Abstract
D-Amino acids are important biological molecules. Improved analytical methods for their resolution and quantification remain of keen interest. In this study, we investigated the use of Marfey’s reagent (chiral) derivatization coupled with LC-MS/MS-based separation and detection of the resulting diastereomers for quantification of the 19 common l- and d-amino acids and glycine. Standard formic acid (pH 2) based separations on reverse phase media were unable to separate all 19 amino acid DL pairs. In contrast, a water/acetonitrile/ammonium acetate (pH 6.5) solvent system allowed all 19 amino acid DL pairs to be chromatographically resolved on a 30 min gradient, with negative mode detection at pH 6.5 giving good sensitivity. Derivatization reaction rates between amino acids varied substantially, with overnight derivatization required for some amino acids. Chromatography at pH 6.5 combined with MS/MS quantification in negative mode demonstrated good linearity over a wide concentration range for all 20 amino acids. Matrix effects, assessed with an MRSA extract, were negligible. Marfey’s derivatized analytes were stable for 24 hrs at room temperature. This method was demonstrated by determining the levels of these analytes in mid-log phase MRSA extracts. This approach provides for the chromatographic resolution and MS/MS-based quantification of all 20 common l- and d-amino acids in complex matrices.
Keywords: Amino acid, Marfey’s reagent, chiral separation, LC-MS/MS
Graphical Abstract
Introduction
There are 20 proteinogenic amino acids (AAs), which are incorporated as their L-enantiomers (except for glycine, which is achiral) in proteins. Their corresponding D-enantiomers also play important roles in many biological systems and processes [1]. The most important D-amino acids in humans are D-Ala and D-Ser. D-Ser is found in the brain [2], where it serves as a co-agonist of the N-methyl-D-aspartate (NMDA) receptor [3, 4]. It has been implicated in a number of diseases, with increased levels observed in Alzheimer’s [5, 6] and amyotrophic lateral sclerosis [7] and with decreased levels observed in schizophrenia [8, 9]. A number of other D-amino acids, including DAsp, D-Cys, D-Leu, D-Pro and D-Glu, have been found in rat brain and pituitary glands [10, 11]. D-Amino acids associated with other diseases include increased D-Ser, D-Pro, and D-Asn with kidney disease and diabetes mellitus [12] increased D-Ala with restricted feeding in rats [13] and decreased D-Glu in heart disease [14].
In food products, the presence of D-Ala in milk can indicate microbial contamination, and DPro in wines and vinegar can be an indicator of age. Fruits, fruit juices, and fermented milk products also contain various D-amino acids including D-Ala, D-Asp, D-Glu, and D-Arg [1]. D-amino acids, including D-Ala, D-Glu, D-Asn and D-Asp, are important components of bacterial cell wall peptidoglycan [15–17]. D-Amino acids also play regulatory roles in bacterial peptidoglycan remodeling, and biofilm and spore formation [18–21]. Given the importance of amino acid chirality to the existence of life on earth, and that amino acids racemize at very slow rates, chiral amino acid analysis is also used to study fossils, archeological artifacts, and meteorites [22, 23].
Given their biological significance, there has been considerable interest in the development and application of analytical methods for D-amino acid quantification (reviewed in [24–26]). Regardless of detection method, DL-amino acid enantiomers must be separated chromatographically to allow separate quantification. Several approaches to this problem have been developed. One approach is to use chiral stationary phases to separate DL-amino acid enantiomers [27]. GC-based analyses using this approach are well established (reviewed in [28]). The alternative approach is to derivatize amino acids with a chiral derivatizing agent, followed by separation of the resulting diastereomers on standard achiral chromatography media [29, 30]
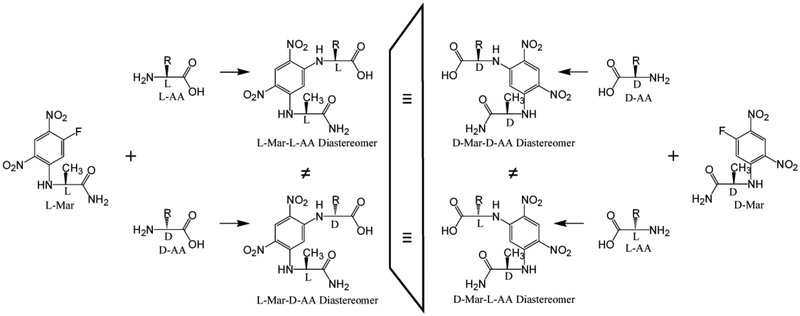
Marfey’s reagent, 1-fluoro-2,4-dinitrophenyl-5-L-alanine amide (L-Mar) is a popular chiral amino acid derivatizing agent [31] (reviewed in [24, 32]) (Fig. 1).
Figure 1.
Reaction scheme of DL-amino acids with l- and d-Marfey’s reagent.
This reagent provides good separation of most DL-amino acid enantiomers as their L-Mar derivative diastereomers on standard reverse phase media with acidic solvent systems. In prior studies using such an approach, we have used Marfey’s reagent and this general approach for the LC-MS/MS based quantification of several key intermediates in the bacterial cell wall peptidoglycan biosynthesis pathway, including D-Ala, L-Ala, D-Ala-D-Ala [33], D-Glu and L-Glu [34], and D-Ala-D-Lac [35, 36]. Marfey’s reagent derivatization combined with multichannel LCMS/MS detection has also been demonstrated for the resolution and quantification of all 8 aminobutyric acid isomers [37], demonstrating the potential of Marfey’s reagent combined with data intensive MS/MS detection and spectral deconvolution for the analysis of complex analyte mixtures.
The present study aims to evaluate and optimize the use of Marfey’s reagent combined with MS/MS detection for the separation and quantification of Gly and the 19 proteinogenic DL-amino acids. This study demonstrates that separation under neutral solvent conditions using negative mode MS/MS detection is an effective approach. Derivatization kinetics for amino acids that can form bis-Marfey’s adducts (Tyr and His) is the principal complicating factor for this approach.
Materials and Methods
General.
Amino acids and amino acid standard mixtures were purchased from Sigma-Aldrich (St. Louis, MO). L-Marfey’s reagent (1-fluoro-2,4-dinitrophenyl-5-L-alanine amide) and DMarfey’s reagent (1-fluoro-2,4-dinitrophenyl-5-D-alanine amide) were purchased from Novobiochem (a division of EMD Chemicals, Gibbstown, NJ). C18 silica gel was obtained from Sep-Pak Cartridges from Waters (Milford, MA). Other reagents were obtained from standard sources and were reagent grade or better. LC-MS/MS was performed on an AB Sciex 3200 QTrap mass spectrometer (Foster City, CA) coupled to a Shimadzu UFLC system (Columbia, MD) using electrospray ionization (ESI) and run with Analyst v.1.4.2 software.
Marfey’s derivative preparation for method development.
An equimolar standard mixture of all 20 L-amino acids was prepared by mixing 400 μL of a 2.5 mM L-amino acid standard solution (Cat. # AAS18) from Sigma-Aldrich (which contains 17 amino acids each at 2.5 mM) with 1300 μL water, and 100 μL each of 10 mM stock solutions of L-Gln, L-Asn and L-Trp. This provided a 2 mL solution with each amino acid at 0.5 mM. To a 100 μL aliquot of this mixture was added 200 μL of 20 mM L-Marfey’s reagent in acetone, followed by 50 μL of 0.5 M triethylamine (TEA) to initiate the reaction. The contents were mixed and kept in the dark at 37°C overnight. The derivatization reaction was quenched by adding 50 μL of 0.5 M HCl, and the sample diluted to 1000 μL with 20% acetonitrile/0.1% formic acid. Marfey’s adducts of individual amino acids, particularly for isobaric Leu and Ile, were prepared similarly. A parallel effort was performed using D-Marfey’s reagent in place of L-Marfey’s reagent, which provided D-Mar-L-AA derivatives as surrogates (physically identical) to L-Mar-D-AA derivatives (Fig. 1).
Marfey’s derivative purification for method development.
To obtain purified desalted samples of individual Mar-AA isomers for MS/MS method optimization, 200 μL samples of the standard mixtures (L-Mar-L-AAs or D-Mar-L-AAs) were fractionated by semi-preparative HPLC on a 4.6 × 250 mm Nucleodur C8 column. (Flow rate of 1 mL/min using a gradient of 5% to 90% of solvent B (30% water/70% acetonitrile/0.1% formic acid) in solvent A (water/0.1% formic acid) over 90 min.) Alternatively, individual Marfey’s reagent derivatized amino acids were cartridge purified over C18-silica to provide desalted samples, as described previously [38].
LC-MS/MS method development.
Initial efforts were based on the use of standard water/acetonitrile/formic acid gradients with MS/MS detection in positive mode. MS/MS detection was optimized for individual Mar-amino acids by infusion of purified desalted fractions into the mass spectrometer and using the Analyst quantitative optimization wizard. For each analyte, the three most intense fragments (Q3) were used in building the final MS/MS method (Table S1). For HPLC method development, a mixture of all 20 l- and d-Mar derivatized L-amino acids was used. The optimized acidic pH solvent system consisted of solvent A (water/0.1 % formic acid) and solvent B (30% water/70% acetonitrile/0.1% formic acid). The optimized gradient was 10–40% B (0–1 min), 40–80% B (1–21 min), 80–100% B (21–22 min), 100–10% B (22–23 min). However, using this gradient, or even shallower gradients, only 15 of 19 Mar-DL-amino acid pairs could be adequately resolved (Fig. S1).
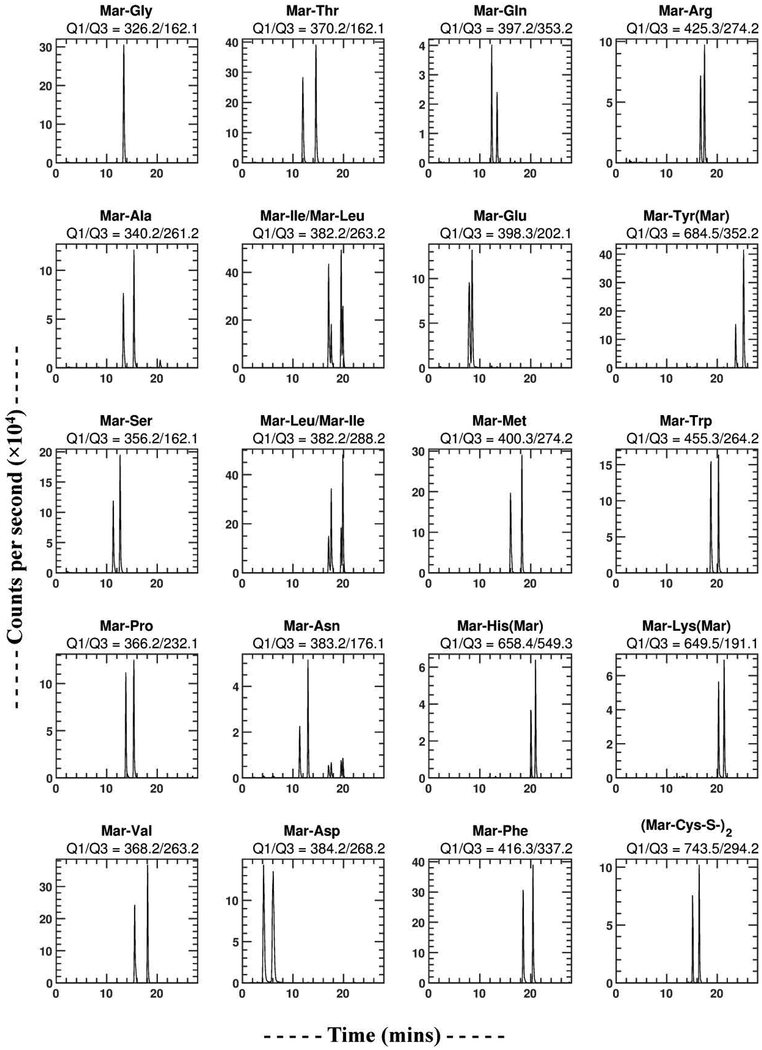
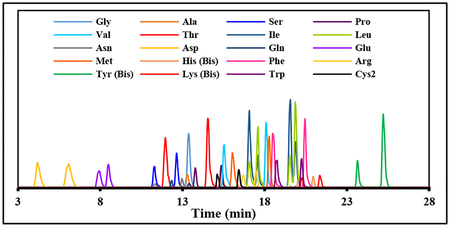
A neutral pH gradient was then tested to see if it could provide improved resolution. This neutral gradient consisted of solvent A (10 mM ammonium acetate pH 6.5 in water) and solvent B (30% A/70% acetonitrile). The optimized neutral pH gradient was 5% B (0–1 min), 5–55% B (1–21 min), 55–100% B (21–22 min), and 100–5% B (22–23 min). This neutral pH gradient was able to separate all enantiomeric Mar-DL-amino acid pairs. However, low sensitivity was observed for these analytes with positive mode MS/MS detection at neutral pH. All Mar-DL-amino acids were then optimized for negative mode MS/MS detection at pH 6.5, and the three most sensitive Q3 fragments for each amino acid (Table S2) used to build a final neutral pH/negative mode detected LC-MS/MS method (Fig. 2).
Figure 2.
LC-MS/MS chromatograms of Marfey’s derivatives of the 20 common DL-amino acids using the neutral (ammonium acetate) pH 6.5 based solvent system on a C8 column and MS/MS detection in negative mode. Each panel represents a single Q1/Q3 channel, and is associated with a particular Mar-AA. Since Leu and Ile have the same molecular weights (isobars), their Marfey’s derivatives both show up in the two Q1=382.2 panels. The Q1/Q3 = 382.2/263.2 channel is selective for Ile (two higher peaks in the Mar-Ile/Mar-Leu panel), and the Q1/Q3 = 382.2/288.2 channel is selective for Leu (two higher peaks in the Mar-Leu/Mar-Ile panel). Note that Mar-Leu/Ile also shows some signal intensity in the Mar-Asn MS/MS channel due to the Mar-Leu/Ile M+1 isotopomers, but since there is good chromatographic resolution of all these L-Mar-DL-AA species they can be individually quantified.
Reaction kinetics of Marfey’s derivatization reactions.
Preliminary studies indicated that most amino acids reacted with Marfey’s reagents relatively quickly, but some – particularly threonine, reacted slowly. Also, some amino acids (His, Tyr, Lys, and Cys2) (Cys2; the S-S linked Cys dimer) can form bis-Marfey’s adducts, and their derivatization kinetics to mono- and bis-adducts needed to be determined to allow proper design of quantitative derivatization conditions. Marfey’s reagent derivatization reaction kinetics were therefore assessed. To 200 μL of the standard amino acid mix of all 20 L-AAs at 0.5 mM was added 400 μL of 20 mM of l- or DMarfey’s reagent in acetone, followed by 100 μL of 0.5 M TEA in water. Samples of 20 μL were removed at different times and quenched with 180 μL 20% acetonitrile/0.1% formic acid. These samples were then analyzed by neutral pH/negative mode LC-MS/MS. Except for His and Tyr, all amino acids underwent derivatization following apparent first order kinetics (including Lys and Cys2). Under the conditions of excess Marfey’s reagent in reaction mixtures (~4-fold), as used in this study, the reactions will be pseudo-first order. For those species which followed apparent first order derivatization kinetics (in the presence of excess Marfey’s reagent), their derivatization kinetics were analyzed by fitting to first order kinetic expressions with the following equations in SPSS.
A simple first order reaction is described by:
| (1) |
A represents the underivatized amino acid, and B represents the derivatized product. The time dependence for the formation of the product is given by:
| (2) |
Where A0 indicates the initial concentration of A, k is the rate constant, t is the time of reaction, and Bt is the concentration of the reaction product B at time t. The observed area for the MS/MS detected Marfey’s adduct is equal to Bt times the sensitivity factor for its MS/MS detection in area units/fmol (AU/fmol), which gives the equation used for fitting the data:
| (3) |
Where SensB is the sensitivity constant for the detection of the reaction product B, and AUB is the detected area units for B. The results of this analysis for those amino acids showing apparent first order kinetics are summarized in Table 2.
Table 2.
Reaction rate constants for 18 amino acids that show apparent first order rection kinetics a
| l-AA | d-AA | |||||
|---|---|---|---|---|---|---|
| l-Mar-AA | k (hr−1) | 4×t1/2 (hr)b | k (hr−1) | 4×t1/2 (hr)b | Max (4×t1/2) (hr)b | Log2 (kd/kl) |
| Gly | 2.92 | 0.95 | 0.95 | |||
| Ala | 0.99 | 2.81 | 1.40 | 1.98 | 2.81 | 0.50 |
| Ser | 0.46 | 6.02 | 0.90 | 3.08 | 6.02 | 0.97 |
| Pro | 40 | 0.29 | 22 | 0.12 | 0.29 | −0.86 |
| Val | 1.90 | 1.46 | 2.92 | 0.95 | 1.46 | 0.62 |
| Thr | 0.17 | 16.31 | 0.29 | 9.56 | 16.72 | 0.77 |
| Ile | 2.55 | 1.09 | 3.01 | 0.92 | 1.09 | 0.24 |
| Leu | 1.88 | 1.48 | 2.05 | 1.35 | 1.48 | 0.12 |
| Asn | 0.84 | 3.30 | 0.92 | 3.02 | 9.86 | 0.13 |
| Asp | 0.31 | 8.94 | 0.51 | 5.44 | 8.97 | 0.72 |
| Gln | 1.11 | 2.49 | 2.11 | 1.32 | 2.49 | 0.93 |
| Glu | 0.36 | 7.70 | 0.56 | 4.95 | 7.69 | 0.64 |
| Met | 1.27 | 2.18 | 1.35 | 2.05 | 2.18 | 0.09 |
| Phe | 4.41 | 0.63 | 3.03 | 0.92 | 0.92 | −0.54 |
| Arg | 1.25 | 2.22 | 1.49 | 1.86 | 2.9 | 0.25 |
| Trp | 7.35 | 0.38 | 4.49 | 0.62 | 0.62 | −0.71 |
| Lys (Bis) | 0.93 | 2.98 | 0.92 | 3.01 | 3.02 | −0.02 |
| Cys2 (Bis) | 0.78 | 3.56 | 0.61 | 4.55 | 4.57 | −0.35 |
For Tyr and His, which show multistep kinetics, see Figure 4.
4×t1/2 is used as indicator of near quantitative (95%) completion of the derivatization reaction.
His and Tyr demonstrated more complex two-step reaction kinetics due to the sequential formation of mono- and bis-Marfey’s derivatives. For the following two-step sequential reaction:
| (4) |
The time dependence of the disappearance of A is described by:
| (5) |
The time dependence of B1, the mono-Marfey’s adduct, is described by the following integrated rate equation [39]:
| (6) |
The observed MS/MS signal for B1 (the mono-Marfey’s adduct) is equal to B1,t × SensB1, where SensB1 is the sensitivity factor for B1 (AU/fmol). The expression for B2, the bis-Marfey’s adduct, is:
| (7) |
The observed MS/MS signal for B2 (the bis-Marfey’s adduct) is equal to B2,t × SensB2, where SensB2 is the MS/MS sensitivity factor for B2 (AU/fmol).
Analytical derivatization procedure.
For analytical sample preparation, 40 μL of 20 mM Marfey’s reagent was added to 20 μL of sample spiked to 100 μM with 13C3-D-Ala as internal standard, followed by 10 μL of 0.5 M TEA. Reaction mixtures were incubated at 37°C for 24 hours, then quenched with 10 μL 0.5 M HCl, and diluted to 200 μL with 20% acetonitrile/0.1% formic acid. For unknown samples, UV-vis (340 nm) monitored HPLC is used to confirm a substantial (at least 3–4 fold) excess of Marfey’s reagent. If not then samples are diluted prior to derivatization.
Analytical method sensitivity and linearity.
To check the sensitivity and linearity of the developed method, a 13 step serial dilution (steps of two) of the 20 L-amino acid mixture was prepared in triplicate in 0.1 N HCl with 100 μM 13C3-D-Ala as internal standard. These serially diluted samples were derivatized with 20 mM Marfey’s reagent as described above, and LCMS/MS analysis performed on 20 μL injections using both the acidic gradient/positive mode detection method, and the neutral pH/negative mode detection method. For each sample, Marfey’s reagent was at least 3-fold in excess of amino acids.
Matrix preparation.
MRSA extract was prepared as described previously using the centrifugation approach from 100 mL of culture [34]. The collected methanol extract was dried under vacuum, and resuspended in 5 mL of water+0.1% formic acid to make a stock matrix solution. A 1:25 fold dilution of this stock solution was made in water+0.1% formic acid to prepare a working matrix solution.
Matrix effect assessment and determination of DL-amino acid levels in MRSA extract.
Matrix effects on analyte quantification were assessed by diluting L-amino acids standards in water, and in matrix, at levels corresponding to 0, 25, 100, and 400 pmol of injected analyte. These samples were derivatized with L-Marfey’s reagent and analyzed by LC-MS/MS using the negative mode/neutral solvent system protocol described above in quadruplicate. Matrix effect for each analyte was calculated from the slopes (S) of the linear regression curves as:
| (8) |
Results and Discussion
Use of D-Mar to provide L-Mar-D-AA standard surrogates.
The goal of this study was to develop a Marfey’s derivatization-based LC-MS/MS analytical approach for the resolution and quantification of the 19 common l- and d-amino acids and Gly. While validated L-amino acid standards are commercially available, similar D-amino acids standards are not. The standard version of Marfey’s reagent is based on L-alanine amide (L-Ala-NH2) (L-Mar). Its D-Ala-NH2 homolog is also available (D-Mar). The approach used in this study was to derivatize an L-AA standard mix with L-Mar to provide an L-Mar-L-AA standard mix, and with D-Mar to provide a DMar-L-AA standard mix. Since a D-Mar-L-AA isomer is the mirror image of an L-Mar-D-AA isomer (Fig. 1) – and therefore physically identical on achiral chromatography media and by MS/MS detection – D-Mar-L-AA standards were used as surrogates for L-Mar-D-AA standards.
LC-MS/MS method development.
Our initial effort focused on developing an LC-MS/MS method using a standard acidic (formic acid based) solvent system and MS/MS detection in positive mode (Fig. S1, Table S1). However, 5 DL-amino acid pairs – Ser, Asn, Gln, His, and Arg – could not be adequately resolved chromatographically using this acidic solvent system. Longer gradients, and incorporation of methanol or isopropanol into the solvent system, also failed to allow their resolution. We then tested HPLC separation using a neutral (pH 6.5) solvent system, which allowed for separation of all DL-amino acid pairs. However, positive mode detection at pH 6.5 was poor, especially for early eluting amino acids (Asp, Glu, Ser). MS/MS optimization for negative mode detection was then performed (Table S2). Negative mode detection with HPLC separation at pH 6.5, provided a good method for the resolution and quantification all 20 proteinogenic DL-amino acids (Fig. 2, Table 1).
Table 1.
Compound dependent parameters and LC-MS/MS characteristics for the most intense MS/MS transitions of the 20 common amino acids in negative mode detectiona.
| tR (min)c | AU/fmol | LLOQ (pmol) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| l-Mar-AAb | Q1 | Q3 | DP (V) | EP (V) | CEP (V) | CE (V) | l-AA | d-AA | RSd | l-AA | d-AA | l-AA | d-AA |
| Gly | 326.2 | 162.1 | −35 | −4.5 | −22 | −28 | 13.38 | 4.62 | 2.7 | ||||
| Ala | 340.2 | 261.2 | −45 | −4 | −18 | −22 | 13.30 | 15.39 | 1.82 | 3.1 | 3.98 | 7.7 | 6.1 |
| Ser | 356.2 | 162.1 | −45 | −5.5 | −18 | −42 | 11.30 | 12.65 | 1.20 | 2.7 | 3.84 | 3.6 | 2.5 |
| Pro | 366.2 | 232.1 | −30 | −6.5 | −18 | −26 | 13.78 | 15.37 | 1.46 | 3.1 | 3.94 | 3.8 | 3.1 |
| Val | 368.2 | 263.2 | −40 | −6.5 | −18 | −18 | 15.53 | 18.09 | 1.82 | 8.8 | 10.98 | 2.3 | 1.9 |
| Thr | 370.2 | 162.1 | −40 | −7 | −16 | −38 | 11.96 | 14.56 | 2.44 | 8.18 | 9.65 | 3.3 | 2.7 |
| Ile | 382.2 | 263.2 | −40 | −3.5 | −16 | −18 | 17.07 | 19.56 | 4.42 | 13.79 | 15.6 | 2.8 | 2.4 |
| Leu | 382.2 | 288.2 | −40 | −3.5 | −16 | −28 | 17.59 | 19.87 | 2.98 | 11.1 | 14.58 | 3.8 | 2.9 |
| Asn | 383.2 | 176.1 | −55 | −7.5 | −14 | −34 | 11.35 | 12.98 | 1.62 | 0.65 | 0.66 | 5.6 | 5.4 |
| Asp | 384.2 | 268.2 | −50 | −3.5 | −54 | −28 | 4.19 | 6.09 | 1.50 | 1.045 | 1.45 | 6.7 | 4.7 |
| Gln | 397.2 | 353.2 | −70 | −6.5 | −20 | −18 | 12.35 | 13.40 | 1.21 | 1.46 | 2.38 | 4.5 | 2.8 |
| Glu | 398.3 | 202.1 | −50 | −5 | −18 | −38 | 7.94 | 8.50 | 0.82 | 2.28 | 2.91 | 7.1 | 5.5 |
| Met | 400.3 | 274.2 | −40 | −6 | −16 | −24 | 16.04 | 18.27 | 2.01 | 9.68 | 12.99 | 3.2 | 2.4 |
| His (Bis) | 658.4 | 549.3 | −90 | −7.5 | −22 | −30 | 20.04 | 20.95 | 1.00 | 14.48 | 20.67 | 5.5 | 7.9 |
| Phe | 416.3 | 337.2 | −45 | −5 | −18 | −20 | 18.50 | 20.45 | 1.79 | 2.25 | 4.5 | 3.3 | 2.2 |
| Arg | 425.3 | 274.2 | −60 | −5 | −16 | −26 | 16.70 | 17.48 | 0.92 | 9.15 | 18.6 | 3.5 | 1.7 |
| Tyr (Bis) | 684.4 | 352.2 | −80 | −8.5 | −26 | −38 | 23.65 | 25.21 | 1.52 | 9.64 | 14.92 | 5.7 | 2.8 |
| Trp | 455.3 | 264.2 | −55 | −7.5 | −20 | −18 | 18.75 | 20.25 | 1.52 | 2.03 | 3.38 | 3.3 | 2.1 |
| Lys (Bis) | 649.5 | 191.1 | −85 | −7.5 | −24 | −58 | 20.27 | 21.36 | 1.04 | 3.14 | 6.48 | 3.5 | 2.1 |
| Cys2 (Bis) | 743.5 | 294.2 | −70 | −4.5 | −34 | −40 | 15.12 | 16.43 | 1.33 | 3.1 | 3.98 | 4.1 | 1.9 |
Global method parameters were: TEM (source temperature) − 300°C; IS (ion spray voltage) – −4500 V; GS1 & GS2 (gas flows) – 50 (arbitrary units); CAD - Medium.
All AAs listed were detected as their mono-Marfey’s adducts except for those indicated as bis-adducts AA(Mar). Cys2 was also detected as its bis-adduct.
The average %CV for retention times was 0.12%.
The average %CV for resolution was 2.4%.
Ile and Leu are isobaric (identical molecular weight) amino acids with similar structures. Their Marfey’s derivatives have identical Q1 masses, and give similar fragmentation patterns, resulting in both Ile and Leu showing up in each other’s MS/MS chromatograms (Fig. 2 and S1). The sequence of elution in the both the neutral (Table 1) and acidic (Table S2) solvent systems was L Ile, L-Leu, D-Ile, D-Leu (Fig. 2), as determined using individual standards of each. Two different Q3 masses selected in negative mode (263.2 and 288.2) provide some selectivity for Ile and Leu respectively. The M+1 isotopomers of Mar-Ile/Leu also showed up in the Mar-Asn channel. However, all of these L-Mar-DL-AAs were well resolved chromatographically (Fig. 2), which allows all of these amino acid isomers to be individually quantified. DL-Glu was the least well resolved of all the DL-amino acids, but sufficiently well resolved for individual isomer quantification as long as their ratio is not too extreme.
Marfey’s derivatization reaction kinetics.
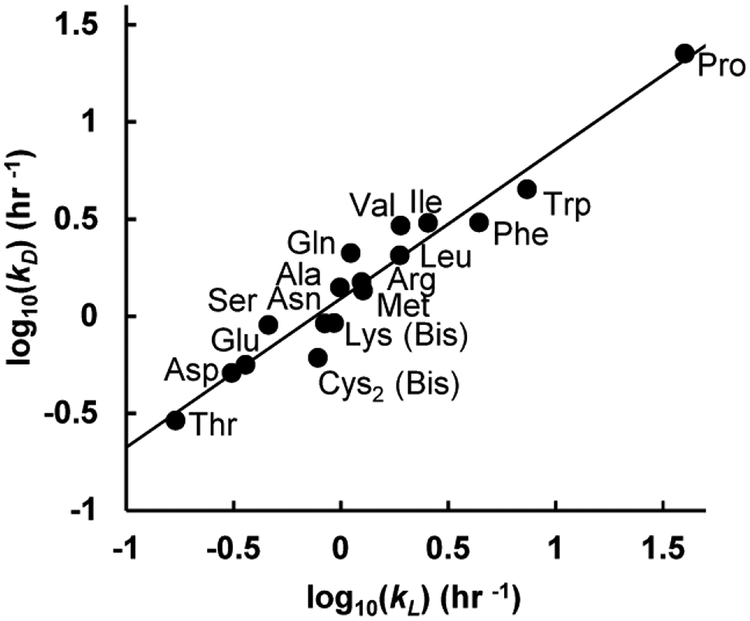
It was evident in preliminary studies that some amino acids react with Marfey’s reagent slowly, or with complex kinetics because of their ability to form both mono- and bis-adducts (e.g. Tyr, His, Lys, and Cys2). The kinetics of derivatization of all 19 DL-amino acids and Gly were therefore analyzed (Table 2), including for bis-adducts for those capable of bis-adduct formation (Fig. 3).
Figure 3.
Reaction rate constants for and correlation between d- and l-amino acids.
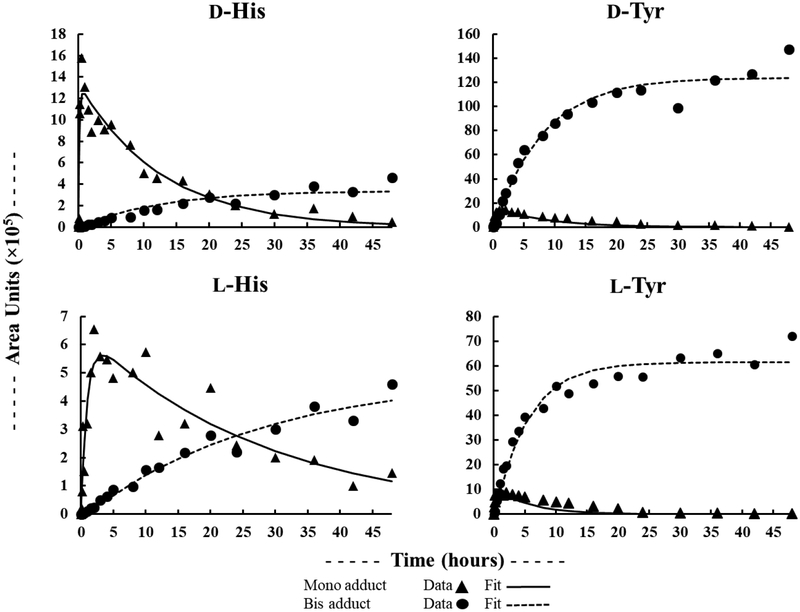
For the majority of mono-adduct forming amino acids, reaction kinetics were rapid under the conditions used here (Table 2). (4×t½ is used to indicate an incubation time for 95% completion of the derivatization reaction.) Pro gave the fastest Marfey’s derivatization reaction kinetics, with a 4×t½ of 30 min. The reaction kinetics of some amino acids were relatively slow, with L-Thr being the slowest reacting of the simple amino acids with a 4×t½ of 17 hrs – likely due to the electron withdrawing effect of the adjacent–OH group. (Gly, not included in this figure, reacted with a rate similar to D-Ile (Table 2).) Given that the reaction of Marfey’s reagent with a given DL-amino acid pair gives different diastereomeric products, and will therefore proceed through different transition-states, there was some potential for different reaction rates for Marfey’s derivatization of d- vs. l-amino acids. A strong correlation between l- and d-AA reaction kinetics was observed (Fig. 3), with D-amino acids reacting only slightly faster than L-amino acids. For bis-adduct forming amino acids, both Lys and Cys2 formed their corresponding bis-adducts quickly following apparent first order reaction kinetics. In contrast, His and Tyr both demonstrated clear two-step reaction kinetics via the mono-Marfey’s derivative to the ultimate bis-Marfey’s derivatives (Fig. 4). For both His and Tyr, formation of bis-adducts was much slower than for mono-adducts.
Figure 4.
Reaction kinetics of l-Mar with l- and d-His, and l- and d-Tyr.
In consideration of these reaction kinetics, a 3 hr incubation would be sufficient for quantitative analysis (>95% conversion) for many DL-amino acid pairs, with the exception of Thr, Asn, Asp, Glu, Cys2, His, and Tyr. A 24 hr incubation would be sufficient for all amino acids except His. DLHis can be determined by using a very long incubation of >78 hrs for formation and quantification of the bis-adduct, or by using isotopically labeled His internal standards with quantification of either the mono- or bis-adduct, or as its mono-adduct using the midpoint time between the mono-adduct maximum for d- and l-His (80 min).
Linearity and sensitivity characteristics.
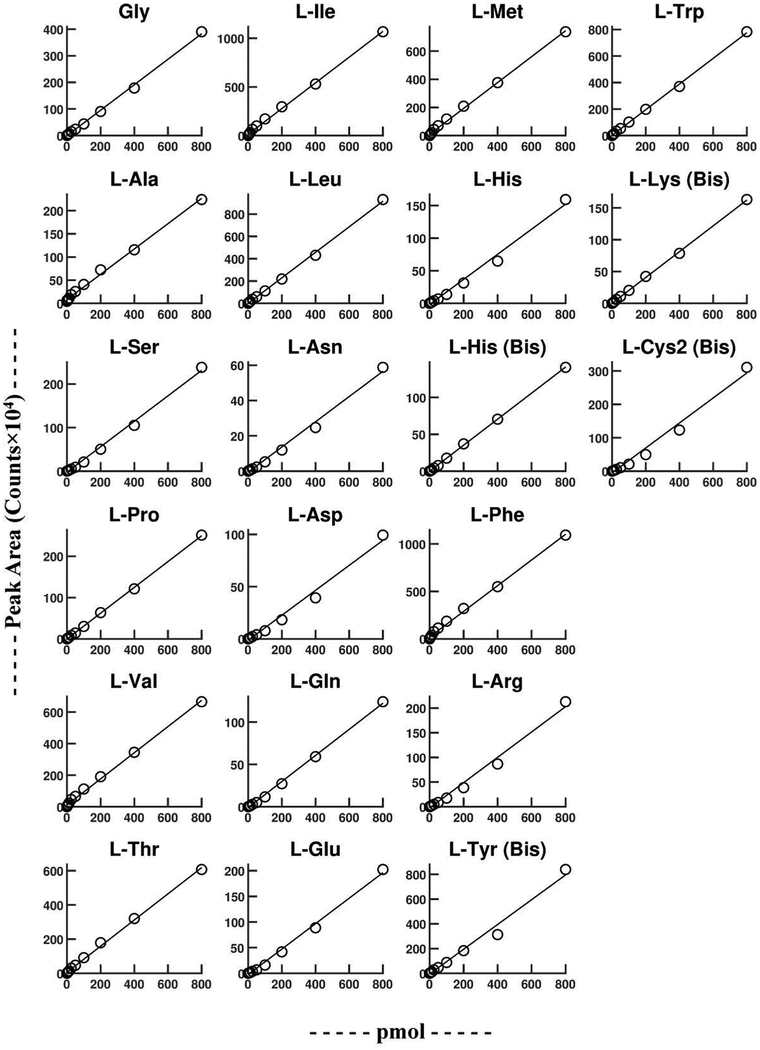
To assess linearity, a serially diluted L-amino acid standard mix in water was derivatized with L-Mar. These samples were then analyzed using both the acidic gradient/positive mode detection method (Fig. S2, Table S3), and the neutral gradient/negative mode detection method (Fig. 5, Table 3). The neutral gradient/negative mode detection method provided good linearity up to 800 pmol (the highest level tested) for all 20 amino acids. Sensitivity fell off slightly at higher analyte concentrations in the acidic gradient/positive mode detection method (Fig. S2, Table S3).
Figure 5.
Linearity of L-Mar-L-AA derivatives using the neutral solvent system and negative mode MS/MS detection.
Table 3.
Linearity (0–800 pmol), matrix effect analysis, and 24 hr stability using the neutral solvent system (pH 6.5) and negative mode MS/MS detection.
| l-Mar-l-AA | Linearity (R2) | Matrix Effect (%ME) | 24 hr Stability (± SE) |
|---|---|---|---|
| Gly | 0.998 | −2.2 | 95.6 ± 6.4 |
| Ala | 0.996 | −7.9 | 96.5 ± 3.7 |
| Ser | 0.996 | −2.8 | 100.8 ± 4.7 |
| Pro | 0.999 | −6.1 | 103.6 ± 2.8 |
| Val | 0.997 | −2.6 | 97.9 ± 3.2 |
| Thr | 0.998 | −3.7 | 96.8 ± 4.0 |
| Ile | 0.998 | −4.3 | 93.7 ± 2.4 |
| Leu | 0.999 | 5.4 | 98.0 ± 1.3 |
| Asn | 0.993 | 2.2 | 91.2 ± 8.3 |
| Asp | 0.997 | −6.0 | 98.7 ± 5.4 |
| Gln | 0.998 | 0.3 | 98.7 ± 5.9 |
| Glu | 0.995 | 5.6 | 107.9 ± 2.9 |
| Met | 0.998 | −4.7 | 100.7 ± 1.7 |
| His (Bis) | 0.999 | −8.4 | 102.6 ± 4.8 |
| Phe | 0.996 | −0.3 | 93.5 ± 3.5 |
| Arg | 0.996 | 2.0 | 101.2 ± 2.5 |
| Tyr (Bis) | 0.994 | −7.2 | 102.1 ± 3.0 |
| Trp | 0.999 | −8.4 | 94.6 ± 2.0 |
| Lys (Bis) | 0.999 | −4.9 | 103.8 ± 3.1 |
| Cys2 (Bis) | 0.988 | −1.6 | 105.2 ± 1.6 |
13C3-D-Ala was used as an internal standard in this study. A lag in response to low analyte levels in the absence of 13C3-D-Ala (data not shown) was observed in both the neutral gradient/negative mode detection method and acidic gradient/positive mode detection method, which was not observed in the presence of 13C3-D-Ala. This lag is likely due to the presence of traces of 1,5-difluoro-2,4-dinitrobenzene (the precursor to Marfey’ reagent [31]) in commercial Marfey’s reagent, which can react with amino acids to give undetectable (in our MRM methods) derivatives. Inclusion of 13C3-D-Ala (or any other suitable unnatural amino acid) as an internal standard competes for reaction with this impurity and restores linearity. The neutral gradient/negative mode detection method was more sensitive (lower LLOQ) than the acidic gradient/positive mode detection method (Table 1 and Table S1). These observations clearly establish the neutral gradient with negative mode detection approach as optimal for these analytes.
Assessment of matrix and storage effects.
Our own area of interest is primarily in the area of bacterial cell wall biosynthesis, which involves several D-amino acids [33–36, 40]. To determine the effect of matrix on the analysis of DL-amino acid levels in bacterial extracts, a pool of bacterial extract was prepared from a 100 mL MRSA culture. Quantification in triplicate of serially diluted amino acids in water vs matrix demonstrated that matrix effects were minimal, with an average %ME of −2.8 ± 4.4 (SD) (Table 3). Samples rerun after 24 hrs at room temperature showed negligible changes in analyte levels (Table 3). After storage at −20 oC for 30 days, most amino acids were at >85% of the originally measure level except for Met (84%), Cys2 (60%), and Tyr (Bis) (13%).
Analysis of DL-amino acid levels in MRSA.
This method was then applied to the determination of these analytes in MRSA (Table 4). Several of these amino acids (d- and l-Ala, d- and l-Glu, l-Lys) have been measured previously in MRSA using an acidic solvent system with positive mode detection [34, 36], with results consistent with those reported in Table 4. L-Pro and L-Glu were the two most abundant amino acids with concentrations of 129 mM and 119 mM respectively. The most abundant D-amino acids were D-Glu and D-Ala, consistent with their important roles as components of bacterial cell wall peptidoglycan. Significant levels D-Asp, DTyr, D-Pro, and D-Ser were also observed.
Table 4.
DL-Amino profile of MRSAa.
| Concentration (mM) ± SEb | |||
|---|---|---|---|
| l-AAs | d-AAs | %d/l | |
| Gly | 15.3 ± 0.4 | ||
| Ala | 67.2 ± 1.7 | 18.5 ± 0.3 | 28 |
| Ser | 15.1 ± 0.3 | 1.26 ± 0.03 | 8.3 |
| Pro | 128.9 ± 1.3 | 1.42 ± 0.03 | 1.1 |
| Val | 34.3 ± 0.5 | 0.51 ± 0.03 | 1.5 |
| Thr | 14.9 ± 0.4 | ND | |
| Ile | 20.1 ± 0.3 | 0.46 ± 0.03 | 2.3 |
| Leu | 22.8 ± 0.1 | 0.56 ± 0.02 | 2.5 |
| Asn | 0.61 ± 0.02 | ND | |
| Asp | 40 ± 4 | 3.93 ± 0.08 | 10 |
| Gln | 24.3 ± 0.5 | ND | |
| Glu | 119 ± 3 | 25.2 ± 0.3 | 21 |
| Met | 21.3 ± 0.2 | 0.29 ± 0.01 | 1.4 |
| His (Bis) | 2.74 ± 0.11 | 0.28 ± 0.03 | 10 |
| Phe | 17.6 ± 0.2 | 0.218 ± 0.004 | 1.2 |
| Arg | 6.79 ± 0.13 | 0.16 ± 0.02 | 2.4 |
| Tyr (Bis) | 14.1 ± 0.9 | 2.93 ± 0.06 | 21 |
| Trp | 0.198 ± 0.005 | ND | |
| Lys (Bis) | 14.4 ± 0.1 | ND | |
| Cys2 | 0.045 ± 0.003 | ND | |
Conclusion
Analytical methods for stereospecific quantification d- and l-amino acids are of interest in many areas. Marfey’s reagent has proven to be an effective and commonly used chiral derivatizing agent for the chromatographic resolution of d- and l- amino acids [24, 31, 32]. Despite its popularity, a systematic study of its application for the resolution and quantification of all 20 common DL-amino acids has not been reported to our knowledge. An LC-MS/MS based approach is ideal for such an analysis given the chromatographic complexity of such a group of analytes (19 DL-amino acid pairs + Gly = 39 species), and the ability of tandem MS to specifically detect and quantify analytes in complex biological samples.
Somewhat surprisingly, since Marfey’s reagent based separations are traditionally performed using acidic solvent systems, a neutral (pH 6.5) solvent system was found superior in that it allowed all diastereomer pairs to be resolved, as well as the Mar-Leu and Mar-Ile isobars (Fig. 2). Sensitivity in positive mode at neutral pH was poor, but sensitivity at neural pH in negative mode (Table 1) was found to be superior to positive mode detection at acidic pH. These observations clearly identified the neutral pH solvent system with negative mode detection as optimal for this application. A kinetic study using this method was then performed (Table 2, Figs 3–4), which demonstrates that a 24 hour derivatization is sufficient for all amino acids except for His (Fig. 4). This method was then validated in terms of linearity, sensitivity, matrix effect, and stability. Finally, this method was demonstrated for DL-amino acid analysis in a complex matrices by the analysis of the DL-amino acid composition of an MRSA extract. This study provides a viable method for the selective and stereospecific quantification of all proteinogenic DL-amino acids by LC-MS/MS spectrometry.
Supplementary Material
Acknowledgments
The authors acknowledge support by grants from National Institute of Health (R21-AI121903 and R15-GM126502) to WGG.
Reference
- 1.Genchi G: An overview on D-amino acids. Amino Acids. 49, 1521–1533 (2017) [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K: The presence of free D-serine in rat brain. FEBS letters. 296, 33–36 (1992) [DOI] [PubMed] [Google Scholar]
- 3.Schell MJ, Brady RO Jr., Molliver ME, Snyder SH: D-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 17, 1604–1615 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mothet JP, Parent AT, Wolosker H, Brady RO Jr., Linden DJ, Ferris CD, Rogawski MA, Snyder SH: D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 97, 4926–4931 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madeira C, Lourenco MV, Vargas-Lopes C, Suemoto CK, Brandao CO, Reis T, Leite RE, Laks J, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Ferreira ST, Panizzutti R: D-serine levels in Alzheimer’s disease: implications for novel biomarker development. Translational psychiatry. 5, e561 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Xing Y, Guo X, Cui Y: Development of an UPLC-MS/MS method for simultaneous quantitation of 11 D-amino acids in different regions of rat brain: Application to a study on the associations of d-amino acid concentration changes and Alzheimer’s disease. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 1058, 40–46 (2017) [DOI] [PubMed] [Google Scholar]
- 7.Paul P, de Belleroche J: The role of D-serine and glycine as co-agonists of NMDA receptors in motor neuron degeneration and amyotrophic lateral sclerosis (ALS). Frontiers in synaptic neuroscience. 6, 10 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantrowitz JT, Epstein ML, Beggel O, Rohrig S, Lehrfeld JM, Revheim N, Lehrfeld NP, Reep J, Parker E, Silipo G, Ahissar M, Javitt DC: Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain : a journal of neurology. 139, 3281–3295 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M: Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 60, 572–576 (2003) [DOI] [PubMed] [Google Scholar]
- 10.Kiriyama Y, Nochi H: D-Amino Acids in the Nervous and Endocrine Systems. Scientifica (Cairo). 2016, 6494621 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weatherly CA, Du S, Parpia C, Santos PT, Hartman AL, Armstrong DW: D-Amino Acid Levels in Perfused Mouse Brain Tissue and Blood: A Comparative Study. ACS chemical neuroscience. 8, 1251–1261 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura T, Hamase K, Miyoshi Y, Yamamoto R, Yasuda K, Mita M, Rakugi H, Hayashi T, Isaka Y: Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Scientific reports. 6, 26137 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morikawa A, Hamase K, Miyoshi Y, Koyanagi S, Ohdo S, Zaitsu K: Circadian changes of D-alanine and related compounds in rats and the effect of restricted feeding on their amounts. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 875, 168–173 (2008) [DOI] [PubMed] [Google Scholar]
- 14.Ariyoshi M, Katane M, Hamase K, Miyoshi Y, Nakane M, Hoshino A, Okawa Y, Mita Y, Kaimoto S, Uchihashi M, Fukai K, Ono K, Tateishi S, Hato D, Yamanaka R, Honda S, Fushimura Y, Iwai-Kanai E, Ishihara N, Mita M, Homma H, Matoba S: D-Glutamate is metabolized in the heart mitochondria. Scientific reports. 7, 43911 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schleifer KH, Kandler O: Peptidoglycan types of bacterial cell walls and their taxinomic implications. Bacteriol Rev. 36, 407–477 (1972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vollmer W, Blanot D, de Pedro MA: Peptidoglycan structure and architecture. FEMS microbiology reviews. 32, 149–167 (2008) [DOI] [PubMed] [Google Scholar]
- 17.Bugg TD, Braddick D, Dowson CG, Roper DI: Bacterial cell wall assembly: still an attractive antibacterial target. Trends in biotechnology. 29, 167–173 (2011) [DOI] [PubMed] [Google Scholar]
- 18.Radkov AD, Moe LA: Bacterial synthesis of D-amino acids. Appl Microbiol Biotechnol. 98, 5363–5374 (2014) [DOI] [PubMed] [Google Scholar]
- 19.Cava F, Lam H, de Pedro MA, Waldor MK: Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 68, 817–831 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto T, Katane M, Saitoh Y, Sekine M, Homma H: Identification and characterization of novel broad-spectrum amino acid racemases from Escherichia coli and Bacillus subtilis. Amino Acids. 49, 1885–1894 (2017) [DOI] [PubMed] [Google Scholar]
- 21.Alvarez L, Aliashkevich A, de Pedro MA, Cava F: Bacterial secretion of D-arginine controls environmental microbial biodiversity. ISME J. 12, 438–450 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demarchi B, Williams MG, Milner N, Russell N, Bailey G, Penkman K: Amino acid racemization dating of marine shells: A mound of possibilities. Quaternary International. 239, 114–124 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elsila JE, Aponte JC, Blackmond DG, Burton AS, Dworkin JP, Glavin DP: Meteoritic Amino Acids: Diversity in Compositions Reflects Parent Body Histories. ACS Central Science. 2, 370–379 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhushan R, Bruckner H: Marfey’s reagent for chiral amino acid analysis: a review. Amino Acids. 27, 231–247 (2004) [DOI] [PubMed] [Google Scholar]
- 25.Ilisz I, Berkecz R, Peter A: Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. Journal of pharmaceutical and biomedical analysis. 47, 1–15 (2008) [DOI] [PubMed] [Google Scholar]
- 26.Szoko E, Vincze I, Tabi T: Chiral separations for D-amino acid analysis in biological samples. Journal of pharmaceutical and biomedical analysis. 130, 100–109 (2016) [DOI] [PubMed] [Google Scholar]
- 27.Piovesana S, Samperi R, Laganà A, Bella M: Determination of Enantioselectivity and Enantiomeric Excess by Mass Spectrometry in the Absence of Chiral Chromatographic Separation: An Overview. Chemistry – A European Journal. 19, 11478–11494 (2013) [DOI] [PubMed] [Google Scholar]
- 28.Schurig V: Gas chromatographic enantioseparation of derivatized alpha-amino acids on chiral stationary phases--past and present. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 879, 3122–3140 (2011) [DOI] [PubMed] [Google Scholar]
- 29.Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R, de Koning TJ: A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of D-amino acids in body fluids. Journal of chromatography. A 1218, 7130–7136 (2011) [DOI] [PubMed] [Google Scholar]
- 30.Tao WA, Zhang D, Nikolaev EN, Cooks RG: Copper(II)-Assisted Enantiomeric Analysis of D,L-Amino Acids Using the Kinetic Method: Chiral Recognition and Quantification in the Gas Phase. Journal of the American Chemical Society. 122, 10598–10609 (2000) [Google Scholar]
- 31.Marfey P: Determination of D-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Research Communications. 49, 591–596 (1984) [Google Scholar]
- 32.Bhushan R, Bruckner H: Use of Marfey’s reagent and analogs for chiral amino acid analysis: assessment and applications to natural products and biological systems. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 879, 3148–3161 (2011) [DOI] [PubMed] [Google Scholar]
- 33.Jamindar D, Gutheil WG: A liquid chromatography-tandem mass spectrometry assay for Marfey’s derivatives of L-Ala, D-Ala, and D-Ala-D-Ala: application to the in vivo confirmation of alanine racemase as the target of cycloserine in Escherichia coli. Analytical biochemistry. 396, 1–7 (2010) [DOI] [PubMed] [Google Scholar]
- 34.Vemula H, Ayon NJ, Gutheil WG: Cytoplasmic peptidoglycan intermediate levels in Staphylococcus aureus. Biochimie. 121, 72–78 (2016) [DOI] [PubMed] [Google Scholar]
- 35.Putty S, Vemula H, Bobba S, Gutheil WG: A liquid chromatography-tandem mass spectrometry assay for D-Ala-D-Lac: a key intermediate for vancomycin resistance in vancomycin-resistant enterococci. Analytical biochemistry. 442, 166–171 (2013) [DOI] [PubMed] [Google Scholar]
- 36.Vemula H, Ayon NJ, Burton A, Gutheil WG: Antibiotic Effects on Methicillin-Resistant Staphylococcus aureus Cytoplasmic Peptidoglycan Intermediate Levels and Evidence for Potential Metabolite Level Regulatory Loops. Antimicrobial agents and chemotherapy. 61, (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vemula H, Kitase Y, Ayon NJ, Bonewald L, Gutheil WG: Gaussian and linear deconvolution of LC-MS/MS chromatograms of the eight aminobutyric acid isomers. Analytical biochemistry. 516, 75–85 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vemula H, Bobba S, Putty S, Barbara JE, Gutheil WG: Ion-pairing liquid chromatography-tandem mass spectrometry-based quantification of uridine diphosphate-linked intermediates in the Staphylococcus aureus cell wall biosynthesis pathway. Analytical biochemistry. 465, 12–19 (2014) [DOI] [PubMed] [Google Scholar]
- 39.Benson SW McGraw-Hill, New York, (1960) [Google Scholar]
- 40.Putty S, Rai A, Jamindar D, Pagano P, Quinn CL, Mima T, Schweizer HP, Gutheil WG: Characterization of D-boroAla as a Novel Broad-Spectrum Antibacterial Agent Targeting D-Ala-D-Ala Ligase. Chemical Biology & Drug Design. 78, 757–763 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.