC analysis showed an AUC = 0.867 (p<0.001), maintaining a high discriminative power. A representative cut-off value was 0.311 [sensitivity 77.78 (95%CI = 52.36–93.59) and specificity 93.33 (95% CI = 68.05–99.83)]. The predictive probabilities for the type of MDD also differed between primary MDD and cocaine-induced MDD patients (U = 36.00, p<0.001).
Discussion
The main findings of this observational study are as follows: (a) There were differences in the plasma concentrations of some inflammatory mediators between CUD (TNF-α, IL-1β and CCL11) and AUD (IL-1β and CXCL12) patients when compared with control subjects; (b) CUD patients diagnosed with comorbid MDD showed significant differences in plasma concentrations of IL-1β, CXCL12, CCL11 and CX3CL1. In contrast, there were no differences in the alcohol group; (c) Plasma CCL11 concentrations were found to be higher in CUD patients with primary MDD than those with cocaine-induced MDD.
Evidence has revealed that the immune system participates in the mechanisms involved in long-term adaptation and neurotoxicity associated with the substance use [35]. As consequence of microglia activation, blood-brain-barrier disruption and neuroinflammation, proinflammatory mediators such as cytokines and chemokines are altered in the brain [36–37]. However, these changes are not restricted to the Central Nervous System, and alterations in these inflammatory signals have been also found in the blood of SUD patients and proposed as biomarkers of such disorders [38]. Thus, our results revealed that abstinent CUD patients had lower concentrations of TNF-α and CCL11, and higher concentrations of IL-1β relative to controls. Accordingly, a previous study in abstinent patients with a pathological use of cocaine reported lower concentrations of TNF-α than controls and elevated concentrations of IL-1β in those patients with a high number of DMS-IV-TR criteria for CUD [11]. These peripheral alterations of relevant cytokines and CCL11 in the plasma of cocaine users could be associated with changes in the expression of immune blood cells. In fact, acute cocaine administration in rats produces increased levels of neutrophils and decreased levels of leukocytes and lymphocytes [39]. Interestingly, a study with cocaine-dependent volunteers reported a decrease in the capacity of monocytes to express TNF-α and IL-6 compared with controls [40]. Unlike the archetypal proinflammatory cytokines TNF-α and IL-1β, there is no literature linking cocaine exposure with changes in plasma levels of CCL11. In contrast, a cross-sectional study has suggested that current cannabis use is linked to increased plasma levels of CCL11 [41]. Regarding AUD patients, significant alterations in the plasma were observed for IL-1β and CXCL12. Similar to CUD patients, high concentrations of IL-1β were found in AUD patients, which has been associated with an altered pattern of production of inflammatory cytokines in peripheral blood monocytes from chronic alcohol patients [42]. In addition to IL-1β, a specific decrease in CXCL12 concentrations in AUD patients was found, which is in agreement with a previous study from our group examining plasma chemokines in abstinent AUD patients in treatment [30].
Among mood disorders, MDD is the comorbid psychiatric disorder more prevalent in SUD patients [7]. Because cytokines play a role in neuronal integrity, neurogenesis and synaptic remodeling, growing evidence suggests that inflammatory mediators are involved in the development of MDD [43]. Moreover, numerous studies have reported changes in circulating proinflammatory cytokines in patients with MDD [44]. In the present study, we explored circulating cytokines and chemokines in SUD patients diagnosed with MDD. Notably, while the diagnosis of comorbid MDD was associated with alterations in plasma concentrations of some cytokines and chemokines in the CUD group, no differences were found in the AUD group. Thus, CUD patients with comorbid MDD showed lower concentrations of IL-1β and CXCL12, and higher concentrations of CX3CL1 and CCL11 as compared with than those CUD patients without this condition. These results suggest that the type of SUD could have a prominent effect on the detection of alterations in inflammatory mediators associated with comorbid MDD. In addition to the type of substance, differences in other clinical and sociodemographic variables between both groups could be affecting the expression of circulating inflammatory mediators, for example age and sex. Previously, we reported that plasma concentrations of IL-1β, CXCL12 and CX3CL1 are positively associated with the number of DSM-IV-TR criteria for cocaine abuse and dependence. However, although cocaine users with severe CUD displayed an elevated prevalence of mood disorders, no differences in the plasma concentrations of these inflammatory mediators were reported [11]. In contrast, another recent study in the same cohort with CUD patients reported no changes of CCL11 concentrations relative to addiction-related variables or diagnosis of psychiatric comorbidity [13]. It is important to point out that in both studies we explored the association of these inflammatory mediators with psychiatric comorbidity at a level more general (mood disorders [11] or psychiatric DSM-IV-TR Axis I disorders and personality disorders [13]). While we observed lower plasma concentrations of IL-1β and CXCL12 in CUD patients with comorbid MDD, other studies conducted in psychiatric patients with no interference of substances have showed opposite results. For example, while increased IL-1β concentrations have been reported in depressed individuals compared with controls [45], another clinical study showed increased plasma levels of CXCL12 in MDD patients with different severities of depressive conditions compared with a control group [46]. These data suggest that the co-occurrence of CUD and MDD could prevent increased plasma concentrations of IL-1β and/or CXCL12 concentrations when CUD or MDD are not comorbid. Unlike IL-1β and CXCL12, chemokines CX3CL1 and CCL11 were found increased in CUD patients with MDD. Accordingly, transient elevated serum levels of CX3CL1 have been described in colorectal cancer patients with anxiety and depression [47]. Regarding CCL11, considerable evidence exists on the role of this chemokine in the progression of neurodegenerative diseases (e.g., schizophrenia or dementia patients) [22, 48], but contradictory results have been reported in relation to MDD. Thus, while changes in CCL11 are observed in women with recurrent MDD with suicidal ideation [24] and in young adults with mood disorders [49], another study in elderly patients with MDD showed no changes [50].
Because there was a strong association between alterations in the plasma expression of certain inflammatory mediators and comorbid MDD, we extended our study in CUD patients diagnosed with primary and cocaine-induced MDD. In fact, the differentiation between primary and substance-induced MDD in dual pathology is a field of study to be explored, and a recent research has showed that platelet IRAS/nischarim (I1-Imidazoline receptor) could discriminate between primary MDD and cocaine-induced MDD in CUD patients [51]. In our study, we have observed differences in the plasma expression of CCL11 and CXCL12 according to the type of MDD. Thus, CCL11 was increased in CUD patients with primary MDD relative to those with cocaine-induced MDD and non-comorbid patients. In the case of CXCL12, CUD patients with cocaine-induced MDD showed decreased CXCL12 concentrations relative to those with primary MDD and non-comorbid patients. Then, the ROC analysis of regression models confirmed that CXCL12 and CCL11, in combination with sex, had a strong discriminative power to discriminate between both types of MDD.
Limitations and strengths
Although our findings support the clinical importance of a differential diagnosis for MDD in CUD and AUD, we are aware of important limitations. First, there is a growing number of additional social, environmental and addiction-related variables (covariates) that could influence our data. Second, because the participants were under current treatment for SUD at the moment of evaluation, we cannot exclude an influence of other psychiatric medications (e.g., anxiolytics and antipsychotics) on these inflammatory signals. Third, the impact of sex/gender in the expression of inflammatory mediators is critical and, therefore, it is required a larger sample size and a better characterization including other variables (e.g., sex hormones). Fourth, a cross-validation of the proposed discriminative models is necessary to ensure reliability and stability of the estimations. Fifth, the inclusion of patients diagnosed with MDD and no history of SUD and healthy controls are needed for future research, even though these psychiatric individuals have been previously described in the literature [45].
The strengths of the study are that we measured inflammatory mediators in a well-phenotyped cohort of subjects characterized by a marked presence of comorbid depressive disorders. Furthermore, we employed a diagnostic procedure with good test-retest reliability, validity, and inner rater reliability in the differentiation between primary and substance-induced MDD [32].
Conclusions
Patients with dual depression have worse clinical evolution and worse therapeutic response. Namely, the efficacy of antidepressant medication and/or psychological therapies in patients with AUD or CUD and comorbid MDD can be influenced by the type of depression (i.e. primary and substance-induced MDD) (DSM-IV-TR). However, there are no biological substrates that can reveal the difference between both mental conditions for a better clinical management of these patients. The present study represents an attempt at the identification of inflammatory biomarkers for the differentiation between primary and substance-induced disorders. Although our findings indicate that plasma concentrations of chemokine CCL11 could act as a potential biological marker to differentiate between primary and substance-induced MDD in CUD patients, further clinical research is necessary to confirm the role of inflammatory mediators in dual depression.
Supporting information
Dataset includes: Sex (man/woman); SUD (no/yes), SUD type (no/AUD/CUD), MDD (no/yes), MDD type (no/primary MDD/induced MDD), and concentrations (pg/mL) of TNF-α, IL-β, CXCL12, CCL2, CCL11 and CX3CL1.
(PDF)
Acknowledgments
The authors are grateful to J. Mateus, R. Rodríguez-Minguela, E. Menoyo, M. Pérez, S. Martín, C. Gibert and G. Rubio for their valuable assistance throughout the clinical part of the study. The authors thank the participants and the psychiatry research support staff for their generosity and interest, which made this study possible.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by RETICS Red de Trastornos Adictivos (RD12/0028/0021, RD12/0028/0009, RD16/0017/0001, RD16/0017/0010, and RD 16/0017/003) funded by Instituto de Salud Carlos III (ISC-III) and European Regional and European Regional Development Funds-European Union (ERDF-EU); Research projects funded by Ministerio de Economía y Competitividad and ISC-III (PI09/02121; PI12/01838, PI16/01698, PI16/01953); Research project funded by Ministerio de Sanidad, Servicios Sociales e Igualdad and Plan Nacional sobre Drogas (043/2017 and 2012I054); Research project funded by Consejería de Economía, Innovación y Ciencia, Junta de Andalucía and ERDF-EU (CTS-433); Suport Grups de Recerca AGAUR Gencat 2017 SGR 316 and SGR530). NGM received a training research grant funded by Consejería de Salud y Bienestar Social, Junta de Andalucía (EF-0202-2017); AS and FJP hold a Miguel Servet research contract funded by ISC-III and ERDF-EU (CP14/00173 and CP14/00212, respectively); PA received a Plan Propio grant from Universidad de Málaga (Incorporación Doctores CI-17-415). These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
References
- 1.Pettinati HM, O'Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. Am J Psychiatry. 2013;170(1):23–30. Epub 2012/12/12. 10.1176/appi.ajp.2012.12010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samet S, Fenton MC, Nunes E, Greenstein E, Aharonovich E, Hasin D. Effects of independent and substance-induced major depressive disorder on remission and relapse of alcohol, cocaine and heroin dependence. Addiction. 2013;108(1):115–23. Epub 2012/07/11. 10.1111/j.1360-0443.2012.04010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrens M, Mestre-Pinto JI, Montanari L, Vicente J, Domingo-Salvany A. Dual diagnosis: an European perspective. Adicciones. 2017;29(1):3–5. Epub 2017/02/09. 10.20882/adicciones.933 . [DOI] [PubMed] [Google Scholar]
- 4.APA. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision Washington, DC: American Psychiatric Association; 2000;75:78–85. [Google Scholar]
- 5.APA. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Association; 2013. [DOI] [PubMed] [Google Scholar]
- 6.WHO. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research: World Health Organization; 1993. [Google Scholar]
- 7.Tirado Munoz J, Farre A, Mestre-Pinto J, Szerman N, Torrens M. Dual diagnosis in Depression: treatment recommendations. Adicciones. 2018;30(1):66–76. Epub 2017/05/12. 10.20882/adicciones.868 . [DOI] [PubMed] [Google Scholar]
- 8.Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8(11):895–903. Epub 2007/10/20. 10.1038/nrn2255 . [DOI] [PubMed] [Google Scholar]
- 9.Teixeira AL, Gama CS, Rocha NP, Teixeira MM. Revisiting the Role of Eotaxin-1/CCL11 in Psychiatric Disorders. Front Psychiatry. 2018;9:241 Epub 2018/07/03. 10.3389/fpsyt.2018.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210(2):349–58. Epub 2008/01/15. 10.1016/j.expneurol.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, Garcia-Marchena N, et al. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict Biol. 2015;20(4):756–72. Epub 2014/05/24. 10.1111/adb.12156 . [DOI] [PubMed] [Google Scholar]
- 12.Pedraz M, Araos P, Garcia-Marchena N, Serrano A, Romero-Sanchiz P, Suarez J, et al. Sex differences in psychiatric comorbidity and plasma biomarkers for cocaine addiction in abstinent cocaine-addicted subjects in outpatient settings. Front Psychiatry. 2015;6:17 Epub 2015/03/13. 10.3389/fpsyt.2015.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maza-Quiroga R, Garcia-Marchena N, Romero-Sanchiz P, Barrios V, Pedraz M, Serrano A, et al. Evaluation of plasma cytokines in patients with cocaine use disorders in abstinence identifies transforming growth factor alpha (TGFalpha) as a potential biomarker of consumption and dual diagnosis. PeerJ. 2017;5:e3926 Epub 2017/10/19. 10.7717/peerj.3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui C, Shurtleff D, Harris RA. Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol. 2014;118:1–12. Epub 2014/09/02. 10.1016/B978-0-12-801284-0.00001-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13(5):398–405. Epub 2011/07/28. 10.1007/s11920-011-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera MI, Kolliker-Frers R, Barreto G, Blanco E, Capani F. Glial Modulation by N-acylethanolamides in Brain Injury and Neurodegeneration. Frontiers in aging neuroscience. 2016;8:81 Epub 2016/05/21. 10.3389/fnagi.2016.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueston CM, Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav. 2014;124:77–91. Epub 2013/11/05. 10.1016/j.physbeh.2013.10.035 . [DOI] [PubMed] [Google Scholar]
- 18.Mora C, Zonca V, Riva MA, Cattaneo A. Blood biomarkers and treatment response in major depression. Expert Rev Mol Diagn. 2018. Epub 2018/04/28. 10.1080/14737159.2018.1470927 . [DOI] [PubMed] [Google Scholar]
- 19.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. Epub 2000/06/03. 10.1146/annurev.immunol.18.1.217 . [DOI] [PubMed] [Google Scholar]
- 20.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70(8):1078–90. Epub 2009/06/06. 10.4088/JCP.08r04505 . [DOI] [PubMed] [Google Scholar]
- 21.Acosta SA, Diamond DM, Wolfe S, Tajiri N, Shinozuka K, Ishikawa H, et al. Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS One. 2013;8(12):e81585 Epub 2013/12/19. 10.1371/journal.pone.0081585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira AL, Reis HJ, Nicolato R, Brito-Melo G, Correa H, Teixeira MM, et al. Increased serum levels of CCL11/eotaxin in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):710–4. Epub 2007/12/22. 10.1016/j.pnpbp.2007.11.019 . [DOI] [PubMed] [Google Scholar]
- 23.Stuart MJ, Singhal G, Baune BT. Systematic Review of the Neurobiological Relevance of Chemokines to Psychiatric Disorders. Front Cell Neurosci. 2015;9:357 Epub 2015/10/07. 10.3389/fncel.2015.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, et al. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev Bras Psiquiatr. 2012;34(1):71–5. Epub 2012/03/07. . [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K. Inflammatory Biomarkers as Differential Predictors of Antidepressant Response. International Journal of Molecular Sciences. 2015;16(4):7796 10.3390/ijms16047796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WQ, Smolik CM, Barba-Escobedo PA, Gamez M, Sanchez JJ, Javors MA, et al. Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology. 2015;90:1–8. 10.1016/j.neuropharm.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trecki J, Unterwald EM. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience. 2009;161(1):13–22. Epub 2009/03/24. 10.1016/j.neuroscience.2009.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44(2):115–27. Epub 2008/10/23. 10.1093/alcalc/agn079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Hutchinson MR, White JM, Somogyi AA, Coller JK. Association of IL-1B genetic polymorphisms with an increased risk of opioid and alcohol dependence. Pharmacogenetics and genomics. 2009;19(11):869–76. Epub 2009/10/06. 10.1097/FPC.0b013e328331e68f . [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Marchena N, Araos PF, Barrios V, Sanchez-Marin L, Chowen JA, Pedraz M, et al. Plasma Chemokines in Patients with Alcohol Use Disorders: Association of CCL11 (Eotaxin-1) with Psychiatric Comorbidity. Front Psychiatry. 2016;7:214 Epub 2017/02/06. 10.3389/fpsyt.2016.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes EV, Liu X, Samet S, Matseoane K, Hasin D. Independent versus substance-induced major depressive disorder in substance-dependent patients: observational study of course during follow-up. J Clin Psychiatry. 2006;67(10):1561–7. Epub 2006/11/17. . [DOI] [PubMed] [Google Scholar]
- 32.Torrens M, Serrano D, Astals M, Perez-Dominguez G, Martin-Santos R. Diagnosing comorbid psychiatric disorders in substance abusers: validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am J Psychiatry. 2004;161(7):1231–7. Epub 2004/07/02. 10.1176/appi.ajp.161.7.1231 . [DOI] [PubMed] [Google Scholar]
- 33.Hasin D, Samet S, Nunes E, Meydan J, Matseoane K, Waxman R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. Am J Psychiatry. 2006;163(4):689–96. Epub 2006/04/06. 10.1176/ajp.2006.163.4.689 . [DOI] [PubMed] [Google Scholar]
- 34.Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. Epub 2014/05/23. S0306-4530(14)00115-2 [pii] 10.1016/j.psyneuen.2014.03.019 . [DOI] [PubMed] [Google Scholar]
- 35.Crews FT. Immune function genes, genetics, and the neurobiology of addiction. Alcohol Res. 2012;34(3):355–61. Epub 2012/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362 Epub 2014/11/19. 10.3389/fncel.2014.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. Epub 2016/03/21. S0889-1591(16)30055-1 [pii] 10.1016/j.bbi.2016.03.010 . [DOI] [PubMed] [Google Scholar]
- 38.Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5(1):83–91. Epub 2009/12/19. 10.1007/s11481-009-9185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jankowski MM, Ignatowska-Jankowska B, Glac W, Swiergiel AH. Cocaine administration increases CD4/CD8 lymphocyte ratio in peripheral blood despite lymphopenia and elevated corticosterone. Int Immunopharmacol. 2010;10(10):1229–34. Epub 2010/07/20. 10.1016/j.intimp.2010.07.003 . [DOI] [PubMed] [Google Scholar]
- 40.Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, et al. Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J Pharmacol Exp Ther. 2007;320(2):507–15. Epub 2006/10/28. 10.1124/jpet.106.112797 . [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Egea E, Scoriels L, Theegala S, Giro M, Ozanne SE, Burling K, et al. Cannabis use is associated with increased CCL11 plasma levels in young healthy volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:25–8. Epub 2013/07/04. 10.1016/j.pnpbp.2013.06.011 . [DOI] [PubMed] [Google Scholar]
- 42.Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom. 2007;72(5):408–15. Epub 2007/02/03. 10.1002/cyto.b.20169 . [DOI] [PubMed] [Google Scholar]
- 43.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. Epub 2010/10/26. 10.1016/j.bbi.2010.10.015 . [DOI] [PubMed] [Google Scholar]
- 44.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. Epub 2009/12/18. 10.1016/j.biopsych.2009.09.033 . [DOI] [PubMed] [Google Scholar]
- 45.Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, et al. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur Neuropsychopharmacol. 2008;18(3):230–3. Epub 2007/08/08. 10.1016/j.euroneuro.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oglodek EA, Szota A, Just MJ, Mos D, Araszkiewicz A. Comparison of chemokines (CCL-5 and SDF-1), chemokine receptors (CCR-5 and CXCR-4) and IL-6 levels in patients with different severities of depression. Pharmacol Rep. 2014;66(5):920–6. Epub 2014/08/26. 10.1016/j.pharep.2014.06.001 . [DOI] [PubMed] [Google Scholar]
- 47.Miranda DO, Anatriello E, Azevedo LR, Santos JC, Cordeiro JFC, Peria FM, et al. Fractalkine (C-X3-C motif chemokine ligand 1) as a potential biomarker for depression and anxiety in colorectal cancer patients. Biomed Rep. 2017;7(2):188–92. Epub 2017/08/15. 10.3892/br.2017.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettcher BM, Fitch R, Wynn MJ, Lalli MA, Elofson J, Jastrzab L, et al. MCP-1 and eotaxin-1 selectively and negatively associate with memory in MCI and Alzheimer's disease dementia phenotypes. Alzheimers Dement (Amst). 2016;3:91–7. Epub 2016/07/28. 10.1016/j.dadm.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, et al. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res. 2014;48(1):13–5. Epub 2013/11/05. 10.1016/j.jpsychires.2013.10.007 . [DOI] [PubMed] [Google Scholar]
- 50.Myung W, Lim SW, Woo HI, Park JH, Shim S, Lee SY, et al. Serum Cytokine Levels in Major Depressive Disorder and Its Role in Antidepressant Response. Psychiatry Investig. 2016;13(6):644–51. Epub 2016/12/03. 10.4306/pi.2016.13.6.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller B, Mestre-Pinto J-I, Álvaro-Bartolomé M, Martinez-Sanvisens D, Farre M, García-Fuster MJ, et al. A Biomarker to Differentiate between Primary and Cocaine-Induced Major Depression in Cocaine Use Disorder: The Role of Platelet IRAS/Nischarin (I1-Imidazoline Receptor). Frontiers in psychiatry. 2017;8:258 10.3389/fpsyt.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset includes: Sex (man/woman); SUD (no/yes), SUD type (no/AUD/CUD), MDD (no/yes), MDD type (no/primary MDD/induced MDD), and concentrations (pg/mL) of TNF-α, IL-β, CXCL12, CCL2, CCL11 and CX3CL1.
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.