Abstract
Purpose
Oliceridine is a novel G protein-biased µ-opioid receptor agonist designed to provide intravenous (IV) analgesia with a lower risk of opioid-related adverse events (ORAEs) than conventional opioids.
Patients and methods
APOLLO-1 (NCT02815709) was a phase III, double-blind, randomized trial in patients with moderate-to-severe pain following bunionectomy. Patients received a loading dose of either placebo, oliceridine (1.5 mg), or morphine (4 mg), followed by demand doses via patient-controlled analgesia (0.1, 0.35, or 0.5 mg oliceridine, 1 mg morphine, or placebo). The primary endpoint compared the proportion of treatment responders through 48 hours for oliceridine regimens and placebo. Secondary outcomes included a composite measure of respiratory safety burden (RSB, representing the cumulative duration of respiratory safety events) and the proportion of treatment responders vs morphine.
Results
Effective analgesia was observed for all oliceridine regimens, with responder rates of 50%, 62%, and 65.8% in the 0.1 mg, 0.35 mg, and 0.5 mg regimens, respectively (all P<0.0001 vs placebo [15.2%]; 0.35 mg and 0.5 mg non-inferior to morphine). RSB showed a dose-dependent increase across oliceridine regimens (mean hours [SD]: 0.1 mg: 0.04 [0.33]; 0.35 mg: 0.28 [1.11]; 0.5 mg: 0.8 [3.33]; placebo: 0 [0]), but none were statistically different from morphine (1.1 [3.03]). Gastrointestinal adverse events also increased in a dose-dependent manner in oliceridine regimens (0.1 mg: 40.8%; 0.35 mg: 59.5%; 0.5 mg: 70.9%; placebo: 24.1%; morphine: 72.4%). The odds ratio for rescue antiemetic use was significantly lower for oliceridine regimens compared to morphine (P<0.05).
Conclusion
Oliceridine is a novel and effective IV analgesic providing rapid analgesia for the relief of moderate-to-severe acute postoperative pain compared to placebo. Additionally, it has a favorable safety and tolerability profile with regard to respiratory and gastrointestinal adverse effects compared to morphine, and may provide a new treatment option for patients with moderate-to-severe postoperative pain where an IV opioid is required.
Keywords: postoperative, analgesia, patient controlled, clinical trial, orthopedic surgery
Plain language summary
Opioids and other pain medications are used to reduce severe pain immediately after medical or surgical procedures. However, opioids can have unwanted side effects that limit their use, such as causing slow and shallow breathing and gastrointestinal problems like nausea or vomiting. Oliceridine is being developed for the short-term treatment of moderate-to-severe pain. It attaches to the same receptors in the body as opioids but activates them in a different way. This study tested three dosing schedules of oliceridine (1.5 mg initially plus 0.1, 0.35, or 0.5 mg on demand) compared with placebo or the opioid morphine (4 mg initially plus 1 mg on demand) in patients who had bunion removal surgery. Drugs were delivered into the vein and patients could control their own use up to a maximum level.
Findings showed that the two higher oliceridine dosing schedules provided similar pain relief to morphine and were fast acting. The most common side effects were similar in nature for oliceridine and morphine, but oliceridine tended to have fewer side effects related to slow and shallow breathing and gastrointestinal side effects. Together with previous findings, these results suggest that oliceridine can reduce moderate to severe pain associated with medical or surgical procedures rapidly and with fewer unwanted side effects than conventional opioids.
Introduction
Acute pain has substantial physiological and psychological consequences and becomes more difficult to manage as severity increases.1 Therefore, early intervention with an effective, fast-acting analgesic is key to the management of acute pain.
Intravenous (IV) opioids are effective analgesics but their use is restricted by a variety of opioid-related adverse events (ORAEs), with the most common including respiratory depression, nausea, vomiting, somnolence, and constipation.2,3 ORAEs can result in dosing interruptions or discontinuations that limit effective analgesia.4,5 In addition, ORAEs increase morbidity and mortality, delay recovery and discharge, and increase overall treatment costs.6–8 Multimodal pain management protocols have been developed in an attempt to optimize acute postoperative analgesia and reduce the use of opioid medications while simultaneously decreasing the risk of ORAEs; however, considerable clinical challenges remain.9,10 IV opioids continue to be an essential component of acute pain management and ORAEs a practical concern, particularly in populations at increased risk.6,11,12
Conventional opioids such as morphine exert their analgesic action by binding to µ-opioid receptors in the brain. This results in a cascade of events within the cell including G protein activation, which is thought to be the principal mediator of analgesia.13,14 Concurrent recruitment of β-arrestin attenuates G protein signaling and has been implicated in the occurrence of ORAEs.15–19 When treated with morphine, β-arrestin knockout mice show enhanced analgesia and fewer respiratory and gastrointestinal ORAEs than wild-type mice.17,20 µ-Opioid receptor ligands that preferentially activate G protein signaling over β-arrestin recruitment may therefore provide more effective analgesia with a lower risk of adverse events (AEs).
Oliceridine (TRV130; Trevena Inc., Chesterbrook, PA, USA) is a novel µ-opioid receptor ligand that acts as a full agonist for G protein activation but exhibits markedly reduced β-arrestin recruitment than conventional opioids such as morphine or fentanyl.18,21 In rodent models, oliceridine is potently analgesic and is associated with less gastrointestinal dysfunction and respiratory suppression than morphine at equianalgesic doses.18 A phase Ib study in healthy volunteers showed that 3 mg and 4.5 mg single-dose IV oliceridine produced greater analgesia than 10 mg morphine.19 Respiratory drive reduction was significantly less with oliceridine compared to morphine, and oliceridine was also associated with less vomiting and less severe nausea. Two single-center phase II studies in patients undergoing bunionectomy or abdominoplasty supported these findings, demonstrating that oliceridine administered as needed (PRN) had similar efficacy compared to morphine22,23 but with a notably reduced incidence of respiratory and gastrointestinal AEs.23
In this report, we extend these observations and present the results of the APOLLO-1 study: a phase III, multicenter, randomized, controlled clinical trial comparing IV oliceridine to placebo for the treatment of moderate-to-severe acute pain following bunionectomy. This model is a standardized and well-characterized model of hard tissue pain. IV morphine was included in the study as an active comparator. On the basis of our earlier observations, we hypothesized that oliceridine would provide superior efficacy vs placebo and comparable efficacy to morphine with a favorable safety/tolerability profile in the acute postoperative setting.
Materials and methods
Study overview
APOLLO-1 (ClinicalTrials.gov; NCT02815709) was a phase III, randomized, double-blind, placebo- and active-controlled study enrolling patients at seven sites in the USA between May 2016 and October 2016. The study was approved by an Institutional Review Board at each investigational site, and patients provided written informed consent before participation. Advarra® provided a centralized institutional review board approval for all sites. APOLLO-1 was conducted in compliance with the Declaration of Helsinki and all International Council for Harmonization Good Clinical Practice guidelines. At the time this study was conducted, oliceridine was an investigational agent studied under an Investigational New Drug application filed with the US Food and Drug Administration.
Patients
Eligible patients were 18–75 years of age and scheduled to undergo primary, unilateral, first-metatarsal bunionectomy with osteotomy and internal fixation. Key preoperative exclusion criteria included body weight <40 kg or body mass index >35 kg/m2; pregnant or breastfeeding; history of opioid hypersensitivity; diagnosis or suspicion of sleep apnea; use of chronic opioid therapy (>15 morphine-equivalent units/day for >3 days/week and for >1 month within 1 year of surgery); use of any analgesic medication within five half-lives (or 48 hours, if unknown) before surgery; chronic nonsteroidal anti-inflammatory drug therapy (daily use for >2 weeks within 6 months before surgery with the exception of aspirin ≤325 mg daily for cardiovascular prophylaxis if the patient was on a stable regimen for ≥30 days); use of agents that could affect analgesic response (central α-adrenergic, antiepileptic, neuroleptic, antidepressant, or antipsychotic agents) that had not been stably dosed for ≥30 days prior to surgery; use of oral or parenteral corticosteroids within 3 months before surgery; or hepatic or renal impairment at screening.
Following surgery, patients were enrolled in the study and received study medication if they reported at least moderate pain, as measured on both a categorical scale (none, mild, moderate, and severe) and on an 11-point numeric rating scale ([NRS]≥4) within 9 hours after discontinuation of regional perineural anesthesia. Key postoperative exclusion criteria included surgical duration >90 minutes, evidence of hemodynamic instability or respiratory insufficiency, or surgical/anesthetic complications or protocol violations that could confound interpretation of study data.
Study treatments
For each regimen, a clinician-administered fixed IV loading dose was followed by demand doses administered PRN via a patient-controlled analgesia (PCA) device. Thus, each dose regimen included a range of actual cumulative drug exposures over the course of the study. The PCA doses were allowed from 10 minutes after the loading dose and were limited by a 6-minute lockout interval. Patients meeting all study entry requirements were randomized in equal allocation to one of five double-blind treatment regimens (Table 1). The oliceridine doses selected for study were expected to confirm earlier clinical observations and establish a range of oliceridine doses with acceptable safety and a range of analgesic efficacy comparable to IV morphine.22,23 Clinician-administered IV supplemental PRN doses were permitted from 1 hour after the loading dose, and then as often as hourly. The dosing limit for all groups was three PCA syringes or six clinician-administered supplemental PRN doses within the first 12 hours (60 mg for oliceridine), after which patients were discontinued and managed conventionally.
Table 1.
Treatment regimens
| Treatment regimens | Clinician-administered loading dose (mg) | Demand dose via PCA (mg) | Clinician-administered supplemental dose (mg) |
|---|---|---|---|
| Placebo | Volume-matched | Volume-matched | Volume-matched |
| Oliceridine 0.1 mg regimen | 1.5 | 0.1 | 0.75 |
| Oliceridine 0.35 mg regimen | 1.5 | 0.35 | 0.75 |
| Oliceridine 0.5 mg regimen | 1.5 | 0.5 | 0.75 |
| Morphine 1 mg regimen | 4 | 1 | 2 |
Notes: Clinician-administered supplemental dosing could start 1 hour after the loading dose and be administered up to hourly, as needed. Further open-label rescue pain medication (oral etodolac 200 mg every 6 hours) was permitted, as needed. PCA had a 6-minute lockout.
Abbreviation: PCA, patient-controlled analgesia.
Concomitant medications
Use of midazolam and/or propofol for initial sedation was at the anesthesia provider’s discretion. A popliteal sciatic nerve block was established with a local anesthetic, and a field block could have been added if the popliteal sciatic nerve block did not sufficiently provide adequate intraoperative anesthesia. Regional anesthesia was maintained during the immediate postoperative period using a continuous infusion of local anesthetic until ~3 AM on postoperative Day 1, during which time patients could have received adjunctive analgesia via optimization of regional anesthetic followed by oral oxycodone 5 mg every 4 hours PRN. If regional anesthesia and adjunctive analgesia were inadequate, the patient was considered a screening failure.
Prophylactic supplemental oxygen was not permitted during the randomized treatment period. Similarly, prophylactic antiemetics were not permitted preoperatively or during the randomized treatment period.
If study medication (including both demand and supplemental PRN doses) provided inadequate pain relief, as determined by an NRS score >4, patients could receive open-label rescue pain medication (oral etodolac 200 mg every 6 hours PRN).
Study endpoints
Primary endpoint
The primary outcome was the percentage of patients in each oliceridine group who met the prespecified response criteria at the end of the randomized 48-hour treatment period. Responders were those who met all of the following criteria: 1) reached a ≥30% improvement in time-weighted sum of pain intensity difference (SPID-48) from baseline; 2) did not receive protocol-specified rescue pain medication; 3) did not discontinue study medication early; and 4) did not reach dosing limits. SPID-48 was determined using NRS pain scores reported at baseline (up to 10 minutes before loading dose [Time 0]), 5, 10, 15, 30, and 45 minutes, and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 18, 24, 32, 40, and 48 hours post-loading dose. Patients who exceeded dosing limits discontinued study medication, and as with patients who took rescue medication or discontinued study medication early, all were included in the safety analysis and the efficacy analysis as non-responders.
Key secondary endpoints
Two key secondary endpoints were prespecified. The first was the respiratory safety burden (RSB) experienced by patients in each treatment group, calculated as the mathematical product of the incidence of a defined set of observed respiratory safety events multiplied by the mean expected cumulative duration of these events (in hours). Observations eligible for consideration as respiratory safety events were changes in respiratory rate, oxygen saturation, and sedation measured using the Moline-Roberts Pharmacologic Sedation Scale.24 The cumulative duration of a respiratory safety event was calculated as the sum of individual observed events. During the randomized treatment period, patients were monitored on a protocol-defined schedule by either a certified registered nurse anesthetist or an anesthesiologist blinded to study medication assignment. The monitoring professional intervened when clinically indicated and determined the onset and resolution of each respiratory safety event. Interventions included provision of supplemental oxygen and dosing interruptions—the incidence and duration of these interventions were assessed as secondary endpoints, as described below.
The second prespecified key secondary endpoint was the proportion of responders in each oliceridine regimen compared to morphine and included both non-inferiority and superiority analyses.
Other secondary endpoints
Other prespecified secondary efficacy endpoints included the proportion of treatment responders over time, cumulative response by time point, and the proportion of patients receiving rescue pain medication over time. Pain intensity was calculated by time point and by SPID scores by time interval. The magnitude of time-weighted average change from baseline in NRS score—including the time to a 1-, 2-, or 3-point change in NRS score—and the magnitude and time to onset of self-reported categorical pain relief were calculated. In addition, the time to meaningful pain relief was assessed using the two-stopwatch method.25 Finally, efficacy was also examined using time to onset, number, and overall proportion of rescue pain medication use, and by clinician- and patient-reported satisfaction with assigned study medication. This measurement was based on score values ranging from “completely dissatisfied” to “completely satisfied” and measured during the pre-discharge period.
Overall safety and tolerability were assessed via the occurrence of treatment-emergent AEs and dosing interruptions due to safety or tolerability reasons. Spontaneously reported AEs were assessed during the randomized treatment and 7-day follow-up period, coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 19.0.26 The severity of each AE and the likely causal relationship to study medication were determined by the clinician. Additional prespecified safety endpoints evaluated the incidence of respiratory and gastrointestinal AEs and the percentage of patients with any respiratory safety event, vomiting, or use of rescue antiemetics. Clinical lab values and vital sign measurements were also conducted as part of the overall safety evaluation.
Statistical analysis
A prespecified Hochberg gatekeeping approach was used to assess the primary and key secondary endpoints in order to control for Type I (false positive) errors when testing a family of multiple treatment groups.27 The order of analysis was: 1) the primary superiority responder analysis vs placebo; 2) the key secondary RSB analysis vs morphine; 3) the key secondary non-inferiority responder analysis vs morphine (at least 50% of the observed effect of morphine); and 4) the key secondary superiority responder analysis vs morphine. Within each analysis step, a Hochberg adjustment was applied to all P-values. The order of the gating was selected according to the specific interest in comparing the respiratory safety profile of oliceridine and morphine, and the significant amount of power expected to be required to achieve non-inferiority between active regimens, which were selected on the basis of their potential equianalgesia.
A planned sample size of 375 patients (75 per treatment group) was estimated to provide 88% power to demonstrate that at least two oliceridine treatment groups were superior to placebo for the primary responder endpoint, superior to morphine for the key secondary RSB endpoint, and non-inferior to morphine for the key secondary responder endpoint. This sample size provided greater than 99% power to demonstrate superiority of all oliceridine groups compared with placebo for the primary endpoint. These power calculations incorporated the Hochberg gatekeeping multiplicity adjustment and were based on results from a phase II clinical trial comparing the efficacy and safety of oliceridine vs placebo and vs morphine following abdominoplasty.23
The primary endpoint of treatment responders (oliceridine superiority vs placebo) was analyzed using a logistic regression model that contained treatment group as a fixed factor and baseline NRS score and study site as covariates. Intermittently missing NRS measures were imputed by linear interpolation. In situations where all NRS values were missing after a certain point (owing to study discontinuation, for example), a model-based multiple imputation method was applied.
The key secondary endpoint of RSB (oliceridine superiority vs morphine) was analyzed using a zero-inflated gamma mixture model, with treatment group as a factor and baseline NRS, body mass index, and study site as covariates.
The key secondary endpoint analysis of treatment responders (oliceridine non-inferiority vs morphine) was analyzed using one-sided linear contrasts on the logarithmic odds scale from a logistic regression model that contained treatment group as a fixed factor and baseline NRS score and study site as covariates. The contrasts were constructed so that any oliceridine treatment regimen shown to have an effect significantly greater than half of the observed morphine effect would be regarded as non-inferior to morphine.
The secondary endpoint of treatment responders (oliceridine superiority vs morphine) was analyzed using the same methods as for the primary comparison of oliceridine vs placebo.
All treatment comparisons were two-sided with an unadjusted (nominal) significance level of α=0.05, with the exception of the primary and key secondary endpoints. Patient demographics and AEs were summarized descriptively.
Results
Patient disposition
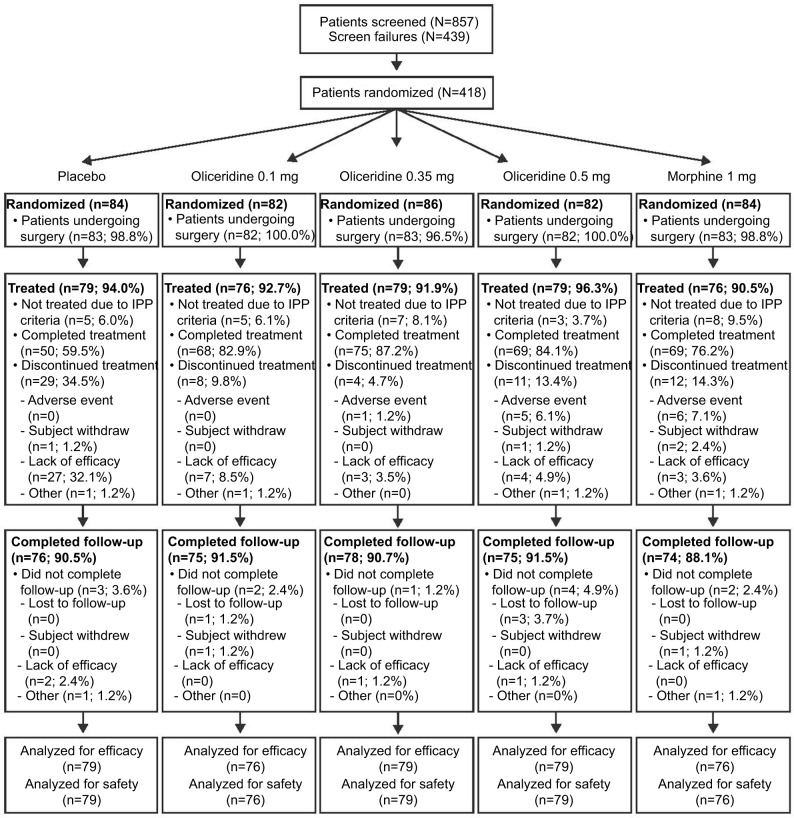
A total of 418 patients were randomized and 389 patients received study medication: placebo, n=79; oliceridine 1.5 mg loading dose/0.1 mg on demand, n=76; oliceridine 1.5 mg loading dose/0.35 mg on demand, n=79; oliceridine 1.5 mg loading dose/0.5 mg on demand, n=79; morphine 4 mg loading dose/1 mg on demand, n=76. Patient disposition throughout the trial is shown in Figure 1. Most patients who received oliceridine completed treatment (90.6%) compared with 63.3% and 84.2% in the placebo and morphine groups, respectively. Patient demographics were comparable across all treatment groups (Table 2). Most patients were female (84.8%). Many reported severe pain at baseline (42.3%), and most had an Apfel risk score of 3 or 4 (77.1%) at enrollment.
Figure 1.
Patient disposition.
Notes: All percentages based on the number of patients randomized. Efficacy and safety analyses include all randomized patients who received ≥1 dose of study medication. Abbreviation: IPP, immediate postoperative period.
Table 2.
Patient demographics and baseline characteristics
| Placebo (n=79) | Oliceridine demand dose regimen | Morphine 1 mg regimen (n=76) | |||
|---|---|---|---|---|---|
| 0.1 mg (n=76) | 0.35 mg (n=79) | 0.5 mg (n=79) | |||
| Gender, n (%) | |||||
| Male | 9 (11.4) | 12 (15.8) | 14 (17.7) | 13 (16.5) | 11 (14.5) |
| Female | 70 (88.6) | 64 (84.2) | 65 (82.3) | 66 (83.5) | 65 (85.5) |
| Mean (SD) age, years | 44.1 (12.6) | 47.5 (12.7) | 43.6 (13.9) | 46.9 (13.8) | 43.3 (14.1) |
| Mean (SD) BMI, kg/m2 | 26.3 (4.3) | 26.4 (4.4) | 26.0 (3.8) | 27.1 (4.3) | 26.5 (4.5) |
| BMI ≥30 kg/m2, n (%) | 19 (24.1) | 19 (25) | 10 (12.7) | 23 (29.1) | 18 (23.7) |
| Race, n (%) | |||||
| White | 56 (70.9) | 47 (61.8) | 56 (70.9) | 61 (77.2) | 50 (65.8) |
| Black/African American | 21 (26.6) | 22 (28.9) | 17 (21.5) | 13 (16.5) | 21 (27.6) |
| Other | 2 (2.5) | 7 (9.2) | 6 (7.6) | 5 (6.3) | 5 (6.6) |
| Mean (SD) surgery duration, minutes | 29.1 (10.4) | 29.9 (10.9) | 30.8 (15.9) | 29.2 (10.2) | 29.2 (11.1) |
| Mean (SD) baseline pain scorea | 7.0 (1.5) | 6.8 (1.8) | 6.6 (1.9) | 6.5 (1.7) | 6.7 (1.6) |
| Baseline categorical pain rating, n (%)b | |||||
| Mild | 0 | 1 (1.3) | 1 (1.3) | 2 (2.5) | 1 (1.3) |
| Moderate | 44 (56.4) | 43 (56.6) | 47 (59.5) | 44 (55.7) | 41 (53.9) |
| Severe | 34 (43.6) | 32 (42.1) | 31 (39.2) | 33 (41.8) | 34 (44.7) |
| Apfel risk score, n (%)c | |||||
| 1 | 1 (1.3) | 3 (3.9) | 1 (1.3) | 5 (6.3) | 0 |
| 2 | 14 (17.7) | 13 (17.1) | 20 (25.3) | 12 (15.2) | 20 (26.3) |
| 3 | 50 (63.3) | 44 (57.9) | 48 (60.8) | 52 (65.8) | 43 (56.6) |
| 4 | 14 (17.7) | 16 (21.1) | 10 (12.7) | 10 (12.7) | 13 (17.1) |
Notes:
Patients self-rated pain on a scale from 0= no pain to 10= worst pain imaginable.
Percentages based on the total number of patients who responded; one patient was missing baseline pain scores in the placebo group.
Apfel score assesses a patient’s risk for postoperative nausea and vomiting based on known risk factors. Total score ranges from 0 to 4, with higher score indicating greater risk, and is the sum of positive responses to the following questions: is the patient female; does the patient have a history of postoperative nausea, vomiting, or motion sickness; is the patient a non-smoker; and does the patient have postoperative opioid use. In this study, all patients were considered as having postoperative opioid use.
Abbreviation: BMI, body mass index.
Study treatment exposure
Across the 48-hour treatment duration, mean (SD) cumulative exposure to study medication was 19.2 mg (11.2), 49.3 mg (27.1), and 57.4 mg (34.6) with the oliceridine 0.1, 0.35, and 0.5 mg demand dose regimens, and 68.0 mg (52.5) with the morphine regimen.
Efficacy
Primary outcome
Patients in each of the oliceridine regimens showed a statistically superior responder outcome compared to those receiving placebo (P<0.0001 for all, with Hochberg adjustment; Figure 2A).
Figure 2.
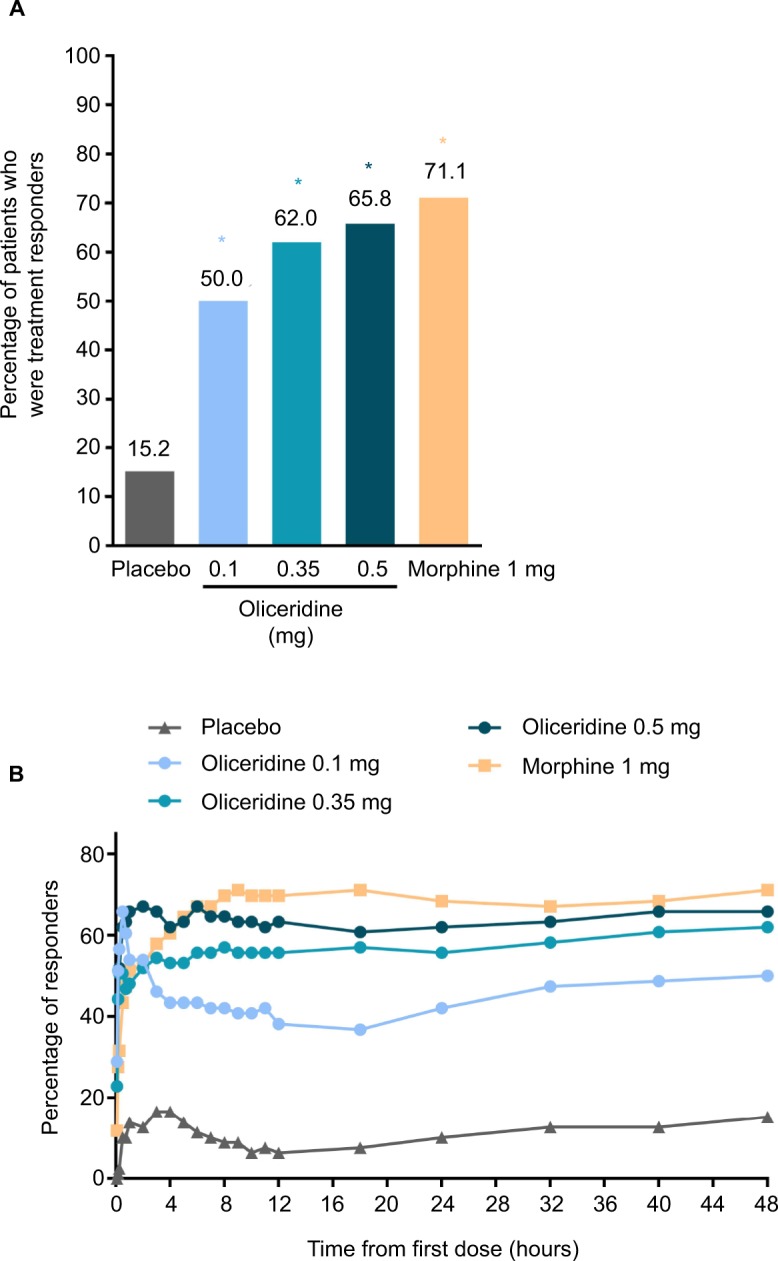
(A) Primary treatment response analysis of oliceridine compared with placebo (treatment responders at 48 hours).
Notes: This primary endpoint analysis compared the percentage of treatment responders in each oliceridine regimen with the percentage of responders in the placebo regimen at 48 hours post-loading dose. (B) Treatment responders over the full treatment period. Responders were those who reached a ≥30% improvement in time-weighted SPID-48 from baseline while not receiving rescue pain medication, discontinuing study medication early, or reaching dosing limits. *P<0.0001 vs placebo (Hochberg adjusted).
Abbreviation: SPID, sum of pain intensity difference.
Secondary outcomes
No formal non-inferiority assessments were conducted between oliceridine and morphine response rates because the predefined statistical analysis failed to show significance at the second gating assessment (ie, RSB). However, exploratory analyses (not corrected for multiplicity) indicated that the oliceridine 0.35 mg and 0.5 mg regimens were non-inferior to morphine (both P<0.01).
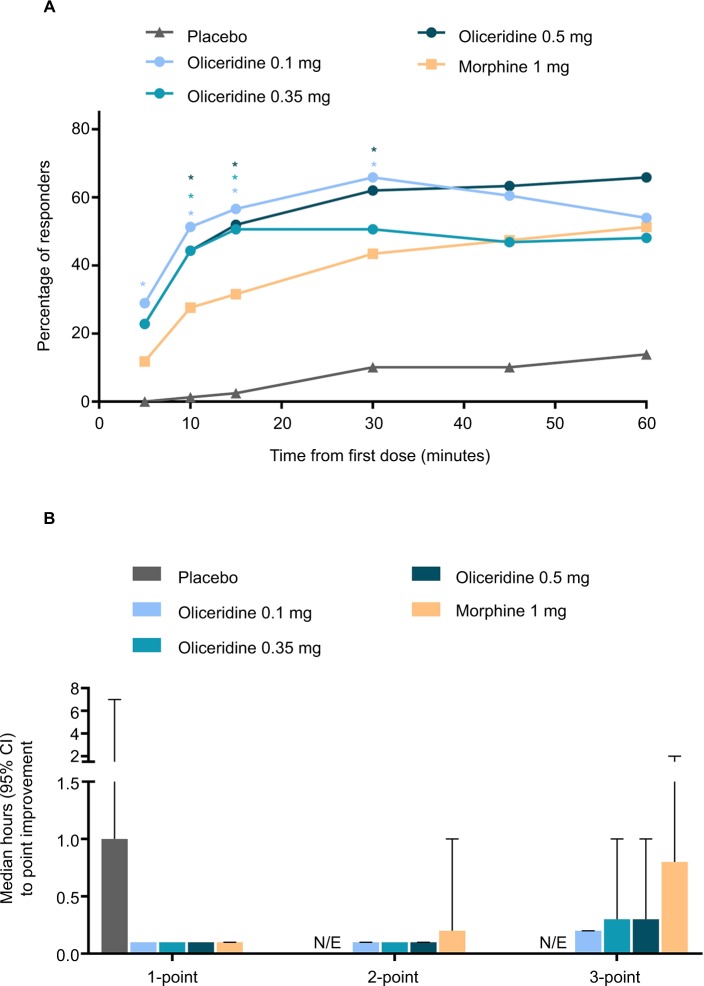
On the secondary outcome measures, oliceridine 0.35 mg and 0.5 mg regimens provide pain relief comparable to the morphine regimen. For example, a similar proportion of patients in all of these groups were treatment responders over the full treatment period (Figure 2B). Also, fewer patients discontinued treatment owing to lack of efficacy in the morphine and oliceridine regimens (Figure 1) compared to those receiving placebo. Likewise, fewer patients in any of the active treatment regimens used rescue medications (Figure 3A). Using the two-stopwatch method, the median time to self-reported “perceptible pain relief ” was 6 minutes in all oliceridine regimens and 6 minutes in the morphine regimen, and the self-reported time to “meaningful pain relief ” was 12 minutes in all oliceridine regimens and 30 minutes in the morphine regimen (P>0.05; Figure 3B). During the first hour of treatment, when supplemental study medication doses were prohibited and rescue pain medication was discouraged, there was a greater proportion of responders with oliceridine regimens vs morphine, particularly during the first 30 minutes (Figure 4A). The time to 1-, 2-, and 3-point change in NRS score from baseline was also shorter in oliceridine groups than with morphine (Figure 4B). Further analyses of pain response over time and clinician-/patient-reported satisfaction with assigned study medication are reported in the Supplementary materials.
Figure 3.
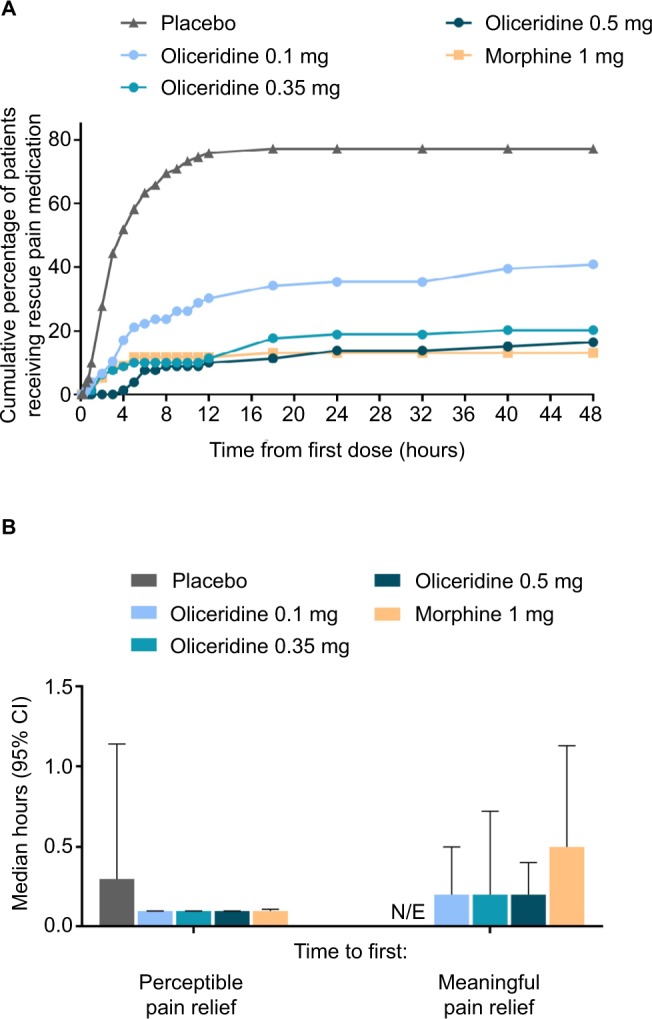
(A) The cumulative percentage of patients using rescue pain medication in each regimen is shown at predefined time points throughout the 48-hour treatment period. Patients could receive open-label rescue pain medication (oral etodolac 200 mg every 6 hours PRN) if they reported a score ≥4 on the pain NRS. (B) Time to first perceptible and first meaningful pain relief. Median time in hours is presented, as reported by patients using the two-stopwatch method. Placebo data were N/E for time to meaningful pain relief.
Abbreviations: N/E, not evaluable; NRS, numeric rating scale; PRN, as needed.
Figure 4.
(A) The proportion of responders over the first 60 minutes of treatment. Responses during this period can largely be attributed to study drug (loading dose at Time 0 and demand doses starting at 10 minutes) since supplemental study medication doses were prohibited and rescue pain medication was discouraged during this time. *P<0.05 for odds ratio vs morphine. (B) Time to 1-, 2-, and 3-point improvement in NRS score. NRS pain scores were reported at baseline (up to 10 minutes before loading dose [Time 0]), 5, 10, 15, 30, and 45 minutes, and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 18, 24, 32, 40, and 48 hours post-loading dose. Median data for the placebo group were not calculable for time to 2- and 3-point improvements due to small number of patients achieving this improvement. Responses in the oliceridine groups were not statistically different from morphine.
Abbreviation: N/E, not evaluable; NRS, numeric rating scale.
Safety
Respiratory safety
There were no statistically significant differences on the composite outcome measure of RSB for any of the oliceridine treatment groups (mean: 0.04–0.80 hours [1–15 minutes from the model-based estimate]) compared to the morphine group (1.10 hours [33 minutes]; Figure 5). The two main components that contribute to the computation of RSB, namely the incidence of respiratory safety events and the mean cumulative duration of those events, increased in a dose-dependent manner across the oliceridine treatment groups (Table 3 and Figure 6). Patients in the 0.1 mg and 0.35 mg regimens had a significantly lower risk of experiencing a respiratory safety event compared with morphine. The highest demand dose regimen of oliceridine (0.5 mg) was not statistically distinguishable from morphine (Table 3). The mean duration of the respiratory safety events waŝ3 hours in the 0.1 mg and 0.35 mg oliceridine regimens, and almost 6 hours in the 0.5 mg and morphine regimens.
Figure 5.
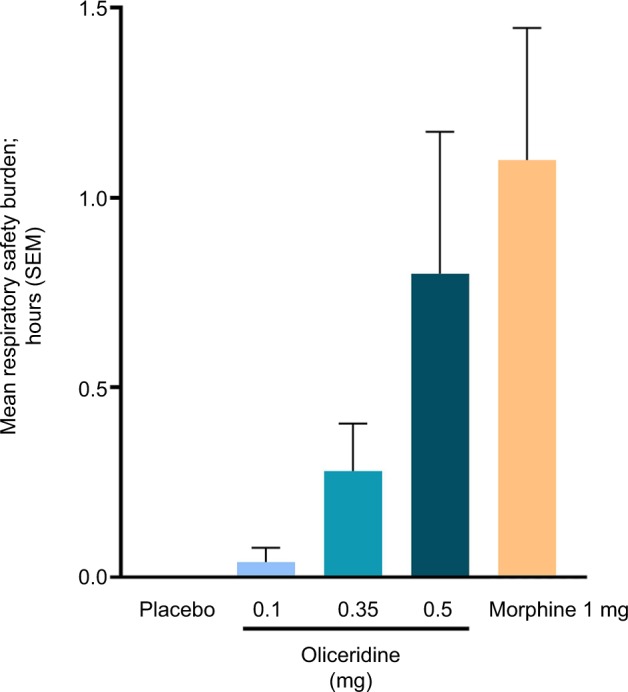
The key prespecified secondary endpoint of cumulative respiratory safety burden.
Notes: During the randomized treatment period, patients were monitored on a protocol-defined schedule by either a certified registered nurse anesthetist or an anesthesiologist blinded to study medication assignment. The monitoring professional intervened when clinically indicated and determined the onset and resolution of each respiratory safety event. Respiratory safety burden was defined as the expected cumulative duration (in hours) of respiratory safety events in a particular treatment group and was calculated as the mathematical product of the prevalence of respiratory safety events and the mean conditional duration (ie, mean duration in affected patients) of such events (Table 3). Mean respiratory safety burden was 1, 1, 9, 15, and 33 minutes for the placebo, oliceridine 0.1 mg, 0.35 mg, 0.5 mg, and morphine groups, respectively (from the model-based estimate). There were no statistically significant differences for this composite outcome measure for any of the oliceridine treatment groups compared to morphine in the prespecified key secondary analysis with Hochberg adjustment.
Abbreviation: SEM, standard error of the mean.
Table 3.
Components of the respiratory safety burden and respiratory safety event measures
| Patients, n (%) unless stated | Placebo (n=79) | Oliceridine demand dose regimen | Morphine 1 mg regimen (n=76) | ||
|---|---|---|---|---|---|
| 0.1 mg (n=76) | 0.35 mg (n=79) | 0.5 mg (n=79) | |||
| Components of the respiratory safety burden | |||||
| Respiratory safety event | 0 | 1 (1.3) | 7 (8.9) | 11 (13.9) | 14 (18.4) |
| P-valuea | – | 0.455 | 0.044 | 0.015 | 0.006 |
| P-valueb | 0.006 | 0.002 | 0.050 | 0.364 | – |
| Duration of event, mean hours (SD) | 0 (N/E) | 2.88 (N/E) | 3.21 (2.24) | 5.72 (7.44) | 5.96 (4.67) |
| P-valuea | – | 0.590 | 0.644 | 0.737 | 0.102 |
| P-valueb | 0.102 | 0.140 | 0.260 | 0.186 | – |
| Respiratory safety event measures | |||||
| Oxygen saturation <90% | 1 (1.3) | 3 (3.9) | 8 (10.1) | 11 (13.9) | 15 (19.7) |
| P-valuea | – | 0.320 | 0.042 | 0.016 | 0.005 |
| P-valueb | 0.005 | 0.006 | 0.100 | 0.352 | – |
| Respiratory rate ≤8 breaths/minute | 0 | 0 | 1 (1.3) | 1 (1.3) | 4 (5.3) |
| P-valuea | – | 1.000 | 0.961 | 0.961 | 0.956 |
| P-valueb | 0.956 | 0.956 | 0.188 | 0.185 | – |
| Sedation (Moline-Roberts Scale ≥3) | 10 (2.7) | 14 (18.4) | 16 (20.3) | 13 (16.5) | 15 (19.7) |
| P-valuea | – | 0.331 | 0.205 | 0.501 | 0.242 |
| P-valueb | 0.242 | 0.838 | 0.926 | 0.610 | – |
Notes:
P-value comparison vs placebo.
P-value comparison vs morphine.
Abbreviation: N/E, not evaluable.
Figure 6.
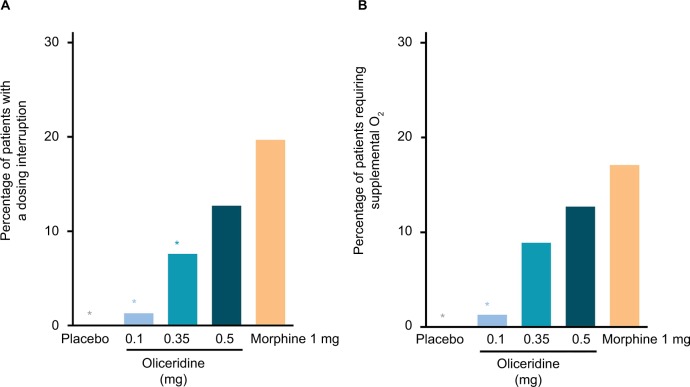
Clinical interventions.
Notes: (A) The proportion of patients in each regimen who experienced any dosing interruption of study medication during the study. Exploratory analyses showed that the odds ratio of an interruption compared to morphine was 0.02 (P=0.0049) for placebo, 0.07 (P=0.0014) for oliceridine 0.1 mg, 0.29 (P=0.0169) for oliceridine 0.35 mg, and 0.56 (P=0.1968) for oliceridine 0.5 mg regimens. (B) The proportion of patients in each regimen who required supplemental oxygen therapy. Exploratory analyses showed that the odds ratio of requiring supplemental oxygen therapy compared to morphine was 0.02 (P=0.0076) for placebo, 0.08 (P=0.0030) for oliceridine 0.1 mg, 0.42 (P=0.0864) for oliceridine 0.35 mg, and 0.66 (P=0.3790) for oliceridine 0.5 mg regimens. *P<0.05 odds ratio vs morphine.
The odds ratio of experiencing a dosing interruption was significantly lower in patients in the 0.1 and 0.35 mg oliceridine regimens compared to the morphine regimen (P<0.05; Figure 6). The odds ratio for requiring supplemental oxygen was significantly lower in the oliceridine 0.1 mg regimens compared to morphine (P<0.01; Figure 6).
Gastrointestinal safety
The proportion of patients experiencing one or more gastrointestinal-related AEs, as defined by MedDRA preferred terms, increased in a dose-dependent manner across the three oliceridine regimens (0.1 mg: 40.8%, 0.35 mg: 59.5%, and 0.5 mg: 70.9% compared to placebo: 24.1% and morphine: 72.4%). This was also observed for the specific terms of nausea and vomiting (Table 4). The odds ratio for rescue antiemetic use was significantly lower in all oliceridine demand dose regimens compared to morphine-treated patients (P<0.05; Figure 7).
Table 4.
Summary of treatment-emergent adverse eventsa
| Placebo (n=79) | Oliceridine demand dose regimen | Morphine 1 mg regimen (n=76) | |||
|---|---|---|---|---|---|
| 0.1 mg (n=76) | 0.35 mg (n=79) | 0.5 mg (n=79) | |||
| Total number of AEs | 166 | 176 | 317 | 349 | 387 |
| Patients with ≥1 AE, n (%) | 54 (68.4) | 56 (73.7) | 68 (86.1) | 72 (91.1) | 73 (96.1) |
| Patients with ≥1 serious AE, n (%) | 0 | 0 | 0 | 0 | 0 |
| Patients with AE by severity, n (%) | |||||
| Mild | 26 (32.9) | 31 (40.8) | 30 (38.0) | 24 (30.4) | 21 (27.6) |
| Moderate | 27 (34.2) | 21 (27.6) | 34 (43.0) | 44 (55.7) | 42 (55.3) |
| Severe | 1 (1.3) | 4 (5.3) | 4 (5.1) | 4 (5.1) | 10 (13.2) |
| Patients discontinued due to AE, n (%) | 0 | 0 | 1 (1.3) | 5 (6.3) | 6 (7.9) |
| Most common AE, n (% of patients)b | |||||
| Nausea | 19 (24.1) | 27 (35.5) | 44 (55.7) | 50 (63.3) | 49 (64.5) |
| Vomiting | 5 (6.3) | 13 (17.1) | 31 (39.2) | 32 (40.5) | 38 (50.0) |
| Headache | 24 (30.4) | 19 (25.0) | 20 (25.3) | 26 (32.9) | 23 (30.3) |
| Dizziness | 8 (10.1) | 21 (27.6) | 25 (31.6) | 28 (35.4) | 26 (34.2) |
| Constipation | 9 (11.4) | 8 (10.5) | 9 (11.4) | 11 (13.9) | 13 (17.1) |
| Somnolence or sedation | 6 (7.6) | 6 (7.9) | 19 (24.1) | 12 (15.2) | 12 (15.8) |
| Pruritus or generalized pruritus | 6 (7.6) | 2 (2.6) | 13 (16.5) | 5 (6.3) | 24 (31.6) |
| Dry mouth | 1 (1.3) | 1 (1.3) | 4 (5.1) | 4 (5.1) | 12 (15.8) |
Notes:
Occurring during the treatment or follow-up period (7 days).
Occurring in ≥10% of patients in any treatment group.
Abbreviation: AE, adverse event.
Figure 7.
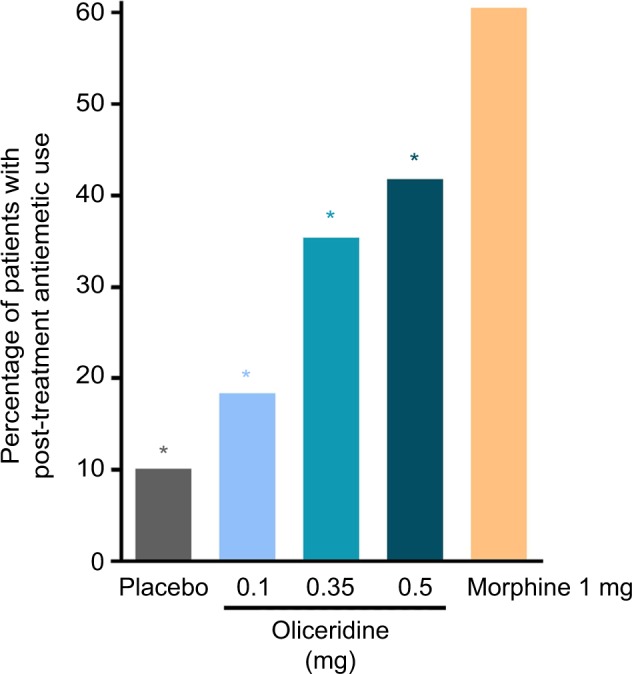
Post-treatment rescue antiemetic use.
Notes: Patients could receive rescue antiemetic medication if they were actively vomiting or if they requested an antiemetic and reported moderate-to-severe nausea on a 4-point scale (none, mild, moderate, severe). Prophylactic antiemetics were not permitted perioperatively or during the randomized treatment period. *P<0.05 for odds ratio for rescue antiemetic use vs morphine.
Overall safety and tolerability
The AEs commonly observed with oliceridine were comparable in nature to those expected from conventional opioids. The most common AEs were nausea, vomiting, headache, dizziness, constipation, somnolence or sedation, pruritus, and dry mouth. No patients experienced a serious AE, few patients reported an AE that was classified as severe, and fewer oliceridine-treated patients discontinued treatment owing to an AE than morphine-treated patients (Table 4).
No deaths were reported during the study and there were no clinically meaningful differences between active treatment regimens in hematology or clinical chemistry parameters, vital sign measurements, or electrocardiogram findings. Likewise, no signal for any significant hepatic abnormalities were evident in patients receiving oliceridine.
Discussion
Important clinical goals in acute pain therapy are to deliver effective analgesia while minimizing opioid dosages and the emergence of AEs.28,29 Oliceridine is a novel, investigational, G protein-selective ligand at the µ-opioid receptor that may address these needs by offering a wider therapeutic window than conventional IV opioids. It exhibits a comparable level of efficacy with clinically meaningful improvements in safety and tolerability. Non-clinical and clinical studies have demonstrated that oliceridine is potently analgesic, with a rapid onset of action and fewer gastrointestinal and respiratory AEs than morphine at equianalgesic-doses.18,19,22,23 APOLLO-1 was a phase III, multicenter, randomized clinical trial comparing IV oliceridine to both placebo and morphine. It was designed to build on earlier findings in patients undergoing bunionectomy or abdominoplasty who received acute postsurgical analgesia via PCA.22,23
Efficacy
Consistent with previous studies, all oliceridine dose regimens were statistically superior to placebo for the primary outcome, namely the proportion of treatment responders over the 48-hour randomized treatment period. An advantage of the primary outcome definition used in this study is that it incorporated important features of both efficacy and tolerability into a clinically relevant outcome. A patient was only considered a responder if they did not require the use of rescue analgesia, if they did not discontinue study medication prior to completion of the randomized treatment period, or if they did not reach predefined study medication dosing limits. Incorporation of rescue analgesia use is a particularly important component of the responder definition. As patients are self-titrating to a level of comfort, this need is a clear indicator of the sufficiency of analgesic effect from the patient’s perspective. This responder definition also complements NRS-based outcomes, which generally measure the intensity of analgesic effect independent of rescue analgesic use. Using the lowest necessary dose of opioids is a consistent goal in contemporary recommendations for acute pain treatment regimens, as they are often down-titrated to offset ORAEs. Therefore, efficacy outcomes that incorporate measures of both treatment sufficiency and tolerability are important considerations for determining the overall clinical effectiveness of opioids.
A key prespecified secondary objective was to compare the efficacy of three PRN oliceridine dose regimens to a standard morphine regimen, providing a broader comparative context to understand the benefit/risk profile of oliceridine in this postsurgical setting. The dose regimens selected for this study were chosen to allow the evaluation of several oliceridine dosages while also permitting safety comparisons between regimens that were previously established to be relatively equianalgesic to morphine.19,22,23 Findings from APOLLO-1 further establish the analgesic efficacy of oliceridine and are consistent with those of previous phase II trials of acute pain in both hard and soft tissues.22,23 While this study examined the clinical effectiveness and safety of oliceridine as a monotherapy, the optimal dose regimen of oliceridine may be different when used as part of multimodal analgesia therapy, where a lower demand dose (associated with fewer AEs) may be adequate. In contrast, a higher demand may be appropriate in clinical settings where pain is more intense. In the APOLLO-1 study, the intermediate 0.35 mg oliceridine demand dose regimen appeared to provide an optimal balance of analgesic efficacy and tolerability, showing comparable efficacy to morphine but with a reduced incidence of AEs.
It is recognized that the speed of pain relief is important in the patient experience of surgery.30 Exploratory analyses showed a rapid onset of effect in all oliceridine regimens with a high proportion of responders at early time points. In addition, the two-stopwatch pain relief assessments and the time to 1-, 2-, and 3-point NRS change showed oliceridine to provide a rapid onset of analgesic effect (Figures 3B and 4B). Combined results from clinical development studies suggest that the analgesic capacity of oliceridine is at least comparable to that of morphine at clinically relevant dosages, with a rapid onset of action.19,22,23
Safety and tolerability
The occurrence of ORAEs can be dose-limiting, may delay recovery and discharge, and are therefore also likely to increase the cost of care.6–8 Some of these AEs can also be life-threatening. A recent survey of physicians who treat postoperative patients showed that effective pain medicines with fewer side effects were perceived to be the most important unmet need in the field of acute pain management.29 Furthermore, ORAEs remain a common challenge, particularly influencing treatment decisions in high-risk patients.29 To the extent that the interfering effect of ORAEs impedes the ability to rapidly achieve effective levels of analgesia, it is of interest to note that a recent report in patients undergoing total knee arthroplasty indicated that inadequate resolution of acute postoperative pain predicted the emergence of chronic pain and a higher likelihood of chronic opioid use.31
In APOLLO-1, few patients who were allocated to oliceridine regimens experienced a severe AE (5.1%), none experienced a serious AE, and few discontinued treatment owing to an AE (2.6% vs 7.9% among morphine-treated patients). Exploratory analyses indicated that oliceridine 0.1 mg and 0.35 mg demand dose regimens were associated with fewer dosing interruptions than morphine; this is particularly noteworthy for the 0.35 mg regimen, which provided a level of efficacy similar to that seen with morphine in this study. Although the 0.5 mg demand dose oliceridine regimen also provided comparable efficacy to morphine, it did not demonstrate any meaningful improvements in overall safety and tolerability compared with the 0.35 mg demand dose oliceridine regimen or morphine.
Oliceridine’s preference for G protein signaling compared to the degree of β-arrestin recruitment at the µ-opioid receptor has been theorized to result in a relatively lower overall incidence of ORAEs than with conventional, unbiased, µ-opioid receptor agonists. This hypothesis has been supported by the non-clinical and clinical data reported to date.18,19,22,23,32 The current study and others, have demonstrated that while oliceridine is associated with dose-related common AEs that are expected from use of a µ-opioid receptor agonist, these are observed at a reduced incidence (particularly gastrointestinal and respiratory AEs) at doses previously demonstrated to be relatively equianalgesic to morphine.22,23 These findings suggest that the therapeutic window for oliceridine may be wider than that of morphine, and that satisfactory analgesia could be achieved with dosages of oliceridine that are associated with fewer AEs.
Opioid-induced respiratory depression (OIRD) is considered to be the most serious of the ORAEs, with potentially fatal consequences.3,7,11 OIRD can advance to cardiopulmonary or respiratory arrest, which has substantial impact on length of stay and overall treatment costs.7 Currently, there are no validated or well-characterized measures of OIRD for use in clinical trials of opioid analgesics. As a new chemical entity with a novel mechanism of action, the potential for improved respiratory safety is of significant interest with oliceridine. In an earlier phase Ib study, oliceridine exerted a reduced effect on the suppression of respiratory drive in the ventilatory response to hypercapnia experimental model, at doses that were at least as analgesic as morphine.19 In a subsequent phase II study, patients treated with oliceridine for the management of acute postoperative pain experienced a significantly reduced incidence of respiratory safety events— such as reductions in respiratory rate, respiratory effort, or hypoxia—compared to patients treated with morphine.23 These initial observations were further explored in the APOLLO-1 study by evaluating the emergence of respiratory safety events, their duration, and the clinical interventions used to manage them. A novel assessment in the APOLLO-1 study attempted to design a more sensitive composite that integrated these various endpoints into a single outcome measure, referred to as RSB. This approach was intended to provide a global index of respiratory safety. Respiratory effects of study medication were frequently monitored in a protocol-specified manner by experienced and trained medical professionals blinded to study medication. The RSB was defined as the cumulative duration of these events, expressed as the mathematical product of their prevalence and their duration. This measure was intentionally multifactorial and designed to identify clinically relevant indicators of respiratory distress. It should be noted that APOLLO-1 was not enriched for patients deemed at high risk for OIRD (eg, those with sleep apnea or obesity).11,12
In APOLLO-1, the reduction in RSB with oliceridine compared with morphine was consistent with the size of the treatment benefit on respiratory safety events observed in the earlier phase I and phase II trials of oliceridine.19,23 However, unlike the earlier results, the differences between groups using this new composite outcome did not reach statistical significance in the APOLLO-1 study population. There are several factors that may have contributed to this outcome. Overall, there was a lower than expected rate of underlying respiratory safety events. Since the prior abdominoplasty study was used in power calculations and sample size estimations for APOLLO-1, the lower than expected incidence of respiratory safety events in the current study may partially explain why statistical significance was not achieved.23 The use of a more intensive method of respiratory safety monitoring in the APOLLO-1 study compared to the approach used in the prior phase IIb abdominoplasty trial may also have contributed to the lower overall event rate. Despite these challenges, the underlying proportion of patients with at least one respiratory safety event was statistically significantly lower in the 0.1 mg and 0.35 mg oliceridine demand dose regimens, but not in the 0.5 mg demand dose regimen, compared with morphine. Furthermore, the smaller proportions of patients experiencing clinically significant reductions in oxygen saturation <90% or requiring supplemental oxygen therapy across the oliceridine dose regimens are consistent with the observed reduction in respiratory safety events. The potentially improved respiratory safety profile of the 0.35 mg oliceridine demand dose regimen compared to morphine was particularly notable given that this regimen provided analgesic efficacy similar to morphine.
Opioid-induced nausea and vomiting are among the most common ORAEs, and patients rank these as among the most distressing complications following surgery.3,33–35 Previous clinical trials have shown evidence of significantly less frequent or less severe nausea and vomiting with oliceridine than with morphine;19,22,23 therefore, these outcomes were also of interest in APOLLO-1. Study findings, reported here, demonstrated a dose-dependent but lower number of patients experiencing upper gastrointestinal-related events of nausea and vomiting, and a reduction in the use of rescue antiemetics, in oliceridine regimens compared to morphine. These relative differences were most evident in oliceridine in the 0.1 mg and 0.35 mg demand dose regimens.
A limitation to the study is that most patients were female, although this is typical for the clinical population seeking treatment for bunion repair. Nevertheless, the findings in APOLLO-1 are similar to those from previous non-clinical studies and in clinical trials of oliceridine for the treatment of acute postsurgical pain in hard and soft tissues.19,22,23 While differences in study population, dose regimens, and respiratory safety endpoints preclude a rigorous comparison of results across clinical trials, findings consistently suggest that oliceridine provides improvements in respiratory safety and the incidence of nausea and vomiting compared to morphine. Additional Phase III trials have been conducted with an aim to further support these findings in other patient groups.36
Conclusion
Results from the APOLLO-1 study demonstrated the efficacy and safety of PRN oliceridine for the management of moderate-to-severe pain following bunionectomy, an established postsurgical model of acute pain. Findings demonstrate superior efficacy compared to placebo. Efficacy, safety, and tolerability data provide important context for evaluating the benefit/risk profile of IV oliceridine compared to morphine, and suggest that oliceridine may provide an important treatment option for the management of patients experiencing moderate-to-severe acute pain.
Data availability
Data from the APOLLO-1 trial are held by Trevena Inc. and are not publicly available. Access to specific data sets, protocols, and reports will be considered on the basis of proposed quality and alignment with the aims of the original study.
Supplementary materials
Pain response by (A) SPID and (B) NRS.
Notes: Pain was assessed at the time points shown up to 48 hours using an 11-point NRS. (A) LS mean SPID for each treatment group. Last observations are carried forward in cases of rescue analgesia, non-protocol analgesia, or discontinuation due to lack of efficacy. Best observation carried forward for discontinuation due to other reasons. Model-based imputation for missing scores. (B) LS mean NRS for each treatment group. Model-based results are from a repeated-measures model with treatment group as the fixed effect, baseline NRS and study site as covariates, time point by treatment as an interaction term, and time point as a repeated effect within patient. An unstructured covariance matrix was used to model the within-patient variance.
Abbreviations: LS mean, least squares mean; NRS, numeric rating scale; SPID, sum of pain intensity difference.
(A) Clinician- and (B) patient-reported satisfaction with assigned study medication.
Notes: Responses were collected at the end of the treatment period. In (A), clinicians did not provide a response for one patient in the placebo group and one patient in the morphine group. In (B), four patients did not provide responses: two in the placebo group and one each in the oliceridine 0.35 mg group and the morphine group.
Acknowledgments
The authors wish to acknowledge the contributions of the patients involved in this study and the scientific contributions from members of Trevena Inc. Medical writing support and manuscript preparation were provided by Jennifer Bodkin, PhD, CMPP, of Engage Scientific Solutions and were funded by Trevena Inc. Trevena Inc. sponsored this study and had a role in the design and conduct of the study and analysis of the data. Medical writing assistance for this manuscript was funded by Trevena Inc. Findings from the APOLLO-1 study have been previously presented as posters at the 36th Annual Scientific Meeting of the American Pain Society (APS), May 17–20, 2017, Pittsburg, PA, USA; and at the 2017 Annual Scientific Meeting of the American Society of Colon and Rectal Surgeons (ASCRS), June 10–14, 2017, Seattle WA, USA. Posters are available online at https://www.jpain.org/article/S1526-5900(17)30148-7/pdf.
Abbreviations
- AE
adverse event
- IV
intravenous
- MedDRA
Medical Dictionary for Regulatory Activities
- NRS
numeric rating scale
- OIRD
opioid-induced respiratory depression
- ORAE
opioid-related adverse event
- PCA
patient-controlled analgesia
- PRN
as needed
- RSB
respiratory safety burden
- SPID
sum of pain intensity difference
Footnotes
Disclosure
David A Burt, Emily Cook, David G Soergel, and Franck Skobieranda were full-time employees and stockholders of Trevena Inc. at the time the research was conducted and manuscript was under preparation. Neil Singla is founder and CEO of Lotus Clinical Research, LLC, an analgesic Clinical Research Organization and research site; in this capacity he has served as a consultant and provided clinical trial services for Trevena Inc. Eugene R Viscusi has served as a consultant to Trevena Inc. The authors report no other conflicts of interest in this work.
References
- 1.National Pharmaceutical Council (NPC) Pain: current understanding of assessment, management, and treatments. Reston, VA: NPC, Joint Commission on Accreditation of Health Care Organization; 2001. [Google Scholar]
- 2.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. [PubMed] [Google Scholar]
- 3.Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. doi: 10.1054/jpai.2002.123652. [DOI] [PubMed] [Google Scholar]
- 4.Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am Fam Physician. 2006;74(8):1347–1354. [PubMed] [Google Scholar]
- 5.Rogers E, Mehta S, Shengelia R, Reid MC. Four strategies for managing opioid-induced side effects in older adults. Clin Geriatr. 2013;21(4) [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391. doi: 10.1002/phar.1223. [DOI] [PubMed] [Google Scholar]
- 7.Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. doi: 10.1371/journal.pone.0150214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habib AS, Chen YT, Taguchi A, Henry Hu X, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr Med Res Opin. 2006;22(6):1093–1099. doi: 10.1185/030079906X104830. [DOI] [PubMed] [Google Scholar]
- 9.Helander EM, Menard BL, Harmon CM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21(1):3. doi: 10.1007/s11916-017-0607-y. [DOI] [PubMed] [Google Scholar]
- 10.Young A, Buvanendran A. Recent advances in multimodal analgesia. Anesthesiol Clin. 2012;30(1):91–100. doi: 10.1016/j.anclin.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Jarzyna D, Jungquist CR, Pasero C, et al. American Society for pain management nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Manag Nurs. 2011;12(3):118–145.e10. doi: 10.1016/j.pmn.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73(1):953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 15.Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 2011;286(36):31731–31741. doi: 10.1074/jbc.M111.248310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 17.Raehal KM, Walker JKL, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314(3):1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 18.DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344(3):708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 19.Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the µ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155(9):1829–1835. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 21.Manglik A, Lin H, Aryal DK, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537(7619):185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viscusi ER, Webster L, Kuss M, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the µ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157(1):264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 23.Singla N, Minkowitz HS, Soergel DG, et al. A randomized, phase 2B study investigating oliceridine (TRV130), a novel µ receptor G protein pathway selective (µ-GPS) modulator, for management of moderate to severe acute pain following abdominoplasty. J Pain Res. 2017;10:2413–2424. doi: 10.2147/JPR.S137952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moline B, Roberts M, Houser J. Validity and interrater reliability of the Moline-Roberts pharmacologic sedation scale. Clin Nurse Spec. 2012;26(3):140–148. doi: 10.1097/NUR.0b013e3182503fd6. [DOI] [PubMed] [Google Scholar]
- 25.Sunshine A, Mulhern SA, Olson N, Elkind A, Almas M, Sikes C. Comparative sensitivity of stopwatch methodology and conventional pain assessment measures for detecting early response to triptans in migraine: results of a randomized, open-label pilot study. Clin Ther. 2006;28(8):1107–1115. doi: 10.1016/j.clinthera.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Medical Dictionary for Regulatory Activities MedDRA version 19.0. Mar, 2016. [Accessed October 2018]. Available from: https://www.meddra.org/how-to-use/support-documentation/english.
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 28.O’Connor AB, Zwemer FL, Hays DP, Feng C. Outcomes after intravenous opioids in emergency patients: a prospective cohort analysis. Acad Emerg Med. 2009;16(6):477–487. doi: 10.1111/j.1553-2712.2009.00405.x. [DOI] [PubMed] [Google Scholar]
- 29.Gan TJ, Epstein RS, Leone-Perkins ML, Salimi T, Iqbal SU, Whang PG. Practice patterns and treatment challenges in acute postoperative pain management: a survey of practicing physicians. Pain Ther. 2018;7(2):205–216. doi: 10.1007/s40122-018-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramia E, Nasser SC, Salameh P, Saad AH. Patient perception of acute pain management: data from three tertiary care hospitals. Pain Res Manag. 2017;2017(1):1–12. doi: 10.1155/2017/7459360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsia H-L, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705–711. doi: 10.1097/AAP.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X-T, Pitis P, Liu G, et al. Structure-activity relationships and discovery of a G protein biased µ opioid receptor ligand, [(3-methoxy-thiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5] decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. doi: 10.1021/jm4010829. [DOI] [PubMed] [Google Scholar]
- 33.Macario A, Weinger M, Carney S, Kim A, Macario A. Which clinical anesthesia outcomes are important to avoid? the perspective of patients. Anesth Analg. 1999;89(3):652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Gan TJ, Sloan F, Dear Gde L, El-Moalem HE, Lubarsky DA. How much are patients willing to pay to avoid postoperative nausea and vomiting? Anesth Analg. 2001;92(2):393–400. doi: 10.1097/00000539-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84(1):6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 36.Minkowitz H, Burt D, Cochrane K, et al. Athena: a Phase 3, open-label safety study of oliceridine for the treatment of moderate-to-severe acute pain; Presented at the 2018 Annual Scientific Meeting of the American Society of Anesthesiologists (ASA); October 13-17, 2018; San Francisco, CA, USA. Abstract No. A2174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pain response by (A) SPID and (B) NRS.
Notes: Pain was assessed at the time points shown up to 48 hours using an 11-point NRS. (A) LS mean SPID for each treatment group. Last observations are carried forward in cases of rescue analgesia, non-protocol analgesia, or discontinuation due to lack of efficacy. Best observation carried forward for discontinuation due to other reasons. Model-based imputation for missing scores. (B) LS mean NRS for each treatment group. Model-based results are from a repeated-measures model with treatment group as the fixed effect, baseline NRS and study site as covariates, time point by treatment as an interaction term, and time point as a repeated effect within patient. An unstructured covariance matrix was used to model the within-patient variance.
Abbreviations: LS mean, least squares mean; NRS, numeric rating scale; SPID, sum of pain intensity difference.
(A) Clinician- and (B) patient-reported satisfaction with assigned study medication.
Notes: Responses were collected at the end of the treatment period. In (A), clinicians did not provide a response for one patient in the placebo group and one patient in the morphine group. In (B), four patients did not provide responses: two in the placebo group and one each in the oliceridine 0.35 mg group and the morphine group.
Data Availability Statement
Data from the APOLLO-1 trial are held by Trevena Inc. and are not publicly available. Access to specific data sets, protocols, and reports will be considered on the basis of proposed quality and alignment with the aims of the original study.