Abstract
Despite considerable efforts to develop cellular, molecular, and structural repair strategies and restore intervertebral disc function after injury, the basic biology underlying intervertebral disc healing remains poorly understood. Remarkably little is known about the origins of cell populations residing within the annulus fibrosus, or their phenotypes, heterogeneity, and roles during healing. This review focuses on recent literature highlighting the intrinsic and extrinsic cell types of the annulus fibrosus in the context of the injury and healing environment. Spatial, morphological, functional, and transcriptional signatures of annulus fibrosus cells are reviewed, including inner and outer annulus fibrosus cells, which we propose to be referred to as annulocytes. The annulus also contains peripheral cells, interlamellar cells, and potential resident stem/progenitor cells, as well as macrophages, T lymphocytes, and mast cells following injury. Phases of annulus fibrosus healing include inflammation and recruitment of immune cells, cell proliferation, granulation tissue formation, and matrix remodeling. However, annulus fibrosus healing commonly involves limited remodeling, with granulation tissues remaining, and the development of chronic inflammatory states. Identifying AF cell phenotypes during health, injury, and degeneration will inform reparative regeneration strategies aimed at improving annulus fibrosus healing.
Keywords: annulus fibrosus, intervertebral disc, cell phenotype, intervertebral disc injury models, healing, regeneration
Graphical abstract
Despite considerable efforts to develop cellular, molecular, and structural repair strategies and restore intervertebral disc function after injury, the basic biology underlying intervertebral disc healing remains poorly understood. This review focuses on recent literature highlighting the intrinsic and extrinsic cell types of the annulus fibrosus in the context of the injury and healing environment, and describes the.spatial, morphological, functional, and transcriptional signatures of annulus fibrosus cells.
Introduction
Acute and chronic injuries of the annulus fibrosus (AF) of the intervertebral disc (IVD) result in spinal pathologies—including herniation and degeneration—that are known causes of back pain, one of the leading causes of global disability.1 AF repair is challenging due to the tissue’s structural complexity, which is key to its biomechanical function. Consequently, injuries such as IVD herniation have a relatively high rate of re-herniation.2 Many studies are developing repair strategies designed to deliver cells, drugs, and biomaterials (alone or in combination) to the IVD, although these strategies remain far from clinical translation. Cell delivery approaches have been especially challenging due to the inexact definition of AF cell phenotypes and the absence of robust differentiation strategies for deriving AF cells.3,4 Designing informed biologic AF repair strategies that aim to restore IVD structure and function requires an improved understanding of the distinct morphological and functional characteristics of the heterogeneous cell types residing in the AF and how they change with injury and degeneration. However, there is relatively little known about AF cell phenotypes.
To date, the majority of IVD cell studies have focused on nucleus pulposus (NP) cell and tissue responses to aging and degeneration, since the NP is regarded as an important signaling center of the IVD. In addition, many of the earliest age- and degeneration-related changes are most prominently displayed in the NP region, suggesting it is an important source of IVD pathologies.5–7 In contrast, there are relatively few studies that have focused on endogenous AF repair capacity even though AF disruption is more commonly associated with pain and disability. Thus, the success of IVD repair strategies relies on the development of effective AF repair and/or regeneration techniques to promote the restoration of IVD function as a whole composite tissue.
Many experimental strategies under development for AF repair consist of combinatorial biomaterial approaches of cells and/or drug delivery that commonly attempt to emulate mature AF structure with relatively little information available on the cell and developmental processes involved in creating the native AF structure. Despite the wide array of experimental approaches, a recent review highlighted that only a small number of investigations focusing on biologic repair strategies to promote AF regeneration have reached the preclinical stage.8 Furthermore, current AF repair design criteria guidelines are often centered primarily on safety, biocompatibility, and biomechanical behaviors, with a focus on preventing reherniation by achieving adhesion and integration with native tissue and/or restoring biomechanical function.9,10 Repair or regenerative potential is often assessed with extracellular matrix protein measurements, with less focus on structure. Most experimental repairs do not match the complex angle-ply lamellar structure of native AF, or its structural or cellular heterogeneity by region. An improved understanding of naive AF cell phenotypes and cellular injury responses may help advance the development of AF repair strategies more rapidly than extensive, iterative screenings of hydrogels, cells, factors, and combinations thereof.
Designing robust AF repair strategies would require improved understanding of the complex and coordinated processes involved in normal AF development as well as the pathological changes occurring with aging, injury, and degeneration. Tissue engineering of biologic laminates demonstrated that cells both develop and respond to the unique cross-ply fiber form of the AF.11 There is also a base knowledge on AF mechanical interlamellar interactions.12–16 Even with these important advances and knowledge, there remains little information on the characteristic phenotypes of different AF cell populations throughout development, maturity, and aging. Identifying cell phenotypes is challenging since very few markers exist and consequently distinction of AF cell phenotypes is currently based on anatomic separation of cells and tissues. Elucidating AF cell responses to injury in both non-regenerative and regenerative contexts is also an important area for research since it may inspire logical design approaches that promote regeneration or reverse injury effects and scarring associated with poor healing in the adult IVD. This literature review therefore specifically focuses on identifying cells of the native AF and synthesizes the known cellular responses to injury using in vivo model systems. We believe this characterization of the cells of the native, healthy AF is an important step in identifying the most promising cell targets for AF repair strategies. This point is highlighted since even nomenclature for AF cells often lacks consistency. Furthermore, the characterization of AF cellular responses to various models of AF injury is intended to help inform the development of repair strategies with potential to advance towards AF regeneration.
Clinical significance
Back and spine pathologies are among the most common sources of pain and disability, and they affect approximately 7.6 million people in the United States.1,17,18 Clinically significant lower back pain has an incidence of 1.39 per 1000 person-years.19 This disability comes at a significant economic cost of approximately $100–200 billion spent on back pain annually, two-thirds of which is a result of lost wages and decreased productivity.20,21 Furthermore, most patients with lower back pain will experience recurrent symptoms, with estimates ranging from 42–80%.22 It is therefore essential to develop successful and lasting treatments for back pain in order to allow patients to return to the workplace and to live without pain and disability.
The etiologies of back pain are diverse and involve the IVD, vertebrae, facet joints, neural elements, as well as surrounding musculature and fascia, or from a combination of these structures.23 One of the most common and best-studied, identifiable sources of back pain is the IVD, which consists of the central NP, surrounded by the AF, and cartilaginous endplates. When the IVD functions properly, it provides flexibility and load transmission to the spine.24–27 After damage through the accumulation of degenerative changes or the acute disruption of AF structure, IVD pathology may be associated with increased pain in patients due to greater rotational motion, 28 instability, loss of IVD height, or NP herniation, with potential to impinge on neural elements, resulting in radiculopathy. Furthermore, the damaged IVD structure is thought to enable neurovascular growth into the normally aneural and avascular regions deep within the IVD, which can be irritated by increased pro-inflammatory signals that enhance nociception and cause pain.29–33 While degenerative changes to the spine and IVD are associated with back pain, the specific phenotype of degenerative changes to the IVD is often difficult to identify and this challenge is confounded since many patients with back pain do not have positive MRI findings for IVD degeneration and since non-painful control subjects often exhibit degenerative changes to their IVDs.34–37 Consequently, it is clear that back pain is a multifactorial condition and that it involves structural injury and degeneration of spinal tissues in addition to multiple competing psychological, social, and economic factors that all require additional research to identify new ways of addressing this global healthcare challenge.18
Annulus fibrosus development
The general development of IVD structures and the key signaling pathways identified to date have been previously reviewed, so here we briefly summarize the main events specific to AF development.38–41 The NP, AF, and vertebral bodies are all mesodermal in origin,6,38,40 although the NP is formed from the notochord (a cartilaginous axis ventral to the neural tube) while the AF is formed from the sclerotome compartment of the somites (repeating paired structures formed on either side of the neural tube).42–46 The patterning of the distinct structures of each vertebral body starts at cranially and proceeds caudally, so that cervical IVDs develop before thoracic and lumbar levels.47 However, within each level, the components develop concurrently, resulting in tightly bound but structurally distinct elements.48 All cells of the AF derive from a population of Scx+/Sox9+ progenitors.49 While AF progenitors are initially disorganized, they subsequently align in the characteristic angle-ply lamellar pattern, forming a network of actin stress fibers coupled via adherens junctions, which may also serve as guides for cell alignment.50,51 Importantly, this cellular alignment precedes the appearance of oriented collagen matrix and is likely critical for the subsequent organization of AF lamellae structure.51 Mechanical loading also plays an important role in IVD development, as a study in chick embryos suggests that fetal movements influence spinal curvature and segmentation.52 These intriguing findings highlight the need to better understand the coordinated formation of neuronal, muscular, and skeletal structures and their interdependencies during development. Although the developmental processes distinguishing inner and outer AF differentiation may inform their postnatal response to injury and healing potential, these mechanisms have yet to be identified.40
Annulus fibrosus structure and function
The AF is a highly fibrous and well-organized tissue surrounding the outer region of the IVD. It is composed of multiple layers of concentric lamellae in an angle-ply fiber orientation that serves to constrain mobility of the IVD and contain the inner NP. This characteristic fibrous organization of the AF confers its ability to withstand circumferential loads and to limit the amount of torsional rotation and bending motions.25,53
The complex AF structure is often separated into two distinct regions: the inner AF containing primarily type II collagen produced by rounded, fibrocartilage cells, and the outer AF containing primarily type I collagen produced by elongated fibroblast-like cells.24,48,51,54–56 The transition between the inner to the outer AF is complex and has been further distinguished at the structural and cellular levels, with regional differences in extracellular matrix composition,12,57,58 cytoskeletal organization,59 and cell types 12,24 that are required to produce matrix and molecules specific to different regions. From the outer to inner AF regions, the ratio of type I collagen to type II collagen decreases.54,55,60 The angle-ply fiber orientation also changes from 65° in the outer AF to 30–45° in the inner AF.61,62 This angle-ply structure contributes to the non-linear and anisotropic mechanical properties of the AF.63 Adding to this structural complexity, individual lamellae are separated by a disorganized interlamellar tissue containing proteoglycan-rich matrix, elastic fibers, and cells,13 and are connected by networks of interlamellar cross-bridges consisting of elastin and type VI collagen, which are thought to be important for maintaining healthy AF function.13,64,65 Elastic fibers are also known to bridge layers, and these have been speculated to be functional tie fibers and/or remnants of vascular channels that regress following IVD development.64,66 The organization of this complex fiber-reinforced material also exhibits excellent mechanical performance, inhibiting crack progression and maintaining excellent stiffness behaviors even after damage has accumulated. 67,68 The distribution and organization of the various components that constitute the native and healthy AF accumulate over decades of life and pose a challenge for the design of repair strategies that aim to mimic this complex and hierarchical tissue.
Cells of the Annulus Fibrosus
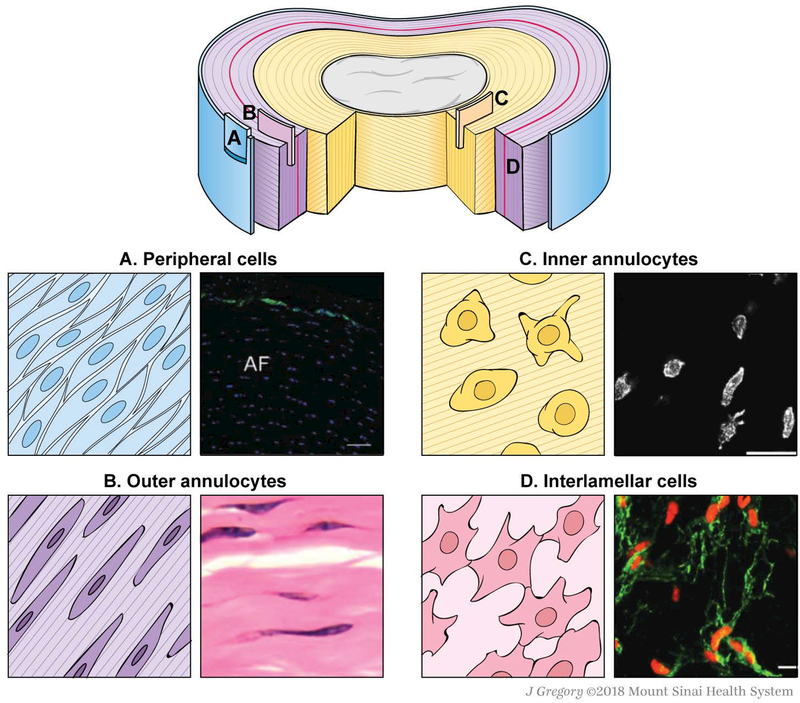
In the human adult, reported AF cell density ranges from approximately 3000 cells/mm3 to 9000 cells/mm3.69–71 This range is markedly lower than that of mature hyaline cartilage, which ranges from 16,000 cells/mm3 to 60,000 cells/mm3.71,72 Despite low AF cell density, there is significant heterogeneity of cell types residing within the AF under healthy conditions. While a few studies have identified possible cell phenotypes within the AF, there are few unique or definitive markers to distinguish intrinsic AF cell populations. Functional definitions of the heterogeneous cell types residing in the AF remain unclear. A recent study of primary and immortalized human AF cells revealed 1161 genes showing higher expression in AF than in NP tissue, and 125 AF-specific genes that encode membrane-associated proteins, providing a substantial set of novel AF membrane-associated markers.73 Thus, these cells are often defined based on regional location, morphology, and/or function (Table 1). We propose that the term “annulocyte” be used to refer to the native, heterogeneous population of sclerotome-derived cells expressing the markers Scx and Tnmd, but should not specifically refer to a single cell type within. Additional cell types that reside within or near the AF, but whose origins are unknown, include peripheral cells, interlamellar cells, and stem/progenitor cells (Fig. 1).
Table 1.
Morphological, mRNA, and protein signatures characteristic of distinct AF cell populations residing within different AF regions.
| Cell type | Morphology | Markers | Species | Location | References | |
|---|---|---|---|---|---|---|
| Annulocyte | Outer | Elongated, fusiform, fibroblast-like | [COL1A1, COL5A1, COL12A1, SFRP2] | Human (14 years*) | Outer AF | 78 |
| Collagen I > Collagen II | Human (27–52 years) | Outer AF | 74 | |||
| - | Bovine (12–24 months) | Outer 20% of AF | 12 | |||
| - | COL5A1 | Rabbit | Outer AF | 76 | ||
| - | COL5A1, TNMD | Human, bovine | Outer AF | 79 | ||
| - | Mkx | Human, mouse | Outer AF | 82 | ||
| Outer/Inner | - | Scx | Mouse | Outer AF (adult), entire AF (young) | 80; 81 | |
| - | Keratan sulfate | Human (69 years), rat (E15–21) | Outer and inner AF) | 57; 77 | ||
| Inner | Rounded, chondrocyte-like | Collagen II | Human (27–52 years) | Inner AF | 74 | |
| Rounded, short processes | - | Bovine (12–24 months) | Inner AF | 12 | ||
| Rounded, long processes | - | Bovine (12–24 months) | Inner AF/NP border | 12 | ||
| Rounded | α-Smooth muscle actin | Canine (adult+) | Inner AF | 86 | ||
| - | Fmod | Mouse (E15.5) | Inner AF | 85 | ||
| Interlamellar | Lace-like network | - | Bovine (12–24 months) | Interlamellar septae (outer AF) | 12; 13 | |
| Peripheral | Elongated$ | CD146, transgelin (SM22α) | Human, mouse (8–9 weeks) | Single-cell layer at outermost AF | 88 | |
| AF stem/progenitor | - | Notch1, Delta4, Jagged1, C-KIT, Stro-1 | Rabbit (3 months), rat (3 months), minipig (6 months) | AF border to ligament zone, perichondrium | 91 | |
| Small/spindle-shaped and polygonal | CD133, CD90, CD73, CD166, P75LNGFR | Human, rat | Unspecified AF | 89 | ||
| - | CD29, CD49e, CD51, CD73, CD90, CD105, CD166, CD184, Stro-1 | Human (adolescent) | Unspecified AF | 90 | ||
| Varied (cobblestone or spindle-like) | Oct-4, nucleostemin, SSEA-4; CD29-, CD44-, CD166-positive; CD4-, CD8-, CD14-negative | Rabbit (6–8 weeks) | Unspecified AF | 95 | ||
| - | CD166, C-KIT, Jagged | Rabbit (6 months) | Unspecified AF | 96 | ||
[ ] collective mRNA signature;
average of 2 donors;
age not specified;
from image
Figure 1.
Schematics and representative microscopic images of the different cell types and their locations in the healthy adult AF. (A) Peripheral cells.88 (B) Outer annulocytes.163 (C) Inner annulocytes.12 (D) Interlamellar cells.12
Annulocytes and other resident cells
The outer AF contains elongated, fusiform, fibroblast-like cells that have previously been referred to as “outer AF cells”, “fibroblast-like AF cells”, “AF-like cells”, “AF fibrochondrocytes”, or simply “AF cells”.12,74,75 We propose that cells residing in the outer portion of the AF be referred to as “outer annulocytes” (Table 1). Vimentin staining for cytoskeletal elements in the bovine AF demonstrated that these elongated cells reside in the outer 20% of the AF and run parallel to oriented collagen fibres with extended lateral processes perpendicular to the lamellae.12 Outer annulocytes have been identified in several species, and a handful of genes and proteins have been proposed as distinct phenotypic markers that can differentiate these outer annulocytes from inner annulocytes and other cells of the IVD. Classically, the extracellular matrix surrounding outer annulocytes is composed of a higher ratio of type I to type II collagen, as demonstrated by immunostaining studies in humans across a range of ages.74 Unsurprisingly, expression of COL5A1, a gene encoding type V collagen, which is thought to regulate type I collagen assembly, is distinctive to the outer AF of rabbit IVDs relative to NP and articular cartilage.76 The glycosaminoglycan keratan sulfate was identified in the inner and outer AF of the developing rat IVD, and has also been identified in human AF tissue as a specific matrix protein of annulocytes.57,77 A transcriptional signature of COL1A1, COL5A1, COL12A1, and SFRP2 was proposed for a population of annulocytes isolated from young human tissue after culture.78 The association of type XII collagen with type I collagen is well established and is thought to modify the interactions between collagen I fibrils and surrounding matrix. SFRP2, a modulator of Wnt signaling, is a putative AF marker specific to outer annulocytes in human and bovine tissue.78,79 Outer annulocytes share similar markers to other dense, fibrous connective tissue cells, such as tenocytes and ligamentocytes, including the genes Tnmd, Mkx, and Scx in human, bovine, and murine AF.79–82
From the outer AF region towards the inner AF and NP, there is a marked change in cell morphology and phenotype from that of the classically defined outer annulocyte to a more rounded cell shape. These cells have been variously termed “chondrocyte-like cell”, “inner-AF cell”, or “discoidal chondrocytes”.83 Like chondrocytes, inner annulocytes produce primarily type II collagen.74,84 Fmod, a gene encoding for fibromodulin, which is a proteoglycan important in collagen assembly, is expressed at high levels in inner annulocytes at embryonic stages and is known to be an AF-specific marker in rodents.85 While these cells are also sometimes described as “NP-like”, we will avoid this terminology since the AF and NP are derived from distinct embryonic progenitors and to date there is no evidence for transdifferentiation of these cells types in vivo. We propose the use of “inner annulocyte” to refer to these rounded, chondrocyte-like cells residing within the inner AF (Table 1). The transition from the fibrous phenotype of outer annulocytes to the chondrogenic phenotype of inner annulocytes may be due to differences in mechanical loading (tensile versus compressive, respectively). The close proximity to the highly pressurized and outward-bulging NP may also provide an additional mechanical cue, as well as direct molecular signals from NP cells. Interestingly, there may be additional heterogeneity within the inner annulocyte population, as one study reported that ~2% of cells isolated from the inner AF also expressed α-smooth muscle actin.86 Staining for cytoskeletal vimentin also showed that inner annulocytes at the NP/inner AF border exhibit longer cellular processes.12 Whether these features are indicative of specific functional activity within the inner annulocyte population is currently unexplored.
The lamellae comprising the AF are separated by interlamellar matrix that functions to maintain AF integrity by integrating the lamellae and providing some interface lubrication between sliding AF structural features.13,14,87 The interlamellar matrix is highly distinct from AF lamellae, with complex elastic fiber and cross-bridge arrangements. This unique structure suggests the existence of a population of specific interlamellar cells that are distinct from inner or outer annulocytes. These interlamellar cells are likely subjected to unique physical cues (such as shearing) that may guide matrix synthesis and homeostasis. To date, interlamellar cells remain poorly characterized but have been described as forming a lace-like network between AF lamellae (Table 1).12
A single layer of cells expressing the markers CD146 and transgelin (SM22α) was identified in the AF periphery in both mouse and human IVD (Table 1).88 While there is speculation that these CD146+ cells may be a population of resident stem cells, their function is still not clear, and they may also serve important functions in wound healing responses and contractility.88
Stem and progenitor cells
Stem and/or progenitor cells have been identified in young, adult, and aged AF tissue (degenerative and non-degenerative); however, there is currently no clear consensus on AF-specific stem/progenitor markers (Table 1). These cells are therefore typically distinguished using surface markers associated with mesenchymal stem cells (MSCs) or pluripotency markers.89,90 IVD stem/progenitor cells reside in a niche at the AF/cartilaginous endplate border and in the nearby perichondrium, and are speculated to migrate into the IVD during homeostasis.89,91–95 With aging, immunohistochemical detection of mesenchymal and pluripotent markers decreases,95,96 suggesting that loss of stem/progenitor cells may be a possible contributor to impaired healing in adults. However, this has not been directly tested. In addition to AF stem/progenitors, putative neural stem cells (expressing the markers nestin and neuron-specific enolase) were identified in young human AF tissue and these progenitors were capable of differentiating into neural cells in vitro; however, their in vivo function remains undetermined.90,97
Cellular responses to injury
The established phases of wound healing include: initial inflammation and extrinsic/inflammatory cell recruitment; cell proliferation; formation of collagenous and fibrotic granulation tissue; and remodeling.98 A thorough characterization of cellular responses to AF injury throughout these wound healing phases without therapeutic intervention is lacking, particularly with age and across various species. It is broadly known that aged and degenerated human IVDs have particularly poor healing capacity due to low cellularity,70 accumulation of structural defects, and chronic inflammation,31,32,99 but a comparison with young human IVD injury responses is difficult because it is uncommon for young human IVDs to be injured and available for study. Injury to the AF leads to a number of pathological changes, including decreased IVD cellularity, upregulated matrix-degrading enzymes, innervation, inflammation, increased growth factor production, and formation of granulation tissue.3,100,101 Delamination of the AF due to excessive loading increased apoptosis of outer annulocytes and it has been suggested that annulocyte apoptotic responses may be associated with excessive cell stretching of annulocytes with limited capacity to reorient and thereby minimize the strain from loading.102,103 Furthermore, aging of IVD tissues is known to induce aberrant cellular responses to injury, including cellular senescence and dysregulated signaling, and lead to the loss of biological structure and function,104 which may affect healing processes. Young and old mice express similar patterns of anabolic cytokines, although differences in the degree of expression suggests different innate responsiveness as a function of age.105 A more thorough characterization of the cellular injury responses and the time course of wound healing in the AF requires further elucidation.
There is extensive literature detailing impaired IVD healing after adult injury, and animal models of IVD injury have been investigated for several decades. The earliest studies of IVD injuries in vivo determined that annular injury produced similar characteristics to human spondylosis deformans, and gross morphological examination demonstrated the first evidence of AF fibrosis following injury.106,107 Models of adult AF injury by using needle puncture of varying sizes result in an impaired, fibrotic healing response to the acute injury that can contribute to slow, progressive degeneration and altered IVD mechanical properties.25,108–114 Such needle puncture injuries are known to result in permanent IVD defects, leading to loss of NP pressurization and accumulation of fibrotic tissue in the NP region, as well as disorganization of AF lamellae and deposition of fibrotic matrix in the puncture tract (Fig. 2). Puncture injuries are also speculated to be at least partly responsible for the accelerated IVD degeneration known to occur in humans following discography.112,115
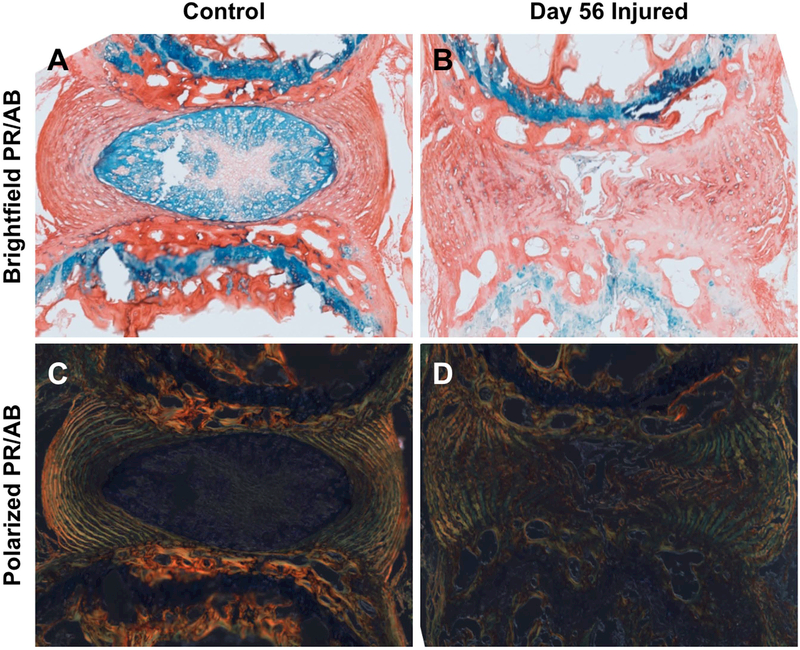
Figure 2.
The AF injury response in a mouse caudal IVD eight weeks following injury. Picrosirius red/alcian blue staining reveals uninjured control IVDs have an intact and proteoglycan-rich NP (A) and highly organized, collagen-rich AF as visualized under polarized light (C). Injury response 8 weeks after puncture consists of NP fibrosis and incomplete healing of puncture tract, with evidence of AF buckling, delamination, and fissures throughout the IVD, as shown by picrosirius red/alcian blue staining (B). Loss of AF lamellae organization is shown by decreased birefringence in polarized light imaging (D). 5 μm thick paraffin sections, 10X magnification.
Inflammation and recruitment of immune cells
After injury, activation of immune cells initiates a phagocytic and pro-inflammatory response to remove cell and tissue debris. Although inflammation is necessary to initiate healing, resolution of initial inflammation is required to initiate fibrosis and remodeling. Annular injury results in the recruitment of irregular cells (which are potentially immune cells) to the wound area that is sustained over time, prolonging the pro-inflammatory environment and promoting degenerative changes (Fig. 3). Multiple studies have identified immune cells after IVD injury and degeneration, including macrophages, T lymphocytes, and mast cells (Table 2), as well as important pro-inflammatory cytokine responses.32,116,117 While some studies demonstrate a weak immune response in herniated tissue 118 and with induced AF injury in animal models,119 repeated injury is known to increase that pro-inflammatory condition.110,113,114,120,121 T lymphocyte and macrophage infiltration is not widely observed in AF injury models with herniation,119 although this may be due to limited reporting since IVD cells are considered immunoprivileged in their healthy state, with few resident inflammatory cells. Similar injury models have demonstrated a poor ability of the AF to heal,122,123 indicating that the weak immune cell response to AF injury without herniation may result in poor healing outcomes. Furthermore, in vivo studies have demonstrated that exposure of NP tissue to AF and to DRG increases macrophage recruitment,124,125 indicating that NP tissue from herniation may be important but not necessarily required for immune cell recruitment following AF injury.
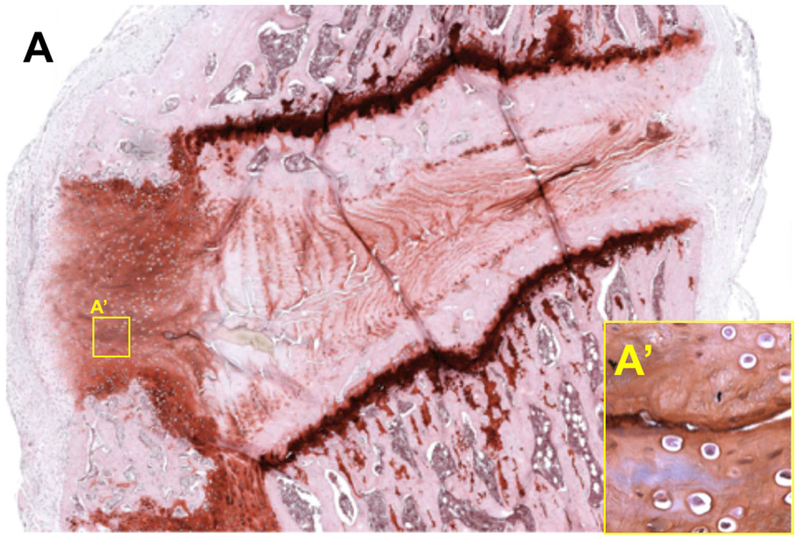
Figure 3.
Recruitment of unidentified and irregular cells in a punctured rat lumbar IVD six weeks following injury. Safranin-O staining reveals a dense, cellular cap surrounding the injury site on the dorsal aspect of a lumbar IVD (A). Rounded, unidentified cells that are potentially inflammatory cells remaining in the repair region over time are observed adjacent to the puncture tract (inset A’ shows higher magnification of the corresponding region in panel A). 5 μm thick paraffin sections, 10X magnification.
Table 2.
Inflammatory cells observed in the AF following injury include macrophages, T lymphocytes, and mast cells.
| Cell type | Markers | Species | Injury type | Location | Time point | Reference |
|---|---|---|---|---|---|---|
| Macrophages | ED1 | Rat | Herniation | Dorsal root ganglion | Day 3 | 124 |
| CD68 | Pig | 5 × 4 mm deep cuts in the center of the AF w/o herniation (L4/5, L5/6) | AF | 1 month post injury | 119 | |
| CD68, Ber-MAC3 | Human | Herniation (excess surgical tissue) | Herniated tissue | Early (time of operation) | 118 | |
| Morph-ology | Mice | Implantation of IVD tissue into peritoneum | AF and NP tissue | 1, 3, 7 days (max at 7 days) | 125 | |
| T lymphocytes | CD3 | Pig | 5 × 4 mm deep cuts in the center of the AF w/o herniation (L4/5, L5/6) | AF | 1 week and 1 month after injury | 119 |
| Mast cells | Tryptase | Human | Herniation | Surgical tissue | Early (time of operation) | 127 |
In humans, macrophages and mast cells have been shown to infiltrate into IVDs with age and degeneration, as well as with herniation.118,126,127 Host IVD cells can be sensitized by chronic inflammatory signals and by the increased presence of macrophages that remain in pro-inflammatory and remodeling states and do not revert to anti-inflammatory healing states.126 Clinical evidence also supports the hypothesis that bacterial infection and low-grade discitis is a potential contributor to IVD degeneration.128–130 Interestingly, endplate changes, recorded as modic changes on MRI, are commonly thought to involve fibrogenic and pro-inflammatory cross-talk between bone marrow and adjacent IVDs.131 Regardless of what the initiator of IVD degeneration is, this inflammatory response, which is required for healing, can become chronic in the IVD, resulting in a frustrated healing state that may predispose the tissue to degeneration and painful conditions.27,32,132
Cell proliferation and granulation tissue formation
In the AF, granulation tissue forms at the outermost layers.123,133,134 Following annular stab in canine IVDs, NP herniation results in the formation of a thin fibrous layer on the outermost layers, with no repair of the interior injury site observed at 20 weeks, while larger block defects resulted in the formation of a solid fibrotic plug throughout the defect.122,134 Similar findings were reported in rabbit, goat, and porcine IVDs, where the most superficial aspect of the AF healed by rapid fibrosis following stab injury, whereas interior regions remained unrepaired even after long time periods.114,133,135,136 In sheep, discrete annular tears or rim lesions without NP herniation resulted in peripheral granulation tissue 18 months after injury, with no bridging of the middle and inner portions of the lesion.123 The presence of granulation tissue staining strongly for TNFα and advanced glycation end products has also been observed, suggesting that granulation tissue can accumulate as a result of disease as well as acute injury and that it is highly pro-inflammatory.137 Furthermore, granulation tissue in human IVDs has been further characterized to contain macrophages as well as other cells that exhibit increased pro-inflammatory and hypertrophic markers.126,138 The source of fibrotic cells in granulation tissue has not been identified.
The early dynamics of cell proliferation/apoptosis, and the contribution of neighboring tissues to AF repair and granulation tissue formation have not been fully defined for most of these injury models. However, poor repair of interior tissues suggest that intrinsic AF cell proliferation is likely minimal following injury. In mice, IVD cells experience a broad decline in proliferative capacity after 9 weeks of age.139 Long-term, end-stage time points at 12 months in ovine IVDs also indicate there is little spontaneous cell recruitment to injured, untreated annular injury sites,140 although definitive lineage tracing experiments have not been carried out.
Matrix remodeling
The remodeling phase of wound healing can occur over a period of years in humans and involves the deposition of type I collagen to replace type III collagen formed during initial wound healing responses.98 Evidence from animal models of annular injury suggest that little matrix remodeling occurs once fibrotic tissue is formed. Indeed, fibrotic matrix can persist from 12 weeks to 25 months post-injury.122,133 In sheep, annular lesions failed to heal and remained void of tissue up to 18 months post-injury, which may be more consistent with human pathology.123 In addition to persistent scar formation within the defect site, the IVD also experiences slow and progressive degeneration after injury. In all animal models, regeneration does not occur, despite prolonged healing times.108,122,123,141 Structural and histological assessments demonstrate evidence of fibrotic and incomplete healing for small injuries, and progressive fibrosis and degeneration in larger injuries.25,109,113,114
Lessons From Models of Mammalian Regeneration
Regenerative medicine is an evolving field that investigates the extent of innate healing capacity of tissues with the goal of understanding the cellular and molecular drivers of cellular, structural, and functional regeneration. Using this knowledge, tissues damaged due to injury, degeneration, or aging may potentially be restored to healthy, regenerated states. This tissue damage may result in the formation of fibrotic scarring, of which the origin and underlying signaling pathways remain largely unknown. Further research on this topic will be required to develop targeted therapies that prevent and/or reverse fibrosis. Regenerative mechanisms vary widely among different mammalian tissues,142 thus indicating the importance of investigating tissue-specific healing and regeneration potential. In particular, the cellular mechanisms involved are highly tissue- and context-specific.143
In the context of designing strategies to restore AF structure and function following injury, understanding the limited capacity of the AF to regenerate has been a topic of many studies. The healing process may result in tissue regeneration, but may also result in non-regenerative, scar-mediated healing. Perfect regeneration of a tissue is generally defined as complete restoration of structure, composition, and function relative to the native, healthy condition. However, regeneration may occur to varying degrees of completeness. To overcome the limited capacity of the adult AF to heal via perfect regeneration, many repair strategies that aim to seal AF defects and deliver cells and factors to promote regeneration are under investigation.3,4,8,100,144 Despite recent advances and approaches designed to overcome the adult AF’s limited healing response and to promote regeneration, achieving perfect regeneration of structure, composition, and function remains difficult. Additionally, the gap in knowledge regarding the molecular pathways that regulate distinct AF cell phenotypes and the paucity of AF regeneration models presents a challenge for the development of cell-based strategies that improve AF healing and promote regeneration.
MSCs reside within the proteoglycan and non-collagenous protein-rich matrix of the IVD, surrounded by the fibrous and cartilaginous outer AF region.140 The presence of MSCs within the IVD may implicate these cells in IVD healing, regeneration, and repair.145 In clinical applications, MSCs injected directly into the IVD space resulted in improvements in IVD hydration and collagenous tissue formation.146 In parallel, decreased structural degeneration-related parameters and patient-reported lumbar pain scores were also observed.140,146 While these results are promising, it is important to note that the functional role of MSCs in healing and repair is not currently known. For example, MSCs may directly differentiate along IVD-specific lineages to replace damaged tissues or MSCs may indirectly modulate the local immune environment. Future investigation is therefore required to elucidate their direct and/or indirect roles in healing. Although true AF regeneration has yet to be achieved, the native AF has the potential for improved healing within certain contexts, such as in the neonatal mouse where IVDs heal via restored function following herniation injury.81 Delivery of cells and factors to promote regeneration show promise in animal models, such as delivery of MSCs overexpressing Mohawk, a transcription factor important in regulating outer AF growth and homeostasis.82 However, several challenges regarding clinically translatable and efficacious delivery remain unresolved. Despite the poor self-healing capacity of adult IVD, the presence of resident cells with stem/progenitor potential poses an attractive target for regenerative therapies. Whether these cells can be activated to promote healing or regeneration remains an unanswered question. Additionally, the paucity of regenerative models presents a challenge for developing cell-based strategies that promote AF healing and restoration of function. Using such a model of intrinsic AF regeneration, key cells and signaling pathways associated with regeneration can provide a roadmap toward engineering successful repair strategies that mimic these biologic cues to improve healing of the human IVD.
Although there are many mammalian models for the repair of complex tissues (such as the IVD), the ability of these models to recapitulate the human condition is a constant challenge and limitation in terms of clinical relevance. The smaller size of some animal models limits the ability to directly compare healing responses that occur in the human anatomical environments; important considerations such as nutrient supply and metabolic activity may be highly dependent on tissue size. It is also worth noting that the IVDs of many animals do not naturally degenerate. This may be due to differing cellular compositions (such as presence of stem/progenitor pools that are absent in humans), biomechanical loading, size, and weight distributions as compared to humans.
Nonetheless, the neonatal mouse is an exciting mammalian model for regeneration of diverse tissues, including the heart,147–151 cochlear hair cells,152,153 and digit tip.154–156 In the context of musculoskeletal tissues, the neonatal IVD 81 and neonatal tendon 157 show improved regenerative capacity relative to adult models. Cell mechanisms underlying neonatal regeneration appear to be tissue- and species-specific; for example, heart and tendon regeneration is driven by mitotically active, differentiated cardiomyocytes and tenocytes, respectively, while cochlear hair cell regeneration is driven by transdifferentiation of supporting cells, and the digit tip is driven by dedifferentiation and redifferentiation of lineage-restricted cells. Collectively, these neonatal murine models identify potential cellular mechanisms and provide a roadmap toward adult mammalian regeneration. In the context of the IVD, neonates demonstrated excellent healing of severe IVD injuries involving large AF defects and complete loss of NP.81 After 8 weeks of healing, neonatal mouse IVDs restored full functional biomechanics and improved collagen organization surrounding the puncture tract. Nevertheless, even in this excellent healing model, there was incomplete repair and persistent collagen disruption. As a result, tissue engineering and other systems that emulate native AF structure are important to both enhance repair and to learn about cell behavior within these defined conditions.11 Furthermore, it remains notable that even with the evolving knowledge about IVD healing processes and enhanced characterization of functional biomechanical and matrix changes following injury, there remains limited knowledge of the identity and function of annulocytes during the injury, repair and healing processes which represents an important open area of future investigation.
Summary
Understanding of AF cell biology during homeostasis and injury has advanced in recent years; however, there is a clear need for further work to validate previously published findings that are highlighted in this review. Understanding the phenotypes and functions of cells residing in the normal, healthy AF, as well as their behaviors following injury, is crucial for developing cellular strategies to repair AF defects and treat IVD degeneration. Developmental processes may inspire reparative regeneration strategies, but developmental and regenerative mechanisms can be distinct, as developmental processes are not always recapitulated in reparative regeneration.142 An improved understanding of development, injury responses, and healing processes can be used to inform repair strategies. Approaches that may be feasibly used to induce reparative regeneration include using knowledge of developmental stages to inform annulus repair, manipulating fibrotic scar formation (which typically interferes with regenerative outcomes), or designing biomimetic tissue-engineering constructs to replace injured tissues. Attempts at in vitro models to better understand developmental processes and develop repair strategies have been made and such models may be key in furthering knowledge of AF development and biology.11
Understanding AF injury and healing is particularly important because AF disruption permits neurovascular invasion, NP herniation, and/or biomechanical instability, which are commonly considered pain generators. The complex structure and composition of the AF, which provides it with excellent mechanical properties and high fracture resistance,68 also makes it a challenging tissue to regenerate. Furthermore, multiple aspects of the microenvironment, including nutrition, oxygenation, acidity, and mechanics are known to influence AF cell behaviors and have been manipulated as part of attempts to develop AF repair strategies.100 The microenvironment and the need to resist high spinal loads are critical factors that must be overcome to achieve successful regeneration and warrant further investigation.100,158,159 To date, there is little information on basic AF cell biology, in particular regarding the cell types residing within the AF and their functional roles in maintaining healthy homeostasis, and the phenotypic shifts after injury or disease. Compared with classical wound-healing phases, the IVD injury response is characterized by unresolved inflammation and formation of granulation tissue that does not remodel over time, a condition that has been referred to as “frustrated healing.”27 The initial inflammatory response of the IVD is limited compared to vascularized tissue, but increases and is maintained over time. This delayed and increased inflammatory response is a likely contributor to the formation of persistent, fibrotic granulation tissue and degenerative changes associated with limited matrix remodeling. In addition to inducing reparative regeneration processes, modulating the inflammatory response may be a potential strategy to prevent fibrosis and promote regeneration in the AF, and it warrants further investigation.
Direct identification of cells involved in AF injury and healing using more precise markers, as well as their cell proliferation responses following injury, remain important areas for future investigation. A major challenge for understanding IVD injury responses is that investigations probe injury responses at varied time points and across multiple species, making direct comparisons difficult. The use of murine models allows for genetic modifications and mechanistic probing of signaling pathways. There is wide availability of antibodies to detect phenotypic markers, which has been an important tool in the identification of the key cellular players of AF biology; however, the relevance of findings from murine models and the relevance of their biomechanical environment to the human condition often comes into question. When normalized for geometry, mechanical parameters such as the torsional stiffness of murine IVDs are comparable to human lumbar IVDs.160 The mouse and rat lumbar and mouse tail IVDs have been shown to be the closest representation of human lumbar IVD geometry in terms of IVD height, anterior-posterior width, and NP area.161 However, small animal models have different nutrient and waste transport dynamics compared to humans, a distinct structural difference (more pronounced NP/AF border), and increased matrix water content.162 The major differences in human and animal cells of the IVD is a potential limiting factor for the development of AF repair strategies; as our understanding of animal AF cell biology increases, the relevance to the human condition should be appropriately considered. However, an improved understanding of these responses may provide clues and targets to mitigate non-regenerative pathways or promote regenerative ones, which can then be incorporated into repair strategies to treat painful conditions associated with IVD herniation.
This review described multiple cell types in the IVD as well as several key areas that are open for further investigation. Four distinct types of AF cells reside in the normal, healthy AF: peripheral cells, outer annulocytes, inner annulocytes, and interlamellar cells. AF stem/progenitor cells and neuronal stem cells in healthy AF tissue have also been identified as being present in the IVD, yet their specific identity and roles during aging, injury, and degeneration are still undetermined. A limited number of investigations reported macrophage, T lymphocyte, and mast cell recruitment after injury and in surgical herniation tissue and IVD degeneration, with the suggestion that they may remain at the site or even promote pro-inflammatory activity of native IVD cells, yet the responses of intrinsic annulocytes and AF stem/progenitor cells to these extrinsic cells are unclear. The cellular responses to IVD injury and healing involve inflammation and recruitment of immune cells, cell proliferation and granulation tissue formation, and matrix remodeling. The very few studies investigating normal AF cellular phenotypes and their responses to injury suggest granulation tissue remains, with limited matrix remodeling and the development of chronic inflammatory states. Neonatal mice demonstrate excellent regeneration in various tissues, and recent research suggests that neonatal AF healing may provide a potential roadmap to promote IVD regeneration with cell-based repair strategies. Consequently, further studies are required, including investigations of AF cell types and using tissue engineering and other regenerative medicine techniques to develop strategies that promote improved healing following AF injury. It remains an open question whether any model or regenerative medicine technique can overcome the high loads and harsh IVD microenvironment to enable reparative regeneration of the AF.
Acknowledgements
This work was supported by U. S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01 AR064157 (to JCI), and R01 AR069537 (to AHH).
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdu RW, Abdu WA, Pearson AM, Zhao W, Lurie JD, Weinstein JN. Reoperation for recurrent intervertebral disc herniation in the spine patient outcomes research trial: analysis of rate, risk factors, and outcome. Spine 2017;42(14):1106–1114. doi: 10.1097/BRS.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guterl CC, See EY, Blanquer SBG, et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater 2013;25:1–21. doi: 10.22203/eCM.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre OM, Cruz MA, Hecht AC, Iatridis JC. Chapter 15: Annulus Fibrosus Repair. In: Härtl R, Bonassar LJ, eds. Biological Approaches to Spinal Disc Repair and Regeneration for Clinicians.; 2017. [Google Scholar]
- 5.Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS ONE 2012;7(4):e35944. doi: 10.1371/journal.pone.0035944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr 2011;21(1):29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xiong C, Kudelko M, et al. Early onset of disc degeneration in SM/J mice is associated with changes in ion transport systems and fibrotic events. Matrix Biol 2018. doi: 10.1016/j.matbio.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Sloan SR, Lintz M, Hussain I, Hartl R, Bonassar LJ. Biologic annulus fibrosus repair: A review of preclinical in vivo investigations. Tissue Eng Part B Rev 2018. doi: 10.1089/ten.TEB.2017.0351. [DOI] [PubMed] [Google Scholar]
- 9.Long RG, Torre OM, Hom WW, Assael DJ, Iatridis JC. Design requirements for annulus fibrosus repair: review of forces, displacements, and material properties of the intervertebral disk and a summary of candidate hydrogels for repair. J Biomech Eng 2016;138(2):021007. doi: 10.1115/1.4032353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Likhitpanichkul M, Dreischarf M, Illien-Junger S, et al. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur Cell Mater 2014;28:25–37; discussion 37. doi: 10.22203/eCM.v028a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater 2009;8(12):986–992. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat 2002;201(2):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavakoli J, Elliott DM, Costi JJ. Structure and mechanical function of the inter-lamellar matrix of the annulus fibrosus in the disc. J Orthop Res 2016;34(8):1307–1315. doi: 10.1002/jor.23306. [DOI] [PubMed] [Google Scholar]
- 14.Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. Measurement of local strains in intervertebral disc anulus fibrosus tissue under dynamic shear: contributions of matrix fiber orientation and elastin content. J Biomech 2009;42(14):2279–2285. doi: 10.1016/j.jbiomech.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connell GD, Vresilovic EJ, Elliott DM. Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J Orthop Res 2011;29(4):547–555. doi: 10.1002/jor.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meakin JR, Hukins DW. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J Biomech 2000;33(5):575–580. doi: 10.1016/S0021-9290(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). Prevalence and most common causes of disability among adults--United States, 2005. MMWR Morb Mortal Wkly Rep 2009;58(16):421–426. [PubMed] [Google Scholar]
- 18.Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. The Lancet 2018;391(10137):2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 19.Waterman BR, Belmont PJ, Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J 2012;12(1):63–70. doi: 10.1016/j.spinee.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 2006;88 Suppl 2:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 21.Duthey B Priority Medicines for Europe and the World - 2013 Update. WHO Headquarters in Geneva: World Health Organization; 2013:1–29. [Google Scholar]
- 22.Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Pract Res Clin Rheumatol 2010;24(6):769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Biyani A, Andersson GBJ. Low back pain: pathophysiology and management. J Am Acad Orthop Surg 2004;12(2):106–115. [DOI] [PubMed] [Google Scholar]
- 24.Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat 2012;221(6):480–496. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J 2013;13(3):243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oxland TR. Fundamental biomechanics of the spine--What we have learned in the past 25 years and future directions. J Biomech 2016;49(6):817–832. doi: 10.1016/j.jbiomech.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 28.Blankenbaker DG, Haughton VM, Rogers BP, Meyerand ME, Fine JP. Axial rotation of the lumbar spinal motion segments correlated with concordant pain on discography: a preliminary study. AJR Am J Roentgenol 2006;186(3):795–799. doi: 10.2214/AJR.04.1629. [DOI] [PubMed] [Google Scholar]
- 29.Stefanakis M, Al-Abbasi M, Harding I, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine 2012;37(22):1883–1891. doi: 10.1097/BRS.0b013e318263ba59. [DOI] [PubMed] [Google Scholar]
- 30.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. The Lancet 1997;350(9072):178–181. doi: 10.1016/S0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 31.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 2005;7(4):R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marras WS, Walter BA, Purmessur D, Mageswaran P, Wiet MG. The Contribution of Biomechanical-Biological Interactions of the Spine to Low Back Pain. Hum Factors 2016;58(7):965–975. doi: 10.1177/0018720816657235. [DOI] [PubMed] [Google Scholar]
- 34.Livshits G, Popham M, Malkin I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 2011;70(10):1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mok FPS, Samartzis D, Karppinen J, Fong DYT, Luk KDK, Cheung KMC. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J 2016;16(1):32–41. doi: 10.1016/j.spinee.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 36.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72(3):403–408. [PubMed] [Google Scholar]
- 37.Adams MA, Dolan P. Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat 2012;221(6):497–506. doi: 10.1111/j.1469-7580.2012.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith LJ, Nerurkar NL, Choi K-S, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech 2011;4(1):31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan WCW, Au TYK, Tam V, Cheah KSE, Chan D. Coming together is a beginning: the making of an intervertebral disc. Birth Defects Res C Embryo Today 2014;102(1):83–100. doi: 10.1002/bdrc.21061. [DOI] [PubMed] [Google Scholar]
- 40.Sivakamasundari V, Lufkin T. Bridging the gap: understanding embryonic intervertebral disc development. Cell & developmental biology 2012;1(2). [PMC free article] [PubMed] [Google Scholar]
- 41.Henriksson HB, Brisby H. Development and regeneration potential of the mammalian intervertebral disc. Cells Tissues Organs (Print) 2013;197(1):1–13. doi: 10.1159/000341153. [DOI] [PubMed] [Google Scholar]
- 42.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 1990;110(1):115–130. [DOI] [PubMed] [Google Scholar]
- 43.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development 2005;132(11):2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 44.Dietrich S, Schubert FR, Gruss P. Altered Pax gene expression in murine notochord mutants: the notochord is required to initiate and maintain ventral identity in the somite. Mech Dev 1993;44(2–3):189–207. [DOI] [PubMed] [Google Scholar]
- 45.Pourquié O, Coltey M, Teillet MA, Ordahl C, Le Douarin NM. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci U S A 1993;90(11):5242–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruggeman BJ, Maier JA, Mohiuddin YS, et al. Avian intervertebral disc arises from rostral sclerotome and lacks a nucleus pulposus: implications for evolution of the vertebrate disc. Dev Dyn 2012;241(4):675–683. doi: 10.1002/dvdy.23750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller F, O’Rahilly R. Segmentation in staged human embryos: the occipitocervical region revisited. J Anat 2003;203(3):297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peacock A Observations on the postnatal structure of the intervertebral disc in man. J Anat 1952;86(2):162–179. [PMC free article] [PubMed] [Google Scholar]
- 49.Sugimoto Y, Takimoto A, Akiyama H, et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 2013;140(11):2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- 50.Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn 1999;215(3):179–189. doi:. [DOI] [PubMed] [Google Scholar]
- 51.Peacock A Observations on the prenatal development of the intervertebral disc in man. J Anat 1951;85(3):260–274. [PMC free article] [PubMed] [Google Scholar]
- 52.Rolfe RA, Bezer JH, Kim T, et al. Abnormal fetal muscle forces result in defects in spinal curvature and alterations in vertebral segmentation and shape. J Orthop Res 2017;35(10):2135–2144. doi: 10.1002/jor.23518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortes DH, Elliott DM. The Intervertebral Disc: Overview of Disc Mechanics. In: Shapiro IM, Risbud MV, eds. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease.; 2014:17–32. [Google Scholar]
- 54.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J 1976;157(1):267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta 1977;492(1):29–42. [DOI] [PubMed] [Google Scholar]
- 56.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol 1995;192(1):53–62. [DOI] [PubMed] [Google Scholar]
- 57.Hayes A Extracellular matrix in development of the intervertebral disc. Matrix Biol 2001;20(2):107–121. doi: 10.1016/S0945-053X(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 58.Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat 2001;198(Pt 1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Duance VC, Blain EJ. Zonal variations in cytoskeletal element organization, mRNA and protein expression in the intervertebral disc. J Anat 2008;213(6):725–732. doi: 10.1111/j.1469-7580.2008.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brickley-Parsons D, Glimcher MJ. Is the chemistry of collagen in intervertebral discs an expression of Wolff’s Law? A study of the human lumbar spine. Spine 1984;9(2):148–163. [DOI] [PubMed] [Google Scholar]
- 61.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res 1989;23(1):75–88. [DOI] [PubMed] [Google Scholar]
- 62.Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990;15(5):402–410. [DOI] [PubMed] [Google Scholar]
- 63.Elliott DM, Setton LA. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: experimental measurement and material model predictions. J Biomech Eng 2001;123(3):256–263. doi: 10.1115/1.1374202. [DOI] [PubMed] [Google Scholar]
- 64.Smith LJ, Elliott DM. Formation of lamellar cross bridges in the annulus fibrosus of the intervertebral disc is a consequence of vascular regression. Matrix Biol 2011;30(4):267–274. doi: 10.1016/j.matbio.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han SK, Chen C-W, Wierwille J, Chen Y, Hsieh AH. Three dimensional mesoscale analysis of translamellar cross-bridge morphologies in the annulus fibrosus using optical coherence tomography. J Orthop Res 2015;33(3):304–311. doi: 10.1002/jor.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu J, Schollum ML, Wade KR, Broom ND, Urban JPG. ISSLS prize winner: A detailed examination of the elastic network leads to a new understanding of annulus fibrosus organization. Spine 2015;40(15):1149–1157. doi: 10.1097/BRS.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 67.Iatridis JC, MaClean JJ, Ryan DA. Mechanical damage to the intervertebral disc annulus fibrosus subjected to tensile loading. J Biomech 2005;38(3):557–565. doi: 10.1016/j.jbiomech.2004.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iatridis JCJC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech 2004;37(8):1165–1175. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomaszewski KA, Walocha JA, Mizia E, Gładysz T, Głowacki R, Tomaszewska R. Age- and degeneration-related variations in cell density and glycosaminoglycan content in the human cervical intervertebral disc and its endplates. Pol J Pathol 2015;66(3):296–309. doi: 10.5114/pjp.2015.54964. [DOI] [PubMed] [Google Scholar]
- 70.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat 1975;120(Pt 1):113–130. [PMC free article] [PubMed] [Google Scholar]
- 71.Roughley PJ. Biology of Intervertebral Disc Aging and Degeneration. Spine 2004;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 72.Stockwell RA. The cell density of human articular and costal cartilage. J Anat 1967;101(Pt 4):753–763. [PMC free article] [PubMed] [Google Scholar]
- 73.van den Akker GGH, Eijssen LMT, Richardson SM, et al. A Membranome-Centered Approach Defines Novel Biomarkers for Cellular Subtypes in the Intervertebral Disc. Cartilage 2018:1947603518764260. doi: 10.1177/1947603518764260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chelberg MK, Banks GM, Geiger DF, Oegema TR. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat 1995;186 (Pt 1):43–53. [PMC free article] [PubMed] [Google Scholar]
- 75.Mackiewicz Z, Salo J, Konttinen YT, et al. Receptor activator of nuclear factor kappa B ligand in an experimental intervertebral disc degeneration. Clin Exp Rheumatol 2009;27(2):299–306. [PubMed] [Google Scholar]
- 76.Clouet J, Grimandi G, Pot-Vaucel M, et al. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford) 2009;48(11):1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- 77.Inerot S, Axelsson I. Structure and composition of proteoglycans from human annulus fibrosus. Connect Tissue Res 1991;26(1–2):47–63. doi: 10.3109/03008209109152163. [DOI] [PubMed] [Google Scholar]
- 78.van den Akker GGH, Surtel DAM, Cremers A, et al. Novel immortal cell lines support cellular heterogeneity in the human annulus fibrosus. PLoS ONE 2016;11(1):e0144497. doi: 10.1371/journal.pone.0144497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther 2010;12(1):R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 2007;236(6):1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 81.Torre OM, Das R, Berenblum RE, Huang AH, Iatridis JC. Neonatal mouse intervertebral discs heal with restored function following herniation injury. FASEB J 2018:fj201701492R. doi: 10.1096/fj.201701492R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakamichi R, Ito Y, Inui M, et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat Commun 2016;7:12503. doi: 10.1038/ncomms12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayes AJ, Isaacs MD, Hughes C, Caterson B, Ralphs JR. Collagen fibrillogenesis in the development of the annulus fibrosus of the intervertebral disc. Eur Cell Mater 2011;22:226–241. [DOI] [PubMed] [Google Scholar]
- 84.Beard HK, Ryvar R, Brown R, Muir H. Immunochemical localization of collagen types and proteoglycan in pig intervertebral discs. Immunology 1980;41(2):491–501. [PMC free article] [PubMed] [Google Scholar]
- 85.Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 2003;130(6):1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- 86.Schneider TO, Mueller SM, Shortkroff S, Spector M. Expression of alpha-smooth muscle actin in canine intervertebral disc cells in situ and in collagen-glycosaminoglycan matrices in vitro. J Orthop Res 1999;17(2):192–199. doi: 10.1002/jor.1100170207. [DOI] [PubMed] [Google Scholar]
- 87.Bruehlmann SB, Matyas JR, Duncan NA. ISSLS prize winner: Collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine 2004;29(23):2612–2620. [DOI] [PubMed] [Google Scholar]
- 88.Nakai T, Sakai D, Nakamura Y, et al. CD146 defines commitment of cultured annulus fibrosus cells to express a contractile phenotype. J Orthop Res 2016;34(8):1361–1372. doi: 10.1002/jor.23326. [DOI] [PubMed] [Google Scholar]
- 89.Risbud MV, Guttapalli A, Tsai T-T, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine 2007;32(23):2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 90.Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am 2010;92(3):675–685. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine 2009;34(21):2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 92.Barreto Henriksson H, Lindahl A, Skioldebrand E, et al. Similar cellular migration patterns from niches in intervertebral disc and in knee-joint regions detected by in situ labeling: an experimental study in the New Zealand white rabbit. Stem Cell Res Ther 2013;4(5):104. doi: 10.1186/scrt315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brisby H, Papadimitriou N, Brantsing C, Bergh P, Lindahl A, Barreto Henriksson H. The presence of local mesenchymal progenitor cells in human degenerated intervertebral discs and possibilities to influence these in vitro: a descriptive study in humans. Stem Cells Dev 2013;22(5):804–814. doi: 10.1089/scd.2012.0179. [DOI] [PubMed] [Google Scholar]
- 94.Henriksson HB, Svala E, Skioldebrand E, Lindahl A, Brisby H. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc: a descriptive study in the New Zealand white rabbit. Spine 2012;37(9):722–732. doi: 10.1097/BRS.0b013e318231c2f7. [DOI] [PubMed] [Google Scholar]
- 95.Liu C, Guo Q, Li J, et al. Identification of rabbit annulus fibrosus-derived stem cells. PLoS ONE 2014;9(9):e108239. doi: 10.1371/journal.pone.0108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yasen M, Fei Q, Hutton WC, et al. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim Biophys Sin (Shanghai) 2013;45(5):368–376. doi: 10.1093/abbs/gmt019. [DOI] [PubMed] [Google Scholar]
- 97.García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat 2010;217(1):1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 99.Kandel R, Roberts S, Urban JPG. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J 2008;17 Suppl 4:480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Iatridis JC, Michalek AJ, Purmessur D, Korecki CL. Localized intervertebral disc injury leads to organ level changes in structure, cellularity, and biosynthesis. Cell Mol Bioeng 2009;2(3):437–447. doi: 10.1007/s12195-009-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walter BA, Korecki CL, Purmessur D, Roughley PJ, Michalek AJ, Iatridis JC. Complex loading affects intervertebral disc mechanics and biology. Osteoarthr Cartil 2011;19(8):1011–1018. doi: 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abbott RD, Howe AK, Langevin HM, Iatridis JC. Live free or die: stretch-induced apoptosis occurs when adaptive reorientation of annulus fibrosus cells is restricted. Biochem Biophys Res Commun 2012;421(2):361–366. doi: 10.1016/j.bbrc.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res 2016;34(8):1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murakami H, Yoon ST, Attallah-Wasif ES, Tsai K-J, Fei Q, Hutton WC. The expression of anabolic cytokines in intervertebral discs in age-related degeneration. Spine 2006;31(16):1770–1774. doi: 10.1097/01.brs.0000227255.39896.f3. [DOI] [PubMed] [Google Scholar]
- 106.Lob A Die zusammenhÄnge zwischen den verletzungen der bandscheiben und der spondylosis deformans im tierversuch. Deutsche Zeitschrift für Chirurgie 1933;240(5–6):421–440. doi: 10.1007/BF02793786. [DOI] [Google Scholar]
- 107.Keyes DC, Compere EL. THE NORMAL AND PATHOLOGICAL PHYSIOLOGY OF THE NUCLEUS PULPOSUS OF THE INTERVERTEBRAL DISC: An Anatomical, Clinical, and Experimental Study. J Bone Joint Surg 1932. [Google Scholar]
- 108.Hsieh AH, Hwang D, Ryan DA, Freeman AK, Kim H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine 2009;34(10):998–1005. doi: 10.1097/BRS.0b013e31819c09c4. [DOI] [PubMed] [Google Scholar]
- 109.Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res 2013;31(8):1276–1282. doi: 10.1002/jor.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rousseau M-AA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC. Stab incision for inducing intervertebral disc degeneration in the rat. Spine 2007;32(1):17–24. doi: 10.1097/01.brs.0000251013.07656.45. [DOI] [PubMed] [Google Scholar]
- 111.Fazzalari NL, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine 2001;26(23):2575–2581. [DOI] [PubMed] [Google Scholar]
- 112.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine 2009;34(21):2338–2345. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 113.Sobajima S, Kompel JF, Kim JS, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine 2005;30(1):15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 114.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 115.Iatridis JC, Hecht AC. Commentary: Does needle injection cause disc degeneration? News in the continuing debate regarding pathophysiology associated with intradiscal injections. Spine J 2012;12(4):336–338. doi: 10.1016/j.spinee.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther 2016;18:3. doi: 10.1186/s13075-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci 2017;1410(1):68–84. doi: 10.1111/nyas.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grönblad M, Virri J, Tolonen J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine 1994;19(24):2744–2751. [DOI] [PubMed] [Google Scholar]
- 119.Kanerva A, Kommonen B, Grönblad M, et al. Inflammatory cells in experimental intervertebral disc injury. Spine 1997;22(23):2711–2715. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Chee A, Shi P, et al. Allogeneic articular chondrocyte transplantation downregulates interleukin 8 gene expression in the degenerating rabbit intervertebral disk in vivo. Am J Phys Med Rehabil 2015;94(7):530–538. doi: 10.1097/PHM.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai A, Moon A, Purmessur D, et al. Assessment of functional and behavioral changes sensitive to painful disc degeneration. J Orthop Res 2015;33(5):755–764. doi: 10.1002/jor.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hampton D, Laros G, McCarron R, Franks D. Healing potential of the anulus fibrosus. Spine 1989;14(4):398–401. [DOI] [PubMed] [Google Scholar]
- 123.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine 1990;15(8):762–767. [DOI] [PubMed] [Google Scholar]
- 124.You C, Zhu K, Liu X, et al. Tumor necrosis factor-α-dependent infiltration of macrophages into the dorsal root ganglion in a rat disc herniation model. Spine 2013;38(23):2003–2007. doi: 10.1097/BRS.0b013e3182a84701. [DOI] [PubMed] [Google Scholar]
- 125.Rand NS, Dawson JM, Juliao SF, Spengler DM, Floman Y. In vivo macrophage recruitment by murine intervertebral disc cells. J Spinal Disord 2001;14(4):339–342. [DOI] [PubMed] [Google Scholar]
- 126.Nakazawa KR, Walter BA, Laudier DM, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J 2017. doi: 10.1016/j.spinee.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wiet MG, Piscioneri A, Khan SN, Ballinger MN, Hoyland JA, Purmessur D. Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain. Sci Rep 2017;7(1):12492. doi: 10.1038/s41598-017-12666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Albert HB, Lambert P, Rollason J, et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 2013;22(4):690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rajasekaran S, Tangavel C, Aiyer SN, et al. ISSLS PRIZE IN CLINICAL SCIENCE 2017: Is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur Spine J 2017;26(5):1384–1400. doi: 10.1007/s00586-017-4972-3. [DOI] [PubMed] [Google Scholar]
- 130.Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J 2013;22(4):697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dudli S, Sing DC, Hu SS, et al. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. Eur Spine J 2017;26(5):1362–1373. doi: 10.1007/s00586-017-4955-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther 2007;9(4):R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith JW, Walmsley R. Experimental incision of the intervertebral disc. J Bone Joint Surg Br 1951;33-B(4):612–625. doi: 10.1302/0301-620X.33B4.612. [DOI] [PubMed] [Google Scholar]
- 134.Key JA, Ford LT. Experimental intervertebral-disc lesions. J Bone Joint Surg Am 1948;30A(3):621–630. [PubMed] [Google Scholar]
- 135.Ethier DB, Cain JE, Yaszemski MJ, et al. The influence of anulotomy selection on disc competence. A radiographic, biomechanical, and histologic analysis. Spine 1994;19(18):2071–2076. [DOI] [PubMed] [Google Scholar]
- 136.Kääpä E, Holm S, Han X, Takala T, Kovanen V, Vanharanta H. Collagens in the injured porcine intervertebral disc. J Orthop Res 1994;12(1):93–102. doi: 10.1002/jor.1100120112. [DOI] [PubMed] [Google Scholar]
- 137.Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS ONE 2013;8(5):e64302. doi: 10.1371/journal.pone.0064302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Illien-Jünger S, Torre OM, Kindschuh WF, Chen X, Laudier DM, Iatridis JC. AGEs induce ectopic endochondral ossification in intervertebral discs. Eur Cell Mater 2016;32:257–270. doi: 10.22203/eCM.v032a17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dahia CL, Mahoney EJ, Durrani AA, Wylie C. Postnatal growth, differentiation, and aging of the mouse intervertebral disc. Spine 2009;34(5):447–455. doi: 10.1097/BRS.0b013e3181990c64. [DOI] [PubMed] [Google Scholar]
- 140.Freeman BJC, Kuliwaba JS, Jones CF, et al. Allogeneic Mesenchymal Precursor Cells Promote Healing in Postero-lateral Annular Lesions and Improve Indices of Lumbar Intervertebral Disc Degeneration in an Ovine Model. Spine 2016;41(17):1331–1339. doi: 10.1097/BRS.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 141.Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J 2010;19(12):2110–2116. doi: 10.1007/s00586-010-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iismaa SE, Kaidonis X, Nicks AM, et al. Comparative regenerative mechanisms across different mammalian tissues. npj Regenerative medicine 2018;3:6. doi: 10.1038/s41536-018-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell 2011;21(1):172–185. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017;129:54–67. doi: 10.1016/j.biomaterials.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells 2016;8(5):185–201. doi: 10.4252/wjsc.v8.i5.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sakai D, Schol J. Cell therapy for intervertebral disc repair: Clinical perspective. Journal of orthopaedic translation 2017;9:8–18. doi: 10.1016/j.jot.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014;124(3):1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Porrello ER, Mahmoud AI, Simpson E, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A 2013;110(1):187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bassat E, Mutlak YE, Genzelinakh A, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017;547(7662):179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fan Y, Zhang Q, Li H, et al. Peptidomics analysis of transient regeneration in the neonatal mouse heart. J Cell Biochem 2017;118(9):2828–2840. doi: 10.1002/jcb.25933. [DOI] [PubMed] [Google Scholar]
- 152.Walters BJ, Liu Z, Crabtree M, Coak E, Cox BC, Zuo J. Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing. J Neurosci 2014;34(47):15751–15763. doi: 10.1523/JNEUROSCI.3200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cox BC, Chai R, Lenoir A, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 2014;141(4):816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lehoczky JA, Robert B, Tabin CJ. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci U S A 2011;108(51):20609–20614. doi: 10.1073/pnas.1118017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lehoczky JA, Tabin CJ. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc Natl Acad Sci U S A 2015;112(43):13249–13254. doi: 10.1073/pnas.1518874112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu L, Han M, Yan M, Lee E-C, Lee J, Muneoka K. BMP signaling induces digit regeneration in neonatal mice. Development 2010;137(4):551–559. doi: 10.1242/dev.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Howell K, Chien C, Bell R, et al. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep 2017;7:45238. doi: 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang Y-C, Urban JPG, Luk KDK. Intervertebral disc regeneration: do nutrients lead the way? Nat Rev Rheumatol 2014;10(9):561–566. doi: 10.1038/nrrheum.2014.91. [DOI] [PubMed] [Google Scholar]
- 159.Jiang L, Yuan F, Yin X, Dong J. Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism. Connect Tissue Res 2014;55(5–6):311–321. doi: 10.3109/03008207.2014.942419. [DOI] [PubMed] [Google Scholar]
- 160.Showalter BL, Beckstein JC, Martin JT, et al. Comparison of animal discs used in disc research to human lumbar disc: torsion mechanics and collagen content. Spine 2012;37(15):E900–7. doi: 10.1097/BRS.0b013e31824d911c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine 2007;32(3):328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 162.Illien-Junger S, Walter BA, Mayer JE, Hecht AC, Iatridis JC. Chapter 22: Intervertebral disc culture models and their applications to study pathogenesis and repair In: Shapiro IM, Risbud M, eds. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease New York: Springer Wien; 2014:353–372. [Google Scholar]
- 163.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]