ABSTRACT
Background
Pareidolic associations are commonly used in medical education to enhance perception of radiological abnormalities. A number of animal‐inspired neuroradiological pareidolias have been defined which should alert clinicians to specific movement disorder diagnoses.
Methods
A review of the published literature detailing neuroradiological abnormalities in movement disorder syndromes was conducted, looking specifically for established animal‐inspired pareidolic associations.
Results
A number of animal‐inspired neuroradiological patterns with specific movement disorder associations have been defined. These include eye of the tiger sign, face of the panda sign, swallow tail sign, hummingbird sign, Mickey Mouse sign, ears of the lynx sign, dragonfly cerebellum, tadpole sign, tigroid/leopard skin sign, and bat wing sign.
Conclusion
Pareidolias represent a quick and easy way of enhancing perception, thereby improving the efficiency and accuracy of image analysis. Movement disorder physicians should keep in mind these associations, given that they will likely facilitate scan analysis.
Keywords: Parkinson's disease, neuroimaging
"Look deep into nature, and then you will understand everything better." ‐ Albert Einstein
Neuroradiological practice requires two basic steps: perception and interpretation. Perception involves identifying an abnormality within an examined image, whereas interpretation refers to correct causal ascription.1 Most misdiagnoses in radiology stem from errors of perception, and there is therefore the most to gain by improving this facet of image evaluation.1
Pareidolia refers to the detection of familiar patterns from superficially innocuous visual stimuli; examples from everyday life include seeing faces in clouds or rocks and religious figures on toast. Pareidolic associations are one of the methods used in medical education to enhance perception, because they are known to improve object detection.2, 3
A number of animal‐inspired pareidolias, alerting clinicians to specific neuroradiological abnormalities, have been defined. This brief review and accompanying illustrations provide an overview of these “animals in the brain” as they relate to movement disorder practice.
Eye of the Tiger Sign
Clinical Correlate: Neurodegeneration With Brain Iron Accumulation Type 1
Arguably the most iconic animal‐inspired neuroradiological abnormality in movement disorder practice, the eye of the tiger sign, was first described by Sethi et al. in 1988 in 2 patients with what was then Hallervorden‐Spatz syndrome (now panthotenate kinase‐associated neurodegeneration [PKAN]/neurodegeneration with brain iron accumulation type 1).4 Classic PKAN presents early (mean age: 3 years), generally with a dystonia‐dominant phenotype beginning in the limbs and cervical region, but progressing axially over time.5 Spasticity, cognitive decline, pigmentary retinopathy, and dystonic opisthotonus are other common findings.5 Atypical PKAN, thought to be due to partial loss of function of the panthotenate kinase 2 protein, is more heterogeneous in its manifestations, presenting later in life with a predominantly parkinsonian phenotype.5 Speech problems (hypophonia, palilalia, dysarthria, and stuttering) and neuropsychiatric symptoms are characteristic of atypical/later‐onset presentations.5
The characteristic eye of the tiger appearance observed in the globus pallidus on T2‐weighted MRI is composed of two elements, an anteromedial focus of high signal, likely due to neuronal loss, gliosis, and increased water content, surrounded by a complete ring of very low signal produced by pathological iron accumulation (Fig. 1).4, 6 Though pathognomonic (present in >95% of cases) for PKAN, the eye of the tiger sign is not specific to this condition, having been reported in other syndromes of neuronal brain iron accumulation and in asymptomatic healthy individuals.7, 8
Figure 1.
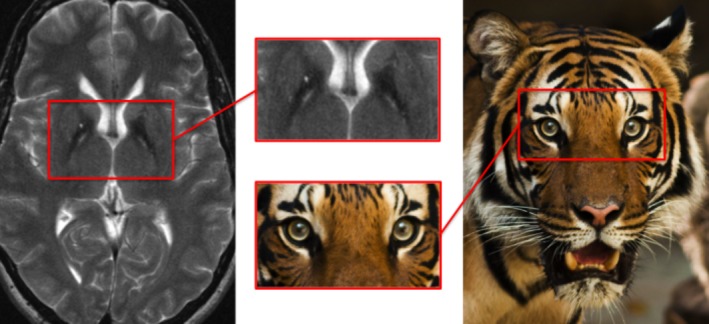
Eye of the tiger sign: neurodegeneration with brain iron accumulation type 1.
Face of the Panda Sign
Clinical Correlate: Wilson's Disease
Wilson's disease, an autosomal‐recessive disorder of copper metabolism caused by mutations in the ATP7B gene, has a myriad of movement disorder presentations. Symptomatic neuropsychiatric Wilson's disease most commonly manifests in early adulthood; tremor, dystonia, choreoathetosis, parkinsonism, and ataxia are all well‐recognized presentations.9 Dysarthria, dysphagia, and drooling are also common and an important clue to the diagnosis.9 MR brain imaging is almost always abnormal at the time of presentation with neuropsychiatric features.9 In the main, this will consist of some combination of T2 signal hyperintensity in the basal ganglia, thalamus, or pons as well as brain atrophy.9 The classic panda faces described below tend to be encountered in more advanced disease.9
The “face of the giant panda” refers to a midbrain appearance caused by signal hyperintensity in the tegmentum with preservation of signal intensity in the red nuclei and pars reticulata of the substantia nigra (SN) and hypointensity of the superior colliculi (Fig. 2).9
Figure 2.
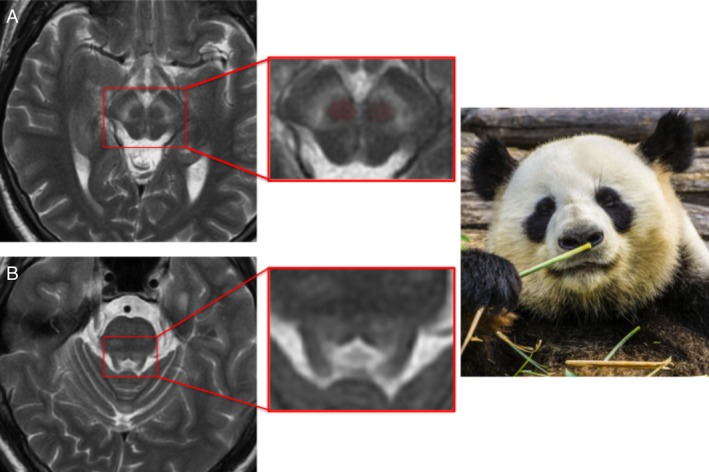
Face of the giant panda (image A) and panda cub (image B) sign: Wilson's disease.
The “face of the panda cub” (Fig. 2), visible on axial pontine imaging, is formed by hypointensity in the central tegmental tracts and medial longitudinal fasciculi (the eyes), the superior cerebellar peduncles (the cheeks), and by signal hyperintensity surrounding the aqueduct as it opens into the fourth ventricle (mouth and nose).9
Swallow Tail Sign
Clinical Correlate: Parkinson's Disease/Parkinson‐Plus Syndromes
The SNpars compacta is the prime target of neurodegeneration in Parkinson's disease (PD). Within the SN, five nigrosomes (clusters of dopaminergic cells) have been defined immunohistochemically, with nigrosome 1, located dorsolaterally, being the largest and the most affected by PD pathology.10 In the healthy state, susceptibility‐weighted MRI (SWI) demonstrating signal hyperintensity in the caudal and mediolateral SN can be used to infer a normal concentration of dopaminergic neurons in nigrosome 1.10 This normal appearance of the SN is described as having a “swallow tail appearance” on SWI, and denotes healthy nigrosome 1 dopaminergic neurons (Fig. 3).
Figure 3.
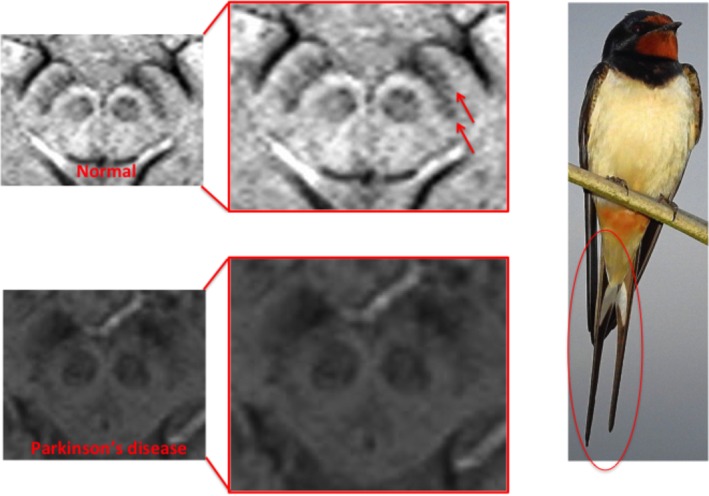
Absence of swallow‐tail sign: PD (tails of swallow denoted by red arrows).
In PD, up to 60% to 80% of nigral dopaminergic neurons are lost by the time patients manifest motor symptoms.10 The radiological correlate of this is the loss of SWI hyperintensity in nigrosome 1 and thus loss of the swallow tail appearance.10 What is better thought of as the “absence of the swallow tail” sign is a sensitive marker for presynaptic dopaminergic deficiency (both PD and Parkinson‐plus syndromes), has high inter‐rater agreement, and is visible even on 1.5 Tesla (T) scans.10, 11 The sign does not, however, differentiate PD from Parkinson‐plus syndromes.11
Hummingbird and Mickey Mouse Sign
Clinical Correlate: Progressive Supranuclear Palsy (PSP)
The hummingbird sign, occasionally called the king penguin sign, is the radiological hallmark of PSP, a four‐repeat tauopathy characterized by axial rigidity, vertical supranuclear gaze palsy, pseudobulbar palsy, early falls, and cognitive change. This distinctive appearance occurs as a result of selective atrophy of the midbrain tegmentum, reflecting involvement of the rostral interstitial nucleus of the medical longitudinal fasciculus, dysfunction of which contributes to the characteristic vertical gaze palsy.12 When viewed in the sagittal plane, preserved pontine volumes resemble the body of the bird whereas the atrophic midbrain projecting anterosuperiorly forms the head and beak (Fig. 4).
Figure 4.
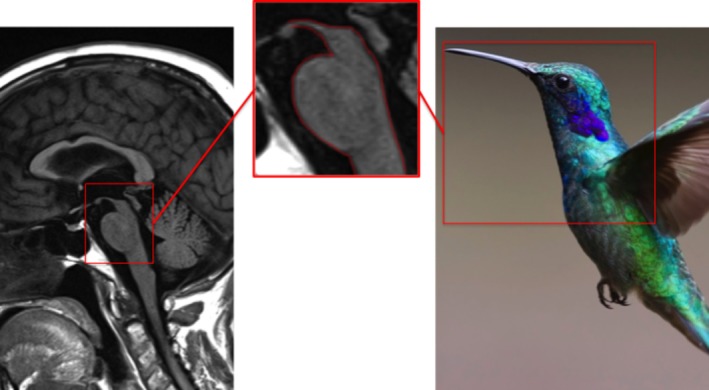
Hummingbird sign: PSP.
The sign is poorly sensitive (especially in early disease), but highly specific, though there have been sparse reports of similar appearances in normal pressure hydrocephalus and fragile‐X tremor ataxia syndrome, both of which cause gait problems.13, 14
While technically a cartoon character, Mickey's status as a mouse earns him a place in this article. Whereas the hummingbird and king penguin signs relate to appearances when viewed in the sagittal plane, the Mickey Mouse sign relates to the shape of the midbrain when viewed on axial imaging (Fig. 5); the atrophic midbrain forms Mickey's face with a pair of cerebral peduncle “ears.”15
Figure 5.
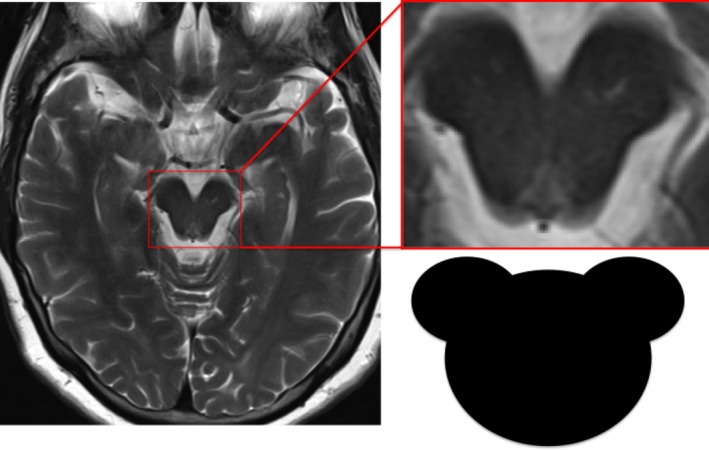
Mickey Mouse sign: PSP.
Ears of the Lynx
Clinical Correlate: Hereditary Spastic Paraplegia (SPG 11 + 15)
The hereditary spastic paraplegias (HSPs) are a group of neurodegenerative conditions which are broadly classified as either pure (isolated lower limb spasticity with or without bladder involvement) or complex, where other neurological features accompany progressive spasticity.16 Complex HSP types 11 and 15 share clinical manifestations of progressive spastic paraplegia, cognitive decline, and, occasionally, a motor neuropathy usually beginning before the second decade of life. A proportion of patients will also manifest levodopa‐responsive parkinsonism or dystonia.16
In addition to callosal thinning, T2‐weighted and fluid‐attenuated inversion recovery (FLAIR) MRI sequences frequently show high signal in the white matter capping the frontal horns of the lateral ventricles in the area of the forceps minor;17 on axial sections, the abnormality resembles the tufts of hair observed on the tips of lynx ears (Fig. 6).17 Though not specific for these conditions (similar changes have been reported in Marchiafava‐Bignami disease, after corpus callosotomy and with developmental anomalies of the calloso‐septal region),17, 18 the sign should specifically be looked for in parkinsonian‐pyramidal syndromes, given that it is present early in the disease course.19
Figure 6.
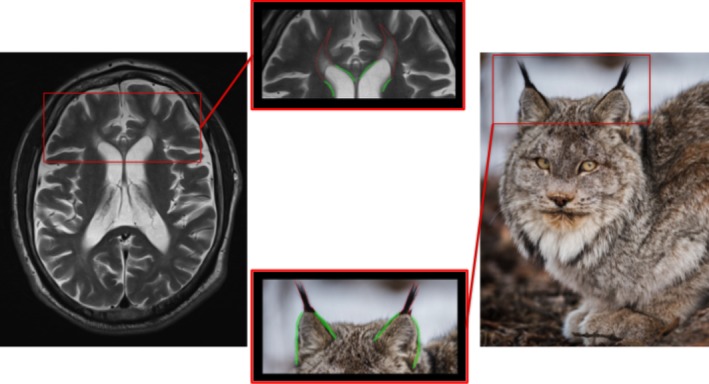
Ears of the lynx sign: HSP 11 + 15.
Dragonfly‐Like Cerebellar Pattern
Clinical Correlate: Pontocerebellar Hypoplasia Type 2
This recently reported sign describes the appearance produced by flattening and hypoplasia of the cerebellar hemispheres with relative preservation of the vermis observed in pontocerebellar hypoplasia type 2 (a rare autosomal‐recessive neurodegenerative disorder), which, when viewed in coronal section, resembles a dragonfly (Fig. 7).20 The condition generally manifests soon after birth with feeding difficulty, developmental regression, and excessive sleepiness.20 Extrapyramidal symptoms (choreoathetosis, dystonia) are common, and the condition is often misdiagnosed as dyskinetic cerebral palsy. Megacisterna magna on brain ultrasound is another clue suggesting the diagnosis.20
Figure 7.
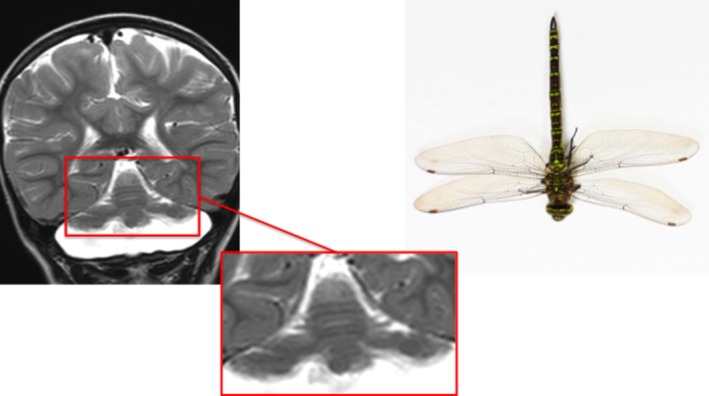
Dragonfly‐like cerebellar pattern: pontocerebellar hypoplasia type 2.
Tadpole Sign
Clinical Correlate: Adult‐Onset Alexander's Disease
The tadpole sign is the classic neuroradiological feature of adult‐onset Alexander's disease. Caused by a mutation in the glial fibrillary acid protein gene, the late‐onset variant of the disease generally presents with brainstem, cerebellar, or myelopathic symptoms.21 The tadpole appearance (Fig. 8) is caused by significant atrophy of the medulla and cervical spinal cord (slim tail of the tadpole), but preservation of pontine volume (head). Other common imaging features include middle cerebellar peduncle signal change and pial FLAIR signal abnormalities.21 Medullary and cervical cord atrophy or signal change (sometimes mimicking neoplastic processes) should always prompt consideration of the diagnosis.21 Palatal tremor, touted as the clinical hallmark of the condition, is frequently absent. 21
Figure 8.
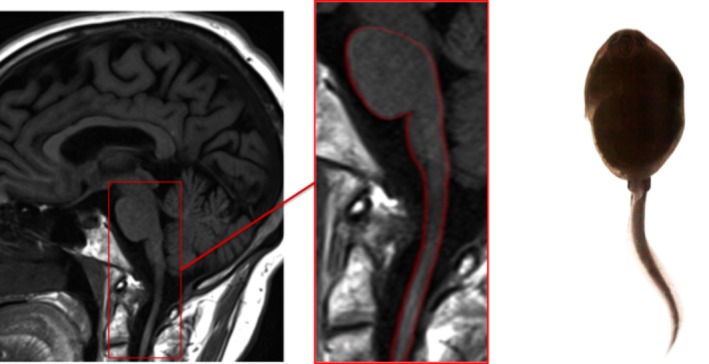
Tadpole sign‐adult‐onset Alexander disease.
Tigroid and Leopard Skin Pattern
Clinical Correlate: Metachromatic Leucodystrophy
Tigroid and leopard skin both describe a specific form of dysmyelination observed in metachromatic leucodystrophy, a recessively inherited lysosomal storage disorder which leads to demyelination in the central and peripheral nervous systems.22 Three subtypes (infantile, juvenile, and adult) are defined, based on the age of onset. Symptoms common to most presentations include developmental or cognitive regression, ataxia, and peripheral neuropathy.22
The neuroradiological features of the condition progress similarly regardless of the subtype.22 Early in the disease course, “sheet‐like” T2 hyperintensities appear in the corpus callosum and periventricular frontal and parietal regions, gradually spreading more posteriorly and into the subcortical regions as the condition progresses.22 The tigroid/leopard‐skin appearance results from areas of normal signal intensity within this sheet, giving the appearance of dark stripes (tigroid) or dots (leopard‐skin) on a white background (Fig. 9).22 The pattern is also observed in other lysosomal storage diseases and in Pelizaeus‐Merzbacher disease, both of which can also produce movement disorder phenomenology.22
Figure 9.
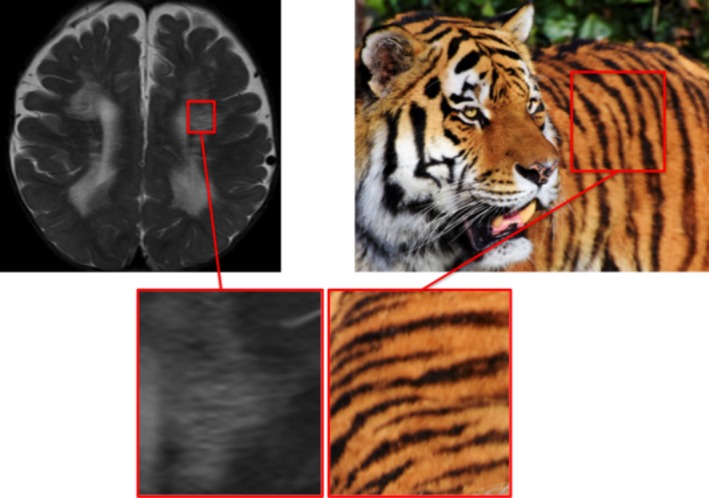
Tigroid and leopard skin pattern: metachromatic leucodystrophy.
Bat Wing Sylvian Fissure
Clinical Correlate: Glutaric Aciduria Type 1
Glutaric aciduria type 1 is a rare disorder of lysine and tryptophan metabolism in which toxic metabolites accumulate in and damage the striatum. Affected children often manifest with dystonic movements, either as part of encephalopathic crises or as an insidious onset movement disorder.23 Delays in diagnosis or treatment can lead to cerebral atrophy, widening of the sylvian fissures, and their characteristic bat wing appearance (Fig. 10).24 The disorder is tested for as part of newborn blood spot screening in many countries, and its consequences completely avoidable with appropriate dietary modifications.24
Figure 10.
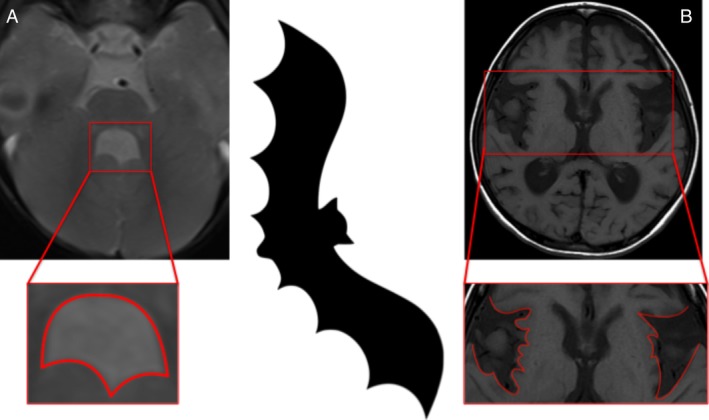
Bat wing fourth ventricle (image A): Joubert syndrome; bat wing sylvian fissure (image B): glutaric aciduria type 1.
Bat Wing Fourth Ventricle
Clinical Correlate: Joubert Syndrome
Joubert syndrome is a polysymptomatic neurogenetic condition generally manifesting in the neonatal period with hypotonia, respiratory difficulties, and oculomotor apraxia, and, later, ataxia, cognitive, and motor delay.25 The rare patients who survive into adulthood may develop a parkinsonian syndrome.25 Neuroimaging characteristically shows vermian aplasia with elongated superior cerebellar peduncles forming a “molar tooth sign” and the resulting enlarged fourth ventricle adopts a “bat wing” appearance (Fig. 10).
Conclusions
Pareidolias represent a quick and easy way of enhancing perception, thereby improving the efficiency and accuracy of image analysis. In addition, as the demands on healthcare systems continue to increase, having in mind familiar radiological patterns specific to distinct clinical movement disorder syndromes (as detailed in this review) facilitates scan evaluation.
Articles of this kind are unavoidably incomplete; each new image forms the basis of future descriptions, and the development of new imaging techniques offers unlimited potential for further descriptions. However, the use of animals as pareidolic associations will likely endure the test of time. Their familiarity to all humans, regardless of nationality, level of training, and experience, make them ideal in this respect. The only limiting factor will remain the creativity of the humanmind and its ability to find new “animals in the brain.”
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
E.M.: 1A, 1B, 1C, 3A
B.B.: 1A, 1B, 3B
M.A.: 1A, 1B, 3B
T.C.: 1A, 1B, 3B
M.M.: 1A, 1B, 3B
K.P.B.: 1A, 1B, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. Informed patient consent was not necessary for this work.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Goodman TR, Kelleher M. Improving novice radiology trainees’ perception using fine art. J Am Coll Radiol 2017;14:1337–1340. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Watanabe K. Seeing objects as faces enhances object detection. Iperception 2015;6:2041669515606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foye P, Abdelshahed D, Patel S. Musculoskeletal pareidolia in medical education. Clin Teach 2014;11:251–253. [DOI] [PubMed] [Google Scholar]
- 4. Sethi KD, Adams RJ, Loring DW, el Gammal T. Hallervorden‐Spatz syndrome: clinical and magnetic resonance imaging correlations. Ann Neurol 1988;24:692–694. [DOI] [PubMed] [Google Scholar]
- 5. Hayflick SJ, Kurian MA, Hogarth P. Neurodegeneration with brain iron accumulation. Handb Clin Neurol 2018;147:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guillerman RP. The eye‐of‐the‐tiger sign. Radiology 2000;217:895–896. [DOI] [PubMed] [Google Scholar]
- 7. van den Bogaard SJ, Kruit MC, Dumas EM, Roos RA. Eye‐of‐the‐tiger‐sign in a 48 year healthy adult. J Neurol Sci 2014;336:254–256. [DOI] [PubMed] [Google Scholar]
- 8. Chang CL, Lin CM. Eye‐of‐the‐tiger sign is not pathognomonic of pantothenate kinase‐associated neurodegeneration in adult cases. Brain Behav 2011;1:55–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Członkowska A, Litwin T, Chabik G. Wilson disease: neurologic features. Handb Clin Neurol 2017;142:101–119. [DOI] [PubMed] [Google Scholar]
- 10. Schwarz ST, Afzal M, Morgan PS, et al. The “swallow tail” appearance of the healthy nigrosome: a new accurate test of Parkinson's disease—a case‐control and retrospective cross‐sectional MRI study at 3T. PLoS One 2014;9:e93814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oustwani CS, Korutz AW, Lester MS, Kianirad Y, Simuni T, Hijaz TA. Can loss of the swallow tail sign help distinguish between Parkinson disease and the Parkinson‐plus syndromes? Clin Imaging 2017;44:66–69. [DOI] [PubMed] [Google Scholar]
- 12. Kato N, Arai K, Hattori T. Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 2003;210:57–60. [DOI] [PubMed] [Google Scholar]
- 13. Immovilli P, Rota E, Morelli N, et al. “humming bird sign” in fragile x‐associated tremor/ataxia syndrome. Mov Disord Clin Pract 2015;2:328–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi Z, Tsuruoka S, Numasawa Y, Tomimitsu H, Shintani S. Disappearance of the hummingbird sign after shunt surgery in a case of idiopathic normal pressure hydrocephalus. Intern Med 2016;55:815–817. [DOI] [PubMed] [Google Scholar]
- 15. Itolikar SM, Salagre SB, Kalal CR. ‘Humming bird sign’, ‘penguin sign’ and ‘mickey mouse sign’ in progressive supranuclear palsy. J Assoc Phys India 2012;60:52. [PubMed] [Google Scholar]
- 16. Kara E, Tucci A, Manzoni C, et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain 2016;139:1904–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riverol M, Samaranch L, Pascual B, et al. Forceps minor region signal abnormality “ears of the lynx”: an early MRI finding in spastic paraparesis with thin corpus callosum and mutations in the spatacsin gene (SPG11) on chromosome 15. J Neuroimaging 2009;19:52–60. [DOI] [PubMed] [Google Scholar]
- 18. Pacheco FT, Rego MM, do Rego JI, da Rocha AJ. “Ears of the lynx” sign in a marchiafava‐bignami patient: structural basis and fiber‐tracking DTI contribution to the understanding of this imaging abnormality. J Neuroimaging 2014;24:205–207. [DOI] [PubMed] [Google Scholar]
- 19. Masdeu J, Pascual B, Franca M, et al. Sensitivity and specificity of the “Ears of the Lynx” MRI sign in spastic paraparesis with SPG mutations (P2.038). Neurology 2015; 84:(14 Suppl) P2.038. [Google Scholar]
- 20. Pacheva IH, Todorov T, Ivanov I, Tartova D, Gaberova K, Todorova A, Dimitrova D. TSEN54 gene‐related pontocerebellar hypoplasia type 2 could mimic dyskinetic cerebral palsy with severe psychomotor retardation. Front Pediatr 2018;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Graff‐Radford J, Schwartz K, Gavrilova RH, Lachance DH, Kumar N. Neuroimaging and clinical features in type II (late‐onset) Alexander disease. Neurology 2014;82:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Rappard DF, Boelens JJ, Wolf NI. Metachromatic leukodystrophy: disease spectrum and approaches for treatment. Best Pract Res Clin Endocrinol Metab 2015;29:261–273. [DOI] [PubMed] [Google Scholar]
- 23. Kölker S, Garbade SF, Greenberg CR, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl‐CoA dehydrogenase deficiency. Pediatr Res 2006;59:840–847. [DOI] [PubMed] [Google Scholar]
- 24. Doraiswamy A, Kesavamurthy B, Ranganathan L. Batwing appearance—a neuroradiologic clue to glutaric aciduria‐type 1. Int J Epilepsy 2015;2:44–48. [Google Scholar]
- 25. Gunzler SA, Stoessl AJ, Egan RA, Weleber RG, Wang P, Nutt JG. Joubert syndrome surviving to adulthood associated with a progressive movement disorder. Mov Disord 2007;22:262–265. [DOI] [PubMed] [Google Scholar]


