Abstract
Rationale:
The evidence regarding the potential health benefits of nut consumption among individuals with type 2 diabetes is limited.
Objective:
To examine intake of total and specific types of nuts, including tree nuts and peanuts, in relation to subsequent risk of cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke, and all-cause and cause-specific mortality among individuals with diabetes.
Methods and Results:
This prospective analysis included 16,217 men and women with diabetes at baseline or diagnosed during follow-up (Nurses’ Health Study: 1980-2014, Health Professionals Follow-Up Study: 1986-2014). Nut consumption was assessed using a validated food frequency questionnaire and updated every 2-4 years. During 223,682 and 254,923 person-years of follow-up, there were 3,336 incident CVD cases and 5,682 deaths. Higher total nut consumption was associated with a lower risk of CVD incidence and mortality. The multivariate-adjusted hazard ratios (95% confidence intervals) for participants who consumed 5 or more servings of total nuts per week (1 serving=28g), compared with those who consumed less than 1 serving per month, were 0.83 (0.71-0.98; P trend=0.01) for total CVD incidence, 0.80 (0.67-0.96; P trend=0.005) for CHD incidence, 0.66 (0.52-0.84; P trend<0.001) for CVD mortality, and 0.69 (0.61-0.77; P trend<0.001) for all-cause mortality. Total nut consumption was not significantly associated with risk of stroke incidence or cancer mortality. For specific types of nuts, higher tree nut consumption was associated with lower risk of total CVD, CHD incidence, and mortality due to CVD, cancer, and all causes, while peanut consumption was associated with lower all-cause mortality only (all P trend<0.001). In addition, compared with participants who did not change the consumption of total nuts from pre- to post-diabetes diagnosis, participants who increased consumption of total nuts after diabetes diagnosis had an 11% lower risk of CVD, a 15% lower CHD risk, a 25% lower CVD mortality, and a 27% lower all-cause mortality. The associations persisted in subgroup analyses stratified by sex/cohort, body mass index at diabetes diagnosis, smoking status, diabetes duration, nut consumption before diabetes diagnosis, or diet quality.
Conclusions:
Higher consumption of nuts, especially tree nuts, is associated with lower CVD incidence and mortality among participants with diabetes. These data provide novel evidence that supports the recommendation of incorporating nuts into healthy dietary patterns for the prevention of CVD complications and premature deaths among individuals with diabetes.
Subject Terms: Cardiovascular Disease; Diet and Nutrition; Diabetes, Type 2; Epidemiology; Secondary Prevention
Keywords: Cardiovascular disease prevention, mortality, diabetes patients, nutrition, peanuts, epidemiology, diabetes mellitus, tree nuts
INTRODUCTION
Nuts are rich in unsaturated fatty acids, plant proteins, fiber, minerals, vitamins, and phytochemicals (e.g., phytosterols, flavonoids, and phenolic acids).1, 2 Several meta-analyses of prospective cohort studies have demonstrated that frequent nut consumption is associated with a lower risk of developing hypertension, cardiovascular disease (CVD), total cancer, and all-cause and cause-specific mortality, primarily in the general population.3-7 Evidence from clinical trials has also suggested the beneficial effects of nut consumption on improving lipid profiles, insulin resistance, oxidative stress, inflammation, and vascular reactivity.8-14 However, among individuals with type 2 diabetes who have elevated risk of developing CVD and mortality,15 evidence regarding the potential health benefits of nut consumption is scarce.
In the only existing prospective study among women with diabetes, total consumption of nuts and peanut butter was associated with a lower risk of CVD,16 although it is unknown whether individual nuts are equally beneficial.4 Indeed, the nutritional composition of peanuts (botanically as legumes) differs from tree nuts (e.g., almonds, walnuts, and hazelnuts),17 and it is of interest to examine the health effects of specific types of nuts among individuals with diabetes. Moreover, the association of nut consumption with all-cause and cause-specific mortality among patients with diabetes remains unclear. Lastly, whether increased nut consumption from pre- to post-diabetes diagnosis may yield health benefits is unknown.
To fill these knowledge gaps, we prospectively investigated total and specific nut (i.e., peanuts and tree nuts) consumption after diabetes diagnosis, as well as changes in nut consumption before and after diabetes diagnosis, in relation to subsequent risk of total CVD, coronary heart disease (CHD), and stroke incidence, and all-cause and cause-specific mortality among individuals with diabetes participating in two large prospective cohort studies.
METHODS
Data availability.
The authors declare that all supporting data are available within the article and its online supplementary files.
Study population.
The Nurses’ Health Study (NHS) is a prospective cohort study established in 1976 with the enrollment of 121,700 female nurses aged 30 to 55 years from 11 U.S. states (New York, California, Pennsylvania, Ohio, Massachusetts, New Jersey, Michigan, Texas, Florida, Connecticut, and Maryland).18 The Health Professionals Follow-Up Study (HPFS) is a prospective cohort study initiated in 1986 with the enrollment of 51,529 male health professionals aged 40 to 75 years from 50 U.S. states.19 Information on lifestyle, medical history, and health conditions was updated every 2 years through validated questionnaires.20 Follow-up rate was over 90% in each 2-year cycle for both cohorts. More details have been documented elsewhere.21, 22
For the current analysis, we included participants with prevalent diabetes at baseline (1980 for the NHS and 1986 for the HPFS, when validated food frequency questionnaires [FFQs] were first administered), as well as incident diabetes cases diagnosed during follow-up through 2014. Participants were excluded if they had CVD or cancer at baseline, reported CVD or cancer before diabetes diagnosis during follow-up, reported implausible daily caloric intake (<500 or >3,500 kcal/day for women, and <800 or >4,200 kcal/day for men), or had missing information on nut consumption at baseline (Online Figure I). These exclusion criteria were based on considerations of minimizing reverse causation bias and reducing the impact of measurement errors and missing data.23 The final analysis included 12,006 women with diabetes in the NHS and 4,211 men with diabetes in the HPFS. For the analysis of changes in nut consumption from pre- to post-diabetes diagnosis, participants with diabetes at baseline or those who had missing data of nut consumption assessed before diabetes diagnosis were further excluded. To increase statistical power, we pooled the participants from the two cohorts in the absence of heterogeneity of results.
The present study was approved by the Institutional Review Boards at the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital, and the return of completed questionnaires was considered implied consent.
Dietary assessment.
Dietary intake was assessed using validated semi-quantitative FFQs with approximately 131 food items administered every 2-4 years.20 In the 1980 and 1984 dietary questionnaires, participants were asked how often, on average, they had consumed nuts (serving size, 28 g [1 ounce]) during the preceding year: never or almost never, 1-3 servings/month, 1 serving/week, 2-4 servings/week, 5-6 servings/week, 1 serving/day, 2-3 servings/day, 4-6 servings/day, or >6 servings/day. In the subsequent FFQs (i.e., 1986 and every four years thereafter), the question regarding nuts was split into two categories: peanuts and tree nuts. Total nut intake was defined as the consumption of tree nuts and peanuts. Tree nuts included walnuts, almonds, Brazil nuts, cashews, pistachios, pecans, macadamias, hazelnuts, and pine nuts (not including peanuts, which are botanically legumes). A validation study of the FFQ demonstrated reasonable validity of the assessment of nut intake; the correlation coefficient was 0.75 between the FFQ and four 1-week diet records for nut intake.24
Our primary exposures of interest were total nut and specific types of nut consumption, including tree nuts and peanuts, assessed after diabetes diagnosis, and changes in nut consumption before and after diabetes diagnosis. The pre-diabetes nut intake was assessed from the most proximal questionnaires before diabetes was ascertained.
Ascertainment of diabetes.
A validated supplementary questionnaire regarding diagnostic tests, symptoms, and hypoglycemic therapy was mailed to participants who reported a physician’s diagnosis of diabetes on any of the biennial questionnaires. Prior to the release of the American Diabetes Association (ADA) criteria in 1997,25 the National Diabetes Data Group criteria were used to diagnose diabetes: 1) fasting glucose concentrations ≥7.8 mmol/l, blood glucose ≥11.1 mmol/l during an oral glucose tolerance test, or random blood glucose ≥11.1 mmol/l, together with one or more diabetes-related symptoms (weight loss, polyuria, excessive thirst, or hunger); 2) elevated glucose levels on more than one occasion in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or an oral hypoglycemic agent). Since 1998, the diagnosis criterion of fasting glucose was lowered to 7.0 mmol/l by ADA criteria. After 2010, HbA1c ≥6.5% was further included in the diagnosis criteria.
In our validation studies, 98% (61/62 cases) of diabetes cases confirmed by the supplementary questionnaire were re-confirmed by medical record review in the NHS, and 97% (57/59 cases) were re-confirmed in the HPFS.26, 27
Ascertainment of CVD and mortality.
The primary outcomes of the current study were CVD incidence and mortality. Incident CVD was defined as fatal and non-fatal CHD (including nonfatal myocardial infarction [MI] and coronary artery bypass graft surgery [CABG]) and fatal and non-fatal stroke. When participants reported cardiovascular events on any biennial questionnaires, permission was requested to access their medical records. Physicians blinded to the participant questionnaire data reviewed all medical records. Non-fatal MI was ascertained according to the World Health Organization criteria, including typical symptoms, elevated cardiac enzyme levels, and electrocardiographic findings.28 Non-fatal stroke was defined based on the National Survey of Stroke criteria, requiring evidence of neurologic deficits with sudden or rapid onset which persisted for at least 24 hours or until death.29 The diagnosis of CABG was based on self-report, for which the validity had been demonstrated.30
Deaths were identified by reports by next of kin, or the U.S. postal authorities, or searching the National Death Index. Using these methods, we were able to ascertain at least 98% of deaths in each cohort.31 Fatal CHD was defined if CHD was listed as the cause of death on the death certificate, and the history of CHD was evident through reviewing hospital records, autopsy reports or other information. Similarly, fatal stroke was identified and confirmed by reviewing death certificates, hospital records, or autopsy records. CVD mortality was defined as ICD-9 (International Classification of Diseases-Ninth Revision) codes of 390-459 and cancer mortality was defined as ICD-9 codes of 140-208.32
Assessment of covariates.
In the biennial follow-up questionnaires, information was updated on demographics, physical activity, cigarette smoking, alcohol consumption, menopausal status and use of postmenopausal hormones (women only), medical history (including use of aspirin and lipid-lowering medication), family history of MI or cancer, presence of hypertension, hypercholesterolemia, CVD, cancer, or other diseases. Body mass index (BMI) was calculated as self-reported weight in kilograms divided by the square of height in meters (kg/m2). Physical activity was estimated as metabolic equivalents (METs) per week based on the average hours spent on various activities, weighted by the intensity level.20
Statistical analysis.
Person-time was calculated from the date of a diabetes diagnosis to occurrence of study outcomes, last return of a valid follow-up questionnaire, or the end of follow-up (June 30, 2014 for the NHS and January 30, 2014 for the HPFS), whichever came first. We stopped updating dietary variables on a report of CVD or cancer because changes in diet after diagnosis of these diseases may confound the associations of interest (only for mortality analysis). Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of total nut and specific types of nut intake with total CVD, CHD, and stroke incidence, and all-cause and cause-specific mortality. Nut consumption after diabetes diagnosis was modeled as a time-varying variable. Changes in nut intake from pre- to post-diabetes diagnosis were defined as the absolute difference in nut consumption, i.e., time-varying post-diabetes nut intake minus pre-diabetes nut intake. Time-varying covariates were considered in the multivariate models. In multivariate models, we adjusted for age (continuous), diabetes duration (years), sex (men or women), Caucasian (yes/no), BMI at diabetes diagnosis (<23.0, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 MET-hours/week), smoking status (never, past, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), family history of MI or cancer (yes, no), current aspirin use (yes, no), presence of hypertension (yes, no), use of lipid-lowering medication (yes, no), diabetes medication use (insulin, oral medication, or others), and intake of total energy, red or processed meat, fruits, and vegetables (all in quartiles). In the analysis of changes in nut consumption from pre- to post-diabetes diagnosis, we further adjusted for nut intake before diabetes diagnosis in the multivariate model. In the current study, the proportional hazards assumption was tested by using a likelihood ratio test comparing models with and without multiplicative interaction terms between exposure and calendar year, and we did not find evidence of violation of the proportional hazards assumption. The linear trend was tested by assigning a median value to each category as a continuous variable. To examine a possible nonlinear relation between nut intake and CVD incidence and mortality, restricted cubic spline regression with 3 knots was used. Tests for nonlinearity were based on the likelihood ratio test comparing two models: one with only the linear term and the other with the linear and the cubic spline terms.
Analyses were further stratified by age at diabetes diagnosis (<65 or ≥65 years), sex/cohort (women/NHS, men/HPFS), BMI at diabetes diagnosis (<25.0, 25.0-29.9, ≥30.0 kg/m2), diabetes duration (<5, 5-9, ≥10 years), smoking status after diabetes diagnosis (never smoker, past smoker, current smoker), alcohol consumption (<5 or ≥5 g/day, approximately the population mean), physical activity (<18 or ≥18 MET-hours/week, approximately the population mean), hypertension or hypercholesterolemia at diabetes diagnosis (yes, no), nut consumption before diabetes diagnosis (≤1 or >1 serving/week), and the Alternate Healthy Eating Index without nut component (<49 or ≥49, approximately the population mean). The P values for the product terms between nut consumption and stratification variables were used to estimate the significance of interactions.
Several sensitivity analyses were conducted to test the robustness of our findings. First, to minimize within-person variation, the average of the last two FFQs was used to estimate the nut consumption. Second, to assess the potential influence of intake of sodium and olive oil on the results, we further adjusted for these variables in the models. Moreover, instead of adjusting for individual foods, we further adjusted for the Alternate Healthy Eating Index without nut component. Third, we continuously updated diet even after participants reported a diagnosis of CVD or cancer. Fourth, although our study population was relatively homogeneous in terms of socioeconomic status, participants’ perception on their standing in U.S. society (top 20%, 30%, 40%, 50%, or >50%) and educational attainment (registered nurse, bachelor’s degree, master’s degree and above, or others) were further adjusted (only in the NHS). In addition, although most women in our study were postmenopausal, menopausal status and use of postmenopausal hormones (premenopausal, postmenopausal never users, postmenopausal past users, or postmenopausal current users) were further included in the model. Fifth, for the analysis of specific types of nuts, we further mutually adjusted for types of nuts. Sixth, because tooth loss might influence nut consumption and is also associated with CVD risk and mortality,33 the number of natural teeth loss was further adjusted. Seventh, we restricted our analyses to incident diabetes cases only (excluding the diabetes cases at baseline). In addition, we stratified the analyses before and after 1998 (the median of follow-up time in the HPFS). Eighth, we excluded deaths that occurred within 4 years after diabetes diagnosis to examine whether the results were impacted by reverse causation bias. Lastly, to control for the potential confounding by glucose control, the self-reported levels of hemoglobin A1c (HbA1c) (<7.0%, 7.0-7.9%, 8.0-9.9%, 10.0-11.9%, and ≥12.0%) were further adjusted in a subgroup of the study participants (only available in supplementary questionnaires administered in 2000 and 2005 in the NHS and 2000, 2004, and 2008 in the HPFS). All statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina). Two-sided P<0.05 was considered statistically significant.
RESULTS
During 223,682 and 254,923 person-years of follow-up, a total of 3,336 incident CVD cases (including 2,567 CHD cases and 789 stroke cases) and 5,682 deaths (including 1,663 CVD deaths and 1,297 cancer deaths) were identified. Compared with participants who consumed total nuts less than 1 serving/month, those who consumed nuts more frequently were older, had a lower BMI and higher level of physical activity, were less likely to smoke, more likely to use aspirin and lipid-lowering medication, and had a higher consumption of total energy, alcohol, fruits, and vegetables (Table 1). Online Table I shows the partial Spearman correlations among types of nut consumption before and after diabetes diagnosis (the correlation coefficients [rs] ranged from 0.42-0.80 between tree nuts, peanuts, and total nuts after diabetes diagnosis, and rs ranged from 0.22-0.43 between individual types of nuts consumed before and after diabetes diagnosis, all P<0.001).
Table 1.
Characteristics of person-years according to total nut consumption among individuals with diabetes *
| Total Nut Consumption |
|||||
|---|---|---|---|---|---|
| <1 serving/month | <1 serving/week | 1 serving/week | 2-4 servings/week | ≥5 servings/week | |
| Person-years | 105778 | 35828 | 29121 | 34593 | 18362 |
| Age, years | 66.2 (10.2) | 64.7 (10.6) | 65.4 (10.0) | 67.7 (9.1) | 69.4 (9.1) |
| Men, % | 9.7 | 16.6 | 23.3 | 28.0 | 33.3 |
| Body Mass Index, kg/m2 | 30.0 (6.3) | 29.7 (6.3) | 29.3 (5.8) | 29.4 (6.0) | 28.5 (5.9) |
| Physical activity, MET-hours/week | 13.6 (21.8) | 15.2 (23.7) | 17.7 (26.8) | 21.3 (31.4) | 23.6 (30.9) |
| Current smoker, % | 10.2 | 9.3 | 8.4 | 7.3 | 6.8 |
| Hypertension, % | 76.3 | 75.3 | 75.6 | 73.9 | 71.1 |
| Hypercholesterolemia, % | 66.7 | 67.0 | 68.0 | 71.0 | 71.1 |
| Family history of MI, % | 21.7 | 23.9 | 25.9 | 24.5 | 24.7 |
| Aspirin use, % | 44.5 | 57.7 | 61.2 | 63.1 | 64.6 |
| Use of anti-hypertensive drug, % | 38.1 | 48.5 | 49.6 | 51.0 | 47.4 |
| Use of lipid-lowering medication, % | 23.0 | 32.0 | 33.2 | 38.4 | 40.3 |
| Total energy intake, kcal/day | 1608.6 (552.0) | 1657.4 (544.5) | 1753.9 (552.0) | 1888.8 (581.3) | 2100.8 (620.2) |
| Alcohol consumption, g/day | 3.2 (8.2) | 4.0 (9.5) | 4.8 (10.1) | 5.4 (10.7) | 6.5 (12.5) |
| Red or processed meat intake, servings/day | 0.9 (0.9) | 1.0 (0.8) | 1.1 (0.8) | 1.1 (0.8) | 1.1 (0.9) |
| Fruit intake, servings/day | 2.2 (1.3) | 2.3 (1.2) | 2.4 (1.2) | 2.4 (1.2) | 2.6 (1.4) |
| Vegetable intake, servings/day | 2.6 (1.3) | 2.8 (1.3) | 3.1 (1.4) | 3.3 (1.5) | 3.5 (1.6) |
| AHEI score, excluding nuts | 48.4 (10.4) | 48.8 (10.5) | 49.7 (10.3) | 51.1 (10.6) | 54.9 (11.2) |
Values are means (SD) or percentages (%). MET: metabolic equivalent. MI, myocardial infarction. AHEI: Alternate Healthy Eating Index.
After multivariate adjustments, including diabetes duration, BMI at diagnosis, other lifestyle and dietary factors, and medication use, higher nut consumption was significantly associated with a lower risk of total CVD and CHD incidence, and a lower CVD mortality and all-cause mortality (Table 2). The HRs and 95% CIs for participants who consumed nuts 5 or more servings/week, as compared with those who consumed nuts less than 1 serving/month, were 0.83 (0.71, 0.98; P trend=0.01) for total CVD, 0.80 (0.67, 0.96; P trend=0.005) for CHD, 0.66 (0.52, 0.84; P trend<0.001) for CVD mortality, and 0.69 (0.61, 0.77; P trend<0.001) for all-cause mortality (Table 2). Total nut consumption was not significantly associated with the risk of stroke incidence and cancer mortality.
Table 2.
Hazard ratios (95% CIs) of CVD incidence and mortality according to total nut consumption after diabetes diagnosis*
| Total Nut Consumption |
P trend | |||||
|---|---|---|---|---|---|---|
| <1 serving/month | <1 serving/week | 1 serving/week | 2-4 servings/week | ≥5 servings/week | ||
| CVD Incidence | ||||||
| Total CVD Incidence | ||||||
| Pearson-years | 105778 | 35828 | 29121 | 34593 | 18362 | |
| Cases | 1708 | 559 | 457 | 422 | 190 | |
| Age-adjusted model | 1.00 | 1.01 (0.92, 1.11) | 1.04 (0.93, 1.15) | 0.83 (0.75, 0.93) | 0.74 (0.63, 0.86) | <0.001 |
| Multivariable model | 1.00 | 1.04 (0.94, 1.15) | 1.08 (0.97, 1.20) | 0.91 (0.81, 1.02) | 0.83 (0.71, 0.98) | 0.01 |
| CHD Incidence | ||||||
| Cases | 1332 | 438 | 347 | 307 | 143 | |
| Age-adjusted model | 1.00 | 1.01 (0.91, 1.13) | 1.01 (0.89, 1.14) | 0.77 (0.68, 0.88) | 0.71 (0.59, 0.84) | <0.001 |
| Multivariable model | 1.00 | 1.03 (0.92, 1.15) | 1.04 (0.92, 1.18) | 0.84 (0.74, 0.96) | 0.80 (0.67, 0.96) | 0.005 |
| Stroke Incidence | ||||||
| Cases | 388 | 125 | 112 | 116 | 48 | |
| Age-adjusted model | 1.00 | 1.00 (0.81, 1.23) | 1.14 (0.92, 1.41) | 1.04 (0.83, 1.28) | 0.84 (0.62, 1.15) | 0.32 |
| Multivariable model | 1.00 | 1.07 (0.86, 1.31) | 1.21 (0.97, 1.51) | 1.15 (0.92, 1.44) | 0.93 (0.68, 1.29) | 0.74 |
| All-Cause and Cause-Specific Mortality | ||||||
| CVD Mortality | ||||||
| Pearson-years | 120820 | 41489 | 33594 | 38784 | 20236 | |
| Cases | 936 | 262 | 200 | 184 | 81 | |
| Age-adjusted model | 1.00 | 0.84 (0.73, 0.97) | 0.79 (0.68, 0.93) | 0.61 (0.52, 0.72) | 0.47 (0.37, 0.59) | <0.001 |
| Multivariable model | 1.00 | 0.97 (0.84, 1.11) | 0.94 (0.80, 1.11) | 0.83 (0.70, 0.98) | 0.66 (0.52, 0.84) | <0.001 |
| Cancer Mortality | ||||||
| Cases | 668 | 193 | 157 | 181 | 98 | |
| Age-adjusted model | 1.00 | 0.86 (0.73, 1.01) | 0.85 (0.71, 1.01) | 0.78 (0.66, 0.93) | 0.74 (0.59, 0.92) | 0.005 |
| Multivariable model | 1.00 | 0.93 (0.79, 1.10) | 0.91 (0.76, 1.09) | 0.89 (0.75, 1.07) | 0.84 (0.67, 1.06) | 0.12 |
| All-Cause Mortality | ||||||
| Cases | 3310 | 792 | 623 | 634 | 323 | |
| Age-adjusted model | 1.00 | 0.73 (0.68, 0.79) | 0.70 (0.65, 0.77) | 0.56 (0.51, 0.61) | 0.48 (0.43, 0.54) | <0.001 |
| Multivariable model | 1.00 | 0.89 (0.82, 0.96) | 0.86 (0.79, 0.95) | 0.78 (0.71, 0.86) | 0.69 (0.61, 0.77) | <0.001 |
Multivariable analyses were adjusted for age (continuous), diabetes duration (years), sex (men or women), Caucasian (yes/no), BMI at diabetes diagnosis (<23.0, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 MET-hours/week), smoking status (never, past, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), family history of MI or cancer (yes/no), current aspirin use (yes/no), presence of hypertension (yes/no), use of lipid-lowering medication (yes/no), diabetes medication use (insulin, oral medication, or others), and intake of total energy, red or processed meat, fruits, and vegetables (all in quartiles).
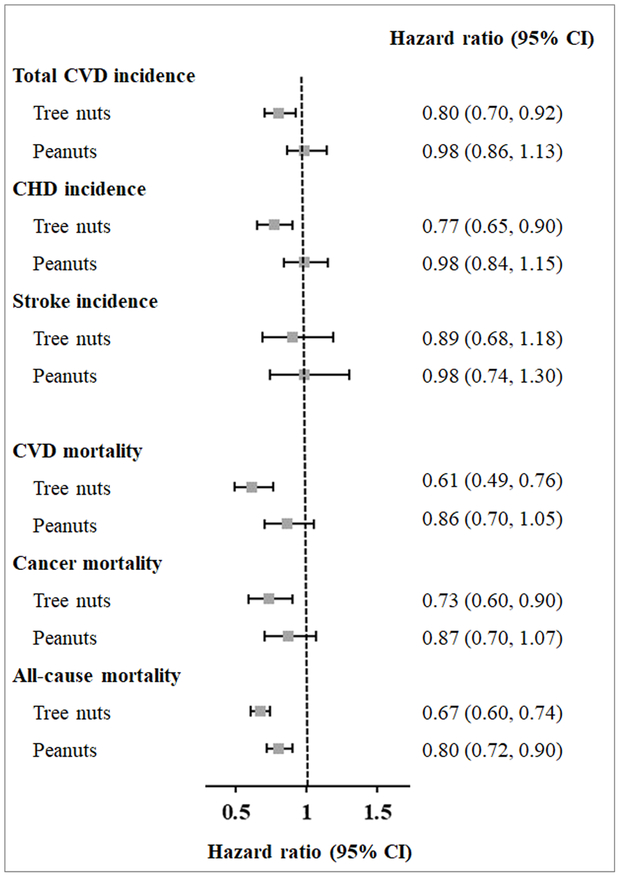
For the analyses of specific types of nuts, when comparing consumption of nuts of 2 or more servings/week with less than 1 serving/month, the HRs and 95% CIs for tree nuts were 0.80 (0.70, 0.92) for total CVD, 0.77 (0.65, 0.90) for CHD, 0.61 (0.49, 0.76) for CVD mortality, 0.73 (0.60, 0.90) for cancer mortality, 0.67 (0.60, 0.74) for all-cause mortality (all P trend<0.001) (Figure 1). Peanut consumption was inversely associated with all-cause mortality (HR [95% CI]: 0.80 [0.72, 0.90]; P trend<0.001), but not with other outcomes.
Figure 1. Hazard ratios (95% CIs) of CVD incidence and mortality according to types of nut consumption after diabetes diagnosis*.
* Multivariate hazard ratio for CVD incidence and mortality among study participants who consumed nuts two or more servings per week versus those who consumed nuts less than one serving per month were adjusted for age (continuous), diabetes duration (years), sex (men or women), Caucasian (yes/no), BMI at diabetes diagnosis (<23.0, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 MET-hours/week), smoking status (never, past, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), current aspirin use (yes/no), family history of MI or cancer (yes/no), presence of hypertension (yes/no), use of lipid-lowering medication (yes/no), diabetes medication use (insulin, oral medication, or others), and intake of total energy, red or processed meat, fruits, and vegetables (all in quartiles).
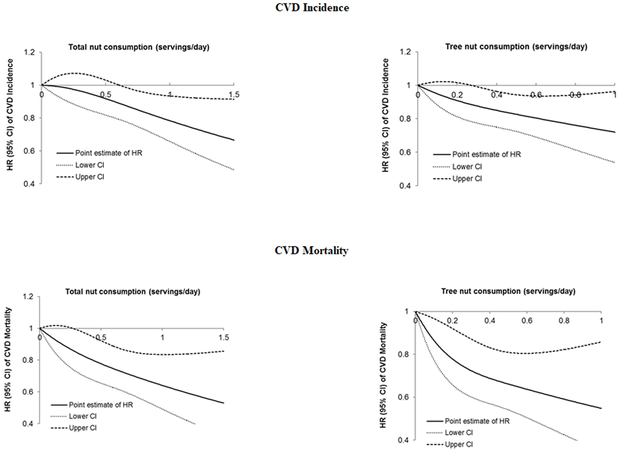
Figure 2 shows the dose-response relationship of total nut and tree nut intake with CVD incidence and CVD mortality. After multivariate adjustments, linear relationships (P linearity <0.001) were demonstrated; each one serving/week increment in total nut consumption was associated with a 3% (95% CI: 1%, 6%) lower risk of CVD incidence and 6% (95% CI: 3%, 10%) lower CVD mortality; and one serving/week increment in tree nut consumption was associated with a 5% (95% CI: 2%, 9%) lower risk of CVD incidence and 11% (95% CI: 5%, 15%) lower CVD mortality (all P trend<0.001). Online Figure II shows the dose-response relationship of total nut and tree nut intake with all-cause mortality (P linearity <0.001).
Figure 2. Associations between total and tree nut consumption and CVD incidence and CVD mortality *.
* Hazard ratios were adjusted for age (continuous), diabetes duration (years), sex (men or women), Caucasian (yes/no), BMI at diabetes diagnosis (<23.0, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0 kg/m2), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 MET-hours/week), smoking status (never, past, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), family history of MI or cancer (yes/no), current aspirin use (yes/no), presence of hypertension (yes/no), use of lipid-lowering medication (yes/no), diabetes medication use (insulin, oral medication, or others), and intake of total energy, red or processed meat, fruits, and vegetables (all in quartiles). All P for non-linearity were <0.001.
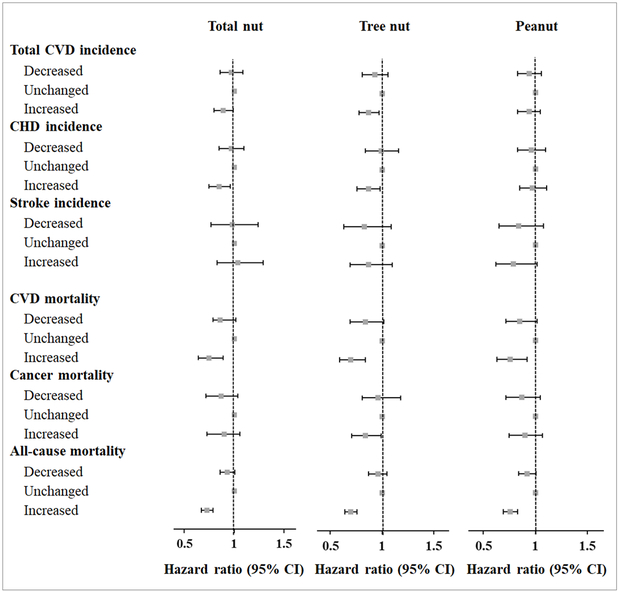
Increment in nut consumption from pre- to post-diabetes diagnosis was also significantly associated with a lower risk of CVD incidence and mortality (Figure 3). Compared with participants without changes in nut intake, those participants who increased nut consumption after diabetes diagnosis had an 11% lower risk of CVD, a 15% lower risk of CHD, a 25% lower CVD mortality, and a 27% lower all-cause mortality. The results were more pronounced for tree nuts (Figure 3).
Figure 3. Hazard ratios (95% CIs) of CVD incidence and mortality according to changes in consumption of total nuts, tree nuts, and peanuts before and after diabetes diagnosis*.
* Multivariable analyses were adjusted for age (continuous), diabetes duration (years), sex (men or women), Caucasian (yes/no), family history of MI or cancer (yes/no), hypertension status (no hypertension, new hypertension, always hypertension), lipid-lowering medication use (never user, new user, always user), aspirin use (never user, new user, always user), changes in smoking status (always never smoker, always past smoker, always current smoker, quit smoking after diabetes diagnosis, others), changes in physical activity (tertiles), changes in alcohol consumption (tertiles), changes in body mass index (tertiles), changes in total caloric intake (tertiles), changes in AHEI excluding nuts (tertiles), and nut consumption before diabetes diagnosis (continuous).
Consistent results were observed when analyses were stratified by age at diabetes diagnosis, sex/cohort, body mass index at diabetes diagnosis, diabetes duration, smoking status, alcohol consumption, physical activity, hypertension or hypercholesterolemia at diabetes diagnosis, nut consumption before diabetes diagnosis, and the Alternate Healthy Eating Index without nut component (Online Figure III). No significant interactions were detected between nut consumption and these stratifying variables (all P interaction>0.1).
In sensitivity analyses, similar results were observed when we used the averages of the last two FFQs to estimate nut consumption after diabetes diagnosis (Online Table II). The results did not materially change when we further adjusted for total intake of sodium and olive oil, the Alternate Healthy Eating Index without nut component, or the number of natural teeth (Online Table III). The results were similar when diet was continuously updated, or when further adjusting for participants’ perception on their standing in U.S. society, educational attainment, or menopausal status and hormone use in the NHS. When specific type of nuts was mutually adjusted, the results remained more pronounced for tree nuts (Online Table IV). Although some of the associations were attenuated probably due to reduced statistical power, most of the results remained significant when analyses were restricted to incident diabetes cases, or stratified before/after 1998, or when deaths occurred within 4 years after diabetes diagnosis were excluded (Online Table V). The inverse associations largely persisted when self-reported HbA1c levels were further controlled for in a subset of the study participants, although some of the associations did not reach statistical significance because of diminished power. For example, comparing extreme categories, the HRs and 95% CIs for tree nuts were 0.60 (0.35, 1.04) for total CVD incidence and 0.69 (0.51, 0.95) for all-cause mortality (both P trend<0.05).
DISCUSSION
In these two large prospective cohort studies among U.S. men and women with diabetes, we found that higher nut consumption, especially tree nuts, was significantly associated with a lower risk of total CVD and CHD incidence, and mortality due to CVD and all causes. The association was independent of established risk factors, including diabetes duration, BMI, lifestyle and dietary factors, medication use, and nut consumption before diabetes diagnosis. In addition, greater increment in nut consumption from pre- to post-diabetes diagnosis was also significantly associated with a lower risk of subsequent CVD events and mortality. Various sensitivity analyses and stratified analyses demonstrated the robustness of these associations.
Comparison with other studies.
Several meta-analyses of prospective observational studies have consistently found that nut consumption is inversely associated with risk of hypertension, CVD, total cancer, and all-cause and cause-specific mortality in the general population.3-6, 32, 34 In the PREDIMED trial among individuals at high cardiovascular risk (n=7447), participants assigned to a Mediterranean diet supplemented with mixed nuts (walnuts, almonds, and hazelnuts) had a 28% reduced risk in developing major cardiovascular events compared with a control diet.35 However, data pertaining to the potential health benefits of nut consumption among persons with diabetes are sparse. It is largely unknown whether nuts should be incorporated into diabetes patients’ diet toward the prevention of CVD events or premature deaths in this high-risk group of individuals. In the only study that examined nut intake in relation to CVD risk among women with diabetes, Li et al., found that total intake of nuts and peanut butter was associated with a lower risk of CVD.16 While our study confirmed the inverse associations for total nuts, we further demonstrated that the intake of tree nuts was more robustly associated with lower CVD incidence and mortality. A similar pattern of associations favoring tree nuts was observed for other disease outcomes. For example, in a prospective study among patients with colon cancer, Fadelu et al., found that tree nuts, but not peanuts, were significantly associated with a lower risk of cancer recurrence or mortality, and all-cause mortality.36 Although the exact reasons underlying these observations are unclear, it may be explained by the difference in nutrient profiles in oil contents between peanuts and tree nuts (e.g., 37.9 g/100g in peanuts vs 50.8 g/100g in walnuts), fatty acid composition (for total saturated fatty acids: 15.5 g/100g in peanuts vs 9.5 g/100g in walnuts; for total unsaturated fatty acids: 84.5 g/100g in peanuts vs 90.3 g/100g in walnuts), and other biologically active compounds (for γ-tocopherol: 60.3 μg/g in peanuts vs 300.5 μg/g in walnuts; for campesterol: 198.3 μg/g in peanuts vs 51.0 μg/g in walnuts; for stigmasterol: 163.3 μg/g in peanuts vs 55.5 μg/g in walnuts).17 Nevertheless, more research is warranted to substantiate the potentially differential associations between tree nuts and peanuts.
Although the exact mechanism underlying the beneficial effects of nut consumption for individuals with diabetes remains to be further elucidated, accumulating evidence from clinical trials among participants with diabetes suggests that nut consumption may improve glycemic control, blood pressure, lipid metabolism, inflammation, and endothelial dysfunction.13, 14, 37-42 These beneficial effects can be at least partially explained by the unique nutritional composition of nuts, including unsaturated fatty acids, fiber, vitamins (such as vitamin E and folate), minerals (such as calcium, potassium, and magnesium), and phytochemicals (such as flavonoids and phytosterols).1, 2 For instance, polyunsaturated fatty acids (PUFAs) in nuts could regulate gene expression in the pathway of lipid metabolism through various mechanisms, including changing membrane composition, eicosanoid production, and intracellular calcium levels.43 Furthermore, PUFAs and their metabolites could affect the transcription of several key genes in lipid metabolism, in conjunction with nuclear receptors and transcription factors, including the nuclear receptors peroxisome proliferator-activated receptor (PPAR), hepatocyte nuclear factor-4α, and liver X receptor (LXR), and the transcription factors sterol-regulatory element binding protein and nuclear factor-κB.43 In addition, α-linolenic acid could induce insulin-like growth factor 1 secretion in hepatocytes via PPAR pathway,44 resulting in a significant improvement in insulin sensitivity.45 Some in vitro studies suggested that phytosterols could reduce cholesterol absorption via the inhibition of 27-hydroxycholesterol generation, LXRα activation, and expression of the ATP-binding cassette transporter A1 in enterocytes.46 In addition, several clinical trials demonstrated that nut supplementation could positively modulate the expression of certain microRNAs (such as miR-192 and miR-375) related with glucose metabolism and insulin sensitivity,47 improve antioxidant status with an increase in oxygen radical absorbance capacity levels,48 favorably modify gut microbiota and bacterial activities via increasing the populations of Bifidobacterium spp. and Lactobacillus spp., and suppressing the growth of Clostridium perfringens.49 In a previous study conducted within the NHS and HPFS, frequent nut consumption was associated with a benign profile of inflammatory biomarkers (i.e., lower levels of plasma C-reactive protein and interleukin 6).50 Of note, despite the high energy density of nuts, there is no evidence for an association between frequent nut consumption and weight gain, possibly explained by the satiating effect of nut consumption.51 Nevertheless, more mechanistic studies are needed to further illustrate potential mechanisms through which nuts play a role in the prevention of morbidity and mortality among patients with diabetes.
Strengths and limitations.
The strengths of the present study include a prospective design, a relatively large sample size comprised of both men and women, long-term follow-up with a high retention rate, repeated assessments of total and specific nuts and other dietary and lifestyle variables before and after diabetes diagnosis, careful adjustments for a multitude of potential risk factors, and analyses of several adjudicated disease outcomes including total CVD, CHD, and stroke incidence, and all-cause and cause-specific mortality.
Several limitations should be considered as well. First, this is an observational study, and therefore causality cannot be proven. In addition, our study participants were all health professionals, and most were Caucasians. Although the relative homogeneity potentially minimizes confounding by socioeconomic status, it could limit generalizability of our findings to other ethnic groups. However, the underlying biological mechanisms are unlikely to differ between health professions and other populations.52 Second, the diabetic patients in our study were diagnosed during an extended period of time since 1980s. The risk profile of persons with diabetes might significantly change over time due to better control of hypertension, blood lipids, and other risk factors in recent years, although similar results were found in analyses stratified by follow-up time. Third, although our validation studies demonstrated reasonable validity of questionnaire assessments for total nut consumption, the validity of self-reported intake of specific types of nuts was not assessed. Measurement errors in self-reported nut intake were inevitable. However, such measurement errors were likely to be non-differential in this prospective study and thus would be more likely to bias the associations towards the null. Fourth, our study did not have direct measurements of glycemic control and severity of diabetes, although the results did not change significantly when further adjusting for duration of diabetes, use of insulin and hypoglycemic medications, or self-reported HbA1c levels. Despite that the validation study indicated excellent accuracy and validity of self-reported diabetes status in our study population who were all health professionals, the validity of self-reported diabetes duration, medication use, and self-reported HbA1c levels has not been illustrated in our cohort. We could not exclude the presence of residual confounding by severity of diabetes or glucose control. Fifth, our study did not have information on how nuts were prepared (e.g., raw, roasted, or salted), and the potential influence of preparation methods could not be tested. In addition, no information on nut allergy was collected in our population, which should be taken into account in future studies. Lastly, the role of confounding by genetic susceptibility or psychosocial stress, residual confounding due to measurement errors of covariates, including other lifestyle factors, or chance in the present study could not be excluded.
Conclusions.
Findings from two large prospective cohort studies suggest that frequent consumption of nuts, especially tree nuts, is associated with a lower risk of CVD incidence and mortality among participants with diabetes. In addition, increased nut consumption before and after diabetes diagnosis is also associated with a lower risk of subsequent CVD events and mortality. These data suggest a potential role of nut intake in the prevention of morbidity and mortality among individuals with diabetes.
Supplementary Material
Novelty and Significance.
What Is Known?
Higher nut consumption is associated with a lower risk of developing hypertension, cardiovascular disease (CVD), and cancer, as well as lower all-cause and cause-specific mortality in populations who are largely healthy.
Evidence regarding the potential health benefits of nut consumption is scarce among individuals with type 2 diabetes who have altered metabolism of macronutrients and elevated risk of CVD and premature deaths.
What New Information Does This Article Contribute?
Higher consumption of nuts, especially tree nuts, is associated with lower CVD incidence and mortality among individuals with diabetes.
Greater increment in nut consumption from pre- to post-diabetes diagnosis is also significantly associated with a lower risk of subsequent CVD incidence and mortality.
Accumulating evidence has demonstrated that more frequent nut consumption is associated with a lower risk of hypertension, CVD, total cancer, and all-cause and cause-specific mortality, primarily in the general population. However, among individuals with type 2 diabetes who have elevated risk of developing CVD and mortality, evidence regarding the potential health benefits of nut consumption is scarce. In addition, whether increased nut consumption from pre- to post-diabetes diagnosis may yield health benefits is unknown. In two large prospective cohort studies among U.S. men and women with diabetes, we found that higher nut consumption, especially tree nuts, was significantly associated with a lower risk of total CVD and CHD incidence, and mortality due to CVD and all causes. The association was independent of established risk factors, including diabetes duration, body mass index, lifestyle and dietary factors, medication use, and nut consumption before diabetes diagnosis. In addition, greater increment in nut consumption after diabetes diagnosis was also significantly associated with a lower risk of subsequent CVD events and mortality. These data provide novel evidence that supports the recommendation of incorporating nuts into healthy dietary patterns for the prevention of CVD complications and premature deaths among individuals with diabetes.
ACKNOWLEDGMENTS
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study that contributed data for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
SOURCES OF FUNDING
This study was supported by research grants from the National Institutes of Health (CA186107, CA176726, CA167552, DK082486, HL35464, HL088521, DK058845, U01 CA167552, and HL034594).
Nonstandard Abbreviations and Acronyms:
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CI
confidence interval
- HR
hazard ratio
Footnotes
DISCLOSURES
Drs. Hu and Li reports grants from the California Walnut Commission. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Kris-Etherton PM, Hu FB, Ros E, Sabate J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J Nutr. 2008;138:1746S–1751S [DOI] [PubMed] [Google Scholar]
- 2.Ros E, Tapsell LC, Sabate J. Nuts and berries for heart health. Curr Atheroscler Rep. 2010;12:397–406 [DOI] [PubMed] [Google Scholar]
- 3.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: A cohort study and meta-analysis. Int J Epidemiol. 2015;44:1038–1049 [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Yu H, He F, Reilly KH, Zhang J, Li S, Zhang T, Wang B, Ding Y, Xi B. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: A systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2014;100:270–277 [DOI] [PubMed] [Google Scholar]
- 6.Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu M, Hu FB, Liu L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am J Clin Nutr. 2014;100:256–269 [DOI] [PubMed] [Google Scholar]
- 7.Mayhew AJ, de Souza RJ, Meyre D, Anand SS, Mente A. A systematic review and meta-analysis of nut consumption and incident risk of cvd and all-cause mortality. Br J Nutr. 2016;115:212–225 [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Alonso P, Salas-Salvado J, Baldrich-Mora M, Juanola-Falgarona M, Bullo M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care. 2014;37:3098–3105 [DOI] [PubMed] [Google Scholar]
- 9.Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827 [DOI] [PubMed] [Google Scholar]
- 10.Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr. 2015;102:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987–2992 [DOI] [PubMed] [Google Scholar]
- 12.Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Cabre-Vila JJ, Salas-Salvado J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21:126–135 [DOI] [PubMed] [Google Scholar]
- 13.Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type ii diabetes. Eur J Clin Nutr. 2009;63:1008–1015 [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care. 2010;33:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: Influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171:404–410 [DOI] [PubMed] [Google Scholar]
- 16.Li TY, Brennan AM, Wedick NM, Mantzoros C, Rifai N, Hu FB. Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J Nutr. 2009;139:1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–178 [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, Monson RR, Stason W, Hennekens CH. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317:1303–1309 [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol. 1991;67:933–938 [DOI] [PubMed] [Google Scholar]
- 20.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Manson JE, Hankinson SE. The nurses’ health study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62 [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468 [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Li Y, Hu Y, Zong G, Li S, Rimm EB, Hu FB, Manson JE, Rexrode KM, Shin HJ, Sun Q. Influence of lifestyle on incident cardiovascular disease and mortality in patients with diabetes mellitus. J Am Coll Cardiol. 2018;71:2867–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867 [DOI] [PubMed] [Google Scholar]
- 25.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 28.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, Lisheng L. World health organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40:139–146 [DOI] [PubMed] [Google Scholar]
- 29.Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke. 1981;12:I13–44 [PubMed] [Google Scholar]
- 30.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900 [DOI] [PubMed] [Google Scholar]
- 31.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and equifax nationwide death search. Am J Epidemiol. 1994;140:1016–1019 [DOI] [PubMed] [Google Scholar]
- 32.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a chinese population-based cohort. Int J Epidemiol. 2005;34:467–474 [DOI] [PubMed] [Google Scholar]
- 34.Guasch-Ferre M, Liu X, Malik VS, Sun Q, Willett WC, Manson JE, Rexrode KM, Li Y, Hu FB, Bhupathiraju SN. Nut consumption and risk of cardiovascular disease. J Am Coll Cardiol. 2017;70:2519–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Fito M, Gea A, Hernan MA, Martinez-Gonzalez MA. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 36.Fadelu T, Zhang S, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson AB, Atienza DM, Messino M, Kindler HL, Venook A, Ogino S, Ng K, Wu K, Willett W, Giovannucci E, Meyerhardt J, Bao Y, Fuchs CS. Nut consumption and survival in patients with stage iii colon cancer: Results from calgb 89803 (alliance). J Clin Oncol. 2018;36:1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulati S, Misra A, Pandey RM. Effect of almond supplementation on glycemia and cardiovascular risk factors in asian indians in north india with type 2 diabetes mellitus: A 24-week study. Metab Syndr Relat Disord. 2017;15:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parham M, Heidari S, Khorramirad A, Hozoori M, Hosseinzadeh F, Bakhtyari L, Vafaeimanesh J. Effects of pistachio nut supplementation on blood glucose in patients with type 2 diabetes: A randomized crossover trial. Rev Diabet Stud. 2014;11:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendall CW, Esfahani A, Josse AR, Augustin LS, Vidgen E, Jenkins DJ. The glycemic effect of nut-enriched meals in healthy and diabetic subjects. Nutr Metab Cardiovasc Dis. 2011;21 Suppl 1:S34–39 [DOI] [PubMed] [Google Scholar]
- 40.Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin a(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;60:1312–1317 [DOI] [PubMed] [Google Scholar]
- 41.Jenkins DJA, Kendall CWC, Lamarche B, Banach MS, Srichaikul K, Vidgen E, Mitchell S, Parker T, Nishi S, Bashyam B, de Souza RJ, Ireland C, Pichika SC, Beyene J, Sievenpiper JL, Josse RG. Nuts as a replacement for carbohydrates in the diabetic diet: A reanalysis of a randomised controlled trial. Diabetologia. 2018;61:1734–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y, Keogh JB, Clifton PM. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: Multiple potential mechanisms of actions. Nutrients. 2017;9:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr. 2005;25:317–340 [DOI] [PubMed] [Google Scholar]
- 44.Fang XL, Shu G, Zhang ZQ, Wang SB, Zhu XT, Gao P, Xi QY, Zhang YL, Jiang QY. Roles of alpha-linolenic acid on igf-i secretion and gh/igf system gene expression in porcine primary hepatocytes. Mol Biol Rep. 2012;39:10987–10996 [DOI] [PubMed] [Google Scholar]
- 45.Clemmons DR. The relative roles of growth hormone and igf-1 in controlling insulin sensitivity. J Clin Invest. 2004;113:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brauner R, Johannes C, Ploessl F, Bracher F, Lorenz RL. Phytosterols reduce cholesterol absorption by inhibition of 27-hydroxycholesterol generation, liver x receptor alpha activation, and expression of the basolateral sterol exporter atp-binding cassette a1 in caco-2 enterocytes. J Nutr. 2012;142:981–989 [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Alonso P, Giardina S, Salas-Salvado J, Arcelin P, Bullo M. Chronic pistachio intake modulates circulating micrornas related to glucose metabolism and insulin resistance in prediabetic subjects. Eur J Nutr. 2017;56:2181–2191 [DOI] [PubMed] [Google Scholar]
- 48.Davis L, Stonehouse W, Loots du T, Mukuddem-Petersen J, van der Westhuizen FH, Hanekom SM, Jerling JC. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr. 2007;46:155–164 [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Lin X, Huang G, Zhang W, Rao P, Ni L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe. 2014;26:1–6 [DOI] [PubMed] [Google Scholar]
- 50.Yu Z, Malik VS, Keum N, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Bao Y. Associations between nut consumption and inflammatory biomarkers. Am J Clin Nutr. 2016;104:722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattes RD, Kris-Etherton PM, Foster GD. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr. 2008;138:1741S–1745S [DOI] [PubMed] [Google Scholar]
- 52.Luu HN, Blot WJ, Xiang YB, Cai H, Hargreaves MK, Li H, Yang G, Signorello L, Gao YT, Zheng W, Shu XO. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern Med. 2015;175:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all supporting data are available within the article and its online supplementary files.