SUMMARY
The fitness of host-associated microbes depends on their ability to access nutrients in vivo. Identifying these mechanisms is significant for understanding how microbes have evolved to fill specific ecological niches within a host. Vibrio fischeri is a bioluminescent bacterium that colonizes and proliferates within the light organ of the Hawaiian bobtail squid, which provides an opportunity to study how bacteria grow in vivo. Here, the transcription factor CysB is shown to be necessary for V. fischeri both to grow on several sulfur sources in vitro and to establish symbiosis with juvenile squid. CysB is also found to regulate several genes involved in sulfate assimilation and to contribute to the growth of V. fischeri on cystine, which is the oxidized form of cysteine. A mutant that grows on cystine but not sulfate could establish symbiosis, suggesting that V. fischeri acquires nutrients related to this compound within the host. Finally, CysB-regulated genes are shown to be differentially expressed among the V. fischeri populations occupying the various colonization sites found within the light organ. Together, these results suggest the biogeography of V. fischeri populations within the squid light organ impacts the physiology of this symbiotic bacterium in vivo through CysB-dependent gene regulation.
Keywords: Vibrionaceae, gene expression, squid, sulfur, symbiosis
ABBREVIATED SUMMARY
The bioluminescent bacterium Vibrio fischeri establishes symbiosis within the light organ of the Hawaiian squid. The transcription factor CysB is used to promote growth on sulfur sources available within the host. The extent of transcriptional regulation by CysB varies among V. fischeri populations within the light organ, suggesting that the host-derived sulfur sources vary among the habitats available to V. fischeri in vivo.
INTRODUCTION
Plants and animals house microbiomes that impact their physiology, development, and evolution (Gilbert et al., 2015). The disruption of a microbiome either through chemical or physical perturbation typically has a negative consequence on the overall fitness of the host (Reid et al., 2011). Despite the increased awareness of the important roles these microbiomes play in regulating states of health and disease, the factors that contribute to the fitness of most microbial species in vivo remain poorly understood. Understanding the factors that impact the fitness of host-associated microbes ultimately depends on determining the mechanisms they use to acquire nutrients and energy for growth in vivo. For microbes that associate with their host in an extracellular manner, the variability in environmental conditions among potential colonization sites within the host represents a challenge for studying those mechanisms (Meadows and Wargo, 2014). However, approaches that account for the spatial organization of host-associated microbial populations, i.e. their biogeography, have the potential to reveal mechanisms that impact microbial fitness at specific colonization sites (Stacy et al., 2016).
An experimental system that is particularly amenable to biogeographical studies is the symbiosis established between Euprymna scolopes (Hawaiian bobtail squid) and the marine bacterium Vibrio fischeri (McFall-Ngai, 2014). Shortly after juvenile squid hatch from their eggs, they acquire V. fischeri cells from the seawater environment, which quickly colonize three epithelium-lined crypt spaces located on either side of the host’s light organ. V. fischeri is a genetically tractable microbe, and the juvenile light organ is amenable to light microscopy, which has led to the use of fluorescent proteins as markers of cell position and gene expression in vivo (Dunn et al., 2006; Sun et al., 2015). The populations resulting from the rapid proliferation of the founder cells that initially colonize the crypt spaces produce bioluminescence that the host uses as a form of camouflage (Jones and Nishiguchi, 2004). Bacterial bioluminescence is a product of the enzyme luciferase, which releases light while consuming reducing power and oxygen (Meighen, 1993). Oxygen is transported from the gills to the bacterial populations via the carrier protein hemocyanin (Kremer et al., 2014). The affinity of hemocyanin for oxygen is positively correlated with pH (Kremer et al., 2014), and, within the crypt spaces of adult animals, V. fischeri promotes the release of oxygen by acidifying the environment through the fermentation of host-derived chitin (Schwartzman et al., 2015).
While chitin fermentation appears to fuel the growth of V. fischeri in the mature symbiosis, the mechanisms that support bacterial growth within the juvenile light organ are less clear. The expression of genes necessary for metabolizing chitin byproducts are repressed by NagC (Sun et al., 2015), suggesting that nutrients other than chitin are accessed by the corresponding V. fischeri populations at this early stage of symbiosis. The current model is that host proteins are degraded into peptides and amino acids that promote V. fischeri growth, which was initially proposed from the successful colonization of juvenile animals by mutants that exhibit auxotrophy for various amino acids (Graf and Ruby, 1998). One amino acid considered in this study was cysteine, which is particularly interesting because its biosynthesis also represents the primary mechanism for a bacterium to convert an inorganic sulfur source, e.g., sulfate, into an organic form that fuels growth. The cysteine auxotroph was supported to 5% of wild-type bacterial levels in vivo (Graf and Ruby, 1998), raising the possibility that V. fischeri can utilize an organic form of sulfur for growth within the light organ. The mutation within the cysteine auxotroph used in this study remains undefined; however, more recently, a ΔcysK mutant, which fails to grow on sulfate due to its inability to synthesize cysteine from the precursors O-acetyl-serine and sulfide, was shown to achieve normal bacterial levels in vivo (Singh et al., 2015), which is consistent with V. fischeri having access to an organic sulfur source within the juvenile light organ that promotes growth in vivo.
In this study, the ability of V. fischeri to grow on various sulfur sources in vitro is used to gain insight into the sulfur sources that facilitate growth of V. fischeri within the squid light organ. The transcription factor CysB is shown to regulate genes necessary for growth on several sulfur sources. Squid colonization assays with defined mutants involving CysB revealed that the ability of V. fischeri to grow on cystine, which is the oxidized form of cysteine, is a trait that is important for symbiosis establishment. Finally, the expression of CysB-regulated genes is shown to differ among the V. fischeri populations that occupy different crypt spaces within the juvenile light organ, suggesting the bioavailability of host-derived sulfur sources varies according to the biogeography of the bacteria in vivo. Together, these findings increase understanding of the molecular mechanisms that promote microbial fitness within host environments.
RESULTS
CysB promotes growth of V. fischeri on various sulfur sources
V. fischeri grows in defined minimal medium containing sulfate as the sole sulfur source (Sun et al., 2015), which shows that V. fischeri has the ability to assimilate sulfate. To determine whether V. fischeri grows on other sulfur sources, we measured the growth yield of the wild-type, symbiotic V. fischeri strain ES114 (Ruby et al., 2005) in sulfur-free medium supplemented with sulfate, thiosulfate, cysteine, cystine, or glutathione, which are sulfur sources commonly used by other γ-proteobacteria (Kredich, 1996). The concentration of each supplemented compound was set so that the concentration of sulfur atoms in each experimental group was 1 mM. After 21 h, some background growth occurred in sulfur-free medium (Fig. 1), suggesting a contaminating sulfur source is present in the base medium, which is similarly observed in studies of sulfate assimilation by other bacteria (Chonoles Imlay et al., 2015). However, supplementation with 1 mM sulfate resulted in a growth yield 3.9-fold higher (Fig. 1), demonstrating that the unknown sulfur source is growth-limiting within the sulfur-free medium and that the assay is sufficient for identifying sulfur-containing compounds that promote bacterial growth. Other compounds that promote the growth of V. fischeri include thiosulfate, cysteine, cystine, and glutathione (Fig. 1), on which other γ-proteobacteria like Escherichia coli and Salmonella typhimurium can grow (Kredich, 1996).
Fig. 1. Impact of sulfur source on growth of V. fischeri.
V. fischeri strain ES114 was grown for 21 h in sulfur-free medium supplemented with the indicated sulfur source (- = no supplement, SO42− = 1 mM sulfate, S2O32− = 0.5 mM thiosulfate, Cys = 1 mM cysteine, Cys2 = 0.5 mM cystine, and GSH = 1 mM glutathione). Reported OD600 values were calculated using a sample containing only media as a blank. Each point represents an independent biological replicate, and the bar represents the mean (n = 3). One-way ANOVA revealed significant differences among means (F5,12 = 361.7, p < 0.0001). A Dunnett’s post-hoc test was performed to statistically compare the means of each group containing a sulfur source to the mean of the no-supplement group, with p-values adjusted for multiple comparisons (**** = p < 0.0001). Experiment was performed three times with similar results obtained among each trial.
In γ-proteobacteria, sulfate assimilation depends on several genes (referred to as cys genes) involved in transporting sulfate into the cell and reducing it to sulfide, which can then be used for anabolic purposes including cysteine biosynthesis (Kredich, 1996). The transcription of cys genes is regulated by the LysR-type transcription factor CysB (Kredich, 1992). Within the genome of ES114, the gene annotated as the cysB homologue is VF_1490 (Fig. 2A). VF_1490 is predicted to encode a 36.1 kDa protein with 324 amino acids and 74% identity to the CysB protein of E. coli. We replaced VF_1490 in ES114 by allelic exchange with a deletion allele (ΔcysB), which lacks the codons for residues 26–323 (Fig. 2A). Unlike wild-type cells, ΔcysB failed to grow on sulfate, but the mutant could grow on cysteine (Fig. 2B), indicating that it is a cysteine auxotroph, as observed with cysB− mutants of other γ-proteobacteria (Kredich, 1996). The mutant failed to grow on thiosulfate and cystine (Fig. 2B), suggesting that VF_1490 regulates genes that permit growth on these sulfur sources. The ΔcysB mutant grew on glutathione (Fig. 2B), indicating that growth of V. fischeri on this compound is independent of VF_1490. We also tested for genetic complementation by introducing the VF_1490 gene with its native promoter in single copy into the genome of the ΔcysB mutant and found using the panel of sulfur-containing compounds that this strain (cysB+) grew similarly to wild-type cells (Fig. 2B). Together, these results suggest that VF_1490 encodes the CysB homologue of V. fischeri that plays a role in promoting growth on various sulfur sources.
Fig. 2. Impact of CysB on growth of V. fischeri on various sulfur sources.
A. Above, genetic locus of VF_1490 (yellow arrow labeled cysB) in the genome of wild-type strain ES114. VF_1489 is predicted to encode a SAM-dependent methyltransferase. Below, corresponding region in the in-frame cysB deletion mutant (ΔcysB) constructed in this study.
B. V. fischeri strains (WT = TIM313, ΔcysB = TIM410, and cysB+ = TIM409) were grown for 21 h in sulfur-free medium supplemented with the indicated sulfur source (- = no supplement, SO42− = 1 mM sulfate, S2O32− = 0.5 mM thiosulfate, Cys = 1 mM cysteine, Cys2 = 0.5 mM cystine, and GSH = 1 mM glutathione). Reported OD600 values were calculated using a media-only sample as a blank. Graphical and error bars represent means and standard deviations of independent biological replicates (n = 3), respectively. Two-way ANOVA revealed significant differences among means due to sulfur source (F5,36 = 1193, p < 0.0001), genotype (F2,36 = 2403, p < 0.0001), and their interaction (F10,36 = 336.6, p < 0.0001). A Dunnett’s post-hoc test was performed to statistically compare the means of each mutant to wild type for each sulfur treatment, with p-values adjusted for multiple comparisons (**** = p < 0.0001; comparisons with p ≥ 0.05 are considered not significant and are not labeled). Experiment was performed three times with similar results obtained among each trial.
CysB promotes the establishment of symbiosis with E. scolopes
To determine whether CysB impacts how V. fischeri establishes symbiosis with E. scolopes, we conducted squid colonization assays (Fig. 3A). These assays require a V. fischeri inoculum in filter-sterilized seawater (FSSW), which contains 27.7 mM sulfate. Because the ΔcysB mutant fails to grow in medium containing sulfate as the sole sulfur source (Fig. 2B), we were concerned that the mutant would exhibit reduced fitness in FSSW, which could complicate interpreting the results from the colonization assay. Therefore, in assays involving the ΔcysB mutant, the initial inoculum of the mutant was elevated up to ten-fold above wild type, which was a level sufficient for the abundance of ΔcysB to stay at or above that of wild type in FSSW for 3.5 h (Fig. 3B).
Fig. 3. Impact of CysB on the ability of V. fischeri strains to establish a symbiotic association with E. scolopes juveniles. Strains used in these experiments are TIM313 (WT), TIM410 (ΔcysB), and TIM409 (cysB+).
A. Timeline of squid colonization assay. Each wash step involves the transfer of the animal to fresh FSSW.
B. Abundance of V. fischeri strains in FSSW over time. Points and error bars represent the means and standard deviations of independent biological replicates (n = 3), respectively. Two-way ANOVA revealed significant differences among means due to genotype (F2,18 = 68.96, p < 0.0001), time (F2,18 = 289.2, p < 0.0001), and their interaction (F4,18 = 121.2, p < 0.0001). A Dunnett’s post-hoc test was performed to statistically compare the means of each mutant to wild type for each time point, with p-values adjusted for multiple comparisons (**** = p < 0.0001, ** = p < 0.01, ns = p ≥ 0.05). Experiment was performed three times with similar results obtained among each trial.
C. Luminescence of animals exposed to indicated V. fischeri strains. Apo = apo-symbiotic. Each point represents the luminescence of each animal (n = 30/group) at 48 h p.i. The dotted line indicates the cutoff above which animals are scored as luminescent (Lum+) and was calculated by performing a one-tailed t-test of Apo group (α = 0.01). Lum+ animal counts are shown below the name of each group. A Kruskal-Wallis test revealed significant differences among group medians (H = 92.93, d.f. = 3, p < 0.0001). A Dunn’s post-hoc test was performed to statistically compare the medians of each group, with p-values adjusted for multiple comparisons. Comparisons between groups with different letters have significantly different medians (p < 0.0001), and comparisons between groups with the same letter do not have significantly different medians (p ≥ 0.05).
D. CFU levels of animals in C. Each point represents the CFU level of one animal. Dotted line indicates the limit of detection (14 CFU/squid). A Kruskal-Wallis test revealed significant differences among group medians (H = 102.1, d.f. = 3, p < 0.0001). A Dunn’s post-hoc test was performed to statistically compare the medians of each group, with p-values adjusted for multiple comparisons. Comparisons between groups with different letters have significantly different medians (a/b and b/c = p < 0.0001, a/c = p < 0.01), and comparisons between groups with the same letter do not have significantly different medians (p ≥ 0.05). Experiment was performed twice with similar results obtained among both trials.
At 48 h post-inoculation (p.i.), all animals exposed to wild-type cells exhibited luminescence (Fig. 3C) and contained CFU (Fig. 3D), consistent with ES114 having colonized each animal’s light organ. In contrast, the majority of animals exposed to the ΔcysB mutant did not exhibit luminescence (Fig. 3C). However, all of these animals contained CFU but at levels approximately 250-fold lower than that of animals containing WT cells (Fig. 3D). One possible reason for decreased abundance of the ΔcysB mutant in vivo is if this mutation impairs the ability of V. fischeri to produce luminescence, which is necessary for the populations of V. fischeri that have resulted from the successful colonization of the squid host to be subsequently maintained (Studer et al., 2014; Verma and Miyashiro, 2013; Visick et al., 2000). However, when grown in the presence of N-3-oxohexanoyl homoserine lactone (3-oxo-C6 HSL), which is a small signaling molecule associated with quorum sensing that stimulates bioluminescence production by V. fischeri (Miyashiro and Ruby, 2012), cultures of the ΔcysB mutant produced bioluminescence (Fig. S1). Thus, these results suggest that the low levels of luminescence associated with animals colonized by the ΔcysB mutant (Fig. 3C) is more likely due to the corresponding populations being too small for quorum sensing to promote detectable bioluminescence. We also found that mutants containing transposon insertions in cysK, cysJ, or cysD are cysteine auxotrophs (Fig. S2) and can establish symbiosis (Figs. S3A–B), suggesting that the growth defect of the ΔcysB mutant in vivo is independent of its failure to grow on sulfate. Finally, animals exposed to the cysB+ mutant displayed levels of luminescence and CFU comparable to animals colonized by WT (Figs. 3C–D), demonstrating genetic complementation with cysB. Together, these results suggest that CysB is necessary for V. fischeri cells to grow to wild-type levels within the environment of the light organ.
Regulation of cys genes in V. fischeri
Our results above suggest that CysB promotes the growth of V. fischeri within the squid light organ. To gain insight into how CysB contributes to symbiosis establishment, we investigated the function of CysB as a transcriptional regulator in V. fischeri. During sulfate assimilation by other γ-proteobacteria, CysB promotes the expression of cys genes to facilitate the transport of sulfate into the cytoplasm and its reduction to sulfide (Kredich, 1992), which can then be used for cysteine biosynthesis (Fig. 4A). Cytoplasmic cysteine indirectly impacts this regulatory function of CysB: cysteine inhibits the enzymatic activity of CysE, which catalyzes the synthesis of the CysB inducer, N-acetyl-serine (NAS) (Kredich and Tomkins, 1966). Thus, at high cysteine levels, NAS levels decrease, which leads to lower transcription of the cys genes. Therefore, the expression level of a cys gene within a V. fischeri population provides a readout for the regulatory activity of CysB within those cells.
Fig. 4. Impact of cysteine on expression of cys genes in V. fischeri.
A. Model of sulfate assimilation and cysteine biosynthesis for V. fischeri ES114. Genes predicted to facilitate enzymatic steps are located next to each arrow. APS = adenosine-5’ phosphosulfate, PAPS = 3’-phosphoadenosine-5’-phosphosulfate. Feedback inhibition of CysE by cysteine is also shown.
B-E. Wild-type ES114 harboring a reporter plasmid for the indicated promoter was grown in sulfate-replete minimal medium ± 1 mM cysteine. Plasmids used are pVF_1893P (B), pSCV26 (C), pVF_0320P (D), and pVF_0310P (E). Gene expression was determined by normalizing the green fluorescence of an individual culture sample with its corresponding OD600 (a.u. = arbitrary units). Each point represents an independent biological replicate, and the bar represents the mean (n = 3). The dotted line indicates the background level of normalized fluorescence for a non-fluorescent control (pVSV105/ES114). For each experiment, an unpaired, two-tailed t test with Welch’s correction was performed to determine statistical differences between group means (n.s. = not significant, ** = p < 0.01, *** = p < 0.001). Each experiment was performed three times with similar results obtained among trials for each promoter.
To study cys gene expression, we constructed reporter plasmids for three promoter regions: cysK (PcysK), cysDN-yfbS-cysD (PcysD), and cysJIH (PcysJ). For each construct, a promoter region was cloned upstream of gfp in the plasmid pTM267 (Miyashiro et al., 2010), which we have previously used for examining gene expression of V. fischeri (Sun et al., 2015). For cultures of cells harboring a reporter plasmid, the GFP/OD600 ratio can be used as a quantitative measure of the transcriptional expression by the corresponding promoter. We first tested the response of these reporter constructs using 1 mM cysteine, which results in decreased expression of cys genes in other γ-proteobacteria (Kredich and Tomkins, 1966). In response to 1 mM cysteine, expression of PcysK in wild-type cells decreased 29.6-fold (Fig. 4B), which suggests the regulatory network controlling cysK transcription in V. fischeri functions similarly to those of other γ-proteobacteria. As a negative control for cys genes, we also used cells containing a reporter for the tetA promoter (PtetA), which is expressed in the absence of the TetR repressor (Hillen and Berens, 1994). Cells harboring the PtetA reporter plasmid showed no change in expression in response to 1 mM cysteine (Fig. 4C), suggesting that the decreased expression of PcysK in response to exogenous cysteine (Fig. 4B) is specific to this promoter and not due to cysteine affecting the fluorescence properties of GFP. In response to exogenous cysteine, expression of PcysD and PcysJ decreased 101- and 157-fold, respectively (Figs 4D & E), which is similar to the effect observed with cysK. Together, these results show that the presence of exogenous cysteine results in repression of cys genes in V. fischeri, which is consistent with their transcription being indirectly controlled by feedback inhibition (Fig. 4A).
We next sought to determine the dependency of cys gene expression on CysB in V. fischeri. However, because the ΔcysB mutant is a cysteine auxotroph (Fig. 2B), it was not possible to grow this mutant using sulfate as the sole sulfur source for measuring cys gene expression under the inducing conditions associated with sulfate assimilation. Therefore, as an alternative approach, we asked whether amino acid substitutions within CysB could be made to induce the expression of cys genes in cells grown under cysteine-replete conditions, which permit the growth of cysteine auxotrophs (Fig. 2B). Residues 86–324 within the CysB homologue of S. typhimurium forms a structure that binds NAS (Mittal et al., 2017), which alters the activity of CysB as a transcription factor via allostery. In E. coli, certain amino acid substitutions within the inducer region impact the ability of CysB to regulate transcription of cys genes (Lochowska et al., 2001). In particular, substitution of Ala-227 with Asp results in a CysB variant (CysBA227D) that shows strong binding to and transcriptional activation of CysB-induced promoters independent of NAS (Lochowska et al., 2001). The Ala-227 residue is conserved in the CysB homologue of V. fischeri (Fig. S4), which enabled construction of a cysBA227D allele by site-directed mutagenesis. This allele was introduced into the genome of the ΔcysB mutant in trans, resulting in the mutant cysBA227D. In the presence of 1 mM cysteine, expression of cysK was 3.0 ± 0.2 times higher in the cysBA227D mutant than in wild-type cells (Fig. 5), suggesting that the CysBA227D variant induces expression of cys genes under cysteine-replete conditions. This result is consistent with V. fischeri using CysB to regulate expression of cys genes in response to cysteine.
Fig. 5. Impact of CysB on expression of cys genes in V. fischeri.
Indicated strains harboring the PcysK::gfp reporter plasmid grown in sulfate-replete minimal medium with 1 mM cysteine. Strains are TIM313 (WT), TIM410 (ΔcysB), TIM409 (cysB+), and TIM411 (cysBA227D). Gene expression was determined by normalizing the green fluorescence of an individual culture sample with its corresponding OD600 (a.u. = arbitrary units). Each point represents an independent biological replicate, and the bar represents the mean (n = 3). The dotted line indicates the background level of normalized fluorescence for a non-fluorescent control (pVSV105/ES114). One-way ANOVA revealed significant differences among means (F3,8 = 127.3, p < 0.0001). A Dunnett’s post-hoc test was performed to statistically compare the means of each mutant group to the mean of the wild-type group, with p-values adjusted for multiple comparisons (**** = p < 0.0001; n.s. = not significant with p ≥ 0.05). Experiment was performed three times with similar results obtained among each trial.
CysBA227D promotes growth of V. fischeri on cystine
Our results with the cysBA227D mutant suggest that CysBA227D promotes some expression of cys genes. To determine whether CysBA227D enables growth on various sulfur sources, we examined the growth yield of the cysBA227D mutant using the panel of sulfur compounds that wild-type cells can utilize (Fig. 1). Similar to the ΔcysB mutant, the cysBA227D mutant could grow on cysteine and glutathione, but not on thiosulfate or sulfate (data not shown and Fig. 6A), with the latter result suggesting that the cys genes are not expressed sufficiently in this mutant for sulfate assimilation. However, the cysBA227D mutant did grow on 0.5 mM cystine (Fig. 6A), which suggests that the CysB regulon was sufficiently induced for growth on this organic sulfur source. The cysBA227D mutant grew similarly to wild type for cystine levels of at least 166 μM (Fig. 6A), and significant growth of the cysBA227D could be detected at 6 μM (Fig. 6A). In an attempt to determine how V. fischeri acquires extracellular cystine, genes predicted to encode factors involved in cystine transport were targeted for deletion. In E. coli, deletion of tcyJ, which encodes a periplasmic cystine-binding protein, and tcyP, which encodes a symporter with lower affinity for cystine, results in a growth defect on cystine (Chonoles Imlay et al., 2015). However, a V. fischeri mutant containing deletion alleles for both genes was able to grow on cystine (Fig. S5), suggesting that V. fischeri has an additional mechanism to access environmental cystine.
Fig. 6. Impact of cysBA227D on growth of V. fischeri in cystine.
A. Growth yield of V. fischeri strains after 21 h of incubation in sulfate-replete media supplemented with the indicated level of cystine. Strains tested were WT = TIM313 (white), cysB+ = TIM409 (gray), and cysBA227D = TIM411 (black). Each bar represents the mean growth yield (n = 3), and error bars represent the standard deviation. Two-way ANOVA revealed significant differences among means due to cystine concentration (F5,36 = 160.9, p < 0.0001), genotype (F2,36 = 1474, p < 0.0001), and their interaction (F10,36 = 296.6, p < 0.0001). A Dunnett’s post-hoc test was performed to statistically compare the means of each mutant to wild type for each concentration of cystine, with p-values adjusted for multiple comparisons (**** = p < 0.0001; comparisons with p ≥ 0.05 are considered not significant and are not labeled). Experiment was performed three times with similar results obtained among each trial.
B. Expression of cysK in indicated V. fischeri strains grown in low (18 μM) or high (500 μM) concentrations of cystine. Strains tested were same as in panel A but harbored PcysK reporter plasmid pVF_1893P. Gene expression was determined by normalizing the GFP fluorescence with OD600. Each point represents an independent biological replicate, and the bar represents the mean (n = 3). Two-way ANOVA revealed significant differences among means due to cystine concentration (F1,12 = 136.8, p < 0.0001), genotype (F2,12 = 25.69, p < 0.0001), and their interaction (F2,12 = 32.53, p < 0.0001). A Sidak’s post-hoc test was performed to statistically compare for each strain the means between cystine concentrations, with p-values adjusted for multiple comparisons (**** = p < 0.0001; comparisons with p ≥ 0.05 are considered not significant and are not labeled). Experiment was performed three times with similar results obtained among each trial.
Based on the growth yields described above, we hypothesized that transcriptional induction of cys genes by CysB is necessary for normal growth on cystine levels below 166 μM. To test this hypothesis, we examined cysK expression in cells grown in sulfate-replete medium containing 18.5 μM (low) and 500 μM (high) cystine. Under conditions of low cystine, wild-type levels of cysK expression were high (Fig. 6B), consistent with cells having exhausted cystine and then growing by assimilating sulfate. The level of cysK expression was 24.3 ± 4.7 times lower in media supplemented with high cystine (Fig. 6B), suggesting that transcriptional induction by CysB is lower in this condition. This result is consistent with the reduction of cystine to two molecules of cysteine within the cytoplasm that promotes feedback inhibition on CysE and, consequently, lower levels of NAS inducer. In the cysBA227D mutant, the level of cysK expression decreased only 1.3-fold between the conditions of low and high cystine concentrations (Fig. 6B), suggesting that CysB mediates the transcriptional response of cysK to cystine. In addition, the level of cysK expression in the cysBA227D mutant was lower than that of wild type for 18.5 μM cystine but higher for 500 μM (Fig. 6B), suggesting that CysBA227D induces expression of cys genes to an intermediate level. Taken together with the growth yield experiments described above, these results suggest that the induction of the CysB regulon by the CysBA227D variant is sufficient to support growth on cystine but not sulfate.
The ability of the cysBA227D mutant to grow on cystine but not sulfate (Fig. 6A) provided a tool to investigate whether such growth properties contribute to symbiosis establishment. Animals exposed to the cysBA227D mutant showed luminescence and CFU levels comparable to animals colonized by the wild-type strain (Figs. S6A–B), suggesting that the CysBA227D variant enables the V. fischeri cells to grow within the light organ. The experimental setup associated with this result is also noteworthy because like the ΔcysB mutant, the cysBA227D mutant decreases in abundance within FSSW (data not shown); however, increasing the initial level of the cysBA227D mutant was unnecessary for the strain to establish symbiosis. These results suggest that V. fischeri can grow in vivo on a sulfur source aside from sulfate in a manner that depends on CysB.
Differential expression of PcysK within the light organ
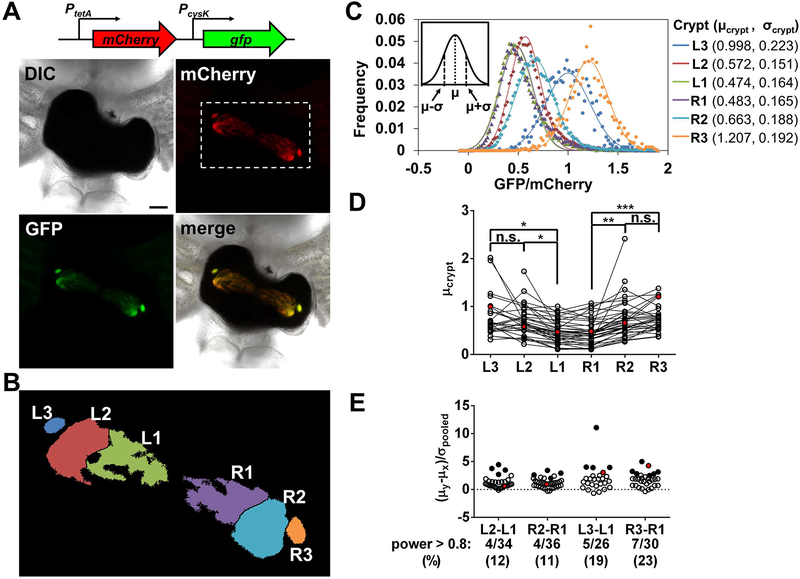
The findings above suggest that CysB promotes growth of V. fischeri on a sulfur source other than sulfate in vivo. Because CysB functions as a transcription factor, a prediction of this model is that CysB-regulated genes will be transcriptionally induced within the host. To test this prediction, the fluorescence-based reporter system for promoter activity (Fig. 4B) was used to quantify gene expression levels of the V. fischeri populations occupying the various crypt spaces within the light organ, in a manner similar to a previous report (Sun et al., 2015). The pTM267 vector used to clone the reporter constructs also contains mCherry downstream of a copy of the tetA promoter (Fig. 7A), which is expressed in V. fischeri (Miyashiro et al., 2010). The mCherry fluorescence provides both a means to locate the populations of V. fischeri and to normalize the corresponding levels of GFP fluorescence in vivo (Sun et al., 2015), which may be affected by factors other than promoter activity, such as population size.
Fig. 7. Impact of location on expression of cysK for V. fischeri populations in vivo.
A. Above, reporter construct in plasmid pVF_1893P for measuring cysK expression in vivo. Not shown are two transcriptional terminators that are present downstream of mCherry. Below, images of a light organ from an experiment with juvenile squid (n = 37) exposed to an inoculum containing the WT ES114 strain harboring the PcysK reporter plasmid pVF_1893P. Scale bar = 100 μm.
B. Regions of interest corresponding to the individual populations that are each labeled according to light organ side (L or R) and crypt type (1–3). The example corresponds to the region indicated by the dotted box in the mCherry panel in A.
C. Histograms of GFP/mCherry values for pixels of individual populations identified in B. Each distribution (points) is fitted by a Gaussian curve (line), which yields parameters μ and σ (inset). Parameter values for each infection are shown to the right.
D. Levels of cysK expression for individual populations. Each point represents μcrypt for an individual population. Lines connecting points indicate populations were within the same animal. Statistical analysis by a Kruskal-Wallis test found significant differences in mean ranks (H = 34.49, 5 d.f., p < 0.0001). A Dunn’s post-hoc analysis was performed to statistically compare the mean ranks between crypt spaces, with p-values adjusted for multiple comparisons (n.s. = not significant, * = p < 0.05, ** = p < 0.01, *** = p < 0.001). Not shown for clarity: L3-R3, L2-R2, and L1-R1, which were n.s., and comparisons between populations located in crypts on different sides of the light organ, which were similar to same-side comparisons. Red points represent the populations within the light organ shown in A.
E. Comparisons of cysK expression distributions between populations within individual light organs. Each point represents the standardized mean difference between the indicated populations. Closed symbols represent comparisons that are significant (α = 0.05, power > 0.8). Red points represent comparisons involving the populations within the light organ shown in A.
Experiment was performed twice with similar results obtained from both trials.
Initial experiments revealed that animals exposed to an inoculum of wild-type cells harboring the PcysK reporter plasmid frequently exhibited higher levels of GFP fluorescence in the V. fischeri populations located within the crypt spaces further from the midline of the light organ, i.e., Crypts 2 and 3 (Fig. 7A), which suggests that PcysK is expressed to levels higher within those crypt spaces relative to populations within Crypt 1. Because our previous method for quantifying gene expression averaged across all of the bacteria throughout the light organ (Sun et al., 2015), we modified this method to quantify gene expression within individual populations (Figs. 7B–C). First, to locate each V. fischeri population within an image set, the region of mCherry-positive pixels was labeled according to its corresponding crypt space (Fig. 7B). For each pixel within the region, the corresponding GFP and mCherry fluorescence levels were used to calculate a GFP/mCherry fluorescence ratio, which yielded a distribution of ratios that represents the expression of cysK throughout that population (Fig. 7C). Each distribution exhibited a single peak that was then fit to a Gaussian curve described by the parameters μcrypt and σcrypt, which correspond to the peak position and spread of GFP/mCherry ratios, respectively (Fig. 7C, inset) and could be used to compare distributions.
For each crypt type, the μcrypt values for cysK expression among the group of animals did not follow a normal distribution (Fig. 7D, Shapiro-Wilk, α = 0.05, p > 0.05), suggesting that cysK is expressed to different levels in the populations that were established within the animal group. Despite this variability among animals, differences were detected within the group between the μcrypt values of populations within Crypt 1 relative to populations in Crypts 2 and 3 (Fig. 7D). In contrast to PcysK, the μcrypt values for animals colonized with wild-type cells harboring the PtetA reporter exhibited normal distributions for each crypt type across the group and no difference between crypts (Fig. S7), suggesting that the observed differences in PcysK expression are specific to that promoter and that the ratio calculations are not intrinsically noisy. To further characterize this pattern of gene expression, we investigated the difference between these crypt types in individual animals by calculating 1) the magnitude of separation between the peaks of each distribution (the standardized mean difference or (μy-μx)/σpooled), and 2) the extent to which the distributions are separate (power). In the latter analysis, given two Gaussian distributions (X and Y, with μy > μx), the differences between X and Y are deemed significant if at least 80% of Y is greater than 95% of X, i.e., power > 0.8 at α = 0.05. For the light organ shown in Fig. 7A, the standardized mean difference between the GFP/mCherry distributions for R3 and R1 is 4.27 with a power of 0.99 (Fig. 7D), suggesting that cysK expression is significantly higher in R3 than in R1. Using this analysis, we observed that 32% of squid (12/37) had at least one population within Crypt 2 or Crypt 3 exhibiting higher levels of cysK expression relative to the population in Crypt 1 (Fig. 7E). Over time, this pattern of differential expression among populations becomes more frequent and exhibits larger differences between crypt spaces (Fig. S8), suggesting that the differences between crypt spaces increases as the symbiosis matures. In addition, the frequency of the expression pattern and the differences in expression were lower in animals exposed to an inoculum at a later time point (Fig. S9), suggesting that the duration of the symbiotic associations is a factor in the development of the pattern of PcysK expression in vivo. Finally, we observed the expression patterns for other CysB-regulated genes are similar to that of PcysK (Fig. S10), suggesting that the crypt environments vary in a manner that impacts how CysB regulates gene expression.
Impact of CysB on gene expression in vivo
Our results described above suggest that CysB-regulated genes are differentially regulated among the crypts spaces within the light organ. To test whether this pattern of gene expression depends on CysB, the expression of PcysK was examined in animals colonized by the cysBA227D mutant. At 72 h p.i., the frequency of cysBA227D populations for each crypt type was comparable to that for the WT strain (Fig. S11), suggesting that the altered regulation of CysB-regulated genes by CysBA227D did not impair the ability of V. fischeri to first access and then grow within each crypt type. Animals containing WT or cysB+ cells generally exhibited higher activity of the cysK promoter in Crypts 2 and 3 relative to Crypt 1 (Fig. 8A–B), which is consistent with the observed differential expression of cysK described above. In contrast, cysK expression in cysBA227D populations within Crypts 2 and 3 were comparable to Crypt 1 (Fig. 8C), suggesting that the ability of CysB to regulate transcription is necessary for the pattern of differential expression of cys genes among the light organ populations. Comparison of the levels of PcysK expression across groups according to crypt type revealed that cysBA227D populations exhibit lower PcysK expression than wild-type for populations within Crypts 2 and 3, but higher expression of PcysK in Crypt 1 (Figs. 8A–C). These results suggest that CysB induces cys gene expression in the populations within the distal crypts but does not induce expression of cys genes in Crypt 1 populations.
Fig. 8. Impact of CysB on expression of cysK in the light organs.
Values of μcrypt for GFP/mCherry distributions (left) and comparisons of GFP/mCherry distributions (right) at 72 h for animals colonized by the indicated strain harboring the cysK reporter plasmid. Each experiment involved three groups of animals (n = 24–28) exposed to the indicated strain. Data were analyzed statistically as described in Fig. 7 legend. For data shown in the left panels, the Kruskal-Wallis test results are listed below and a Dunn’s post-hoc analysis was performed to statistically compare the mean ranks between crypt spaces, with p-values adjusted for multiple comparisons (n.s. = not significant, *** = p < 0.001, **** = p < 0.0001). In right panels, closed symbols represent comparisons that are significant (α = 0.05, power > 0.8). Experiment was performed twice with similar results obtained among both trials.
A. WT = TIM313. (H = 79.54, d.f. = 5, p < 0.0001)
B. cysB+ = TIM409. (H = 75.36, d.f. = 5, p < 0.0001)
C. cysBA227D = TIM411 (H = 14.72, d.f. = 5, p = 0.0116)
DISCUSSION
The fitness of a horizontally transmitted microbe in vivo depends on its access to nutrients within host-associated niches. In this study, the symbiotic bacterium V. fischeri was used to explore the molecular mechanisms to acquire sulfur for growth during the initial colonization of the squid light organ. While V. fischeri can grow on multiple sulfur sources (Fig. 1), the ability to grow on cystine was associated with establishing symbiosis (Figs. 6A & S6). This growth property is controlled by the transcription factor CysB (Fig. 6A), which, through gene regulation, is predicted to modulate the intracellular cysteine pools that serve as the primary source of sulfur for anabolic processes (Kredich, 1996). Furthermore, growth on cystine results in decreased expression of cysteine biosynthesis genes relative to sulfate conditions (Fig. 6B), consistent with cells lowering de novo synthesis of cysteine due to the availability of a compound that can contribute to cysteine pools within the cytoplasm. The finding of different levels of transcriptional induction by CysB among the populations that occupy different crypt types (Fig. 8) suggests that V. fischeri modulates cysteine biosynthesis in accordance with the availability of nutrients within each colonization site in vivo.
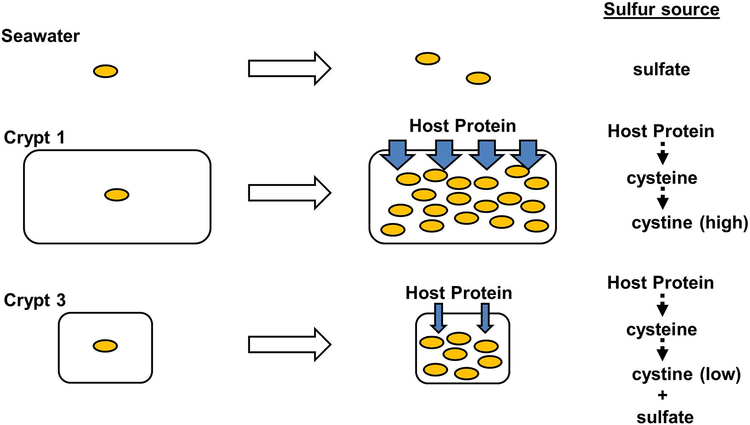
The results obtained during this study have led to the model for acquisition of sulfur by V. fischeri during symbiosis establishment proposed in Fig. 9. While outside of the host, V. fischeri grows on sulfate that is readily available in seawater. This sulfur is converted into an organic form through the biosynthesis of cysteine, which is subsequently available for anabolism. However, when a V. fischeri cell enters a crypt space within the light organ, it gains access to host-derived proteins. Proteolysis of these proteins releases a plethora of amino acids into the crypt spaces. Cleaved cysteine residues autoxidize; resulting in the formation of cystine that is sufficient for CysB-dependent growth of V. fischeri within the crypt space. While V. fischeri may grow on the same type of sulfur source within every crypt space (i.e., host-derived proteins), its availability appears to vary among each type of crypt space, with the lowest concentration present in Crypt 3. Growth on these host-derived nutrients within the crypt spaces establishes the populations that produce bioluminescence during symbiosis.
Fig. 9. Model of sulfur acquisition by V. fischeri during symbiosis.
Growth of V. fischeri in seawater (top) is restricted to sulfate assimilation. For cells that enter Crypt 1, growth is mediated by host-derived compounds, e.g., host protein. Upon degradation of these proteins, cysteine residues are released and undergo auto-oxidation to cystine, which V. fischeri uses as a sulfur source. In Crypts 2 and 3, the concentration of bioavailable cystine is lower, which results in V. fischeri satisfying its sulfur requirements for growth through sulfate assimilation.
The solubility of cystine at physiological ranges of pH suggest it is possible for cystine to be available within the crypt spaces. While the compounds that specifically facilitate V. fischeri growth in vivo are unknown, proteomic analysis of the crypt contents in light organs of adult animals has revealed that V. fischeri is exposed to numerous host proteins in vivo (Schleicher and Nyholm, 2011), which may serve as a source of peptides and amino acids for bacterial growth. A particularly intriguing candidate is hemocyanin, which is a metalloprotein that is expressed in the gills and hypothesized to shuttle oxygen from the gills to the light organ crypt spaces (Kremer et al., 2014), where oxygen serves as a substrate for the light-producing enzyme luciferase. The hemocyanin isoforms of E. scolopes contain numerous cysteines that are predicted to form at least 22 disulfide bonds that contribute to the structure necessary for oxygen carriage (Kremer et al., 2014). If released through degradation and then auto-oxidized, these cysteine residues may serve as an abundant source of sulfur for bacterial growth in vivo. Alternatively, other host-derived factors, such as sulfated glycoproteins or small molecules, may also contribute to the growth of V. fischeri within the light organ. The results presented here warrant future investigation into the growth of V. fischeri on particular host-derived compounds found in the crypt spaces.
Elucidating the mechanisms that enable V. fischeri to grow on cystine in vitro will likely provide insight into the sulfur sources that promote V. fischeri growth within the crypt spaces. The disruption of two genes predicted to each facilitate cystine transport failed to prevent growth of V. fischeri on cystine (Fig. S5), suggesting that V. fischeri has at least one other mechanism for uptake of environmental cystine. Another direction for future studies includes determining the extent to which V. fischeri actively degrades compounds within the host environment to release nutrients for growth. V. fischeri encodes multiple aminopeptidases that may contribute to the degradation of host proteins, with PepN responsible for the majority of aminopeptidase activity observed in vitro (Fidopiastis et al., 2012). This aminopeptidase activity was primarily associated with cells rather than cell-free supernatant (Fidopiastis et al., 2012), suggesting that PepN may contribute to breaking down peptides that have been taken up by the cells expressing PepN. Within the squid, the abundance of a pepN− mutant is initially reduced relative to wild-type cells but then achieves normal levels by 24 h p.i. (Fidopiastis et al., 2012), suggesting PepN activity may contribute to growth in vivo. Four other aminopeptidases are upregulated by 3-oxo-C6 HSL (Antunes et al., 2007), which promotes bioluminescence production by V. fischeri populations within the squid light organ. The expression of these genes through quorum sensing may permit V. fischeri cells to coordinate the expression of aminopeptidases with cell density within each crypt space, thereby optimizing the release of amino acids from host-derived proteins for the amount of cells present within a population. Future studies are necessary to examine any link between aminopeptidase activity of V. fischeri and the response of CysB in vivo.
The striking heterogeneity in the expression of CysB-regulated genes observed for populations in different crypt types provides further evidence that the specific environment experienced by a V. fischeri population depends on the particular crypt that is colonized. Recently, a fluorescence-based reporter derived from PhoB-regulated genes was used as a biosensor for phosphate availability in vivo (Stoudenmire et al., 2018). Fluorescence-based microscopy of light organs colonized by V. fischeri harboring this reporter revealed heterogeneity in expression i) among animals, ii) among crypt spaces in the same animal, and iii) even across individual populations, suggesting that phosphate availability in vivo follows a complex spatiotemporal profile that may be linked to host development. The inferred variation in phosphate within individual populations was not observed for the conditions that stimulate CysB-dependent gene expression, suggesting that the spatial profiles for each process are different. Variation among the crypt spaces impacts the population structure during symbiosis establishment, as populations of non-luminescent strains that initially form in Crypt 1 fail to be maintained (Verma and Miyashiro, 2016). The specific conditions within each crypt type may provide selective pressure that contributes to the genetic variability observed among strains isolated from wild-caught animals (Sun et al., 2016; Wollenberg and Ruby, 2009). Further characterization of the habitat environments within the light organ will enable the testing of hypotheses associated with the adaptation of particular strains in vivo.
This study included the quantification of gene-expression profiles of individual populations in situ. CysB is conserved in other bacteria, including the pathogen Pseudomonas aeruginosa, which was recently shown to use it to regulate transcription of the PqsR receptor that controls expression of virulence factors (Farrow et al., 2015). Further investigation into the CysB regulon of P. aeruginosa may reveal the extent to which cellular responses to sulfur availability impacts its pathogenicity. More generally, approaches based on the biogeography of bacteria within the host will be important for uncovering the mechanisms that impact microbial fitness within host-associated niches. For instance, investigation of Vibrio cholerae cells within the infant-mouse infection model revealed the presence of microcolonies localized to the crypt spaces of proximal small intestine (Millet et al., 2014). However, within the distal region of the small intestine, the cells were not limited only to the crypt spaces. Learning how V. cholerae grows within these different sites may lead to the development of novel therapeutics designed to specifically target V. cholerae within the host. The exclusive nature of the squid-Vibrio association and the well-defined niches for bacterial populations within the light organ have led to the discoveries that the ability to grow on cystine is an important trait for V. fischeri to grow in vivo and that the host-derived sulfur source varies among colonization sites. These findings will serve as the foundation for future investigations into the mechanisms bacterial symbionts use to grow within their hosts.
EXPERIMENTAL PROCEDURES
Media and growth conditions.
V. fischeri strains were grown aerobically at 28°C in LBS medium [1% (wt/vol) tryptone, 0.5% (wt/vol) yeast extract, 2% (wt/vol) NaCl, 50mM Tris-HCl (pH 7.5)], defined-minimal medium (DMM) [50 mM MgSO4, 10 mM CaCl2, 300 mM NaCl, 10 mM KCl, 0.0058% (wt/vol) K2HPO4, 10 μM FeSO4, 50 mM Tris-HCl (pH 7.5)] containing 10 mM GlcNAc, or sulfur-free DMM [50 mM MgCl2, 10 mM CaCl2, 300 mM NaCl, 10 mM KCl, 0.0058% (wt/vol) K2HPO4, 10 μM FeCl3, 50 mM Tris-HCl (pH 7.5)] containing 10 mM GlcNAc. To maintain plasmids, chloramphenicol was added to the medium at a final concentration of 2.5 μg ml−1. Strains that are cysteine auxotrophs were propagated with 1 mM cysteine.
Strains.
All V. fischeri strains used in this study are listed in Table 1 and were derived from ES114 (Ruby et al., 2005).
Table 1.
Strains and plasmids used in this study
| Strains | Genotype | Reference |
|---|---|---|
| ES114 | wild-type V. fischeri | (Ruby et al., 2005) |
| SCV001 | ES114 ΔtcyJ | This study |
| NPW003 | ES114 ΔcysB | This study |
| NPW056 | ES114 ΔtcyJ ΔtcyP | This study |
| EVS102 | ES114 ΔluxCDABEG | (Bose et al., 2008) |
| KL173 | ES114 cysD::Tn5 | This study |
| KL174 | ES114 cysK::Tn5 | This study |
| KL175 | ES114 cysJ::Tn5 | This study |
| TIM313 | ES114 Tn7::[erm] | (Miyashiro et al., 2010) |
| TIM409 | ES114 ΔcysB Tn7::[cysB erm] | This study |
| TIM410 | ES114 ΔcysB Tn7::[erm] | This study |
| TIM411 | ES114 ΔcysB Tn7::[cysBA227D erm] | This study |
| Plasmids | Genotype | Reference |
| pEVS79 | pBC SK (+) oriT cat | (Stabb and Ruby, 2002) |
| pEVS104 | R6Kori RP4 oriT trb tra kan | (Stabb and Ruby, 2002) |
| pEVS107 | R6Kori oriT mini-Tn7 mob erm kan | (McCann et al., 2003) |
| pTM267 | pVSV105 PtetA-mCherry kan::gfp | (Miyashiro et al., 2010) |
| pLosTfoX | pEVS79 tfoX | (Pollack-Berti et al., 2010) |
| pTM392 | pEVS79 ΔtcyJ | This study |
| pNW008 | pEVS79 ΔydjN | This study |
| pTM417 | pEVS79 ΔcysB | This study |
| pTM420 | pEVS107 cysB | This study |
| pTM423 | pEVS107 cysBA227D | This study |
| pSCV26 | pTM267 Δkan PtetA-gfp | This study |
| pUX-BF13 | R6Kori tns bla | (Bao et al., 1991) |
| pVF_0008P | pTM267 Δkan PtcyJ-gfp | This study |
| pVF_0310P | pTM267 Δkan PcysJ-gfp | This study |
| pVF_0320P | pTM267 Δkan PcysD-gfp | This study |
| pVF_1893P | pTM267 Δkan PcysK-gfp | This study |
| pVSV105 | R6Kori ori(pES213) RP4 oriT cat | (Dunn et al., 2006) |
Construction of deletion alleles:
The deletion alleles ΔcysB, ΔtcyJ, and ΔtcyP were individually constructed by amplifying from ES114 genomic DNA by PCR ~1.5 kb on either side of the corresponding gene and cloning the products into pEVS79 to yield plasmids pTM417, pTM392, and pNW008, respectively. Primers and restriction sites are listed in Table 2. To construct a mutant with a particular deletion allele, the corresponding plasmid containing the deletion allele was introduced into a target strain by conjugation using pEVS104 (Stabb and Ruby, 2002) and screening for a double-crossover event, as described elsewhere (Miyashiro et al., 2010).
Table 2.
Primers used in this study
| Primer name | Sequence (5’ -> 3’) |
|---|---|
| Deletion alleles | |
| ΔcysB | |
| ΔcysB-5-SalI-u | GGGTCGACGGATCAGCGTGGTGTGCCTTATCA |
| ΔcysB-5-XbaI-l | GCTCTAGACTCAGCAGTCGATGATACATTCAA |
| ΔcysB-3-XbaI-u | GCTCTAGATAAGATCGCCCTGATATTATTTTC |
| ΔcysB-3-SacI-l | GGGAGCTCGGTATGTTCTCTTTCTCTGGTCTT |
| ΔtcyJ | |
| ΔtcyJ-5-XbaI-u | GGTCTAGAGTTCCTTGTTCGACGATCTTTCCA |
| ΔtcyJ-5-XmaI-l | GGCCCGGGGATTACTCACCATTCATCAAATAA |
| ΔtcyJ-3-XmaI-u | GGCCCGGGGTATTAGGATGATACTTTTTTTGC |
| ΔtcyJ-3-EcoRV-l | GGGATATCGCATTCGCAATAACACGATTTAAT |
| ΔtcypP | |
| ΔtcypP-5-SalI-u | GGGTCGACGATTACCTAACACCCAAATATCAT |
| ΔtcypP-5-HindIII-l | CCGAAGCTTTACGTTGCTGTGTATTAATAAAAAAG |
| ΔtcypP-3-HindIII-u | GGTAGAAAGCGCACAAGCTTAAT |
| ΔtcypP-3-SacI-l | GGGAGCTCGTGCTATACGAGATGTTATTACTC |
| cysB alleles | |
| cysB+ | |
| cysB+-KpnI-u | GGGGTACCGTAGTAATATTTTCCAGTGTAACA |
| cysB+-SpeI-l | GGACTAGTGGTCTATATTTAAAAACTGCTGAG |
| cysBA227D | |
| cysBA227D-u | GTCTTTACCGCAACCGATGATGATGTCATTAAGACTTATG |
| cysBA227D-l | CATAAGTCTTAATGACATCATCATCGGTTGCGGTAAAGAC |
| promoter reporters | |
| PtcyJ-XmaI-u | GGCCCGGGGAAGCCAAAGCGATGAAGACACGG |
| PtcyJ-XbaI-l | GGTCTAGAGCGAGGATATTCTTCGTCCATTTC |
| PcysD-XmaI-u | GGCCCGGGAGAGGTTCATAGTGTGTCTCCAAA |
| PcysD-XbaI-l | GGTCTAGATTGCTGAAGGTGGGTCAATCGTTT |
| PcysJ-XmaI-u | GGCCCGGGGGTAAGAATCAACGAATAAACGAT |
| PcysJ-XbaI-l | GGTCTAGAGAGAGTTCCTTTAATAACATGACG |
| PcysK-XmaI-u | GGCCCGGGGATGTTTACCGTGATACACATAAA |
| PcysK-XbaI-l | GGTCTAGAGAGAATTATCTTCGTAAATCTTTG |
| PtetA-XmaI-XbaI-u | CCGGGTTGACACTCTATCATTGATAGAGTTATTTTACCACTCGCT |
| PtetA-XmaI-XbaI-l | CTAGAGCGAGTGGTAAAATAACTCTATCAATGATAGAGTGTCAACTC |
Construction of cysB+ and cysBA227D alleles:
To generate the cysB+ strain TIM409, the gene VF_1490 was amplified with its promoter region by PCR from ES114 genomic DNA using the primers indicated in Table 2. The resulting amplicon was cloned into pCR-Blunt (Life Technologies, Carlsbad, CA, USA) and then sub-cloned using KpnI/SpeI restriction sites into pEVS107 to yield pTM420. The cysB+ allele was integrated into the large chromosome of the ΔcysB strain NPW003 at the Tn7 site as described elsewhere (Miyashiro et al., 2011). To generate the cysBA227D allele, the pCR-Blunt clone containing the cysB+ allele was used as a template for PCR-based, site-directed mutagenesis with the primer set listed in Table 2. The reaction contained PFU Ultra DNA Polymerase (Agilent Technologies, Santa Clara, CA) with 0.2 mM dNTPs, 125 ng each primer, and 20 nM plasmid template. Amplification was performed in a C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA) with the following steps: 1) 95°C/30 s, 2) 95°C/30 s, 3) 55°C/1 m, 4) 68°C/ 10 m, and 5) 18 cycles of steps 2–4. The PCR product was digested with 10 U DpnI overnight and transformed into Top10 by electroporation. The resulting cysBA227D was sub-cloned into pEVS107 to yield pTM423 and then integrated into the Tn7 site of NPW003 as described above.
Construction of cys Tn5-insertion mutants:
The cysD::Tn5, cysK::Tn5, and cysJ::Tn5 alleles were obtained as part of a different study involving a Tn5-based screen designed to identify mutants with elevated levels of GFP in colonies using the PtcyJ reporter plasmid pVF_0008P (Wasilko NP and Miyashiro T, unpublished). To construct mutants with clean genetic backgrounds, the alleles were introduced into ES114 using a protocol using pLosTfoX (Pollack-Berti et al., 2010), which overexpresses a regulator that enables introduction of markers into strains by natural transformation (Miyashiro et al., 2014).
Promoter reporter plasmids.
To construct reporter plasmids, the promoter regions were amplified from ES114 genomic DNA by PCR using the corresponding primer sets listed in Table 2. The products were cloned into pCR-Blunt, verified by sequencing, and isolated via digestion with XmaI/XbaI. The resulting products were subcloned into the vector fragment of pTM267 (Miyashiro et al., 2010) that was digested with XmaI/XbaI. For constructing pSCV26, which contains the tetA promoter upstream of gfp, the oligonucleotides tetA-XmaI-XbaI-u/-l were initially heated at 95°C for 2 min and then cooled to room temperature. The ends were phosphorylated by polynucleotide kinase (New England Biolabs, Ipswich, MA, USA) and sub-cloned into pTM267 as described above.
Growth yield assays.
Overnight cultures were diluted 1:100 into LBS and incubated aerobically with shaking at 28°C. After 2 h, cells were normalized to OD600 = 1 and a 1-ml sample was centrifuged at 15,000 x g. After 2 min, the supernatant was removed, and the pellets were washed twice with 1 ml DMM (or sulfur-free DMM for assays requiring a negative control for supplementation with a sulfur source). The washed cells were diluted 1:100 into the indicated medium and incubated aerobically with shaking at 28°C. After 21 h, the OD600 of each culture was measured with a BioPhotometer Plus (Eppendorf, Hamburg, Germany).
Culture-based gene expression assay.
For gene expression measurements, cultures grown in LBS overnight were diluted 1:100 into fresh LBS medium and grown aerobically with shaking at 28°C. At OD600 = 1.0, cultures were diluted into DMM supplemented with the indicated sulfur source and grown aerobically with shaking at 28°C. At OD600 = 1.0, 1 ml samples were quickly cooled on an ice-slurry. Cells were pelleted by centrifugation at 4°C for 5 min at 15,000 x g and re-suspended in 350 μl of cold DMM. For each sample, three 100 μl technical replicates were measured with a Tecan M1000Pro fluorescence plate reader (Tecan Group, Mannedorf, Switzerland) for OD600 and green fluorescence (488 ± 5nm excitation/509 ± 5 nm emission). Expression levels were determined by normalizing each green fluorescence measurement by the corresponding OD600 measurement. The background fluorescence associated with the non-fluorescent strain pVSV105/ES114 grown in parallel was subtracted from each expression level prior to calculating fold changes between samples.
Squid colonization assays.
To initiate squid colonization assays, freshly hatched juvenile squid were introduced into plastic tumblers containing filter-sterilized Instant Ocean seawater (FSSW) with inoculums ranging from 3,000–10,000 CFU/ml. At indicated time points, animals were washed by transferring to fresh FSSW. Animal luminescence was measured using a GloMax 20/20 luminometer (Promega, Madison, WI, USA). To determine bacterial abundance in FSSW, samples were serially diluted and plated onto LBS medium supplemented with cysteine. The corresponding CFU counts were used to calculate strain abundance. To determine abundance of bacteria within squid, juvenile squid were first frozen at −80°C for at least 24 h and then homogenized. The homogenate was serially diluted for determining CFU counts as described above. For assays involving fluorescence microscopy, animals were first transferred as a group to a vial on ice. After 10 min, the anesthetized animals were fixed by exposing the animal group to cold 4% paraformaldehyde/marine phosphate buffered saline (mPBS). After 24 h, animals were washed exhaustively in mPBS, and their mantles were dissected to reveal the light organ for microscopy. While the Pennsylvania State University does not require IACUC approval for invertebrate research, the anesthetization steps described above take into account recommendations for humane care and use of laboratory animals.
To quantify fluorescence levels within host-associated populations, light organs were imaged by fluorescence microscopy using a Zeiss 780 confocal microscope (Carl Zeiss AG, Jena, Germany) equipped with a 10x water lens and confocal pinholes set at maximum to mimic quantitative epi-fluorescence conditions. DIC, GFP, and mCherry 12-bit images were collected and processed using the following image-analysis protocol. To identify the location of bacterial populations within the image sets, the mCherry fluorescence images were subjected to thresholding in ImageJ software, version 1.47 (NIH). Populations were defined as particles larger than 100 pixels, and when necessary, a 3-pixel-wide line was drawn to distinguish the infections. For each infection, the GFP and mCherry fluorescence levels of the corresponding pixels were determined by subtracting the background fluorescence associated with host tissue using custom Matlab scripts, version R2013a (MathWorks Inc., Natick, MA, USA). These fluorescence levels were used to calculate the GFP/mCherry fluorescence ratio for each pixel in the defined regions. The subset of GFP/mCherry fluorescence ratios within the average ± 3 standard deviations were fit to a Gaussian model where a, b, and c are the amplitude, centroid, and related to peak width, respectively, that are determined from the fitting function. The peak location (μcrypt) is equal to b, and the spread (σcrypt) was calculated as
Statistical analyses.
Statistical analyses were performed using Prism software (Graphpad, La Jolla, CA, USA).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grant R00 GM097032 (to T.M.) and the Pennsylvania State University Eberly College of Science. The funders had no role in study, design, data collection, and interpretation, or the decision to submit the work for publication. We thank Kyle LaPenna for generating the strains KL173, KL174, and KL175 and members of the Miyashiro lab for constructive criticism of this project. We thank the Statistical Consulting Center at Penn State University for offering advice on statistical analyses.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
REFERENCES
- Antunes LC, Schaefer AL, Ferreira RB, Qin N, Stevens AM, Ruby EG and Greenberg EP (2007). Transcriptome analysis of the Vibrio fischeri LuxR-LuxI regulon. J Bacteriol, 189(22), 8387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Lies DP, Fu H and Roberts GP (1991). An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene, 109(1), 167–8. [DOI] [PubMed] [Google Scholar]
- Bose JL, Rosenberg CS and Stabb EV (2008). Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch Microbiol, 190(2), 169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonoles Imlay KR, Korshunov S and Imlay JA (2015). Physiological Roles and Adverse Effects of the Two Cystine Importers of Escherichia coli. J Bacteriol, 197(23), 3629–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Millikan DS, Adin DM, Bose JL and Stabb EV (2006). New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl Environ Microbiol, 72(1), 802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow JM 3rd, Hudson LL, Wells G, Coleman JP and Pesci EC (2015). CysB Negatively Affects the Transcription of pqsR and Pseudomonas Quinolone Signal Production in Pseudomonas aeruginosa. J Bacteriol, 197(12), 1988–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidopiastis PM, Rader BA, Gerling DG, Gutierrez NA, Watkins KH, Frey MW, Nyholm SV and Whistler CA (2012). Characterization of a Vibrio fischeri aminopeptidase and evidence for its influence on an early stage of squid colonization. J Bacteriol, 194(15), 3995–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF, Bosch TC and Ledon-Rettig C (2015). Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat Rev Genet, 16(10), 611–22. [DOI] [PubMed] [Google Scholar]
- Graf J and Ruby EG (1998). Host-derived amino acids support the proliferation of symbiotic bacteria. Proc Natl Acad Sci U S A, 95(4), 1818–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W and Berens C (1994). Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu Rev Microbiol, 48(345–69. [DOI] [PubMed] [Google Scholar]
- Jones BW and Nishiguchi MK (2004). Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca:Cephalopoda). Mar. Biol, 144(1151–1155. [Google Scholar]
- Kredich NM (1992). The molecular basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol Microbiol, 6(19), 2747–53. [DOI] [PubMed] [Google Scholar]
- Kredich NM (1996). Biosynthesis of Cysteine In Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, and Umbarger HE (eds). Washington, D. C.: American Society for Microbiology, pp. 514–527. [Google Scholar]
- Kredich NM and Tomkins GM (1966). The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem, 241(21), 4955–65. [PubMed] [Google Scholar]
- Kremer N, Schwartzman J, Augustin R, Zhou L, Ruby EG, Hourdez S and McFall-Ngai MJ (2014). The dual nature of haemocyanin in the establishment and persistence of the squid-vibrio symbiosis. Proc Biol Sci, 281(1785), 20140504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochowska A, Iwanicka-Nowicka R, Plochocka D and Hryniewicz MM (2001). Functional dissection of the LysR-type CysB transcriptional regulator. Regions important for DNA binding, inducer response, oligomerization, and positive control. J Biol Chem, 276(3), 2098–107. [DOI] [PubMed] [Google Scholar]
- McCann J, Stabb EV, Millikan DS and Ruby EG (2003). Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl Environ Microbiol, 69(10), 5928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai MJ (2014). The importance of microbes in animal development: lessons from the squid-vibrio symbiosis. Annu Rev Microbiol, 68(177–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows JA and Wargo MJ (2014). Catabolism of host-derived compounds during extracellular bacterial infections. J Cell Biochem, 115(2), 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen EA (1993). Bacterial bioluminescence: organization, regulation, and application of the lux genes. FASEB J, 7(11), 1016–22. [DOI] [PubMed] [Google Scholar]
- Millet YA, Alvarez D, Ringgaard S, von Andrian UH, Davis BM and Waldor MK (2014). Insights into Vibrio cholerae Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria. PLoS Pathog, 10(10), e1004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Singh AK and Kumaran S (2017). Structural and biochemical characterization of ligand recognition by CysB, the master regulator of sulfate metabolism. Biochimie, 142(112–124. [DOI] [PubMed] [Google Scholar]
- Miyashiro T, Klein W, Oehlert D, Cao X, Schwartzman J and Ruby EG (2011). The N-acetyl-D-glucosamine repressor NagC of Vibrio fischeri facilitates colonization of Euprymna scolopes. Mol Microbiol, 82(4), 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T, Oehlert D, Ray VA, Visick KL and Ruby EG (2014). The putative oligosaccharide translocase SypK connects biofilm formation with quorum signaling in Vibrio fischeri. Microbiologyopen, 3(6), 836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T and Ruby EG (2012). Shedding light on bioluminescence regulation in Vibrio fischeri. Mol Microbiol, 84(5), 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T, Wollenberg MS, Cao X, Oehlert D and Ruby EG (2010). A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol Microbiol, 77(6), 1556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack-Berti A, Wollenberg MS and Ruby EG (2010). Natural transformation of Vibrio fischeri requires tfoX and tfoY. Environ Microbiol, 12(8), 2302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R and Busscher HJ (2011). Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol, 9(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Ruby EG, Urbanowski M, Campbell J, Dunn A, Faini M, Gunsalus R, Lostroh P, Lupp C, McCann J, Millikan D, Schaefer A, Stabb E, Stevens A, Visick K, Whistler C and Greenberg EP (2005). Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc Natl Acad Sci U S A, 102(8), 3004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher TR and Nyholm SV (2011). Characterizing the host and symbiont proteomes in the association between the Bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fischeri. PLoS One, 6(10), e25649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman JA, Koch E, Heath-Heckman EA, Zhou L, Kremer N, McFall-Ngai MJ and Ruby EG (2015). The chemistry of negotiation: rhythmic, glycan-driven acidification in a symbiotic conversation. Proc Natl Acad Sci U S A, 112(2), 566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Brooks JF 2nd, Ray VA, Mandel MJ and Visick KL (2015). CysK Plays a Role in Biofilm Formation and Colonization by Vibrio fischeri. Appl Environ Microbiol, 81(15), 5223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV and Ruby EG (2002). RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol, 358(413–26. [DOI] [PubMed] [Google Scholar]
- Stacy A, McNally L, Darch SE, Brown SP and Whiteley M (2016). The biogeography of polymicrobial infection. Nat Rev Microbiol, 14(2), 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoudenmire JL, Essock-Burns T, Weathers EN, Solaimanpour S, Mrazek J and Stabb EV (2018). An iterative synthetic approach to engineer a high-performing PhoB-specific reporter Appl Environ Microbiol, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer SV, Schwartzman JA, Ho JS, Geske GD, Blackwell HE and Ruby EG (2014). Non-native acylated homoserine lactones reveal that LuxIR quorum sensing promotes symbiont stability. Environ Microbiol, 16(8), 2623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, LaSota ED, Cecere AG, LaPenna KB, Larios-Valencia J, Wollenberg MS and Miyashiro T (2016). Intraspecific competition impacts Vibrio fischeri strain diversity during initial colonization of the squid light organ. Appl Environ Microbiol, 82(10), 3082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Verma SC, Bogale H and Miyashiro T (2015). NagC represses N-acetyl-glucosamine utilization genes in Vibrio fischeri within the light organ of Euprymna scolopes. Front Microbiol, 6(741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC and Miyashiro T (2013). Quorum sensing in the squid-Vibrio symbiosis. Int J Mol Sci, 14(8), 16386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC and Miyashiro T (2016). Niche-specific impact of a symbiotic function on the persistence of microbial symbionts within a natural host. Appl Environ Microbiol, 82(19), 5990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Foster J, Doino J, McFall-Ngai M and Ruby EG (2000). Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol, 182(16), 4578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg MS and Ruby EG (2009). Population structure of Vibrio fischeri within the light organs of Euprymna scolopes squid from Two Oahu (Hawaii) populations. Appl Environ Microbiol, 75(1), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.