Abstract
Aims:
Type 2 diabetes mellitus (T2DM), serious and increasingly prevalent among Mexican Americans, produces symptoms related to high and low glucose levels, medication side effects, and long-term complications. This secondary analysis explored symptom prevalence, differences among symptom burden levels, and how symptoms clustered.
Methods:
Clinical measurements and survey data (demographic, quality of life, and the symptom subscale of the Diabetes Symptom Self-Management Inventory) collected from Mexican American adults with T2DM (n=71) were analyzed for symptom prevalence, differences across levels of symptom burden, and symptom clusters. Agglomerative hierarchical and k-means clustering analyses were performed on a Gower matrix. Internal validation methods and rank aggregation were used to identify the best clustering method of the two techniques and to identify symptoms that clustered together.
Results:
Participants reported mean=14 symptoms; tiredness and trouble sleeping were most prevalent. People with high symptom burden had significantly lower quality of life and perceptions of worse diabetes severity. Hierarchical clustering produced three symptom clusters: cluster 1=9 symptoms (e.g. intense thirstiness, dry mouth); cluster 2=9 symptoms (e.g., itching skin, weight gain, noise or light sensitivity); cluster 3=13 symptoms (e.g., nervous, headache, trouble concentrating, and memory loss).
Conclusion:
Mexican Americans with T2DM report several co-occurring symptoms. Quality of life is significantly worse for people with high symptom burden. Three distinct symptom clusters were identified. Studies with larger samples are needed to further diabetes symptom science. Clinicians should assess and address patients’ co-occurring symptoms as a potential means of decreasing symptom burden and improving quality of life.
Keywords: Diabetes, Clusters, Mexican Americans, Quality of Life, Symptoms, Symptom Burden
One in seven Mexican-American adults is diagnosed with type 2 diabetes mellitus (T2DM), an illness with substantial costs, morbidity, and risk of mortality (American Diabetes Association [ADA], 2017; Centers for Disease Control and Prevention [CDC], 2017). The majority of people with T2DM experience a variety of symptoms no matter how long they have been under care for T2DM. In four studies with Mexican Americans with T2DM, 95-97% of participants reported having experienced an average of 4-11 symptoms in the prior week related to hyper- or hypoglycemia, medication side effects, or long-term complications (Brown, Upchurch, García, Barton, & Hanis, 1998; García, 2005; García, 2011; García, Brown, Horner, Zuñiga, & Arheart, 2015). People with more symptoms are more likely to have higher hemoglobin A1c levels and lower quality of life scores (Brown et al. 1998; Garcia, 2005; García, 2011). When asked, people might report having symptoms even though they do not perceive the symptoms as important or bothersome to them. Thus, symptom burden, a combination of number of symptoms, symptom frequency, and perceived importance of the symptoms, may be a more meaningful patient-oriented measure than symptom prevalence alone.
People with chronic diseases often experience more than one symptom at a time. Groups of symptoms that occur together are known as clusters. Symptom clusters are clinically important because the symptoms may have a common etiology and may guide treatment decisions. Identifying diabetes symptom clusters is the first step in the National Institutes of Health Symptom Science Model, with the ultimate goal of advancing “precise and personalized interventions to treat and manage illnesses and improve health” (Cashion, Gill, Hawes, Henderson, & Saligan, 2016, p. 504).
Studies have documented symptom clusters related to cancer (Barsevick, 2007; Barsevick, Whitmer, Nail, Beck, & Dudley, 2006; Miaskowski, Dodd, & Lee, 2004; Van Lancker, Beeckman, Verhaeghe, Van Den Noortgate, & Van Hecke, 2016), kidney disease (Almatary, Douglas, & Bonner, 2016), cardiovascular disease (Bose et al., 2017; McSweeney, Cleves, Zhao, Lefler, & Yang, 2010), and HIV/AIDS (Wilson et al., 2016; Zuñiga, Bose, Park, Lapiz-Bluhm, & García, 2017). However, little is known about diabetes-related symptoms that occur together. Furthermore, the association of diabetes symptom burden with A1c, quality of life, and other patient characteristics have not been explored. Therefore, the purpose of this study was to 1) identify the most common symptoms experienced by Mexican American adults with T2DM; 2) analyze differences among sex, acculturation, education, time since diagnosis, number of comorbid conditions, number of medications, hemoglobin A1c, body mass index (BMI), blood pressure (BP), quality of life and symptom burden; and 3) explore how diabetes symptoms cluster together.
METHODS
Design, Sample and Setting
We conducted a secondary analysis of baseline data that were collected as part of a randomized controlled trial designed to test a symptom-focused diabetes self-management intervention for Mexican Americans with T2DM (Author 1 Publication). After approval by a university institutional review board, the parent study was conducted in a mid-sized city and adjacent suburban and rural areas in Texas where Latinos comprise over one third of the population. Mexican Americans with T2DM were recruited from community settings such as clinics, public libraries, grocery stores, and health fairs via flyers and in-person contact (Author 1, Author 2 Publication). Participants self-identified as Mexican-Americans with T2DM, aged 25-75 years, and spoke English or Spanish. Participants were excluded if they received dialysis, were currently or recently pregnant, had cancer, or were receiving or recently received cancer treatments because those conditions were associated with symptoms similar to T2DM or could affect weight, one of the outcomes of the parent study.
Instrumentation
Data for these analyses include demographic and disease characteristics, clinical measures (A1c, total symptoms, BP, total cholesterol, high density lipoprotein [HDL], low density lipoprotein [LDL], triglycerides, BMI) and quality of life. Data were collected in participants’ homes and included height, weight, vital signs, venous blood, and surveys. Height and weight were measured with a calibrated portable scale and stadiometer. Blood pressure was measured according to American Heart Association guidelines (Pickering et al., 2005). We averaged three readings, each taken one-minute apart. Blood samples were analyzed in a certified, licensed, and accredited commercial laboratory.
The survey questions were read aloud in Spanish or English and the participants’ responses were recorded on the survey by the data collector. Patient and disease descriptors included age, sex, education, marital status, time since diagnosis with diabetes, type of diabetes treatment, concurrent medical conditions, and medications. We used the 38-item symptom subscale of the Diabetes Symptom Self-Management Inventory (DSSCI), which asks participants if they had the symptom in the previous week and how important they feel symptom is. Response choices for symptom prevalence were 0 = “never”, 1 = “rarely”, 2 = “sometimes or a little”, 3 = “occasionally or often” and 4 = “mostly or constantly.” Symptom importance was defined as the respondent’s subjective rating based on whether the symptom happens frequently, is constant, is bothersome, or worrisome. Response choices for symptom importance were 0 = “not at all,” 2 = somewhat important,” and 4 = “very important.” Evidence of validity of DSSCI symptom subscale was demonstrated with correlations between higher total numbers of symptoms reported on the DSSCI with scores on the Illness Perception Questionnaire- revised identity (symptom) subscale (r = 0.57, p < .001) and scores on the Center for Epidemiologic Studies-Depression questionnaire (r = 0.65, p < .001; García et al., 2011). Symptom burden is the sum of the combination of the reported frequency and importance scores for each symptom. The internal consistency reliability for the symptom burden subscale was good, α = .81.
Quality of life was captured with the Diabetes-39 (Boyer & Earp, 1997), which asks respondents to indicate the impact of items pertaining to diabetes control (12 items), anxiety and worry about diabetes (4 items), energy and mobility (15 items), social and peer burden for diabetes management (5 items), and sexual functioning (3 items). Response options ranged from 1 = “not affected at all” to 7 = “extremely affected.” Items were summed and a weighted mean obtained for each subscale and a total standardized summated score obtained, higher scores indicating that diabetes had a significant negative impact on quality of life, i.e. higher scores equate to worse quality of life. In this study, the total internal consistency of the total Diabetes-39 was very good at 0.94 and subscale alphas ranged from .73-.96. In addition, we used a single item measure of perceived diabetes severity (1 = not at all severe; 7 = most severe); diabetes severity scores were standardized to a scale of 0-100; higher scores indicating perceptions that diabetes was more severe.
Statistical Analysis
Data were entered into an SPSS database (v.24) and checked for accuracy. BMI was calculated as kg/meters2. Each participant’s total number of symptoms in the previous week was obtained by summing all symptoms with frequency codes from 1-4. Frequencies, percents, means, and standard deviations were calculated to describe the sample and examine symptom prevalence. We calculated symptom burden by multiplying the frequency score by the importance score for each symptom and then summing the burden score for each symptom. We formed groups for high, medium and low symptom burden based on tertiles and then calculated one-way ANOVAs with Bonferroni post hoc comparisons to assess differences across symptom burden groups in age, sex, acculturation, A1c, BMI, HDL cholesterol, LDL cholesterol, triglycerides, time since diagnosis, number of concurrent medical conditions, quality of life, and perceived diabetes severity. We used a probability level of .004 (= .05/12 ANOVAs) to evaluate statistical significance of ANOVAs.
We conducted a cluster analysis of the symptoms, excluding seven symptoms because at least one case was missing a value for those symptoms, using R, version 3.3.3. We used principal component analysis (PCA) to create components, or sets of related variables, that explain most of the variance in symptoms (Alpaydin, 2014). PCA extracts the most essential information so that clusters are more stable (Husson, Josse, & Pagès, 2010). PCA has been used to find a set of highly correlated symptoms related to pain (Fishbain et al., 2014) and HIV symptom clusters (Zuñiga et al., 2017). We used the generated PCA components to test two clustering techniques: hierarchical and k-means. Both methods modeled clusters by measuring the similarity of observations using Gower distances between data points (Everit, Landau, Leese, & Stahl, 2011). The goal of cluster analysis is to find a solution in which clusters are significantly different from one another and composed of meaningful groups of symptoms (Dunn et al., 2017).
First, we implemented agglomerative hierarchical clustering on Gower distance matrices. Clusters were merged if they resulted in the smallest increase in the overall sum of squares of within-cluster distances. A dendrogram, as shown in Figure 1, provides a visual representation of the distance at which clusters were combined. Next, we implemented clustering using k-means, which involves the pre-specification of the number of clusters, or k. By examining potential cluster solutions from the dendrogram obtained from hierarchical clustering, we chose an initial starting value for k at 3 and also tested solutions for 4 and 5 clusters (Everit, et al., 2011). To confirm the best method (either hierarchical or k-means) and the number of clusters, we used internal validation measures of connectivity and Silhouette width. Connectivity represents the extent to which items are placed in the same cluster as their nearest neighbors in the data space. Connectivity values should be small. The Silhouette value measures the degree of confidence in the clustering assignment of a particular observation, with well-clustered observations having values near 1 and poorly clustered observations having values near −1. These metrics were plotted for 3-, 4-, and 5-cluster solutions. We used an optimal list solution, based on rank aggregation metrics (Pihur, Datta, Datta, 2009) that enables selection of the best method and number of clusters.
Figure 1.

Hierarchical Clustering Clusters. Dendrogram showing cluster distances. The dendrogram is read from bottom to top, with the vertical lines showing joined clusters. The bottom-most row contains symptoms. Cluster distances are plotted with the dendrogram, which is read from bottom to top with the vertical lines showing joined clusters.
RESULTS
The sample of n=71 Mexican American adults with T2DM were predominantly female and middle aged; almost half were Spanish-speaking. Participants ranged from newly diagnosed to having lived with diabetes for 30 years (mean 7 years). Most were prescribed oral hypoglycemic agents (57%); over one quarter of the sample took insulin alone or with oral medication. Mean baseline A1C was 8.4%. Almost all (90%) had at least one concurrent medical condition; the most common was hypertension (53%). See Table 1.
Table 1.
Sample Characteristics (N=71)
| Characteristic | Mean (SD) Range | Frequency n (%) |
|---|---|---|
| Female | 47 (66%) | |
| Age in years | 49.5 (9.1) 29 - 68 | |
| Education in years† | 12.3 (4.3) 3 -24 | |
| Languages read and spoken ‡ | ||
| Only Spanish or Spanish better than English | 31 (44%) | |
| Both equally | 8 (11%) | |
| Only English or English better than Spanish | 31 (44%) | |
| Acculturation § | 11.6 (6.1) 4 - 20 | |
| Married or Partnered | 28 (39%) | |
| Employed full time | 36 (51%) | |
| Years Since Diagnosis | 6.7 (5.9) 0 - 30 | |
| Diabetes treatment | ||
| No medication | 4 (6%) | |
| Oral medication only | 41 (58%) | |
| Injectable (Insulin or Byetta, alone or with oral medication) | 26 (37%) | |
| Number of Medications | 4.5 (4.1) 0 - 24 | |
| BMI † | 36.4 (9.7) 22.5 - 84.5 | |
| Hemoglobin A1c% ‡ | 8.5 (2.3) 5.6 - 16.0 | |
| Number of Comorbidities | 1.8 (1.2) 0 - 5 | |
| Have one or more comorbidity | 64 (90%) |
Note:
N=68
N=70
N=69.
Percentages may not add to 100% due to rounding.
Nearly all respondents (97.3%) reported having at least one symptom in the prior week that could be related to diabetes and most (93.0%) reported having three or more symptoms (mean 14 symptoms, range 0-30). The most commonly reported symptoms were tiredness (67.6%), problems sleeping (59.2%), numbness or tingling in the extremities (57.7%), and dry mouth (56.3). The least commonly reported symptoms were discolored skin (9.9%), infection (14.1%), and weight gain (15.5%).
Symptom burden ranged from 0 to 376 (M = 123.8, SD = 92.2). There were no significant differences in age, sex, acculturation, A1c, BMI, HDL cholesterol, LDL cholesterol, triglycerides, time since diagnosis, number of concurrent medical conditions, or any specific comorbid condition, across the three levels of symptom burden. Quality of life and perceived diabetes severity were significantly different between the low and high symptom burden groups. Quality of life was worse (mean difference = −25.08, standard error = 6.19, F = 8.243, df = 2, p = .002) and perceptions of diabetes severity were higher (mean difference = 33.46, standard error= 4.81, F = 24.187, df = 2, p < .001) for the group with the highest symptom burden.
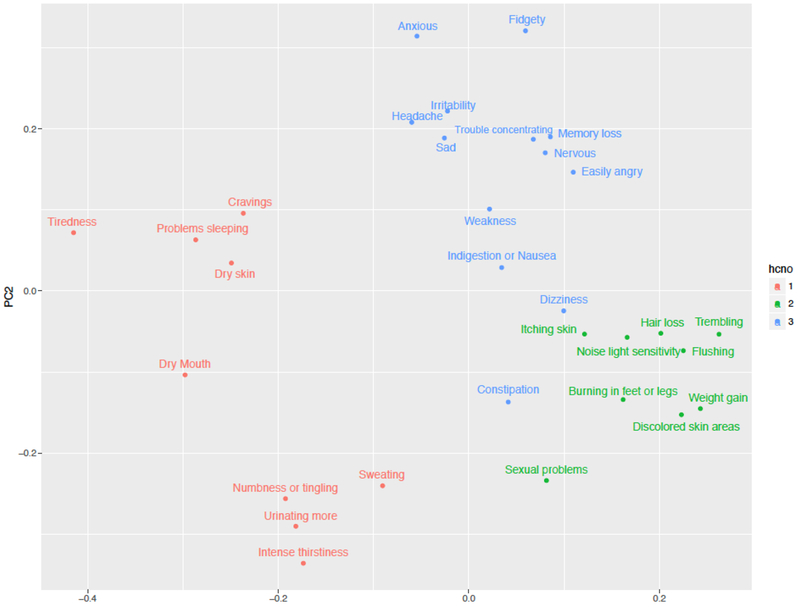
Based on internal validation measures, hierarchical clustering performed better than k-means, achieving lower connectivity values and maximum Silhouette width (Figure 2). Thus, three clusters was the optimal hierarchical clustering solution. As shown in Figure 3, Cluster 1 (C1; n=9 symptoms) is composed of sweating, intense thirstiness, urinating more than usual, cravings, dry mouth, tiredness, numbness or tingling, problems sleeping, and dry skin. Cluster 2 (C2; n=9 symptoms) is composed of itching skin, weight gain, discolored skin areas, noise or light sensitivity, trembling, flushing, sexual problems, burning in feet or legs, and hair loss. Cluster 3 (C3; n=13 symptoms), with the largest number of symptoms, is composed of constipation, dizziness, indigestion or nausea, sadness, irritability, easily angry, nervous, fidgety, weakness, anxiousness, headache, trouble concentrating, and memory loss.
Figure 2.
Internal validation measures of Connectivity and Silhouette Width. Internal validation measures evaluate the performance of the clustering techniques in partitioning the dataset into clusters.
Figure 3:
Implementation of hierarchial cluster cluster number on PCA scores plot
DISCUSSION
Similar to other studies of Mexican American adults with T2DM (Brown et al., 1998; García, 2005; García, 2011) we found that almost all participants reported having symptoms in the prior week that could be related to their T2DM, perhaps due to hyper- or hypoglycemia medication side effects, or long-term complications. Participants reported symptoms even though most had been diagnosed and under treatment on average for four years. The most frequently reported symptoms, tiredness and problems sleeping, and many other reported symptoms could result from T2DM or many other conditions. For this reason, diabetes symptoms are challenging to assess and treat. For example, although people may experience symptoms related to diabetes, they may attribute the symptoms to other conditions or perceive them to be unamenable to diabetes treatments.
High symptom burden was not related to age, sex, acculturation, A1c, BMI, HDL cholesterol, LDL cholesterol, triglycerides, time since diagnosis, or number of concurrent medical conditions. However, high symptom burden was associated with worse quality of life and stronger perceptions of diabetes severity. Thus, even though some symptoms may be perceived as minor or unimportant, symptom burden, calculated from symptom frequency and with people’s perceptions of symptom importance, is an important patient-centered variable.
Mexican American’s symptoms pertaining to T2DM clustered into three clearly delineated clusters. However, we cannot identify a single etiology to account for all the symptoms in a cluster. For example, symptoms in C1 could pertain to hyperglycemia. Thirsty, dry mouth, and urinating more than usual are classic symptoms of diabetes-related hyperglycemia (García, 2011). However, sweating is also included in this cluster and cold sweats are frequently associated with hypoglycemia (García, 2011). Many symptoms in C2 could pertain to long-term effects of diabetes (e.g., itching skin, discolored skin, burning sensation in hands or feet, and sexual problems), while other symptoms in C2 could pertain to hypoglycemia (e.g. trembling) or have no clear origin (e.g., hair loss, flushing, sensitivity to light or noise). Similarly, C3 is composed of symptoms that may represent high blood glucose (e.g., indigestion, constipation, sadness, anger) or low blood glucose (e.g., dizzy, nervous, anxious, fidgety) medication side effects, or perhaps other conditions (García, 2011).
The Diabetes Symptom Self-Care Inventory asks about symptoms experienced in the prior week and it is possible that people reported symptoms they experienced days apart rather than simultaneously. Therefore, clinicians and researchers should assess for symptoms that truly co-occur. Furthermore, to examine symptoms that are specifically linked to hyperglycemia or hypoglycemia, patients should be instructed to record a glucose self-test result when they notice a symptom and clinicians should assess patients’ symptoms at the same time that their glucose is measured. Another challenge is to interpret the significance of ongoing or continuously experienced symptoms, such as dry skin, or numbness and tingling in extremities in terms of glucose levels, symptom clusters and other conditions such as inflammation, and vascular or nerve damage.
A limitation to our analyses is the small sample size. We observed relatively high standard deviation values, which could increase the chance of detecting a minimal effect size. Future analyses should be conducted with larger samples to find smaller but statistically significant differences between clusters. Larger samples would also allow for analyses to include clustering of people, not just symptoms. This is necessary to address the next step of the NIH Symptom Science Model, creating biological, behavioral, and psychological phenotypes of people experiencing the symptom clusters (Cashion et al., 2016; Dunn et al„ 2017).
CONCLUSION
Mexican American adults with T2DM are likely to experience three or more symptoms that could be related to their diabetes at any given time. We found three distinct symptom clusters using the symptom list from the Diabetes Symptom Self-Care Inventory. New measurement strategies are needed to further explore if there are common physiologic underpinnings of symptoms within these clusters and to link symptom clusters to patient outcomes. However, this study adds to the fledgling science of diabetes symptomology and points to the need for clinicians to assess patients’ symptoms and co-occurring symptoms that likely impact symptom burden and quality of life.
Highlights.
Most respondents reported having three or more symptoms in the prior week, with average of 14 symptoms, that could be related to diabetes, despite having been treated for diabetes for several years.
The most commonly reported symptoms were tiredness, problems sleeping, numbness or tingling in the extremities, and dry mouth.
Quality of life was worse and perceptions of diabetes severity were worse for people with the highest symptom burden.
Diabetes-related symptoms formed three distinct clusters however, a clear etiology to account for all symptoms in each cluster is not apparent.
Clinicians should assess patients’ individual and co-occurring symptoms that likely impact symptom burden and quality of life.
Acknowledgments
Funding: This work was supported by a grant (#R21DK076705) from the National Institute of Diabetes and Kidney and Digestive Diseases at the National Institutes of Health. Contents of this manuscript do not necessarily represent the official views of the NIDDK or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflict of interest has been declared by the author(s).
References
- Almatary H, Douglas C, & Bonner A (2016). Multidimensional symptom clusters: An exploratory factor analysis in advanced chronic kidney disease. Journal of Advanced Nursing, 72, 2389–2400. doi: 10.1111/jan.12997. [DOI] [PubMed] [Google Scholar]
- Alpaydin E (2014). Dimensionality Reduction In Introduction to machine learning, 3rd ed. Massachusetts Institute of Technology Press. [Google Scholar]
- American Diabetes Association. (2017). Statistics about diabetes. Accessed August 31, 2017 from http://www.diabetes.org/diabetes-basics/statistics/?loc=db-slabnav.
- Author 1 Publication (to be unblinded after manuscript acceptance)
- Barsevick AM (2007). The elusive concept of the symptom cluster. Oncology Nursing Forum 34(5), 971–980. doi: 10.1188/07.0NF.971-980. [DOI] [PubMed] [Google Scholar]
- Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN (2006). Symptom cluster research: Conceptual, design, measurement, and analysis issues. Journal of Pain and Symptom Management 31(1), 85–95. doi: 10.1016/j.painsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Bose EL, Clermont G, Chen L, Dubrawski AW, Ren D, Hoffman LA,…Hravnak M (2017). Cardiorespiratory instability in monitored step-down unit patients: Using cluster-analysis to identify patterns of change. Journal of Clinical Monitoring and Computing, Epub ahead of print. 10 pages, 10.1007/s10877-017-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JG, & Earp JL (1997). Development of an instrument for assessing the quality of life of people with diabetes: Diabetes-39. Medical Care, 35(5), 440–453. [DOI] [PubMed] [Google Scholar]
- Brown SA, Upchurch SL, García AA, Barton SA, & Hanis CL (1998). Symptom-related self-care of Mexican-Americans with NIDDM: Preliminary findings of the Starr County diabetes education study. Diabetes Educator, 24, 331–339. PMID . [DOI] [PubMed] [Google Scholar]
- Cashion AK, Gill J, Hawes R, Henderson WA, & Saligan L (2016). National Institutes of Health Symptom Science Model sheds light on patient symptoms. Nursing Outlook, 64, 499–506. doi: 10.1016/j.outlook.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). National diabetes statistics report, 2017: fact sheet: Estimates and diabetes and its burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Accessed August 31, 2017 from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [Google Scholar]
- Dunn H, Quinn L, Corbridge SJ, Eldeirawi K, Kapella M, & Collins EG (2017). Cluster analysis in nursing research: An introduction, historical perspective, and future directions. Western Journal of Nursing Research, Epub ahead of print:19 pages. doi: 10.1177/0193945917707705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everit BS, Landau S, Leese M, & Stahl D (2011). Cluster analysis, 5th edition John Wiley & Sons. [Google Scholar]
- Fishbain D, Gao JR, Lewis J, Bruns D, Meyer LJ, & Disorbio JM (2014). Examination of symptom clusters in acute and chronic pain patients. Pain Physician, 17, E349–E357. [PubMed] [Google Scholar]
- García AA (2005). Symptom prevalence and treatments among Mexican Americans with type 2 diabetes. Diabetes Educator, 31, 543–554. PMID . [DOI] [PubMed] [Google Scholar]
- García AA (2011). The Diabetes Symptom Self-Care Inventory for Mexican Americans: Development and pilot testing. Journal of Pain and Symptom Management, 41, 715–727. doi: 10.1016/j.jpainsymman.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García AA, Brown SA, Horner SD, Zuniga J, & Arheart KL (2015). Home-based diabetes symptom self-management education for Mexican Americans with type 2 diabetes. Health Education Research, 30, 484–496. doi: 10.1093/her/cyv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson F, Josse J, & Pages J (September 2010). Principal component methods: hierarchical clustering – partitioning clustering. Why would we need to choose for visualizing data? Applied Mathematics Department (Aggrocampus). Accessed August 14, 2018 at https://pdfs.semanticscholar.org/Q433/5d99d840ac3370f5aeb262828cfl27d3fflc.pdf?ga=2.193831853.184041544.1534275445-405928886.1534275445
- Miaskowski C, Dodd M, & Lee K (2004). Symptom clusters: The new frontier in symptom management research. Journal of the National Cancer Institute Monograph 32, 17–21. [DOI] [PubMed] [Google Scholar]
- McSweeney JC, Cleves MA, Zhao W, Lefler LL, & Yang S (2010). Cluster analysis of women’s prodromal and acute myocardial infarction symptoms by race and other characteristics. Journal of Cardiovascular Nursing, 25, 311–322. doi: 10.1097/JCN.0b013e3181cfbal5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Faulkner BE, Graves J, Hill MN…Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professional from the subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension, 45, 142–161. PMID: . [DOI] [PubMed] [Google Scholar]
- Pihur V, Datta S, Datta S (2009). RankAggreg: An R package for weighted rank aggregation. BMC Bioinformatics, 10, 62–73. doi: 10.1186/1471-2105-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lancker A, Beeckman D, Verhaeghe S, Van Den Noortgate N, & Van Hecke A (2016). Symptom clustering in hospitalised older palliative cancer patients: A cross-sectional study. International Journal of Nursing Studies, 61, 72–81. doi: 10.1016/j.jinurstu.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Wilson NL, Azuero A, Vance DE, Richman JS, Moneyham LD, Raper JL,…Kempf M-C (2016). Identifying symptom patterns in people living with HIV disease. Journal of the Association of Nurses in AIDS Care, 27,121–132. doi: 10.1016/j.jana.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga JA, Bose E, Park J, Lapiz-Bluhm MD, & García AA (2017). Diabetes changes symptom cluster patterns in persons living with HIV. Journal of the Association Nurses in AIDS Care, Epub ahead of print, 9 pages, doi: 10.1016/j.jana.2017.07.004 [DOI] [PubMed] [Google Scholar]