Abstract
The discovery that rapamycin increases lifespan in mice and restores/delays many aging phenotypes has led to the speculation that rapamycin has ‘anti-aging’ properties. The major question discussed in this review is whether a manipulation that has anti-aging properties can alter the onset and/or progression of Alzheimer’s disease, a disease in which age is the major risk factor. Rapamycin has been shown to prevent (and possibly restore in some cases) the deficit in memory observed in the mouse model of Alzheimer’s disease (AD-Tg) as well as reduce Aβ and tau aggregation, restore cerebral blood flow and vascularization, and reduce microglia activation. All of these parameters are widely recognized as symptoms central to the development of AD. Furthermore, rapamycin has also been shown to improve memory and reduce anxiety and depression in several other mouse models that show cognitive deficits as well as in ‘normal’ mice. The current research shows the feasibility of using pharmacological agents that increase lifespan, such as those identified by the National Institute on Aging Intervention Testing Program, to treat Alzheimer’s disease.
Keywords: Rapamycin, Alzheimer’s Disease, Cognition, Behavior
1. Introduction
Rapamycin (also known as Sirolimus) is macrocyclic lactone produced by the bacterium Streptomyces hygroscopicus isolated from soil samples from Easter Island. In 1975, Vezina et al. showed that cultures of Streptomyces hygroscopicus inhibited the growth of fungi with no activity against gram-positive and gram-negative bacteria and low toxicity in mice. Based on the initial studies, rapamycin was developed as an antifungal agent; however, because it was found to have immunosuppressive properties, this line of research was discontinued. In 1988, it was discovered that rapamycin had antirejection properties without the side effects associated with other antirejection agents (Camardo 2003). This discovery led eventually to FDA approving in 1999 the use of rapamycin in combination with other immunosuppressive agents to prevent the rejection of organs in transplant patients (Camardo 2003).
A major breakthrough occurred in 1994, when three groups showed that rapamycin bound a specific protein, Target of Rapamycin (TOR) (Cafferkey et al. 1994; Brown et al. 1994; Sabatini et al. 1994). TOR was subsequently found to be the serine/threonine kinase that was the regulatory nexus in the response of eukaryote cells to nutrients, growth factors, and cellular energy status. In mammals, TOR (mTOR) forms two major complexes: mTORC1, which is inhibited by rapamycin (Caron et al. 2010) and mTORC2, which has been reported to be insensitive to rapamycin; however, recent data suggest that long term rapamycin treatment might inhibit mTORC2 (Sarbassov et al. 2006). The mTORC1 consists of mTOR, Raptor, mLST8, FKBP38, PRAS40, and Deptor, and through specific binding of rapamycin to FKBP12, rapamycin inhibits the activity of mTORC1 leading to a decrease in protein synthesis, increased autophagy and inhibition of cell growth (Stanfel et al. 2009).
2. Effect of Rapamycin on Longevity
The TOR signaling pathway is an attractive candidate to study with respect to aging because it has the potential to affect a large number of processes regulated by TOR signaling that could have a major effect on an organism. In the early 2000s, investigators began studying the effect TOR on lifespan of invertebrates. Mutations in TOR increased the lifespan of yeast (Kaeberlein et al. 2005), C. elegans (Vellai et al. 2003; Jia and Levine 2007; Hansen et al. 2007), and Drosophila (Kapahi et al. 2004). These data provided the first evidence that increased longevity could be achieved by reduced TOR signaling and suggested that rapamycin, which inhibits TOR signaling, might increase lifespan in other species, including mammals. In 2009, the National Institute on Aging Interventions Testing Program reported that feeding rapamycin to mice significantly increased lifespan, both mean and maximum (Harrison et al. 2009). Not only was this the first report to show that a pharmacological agent could increase lifespan of a mammal, the increase in lifespan was observed in both male and female mice on a heterozygous background: (BALB/cByJ × C57BL/6J) F1 females crossed to (C3H/HeJ × DBA/2J) F1 males, which is referred to as UM-HET3 mice. Thus, rapamycin’s effect on lifespan is likely to apply to mice in general, i.e., it is not unique to a specific inbred strain of mice. In addition, the increase in lifespan was replicated at three independent sites, and the increase in lifespan was achieved when administered relatively late in life (e.g., 19 months of age, which would be similar to ~65 years of age in humans). This study was selected by Science as one of the major scientific break-throughs in 2009 (Science 326, 1598–1607). The importance of showing that the increase in lifespan was increased when rapamycin was administered late in life cannot be understated because all previous experimental manipulations that increased the lifespan of mammals (mice or rats) were implemented early in life. Thus, the study by Harrison et al. (Harrison et al. 2009) provided the first proof of principle that a pharmacological agent could be developed to slow down aging in humans, i.e., an antiaging pill was not a dream but theoretically possible.
Since the initial publication in 2009, 15 reports have been published on the effect of rapamycin on the lifespan of mice, and these studies are listed in Table 1. The large number of studies on the lifespan of mice in only five years is impressive and gives us an ability to generalize about how rapamycin affects longevity. The current studies show that rapamycin not only increases the lifespan of various strains of ‘normal’ laboratory mice, but also increases the lifespan of genetically modified mouse models of various human diseases. In studies in which male and female mice have been compared, rapamycin was found consistently to have a greater effect on the lifespan of female mice. Of the 16 studies in which the lifespan of rapamycin treated mice has been studied, only two studies, both with transgenic mouse models of ALS (amyotrophic lateral sclerosis), show no increase in lifespan. Thus, the effect of rapamycin on lifespan is very robust and reproducible in various strains and genetic models of mice. However, the studies with the ALS transgenic mice indicate that rapamycin’s longevity effect may not be universal and that some genotypes do not respond to rapamycin. It is also possible that rapamycin might shorten the lifespan of some genetic models. A recent study by the National Institute on Aging Interventions Testing Program compared the effect of various doses of rapamycin on the lifespan of male and female UM-HET3 mice (Miller et al. 2014). Longevity was shown to be dose dependent from one-third to 3-fold the concentration used initially by Harrison et al. (2009). Thus, the effect of rapamycin on the lifespan of mice occurs over a broad dose range.
Table 1.
Effect of Rapamycin on Lifespan of Mice
| Reference | Mouse Strain | Age Initiated | Increase in Lifespan |
|---|---|---|---|
| Control/Wild Type Mice | |||
| (Harrison et al. 2009) | UM-HET3 | 19 mos | 9% M &14% F |
| (Miller et al. 2011) | UM-HET3 | 9 mos | 10% M &18% F |
| (Anisimov et al. 2011) | 129/Sv | 2 mos | 10% F |
| (Neff et al. 2013) | C57BL/6 | 4, 13, & 20 mos | 11% M |
| (Miller et al. 2014) | UM-HET3 | 9 mos | 3–23% M & 16–26% F |
| (Zhang et al. 2014) | C57BL/6 | 19 mos | nc M & 6% F |
| (Fok et al. 2014) | C57BL/6 | 4 mos | 11% M & 16% F |
| Genetically Modified Mice | |||
| (Fujishita et al. 2008) | ApcD716 | 6–14 wks | 140–220% M/F |
| (Anisimov et al. 2010) | HER-2 | 2 mos | 13% F |
| (Comas et al. 2012) | p53−/− | 2 mos | 30% M |
| (Ramos et al. 2012) | Lmna−/− | 3–4 wks | 23–57% M/F |
| (Komarova et al. 2012) | p53+/− | ~5 mos | 10–28% M |
| (Livi et al. 2013) | Rb1+/− | 8–10 wks | 14% M – 9% F |
| (Johnson et al., 2013) | Ndufs4−/− | ~20 days | 25% M – 38% F |
| (Hasty et al. 2014) | APCMin/+ | 50 days | 280–440% F |
| (Zhang et al. 2011) | G93A | 64 days | nc Sex? |
| (Bhattacharya et al. 2012) | H46R/H48Q | 64 days | nc M/F |
The percent increase in lifespan (nc = no change) is shown for male (M) and female (F) mice or a combination of male and female mice (M/F). The strain of mouse or the genetic mouse model is given with the age that the rapamycin treatment was initiated.
The observation that rapamycin increases lifespan (both mean and maximum) in mice strongly suggests that rapamycin increases lifespan by slowing down aging. In the past two years, several studies have examined the effect of rapamycin on various parameters of healthspan. Currently, it appears that while rapamycin improves some measures of physiological function that decline with age, other functions are not altered by rapamycin. However to date, no physiological function that changes with age is negatively altered by rapamycin (Richardson 2013). One of the major consequences of aging, which leads to reduced quality of life and increased medical costs seen in the elderly, is the occurrence of a wide variety of pathological conditions. Because animal studies in which aging has been altered show a reduced/delayed incidence of most age-related diseases, it is possible that rapamycin might have a broad protection against age-related diseases in humans. The current limited data support this possibility. For example, it is clear that rapamycin has a dramatic effect on cancer in mice (Sharp and Richardson 2011), most likely because if its anti-growth and anti-proliferation properties. In fact, it has even been argued that the life extension seen with rapamycin is due to its anti-cancer action rather than an anti-aging action (Neff et al. 2013). In addition, several studies have shown that rapamycin reduces atherosclerotic plaque formation in mouse models of atherosclerosis (Pakala et al. 2005) (Mueller et al. 2008). Although rapamycin has been reported to improve various motor-tasks in models of Huntington disease (Ravikumar et al. 2004) and Parkinson’s disease (Malagelada et al. 2010), there was no information on the effect of rapamycin on Alzheimer’s disease before 2010 and very little information on the effect of rapamycin on cognition. In fact, the few early studies suggested that rapamycin might have a negative effect on memory, e.g., reduced long-term memory facilitation and consolidation (Casadio et al. 1999; Tischmeyer et al. 2003) and long-term plasticity in the brain (Tang et al. 2002). The focus of this report is to describe the pioneering studies in which the effect of rapamycin on Alzheimer’s disease was tested in transgenic mice genetically manipulated to mimic Alzheimer’s disease (AD-Tg mice). In addition, we review the current studies on the effect of rapamycin on cognition/memory in other mouse models with cognitive impairment as well as ‘normal’ laboratory mice.
3. Effect of Rapamycin on Alzheimer’s Disease
3.1. Cognition/memory
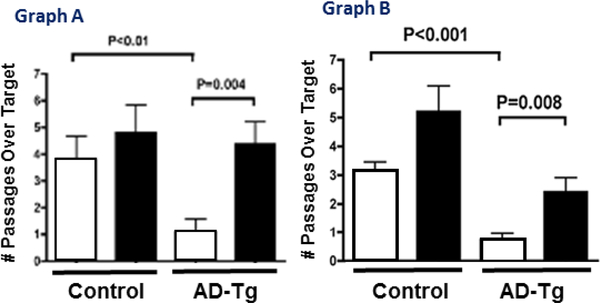
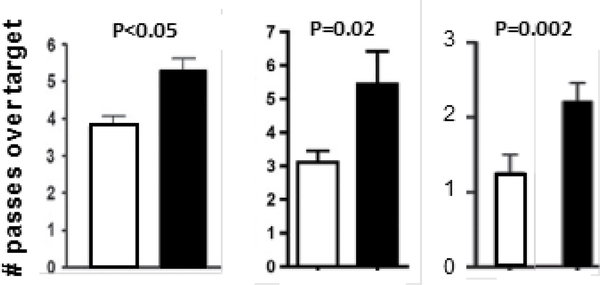
The first studies to test the effect of rapamycin on memory in AD-Tg mice were initiated shortly after the 2009 Nature publication on lifespan and conducted independently by the laboratories of Oddo and Galvan. They found, as shown in Fig. 1, that rapamycin prevented the loss of memory/cognition in two different transgenic mouse models of AD (Caccamo et al. 2010; Spilman et al. 2010). The 3×Tg-AD mice used by Oddo’s laboratory harbors mutant forms of three human genes associated with AD: amyloid protein precursor (APP), tau, and presinilin-1. The 3×Tg-AD mice are the only mouse model of AD that develops both Aβ-containing plaques and tau-containing tangles. The 3×Tg-AD mice show significant memory deficits, which can be first detected around 6 to 8 months of age (Oddo et al. 2003). The hAPP(J20) used by Galvan’s laboratory overexpresses a minigene of APP carrying the Swedish and Indiana FAD mutations. The hAPP(J20) mice show an increase in soluble Aβ levels in brain and synaptic deficits starting at 3 months of age, age-dependent AD-like decline in learning and memory that can be detected at seven months of age, and deposition of Aβ plaques starting at ~10 months of age that steadily increases with increasing age (Mucke et al. 2000) (Hsia et al. 1999). The data in Figure 1 shows that rapamycin administration to young/adult AD-Tg mice for 10 to 13 weeks prevented the loss of cognition observed in the two AD-Tg mouse models studied as measured by the Morris water maze. In fact, there was no significant difference between the control non-treated mice and the AD-Tg mice treated with rapamycin. The dose of the encapsulated rapamycin, which was delivered in the diet fed the AD-Tg mice, was the same as that used in the studies showing rapamycin increased lifespan in UM-HET3 (Harrison et al. 2009) and C57BL/6 mice (Fok et al. 2014). It should also be noted that rapamycin had no detrimental effect on memory/cognition in the control mice as suggested in early studies. In fact, under these experimental conditions, cognition in the control mice appeared to be slightly, but not significantly, improved.
Fig. 1.
Effect of Rapamycin on the Memory of AD-Tg Mice assessed by the Morris Water Maze. Graph A: data taken from Caccamo et al. (2010) for 3XTg-AD (AD-Tg) and non-transgenic (Control) mice fed rapamycin (black bars) or a control diet (white bars) starting at 6 months of age for 10 weeks. Graph B: data were taken from Spilman et al. (2010) for hAPP(J20) (AD-Tg) and non-transgenic (Control) mice fed rapamycin (black bars) or a control (white bars) diet starting at 4 months of age for 13 weeks.
While these studies show that rapamycin was able to prevent the initial loss of memory in the AD-Tg mice, it was of interest to determine whether rapamycin would block ‘established’ memory deficits in symptomatic animals that show cognitive deficits, a stage in the progression of AD-like disease that models mild cognitive impairment (MCI) in humans. Galvan’s laboratory showed that feeding rapamycin for 16 weeks to 7-month-old hAPP(J20) mice, which show AD-like cognitive impairments, improved learning and restored memory to levels indistinguishable from those of wild-type controls (Lin et al. 2013). These data suggest that rapamycin may have the potential to treat cognitive impairment at the earliest stage of AD.
Oddo’s laboratory studied the effect of rapamycin treatment over the lifespan of the AD-Tg mouse to determine if rapamycin could prevent loss of memory. They showed that treating 2-month-old 3×Tg-AD mice with rapamycin for 16 months completely prevented the loss of memory; the rapamycin treated 3×Tg-AG mice at 18-months of age performed as well as 18-month-old control mice (Majumder et al. 2011). However, giving the 3×Tg-AD mice rapamycin for 3 months starting at 15 months of age, an age at which mice show widespread plaques and tangles and profound memory deficits, had no effect on memory indicating that rapamycin might have little effect on cognition if given at later stages of AD. Interestingly, Galvan’s laboratory recently found that rapamycin treatment in young/adults could protect AD-Tg mice from cognitive deficits later in life. They found that hAPP(J20) mice fed rapamycin for 16 weeks starting at 7 months of age, not only improved cognition immediately after feeding rapamycin (Lin et al. 2013) but was also sufficient to maintain cognitive function late in life, such that 28-month-old hAPP(J20) mice given rapamycin for 16 weeks at 7 months of age showed no detectable cognitive deficits in a spatial novelty task (Veronica Galvan personal communication). Therefore, the current data with AD-Tg mice show that the loss of memory can be prevented by rapamycin and that rapamycin-treatment can restore memory if implemented shortly after the onset of memory impairment; however, rapamycin may have little effect when given at late stages of the disease after overt plaque and tangle deposition.
3.2. Plaques, tangles, and autophagy
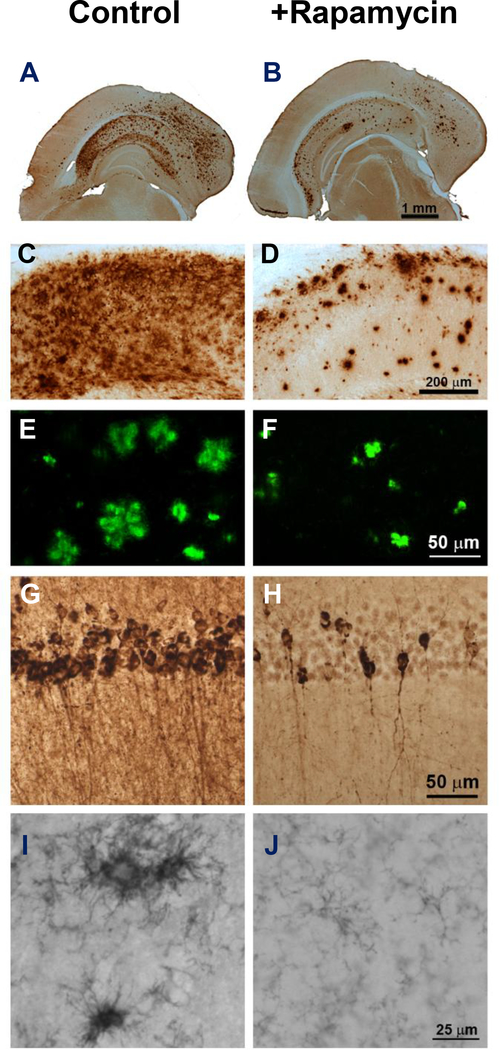
One of the hallmark features that characterizes AD is the accumulation of Aβ-containing plaques and tau-containing tangles, which are believed to play a major role in the decline in memory. As shown in Fig. 2 (A-F), Oddo’s laboratory found that rapamycin treatment reduced the accumulation of Aβ levels and fibrillar aggregates of Aβ approximately 40 to 50% in the 3XAD-Tg mice fed rapamycin for 16 months (Majumder et al. 2011). In addition, rapamycin reduced tau-aggregation as measured by tau phosphorylation (Fig. 2 G,H). Microglia activation, which is an invariable feature of AD pathology, was also observed to be dramatically reduced in the rapamycin treated AD-Tg mice (Fig. 2 I,J). Consistent with these observations, Galvan’s laboratory has also documented that rapamycin reduced levels of Aβ in the brains of hAPP(J20) mice after a few months (Spilman et al. 2010) and amyloid plaques at later stages of the AD-like disease (Lin et al. 2013).
Fig. 2.
Effect of Rapamycin on plaques and tangles in brains of 3XTg-AD mice. The levels of Aβ42 in whole brain (A,B) or hippocampus (C,D), fibrillar aggregates of Aβ (E, F), tau pathology as measured by hyperphosphorylation at Ser212 and Thr214 (G, H), and microglia activation (I, J) were measured in 2-month-old mice fed rapamycin or a control diet for 16 months (data taken from Majumder et al. (2011).
Because rapamycin is known to induce autophagy and because autophagy is the primary pathway cells use to degrade protein aggregates, both Galvan’s and Oddo’s laboratories studied the role of autophagy in the reduction of plaques and tangles in the brains of AD-Tg mice. They showed that markers of autophagy were induced in the brains of hAPP(J20) mice (Spilman et al. 2010) and 3XTg-AD mice (Caccamo et al. 2010). In addition, Oddo’s laboratory showed that rapamycin treated mice show a greater localization of Aβ in lysosomes (60% vs 20%), suggesting a more active degradation of these peptides after rapamycin treatment. Oddo’s laboratory went on to show that the decrease in Aβ levels induced by rapamycin in cells expressing Aβ could be prevented by blocking autophagy. More recently, Oddo’s laboratory showed that rapamycin administration decreased tau pathology in a mouse model of tauopathies, indicating that rapamycin might be beneficial not only for AD but also for other tauopathies (Caccamo et al. 2013). Therefore, it appears that the induction of autophagy by rapamycin plays a major role in the decrease in the accumulation of plaques and tangles in the brains of the AD-Tg mice and possibly the improvement in memory.
Oddo’s laboratory also has taken a genetic approach to determine if the effect of rapamycin on cognition and plaque accumulation occurred through mTOR. Although rapamycin is an mTOR inhibitor, there is growing evidence that it may also have effects independent of mTOR (Malagelada et al. 2010; Thoreen and Sabatini 2009). For example, rapamycin suppresses mTOR-dependent translation of some classes of mRNAs but not others (Choo et al. 2008). In additional, rapamycin binds to L-type voltage-dependent Ca2+ channels, and it is thought that this binding may mediate some of the neuroprotective properties of rapamycin (Ruan et al. 2008). Oddo’s laboratory has selectively removed one copy of the mTOR gene in the brains of the Tg2576 mice, a widely used animal model of AD. They found that genetic suppression of mTOR signaling reduced Aβ deposits and rescued memory deficits (Caccamo et al. 2014), mimicking the rapamycin-mediated improvements. These data strongly suggest that the rapamycin effects on AD-like pathology in mice are mediated through mTOR signaling.
3.3. Cerebral blood flow (CBF) and vascular function
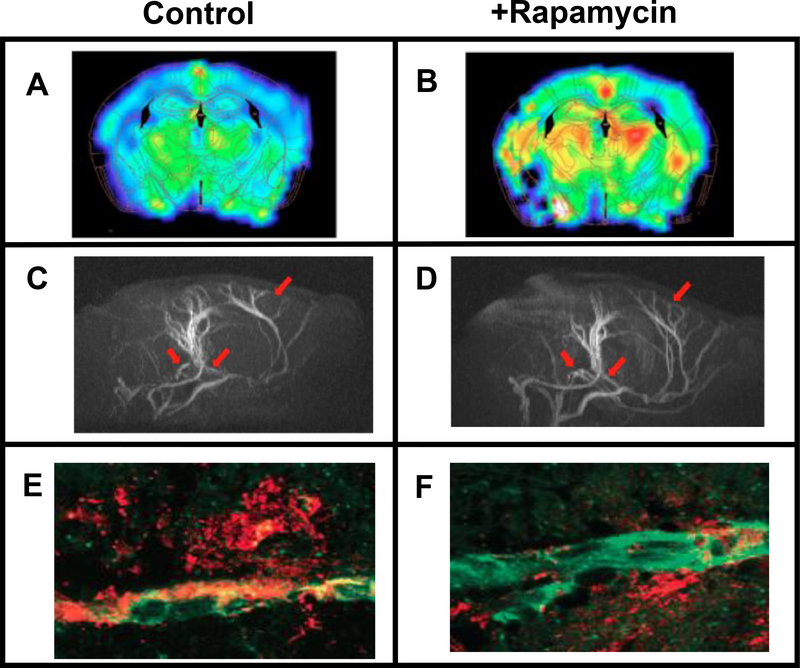
In addition to the accumulation of plaques and tangles, vascular dysfunction is a phenotype of AD (Nagata et al. 2000). Using small animal imaging, Galvan’s laboratory in collaboration with Ai-Ling Lin employed imaging techniques similar to those used in humans to determine the effect of rapamycin on vascular function (Lin et al. 2013). As shown in Fig. 3 (A,B), they found that CBF was restored to wild type levels when the hAPP(J20) were treated with rapamycin for 16 weeks. The restoration of CBF in hAPP(J20) mice was not due to changes in glucose metabolism but appeared to arise from changes in vascular density as shown in Fig. 3 (C,D); rapamycin restored vascular density in the brains of hAPP(J20) mice to the levels found in the brains of control, untreated hAPP(J20). Ai-Ling Lin has also found that rapamycin improves CBF in old rats, LDL-receptor mice fed a high-fat diet, and APOE4 transgenic mice (personal communication); thus, rapamycin’s ability to restore vascular function in brain is not limited to AD.
Fig. 3.
Effect of rapamycin on CBF, vascularization, and cerebral amyloid angiopathy in hAPP(J20) mice. Panels A and B show CBF in various brain regions with brighter colors indicating increased blood flow. Panels C and D show magnetic resonance angiography images of brains showing vasculature with the arrows pointing to regions of the brain where rapamycin restored vascular density. Panels E and F show Aβ (shown in red) associated with brain blood vessels (shown in green). The data were taken from Lin et al. ( 2013) for 7-month-old mice fed rapamycin or a control diet for 16 weeks.
The data obtained by Lin et al. (2013) also indicated that rapamycin acts acutely as an NO-dependent vasodilator, as had been suggested by Cheng et al. (2008). Therefore, they propose that rapamycin’s activation of nitric oxide synthase is critical for the restoration of CBF in hAPP(J20) mice. Because the deposition of Aβ in the brain vasculature has been shown to cause microvessel disruption (Zlokovic 2011), it is possible that the improvement in vascularization in rapamycin-treated treated AD-Tg mice is accompanied by decreased Aβ deposition within brain vasculature. Lin et al. (Lin et al. 2013) measured the amount of Aβ associated with brain blood vessels in and rapamycin-treated and untreated hAPP(J20) mice. Fig. 3 (E,F) shows that the Aβ associated with brain was significantly reduced in rapamycin-treated hAPP(J20) mice, which was correlated to reduced microhemorrhages (see Fig. 3 M,N in (Lin et al. 2013)).
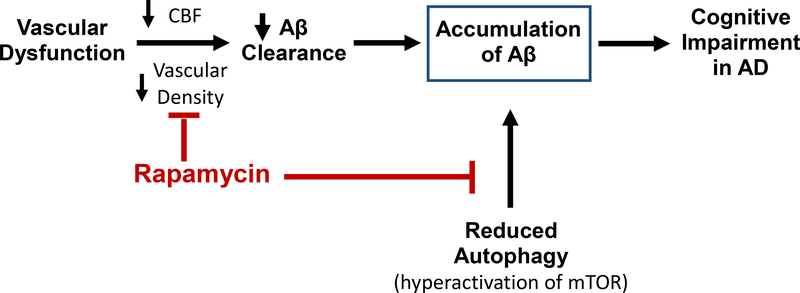
In summary, data generated over the past four years show that rapamycin can prevent, and possibly reverse, the complete AD phenotype observed in transgenic mouse models of AD, e.g., memory is improved, the accumulation of Aβ- and tau-aggregates is reduced, microglia activation is prevented, and CFB and brain vascular density are restored. Based on the current data, we propose that rapamycin prevents AD by acting on several pathways that can potentially lead to cognitive impairment as shown in Fig. 4. First, rapamycin increases the clearance of Aβ by restoring vascular integrity and CBF, and second, rapamycin increases the degradation of Aβ and tau aggregates by inducing autophagy, thereby counteracting the hyperactivation of mTOR that is observed in the brains of AD-Tg mice and patients (Oddo 2012) (Sun et al. 2014).
Fig. 4.
Diagram showing the protective effect of rapamycin in AD using the ‘two-hit’ process described by Zlokovic (2011). Rapamycin prevents the first hit’ by increasing the clearance of Aβ through the restoration of vascular integrity. Rapamycin also blocks the second ‘hit’ by inducing autophagy preventing the accumulation of Aβ in the brain counteracting the hyperactivation of mTOR, which occurs in the brains of AD-Tg mice and patients.
4. Effect of Rapamycin on Mouse Models that are Cognitively Impaired:
Studies over the past 5 years have shown that rapamycin can prevent cognitive impairment that occurs in a number of disorders/conditions that does not involve the accumulation of plaques/tangles. One of the first studies showing the benefits of rapamycin treatment on cognition was reported for mice heterozygous for Tsc2 (tuberous sclerosis protein 2). Tsc1 and Tsc2 form a protein-complex that has tumor suppressor activity and also inhibits mTOR activity. Mutations in these two genes results in hyperactivation of mTOR that is characterized by hamartomas, benign growth of tumors in various organs. However, mutations in these genes also produce changes in the central nervous system separate from tumor growth. Ehninger et al. (2008) showed that Tsc2+/− mice have deficits in learning and memory in the absence of neuropathology and seizures, and rapamycin treatment of these mice rescued the behavioral deficits in the Tsc2+/− mice, including the impairment in memory. Since this report, rapamycin has been shown to prevent/restore the loss of memory arising from a number of other disorders/manipulations, which are associated with the hyperactivation of mTOR, and these studies are listed in Table 2. These disorders/conditions range from drug/alcohol treatment to induce seizures, to genetic manipulations that result in autism-like phenotypes. In addition, Erlich et al. (2007) reported that treating mice with rapamycin after brain trauma enhances the recovery of the brain from injury. Thus, the current literature shows that rapamycin has a broad range of effects on the central nervous system. This should not be surprising because components of the mTOR pathway are widely expressed in the central nervous system, especially Tsc1 and 2, which are highly abundant in the adult central nervous system and play a major role in regulating mTOR activity (Ehninger and Silva 2011).
Table 2.
List of Neurological Disorders Improved by Rapamycin Treatment
| Disorder/Condition | Rapamycin Treatment | Effect of Rapamycin | Reference |
|---|---|---|---|
| Head injury | 4 hours after head trauma | Greater improvement of markers of neurobehavioral function | (Erlich et al. 2007) |
| Tsc2+/− Mice | Treated 3 hr to 5 days continuously before assays. | Corrects deficits in memory/learning (Morris water maze and the 8-arm radial maze) and contextual discrimination. | (Ehninger et al. 2008) |
| THC-cannabinoid treatment | Pre-treatment for 5 days | Prevented decline in performance on the novel object recognition test | (Puighermanal et al. 2009) |
| Alcohol addiction | 1 Dose | Attenuates conditioned preference to alcohol, e.g., decreased binge drinking and motivation to consume alcohol | (Neasta et al. 2010) |
| Deletion of Tsc1 in the cerebellar Purkinje cells | Started at P7 | Prevented deficits in cognition on the water T-maze, pathological phenotypes of autism in the brain, and autistic-like behavior | (Tsai et al. 2012) |
| Pilocarpine-induced seizures | Post-treatment every other day for 8 days | Restoration of cognation as measured on the Morris water maze and the novel object recognition test | (Brewster et al. 2013) |
| Disc1 knockdown in Dentate Granule neurons | Treatment 5 – 13 days after retroviral injection | Reversed deficits in cognition (object-place recognition task) as well anxiety phenotype (elevated plus maze) | (Zhou et al. 2013) |
5. Effect of Rapamycin on Cognition and Behavior of ‘Normal’ Laboratory Mice:
The first study to show that rapamycin increased cognition of ‘normal’ laboratory mice was conducted by Oddo’s laboratory in 2012 (Majumder et al. 2012). They found that 18-month-old mice treated with rapamycin for 16 months showed significantly improved spatial memory on the Morris water maze compared to the mice fed a control diet. Subsequently, Galvan’s laboratory (Halloran et al. 2012) and Neff et al. (Neff et al. 2013) also showed that rapamycin improved spatial memory of mice on the Morris water maze. Fig. 5 compares the Morris water maze data generated by these three groups. In addition, Galvan’s group showed that rapamycin restored the memory of old mice on a passive avoidance task. Majumder et al. (2012) found that the improvement in cognation observed with rapamycin was associated with decreased brain levels of the pro-inflammatory cytokine, IL-1β, and increased NMDA signaling; both have been shown to affect brain function. It should be noted that Erlich et al. (Erlich et al. 2007) found that rapamycin reduced inflammation as shown by decreased numbers of activated microgila in their brain injury model, and several studies suggest that rapamycin reduces inflammation in various tissues (Chen et al. 2009; Bonegio et al. 2005; Dubois et al. 2003). Therefore, some of the benefits arising from rapamycin treatment might occur through rapamycin’s anti-inflammatory actions.
Fig. 5.
Effect of rapamycin of memory on ‘normal’ laboratory mice. Mice were fed rapamycin (black bars) or a control diet (white bars) for various lengths of time and cognitive performance measured by the Morris water Maze. The right graph is 2-month-old mice fed rapamycin for 16 months (data taken from Majumder et al. 2012). The middle graph is 4-month-old mice fed rapamycin for 16 weeks (data taken from Halloran et al., ( 2012). The right graph is 4-month-old mice fed rapamycin for 11 months (data taken from Neff et al. 2013).
In addition to studying the effect of rapamycin on memory, Galvan’s laboratory studied the effect of rapamycin on non-cognitive components of behavior, specifically anxiety- and depressive-like behaviors (Halloran et al. 2012). They found that 4-month-old mice treated with rapamycin for 16 weeks showed reduced anxiety-like behavior, e.g., reduced thigmotaxis (swimming in close proximity to the pool wall) and reduced aversion to open spaces using an elevated plus maze. Rapamycin also was found to reduce depressive-like behavior, e.g., floating during training phase of the Morris water maze and reduced time spent immobile on the tail suspension test. Similar effects of rapamycin on behavior have been reported for genetically modified mice that show autistic-like behavior. Mice with mutations in Tsc2 or Disc1 genes, show increased anxiety, hyperexcitability, abnormal social interaction, repetitive behavior and vocalizations, all of which are attenuated by rapamycin treatment (Tsai et al. 2012; Zhou et al. 2013). Recently, Kolosova et al. (2013) reported that rapamycin treatment decreased anxiety and improved locomotor and exploratory behavior in senescence-accelerated OXYS rats. In addition, Neasta et al. (2010) showed that rapamycin attenuates the conditioned preference of rats and mice to alcohol. Therefore, rapamycin affects a wide variety of cognitive and non-cognitive types of behavior in rodents. Galvan’s laboratory showed that the changes in anxiety- and depressive-like behavior was correlated with increased levels of dopamine and dopamine metabolites in the midbrain.
6. Conclusion:
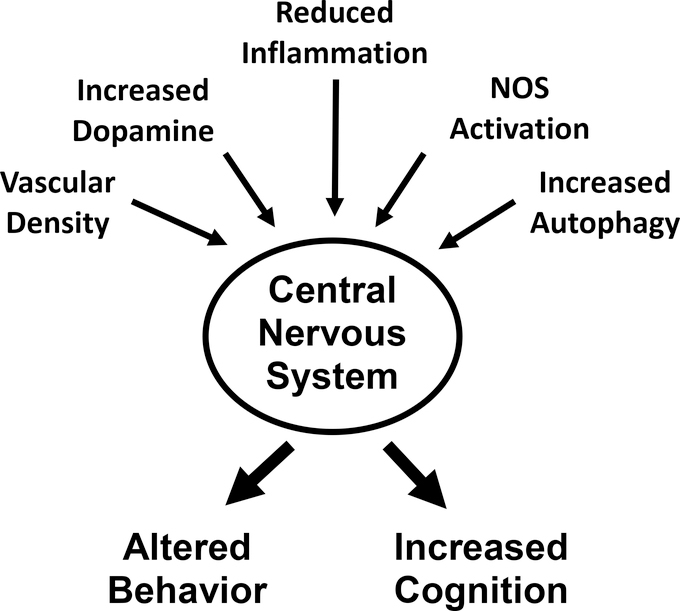
Research over the past four years has shown that rapamycin can have quite profound effects on the central nervous system of mice, resulting in both improved memory and changes in behavior, e.g., anxiety and depression. As shown in Fig. 6, the changes in behavior and cognition observed in mice could arise through a number of pathways that have been reported to be altered by rapamycin. Many of these pathways could also play an important role in preventing the progression of Alzheimer’s disease.
Fig. 6.
Diagram showing pathways reported to change with rapamycin treatment that could impact the changes observed in behavior and cognition observed in laboratory rodents.
The studies from Oddo’s and Galvan’s laboratories show quite convincingly that rapamycin given to mice at a dose shown to increase lifespan and delay many aging phenotypes and pathologies prevented, and may have restored, the loss in cognition observed in two different transgenic mouse models of Alzheimer’s disease. As noted in a recent review, despite over a thousand clinical trials related to AD, only five drugs are currently approved for treatment of the disease (Cavanaugh et al. 2014); unfortunately, these five drugs have no effect on the progression of AD. Also disappointing has been the inability to translate to humans therapies that prevent deficits in cognition in AD-Tg mouse models. It has been argued that the lack of translation from mice to humans is due to species differences in brain structure and complexity. It is also possible that the types of mouse models used do not accurately mimic sporadic AD, which accounts for over 95% of the cases of AD. For example, all the AD transgenic mouse models overexpress in brain various human genes containing mutations associated with familial AD. These AD-Tg mice show a loss of cognition and accumulation of Aβ aggregates early in life, comparable to ~20-year-old human. Therefore, the AD-Tg mice currently used to study AD may not be good models of sporadic AD, which occurs late in life (after 65 years of age). In addition, most of the recent therapies developed to treat AD have focused on reducing the accumulation of Aβ aggregates, either by preventing the processing of Aβ using inhibitors of γ- or β-secretases or using immunization strategies to remove Aβ deposits. While plaques and tangles are correlatively linked to disease symptoms of AD, they may not be causal or key in the mechanism initiating the disease.
We propose that rapamycin has greater likelihood of successfully treating AD than previous therapies studied for three reasons. First, rapamycin appears to have a major effect on aging in rodents, and age is the single greatest risk factor for AD. In fact, the initial study showed that rapamycin was effective in increasing lifespan when implemented late in life (Harrision et al., 2009). Therefore, altering pathways fundamental to aging would be a novel strategy for treating AD, and this strategy is possible now that we have pharmacological intervention into aging with rapamycin that affects a large number of age-related diseases and pathologies in mice (Richardson, 2013). Second, rapamycin appears to have a very broad effect on the central nervous system. It not only reduces the accumulation of both Aβ and tau-containing aggregates, it also improves and restores vascular function and CBF as well as reducing inflammation. Third, rapamycin has an effect on both cognition and behavior in mouse models other than AD-Tg mice, i.e., rapamycin appears to have a very broad effect on the central nervous system. Thus, we predict that rapamycin will potentially be successful in treating sporadic AD patients because of rapamycin’s anti-aging properties, which affect a wide range of pathways (not just Aβ aggregation) that might be involved in the initiation and/or progression of sporadic AD, e.g., such pathways as proteostasis, inflammation, vascular dysfunction, etc.
One major advantage to using rapamycin (or its derivatives, called rapalogues) to treat AD is that it has been approved by the FDA since 1999 for various uses in humans. Therefore, the toxicity profiles of rapamycin and its rapalogues are well characterized (Soefje et al. 2011). The use of rapamycin and rapalogues in treatment of AD are also attractive because these compounds have been given to cancer patients for relatively long periods of time with little change in the quality of life (Mita and Mita 2011). In fact, a pilot study by Lang et al. (Lang et al. 2009) reported that Everolimus, a derivative of rapamycin, affected cognition and depression in humans in a way that is similar to what has been observed in mice. In the study, nine cardiac transplant patients (66 ± 6 years of age) who had initially received a standard immunosuppression regimen of calcineurin inhibitors were followed when they were switched to Everolimus. Four weeks after Everolimus treatment, significant improvements were observed in memory (Wechsler Memory Scale-Revised), depression (Beck Depression Inventory), and executive function (Trail Making Tests A and B).
HIGHLIGHTS.
Rapamycin prevents the deficit in cognition observed in Alzheimer’s transgenic mice
Rapamycin blocks the aggregation of Aβ and tau in Alzheimer’s transgenic mice
Rapamycin restores cerebral blood flow and vascular density in transgenic mice
Rapamycin prevents behavior changes in various mouse models
Anti-aging manipulations have the potential to prevent Alzheimer’s disease
Acknowledgments
This work was supported in part by a RC2AG036613 NIH Recovery Act Great Opportunities grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV 2010. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 176,2092–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV 2011. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 10,4230–4236. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Bokov A, Muller FL, Jernigan AL, Maslin K, Diaz V, Richardson A, Van Remmen H 2012. Dietary restriction but not rapamycin extends disease onset and survival of the H46R/H48Q mouse model of ALS. Neurobiol Aging. 33,1829–1832. [DOI] [PubMed] [Google Scholar]
- Bonegio RG, Fuhro R, Wang Z, Valeri CR, Andry C, Salant DJ, Lieberthal W 2005. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol. 16,2063–2072. [DOI] [PubMed] [Google Scholar]
- Brewster AL, Lugo JN, Patil VV, Lee WL, Qian Y, Vanegas F, Anderson AE 2013. Rapamycin reverses status epilepticus-induced memory deficits and dendritic damage. PLoS ONE. 8,e57808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL 1994. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 369,756–758. [DOI] [PubMed] [Google Scholar]
- Caccamo A, De Pinto V, Messina A, Branca C, Oddo S 2014. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci. 34,7988–7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, Oddo S 2013. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell. 12,370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S 2010. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 285,13107–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferkey R, McLaughlin MM, Young PR, Johnson RK, Livi GP 1994. Yeast TOR (DRR) proteins: amino-acid sequence alignment and identification of structural motifs. Gene. 141,133–136. [DOI] [PubMed] [Google Scholar]
- Camardo J 2003. The Rapamune era of immunosuppression 2003: the journey from the laboratory to clinical transplantation. Transplant Proc. 35,18S–24S. [DOI] [PubMed] [Google Scholar]
- Caron E, Ghosh S, Matsuoka Y, Ashton-Beaucage D, Therrien M, Lemieux S, Perreault C, Roux PP, Kitano H 2010. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 6,453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER 1999. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 99,221–237. [DOI] [PubMed] [Google Scholar]
- Cavanaugh SE, Pippin JJ, Barnard ND 2014. Animal models of Alzheimer disease: historical pitfalls and a path forward. Altex. 31,279–302. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Zhong L, Zhang L, Ji XP, Zhang M, Zhao YX, Zhang C, Zhang Y 2009. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br J Pharmacol. 156,941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Tempel D, Oostlander A, Helderman F, Gijsen F, Wentzel J, van Haperen R, Haitsma DB, Serruys PW, van der Steen AF, de Crom R, Krams R 2008. Rapamycin modulates the eNOS vs. shear stress relationship. Cardiovasc Res. 78,123–129. [DOI] [PubMed] [Google Scholar]
- Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J 2008. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 105,17414–17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP 2012. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53−/− mice by delaying carcinogenesis. Aging. 4,715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Shou W, Haneline LS, Fleischer S, Waldmann TA, Muller JR 2003. Distinct pathways involving the FK506-binding proteins 12 and 12.6 underlie IL-2-versus IL-15-mediated proliferation of T cells. Proc Natl Acad Sci U S A. 100,14169–14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ 2008. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 14,843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Silva AJ 2011. Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol Med. 17,78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R 2007. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 26,86–93. [DOI] [PubMed] [Google Scholar]
- Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH 3rd, Zhang Y, Becker KG, Perez VI, Richardson A 2014. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS ONE. 9,e83988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM 2008. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci U S A. 105,13544–13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, Galvan V 2012. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 223,102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C 2007. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 6,95–110. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 460,392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P, Livi CB, Dodds SG, Jones D, Strong R, Javors M, Fischer KE, Sloane L, Murthy K, Hubbard G, Sun L, Hurez V, Curiel TJ, Sharp ZD 2014. eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila). 7,169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L 1999. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 96,3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Levine B 2007. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 3,597–599. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Kayser E-G, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VX, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M 2013. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 342,1524–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK 2005. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 310,1193–1196. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S 2004. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 14,885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova NG, Vitovtov AO, Muraleva NA, Akulov AE, Stefanova NA, Blagosklonny MV 2013. Rapamycin suppresses brain aging in senescence-accelerated OXYS rats. Aging. 5,474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV 2012. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging. 4,709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang UE, Heger J, Willbring M, Domula M, Matschke K, Tugtekin SM 2009. Immunosuppression using the mammalian target of rapamycin (mTOR) inhibitor everolimus: pilot study shows significant cognitive and affective improvement. Transplant Proc. 41,4285–4288. [DOI] [PubMed] [Google Scholar]
- Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V 2013. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 33,1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, Hubbard G, Hasty P, Sharp ZD 2013. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging. 5,100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S 2012. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 11,326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S 2011. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE. 6,e25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA 2010. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 30,1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R 2011. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 66,191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R 2014. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 13,468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita M, Mita A 2011. Are we ready to move away from nature?: the rapamycin story. Target Oncol. 6,63–64. [DOI] [PubMed] [Google Scholar]
- Mucke L, Yu GQ, McConlogue L, Rockenstein EM, Abraham CR, Masliah E 2000. Astroglial expression of human alpha(1)-antichymotrypsin enhances alzheimer-like pathology in amyloid protein precursor transgenic mice. Am J Pathol. 157,2003–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J 2008. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR−/− mice despite severe hypercholesterolemia. Atherosclerosis. 198,39–48. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kondoh Y, Atchison R, Sato M, Satoh Y, Watahiki Y, Hirata Y, Yokoyama E 2000. Vascular and metabolic reserve in Alzheimer’s disease. Neurobiol Aging. 21,301–307. [DOI] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D 2010. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 107,20093–20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Holter SM, Moreth K, Prehn C, Puk O, Racz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Hofler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Ehninger D 2013. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 123,3272–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S 2012. The role of mTOR signaling in Alzheimer disease. Front Biosci (Schol Ed). 4,941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM 2003. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 39,409–421. [DOI] [PubMed] [Google Scholar]
- Pakala R, Stabile E, Jang GJ, Clavijo L, Waksman R 2005. Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J Cardiovasc Pharmacol. 46,481–486. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A 2009. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 12,1152–1158. [DOI] [PubMed] [Google Scholar]
- Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK 2012. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 4,144ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 36,585–595. [DOI] [PubMed] [Google Scholar]
- Richardson A 2013. Rapamycin, anti-aging, and avoiding the fate of Tithonus. J Clin Invest. 123,3204–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan B, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, Liang S, Chen Y, Mercado ML, Feng X, Bennett F, von Schack D, McDonald L, Zaleska MM, Wood A, Reinhart PH, Magolda RL, Skotnicki J, Pangalos MN, Koehn FE, Carter GT, Abou-Gharbia M, Graziani EI 2008. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci U S A. 105,33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH 1994. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 78,35–43. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 22,159–168. [DOI] [PubMed] [Google Scholar]
- Sharp ZD, Richardson A 2011. Aging and cancer: can mTOR inhibitors kill two birds with one drug? Target Oncol. 6,41–51. [DOI] [PubMed] [Google Scholar]
- Soefje SA, Karnad A, Brenner AJ 2011. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol. 6,125–129. [DOI] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V 2010. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. 5,e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK 2009. The TOR pathway comes of age. Biochim Biophys Acta. 1790,1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Ji X, Mao X, Xie L, Jia J, Galvan V, Greenberg DA, Jin K 2014. Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimers Dis. 38,437–444. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM 2002. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 99,467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Sabatini DM 2009. Rapamycin inhibits mTORC1, but not completely. Autophagy. 5,725–726. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H, Gundelfinger ED 2003. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur J Neurosci. 18,942–950. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M 2012. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 488,647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F 2003. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 426,620. [DOI] [PubMed] [Google Scholar]
- Vezina C, Kudelski A, Sehgal SN 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 28,721–726. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li L, Chen S, Yang D, Wang Y, Zhang X, Wang Z, Le W 2011. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy. 7,412–425. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K 2014. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 69,119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Li W, Huang S, Song J, Kim JY, Tian X, Kang E, Sano Y, Liu C, Balaji J, Wu S, Zhou Y, Zhou Y, Parivash SN, Ehninger D, He L, Song H, Ming GL, Silva AJ 2013. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron. 77,647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV 2011. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 12,723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]