Abstract
Rationale:
Inhibition of miR-33 reduces atherosclerotic plaque burden, but miR-33 deficient mice are predisposed to the development of obesity and metabolic dysfunction. The pro-atherogenic effects of miR-33 are thought to be in large part due to its repression of macrophage cholesterol efflux, through targeting of ATP Binding Cassette Subfamily A Member 1 (Abca1). However, targeting of other factors may also be required for the beneficial effects of miR-33 and currently available approaches have not allowed researchers to determine the specific impact of individual miRNA target interactions in vivo.
Objective:
In this work, we sought to determine how specific disruption of Abca1 targeting by miR-33 impacts macrophage cholesterol efflux and atherosclerotic plaque formation in vivo.
Methods and Results:
We have generated a novel mouse model with specific point mutations in the miR-33 binding sites of the Abca1 3’UTR, which prevents targeting by miR-33. Abca1 binding site mutant (Abca1BSM) mice had increased hepatic ABCA1 expression but did not show any differences in body weight or metabolic function after high fat diet feeding. Macrophages from Abca1BSM mice also had increased ABCA1 expression, as well as enhanced cholesterol efflux and reduced foam cell formation. Moreover, LDLR deficient animals transplanted with bone marrow from Abca1BSM mice had reduced atherosclerotic plaque formation, similar to mice transplanted with bone marrow from miR-33 knockout mice.
Conclusion:
Although the more pronounced phenotype of miR-33 deficient animals suggests that other targets may also play an important role, our data clearly demonstrate that repression of ABCA1 is primarily responsible for the pro-atherogenic effects of miR-33. This work shows for the first time that disruption of a single miRNA/target interaction can be sufficient to mimic the effects of miRNA deficiency on complex physiologic phenotypes in vivo and provides an approach by which to assess the impact of individual miRNA targets.
Keywords: Atherosclerosis, Basic Science Research, Genetically Altered and Transgenic Models, Lipids and Cholesterol, Metabolism
Keywords: miR-33, ABCA1, atherosclerosis, cholesterol efflux, ABC transporter, atherogenesis, cholesterol homeostasis, miRNAs, foam cells
INTRODUCTION
microRNAs (miRNAs) and other non-coding RNAs have been demonstrated to be critical regulators of numerous physiologic functions including regulation of lipid metabolism1, 2. The miR-33 family of miRNAs, consisting of miR-33a and miR-33b, are intronic miRNAs encoded within the sterol response binding protein 2 (SREBP2) and SREBP1 genes3. Like many intronic miRNAs, miR-33a and miR-33b are co-transcribed with their host genes. While SREBP2 induces the expression of genes involved in cholesterol uptake and synthesis, miR-33a and miR-33b target genes involved in cholesterol export, including the ATP Binding Cassette Subfamily A Member 1 (Abca1) and ATP Binding Cassette Subfamily G Member 1 (Abcg1)3–5. In the liver, miR-33 also impedes bile acid synthesis and secretion by targeting cytochrome P450 family 7 subfamily A member 1 (Cyp7a1), ATP binding cassette subfamily B member 11 (Abcb11), and ATPase phospholipid transporting 8B1 (Atp8b1)6. These regulatory functions of miR-33 help ensure that cells are protected from additional sterol loss under low sterol conditions3–5. However, these miR-33 targets are also key regulators of reverse cholesterol transport, the primary mechanism by which cholesterol is removed from peripheral tissues, including the cells that make up atherosclerotic plaques. As such, inhibition of miR-33 has emerged as a promising approach for the treatment of CVD, and miR-33 antagonism has been demonstrated to increase circulating high-density lipoprotein cholesterol (HDL) in both rodents and non-human primates and to reduce atherosclerotic plaque burden7–11.
However, concerns have been raised over potential unintended consequences of miRNA based therapies and the insufficient methods through which researchers identify miRNA targets and assess their physiologic relevance12. These concerns arise largely from the ability of miRNAs to target many different mRNAs and are especially valid for miR-33, as it has been shown to affect numerous cellular processes in addition to regulating lipid metabolism. These effects include regulation of mitochondrial respiration, autophagy, fatty acid oxidation, inflammatory response, cellular proliferation, β-amyloid deposition, adipocyte differentiation, and state dependent memory7, 13–18. Moreover, our recent work has demonstrated that miR-33 knockout mice (miR-33−/−) are predisposed to the development of obesity and metabolic dysfunction when fed a high fat diet (HFD)18, 19. These findings warrant a more in-depth elucidation of the specific mechanisms by which miR-33 regulates obesity and metabolic function before further development of anti-miR-33 therapeutic approaches for treatment of CVD in humans. In this work, use of recently developed genome editing techniques allows us to assess the specific mechanisms by which miRNAs function through genetic disruption of specific a miRNA/target interaction. Using a novel mouse model in which targeting of Abca1 by miR-33 is selectively impaired we demonstrate that the pro-atherogenic effects of miR-33 are primarily due to repression of ABCA1.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
A detailed description of the Materials and Methods used in this study are available in the Online Data Supplement. Abca1 binding site mutant (Abca1BSM) animals were generated with the assistance of the Yale Genome Editing Center. Genetic modifications in ES cells were tested using standard techniques and confirmed in mutant mice by direct sequencing and PCR amplification. Accelerated atherosclerosis or diet induced obesity were induced by feeding a Western diet (1.25% cholesterol; D12108) or high fat diet (60% Fat; D12492), respectively (Research Diets, Incorporated, New Brunswick, NJ, USA). Bone marrow transplant (BMT) experiments, in vivo foam cell formation, plaque characterization, glucose tolerance tests, and measurements of body composition, circulating and cellular lipids, cholesterol efflux, efferocytosis, and reverse cholesterol transport were carried out using established techniques18, 19. All animals were identified by four digit numerical codes throughout the completion and analysis of these studies to ensure that researchers were blinded to the genotypes of the animals. Statistical differences were measured using an unpaired two-sided Student’s t-test, or one-way ANOVA with Bonferroni correction for multiple comparisons. Normality was checked using the Kolmogorov-Smirnov test. A nonparametric test (Mann-Whitney) was used when data did not pass the normality test. A value of P≤0.05 was considered statistically significant.
RESULTS
Generation and characterization of Abca1 binding site mutant mice.
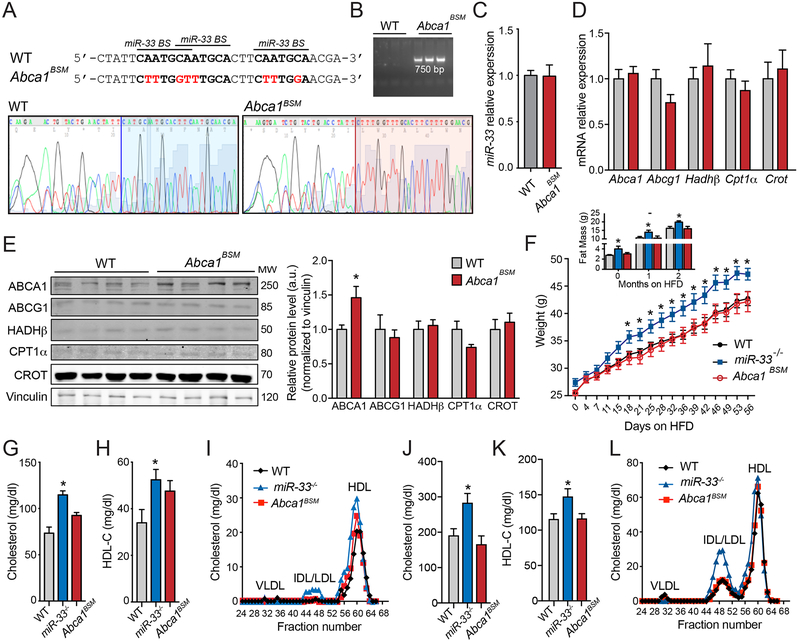
To determine the extent to which repression of ABCA1 mediates the effects of miR-33 in vivo, we utilized CRISPR/Cas9 technology to generate mice in which 3 miR-33 binding sites within the Abca1 3’UTR were edited to prevent miR-33 binding. These specific mutations have been demonstrated to disrupt the effects of miR-33 on the Abca1 3’UTR using luciferase assays3. The presence of these specific mutations in Abca1 binding site mutant (Abca1BSM) mice was confirmed by direct sequencing (Figure 1A) as well as PCR amplification with primers specific to this region (Figure 1B). The disruption of miR-33 binding sites in the Abca1 3’UTR did not alter the expression levels of miR-33 (Figure 1C). To confirm that these mutations were sufficient to selectively derepress ABCA1 in vivo, we assessed the expression of ABCA1 and other miR-33 targets in the liver of wildtype (WT) and Abca1BSM mice. While the mRNA expression level of Abca1 was not altered (Figure 1D), ABCA1 protein levels were significantly increased in the liver of Abca1BSM mice (Figure 1 E). This is likely because miRNAs can impair translation as well as reducing mRNA stability and therefore often have a more pronounced effect on protein levels. This effect was specific to ABCA1, as neither the mRNA nor the protein levels of other miR-33 targets were not significantly increased (Figure 1D and1E). Our prior work has demonstrated that miR-33−/− mice are predisposed to the development of obesity and metabolic dysfunction19, which can offset the beneficial effects of miR-33 within plaque macrophages18. These effects of miR-33 do not appear to be mediated by ABCA1, as Abca1BSM mice do not show any differences in body weight, fat mass, or regulation of glucose homeostasis after HFD (60% fat) feeding (Figure 1F and Online Figure I). Surprisingly, the increased expression of ABCA1 in the livers of Abca1BSM mice did not result in a significant increase in circulating levels of cholesterol or HDL. While there is a strong trend toward increased cholesterol and HDL in chow diet fed mice, these changes were less pronounced that those observed in miR-33−/− mice and did not reach statistical significance (Figure 1G–I). Additionally, under HFD fed conditions Abca1BSM mice did not show any differences in total cholesterol or HDL levels, likely because these effects are secondary to the overall obesity and dyslipidemia observed in miR-33−/− mice (Figure 1J–L).
Figure 1. Generation of Abca1 binding site mutant mice results in derepression of ABCA-1 in vivo.
A-B) CRISPR/Cas9 mediated mutation of the 3 binding sites (BS) for miR-33 (black bars) in the genomic region of Abca1 was performed to generate Abca1BSM mice (mutated sequences in red). This was confirmed by direct sequencing A) and PCR amplification B) in DNA isolated from WT and Abca1BSM mice. C-D) qPCR analysis of the expression of miR-33 normalized to U6 (n=5) C) and the mRNA expression of miR-33 target genes normalized to 18s (n=4–5) D) in liver samples isolated from WT and Abca1BSM mice. E) Representative western blot analysis of miR-33 targets in liver samples isolated from WT and Abca1BSM mice. Right panel shows the quantification of band densitometry values and are expressed in a.u. after correction for loading control vinculin (n=4). F) Body weight and fat mass after high fat diet (60% fat) feeding in WT, miR-33−/−, and Abca1BSM mice (n=6–9 per group). G-H) Measurements of G) cholesterol and H) HDL-cholesterol in plasma of chow diet fed WT, miR-33−/− and Abca1BSM mice (n=7). I) Lipoprotein profile from FPLC fractionation of pooled plasma of WT, miR-33−/− and Abca1BSM mice on a chow diet. J-K) Measurements of J) cholesterol and K) HDL-cholesterol in plasma of HFD fed WT, miR-33−/− and Abca1BSM mice (n=7). L) Lipoprotein profile from FPLC fractionation of pooled plasma of WT, miR-33−/− and Abca1BSM mice on a HFD. All data are represented as the mean ± SEM. *P≤0.05 compared with WT mice under the same conditions.
Macrophages from Abca1BSM mice have increased cholesterol efflux capacity and decreased foam cell formation.
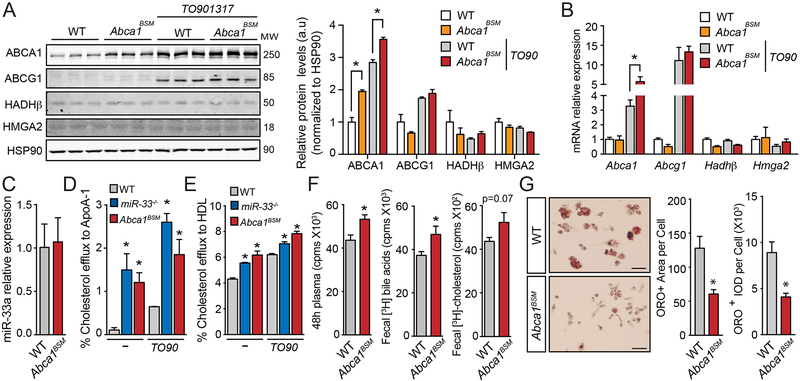
Peritoneal macrophages (PM) from Abca1BSM mice had increased ABCA1 protein expression both under basal conditions and after treatment with the LXR ligand TO901317 (TO90), while the expression of other miR-33 targets was unaffected (Figure 2A). While the mRNA expression levels of Abca1 in PM under basal conditions was not altered, the induction of Abca1 mRNA expression after TO90 treatment was significantly increased (Figure 2B). Once again, the expression of other miR-33 targets was not found to be elevated in Abca1BSM mice confirming that these animals have selective derepression of ABCA1. As in the liver the expression levels of miR-33 in PM from Abca1BSM mice were not altered (Figure 2C). Consistent with the increased expression of ABCA1, the ability of PM from Abca1BSM mice to efflux cholesterol to APOA1 or HDL was enhanced both under basal and TO90 stimulated conditions, similar to PM from miR-33−/− animals (Figure 2D and2E).
Figure 2. Macrophages from Abca1BSM mice have increased ABCA1 expression, enhanced cholesterol efflux, and reduced foam cell formation.
A-B) Protein and mRNA expression of miR-33 target genes (Abca1, Abcg1, Hadhβ and Hmga2) from peritoneal macrophages isolated from WT and Abca1BSM mice, untreated or stimulated with the LXR ligand TO901317 (TO90). A) Representative western blot analysis out of three with similar results. Relative protein levels were determined by band densitometry and are expressed in a.u after correction for loading control HSP90 (n=3). B) Relative mRNA expression levels normalized to 18S rRNA (n=4–5). C) Expression levels of miR-33 in PM from WT and Abca1BSM mice normalized to U6 (n=7–8). D-E) Cholesterol efflux to APOA1 D) and HDL E) in peritoneal macrophages isolated from WT, miR-33−/− and Abca1BSM mice, untreated or stimulated with TO90 (n=3). F) [3H]-cholesterol in plasma (left) fecal bile acids (middle) and total fecal cholesterol (right) from WT mice injected intraperitoneally with [3H]-cholesterol-loaded bone marrow derived macrophages from WT and Abca1BSM mice (n=11–14). G) Representative high magnification images of Oil red O (ORO) staining in foam cells generated in vivo from Ldlr−/− mice transplanted with bone marrow from WT and Abca1BSM mice after feeding a Western diet (1.25% cholesterol). Quantification of the ORO-positive area per cell (left) and integrated optical density (IOD) (Right) is shown in the right panel. For each animal quantification was performed in at least six low magnification images from different fields (n=4) Scale bars, 100μm. All data are represented as the mean ± SEM. *P≤0.05 compared with macrophages from WT mice under the same conditions.
To determine the impact of disrupting Abca1 targeting by miR-33 in vivo, we preloaded bone marrow derived macrophages from WT and Abca1BSM mice with radioactively labeled cholesterol and injected these cells into the peritoneal cavity of WT mice18. After 48 hours, we observed increased accumulation of radioactivity in the plasma of animals injected with PM from Abca1BSM mice, as well as the bile acids present in the feces of these animals. Total fecal cholesterol also showed a trend toward being increased, but this did not reach statistical significance. These findings demonstrate that the cholesterol efflux capacity of macrophages from Abca1BSM mice was increased in vivo (Figure 2F). To further assess whether this increased cholesterol efflux capacity can influence macrophage lipid accumulation, we utilized a model of in vivo foam cell (IVFC) formation, by performing bone marrow transplants (BMT) from WT or Abca1BSM mice into the Ldlr−/− mouse model of atherosclerosis. After feeding a western diet (WD) with 1.25% cholesterol, PM from Ldlr−/− mice showed substantial accumulation of neutral lipids visualized by oil red O (ORO) staining. However, in animals reconstituted with bone marrow (BM) from Abca1BSM mice, we observed a substantial reduction in both the area and intensity of ORO in PM following WD feeding (Figure 2G and Online Figure II). Since these results indicate that the increased levels of ABCA1 and enhanced cholesterol efflux result in reduced foam cell formation and lipid accumulation, transcription factors that are known to be responsive to intracellular lipid levels, such as SREBPs and LXR, would be expected to have increased and decreased activity, respectively. Consistent with this, we see that the mRNA expression of SREBP2 target genes tended to be elevated, while expression of LXR target genes showed a trend toward being reduced in PM from our Abca1BSM mice (Figure 2B and Online Figure III).
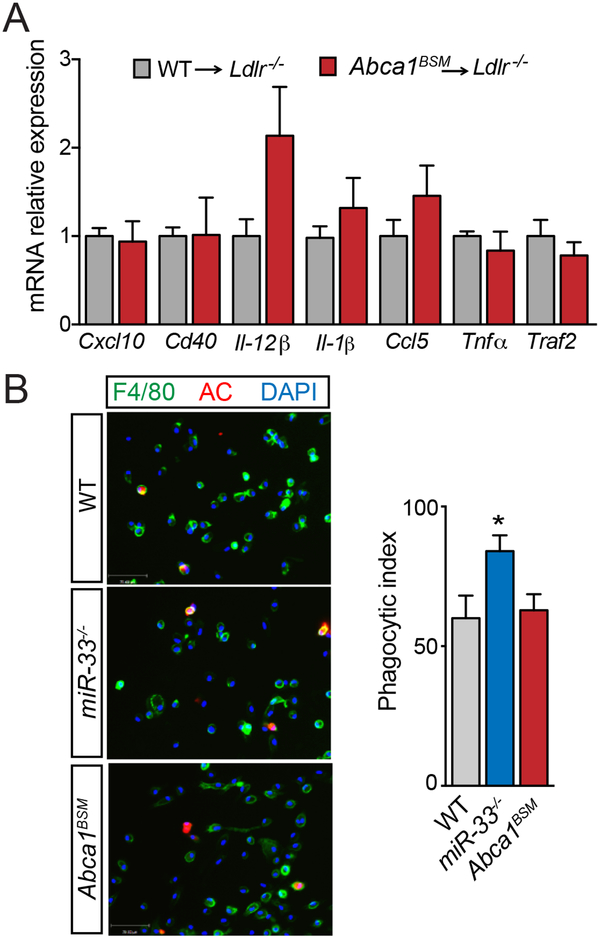
In addition to reduced lipid accumulation, our previous work showed that IVFCs from miR-33 deficient animals had reduced expression of a number of inflammatory genes. As ABCA1 plays an important role in the regulation of inflammatory response through changes in membrane lipid composition20, we sought to determine whether these changes could also be attributed to derepression of ABCA1. However, the expression of inflammatory genes downregulated in miR-33−/− mice was either unaltered or in some cases increased in IVFCs from Abca1BSM mice (Figure 3A). Similarly, PM from miR-33−/− mice have an improved ability to take up apoptotic cells, but no differences in efferocytosis capacity were observed in PM from Abca1BSM mice (Figure 3B). Together these findings show that derepression of ABCA1 is responsible for some but not all of the effects of miR-33 on macrophages, suggesting that disruption of ABCA1 targeting may be able to at least in part mimic the atheroprotective effects of macrophage miR-33 deficiency in vivo.
Figure 3. Expression of inflammatory genes and efferocytosis capacity in macrophages from Abca1BSM mice.
A) mRNA expression of a number of inflammatory genes in foam cells generated in vivo from Ldlr−/− mice transplanted with bone marrow from WT and Abca1BSM mice after feeding a Western diet (1.25% cholesterol) (n=3–4). B) Representative images of the in vitro engulfment of CellTracker Red labeled apoptotic Jurkat cells by peritoneal macrophages isolated from WT and Abca1BSM mice. Right, quantification of phagocytic index, which is the number of apoptotic cells (AC, red) ingested in 1h per F4/80-positive macrophage (green) x 100. Quantification performed in at least five images from different fields (n=3). Scale bars, 70μm. All data are represented as the mean ± SEM. *P≤0.05 compared with macrophages from WT mice under the same conditions.
Disruption of Abca1 binding by miR-33 is sufficient to reduce atherosclerotic plaque formation.
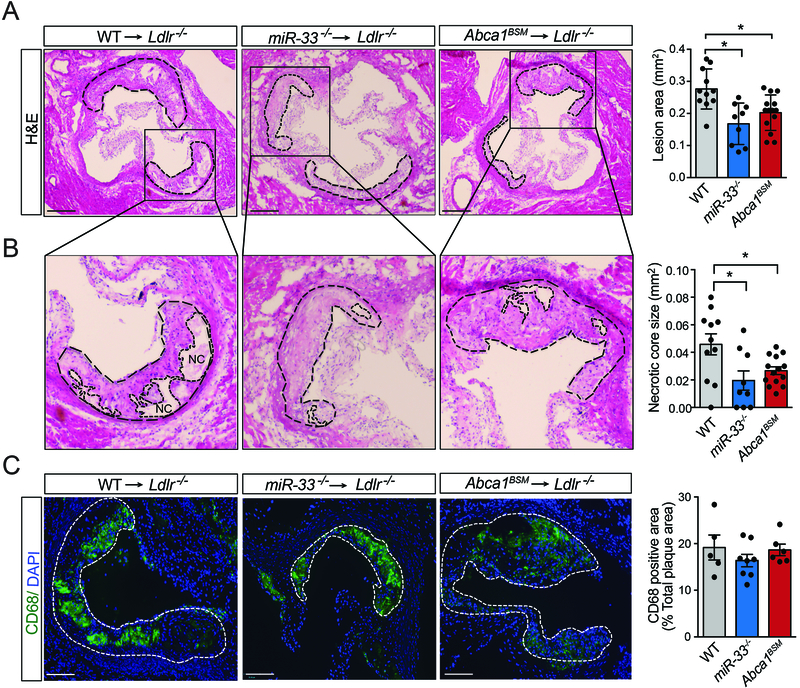
To determine whether the effects observed in Abca1BSM macrophages are sufficient to mimic the effects of miR-33 deficiency on atherosclerosis, we again performed BMT experiments into Ldlr−/− mice. Following 3 months of WD feeding, we did not observe any differences in the circulating lipids of animals transplanted with WT, Abca1BSM, or miR-33−/− BM (Online Figure IV). As in our previous work, histological analysis of the aortic root demonstrated that reconstitution with BM from miR-33−/− mice reduced atherosclerotic plaque size, and similar changes were observed in animals reconstituted with BM from Abca1BSM mice (Figure 4A). Additionally, the size of the necrotic cores were reduced in plaques from animals transplanted with either Abca1BSM or miR-33−/− BM (Figure 4B). Consistent with our prior work we did not observe any differences in the percentage of plaque area that stained positive for the macrophage marker CD68 in animals transplanted with miR-33−/− BM, and this was also unaffected in animals reconstituted with BM from Abca1BSM mice (Figure 4C).
Figure 4. Disruption of Abca1 binding by miR-33 is sufficient to reduce atherosclerotic plaque size.
Representative histological analysis of cross-sections of the aortic sinus stained from Ldlr−/− mice transplanted with WT, miR-33−/− and Abca1BSM bone marrow after 12 months of western diet (1.25% cholesterol) feeding. A-B) Representative images of H&E stained sections with quantification of total plaque area A) and necrotic core size B). Dashed lines show the boundary of the developing necrotic core (NC) (n=9–14 animals). C) Representative images of CD68 stained sections with quantification of percent CD68 positive area (n=5–8). All data are represented as the mean ± SEM. *P≤0.05 compared with mice reconstituted with WT bone marrow. Each dot is based on quantification of ≥ 9 sections from an individual animal. Scale bars, 200μm.
DISCUSSION
While the less pronounced effects of transplantation with Abca1BSM BM suggest that other targets may also be involved in the pro-atherogenic effects of miR-33, our data clearly demonstrate that specific loss of the ability to target Abca1 largely mimics the effects of miR-33 deficiency on macrophage cholesterol efflux, and atherosclerotic plaque formation. Previous work has suggested that derepression of miR-33 targets regulating cellular bioenergetics, including factors involved autophagy and mitochondrial respiration, are necessary for enhanced cholesterol efflux in response to miR-33 deficiency. However, these conclusions are largely based on findings showing that the ability of miR-33 inhibitors to enhance cholesterol efflux is lost under conditions in which mitochondrial function or autophagy are impaired21, 22. While it is likely that the derepression of genes related to autophagy and mitochondrial function upon inhibition or genetic deletion of miR-33 helps macrophages and other cells to maintain high levels of cholesterol efflux, our findings indicate that this is not required to increase cholesterol efflux and decrease foam cell formation in macrophages, or to reduce atherosclerotic plaque size. In the future, it will be important to determine the specific mechanisms by which miR-33 regulates feeding, obesity, and metabolic function. In this work we can conclude that these effects are independent of Abca1 targeting, but a more thorough exploration of the specific mechanisms behind these effects will first require a better understanding of what organs are primarily responsible for mediating these effects. As our data also demonstrates that derepression of ABCA1 is the primary mechanism by which miR-33 regulates macrophage cholesterol efflux and atherosclerotic plaque formation, more targeted approaches to pharmacologically disrupt targeting of Abca1 by miR-33 may provide a more promising approach for the development of new therapeutic strategies.
In this work, we employ recently developed genome editing techniques to selectively disrupt an individual miRNA/target interaction in vivo, and demonstrate that this is sufficient to mimic some but not all of the effects of miRNA deficiency. To our knowledge this is the first time anyone has been able to demonstrate that an individual miRNA target is responsible for mediating complex physiologic effects in vivo. These findings indicate that approaches to disrupt binding of specific miRNA targets may have strong therapeutic value. Target site blockers are short oligoes designed to bind to specific regions in the 3’UTR of target genes and prevent miRNA binding. However currently available products have not shown the necessary efficacy, and/or specificity to be utilized either for research purposes or therapeutic applications. While our work highlights the importance of improving this technology, it also provides a genetic approach by which researchers can probe more deeply into the specific mechanisms by which miRNAs exert their effects. This will be critical for the development of miRNA based therapeutic approaches, as one of the biggest impediments in this field has been the inability to determine the specific mechanisms by which miRNAs exert their effects in vivo.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Inhibition of miR-33 has been shown to increase circulating HDL, promote macrophage cholesterol efflux and reduce atherosclerotic plaque burden.
LDLR deficient mice reconstituted with bone marrow from animals lacking miR-33 have smaller atherosclerotic plaques, while whole body loss of miR-33 promotes obesity and metabolic dysfunction.
The effects of miR-33 on HDL biogenesis, cholesterol efflux, and atherosclerosis may be due at least in part to its ability to repress the cholesterol transporter ABCA1.
What New Information Does This Article Contribute?
Generation of mice in which the binding sites for miR-33 in the Abca1 gene have been modified (Abca1BSM) results in increased expression of ABCA1 in the liver and macrophages.
Abca1BSM mice do not have any differences in body weight or metabolic function, but macrophages from these animals have increased cholesterol efflux both in vitro and in vivo.
Reconstitution of LDLR deficient mice with bone marrow from Abca1BSM animals reduces atherosclerotic plaque size.
Inhibition of miR-33 has been shown to reduce atherosclerotic plaque burden, but genetic deletion results in obesity and metabolic dysfunction. While the ability of miR-33 to regulate atherosclerosis is thought to be at least in part due to its ability to target Abca1, an important factor in HDL biogenesis and macrophage cholesterol efflux, previous work has not been able to determine to what extent changes in Abca1 are responsible for mediating these effects. In this work, we have generated a novel mouse model in which the interaction between Abca1 and miR-33 has been disrupted (Abca1BSM mice), resulting in increased expression of ABCA1. Macrophages from Abca1BSM mice have an increased capacity to efflux cholesterol, and reconstitution with bone marrow from Abca1BSM mice results in smaller atherosclerotic plaques in LDLR deficient animals. Our findings indicate that Abca1 is the primary target of miR-33 responsible for its ability to regulate macrophage cholesterol efflux and atherosclerosis but is not responsible for its effects on obesity and metabolic function. While miR-33 like many micro-RNAs can mediate complex physiologic effects in vivo, this study shows that disruption of binding to specific miRNA targets may have strong therapeutic value.
Acknowledgments
SOURCE OF FUNDING
This work was supported by grants from the National Institutes of Health (R35HL135820 to CF-H), the American Heart Association (16EIA27550005 to CF-H; and 17SDG33110002 to NR), the American Diabetes Association (1–16-PMF-002 to AC-D), and the Foundation Leducq Transatlantic Network of Excellence in Cardiovascular Research MIRVAD (to CF-H).
Nonstandard Abbreviations and Acronyms:
- BM
Bone marrow
- BMT
Bone marrow transplant
- PM
Peritoneal macrophages
- BSM
Binding site mutant
- miRNA
microRNA
- HFD
High fat diet
- WD
Western diet
- ORO
Oil red O
Footnotes
DISCLOSURE
None.
REFERENCES
- 1.Aryal B, Singh AK, Rotllan N, Price N, Fernandez-Hernando C. Micrornas and lipid metabolism. Curr Opin Lipidol. 2017;28:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Price NL, Fernandez-Hernando C. Non-coding rnas in lipid metabolism. Vascul Pharmacol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. Mir-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquart TJ, Allen RM, Ory DS, Baldan A. Mir-33 links srebp-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. Microrna-33 and the srebp host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, Ford DA, Baldan A. Mir-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med. 2012;4:882–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price NL, Ramirez CM, Fernandez-Hernando C. Relevance of microrna in metabolic diseases. Crit Rev Clin Lab Sci. 2014;51:305–320 [DOI] [PubMed] [Google Scholar]
- 8.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, Khatsenko OG, Kaimal V, Lees CJ, Fernandez-Hernando C, Fisher EA, Temel RE, Moore KJ. Inhibition of mir-33a/b in non-human primates raises plasma hdl and lowers vldl triglycerides. Nature. 2011;478:404–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microrna-33 inhibits the progression of atherosclerosis in ldlr−/− mice--brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rottiers V, Obad S, Petri A, McGarrah R, Lindholm MW, Black JC, Sinha S, Goody RJ, Lawrence MS, deLemos AS, Hansen HF, Whittaker S, Henry S, Brookes R, Najafi-Shoushtari SH, Chung RT, Whetstine JR, Gerszten RE, Kauppinen S, Naar AM. Pharmacological inhibition of a microrna family in nonhuman primates by a seed-targeting 8-mer antimir. Sci Transl Med. 2013;5:212ra162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson EN. Micrornas as therapeutic targets and biomarkers of cardiovascular disease. Sci Transl Med. 2014;6:239ps233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirera-Salinas D, Pauta M, Allen RM, Salerno AG, Ramirez CM, Chamorro-Jorganes A, Wanschel AC, Lasuncion MA, Morales-Ruiz M, Suarez Y, Baldan A, Esplugues E, Fernandez-Hernando C. Mir-33 regulates cell proliferation and cell cycle progression. Cell Cycle. 2012;11:922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, Esplugues E, Fisher EA, Penalva LO, Moore KJ, Suarez Y, Lai EC, Fernandez-Hernando C. Mir-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Yoon H, Horie T, Burchett JM, Restivo JL, Rotllan N, Ramirez CM, Verghese PB, Ihara M, Hoe HS, Esau C, Fernandez-Hernando C, Holtzman DM, Cirrito JR, Ono K, Kim J. Microrna-33 regulates apoe lipidation and amyloid-beta metabolism in the brain. J Neurosci. 2015;35:14717–14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouimet M, Ediriweera H, Afonso MS, Ramkhelawon B, Singaravelu R, Liao X, Bandler RC, Rahman K, Fisher EA, Rayner KJ, Pezacki JP, Tabas I, Moore KJ. Microrna-33 regulates macrophage autophagy in atherosclerosis. Arterioscler Thromb Vasc Biol. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price NL, Holtrup B, Kwei SL, Wabitsch M, Rodeheffer M, Bianchini L, Suarez Y, Fernandez-Hernando C. Srebp-1c/microrna 33b genomic loci control adipocyte differentiation. Mol Cell Biol. 2016;36:1180–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price NL, Rotllan N, Canfran-Duque A, Zhang X, Pati P, Arias N, Moen J, Mayr M, Ford DA, Baldan A, Suarez Y, Fernandez-Hernando C. Genetic dissection of the impact of mir-33a and mir-33b during the progression of atherosclerosis. Cell Rep. 2017;21:1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price NL, Singh AK, Rotllan N, Goedeke L, Wing A, Canfran-Duque A, Diaz-Ruiz A, Araldi E, Baldan A, Camporez JP, Suarez Y, Rodeheffer MS, Shulman GI, de Cabo R, Fernandez-Hernando C. Genetic ablation of mir-33 increases food intake, enhances adipose tissue expansion, and promotes obesity and insulin resistance. Cell Rep. 2018;22:2133–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. Lxrs link metabolism to inflammation through abca1-dependent regulation of membrane composition and tlr signaling. Elife. 2015;4:e08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper ME, Rayner KJ. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-mir33 in atherosclerosis. Circ Res. 2015;117:266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Loke P, Moore KJ. Microrna-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.