Abstract
Background:
Frontoparietal functional connectivity decreases with multiple anesthetics using electrophysiology and functional imaging. This decrease has been proposed as a final common functional pathway to produce anesthesia. Two alternative measures of long-range cortical interaction are coherence and phase-amplitude coupling. While phase-amplitude coupling within frontal cortex changes with propofol administration, effects of propofol on phase-amplitude coupling between different cortical areas have not previously been reported. Based on phase-amplitude coupling observed within frontal lobe during the anesthetized period, we hypothesize that between-lead phase-amplitude coupling analysis should decrease between frontal and parietal leads during propofol anesthesia.
Methods:
We use a published monkey electrocorticography dataset (N=2 animals) to test for interactions in the cortical oculomotor circuit, which is robustly interconnected in primates, and in the visual system during propofol anesthesia using coherence and inter-area phase-amplitude coupling.
Results:
Propofol induces coherent slow oscillations in visual and oculomotor networks made up of cortical areas with strong anatomic projections. Frontal eye field within-area phase-amplitude coupling increases with a time course consistent with a bolus response to intravenous propofol (Modulation Index increase of 12.6 fold). Contrary to our hypothesis, inter-areal phase-amplitude coupling also increases with propofol, with the largest increase in phase-amplitude coupling in frontal eye field low frequency phase modulating lateral intraparietal area beta power (27 fold increase) and Visual Area V2 (V2) low frequency phase altering Visual Area V1 (V1) beta power (19 fold increase).
Conclusions:
Propofol anesthesia induces coherent oscillations and increases certain frontoparietal interactions in oculomotor cortices. Frontal eye field and lateral intraparietal area show increased coherence and phase-amplitude coupling. V2 and V1, which have similar anatomic projection patterns, show similar increases in phase-amplitude coupling, suggesting higher order feedback increases in influence during propofol anesthesia relative to wakefulness. This suggests that functional connectivity between frontal and parietal areas is not uniformly decreased by anesthetics.
Introduction
Multiple studies with multiple anesthetics report decreased corticocortical functional connectivity during general anesthesia, using both electrophysiology and fMRI1–5. A particularly attractive integration of these data is an hypothesis that anesthesia results from disruption of frontoparietal functional connectivity, which occurs even with agents that act on distinct receptor systems, and hence might represent a final common functional pathway to produce an anesthetic state.6,7
Functional connectivity quantifies a probabilistic relationship between two signals: two signals are “functionally connected” if a signal at sensor A can be used to predict the signal from sensor B. This statistical relationship can result from a number of anatomic relationships. Cross-frequency coupling, including phase-amplitude coupling -- or the gating of a high frequency oscillation’s amplitude by the phase of a low frequency oscillation -- has been proposed as a mechanism to modulate long range neuronal communication and hence functional connectivity.8,9 Phase-amplitude coupling can be generically produced by interconnected populations of excitatory and inhibitory neurons,10 and can be observed between different frequency bands recorded in a single area or between two different areas. Within a single cortical area, propofol alters slow oscillation-alpha band phase-amplitude coupling11 and propofol, sevoflurane, and ketamine have all been shown to increase delta-gamma and theta-gamma phase-amplitude coupling.12 Inter-area phase-amplitude coupling has not been widely reported in anesthesia studies to date, though it has been used in other contexts.13
Many studies of anesthetic effects on cortical functional connectivity have utilized electroencephalography (EEG) data for practical reasons: EEG is easy to record, noninvasive, and shows characteristic shifts with anesthetic depth.14 Yet as a volume averaged, low pass filtered readout of the cortical areas underlying relatively large leads, EEG has specific disadvantages for relating shifts in functional connectivity to anatomic connectivity.15 More finely resolved electrocorticography data provided by smaller leads placed directly on the surface of cortex may offer a better scale for connectivity analyses. While one can still critique electrocorticography as a macroscopic measure of neuronal activity, the main limitation of electrocorticography is its inherent invasiveness, which prevents the practical acquisition of such data from normal human brains.
Here we analyze data from a publically available repository of macaque monkey electrocorticography to ask whether propofol, which decreases frontoparietal functional connectivity, also dissociates activity in two cortical areas involved in oculomotor behavior that have particularly potent reciprocal anatomic projections: frontal eye field and lateral intraparietal area. This circuit seems appropriate to query with phase-amplitude coupling as stimulation of frontal eye field evokes gamma band oscillations within lateral intraparietal area.16 To compare with other corticocortical interactions, we compare the relationships between frontal eye field and lateral intraparietal area to those with two occipital visual cortical areas, V1 and V2, which also project to frontal eye field and lateral intraparietal area. Counter the hypothesis of loss of frontoparietal connectivity, here we show that phase-amplitude coupling between frontal eye field and lateral intraparietal area increases during anesthesia produced by bolus intravenous administration of propofol, and a similar pattern occurs between V2 and V1.
Materials and Methods
Subjects and data recordings
This study analyzes previously published neurophysiology data from a publicly available repository, available via the Neurotycho website (http://neurotycho.org/). For experimental details of data acquisition, the reader is referred to the original report, which includes the statement: “All experimental and surgical procedures were performed in accordance with the experimental protocols (No. H24-2-203(4)) approved by the RIKEN ethics committee and the recommendations of the Weatherall report, ‘The use of non-human primates in research’.”17
Briefly, high density electrocorticography signals were recorded from 2 adult Macaca fuscata macaque monkeys (Monkeys C & G) by investigators at the Brain Science Institute, RIKEN, Japan, using a subdural 128-channel electrocorticography electrode array (Unique Medical, Japan),18 covering the cortical surface of the left hemisphere (Figure 1A). A reference electrode consisting of a rectangular platinum plate was placed in the subdural space between the electrocorticography array and dura mater, and a ground electrode was placed in the epidural space. Electrocorticography data sampled at 1 kHz using a Cerebus data acquisition system (Blackrock, UT, USA). The sample size was based on the available data.
Figure 1. Experimental Design.
A. Representative electrocorticography leads were selected from each monkey’s grid layout as indicated for further analysis. Note frontal eye field and lateral intraparietal area are separated by approximately 30mm, LIP and V2 are separated by approximately 10mm, and V1 and V2 are separated by approximately 15 mm. B. Block Design. On each experimental day, baseline data were first recorded with eyes open, before covering the monkey’s eyes with a blindfold. An anesthesia session was then recorded. After the monkey recovered responsiveness to somatosensory stimulation, the recovery period was then recorded in an eyes closed epoch, and finally the blindfold was removed. C. The anesthesia data was recorded to capturing the onset of a bolus dose of propofol (~5 mg Kg−1), with documentation of the time of loss of responsiveness to sensory stimulation and recovery of responsiveness to sensory stimulation.
Experimental procedure
The monkeys were restrained in a primate chair with careful monitoring of heart rate and respirations throughout the experiment. For baseline data, recordings began with the animal awake with eye open. After 10-20 minutes of data acquisition, the eyes were covered and recording continued for another 10-20 minutes (Figure 1B). For the anesthesia period, a bolus dose of propofol (5 or 5.2 mg/kg) was injected intravenously. Loss of responsiveness was determined by hand manipulation and by touching the nostril and philtrum with a cotton swab. Slow wave oscillations in the electrocorticography served as an additional confirmation of loss of consciousness. Recording continued until the monkey regained responsiveness to manipulation of the hand or stimulation of the philtrum with a cotton swab. After 10-20 minutes of recording with the eyes covered, the eye covering was removed and a final period of data acquired. For our analysis we compared the baseline eyes-closed state to the period of unconsciousness and the recovery state with eyes closed. Each monkey was administered propofol on two separate days.
Data analysis
Electrocorticography signals were re-referenced using a common average. A 10th order notch filter (49-51 Hz) reduced line noise contamination, and the resulting data were detrended prior to analysis. Artifacts were detected by eye after z-score normalization and excluded from further analysis.
Spectral Analysis
We computed spectrograms for 500s following induction with propofol using the multi-taper method as implemented in the Chronux toolbox 19. For the analysis, spectral estimates using 20 second sliding windows with a 1 second step were computed using 7 tapers. Thus time resolution of our spectrograms is 20 seconds and the frequency resolution is 0.4 Hz. After inspecting to confirm no artifacts contaminated the analysis, a group level spectrogram was calculated by averaging over all experiments. Coherence offers one method for determining long range cortical interactions.20 Time-varying coherence, or cohereograms, were calculated similarly using the multi-taper method with the same parameters and hence same time-frequency bandwidth.
Phase-amplitude coupling
Phase-amplitude coupling (Figure 2) can be quantified by many methods,13,21–27 each with specific advantages and disadvantages.21,28 However, the Kullback-Leibler modulation index proposed by Tort et al. performs better than or equivalent to other methods in terms of tolerance to noise, independence of amplitude, and sensitivity to modulation width28 and hence used here. To quantify the modulation of the amplitude of activity in a high frequency band fA, as a function of the phase of the rhythm in a lower frequency band fp, modulation index is computed from a phase-amplitude histogram distribution, obtained as follows.
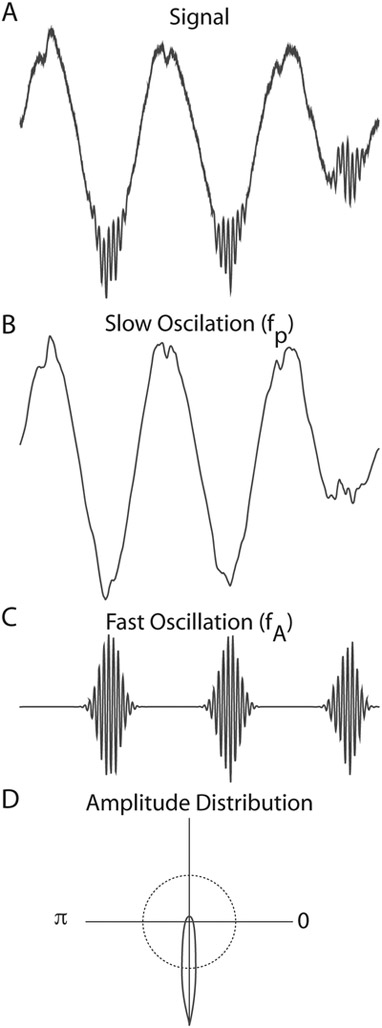
Figure 2. Phase-amplitude coupling.
A. A signal with within-area phase-amplitude coupling. B. A lowpass filtered version of signal A isolates the slow-wave envelope (fp). C. A bandpass filtered version of signal A isolates the faster oscillation (fA). Phase amplitude coupling is evident in that the amplitude of fA is largest during one phase of fp, the trough. D. This can be clearly seen in the amplitude distribution, where the average amplitude of fA is plotted as a function of the phase angle of fp. These data demonstrate a large deviation from the uniform distribution (shown as the dotted line).
First, a raw signal Xraw(t) is band pass filtered to obtain two frequency ranges for analysis: xfp(t) and xfA(t). The Hilbert transform is applied to the filtered signals xfp(t) and xfA(t) in order to extract the phase of xfp(t) as φfp(t) and the amplitude of xfA(t) as AfA(t). Then, the composite time series Ψ[φfp(t), AfA(t)] is constructed, which gives the amplitude of the oscillation fA at each corresponding phase of the rhythm fp. The phases φfp are binned into j bins and the mean of AfA over each phase bin j, , is calculated and then normalized by dividing each bin value by the sum over the bins :
where N is the number of phase bins. Note and P(J) ≥ 0 ∀ j and . This distribution-like function is referred to as the “amplitude distribution.” It is obtained by plotting P as a function of the phase bin.
The modulation index is derived from the Kullback-Leibler divergence 29 between the observed P(j) and the uniform distribution, as the amplitude distribution P(j) over phase bins is expected to be uniform in the absence of phase-amplitude coupling. The Kullback-Leibler divergence of a discrete distribution P from a distribution Q is defined as
Note that DKL(P, Q) ≥ 0 and DKL(P, Q) = 0 if and only if P = Q. Furthermore, DKL is related to the Shannon entropy (H) of a distribution P:
So, the Kullback-Leibler divergence between the observed P(j) and the uniform distribution is:
Where U is the uniform distribution. Note that log(N) is the maximum entropy value possible over the discrete distribution, which only occurs for a uniform distribution.
The modulation index (MI) is calculated by dividing the Kullback-Leibler divergence of the observed P phase amplitude distribution from the uniform distribution by log(N):
If the mean phase amplitude distribution is uniform over phase bin, MI = 0 (i.e., there is no coupling). If P is entirely concentrated in one bin like a Dirac delta distribution, then MI = 1.
In this study, we filtered the electrocorticography signal to extract frequency bands of interests: slow wave (0.1–1 Hz) and beta band (14–25 Hz) oscillations with eegfilt function implemented in EEGLab 30. We computed modulation index by assigning each temporal sample to one of N = 18 equally spaced phase bins based on the instantaneous value of φfp(t) within a 2 min epoch. Note that the minimum data length needed for a reliable measurement depends on the frequency because slower oscillations will have fewer cycles sampled than faster rhythms.28 In order to minimize the contamination of our phase-amplitude coupling calculation by response transients, we compared phase-amplitude coupling during the middle 2 minutes of each phase of the experiment.
Statistical Analysis
Modulation indices for all the frontal channels over three different stages are statistically compared for each experiment by two-tailed two sample paired Student’s t test with a significance criterion of p<0.05. The stages include (1) pre-anesthesia baseline waking state, (2) anesthetic-induced unconsciousness, and (3) post-anesthetic recovery wakefulness. The 2 minute time-resolved MI series (16 analysis channels × 2 animals × 2 experiments) were bootstrapped to determine 95% confidence intervals (2000 replicates) and averaged across all channels, subjects, and experiments for each time window. For cross-channel (frontal eye field, lateral intraparietal area, V1 and V2) phase amplitude coupling, we computed the four-channel coupling matrix for all subjects and then constructed bootstrap 95% confidence intervals (2000 replicates), then averaged within experiment stage to obtain the mean cross-channel coupling matrix. All statistical analysis was performed in Matlab (Mathworks, MA, US). There were no missing data.
Results
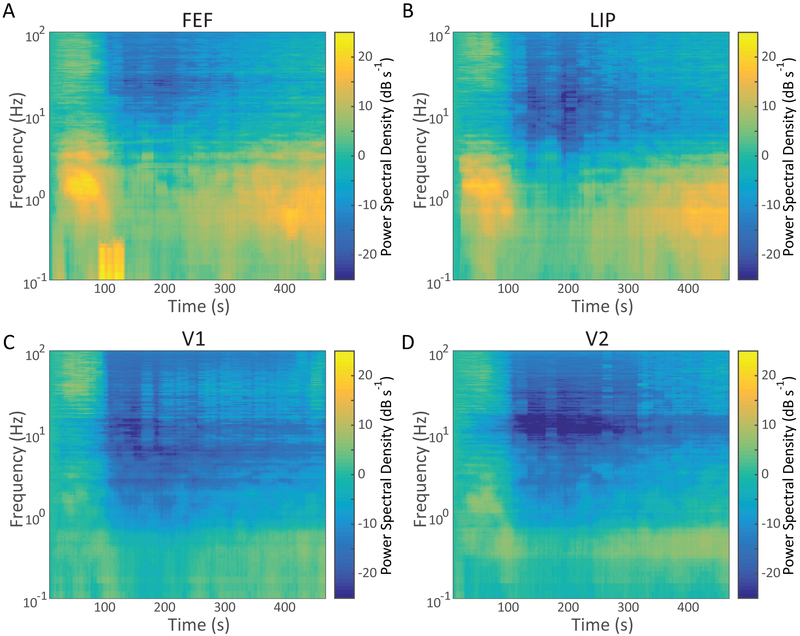
To characterize the power spectral density response to bolus propofol administration, we computed average spectrograms aligned to the administration of propofol normalized to the pre-propofol baseline, eyes-closed spectrum.31 These spectrograms revealed an early development of an ~1 Hz oscillation in frontal eye field with diffuse high frequency power approximately 30s after the intravenous bolus that then decreases within 100s (Figure 3A). At approximately 225s post bolus, a slow oscillation returns with gradual return of higher frequency power. The lateral intraparietal area average spectrogram response aligned to the the administration of propofol revealed a similar pattern (Figure 3B). V1 and V2 averaged spectrograms revealed a similar low frequency power pattern, with early termination of the intrinsic eyes-closed alpha rhythm (Figure 3C & D), though the magnitude of the shift in low frequency power was substantially smaller than in the frontal and parietal channels.
Figure 3. Time Evolution of Effects of Propofol on the Power Spectrum.
Sliding window Thomson multitaper estimate of the spectrogram after IV bolus of propofol, by area: (A) Frontal Eye Field (FEF), (B) Lateral Intraparietal Area (LIP), (C) V1, and (D) V2. Each plot shows the average across all animals and experimental sessions of the power spectral density as a function of frequency and time, aligned to the IV push of propofol. These estimates have a temporal resolution of 20 seconds and a 0.4 Hz frequency resolution.
Coherent slow oscillations occur in anatomically connected regions of the cortical circuit during propofol anesthesia
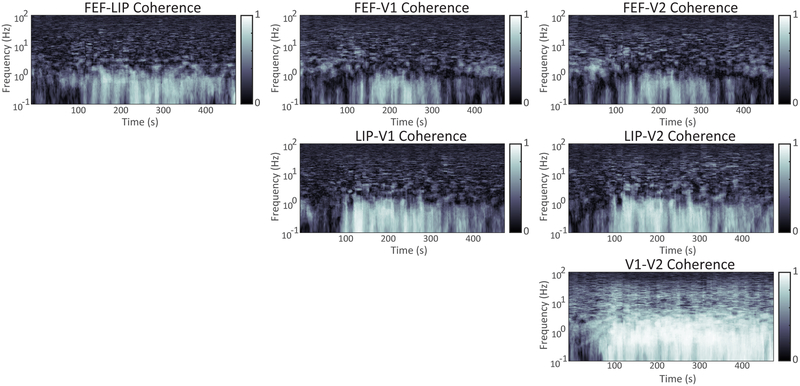
The dominant feature in the average cohereograms following the propofol bolus is a peak in the slow-oscillation range. Coherence between frontal eye field and lateral intraparietal area (Figure 4A) and between V1 and V2 (Figure 4F) is established at 1 Hz before the low frequency power transient seen in the spectrogram decreases at 100 seconds. After approximately 100s, the transient in the spectrogram has passed and coherent slow wave activity can be detected between all of the cortical areas tested (Figure 4A-F), and persists for several hundred seconds. There is an additional coherence peak in the delta frequency range between frontal eye field-V1 and frontal eye field-V2 during the initial power transient response from roughly 50-75 seconds, but it does not recur until the slow oscillation coherence decreases approximately 400 seconds into the anesthetized period.
Figure 4: Time Evolution of Coherence Following Propofol Bolus.
Mean over all subjects of sliding window multitaper estimate of coherence as a function of frequency and time after propofol bolus. Estimation parameters, and hence time and frequency resolution, are the same as Figure 4: 20 seconds and 0.4 Hz.
Bolus intravenous propofol increases within-lead phase-amplitude coupling
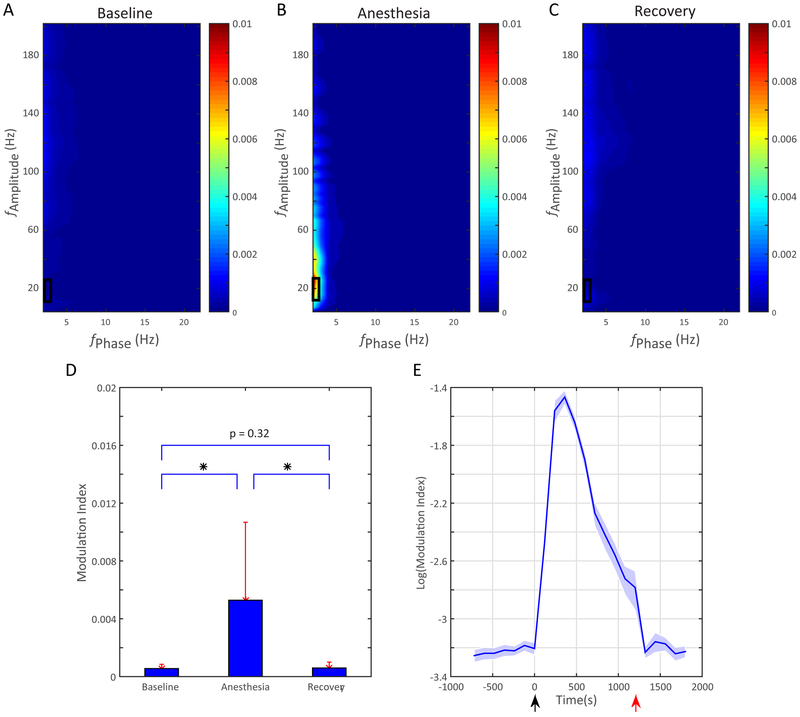
Propofol anesthesia has been reported to increase within-area phase-amplitude coupling.12 We also find that propofol increases modulation index (Figure 5B), and the modulation index returns to baseline with emergence from anesthesia within the frontal leads on the electrocorticography (Figure 5A,C). This increase is most prominent between beta power(15-25 Hz) and slow oscillation (0.1-1Hz) phase, as indicated by the boxes in Figure 5A-C. Comparing the modulation index in this slow oscillation-beta range shows a significant elevation only during the propofol period (Figure 5D). The time course of the increased phase-amplitude coupling between beta power and slow oscillation phase is consistent with an impulse response to the IV bolus (Figure 5E).
Figure 5. Intra-areal Phase Amplitude Coupling Increases with Propofol.
The mean comodulograms across frontal channels are shown for the (A) baseline, (B) the anesthetized period; and (C), the recovery period. The slow oscillation phase modulating beta band amplitude during the anesthetized period is indicated by the box. (D) Slow-oscillation phase-beta amplitude Modulation Index from the boxed region of panels A-C over three stages across frontal channels. Values shown as mean, error bars are SD, * indicates p<0.05. (E) Time course of frontal channels intra area Modulation Index response to propofol calculated in 2 minute increments. Black arrow: injection of anesthetic agent; red arrow: emergence from unconsciousness. Shaded area: bootstrap 95% confidence interval.
IV propofol selectively increases between-lead phase-amplitude coupling, consistent with anatomic connectivity
Given the presence of within-lead phase-amplitude coupling between the slow oscillation and beta activity, we chose this frequency pair to test for phase-amplitude coupling between the different oculomotor and visual cortical areas. We performed a 4×4 computation of cross-lead phase-amplitude coupling between frontal eye field, lateral intraparietal area, V1, and V2 (Figure 6, Table 1). During the anesthetized period (Figure 6B), the phase amplitude coupling between the slow oscillation in frontal eye field and beta power in lateral intraparietal area was as high in magnitude as the within lateral intraparietal area modulation of beta amplitude by slow oscillation phase. V2 phase similarly showed a strong modulatory influence on V1 beta power, with much lower average modulation index for other interactions. Again, by emergence the phase-amplitude coupling (Figure 6C) had essentially returned to baseline.
Figure 6. Inter-areal Phase Amplitude Coupling Increases with Propofol.
Mean of cross-channel modulation index between lateral intraparietal area (LIP), frontal eye field (FEF), V1, and V2 from all four experiments, by interval: A. Baseline, B. Anesthesia, C. Recovery. The strongest modulation is lateral intraparietal area beta amplitude modulated by frontal eye field slow-wave phase, followed by V1 beta amplitude modulated by V2 slow-wave phase. See Table 1 for bootstrap confidence limits.
Table 1.
Inter-areal Phase Amplitude Coupling variations. Values are means (95 % confidence interval). Confidence intervals are computed using the bootstrap method (2000 replicates). All values are modulation indices ×10^-4. Coupling strength increases dramatically under anesthesia.
| Baseline | Anesthetized | Recovery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEF Amp |
LIP Amp |
V1 Amp |
V2 Amp |
FEF Amp |
LIP Amp |
V1 Amp |
V2 Amp |
FEF Amp |
LIP Amp |
V1 Amp |
V2 Amp |
|
| FEF phase | 5.4 (4.7 - 6.3) | 5.4 (4.8 - 5.9) | 5.4 (4.8 - 6.1) | 5.5 (4.7 - 6.5) | 68.1 (45.4 - 104.6) | 145.9 (121.3 - 186.1) | 68.0 (49.0 - 91.0) | 89.7 (58.9 - 113.4) | 6.5 (5.8 - 7.5) | 6.9 (5.8 - 8.1) | 13.5 (9.4 - 16.8) | 6.7 (5.3 - 7.8) |
| LIP phase | 5.5 (4.7 - 6.4) | 5.9 (5.1 - 6.6) | 5.6 (4.7 - 6.6) | 5.7 (4.6 - 6.6) | 23.2 (17.3 - 31.1) | 138.8 (108.8 - 161.6) | 61.0 (31.5 - 96.7) | 57.8 (46.8 - 69.5) | 6.5 (5.7 - 7.7) | 6.6 (5.3 - 8.1) | 10.6 (7.4 - 14.4) | 5.8 (4.8 - 6.4) |
| V1 phase | 5.8 (5.0 - 6.7) | 5.5 (4.6 - 6.5) | 9.0 (7.4 - 10.8) | 6.0 (5.3 - 6.9) | 42.2 (28.8 - 57.0 ) | 89.1 (56.7 - 122.9) | 69.2 (46.0 - 89.1) | 80.0 (61.1 - 103.7) | 6.9 (5.4 - 8.4) | 6.5 (5.5 - 7.5) | 10.7 (8.0 - 12.5) | 6.4 (5.5 - 7.4) |
| V2 phase | 4.8 (4.3 - 5.6) | 5.4 (4.4 - 6.2) | 6.6 (5.5 - 7.7) | 5.7 (4.9 - 6.5) | 62.0 (30.4 - 107.4) | 82.0 (46.9 - 125.1) | 126.0 (94.3 - 151.3) | 109.2 (73.6 - 146.6) | 6.7 (5.8 - 7.5) | 6.1 (5.0 - 7.0) | 15.7 (10.5 - 20.7) | 7.0 (6.0 - 8.3) |
Discussion
Multiple EEG studies have reported anesthetics, including propofol, functionally disconnect frontal cortical inputs from other cortical areas, particularly parietal lobe.6,7 This drop in functional connectivity has been interpreted as suppression of cortical feedback contributing to the anesthetized state, which might suggest anesthetics selectively suppress synapses from higher order areas. It has also been reported in human LFP data that slow wave oscillations are fragmented and incoherent at a distance.32 Yet here we show the opposite with electrocorticography: certain interactions, including coherent slow wave oscillations and slow wave-beta phase-amplitude coupling, increase in cortical areas with known anatomic projections from one to the other.
Importantly, these increases in coupling between cortical areas appear to depend upon the projection anatomy. Coherence increases around 1 Hz occur earlier and persist longer between frontal eye field and lateral intraparietal area and between V2 and V1 than between the visual and oculomotor cortices, and changes in interareal phase-amplitude coupling are largest with higher-order feedback projections, namely frontal eye field to lateral intraparietal area and V2 to V1. These coherent slow waves, recorded by electrodes separated by ~30mm, contrast with the drop in phase locking with distance previously reported with propofol anesthesia.32 We have not computed phase locking factors between all possible leads for direct comparison to that study, but several observations may explain the differences. Lewis and colleagues comment that low frequency correlations that existed pre-loss of consciousness persist or increase after loss of consciousness, so some coherence was observed in areas that were coherent pre loss of consciousness, which was intermittently true between frontal eye field and lateral intraparietal area in our data. Lewis and colleagues also did not compare electrodes to projection anatomy, which suggests that one way to reconcile this discrepancy might be to consider distance not in Euclidean terms but in terms of synaptic distance between two areas. On average, with a randomly placed electrode grid, the number of synapses between recorded neuronal populations will increase with the Euclidean distance between electrodes on the grid, but this relationship will occasionally be violated by long range projections (that might not be robustly sampled by grids placed for clinical reasons). This would accord with several recent fMRI based studies of functional connectivity during anesthesia which show that during anesthesia the functional connectivity maps show a diminished dynamic repertoire dominated by anatomical connectivity.33,34
Interareal phase-amplitude coupling should not be a surprise given the presence of within-area phase-amplitude coupling and coherence in the slow oscillation. Notably, however, these phase-amplitude coupling changes are markedly asymmetric, in that lateral intraparietal area slow wave oscillation modulation of frontal eye field beta power and V1 slow wave oscillation modulation of V2 beta power is not as prominent as the reverse (Table 1). Thus phase-amplitude coupling between slow oscillation phase in the “higher order” cortical area and the beta amplitude in the “lower order” area increases during anesthesia, and that increase is larger than the phase-amplitude coupling seen in the reverse direction. This is the opposite of the predicted result from the frontoparietal connectivity hypothesis of general anesthesia.
Although all four of these cortical areas project to each other, there are differences in projection anatomy that mirror the observed asymmetry in the phase-amplitude coupling result. Frontal eye field has monosynaptic connections to lateral intraparietal area35, V136, and V237. The frontal eye field projection to lateral intraparietal area appears to involve all layers35, whereas the projection to V2 targets layers 1 or 5/637. Lateral intraparietal area has a reciprocal connection with frontal eye field38,39. V1 projects primarily to layer 4 of V2, while projections from V2 form primarily excitatory synapses in layers 1, ⅔, and 540. Peripheral but not foveal V2 projects to lateral intraparietal area41, though other extrastriate visual areas are also closely connected with lateral intraparietal area42,43. While it is tempting to attribute asymmetry in the phase-amplitude coupling results to differences in laminar projection pattern, more work needs to be done to identify the nature of the differences of the projection anatomy and then perturb those specific connections to test whether causality can be attributed to anatomy.
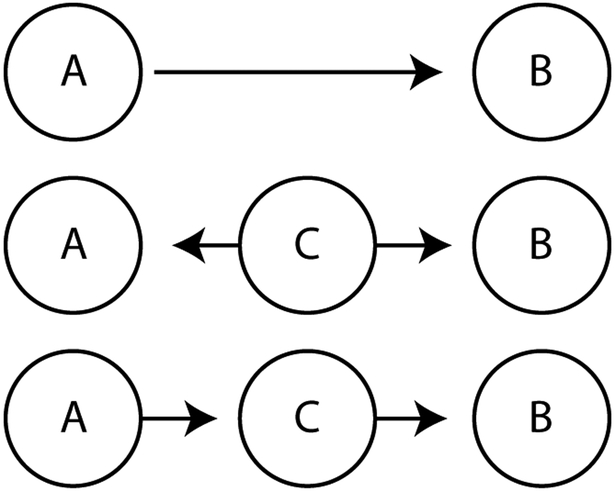
There are several interpretations of this discordance between our analysis and the functional connectivity data. Functional connectivity is a statistical relationship, and hence separate from anatomic connectivity, namely, axons from cortical area A synapsing on neurons in cortical area B. The simplest case of functional connectivity decreases with anesthesia would be when the anesthetic decreases synaptic transmission between two anatomically connected areas. In this case, however, we would expect phase-amplitude coupling between A and B to decrease during anesthesia, contrary to what we observe. Alternatively, functional connectivity does not need to arise from anatomic connectivity between A and B. Two examples of this are shown in Figure 7: if a third area C projects to and influences firing in areas A and B, then A and B will be functionally connected even though the coupling does not occur through a direct anatomic projection; or if the connection between A and B results from a cascade, where area A affects area C, which then affects area B. Thus, the drop in frontoparietal functional connectivity detected with anesthesia might result from suppression of a common input to both areas, such as a brainstem or thalamic modulatory input, or it might result from degradation of communication along a relay cascade. Dropout of frontoparietal functional connectivity measured with EEG between dorsolateral prefrontal cortex and inferior parietal lobule was shown together with fMRI evidence for suppression of an intermediate area, dorsal anterior insula by Warnaby and colleagues.5 This suggests suppression of intervening projections (including insula, thalamus, and brainstem) might contribute to the reported decrease in frontoparietal functional connectivity with anesthesia when cortical areas do not have dense direct projections between them. Finally, though perhaps less likely, the filtered and volume averaged EEG may simply show more functional connectivity at baseline than the more focal electrocorticography, and the resultant averaging could blur out most of the increased interaction that we report in this paper.
Figure 7. Network coupling architecture.
Networks that can produce coupling between two neuron populations, A and B. While anatomic projections between A and B will produce detectable functional connectivity, functional connectivity could also be detected if a common area C projects to both A and B, or if a cascade of connections exists such that A projects to C which then projects to B.
It is unlikely that the observed effect is a signal processing artifact. While within area delta-gamma and theta-gamma phase-amplitude coupling has been reported to increase with anesthesia,12 the inter-areal slow wave-beta phase-amplitude coupling increase we observe is of the same magnitude or larger than the within-area slow wave-beta phase-amplitude coupling increase we see in these areas. The increase in phase-amplitude coupling follows a time-course that matches the expected bolus response to IV administration of an anesthetic and returns to baseline by the time the animal has emerged from anesthesia. Increases in phase-amplitude coupling are directional and consistently larger from a “higher order” area to a “lower order” area, and they are not universal -- the visual occipital areas show minimal phase-amplitude coupling with the oculomotor frontal and parietal areas, despite comparable physical distances between the locations.
A potential limitation of this report stems from our reliance on an existing published electrocorticography dataset, with only four propofol experiments, though two administrations were performed in two animals. As a result, we cannot fully characterize the full population variability in the responsiveness of phase-amplitude coupling as a measure of corticocortical interaction during anesthesia, but there was no instance in which the rise in phase-amplitude coupling between frontal eye field and lateral intraparietal area or between V2 and V1 was absent. Additionally, because our analysis was limited to propofol, which has previously been shown to affect phase-amplitude coupling, the observed increase in phase-amplitude coupling between frontal eye field and lateral intraparietal area and V2 to V1 may represent drug-specific effects rather than correlates of the anesthetized state per se.
Limitations in our approach notwithstanding, our observations complicate current theories of anesthesia that posit a suppression of frontoparietal interactions produces the anesthetized state. While this theory may prove true for nonspecific interactions between frontal and parietal regions, anatomically connected areas show increased interactions as measured by phase-amplitude coupling during propofol anesthesia. This effect is directional, in a fashion that correlates with the laminar nature of known anatomic projections, suggesting that GABA-ergic agents like propofol may differentially disturb processing in different cortical lamina.
Significantly, frontal eye field is part of dorsolateral prefrontal cortex, which is hypothesized to be central to the conscious perception of stimuli.44 Certainly frontal eye field and lateral intraparietal area are involved in visual working memory and attention,45–47 which are necessary for the transition from phenomenal to access consciousness.48,49 The ability of propofol to alter coupling between frontal eye field and lateral intraparietal area thus might be key to the disruption of conscious experience. We have shown that propofol induces an interaction between frontal eye field and lateral intraparietal area that is absent during conscious states. One could hypothesize that this aberrant coupling prevents frontal eye field from stabilizing distributed representations across multiple cortical areas, and hence precludes the dynamic formation of multiple cortical areas into transient assemblies conjectured to underlie conscious perception. Further work is necessary to understand how increased phase-amplitude coupling relates to drops in functional connectivity previously reported with multiple anesthetics and multiple methodologies.
Summary.
What is known:
A decrease in frontoparietal functional connectivity has been demonstrated with multiple anesthetic agents, and this decrease has been proposed as a final common functional pathway to produce anesthesia.
Two alternative measures of long-range cortical interaction are coherence and phase amplitude coupling. While phase-amplitude coupling within frontal cortex changes with propofol administration, effects of propofol on phase amplitude coupling between different cortical areas have not previously been reported.
What this study adds that is new:
Using a previously published monkey electrocorticography dataset it was found that propofol induced coherent slow oscillations in visual and oculomotor networks made up of cortical areas with strong anatomic projections.
Frontal eye field within-area phase-amplitude coupling increased.
Contrary to expectations from previous functional connectivity studies, inter-areal phase-amplitude coupling also increased with propofol.
Acknowledgments
Funding Statement: Supported by National Natural Science Foundation of China (Beijing, China) grant No.51675389 (to Dr. Ma), grants from California Capital Equity LLC (Culver City, California; to Dr. Liu) and National Institute of General Medical Sciences (Bethesda, Maryland) grant No. 1K08GM121961 (to Dr. Hudson).
Footnotes
Clinical trial number and registry URL: N/A
Prior Presentations: None
Conflicts of Interest: The authors declare no competing interests.
References
- 1.Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Berger S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schröter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschläger A, Kochs EF, Schneider G: Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top-down processing in association with anesthetic-induced unconsciousness. Anesthesiology 2013; 119:1031–42 [DOI] [PubMed] [Google Scholar]
- 2.Palanca BJA, Mitra A, Larson-Prior L, Snyder AZ, Avidan MS, Raichle ME: Resting-state Functional Magnetic Resonance Imaging Correlates of Sevoflurane-induced Unconsciousness. Anesthesiology 2015; 123:346–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boveroux P, Vanhaudenhuyse A, Bruno M-A, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant J-F, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M: Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 2010; 113:1038–53 [DOI] [PubMed] [Google Scholar]
- 4.Ranft A, Golkowski D, Kiel T, Riedl V, Kohl P, Rohrer G, Pientka J, Berger S, Thul A, Maurer M, Preibisch C, Zimmer C, Mashour GA, Kochs EF, Jordan D, Ilg R: Neural Correlates of Sevoflurane-induced Unconsciousness Identified by Simultaneous Functional Magnetic Resonance Imaging and Electroencephalography. Anesthesiology 2016; 125:861–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warnaby CE, Seretny M, Ní Mhuircheartaigh R, Rogers R, Jbabdi S, Sleigh J, Tracey I: Anesthesia-induced Suppression of Human Dorsal Anterior Insula Responsivity at Loss of Volitional Behavioral Response. Anesthesiology 2016; 124:766–78 [DOI] [PubMed] [Google Scholar]
- 6.Ku S-W, Lee U, Noh G-J, Jun I-G, Mashour GA: Preferential Inhibition of Frontal-to-Parietal Feedback Connectivity Is a Neurophysiologic Correlate of General Anesthesia in Surgical Patients. PLoS One 2011; 6:e25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA: Disruption of Frontal–Parietal Communication by Ketamine, Propofol, and Sevoflurane. Anesthesiology 2013; 118:1264–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meij R, Kahana M, Maris E: Phase-amplitude coupling in human electrocorticography is spatially distributed and phase diverse. J Neurosci 2012; 32:111–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canolty RT, Knight RT: The functional role of cross-frequency coupling. Trends Cogn Sci 2010; 14:506–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onslow ACE, Jones MW, Bogacz R: A canonical circuit for generating phase-amplitude coupling. PLoS One 2014; 9:e102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukamel EA, Pirondini E, Babadi B, Wong KFK, Pierce ET, Harrell PG, Walsh JL, Salazar-Gomez AF, Cash SS, Eskandar EN, Weiner VS, Brown EN, Purdon PL: A transition in brain state during propofol-induced unconsciousness. J Neurosci 2014; 34:839–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal D, Silverstein BH, Sharba L, Li D, Hambrecht-Wiedbusch VS, Hudetz AG, Mashour GA: Propofol, Sevoflurane, and Ketamine Induce a Reversible Increase in Delta-Gamma and Theta-Gamma Phase-Amplitude Coupling in Frontal Cortex of Rat. Front Syst Neurosci 2017; 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruns A, Eckhorn R: Task-related coupling from high- to low-frequency signals among visual cortical areas in human subdural recordings. Int J Psychophysiol 2004; 51:97–116 [DOI] [PubMed] [Google Scholar]
- 14.Brown EN, Lydic R, Schiff ND: General anesthesia, sleep, and coma. N Engl J Med 2010; 363:2638–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunez PL, Srinivasan R: Electric Fields of the Brain: The Neurophysics of EEG. Oxford University Press, USA, 2006 [Google Scholar]
- 16.Premereur E, Vanduffel W, Roelfsema PR, Janssen P: Frontal eye field microstimulation induces task-dependent gamma oscillations in the lateral intraparietal area. J Neurophysiol 2012; 108:1392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagawa T, Chao ZC, Hasegawa N, Fujii N: Large-scale information flow in conscious and unconscious states: an ECoG study in monkeys. PLoS One 2013; 8:e80845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagasaka Y, Shimoda K, Fujii N: Multidimensional Recording (MDR) and Data Sharing: An Ecological Open Research and Educational Platform for Neuroscience. PLoS One 2011; 6:e22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP: Chronux: a platform for analyzing neural signals. J Neurosci Methods 2010; 192:146–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malekmohammadi M, AuYong N, Price CM, Tsolaki E, Hudson AE, Pouratian N: Propofol-induced Changes in α-β Sensorimotor Cortical Connectivity. Anesthesiology 2018; 128:305–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MX: Assessing transient cross-frequency coupling in EEG data. J Neurosci Methods 2008; 168:494–9 [DOI] [PubMed] [Google Scholar]
- 22.Kramer MA, Eden UT: Assessment of cross-frequency coupling with confidence using generalized linear models. J Neurosci Methods 2013; 220:64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyakoshi M, Delorme A, Mullen T, Kojima K, Makeig S, Asano E: Automated detection of cross-frequency coupling in the electrocorticogram for clinical inspection. Conf Proc IEEE Eng Med Biol Soc 2013; 2013:3282–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser M-B, Moser EI: Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009; 462:353–7 [DOI] [PubMed] [Google Scholar]
- 25.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT: High gamma power is phase-locked to theta oscillations in human neocortex. Science 2006; 313:1626–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amiri M, Frauscher B, Gotman J: Phase-Amplitude Coupling Is Elevated in Deep Sleep and in the Onset Zone of Focal Epileptic Seizures. Front Hum Neurosci 2016; 10:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penny WD, Duzel E, Miller KJ, Ojemann JG: Testing for nested oscillation. J Neurosci Methods 2008; 174:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tort ABL, Komorowski R, Eichenbaum H, Kopell N: Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 2010; 104:1195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullback S, Leibler RA: On Information and Sufficiency. Ann Math Stat 1951; 22:79–86 [Google Scholar]
- 30.Delorme A, Makeig S: EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004; 134:9–21 [DOI] [PubMed] [Google Scholar]
- 31.Hudson AE: Metastability of Neuronal Dynamics during General Anesthesia: Time for a Change in Our Assumptions? Front Neural Circuits 2017; 11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL: Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A 2012; 109:E3377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barttfeld P, Bekinschtein TA, Salles A, Stamatakis EA, Adapa R, Menon DK, Sigman M: Factoring the brain signatures of anesthesia concentration and level of arousal across individuals. Neuroimage Clin 2015; 9:385–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhrig L, Sitt JD, Jacob A, Tasserie J, Barttfeld P, Dupont M, Dehaene S, Jarraya B: Resting-state Dynamics as a Cortical Signature of Anesthesia in Monkeys. Anesthesiology 2018. doi: 10.1097/ALN.0000000000002336 [DOI] [PubMed] [Google Scholar]
- 35.Anderson JC, Kennedy H, Martin KAC: Pathways of attention: synaptic relationships of frontal eye field to V4, lateral intraparietal cortex, and area 46 in macaque monkey. J Neurosci 2011; 31:10872–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clavagnier S, Falchier A, Kennedy H: Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn Affect Behav Neurosci 2004; 4:117–26 [DOI] [PubMed] [Google Scholar]
- 37.Stanton GB, Bruce CJ, Goldberg ME: Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol 1995; 353:291–305 [DOI] [PubMed] [Google Scholar]
- 38.Ferraina S, Paré M, Wurtz RH: Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. J Neurophysiol 2002; 87:845–58 [DOI] [PubMed] [Google Scholar]
- 39.Schall JD: Visuomotor Areas of the Frontal Lobe, Cerebral Cortex. 1997, pp 527–638 [Google Scholar]
- 40.Anderson JC, Martin KAC: The synaptic connections between cortical areas V1 and V2 in macaque monkey. J Neurosci 2009; 29:11283–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baizer JS, Ungerleider LG, Desimone R: Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci 1991; 11:168–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen RA, Asanuma C, Essick G, Siegel RM: Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 1990; 296:65–113 [DOI] [PubMed] [Google Scholar]
- 43.Blatt GJ, Andersen RA, Stoner GR: Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol 1990; 299:421–45 [DOI] [PubMed] [Google Scholar]
- 44.van Vugt B, Dagnino B, Vartak D, Safaai H, Panzeri S, Dehaene S, Roelfsema PR: The threshold for conscious report: Signal loss and response bias in visual and frontal cortex. Science 2018; 360:537–42 [DOI] [PubMed] [Google Scholar]
- 45.Noudoost B, Chang MH, Steinmetz NA, Moore T: Top-down control of visual attention. Curr Opin Neurobiol 2010; 20:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark KL, Noudoost B, Moore T: Persistent spatial information in the frontal eye field during object-based short-term memory. J Neurosci 2012; 32:10907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinen K, Feredoes E, Ruff CC, Driver J: Functional connectivity between prefrontal and parietal cortex drives visuo-spatial attention shifts. Neuropsychologia 2017; 99:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chica AB, Bartolomeo P: Attentional routes to conscious perception. Front Psychol 2012; 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chica AB, Valero-Cabré A, Paz-Alonso PM, Bartolomeo P: Causal contributions of the left frontal eye field to conscious perception. Cereb Cortex 2014; 24:745–53 [DOI] [PubMed] [Google Scholar]