Abstract
Alcohol (ethyl alcohol; ethanol) and caffeine are the two most widely used psychoactive substances in the world. Caffeine and ethanol have both been reported to constrict cerebral arteries in several species, including humans. We have recently shown that application of 10 μM caffeine mixed with 50 mM ethanol to in vitro pressurized cerebral arteries of rat reduced ethanol-induced constriction. This effect was dependent on the presence of nitric oxide (NO•) and could be observed in de-endothelialized arteries supplied with the NO• donor sodium nitroprusside (SNP). The molecular target(s) of ethanol-caffeine interaction in cerebral arteries has remained unknown. In the present work, we used rat and mouse middle cerebral arteries (MCA) to identify the extra-endothelial effectors of NO•-mediated, caffeine-induced protection against ethanol-evoked arterial constriction. Constriction of intact MCA of rat by either 50 mM ethanol or 10 μM caffeine was ablated in the presence of a selective TRPV1 pharmacological blocker. TRPV1 pharmacological block, but not block of TRPA1, PKG or BK channels, removed caffeine-induced protection against ethanol-evoked rat MCA constriction, whether evaluated in arteries with intact endothelium or in SNP-supplemented, de-endothelialized arteries. In mouse arteries, caffeine-induced protection against ethanol-induced MCA constriction was significantly amplified, resulting into actual vasodilation, upon pharmacological block of TRPV1, and in TRPV1 knock-out arteries. Despite some species-specific differences, our study unequivocally demonstrates the presence of functional, extra-endothelial TRPV1 that participates in both endothelium-independent MCA constriction by separate exposure to ethanol or caffeine and caffeine-induced protection against ethanol-evoked MCA constriction.
Keywords: ethanol, caffeine, TRPV1 channel, arterial smooth muscle, cerebral artery, TRPV1 knockout mouse
Introduction
Alcohol (ethyl alcohol; ethanol) and caffeine are the two most widely used psychoactive substances in the world (Ferreira & Willoughby, 2008; Cappelletti et al., 2015; Peacock et al., 2017). Episodic, moderate-to-heavy alcohol intake, such as during “binging,” constitutes the primary form of excessive alcohol consumption in the US. Indeed, ~29% of females and 43% of males have had binge drinking experience (CDC, 2014). In turn, >90% of adults in the US consume caffeine regularly (Frary et al., 2005; Juliano et al., 2012). The combined use of alcohol and caffeine, the latter as the main active ingredient of “power/energy supplements”, such as chewing bars, gums, gels, and beverages (i.e., “energy drinks”) is increasing sharply (Malinauskas et al., 2007). Moreover, the sequential and/or simultaneous exposure of humans to caffeine and alcohol within the culture of “pre-party and party events” has reached epidemic proportions across US College campuses (Striley & Khan, 2014; Cobb et al., 2015; Linden-Carmichael & Lau-Barraco, 2017). Both the cardiovascular and the central nervous system have been identified as major targets of alcohol-caffeine exposure (Verster et al., 2012; Chrysant & Chrysant, 2015). In spite of its critical role for normal brain function, however, very little is known about the effect of alcohol-caffeine on the cerebral circulation. It is noteworthy to underscore that caffeine does not significantly alter ethanol metabolism in the body (Ferreira et al., 2006). Therefore, the impact of ethanol-caffeine interactions on organ and system function is primarily the result of these drugs’ actions on specific receptors in target organs.
Regarding alcohol and the cerebral circulation, numerous epidemiological studies have described the deleterious effect of alcohol on cerebrovascular health (Altura & Altura, 1984; Puddey et al., 1999; Reynolds et al., 2003; Patra et al., 2010). Episodic drinking with blood alcohol levels reaching 35–80 mM is associated with an increased risk for cerebrovascular ischemia (Puddey et al., 1999), stroke, and death from ischemic stroke (Reynolds et al., 2003). Cerebrovascular ischemia may result from impaired vasodilation and/or enhanced constriction of cerebral arteries. In a rat model widely used to mimic human cerebral artery function, we previously demonstrated that cerebrovascular constriction evoked by 10–100 mM ethanol in vivo and in vitro involves drug-induced reduction of ionic current mediated by voltage-/calcium-gated, large conductance K+ (BK) channels in the vascular smooth muscle (SM) (Liu et al., 2004; Bukiya et al., 2014). This inhibition may result from direct sensing of ethanol by the BK proteins (Bukiya et al., 2009; Kuntamallappanavar & Dopico, 2017) and/or BK channel modulation by ethanol-sensitive parties other than BK itself, e.g., ryanodine receptors (Ye et al., 2014).
In turn, in vivo studies on humans demonstrate a decrease in cerebral blood flow following acute intake of caffeine (Mathew & Wilson, 1985; Cameron et al., 1990; Addicott et al., 2009). This decrease in cerebral blood flow is consistent with caffeine-induced cerebral artery constriction. Indeed, we have recently demonstrated that caffeine at physiologically relevant concentrations (0.3–50 μM) constricted rat cerebral arteries in vivo and in vitro (Chang et al., 2016). A wide variety of targets has been identified to participate in caffeine action on arterial diameter. These targets include several types of adenosine receptors, phosphodiesterase enzymes, ryanodine receptors and nitric oxide (NO•) synthase (NOS)-mediated activation of protein kinase G (PKG) (Umemura et al., 2006; Echeverri et al., 2010; Jin et al., 2012).
In contrast to the solid documentation of modification of cerebrovascular function by either alcohol or caffeine, the studies on alcohol-caffeine combinations on cerebrovascular health are extremely scarce (Verster et al., 2012; Lapchak et al., 2004). We recently demonstrated that caffeine co-administration blunted ethanol-induced constriction of rat or mouse middle cerebral arteries (MCA) (Chang et al., 2016). Caffeine protective action against ethanol-induced MCA constriction was lost in arteries from endothelial NOS (eNOS) knockout (KO) mouse, and rat MCA where the endothelium was mechanically removed, but restored upon perfusion of de-endothelialized vessels with sodium nitroprusside (Chang et al., 2016), an NO• donor (Bates et al., 1991).
The cellular targets of NO• that mediate caffeine-induced protection against ethanol-evoked cerebrovascular constriction remain unknown. Upon endothelium removal, the overwhelming majority of the remaining cell elements in the cerebral artery are SM cells (Lee, 1995). Therefore, we hypothesized that an NO•-sensitive target(s) present in the arterial myocyte itself participates in ethanol-caffeine interaction on the cerebral circulation. Given its presence in arterial SM in both rat and mouse (Baylie & Brayden, 2011; Cavanaugh et al., 2011; Tóth et al., 2014), and its sensitivity to ethanol and caffeine (albeit in primary sensory neurons) (Nicoletti et al., 2008; Daher et al., 2009), we focus our investigation of ethanol and caffeine cerebrovascular targets on the transient receptor potential cation channel subfamily V member 1 (vanilloid receptor 1; TRPV1). Thus, using in vitro artery diameter determinations in rat and TRPV1 KO mouse models, and selective pharmacology, this study documents for the first time that TRPV1 channels are involved in the cerebrovascular responses evoked by ethanol and caffeine, whether administered individually or in combination.
Material and Methods
Ethical considerations
Care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Measurements of cerebral artery diameter
Adult male Sprague-Dawley rats (≈250 g) were purchased from Harlan Laboratories (Indianapolis, IN). Adult 6–12 weeks old, male homozygous TRPV1 (B6.129X1-Trpv1tm1Jul/J) KO and C57BL/6 control mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Rats and mice were decapitated using a guillotine and sharp scissors, respectively. Middle cerebral arteries (<260 μm and <150 μm external diameter in rat and mouse species, respectively) were isolated and cannulated as described in earlier work (Bukiya et al., 2013). Endothelium was removed by passing an air bubble into the vessel lumen for 90 seconds before vessel cannulation. The presence/absence of functional endothelium was experimentally verified by the MCA diameter response to 10 μM acetylcholine, an endothelium-dependent vasodilator, versus the MCA diameter response to 10 μM sodium nitroprusside (SNP), an endothelium-independent vasodilator (Liu et al., 2004; Bukiya et al., 2007). The chamber containing the cannulated artery was continuously perfused at a rate of 3.75 ml/min with physiologic saline solution (PSS) (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose, and 24 NaHCO3. PSS was continuously gassed with 21%/5%/74% O2/CO2/N2 mixture to equilibrate pH at 7.4. PSS perfused through the chamber was maintained at 35–37°C. Arteries were monitored with a camera (Sanyo VCB-3512T; Sanyo Electric Co., Moriguchi, Japan) attached to an inverted EclipseTS100 microscope (Nikon, Tokyo, Japan). The external diameter of the arterial wall was measured and digitized using the automatic edge-detection function of IonWizard software (IonOptics, Milton, MA).
Experiments were performed on arteries subjected to 60 mm Hg intravascular pressure to ensure both development and maintenance of myogenic tone (Bukiya et al., 2007; 2011; 2013). Once myogenic tone developed and artery diameter remained stable for at least 10 minutes, drugs were applied to the artery by chamber perfusion. The effect of a given drug application was evaluated immediately before the solution was changed to drug-free PSS, or to the next drug combination in the sequence, depending on experimental design. At the end of each experiment, passive arterial diameter (for myogenic tone calculation) was determined by exposing the vessel to Ca2+-free solution (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2 EGTA, 11 glucose, 24 NaHCO3; pH 7.4.
Western blotting
TRPV1 and beta actin protein detection was performed in tissue lysates from wild type C57BL/6 (positive control) and TRPV1 KO on C57BL/6 background (negative control) mouse whole brains, rat de-endothelialized middle cerebral arteries, and rat brain (MCA removed). Tissue lysate fractions were separated on a 4–15% SDS–polyacrylamide gel and transferred onto polyvinylidene difluoride (PVDF) membranes. PVDF membranes were then blocked with 5% nonfat dry milk made in Tris-buffered saline containing 0.1% Tween 20 for 1 h. The membranes were then incubated with mouse polyclonal anti-TRPV1 antibody (ab6166, 1:500 dilution, Abcam) overnight at 4°C in TBS that was supplemented with 0.1% Tween 20 (TBS-T) and 5% nonfat dry milk. Membranes were next incubated with horseradish peroxidase–conjugated secondary antibodies at room temperature for 1 hour. Protein was then visualized using the SuperSignal West Pico 34080 Chemiluminescent Substrate kit (Thermo Fisher Scientific). A rabbit polyclonal anti-TRPV1 antibody (1:500 dilution; ab6166; Abcam) and mouse monoclonal anti-beta-actin antibody (1:1,000 dilution; ab8226, Abcam) were used to detect TRPV1 and beta-actin proteins, respectively. Goat anti-rabbit (1:5,000, ab97080, Abcam) and goat anti-mouse (1:5,000, 62-6520, Invitrogen) antibodies were used as secondary antibodies. Optical density of the protein-associated signal on Western blot was quantified using ImageJ software.
Chemicals
Ethanol was purchased from American Bioanalytical (Natick, MA). A 784168 and HC 030031 were purchased from Tocris Bioscience (Bristol, United Kingdom); PKG inhibitor peptide DT-3 was purchased from Axxora LLC (Farmingdale, NY). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). For AA 784168 and HC 030031, 10 mM stock solutions were prepared in DMSO, aliquoted and stored at −20°C. On the day of the experiment, aliquots were thawed and used to prepare blocker-containing solutions at final concentration. For capsaicin-containing solutions, 1 mM capsaicin stock solution was prepared in DMSO and further diluted in PSS immediately before experiment to render the desired final concentration. For SNP and caffeine, 1 mM stock solutions were prepared in PSS daily, and further diluted in PSS to render final concentrations.
Data Analysis
Final plotting, fitting and statistical analysis of data were conducted using Origin 8.5.1 (OriginLab, Northampton, MA) and InStat 3.0 (GraphPad, La Jolla, CA). Statistical analysis was conducted using either Student’s t test (for comparison of two groups) or one-way analysis of variance followed by Bonferroni’s multiple comparison test (for comparison of multiple groups), according to experimental design. P < 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M., and n = number of arteries. Within a given experimental group, each artery was obtained from a separate animal.
Results
Ethanol- and caffeine-induced constriction of cerebral arteries with intact endothelium is ablated by pharmacological block of TRPV1
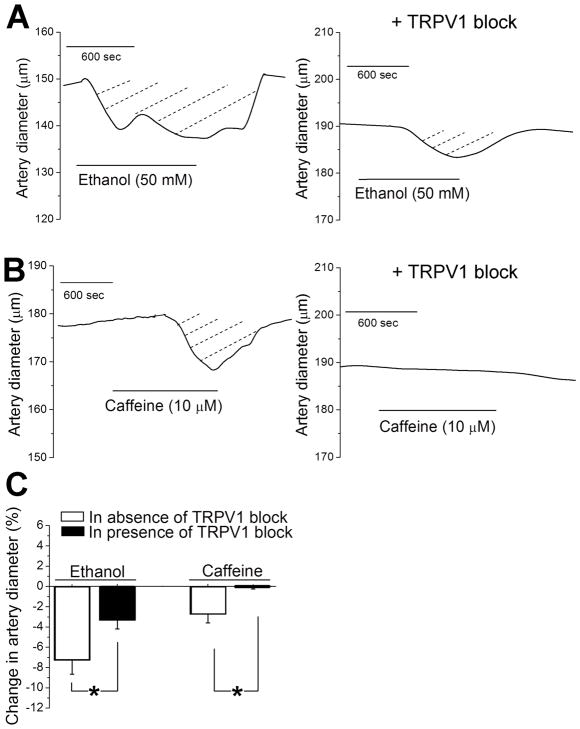
Before addressing any possible contribution of TRPV1 to the cerebrovascular action of an ethanol-caffeine combination, we investigated the role of this receptor in the individual actions of these agents on cerebral artery diameter. Following myogenic tone development, a 20-min application of 50 mM ethanol evoked a decrease in MCA diameter by 14.9±3 μm or 7±1% (P<0.05), which was fully reversible upon ethanol washout (Fig. 1A, left panel). The possible contribution of TRPV1 to ethanol-induced constriction of rat MCA was determined by probing alcohol in presence of 100 nM A 784168. At this concentration, A 784168 has been reported to block TRPV1 but not other receptors (including TRPs different from TRPV1; Bianchi et al., 2007). Application of A 784168 did not modify MCA diameter significantly (increase in artery diameter by 1±1.3 μm or 0.4±0.8 % change compared to pre-A 784168 diameter; P>0.05), suggesting that TRPV1’s contribution to the regulation of basal MCA diameter is minimal, if any. In contrast, the TRPV1 blocker significantly blunted ethanol-induced constriction (decrease in diameter by only 5.3±1.5 μm or 3±1% from pre-ethanol values; P<0.05) (Fig. 1A, right panel), indicating major participation of TRPV1 in ethanol-evoked constriction of rat MCA. The fact that there is a remnant ethanol-induced constriction in the presence of TRPV1 blockade, however, suggests that TRPV1 is not the sole key effector of such ethanol action (see Discussion).
Figure 1. Pharmacological block of TRPV1 blunts ethanol- and caffeine-induced constriction of cerebral arteries when these compounds are applied separately.
A. Diameter traces from pressurized middle cerebral artery segments of rat showing blunting of ethanol-induced constriction of rat middle cerebral artery in presence of TRPV1 blocker 100 nM A 784168. B. Artery diameter traces showing blunting of caffeine-induced constriction of rat middle cerebral artery in presence of TRPV1 blocker 100 nM A 784168. C. Averaged percent changes in artery diameter evoked by 50 mM ethanol in absence (n=8) versus presence (n=4) of TRPV1 blocker, and by 10 μM caffeine in absence (n=11) versus presence (n=6) of TRPV1 blocker. Ethanol and caffeine data were tested separately using non-paired Student’s t-test.
*Statistically significant difference; P =0.0493 for ethanol; P=0.048 for caffeine.
In turn, a 20-min exposure of rat MCA to 10 μM caffeine evoked a reduction in artery diameter by 6.5±2.6 μm or 3±1% (P<0.05), which was fully reversible upon caffeine washout (Fig. 1B, left panel). Application of A 784168 totally suppressed caffeine-induced constriction (Fig. 1B, right panel), unveiling a predominant role for TRPV1 in caffeine-evoked constriction to rat MCA. Since we previously demonstrated that ethanol- and caffeine-induced MCA constrictions in the rat were largely endothelium-independent (Liu et al., 2004; Chang et al., 2016), we hypothesize that the TRPV1 involved in MCA constriction by ethanol or caffeine has an extra-endothelial location.
The presence of TRPV1 in rat de-endothelialized middle cerebral arteries is confirmed experimentally
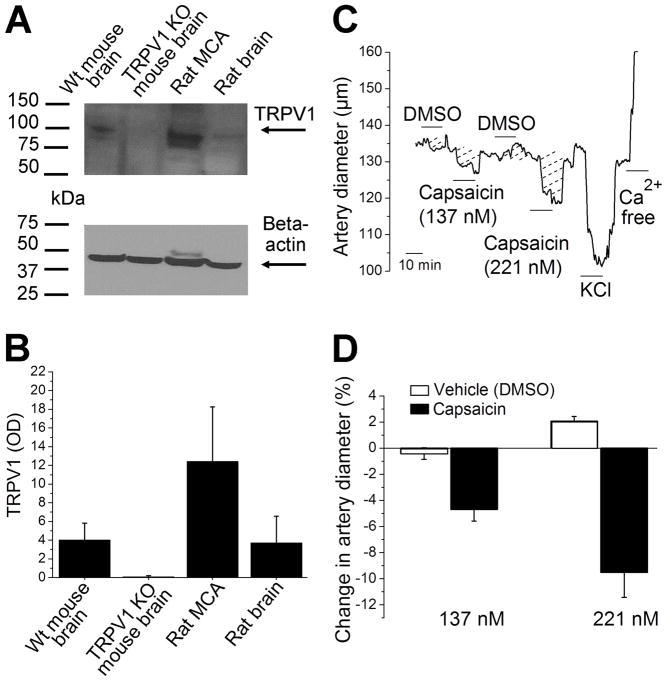
To actually determine the presence of TRPV1 protein in rat de-endothelialized cerebral arteries, we performed Western blotting on lysates from this preparation using wild type and TRPV1 KO mouse brains as positive and negative controls, respectively (Fig. 2A). Data show a band around 100 kDa, which was present in wild type mouse brain lysate and in rat de-endothelialized MCA but absent in brain lysate from TRPV1 KO mouse (Fig. 2A–B; Supplementary Fig. 1). Thus, the band under study most likely labels TRPV1 protein.
Figure 2. TRPV1 detection in rat de-endothelialized middle cerebral arteries.
A. Western blot films showing TRPV1 and beta-actin bands. Here and in B, MCA: middle cerebral artery (de-endothelialized). B. Averaged data showing the optical density (OD) of TRPV1-associated band after background subtraction in Western blots. Blots were loaded 4 times, in two independent experimental occasions (Supplementary Fig. 1). C. Diameter trace from rat de-endothelialized and pressurized middle cerebral artery segments showing constriction in response to the TRPV1 selective agonist capsaicin (137 and 221 nM). D. Averaged percent changes in artery diameter evoked by 137 nM (n=8) and 221 nM capsaicin (n=8), and corresponding vehicle (DMSO-containing) solutions (n=8 for each).
Biochemical determination of TRPV1 was followed by functional assays. Thus, rat de-endothelized MCA segments were probed with the selective TRPV1 opener capsaicin (Caterina et al., 2000). Consistent with the existence of extra-endothelial TRPV1 that mediates vessel constriction, application of increasing concentrations of capsaicin (137 and 221 nM) evoked an incremental decrease in cerebral artery diameter (Fig. 2C–D). Together, data shown in Fig. 2 document for the first time the existence of functional TRPV1 in rat MCA. Moreover, this receptor(s) has an extra-endothelial location.
Caffeine-induced protection against ethanol-induced constriction of rat middle cerebral arteries is removed by selective TRPV1 block
MCA constriction in response to acute, in vitro application of 50 mM ethanol was drastically blunted in the presence of 10 μM caffeine (Fig. 3A, top panel; Fig. 3B, third bar from left), this caffeine action being linked to NO• availability to the vascular SM (Chang et al., 2016). Moreover, Fig. 3B demonstrates that caffeine-induced protection against ethanol-evoked MCA constriction was retained when the ethanol-caffeine mixture was applied under selective pharmacological agents that block SM targets of NO• action on vascular diameter: TRPA1 (10 μM HC 030031; Taylor-Clark et al., 2008), PKG (1 μM inhibitor peptide DT-3; Dostmann et al., 2000) or BK channels (1 μM paxilline; Zhou & Lingle, 2014) (Fig. 3B). In contrast, caffeine-induced protection against ethanol-evoked MCA constriction was removed under selective pharmacological block of TRPV1 with 100 nM A 784168 (Fig. 3A, bottom trace; Fig. 3B, fourth bar). Thus, TRPV1 block in the presence of ethanol and caffeine decreases the “vasodilatory” effect of ethanol and caffeine co-administration, i.e., the blocker favors vasoconstriction. These data strongly suggest that TRPV1 mediates, at least in part, caffeine-ethanol vasodilatory action.
Figure 3. Pharmacological block of TRPV1 removes caffeine protection against ethanol-induced constriction.
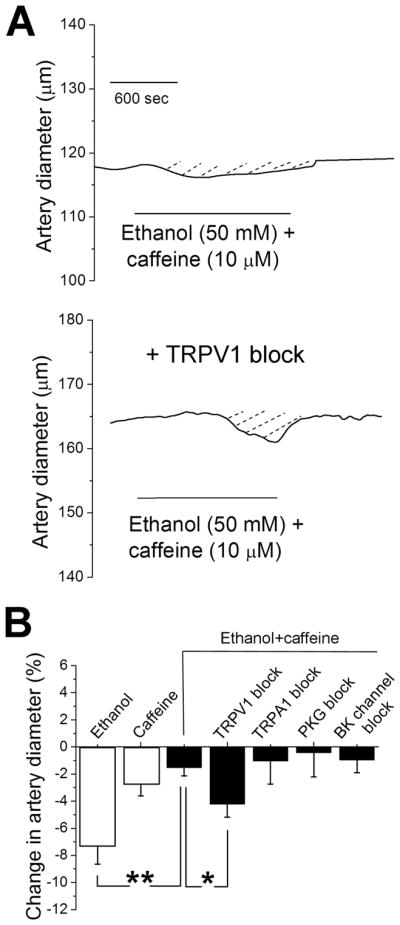
A. Top diameter trace of pressurized middle cerebral artery segment shows caffeine protection against ethanol-induced constriction when 50 mM ethanol and 10 μM caffeine are applied concurrently. In contrast, artery is constricted when ethanol mixture with caffeine is applied in presence of TRPV1 blocker 100 nM A 784168. C. Averaged percent changes in artery diameter evoked by 50 mM ethanol (n=8), 10 μM caffeine (n=7), or their mixture in absence (n=5) versus presence of either TRPV1 (n=4), TRPVA1 (n=4), PKG (n=4), or BK channel blocker (n=4). Each experimental group was individually compared to artery constriction in presence of ethanol mixture with caffeine using unpaired Student’s t-test.
*Statistically significant difference; P=0.0083 for ethanol-induced constriction in absence versus presence of caffeine, P=0.0376 for ethanol mixture with caffeine in absence versus presence of TRPV1 antagonist.
TRPV1-mediated control over ethanol and caffeine effects on middle cerebral artery diameter has endothelium dependent and independent components
Data obtained in intact MCA from rat led us to hypothesize that SM TRPV1 participates in MCA contraction evoked by either caffeine or ethanol. On the other hand, both endothelial and peripheral nerve TRPV1s may participate in vasodilation (Baylie & Brayden, 2011). Thus, we can also hypothesize that endothelial or neural TRPV1 participate in caffeine-induced protection against ethanol-evoked constriction (see above). To test the relative role of endothelial vs. extra-endothelial TRPV1s and also to rule out the participation of endothelial factors other than NO• in ethanol and caffeine actions, we next investigated ethanol and caffeine actions on de-endothelialized MCA, using SNP as NO• donor. Rat arteries were de-endothelialized prior to cannulation and pressurization, and the presence or absence of a functional endothelium was determined via pharmacologic means (see Material and Methods). Vessels were constantly perfused with 10 μM SNP. Under these experimental conditions, ethanol-induced constriction reached 10.4±0.7 μm or 6±1% of pre-ethanol diameter values, and was totally blunted in presence of TRPV1 antagonist (Fig. 4A, two left bars). This outcome is similar to that obtained in MCA with intact endothelium (Fig. 1A,C), consistent with our hypothesis that TRPV1 contributing to ethanol-induced constriction in intact arteries are located in the vascular SM. On the other hand, caffeine-induced constriction, which could be observed in de-endothelialized MCA (Chang et al., 2016), was absent in de-endothelialized vessels perfused with 10 μM SNP (Fig. 4A, third bar from left). This outcome underscores an antagonistic role of SNP onto caffeine action, which appears to be independent of extra-endothelial TRPV1: in the presence of SNP, caffeine-induced constriction is lost in both presence and absence of TRPV1 block (Fig. 4A, two right bars).
Figure 4. Ethanol and caffeine effects on diameter of de-endothelialized cerebral arteries in presence of TRPV1 antagonist.
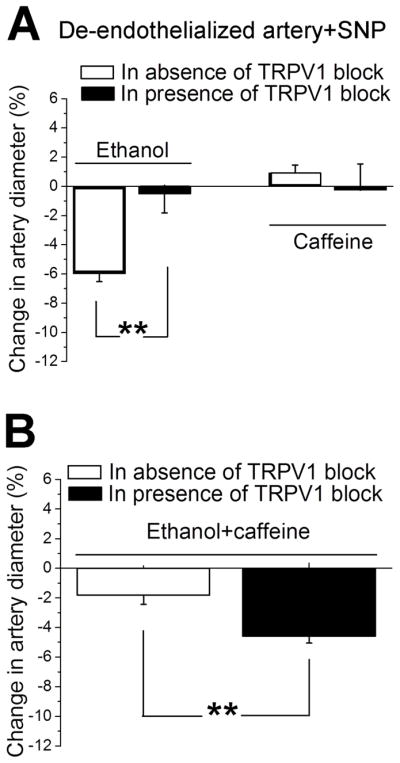
A. Averaged percent changes in artery diameter evoked by 50 mM ethanol in absence (n=7) versus presence (n=5) of TRPV1 blocker, and by 10 μM caffeine in absence (n=11) versus presence (n=4) of TRPV1 blocker. Ethanol and caffeine data were tested separately using non-paired Student’s t-test. **Statistically significant difference; P =0.0025 for ethanol; P=0.0973 for caffeine. B. Averaged percent changes in artery diameter evoked by 50 mM ethanol mixture with 10 μM caffeine in absence (n=4) versus presence (n=5) of TRPV1 blocker 100 nM A 784168. Data were tested using non-paired Student’s t-test.
**Statistically significant difference; P =0.0076.
Finally, we evaluated in our rat MCA model a possible functional interaction between SNP and extra-endothelial TRPV1 on the combined administration of ethanol and caffeine and its effect on vessel diameter. As found in intact arteries (Fig. 1B), co-application of caffeine with ethanol resulted in significant ablation of ethanol-induced constriction: from 10.4±0.7 μm or 6±1% (ethanol alone) to 2.9±0.9 μm or 2±1% (ethanol mixed with caffeine) (P=0.0052). Moreover, testing the ethanol-caffeine mixture in presence of TRPV1 antagonist removed caffeine protection against ethanol-induced constriction, the latter reaching 7.2±0.8 μm or 5±1% and thus becoming similar to the effect of ethanol alone (P=0.0973) (Fig. 4B). This suppression of caffeine-induced protection is similar to that observed in the intact MCA (Fig. 1B), indicating that extra-endothelial TRPV1 participates in a SNP-mediated, extra-endothelial mechanism(s) that mediates caffeine-induced protection against ethanol-induced constriction of rat MCA.
TRPV1 is an effector of sodium nitroprusside in de-endothelialized arteries
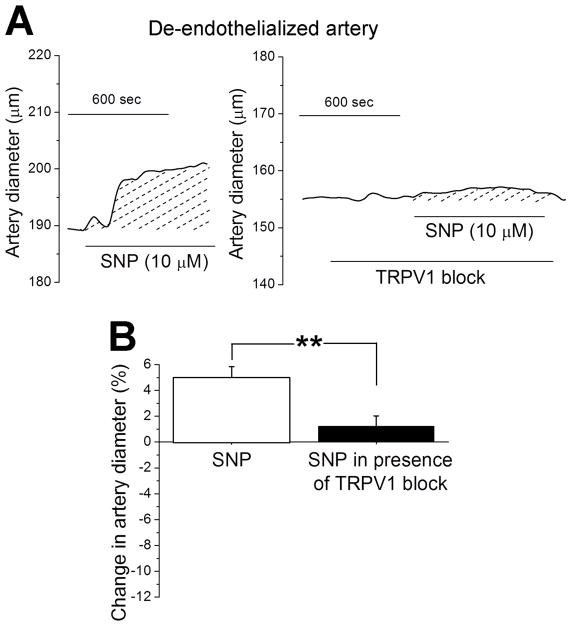
To determine whether extra-endothelial also TRPV1 participates in an SNP-mediated, endothelium-independent dilatory mechanism in rat MCA, we next applied 10 μM SNP to de-endothelialized MCA from rat in the absence and presence of 100 nM A 784168. Fig. 5A shows that bath application of the TRPV1 blocker prevented SNP-induced dilation. On average, SNP-driven increase in rat MCA diameter reached 8.3±1.7 μm or 5±1% and 0.9±1.2 μm or 0.7±0.8% (P<0.01) in the absence and presence of 100 nM A 784168 (Fig. 5B). Thus, extra-endothelial TRPV1 is a major effector of SNP-induced MCA dilation.
Figure 5. Sodium nitroprusside (SNP) dilation of de-endothelialized cerebral arteries is blunted in presence of TRPV1 antagonist.
A. Diameter traces from de-endothelialized pressurized middle cerebral artery segments of rat showing that dilation evoked by 10 μM SNP is blunted in presence of the TRPV1 antagonist 100 nM A 784168. B. Averaged percent changes in artery diameter evoked by 10 μM SNP in absence (n=15) versus presence (n=10) of TRPV1 blocker 100 nM A 784168. Data were tested using non-paired Student’s t-test.
**Statistically significant difference; P =0.005.
Genetic ablation of TRPV1 drastically affects ethanol and caffeine actions on MCA diameter
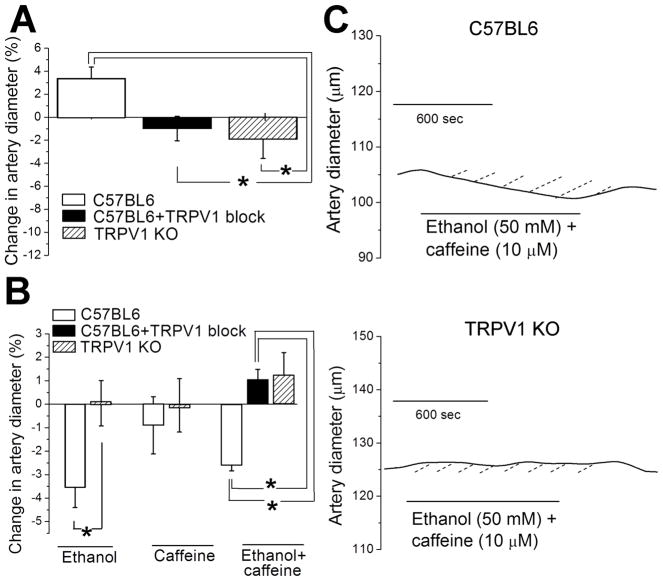
Data using selective pharmacological block seem to indicate that TRPV1s (mainly extra-endothelial) are key participants in ethanol and caffeine actions on rat MCA. To further test this hypothesis, we evaluated drug actions in MCA from TRPV1−/− mice. Mouse MCAs were dissected out and pressurized at 60 mmHg following a procedure identical to that used on rat MCA segments. Application of 10 μM SNP to de-endothelialized cerebral arteries of control (wild type C57BL6) mouse evoked on average a 2.5±1.5 μm or 3±1% increase in diameter (Fig. 6A, left bar). As found in de-endothelialized MCA from rat (Fig. 5), SNP-induced, endothelium-independent vasodilation was drastically blunted in the presence of pharmacological block of TRPV1 with 100 nM A 784168 (Fig. 6A, middle bar), indicating that participation of extra-endothelial TRPV1 in SNP-induced dilation of MCA is conserved in rats and mice. Consistent with results based on pharmacological block of TRPV1, genetic ablation of this receptor drastically blunted SNP-induced, endothelial-independent MCA dilation (Fig. 6A, right bar).
Figure 6. TRPV1 deletion or pharmacological block antagonizes effects of SNP, ethanol, and ethanol mixture with caffeine in de-endothelialized cerebral arteries of mouse.
A. Averaged percent changes in diameter evoked by 10 μM SNP in arteries of C57BL6 in absence (n=8) and presence of TRPV1 blocker (n=4) when compared to TRPV1 KO (n=8) mouse line. Data were tested using non-paired Student’s t-test. *Statistically significant difference from C57BL6 in absence of TRPV1 blocker; P =0.027. B. Averaged percent changes in diameter evoked by 50 mM ethanol in arteries from C57BL6 (n=5) versus TRPV1 KO (n=3) mouse lines, by 10 μM caffeine in arteries from C57BL6 (n=6) versus TRPV1 KO (n=4) mouse lines, and by ethanol mixture with caffeine in arteries from C57BL6 in absence of TRPV1 blocker (n=3) and in presence of TRPV1 blocker (n=4) when compared to TRPV1 KO (n=4) mouse line. Data for each drug were tested separately using non-paired Student’s t-test.
*Statistically significant difference; P=0.0383 for ethanol; P=0.0114 for ethanol mixture with caffeine. C. Diameter traces from de-endothelialized pressurized middle cerebral artery segments showing effect of ethanol co-application with caffeine in artery from C57BL6 (top panel) and TRPV1 KO (bottom panel) mice.
In the presence of SNP, administration of 50 mM ethanol evoked constriction of de-endothelialized MCA from control mouse (Fig. 6B, first bar from left). This outcome is consistent with that observed in de-endothelialized, SNP-exposed MCA from rats (Fig. 3A), which points to a conserved mechanism(s) mediating endothelium-independent, ethanol-induced constriction of MCA. Moreover, ethanol action was fully lost in de-endothelialized arteries from TRPV1−/− mice (Fig. 6B, second bar from left). This outcome mimics that observed in rat MCA exposed to ethanol in presence of pharmacological TRPV1 block (Fig. 3A). This similarity confirms the selectivity of 100 nM A 784168 to block TRPV1, and also indicates that the participation of extra-endothelial TRPV1 in ethanol action on MCA diameter is conserved between rat and mouse.
In the presence of SNP, administration of 10 μM caffeine evoked highly variable responses in diameter of MCA from control mouse, with averaged values being statistically not different from pre-caffeine data (Fig. 6B, third bar from left). Similar refractoriness to caffeine was observed in MCA from TRPV1−/− mouse. The lack of significant change in MCA diameter after caffeine exposure in mice (whether control or TRPV1−/−) is similar to the results obtained with rat de-endothelialized, SNP-exposed MCA in presence or absence of TRPV1 pharmacological block (Fig. 3A). This outcome strongly suggests that, as found in the rat, SNP antagonizes caffeine-induced constriction of MCA by a mechanism that does not require the presence of a functional endothelium, yet it is also independent of extra-endothelial TRPV1.
In contrast to the consistency in outcomes between rat and mouse when ethanol and caffeine were individually tested in de-endothelized MCA (see above), co-application of ethanol and caffeine to mouse arteries rendered results that differed from those obtained in rats, as follows. First, in control (C57BL6) mice, the ethanol-caffeine mixture evoked a contraction that was smaller (1.8±0.7 μm or ~2% reduction in MCA diameter) than that evoked by ethanol alone (3.7±0.8 μm or ~3.5% reduction in MCA diameter) (compare first and fifth bar from left in Fig. 6B). However, ethanol-caffeine induced contraction still represented a significant fraction of ethanol action alone (~50–70%) when compared to the rat ratio: caffeine-ethanol, ~2%; ethanol alone, ~6%; ratio, ~33%. Thus, caffeine-induced protection against ethanol-evoked constriction is reduced in the mouse species. Second, TRPV1 pharmacological block in C57BL6 mouse arteries favored caffeine-induced protection against ethanol-evoked constriction (Fig. 6B, fifth and sixth bars from left). This effect of TRPV1 blockade is opposite to that observed in de-endothelialized rat MCA (Fig. 3B), and could be explained by differential participation of extra-endothelial TRPV1s in the two species (see Discussion). As found with ethanol and caffeine when applied alone, however, TRPV1 genetic ablation on co-application of ethanol and caffeine rendered an outcome that mimicked that obtained when the ethanol-caffeine mixture was probed with TRPV1 selective pharmacological block: in this case, mild vasodilation (Fig. 6B, bars sixth and seventh from left; Fig. 6C).
Discussion
Despite the high prevalence (Cobb et al., 2015) and widespread pathophysiological consequences (Attwood, 2012; Heinz et al., 2013; Marczinski & Fillmore, 2014) of alcohol (ethanol)-caffeine joint intake, there are very few studies documenting the biological targets and mechanisms that participate in ethanol+caffeine actions in the body. In a recent study (Chang et al., 2016), we demonstrated that while individual administration of caffeine at concentrations reached in human circulation after a single cup of coffee (Noguchi et al., 2015) evoked MCA constriction, such caffeine dose reduced the MCA constriction evoked by 50 mM (0.23 g/dL) ethanol, that is, a concentration corresponding to BAL reached during moderate to heavy alcohol intoxication in humans (Diamond, 1992). The time course and magnitude of rat MCA constriction in response to individual application of ethanol or caffeine shown in the current study are similar to those reported in adult rats and mice (Liu et al., 2004; Bukiya et al., 2009; 2011; Chang et al., 2016; Simakova et al., 2017) and, according to Poiseuille’s law, would result in robust decreases in local blood flow: ~31% and ~13% for alcohol and caffeine, respectively. While ethanol and caffeine individual actions are largely endothelium-independent, caffeine-induced protection against ethanol-induced constriction is dependent on the presence of vascular endothelium, and requires the availability of NO• to extra-endothelial targets, most likely located in the vascular SM (Chang et al., 2016). The targets of NO• that mediate caffeine-induced protection against ethanol-evoked constriction of MCA have remained unknown. In the present study, using both pharmacological blockade (100 nM A 784168) and genetic ablation (TRPV1−/− mouse), we document for the first time that TRPV1 participates in ethanol and caffeine actions on MCA diameter. A major limitation of our study, however, is that all data were collected in male animals (rat and mouse). Thus, it remains to be determined whether sex plays a significant role on the participation of TRPV1 in the cerebrovascular actions of ethanol and/or caffeine.
The use of 100 nM A 784168 and genetic ablation of TRPV1 rendered same outcomes when we probed arteries with ethanol alone or in combination with caffeine, which underscores the efficacy of the pharmacological in vitro tool to block TRPV1-mediated biological responses. On the other hand, the fact that 100 nM A 784168 but not 10 μM HC 030031 (a TRPA1 antagonist; Taylor-Clark et al., 2008) blocked a TRPV1-mediated response (caffeine protective effect) underscores the selectivity of our pharmacological approach to target TRPV1.
TRPV1 has been identified in all major components of arterial vessels, including endothelium, peripheral nerve endings that innervate the artery, and the SM itself (Baylie & Brayden, 2011; Cavanaugh et al., 2011; Tóth et al., 2014). Thus, it has traditionally been difficult to ascribe to a given TRPV1 population its specific participation in arterial function and pharmacology (reviewed in Baylie & Brayden, 2011). Present data confirm our initial result (Chang et al., 2016) documenting that rat MCA constriction in response to separate administration of ethanol and caffeine is endothelium-independent (Figs. 2B, 3A), and show for the first time that pharmacological suppression of TRPV1 significantly reduces MCA constriction in response to either psychotropic in rat cerebral arteries. Extra-endothelial TRPV1s have been mapped to perivascular nerves, and their stimulation leads to vasodilation (albeit in mesenteric arteries) (Wu et al., 2006). Therefore, pharmacological block of neural TRPV1 unlikely serves as a major mechanism leading to reduction of MCA constriction by ethanol or caffeine (Fig. 1). On the other hand, TRPV1 mRNA and protein expression have both been reported in the SM of human pulmonary arteries (Wang et al., 2006) and mouse arterioles (Cavanaugh et al., 2011). Our data document for the first time the presence of TRPV1 protein in de-endothelized MCA from rats (Fig. 2A–B), a preparation where ≫50% of cellular content corresponds to SM (Lee, 1995). Moreover, the TRPV1 agonist capsaicin causes Ca2+ uptake and constriction of arterioles (Kark et al., 2008; Cavanaugh et al., 2011), and of carotid and skeletal muscle arteries (Toth et al., 2014). Moreover, TRPV1 block reduces niacin-induced dilation of cutaneous arteries (Clifton et al., 2015) and our current data demonstrate that the TRPV1 selective agonist capsaicin constricts rat MCA (Fig. 2B–C). Therefore, endothelium-independent, TRPV1-sensitive, ethanol-induced contraction of MCA is highly dependent on TRPV1 receptors likely present in the SM itself. The simplest explanation for our ethanol data is the alcohol itself activates MCA SM TRPV1. This is consistent with previous findings documenting that ethanol sensitizes TRPV1 in pancreatic primary sensory neurons (Vigna et al., 2014) and potentiates capsaicin activation of TRPV1 expressed in HEK293 cells (Trevisani et al., 2002) while capsazepine, a TRPV1 blocker, blunts ethanol-induced potentiation of carotid artery contraction evoked by heat (Mustafa & Ismael, 2017).
Our current data, however, also show that TRPV1 block is not sufficient to fully suppress ethanol-induced constriction of MCA, suggesting that extra-endothelial TRPV1 are not the sole key effectors of such ethanol action. We have previously demonstrated that pharmacological block of BK channels results in loss of ethanol-induced constriction of rat MCA (Liu et al., 2004). TRPV1 serves as a critical contributor to intracellular Ca2+ homeostasis (Earley, 2010), and Ca2+ entry via TRPV1 could directly stimulate BK channel activity in SM, as demonstrated in rat dorsal root ganglion neurons where TRPV1 and BK channels form a protein complex (Wu et al., 2013). If BK channels are located downstream of TRPV1 influx in MCA SM, the ethanol-induced constriction that is resistant to TRPV1 block may involve SM targets rather independent of TRPV1, yet coupled to BK function. In rat cerebral SM, BK currents are largely determined by local, vasodilatory Ca2+ signals termed “sparks”, which reflect activity of ryanodine receptors (RyR) (Pérez et al., 1999). We have previously shown that the frequency of BK currents and Ca2+ sparks are both inhibited by 50 mM ethanol (Liu et al., 2004). Moreover, we identified RyR2 as a prevalent RyR type in rat cerebral artery SM (Vaithianathan et al., 2010) and documented that 50 mM ethanol inhibited RyR2 activity (Ye et al., 2014).
Present results also show that TRPV1 is a major determinant of rat MCA constriction evoked by 10 μM caffeine (Fig. 1C), that is, a concentration found in human circulation after a single cup of coffee (Noguchi et al., 2015). This caffeine action is largely endothelium-independent (Chang et al., 2016). Thus, using arguments similar to those applied to advance a location for the extra-endothelial TRPV1 involved in ethanol action on MCA (see above), we interpret that the TRPV1 mediating caffeine action on de-endothelialized MCA is likely located in the vascular SM. The simplest explanation for our data on caffeine-TRPV1 interaction on MCA is that caffeine activates SM TRPV1. This interpretation is consistent with previous data obtained in rabbit nodose ganglion neurons in which TRPV1 antagonists reduced the amplitude of caffeine-induced Ca2+ transients (Daher et al., 2009).
There are multiple targets in arterial SM that may serve as effectors of SNP-mediated, NO• action on vessel diameter, including TRPA, PKG, BK and TRPV1 channels (Koh et al., 1995; Yoshida et al., 2006; Miyamoto et al., 2009; Tsai & Kass, 2009). However, our study also demonstrates that extra-endothelial TRPV1s themselves are the primary effectors of SNP-mediated, caffeine-induced protection against ethanol-induced MCA constriction in the rat species. As this protection reflects a vasodilatory action of caffeine, we hypothesized that vasodilating TRPV1 located in perivascular nerves (Jin et al., 2012) could be involved. This interpretation is in line with our speculation that the differential protection of caffeine against ethanol-evoked constriction in rat vs. mouse could be the result of a different inter-species prevalence of extra-endothelial TRPV1s: vasoconstricting in SM and vasodilating in perivascular nerves in mouse and rat, respectively. It is also possible that caffeine-induced protection against ethanol-evoked constriction of MCA reflects competition between caffeine and ethanol at the TRPV1 itself, yet participation of caffeine targets (for a review, see: Echeverri et al., 2010) additional to those involved in caffeine-induced constriction itself cannot be ruled out. These additional targets could include RyR, as crosstalk between TRPV1 and RyR-mediated Ca2+ release from endoplasmic reticulum has been reported (albeit in skeletal muscle; Lotteau et al., 2013). Lastly, caffeine-induced protection against ethanol-evoked constriction of MCA was restored upon supplementation of de-endothelialized arteries with the NO• donor SNP (Chang et al., 2016). Considering that NO•-driven activation of TRPV1 has been reported in several experimental preparations (Miyamoto et al., 2009; Zhao et al., 2016), it becomes apparent that NO• could serve as an activator of extra-endothelial TRPV1 in MCA.
Our present work conducted in de-endothelialized MCA in the presence of SNP demonstrates that caffeine-induced protection against ethanol-evoked constriction in mouse (Fig. 6B) was drastically smaller than that in rats under similar experimental conditions (Fig. 3B). However, this caffeine effect is similar in the two species when evaluated in presence of an intact endothelium (Chang et al., 2016). Current experimental conditions underscore that NO• itself could not replicate such similar caffeine-induced protection in the two species. This difference strongly suggests that an endothelial, yet to be isolated, factor(s) prevails over any NO• modulatory action and thus, ensures a similar caffeine-induced protection against ethanol-evoked constriction of MCA in rat and mouse. Our species-specific findings are in line with a recent report showing significant differences in vascular parameters between rat and mouse, thus cautioning against interchangeable use of these species for experimental vascular work (Boswell et al., 2014). Despite the species-specific nuances on caffeine-induced protection against alcohol-evoked constriction of MCA, our work has unequivocally identified TRPV1 as a major contributor to a) ethanol-induced constriction, b) caffeine-induced constriction, and c) caffeine-induced protection against ethanol action on MCA. The relative contribution of endothelial vs. extra-endothelial TRPV1 to the cerebrovascular actions of alcohol and/or caffeine are very likely to be affected by conditions where endothelial function is reduced, such as ageing, atherosclerosis, peripheral vascular disease and diabetes (Davignon & Ganz, 2004; Seals et al., 2011; Rajendran et al., 2013).
Supplementary Material
Original films showing Western blot data. Arrows depicts a line of bands (≈100 kDa) that are present in rat middle cerebral artery (MCA) lysate but absent in TRPV1 knock-out (KO) mouse brain. All samples in each experimental run contained equal amounts of total protein. Staining against beta-actin was used as loading control.
Highlights.
Functional, extra-endothelial TRPV1 is found in rat middle cerebral artery
Extra-endothelial TRPV1 participates in cerebral artery constriction by ethanol
Extra-endothelial TRPV1 is a main mediator of cerebral artery constriction by caffeine
Extra-endothelial TRPV1 participates in caffeine-induced protection against ethanol-evoked constriction of cerebral arteries
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism by grant R37-AA11560 (A.M.D.). Authors deeply thank Shivantika Bisen for excellent technical assistance.
Abbreviations
- BAL
blood alcohol level
- BK
voltage-/calcium gated potassium (channel) of large conductance
- KO
knockout
- MCA
middle cerebral artery
- NOS
nitric oxide synthase
- PKG
protein kinase G
- PSS
physiologic saline solution
- RyR
ryanodine receptor
- SM
smooth muscle
- SNP
sodium nitroprusside
- TRPA1
transient receptor potential cation channel subfamily A member 1 (ankyrin receptor 1)
- TRPV1
transient receptor potential cation channel subfamily V member 1 (vanilloid receptor 1)
Footnotes
Conflicts of interest. None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addicott MA, Yang LL, Peiffer AM, Burnett LR, Burdette JH, Chen MY, Hayasaka S, Kraft RA, Maldjian JA, Laurienti PJ. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Human Brain Mapping. 2009;30:3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:325–331. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- Attwood AS. Caffeinated alcohol beverages: a public health concern. Alcohol and Alcoholism. 2012;47:370–371. doi: 10.1093/alcalc/ags062. [DOI] [PubMed] [Google Scholar]
- Bates JN, Baker MT, Guerra R, Jr, Harrison DG. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochemical Pharmacology. 1991;42:S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiologica (Oxford) 2011;203:99–116. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi BR, El Kouhen R, Neelands TR, Lee CH, Gomtsyan A, Raja SN, Vaidyanathan SN, Surber B, McDonald HA, Surowy CS, Faltynek CR, Moreland RB, Jarvis MF, Puttfarcken PS. [3H]-A-778317 [1-((R-5-tert-butyl-indan-1-yl)-3-isoquinolin-5-yl-urea]: a novel, stereoselective, high-affinity antagonist is a useful radioligand for the human transient receptor potential vanilloid-1 (TRPV1) receptor. Journal of Pharmacology and Experimental Therapeutics. 2007;323:285–293. doi: 10.1124/jpet.107.124305. [DOI] [PubMed] [Google Scholar]
- Boswell CA, Mundo EE, Ulufatu S, Bumbaca D, Cahaya HS, Majidy N, Van Hoy M, Schweiger MG, Fielder PJ, Prabhu S, Khawli LA. Comparative physiology of mice and rats: radiometric measurement of vascular parameters in rodent tissues. Molecular Pharmacology. 2014;11:1591–1598. doi: 10.1021/mp400748t. [DOI] [PubMed] [Google Scholar]
- Bukiya A, Dopico AM, Leffler CW, Fedinec A. Dietary cholesterol protects against alcohol-induced cerebral artery constriction. Alcoholism Clinical and Experimental Research. 2014;38:1216–1226. doi: 10.1111/acer.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. Federation of European Biochemical Societies Letters. 2009;583:2779–2784. doi: 10.1016/j.febslet.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Molecular Pharmacology. 2007;72:359–369. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, Parrill AL, Dopico AM. Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Molecular Pharmacology. 2013;83:1030–1044. doi: 10.1124/mol.112.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Kuntamallappanavar G, Asuncion-Chin M, Dopico AM. Smooth muscle cholesterol enables BK β1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arteriosclerosis Thrombosis and Vascular Biology. 2011;31:2410–2423. doi: 10.1161/ATVBAHA.111.233965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Modell JG, Hariharan M. Caffeine and human cerebral blood flow: a positron emission tomography study. Life Sciences. 1990;47:1141–1146. doi: 10.1016/0024-3205(90)90174-p. [DOI] [PubMed] [Google Scholar]
- Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Current Neuropharmacology. 2015;13:71–88. doi: 10.2174/1570159X13666141210215655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. Journal of Neuroscience. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Fedinec AL, Kuntamallappanavar G, Leffler CW, Bukiya AN, Dopico AM. Endothelial Nitric Oxide Mediates Caffeine Antagonism of Alcohol-Induced Cerebral Artery Constriction. Journal of Pharmacology and Experimental Therapeutics. 2016;356:106–115. doi: 10.1124/jpet.115.229054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysant SG, Chrysant GS. Cardiovascular complications from consumption of high energy drinks: recent evidence. Journal of Human Hypertension. 2015;29:71–76. doi: 10.1038/jhh.2014.47. [DOI] [PubMed] [Google Scholar]
- Clifton HL, Inceoglu B, Ma L, Zheng J, Schaefer S. TRPV1 channels are involved in niacin-induced cutaneous vasodilation in mice. Journal of Cardiovascular Pharmacology. 2015;65:184–191. doi: 10.1097/FJC.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Nasim A, Jentink K, Blank MD. The role of caffeine in the alcohol consumption behaviors of college students. Substance Abuse. 2015;36:90–98. doi: 10.1080/08897077.2013.835763. [DOI] [PubMed] [Google Scholar]
- Daher JP, Gover TD, Moreira TH, Lopes VG, Weinreich D. The identification of a caffeine-induced Ca2+ influx pathway in rat primary sensory neurons. Molecular and Cellular Biochemistry. 2009;327:15–19. doi: 10.1007/s11010-009-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- Diamond I. Cecil Textbook of Medicine. 1992:44–47. [Google Scholar]
- Dostmann WRG, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge W. Highly Specific, Membrane-permeant Peptide Blockers of cGMP-dependent Protein Kinase I Alpha Inhibit NO-induced Cerebral Dilation. Proceedings of the National Academy of Sciences USA. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S. Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle. Microcirculation. 2010;17:237–249. doi: 10.1111/j.1549-8719.2010.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A. Caffeine’s Vascular Mechanisms of Action. International Journal of Vascular Medicine. 2010:834060. doi: 10.1155/2010/834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MP, Willoughby D. Alcohol consumption: the good, the bad, and the indifferent. Applied Physiology, Nutrition, and Metabolism. 2008;33:12–20. doi: 10.1139/H07-175. [DOI] [PubMed] [Google Scholar]
- Ferreira SE, de Mello MT, Pompéia S, de Souza-Formigoni ML. Effects of energy drink ingestion on alcohol intoxication. Alcoholism Clinical and Experimental Research. 2006;30:598–605. doi: 10.1111/j.1530-0277.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. Journal of American Dietetic Association. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, de Wit H, Lilje TC, Kassel JD. The combined effects of alcohol, caffeine, and expectancies on subjective experience, impulsivity, and risk-taking. Experimental and Clinical Psychopharmacology. 2013;21:222–234. doi: 10.1037/a0032337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Sun P, Takatori S, Koyama T, Zamami Y, Tangsucharit P, Kitamura Y, Kawasaki H. Involvement of perivascular nerves and transient receptor potential vanilloid 1 (TRPV1) in vascular responses to histamine in rat mesenteric resistance arteries. European Journal of Pharmacology. 2012;680:73–80. doi: 10.1016/j.ejphar.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Jin Y, Kim J, Kwak J. Activation of the cGMP/Protein Kinase G Pathway by Nitric Oxide Can Decrease TRPV1 Activity in Cultured Rat Dorsal Root Ganglion Neurons. Korean Journal of Physiology and Pharmacology. 2012;16:211–217. doi: 10.4196/kjpp.2012.16.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Huntley ED, Harrell PT, Westerman AT. Development of the caffeine withdrawal symptom questionnaire: caffeine withdrawal symptoms cluster into 7 factors. Drug and Alcohol Dependence. 2012;124:229–234. doi: 10.1016/j.drugalcdep.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Molecular Pharmacology. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. Journal of Physiology. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntamallappanavar G, Dopico AM. BK β1 subunit-dependent facilitation of ethanol inhibition of BK current and cerebral artery constriction is mediated by the β1 transmembrane domain 2. British Journal of Pharmacology. 2017 doi: 10.1111/bph.14046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Song D, Wei J, Zivin JA. Pharmacology of caffeinol in embolized rabbits: clinical rating scores and intracerebral hemorrhage incidence. Experimental Neurology. 2004;188:286–291. doi: 10.1016/j.expneurol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lee RM. Morphology of cerebral arteries. Pharmacology and Therapeutics. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Lau-Barraco C. Alcohol Mixed with Energy Drinks: Daily Context of Use. Alcoholism Clinical and Experimental Research. 2017;41:863–869. doi: 10.1111/acer.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proceedings of the National Academy of Sciences USA. 2004;101:18217–18222. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotteau S, Ducreux S, Romestaing C, Legrand C, Van Coppenolle F. Characterization of functional TRPV1 channels in the sarcoplasmic reticulum of mouse skeletal muscle. PLoS One. 2013;8:e58673. doi: 10.1371/journal.pone.0058673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutrition Journal. 2007;6:35. doi: 10.1186/1475-2891-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT. Energy drinks mixed with alcohol: what are the risks? Nutrition Reviews. 2014;72:98–107. doi: 10.1111/nure.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Caffeine induced changes in cerebral circulation. Stroke. 1985;16:814–817. doi: 10.1161/01.str.16.5.814. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S, Ismael HN. Ethanol potentiates heat response in the carotid artery via TRPV1. Life Sciences. 2017;188:83–86. doi: 10.1016/j.lfs.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Nicoletti P, Trevisani M, Manconi M, Gatti R, De Siena G, Zagli G, Benemei S, Capone JA, Geppetti P, Pini LA. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia. 2008;28:9–17. doi: 10.1111/j.1468-2982.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Matsuzaki T, Sakanashi M, Hamadate N, Uchida T, Kina-Tanada M, Kubota H, Nakasone J, Sakanashi M, Ueda S, Masuzaki H, Ishiuchi S, Ohya Y, Tsutsui M. Effect of caffeine contained in a cup of coffee on microvascular function in healthy subjects. Journal of Pharmacological Sciences. 2015;127:217–222. doi: 10.1016/j.jphs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, Rehm J. Alcohol consumption and the risk of morbidity and mortality for different stroke types--a systematic review and meta-analysis. BMC Public Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Ferris J, Winstock A. Energy drink use frequency among an international sample of people who use drugs: Associations with other substance use and well-being. Drug and Alcohol Dependence. 2017;174:70–79. doi: 10.1016/j.drugalcdep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Pérez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. Journal of General Physiology. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddey IB, Rakic V, Dimmitt SB, Beilin LJ. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors--a review. Addiction. 1999;94:649–663. doi: 10.1046/j.1360-0443.1999.9456493.x. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. International Journal of Biological Sciences. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. The Journal of the American Medical Association. 2003;289:579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clinical Sciences (London) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakova MN, Bisen S, Dopico AM, Bukiya AN. Statin therapy exacerbates alcohol-induced constriction of cerebral arteries via modulation of ethanol-induced BK channel inhibition in vascular smooth muscle. Biochemical Pharmacology. 2017;145:81–93. doi: 10.1016/j.bcp.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striley CW, Khan SR. Review of the energy drink literature from 2013: findings continue to support most risk from mixing with alcohol. Current Opinions in Psychiatry. 2014;27:263–268. doi: 10.1097/YCO.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Molecular Pharmacology. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Tóth A, Czikora A, Pásztor ET, Dienes B, Bai P, Csernoch L, Rutkai I, Csató V, Mányiné IS, Pórszász R, Edes I, Papp Z, Boczán J. Vanilloid receptor-1 (TRPV1) expression and function in the vasculature of the rat. Journal of Histochemistry and Cytochemistry. 2014;62:129–144. doi: 10.1369/0022155413513589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nature Neuroscience. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Tsai E, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacology and Therapeutics. 2009;122:216–236. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, Ueda K, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Jitsuiki D, Soga J, Goto C, Chayama K, Yoshizumi M, Higashi Y. Effects of acute administration of caffeine on vascular function. American Journal of Cardiology. 2006;98:1538–1541. doi: 10.1016/j.amjcard.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Vaithianathan T, Narayanan D, Asuncion-Chin MT, Jeyakumar LH, Liu J, Fleischer S, Jaggar JH, Dopico AM. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. American Journal of Physiology Cell Physiology. 2010;299:C264–C278. doi: 10.1152/ajpcell.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Aufricht C, Alford C. Energy drinks mixed with alcohol: misconceptions, myths, and facts. International Journal of General Medicine. 2012;5:187–198. doi: 10.2147/IJGM.S29313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna SR, Shahid RA, Liddle RA. Ethanol contributes to neurogenic pancreatitis by activation of TRPV1. Federation of American Societies for Experimental Biology Journal. 2014;28:891–896. doi: 10.1096/fj.13-236208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Qin C, Foreman RD, Farber JP. Transient receptor potential vanilloid receptor-1 does not contribute to slowly adapting airway receptor activation by inhaled ammonia. Autonomic Neuroscience. 2007;133:121–127. doi: 10.1016/j.autneu.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu Y, Hou P, Yan Z, Kong W, Liu B, Li X, Yao J, Zhang Y, Qin F, Ding J. TRPV1 channels are functionally coupled with BK(mSlo1) channels in rat dorsal root ganglion (DRG) neurons. PLoS One. 2013;8:e78203. doi: 10.1371/journal.pone.0078203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Jian K, Jaggar JH, Bukiya AN, Dopico AM. Type 2 ryanodine receptors are highly sensitive to alcohol. Federation of European Biochemical Societies Letters. 2014;588:1659–1665. doi: 10.1016/j.febslet.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nature Chemical Biology. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- Zhao JF, Shyue SK, Lee TS. Excess Nitric Oxide Activates TRPV1-Ca(2+)-Calpain Signaling and Promotes PEST-dependent Degradation of Liver X Receptor α. International Journal of Biological Sciences. 2016;12:18–29. doi: 10.7150/ijbs.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lingle CJ. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. Journal of General Physiology. 2014;144:415–440. doi: 10.1085/jgp.201411259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Original films showing Western blot data. Arrows depicts a line of bands (≈100 kDa) that are present in rat middle cerebral artery (MCA) lysate but absent in TRPV1 knock-out (KO) mouse brain. All samples in each experimental run contained equal amounts of total protein. Staining against beta-actin was used as loading control.