Abstract
Objectives:
The objective of this study was to evaluate the changes in three-dimensional (3D) speckle-tracking echocardiography (STE)-derived measures of mechanics and their associations with systolic and diastolic dysfunction after anthracyclines.
Background:
An improved understanding of the changes in 3D cardiac mechanics with anthracyclines may provide important mechanistic insight and identify new metrics to detect cardiac dysfunction.
Methods:
142 women with breast cancer receiving doxorubicin (240 mg/m2) with or without trastuzumab underwent 3D STE at standardized intervals prior to, during, and annually after chemotherapy. Left ventricular ejection fraction (LVEF), global circumferential strain (GCS), global longitudinal strain (GLS), principal strain, twist, and torsion were quantified. Linear regression analyses defined the associations between clinical factors and 3D parameters. Linear regression models with cluster robust variance estimators determined the associations between 3D measures and 2D LVEF and Doppler-derived E/e’ over time.
Results:
There were significant abnormalities in 3D LVEF, GCS, GLS, and principal strain post-doxorubicin as compared to controls (p<0.001). 3D parameters worsened post-anthracyclines and only partially recovered to baseline over a median of 2.1 years (interquartile range 1, 4). Higher blood pressure and body mass index were associated with worse post-anthracycline 3D GCS and GLS, respectively. All 3D measures were associated with 2D LVEF at the same visit; only 3D LVEF, GCS, GLS, and principal strain were associated with 2D LVEF at subsequent visits (p<0.05). In exploratory analyses, 3D LVEF and GCS were associated with subsequent systolic function independent of their corresponding 2D measures. 3D LVEF, GCS, principal strain, and twist were significantly associated with concurrent, but not subsequent, E/e’.
Conclusions:
Anthracyclines result in early and persistent abnormalities in 3D mechanics. 3D LVEF and strain measures are associated with concurrent and subsequent systolic dysfunction, and concurrent diastolic dysfunction. Future research is needed to define the mechanisms and clinical relevance of abnormal 3D mechanics.
Keywords: cardio-oncology, 3D echocardiography, anthracyclines
INTRODUCTION
Anthracycline-induced cardiotoxicity is a leading cause of cardiovascular morbidity among cancer survivors (1). Although heart failure may take months or years to manifest clinically, cardiomyocyte apoptosis can occur within days of doxorubicin exposure (2). In fact, circulating measures of troponin are detectable in some patients immediately after anthracycline therapy, indicative of early myocardial injury (3,4). However, the temporal changes in cardiac mechanics early after anthracycline exposure are not comprehensively understood.
Left ventricular ejection fraction (LVEF) derived from two-dimensional (2D) echocardiography is the primary parameter used to assess cardiac function after cancer therapy. Although a valid measure, LVEF is limited by temporal and observer variability (5) and declines may occur late, after substantial cardiac injury. As such, there is an active body of research evaluating the use of more sensitive echocardiographic measures to detect early subclinical changes in cardiac function. Multiple prior studies, including one from our own research group, have demonstrated potential utility for 2D longitudinal and circumferential strain in the diagnosis and prediction of cardiac dysfunction after cancer therapy (6,7). 2D speckle-tracking echocardiography (STE), however, may be limited by suboptimal tracking of the endocardial border, sensitivity to acoustic shadowing, and the inability to detect out-of-plane motion of speckles (8).
Three-dimensional (3D) STE has the potential to overcome some of the technical limitations of 2D STE by tracking the movement of speckles within the entire scan volume (8). Compared with 2D STE, 3D STE may have greater accuracy for the quantitation of strain and left ventricular (LV) volumes. Furthermore, 3D STE measurements have been noted to be more reproducible than corresponding 2D STE measurements (9,10), although temporal resolution and image quality may limit the feasibility of 3D STE (11,12). 3D STE also allows for the quantitation of principal strain, the change in myocardial length along the axis of maximal shortening, as well as twist and torsion, which reflect rotational displacement along the LV long axis. Twist is defined as the absolute apex-to-base difference in LV rotation, and torsion is the base-to-apex gradient in rotation angle along the long axis of the left ventricle, expressed in degrees per centimeter (8). Despite these potential advantages of 3D STE technology for the assessment of cardiac function and mechanics, the clinical role for 3D STE remains to be proven. While 2D STE has been studied in the setting of anthracycline chemotherapy, there are only a few small studies reporting changes in 3D STE parameters with chemotherapy (11,13–15). Importantly, a comprehensive understanding of the changes in 3D LV mechanics with anthracyclines and their ability to predict subsequent cardiac dysfunction may inform and improve the management of cancer patients.
The overall objectives of this study were to characterize the changes in 3D LV mechanics over time with a specific focus on the post-anthracycline chemotherapy timepoint; to identify the clinical characteristics that predispose to abnormalities in these parameters; to determine the associations between 3D LV mechanics and concurrent and subsequent systolic and diastolic dysfunction during and after anthracycline therapy; and to explore the potential incremental benefit of 3D STE over 2D STE. These analyses were performed in a subcohort of participants from an ongoing prospective, longitudinal breast cancer cohort of women receiving doxorubicin with or without trastuzumab.
METHODS
Study Participants
The Cardiotoxicity of Cancer Therapy study is a prospective, longitudinal cohort study of women with breast cancer receiving treatment at the Rena Rowan Breast Center at the University of Pennsylvania (Philadelphia, Pennsylvania) (7). Eligible participants were women age ≥ 18 receiving anthracyclines with or without trastuzumab for the treatment of breast cancer. Pregnancy and inability to provide informed consent were the only exclusion criteria.
Chemotherapy regimens were prescribed at the discretion of the oncology providers and consisted of doxorubicin (240 mg/m2) and cyclophosphamide followed by paclitaxel with or without trastuzumab. Trastuzumab was dosed according to standard guidelines. Echocardiograms were obtained after completion of anthracyclines and annually thereafter in participants receiving anthracyclines alone. Participants treated with trastuzumab underwent echocardiograms every 3 months during therapy and annually thereafter (7). Detailed clinical data were obtained at baseline and at each subsequent visit. The current analysis is restricted to participants with analyzable 3D echocardiograms at the post-anthracycline visit and at least one additional follow-up 2D echocardiogram.
In addition to the participants with breast cancer, a control group of healthy female volunteers (N=21) determined to be free of any medical illness by history and physical examination underwent a single echocardiogram with 3D imaging. This study was approved by the University of Pennsylvania Institutional Review Board; all participants provided written informed consent.
Transthoracic Echocardiography
All echocardiographic studies were acquired on a commercially available system (Vivid 7 or Vivid E9, GE Healthcare, Milwaukee, Wisconsin) by a dedicated sonographer team. Two-dimensional echocardiographic images were acquired in the parasternal short-axis view at the mid-papillary level and in the apical views at 60–80 frames/second. Real-time 3D echocardiographic imaging was then performed in the same apical position using a matrix-array transducer. Full-volume acquisition, in which 6 adjacent subvolumes were captured over 6 consecutive cardiac cycles, was performed during a breath-hold to minimize artifact between subvolumes. Special care was taken to include the entire left ventricular cavity within the pyramidal data set. 3D images were acquired at a minimum of 22 frames/second, and all images were digitally archived at the acquisition frame rate for offline analysis.
Two-dimensional LV volumes were measured using commercially available, vendor-independent software (TomTec Imaging Systems, Unterschleissheim, Germany). The endocardial contour was manually traced at end-diastole and end-systole; LV end-diastolic volume (EDV) and LV end-systolic volume (ESV) were determined using Simpson’s method of disks and used to derive LVEF (16). Early (E) and late (A) mitral inflow velocities were quantified using pulsed wave Doppler. The early diastolic annular velocity (e’) was quantified via pulsed wave tissue Doppler of the septal and lateral mitral annuli, and average values were derived. The E/e’ ratio was used to quantify diastolic function.
Three-dimensional LVEF and strain were quantified using dedicated, semi-automated software (4D-LV Analysis, TomTec Imaging Systems). Nonforeshortened apical views were identified at end-diastole and LV endocardial and epicardial boundaries were initialized. The 3D endocardial surface was then automatically detected with manual adjustments made as necessary, and automatically tracked over time in 3D space with derivation of 3D LVEF, GCS, GLS, principal strain, twist, and torsion. Studies were excluded if either dropout of ≥4 wall segments or stitch artifact was present. To improve the reliability of tracking outcomes, each 3D echocardiogram was quantitated twice by a single observer and the average of the two observations was used for analysis.
All quantitation was performed by observers blinded to subject characteristics and timing of echocardiograms.
Reproducibility Analyses
Two-dimensional LVEF was quantified by a dedicated research sonographer (T.P.), with an intra-observer coefficient of variation of 4.9%. Similarly, 2D strain was quantified by a single sonographer (T.P.) with intraobserver CVs for longitudinal and circumferential strain of 10.9% and 9.4%, respectively. Three-dimensional echocardiograms were quantified by two dedicated, trained physicians (K.W.Z. and G.G.), each blinded to the results of the other’s measurements. Inter-observer coefficients of variation for 3D measures were as follows: LVEF, 3.3%; GLS, 6.8%; GCS, 5.5%; twist, 20.7%; torsion, 13.6%.
Statistical Analyses
Descriptive statistics were used to summarize study participant and healthy control characteristics at baseline and 3D echocardiographic parameters at the post-anthracycline visit. Mean values of 3D-derived measures of cardiac mechanics over time were assessed graphically using LOESS smoothing functions, and compared graphically to the corresponding 2D parameters. The distributions of 3D parameters in the study cohort at the post-anthracycline timepoint and in the healthy control cohort were compared via the Wilcoxon rank sum test to account for the non-normal distribution of some parameters. Univariable associations between baseline clinical factors and abnormal post-anthracycline 3D measures of myocardial deformation were determined using linear regression.
We first focused on 3D parameters obtained at the post-anthracycline timepoint as potential predictors of subsequent 2D cardiac function. The associations between each post-anthracycline 3D parameter and subsequent changes in 2D LVEF and E/e’ quantified at each follow-up visit over the entire study duration were estimated using linear regression, with cluster robust variance to account for repeated 2D measures within a given individual. Each of these models was adjusted for 2D LVEF or E/e’ at the post-anthracycline visit, age, body mass index (BMI), heart rate (time-varying), systolic blood pressure (time-varying), and time since the post-anthracycline visit (modeled non-parametrically using cubic splines). All 3D parameters were standardized based on the interquartile range (IQR) at the post-anthracycline visit. To explore the incremental utility of 3D STE parameters, we repeated these analyses with adjustment for the corresponding 2D parameters in the 3D analyses.
Next, we evaluated the associations between 3D-derived parameters obtained across all timepoints and the associations with 2D LVEF and E/e’ at both the same (cross-sectional) and at the immediate subsequent follow-up visit (lagged). These were estimated using linear regression with cluster robust variance. All models were adjusted for the same clinical and analogous 2D echocardiographic parameters noted above, including similar sensitivity analyses of 2D STE parameters, and all 3D parameters were standardized based on the IQR at the post-anthracycline visit.
Analyses were performed using R 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population: Cardiotoxicity of Cancer Therapy Subcohort and Healthy Female Control Participants
3D echocardiography analyses were quantified in 142 breast cancer participants (median age 49 [IQR 41, 56]; 65% Caucasian). A median of three 3D echocardiograms were quantitated per individual (IQR 2, 4), resulting in a total of 429 3D echocardiograms for this study. There were a total of 253 analyzed echocardiograms in the first year post cancer therapy initiation, followed by 63 in the second year, 56 in the third year, and 57 in the fourth year and beyond. Ninety-four percent of the acquired 3D images were considered analyzable. Baseline characteristics are shown in Table 1. Ninety-one percent received anthracyclines without trastuzumab; 9% received anthracyclines with trastuzumab.
Table 1.
Baseline cohort characteristics
| Cardiotoxicity of Cancer Therapy Subcohort | Female Healthy Control Participants | |
|---|---|---|
| N = 142 | N = 21 | |
| Age (years) | 49 (41, 56)* | 36 (27, 51) |
| Race | ||
| Caucasian | 93 (65) | 9 (43) |
| Black | 35 (25) | 7 (33) |
| Other or unknown | 14 (10) | 5 (23) |
| Body Mass Index (kg/m2) | 26 (24, 30) | 24 (22, 27) |
| Systolic blood pressure (mmHg) | 125 (116, 134) | 116 (110, 127) |
| Diastolic blood pressure (mmHg) | 74 (68, 81) | 76 (68, 80) |
| Heart rate (beats per minute) | 77 (72, 87) | 71 (63, 77) |
| Chemotherapy regimen | ||
| Doxorubicin | 129 (91) | |
| Doxorubicin + Trastuzumab | 13 (9) | |
| Radiation therapy | 84 (59) | |
| History of diabetes | 11 (8) | |
| History of hypertension | 33 (23) | |
| History of hyperlipidemia | 30 (21) | |
| Smoking status | ||
| Never | 82 (58) | 18 (86) |
| Former | 48 (34) | 3 (15) |
| Current | 12 (8) | 1 (5) |
| Use of any cardiac medication | 21 (15) | |
| ACE inhibitor | 11 (8) | |
| Angiotensin receptor blocker | 5 (4) | |
| Beta blocker | 9 (6) | |
| 3D LVEF (%)† | 49.9 (43.6, 54.6) † | 57.0 (54.3, 62.0)* |
| GCS (%)† | −22.0 (−25.7, −18.5) † | −27.2 (−30.9, −24.7)* |
| GLS (%)† | −14.6 (−17.0, −12.3) † | −18.7 (−20.7, −17.5)* |
| Principal strain (%)† | −25.4 (−29.0, −21.7) † | −30.1 (−32.4, −27.2)* |
| Twist (°)† | 9.0 (6.3, 12.5) † | 10.3 (7.4, 13.9) |
| Torsion (°/cm) † | 1.1 (0.8, 1.6) † | 1.3 (1.0, 1.8) |
Variables displayed as median (IQR) or N (%)
Echocardiographic values are post-anthracycline for the cancer subcohort
P<0.001 comparison of post-anthracycline time point and healthy control via Wilcoxon rank sum test.
3D indicates three-dimensional; LVEF, left ventricular ejection fraction; GCS, global circumferential strain; and GLS, global longitudinal strain.
As a comparison, we included 21 female healthy control participants (Table 1). The median age was 36 (IQR 27, 51) and 43% were Caucasian.
Abnormalities in 3D-Derived Measures of Cardiac Mechanics Post-Anthracyclines
We first sought to define the early changes in 3D echocardiography parameters post-anthracyclines, given data from our own group and others suggesting that functional changes detected at this timepoint are reflective of increased cardiac stress and may be associated with subsequent cardiac dysfunction (3,4,6). Summary statistics for 3D echocardiography parameters in the 142 participants after anthracycline therapy are shown in Table 1. Compared to the group of healthy female controls, there were significant abnormalities in multiple measures of cardiac mechanics after anthracycline therapy including 3D LVEF, GCS, GLS, and principal strain (all p<0.001). There were no statistically significant abnormalities in twist or torsion after anthracycline chemotherapy, although these tended to be worse. Among 47 participants for whom both baseline and post-anthracycline 3D quantifiable echocardiograms were available, baseline values were not significantly different from controls with the exception of longitudinal strain, which was worse in the breast cancer group (Supplementary Table 1). In participants with both baseline and post-anthracycline quantifiable 3D echocardiograms, 3D LVEF, principal strain, GLS, and GCS were significantly worse following anthracyclines, as compared to baseline (Supplementary Figure 1).
Associations with Post-Anthracycline 3D-Derived Measures of Cardiac Mechanics
Next, we used linear regression analyses to identify associations between baseline clinical characteristics and abnormal post-anthracycline 3D echocardiographic parameters. In univariable analyses, higher baseline diastolic blood pressure was significantly associated with worse GCS and 3D LVEF (p<0.05; Supplementary Table 2). African American race was significantly associated with reduced 3D LVEF as compared to Caucasians. Higher baseline BMI was associated with worse GLS and 3D LVEF; older age was associated with worse GLS. There were no statistically significant associations observed between baseline systolic blood pressure, heart rate, use of cardiac medications, diabetes, hypertension, or hyperlipidemia with other post-anthracycline 3D parameters. Similarly, no clinical characteristics were associated with twist or torsion following anthracycline therapy.
We then determined the associations between post-anthracycline 3D echocardiographic parameters and changes in 2D systolic and diastolic function over the entire duration of follow-up (Table 2). Worse post-anthracycline 3D LVEF and 3D GCS were associated with worse subsequent 2D LVEF over a median follow-up time of 1.7 years following the post-anthracycline visit (IQR 0.3, 3.4 years). For each IQR worsening in 3D LVEF post-anthracyclines (adjusted for 2D LVEF), there was a 1.2% worsening of subsequent 2D LVEF (p=0.045). Moreover, each interquartile range worsening in 3D GCS was associated with a 1.3% decrease in subsequent 2D LVEF (p=0.032). This association between post-anthracycline 3D GCS and subsequent 2D LVEF remained significant after adjusting for 2D GCS (β −1.2, 95% CI −2.2 to −0.1, p=0.033). Post-anthracycline 3D GLS was not associated with subsequent 2D LVEF (p=0.23), irrespective of adjustment for 2D GLS (p=0.31). There were no significant associations between post-anthracycline 3D echocardiographic parameters and subsequent measures of E/e’.
Table 2.
Association between post-anthracycline 3D-derived measures of cardiac mechanics and subsequent 2D LVEF or E/e’
| Median (IQR) | 2D LVEF Beta (95% CI) | P-value* | E/e’ Beta (95% CI) | P-value* | |
|---|---|---|---|---|---|
| 3D LVEF (%) | 49.9 (43.6, 54.6) | 1.2 (0.0, 2.3) | 0.045 | 0.4 (−0.1, 0.8) | 0.10 |
| GCS (%) | −22.0 (−25.7, −18.5) | −1.3 (−2.5, −0.1) | 0.032 | −0.3 (−0.8, 0.1) | 0.15 |
| GLS (%) | −14.6 (−17.0, −12.3) | −1.0 (−2.5, 0.6) | 0.23 | −0.2 (−0.7, 0.2) | 0.31 |
| Principal strain (%) | −25.4 (−29.0, −21.7) | −1.0 (−2.1, 0.2) | 0.10 | −0.4 (−0.8, 0.1) | 0.11 |
| Twist (°) | 9.0 (6.3, 12.5) | −0.1 (−1.1, 0.9) | 0.82 | −0.0 (−0.5, 0.5) | 0.96 |
| Torsion (°/cm) | 1.1 (0.8, 1.6) | −0.0 (−0.9, 0.8) | 0.93 | 0.1 (−0.4, 0.5) | 0.74 |
P-values are based on the Wald test.
All models were adjusted for 2D LVEF or E/e’ (depending upon the outcome of interest) at the post-anthracycline visit, age, BMI, heart rate (time-varying), systolic blood pressure (time-varying), and time since the post-anthracycline visit (modeled nonparametrically using cubic splines). All echocardiographic parameters were standardized based on the interquartile range at the post-anthracycline visit. As an example, an interquartile increase from −25.7% to −18.5% for GCS represents a worsening of strain and is associated with a 1.3% decline in LVEF.
3D indicates three-dimensional; LVEF, left ventricular ejection fraction; GCS, global circumferential strain; and GLS, global longitudinal strain.
Changes in 3D-Derived Measures of Cardiac Mechanics Over Time
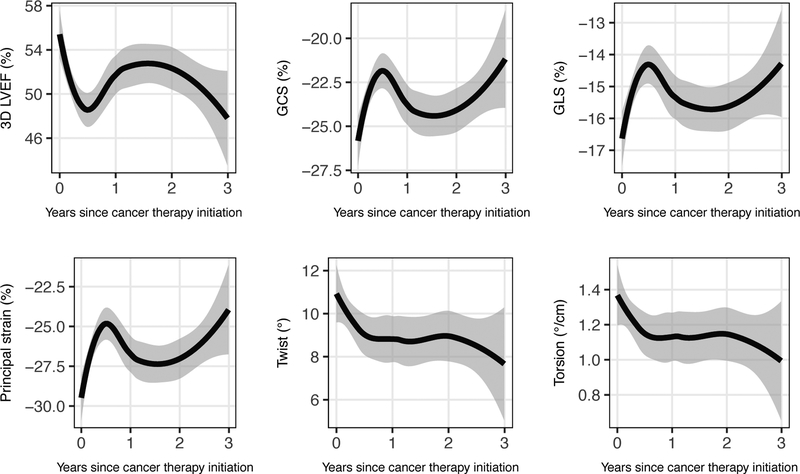
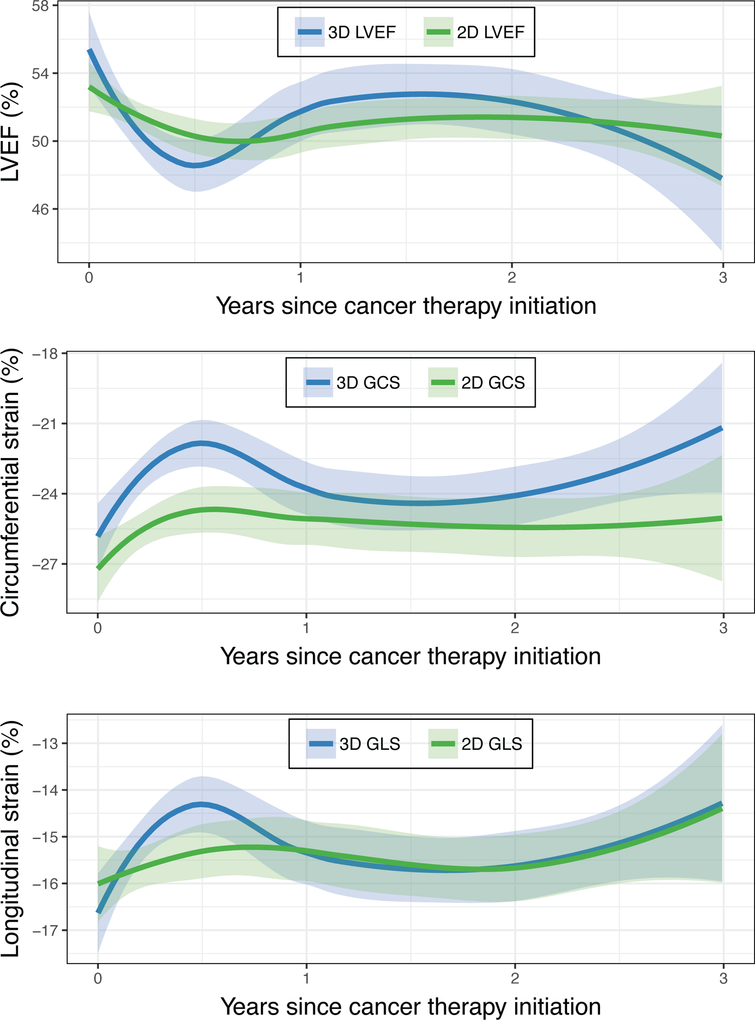
After focusing on 3D echocardiographic measures at the post-anthracycline timepoint, we next examined changes in 3D measures of cardiac mechanics over the duration of follow-up (Figure 1). For each parameter, we observed an early worsening that reached a nadir approximately 6 months after chemotherapy initiation. For 3D LVEF, GCS, and GLS, this initial decrement was followed by modest improvement, though at 2 years these values remained worse than baseline. In contrast, values for 3D twist and torsion plateaued at six months without substantial subsequent change. As shown in Figure 2, changes in 3D LVEF, GCS, and GLS tended to track with changes in the corresponding 2D echocardiographic parameters, although the 6-month nadir for the 3D parameters was worse than those of the corresponding 2D parameters. Findings were similar in a sensitivity analysis restricted to the 47 participants with a baseline 3D echocardiogram available (Supplementary Figure 1).
Figure 1. 3D-Derived Measures of Cardiac Mechanics over Time.
The mean absolute value of each 3D echocardiographic parameter is shown through 3 years of follow-up, as estimated by a LOESS smoothing function. Grey bands represent 95% confidence intervals. The 3D parameters are 3D LVEF, global circumferential strain (GCS), global longitudinal strain (GLS), principal strain, twist, and torsion. Changes in 3D parameters reached nadir at 6 months after anthracyclines, without recovery to baseline at 2.5 years.
Figure 2. Comparison of 3D- and 2D-Derived Measures of Cardiac Mechanics over Time.
Mean absolute values of 3D and 2D LVEF, circumferential strain, and longitudinal strain are shown through 3 years of follow-up, as estimated by a LOESS smoothing function. Shaded bands represent 95% confidence intervals. 3D parameters are shown in blue and 2D parameters are shown in green. Changes in 3D parameters were more pronounced than the changes in 2D parameters after anthracyclines, suggesting that 3D echocardiography may provide more sensitive detection of cardiac dysfunction.
Associations between 3D-Derived Measures of Cardiac Mechanics and Systolic and Diastolic Function
Lastly, we determined the cross-sectional and longitudinal associations between 3D measures of cardiac mechanics and 2D systolic (LVEF) and diastolic (E/e’) function over the entire duration of follow-up. Median follow-up by 2D echocardiography for these individuals was 2.1 years (IQR 1, 4) from the initiation of chemotherapy. 3D LVEF, GCS, GLS, principal strain, twist, and torsion were each cross-sectionally associated with 2D LVEF at the same visit (p<0.05) (Table 3). In longitudinal analyses incorporating “lagged” analyses, 3D LVEF, GCS, GLS, and principal strain were also significantly associated with 2D LVEF at the immediate subsequent follow-up visit (Table 4). When adjusting for the corresponding 2D strain parameter, 3D GCS remained significant (β −1.2, 95% CI −2.2 to −0.4, p=0.004). Moreover, 3D LVEF, GCS, principal strain, and twist were cross-sectionally associated with E/e’ at the same visit (Table 3), but not at the subsequent visit (Table 4).
Table 3.
Cross-sectional associations between 3D measures of cardiac mechanics and 2D LVEF or E/e’ at the same visit
| Median (IQR) | 2D LVEF Beta (95% CI) | P-value* | E/e’ Beta (95% CI) | P-value* | |
|---|---|---|---|---|---|
| 3D LVEF (%) | 49.9 (43.6, 54.6) | 2.5 (1.9, 3.1) | 0.001 | −0.3 (−0.6, −0.0) | 0.026 |
| GCS (%) | −22.0 (−25.7, −18.5) | −2.4 (−3.0, −1.8) | 0.001 | 0.4 (0.1, 0.6) | 0.009 |
| GLS (%) | −14.6 (−17.0, −12.3) | −2.0 (−2.8, −1.2) | <0.001 | 0.1 (−0.2, 0.5) | 0.50 |
| Principal strain (%) | −25.4 (−29.0, −21.7) | −2.6 (−3.2, −1.9) | <0.001 | 0.4 (0.1, 0.7) | 0.008 |
| Twist (°) | 9.0 (6.3, 12.5) | 0.7 (0.1, 1.3) | 0.019 | −0.2 (−0.4, −0.0) | 0.026 |
| Torsion (°/cm) | 1.1 (0.8, 1.6) | 0.6 (0.1, 1.2) | 0.029 | −0.2 (−0.4, 0.0) | 0.071 |
P-values are based on the Wald test.
All models were adjusted for 2D LVEF or E/e’ (depending upon the outcome of interest) at baseline, age, BMI, heart rate (time-varying), systolic blood pressure (time-varying), and time since baseline (modeled non-parametrically using cubic splines).
All echocardiographic parameters were standardized based on the interquartile range at the post-anthracycline visit. An example of the interpretation is noted in Table 2.
3D indicates three-dimensional; LVEF, left ventricular ejection fraction; GCS, global circumferential strain; and GLS, global longitudinal strain.
Table 4.
Lagged associations between 3D measures of cardiac mechanics and 2D LVEF or E/e’ at subsequent visit
| Median (IQR) | 2D LVEF Beta (95% CI) | P-value* | E/e’ Beta (95% CI) | P-value* | |
|---|---|---|---|---|---|
| 3D LVEF (%) | 49.9 (43.6, 54.6) | 1.6 (0.7, 2.6) | 0.001 | −0.1 (−0.4, 0.2) | 0.54 |
| GCS (%) | −22.0 (−25.7, −18.5) | −1.6 (−2.4, −0.7) | 0.001 | 0.1 (−0.2, 0.5) | 0.39 |
| GLS (%) | −14.6 (−17.0, −12.3) | −1.3 (−2.3, −0.3) | 0.010 | −0.2 (−0.6, 0.2) | 0.31 |
| Principal strain (%) | −25.4 (−29.0, −21.7) | −1.5 (−2.5, −0.6) | <0.001 | 0.2 (−0.2, 0.5) | 0.34 |
| Twist (°) | 9.0 (6.3, 12.5) | 0.5 (−0.3, 1.4) | 0.24 | −0.2 (−0.6, 0.1) | 0.15 |
| Torsion (°/cm) | 1.1 (0.8, 1.6) | 0.5 (−0.3, 1.3) | 0.20 | −0.2 (−0.5, 0.1) | 0.25 |
P-values are based on the Wald test.
All models were adjusted for 2D LVEF or E/e’ (depending upon the outcome of interest) at baseline, age, BMI, heart rate (time-varying), systolic blood pressure (time-varying), and time since baseline (modeled non-parametrically using cubic splines).
All echocardiographic parameters were standardized based on the interquartile range at the post-anthracycline visit. An example of the interpretation is noted in Table 2.
3D indicates three-dimensional; LVEF, left ventricular ejection fraction; GCS, global circumferential strain; and GLS, global longitudinal strain.
DISCUSSION
In this prospective longitudinal subcohort study of 142 women with breast cancer, we comprehensively characterized 3D measures of myocardial deformation early after anthracycline therapy and over a median of 2.1 years of follow-up. We report four key findings from this study. First, there were abnormalities in 3D LVEF, GCS, GLS, and principal strain early after anthracycline chemotherapy that reached a nadir within 6 months and persisted at 2 years of follow-up. Second, higher baseline blood pressure and BMI were associated with worse post-anthracycline GCS and GLS, respectively. Third, 3D GCS, GLS, and principal strain were associated with concurrent and subsequent changes in systolic function, and 3D GCS and principal strain with concurrent changes in diastolic function over the duration of follow-up. Fourth, in exploratory analyses, post-anthracycline 3D LVEF and 3D GCS were associated with subsequent 2D LVEF across all timepoints even after adjusting for the corresponding 2D parameters. Our data would suggest that 3D measures obtained after anthracyclines and over time are associated with subsequent declines in LVEF, and 3D LVEF and circumferential strain, in particular, may hold incremental value. These findings provide further insight into the mechanics of cardiac dysfunction following anthracycline therapy and suggest utility for 3D assessment. However, they also highlight the need for rigorous translational studies to understand the mechanistic significance of changes in 3D measures and clinical trials to define their relevance and feasibility in everyday practice.
Abnormalities in 2D GLS and GCS have been demonstrated in multiple studies after cardiotoxic cancer therapy, including by our own group (6,7). However, there are fewer studies of 3D STE. Two studies of 3D STE in adults treated for breast cancer (11) or lymphoma (15) reported reductions in 3D parameters with anthracyclines, but these studies were smaller, of shorter follow up duration, and did not investigate the association between changes in 3D parameters and subsequent systolic and diastolic function. While 3D STE has the potential to overcome some of the technical limitations of 2D STE by tracking the movement of speckles within the entire scan volume (9,10), image quality and temporal resolution may limit 3D interpretability (11,12). In a breast cancer cohort, Santoro and colleagues found that feasibility of 3D STE was only 60% while 2D STE feasibility was 90%. In our study, the reproducibility of 3D measures was greater than 2D and the analyzability of 3D STE in acquired images was 94%, similar to the 90% feasibility in a healthy population study (12). However, the availability and feasibility of 3D STE in clinical practice require further study.
Our analyses also demonstrated that specific baseline cardiovascular risk factors, namely blood pressure and obesity are associated with the development of abnormal 3D myocardial deformation early after anthracycline therapy, including GCS and GLS. Changes in 3D parameters were more pronounced than 2D changes, and with respect to 3D LVEF and circumferential strain, this measure was independently associated with subsequent LVEF changes even when accounting for the 2D analogues, suggesting incremental utility. We also found early abnormalities in 3D GCS, GLS, and principal strain that improved over multiple years of follow-up, but did not return to baseline. The lack of complete recovery to baseline in 3D LVEF, circumferential or longitudinal strain support the hypothesis that anthracyclines result in a modest, persistent decrease in cardiac function (17). The temporal changes in cardiac mechanics after cardiotoxic chemotherapy in the short and long term are important to further understand the natural history of cardiac dysfunction and recovery after anthracyclines.
3D LVEF and several myocardial deformation parameters were associated with concurrent and subsequent LVEF declines. There were significant associations between 3D LVEF and concurrent and subsequent 2D LVEF declines. This finding, along with the pronounced changes observed in 3D LVEF (Figure 1), suggest that 3D LVEF may also be clinically useful. Multiple studies have also shown that abnormal 2D GLS and GCS after anthracyclines is associated with subsequent systolic dysfunction (6,7,18). In our study, 3D GCS remained significantly associated with subsequent reductions in LVEF even after adjusting for 2D GCS, findings that were not seen with GLS. This is consistent with prior modeling which found that LVEF is more strongly determined by GCS than GLS (19). Principal strain in the subendocardium has been shown to align in the circumferential direction and not along the right-handed helix of the subendocardial muscle fibers (20). Our finding that principal strain is associated with systolic function after anthracyclines also supports the importance of circumferential mechanics to global systolic function after anthracycline exposure.
There were no statistically significant abnormalities in twist or torsion early after anthracyclines, though both rotational parameters were associated with concurrent changes in 2D LVEF throughout follow-up. As such, we hypothesize that circumferential mechanics may deteriorate earlier than twist mechanics after anthracyclines. This finding may also reflect relative sparing of the subepicardial fibers, which primarily determine twist mechanics, from early anthracycline-induced damage (21).
We acknowledge the potential limitations of this study. First, our sample size of 142 participants may have limited our ability to detect smaller effect sizes. Nevertheless, the present study represents the largest cohort of women to date to undergo evaluation of changes in 3D cardiac mechanics. Second, the median follow-up time in the cohort was limited to 2.1 years. As a result, there were few clinical HF events in the cohort, preventing an assessment of the correlation between changes in 3D parameters with longer term clinical outcomes and more comprehensively assessing their potential incremental value. In the same way, we are limited in our assessment of the potential incremental value of 3D strain measures over 3D LVEF; this is an important area of future study. Third, inter-vendor variability in 3D strain and limitations in spatial and temporal resolution are well-documented. Vendor-independent analysis software has been shown to mitigate this variability, but there remains an important need for improved technology. Moreover, there is an important need to further reduce inter-vendor variability across various 3D platforms; with current recommendations suggesting that the same analyses platform be used when comparing studies (22). Finally, while our results suggest that 3D acquisition may be feasible in a cohort of breast cancer patients, and 3D LVEF and 3D GCS may provide incremental value above corresponding 2D parameters, additional research is needed to comprehensively determine the utility of 3D measures in cardio-oncology. Building upon ongoing efforts with 2D echocardiography (23), future trials could also evaluate the clinical utility of 3D-derived measures of cardiac mechanics in guiding patient management as part of an imaging marker-guided strategy.
In conclusion, comprehensive 3D STE evaluation after anthracycline chemotherapy identified abnormalities in 3D LVEF, GCS, GLS, and principal strain that persisted over time in this longitudinal breast cancer cohort. 3D LVEF, GCS, GLS, and principal strain were associated with concurrent and subsequent changes in systolic function, with 3D LVEF and GCS being associated with subsequent systolic function independent of the 2D counterpart. Further study is needed to understand the mechanisms of abnormal 3D GCS, GLS, and principal strain after anthracycline chemotherapy.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge
Abnormalities in 3D measures of cardiac function and mechanics, as defined by left ventricular ejection fraction, global circumferential strain, global longitudinal strain, and principal strain were observed in breast cancer patients early after exposure to doxorubicin chemotherapy. These abnormalities persisted over a median followup of 2.1 years. 3D-derived measures were associated with concurrent and subsequent systolic dysfunction, and concurrent diastolic dysfunction.
Translational Outlook
Additional research is needed to understand the pathophysiologic mechanisms and clinical relevance of changes in 3D mechanics with anthracycline chemotherapy. Our findings also suggest that abnormal circumferential mechanics may play an important role in the development of left ventricular ejection fraction declines. While our study indicates 3D echocardiographic assessment is feasible and reproducible, larger studies are needed to further define their utility in cardio-oncology.
Funding:
This work was supported by the National Heart, Lung, and Blood Institute grants R01-HL118018 and K23-HL095661, the McCabe Fellow Award, and the American Cancer Society Institutional Research Grant 78–002–30 to Dr. Ky. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS LIST
- IQR
interquartile range
- GCS
global circumferential strain
- GLS
global longitudinal strain
- LVEF
left ventricular ejection fraction
- STE
speckle-tracking echocardiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Doyle JJ., Neugut AI, Jacobson JS, Grann VR, Hershman DL Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. J Clin Oncol 2005;23(34):8597–605. [DOI] [PubMed] [Google Scholar]
- 2.Childs AC., Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res 2002;62(16):4592–8. [PubMed] [Google Scholar]
- 3.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63(8):809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109(22):2749–54. [DOI] [PubMed] [Google Scholar]
- 5.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61(1):77–84. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan HK., French B, Khan AM, et al. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics–related cardiac dysfunction. JACC Cardiovasc Imaging 2016;9(10):1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. Eur J Echocardiogr 2011;12:167–205. [DOI] [PubMed] [Google Scholar]
- 9.Maffessanti F, Nesser H-J, Weinert L, et al. Quantitative evaluation of regional left ventricular function using three-dimensional speckle tracking echocardiography in patients with and without heart disease. Am J Cardiol 2009;104(12):1755–62. [DOI] [PubMed] [Google Scholar]
- 10.Nesser H-J, Mor-Avi V, Gorissen W, et al. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: comparison with MRI. Eur Heart J 2009;30(13):1565–73. [DOI] [PubMed] [Google Scholar]
- 11.Santoro C, Arpino G, Esposito R, et al. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients : a balance with feasibility 2017;(November):930–6. [DOI] [PubMed] [Google Scholar]
- 12.Muraru D, Cucchini U, Mih S Left Ventricular Myocardial Strain by Three-Dimensional Speckle-Tracking Echocardiography in Healthy Subjects : Reference Values and Analysis of Their Physiologic and Technical Determinants n.d:858–72. [DOI] [PubMed]
- 13.Okuma H, Noto N, Tanikawa S Impact of persistent left ventricular regional wall motion abnormalities in childhood cancer survivors after anthracycline therapy : Assessment of global left ventricular myocardial performance by 3D speckle-tracking echocardiography. J Cardiol 2017;70(4):396–401. [DOI] [PubMed] [Google Scholar]
- 14.Yu HK, Yu W, Cheuk DKL, Wong SJ, Chan GCF, Cheung YF New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr 2013;26(8):846–52. [DOI] [PubMed] [Google Scholar]
- 15.Song FY, Shi J, Guo Y, et al. Assessment of biventricular systolic strain derived from the two-dimensional and three-dimensional speckle tracking echocardiography in lymphoma patients after anthracycline therapy. Int J Cardiovasc Imaging 2017;33(6):857–68. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16(3):233–71. [DOI] [PubMed] [Google Scholar]
- 17.Narayan HK., Finkelman B, French B, et al. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations with Ejection Fraction Decline, Recovery, and Heart Failure Symptoms over 3 Years of Follow-Up. Circulation 2017;135(15):1397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26(5):493–8. [DOI] [PubMed] [Google Scholar]
- 19.Stokke TM, Hasselberg NE, Smedsrud MK, et al. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison Between Ejection Fraction and Strain. J Am Coll Cardiol 2017;70(8):942–54. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista A, Gabriele S, Nardinocchi P, et al. Non-invasive assessment of functional strain lines in the real human left ventricle via speckle tracking echocardiography. J Biomech 2015;48(3):465–71. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc Imaging 2008;1(3):366–76. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzini C, Lamberti C, Aquilina M, Rocca A, Cortesi P, Corsi C Reliability of Left Ventricular Ejection Fraction from Three-Dimensional Echocardiography for Cardiotoxicity Onset Detection in Patients with Breast Cancer. J Am Soc Echocardiogr 2017;30(11):1103–10. [DOI] [PubMed] [Google Scholar]
- 23.Strain Surveillance During Chemotherapy for Improving Cardiovascular Outcomes (SUCCOUR), ACTRN12614000341628. Available at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366020&isReview=true. Accessed June 11, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.