Abstract
During the past decade, a conceptual shift occurred in the field of Alzheimer’s disease (AD) considering the disease as a continuum. Thanks to evolving biomarker research and substantial discoveries, it is now possible to identify the disease even at the preclinical stage before the occurrence of the first clinical symptoms. This preclinical stage of AD has become a major research focus as the field postulates that early intervention may offer the best chance of therapeutic success. To date, very little evidence is established on this “silent” stage of the disease. A clarification is needed about the definitions and lexicon, the limits, the natural history, the markers of progression, and the ethical consequence of detecting the disease at this asymptomatic stage. This article is aimed at addressing all the different issues by providing for each of them an updated review of the literature and evidence, with practical recommendations.
Keywords: Alzheimer’s disease, Preclinical, Asymptomatic, Diagnostic criteria, Biomarkers, Pathophysiology, Genetics, CSF biomarkers, Blood biomarkers, MRI, Amyloid PET, Tau PET
The preclinical Alzheimer’s disease (AD) concept emerged in the late 20th century initially defined as cognitively unimpaired individuals who displayed AD brain lesions on postmortem examination [1]. With the development of AD pathologic markers, the concept evolved and preclinical AD are now considered when these markers are present in cognitively normal individuals. However, the challenges to provide a unified definition for cognitive health, for cognitive decline, and for the best signature of in vivo AD pathology remain to be resolved. The great heterogeneity of methodologies used in different studies referring to different definitions of preclinical AD has created confusion. Standardizing of these definitions is important to future AD research.
In the last decade, a conceptual shift has occurred in the field of AD with a new diagnostic framework having been developed. Associated with this scheme is a new disease model that begins with risk factor assessment (directed at the potential for primary prevention), advances to screening (for early detection and early intervention of disease—secondary prevention), proceeds through diagnosis and staging, and leads to treatments and monitoring of treatment effects. This approach includes screening with tests with high sensitivity, lower specificity, and low cost, to those with higher specificity and value with potential for longitudinal quantification. Individuals enter into the algorithm at different points according to the manner in which they present clinically. Broadly, this can be divided into those who are asymptomatic and those who are symptomatic [2]. The former provides the basis for preventive approaches.
This new approach of AD mainly results from unprecedented growth of interest in elucidating the preclinical stage. Individuals can now be identified as being in the preclinical state by the in vivo evidence of Alzheimer pathology (AP), for example, by a biological or molecular “signature” of AD. The existence of AP biomarkers in preclinical AD has been validated through the study of presymptomatic autosomal dominant mutation carriers where AD pathophysiological markers have been analyzed in the cerebrospinal fluid (CSF) or in brain with amyloid positron emission tomography (PET) imaging [3].
CSF tau changes have been shown to occur ~15 years before the onset of clinical AD [3,4], and decline in CSF Ab42 is extrapolated within subject longitudinal analysis up to 20 years before symptom onset. This construct has also been validated in those asymptomatic at risk for clinical AD (AR-AD), where altered CSF levels of AP biomarkers or brain amyloidopathy as seen on PET can precede the occurrence of the prodromal or dementia stage by several years [5]. These data are in agreement with postmortem evidence of the presence of AP in cognitively normal individuals [6]. Based on both in vivo and postmortem evidence, a hypothetical model outlining potential dynamic changes and a temporal ordering of AD biomarker classes have been postulated [7]. According to this model, lowering of CSF Aβ42 followed by abnormal brain amyloid tracer uptake is expected to precede the presence of biomarkers of neuronal injury, regional structural brain changes, and ultimately clinical changes such as decline of memory and cognitive functions with a significant impact on activities of daily living. This preclinical AD stage is important for studies aimed at prevention of progression to the clinical state, as well as for research into novel bio-markers that might verify therapies with early disease modification. A better understanding of the natural history of the preclinical stage, the evolution of pathophysiology and structural brain alterations, the influencing factors (i.e., triggers) of disease progression, and related ethical issues is needed.
1. Issue 1—the definition of the preclinical stage of AD
The fact that the disease process starts many years before the development of symptoms and that effective interventions could be initiated at this time in the future, make the definition of the preclinical stage necessary. Conceptually, the definition of preclinical AD would theoretically span from the first neuropathologic brain lesions to the onset of the first clinical symptoms of AD. In practice, however, these boundaries are challenging. Although there is a seamless continuum between the different AD states, the definition of the clinical onset of the disease requires attention as it relates most specifically to the development of preventive strategies. Another key issue in relationship to the preclinical stage is the definition of AD. A particular point of contention is whether AD should be defined by the expression of the clinical symptoms such as the first cognitive changes or only by the presence of AP biomarkers including CSF and or PET even in absence of any clinical symptoms. How this issue is addressed is of considerable importance to therapeutic development strategies.
1.1. Classification and stratification of preclinical states of AD
Different classifications of preclinical states have been proposed. The international working group (IWG) has defined two different preclinical states: the presymptomatic and the asymptomatic at risk state [8]. The entity named pre-symptomatic AD recognizes the fact that some individuals are virtually destined to develop full clinical AD, because they are known to carry an autosomal dominant monogenic mutation. The disease, whatever its stage, can be diagnosed with the identification of the mutation. An “asymptomatic at risk” state is more controversial. To be classified as asymptomatic at risk, by definition, individuals must not have clinical evidence of prodromal AD. According to the recent IWG revision, preclinical states of AD require the absence of clinical signs and symptoms of AD (both typical or atypical phenotypes) and the presence of at least one biomarker of Alzheimer’s pathology.
A staging classification for the asymptomatic at risk state may also be considered aiming at stratifying patients on the basis of biomarkers. The National Institute on Aging/Alzheimer Association (NIA/AA) approach, based on the biomarker model of Jack [9,10] has proposed two hypothetical subgroups and/or stages as a function of the biomarker profile: stage 1 showing in vivo evidence of amyloidosis in the brain by either PET or CSF biomarkers and stage 2 showing in vivo evidence of both amyloidosis and neurodegeneration. The proposed staging classification introduces, for stage 1, a slight deviation from the IWG2 rule that requires the coexistence of tau (p- or t-tau) changes in the CSF for corroborating Alzheimer pathology [11]. The latter decreases the sensitivity of the definition but reinforces the diagnostic accuracy by certifying the existence of an ongoing neurodegenerative process of AD. In addition, the model is largely conceptualized from cross-sectional observations in autosomal dominant AD (ADAD) subjects [3]. When applied to sporadic AD, these stages may not reflect the heterogeneity of this form of AD. There are circumstances where pathologic tau hyperphosphorylation and the related neurodegeneration process begin ahead of brain amyloidopathy (tau first) [12,13]. The multiplicity of hypothetical models is useful to capture the phenotypic variants of AD that are likely to result from different pathogenic processes (e.g., different strains of Aß [14,15]; mixed pathology [especially in elderly subjects][16]; different genetic backgrounds [17–19]; and different relevant risk factors of AD [20]).
Another staging classification can be determined based on a low and/or high risk dichotomy to further develop clinical AD. The risk—defined as the probability for a patient to develop the clinical symptoms in the rest of his or her lifetime—is due to, on the one hand, how fast the patient is progressing (which is determined by established risk-enhancing modifiable or nonmodifiable factors, such as age, modifying genes, cognitive reserve, comorbidities, and so forth) and, on the other hand, how advanced the subject and/or patient is on his/her curve of progression (stage of biomarker expression). In this regard, for APOE ε4 homozygotes (ε4/ε4) cognitively normal elderly individuals are at very high risk to develop clinical AD and present a particularly meaningful target population for research projects on asymptomatic at risk state. Observational studies are needed to better know the influencing factors (factors of prevention and risk factors) that may determine the staging of risk. Today, the combination of CSF or PET Aβ and tau biomarkers may best stratify low-risk and high-risk individuals [21], but imaging markers of Aβ and tau provide additional information about spatial distribution and longitudinal changes (particularly for tau PET) that would allow an “in vivo” staging of AD pathology [22]. This would facilitate the selection of a biomarker “threshold” of AD changes that can be tailored to individual studies. For instance, observational studies or low-risk interventions (e.g., lifestyle modifications such as exercise or diet) may select individuals with low levels of AD pathology, whereas interventions that involve greater cost or risk or require short-term cognitive changes as an outcome, may select individuals with intermediate or greater AD biomarker changes.
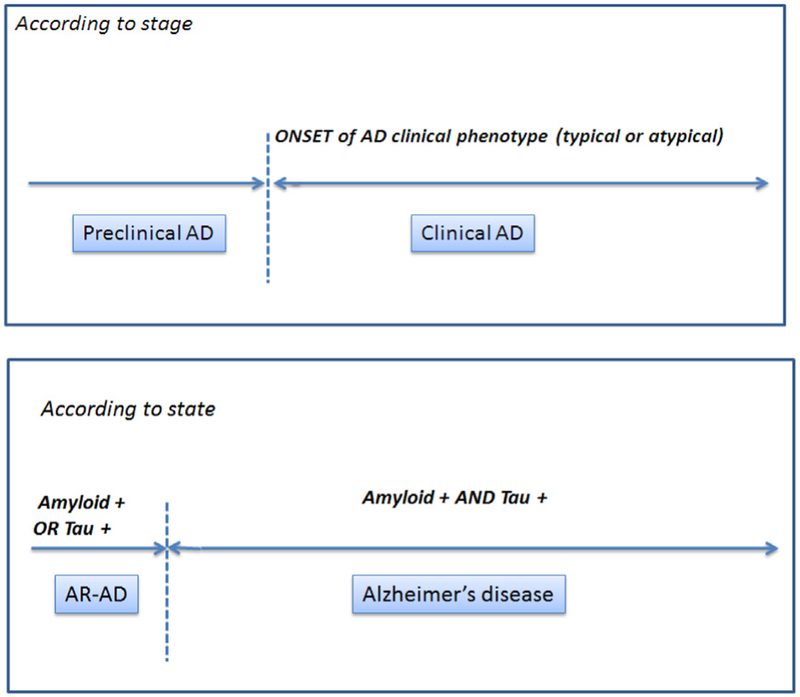
In summary, we propose an arbitrary dichotomy between: (1) an already developed AD pathology evidenced by the co-occurrence of amyloid and tau pathology (that can be inferred in vivo with the use of pathophysiological biomarkers), whatever the stage (preclinical stage or symptomatic/prodromal and dementia stage); and (2) a situation at risk of AD mainly in asymptomatic individuals exhibiting an isolated brain amyloidopathy (asymptomatic A+) or tauopathy (asymptomatic T+; Fig 1 and Table 1). This reflects the separation between on one hand the disease AD and on the other hand a risk factor. The contribution of neurodegeneration and/or topographic biomarkers (magnetic resonance imaging [MRI] and FDG PET) in this framework of asymptomatic individuals, and their relationships with tauopathy remain to be determined in longitudinal studies on cognitively healthy elderly individuals.
Fig. 1.
Proposal for a unified lexicon for preclinical AD. Abbreviation: AD, Alzheimer’s disease.
Table 1.
Toward a unified conception of preclinical AD
| Proposed definition | NIA-AA, 2011 | IWG-2, 2014 | Proposed criteria, 2016 |
|---|---|---|---|
| AD starts | |||
| With the first brain lesion | + | ||
| With the first symptom of AD | + | ||
| When there is evidence of Aß and Tau pathology |
+ | ||
| Preclinical AD can be detected in asymptomatic individuals | |||
| When there is evidence of Aß pathology | + (stage 1) | + (PET) | |
| When there is evidence of Aß and Tau pathology |
+ (stage 2)* | + (CSF) | + |
| Asymptomatic at risk for AD can be detected in cognitively normal individuals | |||
| When there is evidence of Aß pathology (“Asymptomatic A+”) OR evidence of Tau pathology (“Asymptomatic T+”) |
+ | ||
Abbreviations: AD, Alzheimer’s disease; NIA-AA, National Institute on Aging/Alzheimer Association; IWG, international working group.
NOTE. The criteria now stipulate that the Aß+ group (A+) is asymptomatic at risk for AD, whereas the Aß+/Tau+ group (A+, T+) is considered as having preclinical AD.
In the NIA-AA criteria, markers on neurodegeneration (i.e., brain atrophy on MRI or hypo-metabolism on FDG PET) were also considered instead of tau markers to diagnose preclinical AD.
Recommendation—
Based on the high-risk or low-risk dichotomy for a further progression to clinical AD, we propose to consider the terms of “preclinical AD” when the risk is particularly high (e.g., both Aß and Tau markers beyond pathologic thresholds) and that of AR-AD when the evolution to a clinical AD is less likely or still needs to be determined (only one pathophysiological marker considered abnormal).
1.2. The starting point of Alzheimer’s disease
It has been proposed that the presence of at least one marker of brain amyloidosis in CSF or PET in cognitively normal individuals may be sufficient to establish the diagnosis of AD, even in the absence of any clinical manifestations [21,23,24]. In line with this consideration, any individual with brain amyloidosis (as defined by currently defined thresholds) might be treated with disease-modifying drugs in the future although there is no definitive evidence that all these individuals will eventually develop the disease at a later time. An alternative consideration is that amyloidosis is at least necessary and obligatory for an AD diagnosis, but not sufficient to reliably predict further progression to a symptomatic stage of disease. Based on postmortem evidence, there is a significant proportion of individuals with AP in their brain sufficient to meet neuropathologic diagnostic criteria who did not have evidence of clinically expressed disease antemortem [25].
Defining disease start is important in light of preventive intervention. Thus, it is important to ascertain what proportion of cognitively healthy individuals, with positive AP bio-markers, will progress to the clinical state of AD. There are data indicating that the speed of progression is variable from one subject to the next: elderly patients with a predominant limbic form of the disease have generally a less-aggressive disease dynamic compared to the neocortical form with hippocampal sparing type that can present in younger patients particularly with focal cognitive signs [26]. The concept of “cognitive reserve” has been proposed to describe the apparently good tolerance (or perhaps resistance) of developing neuropathologic lesions in some individuals.
To summarize
Studies on the evolution of preclinical AD may hold the key to determining whether and when there is sufficient AP to know that clinical evolution is inevitable.
At present, the data are insufficient to ascertain that the presence of an isolated brain amyloidopathy implies the further development of clinical AD, although it is highly related. There is a need for further data on outcome of individuals at a preclinical stage with amyloid positive PET scans and to address the long-term progression to a clinically symptomatic state.
By contrast, the additional presence of a biomarker of tau pathology is associated with a more rapid progression to clinical AD (Vos et al, 2013 Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study). Therefore, AD can be certified and defined by positive markers of both tauopathy and amyloidopathy, recognizing that in the future amyloid positivity may turn out to be sufficient to define preclinical AD. An agnostic descriptive AP biomarker nomenclature, for example, brain amyloidopathy + (A+), brain tauopathy + (T+)[27] may assist in framing this possibility.
AD becomes symptomatic when neuronal lesions reach a given threshold and within a unique host susceptibility likely modulated by their genetic and epigenetic factors, other brain injuries, and other resilience factors.
Recommendations concerning the definition of AD—
We may consider that AD exists and can be recognized before the onset of cognitive symptoms when there is little doubt about progression to clinical disease over a short period. This is the case when both tauopathy (tau PET ligand uptake spread to neocortex or CSF) and amyloidopathy (PET evidence of AD pattern or CSF measured) are present. Isolated low CSF levels of Aβ or high PET retention define only amyloidosis, which is a risk factor for AD. PET Tau retention in the medial temporal lobe (MTL), in the absence of Aβ changes, does not allow the diagnosis of AD. Only the association of both pathologic hallmarks defines AD even in the absence of cognitive symptoms.
1.3. The starting point of AR-AD
Notwithstanding the heterogeneity of sporadic AD, physiological age-related alterations have to be separated from pathologic changes occurring in the brain that are caused by the underlying AD process. This is complicated by the fact that there is likely an apparent dynamic and overlapping “continuum” between AD and aging. For instance, neurofibrillary tangles are the first neuropathologic hallmarks to appear in AD in the MTL, that is, transentorhinal and entorhinal cortex and the hippocampus. These lesions, however, also accumulate in MTL in aging as well as shown both in postmortem studies, in which almost all individuals have some evidence of tauopathy [28,29]. Early tau PET imaging studies support this neuropathologic observation [30]. These changes were recently designated as primary age-related tauopathy [31]. This issue raises the question of the exact point in time when Alzheimer pathology does actually start developing. No specific aspect of the tau pathology of the MTL makes it possible to predict either a limited progression or the development of AD [32]. Amyloid deposition in the brain seems to have a closer link to AD-related pathophysiology and may be a better disease marker though it too has an increasing prevalence with age on both PET and neuropathology. Its age-related increase advances to a point where it is nearly always described to some extent in healthy normal individuals on postmortem analysis [25,33]. If we define the disease as starting with the appearance of the brain lesions, the prevalence of the disease, based on neuropathologic evidence, would excessively increase as almost all postmortem assessment >70 years shows both types of AD brain lesions [34]. Pathologic criteria have tried to avoid this bias by establishing thresholds for the number of lesions needed to establish the diagnosis of AD [35,36].
Recommendation—
An at “risk state” can be identified before AD. AR-AD starts with the presence of either brain amyloidopathy or of an isolated tau pathology spreading outside the MTL. It is not established today that, when isolated, either of these changes are sufficient to certify AD in a preclinical stage.
1.4. Comparison of asymptomatic at risk (sporadic) and presymptomatic (genetic) states
AD in general has a multifaceted pathophysiology displaying a highly complex genetic heterogeneity. This implies both that the same phenotype can be generated or altered by a number of different genetic loci and alleles and that mutations or polymorphisms at different positions in the same gene result in the same clinical syndrome [17]. In addition, different mutations in the same gene can result in clinically distinct syndromes (phenotypes). For these reasons, AD is defined as a “genetically complex” disease.
AD can be expressed with both the existence of (1) dominantly inherited and (2) nondominantly inherited forms of the disease. The former, owing to a mutation in amyloid precursor protein (APP, located at chromosome region 21q21.2), presenilin 1 (PSEN1, located at 14q24.3) or presenilin 2 (PSEN2, located at 1q42.13), is referred to as familial AD (fAD). fAD accounts for <1% of all AD cases and presents as classic Mendelian ADAD, often with early (<65 years) age of onset. The latter, commonly defined as “sporadic” AD (sAD) as it does not always display obvious familial aggregation, accounts for >99% of all AD cases [17,37]. These individuals develop late-onset AD (LOAD), which usually presents after 65 years. The rare form of dominantly inherited fAD has been traditionally considered as a model of AD mechanisms that may underlie the much more common sAD. Presymptomatic mutation carrier individuals may provide important clues on biomarkers associated with the preclinical state of the disease. They are also of potential utility to investigate the efficacy of disease-modifying agents in delaying the clinical onset of the disease as it is possible to estimate when the clinical signs of the disease will appear based on the family history of the carriers. It remains uncertain whether it is possible to transpose data derived from familial AD on the whole spectrum of sporadic AD. Familial cases of the disease appear to have the same clinical and pathologic phenotypes as sporadic cases. Notably, a study designed to investigate the existence of specific differences in the clinical features of fAD and sAD revealed that—apart from the age of onset, where a positive family history of dementia was associated with an earlier beginning—no major differences in terms of clinical phenotype—including rate of cognitive decline, duration of illness, and presence of non-cognitive symptoms—were reported between fAD patients compared to patients with sporadic disease [38,39]. In another study, AD patients were examined to test the hypothesis that cases with a familial aggregation differ from cases without such an aggregation with reference to cognitive impairment. After evaluating cognitive function, the results did not yield statistically significant differences between the two groups for any of the neurocognitive domains analyzed. Therefore, the hypothesis that the presence of a familial aggregation might result in a distinct phenotype in AD was not confirmed [40]. The neuropathology of fAD also appears to be similar to that of sporadic cases [41]. In a study by Nochlin et al. [42], the density of neurofibrillary tangles and senile plaques was compared in different cortical areas, the amygdala, the hippocampus, the parahippocampal gyrus, and the cerebellum in fAD patients and sAD patients with early, intermediate, and late ages of onset of dementia and in age-matched controls. In all brain regions, cases showed more severe alterations than controls. Of note, no substantial differences were observed in the severity scores of neurofibrillary tangles and senile plaques between fAD and sAD. An inverse correlation between age of onset of dementia and the density of neurofibrillary tangles and senile plaques was reported in all regions in fAD and sAD combined. However, the pathophysiological mechanisms underlying amyloid accumulation may differ, with overproduction of Aβ42 in fAD and reduced clearance of Aβ42 in sAD. In summary, no compelling indication that the neuropathologic features of fAD differ from those of sAD was found.
In conclusion, the two general genetic conditions of the disease—namely, mutation carrier variants and sporadic forms—appear to be comparable, and we may consider that most of the data drawn from fAD, except in the genetic domain, can be translated to sAD. The absence of comorbid aging changes, more rapid progression, and age-determined pathology abundance may affect trial design and treatment approaches for fAD subjects.
Recommendation—
Although the final outcomes (i.e., the neuropathologic and clinical presentation) of sAD and fAD seem to be similar, the pathophysiology of Aβ accumulation may differ. Therefore, an extrapolation of drug efficacy from one model to the other should be considered with caution.
2. Issue 2—the biomarkers required for identifying preclinical AD
Several biomarkers are currently proposed as indicators of the “asymptomatic at risk” state candidates. These bio-markers have recently divided into (1) pathophysiological/diagnostic markers, reflecting AD pathology at any point on the disease continuum and (2) topographical and/or prognostic markers, reflecting “downstream” damage [11].
The pathophysiological markers of AD are those indicating the specific presence of tau pathology (CSF or PET tau) and amyloid pathology (CSF Aβ42 or PET amyloid), whereas the topographical markers include volume changes in the brain (hippocampal atrophy, cortical thickness, and others…) assessed by MRI and hypometabolism of neocortical regions measured by fluorodeoxyglucose (FDG)—PET. The consideration of how these biomarkers can be used to define preclinical AD requires further consideration.
2.1. Topographical markers
Topographical markers alone are insufficient for identifying the presence of preclinical AD stage, although functional changes have been reported to occur in the brains of healthy subjects who are biomarker positive. This is the case for both FDG-PET, displaying a regional hypometabolism in temporo-parietal or in precuneus cortical areas [43], and fMRI, showing changes in functional connectivity between specific brain regions [44,45]. Although such alterations are suggestive of an ongoing pathologic process, there is no way to ascertain that this downstream process corresponds to AD pathology in asymptomatic at-risk individuals. The same consideration may apply for MRI-related morphologic changes (cortical thinning, enlargement of sulci, hippocampal atrophy), as it has been recognized that these alterations generally occur later in the continuum of AD pathology and that they are not specific for AD. However, the identification of a specific network of atrophy involving different connected areas by automated classifier may turn out to be an accurate and early marker of AD. This needs to be demonstrated.
Recommendation—
Downstream topographical bio-markers (MRI and FDG-PET) are not suitable for defining preclinical AD. However, they may be useful for the screening of subjects at risk.
2.2. Pathophysiological biomarkers
AD is conceptually defined by the presence of biomarkers of AD pathology. It remains to be shown that this holds true for the preclinical stage of AD. A recent meta-analysis estimated the prevalence of Aβ biomarker positivity (as defined by either CSF Aβ42 or amyloid PET) in nearly 3000 cognitively normal individuals [46]. The prevalence of amyloid positivity was estimated at 10.4% at age 50 years, increasing by 3%–5% every 5 years of life to an estimated 43.8% at age 90 years. Amyloid positivity correlated very strongly with presence of the APOE ε4 allele, with ε4 carriers 2–3 times more likely to show positive amyloid biomarkers than noncarriers in each age stratification (e.g., 47.9% of ε4 carriers were Aβ+ at age 75 years vs 17.1% of noncarriers).
2.2.1. CSF changes in preclinical AD
Longitudinal studies in cognitively healthy individuals showed a correlation between baseline levels of CSF bio-markers and further development of prodromal AD for Aβ42 alone [47] or in combination with total tau (T-tau) [48] or phospho-tau (P-tau) [49]. Results in preclinical PSEN1 mutation carriers [50] or subjects with subjective memory complaints show that low levels of Aβ42 are the best indicator of progression in asymptomatic at-risk patients [50,51]. In addition, cognitively healthy subjects having only low concentrations of Aβ42 show significant changes in cortical thickness [52] and changes in the resting state network as shown with fMRI [53]. Taken together, these data suggest that at the beginning of the preclinical state, amyloid may be the first positive marker, as proposed by Jack et al. [13]. Other studies showed that in subjects with low-CSF Aβ42, the additional presence of MRI or CSF tau alterations was indicative of faster progression to a clinical state [54–56]. Furthermore, a recent large meta-analysis on cross-sectional data showed that Aβ deposition occurs in an age-dependent fashion and also inferred a 20- to 30-year interval between first development of amyloid positivity and onset of dementia [46]. This would put CSF Aβ42 alone in the category for best “state” marker for AR-AD. From the tau protein perspective, one study showed that alterations in CSF levels of p-tau may precede those of Aβ [57], which correlates with postmortem evidence showing the presence of neurofibrillary tangles in the entorhinal cortex and in hippocampal-related structures [58,59] as the first neuropathologic event in AD. This suggests that in AD, CSF tau changes may not only be considered as nonspecific (downstream) markers of neuronal death, as seen in other neurodegenerative diseases [60], but also as a more-specific pathophysiological marker of AD in relation to neurofibrillary tangles [61,62]. Based on the above considerations, the presence of both CSF biomarkers (increased levels of tau and low levels of Aβ42) significantly increases the specificity for the diagnosis of an “asymptomatic at risk” state, similar to the use in the clinical phase of the disease [63,64]. However, as changes in tau levels may generally occur at a later time, the selection of subjects based on the presence of isolated low-CSF Aβ levels for specific research purposes could be considered with the risk of a lower diagnostic accuracy.
2.2.2. Amyloid PET changes in preclinical AD
Amyloid PET has a high specificity for AD plaques. There is increasing evidence from PET-to-autopsy studies to suggest that amyloid PET is detecting “Consortium to Establish a Registry for AD” moderate-to-frequent neuritic plaques and is typically negative in individuals with absent to sparse plaques [65–69]. Fewer studies have correlated PET with Thal amyloid staging, suggesting earliest detection around stage 1 [66,70]. Overall, these studies suggest that amyloid PET is detecting early stages of amyloid deposition as defined by either spatial extent of any amyloid pathology or highest regional density of neuritic plaques. Quantification methods seem to provide a more efficient and objective measure for subject classification in research studies and clinical trials. The optimal quantitative threshold for early detection is highly dependent on the specific radiotracer used, as well as on the reference region applied to standardize measurements across subjects, and the pipeline used for image analysis [71]. A method for standardizing measurements to a uniform “Centiloid” scale has been recently proposed to facilitate comparisons between studies and across radiotracers [72]. Every threshold implicates the issue of a gray zone. Conversely, in clinical practice, amyloid PET is interpreted by visual reads. Each method has its own pitfalls and sources of error, and most studies have found high concordance between visual reads and quantitative classification (κ values ranging from 0.63–0.90 depending on the radiotracer and population [73–76]).
2.2.3. Comparing amyloid PET and CSF changes
Amyloid PET and CSF Aβ42 are measuring different aspects of amyloid biology: (1) fibrillar aggregates of Aβ for PET and (2) soluble Aβ42 monomer levels, which are only indirectly related to plaques, for CSF Aβ42. Despite these biological differences, CSF Aβ42 and amyloid PET are highly concordant when used to dichotomize individuals as amyloid positive or amyloid-negative, showing 80%–90% agreement across studies [77–80]. Discordant results in studies are more often attributable to positive-CSF Aβ42 with negative PET. When both CSFAβ42 and amyloid PET are included in the same models, amyloid PET appears to be more strongly predictive of cognitive decline and longitudinal brain atrophy, thus supporting the notion that PET is capturing more advanced pathology or that amyloid PET is a more precise measure [80]. Despite these differences, the Jansen meta-analysis did not find significant discrepancies in the estimation of prevalence of amyloid positivity across the lifespan when assessed by CSF versus PET [46]. Based on current literature, it is thus reasonable to conclude that amyloid positivity can be established using either CSF or amyloid PET. There is a modest evidence to support the notion that CSF may be more sensitive to the earliest stages of amyloid deposition, whereas by virtue of capturing more advanced pathology, amyloid PET may be more specific for detecting individuals who are truly on an AD trajectory.
2.2.4. Tau PET
AD is a dual proteinopathy defined pathologically by the deposition of both Aβ and tau. However, there is a debate on the significance of an in vivo evidence of tauopathy. When changes in tau proteins occur without depressed Aβ42, it constitutes a biomarker pattern of “suspected non-Alzheimer’s disease pathophysiology” (SNAP). Little is known about these tau-positive and/or amyloid-negative cognitively normal subjects. It may correspond, in most of the cases, to physiological aging and to non-AD pathologies as well (such as tauopathies, vascular brain disorders, repeated brain injury, and so forth) [46,56–58]. As discussed above, it may also be the first stage of AD pathology. Ongoing longitudinal studies indicate that a non-negligible percentage of subjects, classified as SNAP, will evolve to clinically diagnosed AD (5% in [21]). Although CSF hypothetically allows the assessment of both proteins in vivo, the lack of specificity of total-tau—and to a much less extent p-tau—measurements to AD, as well as the apparent limited dynamic range of CSF tau measurements for longitudinal change suggest that CSF tau is not a straightforward representation of brain neurofibrillary tangles (NFT) load. The recent emergence of multiple-candidate radiotracers for imaging tau pathology in vivo offers tremendous hope in terms of furthering our understanding of the pathogenesis of AD and of improving our ability to diagnose and treat the disease, including in the presymptomatic phase.
Tau PET is a rapidly evolving field. Although biochemically distinct, these putative tau tracers all share a high selectivity for tau-protein aggregates over aggregated Aβ in postmortem AD brain samples, and early in vivo binding patterns that correspond to Braak staging of NFTs across the aging-AD dementia continuum [22,81–85]. Early human studies show (even with small numbers) the expected correlations with cognitive state and brain atrophy [83,85,86]. The combination of Aβ and tau positivity on molecular imaging may prove to be a particularly powerful method to detect individuals who truly show the dual proteinopathy signature of AD in a preclinical stage. Beyond merely defining individuals as “positive” or “negative” for Aβ and tau, one can envision biomarker-based “staging” of AD neuropathologic changes, analogous to the NIA-AA neuropathologic criteria [87], based on the spatial extent of amyloid and tau tracers. For example, a cognitively normal individual with diffuse cortical amyloid tracer binding but tau PET binding restricted to MTL would be classified as asymptomatic A (+), whereas an individual with diffuse cortical amyloid tracer binding and tau PET binding extending outside the MTL would be classified as preclinical AD.
Provisional conclusion for pathophysiological bio-markers—
In vivo evidence of biomarkers of both tauopathy and amyloidopathy allows diagnosis of AD at preclinical stage. The existing literature confirms that the presence of an isolated amyloidopathy (in the brain or in the CSF) or tauopathy (in the brain) characterize only an asymptomatic at risk state for AD although we acknowledge that CSF tau changes may occur late in the preclinical stage.
2.3. Standardization operating procedures (SOPs), thresholds, and cut-off
In the absence of any clinical changes in asymptomatic subjects, the unique link with the underlying disease in a given individual is the evidence of score in biomarkers above the reference threshold. Therefore, the validity of the measure is essential because of ethical consequences of disclosure of a wrong condition. Implementation of standardized procedures (SOP) is of special importance for preclinical AD diagnosis. However, both amyloid PET and CSF biomarker measures are subject to significant methodologic variations, which can substantially affect the different cut-off values.
2.3.1. CSF standardization
With reference to the CSF biomarkers, differences in pre-analytical protocols, analytical procedures, assays quality together with discrepancies in absolute levels between assay formats, and not the least batch-to-batch differences during assay production introduce variability and warrant assay-specific cut-offs [88,89]. At present, each laboratory has to establish their own internally validated cut-off values and a rigorous analytical quality system, including certified procedures, methodologies, and bridging of batches, to guarantee longitudinal stability in its measurements [90]. For this reason, international standardization efforts have been initiated: the Alzheimer’s Association Global Biomarkers Standardization Consortium (GBSC) and International Federation of Clinical Chemistry Working Group for CSF proteins (IFCC WG-CSF) [91]. The aim was to propose protocols harmonizing laboratory practices [92], defining procedures on CSF collection and handling [93,94], creating certified reference materials for assay calibration [95], and establishing reference measurement procedures (RMP) [96]. Great progress has been made recently with the first RMP for CSF Aβ42 [97] and the first fully automated method for quantification of CSF Aβ42 [98]. This method shows minimal between-lab and between-batch variability and will be essential to establish uniform global cut-off levels for the CSF biomarkers.
2.3.2. Amyloid PET standardization
The type of amyloid ligand used, thresholds established, methods used for amyloid PET imaging processing, target and reference regions used, partial volume correction, acquisition time duration, and potential head movements represent possible confounding factors and introduce variability, thus substantially affecting the cut-off points used. An amyloid PET scan can be considered either positive or negative based on specific cut-off values that differ according to the amyloid ligand used, the relevant brain regions or the method of calculation used. Given the pressing importance of the need for standardization, a working group headed by William E Klunk (University of Pittsburgh, Pennyslvania, USA) has been set up to standardize quantitative amyloid imaging measures by scaling the outcome of each particular tracer or analysis method to a 0-to-100 scale, anchored by young controls (≤45 years), and typical AD patients. The “centiloid” (CL) has been proposed as unit of measure of this 100-point unified scale for all Aβ imaging tracers used [72]. The CL standardization method is expected to support clear definition of cutoffs for the earliest signs of amyloid-positivity in cognitively healthy controls and further establishment of the range of amyloid positivity typical of AD (AD-like levels vs earliest evidence of positivity in controls).
Recommendation—
The validity of measures is even more essential in preclinical stages, that is, in conditions where there are no other relevant signs helpful for diagnosing the disease. The quality of the measures should be unquestionable.
3. Issue 3—the natural history of preclinical stage and influencing factors
In vivo evidence of AD pathology is a fundamental feature accounting for the progression and/or conversion to a clinical disease. It is necessary but is it sufficient? Will all cognitively healthy subjects, who are biomarker positive, develop the clinical disease during their lifetime and if so, can the timing be predicted?
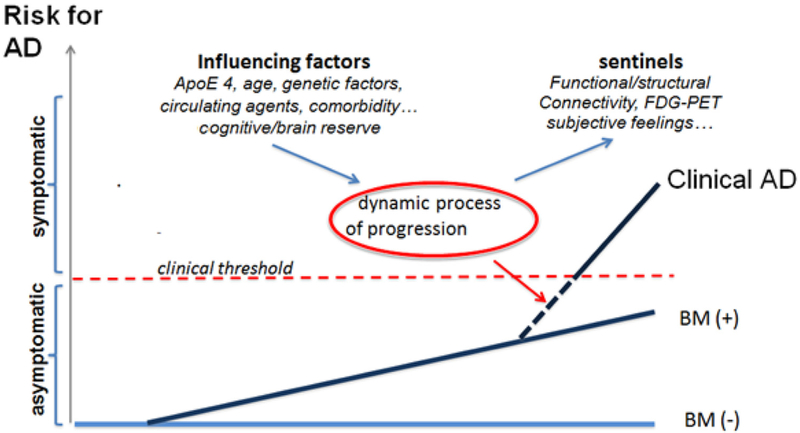
The identification of AR-AD subjects should rely only on the presence of a pathophysiological biomarker. These changes implicate the existence of AD pathology, which is necessary for further developing the clinical disease; it is not clear, however, if such alterations are sufficient. On one hand, it is possible to hypothesize that, when a definite threshold of amyloid burden is reached, or when specific anatomic structures of the brain are affected by the putative AD pathology, the dynamic process of progression is activated. However, even with high levels of ligand retention as typically seen in subjects with AD, some elderly individuals show little if any cognitive disturbances, therefore suggesting the influence of additional mediators such as cognitive reserve. On the other hand, the process can be accelerated by several factors affecting the risk and/or the rate of progression. It can be speculated that the occurrence of clinical onset of AD is the expression of a complex algorithm where the presence of AD brain lesions plays a key role and additional positive and/or negative factors need to be considered (Fig. 2); for instance, a number of variables may have a negative effect. If the sequential appearance of existing biomarkers and the polygenic and environmental protective/risk factors is considered, it should be possible, at some point, to predict the risk spectrum of a given individual and the putative time to the onset of a clinical disease, that is, to determine his condition along a risk spectrum, ranging from negligible risk to immediate risk.
Fig. 2.
The risk of clinical AD–hypothetical model. Abbreviation: AD, Alzheimer’s disease; BM, pathophysiological biomarkers.
3.1. Will all BM(+) cognitively normal subjects progress to AD?
Converging data from multiple longitudinal studies have consistently found that cognitively normal individuals with a positive Aβ biomarker show, as a group, accelerated cognitive decline compared to Aβ-negative individuals [99–104]. However, individual trajectories within Aβ+ individuals are quite variable, with some individuals showing little or no change in cognitive performance even with extended follow-up [105]. Furthermore, the rates of incident clinically significant cognitive impairment (i.e., progression from a diagnosis of cognitively normal to prodromal AD or AD dementia) in those defined as Aβ positive by levels of CSF Aβ42 alone appear to be relatively low, ranging from 13.5%–22.9% in studies with median follow-up of 15–54 months [21] (Washington University), [106–108]. The question of whether all individuals with a positive Aβ biomarker would develop AD if they lived long enough is purely theoretical. From a practical point of view, the estimated 15–20-year lag time between incident amyloid positivity and the median onset of early cognitive impairment [3,109], along with the increased incidence of Aβ-positivity with age essentially guarantees that a sizable minority of cognitively normal elderly individuals will die with a high-amyloid burden but without experiencing discernable cognitive impairment during life, and in fact neuropathologic studies of aging support this [110].
Across studies, those individuals showing abnormalities in both Aβ and additional biomarkers that demonstrate more rapid cognitive decline and higher rates of conversion to prodromal and/or dementia, whereas individuals with an isolated abnormality in Aβ show marginal or no difference from Aβ-negative individuals in their cognitive trajectories over a 5–10-year period [21,101,106,111,112]. For example, in the study by Vos et al., the conversion rate from CDR 0 to 0.5 was only 12% in Aβ-positive individuals with normal CSF tau and p-Tau versus 32.7% in Aβ-positive subjects who also had elevated CSF tau or p-tau levels. Similarly, Knopman et al. reported 18% progression from normal to prodromal/dementia >15 months in Aβ-positive versus 5% progression in Aβ-negative subjects enrolled in the Mayo Clinic Study of Aging, but the progression rate was 11% in Aβ+ subjects with normal hippocampal volumes and brain metabolism (not significantly different from the Aβ-negative reference group) versus 24% in individuals with abnormal MRI/FDG. On-going studies on preclinical subjects should provide more information in the future (see Table 2).
Table 2.
On-going cohorts useful for the study of preclinical AD
| Cohort acronym | Cohort name | Start date | End date | Ref | Website |
|---|---|---|---|---|---|
| ADNI | Alzheimer’s Disease Neuroimaging Initiative | 2004 | 2009 | Weiner et al., 2015 | http://www.adni-info.org/Home.aspx |
| ADNI-GO | Alzheimer’s Disease Neuroimaging Initiative Grand Opportunities |
2009 | 2011 | Weiner et al., 2015 | http://www.adni-info.org/Home.aspx |
| ADNI-2 | Alzheimer’s Disease Neuroimaging Initiative-2 | 2011 | Ongoing | Weiner et al., 2015 | http://www.adni-info.org/Home.aspx |
| AIBL | Australian Imaging, Biomarkers and Lifestyle (AIBL) Flagship Study of Ageing |
2006 | Ongoing | Ellis et al., 2009 | http://aibl.csiro.au/ |
| Amsterdam cohort | Amsterdam clinical research cohort on dementia | 2000 | Ongoing | van der Flier et al., 2014 | NA |
| BIOCARD | Biomarkers of Cognitive Decline Among Normal Individuals |
1995 Restarted in 2009 |
2005 ongoing |
Soldan et al., 2013 | http://www.alzresearch.org/biocard.cfm |
| HABS | Harvard Aging Brain Study | 2009 | Ongoing | Mormino et al., 2014; Dagley et al., 2015 |
http://nmr.mgh.harvard.edu/lab/harvardagingbrain |
| INSIGHT | INveStIGation of AlzHeimer’s PredicTors in Subjective Memory Complainers |
2013 | Ongoing | NA | NA |
| MCSA | Mayo Clinic Study of Aging | 2004 | Ongoing | Roberts et al., 2008 & 2012 |
http://www.mayo.edu/research/centers-programs/ alzheimers-disease-research-center/about |
| MEMENTO | DeterMinants and Evolution of AlzheiMer’s disEase aNd relaTed disOrders |
2011 | Ongoing | NA | http://www.memento-cohort.org/memento |
| NACC | National Alzheimer’s Coordinating Center database | 2005 | Ongoing | Beekly et al., 2004 & 2007 | https://www.alz.washington.edu/ |
| Nun Study | Nun Study | 1986 | Ongoing | Iacono et al., 2015 |
https://web.archive.org/web/20131229163933/https:// www.healthstudies.umn.edu/nunstudy/ |
| WU-ADRC | Charles and Joanne Knight Alzheimer’s Disease Research Center at Washington University in Saint Louis |
2004 | Ongoing | Morris et al., 2009 | http://www.adrc.wustl.edu/ |
| UK-ADC | University of Kentucky, Alzheimer Disease Center | 1985 | Ongoing | Jicha et al., 2012 | http://www.uky.edu/coa/adc |
| WRAP | Wisconsin Registry for Alzheimer’s Prevention | 2001 | Ongoing | La Rue A et al., 2008; Sager MA., 2005 |
http://www.wai.wisc.edu/research/wrap.html |
Beyond the burden of neuropathology, structural/functional brain changes, and baseline cognitive performance, the trajectory of Aβ+ normal individuals may further be modified by genetic factors (e.g., APOE ε4 genotype) [108,113], cognitive reserve [114,115], medical comorbidities that contribute to cognitive function (mood, sleep, endocrine and primary cardiopulmonary, renal and hepatic disorders), lifestyle factors (such as exercise and diet) [116–118] and possibly cognitive training. Other factors may have a significant impact. Given the relatively small number of the neurons in these structures, the initial size of the temporal cortex and hippocampus may affect the consequences of neuronal death before the onset of pathologic changes. Additionally, the co-occurrence of AD neuropathology and small cerebrovascular lesions (lacunar infarcts) is common in advanced age, and the cerebrovascular burden has an impact on the onset and severity of the clinical symptoms of AD [119].
Recommendation—
As progression rates significantly increase with the duration of the follow-up, longer follow-ups are needed to establish if all research participants will progress to clinical AD and which factors are the most important for progression.
3.2. Age and the risk of progression to clinical AD
Influencing factors of progression can be categorized into two groups: (1) nonmodifiable risk factors, such as age and genetic risk factors and (2) modifiable risk factors, such as cardiovascular risk factors and lifestyle.
Age is the most important risk factor. In a recent study, analyzing 1246 subjects aged 30–95 years, the risk increased with age particularly after 70 years and in APOE ε4 carriers [120]. As a result, it can affect the likelihood of developing AD with age [33,121,122]. Age significantly increases the risk of ligand retention in amyloid PET in cognitively normal individuals. In previous series, the frequency of individuals with amyloid load was 18%–23.1% at age 60–69 years, 25.8%–37.5% at age 70–79 years, and >30.3% to 65% after 80 years [121,123,124]. A recent meta-analysis showed that the prevalence of amyloid pathology increased from 10% (at age 50 years) to 44% (at age 90 years) among participants with normal cognition with a 2 to 3 times higher prevalence estimates in APOE ε4 carriers [46].
Aging may also have an impact on other biomarkers as pathologic features of AD [125]. Smaller baseline hippocampal volumes were significantly associated with age in normal subjects, in MCI subjects, and in AD patients [126]. In a sample of 985 cognitively normal individuals, neurodegenerative changes assessed with hippocampal volume (MRI) or PET-FDG (18F) were noticed in 0% at age 50–59 years; 7.8% at age 60–69 years; 28.4% at age 70–79 years; and 52.4% after 80 years [124]. With regard to CSF biomarkers, a significant association with age was found in a cohort of cognitively normal adults aged from 21 to 88 years. Moreover, older subjects (i.e.>.65 years) had 20% lower CSF Aβ42, 47% higher total tau, and 33% higher p-tau levels relative to young subjects (<65 years), with corresponding increase of tau/Aβ42 and p-tau/Aβ42 ratios [127]. In addition, investigating the presence of both markers of amyloidosis and neurodegeneration at the same time, Jack et al. [120] found that their frequency increase from 2.5% at age 60–69 years, 13.2% at age 70–79 years, and 31% after 80 years.
Temporary conclusion concerning the influence of age—
Age is a major risk factor for the progression to a clinical AD in AS-AD subjects. Age has a direct influence on progression and also plays an indirect role via associated comorbidities. Mean population age of a study may impact the conversion rates more than the study duration.
3.3. APOE ε4 and other genetic risk factors of progression to clinical AD
To date, APOE ε4 represents the strongest and best-established genetic risk factor for sporadic AD [37,128,129]. Subjects with one or two copies of the ε4 allele display higher risk of developing AD as compared to noncarriers. The same allele also significantly reduces the mean age for AD onset [129–131]. A recent study estimated the mean age at clinical onset to be 68 years in ε4 homozygotes, 76 years in ε4 heterozygotes, and 84 years in ε4 noncarriers [129,132]. As the proportion of subjects who will develop AD is higher among APOE ε4 carriers than among noncarriers, cognitively healthy APOE ε4 carriers offer a great opportunity to investigate brain changes in the asymptomatic stages of AD [133]. Along these lines, it has been reported that APOE ε4 directly affects MRI, CSF, and cognitive biomarkers in AD [134]. The apolipoprotein E (APOE) ε4 allele and high levels of beta-amyloid (Aβ) are associated with episodic memory decline and increased risk for clinical AD. In healthy individuals, Aβ+ ε4 carriers have shown significantly faster decline on memory tasks than Aβ+ ε4 noncarriers >a 54-month assessment period, suggesting that, in the preclinical stages of AD, the manifestation of memory decline in older adults with high Aβ is exacerbated by the presence of APOE ε4 [108]. Finally, it should be mentioned that APOE ε4 carriers may be more vulnerable for harmful lifestyle factors suggesting complex gene-environmental interactions [135].
In recent years, other genes have been established to significantly modify the risk for AD on a genome-wide scale in addition to APOE. Variants in genes involved in lipid metabolism, inflammatory response, and endocytosis have been identified through genome-wide associated studies. Polymorphisms in or near several genes that are associated with AD risk were identified, including: ABCA7, CLU, CR1, CD33, CD2AP, EPHA1, BIN1, PICALM, MS4A, CASS4, CELF1, DSG2, FERMT2, HLA-DRB5-DBR1, INPP5D, MEF2C, NME8, PTK2B, SLC24H4-RIN3, SORL1, and ZCWPW1 [128–132,136–140]. The risk conferred by single genes is small and currently only one locus (a rare amino-acid changing polymorphism in the TREM2 gene) shows convincing evidence for playing a significant role in modifying genetic predisposition. Although the findings uncovered by these powerful new technologies have considerably advanced our understanding of the pathophysiological mechanisms underlying neuronal degeneration in AD (e.g., by highlighting dysfunction of endocytotic pathways and immune system response as important primary risk factors), their utility in predicting progression to clinical AD is currently under investigation.
Provisional conclusion concerning the influence of APOE status—
At present, APOE ε4 is the main genetic risk factor of progression to clinical AD.
3.4. Modifiable and lifestyle risk factors of progression to a clinical AD
AD is increasingly recognized as a complex multifactorial disease given the interconnection of the genetic component with other risk factors such as co-morbidity, vascular risk factors, environmental, and lifestyle factors [141]. A recent meta-analysis on lifestyle modifiable risk factors made use of a mixed-method approach combining findings from a systematic literature review and a Delphi consensus study [142]. The authors revealed several somatic and lifestyle factors for AD including depression, (midlife) hypertension, (midlife) obesity, diabetes, physical inactivity, smoking, and low education. It has been estimated that about one-third of AD dementia cases worldwide might be attributable to the above-mentioned modifiable risk factors. This estimate accounts for the frequent co-occurrence of these risk factors in the same individual [20]. Two main aspects need to be considered when weighting the role of these risk factors in relation to clinical expression of AD. One is time at exposure because effect of specific risk/protective factors largely depends on age at which they intervene. Risk factors are unlikely to occur isolated but might interact in a synergistic or antagonistic way or form clusters (e.g. metabolic syndrome) [143]. For most of these risk factors, the mediating pathways are not completely known, for example, whether they are acting on the amyloid process, on the reserve capacities, or on the inflammatory pathway.
Recent studies are in favor of a decrease in age-specific prevalence or incidence rate of dementia and AD during the last 10–15 years [144,145], and beside a better management of vascular factors and a progression in educational level, the global improvement in lifestyle could explain these findings.
Temporary conclusion concerning other influencing factors—
Several modifiable risk factors have been recently identified that may influence the development of clinical AD.
3.5. Factors of prevention
Preventive factors should be critically considered as they may slow down or postpone the progression to a clinical AD [146]. The major interest for identifying vascular and lifestyle modifiable risk factors is the possibility of impacting the onset of a clinical AD by advising and intervening subjects at risk. The multifactorial nature of AD suggests that multicomponent interventions targeting several risk factors simultaneously might be needed for optimal preventive effects. In the last three decades, several studies, most observational, have underlined the role of modifiable factors in delaying the clinical onset of AD [147]. Among these studies, FINGER [148] is a landmark randomized controlled trial, which showed that a multicomponent approach targeting several vascular and lifestyle-related risk factors simultaneously in elderly people at risk of dementia can improve or maintain cognitive functioning. The study implemented a 2-year intervention program, which included nutritional guidance, physical exercise, cognitive training, social stimulation, and management of vascular and metabolic risk factors [148].
Other studies showed that adherence to Mediterranean diet and programs of physical exercise were associated with decreased brain AD-burden among cognitive intact individuals, thus indicating that lifestyle factors may modulate AD risk [149–152]. Both greater lifetime cognitive activity and physical exercise have shown to be related to lower brain Aβ burden [117,153] and fewer cerebrovascular lesions [118]. There is extensive epidemiologic and experimental evidence demonstrating the concept of cognitive reserve, identified in those individuals with high educational and occupational attainment, and engagement in leisure and social activities. Each of these factors has been associated in observational studies with decreased risk of developing dementia [154–157] and more successful aging [158], and they are able to modulate the neuropathologic process of AD detected through in vivo AD biomarkers [159].
Genetic factors may also have a protective effect. A rare variant in the APP gene that protects against AD has recently been disclosed [160].
Temporary conclusion concerning factors of prevention—
Several genetic and lifestyle factors have been identified that may delay the onset of clinical AD.
4. Issue 4—the transition phase to clinical AD
After identifying subjects at risk for AD based on the presence of specific biological predictors, the main issue is to detect those with the highest likelihood to progress to definite clinical AD in the forthcoming months to years. Full-blown AD may arise several years (>15 years) after the initial detection of a positive biomarker [46,161]. Consequently, the long-term treatment of cognitively normal individuals with medications having potentially significant and serious side effects (e.g., amyloid related imaging abnormalities [ARIA] [162]) remains an uncertain value proposition. Owing to ethical, financial, and medical constraints, it may be important to identify and treat only subjects having the highest risk of developing clinical AD within a short time frame. For that reason, the identification of markers, able to detect disease progression in a relative short time frame before the onset of clinical symptoms, becomes of crucial importance.
4.1. The end of the preclinical stage
The impact of AD on objective measures of cognition and clinical manifestations is a gradual process beginning in the preclinical phase and continuing progressively but inexorably into the prodromal and dementia stages, making it impossible to define a discrete onset of the clinical state. The preclinical stage progresses imperceptibly; clinical manifestations are eventually apparent but without a discrete onset. Growing evidence of neuropathologic progression as well as subtle cognitive decline among asymptomatic individuals with brain amyloid [109,163] supports this view. A minimum threshold of changes can be determined on objective reliable measures of cognition. However, this requires assessment of intra-individual change. A cognitive composite score, which integrates scores from different episodic memory tests, executive functions and orientation, has been recently proposed as an outcome for clinical trials applied to preclinical stages [164–166]. A decline in a composite score presents the advantage of avoiding the problems related to “time to event” assessment but raises the question of its clinical significance in a clinically healthy population. Several critically relevant questions still need to be answered: should it be based on specific cut-off values or on a decline from a previous score? Should episodic memory performance be primarily considered?
Recommendations—
As cognitive symptoms are the first clinical changes to appear in typical AD, an objective impairment or decline in an episodic memory test or in a cognitive composite score is recommended as a marker of clinical onset of AD. The threshold to which the performance is impaired and the amount of change that should be considered for a decline and the delay between both evaluations remain to be defined or validated.
4.2. Structural neuroimaging and progression to a clinical AD
Abnormalities in structural MRI become clearly detectable before the first clinical signs of the disease. Hippocampal volume represents one of the best-established structural imaging markers for AD. Regional analysis, aimed to investigate hippocampal subfields, has shown that CA1 region and subiculum compared with the total volume of hippocampus are more closely associated with progression to MCI in cognitive intact individuals [167,168]. Moreover, rates of atrophy were faster, especially in the temporal lobes, in cognitive intact Aβ positive elderly individuals [169]. Other medial temporal lobe regions, besides the hippocampus, showed volume reduction in cognitive intact elderly at risk for AD. Decreased entorhinal cortex volume was shown to precede significant cognitive decline by 4 years in cognitively intact elderly [167]. Recently, early structural abnormalities in the neocortex and cortical thickness have aroused growing interest [169,170]. In cognitively intact elderly subjects, high brain Aβ deposition levels were associated with fast gray matter atrophy in the posterior cingulate and/or precuneus and hippocampus [169]. Decreased gray-matter volume in the parietal lobe, notably in the angular gyrus, has been described in cognitively intact individuals in advance of progression toward mild cognitive impairment (MCI) [171]. Moreover, prefrontal cortex atrophy in cognitively intact individuals was found to precede dementia onset over a 6-year period [172]. These results support the hypothesis that early changes of structural imaging markers might represent a good topographical marker of progression in the preclinical stage [173].
Recommendations—
Rate of longitudinal change assessed by structural MRI increases with progression to clinical disease in asymptomatic biomarker positive subjects and can be used for staging of high risk of progression.
4.3. Molecular neuroimaging and progression to clinical AD
It is not yet established that progressive amyloidosis in the brain, through positive amyloid PET, reliably predicts further progression to clinical AD. Development of tau PET may become a useful tool for this prediction. It is assumed that a spreading of the metabolic tau PET pattern out the medial temporal structures to lateral temporal and frontal neocortical areas may indicate an active progression to a clinical AD [22]. We may also take into account surrogate markers of tau/neuronal injury such as glucose metabolism. In the AD Neuroimaging Initiative (ADNI) cohort, models with baseline features derived from MRI and FDG-PET were capable of successfully predicting whether an individual will progress and convert to MCI within 48 months or remain cognitively stable, with 81.2% accuracy [174]. Another study [175] showed that reduced FDG-PET brain metabolism and executive function, predict clinical progression in elderly healthy subjects with a sensitivity = 82% and specificity = 93%.
Recommendation—
Changes in FDG-PET track progression to clinical disease in asymptomatic biomarker positive subjects.
4.4. Functional connectivity and progression to clinical AD
New neuroimaging and magneto-encephalography (MEG)/electroencephalography (EEG) tools can target pre-clinical disease evolution through structural, metabolic, or functional measurements [9,176].
4.4.1. MRI functional connectivity markers
AD is characterized by alterations in multiple brain regions such as hippocampal and medial temporal regions involved in memory, and cortical association areas in frontal, temporal, and parietal lobes. The latter contains regions that are deactivated during many cognitive tasks but display increased activity in the resting state on functional MRI (rs-fMRI) studies, targeting the “Default Mode Network” (DMN) [177]. Functional connectivity between the hippocampus and the posterior part of the DMN is significantly reduced in AD patients [178]. This finding is consistent with the reported altered hippocampal connectivity with several cortical DMN areas during the early stages of AD [179]. Interestingly, not only was connectivity reduced in rs-fMRI studies in patients with AD and MCI, but a reduction was also observed in older individuals, especially those with poor memory ability while performing memory encoding tasks [180]. In studying the resting state network (RSN) in AD and MCI, Seeley et al. [181] showed that the functional networks in the brain exhibit a “small world” architecture (i.e., any two network nodes are connected via a small number of nearest neighbor nodes) revealing that the disease alterations were related to the underlying anatomy and loss of functional connectivity. Subsequent connectivity studies showed that the functional integrity in RSNs including the DMN is lower in AD patients than in controls [182]. Together, these observations demonstrate structural and functional network disruptions in normal elderly controls and AD patients, but it is still unknown whether these alterations emerge predictably during early and preclinical stages of the disease. Confirmation of the RSN degeneration hypothesis raises important questions about the impact of specific syndromes on RSN structure and function. Buckner et al. [183] found, using RSN and 11C PIB-PET, Aβ positivity in regions of the DMN. Sperling et al. [184] showed that cognitively intact individuals with Aβ pathology in the DMN fail to deactivate it during memory tasks. These findings suggest that Aβ pathology is linked to functional network connectivity dysfunction.
4.4.2. Functional connectivity markers
Among the established EEG/MEG analytical approaches in AD research, resting state spectral measures indicate a phenomenon of abnormal diffuse slowing of the spontaneous brain activity [185]. In contrast to resting states, goal-driven EEG/MEG brain activity engages task-related and attention-related brain networks. Event-related potentials, such as P300, reveal systematically delayed and suppressed responses to sensory and cognitive stimuli. The fundamental utility of EEG to detect very early preclinical brain signal changes even in the presymptomatic stage of the disease has been demonstrated in familial AD [161,186,187].
New specific strategies using sensitive neurodynamics measures may be developed for the detection of preclinical AD in the future. The pathologic changes that occur in the brain many years before the clinical onset should induce functional changes in brain structures, which can be identified by EEG/MEG. The sensitivity of functional connectivity EEG measures to clinical AD has been repeatedly demonstrated [188–192], notably in a recent large-scale study with 318 AD patients and 133 age-matched controls [193]. To our knowledge, no neurodynamics (EEG/MEG) functional connectivity studies have yet investigated the crucial preclinical progression process to pursue very early prediction of AD.
Temporary conclusion on functional connectivity markers—
At present, there is insufficient evidence for a distinct pattern of changes in functional connectivity as a predictor of a further progression to a clinical AD.
4.5. The earliest clinical (cognitive, functional, or behavioral) changes indicative of the clinical state of AD
On traditional neuropsychological testing, differences between cognitively healthy subjects who will develop a dementia and those who will not can be observed 10 to 17 years before the diagnosis of dementia [194]. At a group level, it has been shown that cognitive changes initially consist of subtle decreases in episodic memory, psychomotor speed, verbal fluency, and concept formation. On an individual basis, however, these changes cannot be considered pathognomonic manifestations of clinical AD. This is due to their lack of specificity as they can be seen among other conditions such as depression [195,196], drug abuse [197], and Parkinson’s disease [198]. Another consideration is the very small size of observed changes. Compared with individuals without pathology, those with AD pathology had an accelerated rate of decline on a variety of measures, with an annual change of 10%–15% of a SD [199]. The meta-analytic difference between cognitively normal amyloid+ and amyloid individuals on episodic memory measures was d = 0.25, that is, a quarter of a standard deviation [200].
After decline on episodic memory, psychomotor speed, and verbal fluency measures, global cognition (MMSE), subjective appraisal, and IADLs begin to decline 5–8 years before dementia onset [3,201]. Differences in subjective appraisal of cognition and function, also referred to as Subjective Cognitive Concerns, have been reported for individuals classified according to the 2011 NIA-AA pre-clinical AD stages [164], as amyloid+ versus amyloid [202]; and for those who experience a diagnostic progression (e.g., on the Clinical Dementia Rating (CDR) global staging 0 to 0.5) [164]. Increasing scores on the Cognitive Function Instrument predicted progression on the CDR independently of objective performance [164]. Self-report may be particularly useful very early in the process of decline [164]. Depressive features may also be an early marker of clinically relevant change [201,203].
An approach to tracking cognitive change in the preclinical stage (used in the A4 study and the Alzheimer’s Prevention initiative) has been to use cognitive composite scores as outcome measures of preclinical AD [163,204]. For example, the Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite (ADCSPACC) [163] consists of summed standardized change scores on the Free and Cued Selective Reminding Test (FCSRT) total recall, logical memory delayed recall, Digit Symbol Substitution, and MMSE. This composite score differentiated between amyloid+ and amyloid and between CDR progressors and CDR stable. The composite difference between amyloid+ and amyloid at 24 months was −1.2, suggesting that a summed metric may provide a more robust measure of change than single scores. Another approach is to consider that a decline over time in healthy cognitively normal subjects in specific highly demanding cognitive tests, i.e., the Digit Substitution Subtest of the Wechsler Adult Intelligence Scale, may indicate that a pathologic process is developing silently. This may be used to predict amyloid PET positivity. Long-term longitudinal evaluations of these new tools are required to establish their ability to accurately indicate a progression to clinical AD. Finally, there have been recent instrument development efforts targeting specific components of memory including associative binding (e.g., the MCT), feature binding (e.g., the Short-Term Memory Binding Test), and pattern separation (Behavioral Pattern Separation-Object test) [205]. These tasks have shown promise in identifying amyloid+ individuals and carriers of autosomal dominant mutations for AD. The utility of novel measures including test–retest reliability, good signal-to-noise properties, and relative insensitivity to cognitive reserve variables must still be established invalidation studies. Until then, validated clinical markers of the clinical phenotype of AD, including free and cued recall measures [206–208] and the temporary memory binding task [209] among other scales, should be used to distinguish preclinical and prodromal AD.
Temporary conclusion on the clinical markers of progression—
A sustained and significant decline in a cognitive or memory test or in a composite measure with established specificity for AD may be considered as a marker of a clinical onset.
5. Issue 5—where are we today and how to progress?
The identification of AR-AD or preclinical AD subjects becomes important with the development of disease-modifying drugs that should reduce brain amyloid lesions. Three antibodies targeting Aβ, gantenerumab bapineuzumab, and aducanumab have been recently reported to significantly decrease brain amyloid ligand retention when compared to placebo in PET studies [210,211] in patients with clinical AD. Several studies in AR-AD (ADCS-A4 trials in older adults with amyloid-positive brain scans; Zinfandel-Takeda prevention study in older adults carriers of APOE ε4 and TOMM40 alleles; API-APOE in older adults homozygotes APOE ε4 and EPAD) are currently on going that are aimed at delaying the accumulation of AD neuropathology. If delaying the disease onset of the clinical manifestations of the disease by such interventions is demonstrated, there will be a significant incentive to identify from the general population those at risk of developing AD and to select those that may require more invasive (CSF sampling) or expensive (amyloid PET) investigations. In addition, specific designs of such trials, inclusion criteria, and outcomes will have to be elaborated.
5.1. The significance of subjective cognitive decline in the context of preclinical state
Subjective cognitive complaints and the more recent concept of subjective cognitive decline (SCD) have been proposed as potential indicators of a preclinical state of AD [212] because they may increase the risk of progressing to clinical AD. In some studies, the presence of SCD is associated with subsequent cognitive decline and progression to dementia [213] and may be related to brain amyloid deposition on amyloid PET [214]. However, the existence of cognitive complaints does not necessarily imply a progression to clinical AD: they were present in only 16% of the cases progressing to clinical AD in the AMSTERDAM study and in 7%–37% of the Mayo Clinic Study on Aging (MCSA) depending mostly on the age of the subjects [214]. In a recent meta-analysis, Mitchell et al. [215] demonstrated that the annual rate of progression of subjects with subjective memory complaint (i.e., SCD) to dementia is 2.33% with a relative risk compared to subjects without SCD (ACR of 1%) of 2.07, 95% CI (1.77–2.44). They also describe a cumulative conversion proportion of SCD to MCI of 24.5% >4.1 years and to Dementia of 11% >4.8 years. In subjects without SCD, comparative estimates are that 4.6% progress to dementia over 4 years. Therefore, even if the data suggest a two-fold increase in the risk of dementia in subjects with SCD, the relative risk remains low and SCD cannot be a proxy to preclinical AD. In that sense, a study derived from the AIBL cohort showed that the percentage of memory complainers was similar in the PIB-positive (55%) and PIB-negative groups (53%) [216].
Subjective memory complaints are found in the vast majority of adults >60 years [217] and only a small proportion of them are biomarker positive (<30% at that age). Changes in metamemory may result from many other causes frequently observed in the aging population, including attention difficulties, depressed mood, sleep disorders, various neurodegenerative diseases, and drug side effects. In contrast, the risk of prediction of a positive AD biomarker increases when SCD is enriched with specific properties, which defines the “SCD+” [212].
Recommendations—
(1) SCD is not a proxy for preclinical AD, as individuals with SCD only present with a small increased risk when compared to noncomplainers; (2) individuals with a positive Aβ marker associated with SCD are only “at risk for AD” and should not be considered as having clinical AD.
5.2. Blood markers and the screening of preclinical AD
The biological approach consists of the identification of blood-based biomarkers that may support the detection of at-risk individuals and who will consequently be suitable targets for preventive and symptomatic pharmacological intervention trials [218–222]. The criteria of the Biomarkers Definitions Working Group of the National Institutes of Health (NIH) emphasize the urgent need for an easily obtained sample of peripheral tissue that can be analyzed either in the office or at a central location and that enable an accurate diagnosis [223]. Recent diagnostic blood-based biomarker work has been developed across cohorts [219,221,224], assay platforms [225,226], and even translated back into AD animal models for validation purposes [221,225]. With regard to AR-AD, there is evidence for the utility of blood-based biomarkers in the prediction of future incidence of MCI–prodromal AD [227–229] as well as detection of neocortical Aβ burden from both the ADNI and AIBL cohorts burden [230,231]. Given the heterogeneity of biological processes leading to cognitive loss and accumulation of Aβ, it is possible that disease-specific subgroups may offer novel pathways forward for predicting preclinical AD status.
However, despite the current progress, the search for optimal and reliable blood-based biomarkers for the screening of subjects at a preclinical stage of AD has been limited by the current state of the science and methodological issues. As a result, these markers are not yet ready for clinical application [227,232] as they have not yet been evaluated specifically within the context for which they are intended (i.e., fit for purpose). That is, the vast majority of the work examining blood-based AD biomarkers (including work in the preclinical stage) has been conducted within dementia clinics rather than among population-based or primary care clinic settings where the substantially lower base rates will have a significant impact on the study findings [225,233,234]. As such, all current methods are research use only with some work moving toward laboratory developed test status. Another barrier to this work that requires attention is the lack of consistent methods used across laboratories. The blood-based biomarker research raises the same issues of establishment of SOPs as for CSF investigations [235–237].
The challenge is to conceptualize the emerging approaches and standardize methods and protocols for sample collection both at baseline and at follow-up, to analyze and integrate large-scale complex data sets. One of the major benefits from a successful blood-based biomarker strategy will be to provide an inexpensive and minimally invasive way to enrich the population to screen and possibly to monitor changes over time and responses to clinical interventions [232,236,237].
Recommendation—
To date, evidence from studies on blood-based biomarkers indicates a limited value for the characterization of preclinical stage of AD. Further studies are needed to justify their use for enrichment or for screening.
5.3. Application for clinical trials in preclinical states
The definition of the preclinical state is especially important for the design of clinical trials of disease-modifying agents. Intervention at such an early stage would offer the best chance of therapeutic success because the intervention would target less established and extensive pathologic processes and that are potentially reversible.
5.3.1. Overview of the regulatory situation
In a commendable effort, regulatory agencies have made significant strides in integrating the recent paradigm shift engaged by the AD scientific community. Both the United States Food and Drug Administration (FDA) and European Medicines Agencies (EMA) have discussed the use of in vivo biomarkers and their implementation in study designs in AD clinical trials. In Feb 2013, the FDA released draft guidance for the treatment of early state AD [238,239]. The FDA acknowledges the difficulty in demonstrating a functional benefit in the preclinical stages of the disease. They allow for an accelerated approval mechanism (i.e., a provisional approval) based on the demonstration of efficacy on a single-cognitive measure that is contingent on postapproval studies to demonstrate a relevant clinical benefit. After postponing the preparation of a new guideline in 2012, the EMA (European Medicines Agencies) has recently released a discussion article on the same topic [240].
The EMA document distinguishes between (1) disease modification with slowing or arrest of symptom progression and (2) primary prevention of symptomatic disease by intervention in suspected pathogenic mechanisms at a presymptomatic stage. The latter seems to cover studies conducted in preclinical stage, generally described as secondary prevention in the scientific literature [241]. However, there is little said about the clinical trials designs. For “prevention” trials, the EMA suggests 5-year duration studies but recommends getting scientific advice before embarking into this type of development. The FDA document does not specifically deal with design across specific disease stages but only refers in a separate section to the “randomized start” or “randomized withdrawal” designs as ways to differentiate a symptomatic effect from a disease-modifying effect.
Concerning in vivo biomarkers, both agencies have approved three β-amyloid isotopic ligands (florbetapir, flutemetamol, and florbetaben). The indication section in the US labeling, identical for the three compounds, is to estimate Aβ plaque density in patients with cognitive impairment who are being evaluated for AD or other causes of cognitive decline and essentially to rule out AD in case of a negative examination. The European labeling is insisting on the need to use them in conjunction with a clinical evaluation and expresses caution in the interpretation of a positive result.
5.3.2. Overview on the recently completed or ongoing studies
To put preclinical AD trials in perspective, it is useful to get an overview of the range of AD prevention trials. Table 3 reports the on-going or recently completed clinical trials, as documented from a survey conducted on ClinicalTrials.gov and the International Standard Randomized Controlled Trials Number (ISRCTN) registries (key words: Alzheimer disease, prevention) [148,165,242–259]. The 19 retrieved studies, also including a few studies with small sample size and short duration (which are pilot trials), may be classified under four different categories
Studies in populations of asymptomatic at risk subjects with positive biomarkers of AD (3 studies) [165,242,243]. The search for positive biomarkers among a large population is not feasible; hence, the trend to focus on subgroups of people where the prevalence of positive biomarkers is a priori higher [165,253].
Studies in populations of presymptomatic subjects with autosomal dominant mutation at a preclinical stage (two studies) [244,245].
One study in those at high risk because of APOE ε4 and TOMM40 alleles [2,246].
Prevention studies for participants with or without various suspected risk factors (e.g. age, population of children from AD patients, elderly persons with frailty and/or subjective memory complaints [SMC]) (12 studies) [148,248–258]. To conduct studies in populations without cognitive complaints or symptoms, very large sample sizes are required. The necessary number of participants, however, decreases to 1000–3000 after introducing more risk factors, for example, SMC or frailty [250–253].
Table 3.
Completed and ongoing Alzheimer prevention randomized controlled trials
| Name and NCT | Status | Intervention | Inclusion | N | Primary evaluation criterion |
Secondary evaluation criteria |
Treatment duration | Dates |
|---|---|---|---|---|---|---|---|---|
| Alzheimer Disease Prevention Trial (PREPARE AD) NCT00000176 |
Completed | Estrogens | ≥65 y, healthy female | NA | Onset of memory loss | — | 3 y | 1999-Sept 2007 |
| Alzheimer’s Disease Anti-Inflammatory Prevention Trial ADAPT NCT00007189 | Completed | Naproxen, celecoxib | ≥70 y, first degree relative with dementia | 2625 | NR | NR | 5–7 y | Jan 2001-Jan 2007 |
| The Ginkgo Evaluation of Memory (GEM) study NCT00376510 | Completed | Tanakan | ≥70 y, SMC, MMSE>25, CDR 0 to 0.5 | 2878 | Conversion to AD | Rate of cognitive decline | 60 mo | 2001–2009, completed |
| Prevention of Dementia by Intensive Vascular Care (PreDIVA) ISRCTN29711771 | Ongoing | CV prevention by nurse monitoring, multidomain intervention, cluster randomization | 70–78 y, MMSE >23, not demented | 3535 | dementia, disability | Cognitive decline (MMSE, VAT), depression, cardiovascular events |
6y | Start: 2006 |
| The Multidomain Alzheimer Preventive Trial (MAPT) NCT00672685 |
Completed | Omega-2 fatty acids; multidomain intervention, placebo; factorial design |
≥70 y; frail at least one cirteria: SMC, difficulty performing one ADL, or walking speed≤0,77 m/s, not AD, MMSE≥25 |
1680 | Cognitive function (Grober & Buschke) |
FDG-PET; MRI; A-beta PET (AV45) |
36 mo | 2008–2014 |
| Exercise for Cognition and Everyday Living for Seniors With Memory Complaints (EXCEL) NCT00958867 |
Completed | Aerobic training, resistance training, strech and relaxation |
Seniors with subjective memory complaint; MMSE>24, MOCA<26; ≥70 y |
86 | Cognitive performance | Everyday problem solving ability | 6 mo | Aug 2009-Mar 2010 |
| The Finnish Geriatric Intervention Study to prevent to Cognitive Impairment and Disability (FINGER) NCT01041989 |
Ongoing | Lifestyle | Increased dementia risk; 60–77 year-old, MMSE 20–26, delayed recall ≤75%; word list memory 14 ≤ 19; dementia risk factor>6 | 1200 | NTB, stroop, TMT A&B at 7 years |
Zung depression ADCS-ADL, RAND-36 (QoL) | 2y | Sept 2009-Dec 2018 |
| ASPirin in Reducing Events in the Elderly (ASPREE). NCT01038583 |
Ongoing | aspirin | Elderly, that is, ≥65 y for African American and hispanic (70 for caucasian); without dementia, severe HY, and able to perform 6 Katz ADLs |
19,000 | Death from any cause or incident, dementia, or persistent physical disability. | Jan 2010-Jan 2018 | ||
| Effect of simvastatin on biomarkers (SimBio) NCT01142336 |
Ongoing | Simvastatin | 45–64 y, cognitively normal (MMSE>26) logical memory recall.6, CDR = 0 |
120 | CSF: A-beta, tau and BDNF |
Markers of inflamation of oxidative stress in CSF |
1 y | June 2010–Oct 2015 |
| Ginkgo Biloba Prevention Trial in older individuals NCT00010803 |
Completed | Ginko | ≥75 y, nondemented | 3069 | Incidence of dementia, specifically Alzheimer’s disease based on DSM-IV criteria as determined by an expert panel of clinicians using an adjudication process. |
Incidence of vascular disease, changes in cognitive function scores over time (CDR, MMSE, ADAS), total mortality, and changes in functional status. |
6 y on average | Oct 2010–July 2011 |
| TOP-COG ISRCTN67338640 |
Completed | Simvastatin | Down syndrome aged >50 years |
60 | Pilot study: feasibility, patient inclusion, and retention |
Neuropsychological tests battery |
Up to 2 y | Apr 2012–March 2014 |
| DIAN-TU NCT01760005 | Ongoing | Ongoing Gantenerumab (s.c.), solanezumab (i.v.) |
PS and mutation carriers or belonging to family −15 to +10 years of parental symptom onset, normal cognition, or mild impairment CDR 0 to 1 |
210 | Gantenerumab arm: firillar amyloid deposit (11C PIB-PET) Solanezumab: A-beta CSF concentration |
Other biomarker: CSF tau MRI, FDG-PET, exploratory clinical measures: CDR, MMSE, NPI, FAQ |
2 y | Dec 2012–Mar 2017 |
| Pilot Study: lipoic acid and omega-3 fatty acids for Alzheimer’s prevention NCT01780974 |
Ongoing | Lipoic acid and omega-3 fatty acids |
≥55 years Nondemented: Montreal Cognitive Assessment > 26 and Clinical Dementia Rating = 0 Diagnosis of Essential Hypertension with systolic 90–160mmHg and diastol |
30 | TMT-B | Brain hyperintensity volume on brain MRI |
1 year | Apr 2013–Sept 2015 |
| TOMMOROW NCT01931566 |
Ongoing | Pioglitazone | 65–83 y; CDR = 0; One memory test >−1,5 SD, MMSE>25 |
5800 | Time to MCI | Cognitive test battery: episodic memory, executive function, language, attention; ADCS-ADL |
48 mo | Aug 2013–Apr 2019 |
| Alzheimer prevention through exercise APEX NCT02000583 |
Ongoing | Aerobic exercise | Clinical Dementia Rating 0 (nondemented) Age ≥65 years Florbetapir PET evidence of cerebral amyloidosis |
100 | Brain amyloid load (florbetapir) |
Structural brain MRI, cognition |
52 wk | Nov 2013–Jan 2018 |
| API NCT01998841 | Ongoing | Crenezumab | Carriers of PSEN-1, E280 A mutation, non- MCI, nondemented |
300 | Composite: cognitive: world list recall, multilingual naming test, MMSE, CERAD constructional praxis, Raven progressive matrices |
Progression to MCI or dementia; change in CDR-SB, FCSRT, RBANS, CMRrl, cerebral amyloid, volumetric MRI, CSF tau |
260 wk | Dec 2013–Sep 2020 |
| Modulation of Micro- RNA Pathways by Gemfibrozil in Predementia Alzheimer Disease NCT02045056 |
Ongoing | Gemfibrozil | Men or women aged 65– 90 years 48 cognitively intact subjects, and 24 subjects with early cognitive decline (CDR 0.5) predementia |
70 | Safety, micro-RNA107 levels in blood and CSF; Beta-amyloid concentration in CSF |
FCSRT, paired asociated learning |
48 wk | Jan 2014–June 2016 |
| Clinical Trial of Solanezumab for Older Individuals Who May be at Risk for Memory Loss (A4) NCT02008357 |
Ongoing | Solanezumab | MMSE, 25–30 CDR = 0; logicalmemory II: 6–18, florbetapir+, 65–85 y |
1150 | ADCS-PACC | Cognitive function index, ADCS-ADL, SUVr, CSF tau and beta- amyloid |
168 wk | Feb 2014–Apr 2020 |
| J&J-54861911 NCT02260674 |
Ongoing | JNJ-54861911 | Predementia, CDR 0 to 0.5 and either low cerebrospinal fluid (CSF) ABeta 1–42 levels or positive- amyloid PET scan |
100 | AES | Drug concentration blood ± CSF CSF A-beta, PK |
6 mo | Nov 2014–Mar 2016 |
With respect to inclusion criteria, subjects should be asymptomatic although how this is defined may differ among trials. Individuals enrolled in these trials will need to comply with potentially cumbersome or invasive examinations. Some trials include patients at different stages [243,244,251,256,258], other are focused only on preclinical stages. In the latter case, the selection criteria are based on CDR, MMSE, and memory tests (FCSRT or Rey Auditory Verbal Learning Test). In the case of sporadic forms of the disease, the biomarkers used for selection criteria are typically CSF markers (low amyloid with or without elevated tau) or amyloid PET, representing the pathophysiological markers of Alzheimer [165,242,243].
Building a clinical trial with precharacterized cohorts allows accumulation of knowledge of the course before randomization and provides a de facto run-in period before the randomization. For instance, participant selection can be based on prior clinical or biomarker rate of progression, as it is proposed for the EPAD project [260,261]. This helps to (1) ensure that a pathophysiological process is occurring and (2) allow stratification of patients based on biomarker status or their rate of progression. The need for collaboration among academic institutions, governmental organizations, and industrial partners finds an application in the building of large cohorts [2,262]. These design considerations help to fill in the existing gaps in our detailed understanding of the disease progression; moreover, they may also serve as infrastructure for conducting clinical trials.
Concerning endpoints of clinical trials, only four studies [241,244,250,255,258,259] use alterations on a biomarker as primary endpoint. In contrast, most of the recent studies have a primary clinical end point: a change on a cognitive performance measure by a single test (e.g., MAPT using FCSRT [252,263]); a composite score [244,245,253]; or a conversion to MCI [2,246] or dementia [250,256,264,265]. Trials using MCI or dementia as endpoints have the longest durations (4–6 years) and the largest sample sizes. For instance, ASPREE with a combined endpoint of death, dementia, or physical disability has a planned treatment duration of 3 years and sample size of 19,000 [254,266].
5.3.3. Recommendations for future clinical trials in preclinical state
Several recommendations for future prevention clinical trials have been released [147,261–269]. In particular, they report indications concerning (1) lexicon; (2) target population; (3) selection criteria; (4) design; and (5) outcomes.
Regarding the lexicon, as there are no discrete clinical events in the early stages, the definitions of primary prevention and secondary prevention need to be carefully discussed. Primary prevention should describe interventions on general populations presenting variable risk factors (e.g., age or APOE ε4 allele carrier status) but without identified markers of the presence of an AD-related process. Consequently, secondary prevention should refer to interventions in “at risk” populations, that is, subjects where there is a suspicion than an AD-related process has begun and can be assessed. This will cover studies in preclinical stages.
Concerning the target population, it is proposed that identification of risk factors may be a crucial step in AD research for enriching the population including biomarker status. Enriching the population based on risk factors may be a double-edged sword: on one side, it can help in identifying subjects more likely to progress, and on the other side, these individuals will not be necessarily the most responsive to treatment if this reflects a multiplicity of concomitant pathological processes not all amenable to the mechanism of action of the compound under study. Expressions such as “low risk” and “high risk”, moreover, may generate some ambiguity. They could define where an individual is on his/her way toward the clinical expression of the disease—that is, the staging—or alternatively describes if the patient is on a slow or fast path of progression according to all his or her risk factors. In practice, both dimensions should be integrated in the concept of “risk” as the probability for a patient to develop the pathology during the rest of his or her lifetime or during the trial. Comorbidities and lifestyle factors that may influence disease progression, and outcomes should be carefully monitored.
Regarding the selection criteria, the inclusion criteria should require the positivity of pathophysiological markers (see corresponding paragraph). To select participants with a known rate of progression, it may also be important to focus on measures that reflect disease progression. Markers of neuro-degeneration may be used including brain atrophy, hippocampal volume, and brain hypometabolism on FDG-PET.
Concerning the design, the randomized start or randomized withdrawal designs are attractive but not pragmatic; moreover, they have never been successfully implemented. A practical alternative is to conduct clinical studies, with a classical parallel groups design within well-known cohorts where the progression of a biomarker and a clinical outcome are monitored.
Concerning outcomes, a measure of change rather than reaching a new stage of the disease (e.g. moving from asymptomatic at risk to prodromal AD) is less challenging in terms of sample size and duration. It also avoids the difficulties in assessing if and when the patient reached this new stage. A pure neuropsychological scale or a combination of neuropsychological scales may be recommended for preclinical AD and accelerated approval, whereas composites such as the Clinical Dementia Rating (CDR) scale are more suitable for the prodromal phase of the disease.
Temporary conclusion on the design of RCT—
For clinical trials in preclinical state, the major challenge is to be able to recognize specific cognitive changes with outcome measures.
5.4. Ethical issues and the preclinical stage
Informing individuals about risk biomarkers when it is uncertain if all will progress to clinical AD raises important ethical challenges.
Arguments in favor of a preclinical characterization and disclosure can be analyzed from the point of view of preclinical subjects, families, clinicians, and society. Biomarker disclosure may serve as an argument of respect for individual autonomy, of moral and legal right to receive a specific diagnosis [270], for open and honest discussion of expression of feelings and fears [271], to facilitate the recruitment to preclinical trials AD [272], and to formulate advance directives [273]. In a study of public perceptions of presymptomatic biomarker testing for AD, 90.5% of participants endorsed that they would “pursue a healthier lifestyle” in the case of high risk for AD [274]. Evidence indicate that awareness of personal AD risk may motivate individuals to engage in beneficial, risk-lowering behaviors [275]. For exceptional cases of individuals with a high measure of social responsibility and cognitive demand (aging pilots, physicians and nurses), preclinical AD screening may find policy applications in the future [276].
In contrast, arguments against disclosure of biomarker status are numerous, and it is a pressing issue. For clinicians, the disclosure of diagnostic information about AD has long been recognized as a challenging task. Moreover, the accelerating paradigm shift toward the preclinical stage should enhance the concern about this difficulty. For patients, biomarker status is potentially harmful knowledge as a subject can develop anxiety or depression or even suicidal ideation [277]. Fifty-five years and older Americans fear AD more than any other disease, such as, for example, cancer [278]. Given these intense emotions, people are likely to strongly differ both in their desire to know whether they are at risk to develop AD and, if they do learn the diagnosis, how they react to it. Also, family’s emotions could impact on the interpretation and reactions of the individual-labeled preclinical AD. At a practical level, it is possible that disclosure may have ramifications well beyond the individual and his or her family. For instance, friendship networks may shift when peers distance themselves. Even broader are the implications for driving privileges, employment and insurance coverage, as individuals with AR-AD may be more vulnerable to discrimination because of their diagnosis. Finally, bio-markers are not yet able to provide meaningful information for care [277].
Would it be ethically acceptable treating an individual for a disease that will not be clinically developed by the subjects? The justification of trials that enroll AR-AD subjects is to test interventions before neurodegeneration reaches a point at which therapy is less likely to succeed. Most cognitively normal participants in AD preclinical trials declare unwillingness to take a study drug or site fear, anxiety, or hesitation related to medical procedures as deterrents to a decision to enroll [272]. Subjects considering whether to accept preclinical interventions in the scenario of being told they were at increased risk for AD, were much more likely to express a desire to reduce this risk, and less likely to cite barriers, compared to normal risk subjects [272]. Likewise, subjects with increased genetic risk for AD (carriers of the ε4 allele), who know their status, more frequently desire to reduce that risk (taking vitamins or supplements) than did counterparts who learned they were noncarriers [279,280]. These findings suggest that people who learn they have increased risk for AD may be more likely to desire preclinical interventions.
Recommendation—
In the absence of definitive answers concerning the algorithm of progression to a clinical disease, we consider that the knowledge of biomarker status should not be disclosed at a preclinical stage, except when well-informed subjects request the information, in cases of high level of social responsibility and cognitive demand or in case of inclusion in research protocols and clinical trials.
5.5. Future direction for research in the field
Currently, substantial advances in discovery, development, and validation of AD-related biomarkers have paved the way for the era of multimodal investigations integrating modalities and different biological fluids [263,281–283]. They are derived from neurogenetics [284–286], structural or functional or metabolic neuroimaging and neurophysiology [287,288], neurochemistry on biofluids [289–291], namely CSF [94,292,293] and blood (plasma/serum) [218,232,236,237]. The predictive performance of this multimodal approach is not definitely established and should be analyzed in light of our expectation (in terms of sensitivity and/or specificity) and their condition (i.e. isolated or in combination [294,295]. Additionally, several critical opinions of regulatory agencies and industry stakeholders in AD biomarker discovery area have been reported [2,296,297].
5.5.1. Future perspectives
Any prospective plan to substantially promote scientific progress toward improved early characterization of preclinical AD, through improved detection and subsequent “therapy development”, must address some crucial challenges. The major scientific-technical barriers that must be surmounted include
The formulation of new ideas and/or conceptual models on the origins of AD. Current treatments are woefully inadequate to provide lasting symptomatic relief. Also present approaches and/or paradigms for therapy development, primarily based on prevailing ideas on etiology have not been productive; thus, there is a need to re-evaluate our thinking and assumptions about the pathogenesis of dementia/AD.
Untangling the biologic complexities of a polygenic brain condition. AD is manifested in different forms, where multiple factors (genes, life-style, co-morbid conditions, and so forth] interact to generate disease/syndrome. Thus, one of the important challenges is the need for: (1) discoveries-knowledge to understand the new biology of complex brain disorders and (2) the development of appropriate computational resource-tools to solve the interactions among all key variables from the perspective of systems biology and systems neurophysiology.
The development-validation of novel technologies/computational algorithms for accurate identification of asymptomatic individuals at risk. The actual degenerative process of AD starts several decades before the clinical symptoms appear or before any behavioral and/or clinical signs can be detected with the current technologies. Thus, one of the important challenges is to detect or measure earliest and smallest changes that will accurately predict with high degree of certainty an individual’s risk profile, that is, prognosis for future expression of symptom or disease onset. This problem will require new computational capabilities/resources for accurately predicting the consequences of early molecular changes [biomarkers] on subsequent expression of clinical feature-symptoms, that is, understand the causal relationship between early biological indicators and clinical features of the disorder.
The creation of large international databases with shared research resources and infrastructures. To attain the strategic objectives of a well-harmonized international initiative to accelerate therapy development will require, as an essential prerequisite, ready availability of very large numbers of well-characterized and well-diversified subjects-volunteers—thus, the need to create an international-shared resource capability for collecting multinational longitudinal data on large numbers of healthy aging and preclinical AD subjects. This broad social-scientific initiative should proceed in concert with advances in basic understanding of the biology of AD that includes developing animal models that better reflect the disease in humans, understanding interactions between b-amyloid and tau, understanding tau templating, and transmission and cell-signaling pathways.
GLOSSARY.
Lexicon used in the article.
State versus stage
“State” refers to a given pathophysiological framework (state of asymptomatic at-risk versus state of Alzheimer’s disease), whereas “stage” refers to a degree of disease progression within a given state (preclinical, prodromal, and dementia for AD).
Alzheimer’s disease
AD is defined by the positivity of biomarkers of both amyloidopathy (A1) and tauopathy (T1) in line with the pathologic definition of the disease. Therefore, two phases of the disease can be distinguished in the continuum:
A clinical stage (“clinical AD”) defined by the occurrence of the clinical phenotype of AD (either typical or atypical) and which encompasses both the prodromal and the dementia stages;
A preclinical stage (“preclinical AD”) before the onset of the clinical phenotype. The development of biomarkers of Alzheimer pathology makes possible to recognize AD before the onset of the specific clinical phenotype.
Asymptomatic at risk for AD
This state consists of cognitively normal individuals for whom the biomarker pattern is insufficient to reach the above definition of AD. They can be characterized by the positivity of the pathophysiological biomarker (i.e. either “Asymptomatic A+” or “Asymptomatic T+”).
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed the literature using traditional (PubMed) sources and meeting abstracts. During the past decade, a conceptual shift occurred in Alzheimer’s disease (AD) area considering the disease as a continuum. Thanks to evolving biomarker research and substantial discoveries, it is now possible to identify the disease at the preclinical stage, before the occurrence of the first clinical symptoms.
Interpretation: The preclinical stage of AD has become a major research focus as the field postulates that early intervention may offer the best chance of therapeutic success. A clarification is needed about the definitions and lexicon, the natural history, the markers of progression at this asymptomatic stage. This article aims at addressing all these issues by providing for each of them an updated review of the literature.
Future directions: Any prospective plan to substantially promote scientific progress toward improved early characterization of preclinical AD must address crucial challenges.
Acknowledgments
H.H. is supported by the AXA Research Fund, the Fondation Université Pierre et Marie Curie, and the “Fondation pour la Recherche sur Alzheimer”, Paris, France. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06. S.L. is supported by the European Medical Information Framework (EMIF). R.S. is supported by the National Institute on Aging and Alzheimer’s Association.
The authors acknowledge the helpful contribution of Zaven Khachaturian as an advisor to the Working Group in charge of the article. The meeting was supported by the French International Foundation for Research in Alzheimer’s disease (IFRAD).
B.D. serves as member of advisory board for Avid/Lilly, Roche, and Boehringer Ingelheim, and he receives research support from Amivid and Pfizer for his institution. H.H. serves as Senior Associate Editor for the journal Alzheimer’s & Dementia; he has been a scientific consultant and/or speaker and/or attended scientific advisory boards of Axovant, Anavex, Eli Lilly and company, GE Healthcare, Cytox, Jung Diagnostics, Roche, Biogen-Idec, Takeda-Zinfandel, and Oryzon and receives research support from the Association for Alzheimer Research (Paris), Pierre and Marie Curie University (Paris), Pfizer & Avid (paid to institution) and has patents as co-inventor but received no royalties. H.F. is a co-investigator on clinical trials sponsored by TauRx, Hoffmann LaRoche, and US National Institutes of Health/Lilly Pharmaceuticals with funding to the UBC Alzheimer Clinic. He receives peer reviewed funding from the Canadian Institutes of Health Research, National Institute on Aging and Brain Canada MIRI. He has received personal fees for consulting for the Bluefield Foundation to Cure Frontotemporal dementia advisory board (2012), for Danone Nutricia (2012), for the Innovative Medicines Initiative 2014 and for being a member of the Scientific Advisory Board of the Tau Consortium 2015–2016. He has provided consultations through UBC service agreements for the following: Hakko Kyowa Kirin Pharmaceuticals, Lilly Pharmaceutical, General Electric, Biogen-Idec, Eisai Pharmaceuticals, Banner Institute and Genentech Pharmaceuticals, Merck Pharmaceuticals, Arena Pharmaceuticals, ISIS Pharmaceuticals, and Eisai DSMB. No personal compensation was received; all funds received by UBC. He has a patent issued for Detecting and Treating Dementia Serial #12/3–2691 US Patent no. PCT US2007/07008 Washington DC. No funds received. He receives royalties from the book Atlas of Alzheimer’s Disease (2007) Informa Health London. P.S. has received research support from Merck, GE Healthcare, and Piramal (paid to institution) and has consulting agreements with Takeda, Lilly, Nutricia, Probiodrug, and EIP Pharma (paid to institution). He reports no conflicts with the present work. P.A. has served as a consultant to the following companies: NeuroPhage, Eisai, Bristol-Myers Squibb, Eli Lilly, Merck, Roche, Genentech, Abbott, Pfizer, Novartis, AstraZeneca, Janssen, Ichor, Lundbeck, Biogen, iPerian, Probiodrug, Anavex, Abbvie, Janssen, Cohbar, aTyr, Axovant, and CogRx. P.A. receives research support from Eli Lilly, Janssen, the Alzheimer’s Association, and the NIH. S.A. reported support from Beaufour Ipsen Pharma, Pierre Fabre, Lilly, Nestle, Servier, and Sanofi outside the submitted work. H.Ba., H.Be., and L.B. declare no conflicts of interest. K.Bl. has served as a consultant for Alzheon, Eli Lilly, Novartis, Roche Diagnostics, and Sanofi-Aventis, at Advisory Boards for Amgen and IBL International, and given lectures for Fujirebio Europe and Lundbeck. K.Bl.’s research team has received grants for collaborative research projects from Eli Lilly and Roche Diagnostics. K.Br. declares no conflict of interests in relation with the article. Personal views are presented, which cannot be regarded as official opinion of the BfArM or EMA. E.C. has received funding from the grant Conv. n.130/GR-2011–02350494 funded by the Italian Ministry of Health (Bando Giovani Ricercatori 2011–2012). S.C. is supported by an Alzheimer’s Research UK Senior Research Fellowship and ESRC/NIHR (ES/L001810/1) and EPSRC (EP/M006093/1) grants. J.-F.D. has received research grants from IPSEN and Roche. C.D. is the administrator of the Brain Bank GIE NeuroCEB funded by the patients’ associations Fondation ARSEP, France Alzheimer, France Parkinson, Comprendre les syndromes cérébelleux.
He has received financial supports from grants of the Agence Nationale de la Recherche (ANR) and from the foundation Plan Alzheimer. S.E. has no conflict of interest. G.F. has served in advisory boards for Lilly, BMS, Bayer, Lundbeck, Elan, Astra Zeneca, Pfizer, TauRx, Wyeth, GE, Baxter and received unrestricted grants from Wyeth Int.l, Lilly Int.l, Lundbeck Italia, GE Int.l, Avid/Lilly, Roche, Piramal, and the Alzheimer’s Association. S.G. is a member of scientific advisory board of TauRx, Roche, Boeringer, Takeda, and he has done paid lectures for EverPharma, Schwabe. R.G. declares no conflict of interest on the current work. He is a former employee of sanofi, holding stocks of sanofi. A.A.G. receives research support from Probiodrug AG and Boehringer Ingelheim via the VUmc Alzheimer center. She reports no conflicts with the present work. M.-O.H. received honoraria from MIRA-MAL and ROCHE as a speaker and from GE Healthcare as a consultant. D.H. is a co-founder, has equity interests, and is on the scientific advisory board of C2N Diagnostics, LLC. He consults for Genentech, AbbVie, Eli Lilly, Denali, Novartis, and Neurophage. M.K. declares grant support from Academy of Finland, Swedish Research Council, Center of Innovative Medicine (CIMED), EU Joint Programme—Neurodegenerative Disease Research (JPND), Innovative Medicine Initatives (IMI), AXA Research Fund, Alzheimer foundation. M.K. has served as a consultant for Pfizer, Merz, Alzheon, Nutricia, Nestle, and Neurotrack. She reports no conflicts with the present work. S.L. has received lecture honoraria from Roche. J.L.M. has no conflict of interests. S.E.O. has no conflicts of interest in relation with the article. G.D.R. receives research support from Avid Radiopharmaceuticals and has received speaking honoraria from GE Healthcare, Piramal Imaging, and Medscape. C.R. is a member of Advisory Board for Janssen and Grant recipient—GE Healthcare, Avid Radiopharmaceuticals, Piramal Imaging, Navidea Biopharmaceuticals, Astra Zeneca. S.S. received research support and consulting fees from Biogen, Merck, Genentech, Roche, and Lilly and research support from Avid, Novartis, and Functional Neuromodulation. He holds no patents and receives no royalties, and he reports no direct conflicts with this work. L.S.S. (within the past 3 years) has received grant or research support for industry-sponsored studies from Pfizer, Baxter, Eli Lilly, Forum, Lundbeck, Merck, Novartis (Alzheimer Prevention Initiative and NIH), Roche/Genentech, TauRx; for NIH sponsored research, USC ADRC, ADCS (UCSD), ADNI (NCIRE), Banner Alzheimer Prevention Initiative, P50 AG05142, R01 AG033288, R01 AG037561, UF1 AG046148; from the State of California, the California Alzheimer Disease Center (CADC), and California Institute for Regenerative Medicine (CIRM). L.S.S. within the past 3 years, has consulted or served on committees for AC Immune, Accera, Avraham, Axovant, Baxter, Boehringer Ingelheim, Bracket, Cerespir, Cognition, Forum, Insys, Medavante, Merck, Neurim, Novartis, Roche, Stemedica, Takeda, TauRx, Transition, vTv Therapeutics, Toyama/FujiFilm, Zinfandel. R.S. has research support from Eli Lilly and Co. and Janssen Pharmaceuticals. She has served as a consultant for Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. M.T. and M.C. declares no conflicts of interest. J.C. has provided consultation to Abbvie, Acadia, Actinogen, ADAMAS, Alzheon, Anavex, Avanir, Biogen-Idec, Biotie, Boehinger-Ingelheim, Chase, Eisai, Forum, Genentech, Grifols, Intracellular Therapies, Lilly, Lundbeck, Merck, Neurotrope, Novartis, Nutricia, Otsuka, QR Pharma, Resverlogix, Roche, Servier, Suven, Takeda, and Toyoma companies and to GE Health-care and MedAvante. J.C. owns stock in ADAMAS, Prana, Sonexa, MedA-vante, Neurotrax, and Neurokos and the copyright of the Neuropsychiatric Inventory. Cliff Jack provided consultation to Eli Lily.
References
- [1].Hubbard BM, Fenton GW, Anderson JM. A quantitative histological study of early clinical and preclinical Alzheimer’s disease. Neuropathol Appl Neurobiol 1990;16:111–21. [DOI] [PubMed] [Google Scholar]
- [2].Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 2010;9:560–74. [DOI] [PubMed] [Google Scholar]
- [3].Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med 2014; 6:226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 2012;69:98–106. [DOI] [PubMed] [Google Scholar]
- [6].Delaere O, Mennen U, Van Heerden W, Raubenheimer E, Wiese AM, Boomker D. Side-effects of rinsing fluids on the rat femoral artery. A histological and electron microscopic study. J Hand Surg Br 1997; 22:568–73. [DOI] [PubMed] [Google Scholar]
- [7].Jack CR Jr, Vemuri P, Wiste HJ, Weigand SD, Aisen PS, Trojanowski JQ, et al. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol 2011;68:1526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 2010;9:1118–27. [DOI] [PubMed] [Google Scholar]
- [9].Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010;9:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–29. [DOI] [PubMed] [Google Scholar]
- [12].Jack CR Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron 2013;80:1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 2013;81:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 2015;16:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heilbronner G, Eisele YS, Langer F, Kaeser SA, Novotny R, Nagarathinam A, et al. Seeded strain-like transmission of beta-amyloid morphotypes in APP transgenic mice. EMBO Rep 2013; 14:1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011; 121:571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci 2012;107:79–100. [DOI] [PubMed] [Google Scholar]
- [18].Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One 2010;5:e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].International Genomics of Alzheimer’s Disease Consortium. Convergent genetic and expression data implicate immunity in Alzheimer’s disease. Alzheimers Dement 2015;11:658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 2014;13:788–94. [DOI] [PubMed] [Google Scholar]
- [21].Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study. Lancet Neurol 2013;12:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau PET imaging in aging and early Alzheimer’s disease. Ann Neurol 2016;79:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic bio-markers. Lancet Neurol 2013;12:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Delaere P, He Y, Fayet G, Duyckaerts C, Hauw JJ. Beta A4 deposits are constant in the brain of the oldest old: An immunocytochemical study of 20 French centenarians. Neurobiol Aging 1993;14:191–4. [DOI] [PubMed] [Google Scholar]
- [26].Cavedo E, Pievani M, Boccardi M, Galluzzi S, Bocchetta M, Bonetti M, et al. Medial temporal atrophy in early and late-onset Alzheimer’s disease. Neurobiol Aging 2014;35:2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jack CR, Bennett DA, Blennow K, Carrillo M, Feldman H, Frisoni G, et al. Amyloid/tau/neurodegeneration (A/T/N): An unbiased descriptive classification scheme for biomarkers commonly used in Alzheimers disease research. submitted.
- [28].Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 2011;121:171–81. [DOI] [PubMed] [Google Scholar]
- [29].Duyckaerts C Tau pathology in children and young adults: can you still be unconditionally baptist? Acta Neuropathol 2011;121:145–7. [DOI] [PubMed] [Google Scholar]
- [30].Mukaetova-Ladinska EB, Harrington CR, Roth M, Wischik CM. Alterations in tau protein metabolism during normal aging. Dementia 1996;7:95–103. [DOI] [PubMed] [Google Scholar]
- [31].Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014; 128:755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M, et al. PART is part of Alzheimer disease. Acta Neuropathol 2015; 129:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 2011;69:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997;18:351–7. [DOI] [PubMed] [Google Scholar]
- [35].Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol 1985;42:1097–105. [DOI] [PubMed] [Google Scholar]
- [36].Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991;41:479–86. [DOI] [PubMed] [Google Scholar]
- [37].Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron 2010;68:270–81. [DOI] [PubMed] [Google Scholar]
- [38].Holmes C, Lovestone S. The clinical phenotype of familial and sporadic late onset Alzheimer’s disease. Int J Geriatr Psychiatry 2002; 17:146–9. [DOI] [PubMed] [Google Scholar]
- [39].Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014;83:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haupt M, Kurz A, Pollmann S, Romero B. Alzheimer’s disease: Identical phenotype of familial and non-familial cases. J Neurol 1992; 239:248–50. [DOI] [PubMed] [Google Scholar]
- [41].Marechal L, Campion D, Hannequin D. Familial forms of Alzheimer’s disease. Presse Med 2003;32:756–63. [PubMed] [Google Scholar]
- [42].Nochlin D, van Belle G, Bird TD, Sumi SM. Comparison of the severity of neuropathologic changes in familial and sporadic Alzheimer’s disease. Alzheimer Dis Assoc Disord 1993;7:212–22. [PubMed] [Google Scholar]
- [43].Bohnen NI, Djang DS, Herholz K, Anzai Y, Minoshima S. Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med 2012;53:59–71. [DOI] [PubMed] [Google Scholar]
- [44].Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 2009;29:1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jagust W Amyloid + activation = Alzheimer’s? Neuron 2009; 63:141–3. [DOI] [PubMed] [Google Scholar]
- [46].Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015;313:1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1–42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry 2007; 78:461–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007;64:343–9. [DOI] [PubMed] [Google Scholar]
- [49].Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebro-spinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord 2007;24:118–24. [DOI] [PubMed] [Google Scholar]
- [50].Fortea J, Llado A, Bosch B, Antonell A, Oliva R, Molinuevo JL, et al. Cerebrospinal fluid biomarkers in Alzheimer’s disease families with PSEN1 mutations. Neurodegener Dis 2011;8:202–7. [DOI] [PubMed] [Google Scholar]
- [51].van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid Abeta42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement 2013;9:481–7. [DOI] [PubMed] [Google Scholar]
- [52].Fortea J, Sala-Llonch R, Bartres-Faz D, Llado A, Sole-Padulles C, Bosch B, et al. Cognitively preserved subjects with transitional cerebrospinal fluid ss-amyloid 1–42 values have thicker cortex in Alzheimer’s disease vulnerable areas. Biol Psychiatry 2011;70:183–90. [DOI] [PubMed] [Google Scholar]
- [53].Rami L, Sala-Llonch R, Sole-Padulles C, Fortea J, Olives J, Llado A, et al. Distinct functional activity of the precuneus and posterior cingulate cortex during encoding in the preclinical stage of Alzheimer’s disease. J Alzheimers Dis 2012;31:517–26. [DOI] [PubMed] [Google Scholar]
- [54].Roe CM, Fagan AM, Grant EA, Holtzman DM, Morris JC. CSF bio-markers of Alzheimer disease: “Noncognitive” outcomes. Neurology 2013;81:2028–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].van Rossum IA, Vos SJ, Burns L, Knol DL, Scheltens P, Soininen H, et al. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology 2012;79:1809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Rossum IA, Visser PJ, Knol DL, van der Flier WM, Teunissen CE, Barkhof F, et al. Injury markers but not amyloid markers are associated with rapid progression from mild cognitive impairment to dementia in Alzheimer’s disease. J Alzheimers Dis 2012;29:319–27. [DOI] [PubMed] [Google Scholar]
- [57].Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-beta changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol 2013;126:631–41. [DOI] [PubMed] [Google Scholar]
- [58].Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992;42:631–9. [DOI] [PubMed] [Google Scholar]
- [59].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- [60].van Harten AC, Kester MI, Visser PJ, Blankenstein MA, Pijnenburg YA, van derFlier WM, et al. Tauand p-tau as CSF biomarkers in dementia:A meta-analysis. Clin Chem Lab Med 2011;49:353–66. [DOI] [PubMed] [Google Scholar]
- [61].Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 2006; 129:3035–41. [DOI] [PubMed] [Google Scholar]
- [62].Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009;66:382–9. [DOI] [PubMed] [Google Scholar]
- [63].Duits FH, Teunissen CE, Bouwman FH, Visser PJ, Mattsson N, Zetterberg H, et al. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement 2014; 10:713–7232. [DOI] [PubMed] [Google Scholar]
- [64].Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, et al. CSF biomarkers for the differential diagnosis of Alzheimer’s disease: A large-scale international multicenter study. Alzheimers Dement 2015;11:1306–15. [DOI] [PubMed] [Google Scholar]
- [65].Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: Statistical and pathological evaluation. Brain 2015;138:2020–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 2015;138:1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: A prospective cohort study. Lancet Neurol 2012; 11:669–78. [DOI] [PubMed] [Google Scholar]
- [68].Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol 2015;72:287–94. [DOI] [PubMed] [Google Scholar]
- [69].Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: Phase 3 study. Alzheimers Dement 2015; 11:964–74. [DOI] [PubMed] [Google Scholar]
- [70].Thal DR, Beach TG, Zanette M, Heurling K, Chakrabarty A, Ismail A, et al. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement 2015;11:975–85. [DOI] [PubMed] [Google Scholar]
- [71].Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, et al. Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging 2014;41:1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MD Sr, Jagust WJ, et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 2015; 11:1–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011;77:2034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: Concordance with visual image reads. J Nucl Med 2014;55:1623–8. [DOI] [PubMed] [Google Scholar]
- [75].Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, et al. Classification of amyloid-positivity in controls: Comparison of visual read and quantitative approaches. Neuroimage 2013;71:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schreiber S, Landau SM, Fero A, Schreiber F, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative. Comparison of visual and quantitative Florbetapir F 18 positron emission tomography analysis in predicting mild cognitive impairment outcomes. JAMA Neurol 2015;72:1183–90. [DOI] [PubMed] [Google Scholar]
- [77].Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, et al. Concordance between cerebrospinal fluid bio-markers and [11C]PIB PET in a memory clinic cohort. J Alzheimers Dis 2014;41:801–7. [DOI] [PubMed] [Google Scholar]
- [78].Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol 2014;71:1282–9. [DOI] [PubMed] [Google Scholar]
- [79].Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 2013;74:826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease. Brain 2015; 138:772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 2013; 9:666–76. [DOI] [PubMed] [Google Scholar]
- [82].Okamura N, Furumoto S, Harada R, Tago T, Yoshikawa T, Fodero-Tavoletti M, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med 2013;54:1420–7. [DOI] [PubMed] [Google Scholar]
- [83].Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis 2013;34:457–68. [DOI] [PubMed] [Google Scholar]
- [84].Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alz heimer patients compared to normal controls. Neuron 2013; 79:1094–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Okamura N, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Harada R, Yates P, et al. Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain 2014; 137:1762–71. [DOI] [PubMed] [Google Scholar]
- [86].Ossenkoppele R, Schonhaut DR, Baker SL, O’Neil JP, Janabi M, Ghosh PM, et al. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann Neurol 2015;77:338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, Anckarsater R, et al. Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Vanderstichele H, Stoops E, Vanmechelen E, Jeromin A. Potential sources of interference on Abeta immunoassays in biological samples. Alzheimers Res Ther 2012;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement 2013;9:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Carrillo MC, Blennow K, Soares H, Lewczuk P, Mattsson N, Oberoi P, et al. Global standardization measurement of cerebral spinal fluid for Alzheimer’s disease: an update from the Alzheimer’s Association Global Biomarkers Consortium. Alzheimers Dement 2013; 9:137–40. [DOI] [PubMed] [Google Scholar]
- [92].Teunissen CE, Verwey NA, Kester MI, van Uffelen K, Blankenstein MA. Standardization of Assay Procedures for Analysis of the CSF Biomarkers Amyloid beta((1–42)), Tau, and Phosphorylated Tau in Alzheimer’s Disease: Report of an International Workshop. Int J Alzheimers Dis 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 2012;8:65–73. [DOI] [PubMed] [Google Scholar]
- [94].Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010; 6:131–44. [DOI] [PubMed] [Google Scholar]
- [95].Mattsson N, Zetterberg H. What is a certified reference material? Biomark Med 2012;6:369–70. [DOI] [PubMed] [Google Scholar]
- [96].Mattsson N, Zegers I, Andreasson U, Bjerke M, Blankenstein MA, Bowser R, et al. Reference measurement procedures for Alzheimer’s disease cerebrospinal fluid biomarkers: definitions and approaches with focus on amyloid beta42. Biomark Med 2012;6:409–17. [DOI] [PubMed] [Google Scholar]
- [97].Leinenbach A, Pannee J, Dulffer T, Huber A, Bittner T, Andreasson U, et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin Chem 2014;60:987–94. [DOI] [PubMed] [Google Scholar]
- [98].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement 2015. [DOI] [PubMed] [Google Scholar]
- [99].Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 2007;130:2837–44. [DOI] [PubMed] [Google Scholar]
- [100].Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ. The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement 2013; 9:687–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol 2014;71:1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014;137:221–31. [DOI] [PubMed] [Google Scholar]
- [103].Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 2012;72:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol 2009; 66:1476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, et al. Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. Neurobiol Aging 2015; 36:1231–8. [DOI] [PubMed] [Google Scholar]
- [106].Knopman DS, Jack CR Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Toledo JB, Weiner MW, Wolk DA, Da X, Chen K, Arnold SE, et al. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun 2014;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lim YY, Villemagne VL, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. APOE epsilon4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging 2015;36:1239–44. [DOI] [PubMed] [Google Scholar]
- [109].Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol 2013;12:357–67. [DOI] [PubMed] [Google Scholar]
- [110].Jicha GA, Abner EL, Schmitt FA, Kryscio RJ, Riley KP, Cooper GE, et al. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol Aging 2012;33:622.e1–62216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Toledo JB, Da X, Weiner MW, Wolk DA, Xie SX, Arnold SE, et al. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol 2014;127:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009; 132:1310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, et al. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 2014; 82:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010;67:353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Roe CM, Fagan AM, Grant EA, Marcus DS, Benzinger TL, Mintun MA, et al. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Arch Neurol 2011;68:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, et al. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol 2012;69:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol 2010;68:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 2014;35:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997;277:813–7. [PubMed] [Google Scholar]
- [120].Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA Neurol 2015;72:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010; 31:1275–83. [DOI] [PubMed] [Google Scholar]
- [122].Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci 2001;17:101–18. [DOI] [PubMed] [Google Scholar]
- [123].Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Jack CR Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol 2014;13:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, et al. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302–9. [DOI] [PubMed] [Google Scholar]
- [126].Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 2009;132:1067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Paternico D, Galluzzi S, Drago V, Bocchio-Chiavetto L, Zanardini R, Pedrini L, et al. Cerebrospinal fluid markers for Alzheimer’s disease in a cognitively healthy cohort of young and old adults. Alzheimers Dement 2012;8:520–7. [DOI] [PubMed] [Google Scholar]
- [128].Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci 2014;37:79–100. [DOI] [PubMed] [Google Scholar]
- [129].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261:921–3. [DOI] [PubMed] [Google Scholar]
- [130].Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993;43:1467–72. [DOI] [PubMed] [Google Scholar]
- [131].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–56. [PubMed] [Google Scholar]
- [132].Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 1993;11:575–80. [DOI] [PubMed] [Google Scholar]
- [133].Chetelat G, Fouquet M. Neuroimaging biomarkers for Alzheimer’s disease in asymptomatic APOE4 carriers. Rev Neurol (Paris) 2013; 169:729–36. [DOI] [PubMed] [Google Scholar]
- [134].Leoni V The effect of apolipoprotein E (ApoE) genotype on bio-markers of amyloidogenesis, tau pathology and neurodegeneration in Alzheimer’s disease. Clin Chem Lab Med 2011;49:375–83. [DOI] [PubMed] [Google Scholar]
- [135].Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med 2008; 12:2762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 2009;41:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bu G Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009; 10:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Schipper HM. Apolipoprotein E: implications for AD neurobiology, epidemiology and risk assessment. Neurobiol Aging 2011; 32:778–90. [DOI] [PubMed] [Google Scholar]
- [139].Sando SB, Melquist S, Cannon A, Hutton ML, Sletvold O, Saltvedt I, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BMC Neurol 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 2013; 45:1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Imtiaz B, Tolppanen AM, Kivipelto M, Soininen H. Future directions in Alzheimer’s disease from risk factors to prevention. Biochem Pharmacol 2014;88:661–70. [DOI] [PubMed] [Google Scholar]
- [142].Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Munoz Sanchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry 2015;30:234–46. [DOI] [PubMed] [Google Scholar]
- [143].Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015; 138:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 2013;80:1888–94. [DOI] [PubMed] [Google Scholar]
- [146].Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, Erickson KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol Aging 2014;35(Suppl 2):S74–8. [DOI] [PubMed] [Google Scholar]
- [147].Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M, et al. Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med 2014;275:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a rand-omised controlled trial. Lancet 2015;385:2255–63. [DOI] [PubMed] [Google Scholar]
- [149].Matthews DC, Davies M, Murray J, Williams S, Tsui WH, Li Y, et al. Physical Activity, Mediterranean Diet and Biomarkers-Assessed Risk of Alzheimer’s: A Multi-Modality Brain Imaging Study. Adv J Mol Imaging 2014;4:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 2011;108:3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 2014;83:1753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Mosconi L, Murray J, Tsui WH, Li Y, Davies M, Williams S, et al. Mediterranean Diet and Magnetic Resonance Imaging-Assessed Brain Atrophy in Cognitively Normal Individuals at Risk for Alzheimer’s Disease. J Prev Alzheimers Dis 2014;1:23–32. [PMC free article] [PubMed] [Google Scholar]
- [153].Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O’Neil JP, et al. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol 2012;69:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 2001; 57:2236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wilson RS. Elderly women with larger social networks are less likely to develop dementia. Evid Based Ment Health 2009;12:22. [DOI] [PubMed] [Google Scholar]
- [157].Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med 2006;36:441–54. [DOI] [PubMed] [Google Scholar]
- [158].Suchy Y, Kraybill ML, Franchow E. Instrumental activities of daily living among community-dwelling older adults: discrepancies between self-report and performance are mediated by cognitive reserve. J Clin Exp Neuropsychol 2011;33:92–100. [DOI] [PubMed] [Google Scholar]
- [159].Lo RY, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord 2013;27:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012;488:96–9. [DOI] [PubMed] [Google Scholar]
- [161].Ally BA, Jones GE, Cole JA, Budson AE. The P300 component in patients with Alzheimer’s disease and their biological children. Biol Psychol 2006;72:180–7. [DOI] [PubMed] [Google Scholar]
- [162].Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 2012;11:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014;71:961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol 2015;72:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014;6:228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Donohue MC, Gamst AC, Thomas RG, Xu R, Beckett L, Petersen RC, et al. The relative efficiency of time-to-threshold and rate of change in longitudinal data. Contemp Clin Trials 2011; 32:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging 2010; 31:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging 2010;31:1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, et al. Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol 2013;70:903–11. [DOI] [PubMed] [Google Scholar]
- [170].Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, et al. Selective disruption of the cerebral neocortex in Alzheimer’s disease. PLoS One 2010;5:e12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, et al. Brain structural alterations before mild cognitive impairment. Neurology 2007;68:1268–73. [DOI] [PubMed] [Google Scholar]
- [172].Burgmans S, van Boxtel MP, Smeets F, Vuurman EF, Gronenschild EH, Verhey FR, et al. Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiol Aging 2009;30:1413–9. [DOI] [PubMed] [Google Scholar]
- [173].Lazarczyk MJ, Hof PR, Bouras C, Giannakopoulos P. Preclinical Alzheimer disease: identification of cases at risk among cognitively intact older individuals. BMC Med 2012;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Rizk-Jackson A, Insel P, Petersen R, Aisen P, Jack C, Weiner M. Early indications of future cognitive decline: stable versus declining controls. PLoS One 2013;8:e74062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ewers M, Brendel M, Rizk-Jackson A, Rominger A, Bartenstein P, Schuff N, et al. Reduced FDG-PET brain metabolism and executive function predict clinical progression in elderly healthy subjects. Neuroimage Clin 2014;4:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, et al. Clinical Core of the Alzheimer’s Disease Neuro-imaging Initiative: progress and plans. Alzheimers Dement 2010; 6:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004;101:4637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 2007; 104:18760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Zhang D, Wang Y, Zhou L, Yuan H, Shen D, Alzheimer’s Disease Neuroimaging Initiative. Multimodal classification of Alzheimer’s disease and mild cognitive impairment. Neuroimage 2011; 55:856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A 2008;105:2181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neuro-degenerative diseases target large-scale human brain networks. Neuron 2009;62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Toussaint PJ, Maiz S, Coynel D, Doyon J, Messe A, de Souza LC, et al. Characteristics of the default mode functional connectivity in normal ageing and Alzheimer’s disease using resting state fMRI with a combined approach of entropy-based and graph theoretical measurements. Neuroimage 2014;101:778–86. [DOI] [PubMed] [Google Scholar]
- [183].Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Pihlajamaki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals. Behav Neurol 2009;21:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].van Straaten EC, Scheltens P, Gouw AA, Stam CJ. Eyes-closed task-free electroencephalography in clinical trials for Alzheimer’s disease: an emerging method based upon brain dynamics. Alzheimers Res Ther 2014;6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Golob EJ, Ringman JM, Irimajiri R, Bright S, Schaffer B, Medina LD, et al. Cortical event-related potentials in preclinical familial Alzheimer disease. Neurology 2009;73:1649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Quiroz YT, Ally BA, Celone K, McKeever J, Ruiz-Rizzo AL, Lopera F, et al. Event-related potential markers of brain changes in preclinical familial Alzheimer disease. Neurology 2011;77:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Alonso JF, Poza J, Mananas MA, Romero S, Fernandez A, Hornero R. MEG connectivity analysis in patients with Alzheimer’s disease using cross mutual information and spectral coherence. Ann Biomed Eng 2011;39:524–36. [DOI] [PubMed] [Google Scholar]
- [189].Bajo R, Maestu F, Nevado A, Sancho M, Gutierrez R, Campo P, et al. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J Alzheimers Dis 2010;22:183–93. [DOI] [PubMed] [Google Scholar]
- [190].Hsiao FJ, Wang YJ, Yan SH, Chen WT, Lin YY. Altered oscillation and synchronization of default-mode network activity in mild Alzheimer’s disease compared to mild cognitive impairment: an electro-physiological study. PLoS One 2013;8:e68792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, et al. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int J Psychophysiol 2015. [DOI] [PubMed] [Google Scholar]
- [192].Stam CJ, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, et al. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuro-image 2006;32:1335–44. [DOI] [PubMed] [Google Scholar]
- [193].Engels MM, Stam CJ, van der Flier WM, Scheltens P, de Waal H, van Straaten EC. Declining functional connectivity and changing hub locations in Alzheimer’s disease: an EEG study. BMC Neurol 2015; 15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, Fou-bert-Samier A, et al. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain 2014;137:1167–75. [DOI] [PubMed] [Google Scholar]
- [195].Henry J, Crawford JR. A meta-analytic review of verbal fluency deficits in depression. J Clin Exp Neuropsychol 2005;27:78–101. [DOI] [PubMed] [Google Scholar]
- [196].Beheydt LL, Schrijvers D, Docx L, Bouckaert F, Hulstijn W, Sabbe B. Psychomotor retardation in elderly untreated depressed patients. Front Psychiatry 2014;5:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev 2011; 4:42–56. [DOI] [PubMed] [Google Scholar]
- [198].Koster DP, Higginson CI, MacDougall EE, Wheelock VL, Sigvardt KA. Subjective Cognitive Complaints in Parkinson Disease Without Dementia: A Preliminary Study. Appl Neuropsychol Adult 2015;22:287–92. [DOI] [PubMed] [Google Scholar]
- [199].Riley KP, Jicha GA, Davis D, Abner EL, Cooper GE, Stiles N, et al. Prediction of preclinical Alzheimer’s disease: longitudinal rates of change in cognition. J Alzheimers Dis 2011;25:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013;80:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol 2008;64:492–8. [DOI] [PubMed] [Google Scholar]
- [202].Buckley RF, Ellis KA, Ames D, Rowe CC, Lautenschlager NT, Maruff P, et al. Phenomenological characterization of memory complaints in preclinical and prodromal Alzheimer’s disease. Neuropsychology 2015;29:571–81. [DOI] [PubMed] [Google Scholar]
- [203].Ringman JM, Liang LJ, Zhou Y, Vangala S, Teng E, Kremen S, et al. Early behavioural changes in familial Alzheimer’s disease in the Dominantly Inherited Alzheimer Network. Brain 2015; 138:1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimers Dement 2014;10:666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Rentz DM, Parra Rodriguez MA, Amariglio R, Stern Y, Sperling R, Ferris S. Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: a selective review. Alzheimers Res Ther 2013;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Derby CA, Burns LC, Wang C, Katz MJ, Zimmerman ME, L’Italien G, et al. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology 2013;80:1307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology 1988;38:900–3. [DOI] [PubMed] [Google Scholar]
- [208].Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology 2007;69:1859–67. [DOI] [PubMed] [Google Scholar]
- [209].Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain 2010;133:2702–13. [DOI] [PubMed] [Google Scholar]
- [210].Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012; 50:2880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol 2010;67:317–24. [DOI] [PubMed] [Google Scholar]
- [212].Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 2014;10:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Gallassi R, Oppi F, Poda R, Scortichini S, Stanzani Maserati M, Marano G, et al. Are subjective cognitive complaints a risk factor for dementia? Neurol Sci 2010;31:327–36. [DOI] [PubMed] [Google Scholar]
- [214].Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 2012; 79:1570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand 2014;130:439–51. [DOI] [PubMed] [Google Scholar]
- [216].Pike KE, Ellis KA, Villemagne VL, Good N, Chetelat G, Ames D, et al. Cognition and beta-amyloid in preclinical Alzheimer’s disease: data from the AIBL study. Neuropsychologia 2011;49:2384–90. [DOI] [PubMed] [Google Scholar]
- [217].Berman S, Lowenthal JP, Sorrentino JV, White AB. A safety test for Eastern equine encephalomyelitis vaccine. Appl Microbiol 1967; 15:968–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Gupta VB, Sundaram R, Martins RN. Multiplex biomarkers in blood. Alzheimers Res Ther 2013;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology 2012;79:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].O’Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, et al. A serum protein-based algorithm for the detection of Alzheimer disease. Arch Neurol 2010;67:1077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Doecke J, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, et al. Blood-based protein biomarkers for the diagnosis of Alzheimer’s disease. Arch Neurol 2012;69:1318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Sattlecker M, Kiddle SJ, Newhouse S, Proitsi P, Nelson S, Williams S, et al. Alzheimer’s disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement 2014; 10:724–34. [DOI] [PubMed] [Google Scholar]
- [223].Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- [224].O’Bryant SE, Xiao G, Barber R, Huebinger R, Wilhelmsen K, Edwards M, et al. A Blood-Based Screening Tool for Alzheimer’s Disease That Spans Serum and Plasma: Findings from TARC and ADNI. PLoS One 2011;6:e28092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].O’Bryant SE, Xiao G, Zhang F, Edwards M, German DC, Yin X, et al. Validation of a serum screen for alzheimer’s disease across assay platforms, species, and tissues. J Alzheimers Dis 2014;42:1325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Kiddle SJ, Sattlecker M, Proitsi P, Simmons A, Westman E, Bazenet C, et al. Candidate blood proteome markers of Alzheimer’s disease onset and progression: A systematic review and replication study. J Alzheimers Dis 2014;38:515–31. [DOI] [PubMed] [Google Scholar]
- [227].Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine 2014;20:415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Hye A, Riddoch-Contreras J, Baird AL, Ashton NJ, Bazenet C, Leung R, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease [erratum appears in Arch Neurol. 2007 Sep;64(9):1246]. Arch Neurol 2007;64:354–62. [DOI] [PubMed] [Google Scholar]
- [230].Burnham SC, Faux NG, Wilson W, Laws SM, Ames D, Bedo J, et al. A blood-based predictor for neocortical Aβ burden in Alzheimer’s disease: Results from the AIBL study. Mol Psychiatry 2014; 19:519–26. [DOI] [PubMed] [Google Scholar]
- [231].Apostolova LG, Hwang KS, Avila D, Elashoff D, Kohannim O, Teng E, et al. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology 2015; 84:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].O’Bryant SE, Gupta V, Henriksen K, Edwards M, Jeromin A, Lista S, et al. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer’s disease research. Alzheimers Dement 2015;11:549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Evaluation Mayeux R. and use of diagnostic tests in Alzheimer’s disease. Neurobiol Aging 1998;19:139–43. [DOI] [PubMed] [Google Scholar]
- [234].Breteler M Mapping out biomarkers for Alzheimer’s disease. JAMA 2011;305:304–5. [DOI] [PubMed] [Google Scholar]
- [235].Apweiler R, Aslanidis C, Deufel T, Gerstner A, Hansen J, Hochstrasser D, et al. Approaching clinical proteomics: current state and future fields of application in cellular proteomics. Cytometry A 2009;75:816–32. [DOI] [PubMed] [Google Scholar]
- [236].Henriksen K, O’Bryant SE, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement 2014;10:115–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Snyder HM, Carrillo MC, Grodstein F, Henriksen K, Jeromin A, Lovestone S, et al. Developing novel blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement 2014;10:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].FDA. Guidance for industry: Alzheimer’s disease: developing drugs for the treatment of early stage disease. In: Research CfDEa, editor. Washington, DC: 2013. [Google Scholar]
- [239].Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med 2013;368:1169–71. [DOI] [PubMed] [Google Scholar]
- [240].EMA. EMA: Discussion paper on the clinical investigation of medicines for the treatment of Alzheimer s disease and other dementias; 2014. London, UK. [Google Scholar]
- [241].Kryscio RJ. Secondary prevention trials in Alzheimer disease: the challenge of identifying a meaningful end point. JAMA Neurol 2014;71:947–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].APEX. Effect of Aerobic Exercise on Pathophysiology of PreClinical Alzheimer’s Disease.
- [243].A Safety and Tolerability Study of JNJ-54861911 in Participants With Early Alzheimer’s Disease; 2016
- [244].Mills SM, Mallmann J, Santacruz AM, Fuqua A, Carril M, Aisen PS, et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris) 2013;169:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 2011;26(Suppl 3):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Takeda. Biomarker Qualification for Risk of Mild Cognitive Impairment (MCI) Due to Alzheimer’s Disease (AD) and Safety and Efficacy Evaluation of Pioglitazone in Delaying Its Onset (TOMMORROW); 2016.
- [247].Cooper SA, Caslake M, Evans J, Hassiotis A, Jahoda A, McConnachie A, et al. Toward onset prevention of cognitive decline in adults with Down syndrome (the TOP-COG study): study protocol for a randomized controlled trial. Trials 2014;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Sano M, Jacobs D, Andrews H, Bell K, Graff-Radford N, Lucas J, et al. A multi-center, randomized, double blind placebo-controlled trial of estrogens to prevent Alzheimer’s disease and loss of memory in women: design and baseline characteristics. Clin Trials 2008; 5:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Group AR. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s Disease Anti-Inflammatory Prevention Trial (ADAPT). PLoS Clin Trials 2006;1:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B, et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol 2012;11:851–9. [DOI] [PubMed] [Google Scholar]
- [251].Richard E, Van den Heuvel E, Moll van Charante EP, Achthoven L, Vermeulen M, Bindels PJ, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord 2009;23:198–204. [DOI] [PubMed] [Google Scholar]
- [252].Gillette-Guyonnet S, Andrieu S, Dantoine T, Dartigues JF, Touchon J, Vellas B, et al. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer’s disease. Alzheimers Dement 2009;5:114–21. [DOI] [PubMed] [Google Scholar]
- [253].ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med 2015; 49:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Group AI. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013;36:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Uo Washington. Effects of Simvastatin on Biomarkers (SimBio); 2015.
- [256].DeKosky ST, Fitzpatrick A, Ives DG, Saxton J, Williamson J, Lopez OL, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials 2006;27:238–53. [DOI] [PubMed] [Google Scholar]
- [257].University OHaS. Pilot Study: Lipoic Acid and Omega-3 Fatty Acids for Alzheimer’s Prevention; 2014.
- [258].Uo Kentucky. Modulation of Micro-RNA Pathways by Gemfibrozil in Predementia Alzheimer Disease; 2015.
- [259].Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, et al. Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement 2013;9:438–44. [DOI] [PubMed] [Google Scholar]
- [260].Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S, et al. Development of interventions for the secondary prevention of Alzheimer’s dementia: the European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry 2016;3:179–86. [DOI] [PubMed] [Google Scholar]
- [261].Vellas B, Hampel H, Rouge-Bugat ME, Grundman M, Andrieu S, Abu-Shakra S, et al. Alzheimer’s disease therapeutic trials: EU/US Task Force report on recruitment, retention, and methodology. J Nutr Health Aging 2012;16:339–45. [DOI] [PubMed] [Google Scholar]
- [262].Vellas B, Aisen PS, Sampaio C, Carrillo M, Scheltens P, Scherrer B, et al. Prevention trials in Alzheimer’s disease: an EU-US task force report. Prog Neurobiol 2011;95:594–600. [DOI] [PubMed] [Google Scholar]
- [263].Hampel H, Lista S, Teipel SJ, Garaci F, Nistico R, Blennow K, et al. Perspective on future role of biological markers in clinical therapy trials of Alzheimer’s disease: a long-range point of view beyond 2020. Biochem Pharmacol 2014;88:426–49. [DOI] [PubMed] [Google Scholar]
- [264].Carrillo MC, Brashear HR, Logovinsky V, Ryan JM, Feldman HH, Siemers ER, et al. Can we prevent Alzheimer’s disease? Secondary “prevention” trials in Alzheimer’s disease. Alzheimers Dement 2013;9:123–1311. [DOI] [PubMed] [Google Scholar]
- [265].Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med 2014;275:251–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Peters KR, Lynn Beattie B, Feldman HH, Illes J. A conceptual framework and ethics analysis for prevention trials of Alzheimer Disease. Prog Neurobiol 2013;110:114–23. [DOI] [PubMed] [Google Scholar]
- [267].Hampel H, Wilcock G, Andrieu S, Aisen P, Blennow K, Broich K, et al. Biomarkers for Alzheimer’s disease therapeutic trials. Prog Neurobiol 2011;95:579–93. [DOI] [PubMed] [Google Scholar]
- [268].Holland D, McEvoy LK, Desikan RS, Dale AM, Alzheimer’s Disease Neuroimaging Initiative. Enrichment and stratification for predementia Alzheimer disease clinical trials. PLoS One 2012;7:e47739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Richard E, Andrieu S, Solomon A, Mangialasche F, Ahtiluoto S, Moll van Charante EP, et al. Methodological challenges in designing dementia prevention trials - the European Dementia Prevention Initiative (EDPI). J Neurol Sci 2012;322:64–70. [DOI] [PubMed] [Google Scholar]
- [270].Post SG. The Moral Challenge of Alzheimer Disease. Baltimore, MD: The Johns Hopkins University Press; 1995. [Google Scholar]
- [271].Gauthier S, Leuzy A, Racine E, Rosa-Neto P. Diagnosis and management of Alzheimer’s disease: past, present and future ethical issues. Prog Neurobiol 2013;110:102–13. [DOI] [PubMed] [Google Scholar]
- [272].Grill JD, Karlawish J, Elashoff D, Vickrey BG. Risk disclosure and preclinical Alzheimer’s disease clinical trial enrollment. Alzheimers Dement 2013;9:356–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Fisk JD, Sadovnick AD, Cohen CA, Gauthier S, Dossetor J, Eberhart A, et al. Ethical guidelines of the Alzheimer Society of Canada. Can J Neurol Sci 1998;25:242–8. [DOI] [PubMed] [Google Scholar]
- [274].Caselli RJ, Langbaum J, Marchant GE, Lindor RA, Hunt KS, Henslin BR, et al. Public perceptions of presymptomatic testing for Alzheimer disease. Mayo Clin Proc 2014;89:1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Schipper HM. Presymptomatic apolipoprotein E genotyping for Alzheimer’s disease risk assessment and prevention. Alzheimers Dement 2011;7:e118–23. [DOI] [PubMed] [Google Scholar]
- [276].Taylor JL, Kennedy Q, Adamson MM, Lazzeroni LC, Noda A, Murphy GM, et al. Influences of APOE epsilon4 and expertise on performance of older pilots. Psychol Aging 2011;26:480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Karlawish J Addressing the ethical, policy, and social challenges of preclinical Alzheimer disease. Neurology 2011;77:1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].MetLife_Foundation. MetLife Foundation Alzheimer’s Survey: What America Thinks Harris Interactive; 2006.
- [279].Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis Assoc Disord 2008;22:94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Vernarelli JA, Roberts JS, Hiraki S, Chen CA, Cupples LA, Green RC. Effect of Alzheimer disease genetic risk disclosure on dietary supplement use. Am J Clin Nutr 2010;91:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Dubois B, Epelbaum S, Santos A, Di Stefano F, Julian A, Michon A, et al. Alzheimer disease: from biomarkers to diagnosis. Rev Neurol (Paris) 2013;169:744–51. [DOI] [PubMed] [Google Scholar]
- [282].Hampel H, Lista S. Use of biomarkers and imaging to assess patho-physiology, mechanisms of action and target engagement. J Nutr Health Aging 2013;17:54–63. [DOI] [PubMed] [Google Scholar]
- [283].Hampel H, Lista S, Khachaturian ZS. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement 2012;8:312–36. [DOI] [PubMed] [Google Scholar]
- [284].Hampel H, Lista S. Alzheimer disease: from inherited to sporadic AD-crossing the biomarker bridge. Nat Rev Neurol 2012; 8:598–600. [DOI] [PubMed] [Google Scholar]
- [285].Bertram L, Hampel H. The role of genetics for biomarker development in neurodegeneration. Prog Neurobiol 2011;95:501–4. [DOI] [PubMed] [Google Scholar]
- [286].Zetzsche T, Rujescu D, Hardy J, Hampel H. Advances and perspectives from genetic research: development of biological markers in Alzheimer’s disease. Expert Rev Mol Diagn 2010; 10:667–90. [DOI] [PubMed] [Google Scholar]
- [287].Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H. Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease. Med Clin North Am 2013;97:399–424. [DOI] [PubMed] [Google Scholar]
- [288].Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuro-imaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci 2011;34:430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Lista S, O’Bryant SE, Blennow K, Dubois B, Hugon J, Zetterberg H, et al. Biomarkers in sporadic and familial Alzheimer’s Disease. J Alzheimers Dis 2015;47:291–317. [DOI] [PubMed] [Google Scholar]
- [290].Rosen C, Hansson O, Blennow K, Zetterberg H. Fluid biomarkers in Alzheimer’s disease - current concepts. Mol Neurodegener 2013;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Blennow K, Zetterberg H, Fagan AM. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med 2012;2:a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Hampel H, Shen Y, Walsh DM, Aisen P, Shaw LM, Zetterberg H, et al. Biological markers of amyloid beta-related mechanisms in Alzheimer’s disease. Exp Neurol 2010;223:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer’s disease. Exp Gerontol 2010;45:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Edwards M, Balldin VH, Hall J, O’Bryant S. Combining select neuropsychological assessment with blood-based biomarkers to detect mild Alzheimer’s disease: a molecular neuropsychology approach. J Alzheimers Dis 2014;42:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Lista S, Garaci FG, Ewers M, Teipel S, Zetterberg H, Blennow K, et al. CSF Abeta1–42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer’s disease. Alzheimers Dement 2014;10:381–92. [DOI] [PubMed] [Google Scholar]
- [296].Broich K, Weiergraber M, Hampel H. Biomarkers in clinical trials for neurodegenerative diseases: regulatory perspectives and requirements. Prog Neurobiol 2011;95:498–500. [DOI] [PubMed] [Google Scholar]
- [297].Hampel H, Lista S. The rising global tide of cognitive impairment. Nat Rev Neurol 2016; 10.1038/nrneurol.2015.250. [DOI] [PubMed] [Google Scholar]