Abstract
Rationale:
Postconditioning (PostC) at the time of primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI) may reduce infarct size and improve myocardial salvage. However, clinical trials have shown inconsistent benefit.
Objective:
We performed the first NHLBI-sponsored trial of PostC in the United States utilizing strict enrollment criteria to optimize the early benefits of PostC and assess its long-term effects on LV function.
Methods and Results:
We randomized 122 STEMI patients to PostC (Four, 30-sec. PTCA inflations / deflations) + PCI(n=65) vs. routine PCI (n=57). All subjects had an occluded major epicardial artery (TIMI= 0) with ischemic times between 1 and 6 hours with no evidence of pre-infarction angina or collateral blood flow. Cardiac MRI measured at 2 days post-PCI showed no difference between the PostC group and Control in regards to infarct size (22.5 ± 14.5 g vs. 24.0 ± 18.5 g), myocardial salvage index (MSI) (30.3 ± 15.6% vs. 31.5 ± 23.6%) or mean LVEF. MRI at 12-months showed a significant recovery of LVEF in both groups (61.0 ± 11.4% and 61.4± 9.1%; p < 0.01). Subjects randomized to PostC experienced more favorable remodeling over 1 year (LVEDV = 157 ± 34 to 150 ± 38 ml) compared to the Control group (157 ± 40 to 165 ± 45 ml) (p < 0.03) and reduced microvascular obstruction (MVO) (p=0.05) on baseline MRI and significantly less adverse LV remodeling compared to Control subjects with MVO (p < 0.05). No significant adverse events were associated with the PostC protocol and all patients but one (hemorrhagic stroke) survived thru one-year of follow-up.
Conclusions:
We found no early benefit of PostC on infarct size, MSI and LV function compared to routine PCI. However, PostC was associated with improved LV remodeling at one year of follow-up, especially in subjects with MVO.
Keywords: Postconditioning, ST-segment elevation myocardial infarction, MRI, microvascular obstruction, ischemia reperfusion injury
Subject Terms: Ischemia, Myocardial Biology, Physiology, Translational Studies, Vascular Biology
INTRODUCTION
Although the rapid restoration of coronary blood flow during ST-elevation myocardial infarction (STEMI) is the most effective means of reducing infarct size and the development of heart failure (1), it is frequently associated with reperfusion injury to the myocardium and vasculature (2,3). Thus, mitigating reperfusion injury in humans is an important remaining target to reduce infarct size in STEMI patients (4). Unfortunately, efforts at reducing reperfusion injury by methods successful in animal models have largely been unsuccessful in clinical trials (5) despite the targeting of multiple salvage pathways. The reasons for these disparities in humans and animals are likely multifactorial but may include the multiple co-morbidities in patients and concomitant medication use that can abrogate cardiac protection. (6,7).
In 2003 Zhao and Vinten-Johansen (8) described a modified reperfusion technique called postconditioning (PostC) that reduced infarct size in dogs by 44% following a 60-minute occlusion of the left anterior descending (LAD) coronary artery. The technique utilized repeated, brief occlusions of the artery (30 s) followed by reperfusion (30 s) over several cycles implemented immediately upon reperfusion. PostC likely shares several common pathways of myocardial protection with ischemic preconditioning that converge on the main end-stream effector of reperfusion injury, the mitochondrial permeability transition pore (2,9). PostC has great clinical potential since it can be performed in the cardiac catheterization laboratory using a percutaneous transluminal angioplasty balloon during the setting of primary percutaneous coronary intervention (PCI) for STEMI (10–14). Unfortunately, the initial benefit of PostC has been mixed with several trials demonstrating reduced infarct size (10,11) while other trials showing no benefit (12,13). Importantly, very few studies have examined the long-term effects of PostC on LV function.
We hypothesized that one reason for the inconsistency of benefit of infarct size reduction and myocardial salvage in clinical trials was related to patient selection. Previous trials frequently included patients with partial reperfusion (TIMI 1 or 2) that may abrogate the benefit of PostC (15). Additionally, they often included patients with prolonged ischemic times (up to 12 hours) where the chance of salvage is negligible, or failed to exclude patients with pre-infarction angina, a strong mitigator of infarct size that may occur in up to 30% of patients with STEMI (16).
We developed a single center trial funded by the National Heart Lung and Blood Institute with MRI imaging and strict enrollment criteria to provide a more homogeneous population using a standardized protocol to determine if PostC has long-term favorable effects on LV remodeling and infarct size reduction.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study population.
This trial was a single center study performed at the Minneapolis Heart Institute at Abbott Northwestern Hospital enrolling STEMI patients who presented to the cardiac catheterization laboratory as part of our Level 1 program (17). This primary PCI network consists of 31 hospitals and emergency departments throughout Minnesota and Western Wisconsin. Enrollment began in April 2011 and concluded in March 2016. The trial was registered at ClinicalTrials.gov (NCT01324453). Five interventional cardiologists were trained in the protocol and agreed to participate in the study.
Experimental protocol.
Enrollment was a two-step process. Initial eligibility for the trial was assessed in the catheterization laboratory following coronary angiography but before PCI and required subjects to have: a.) 100% occlusion of a major epicardial artery (TIMI 0) in the proximal or mid-segment; b.) no evidence of collateral blood flow to the infarct region; c.) ischemic times between 1 and 6 hours; d.) no evidence of pre-infarction angina. Important exclusion criteria included cardiac arrest, renal failure, history of coronary artery bypass surgery, previous infarct in same territory, cardiogenic shock or age > 80 years. Patients meeting initial eligibility criteria and who provided verbal consent to the interventional cardiologist performing the procedure were then randomized by a computer-generated randomization code created by a biostatistician placed in sealed envelope to PostC followed by PCI with stent placement or to routine PCI and stenting. The envelope containing the assigned treatment was procured by a catheterization lab technician not affiliated with the study. The PostC protocol consisted of 30-sec inflations of an appropriately sized PTCA balloon followed by 30 sec. of reperfusion repeated over 4 cycles as used in our feasibility trial (14). PostC was commenced immediately upon reperfusion with the guide wire. All patients were pre-treated with thienopyridines and heparin in their referring emergency departments prior to transfer. Additional heparin was administered to maintain the activated clotting time> 200 sec. and glycoprotein IIB IIIA inhibitors were left to the discretion of the interventional cardiologist. Use of aspiration thrombectomy could not be performed until after the PostC protocol was completed.
Final enrollment criteria in the trial was pre-specified to require all patients to undergo cardiac MRI prior to discharge. Records were reviewed to ensure that they met eligibility criteria including stipulated ischemic times and absence of pre-infarction angina. Those patients enrolled in the catheterization laboratory that could not undergo MRI or then met exclusion criteria were not formally enrolled in the trial but were followed for safety over 30 days. All patients were followed for 1-year for major adverse cardiovascular events and were required to take aspirin and thienopyridines and recommended post-infarct medications including beta-blockers, angiotensin converting enzyme inhibitors and statins for duration of the trial. To determine if a long-term benefit of PostC occurred, patients underwent repeat cMRI measurements of LV function and infarct size at 3 and 12 months following reperfusion.
Consent process.
This study was initially approved by the Institutional Review Boards (IRB) of Allina Health System and the University of Minnesota who permitted the use of an abbreviated verbal consent process in the catheterization laboratory followed by full, informed written consent within 24 hrs. However, in 2014, Allina Health System began utilizing an outside IRB agency to approve the renewal of clinical trials. They now stipulated that our initial verbal consent process was no longer permissible and the only way to continue the trial was to obtain an “emergency waiver of informed consent”. Although no safety issues had been identified by the DSMB, the trial was placed on clinical hold for over one-year despite not having a single complication or adverse event while we fulfilled the requirements for the waiver (BRANY IRB # 15-13-43) (17). Subsequently, enrollment in the trial commenced with the waiver although all patients were given the opportunity within 24 hours of their PCI to decline participation.
Measurement of global LV function and volumes, infarct size, microvascular obstruction and myocardial salvage index with cardiac MRI.
A cMRI 1.5 T scanning unit (Avanto, Cardiac MR Scanner, Siemens Medical Solutions, Malvern, PA) was utilized for cMRI imaging of cardiac function and tissue contrast enhancement. Following short-axis cine acquisition for left-ventricular function assessment, T2-weighted images were acquired for the evaluation of edema in the identical short-axis image planes. Gadolinium was then administered using a 0.2 mmol/kg dose for both early and late contrast imaging. Two minutes after contrast administration, single shot Tru-FISP short axis images covering the left ventricle were acquired in a single breath hold for evaluation of microvascular obstruction (MVO) indicated by signal hypo-enhancement. After 10–15 minutes, late contrast enhancement imaging was performed with diastolic 2D flash imaging. The entire left ventricle was covered using short-axis images with breath holds using a slice thickness of 5 mm (to minimize partial volume averaging) and no interslice gap. Myocardial mass was planimetered from the cine images using the Siemens Argus analysis software. A second analysis planimetered only the areas of scar in each 2D slice from the late contrast enhanced images. The total mass of scar tissue was reported as a percentage of the entire myocardial mass or the percentage of infracted myocardium. MVO was manually calculated as the hypoenhanced region within the delayed hyperenhanced infarct region and expressed as grams of myocardium and percentage of the LV mass. Quantitative estimation of myocardium at risk (AAR) was measured as the hyperintense region on T2-weighted images. Measurements were performed using the QMass software package (Medis Inc., Raleigh NC) by a single investigator who was blinded to treatment. The endocardial and epicardial borders were manually identified and the regions of interest (edema or scar) are automated as 2 standard deviations above the mean intensity of the remote myocardium. The myocardial salvage index (MSI) was calculated using the formula: MSI = (AAR – Infarct size) / AAR × 100 %.
Study end-points and statistical analysis.
Primary end-points of the trial were developed for both the patient’s initial baseline STEMI presentation and their long-term follow-up. Primary endpoints at baseline included cMRI-derived infarct size and MSI between the PostC and Control groups. Primary long-term endpoints included the change in LVEF and LV volumes in the PostC and Control groups between baseline and one year and the influence of MVO. Safety was assessed by recording major adverse cardiac events rates defined as death, recurrent MI, heart failure readmission and non-fatal stroke to one year. Pre-specified exploratory endpoints included the effect of age, gender and ischemic time on infarct size and MSI
The sample size was based on our hypothesis that PostC would reduce infarct size by 20% compared to control based on the study of Lonborg et al (11) which was the first PostC trial to utilize cMRI for endpoint analysis. We assumed a mean infarct size of 20% of LV mass with a standard deviation of 8%. For a statistical power of 80% and an alpha of 0.05 we required a total of 64 subjects in each of the two groups. We estimated a 10% loss of patients to cMRI contraindications or late exclusion criteria resulting in a required estimate of 140 total patients for the trial. Categorical variables were compared by Fisher exact test and continuous variables were compared by Student t-test.
RESULTS
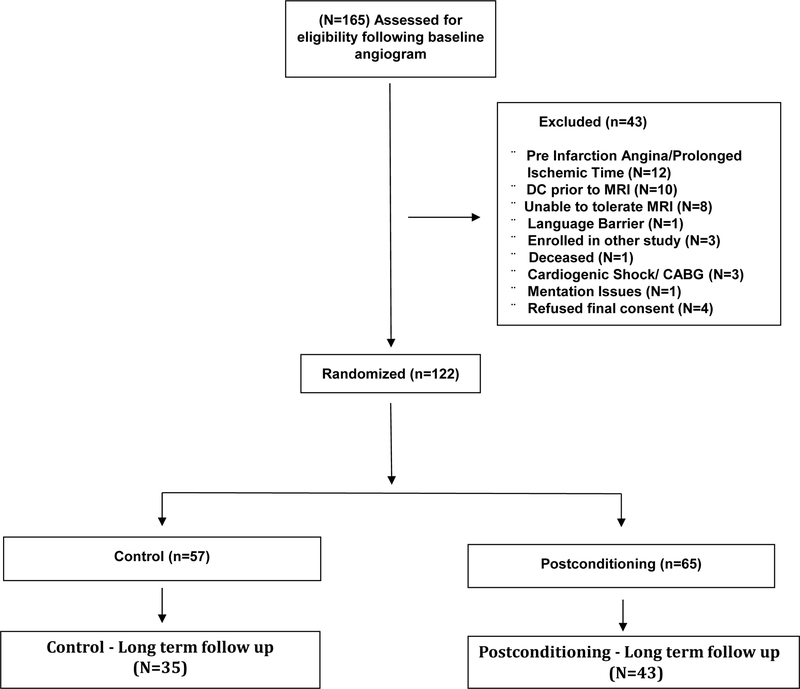
Between April 2011 and March 2016 (including 18 months of clinical hold) we provisionally enrolled 165 patients that met entry criteria following baseline angiography (PostC=82; CON=83) into the trial. (Figure 1). A total of 43 patients did not meet the stipulated final enrollment criteria and were only followed for safety over 30 days. Reasons for exclusion included: discharged prior to MRI (n=10); Could not tolerate MRI (n=8); enrolled in other research study (n=3); refused final consent because of inability to return for follow-up visits (n=4); pre-infarction angina (n=12); contrast allergy (n=1); coronary artery bypass surgery (n=1); deceased (n=1; Control); language barrier (n=1); mentation issues (n=1); cardiogenic shock (n=2). Thus, the final randomized cohort included 122 patients (PostC=65; Control=57). A total of 78 patients returned for long-term cMRI follow-up with scans performed at 3 and 12-months (PostC = 43, Control = 35).
Figure 1-.
CONSORT Diagram
Baseline data.
The majority of the final randomized subjects were male (85%) with an average age of 59 years. (Table 1). The average ischemic duration was 150 minutes and did not differ between the PostC and Control groups. Peak creatine kinase muscle/brain (CK-MB) was 133 U/L in the PostC group and 159 U/L in the Control group (p=0.59) with no difference in the area-under-the-curve measurements in CK over the first 24 hrs. following reperfusion (Table 2). Baseline cMRIs were performed at a mean of 2.1 days post-PCI in the PostC group and 1.8 days in the Control group. Baseline was 49.7 ± 9.9% in the PostC group and 48.0 ±12.3% in the Control group. LV end-diastolic volume (LVEDV) was 158 ± 40 ml in the PostC group and 163 ± 41 ml in the Control group (Table 2).
TABLE 1-.
Baseline Demographics
| All Patients (n=122) |
Control (n=57) |
Post Conditioning (n=65) |
P-Value | |
|---|---|---|---|---|
| Age (Years) | 59.3 ± 10.0 | 59.1 ± 10.1 | 59.4 ± 10.1 | 0.84 |
| Male, (%) | 102 (85.0) | 47 (82.5) | 55 (87.3) | 0.46 |
| Body mass index, kg/m | 29.8 ± 4.5 | 29.9 ± 4.4 | 29.6 ± 4.6 | 0.72 |
| Diabetes, (%) | 22 (18.3) | 9 (15.8) | 13 (20.6) | 0.49 |
| Family history of coronary artery disease, (%) | 44 (40.4) | 26 (48.2) | 18 (32.7) | 0.10 |
| Hypertension, (%) | 64 (53.3) | 28 (49.1) | 36 (57.1) | 0.38 |
| Hyperlipidemia, (%) | 67 (56.3) | 29 (50.9) | 38 (61.3) | 0.25 |
| Current smoker, (%) | 44 (37.0) | 22 (39.3) | 22 (34.9) | 0.62 |
| History of stroke, (%) | 1 (0.8) | 0 (0) | 1 (1.8) | 0.47 |
| Previous PCI, (%) | 12 (10.3) | 6 (10.7) | 6 (9.8) | 0.88 |
| Use of glycoprotein IIb/IIIa inhibitor, (%) | 48 (42.1) | 20 (36.4) | 28 (47.5) | 0.23 |
| RCA, (%) LAD, (%) LCx/PDA/PLA, (%) |
54 (46.2) 52 (44.4) 11 (9.4) |
28 (50.0) 22 (39.3) 6 (10.7) |
26 (42.6) 30 (49.2) 5 (8.2) |
0.55 |
| BMS, (%) DES, (%) |
6 (5.2) 110 (94.8) |
5 (9.1) 50 (90.9) |
1 (1.6) 60 (98.4 |
0.070 |
| Thrombectomy, (%) | 39 (34.5) | 24 (44.4) | 15 (25.4) | 0.034 |
| TIMI grade 3 post-PCI, (%) | 117 (97.5) | 55 (96.5) | 62 (98.4) | 0.50 |
| EF (%) | 49.4 ± 11.0 | 48.0 ± 12.3 | 49.7 ± 9.9 | 0.72 |
| Ischemic Duration (mins) | 150 (112, 217) | 159 (115, 221) | 145 (112, 214) | 0.55 |
| Pre Cath Vitals | ||||
| Heart Rate | 73 (64, 87) | 73 (63, 85) | 74 (64, 93) | 0.41 |
| Respirations | 17 (15, 20) | 18 (14, 20) | 17 (16, 21) | 0.57 |
| Systolic BP | 137 (119, 145) | 138 (119, 144) | 136 (125, 146) | 0.61 |
| Diastolic BP | 83 (75, 94) | 79 (74, 93) | 85 (78,96) | 0.33 |
| O2 Saturation | 98 (95, 100) | 97 (95, 100) | 98 (95, 100) | 0.89 |
Table 2-.
CK Area-under-the-Curve Measurements
| CK (U/L) | 217 ± 430 | 1721 ± 1605 | 1770 ± 1601 | 1298 ± 1307 | 986 ± 1263 | 810 ± 1313 | 680 ± 1124 |
| CKMB(U/L) (mean) |
168 | 108 | 65.5 | 32.1 | 20.1 | 19.5 | |
| Time (hrs) Post-PCI |
Baseline | 4.5 | 12.8 | 21.3 | 29 | 40 | 45 |
| CK (U/L) | 227 ± 339 | 1633 ± 1592 | 1664 ± 1339 | 1238 ± 935 | 943 ± 699 | 667 ± 457 | 527 ± 400 |
| CKMB (U/L) (Mean) |
183 | 128 | 77.5 | 40.2 | 19.5 | 17 | |
| TIME (hrs) Post-PCI |
Baseline | 4.0 | 13.3 | 21.5 | 29.9 | 40 | 45.8 |
Infarct size and myocardial salvage.
Mean infarct size measured by cMRI was 22.5 ± 14.5 g in the PostC group and 24.0 ± 18.5 g in the Control group (p=NS). The mean AAR was 31.9 ± 16.9 g in the PostC group and 33.4 ± 15.4 g in the control group. As a result, there was no difference in the myocardial salvage index between the two groups which was 30.3 % in the PostC group and 31.5% in the Control group (Table 3). Mean infarct size significantly declined in both groups over the 12 months of follow-up (p < 0.01) (Table 4A) and was accompanied by a significant decline in LV mass (Table 4B). In pre-specified subgroup analyses, no difference between the two groups were observed for any of these parameters when patients were stratified by age (< or > 65 yrs.), gender or ischemic time (< or > 3 hrs.).
TABLE 3-.
cMRI Baseline Data
| Postconditioning (n=65) | Control (n=57) | |
|---|---|---|
| LV mass (g) | 136 ± 33 | 149 ± 42* |
| LVEF (%) | 54.0 ± 10.7 | 53.5 ± 9.8 |
| LVEDV (ml) | 158 ± 40 | 163 ± 41 |
| LVESV (ml) | 72 ± 29 | 75 ± 28 |
| Infarct Size (g) | 22.5 ± 14.5 | 24.0 ± 18.5 |
| AAR (g) | 31.9 ± 16.9 | 33.4 ± 15.4 |
| MSI (%) | 30.3 ± 15.6 | 31.5 ± 23.6 |
| MVO (n) | 29 | 22 |
p < 0.07; AAR = Area-at-Risk; MSI = Myocardial Salvage Index;
MVO = Microvascular Obstruction.
TABLE 4A-.
Long Term cMRI Follow-up Data
| Baseline | 3-mo | 12-mo | Baseline | 3-mo | 12-mo | |
|---|---|---|---|---|---|---|
| LVEF (%) | 55 ± 10.4 | 61.3±10.6* | 61.4±9.1* | 53.8±10.0 | 61.1±10.5* | 61±11.4* |
| LVEDV (ml) | 157 ± 34 | 154 ±37 | 150 ± 38k | 157 ± 40 | 161 ± 45 | 165 ± 45 |
| LVESV (ml) | 70 ± 25 | 60.5 ± 26u | 58 ± 28u | 72 ± 30 | 63 ± 30u | 66 ± 34 |
| Infarct Size (g) | 22.9±12.4 | 14.6±6.9t | 10.9±7.8t | 27.4±22.7 | 15.8±12.1t | 14.7±10.5t |
p< 0.01 vs. Baseline Infarct Size;
p < 0.01 vs. Baseline LVEF;
p < 0.001 vs Baseline LVESV;
p < 0.05 vs. 12-month Control LVEDV Group.
Table 4B-.
Baseline and 12-month LV Mass in Subjects who had both baseline and 12-month measurements
| Baseline LV mass (g) | Baseline LV viable mass (g) | 12-Mo LV mass (g) | 12-mo LV viable mass (g) | |
|---|---|---|---|---|
| Postconditioning | 139 ± 30 | 111 ± 28* | 129 ± 25 | 113 ± 24 |
| Control | 145 ± 39 | 119 ± 30* | 129 ± 33 | 111 ± 37 |
P < 0.05
Late effects of postconditioning on LV function and remodeling.
A total of 43 subjects in the PostC group and 35 subjects in the Control group underwent additional cMRI imaging at 3 and 12-months to determine if a delayed benefit of PostC occurs (Table 4A). LVEF significantly increased in both groups from baseline to 3 months (p < 0.05) with no further change out to one year. However, there was no difference in LVEF between the PostC and Control groups. Left ventricular end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were significantly smaller when measured at 12 months in the PostC group compared to the Control group (Table 4A). LVEDV decreased from 157 ± 34 ml to 150 ± 38 ml in the PostC group while it increased from 157 ± 40 ml to 165 ± 45 ml in the Control group (p < 0.03). Similar findings occurred for LVESV, which significantly decreased in both groups at 3 months. However, LVESV was significant smaller at 12 months in the PostC group (58 vs. 66 ml) (p < 0.05). There were no differences in medication usage between the two groups at 12 months including ACE inhibitors, beta-blockers, statins or aspirin.
Postconditioning and microvascular obstruction (MVO).
A total of 51 patients with 12-month MRIs had “late” MVO on their baseline scans. PostC did not reduce the incidence of MVO. However, patients randomized to PostC tended to have less MVO than those randomized to Control (9.6 vs. 11.5 g) and had significantly less MVO as percentage of infarct size (30.5 vs. 37.1%; p=0.05) (Table 5). Subjects with MVO randomized to PostC (n=29) experienced significantly less adverse remodeling at 12 months of follow-up compared to those subjects with MVO who were randomized to Control (n=22) (Table 6).
TABLE 5-.
Measurements of Microvascular Obstruction on Baseline MRI scan
| MVO mass (g) | % of LV mass | % of Infarct Size | % of AAR | |
|---|---|---|---|---|
| Postconditioning | 9.6 ± 10.0 | 6.0 ± 5.5 | 30.5 ± 19.5 | 26.7 ± 16.8 |
| Control | 11.5 ± 7.2 | 8.4 ± 4.1 | 37.1 ± 17.0* | 30.2 ± 15.7 |
p = 0.05
TABLE 6-.
Long-term MRI Follow-up of Subjects who had Microvascular Obstruction (MVO) on Baseline MRI scan
| Baseline | 12-month | Baseline | 12-month | |
|---|---|---|---|---|
| LVEF (%) | 51.1± 9.4 | 59.2 ± 12.6t | 48.5 ± 8.2 | 55.7 ± 10.3t |
| LVEDV (ml) | 156 ± 41 | 153 ± 44u | 155 ± 38 | 174 ± 46 |
| LVESV (ml) | 78 ± 29 | 65 ± 34* | 79 ± 27 | 79 ± 36 |
| Infarct Size (g) | 27.8 ± 15.6 | 15.5 ± 8.2** | 33.1 ± 18.7 | 19.2 ± 8.9** |
p < 0.05 vs. baseline LVESV;
p < 0.05 change in LVEDV from baseline to 12-months PostC vs. Control Group
p < 0.05 vs. Baseline LVEF
p < 0.01 vs. Baseline infarct size
Safety and serious adverse events.
During the procedure, 2 patients in the PC group underwent successful DC cardioversion for ventricular fibrillation. One occurred during injection of the right coronary artery following the PostC protocol and successful LAD stent placement. A second patient experienced 2 episodes of VF during balloon inflation of the PC protocol of the LAD and was successfully cardioverted. There were only 3 major adverse cardiac events in the trial over 12 months of follow-up in the 122 subjects. One patient in the PostC group experienced acute stent thrombosis 2 hours following PCI and underwent thrombectomy and placement of a second stent. One patient died in the Control group due to a hemorrhagic stroke that occurred 100 days following his PCI and 1 patient in the PostC group was admitted for acute heart failure 345 days following PCI.
DISCUSSION
In this first randomized, controlled trial of PostC in the United States funded by the National heart, Lung and Blood Institute, PostC did not significantly reduce infarct size or improved myocardial salvage as measured by early cardiac MRI in STEMI patients undergoing primary PCI. This neutral finding occurred in all subgroups analyzed despite utilizing a design specifically created to optimize the enrollment criteria of the subjects by eliminating confounders that are known to modify infarct size or reperfusion injury; a strategy lacking in most PostC trials. This is the 3rd largest PostC trials to utilize cMRI (4), but is the largest to provide long-term imaging follow-up with cMRIs at 3 and 12 months to determine if a late benefit of PostC occurs. Indeed, we observed that long-term benefit of LV remodeling occurred in the PostC group at 12 months, as LV volumes were significantly smaller than the Control group. Patients in the PostC group had significantly less MVO as percent of infarct size compared to Control that may have contributed to the improvement in LV remodeling observed at one year. Given that MVO is associated with increased morbidity and mortality in STEMI patients, longer-term follow-up of these patients is warranted. These findings suggest that PostC may initiate some type of delayed protection that reduces adverse LV remodeling that is not appreciated in the acute setting shortly after PCI that may be missed in trials that do not incorporate long-term imaging.
Our protocol enrolled an ideal population to study ischemia-reperfusion injury by eliminating the major known controllable confounders on infarct size and salvage. Thus, we limited ischemic times to 1 to 6 hours, excluded all patients with visible collaterals or pre-infarction angina (16,19) and mandated that all patients have a 100% occluded major epicardial artery at presentation (TIMI 0). Importantly, the PostC algorithm was instituted immediately at reperfusion, a technique missing in many PostC trials that have first performed aspiration thrombectomy that may result in the establishment of reperfusion but delays the initiation of the PostC protocol. All aspiration thrombectomy performed in our trial was mandated to occur after the PostC algorithm. We designed our strategy to hopefully reconcile the conflicting results of previous PostC trials on infarct size and myocardial salvage by avoiding the loss of the protection afforded by PostC when not initiated within 1 minute of reperfusion (8).
Previous postconditioning trials.
The first randomized trial of postconditioning reported by Staat et al (10) in 2005 (n=30) demonstrated that patients randomized to PostC had a significant reduction in serial CK release and improved myocardial blush grade at completion of the procedure. In that trial, direct stenting was utilized in all patients who were required to have TIMI 0 flow during the presenting angiogram and no evidence of visible collaterals or pre-infarction angina. The mean ischemic time was > 5 hours and the PostC algorithm was notable for utilizing four, 60-second inflation / deflations as opposed to 30 second inflations used in the seminal pre-clinical study of Zhao and Vinten-Johansen (8) and in our present clinical trial. This initial study (10) was followed by multiple small, single center studies, including our own pilot study (14), that confirmed a benefit of PostC on infarct size reduction. These studies include the first PostC trial to use cMRI to measure infarct size and MSI by Longborg et al. (11) in 86 STEMI patients using a 30-sec algorithm. However, in that study, patients with pre-infarction angina or visible collaterals were not excluded and ischemic times up to 12 hours were allowed. Still, infarct size as a percent of LV mass decreased by 18% with PostC compared to routine PCI when measured by cMRI 3 months after reperfusion. There was no difference in peak troponin or LVEF or LV volumes between the two groups.
More recently, there have been several large trials that have failed to confirm a benefit of PostC in the setting of primary PCI for STEMI. The LIPSIA Conditioning trial (20) randomized 696 STEMI patients to a combination of remote ischemic preconditioning and PostC versus PostC alone (4 cycles of 30 sec I/R) compared to a control group that underwent standard primary PCI. They reported the combination of the 2 protocols (Remote + PostC) but not PostC alone, significantly improved myocardial salvage as measured by cardiac MRI within 3 days following reperfusion compared to Control (49 vs. 40%). Their neutral findings of Post C alone is not surprising given that over 30% of their patients had already reperfused (TIMI 2 or 3 flow) on initial angiography that would render any PostC intervention ineffective (15).
The second largest PostC trial was the POST randomized trial (12) performed in 17 centers in South Korea which randomized 700 STEMI patients to primary PCI and stenting + PostC versus routine Primary PCI and stenting. The primary endpoint of ST-segment resolution by electrocardiogram at 24 hours was not improved by PostC that utilized 4, 1-minute occlusions / reperfusions. Notably, over 50% of patients underwent aspiration thrombectomy prior to PostC making the requirement to initiate PostC within 1 minute of reperfusion unlikely in many patients. At 1-year (21), the authors reported no clinical benefit of PostC on MACE.
The recently published DANAMI-3 – iPOST trial randomized 1234 STEMI patients from Denmark to PostC and stenting vs. stenting alone (13). The primary endpoint of all-cause death (p=0.18) and hospitalization for heart failure (p=0.96) was not different between the two groups at a mean follow-up of 38 months. In a subgroup of patients that underwent cMRI imaging (n=358) at Day 1 and at 3 months, there was no difference in infarct size, myocardial salvage, MVO or LVEF. At 18 months, LVEF by echocardiography was higher in the PostC group vs. Control (52.7 vs. 50.8%). However, the benefits of PostC may have been blunted by the protocol that permitted TIMI 1 flow and ischemic times up to 12 hours. Following initial reperfusion to demonstrate TIMI 2 or 3 flow, aspiration thrombectomy was performed in over half the subjects and a new balloon, sized to the vessel, was then procured to perform the postC algorithm. This did not occur in our trial as the postC balloon was used to obtain initial reperfusion such that there was no delay in starting the postC algorithm.
Delayed benefit of postconditioning?
We observed significantly less adverse LV remodeling at one year in those subjects randomized to PostC compared to Control. This observation suggests a delayed benefit of PostC following STEMI. Reperfusion injury is a trigger for apoptosis which can be ongoing following STEMI and contribute to adverse LV remodeling (22). PostC has been shown to inhibit apoptosis through several pathways including a reduction in oxidative stress and inhibition of the translocation of nuclear factor kappa-B from the cytoplasm to the nucleus. This translocation results in the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (23) a known trigger for apoptosis. Thus, PostC could potentially reduce stimuli for ongoing apoptosis that would be reflected in improved remodeling over time but not be seen acutely. Additionally, a variety of other factors could improve remodeling following PostC. This may include improved microvascular function and neovascularization in the infarct border zone and blunting of the renin-angiotensin system (24). Additionally, the ability of PostC to reduce the extent of MVO as we and others (25) observed, can be associated with marked improvements in long-term LV remodeling as observed in the TIME trial (26)
Several PostC trials have performed imaging studies at later time points to examine potential long-term effects of PostC. The POSTEMI Trial found no improvement in LVEF or infarct size by cMRI (13.5 vs. 14.4%) with PostC in 249 patients when measured at 4 months of follow-up (27). However, the PostC protocol was not initiated until at least 1 minute after reperfusion which could have rendered the PostC less effective (28) and no measurements of LV volumes were reported. Additionally, all subjects received eptifibatide and the influence of glycoprotein IIB IIIA receptor blockade in PostC trials is unknown. Freixa et al. (29) observed no benefit of PostC on LVEF and change in LV volumes by cMRI in 62 patients between 7 days and 6 months. However, the prolonged mean ischemic times of nearly 6 hours may have abrogated any benefit of PostC.
Only one other previous PostC study has performed MRI analysis at baseline and 12-months (30) and found no significant benefit of PostC on IS or MSI in 76 patients measured 6–9 days post-STEMI. However, PostC patients with the largest area-at-risk had smaller infarcts and greater LVEF. At 12 months (n=68), the benefit in the PostC subgroup with the greatest AAR was maintained. In contrast to our findings, they observed no benefit of PostC on LV remodeling at 12 months.
Postconditioning and microvascular obstruction (MVO).
A novel finding from our study was the interaction of PostC and the presence of MVO. Patients with MVO randomized to PostC had smaller infarcts at baseline and significantly less adverse remodeling at one year than those patients with MVO randomized to control (Table 6). To our knowledge, the ability of PostC to mitigate the long-term deleterious effects of MVO has not previously been described in any PostC trial and this finding will require further investigation. In a previous small clinical trial of PostC (n=50), Mewton et al. (25) observed that patients randomized to PostC had significantly smaller amounts of early and late MVO compared to control, although the incidence of MVO was similar between the two groups as we observed in our trial. No long-term follow-up of these patients was provided. However, given the smaller infarct size in their PostC group (18 vs. 28% of LV), it is likely that they too would have found more favorable remodeling.
The presence of MVO is known to be a powerful predictor of mortality and adverse LV remodeling following STEMI (31). MVO was observed on the baseline MRIs in nearly half of our cohort with analyzable MRIs for infarct size. We observed that PostC tended to reduce MVO mass and significantly reduce its extent as a percentage of infarct size compared to Control. Protection of the coronary microcirculation and improved blood flow in the infarct region via reduced MVO may be an important, yet underreported benefit of PostC and may have contributed to the favorable remodeling effects we observed in this cohort. In a recent cell therapy study of similar STEMI patients (26) we reported that subjects with MVO experienced reduced recovery of LVEF and greater adverse LV remodeling compared to similar subjects without MVO. However, in contrast to PostC, cell therapy did not improve LV remodeling at one and two years of follow-up. In the current study, we demonstrate that patients with MVO who underwent PostC had smaller LV volumes and improved LVEF at one year compared to patients with MVO randomized to Control. How PostC mitigates MVO is not known but a potential mechanism may involve PostC’s reported benefits on microvascular function that could improve endothelial integrity and reduce the development of MVO post-STEMI (32, 33).
Is there a role for postconditioning in STEMI?
Reducing ischemia-reperfusion injury in the setting of STEMI is an important remaining target to reduce infarct size. Unfortunately, the failure of the early results of this trial is consistent with many other studies of myocardial protection unable to recapitulate the findings of infarct size reduction in animals. The outcomes of PostC clinical trials bear a striking resemblance to the initial studies of cardiovascular cell therapy on improving LV function following STEMI. Initial positive trials using bone marrow mononuclear cells and subsequent positive meta-analyses give way to larger trials using cMRI imaging that were neutral despite a similar patient population (26, 34). Although recent meta-analyses (35) suggest a clinical benefit of PostC in reducing infarct size by serial cardiac enzymes, many subsequent larger trials using advanced cMRI imaging such as ours, have found no acute benefit of this intervention. Similar to cell therapy, the failure of the PostC studies to define basic methodology such as the optimum PostC algorithm and other factors such as the role of direct stenting, aspiration thrombectomy and the duration of index ischemia that is most responsive to PostC (36,37) remain a major limitation.
To date, almost 4000 patients have been randomized in 26 PostC trials (5). Unfortunately, half of the patients were enrolled in just 2 trials (12,13), where cMRI was utilized only selectively in just 20% of the 1700 patients enrolled. Although combining studies through meta-analysis may be helpful to provide insights into clinical mechanisms, that has not been the case in PostC trials as there appears to be too many variables to consider that affect infarct size and myocardial salvage. There are the obvious factors of ischemic time, age, sex, TIMI flow and pre-infarction angina, but there are other factors intrinsic to the procedure to consider such as the PostC algorithm (30 sec vs. 60 sec), the role of direct stenting, aspiration thrombectomy and the timing of the cMRI post-reperfusion for T2 MRI-measurements of AAR. The potential variations in myocardial edema that may occur over several days following reperfusion remains problematic in accurately determining AAR for myocardial salvage measurements (38). Analysis of patient-specific data across trials may provide much greater insight than traditional meta-analyses given all the variables that influence infarct size and myocardial salvage.
Limitations.
Our findings of the influence of PostC on MVO and late remodeling is tempered by the relatively small sample size of the cohort due to a significant number of patients not participating in the 12-month MRI analysis. Unfortunately, this attrition is quite common in studies involving long-term cMRI imaging. For example, in the 2-year Follow-up of the TIME Trial (26), only 85 out of 120 patients complete their 2-year cMRI. Similarly, the SWISS-AMI Trial (34) had only 150 out of 200 patients complete their 1-year cMRIs. Importantly, the subjects in our Trial that underwent long-term cMRI follow-up was very representative of the total cohort in regards to infarct size, LVEF and LV volumes at study entry. However, we acknowledge that the small sample size will increase the risk that our findings were related to chance and thus confirmatory studies will be required. We did not utilize direct stenting (10) in our protocol as it is our practice to first visualize part of the distal vessel with the guidewire and an initial PTCA inflation incorporated into the PostC algorithm. We cannot rule out that the guidewire caused some initial gentle reperfusion that could have slightly abrogated any infarct-sparing effect of the PostC protocol (39).
Conclusions.
Despite our best efforts to minimize confounders that are known to influence infarct size and reperfusion injury, we were unable to demonstrate an acute benefit of PostC in this cohort of STEMI patients from the United States as assessed by cMRI. However, PostC reduced the extent of MVO in the infarct zone and did produce a significant benefit on LV remodeling over time, which potentially could reduce the development of heart failure in STEMI patients. The benefits of PostC on remodeling was particularly noticeable in those subjects with MVO who are at the highest risk of mortality and developing heart failure. Our study suggests that the effectiveness of PostC may not be accurately judged by its acute results and that longer-term imaging and follow-up should be incorporated in future studies, particularly in those patients with MVO.
NOVELTY AND SIGNIFICANCE.
What Is Known?
Modifying reperfusion by postconditioning (postC) may reduce infarct size in the setting of ST-elevation myocardial infarction (STEMI). However, this has not been consistently observed in all clinical trials.
The failure to exclude mitigators of infarct size on enrollment such as pre-infarction angina or collateral blood flow may contribute to these disparate results.
Little clinical data are available that describe the long-term effects of PostC on LV function and volumes.
What New Information Does this Article Contribute?
In comparison with control patients, in 122 STEMI patients, PostC failed to reduce infarct size or improve myocardial salvage as measured by cardiac MRI, 2 days after reperfusion with primary PCI.
At one year, patients randomized to PostC experienced improved LV remodeling in comparison with control patients.
PostC reduced microvascular obstruction (MVO) as a percent of infarct size compared with the control group.
Patients with MVO randomized to PostC demonstrated better recovery of LV function and volumes than control patients with MVO.
PostC may reduce infarct size and improve myocardial salvage at the time of primary percutaneous coronary intervention (PCI) in patients with STEMI. However, clinical trials have shown inconsistent benefit. Using a strict enrollment criteria to optimize the early benefits of PostC, we performed the first NHLBI-sponsored trial of PostC to determine the acute and long-term effects of PostC. We randomized 122 STEMI patients with TIMI 0 flow in a major epicardial artery and ischemic times between 1 and 6 h with no evidence of pre-infarction angina or collateral blood flow. PostC (n=65) failed to reduce infarct size or improve myocardial salvage when measured by cardiac MRI at Day 2 compared with control (n=57). However, at one year of follow-up, patients randomized to PostC experienced less adverse remodeling than patients in the control group, and had significantly reduced MVO as a percent of infarct size. These findings demonstrate that the benefits of PostC may not be manifest early after STEMI and suggest that long-term clinical and imaging follow-up of these patients should be incorporated into future clinical trials. PostC may improve the integrity or function of the microcirculation such that MVO is reduced, which may result in a long-term benefit of this intervention.
SOURCES OF FUNDING
Funded by the National Heart Lung and Blood Institute; RO1 HL103927
Nonstandard Abbreviations and Acronyms:
- cMRI
cardiac magnetic resonance imaging
- IRB
Institutional Review Board
- LAD
left anterior descending
- LVEDV
left ventricular end-diastolic volume
- LVESV
left ventricular end-systolic volume
- LVEF
left ventricular ejection fraction
- MVO
microvascular obstruction
- MSI
myocardial salvage index
- PCI -
percutaneous coronary intervention
- PostC
postconditioning
- STEMI
ST-elevation myocardial infarction
- TIMI
thrombolysis in myocardial infarction
Footnotes
DISCLOSURES
The authors have nothing to disclose
Clinical Trial Registration: NCT01324453.
REFERENCES
- 1.Loh JP, Tan L-L, Zheng H, Lau YH, Chan SP, Tan KB, Chua T, Tan HC, Foo D, Lee CW, Tong KL, Foo LL, Hausenloy D, Sahlen A, Yeo KK, Fox KAA, Wang TY, Richards AM, Chan MY. First medical contact-to-device time and heart failure outcomes among patients undergoing primary percutaneous coronary intervention. Circulation: Quality and Outcomes. 2018;11:e004699. [DOI] [PubMed] [Google Scholar]
- 2.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Post-conditioning inhibits mitochondrial permeability transition. Circulation 2005;111:194–197. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–35. [DOI] [PubMed] [Google Scholar]
- 4.Heusch G and Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 2017;38:774–784. [DOI] [PubMed] [Google Scholar]
- 5.Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post- and remote conditioning. Circ Res 2016;119:676–695. [DOI] [PubMed] [Google Scholar]
- 6.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemic reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G Critical issues for the translation of cardioprotection. Circ Res 2017;120:1477–86. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z-Q, Corvera JS, Halkos ME, Kerendib F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol 2003;285:H579–H588. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 2004;61:372–385. [DOI] [PubMed] [Google Scholar]
- 10.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation 2005;112:2143–2148. [DOI] [PubMed] [Google Scholar]
- 11.Lonborg J, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, Engstrom T. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv 2010;3:34–41. [DOI] [PubMed] [Google Scholar]
- 12.Hahn J-Y, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, Choi SH, Choi JH, Bae JH, An KJ, Park JS, Oh JH, Kim SW, Hwang JY, Ryu JK, Park HS, Lim DS, Gwon HC. Ischemic postconditioning during primary percutaneous coronary intervention: The effects of postconditioning on myocardial reperfusion in patients with ST-segment evelation myocardial infarction (POST) randomized trial. Circulation 2013;128:1889–1896. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Clemmensen P, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Ravkilde J, Tilsted HH, Villadsen A, Aaroe J, Jensen SE, Raungaard B, Botker HE, Terkelsen CJ, Maeng M, Kaltoft A, Krussell LR, Jensen LO, Veien KT, Kofoed KF, Torp-Pedersen C, Kyhl K, Nepper-Christensen L, Treiman M, Vejlstrup N, Ahtarovski K, Lonborg J, Kober L,Third Danish Study of Optimal Acute Treatment with ST Elevation Myocardial Infarction-Ischemic Postconditioning (DANAMI-3-iPOST) Investigators. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol 2017;2:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia S, Henry TD, Wang YL, Chavez IJ, Pedersen WR, Lesser JR, Shroff GR, Moore L, Traverse JH. Long term follow-up of patients undergoing postconditioning during ST-elevation myocardial infarction. J Cardiovasc Transl Res 2011; 4:92–8. [DOI] [PubMed] [Google Scholar]
- 15.Roubille F, Mewton N, Elbaz M, Roth O, Prunier F, Cung TT, Piot C, Roncalli J, Rioufol G, Bonnefoy-Cudraz E, Wiedemann JY, Furber A, Jacquemin L, Willoteaux S, Abi-Kahllil W, Sanchez I, Finet G, Sibellas F, Ranc S, Boussaha I, Croisille P, Ovize M. No postconditioning in the human heart with TIMI flow 2–3 on admission. Eur Heart J 2014;35:1675–1682. [DOI] [PubMed] [Google Scholar]
- 16.Reiter R, Henry TD, Traverse JH. Preinfarction angina reduces infarct size in ST-elevation myocardial infarction treated with percutaneous coronary intervention. Circ Cardiovasc Interv 2013;6:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry TD, Sharkey SW, Burke MN, Chavez IJ, Graham KJ, Henry CR, Lips DL, Madison JD, Menssen KM, Mooney MR, Newell MC, Pedersen WR, Poulose AK, Traverse JH, Unger BT, Wang YL, Larson DM. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007;116:721–8. [DOI] [PubMed] [Google Scholar]
- 18.Traverse JH. The misguided regulation of cardiac emergencies: The rise of the IRB-industrial complex and the increasing risk to cardiovascular research and our patients. Circ Res 2016;119:1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonborg J, Kelbaek H, Vejlstrup N, Botker HE, Kim WY, Holmvang L, Jorgensen E, Helqvist S, Saunamaki K, Thuesen L, Krusell LR, Jensen JS, Korber L, Treiman M, Holst JJ, Engstrom T. Influence of pre-infarction angina, collateral flow, and pre-procedural TIMI flow on myocardial salvage index by cardiac magnetic resonance in patients with ST-segment elevation myocardial infarction. Eur Heart J-Cardiovasc Imag 2012;13:433–443. [DOI] [PubMed] [Google Scholar]
- 20.Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischemic preconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 2015;36:3049–3057. [DOI] [PubMed] [Google Scholar]
- 21.Hahn J-Y, Yu CW, Park HS, Song YB, Kim EK, Lee HJ, Bae JW, Chung WY, Choi SH, Choi JH, Bae JH, An KJ, Park JS, Oh JH, Kim SW, Hwang JY, Ryu JK, Lim DS, Gwon HC. Long-term effects of postconditioning on clinical outcomes: 1 year follow-up of the POST randomized trial. Am Heart J 2015; 169:639–646. [DOI] [PubMed] [Google Scholar]
- 22.Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, Dobrina A, Rossiello R, Silvestri F, Baldi F, Di Sciascio G. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol 2002;34:165–174. [DOI] [PubMed] [Google Scholar]
- 23.Kin H, Wang N-P, Mykytenko J, Reeves J, Deneve J, Jiang R, Zatta AJ, Guyton RA, Vinten-Johansen J, Zhao ZQ. Inhibition of myocardial apoptosis by postconditioning is associated with attenuation of oxidative stress-mediated nuclear factor-kappaB translocation and TNF-alpha release. Shock 2008;29:761–68. [DOI] [PubMed] [Google Scholar]
- 24.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodeling in coronary artery disease and heart failure. Lancet 2014;383:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mewton N, Thibault H, Roubille F, Lairez O, Rioful G, Sportouch C, Sanchez I, Bergerot C, Cung TT, Finet G, Angoulvant D, Revel D, Bonnefoy-Cudraz E, Elbaz M, Piot C, Sahroui I, Croisille P, Ovize M. Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol 2013;108:383–392. [DOI] [PubMed] [Google Scholar]
- 26.Traverse JH, Henry TD, Pepine CJ,Willerson JT, Chugh A, Yang PC, Zhao DXM, Ellis SG, Forder JR, Perin EC, Penn MS, Hatzopoulos AK, Chambers JC, Baran KW, Raveendran G, Gee AP, Taylor DA, Moye’ L, Ebert RF, Simari RD. TIME Trial: Effect of timing of stem cell delivery following ST-elevation myocardial infarction on the recovery of global and regional left ventricular function. Final 2-year analysis. Circ Res 2018;122:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limalanathan S, Andersen GO, Klow NE, Abdelnoor M, Hoffmann P, Eritsland J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI. Results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J Am Heart Assoc. 2014;3:e000679 Doi: 10.1161/JAHA.113.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 2004;62:74–85. [DOI] [PubMed] [Google Scholar]
- 29.Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, De Caralt TM, Betriu A, Masotti M. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J 2012;33:103–12. [DOI] [PubMed] [Google Scholar]
- 30.Sorensson P, Ryden L, Saleh N, Tornvall P, Arheden H, Pernow J. Long-term impact of postconditioning on infarct size and left ventricular ejection fraction in patients with ST-elevation myocardial infarction. BMC Cardiovascular Disorders 2013;13:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systemic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Zhang X, Li C, Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function and LV recovery after myocardial infarction. J Int Cardiol. 2006;5:367–375. [DOI] [PubMed] [Google Scholar]
- 33.Heusch G The coronary circulation as a target of cardioprotection. Circ Res 2016;118:1643–1658. [DOI] [PubMed] [Google Scholar]
- 34.Surder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, Soncin S, Turchetto L, Radrizzani M, Zuber M, Windecker S, Moschovitis A, Buhler I, Erne P, Luscher TF, Corti R Effect of bone marrow-derived mononuclear cell treratment, early or late after acute myocardial infarction: Twelve months CMR and long-term clinical results. Circ Res 2016;119:481–90. [DOI] [PubMed] [Google Scholar]
- 35.Touboul C, Angoulvant D, Mewton N, Ivanes F, Muntean D, Prunier F, Ovize M, Bejan-Angoulvant T. Ischemic postconditioning reduces infarct size: systematic review and meta-analysis of randomized controlled trials. Arch Cardiovasc Dis 2015; 108:39–49. [DOI] [PubMed] [Google Scholar]
- 36.Azzalini L, Millan X, Ly HQ, L’Allier PL, Jolicoeur EM. Direct stenting versus pre-dilation in ST-elevation myocardial infarction: a systemic review and Meta-analysis. Journal of Interventional Cardiology 2015;28:119–131. [DOI] [PubMed] [Google Scholar]
- 37.Manintveld OC, Te Lintel Hekkert M, van den Bos EJ, Suurenbroek GM, Dekkers DH, Verdouw PD, Lamers JM, Duncker DJ. Cardiac effects of postconditioning depend critically on the duration of index ischemia. Am J Physiol Heart Circ Physiol 2007;292:H1551–H1560. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Jimenez R, Barreiro-Perez M, Martin-Garcia A, Sanchez-Gonzalez J, Aguero J, Galan-Arriola C, Garcia-Prieto J, Diaz-Pelaez E, Vara P, Marinez I Zamarro I, Garde B, Sanz J, Fuster V, Sanchez PL, Ibanez B. Dynamic edematous response of the human heart to myocardial infarction: implications for assessing myocardial area at risk and salvage. Circulation 2017;136:1288–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musiolik J, van Caster P, Skyschally A, Boengler K, Gres P, Schulz R, Heusch G. Reduction of infarct size by gentle reperfusion without activation of reperfusion injury salvage kinases in pigs. Cardiovascular Res 2010;85:110–117. [DOI] [PubMed] [Google Scholar]