SUMMARY
Surface properties, such as adhesion and hydrophobicity, constrain dispersal of bacterial spores in the environment. In Bacillus subtilis, these properties are influenced by the outermost layer of the spore, the crust. Previous work has shown that two clusters, cotVWXYZ and cgeAB, encode the protein components of the crust. Here, we characterize the respective roles of these genes in surface properties using Bacterial Adherence to Hydrocarbons assays, negative staining of polysaccharides by India ink, and Transmission Electron Microscopy. We showed that inactivation of crust genes caused increases in spore relative hydrophobicity, disrupted the spore polysaccharide layer, and impaired crust structure and attachment to the rest of the coat. We also found that cotO, previously identified for its role in outer coat formation, is necessary for proper encasement of the spore by the crust. In parallel, we conducted fluorescence microscopy experiments to determine the full network of genetic dependencies for subcellular localization of crust proteins. We determined that CotZ is required for the localization of most crust proteins, while CgeA is at the bottom of the genetic interaction hierarchy.
Keywords: sporulation, Bacillus subtilis, spore coat, spore crust, spore surface, morphogenetic proteins
Graphical Abstract
A group of Bacillus subtilis coat proteins, encoded by cotO, cotVW, cotXYZ and cgeAB, influence spore crust structure, relative surface hydrophobicity and anchoring of a polysaccharide layer. Crust assembly is controlled by a complex set of interactions, with CotZ acting as the principal morphogenetic crust protein and cgeA at the bottom of the genetic hierarchy. CotO promotes encasement of the spore by the crust.

INTRODUCTION
Many bacteria adopt a dormant life style to survive extreme environmental challenges. Several species among the Firmicutes, including those that populate the human gastrointestinal tract (Browne et al., 2016), can form highly resistant dormant cells called endospores (hereafter spores). In addition to providing a key to survival, an important property of these spores is their ability to disperse in the environment to colonize new hosts or niches (Abe et al., 2014; Browne, Neville, Forster, & Lawley, 2017; McKenney, Driks, & Eichenberger, 2013). While spore resistance has been investigated in detail, the process of spore dispersal, the structural basis for spore surface and specific properties such as adhesion, hydrophobicity or hydrophilicity, are much less well understood (Chen, Driks, Tawfiq, Mallozzi, & Patil, 2010; Faille et al., 2010; Setlow, 2014). In this study, we report a detailed analysis of spore properties provided by a group of genes that encode components of the outermost layer of the spore in Bacillus subtilis. In this bacterium, established long ago as the model for the study of sporulation, the genome is found in the spore core, surrounded by a membrane, a cortex made of peptidoglycan, and a complex coat composed of over 80 proteins (Driks & Eichenberger, 2016; Henriques & Moran, 2007). These proteins are separated into four concentric layers: the basement layer, inner coat, outer coat and crust (McKenney et al., 2013).
Sporulation is triggered by prolonged nutrient deprivation, which leads to the asymmetric division of the sporulating cell generating a forespore and a larger mother cell (Errington, 2003; Higgins & Dworkin, 2012; Stragier & Losick, 1996; Tan & Ramamurthi, 2014). Each of these cells will launch a distinct program of gene expression controlled by cell-specific σ factors (σF followed by σG in the forespore; σE followed by σK in the mother cell) (de Hoon, Eichenberger, & Vitkup, 2010). Shortly after asymmetric division, the mother cell begins to engulf the forespore in a phagocytosis-like process. Coat morphogenesis commences at this stage, with the first group of coat proteins synthesized in the mother cell cytoplasm under the control of σE. These proteins are recruited to the forespore surface by a group of coat morphogenetic proteins that assemble early to form a coat scaffold, with at least one morphogenetic protein per coat layer; i.e. SpoIVA for the basement layer, SafA for the inner coat and CotE for the outer coat (Driks, Roels, Beall, Moran, & Losick, 1994; Ozin, Henriques, Yi, & Moran, 2000; Roels, Driks, & Losick, 1992; Takamatsu, Kodama, Nakayama, & Watabe, 1999; Zheng, Donovan, Fitz-James, & Losick, 1988). Once engulfment is complete, the forespore becomes a cell within the mother cell cytoplasm, and a second group of coat proteins is expressed under the control of σK. Thus, coat morphogenesis relies on the timely synthesis and correct localization of proteins to the appropriate coat layer (Driks et al., 1994; McKenney & Eichenberger, 2012).
The crust is the outermost layer of the spore (Imamura, Kuwana, Takamatsu, & Watabe, 2010; McKenney et al., 2010). Previous work suggested that crust assembly relies on coat proteins CotX, CotY and CotZ, which were defined as the crust morphogenetic proteins (McKenney et al., 2010), and originally identified as components of the spore coat insoluble fraction (Zhang, Fitz-James, & Aronson, 1993). Based on epifluorescence microscopy data, the coat proteins CotV, CotW and CgeA were also characterized as components of the crust (Imamura et al., 2010; Imamura, Kuwana, Takamatsu, & Watabe, 2011; McKenney et al., 2010). In addition, two-hybrid analyses revealed a complex network of putative interactions between these proteins (Krajcikova, Forgac, Szabo, & Barak, 2017). Furthermore, CotV, CotW and CotY have the capacity to self-assemble and form highly ordered structures, including fibers and stacks (Jiang et al., 2015). As the spore outermost layer, the crust influences how the spore interacts with its environment (Abe et al., 2014; McKenney et al., 2013). Spore surface properties determine spore to spore and spore to host interactions, adhesion to other surfaces, and dispersal in aqueous environments. In some species, such as those in the Bacillus cereus group (which includes B. cereus, B. thuringiensis and B. anthracis (Helgason et al., 2000)), an exosporium consisting of glycoproteins balloons away from the spore outer coat and constitutes the outermost layer of the spore (Driks, 2002; Driks & Eichenberger, 2016; Stewart, 2015). While the crust and the exosporium are distinct structures, some B. subtilis crust genes (cotY and cotZ) are homologous to B. cereus/B. anthracis exosporium genes (cotY and exsY).
The crust and exosporium are adorned with polysaccharides (PS) (Waller, Fox, Fox, Fox, & Price, 2004) and the overall composition of the spore surface carbohydrates in B. subtilis and its close relatives (sometimes referred as the B. subtilis group) and the B. cereus group have at least some sugars in common, with rhamnose being one of the mutual sugars produced (Wunschel, Fox, Black, & Fox, 1994). Nevertheless, several species appear to synthesize at least one species-specific sugar during sporulation; i.e. anthrose in B. anthracis, cereose in B. cereus and quinovose in B. subtilis (Daubenspeck et al., 2004; Faille et al., 2014; Li et al., 2017). Carbohydrate production during sporulation in B. subtilis is dependent on σK, which controls expression of the spore polysaccharide synthesis (sps) operon (Eichenberger et al., 2004). The four distal genes in the operon, spsIJKL, encode the enzymes necessary for rhamnose synthesis (Cangiano et al., 2014; Plata, Fuhrer, Hsiao, Sauer, & Vitkup, 2012). Even though the corresponding operon, rmlACBD, has a different name in the B. cereus group, its function appears to be conserved (Stewart, 2015). Interestingly, the rhamnose-producing operon is flanked by genes involved in exosporium assembly (bclA and genes encoding putative glycosyltransferases on one side; cotO and exsY on the other side). We have previously determined that a GFP fusion to SpsI, a glucose-1-phosphate thymidylyltransferase, localized to the outer coat under the control of cotE (Arrieta-Ortiz et al., 2015). Hence, if rhamnose is produced near the outer coat, glycosyltransferases must be available to transfer carbohydrates and anchor them to specific coat/crust proteins.
Rhamnose is not the sole carbohydrate present on the spore surface (Wunschel et al., 1994). In B. subtilis, another contributor to spore polysaccharide synthesis is the spsM gene, which is reconstituted specifically through a rearrangement of the mother cell genome during late sporulation (Abe et al., 2014; Abe, Takamatsu, & Sato, 2017), and two σK-controlled paralogs of spsI, yfnH and ytdA, although the identity of the sugars produced remains unclear (Arrieta-Ortiz et al., 2015). It has also been suggested that the presence of a mucous layer surrounding spores in certain strains of B. subtilis influenced hydrophobicity and adhesion properties (Faille et al., 2014). Adhesion properties vary greatly among spore-forming species (Ronner, Husmark, & Henriksson, 1990; Wiencek, Klapes, & Foegeding, 1990). For instance, B. anthracis and B. cereus spores are considered hydrophobic compared to B. subtilis, suggesting that the presence of an exosporium could account for this difference (Ankolekar & Labbe, 2010; Koshikawa et al., 1989; Ronner et al., 1990). Variations between strains have also been reported even in the absence of an exosporium (Faille et al., 2010). Another study concluded that both B. subtilis and B. anthracis spores were hydrophilic (Chen et al., 2010), which further suggests variation in surface properties within species. Studying changes in adherence among closely related strains will help uncover how subtle modifications in the molecular composition of the spore surface were selected for to optimize interaction with specific environments.
In this study, we have utilized three complementary methods to study spore surface properties: Bacterial Adherence to Hydrocarbons (BATH) assays, negative staining of PS with India ink, and Transmission Electron Microscopy (TEM) in the presence of Ruthenium red. The BATH assay takes isolated spores resuspended in Phosphate Buffer Saline (PBS) and exposes them to hexadecane, an apolar solvent, allowing them to settle in the phase (aqueous or organic) which more strongly interacts with the spore surface (Faille et al., 2010). This semi-quantitative approach provides evidence of the surface composition of the spore via their interactive properties. Negative staining is a method by which spores are stained with India ink, where the stain is unable to penetrate through a PS layer, thus producing a white halo around the spores. This approach allows us to identify defects in PS deposition in mutant spores and screen for the presence of a PS layer in natural isolates. Lastly, TEM imaging with Ruthenium red, a stain that reveals the presence of the crust (McKenney et al., 2010; Waller et al., 2004), was used to study the ultrastructure of the spore and the effect caused by deletion of specific crust genes. In combination, these approaches reveal the respective contributions of each known crust gene to crust assembly and surface properties. Characterizing the structural properties of the crust will help us to understand the mechanisms of spore dispersal as well as the molecular basis for interaction between spores, with hosts and in the environment.
RESULTS
Variability in spore surface properties
We used BATH assays to compare the relative hydrophobicity profiles of spores from a selection of B. subtilis strains (all strains used in this study are listed in Table S1). Comparing the laboratory strains B. subtilis (168 and PY79) to two strains of B. subtilis natto (BEST 195 and Takahashi), spores from the natto strains are considerably more hydrophobic (Figure 1A). BEST195 spores settled at an average of 4.8±0.8% spores remaining in the aqueous layer compared to 98.0±1.3% for strain 168 spores. BATH assays for additional B. subtilis strains, including the wild isolate B. subtilis NCIB 3610 (Figure S1A), and closely related species (Figure S1B) can be found in the supporting information. Negative staining of PS with India ink also revealed variation in spore properties (Figure 1B). While 168 and PY79 spores are surrounded by a large halo, spores from the BEST 195 strain have a thinner halo, whereas Takahashi spores do not exhibit a visible halo. Measurements of halo areas can be found in Figure S2A (along with the fields of spores used in the counting, Figure S2B), suggesting that PS deposition to the spore surface is impaired in the natto strains. The India ink staining procedure is, however, not quantitative enough to precisely measure the extent of the defect. To better compare the morphologies of the spore outermost layers in these strains, we collected TEM images in the presence of Ruthenium red, which is regarded as a promiscuous stain, but can reveal PS-containing structures in spores (Waller et al., 2004). As previously reported (McKenney et al., 2010), the multilayered structure of the coat, including the inner coat, outer coat and crust, is evident in the PY79 strain (Fig. 1C). By contrast, in natto spores, the crust layer is disrupted, although the rest of the coat appears unaffected. A thin crust is detected in most BEST195 spores, but it rarely encircles the entire spore. The difference is more pronounced for the Takahashi strain, whose spores do not exhibit any crust or crust-like material. Thus, even when considering the possibility that Ruthenium red and India ink staining could be sensitive to different forms of PS, both approaches highlighted differences between the laboratory and natto strains, consistent with the phenotypes revealed in the BATH assays.
Figure 1: Spore surface properties differ greatly among strains of B. subtilis.
A. Analysis by BATH assay of spore relative hydrophobicity in strains of B. subtilis (168, PY79, natto BEST195, and natto Takhashi). The graph shows the percentage of spores remaining in the aqueous layer (PBS) after addition of hexadecane and vortexing for the indicated times. Experiments were performed in triplicate. Spores from both natto strains are more hydrophobic than spores from the reference laboratory strains 168 and PY79. BATH assays for other B. subtilis strains and closely related species are provided in Figures S1A and S1B. B. Negative staining of B. subtilis spores with India Ink reveals halos corresponding to PS layers in 168 and PY79, a reduced halo in BEST195, and no halo in Takahashi (scale bars=4 μm). Measurements of the halo areas are provided in Figure S2. C. Analysis of coat and crust morphology by TEM in the presence of Ruthenium red. Scale bars are 200 nm. Wild type spores (PY79) have a crust (Cr, dark red arrow), an electron dense outer coat layer (Oc, orange arrow), a lamellar inner coat layer (Ic, yellow arrow), and a cortex (Cx, white arrow). BEST 195 spores have a disrupted crust (i.e. a thinner crust that does not fully encircle the spore). Takahashi spores have no detectable crust. D. The chromosomal regions near the spore crust genes are shown for B. subtilis 168 and B. subtilis natto BEST195. Genes are represented as arrows indicating the direction of transcription. Genes expressed during sporulation are colored: crust genes are dark red, the outer coat gene cotO is orange, the sporulation gene yjcA is olive; promoters are also indicated. For B. subtilis natto BEST 195, crust gene orthologs with a lower degree of conservation (<90% amino acid sequence identity) are indicated with a paler color.
There are at least two gene regions necessary for crust formation in B. subtilis 168, one centered around the cotXYZ gene cluster and another that includes the cgeAB operon (Figure 1D). A comparative analysis (Table S2) confirms that all crust genes are present and highly conserved in BEST 195 (>94% amino acid sequence identity), except cotX (54%), cotV (45%) and cotW (39%). No sequence data is currently available for the Takahashi strain. A protein sequence alignment for CotX reveals that two stretches are absent in the N-terminal portion of CotX in BEST 195, a heavily charged sequence (DKEKHERK) and a palindromic stretch (NGFFGN) (Figure S3). Additional work will be necessary to determine if these differences in protein sequence are causing the observed modifications in crust morphology or if other factors (e.g. differences in gene expression) are more important. By contrast, enzymes involved in PS synthesis (cgeCDE operon, sps operon, spsM, yfnHGF operon and ytdA) are nearly identical in all B. subtilis strains (>98% amino acid sequence identity, not shown). To better understand the molecular basis for variability in spore hydrophobicity and PS layer thickness, we decided to explore the individual contributions of each crust gene to spore surface properties in the laboratory strain PY79.
Respective contributions of CotX, CotY, and CotZ to spore surface properties
It is already known that assembly of the outermost layer of the coat is impaired in ΔcotXYZ spores. Therefore, CotX, CotY, and CotZ have been defined as the crust morphogenetic proteins (McKenney et al., 2013). Here we analyze by BATH assay, India Ink staining, and TEM in the presence of Ruthenium red, the phenotypes for each single mutant in the cotXYZ gene cluster. As previously observed (McKenney et al., 2010), the crust is notably absent in ΔcotXYZ spores (Figures 2A and 3A-B). This morphological difference is echoed phenotypically in the BATH assay (Figure 2B), where crust deficient ΔcotXYZ spores are found to be much more hydrophobic than wild type spores and settle in the aqueous phase at an average of 17±7%. By contrast, the difference observed between wild type (PY79) and ΔcotXYZ spores in the India ink assay is more modest. In the ΔcotXYZ mutant, the halo of negative staining surrounding the spores is thinner, but still present (Figure 2C and Figure S4), implying that the crust is not the sole anchoring site for PS. This observation further suggests that some PS could be added directly to the outer coat. It remains unclear, however, if some fraction of the PS is always added to the outer coat or if it is only added when the crust is absent (i.e. the outer coat would become an anchor site by default). These results also suggest that hydrophobicity is not systematically correlated to absence of PS, given that certain types of relatively hydrophobic spores retain at least some PS. To test the independent contributions of each gene on the observed phenotypes, we repeated these experiments with strains bearing individual gene deletions.
Figure 2: Contributions of CotX, CotY and CotZ to spore surface morphology and hydrophobicity.
A. Analysis of coat and crust morphology by TEM in the presence of Ruthenium red. Wild type spores (PY79) have a crust (Cr, dark red arrow) as the outermost layer of the coat and an outer coat (Oc, orange arrow). ΔcotXYZ (PE620) spores are missing the crust, but the outer coat is unaffected. ΔcotX spores (PE2974) have also lost the crust, ΔcotY spores (PE2975) show gaps (black arrow) in the outer coat but a thin layer of crust material is still present (dark red arrow), ΔcotZ spores (PE2976) have no crust and additional outer coat defects (black arrow). Scale bars are 200 nm. B. Analysis of the degree of spore hydrophobicity by BATH assay: ΔcotX (PE3120), ΔcotY (PE3121), and ΔcotZ (PE3122) spores are all more hydrophobic than wild type (PY79) spores but less hydrophobic than ΔcotXYZ (PE620) spores. Experiments were performed in triplicate. C. Analysis of the PS layer by negative staining with India ink. In contrast to wild type spores (PY79), ΔcotXYZ spores (PE620), ΔcotX spores (PE3120), ΔcotY spores (PE3121) and ΔcotZ spores (PE3122) have reduced PS layers. Scale bars are 4 μm. Measurements of the halo areas are provided in Figure S4.
Figure 3: Ultrastructural analysis by TEM of crust mutant spores, including extracellular milieu.
A. Wild type spores (PY79) exhibit all layers of the coat including the crust (arrows) with little extracellular material in the field. Β. ΔcotXYZ spores (PE620) are missing the crust, some extracellular crust-like structures (arrows) are observed. C. ΔcotX spores (PE2974) have no crust but extracellular “beads on a string” crust-like structures are seen in the milieu or attached to spores (arrows, left panel). Additional examples of “beads on a string” structures (right panel). D. ΔcotZ spores (PE2976) display outer coat and crust defects with debris in the extracellular milieu (arrows). Scale bars are 1 μm.
Testing adhesive properties by BATH assays, ΔcotX spores are the most hydrophobic (30±0.5% of spores remaining in the aqueous layer; Figure 2B), followed by ΔcotZ spores (41.7±11%) and ΔcotY spores (61.2±1.7%). India Ink staining reveals that ΔcotX, ΔcotY and ΔcotZ spores all exhibit some reduction of the PS layer (Figure 2C, see Figure S4 for measurements of halo area). A greater variability is observed for ΔcotZ spores, which harbor a faint and diffuse PS layer of non-uniform thickness (Figure S4).
By TEM, ΔcotY spores sometimes exhibit gaps in the outer coat (Figure 2A, black arrow) with no apparent crust, except for a thin layer (“beads on a string” structure) attached to the rest of the coat (Figure 2A, dark red arrow). By comparison, ΔcotX spores have a normal outer coat and usually lack the crust. Some spores show “beads on a string” structures attached to the spore surface (Figure 2A, dark red arrow), but these crust-like structures are more frequently observed in the extracellular milieu surrounding the spores (Figure 3C, arrows and left panel). Because spores subjected to BATH or TEM experience harsh mechanical perturbations during sample preparation, it is possible that any loosely associated structure (crust, PS or both) disassociates from the spore. This could explain why PS remnants are still visible on India ink-stained ΔcotX spores (Figure 2C) but are likely removed during sample preparation for TEM (Figure 2A). As a control, we repeated the BATH assays with spores produced on plates (rather than in liquid medium with shaking) and obtained very similar results (Figure S5), suggesting that differences in spore production methods are unlikely to cause significant variability in crust morphology. Considering that, in TEM, wild type spores retain their crust, while ΔcotX spores do not, we propose that CotX plays a role in maintaining the tight association between the outer coat and crust.
Deletion of cotZ causes the largest disruption in spore morphology. TEM images show spores that are no longer encased by the crust and bear structural defects (gaps) in the outer coat (Figure 2A, black arrow). Putative crust and coat debris are observed in the milieu (Figure 3D). Tail-like structures attached to one spore pole are also detected. These structures could correspond to strands of crust affixed to the spores. Unexpectedly, ΔcotZ spores exhibit more pronounced coat defects than ΔcotXYZ spores, considering that the structure of the outer coat is also impaired. This suggests that completing coat and crust assembly in the absence of CotZ is more damaging than the combined absence of CotX, CotY and CotZ. We propose that the relatively hydrophilic character of the ΔcotZ spores compared to ΔcotXYZ spores in BATH assays may be due to the presence of gaps in the outer coat. Taken together (Table S3), our data confirm that each crust morphogenetic protein plays a role in crust assembly. Previous work has signaled, however, that CotX, CotY and CotZ are not the sole proteins of the crust. A comprehensive characterization of the mechanisms of crust assembly requires an understanding of the roles of the σK-controlled cgeAB and cotVW operons.
Contributions of other crust proteins to spore surface properties
Gene operon cgeAB was originally identified as a transcription unit under the dual control of σK and GerE (cge stands for controlled by GerE), two regulators of late sporulation gene expression in the mother cell (Roels & Losick, 1995). The same authors reported that cge mutant spores tended to clump, leading to the conclusion that these genes influenced spore surface properties. CgeA, was later characterized as a crust protein (Imamura et al., 2010), while the function of CgeB is not well defined, although it contains a putative glycosyltransferase domain (PFAM13524). To detect the contributions of CgeA and CgeB to crust morphology and spore adhesive properties, we used the same strategy as above. In the BATH assay, ΔcgeA and ΔcgeB spores exhibit strong relative hydrophobicity phenotypes settling in the aqueous phase at 2.8±1.2% and 6.0±1.5% (Figure 4A). India Ink staining shows that ΔcgeA and ΔcgeB spores lack the PS layer (Figure 4B). By TEM, ΔcgeB spores do not have a crust and no crust-like structures were detected in the extracellular milieu or attached to the spore coat (Figure 4C). In contrast, a crust-like structure is visible on ΔcgeA spores, where it appears to balloon away from the spore surface. Although the crust is assembled, it is loosely attached, i.e., it seems to contact the outer coat at a limited number of sites, suggesting that CgeA, like CotX, plays a role in the interaction of the crust with the rest of the coat. Taken together (Table S3), both ΔcgeA and ΔcgeB spores have crust morphology defects and altered surface properties, but the phenotypes for ΔcgeB and ΔcgeA spores are not identical.
Figure 4: Contributions of CgeA and CgeB to spore surface morphology and hydrophobicity.
A. BATH assay reveals that in comparison to wild type (PY79) spores, ΔcgeA (PE2765) and ΔcgeB (PE2916) spores are as hydrophobic as ΔcotXYZ spores (PE620). B. By India Ink staining, the PS layer is greatly reduced in ΔcgeA (PE2765) and ΔcgeB (PE2916) spores (scale bars=4 μm). C. By TEM in the presence of Ruthenium red. Scale bars are 200 nm. ΔcgeA (PE2765) spores have a ballooned crust, ΔcgeB (PE2916) spores have no crust.
CotW was among the first identified crust proteins (McKenney et al., 2010) and the σK-controlled cotVW operon is adjacent to the cotXYZ gene cluster. By BATH assay, ΔcotV, ΔcotVW, and ΔcotW spores show an intermediate phenotype (Figure 5A), settling in the aqueous phase at 58.5±12.6%, 35.4±7.1% and 16.8±2.9%, respectively. By India Ink staining, ΔcotVW, ΔcotV and ΔcotW spores have non-uniformly distributed PS layers, implying that CotV and CotW are both required for proper deposition of PS on the crust (Figure 5B and Figure S6). TEM images of ΔcotVW, ΔcotV, ΔcotW spores show a range of impaired crust phenotypes (Figure 5C). Spores of ΔcotVW exhibit the strongest defect since they have no detectable crust. The least severe phenotype is observed for ΔcotV spores that have slightly thinner crusts, fully encircling the spore (dark red arrow, Figure 5C). However, in some spores, the crust appears to have separated from the rest of the coat, i.e. there is a larger space between the outer coat and the crust (black arrow, Figure 5C). Paradoxically, by India ink staining (Figure S6), ΔcotV spores appear to have a more pronounced phenotype and it was not possible to measure the halo area because it was too diffuse. By TEM analysis, it can be observed that ΔcotW spores retain a lightly stained, disorganized crust that also separated from the rest of the coat (black arrow, Figure 5C). Furthermore, the crust often does not fully surround the spore, with patches of crust material on the surface (dark red arrow, Figure 5C). Taken together (Table S3), these data confirm that CotV and CotW are also necessary for proper crust structure. To more precisely determine the genetic hierarchy controlling crust assembly during sporulation, we analyzed the subcellular localization of the crust proteins fused to fluorescent proteins and how deletion of the other crust proteins may impact localization patterns.
Figure 5: Contributions of CotV and CotW to spore surface morphology and hydrophobicity.
A. BATH assay reveals that ΔcotW (PE2942), ΔcotV (PE3073) and ΔcotVW (PE566) spores are more hydrophobic than wild type (PY79) spores. B. India Ink staining reveals disruption of the PS layer in ΔcotVW (PE566), ΔcotV (PE3073) and ΔcotW (PE2942) spores (scale bars=4 μm). Measurements of the halo areas are provided in Figure S6. C. Ultrastructural analysis by TEM in the presence of Ruthenium red reveals that ΔcotVW (PE566) spores have no crust. ΔcotV (PE3073) spores have a lightly stained crust (dark red arrow) that, in some places (black arrows), has separated from the rest of the coat. ΔcotW (PE2942) spores have lightly stained, disorganized, crust-like filaments (dark red arrow) separated from the rest of the coat (black arrow). Scale bars are 200 nm.
Epifluorescence microscopy uncovers the crust assembly hierarchy
While the analyses described above have focused on the surface properties and morphology of mature spores, we also aimed to gather information during the crust assembly process inside the mother cell. The subcellular localization of most crust proteins was analyzed by epifluorescence microscopy at hour 7 after suspension in Sterlini-Mandelstam medium (Figure 6). As controls, we performed BATH assays on strains expressing GFP fusions and observed that their hydrophobicity profiles matched that of the wild type strain (Figure S7), suggesting that the fusions are functional. For each deletion mutant, the localization of CgeA-GFP was also analyzed in mature 2-day-old spores (Figure S8). In wild type cells, CgeA-GFP, CotW-GFP, CotX-GFP, CotY-GFP and CotZ-GFP localize to the spore surface during the late stages of sporulation. Intermediate stages of localization may include bright foci and single caps on the mother cell proximal (MCP) pole of the forespore (McKenney & Eichenberger, 2012), but after forespores transition from phase dark to phase bright, most cells exhibit a fluorescent ring fully encasing the forespore. Potential interdependencies were mapped by determining if localization patterns were impaired by deletion of specific crust genes. We first sought to determine the impact of deleting entire gene clusters, i.e. ΔcotXYZ and ΔcgeAB. The localization of all the fusions, except CotZ-GFP, was severely disrupted by deletion of cotXYZ (scoring of the cells is provided in Table S4). By contrast, deletion of cgeAB does not perturb the localization pattern of a single fusion, which is consistent with previous fluorescence microscopy experiments on mature spores by (Imamura et al., 2011). In ΔcotXYZ sporulating cells, the signal for CgeA-GFP is diffuse in the mother cell cytoplasm. As previously shown (McKenney et al., 2010), the localization of CotW-GFP is similarly disrupted with mother cells exhibiting diffuse fluorescence in their cytoplasm. Localization of CotX-GFP and CotY-GFP is also impaired in the triple mutant, with focal points seen on the surface of the spore, implying that CotX-GFP requires CotY and/or CotZ, while CotY-GFP requires CotX and/or CotZ for recruitment to the crust. Taken together (Table S6A), these data are consistent with the previous characterization of CotX, CotY and CotZ as crust morphogenetic proteins, but also suggest that CotZ is at the top of the localization hierarchy since it can localize independently of the other crust proteins. Previous work has shown that subcellular localization of CotZ-GFP is influenced by the outer coat morphogenetic protein CotE and the transcription factor GerE (McKenney et al., 2010; McKenney & Eichenberger, 2012).
Figure 6: Genetic requirements for the localization of fluorescently labeled crust proteins.
Fluorescent images were acquired at hour 7 after resuspension at 37°C in Sterlini-Mandelstam medium. The subcellular localization of CgeA-GFP (PE3260), CotW-GFP (PE599), CotX-GFP (PE3151), CotY-GFP (PE3158), and CotZ-GFP (PE3286) was determined in the wild type strain (PY79), ΔcotXYZ (PE620), ΔcgeAB (PE2425), ΔcotX (PE3120), ΔcotY (PE3121), ΔcotZ (PE3122) mutants. Proper localization of all fusions, except CotZ-GFP, is impaired in a ΔcotXYZ sporulating cell. No crust protein appears to be dependent on cgeAB and CgeA-GFP relies on CotX, CotZ, and less strictly on CotY, for proper subcellular localization. CotY-GFP is minimally dependent on CotX and CotX-GFP is minimally dependent on CotY. All fusions, except CotW-GFP, rely on cotZ for proper localization. A detailed description of all strain genotypes can be found in Table S1. Scoring of the protein localization phenotypes is provided in Table S4. A figure showing the localization of the CgeA-GFP fusion in mature (2-day old) spores is provided in the supporting information (Figure S8).
To better define which of the three crust morphogenetic proteins are principally responsible for the observed localization phenotypes, we compared the localization of each protein fusion in individual deletion strains. In the ΔcotX strain, localization of CotW-GFP and CotZ-GFP appears to be unaffected, while the patterns for CgeA-GFP and CotY-GFP are disrupted. The disruption is more severe for CgeA-GFP, where fluorescence is diffuse in the mother cell cytoplasm. CotY-GFP can still form uneven rings of fluorescence around the forespore, with brighter intensity at the poles and a significant decrease in fluorescence near the forespore short axis. This pattern of localization is consistent with that previously observed in mature spores by (Imamura et al., 2011). In total, these data suggest that CotY (partially) and CgeA (fully) are dependent on CotX for proper localization to the crust. In contrast, deletion of cotY does not seem to strongly affect localization of other crust proteins. In the ΔcotY strain, CgeA-GFP fluorescence is found mostly at the polar caps and is not fully encasing the mature spores (Figure S8). Similarly, CotX-GFP fluorescence is brighter at the poles, whereas CotW-GFP and CotZ-GFP localization is unchanged. Proper localization of CgeA-GFP, CotX-GFP, and CotY-GFP was disrupted in the ΔcotZ background. Because CgeA-GFP localization is affected by all three of the individual morphogenetic proteins, we place it at the bottom of the crust assembly hierarchy.
CotW-GFP remained able to form rings of fluorescence fully encasing forespores in the ΔcotZ mutant. This was unexpected, considering that CotW-GFP localization was disrupted in the cotXYZ triple mutant and unaffected in the ΔcotX and ΔcotY single mutants. It is therefore likely that the three crust morphogenetic proteins have redundant effects in guiding CotW-GFP localization. It should also be noted that CotW-GFP localization is perturbed in a ΔcotE mutant (McKenney et al., 2010).
We have demonstrated above that the cotVW operon, which is adjacent to the cotXYZ cluster, is necessary for proper crust morphology and surface properties in mature spores. We thus sought to determine if the localization patterns of crust proteins were affected by the absence of cotV, cotW or both. At hour 8 of sporulation, CgeA-GFP formed rings of fluorescence around maturing forespores in both wild type and mutant sporulating cells (Figure 7 and scoring in Table S5). However, 2-day-old ΔcotW and ΔcotVW spores were no longer surrounded by a ring of fluorescence, although fluorescent signals were detected in the nearby debris (Figure 7, arrows). By contrast, the signal persisted in ΔcotV and wild type spores. Thus, cotW appears to play a role in maintaining the structural integrity of the crust by ensuring that CgeA remains associated with the spore surface. Similar experiments were conducted with CotX-GFP. In that case, cotVW was necessary for CotX-GFP stabilization, as the protein appears degraded (loss of fluorescence) in ΔcotW sporulating cells and mature spores. The destabilization of CotX-GFP is also observed in ΔcotV sporulating cells, but to a lesser extent, since some CotX-GFP fluorescence is detected surrounding mature ΔcotV spores. CotY-GFP localization at hour 8 and in mature spores was also perturbed in the cotVW mutant strain. However, a fluorescent signal was detected around mature spores in both the cotV and cotW single mutants, suggesting that the two genes are redundant for CotY-GFP localization. At hour 8 in ΔcotV sporulating cells, localization of CotY-GFP is like wild type but appears to be delayed in ΔcotW sporulating cells. Lastly, CotZ-GFP localization is independent of CotW or CotV.
Figure 7: Localization of several fluorescently labeled crust proteins is dependent on cotW.
The subcellular localization of CgeA-GFP (PE3260), CotX-GFP (PE3151), CotY-GFP (PE3158) and CotZ-GFP (HS176) was determined in the wild type (PY79) strain, ΔcotVW (PE566), ΔcotV (PE3073), and ΔcotW (PE2942) mutants. Fluorescent images were acquired at hour 8 and two days after resuspension and incubation at 37°C in Sterlini-Mandelstam medium. The localization of CgeA-GFP is mostly unaffected in the mutants during sporulation; however, the ring of fluorescence is lost in ΔcotW and ΔcotVW 2-day-old spores, indicating that CotW, but not CotV, is necessary for maintaining proper subcellular localization of CgeA-GFP in mature spores (fluorescent debris are indicated with arrows). CotW and, to a lesser extent, CotV are required for CotX-GFP stability, as fluorescence is significantly reduced in their absence. The localization of CotY-GFP is affected by the combined deletion of cotW and cotV. CotZ-GFP is independent of CotV and CotW for localization. A detailed description of all strain genotypes can be found in Table S1. Scoring of the protein localization phenotypes is provided in Table S5.
Although a hierarchical pattern emerges from the fluorescence microscopy dataset (Table S7A), with CotZ at the top and CgeA at the bottom, there are many unanticipated interactions indicating that this is not a strict linear assembly pathway and that each crust protein may interact with several other crust proteins. This observation is echoed by recent studies from the Barak lab using two-hybrid assays (Table S7B) that revealed a complex network of interactions between crust and coat proteins (Krajcikova et al., 2017).
CotO promotes encasement of the spore by the crust
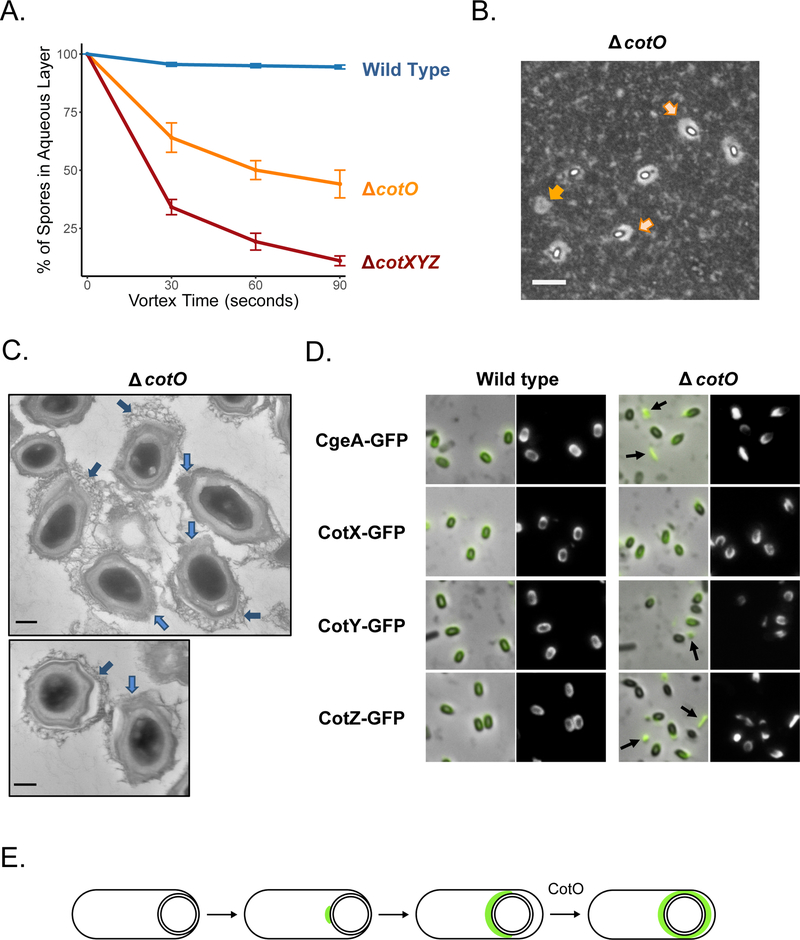
The cotXYZ gene cluster is flanked by the cotVW operon on one side and cotO on the other. CotO, which is widely conserved among the Bacillales, including the B. cereus group, has been characterized as an outer coat protein in B. subtilis, where it is involved in the late stages of outer coat assembly and in spore surface topography (McPherson et al., 2005). By BATH assay, ΔcotO spores display a relatively intermediate hydrophobic character wherein 44.1±5.9% of spores remain in the aqueous layer (Figure 8A). By India Ink staining, most spores appear to have PS, but the layer is often seen to be non-uniform with significantly more accumulation of PS material at one pole of the spore (Figure 8B; light orange arrows). There also appears to be some detached PS material in the milieu (Figure 8B; dark orange arrow). Measurements of halo areas are provided in Figure S9. The TEM images are consistent with the other assays. As previously reported (McPherson et al., 2005), all ΔcotO spores have a disorganized outer coat (Figure 8C; light blue arrows); however, some spores retain a crust, while others do not. When a crust is present, it does not fully encase the spore but accumulates web-like material on one pole (Figure 8C; dark blue arrows). These observations suggest that CotO is required to prepare the outer coat surface for crust deposition. Furthermore, considering the asymmetric deposition of the crust observed by TEM and PS layer by Indian ink staining, we propose that CotO is necessary to promote spore encasement by the crust.
Figure 8: CotO influences crust assembly and spore surface properties.
A. BATH assays reveal an intermediate hydrophobicity profile for ΔcotO (PE250) spores, i.e. less hydrophilic than wild type (PY79) and less hydrophobic than ΔcotXYZ (PE620) spores. B. India Ink staining of ΔcotO (PE250) spores displays an asymmetric distribution of the PS layer favoring one pole (light orange arrows) and apparent PS debris in the milieu (dark orange arrow); scale bar=4 μm. Measurements of the halo areas can be found in Figure S9. C. TEM in the presence of ruthenium red reveals that some ΔcotO (PE250) spores display a disorganized and web-like crust that is primarily attached to one pole (dark blue arrows) and there are gaps in the outer coat observable at the opposite pole or in spores that have lost their crust (light blue arrows). Scale bars are 200 nm. D. Localization of CgeA-GFP (PE3260), CotX-GFP (PE3151), CotY-GFP (PE3158), and CotZ-GFP (PE3301) in wild type and ΔcotO (PE3259, PE3150, PE3156 and PE3302, respectively) 2-day-old spores. Fusion proteins are unable to complete spore encasement in the absence of CotO. Arrows indicate fluorescent debris. Scoring of the protein localization phenotypes is provided in Table S6. E. Model of CotO contribution to crust assembly by promoting spore encasement in the late stages of sporulation. A figure showing the localization of the same fusions in wild type and cotO mutant spores at successive time points after resuspension in sporulation medium can be found in the supporting information (Figure S10).
We conducted epifluorescence microscopy experiments to determine whether the localization of the GFP fusions to the crust proteins was affected in ΔcotO sporulating cells. CgeA-GFP, CotX-GFP, CotY-GFP, and CotZ-GFP were unable to complete spore encasement (Figure 8D for 2-day-old spores after induction of sporulation by exhaustion and Table S6 for scoring; Figure S10 for sporulating cells at successive time points after induction of sporulation by resuspension in Sterlini Mandelstam medium). Like for the India ink assays, fluorescent crust debris (arrows, Figure 8D) were sometimes observed in the milieu near ΔcotO spores. Taken together, these experiments suggest that CotO assists in the interactions of the crust with the outer coat and is necessary for the later stages of crust assembly, promoting full encasement of the spore by the crust. Because encasement is incomplete, the crust is likely to be loosely attached to the rest of the coat. A significant fraction of mature spores appears crust-less, most likely because the crust has been shed. Fluorescence microscopy data during spore formation (Figure S10) suggest that most sporulating cells progress to the single cap stage but are unable to form the second cap (Figure 8E), a characteristic encasement block, similar to that observed for other coat protein-GFP fusions in a spoVID mutant (Wang et al., 2009). We propose that the heterogeneity observed in the mature spore population (Figure 8D, Table S6) is caused by the fact that several spores kept their cap of crust attached to the spore pole, while the rest of the spores lost it because it was loosely attached. Furthermore, we checked if other coat proteins known to be required for outer coat assembly (CotG, CotH, and CotM) (Henriques, Beall, & Moran, 1997; Zilhao et al., 1999; Zilhao et al., 2004) also played a role in the localization of crust proteins (Figure S11). Absence of these other outer coat proteins did not influence localization of CotX-GFP and CotZ-GFP, implying that outer coat assembly perturbations do not systematically result in crust localization defects and that the role of CotO in crust assembly is specific.
DISCUSSION
In this study, we characterize the relative contributions of previously identified crust proteins (CgeA, CotV, CotW, CotX, CotY and CotZ) toward assembly of the spore outermost layer and how they influence the surface properties of mature spores. In Figure 9, we propose a model, derived from epifluorescence microscopy experiments, that compiles genetic interactions among crust proteins, as well as the contributions of two morphogenetic outer coat proteins, CotE and CotO (Table S7A). CotZ, which requires CotE and CotO but none of the crust proteins for localization, is at the top of the crust assembly hierarchy and is integral to the localization of all other crust proteins, except perhaps CotW (considering that localization of CotW-GFP is impaired in a ΔcotXYZ mutant, but not in a ΔcotZ mutant). Previous work suggested that CotY was also necessary for CotZ localization (Imamura et al., 2011); however, we were unable to confirm this result with our deletion mutants and CotZ-GFP expressing strains. By contrast, CgeA directly or indirectly relies on all other crust proteins for proper localization and does not affect the ability of the spore to assemble the major crust components. Therefore, CgeA is at the bottom of the hierarchy. In general, the relationships between crust proteins appear intricate, with each protein interacting with more than one protein. Our genetic interaction dataset is consistent with the protein interaction data obtained by two-hybrid assays (Krajcikova et al., 2017) (Table S7B). We have also found that CotO promotes encasement of the spore by CgeA, CotX, CotY and CotZ. Thus, crust assembly seems to follow the logic previously uncovered for the rest of the coat, with a first morphogenetic protein dedicated to the recruitment of all other coat proteins in a specific layer (SpoIVA for the basement layer, SafA for the inner layer, CotE for the outer layer and CotZ for the crust) and a second morphogenetic protein (SpoVID for most layers and CotO specifically for the crust) required at a later stage for spore encasement (McKenney et al., 2013; McKenney & Eichenberger, 2012; Nunes et al., 2018; Wang et al., 2009). CotX, CotY and CotZ had been previously defined as crust morphogenetic proteins, and the data presented here confirm that all these proteins play a major role in crust assembly.
Figure 9: Hierarchical network of genetic dependencies in crust assembly.
Weight of lines indicated strength of interaction. Red: Crust proteins. Orange: outer coat protein required for encasement by the crust. Purple: outer coat protein required for recruitment of CotZ and CotW. Red arrow: Required for proper localization. Dash olive arrow: required for protein stabilization. Olive arrow: required to maintain the structure. Orange arrow: required for spore encasement.
It has been previously shown that the spore surface is composed of both proteins and polysaccharides (Waller et al., 2004). Thus, in addition to an updated genetic hierarchy for coat and crust assembly, we report how individual deletions of crust genes affect spore surface phenotypes, including presence or absence of a PS layer, crust morphology, and degree of hydrophobicity. From TEM in the presence of Ruthenium red, we found that when crust morphology was severely disrupted (e.g. leaving debris as for ΔcotZ spores), the polysaccharide layer was also impaired, as determined by negative staining with India ink. Yet, in mutants like ΔcotXYZ, spores still exhibited a PS layer even in the absence of a detectable crust. A possible interpretation of this result is that some PS could attach to the outer coat, either naturally or to compensate for a missing crust. An alternative explanation would be that the Ruthenium red and India ink staining procedures are sensitive to different types of PS. Nevertheless, and keeping in mind that CotV, CotW and CotY are capable of self-assembly (Jiang et al., 2015), attempted crust assembly with an incomplete set of crust proteins appears to be more disruptive to overall coat morphology than if the crust is removed completely. For example, in ΔcotZ spores, and in contrast to ΔcotXYZ spores, the presence of CotX and CotY may be enough to initiate crust assembly and this aborted attempt ends up disrupting both the outer coat and the crust. Another important result from the TEM analysis was the detection of crust like-material in the form of filaments or “beads-on-a-string” structures. This phenotype was observable in the extracellular milieu (e.g. ΔcotX spores) or still attached to the spore (e.g. ballooned crust morphology of ΔcgeA spores), pointing to a possible role for CotX and CgeA in attaching the crust to the rest of the coat. It should be noted that these proteins are absent or have diverged greatly in strains of the B. cereus group that have exosporia instead of crusts. On the other hand, CotX is required, along with CotW, for exosporium formation in B. megaterium QMB 1551, which does not have orthologs of CotY and CotZ (Manetsberger, Ghosh, Hall, & Christie, 2018). CotO is conserved in Bacillus species that also have orthologs of CotY and CotZ. It was recently shown that cotO plays a related role in B. anthracis, where it is involved in the coordination of coat and exosporium assembly (Boone et al., 2018).
For PS to be added to the spore surface, they must be produced during sporulation and covalently linked to one or several crust proteins. A candidate for this role is the putative glycosyltransferase, CgeB, considering that by TEM no crust is visible for ΔcgeB spores stained with Ruthenium red. Because CgeA is at the bottom of the assembly hierarchy and is produced from the same operon as CgeB, it is a candidate for glycosylation. In any case, several putative glycosyltransferases are expressed in the mother cell during sporulation (Eichenberger et al., 2004) and much work remains to be done to determine which type of PS, and at what proportion, are displayed on the spore crust and how exactly they anchor there. In B. anthracis and B. cereus, the glycoprotein BclA has been identified as an anchoring point for PS to the spore surface and its disruption results in modifications of the spore surface properties (Daubenspeck et al., 2004; Lequette et al., 2011; Sylvestre, Couture-Tosi, & Mock, 2002). BclA is, however, not conserved in B. subtilis strain 168, and no BclA-like protein has yet been discovered. Understanding how PS anchor to the spore surface in B. subtilis is a concrete goal for future research.
Our data also indicate that even though B. subtilis 168 and natto have high sequence similarity, there is variability in their spore PS layers and the chemical properties of their surfaces. More generally, PS composition and its influence on relative hydrophobicity likely impacts dispersal in the environment and determines whether spores will stick to one another (leading to clumping). The chemical properties of the spore surface remain incompletely documented. Many studies report that adhesion properties vary greatly among spore-forming species, suggesting, specifically, that B. anthracis and B. cereus spores are more hydrophobic than B. subtilis (Ankolekar & Labbe, 2010; Koshikawa et al., 1989; Ronner et al., 1990; Wiencek et al., 1990). However, another study argues that B. subtilis and B. anthracis spores are largely hydrophilic (Chen et al., 2010). These differences in interpretation may reflect the methods used. We note that here, we are relying on the BATH assay only to identify differences in relative hydrophobicity between strains, not to make a definitive claim about the absolute surface characteristics of a given mutant.
The discoveries of the sugars anthrose and cereose on the surface of B. anthracis and B. cereus spores, respectively, implies that the carbohydrate profiles of spores can be highly species-specific (Daubenspeck et al., 2004; Li et al., 2017; Maes et al., 2016). These properties are especially important for understanding pathogenic spore forming bacteria and could significantly influence strategies deployed for their elimination. Expanding our work to other spore-forming species, either closely or more distantly related, will provide clues about how bacteria adapt their spore surfaces to specific environments. Importantly, our results suggest that minor genetic differences can lead to major changes in surface properties, implying that small evolutionary changes could turn a hydrophilic spore into a hydrophobic spore and vice versa. Knowing how natural isolates vary in adhesion can help us to better understand how this property drives ecologically critical characteristics: the survivability, pathogenicity, and dispersal of the spore in an environment.
EXPERIMENTAL PROCEDURES
Bacterial Strains
Strains used in this study are listed in Table S1. Gene deletion strains were either previously constructed or obtained from the whole genome deletion library (BKE strains) available at the Bacillus Genetic Stock Center (Koo et al., 2017). For gene deletions within operons in BKE strains (e.g. cotV in the cotVW operon), the antibiotic resistance cassette was removed using the pDR244a plasmid, thus generating a deletion with a scar.
Fluorescent Protein Fusions
CgeA-GFP (NY17), CotX-GFP (NY6), CotY-GFP (NY10) and CotZ-GFP (NY15) expressing strains were constructed as described in the supporting information (Figures S12-S14; with corresponding primers in Table S8). These gfp fusions (to the 3’end of the respective crust genes) are expressed from the native promoter of the crust gene and integrated at the non-essential amyE locus. Stratins expressing CotW-GFP and CotZ-GFP from their native locus have been described previously (Kim et al., 2006; McPherson et al., 2005). Functionality of all fusions was tested in BATH assays (Figure S7), all fusions expressing strains produce spores that are as hydrophilic as wild type spores (as merodiploids for strains expressing fusions from amyE, i.e. a wild type copy of the gene remains present at the native locus).
Sporulation conditions
For sporulation by exhaustion, 5 ml of Difco Sporulation Medium (DSM) are inoculated from a single colony and grown 18 hours at 37° C. For sporulation by resuspension, 5ml of Growth Medium (25% LB with trace elements) inoculated from a single colony undergo serial dilutions and each preculture is incubated overnight at 30° C. Next day, dilution with OD600 closest to 0.6 is selected to inoculate 20ml of Growth Medium. The culture is incubated in shaking water bath at 37° C and its OD600 measured hourly until samples reach 0.6–0.9. Cells are pelleted and resuspended into 20ml of Sterlini-Mandelstam (SM) medium (defined as hour 0 of sporulation). The cultures are incubated in shaking water bath at 37° C and samples collected at the desired time into sporulation.
Bacterial Adhesion to Hydrocarbons (BATH) assay
The protocol for BATH assays was adapted from a study by (Faille et al., 2010). Bacterial strains are grown overnight on LB agar plates at 37° C. Single colonies are inoculated into 20ml of Difco Sporulation Medium (DSM) in 250ml aerated Erlenmeyer flasks. Samples are then incubated for 3 days in a water bath at 37° C. After incubation, cells are pelleted by centrifugation at 5000 rpm and washed 3 times in cold dH2O. Supernatant is then vacuumed out and pellet is resuspended in 400μl of 20% Histodenz and layered over 200μl of 50% Histodenz in microcentrifuge tubes. Samples are centrifuged, and top layers are removed while bottom layers are washed 3 times and resuspended into 1ml of cold dH2O. 100μl are used to measure the OD600 and based on reading, to calculate amount of 1X PBS required to dilute sample to OD600 of 0.5 (using the calculation: ). Diluted samples are measured to collect initial OD600 for samples prior to hexadecane treatment (A0). 3ml of sample are aliquoted into glass test tubes for the collection of three timepoints after exposure to hexadecane (30, 60, and 90 seconds). 500μl of Hexadecane are layered onto each sample and then each tube is vortexed at an intensity of 8 (Vortex Genie 2) for the respective time points. Samples then settle for 30 minutes and 1ml of the aqueous layer is used to measure the OD600 of each timepoint for each sample. Using absorbance measurements, we then calculate the percentage of spores remaining in the aqueous layer using the calculation: .
India ink staining
Spores were resuspended in dH2O. Two μl were mixed with an equal amount of India ink (Daiso Soygo, Japan) and observed by phase-contrast microscopy as described previously (Abe et al., 2014). The area of halo was measured using ImageJ (the bright spore area was subtracted) and plotted in a box-and-whisker plot (Figures S2, S4, S6 and S9). The box is delimited by the 1st and 3rd quartiles, the line crossing the box is the median and x is the average, the whiskers correspond to the minimum and maximum areas.
Transmission electron microscopy
Spores were fixed, stained with Ruthenium red, and analyzed by thin section TEM as described in (McKenney et al., 2010).
Fluorescence microscopy
500μl of sample from the sporulating culture are spun down and resuspended in 50μl of 1X PBS and membranes are stained with 1μl of 1μg/μl FM4–64. Images are collected as described before (McKenney & Eichenberger, 2012).
Supplementary Material
ACKNOWLEDGMENTS
We thank Julia Bartels and Thorsten Mascher for sharing results prior to publication. We are grateful to Carol Gross, Byoung-Mo Koo, Rich Losick and Dan Zeigler for strains. We thank Brian Liu for building the CgeA-YFP fusion used in some of the pilot experiments. This work was supported by funds from the Zegar Family Foundation and originally from NIH GM081571 to PE. Additional support was provided by Grant-in-Aid for Scientific research from the Japan Society for the Promotion of Science (KAKENHI) [15K18675 to K.A. and 15K07371 to T.S.]. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- Abe K, Kawano Y, Iwamoto K, Arai K, Maruyama Y, Eichenberger P, & Sato T (2014). Developmentally-regulated excision of the SPbeta prophage reconstitutes a gene required for spore envelope maturation in Bacillus subtilis. PLoS Genet, 10(10), e1004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Takamatsu T, & Sato T (2017). Mechanism of bacterial gene rearrangement: SprA-catalyzed precise DNA recombination and its directionality control by SprB ensure the gene rearrangement and stable expression of spsM during sporulation in Bacillus subtilis. Nucleic Acids Res, 45(11), 6669–6683. doi: 10.1093/nar/gkx466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankolekar C, & Labbe RG (2010). Physical characteristics of spores of food-associated isolates of the Bacillus cereus group. Appl Environ Microbiol, 76(3), 982–984. doi: 10.1128/AEM.02116-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta-Ortiz ML, Hafemeister C, Bate AR, Chu T, Greenfield A, Shuster B, … Eichenberger P (2015). An experimentally supported model of the Bacillus subtilis global transcriptional regulatory network. Mol Syst Biol, 11(11), 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone TJ, Mallozzi M, Nelson A, Thompson B, Khemmani M, Lehmann D, … Driks A (2018). Coordinated Assembly of the Bacillus anthracis Coat and Exosporium during Bacterial Spore Outer Layer Formation. MBio, 9(6). doi: 10.1128/mBio.01166-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, … Lawley TD (2016). Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature, 533(7604), 543–546. doi: 10.1038/nature17645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HP, Neville BA, Forster SC, & Lawley TD (2017). Transmission of the gut microbiota: spreading of health. Nat Rev Microbiol, 15(9), 531–543. doi: 10.1038/nrmicro.2017.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano G, Sirec T, Panarella C, Isticato R, Baccigalupi L, De Felice M, & Ricca E (2014). The sps gene products affect the germination, hydrophobicity, and protein adsorption of Bacillus subtilis spores. Appl Environ Microbiol, 80(23), 7293–7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Driks A, Tawfiq K, Mallozzi M, & Patil S (2010). Bacillus anthracis and Bacillus subtilis spore surface properties and transport. Colloids Surf B Biointerfaces, 76(2), 512–518. [DOI] [PubMed] [Google Scholar]
- Daubenspeck JM, Zeng H, Chen P, Dong S, Steichen CT, Krishna NR, … Turnbough CL Jr. (2004). Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J Biol Chem, 279(30), 30945–30953. doi: 10.1074/jbc.M401613200 [DOI] [PubMed] [Google Scholar]
- de Hoon MJ, Eichenberger P, & Vitkup D (2010). Hierarchical evolution of the bacterial sporulation network. Curr Biol, 20(17), R735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driks A (2002). Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol, 10(6), 251–254. [DOI] [PubMed] [Google Scholar]
- Driks A, & Eichenberger P (2016). The Spore Coat. Microbiol Spectr, 4(2). doi: 10.1128/microbiolspec.TBS-0023-2016 [DOI] [PubMed] [Google Scholar]
- Driks A, Roels S, Beall B, Moran CP Jr., & Losick R (1994). Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev, 8(2), 234–244. [DOI] [PubMed] [Google Scholar]
- Eichenberger P, Fujita M, Jensen ST, Conlon EM, Rudner DZ, Wang ST, … Losick R (2004). The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol, 2(10), e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J (2003). Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol, 1(2), 117–126. doi: 10.1038/nrmicro750 [DOI] [PubMed] [Google Scholar]
- Faille C, Lequette Y, Ronse A, Slomianny C, Garenaux E, & Guerardel Y (2010). Morphology and physico-chemical properties of Bacillus spores surrounded or not with an exosporium: consequences on their ability to adhere to stainless steel. Int J Food Microbiol, 143(3), 125–135. [DOI] [PubMed] [Google Scholar]
- Faille C, Ronse A, Dewailly E, Slomianny C, Maes E, Krzewinski F, & Guerardel Y (2014). Presence and function of a thick mucous layer rich in polysaccharides around Bacillus subtilis spores. Biofouling, 30(7), 845–858. [DOI] [PubMed] [Google Scholar]
- Helgason E, Okstad OA, Caugant DA, Johansen HA, Fouet A, Mock M, … Kolsto. (2000). Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis--one species on the basis of genetic evidence. Appl Environ Microbiol, 66(6), 2627–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques AO, Beall BW, & Moran CP Jr. (1997). CotM of Bacillus subtilis, a member of the alpha-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol, 179(6), 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques AO, & Moran CP Jr. (2007). Structure, assembly, and function of the spore surface layers. Ann Rev Microbiol, 61, 555–588. [DOI] [PubMed] [Google Scholar]
- Higgins D, & Dworkin J (2012). Recent progress in Bacillus subtilis sporulation. FEMS Microbiol Rev, 36(1), 131–148. doi: 10.1111/j.1574-6976.2011.00310.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura D, Kuwana R, Takamatsu H, & Watabe K (2010). Localization of proteins to different layers and regions of Bacillus subtilis spore coats. J Bacteriol, 192(2), 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura D, Kuwana R, Takamatsu H, & Watabe K (2011). Proteins involved in formation of the outermost layer of Bacillus subtilis spores. J Bacteriol, 193(16), 4075–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Wan Q, Krajcikova D, Tang J, Tzokov SB, Barak I, & Bullough PA (2015). Diverse supramolecular structures formed by self-assembling proteins of the Bacillus subtilis spore coat. Mol Microbiol, 97(2), 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Hahn M, Grabowski P, McPherson DC, Otte MM, Wang R, … Driks A (2006). The Bacillus subtilis spore coat protein interaction network. Mol Microbiol, 59(2), 487–502. [DOI] [PubMed] [Google Scholar]
- Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, … Gross CA (2017). Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst, 4(3), 291–305 10.1016/j.cels.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa T, Yamazaki M, Yoshimi M, Ogawa S, Yamada A, Watabe K, & Torii M (1989). Surface hydrophobicity of spores of Bacillus spp. J Gen Microbiol, 135(10), 2717–2722. doi: 10.1099/00221287-135-10-2717 [DOI] [PubMed] [Google Scholar]
- Krajcikova D, Forgac V, Szabo A, & Barak I (2017). Exploring the interaction network of the Bacillus subtilis outer coat and crust proteins. Microbiol Res, 204, 72–80. doi: 10.1016/j.micres.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Lequette Y, Garenaux E, Tauveron G, Dumez S, Perchat S, Slomianny C, … Faille C (2011). Role played by exosporium glycoproteins in the surface properties of Bacillus cereus spores and in their adhesion to stainless steel. Appl Environ Microbiol, 77(14), 4905–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mukherjee T, Bowler K, Namdari S, Snow Z, Prestridge S, … Bar-Peled M (2017). A four-gene operon in Bacillus cereus produces two rare spore-decorating sugars. J Biol Chem, 292(18), 7636–7650. doi: 10.1074/jbc.M117.777417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes E, Krzewinski F, Garenaux E, Lequette Y, Coddeville B, Trivelli X, … Guerardel Y (2016). Glycosylation of BclA Glycoprotein from Bacillus cereus and Bacillus anthracis Exosporium Is Domain-specific. J Biol Chem, 291(18), 9666–9677. doi: 10.1074/jbc.M116.718171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetsberger J, Ghosh A, Hall EAH, & Christie G (2018). Orthologues of Bacillus subtilis spore crust proteins have a structural role in the Bacillus megaterium QM B1551 spore exosporium. Appl Environ Microbiol doi: 10.1128/AEM.01734-18 [DOI] [PMC free article] [PubMed]
- McKenney PT, Driks A, & Eichenberger P (2013). The Bacillus subtilis endospore: assembly and functions of the multilayered coat. Nat Rev Microbiol, 11(1), 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney PT, Driks A, Eskandarian HA, Grabowski P, Guberman J, Wang KH, … Eichenberger P (2010). A distance-weighted interaction map reveals a previously uncharacterized layer of the Bacillus subtilis spore coat. Curr Biol, 20(10), 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney PT, & Eichenberger P (2012). Dynamics of spore coat morphogenesis in Bacillus subtilis. Mol Microbiol, 83(2), 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DC, Kim H, Hahn M, Wang R, Grabowski P, Eichenberger P, & Driks A (2005). Characterization of the Bacillus subtilis spore morphogenetic coat protein CotO. J Bacteriol, 187(24), 8278–8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes F, Fernandes C, Freitas C, Marini E, Serrano M, Moran CP Jr., … Henriques AO (2018). SpoVID functions as a non-competitive hub that connects the modules for assembly of the inner and outer spore coat layers in Bacillus subtilis. Mol Microbiol doi: 10.1111/mmi.14116 [DOI] [PMC free article] [PubMed]
- Ozin AJ, Henriques AO, Yi H, & Moran CP Jr. (2000). Morphogenetic proteins SpoVID and SafA form a complex during assembly of the Bacillus subtilis spore coat. J Bacteriol, 182(7), 1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata G, Fuhrer T, Hsiao TL, Sauer U, & Vitkup D (2012). Global probabilistic annotation of metabolic networks enables enzyme discovery. Nat Chem Biol, 8(10), 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels S, Driks A, & Losick R (1992). Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol, 174(2), 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels S, & Losick R (1995). Adjacent and divergently oriented operons under the control of the sporulation regulatory protein GerE in Bacillus subtilis. J Bacteriol, 177(21), 6263–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronner U, Husmark U, & Henriksson A (1990). Adhesion of Bacillus spores in relation to hydrophobicity. J Appl Bacteriol, 69(4), 550–556. [DOI] [PubMed] [Google Scholar]
- Setlow P (2014). Spore resistance properties. Microbiol Spectr, 2(5). [DOI] [PubMed] [Google Scholar]
- Stewart GC (2015). The Exosporium Layer of Bacterial Spores: a Connection to the Environment and the Infected Host. Microbiol Mol Biol Rev, 79(4), 437–457. doi: 10.1128/MMBR.00050-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P, & Losick R (1996). Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet, 30, 297–241. [DOI] [PubMed] [Google Scholar]
- Sylvestre P, Couture-Tosi E, & Mock M (2002). A collagen-like surface glycoprotein is a structural component of the Bacillus anthracis exosporium. Mol Microbiol, 45(1), 169–178. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Kodama T, Nakayama T, & Watabe K (1999). Characterization of the yrbA gene of Bacillus subtilis, involved in resistance and germination of spores. J Bacteriol, 181(16), 4986–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IS, & Ramamurthi KS (2014). Spore formation in Bacillus subtilis. Environ Microbiol Rep, 6(3), 212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LN, Fox N, Fox KF, Fox A, & Price RL (2004). Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore of Bacillus anthracis and Bacillus subtilis. J Microbiol Methods, 58(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Wang KH, Isidro AL, Domingues L, Eskandarian HA, McKenney PT, Drew K, … Eichenberger P (2009). The coat morphogenetic protein SpoVID is necessary for spore encasement in Bacillus subtilis. Mol Microbiol, 74(3), 634–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiencek KM, Klapes NA, & Foegeding PM (1990). Hydrophobicity of Bacillus and Clostridium spores. Appl Environ Microbiol, 56(9), 2600–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunschel D, Fox KF, Black GE, & Fox A (1994). Discrimination among the B. cereus group, in comparison to B. subtilis, by structural carbohydrate profiles and ribosomal RNA spacer region PCR. System. Appl. Microbiol, 17, 625–635. [Google Scholar]
- Zhang J, Fitz-James PC, & Aronson AI (1993). Cloning and characterization of a cluster of genes encoding polypeptides present in the insoluble fraction of the spore coat of Bacillus subtilis. J Bacteriol, 175(12), 3757–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LB, Donovan WP, Fitz-James PC, & Losick R (1988). Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev, 2(8), 1047–1054. [DOI] [PubMed] [Google Scholar]
- Zilhao R, Naclerio G, Henriques AO, Baccigalupi L, Moran CP Jr., & Ricca E (1999). Assembly requirements and role of CotH during spore coat formation in Bacillus subtilis. J Bacteriol, 181(8), 2631–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhao R, Serrano M, Isticato R, Ricca E, Moran CP Jr., & Henriques AO (2004). Interactions among CotB, CotG, and CotH during assembly of the Bacillus subtilis spore coat. J Bacteriol, 186(4), 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.