Abstract
Baroreceptors are mechanosensitive elements of the peripheral nervous system that maintain homeostasis by coordinating physiological responses to external and internal stimuli. While it is recognized that carotid and cardiopulmonary baroreceptor reflexes modulate autonomic output to mitigate excessive fluctuations in arterial blood pressure and to maintain intravascular volume, increasing evidence suggests that baroreflex pathways also project to key regions of the central nervous system (CNS) that regulate somatosensory, somatomotor and CNS arousal. In addition to maintaining autonomic homeostasis, baroreceptor activity modulates the perception of pain, as well as neuroimmune, neuroendocrine, and cognitive responses to physical and psychological stressors. In this review, we summarize the role that baroreceptor pathways play in modulating acute and chronic pain perception. The contribution of baroreceptor function to postoperative outcomes is also presented. Finally, methods that enhance baroreceptor function, which hold promise in improving postoperative and pain management outcomes are presented.
Summary Statement:
We discuss the evidence that baroreceptor function modulates acute and chronic pain perception and contributes to perioperative outcomes. As such, these little-studied associations represent an opportunity to investigate a novel process that impacts: 1) our understanding of physiological factors that mediate chronic pain and perioperative outcomes and 2) the implement novel interventions that will improve pain management and perioperative outcomes.
Introduction
The central (CNS) and peripheral (PNS) nervous systems work in concert to maintain homeostasis in response to psychological and physical stressors. An ensemble of coordinated biological processes modulates sensory, emotional, motor, autonomic, neuroendocrine, and immune responses to tissue damage, including surgery.1–5 Mechanosensitive baroreceptor afferents mediate physiological responses to internal stimuli by integrating and modulating PNS and CNS responses to internal stimuli and stressors to maintain homeostasis. These receptors respond to changes in arterial pressure (AP), venous pressure, and respiratory dynamics.6–8 Baroreceptor afferents transmit information to discrete regions of the brain stem nucleus tractus solitarius (NTS), via afferents coursing in or with the vagus nerve (aortic depressor nerve and cardiopulmonary afferents) and glossopharyngeal nerve (carotid arterial baroreceptors). Carotid and cardiopulmonary baroreceptor reflexes modulate autonomic output to maintain resting blood pressure, buffer excessive fluctuations in AP (carotid sinus baroreceptors), and to maintain intravascular volume (cardiopulmonary baroreceptors). Baroreceptor activity engages CNS networks that regulate somatosensory, somatomotor, CNS arousal, as well as autonomic, neuroimmune, and neuroendocrine responses to physical and psychological stressors.2 An important, but under-investigated, area of study is whether changes in baroreceptors contribute to pathological conditions in a variety of clinical settings. In this review, we summarize the neurobiology of the baroreceptor function and how baroreflex mechanisms are thought to contribute to acute and chronic pain conditions as well as perioperative outcomes.
Baroreceptor Reflex
Baroreceptor activation:
Arterial, carotid sinus, baroreceptors are mechanoreceptors that are located in the aortic arch and carotid sinuses and are “tuned” to changes in systemic arterial pressure. These receptors have terminals associated with both myelinated (Aδ) and unmyelinated (C) afferent fibers in the inner adventitial layer of the arterial wall that respond to stretch generated by transmural pressure on a beat-to-beat basis.8 Stimulation of arterial baroreceptors modulates transient changes in blood pressure to maintain a homeostatic set point for AP by dynamically adjusting sympathetic and parasympathetic output to the heart and the peripheral vascular system. Arterial baroreceptor mechanoreceptors respond to increases in intramural pressure depending on resting AP, mainly systolic and pulse pressure. As resting AP increases, a given incremental change in AP evokes greater activation of carotid sinus baroreceptor afferents, thereby promoting a greater increase in parasympathetic tone and a decrement in sympathetic tone. For normotensive individuals, the threshold for baroreceptor activation is at a carotid mean arterial pressure of approximately 60 mmHg9, and it is active across the whole range of normal blood pressures.10 This threshold changes with aging with lower carotid sinus pressure thresholds observed for young subjects (45 mmHg at 22 ± one year) compared to older subjects (80 mmHg at 61 ± two years).11
Nucleus tractus solitarius and cardiovascular response:
The NTS is the central projection site for baroreceptors and modulates the activity of spinal and supraspinal networks that coordinate the responses to environmental stressors. The NTS sends excitatory glutamatergic projections to the caudal ventrolateral medulla12, which projects GABAergic inhibitory fibers to the rostral ventrolateral medulla.13 This short neural circuit converts the baroreceptor excitatory input to NTS into an inhibitory output that reduces the descending excitatory tone originating in the rostral ventrolateral medulla that projects to the intermediolateral region of the spinal cord.12,13 Activation of this pathway produces a reduction in cardiosympathetic tone and vascular resistance.14 The NTS also sends direct excitatory projections to the dorsal vagal motor nucleus and nucleus ambiguus, which enhances parasympathetic output.15,16 This baroreceptor-elicited shift in autonomic balance towards the parasympathetic side results in a reduction in heart rate, AP, and adrenal secretion of adrenaline.17,18
Assessment of Baroreflex Sensitivity and Influencing Factors
One way of assessing baroreceptor function is through the measurement of the baroreceptor sensitivity (BRS), which is typically defined by the relationship between the change in AP and the associated effect on inter-beat interval19,20 and most procedures measure the change in heart rate as a function of the change in systolic AP (SAP). The development of reliable methods to estimate BRS has opened a window that enables the investigation of the role of baroreflex dysfunction in many medical conditions. The gold-standard procedure for assessing BRS is to measure the ratio of change in heart rate to the change in SAP in responses to the intravenous administration of a low dose of a vasopressor agent (e.g., phenylephrine).21–26 In addition, non-invasive methods have been developed to allow for the assessment of BRS in response to the small natural continuous variations in blood pressure, i.e., ‘spontaneous BRS.’27 In Fig. 1 illustrates how the simultaneous recording of beat-to-beat SAP and heart rate is used to estimate BRS by the sequence method (For more details on the methods for the estimation of BRS see the Supplemental Digital Content 1).
FIGURE 1:
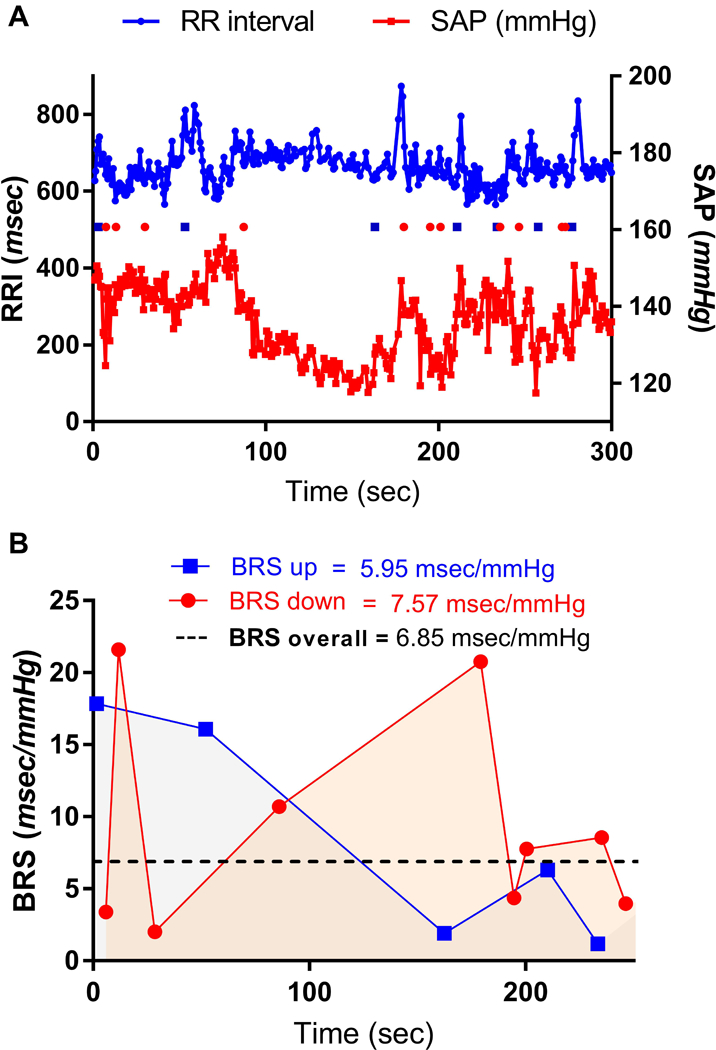
Estimation of BRS using the sequence method. ECG and SAP waveforms are recorded simultaneously, then beat-to-beat SAP and the calculated R-R intervals (RRI) are plotted against time course. Panel A: SAP and RR interval signals and the baroreflex sequences acquired using the sequence method. Closed squares indicate UP sequences. Open squares indicate DOWN sequences. Sequence selection criteria: SAP > 0.5 mmHg, RRI > 1 ms, sequence > 3, a significant correlation coefficient (r > 0.9). Note that the significant sequences cluster in segments where SAP and RRI signals apparently oscillate more coherently (in this case, at the start and the end of this recording.). Panel B: Within-subject variability of the BRS. Mean UP, DOWN, and overall BRS calculated from the sequences shown in panel A.
Thresholds for normal and abnormal spontaneous BRS have been proposed by the Autonomic Tone and Reflexes After Myocardial Infarction Study.25,28 In general, a normal BRS is defined as > 6 ms/mmHg a moderate dysfunction as 3–6 ms/mmHg, and severe dysfunction as < 3 ms/mmHg. However, estimates of BRS must be interpreted in the context of gender, age, and circadian rhythm. Female subjects have 50% lower cardiac baroreflex sensitivity than men29, which is associated with lower AP and estrogen-mediated central sympathoinhibition and peripheral vasodilation.30 BRS fluctuates across the reproductive cycle with increases during the mid-luteal phase when estrogen and progesterone levels are elevated31,32 and around the ovulation33 whereas it is markedly suppressed during pregnancy34, contributing to pregnancy complications such as orthostatic hypotension and severe hypotension with peripartum hemorrhage.31 Also, there is an age-related decline in BRS that results from increased arterial wall stiffness and a subsequent reduction in the ability of baroreceptor mechanoreceptors to process changes in arterial pressure.35 This leads to increases in sympathetic nerve activity and SAP with aging.36 Finally, diurnal variations in BRS have been identified in humans, with reduced sensitivity after waking compared to sleep, although other more complex patterns have also been described.37
Baroreflex Regulation of Pain Perception
Influence of arterial and venous blood pressure on pain perception:
To date, most studies have indirectly examined the relationship between BRS and pain perception by examining the association of pain perception with experimentally-induced changes in AP and venous blood pressure (i.e., physiological events that activate baroreceptor afferent activity). In animals, vasopressor mediated arterial hypertension in response to vasopressor agents38 or abdominal aortic occlusion produce hypoalgesia or antinociceptive behaviors.39 Similarly, genetically hypertensive rats are hypoalgesic, which is reversed by lowering arterial blood pressure via ganglionic blockade or by right vagatomy.40 Similarly, chronic hypertension induced by renal artery clipping or increasing dietary salt in salt-sensitive rats41 induces a hypoalgesia that is reversed by lowering blood pressure.42 Noteworthy, spinal nociceptive transmission is diminished in genetically hypertensive rats43, and the observed hypoalgesia can be reversed with pharmacologic procedures that lower AP. Similarly, elevating arterial pressure in normotensive rats impairs spinal nociceptive transmission39 and in normotensive animals lowering AP induces hyperalgesia.40 Elevating venous pressure by volume expansion activates cardiopulmonary volume vagal afferents and evokes a profound hypoalgesia in rats.44–46 In addition to venous blood pressure, the activation of vagal afferents with intravenously administered morphine, met-enkephalimamide, or other vagal afferent stimulants, produces an almost immediate cardiopulmonary mediated hypoalgesia in rats that appears to be independent of CNS penetration.46–48
An association between AP and pain perception has also been demonstrated in humans, with evidence that healthy normotensive individuals experience decreased pain sensitivity as a function of increasing resting AP.49–58 In contrast, individuals with chronically low resting AP are prone to thermal hyperalgesia.59 As observed in rats, hypertension-associated hypoalgesia in humans is correlated with systolic AP as opposed to diastolic AP.57 The processing of nociceptive stimuli also varies throughout the cardiac cycle such that during systole (e.g., maximal baroreceptor load) pain sensitivity is diminished compared to during diastole.60–63 There is a greater effect size of systolic AP compared to diastolic AP on pain sensitivity.49,50,56,64–67 This further suggests a pain-modulatory role for arterial baroreceptors although the relative contribution of slowly and rapidly adapting baroreceptor afferents to sustained versus phasic changes in blood pressure on pain perception remains an open question.
Changes in AP have also been reported to contribute to the suppression of pain perception measured in conditioned pain modulation paradigms, procedures which assesses the strength of endogenous pain regulatory systems.68,69 The strength of endogenous inhibitory pain positively correlate with increases in AP elicited by a noxious conditioning stimulus.70 Considering that reduced conditioned pain suppression has been linked with the development of chronic pain71, it remains to be determined if reduced baroreceptor function and blood pressure are risk determinates for acute perioperative and chronic pain.
It should also be noted that some studies have not observed a reciprocal relationship between AP and pain sensitivity. The hypoalgesia exhibited by hypertensive patients persists after reductions of AP with medical treatment.72 Hypoalgesia is already present in borderline hypertension and is antecedent to established hypertension.72 Normotensives with a family history of hypertension, and a presumed genetic risk for hypertension, also have reduced responsiveness to acute pain despite having a normal resting AP73–76, although this has been reported for males but not for females77 and has not been observed by others investigators.72 Moreover, pain tolerance measured in normotensive individuals at age 14 predicts ambulatory blood pressure later in life.78 It has been proposed that mechanisms independent of AP, such as venous hypertension, which is antecedent to the expression of essential hypertension, may explain the temporal discordance between early life AP and pain sensitivity.40 In support of this hypothesis, an increase in venous pressure – a stimulus that stimulates low-pressure cardiopulmonary baroreceptors and induces hypoalgesia - occurs before the onset of arterial hypertension in genetically hypertensive rats.40 Furthermore, low-pressure cardiopulmonary baroreceptors, unlike arterial baroreceptors, do not reset and continue exerting a modulatory influence on pain processing in the presence of sustained elevation in AP.79
In summary, there is evidence that supports an association between AP and pain perception: pain sensitivity is correlated with responses to acute episodic changes in AP, is diminished in chronic hypotension, and is inversely correlated with resting AP in normotensive individuals. Also, a substantial body of functional and anatomical evidence supports the causal nature of this association. The temporal and causal relation between AP, BRS, and pain perception requires further investigation that promises to reveal a better understanding of the pathophysiological processes that contribute to aberrant pain perception and autonomic function.
Baroreflex stimulation and pain perception:
The demonstrated relationship between pain perception and carotid sinus and cardiopulmonary baroreceptor activation1,46,58,80,81 implies that this relationship can be affected by changes in BRS; however, the relationship between BRS and pain perception has been much less studied. Spontaneous BRS has been shown to be inversely correlated with ischemic and thermal pain responses in normotensive human subjects.82 Similarly, a reciprocal relationship between BRS assessed during cold noxious stimulation has been reported in normotensive human subjects.83 It should be noted that the relationship between BRS and pain is temporally dynamic and influenced by the individual’s physiological and emotional status. For example, the magnitude of the relationship between BRS and cold pain perception is inversely associated with resting AP.83 Moreover, BRS assessed with rises in AP increases versus decreases in AP are differentially associated with experimentally-evoked pain in normotensive subjects.82
In an attempt to provide insight into the causal nature of these associations, investigators have used direct mechanical or electrical manipulations of baroreceptors, which allow more stimulus control than other indirect methods of stimulation (e.g., tilt-table, pharmacological, and volume-induced AP changes). The mechanical stimulation of carotid baroreceptors with external neck suction, which simulates an AP increase, reduces mechanical pain84 although it has no effect on thermal84, electrically-induced pain61, or experimentally-induced ischemic pain85 in normotensive human subjects. In contrast, external neck compression, which mimics a reduction in AP, reduces electrically-induced pain ratings in normotensive adults.61 The electrical stimulation of the cervical vagus, which activates baroreceptor afferents, produces antinociceptive effects at high intensities and pronociceptive effects low intensities of stimulation in rats86, cats87, and humans.88 Thus, carotid baroreceptors and vagal afferents exhibit a complex and dynamic influence on nociceptive processing.
Collectively, these findings provide evidence to support the view that baroreflex function modulates pain perception. The occurrence, efficacy, and directionality of baroreceptor reflex activity as indexed by BRS on pain perception is influenced by many factors, such as level of resting AP, pain modality, method of baroreceptor stimulation, among many other factors. At present, we do not know if alterations in baroreflex function contribute to chronic pain syndromes where BRS is known to be substantially reduced (see below section ‘Clinical Implications of Impaired Baroreceptor-Mediated Pain Modulation’).
Physiological mechanisms mediating baroreflex inhibition of pain:
The mechanisms and pathways by which elevations in arterial and venous blood pressure decreases pain sensitivity are not fully understood. Arterial and venous blood pressure-related hypoalgesia have been associated with carotid sinus46,62,89,90 and cardiopulmonary baroreceptors.47,91,92 Several studies have documented an attenuation of hypertension-associated hypoalgesia by decreasing or interrupting the sinoaortic afferent limb of the baroreflex.38,45,91,93 Volume expansion induces hypoalgesia that is partially reversed by right vagotomy.44–46 In addition, noxious heat-evoked responses of wide-dynamic-range and high-threshold lumbosacral spinal dorsal horn neurons are reduced in spontaneously hypertensive rats compared to normotensive controls.43 In agreement with this observation, spontaneous BRS correlates with the temporal summation of pain since higher resting systolic AP and greater BRS are associated with significantly lower temporal “wind-up” of heat pain in healthy human subjects.94 These findings suggest that (a) AP-mediated hypoalgesia requires an intact baroreceptor afferent input and (b) the activation of second-order spinal nociceptive neurons by primary nociceptive afferents is inhibited by baroreceptor stimulation evoked by increases in AP.
Animal studies support a role for endogenous opioid activity as one of many possible endogenous neurotransmitter systems involved in hypertension associated hypoalgesia.41,42,95–98 Maixner and colleagues demonstrated that naloxone reverses the hypoalgesia observed in spontaneously hypertensive rats in both pre-hypertensive neonatal and hypertensive adult animals.40 Similarly, sympathetic inhibition resulting from baroreceptor stimulation is mediated by endogenous opioid networks in rabbits99 that appear to originate in the NTS and rostral ventrolateral medulla.100 Hypertensive rats exhibit neurochemical markers of elevated opioid activity in the spinal cord and other CNS nuclei.41,101 Interestingly, it has been proposed that in the presence of essential hypertension there is reduced hypothalamic sensitivity to endogenous opioids, which leads to (a) a reduction in baroreflex-inhibition of the sympathetic output, (b) an increase and prolongation of AP response to environmental stimuli, (c) prolonged baroreceptor stimulation, and finally, (d) an excessive release of endogenous opioids.102 The proposed excessive release of endogenous opioids, whether elicited by baroreflexes or by non-baroreflex mechanisms (e.g., a primary brain stem nuclei dysfunction103), may mediate the hypoalgesia seen in hypertensive conditions. In human studies, hypertensive subjects exhibit enhanced levels of circulating endorphins and diminished sensitivity to noxious thermal stimuli.104 Of note, pain-relieving actions of angiotensin II, which is increased several hypertensive conditions, have been related to the AT2-receptor-mediated central release of endogenous opioids.105 However, a conclusive role for endogenous opioids in hypertension-associated hypoalgesia remains to be established, as naloxone fails to reverse hypoalgesia in hypertensive humans.50,106
A second likely mediator of hypoalgesia under hypertensive conditions and baroreceptor stimulation is the activation of α2-adrenergic receptors in brain regions involved in both autonomic and sensory processing. Noteworthy, NTS, rostral ventrolateral medulla, and caudal ventrolateral medulla contain noradrenergic and adrenergic neurons.100,107 Microinjection of the α2-adrenergic receptor agonist clonidine into NTS produces analgesia mediated by opioid receptors in normotensive rats and spontaneously hypertensive rats.108 Of note, morphine administration to the region of the NTS produces naloxone-reversible analgesia in rats.109 Both analgesia induced by increased AP and hypoalgesia in spontaneously hypertensive animals are abolished following α-adrenergic blockade.46,97,110 Again, the translation to humans is lacking, although there is indirect evidence that subjects with elevated AP within the normotensive range demonstrate increased pain tolerance along with higher circulating levels of norepinephrine.111
Clinical Implications of Impaired Baroreceptor-Mediated Pain Modulation
Baroreceptor Dysfunction and Chronic Musculoskeletal Pain:
Emerging evidence suggests that diminished BRS not only augments the perception pain to experimental noxious stimuli but also contributes to the etiology of chronic musculoskeletal pain conditions, such as fibromyalgia, temporomandibular disorders, and chronic back pain.58,64,112,113 In these patients, changes in the sensitivity to experimental pain and the perceived intensity of ongoing clinical pain correlates with diminished BRS and resting AP.94,112,114 These chronic pain states share several features including altered autonomic nervous system function.115 Specifically, many fibromyalgia patients show a high prevalence of orthostatic hypotension.116 Fibromyalgia patients also exhibit a negative correlation between BRS sensitivity and clinical pain intensity and the severity of clinical complaints, and there is a reduction in resting BRS by nearly 35% in fibromyalgia patients compared to healthy control women.113 Furthermore, systolic, diastolic, and mean arterial pressures are correlated with thermal and ischemic pain in males but not females, with higher blood pressure associated with lower pain sensitivity in males.117,118 The observed sex difference in the blood pressure-pain sensitivity relationships coupled with a reduction in BRS in females may represent an important risk pathway that partially explains the female predominance of common chronic pain conditions like fibromyalgia119 and sex differences in responses to both pharmacologic and non-pharmacologic interventions for pain.120
At the population level, there is a lower incidence and prevalence of common musculoskeletal pain conditions in individuals with elevated AP, supporting a relationship between hypertension and hypoalgesia in various chronic pain states. In a large headache study, higher systolic and diastolic blood pressures were associated with a reduced risk for non- migrainous headache121 and chronic musculoskeletal complaints.122 Moreover, there is a significant negative relationship between several self-reported chronic pain conditions and hypertension.123 The association between AP and the prevalence/incidence of chronic pain could be mediated by an impaired baroreceptor function that disrupts the normal modulatory effect of AP on pain processing. Whether altered baroreflex function represents a risk factor for the onset and persistence of chronic musculoskeletal pain and whether strengthening of baroreflex function represents a resilience factor that protects from common chronic pain conditions are questions that remain to be answered.
Baroreflex Dysfunction and Inflammatory Mediated Pain:
BRS is inversely associated with carotid atherosclerosis inflammatory markers124, subclinical hypothyroidism125, and pregnancy-induced hypertension.126 Baroreflex dysfunction can occur secondary to autonomic dysfunction in response to focal or systemic pathologies.127 While autonomic dysfunction is generally thought to be a consequence of chronic inflammation, new research indicates that in many cases autonomic dysfunction actually precedes the development of some of these conditions. Compared to healthy controls, patients at risk of developing rheumatoid arthritis (i.e., positive for multiple auto-antibodies) have lower cardio-parasympathetic activity and elevated cardio-sympathetic activity, manifested by reduced HRV and elevated resting heart rate. Individuals at risk for rheumatoid arthritis display a cardio-parasympathetic/sympathetic profile similar to patients with established rheumatoid arthritis as well as higher serum levels of norepinephrine, an indicator of augmented sympathetic nervous activity.128 In patients with rheumatoid arthritis who have suffered a stroke, inflammation is reduced on the paralyzed side.129 Moreover, sympathetic tone is positively correlated with plasma IL-6 levels in hypertensive postmenopausal women.130 Consistently, central sympathetic inhibition in hypertensive patients reduces systemic TNFα levels in young healthy non-pregnant women.131 Thus, these clinical studies demonstrate a clear baroreflex and systemic autonomic dysfunction association with inflammation.
Animal studies suggest that the association between autonomic dysfunction and inflammation depends on the bi-directional communication between the autonomic nervous system, neuroimmune, and inflammatory processes (reviewed in detail elsewhere132,133). Thus, the severity of inflammation is not merely immune-mediated but is also modulated by the nervous and endocrine systems. Peripheral mediators of inflammation, specifically interleukin IL-1β and TNFα activate vagal afferents. The efferent limb of this reflex involves vagal parasympathetic fibers that release acetylcholine, which deactivates macrophages, preventing the secretion of inflammatory cytokines, and inhibits the synthesis of TNF-α in innervated immune organs, including the liver, spleen, and heart.23,134 Moreover, baroreflex activation diminishes neutrophil migration and synovial concentrations of inflammatory cytokines TNFα, IL-1β, and IL-6 in the rat by inhibiting sympathetic drive to the knee in an experimental arthritis model.135 Unlike parasympathetic anti-inflammatory effects, sympathetic stimulation is associated with pro-inflammatory effects mediated by that activation of adrenergic receptors on immune cells or indirectly via numerous mechanisms, including the production and distribution of lymphocytes and modulation of the release of pro-inflammatory peptides.136 In a rat model of stroke, infection rates are reduced after sympathectomy, which attenuates sympathetically-mediated immunosuppression.137
Emerging evidence suggests a direct causal relationship between baroreflex function and inflammatory reflex arcs. The electrical activation of baroreflex pathways attenuates joint inflammation in experimental arthritis induced by the administration of zymosan into the femorotibial cavity in rats with lumbar sympathectomy, adrenalectomy, celiac subdiaphragmatic vagotomy or splenectomy.135 Baroreflex activation attenuates neutrophil migration and the synovial levels of pro-inflammatory cytokines TNF, IL-1β, and IL-6 but not anti-inflammatory cytokine IL-10.135 Baroreflex and autonomic dysfunctions also modulate local and systemic inflammatory in animal inflammatory models.138 The autonomic regulation of inflammatory mediators act either directly on nociceptors or indirectly on sympathetic nerve terminals to produce and release inflammatory substances that contribute to the perception of pain139 and chronic inflammatory pain conditions like arthritis.140 In addition, afferent pain signaling can directly modulate other components of inflammation, including plasma extravasation and neutrophil function, which are modulated by vagal afferent activity.141 The sympathetic contribution to hyperalgesia has also been demonstrated in humans142, and it is well-described in neuropathic pain conditions such as complex regional pain syndromes.
Baroreflex Dysfunction and Perioperative Pain:
The putative causal relationship between baroreflex dysfunction and exaggerated inflammation in human subjects is of substantial relevance to perioperative outcomes. Baroreflex dysfunction is observed preoperatively in patients with several comorbidities and postoperatively, particularly after endarterectomy or other neck surgeries affecting the carotid sinus nerve.127 In a prospective surgical study 143 that examined thirty patients undergoing carpal tunnel surgery who underwent preoperative BRS testing and postoperative pain assessments at 6 weeks (acute pain) and ~1 year (persistent pain), there was a significant negative correlation between a measure of heart-rate variability (i.e., the square root of the mean squared differences of successive R-R intervals, RMSSD) and acute postoperative pain. Preoperative resting AP, and presumably, baroreceptor activation, has also been reported to be associated with postoperative pain intensity at 24 h and 48 h postoperatively in men undergoing prostatectomy, even after accounting for patient-controlled opioid use.144 Similarly, there is a negative correlation between resting preoperative AP and postoperative pain after cesarean section.145
Baroreceptor dysfunction associated with impaired autonomic homeostasis increases the vulnerability to the hypotensive effects of general anesthesia.146,147 Chronic hypertensive patients have lower baseline values of BRS and exhibit a more pronounced decrease in both systolic and diastolic AP following propofol administration.148 Furthermore, endotracheal intubation, which is a sympathetic stimulus that should raise AP, decreases AP in chronic hypertensive patients.148 Intraoperative hypotension caused by diminished baroreceptor activation is likely to contribute to augmented inflammatory reactions to surgical trauma, and as a result, to exaggerate acute postoperative pain and an increased vulnerability to chronic postoperative pain. Further work is required to establish the contribution of diminished BRS to perioperative adverse events, postoperative pain and the likelihood of developing persistent pain following common surgical procedures.
The Association of Medical and Health Conditions with BRS
Significant alterations in BRS have been observed in several diseases and health conditions (Fig. 2). Low BRS is commonly seen in patients with hypertension and diabetes149,150, carotid atherosclerosis151,152, obesity153, in smokers154, and high alcohol consumption.155 Patients with obstructive sleep apnea have an attenuated BRS156, which is associated with increased blood pressure variability157, increased sympathetic activity158, desensitization of vascular adrenergic receptors, and decreased peripheral vascular adrenergic responses.159 In the context of perioperative outcomes, this autonomic dysfunction is of relevance since it is linked to cardiovascular morbidity and obstructive sleep apnea.160 Autonomic dysfunction is also prevalent in patients with chronic kidney disease who have an increased risk of sudden cardiac death associated with reduced spontaneous BRS.161 Interestingly, BRS dysfunction is correlated with glomerular filtration rate162, suggesting that there is a direct association between reduced BRS and declining renal function. All of these BRS-associated events can be further aggravated by the fact that prolonged bed rest induces rapid detrimental changes in baroreflex function.163 Congruent with a baroreflex-mediated modulation of pain, there is an increased prevalence of chronic pain and/or greater pain perception in patients with conditions with reduced BRS as well as obstructive sleep apnea164, diabetes165, obesity166, chronic kidney disease 167, smoking168, alcoholism169, and hypertension170, although the mechanisms mediating these associations are not yet well understood. (see Fig. 2)
FIGURE 2:
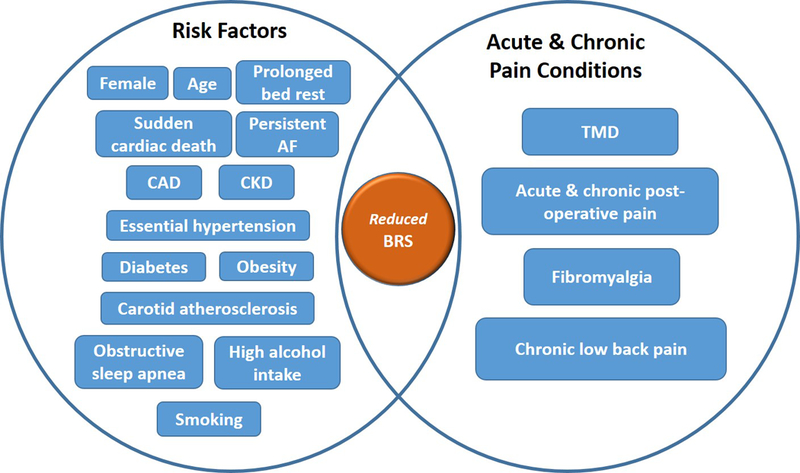
Reduced BRS is frequently observed in patients with cardiovascular, renal, sleep, and metabolic disorders, a feature that is shared with acute and chronic pain conditions, and that is associated with high prevalent chronic pain in these disorders. AF: atrial fibrillation. CAD: coronary artery disease. CKD: chronic kidney diseases. TMD: Temporal mandibular disorders.
Baroreflex dysfunction expressed as a reduced BRS has been reported in several cardiovascular conditions such as essential hypertension, impaired cardiac contractility171, post-myocardial infarction sudden death172, heart failure173,174, coronary artery disease25, and atrial fibrillation.175 Baroreflex function has also been implicated in modulating muscle tone62, sensorimotor performance176, startle reflex177, cortical activity3, and sleep178, cognitive performance179–182, and cortical arousal.80 Of note, about fifty percent of cardiac surgery patients183,184 and up to 26% of elderly non-cardiac surgery patients185 experience early postoperative cognitive dysfunction, which can persist in the long-term, significantly diminishing the life quality. The etiology of postoperative cognitive dysfunction is multifactorial and known to be associated with several patient-related factors.186 It is not known if BRS contributes to postoperative changes in cognition. The pathophysiological processes involved in postoperative surgical outcomes are complex; however, it is clear that postoperative outcomes do not solely result from surgical insult but instead are strongly influenced by the patient’s preoperative physiologic status that is regulated, at least in part, by baroreceptor function and BRS.
Is BRS a Modifiable Risk Factor?
While there are several interventions that can modify BRS, the most extensively assessed approach is vagal nerve stimulation, which has been evaluated for the treatment of a variety of conditions: neurological (partial seizures187, drug-resistant epilepsy188, tinnitus189,190, traumatic brain injury191,192, stroke193), psychiatric (Alzheimer’s disease194,195, cognitive decline196, posttraumatic stress disorder197, treatment-resistant anxiety disorders198), painful/inflammatory (headaches199,200, rheumatoid arthritis201,202, fibromyalgia203, chronic pelvic pain204, Crohn’s disease205), and cardiovascular/metabolic (coronary artery disease206, heart failure207, hypertension208–211, obesity212). Among these conditions where pain, inflammation, cognitive impairment, and cardiovascular events are likely to occur, vagal nerve stimulation has been proposed as a preoperative optimization procedure with the goal of reducing the incidence of adverse postoperative outcomes.
Other less invasive procedures that have been suggested to increase BRS in a clinically meaningful way include increasing venous pressure via fluid management45,213,214, acupuncture or somatic afferent stimulation215–218, the stimulation of cranial vagal afferents arising from the ear’s concha219,220, cardiovascular conditioning221–223, operant learning procedures224, intraoral appliances114, and relaxation/biofeedback therapies225–228. Future studies are required to assess the effects of these procedures on BRS, acute and chronic pain perception and perioperative surgical outcomes.
Conclusions
We have summarized the evidence suggesting a role for baroreceptor function in both acute and chronic pain conditions as well as perioperative outcomes. While the measurement of baroreflex function in the perioperative period currently remains mostly relegated to the research environment, the assessment of perioperative BRS is highly likely to yield important clinically meaningful information that leads to novel strategies for organ protection and pain management. Preoperative recognition of impaired baroreflex function as an important modifiable risk factor requires exploration. Further research in this field is warranted since it is likely to provide actionable information that will reduce the sequelae of surgical stress and improve the management of chronic pain, and adverse surgical outcomes. Existing non-invasive interventions known to increase BRS should be explored for managing patients with chronic pain and implemented preoperative to optimization surgical outcomes.
Supplementary Material
TABLE 1:
Association between BRS and AP with persistent postoperative pain
| AP or BRS measurements | Surgical procedure | Outcome |
|---|---|---|
| Preoperative BRS | Carpal tunnel release | Negative correlation with both acute and persistent postoperative pain.143 |
| Presurgical systolic AP | Prostatectomy | Negative correlation with 24h and 48h postoperative acute pain.144 |
| Resting preoperative blood pressure | Cesarean section | Negative correlation with postoperative pain.145 |
Acknowledgments:
We thank Dr. Lana Watkins, Associate Professor, Department of Psychiatry and Behavioral Science, Duke University, for her comments and assistance with the Supplemental Digital Content on methods to estimate BRS.
Funding Statement:
This work was supported by NIH grant U01DE017018 and P01NS045685 and institutional support from the Department of Anesthesiology at Duke University.
Footnotes
Conflicts of Interest: The authors declare no competing interests.
Clinical trial number and registry URL: Not applicable
Prior Presentations: Not applicable
REFERENCES
- 1.Maixner W, Sigurdsson A, Fillingim R, Lundeen T, Booker D: Regulation of acute and chronic orofacial pain, Orofacial Pain and Temporomandibular Disorders. Edited by Fricton JR, Dubner RB. New York, Raven Press, Ltd, 1995, pp 85–102 [Google Scholar]
- 2.Saper CB: The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 2002; 25: 433–69 [DOI] [PubMed] [Google Scholar]
- 3.Bonvallet M, Dell P, Hiebel G: Tonus sympathique et activite electrique corticale. Electroencephalography and clinical neurophysiology 1954; 6: 119–144 [DOI] [PubMed] [Google Scholar]
- 4.Duschek S, Worsching J, Reyes Del Paso GA: Autonomic cardiovascular regulation and cortical tone. Clin Physiol Funct Imaging 2015; 35: 383–92 [DOI] [PubMed] [Google Scholar]
- 5.Dobson GP: Addressing the Global Burden of Trauma in Major Surgery. Front Surg 2015; 2: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abboud FM, Thames MD: Interaction of cardiovascular reflexes in circulatory control, Handbook of Physiology - The Cardiovascular System. Edited by Shepherd JT, Abboud FM. Bethesda, MD, American Physiological Society, 1984, pp 675–753 [Google Scholar]
- 7.Mark AL, Mancia G: Cardiopulmonary baroreflexes in humans. Compr Physiol 2011; Supl 8: 795–813 [Google Scholar]
- 8.Kumada M, Terui N, Kuwaki T: Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Prog Neurobiol 1990; 35: 331–61 [DOI] [PubMed] [Google Scholar]
- 9.Rau H, Elbert T: Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol Psychol 2001; 57: 179–201 [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Ferrari A, Leonetti G, Pomidossi G, Zanchetti A: Carotid sinus baroreceptor control of arterial pressure in renovascular hypertensive subjects. Hypertension 1982; 4: 47–50 [DOI] [PubMed] [Google Scholar]
- 11.Fisher JP, Kim A, Young CN, Fadel PJ: Carotid baroreflex control of arterial blood pressure at rest and during dynamic exercise in aging humans. Am J Physiol Regul Integr Comp Physiol 2010; 299: R1241–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weston M, Wang H, Stornetta RL, Sevigny CP, Guyenet PG: Fos expression by glutamatergic neurons of the solitary tract nucleus after phenylephrine-induced hypertension in rats. J Comp Neurol 2003; 460: 525–41 [DOI] [PubMed] [Google Scholar]
- 13.Willette RN, Punnen S, Krieger AJ, Sapru HN: Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Res 1984; 321: 169–74 [DOI] [PubMed] [Google Scholar]
- 14.Sved AF, Ito S, Sved JC: Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep 2003; 5: 262–8 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal SK, Calaresu FR: Electrical stimulation of nucleus tractus solitarius excites vagal preganglionic cardiomotor neurons of the nucleus ambiguus in rats. Brain Res 1992; 574: 320–4 [DOI] [PubMed] [Google Scholar]
- 16.Jordan D, Khalid ME, Schneiderman N, Spyer KM: The location and properties of preganglionic vagal cardiomotor neurones in the rabbit. Pflugers Arch 1982; 395: 244–50 [DOI] [PubMed] [Google Scholar]
- 17.Sved AF: Peripheral pressor systems in hypertension caused by nucleus tractus solitarius lesions. Hypertension 1986; 8: 742–7 [DOI] [PubMed] [Google Scholar]
- 18.Sved AF, Ito S, Madden CJ: Baroreflex dependent and independent roles of the caudal ventrolateral medulla in cardiovascular regulation. Brain Res Bull 2000; 51: 129–33 [DOI] [PubMed] [Google Scholar]
- 19.Parati G, Di Rienzo M, Mancia G: How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens 2000; 18: 7–19 [PubMed] [Google Scholar]
- 20.Reyes Del Paso GA, Gonzales MI, Hernandez JA: Comparison of baroreceptor cardiac reflex sensivity estimates from intersystolic and ECG R-R intervals. Psychophysiology 2010; 47: 1102–1108 [DOI] [PubMed] [Google Scholar]
- 21.Smyth HS, Sleight P, Pickering GW: Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ Res 1969; 24: 109–21 [DOI] [PubMed] [Google Scholar]
- 22.Kuusela TA: Methodological Aspects of Baroreflex Sensitivity Analysis, Heart Rate Variability (HRV) Signal Analysis: Clinical Applications. Edited by Kamath MW M; Upton A, CRC Press; 2012, pp 43–58 [Google Scholar]
- 23.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ: Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–62 [DOI] [PubMed] [Google Scholar]
- 24.Davies LC, Francis DP, Scott AC, Ponikowski P, Piepoli M, Coats AJ: Effect of altering conditions of the sequence method on baroreflex sensitivity. J Hypertens 2001; 19: 1279–87 [DOI] [PubMed] [Google Scholar]
- 25.La Rovere MT, Pinna GD, Raczak G: Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 2008; 13: 191–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watkins LL, Fainman C, Dimsdale J, Ziegler MG: Assessment of baroreflex control from beat-to-beat blood pressure and heart rate changes: a validation study. Psychophysiology 1995; 32: 411–4 [DOI] [PubMed] [Google Scholar]
- 27.Duschek S, Dietel A, Schandry R, Reyes Del Paso GA: Increased baroreflex sensitivity and reduced cardiovascular reactivity in individuals with chronic low blood pressure. Hypertens Res 2008; 31: 1873–8 [DOI] [PubMed] [Google Scholar]
- 28.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr., Camm AJ, Schwartz PJ, Tone AIA, Reflexes After Myocardial I: Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 2001; 103: 2072–7 [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Rahman AR, Merrill RH, Wooles WR: Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J Appl Physiol (1985) 1994; 77: 606–13 [DOI] [PubMed] [Google Scholar]
- 30.Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N: Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 2015; 5: 193–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks VL, Cassaglia PA, Zhao D, Goldman RK: Baroreflex function in females: changes with the reproductive cycle and pregnancy. Gend Med 2012; 9: 61–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minson CT, Halliwill JR, Young TM, Joyner MJ: Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 2000; 101: 862–8 [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Sato M, Umehara S, Nishikawa T: Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol 2003; 285: R1091–7 [DOI] [PubMed] [Google Scholar]
- 34.Brooks VL, Dampney RA, Heesch CM: Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 2010; 299: R439–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H: Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 2001; 281: H284–9 [DOI] [PubMed] [Google Scholar]
- 36.Rowe JW, Troen BR: Sympathetic nervous system and aging in man. Endocr Rev 1980; 1: 167–79 [DOI] [PubMed] [Google Scholar]
- 37.Taylor CE, Atkinson G, Willie CK, Jones H, Ainslie PN, Tzeng YC: Diurnal variation in the mechanical and neural components of the baroreflex. Hypertension 2011; 58: 51–6 [DOI] [PubMed] [Google Scholar]
- 38.Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, Pickering TG: Baroreceptor activation reduces reactivity to noxious stimulation: implications for hypertension. Science 1979; 205: 1299–1301 [DOI] [PubMed] [Google Scholar]
- 39.Thurston CL, Randich A: Acute increases in arterial blood pressure produced by occlusion of the abdominal aorta induces antinociception: peripheral and central substrates. Brain research 1990; 519: 12–22 [DOI] [PubMed] [Google Scholar]
- 40.Maixner W, Touw KB, Brody MJ, Gebhart GF, Long JP: Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain research 1982; 237: 137–145 [DOI] [PubMed] [Google Scholar]
- 41.Zamir N, Simantov R, Segal M: Pain sensitivity and opioid activity in genetically and experimentally hypertensive rats. Brain Res 1980; 184: 299–310 [DOI] [PubMed] [Google Scholar]
- 42.Zamir N, Segal M: Hypertension-induced analgesia: changes in pain sensitivity in experimental hypertensive rats. Brain Res 1979; 160: 170–3 [DOI] [PubMed] [Google Scholar]
- 43.Randich A, Robertson JD: Spinal nociceptive transmission in the spontaneously hypertensive and Wistar-Kyoto normotensive rat. Pain 1994; 58: 169–83 [DOI] [PubMed] [Google Scholar]
- 44.Morgan MM, Fields HL: Activity of nociceptive modulatory neurons in the rostral ventromedial medulla associated with volume expansion-induced antinociception. Pain 1993; 52: 1–9 [DOI] [PubMed] [Google Scholar]
- 45.Maixner W, Randich A: Role of the right vagal nerve trunk in antinociception. Brain Res 1984; 298: 374–7 [DOI] [PubMed] [Google Scholar]
- 46.Randich A, Maixner W: Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev 1984; 8: 343–67 [DOI] [PubMed] [Google Scholar]
- 47.Randich A, Gebhart GF: Vagal afferent modulation of nociception. Brain Res Brain Res Rev 1992; 17: 77–99 [DOI] [PubMed] [Google Scholar]
- 48.Randich A, Maixner W: D-Ala2]-methionine enkephalinamide reflexively induces antinociception by activating vagal afferents. Pharmacology, biochemistry, and behavior 1984; 21: 441–448 [DOI] [PubMed] [Google Scholar]
- 49.Bruehl S, Carlson CR, McCubbin JA: The relationship between pain sensitivity and blood pressure in normotensives. Pain 1992; 48: 463–7 [DOI] [PubMed] [Google Scholar]
- 50.McCubbin JA, Bruehl S: Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain 1994; 57: 63–7 [DOI] [PubMed] [Google Scholar]
- 51.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB: Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain 1996; 12: 260–9 [DOI] [PubMed] [Google Scholar]
- 52.Fillingim RB, Maixner W, Bunting S, Silva S: Resting blood pressure and thermal pain responses among females: effects on pain unpleasantness but not pain intensity. Int J Psychophysiol 1998; 30: 313–8 [DOI] [PubMed] [Google Scholar]
- 53.Pfleeger M, Straneva PA, Fillingim RB, Maixner W, Girdler SS: Menstrual cycle, blood pressure and ischemic pain sensitivity in women: a preliminary investigation. Int J Psychophysiol 1997; 27: 161–6 [DOI] [PubMed] [Google Scholar]
- 54.Ditto B, Seguin JR, Boulerice B, Pihl RO, Tremblay RE: Risk for hypertension and pain sensitivity in adolescent boys. Health Psychol 1998; 17: 249–54 [DOI] [PubMed] [Google Scholar]
- 55.Ditto B, France J, France CR: Risk for hypertension and pain sensitivity in women. Int J Behav Med 1997; 4: 117–30 [DOI] [PubMed] [Google Scholar]
- 56.Guasti L, Zanotta D, Petrozzino MR, Grimoldi P, Diolisi A, Garganico D, Gaudio G, Grandi AM, Bertolini A, Venco A: Relationship between dental pain perception and 24 hour ambulatory blood pressure: a study on 181 subjects. J Hypertens 1999; 17: 1799–804 [DOI] [PubMed] [Google Scholar]
- 57.Zamir N, Shuber E: Altered pain perception in hypertensive humans. Brain Res 1980; 201: 471–4 [DOI] [PubMed] [Google Scholar]
- 58.Bruehl S, Chung OY: Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 2004; 28: 395–414 [DOI] [PubMed] [Google Scholar]
- 59.Duschek S, Dietel A, Schandry R, del Paso GA: Increased sensitivity to heat pain in chronic low blood pressure. European journal of pain (London, England) 2009; 13: 28–34 [DOI] [PubMed] [Google Scholar]
- 60.Edwards L, Ring C, McIntyre D, Carroll D: Modulation of the human nociceptive flexion reflex. Psychophysiology 2001; 38: S38–S38 [PubMed] [Google Scholar]
- 61.Edwards L, McIntyre D, Carroll D, Ring C, France CR, Martin U: Effects of artificial and natural baroreceptor stimulation on nociceptive responding and pain. Psychophysiology 2003; 40: 762–769 [DOI] [PubMed] [Google Scholar]
- 62.Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, Brunia CH: Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proc Natl Acad Sci U S A 1994; 91: 6329–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elbert T, Rockstroh B, Lutzenberger W, Kessler M, Pietrowsky R: Baroreceptor stimulation alters pain sensation depending on tonic blood pressure. Psychophysiology 1988; 25: 25–29 [DOI] [PubMed] [Google Scholar]
- 64.Bruehl S, Chung OY, Ward P, Johnson B, McCubbin JA: The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade. Pain 2002; 100: 191–201 [DOI] [PubMed] [Google Scholar]
- 65.Myers CD, Robinson ME, Riley JL, 3rd, Sheffield D: Sex, gender, and blood pressure: contributions to experimental pain report. Psychosom Med 2001; 63: 545–50 [DOI] [PubMed] [Google Scholar]
- 66.Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS: Race and sex differences in cutaneous pain perception. Psychosom Med 2000; 62: 517–23 [DOI] [PubMed] [Google Scholar]
- 67.Guasti L, Gaudio G, Zanotta D, Grimoldi P, Petrozzino MR, Tanzi F, Bertolini A, Grandi AM, Venco A: Relationship between a genetic predisposition to hypertension, blood pressure levels and pain sensitivity. Pain 1999; 82: 311–7 [DOI] [PubMed] [Google Scholar]
- 68.Yarnitsky D: Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010; 23: 611–5 [DOI] [PubMed] [Google Scholar]
- 69.Le Bars D, Villanueva L, Bouhassira D, Willer JC: Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol Fiziol Eksp Ter 1992: 55–65 [PubMed] [Google Scholar]
- 70.Chalaye P, Devoize L, Lafrenaye S, Dallel R, Marchand S: Cardiovascular influences on conditioned pain modulation. Pain 2013; 154: 1377–82 [DOI] [PubMed] [Google Scholar]
- 71.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M: Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008; 138: 22–8 [DOI] [PubMed] [Google Scholar]
- 72.Ghione S, Rosa C, Mezzasalma L, Panattoni E: Arterial hypertension is associated with hypalgesia in humans. Hypertension 1988; 12: 491–497 [DOI] [PubMed] [Google Scholar]
- 73.France CR, Stewart KM: Parental history of hypertension and enhanced cardiovascular reactivity are associated with decreased pain ratings. Psychophysiology 1995; 32: 571–578 [DOI] [PubMed] [Google Scholar]
- 74.al’Absi M, Petersen KL, Wittmers LE: Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain 2002; 96: 197–204 [DOI] [PubMed] [Google Scholar]
- 75.Al’Absi M, Buchanan T, Lovallo WR: Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology 1996; 33: 655–661 [DOI] [PubMed] [Google Scholar]
- 76.France CR, Suchowiecki S: Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiology 2001; 38: 107–13 [PubMed] [Google Scholar]
- 77.al’Absi M, Buchanan TW, Marrero A, Lovallo WR: Sex differences in pain perception and cardiovascular responses in persons with parental history for hypertension. Pain 1999; 83: 331–8 [DOI] [PubMed] [Google Scholar]
- 78.Campbell TS, Ditto B, Seguin JR, Sinray S, Tremblay RE: Adolescent pain sensitivity is associated with cardiac autonomic function and blood pressure over 8 years. Hypertension 2003; 41: 1228–33 [DOI] [PubMed] [Google Scholar]
- 79.Zamir N, Maixner W: The relationship between cardiovascular and pain regulatory systems. Annals of the New York Academy of Sciences 1986; 467: 371–384 [DOI] [PubMed] [Google Scholar]
- 80.Duschek S, Werner NS, Reyes Del Paso GA: The behavioral impact of baroreflex function: a review. Psychophysiology 2013; 50: 1183–93 [DOI] [PubMed] [Google Scholar]
- 81.Maixner W: Interaction between cardiovascular and pain modulatory systems: physiological and pathophysiological implications. Journal of Cardiovascular Electrophysiology 1991; 2: S3–S12 [Google Scholar]
- 82.Chung OY, Bruehl S, Diedrich L, Diedrich A, Chont M, Robertson D: Baroreflex sensitivity associated hypoalgesia in healthy states is altered by chronic pain. Pain 2008; 138: 87–97 [DOI] [PubMed] [Google Scholar]
- 83.Duschek S, Mück I, Del Paso GR: Relationship between baroreceptor cardiac reflex sensitivity and pain experience in normotensive individuals. International Journal of Psychophysiology 2007; 65: 193–200 [DOI] [PubMed] [Google Scholar]
- 84.Rau H, Brody S, Larbig W, Pauli P, Vohringer M, Harsch B, Kroling P, Birbaumer N: Effects of PRES baroreceptor stimulation on thermal and mechanical pain threshold in borderline hypertensives and normotensives. Psychophysiology 1994; 31: 480–485 [DOI] [PubMed] [Google Scholar]
- 85.France C, Ditto B, Adler P: Pain sensitivity in offspring of hypertensives at rest and during baroreflex stimulation. J Behav Med 1991; 14: 513–25 [DOI] [PubMed] [Google Scholar]
- 86.Ren K, Randich A, Gebhart GF: Modulation of spinal nociceptive transmission from nuclei tractus solitarii: a relay for effects of vagal afferent stimulation. J Neurophysiol 1990; 63: 971–86 [DOI] [PubMed] [Google Scholar]
- 87.Bossut DF, Maixner W: Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain 1996; 65: 101–109 [DOI] [PubMed] [Google Scholar]
- 88.Ness TJ, Fillingim RB, Randich A, Backensto EM, Faught E: Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain 2000; 86: 81–5 [DOI] [PubMed] [Google Scholar]
- 89.Angrilli A, Mini A, Mucha RF, Rau H: The influence of low blood pressure and baroreceptor activity on pain responses. Physiol Behav 1997; 62: 391–7 [DOI] [PubMed] [Google Scholar]
- 90.Dworkin B: Hypertension as a learned response, Behavioral medicine in cardiovascular disorders. Edited by Elbert T, Langosh W, Steptoe A, Vaitl D. Chichester, UK, Wiley, 1988 [Google Scholar]
- 91.Randich A, Maixner W: The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nociception and stress-induced analgesia. Ann N Y Acad Sci 1986; 467: 385–401 [DOI] [PubMed] [Google Scholar]
- 92.D’Antono B, Ditto B, Sita A, Miller SB: Cardiopulmonary baroreflex stimulation and blood pressure-related hypoalgesia. Biol Psychol 2000; 53: 217–31 [DOI] [PubMed] [Google Scholar]
- 93.Meller ST, Lewis SJ, Brody MJ, Gebhart GF: Nociceptive afferent vagal input is enhanced after transection of the aortic depressor nerve. Hypertension 1990; 15: 797–802 [DOI] [PubMed] [Google Scholar]
- 94.Chung OY, Bruehl S, Diedrich L, Diedrich A: The impact of blood pressure and baroreflex sensitivity on wind-up. Anesthesia and Analgesia 2008; 107: 1018–1025 [DOI] [PubMed] [Google Scholar]
- 95.Naranjo JR, Fuentes JA: Association between hypoalgesia and hypertension in rats after short-term isolation. Neuropharmacology 1985; 24: 167–71 [DOI] [PubMed] [Google Scholar]
- 96.Saavedra JM: Naloxone reversible decrease in pain sensitivity in young and adult spontaneously hypertensive rats. Brain Res 1981; 209: 245–9 [DOI] [PubMed] [Google Scholar]
- 97.Saavedra JM: Spontaneously (genetic) hypertensive rats: naloxone-reversible and propranolol-reversible decrease in pain sensitivity. Experientia 1981; 37: 1002–3 [DOI] [PubMed] [Google Scholar]
- 98.Sitsen JM, de Jong W: Hypoalgesia in genetically hypertensive rats (SHR) is absent in rats with experimental hypertension. Hypertension 1983; 5: 185–90 [DOI] [PubMed] [Google Scholar]
- 99.Weinstock M, Schorer-Apelbaum D, Rosin AJ: Endogenous opiates mediate cardiac sympathetic inhibition in response to a pressor stimulus in rabbits. J Hypertens 1984; 2: 639–46 [DOI] [PubMed] [Google Scholar]
- 100.Pilowsky PM, Goodchild AK: Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 2002; 20: 1675–88 [DOI] [PubMed] [Google Scholar]
- 101.Zamir N, Segal M, Simantov R: Opiate receptor binding in the brain of the hypertensive rat. Brain Res 1981; 213: 217–22 [DOI] [PubMed] [Google Scholar]
- 102.McCubbin JA: Diminished opioid inhibition of blood pressure and pituitary function in hypertension development, Stress, neuropeptides, and systemic disease. Edited by McCubbin JA, Kaufmann PG, Nemeroff CB. San Diego, Academic Press, 1991 [Google Scholar]
- 103.Smith JK, Barron KW: The rostral and caudal ventrolateral medulla in young spontaneously hypertensive rats. Brain Res 1990; 506: 153–8 [DOI] [PubMed] [Google Scholar]
- 104.Sheps DS, Bragdon EE, Gray TF, 3rd, Ballenger M, Usedom JE, Maixner W: Relation between systemic hypertension and pain perception. The American Journal of Cardiology 1992; 70: 3F–5F [DOI] [PubMed] [Google Scholar]
- 105.Bali A, Singh N, Jaggi AS: Renin-angiotensin system in pain: existing in a double life? J Renin Angiotensin Aldosterone Syst 2014; 15: 329–40 [DOI] [PubMed] [Google Scholar]
- 106.Schobel HP, Handwerker HO, Schmieder RE, Heusser K, Dominiak P, Luft FC: Effects of naloxone on hemodynamic and sympathetic nerve responses to pain in normotensive vs. borderline hypertensive men. J Auton Nerv Syst 1998; 69: 49–55 [DOI] [PubMed] [Google Scholar]
- 107.Sved AF, Tsukamoto K, Schreihofer AM: Stimulation of alpha 2-adrenergic receptors in nucleus tractus solitarius is required for the baroreceptor reflex. Brain Res 1992; 576: 297–303 [DOI] [PubMed] [Google Scholar]
- 108.Kunos G, Mosqueda-Garcia R, Mastrianni JA, Abbott FV: Endorphinergic mechanism in the central cardiovascular and analgesic effects of clonidine. Can J Physiol Pharmacol 1987; 65: 1624–32 [DOI] [PubMed] [Google Scholar]
- 109.Oley N, Cordova C, Kelly ML, Bronzino JD: Morphine administration to the region of the solitary tract nucleus produces analgesia in rats. Brain Res 1982; 236: 511–5 [DOI] [PubMed] [Google Scholar]
- 110.Ren K, Randich A, Gebhart GF: Vagal afferent modulation of a nociceptive reflex in rats: involvement of spinal opioid and monoamine receptors. Brain Res 1988; 446: 285–94 [DOI] [PubMed] [Google Scholar]
- 111.Rosa C, Ghione S, Mezzasalma L, Pellegrini M, Basile Fasolo C, Giaconi S, Gazzetti P, Ferdeghini M: Relationship between pain sensitivity, cardiovascular reactivity to cold pressor test and indexes of activity of the adrenergic and opioid system. Clin Exp Hypertens A 1988; 10 Suppl 1: 383–90 [DOI] [PubMed] [Google Scholar]
- 112.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, Harris MB: Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med 1997; 59: 503–11 [DOI] [PubMed] [Google Scholar]
- 113.Reyes del Paso GA, Garrido S, Pulgar A, Duschek S: Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res 2011; 70: 125–34 [DOI] [PubMed] [Google Scholar]
- 114.Maixner W, Greenspan JD, Dubner R, Bair E, Mulkey F, Miller V, Knott C, Slade GD, Ohrbach R, Diatchenko L: Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. The Journal of Pain 2011; 12: T75–T91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W: Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain 2009; 10: 542–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bou-Holaigah I, Calkins H, Flynn JA, Tunin C, Chang HC, Kan JS, Rowe PC: Provocation of hypotension and pain during upright tilt table testing in adults with fibromyalgia. Clinical and experimental rheumatology 1997; 15: 239–246 [PubMed] [Google Scholar]
- 117.Fillingim RB, Maixner W: The influence of resting blood pressure and gender on pain responses. Psychosomatic medicine 1996; 58: 326–332 [DOI] [PubMed] [Google Scholar]
- 118.Fillingim RB, Maixner W: Gender differences in the responses to noxious stimuli. Pain Forum 1995; 4: 209–221 [Google Scholar]
- 119.Heidari F, Afshari M, Moosazadeh M: Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017; 37: 1527–1539 [DOI] [PubMed] [Google Scholar]
- 120.Bartley EJ, Fillingim RB: Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013; 111: 52–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hagen K, Stovner LJ, Vatten L, Holmen J, Zwart JA, Bovim G: Blood pressure and risk of headache: a prospective study of 22 685 adults in Norway. J Neurol Neurosurg Psychiatry 2002; 72: 463–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hagen K, Zwart JA, Holmen J, Svebak S, Bovim G, Stovner LJ, Nord-Trondelag Health S: Does hypertension protect against chronic musculoskeletal complaints? The Nord-Trondelag Health Study. Arch Intern Med 2005; 165: 916–22 [DOI] [PubMed] [Google Scholar]
- 123.Olsen RB, Bruehl S, Nielsen CS, Rosseland LA, Eggen AE, Stubhaug A: Chronic pain and cardiovascular stress responses in a general population: the Tromso Study. J Behav Med 2014; 37: 1193–201 [DOI] [PubMed] [Google Scholar]
- 124.Ulleryd MA, Prahl U, Borsbo J, Schmidt C, Nilsson S, Bergstrom G, Johansson ME: The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One 2017; 12: e0174974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Syamsunder AN, Pal P, Pal GK, Kamalanathan CS, Parija SC, Nanda N, Sirisha A: Decreased baroreflex sensitivity is linked to the atherogenic index, retrograde inflammation, and oxidative stress in subclinical hypothyroidism. Endocr Res 2017; 42: 49–58 [DOI] [PubMed] [Google Scholar]
- 126.Subha M, Pal P, Pal GK, Habeebullah S, Adithan C, Sridhar MG: Decreased baroreflex sensitivity is linked to sympathovagal imbalance, low-grade inflammation, and oxidative stress in pregnancy-induced hypertension. Clin Exp Hypertens 2016; 38: 666–672 [DOI] [PubMed] [Google Scholar]
- 127.Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson RM: The diagnosis and treatment of baroreflex failure. N Engl J Med 1993; 329: 1449–55 [DOI] [PubMed] [Google Scholar]
- 128.Koopman FA, Tang MW, Vermeij J, de Hair MJ, Choi IY, Vervoordeldonk MJ, Gerlag DM, Karemaker JM, Tak PP: Autonomic Dysfunction Precedes Development of Rheumatoid Arthritis: A Prospective Cohort Study. EBioMedicine 2016; 6: 231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thompson M, Bywaters EG: Unilateral rheumatoid arthritis following hemiplegia. Ann Rheum Dis 1962; 21: 370–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poyhonen-Alho MK, Manhem K, Katzman P, Kibarskis A, Antikainen RL, Erkkola RU, Tuomilehto JO, Ebeling PE, Kaaja RJ: Central sympatholytic therapy has anti-inflammatory properties in hypertensive postmenopausal women. J Hypertens 2008; 26: 2445–9 [DOI] [PubMed] [Google Scholar]
- 131.Bernstein IM, Damron D, Schonberg AL, Sallam RM, Shapiro R: The relationship of plasma volume, sympathetic tone, and proinflammatory cytokines in young healthy nonpregnant women. Reprod Sci 2009; 16: 980–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tracey KJ: The inflammatory reflex. Nature 2002; 420: 853–9 [DOI] [PubMed] [Google Scholar]
- 133.Tracey KJ: Reflex control of immunity. Nat Rev Immunol 2009; 9: 418–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ: Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med 2002; 195: 781–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bassi GS, Brognara F, Castania JA, Talbot J, Cunha TM, Cunha FQ, Ulloa L, Kanashiro A, Dias DP, Salgado HC: Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Brain Behav Immun 2015; 49: 140–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pongratz G, Straub RH: The sympathetic nervous response in inflammation. Arthritis Res Ther 2014; 16: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A: Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 2003; 198: 725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brognara F, Castania JA, Dias DPM, Lopes AH, Fazan R, Jr., Kanashiro A, Ulloa L, Salgado HC: Baroreflex stimulation attenuates central but not peripheral inflammation in conscious endotoxemic rats. Brain Res 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taiwo YO, Goetzl EJ, Levine JD: Hyperalgesia onset latency suggests a hierarchy of action. Brain Res 1987; 423: 333–7 [DOI] [PubMed] [Google Scholar]
- 140.Aley KO, Messing RO, Mochly-Rosen D, Levine JD: Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 2000; 20: 4680–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levine JD, Khasar SG, Green PG: Neurogenic inflammation and arthritis. Ann N Y Acad Sci 2006; 1069: 155–67 [DOI] [PubMed] [Google Scholar]
- 142.Drummond PD: The effect of sympathetic activity on thermal hyperalgesia in capsaicin-treated skin during body cooling and warming. Eur J Pain 2001; 5: 59–67 [DOI] [PubMed] [Google Scholar]
- 143.Nielsen R, Nikolajsen L, Kroner K, Molgaard H, Vase L, Jensen TS, Terkelsen AJ: Pre-operative baroreflex sensitivity and efferent cardiac parasympathetic activity are correlated with post-operative pain. Acta Anaesthesiol Scand 2015; 59: 475–85 [DOI] [PubMed] [Google Scholar]
- 144.France CR, Katz J: Postsurgical pain is attenuated in men with elevated systolic blood pressure. Pain Res Manage 1999; 4: 100–103 [Google Scholar]
- 145.Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, Washburn SA, Harris L, Eisenach JC: Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology 2006; 104: 417–25 [DOI] [PubMed] [Google Scholar]
- 146.Stirt JA, Frantz RA, Gunz EF, Conolly ME: Anesthesia, catecholamines, and hemodynamics in autonomic dysfunction. Anesth Analg 1982; 61: 701–4 [PubMed] [Google Scholar]
- 147.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ: Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007; 107: 213–20 [DOI] [PubMed] [Google Scholar]
- 148.Dorantes Mendez G, Aletti F, Toschi N, Canichella A, Dauri M, Coniglione F, Guerrisi M, Signorini MG, Cerutti S, Ferrario M: Baroreflex sensitivity variations in response to propofol anesthesia: comparison between normotensive and hypertensive patients. J Clin Monit Comput 2013; 27: 417–26 [DOI] [PubMed] [Google Scholar]
- 149.Ding W, Zhou L, Bao Y, Zhou L, Yang Y, Lu B, Wu X, Hu R: Autonomic nervous function and baroreflex sensitivity in hypertensive diabetic patients. Acta Cardiol 2011; 66: 465–70 [DOI] [PubMed] [Google Scholar]
- 150.Dauphinot V, Gosse P, Kossovsky MP, Schott AM, Rouch I, Pichot V, Gaspoz JM, Roche F, Barthelemy JC: Autonomic nervous system activity is independently associated with the risk of shift in the non-dipper blood pressure pattern. Hypertens Res 2010; 33: 1032–7 [DOI] [PubMed] [Google Scholar]
- 151.Nasr N, Czosnyka M, Pavy-Le Traon A, Custaud MA, Liu X, Varsos GV, Larrue V: Baroreflex and cerebral autoregulation are inversely correlated. Circ J 2014; 78: 2460–7 [DOI] [PubMed] [Google Scholar]
- 152.Simula S, Laitinen T, Vanninen E, Pajunen P, Syvanne M, Hedman A, Hartikainen J: Baroreflex sensitivity in asymptomatic coronary atherosclerosis. Clin Physiol Funct Imaging 2013; 33: 70–4 [DOI] [PubMed] [Google Scholar]
- 153.Schutte AE, Van Rooyen JM, Huisman HW, Mukuddem-Petersen J, Oosthuizen W, Hanekom SM, Jerling JC: Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. Am J Hypertens 2006; 19: 629–36 [DOI] [PubMed] [Google Scholar]
- 154.Gerhardt U, Vorneweg P, Riedasch M, Hohage H: Acute and persistant effects of smoking on the baroreceptor function. J Auton Pharmacol 1999; 19: 105–8 [DOI] [PubMed] [Google Scholar]
- 155.Kardos A, Watterich G, de Menezes R, Csanady M, Casadei B, Rudas L: Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension 2001; 37: 911–6 [DOI] [PubMed] [Google Scholar]
- 156.Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG: Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 1996; 154: 1490–6 [DOI] [PubMed] [Google Scholar]
- 157.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK: Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998; 98: 1071–7 [DOI] [PubMed] [Google Scholar]
- 158.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK: Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 1998; 98: 772–6 [DOI] [PubMed] [Google Scholar]
- 159.Grote L, Kraiczi H, Hedner J: Reduced alpha- and beta(2)-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2000; 162: 1480–7 [DOI] [PubMed] [Google Scholar]
- 160.Baguet JP, Barone-Rochette G, Tamisier R, Levy P, Pepin JL: Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol 2012; 9: 679–88 [DOI] [PubMed] [Google Scholar]
- 161.Johansson M, Gao SA, Friberg P, Annerstedt M, Carlstrom J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen FD, Strombom U: Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens 2007; 25: 163–8 [DOI] [PubMed] [Google Scholar]
- 162.Lacy P, Carr SJ, O’Brien D, Fentum B, Williams B, Paul SK, Robinson TG: Reduced glomerular filtration rate in pre-dialysis non-diabetic chronic kidney disease patients is associated with impaired baroreceptor sensitivity and reduced vascular compliance. Clin Sci (Lond) 2006; 110: 101–8 [DOI] [PubMed] [Google Scholar]
- 163.Convertino VA, Doerr DF, Eckberg DL, Fritsch JM, Vernikos-Danellis J: Head-down bed rest impairs vagal baroreflex responses and provokes orthostatic hypotension. J Appl Physiol (1985) 1990; 68: 1458–64 [DOI] [PubMed] [Google Scholar]
- 164.Lam KK, Kunder S, Wong J, Doufas AG, Chung F: Obstructive sleep apnea, pain, and opioids: is the riddle solved? Curr Opin Anaesthesiol 2016; 29: 134–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krein SL, Heisler M, Piette JD, Makki F, Kerr EA: The effect of chronic pain on diabetes patients’ self-management. Diabetes Care 2005; 28: 65–70 [DOI] [PubMed] [Google Scholar]
- 166.Narouze S, Souzdalnitski D: Obesity and chronic pain: systematic review of prevalence and implications for pain practice. Reg Anesth Pain Med 2015; 40: 91–111 [DOI] [PubMed] [Google Scholar]
- 167.Davison SN, Koncicki H, Brennan F: Pain in chronic kidney disease: a scoping review. Semin Dial 2014; 27: 188–204 [DOI] [PubMed] [Google Scholar]
- 168.Goesling J, Brummett CM, Hassett AL: Cigarette smoking and pain: depressive symptoms mediate smoking-related pain symptoms. Pain 2012; 153: 1749–54 [DOI] [PubMed] [Google Scholar]
- 169.Zale EL, Maisto SA, Ditre JW: Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev 2015; 37: 57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ohayon MM, Stingl JC: Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res 2012; 46: 444–50 [DOI] [PubMed] [Google Scholar]
- 171.Ackland GL, Whittle J, Toner A, Machhada A, Del Arroyo AG, Sciuso A, Jenkins N, Dyson A, Struthers R, Sneyd JR, Minto G, Singer M, Shah AM, Gourine AV: Molecular Mechanisms Linking Autonomic Dysfunction and Impaired Cardiac Contractility in Critical Illness. Crit Care Med 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD: Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation 1988; 78: 969–79 [DOI] [PubMed] [Google Scholar]
- 173.Floras JS, Ponikowski P: The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 2015; 36: 1974–82b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L: Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96: 3450–8 [DOI] [PubMed] [Google Scholar]
- 175.Field ME, Wasmund SL, Page RL, Hamdan MH: Restoring Sinus Rhythm Improves Baroreflex Function in Patients With Persistent Atrial Fibrillation. J Am Heart Assoc 2016; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McIntyre D, Ring C, Hamer M, Carroll D: Effects of arterial and cardiopulmonary baroreceptor activation on simple and choice reaction times. Psychophysiology 2007; 44: 874–9 [DOI] [PubMed] [Google Scholar]
- 177.Nyklicek I, Wijnen V, Rau H: Effects of baroreceptor stimulation and opioids on the auditory startle reflex. Psychophysiology 2005; 42: 213–22 [DOI] [PubMed] [Google Scholar]
- 178.Romero-Osorio O, Gil-Tamayo S, Narino D, Rosselli D: Changes in sleep patterns after vagus nerve stimulation, deep brain stimulation or epilepsy surgery: Systematic review of the literature. Seizure 2018; 56: 4–8 [DOI] [PubMed] [Google Scholar]
- 179.Duschek S, Muckenthaler M, Werner N, del Paso GA: Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol 2009; 81: 110–7 [DOI] [PubMed] [Google Scholar]
- 180.Reyes Del Paso GA, Gonzalez MI, Hernandez JA, Duschek S, Gutierrez N: Tonic blood pressure modulates the relationship between baroreceptor cardiac reflex sensitivity and cognitive performance. Psychophysiology 2009; 46: 932–938 [DOI] [PubMed] [Google Scholar]
- 181.Reyes Del Paso GA, Mata JL, Martin-Vazquez M: Relationships between baroreceptor cardiac reflex sensitivity and cognitive performance: Modulations by gender and blood pressure. Psychophysiology 2012; 49: 138–144 [DOI] [PubMed] [Google Scholar]
- 182.Yasumasu T, Reyes Del Paso GA, Takahara K, Nakashima Y: Reduced baroreflex cardiac sensitivity predicts increased cognitive performance. Psychophysiology 2006; 43: 41–5 [DOI] [PubMed] [Google Scholar]
- 183.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA: Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001; 344: 395–402 [DOI] [PubMed] [Google Scholar]
- 184.Hogue CW Jr., Palin CA, Arrowsmith JE: Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg 2006; 103: 21–37 [DOI] [PubMed] [Google Scholar]
- 185.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS: Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998; 351: 857–61 [DOI] [PubMed] [Google Scholar]
- 186.Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, Whitson HE, Mathew JP: Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol Clin 2015; 33: 517–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM: The influence of vagus nerve stimulation on memory. Cogn Behav Neurol 2006; 19: 119–22 [DOI] [PubMed] [Google Scholar]
- 188.DeGiorgio CM, Krahl SE: Neurostimulation for drug-resistant epilepsy. Continuum (Minneap Minn) 2013; 19: 743–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.De Ridder D, Vanneste S, Engineer ND, Kilgard MP: Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation 2014; 17: 170–9 [DOI] [PubMed] [Google Scholar]
- 190.Lehtimaki J, Hyvarinen P, Ylikoski M, Bergholm M, Makela JP, Aarnisalo A, Pirvola U, Makitie A, Ylikoski J: Transcutaneous vagus nerve stimulation in tinnitus: a pilot study. Acta Otolaryngol 2013; 133: 378–82 [DOI] [PubMed] [Google Scholar]
- 191.Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA: Vagus Nerve Stimulation and Other Neuromodulation Methods for Treatment of Traumatic Brain Injury. Neurocrit Care 2016; 24: 308–19 [DOI] [PubMed] [Google Scholar]
- 192.Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL: Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J Neurotrauma 2016; 33: 871–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, Hilmi O, McLean J, Forbes K, Kilgard MP, Rennaker RL, Cramer SC, Walters M, Engineer N: Safety, Feasibility, and Efficacy of Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Ischemic Stroke. Stroke 2016; 47: 143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sjogren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E: Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psychiatry 2002; 63: 972–80 [DOI] [PubMed] [Google Scholar]
- 195.Merrill CA, Jonsson MA, Minthon L, Ejnell H, CsS H, Blennow K, Karlsson M, Nordlund A, Rolstad S, Warkentin S, Ben-Menachem E, Sjogren MJ: Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J Clin Psychiatry 2006; 67: 1171–8 [DOI] [PubMed] [Google Scholar]
- 196.Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT: Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging 2015; 36: 1860–7 [DOI] [PubMed] [Google Scholar]
- 197.Cimpianu CL, Strube W, Falkai P, Palm U, Hasan A: Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm (Vienna) 2017; 124: 145–158 [DOI] [PubMed] [Google Scholar]
- 198.George MS, Ward HE Jr., Ninan PT, Pollack M, Nahas Z, Anderson B, Kose, Howland RH, Goodman WK, Ballenger JC: A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul 2008; 1: 112–21 [DOI] [PubMed] [Google Scholar]
- 199.Silberstein SD, Calhoun AH, Lipton RB, Grosberg BM, Cady RK, Dorlas S, Simmons KA, Mullin C, Liebler EJ, Goadsby PJ, Saper JR: Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 2016; 87: 529–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Trimboli M, Al-Kaisy A, Andreou AP, Murphy M, Lambru G: Non-invasive vagus nerve stimulation for the management of refractory primary chronic headaches: A real-world experience. Cephalalgia 2017: 333102417731349. [DOI] [PubMed] [Google Scholar]
- 201.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP: Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.De Herdt V, Bogaert S, Bracke KR, Raedt R, De Vos M, Vonck K, Boon P: Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J Neuroimmunol 2009; 214: 104–8 [DOI] [PubMed] [Google Scholar]
- 203.Lange G, Janal MN, Maniker A, Fitzgibbons J, Fobler M, Cook D, Natelson BH: Safety and efficacy of vagus nerve stimulation in fibromyalgia: a phase I/II proof of concept trial. Pain Med 2011; 12: 1406–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Napadow V, Edwards RR, Cahalan CM, Mensing G, Greenbaum S, Valovska A, Li A, Kim J, Maeda Y, Park K, Wasan AD: Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med 2012; 13: 777–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bonaz B, Sinniger V, Hoffmann D, Clarencon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocme C, Faure P, Cracowski JL, Pellissier S: Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil 2016; 28: 948–53 [DOI] [PubMed] [Google Scholar]
- 206.Zamotrinsky A, Afanasiev S, Karpov RS, Cherniavsky A: Effects of electrostimulation of the vagus afferent endings in patients with coronary artery disease. Coron Artery Dis 1997; 8: 551–7 [PubMed] [Google Scholar]
- 207.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ, CardioFit Multicenter Trial I: Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011; 32: 847–55 [DOI] [PubMed] [Google Scholar]
- 208.Bakris GL, Nadim MK, Haller H, Lovett EG, Schafer JE, Bisognano JD: Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens 2012; 6: 152–8 [DOI] [PubMed] [Google Scholar]
- 209.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA: Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J Am Coll Cardiol 2011; 58: 765–73 [DOI] [PubMed] [Google Scholar]
- 210.Hoppe UC, Brandt MC, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovett EG, Haller H: Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens 2012; 6: 270–6 [DOI] [PubMed] [Google Scholar]
- 211.Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T, de Leeuw PW: Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol 2010; 56: 1254–8 [DOI] [PubMed] [Google Scholar]
- 212.Val-Laillet D, Biraben A, Randuineau G, Malbert CH: Chronic vagus nerve stimulation decreased weight gain, food consumption and sweet craving in adult obese minipigs. Appetite 2010; 55: 245–52 [DOI] [PubMed] [Google Scholar]
- 213.Convertino VA, Reister CA: Effect of G-suit protection on carotid-cardiac baroreflex function. Aviat Space Environ Med 2000; 71: 31–6 [PubMed] [Google Scholar]
- 214.Ditto B, Lewkowski MD, Rainville P, Duncan GH: Effects of cardiopulmonary baroreceptor activation on pain may be moderated by risk for hypertension. Biol Psychol 2009; 82: 195–197 [DOI] [PubMed] [Google Scholar]
- 215.Hotta H, Uchida S: Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int 2010; 10 Suppl 1: S127–36 [DOI] [PubMed] [Google Scholar]
- 216.Lima JW, Hentschke VS, Rossato DD, Quagliotto E, Pinheiro L, Almeida E, Jr., Dal Lago P, Lukrafka JL: Chronic electroacupuncture of the ST36 point improves baroreflex function and haemodynamic parameters in heart failure rats. Auton Neurosci 2015; 193: 31–7 [DOI] [PubMed] [Google Scholar]
- 217.Michikami D, Kamiya A, Kawada T, Inagaki M, Shishido T, Yamamoto K, Ariumi H, Iwase S, Sugenoya J, Sunagawa K, Sugimachi M: Short-term electroacupuncture at Zusanli resets the arterial baroreflex neural arc toward lower sympathetic nerve activity. Am J Physiol Heart Circ Physiol 2006; 291: H318–26 [DOI] [PubMed] [Google Scholar]
- 218.Thieme K, Meller T, Evermann U, Malinowski R, Mathys M, Gracely RH, Maixner W, Turk DC: Efficacy of Systolic Extinction Training (SET) in Fibromyalgia Patients with elevated Blood Pressure Response to Stress - A Tailored RCT Study. Arthritis Care Res (Hoboken) 2018 [DOI] [PubMed] [Google Scholar]
- 219.He W, Wang X, Shi H, Shang H, Li L, Jing X, Zhu B: Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med 2012; 2012: 786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yuan H, Silberstein SD: Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part III. Headache 2016; 56: 479–90 [DOI] [PubMed] [Google Scholar]
- 221.Convertino VA, Adams WC: Enhanced vagal baroreflex response during 24 h after acute exercise. Am J Physiol 1991; 260: R570–5 [DOI] [PubMed] [Google Scholar]
- 222.Jennings GL: Mechanisms for reduction of cardiovascular risk by regular exercise. Clin Exp Pharmacol Physiol 1995; 22: 209–11 [DOI] [PubMed] [Google Scholar]
- 223.Mack GW, Convertino VA, Nadel ER: Effect of exercise training on cardiopulmonary baroreflex control of forearm vascular resistance in humans. Med Sci Sports Exerc 1993; 25: 722–6 [PubMed] [Google Scholar]
- 224.Meller T, Stiehm F, Malinowski R, Thieme K: [Baroreflex sensitivity and chronic pain : Pathogenetic significance and clinical implications]. Schmerz 2016; 30: 470–476 [DOI] [PubMed] [Google Scholar]
- 225.del Paso GA, Gonzalez MI: Modification of baroreceptor cardiac reflex function by biofeedback. Appl Psychophysiol Biofeedback 2004; 29: 197–211 [DOI] [PubMed] [Google Scholar]
- 226.Lehrer P, Vaschillo E, Lu SE, Eckberg D, Vaschillo B, Scardella A, Habib R: Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest 2006; 129: 278–84 [DOI] [PubMed] [Google Scholar]
- 227.Lin G, Xiang Q, Fu X, Wang S, Wang S, Chen S, Shao L, Zhao Y, Wang T: Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. J Altern Complement Med 2012; 18: 143–52 [DOI] [PubMed] [Google Scholar]
- 228.Reyes del Paso GA: A biofeedback system of baroreceptor cardiac reflex sensitivity. Appl Psychophysiol Biofeedback 1999; 24: 67–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


