Abstract
The International Endocervical Adenocarcinoma Criteria and Classification (IECC) categorizes endocervical adenocarcinomas (ECAs) based on morphological features linked to etiology (i.e. human papilloma virus (HPV) infection), resulting in separation of ECAs into HPV-associated (HPVA) and unassociated, or non-HPVA (NHPVA) types. NHPVAs are reported to be large and present at high stage in older individuals. Our aim was to examine the clinical outcomes in these tumor types.
Full slide sets of 205 ECAs were collected from 7 institutions worldwide and classified based on IECC criteria and the presence or absence of HPV. Clinical and morphological parameters were correlated with follow-up data. Statistical analysis of overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) were conducted using the Kaplan-Meier survival analysis and compared using the log-rank test for univariate analysis. Multivariate survival analysis was conducted, and the survival endpoints considered were OS, disease-free survival (DFS), and PFS.
Statistically significant survival differences (overall survival [OS], disease-free survival [DFS] and progression-free survival [PFS] were found when comparing the following categories: HPVA>NHPVA (i.e., survival was superior in the setting of HPVAs), including patients treated with surgery followed by adjuvant therapy; usual-type HPVA>mucinous HPVA; FIGO grade 3 HPVA>NHPVA; HPVA>NHPVA, both with lymphovascular invasion (LVI); and HPVA>NHPVA in patients with pelvic recurrences. Although there were trends favoring HPVA outcomes over those of NHPVA, these differences were not statistically significant in the following categories: mucinous HPVA versus NHPVA; HPVA versus NHPVA, both with lymph node metastases at presentation; and HPVA versus NHPVA in patients with distant metastasis. Survival for both HPVA and NHPVA were similar when surgery without adjuvant therapy was used. FIGO grading did not have prognostic significance in HPVAs. Multivariable analysis of HPVAs indicated nearly significant statistical associations between stage and both OS and DFS (p=0.07 and 0.06, respectively), and between Silva invasion pattern and OS (p=0.09). Multivariate analysis of NHPVAs indicated statistically significant association between OS and age (p=0.03), stage (p=0.02) and tumor size (p=0.002), and between DFS and stage (p=0.004) and tumor size (p=0.004). Multivariate analysis of HPVAs and NHPVAs together revealed nearly significant associations between OS and HPV status and stage (both [p=0.06]). For DFS, stage was a significant variable (p=0.04), while HPV status and tumor size were nearly significant (p=0.06 and 0.07, respectively).
Clinical outcome studies support the idea that the IECC classification not only separates ECAs based on HPV status (usually assessed on H&E slides), but also has important clinical relevance.
Keywords: Endocervical adenocarcinoma, HPV, Clinical outcomes, Management
INTRODUCTION
The International Endocervical Adenocarcinoma Criteria and Classification (IECC) has been proposed as an alternative to the WHO 2014 classification [1, 2]. IECC classifies endocervical adenocarcinomas (ECAs) based on morphological features linked to HPV infection as assessed on H&E slides, resulting in separation of ECAs into two broad categories: HPV-associated (HPVA) and unassociated, or non-HPVA (NHPVA) adenocarcinomas. The most common HPVA is usual-type; 95% of these are determined to be HPV-positive through use of an RNA-based in situ hybridization assay that recognizes 18 different types of high-risk HPV. Gastric-type is the most common NHPVA and is HPV-negative in all cases [2]. The validity of this new classification is supported by HPV status and, at present, by limited clinical data [2, 3]. In this report, we aim to further explore clinical outcomes in ECAs classified by IECC. This information should have practical relevance in the management of patients with endocervical adenocarcinoma.
MATERIALS AND METHODS
Institutional approval for this study was obtained from each of the participating centers.
Full slide sets of 409 invasive ECAs were collected from 7 institutions worldwide (USA: Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, and Massachusetts General Hospital, Boston, MA; Canada: Vancouver General Hospital and *OVCARE and British Columbia Cancer Research Centre, Vancouver, BC; Romania: University of Medicine and Pharmacy of Targu Mures and Regional Institute of Oncology, Iasi; Japan: The Jikei University School of Medicine, Tokyo; Mexico: Hospital de Oncología Mexico City, Mexico City; Israel: Sheba Medical Center, Tel-Hashomer, Ramat Gan; Italy: Ospedale Sacro Cuore Don Calabria, Negrar). In-situ carcinomas, squamous carcinomas, adenosquamous carcinomas, tumors with a neuroendocrine component, carcinosarcomas, and any tumor demonstrating clinical, macroscopic or microscopic features suggesting a lower uterine segment, uterine corpus, or adnexal primary, were excluded. Tumors treated with neoadjuvant chemotherapy and/or radiotherapy, were also excluded. Types of specimens included were conizations/trachelectomies/hysterectomies with lymph node dissection; however, biopsy and LEEP specimens were excluded. Clinical stage was assigned for FIGO stages > IB1 and microscopic/surgico-pathological staging was used for FIGO stages < IB1. Stage IB1 contained a mixture of clinically staged and surgico-pathologically staged cases, per FIGO. Tumor size (largest dimension) was measured macroscopically when tumor was grossly visible at prosection, and microscopically when a macroscopic abnormality was not appreciable.
All subtypes of ECA were included in this study (Figure 1). Assessment of morphology required examination of all H&E slides with tumor (an average of 12 slides per case). A consensus diagnosis was reached in every case, with at least two and as many as four study pathologists reviewing slides at a multi-head microscope. All tumors were classified according to the IECC [2], and microscopic grading was performed according to the FIGO grading system as used for endometrioid endometrial carcinomas (grade 1: <5% solid growth, grade 2: 6–50% solid growth, grade 3: >50% solid growth). [1]. Briefly, the presence of apical mitotic figures and apoptotic bodies easily appreciable at scanning magnification were sufficient to place a tumor in the HPVA category; ECAs lacking these features were assigned to the NHPVA category [2]. As both usual and mucinous HPV-associated adenocarcinomas can exhibit cytoplasmic mucin, we diagnosed usual-type HPVA when a tumor demonstrated fewer than 50% of neoplastic cells with obvious intracytoplasmic mucin, as seen on H&E sections; mucinous HPVA, not otherwise specified (NOS) had features similar to usual-type adenocarcinoma, but had more than 50% of neoplastic cells with appreciable intracytoplasmic mucin. HPV-associated mucinous carcinomas were further subclassified into: intestinal-type mucinous carcinoma, in which more than 50% of glands contained goblet cells; and invasive stratified mucin-producing carcinoma (iSMILE), in which a lesion was composed almost entirely (> 90%) [4] of invasive nests of stratified columnar cells with peripheral palisading and variable amounts of intracytoplasmic mucin [5], resembling its in-situ counterpart (SMILE) [6]. Gastric-type NHPVAs were diagnosed according to existing criteria [7].
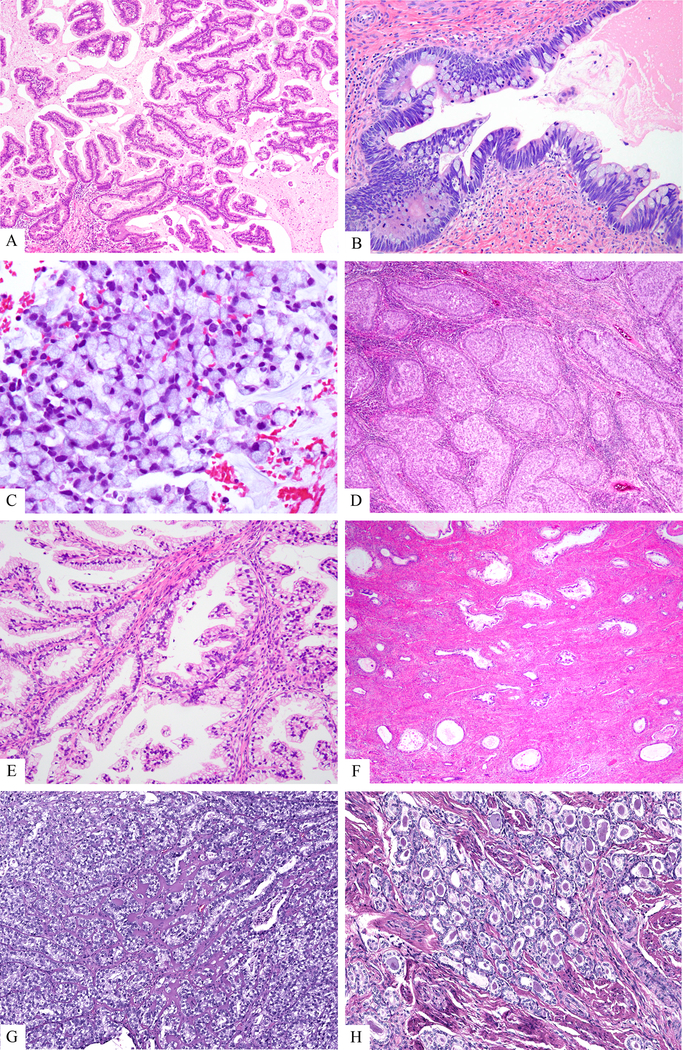
Figure 1:
A. Mucinous HPVA, not otherwise specified, is characterized by a glandular proliferation with neoplastic cells showing easily identified mitoses and apoptotic bodies (HPVA-defining) as well as obvious intracytoplasmic mucin constituting > 50% of cells. B. Mucinous HPVA, intestinal-type demonstrates features of HPVAs and, in addition, shows obvious goblet cells. C. Mucinous HPVA, signet ring type. This tumor tested positive with the HR-HPV probe. D. Invasive stratified mucinous carcinoma (HPVA). This tumor is typified by invasive nests of stratified mucin-containing neoplastic cells surrounded by a peripheral rim of basaloid/reserve-like cells. E. Gastric-type carcinoma (NHPVA). This tumor is composed of neoplastic glands lined by high columnar cells, with pink-to-clear cytoplasm with a “plant cell-look”, and mostly basally placed atypical nuclei. Easily identified mitoses and apoptotic bodies are not appreciated. F. Minimal deviation adenocarcinoma (‘adenoma malignum” of mucinous type or gastric-type adenocarcinoma, well differentiated), in NHPVA. Highly differentiated glands, some irregularly shaped, lined by high columnar cells, with pink-to-clear cytoplasm with a “plant cell-look”, and mostly basally placed atypical nuclei. Only a minimal stromal reaction to invasion is present, while easily identified mitoses and apoptotic bodies are not appreciated. G. Clear cell carcinoma (NHPVA) resembles clear cell carcinomas of endometrium and ovary. H. Mesonephric carcinoma (NHPVPA) can display many patterns, but the example displayed here shows one of the most common patterns: neoplastic glands containing intraluminal eosinophilic secretions. Easily identified mitoses and apoptotic bodies are not appreciated
HPV status was determined by HPV in-situ hybridization in tissue microarrays, using the Advanced Cell Diagnostics (ACD) (Hayward, CA) RNAscope® system (catalogue no. 312598). The RNAscope® Probe HPV-HR18 contains probes targeting E6 and E7 mRNA for the following high-risk subtypes: HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82. The methodology and interpretation have previously been discussed in detail [2].
Clinical data and follow-up were collected. Of the 409 cases, 205 were tested with the HPV-HR18 probe; these constituted the study set. To exclude the possibility of ascertainment bias, we verified that the study cases did not differ statistically from the remaining 204 cases with respect to the clinicopathological variables studied, the only exception being more high-grade cases among the cases not tested for HPV. Of the 409 cases, 205 were reviewed for both morphological features and HPV status. “Local recurrence” was defined as recurrent disease confined to the pelvis, including pelvic lymph nodes and para-aortic lymph nodes, while “Distant recurrence” was defined as an extrapelvic recurrence.
Pelvic and paraaortic lymph nodes surgically removed at hysterectomy were recorded as lymph node metastases (LNM), while recurrence in pelvic lymph node(s) was classified as a local recurrence.
One hundred eighty-seven cases of the 205 cases had at least 5-year follow-up and were retained for statistical analysis, using GraphPad Prism 6 for Windows. The survival curves were determined by Kaplan-Meier survival analysis, and the log-rank test was used to compare clinical outcomes in the different tumor categories. Adjusted recurrence and survival curves were estimated by overall hazard ratio power transformations of the baseline comparison curves for univariate analysis. For the multivariate analysis, the covariates age, stage, grade, tumor size and Silva pattern, were compared to the end-points OS, PFS and DFS. In the NHPVA-only group, we excluded Silva pattern as a covariate because those patients are all classified as “C”. The statistical significance of each covariate was assessed using their respective likelihood ratio test p-values from the multivariate survival model. We considered the value p<0.05 to be statistically significant.
RESULTS
Among the 205 cases with known HPV status, the most common HPVA type was usual-type (71.7%), and the most common NHPVA type was gastric-type (11.7%) (Table 1). Less common HPVAs were mucinous HPVAs of various subtypes (mucinous not otherwise specified [NOS], iSMILE, and intestinal type). Clear cell carcinoma was the next most common NHPVA (3.4% of the entire cohort). The following types (all NHPVAs) were rarely identified: endometrioid (01.0%), serous (0.5%) and mesonephric (0.5%) (Table 1).
Table 1.
Distribution of cohort: IECC subtypes, patient age, tumor size, FIGO stage, presence of LVI, LNM, and local (pelvic) and distant (extrapelvic) recurrences
| Subtypes | Cases nr/% | Age* (IQR) | Tumor size* (mm) (IQR) | FIGO stage I nr/% | FIGO stage II nr/% | FIGO stage III nr/% | FIGO stage IV nr/% | LVI nr/% | LNM nr/% | Local recurrence nr/% | Distant recurrence nr/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual (HPVA) | 147 (71.7) | 42 (16–82) | 20 (1–144) | 123 (83.7) | 7 (4.76) | 2 (1.36) | 1 (0.68) | 73 (49.8) | 18 (12.2) | 13 (8.8) | 8 (5.4) |
| Mucinous NOS (HPVA) | 3 (1.46) | 41 (41–44) | 6 (6–21) | 2 (66.7) | 1 (33.3) | 0 | 0 | 2 (66.7) | 0 | 1 (33.3) | 0 |
| Mucinous Intestinal (HPVA) | 4 (1.95) | 37.5 (30–67) | 8.5 (0.5–1.5) | 3 (75) | 1 (25) | 0 | 0 | 0 | 0 | 1 (25) | 0 |
| iSMILE (HPVA) | 9 (4.39) | 50 (25–66) | 9 (3–36) | 8 (88.9) | 0 | 1 (11.1) | 0 | 2 (22.2) | 2 (22.2) | 0 | 2 (22.2) |
| Villoglandular (HPVA) | 2 (0.97) | 31 (31–31) | 6 (4–8) | 2 (100) | 0 | 0 | 0 | 2 (100) | 2 (100) | 0 | 0 |
| Adenocarcinoma NOS (HPVA) | 4 (1.95) | 57 (35–68) | 38.5 (15–45) | 0 | 0 | 4 (100) | 0 | 2 (50) | 2 (50) | 0 | 2 (50) |
| Gastric (NHPVA) | 24 (11.7) | 49.5 (36–78) | 40 (10–90) | 9 (37.5) | 11 (45.8) | 2 (8.33) | 0 | 20 (83.3) | 4 (16.7) | 8 (33.3) | 2 (8.33) |
| Clear cell (NHPVA) | 7 (3.41) | 65 (33–79) | 43 (5.5–45) | 4 (57.1) | 3 (42.9) | 0 | 0 | 5 (71.4) | 1 (14.3) | 0 | 3 (42.9) |
| Endometrioid (NHPVA) | 2 (0.97) | 60.5 (55–65) | 17.2 (4.3–30) | 1 (50) | 0 | 0 | 0 | 1 (50) | 1 (50) | 0 | 0 |
| Serous (NHPVA) | 1 (0.48) | 69 | 44 | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | 0 | 1 (100) |
| Mesonephric (NHPVA) | 1 (0.48) | 52 | 0.2 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adenocarcinoma NOS (NHPVA) | 1 (0.48) | 64 | 35 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
Median
Abbreviations: nr, number of cases; %, percentage of cases; LVI, lymphovascular invasion; LNM, lymph node metastases
As previously reported [2], NHPVAs tended to be larger (p=0.076) and occurred in older patients, compared to HPVAs (Table 1). 62.5% of gastric-type cases were Stage II or higher, while 83.7% of usual-type ECAs were Stage I at diagnosis (Table 1). NHPVAs were more frequently associated with lymphovascular invasion (LVI), LNM, and local and distant recurrence, compared to HPVAs (Table 1). 49.8% of usual-type HPVAs were associated with LVI and 12.2% with LNM, compared to gastric-type ECAs in which LVI was present in 83.3% of cases and LNM in 16.7% of cases. Only 8.8% of patients with usual-type ECAs developed local recurrences and 5.4% developed distant recurrences. One-third of patients with gastric-type ECAs experienced local recurrences and 8.3% suffered distant recurrences (Table 1).
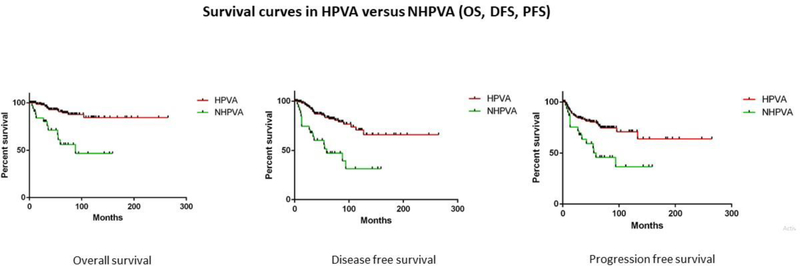
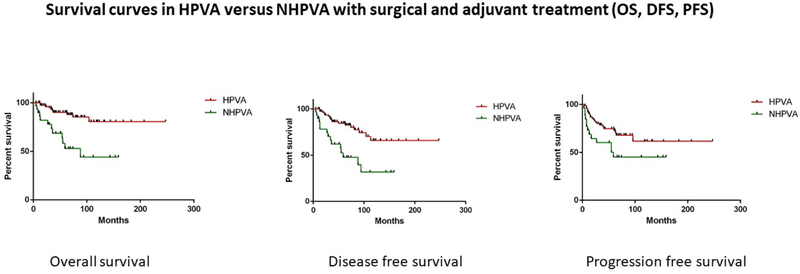
One hundred eighty-seven cases with both at least 5-year follow up and ascertained HPV status were used for outcomes analyses. HPVAs were associated with better overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) compared to NHPVAs (p<0.0001, p< 0.0001 and p=0.0002, respectively) (Table 2) (Figure 2). HPVAs demonstrated better OS, DFS and PFS, compared to NHPVAs, in patients who received surgical and adjuvant treatment (p<0.0001, p=0.0002 and p=0.03 respectively); however, outcomes were comparable in patients who received only surgical treatment (Table 2) (Figure 3). Adjuvant treatment consisted primarily of combined radiation and chemotherapy; only a minority of patients received adjuvant radiotherapy alone.
Table 2.
Univariate analysis of OS, DFS and PFS in IECC cohort with respect to IECC subtype, microscopic grade, presence of LVI, LNM, treatment, local (pelvic) and distant (extrapelvic) recurrences.
| Test | OS | P value | HR | CI | DFS | P value | HR | CI | PFS | P value | HR | CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPVA vs. NHPVA | **** | < 0.0001 | 0.06 | 0.017– 0.17 | **** | < 0.0001 | 0.18 | 0.07– 0.41 | *** | 0.0002 | 0.20 | 0.08– 0.47 |
| HPVA usual vs. HPVA mucinous | NS | 0.08 | 0.18 | 0.02– 1.19 | NS | 0.28 | 0.48 | 0.12– 1.82 | * | 0.04 | 0.20 | 0.03– 0.98 |
| HPVA mucinous vs. NHPVA gastric | NS | 0.10 | 0.40 | 0.13– 1.19 | NS | 0.34 | 0.63 | 0.2432–1.620 | NS | 0.67 | 0.78 | 0.25– 2.41 |
| Grade 3: HPVA vs. NHPVA | **** | <0.0001 | 0.02 | 0.005– 0.16 | ** | 0.0097 | 0.16 | 0.03–0.63 | NS | 0.41 | 0.52 | 0.11–2.44 |
| Grade 1 vs. 2 vs. 3: HPVA | NS | 0.57 | NS | 0.59 | NS | 0.38 | ||||||
| Grade 1 vs. 2 vs. 3: NHPVA | NS | 0.22 | NS | 0.3 | NS | 0.99 | ||||||
| LVI: HPVA vs. NHPVA | ** | 0.003 | 0.21 | 0.07–0.61 | * | 0.02 | 0.42 | 0.19–0.88 | NS | 0.06 | 0.49 | 0.23–1.04 |
| LNM: HPVA vs. NHPVA | NS | 0.26 | 0.37 | 0.06–2.04 | NS | 0.46 | 0.62 | 0.16–2.28 | NS | 0.28 | 0.56 | 0.20–1.59 |
| Surgical treatment: HPVA vs. NHPVA | NS | 0.73 | 2.8 | 0.007– 10 | ** | 0.06 | 0.009 | 0.0003–0.26 | ** | 0.007 | 0.01 | 0.0004–0.30 |
| Surgical + oncologic treatment: HPVA vs. NHPVA | **** | < 0.0001 | 0.13 | 0.04–0.36 | *** | 0.0002 | 0.18 | 0.07–0.44 | * | 0.03 | 0.41 | 0.18–0.93 |
| Pelvic recurrence: HPVA vs. NHPVA | *** | 0.0005 | 0.05 | 0.01–0.28 | ** | 0.005 | 4.58 | 1.90–11.06 | * | 0.01 | 0.21 | 0.06–0.69 |
| Distant recurrence: HPVA vs. NHPVA | NS | 0.09 | 3.404 | 0.82–14.08 | NS | 0.58 | 1.39 | 0.43–4.46 | NS | 0.48 | 1.03 | 0.35–3.03 |
Abbreviations: NS, not significant
Figure 2: Survival curves in HPVA versus NHPVA.
Kaplan-Meier rates of overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) in IECC cases (HPVA vs. NHPVA)
Figure 3: Survival curves in HPVA versus NHPVA with surgical and adjuvant treatment.
Kaplan-Meier rates of overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) in HPVAs and NHPVAs with surgical treatment alone versus surgical and adjuvant treatment
Mucinous HPVAs (represented by mucinous NOS, iSMILE, and mucinous intestinal types) had a worse PFS (p=0.04) compared to usual-type HPVAs. Although mucinous HPVAs had a better OS (p=0.10) than gastric-type NHPVAs, this was not statistically significant (Table 2).
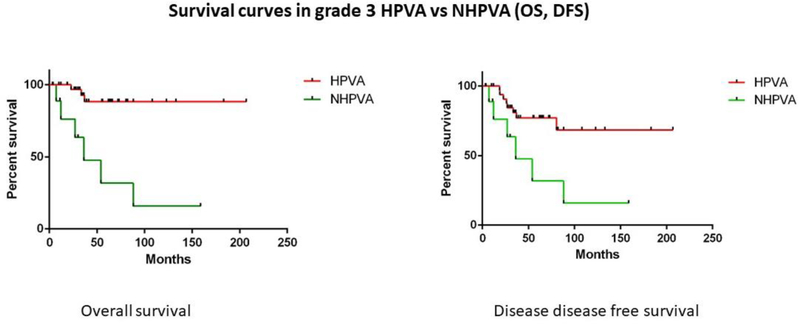
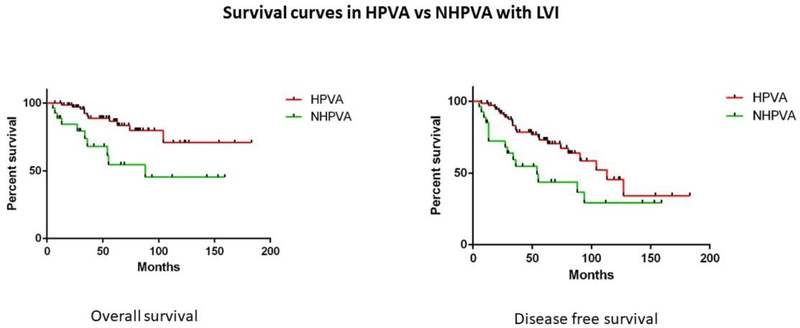
In the univariate analysis, no statistically significant differences in OS, DFS or PFS were found between FIGO grades 1, 2, and 3 HPVAs. Similarly, no statistically significant differences were found in OS, DFS or PFS between FIGO grades 1, 2, and 3 NHPVAs (Table 2). However, grade 3 HPVAs had better OS and DFS than grade 3 NHPVAs (p<0.0001 and p=0.0097, respectively) (Table 2) (Figure 4). HPVAs with LVI had superior OS and DFS compared to NHPVAs with LVI (p=0.003 and p=0.02, respectively) (Table 2) (Figure 5). No significant differences were found between HPVAs with LNM and NHPVAs with LNM (Table 3).
Figure 4: Survival curves in grade 3 HPVA versus NHPVA.
Kaplan-Meier rates of overall survival (OS) and disease-free survival (DFS) in grade 3 HPVAs versus grade 3 NHPVAs
Figure 5: Survival curves in HPVA versus NHPVA with LVI.
Kaplan-Meier rates of overall survival (OS) and disease-free survival (DFS) in HPVAs with LVI versus NHPVAs with LVI
Table 3.
Multivariate analysis of OS, DFS and PFS in IECC cohort with respect to covariates age, FIGO stage, grade, tumor size and Silva pattern in the entire cohort, HPVA only and NHPVA groups.
| Covariate | OS | DFS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| p value | HR | CI | p value | HR | CI | p value | HR | CI | |
| All (HPVA and NHPVA) | |||||||||
| HPV status | 0.060 | 0.14 | 0.03–0.95 | 0.064 | 0.14 | 0.02–1.02 | 0.81 | 1.3 | 0.14–11 |
| Age | 0.95 | 1 | 0.96–1.05 | 0.77 | 1.01 | 0.96–1.05 | 0.97 | 1 | 0.96–1.04 |
| Stage (I/II vs III/IV) | 0.056 | 7.73 | 1.32–33.8 | 0.044 | 8.43 | 1.47–37.3 | 0.65 | 1.45 | 0.3–7.09 |
| Grade (1 vs 2 vs 3) | 0.34 | 3.42, 3.51 | 0.21–520, 0.27–494 | 0.39 | 2.55, 2.98 | 0.16–393, 0.23–416 | 0.067 | 0.37 | 0.13–1.08 |
| Size | 0.10 | 1.03 | 0.99–1.07 | 0.067 | 1.04 | 1–1.08 | 0.48 | 0.99 | 0.96–1.02 |
| Silva pattern (A/ B vs C) | 0.09 | 3.5 | 0.4–458 | 0.18 | 2.29 | 0.24–304 | - | - | - |
| HPVA only | |||||||||
| Age | 0.83 | 1.01 | 0.96–1.05 | 0.63 | 1.02 | 0.96–1.06 | 0.96 | 1 | 0.96–1.04 |
| Stage (I/II vs III/IV) | 0.072 | 6.98 | 1.17–30 | 0.056 | 7.48 | 1.31–32 | 0.73 | 1.32 | 0.27–6.4 |
| Grade (1 vs 2 vs 3) | 0.95 | 0.33, 0.3 | 0–64, 0–65 | 0.99 | 0.32, 0.33 | 0–63, 0–74 | 0.072 | 0.38 | 0.13–1.1 |
| Size | 0.19 | 1.03 | 0.99–1.07 | 0.10 | 1.03 | 0.99–1.08 | 0.44 | 0.99 | 0.96–1.02 |
| Silva pattern (A/B vs C) | 0.086 | 4.14 | - | 0.18 | 2.58 | - | - | - | - |
| NHPVA only | |||||||||
| Age | 0.032 | 1.06 | 1–1.1 | 0.052 | 1.09 | 1–1.18 | 0.51 | 1.02 | 0.96–1.1 |
| Stage (I/II vs III/IV) | 0.022 | 8×109 | - | 0.0044 | 5×1022 | - | 1 | 9×108 | - |
| Grade (1 vs 2 vs 3) | 0.54 | 0.35, 1.17 | 0.02–5.5, 0.18–7.4 | 0.098 | 0, 0.74 | 0.09–6.06 | 0.44 | 0.4 | 0.04–4.03 |
| Size | 0.0022 | 1.1 | 1.01–1.19 | 0.0035 | 1.1 | 1.01–1.2 | 0.27 | 1.04 | 0.97–1.1 |
| Silva pattern (A/B vs C) | - | - | - | - | - | - | - | - | - |
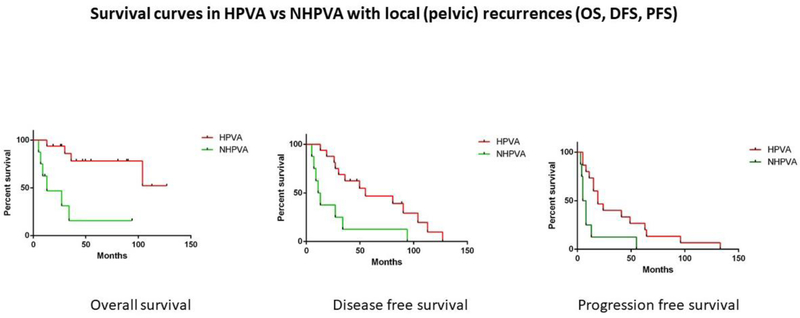
Sites of local recurrences included ovary, vagina, vulva, bladder, pelvic cavity, pelvic/paraaortic lymph nodes, pelvic sidewall, ureter, sacrum, and anus. Sites of distant recurrences included spine, lung, mediastinal lymph nodes, retroperitoneum, chest wall, brain, small bowel, abdominal wall, liver, and colon. HPVAs with pelvic recurrence had better OS, DFS and PFS than NHPVAs with pelvic recurrence (p=0.0005, p=0.005, p=0.01) (Table 2) (Figure 6). However, when HPVAs and NHPVAs with distant recurrences were compared, no statistical significance was found in OS, DFS, or PFS (p=0.09, p=0.58, p=0.48 respectively) (Table 2). Five patients had a lymph node recurrence: 3 of these patients had usual type HPVAs and 2 had gastric-type NHPVAs. All 5 affected patients were initially treated with surgery and adjuvant therapy; however, no data regarding oncologic treatment for the lymph node recurrences were available. In these 5 patients, the lymph node recurrences were pelvic, paraaortic, paraaortic, pelvic and paraaortic, and were documented at 41, 7, 10, 5 and 27 months, respectively, after completion of initial therapy. Four of the 5 patients died of disease. The 1 patient who remains alive with no evidence of disease had a gastric-type ECA, with a para-aortic lymph node recurrence identified 27 months after her initial treatment.
Figure 6: Survival curves in HPVA versus NHPVA with local (pelvic) recurrences.
Kaplan-Meier rates of overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS) in HPVAs associated with pelvic recurrence versus NHPVAs associated with pelvic recurrence
Multivariate analysis (Table 3) of HPVAs indicated nearly significant statistical associations between patient stage and DFS (p=0.06) and between Silva invasion pattern and OS (p=0.09). Multivariate analysis of NHPVAs indicated statistically significant associations between OS and age (p=0.03), stage (p=0.02) and tumor size (p=0.002), as well as between DFS and stage (p=0.004) and tumor size (p=0.004). Multivariate analysis of HPVAs and NHPVAs together revealed nearly significant associations between OS and HPV status and stage (both p=0.06). For DFS, stage was a significant variable (p=0.04), while HPV status and tumor size were nearly significant (p=0.06 and 0.07, respectively). FIGO grade was not statistically significant for survival in any of the analyses (entire cohort combined, HPVA alone or NHPVA alone).
DISCUSSION
Unlike cervical squamous cell carcinoma, ECAs are less common and are not always associated with HPV infection [1, 8]. The current WHO 2014 classification [1] is based on morphological (specifically cytoplasmic) features, using subjective criteria that lack strong correlations with pathogenesis or clinical outcomes.
The IECC was specifically developed to help practitioners recognize HPV-associated and –unassociated variants of ECA [2]. The IECC segregates ECAs into HPVA and NHPVA using H&E slides alone. Both HPVA and NHPVA categories can be further stratified using existing criteria. The validity of the HPVA and NHPVA categories is supported by p16/HPV status [2, 9]. The IECC demonstrates superior inter-observer agreement compared to the WHO classification, and facilitates distinction between HPVA and NHPVA ECAs with good reproducibility and excellent prediction of HPV status [9]. Multivariate analysis indicated that HPV status was nearly statistically significantly associated with OS (HR=0.14 [0.02–0.99], p=0.06) and DSS (HR=0.15 [0.02–1.06], p=0.06). The most likely explanation for these nearly-significant results is the relative paucity of recurrences and deaths, particularly in the HPVA category.
Clinical and demographic parameters differ between the two main IECC categories. NHPVAs are generally larger tumors that occur in older patients and are diagnosed at FIGO Stage II or higher in more than 50% of cases. Similar results have been reported by Karamurzin et al [10]. In that study, 59% of patients with gastric-type NHPVAs were FIGO Stage II or higher. Multivariate analysis of NHPVAs indicated statistically significant association between OS and age (p=0.02), stage (p=0.02) and tumor size (p=0.002), and between DSS and stage (p=0.004) and tumor size (p=0.004). For NHPVA, age, stage and tumor size were significantly associated with overall survival; however, for HPVA, none of the variables studied reached statistical significance for overall survival on multivariate analysis.
Moreover, the two main IECC categories differ with respect to prognostic parameters assessed on histologic examination. Instead of relying upon associations between the microscopic substage (FIGO stage IA2, for example) and LNM, the Silva system [11] has correlated LNM with the pattern of stromal invasion of HPVAs with superior results. Irrespective of microscopic substage, Silva A (non-destructive invasion) is not associated with LNM, Silva B (limited destructive invasion) is usually not associated with LNM, while Silva C (diffuse destructive invasion) may be associated with LNM in up to 25% of cases [11]. NHPVAs are always of Silva C type [3] and are more frequently associated with LVI and LNM compared to HPVAs, rendering Silva classification irrelevant for prognostication in NHPVAs. An exploration of the significance of the Silva pattern of invasion in the context of the IECC has been described in a recent publication. In the current study, the Silva invasion pattern was assessed only in HPVAs and was found to have nearly statistically significant associations with overall survival (p=0.09) [12].
We have demonstrated that HPVAs (including usual and mucinous types) have superior OS, DFS and PFS compared to NHPVAs (predominantly gastric-type ECAs in this cohort) on univariate analysis. Similar results have been reported by other investigators. Kojima demonstrated that patients with gastric-type ECAs had significantly decreased 5-year DSS compared with non-gastric type (30% vs. 77%, p<0.0001), and that gastric-type morphology was associated with a significant risk for disease recurrence compared to non-gastric type (p=0.001; HR 4.5, 95% CI, 1.42–14.2) [7]. The more recent study by Karamurzin and colleagues reported that DSS at 5 years was 42% for gastric-type ECAs compared to 91% for usual-type ECAs [10]. A recent report validating the IECC criteria included 82 ECAs from a single institution [9]. HPVAs comprised 87% of the cohort; 10% were NHPVAs; 3% were unclassifiable [9]. NHPVAs demonstrated significantly higher frequencies of destructive invasive patterns (p=0.009), LVI (p=0.02), and advanced stage (p < 0 .001). Worse RFS and DSS were observed in NHPVAs, compared to HPVAs [9].
Our data also suggest that the use of adjuvant therapy may be prognostically significant in HPVAs, as recently reported [13]. Survival did not differ between HPVAs and NHPVAs in patients who underwent surgery alone, but significant differences between HPVAs and NHPVAs were evident in patients treated with a combination of surgery and adjuvant therapy. Previous publications have demonstrated that NHPVAs are frequently associated with local and distant recurrences, including sites that are only rarely involved by recurrent HPVAs [10]. In the present study, HPVAs with pelvic recurrence had a better OS, DFS and PFS than NHPVAs with pelvic recurrence, but no differences were observed in the setting of distant recurrence. The benefits of currently available chemo/radiation protocols for treating NHPVA patients should be investigated further.
We compared clinical outcomes between two types of ECAs containing mucin: mucinous HPVA and gastric-type NHPVA. The mucinous HPVA category, defined by the IECC as an HPVA in which more than 50% of tumor cells harbor easily identified cytoplasmic mucin [2], is a heterogeneous collection of sub-variants: mucinous NOS, intestinal, signet-ring cell, and iSMILE (a newly described subtype) (Figure 1) [5]. Despite the presence of detectable HPV in usual-type and mucinous HPVAs, there are some immunohistochemical differences between the two. MUC6, CDX2, p53, vimentin and ER expression differ among these types, especially when comparing intestinal-type mucinous HPVA and usual-type HPVA [14]. We previously reported that iSMILE frequently express MUC6 (evidence of glandular differentiation), but they are less frequently positive for PAX8 (possibly indicating reserve cell origin), with more frequent p53 overexpression compared to usual-type ECAs [4]. The current study demonstrates that mucinous HPVAs portend a worse PFS than usual-type HPVAs, but a better (although non-significant) OS compared to gastric-type NHPVAs, indicating that mucinous HPVAs should be separated from both non-mucinous HPVAs and NHPVAs if confirmed in other studies. These analyses were admittedly limited by the relative rarity of mucinous HPVAs and, possibly, by a classification that is not entirely reproducible. In the work published by Hodgson, et al [9] the distinction between HPVA and NHPVA was reproducible, whereas distinction among the various types of HPVA was not. iSMILE adenocarcinomas have sufficiently characteristic histological and immunohistochemical features that should enable distinction from other HPVAs, however we think that the differences between usual-type and mucinous NOS HPVAs are rather subjective despite the criteria proposed by the IECC
Microscopic grade is not an important prognostic parameter in ECAs and is not usually taken into account in patient management [15]. There were no statistically significant differences in OS and DFS among FIGO grades 1, 2 and 3 HPVAs, and these findings were similar among NHPVAs, using both univariate and multivariate analysis. Therefore, HPVAs should not be assigned a grade using existing grading schemes; rather, the Silva invasion pattern is informative with respect to OS. In NHPVAs, however, there were no apparent associations between histologically-assessed features and clinical outcomes (based on this series in which gastric-type NHPVAs are more prevalent than other NHPVAs).
Clinical outcome studies support the idea that the IECC classification not only categorizes ECAs based on HPV status, but also has important clinical relevance along with stage, tumor size and Silva pattern. For HPVAs alone, stage and Silva pattern are clinically significant; whereas for NHPVAs, age, stage and tumor size are significantly associated with survival. Adjuvant therapy may preferentially benefit patients with HPVAs, but this should be confirmed in further studies. Our findings support rigorous separation of HPVAs from NHPVAs, and suggest that mucinous HPVAs should continue to be distinguished from non-mucinous HPVAs, especially if further studies support differences in clinical outcomes. FIGO grading of HPVAs and NHPVAs is not informative, while the Silva system performs extremely well for HPVAs, but is not applicable to NHPVAs, suggesting that Silva pattern might be viewed as an adjunct to or surrogate for HPVA grade. Future management strategies should be designed in accordance with these findings.
Acknowledgments
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748 (Dr. Soslow, Dr. Park, Dr. Abu-Rustum).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kurman RJ, Carcangiu M, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs Lyon, France: World Health Organization. WHO Press. IARC; 2014. [Google Scholar]
- 2.Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol 2018;42:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolnicu S, Barsan I, Hoang L, et al. Stromal invasion pattern identifies patients at lowest risk of lymph node metastasis in HPV-associated endocervical adenocarcinomas, but is irrelevant in adenocarcinomas unassociated with HPV. Gynecol Oncol 2018;150:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolnicu S, Hoang L, Hanko-Bauer O, et al. Cervical adenosquamous carcinoma: detailed analysis of morphology, immunohistochemical profile, and clinical outcomes in 59 cases. Mod Pathol 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Lastra RR, Park KJ, Schoolmeester JK. Invasive stratified mucin-producing carcinoma and stratified mucin-producing intraepithelial lesion (SMILE): 15 cases presenting a spectrum of cervical neoplasia with description of a distinctive variant of invasive adenocarcinoma. Am J Surg Pathol 2016;40:262–269. [DOI] [PubMed] [Google Scholar]
- 6.Park JJ, Sun D, Quade BJ, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol 2000;24:1414–1419. [DOI] [PubMed] [Google Scholar]
- 7.Kojima AK, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 2007;31:664–672. [DOI] [PubMed] [Google Scholar]
- 8.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol 2011;35:633–646. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson A, Park KJ, Djordjevic B, et al. International Endocervical Adenocarcinoma Criteria and Classification: validation and interobserver reproducibility. Am J Surg Pathol 2019;43:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric-type endocervical adenocarcinoma: an aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol 2015;39:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz De Vivar A, Roma AA, Park KJ, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol 2013;32:592–601. [DOI] [PubMed] [Google Scholar]
- 12.Stolnicu S, Barsan I, Hoang L, et al. Stromal invasion pattern identifies patients at lowest risk of lymph node metastasis in HPV-associated endocervical adenocarcinomas, but is irrelevant in adenocarcinomas unassociated with HPV. Gynecol Oncol 2018;150:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima A, Shimada M, Mikami Y, et al. Chemoresistance of gastric-type mucinous carcinoma of the uterine cervix: a study of the Sankai Gynecology Study Group. Int J Gynecol Cancer 2018;28:99–106. [DOI] [PubMed] [Google Scholar]
- 14.Stolnicu S, et al. Diagnostic algorithmic proposal based on comprehensive immunohistochemical evaluation of 297 invasive endocervical adenocarcinomas. Am J Surg Pathol 2018;42:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh WJ, Abu-Rustum N, Bean S, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:170–199. [DOI] [PubMed] [Google Scholar]