Abstract
Background
A positive association between birth weight (BW) and body mass index (BMI) has been shown among children in many populations. The aim of this study was to investigate BMI trajectory according to BW status and the protective effect of breastfeeding on the prevalence of overweight/obesity in children 6 years of age.
Methods
A retrospective cohort study was conducted between January 1, 2008 and December 31, 2016 utilizing data from the National Health Information Database of Korea. The 38,049 subjects were followed until the end of 2016, providing that subjects were completely eligible for all health check-ups from birth to 6 years of age. At each check-up period, multiple logistic regressions were used to investigate the association between BW status (low birth weight [LBW], normal birth weight [NBW], high birth weight [HBW]) and growth development.
Results
HBW infants were highly likely to be overweight/obese compared to NBW infants (odds ratio [OR], 1.70–2.35) and LBW infants were highly likely to be underweight (OR, 1.69–2.20) through 6 years of age. The risk of overweight/obesity decreased significantly if HBW infants were breast-fed for 6 months (OR, 0.54–0.76).
Conclusion
HBW status is associated with overweight/obesity during early childhood. Exclusive breastfeeding is a significant protective factor against overweight/obesity in children with HBW.
Keywords: Exclusive Breastfeeding, Overweight, Obesity, High Birth Weight, Children
Graphical Abstract
INTRODUCTION
The increase in the prevalence of childhood overweight and obesity, which began in the 1970s, has grown into a global epidemic.1,2 Obesity persists from childhood to adolescence and into adulthood and is a leading cause of health problems. Extensive epidemiological studies have demonstrated that obesity is an important risk factor for many chronic diseases in adulthood, including diabetes mellitus, stroke, ischemic heart disease, hypertension, and several cancers.3
Early childhood may be a particularly sensitive period in which there is an increase in variation in levels of fatness.4 The development of overweight and obesity among 4- to 5-year-old children may be associated with the timing of the adiposity rebound, the point at which body mass index (BMI) begins to rise from the childhood trough.5 Several studies have indicated that high birth weight (HBW) is associated with an increased risk of childhood and adolescent obesity.6,7,8
Children in the low ranges of BW tended to show rapid weight gain in early life, which ultimately may lead to obesity in adulthood. Children in the upper ranges of BW also had an increased BMI in adulthood, showing signs of tracking.9 The tendency of BW to determine growth status throughout childhood has been demonstrated in several studies.10 Over the previous decade, many studies have indicated an association between breastfeeding and childhood obesity.11,12 However, literature regarding the preventive effect of breastfeeding on childhood obesity is still inconsistent.
Therefore, the aim of this study was to investigate BMI trajectory according to BW status and to study the protective effect of breastfeeding on obesity in a cohort of children who were followed longitudinally from birth to 6 years of age utilizing the National Health Information Database (NHID).
METHODS
Study design
A cohort study was conducted with 273,121 subjects born in 2008 who underwent the first infant medical check-up provided by NHID in 2008 or 2009. NHID is a public database which catalogs health care utilization, medical check-ups, socio-demographic variables, and mortality for the whole population of South Korea, and was formed by the National Health Insurance Service (NHIS). We utilized the qualification database and infant medical check-up data from the NHID database.
The selected 38,039 subjects had completed all seven infant medical check-ups: the 1st check-up at 4 to 6 months of age, 2nd check-up at 9 to 12 months of age, 3rd check-up at 18 to 24 months of age, 4th check-up at 30 to 36 months of age, 5th check-up at 42 to 48 months of age, 6th check-up at 54 to 60 months of age, and 7th check-up at 66 to 72 months of age. At each medical check-up, the height (cm) and weight (kg) of children were measured and BMI (kg/m2) was calculated accordingly. In the qualification database, information regarding socioeconomic status (SES) was retrieved from the monthly contribution, which was calculated based on household income. We categorized SES into five groups: one group of medical aid beneficiaries and four groups with similar population size. Through questionnaires in medical check-ups, BW and feeding behavior were obtained. Feeding behavior was categorized into breastfeeding only, formula feeding only and mixed feeding based on the response to the questionnaire at the1st check-up.
Exclusive breastfeeding was defined as infants who were fed no food other than breast milk for 6 months. The data included the infant's BW, but not the maternal gestational age. Therefore, infants were categorized into three groups by BW; low birth weight (LBW) was defined as BW less than or equal to 2,500 g,13 normal birth weight (NBW) was defined as BW greater than 2,500 g and less than 4,000 g, and HBW was defined as BW greater than or equal to 4,000 g. For the growth development outcome, underweight, overweight and obesity were defined based on BMI, according to criteria established by Korean National Growth Charts.14 Subjects with a BMI less than or equal to the 10th percentile were classified as underweight; those with BMI greater than or equal to the 90th percentile were classified as overweight, and those with BMI greater than or equal to the 97th percentile, as obese. For subjects over 2 years of age, we referenced the cut-off BMI from the Korean National Growth Charts by their age and gender. As there was no reference for subjects from birth up to 2 years of age, we applied the same standard for the growth development outcome for this group as well.
Statistical analyses
General characteristics of the subjects of each check-up period are presented as numbers and percentages, and the differences were compared using the χ2 test. At each check-up period, multinomial logistic regression was used to investigate the association between three BW groups (LBW, NBW, and HBW) and three groups of growth development (overweight/obese, normal, and underweight), adjusting for sex and parental SES at infant's birth. These adjustments are selected from the available variables based on a directed acyclic graph. For each check-up, multiple logistic regression was used to investigate the effect of breastfeeding behavior on overweight. All analyses were performed with SAS statistical package version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board (IRB) of Ewha Womans University College of Medicine (approval No. 2017-01-029) and National Health Insurance Service (research management No. NHIS-2017-1-069). Informed consent was waived by the board.
RESULTS
Table 1 shows the general characteristics of the subjects by LBW, NBW, and HBW groups. Of 38,049 subjects, 19,380 (51.0%) were male and 18,659 (49.0%) were female. The mean body weight were 2.25 ± 0.31 kg, 3.23 ± 0.32 kg and 4.14 ± 0.17 kg in LBW, NBW, and HBW groups, respectively. There were more boys than girls in the HBW group, and LBW infants received formula milk more than breast milk (P < 0.001).
Table 1. General characteristics at each check-up period by BW groups.
| BW group | No. (%) or mean (± SD) | ||||
|---|---|---|---|---|---|
| LBW (n = 2,312) | NBW (n = 34,332) | HBW (n = 1,405) | P value | ||
| BW, kg | 2.25 ± 0.31 | 3.23 ± 0.32 | 4.14 ± 0.17 | ||
| Sex | < 0.001 | ||||
| Male | 1,012 (44.0) | 17,469 (50.9) | 899 (64.0) | ||
| SES | 0.960 | ||||
| 1 | 67 (2.9) | 967 (2.8) | 39 (2.8) | ||
| 2 | 514 (22.3) | 7,612 (22.2) | 326 (23.2) | ||
| 3 | 643 (27.9) | 9,831 (28.6) | 407 (29.0) | ||
| 4 | 584 (25.4) | 8,663 (25.2) | 334 (23.8) | ||
| 5 | 494 (21.5) | 7,259 (21.1) | 299 (21.3) | ||
| Feeding | < 0.001 | ||||
| Breastfeed | 724 (31.9) | 16,487 (48.4) | 673 (47.9) | ||
| Formula milk | 1,071 (47.1) | 10,780 (31.6) | 416 (29.6) | ||
| Mixed | 477 (21.0) | 6,816 (20.0) | 303 (22.5) | ||
BW = birth weight, SES = socioeconomic status, SD = standard deviation, LBW = low birth weight, NBW = normal birth weight, HBW = high birth weight.
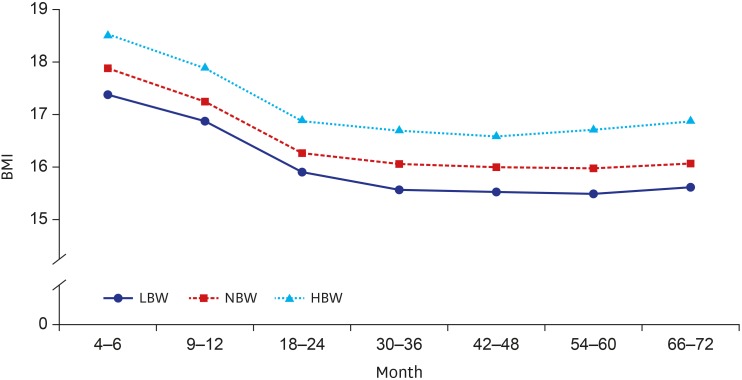
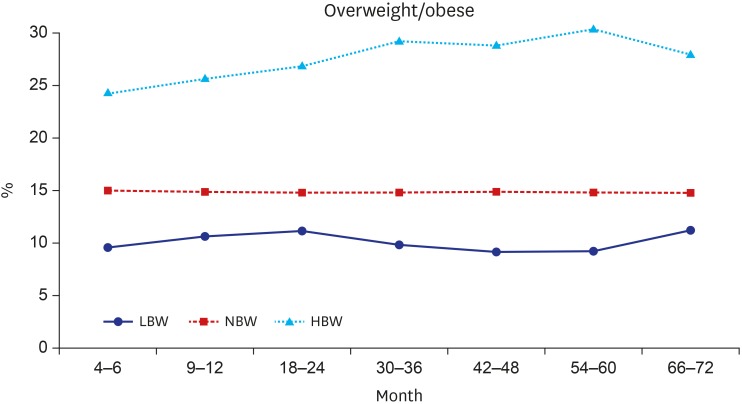
Fig. 1 presents the trajectory of mean BMI by birthweight status according to age. Overall, infants' BMI decreased until 24 months after birth and stayed relatively constant afterwards. At each check-up period, the BMIs between three groups showed statistically significant difference (P < 0.001). Children born with HBW had higher BMIs and children born with LBW had lower BMIs compared to NBW children throughout the follow-up period. Fig. 2 shows the trend of prevalence of overweight/obesity by BW group with time. The percentage of overweight/obesity among those three BW groups represents statistically significant difference at each check-up period (P < 0.001). About 10% of infants in the LBW group and 15% of infants in the NBW group became overweight/obese. However, more than 25% of HBW infants became overweight/obese.
Fig. 1. The mean BMI trajectory of the BW groups at each check-up period.
BMI = body mass index, BW = birth weight, LBW = low birth weight, NBW = normal birth weight, HBW = high birth weight.
Fig. 2. The prevalence of overweight/obesity at each check-up period in each group.
LBW = low birth weight, NBW = normal birth weight, HBW = high birth weight.
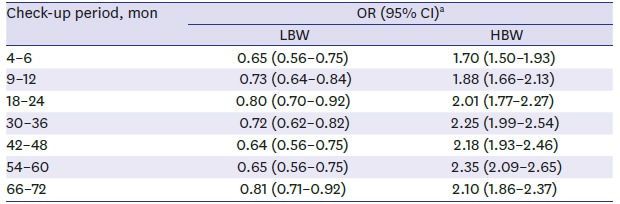
Table 2 presents the odds ratio (OR) of overweight/obesity for the LBW or HBW groups compared to the NBW group at each check-up. Until 72 months after birth, LBW infants have statistically significant lower risk of becoming overweight/obese, with ORs ranging from 0.64 to 0.81. On the contrary, HBW infants showed higher risk of overweight/obesity compared to the NBW group (1.70–2.35) throughout the first 6 years of their lives.
Table 2. The effect of BW on the risk of early childhood overweight/obesity at each check-up period comparing to the NBW group.
| Check-up period, mon | OR (95% CI)a | |
|---|---|---|
| LBW | HBW | |
| 4–6 | 0.65 (0.56–0.75) | 1.70 (1.50–1.93) |
| 9–12 | 0.73 (0.64–0.84) | 1.88 (1.66–2.13) |
| 18–24 | 0.80 (0.70–0.92) | 2.01 (1.77–2.27) |
| 30–36 | 0.72 (0.62–0.82) | 2.25 (1.99–2.54) |
| 42–48 | 0.64 (0.56–0.75) | 2.18 (1.93–2.46) |
| 54–60 | 0.65 (0.56–0.75) | 2.35 (2.09–2.65) |
| 66–72 | 0.81 (0.71–0.92) | 2.10 (1.86–2.37) |
BW = birth weight, OR = odds ratio, CI = confidence interval, LBW = low birth weight, HBW = high birth weight, NBW = normal birth weight.
aORs and its associated 95% CIs from multinomial logistic regression model after adjusting for sex, socioeconomic status, and breastfeeding behavior.
Lastly, we explored the effect of breastfeeding behavior on overweight/obesity. We compared infants who had been only breastfed, only received formula milk, and received mixed feeding for up to 6 months after birth. The adjusted ORs with 95% confidence intervals (CIs; log scale) of overweight/obesity based on feeding behavior in different BW groups are presented in Table 3. In all three groups (NBW, HBW and LBW), infants who received only breast milk showed lower tendency of overweight/obesity than infants with formula milks in every check-up periods. There were some contrary results. Among infants in HBW group, breast only at 4–6 months, mixed and breast milk only at 66–72 months and LBW infants with breast milk only at 54–60 months showed increased overweight/obesity over formula milk infants. However, there was no statistical significance (P > 0.05). In short, for both HBW and NBW infants, exclusive breastfeeding generally had a statistically significant protective effect against overweight/obesity, and the effect was higher in the HBW group (P < 0.001).
Table 3. The effect of breastfeeding on the risk of early childhood overweight/obesity by BW group at each check-up period.
| Check-up period, mon | OR (95% CI)a | |||
|---|---|---|---|---|
| LBW | NBW | HBW | ||
| 4–6 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.80 (0.54–1.16) | 0.98 (0.90–1.07) | 0.94 (0.66–1.34) | |
| Breastmilk only | 0.83 (0.60–1.15) | 1.05 (0.98–1.12) | 1.14 (0.85–1.51)b | |
| 9–12 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.76 (0.53–1.09) | 0.85 (0.78–0.92) | 0.78 (0.56–1.09) | |
| Breastmilk only | 0.74 (0.54–1.01) | 0.74 (0.69–0.79) | 0.69 (0.52–0.91) | |
| 18–24 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.95 (0.68–1.33)b | 0.83 (0.77–0.90) | 0.71 (0.51–0.98) | |
| Breastmilk only | 0.68 (0.50–0.94) | 0.61 (0.57–0.65) | 0.54 (0.41–0.71) | |
| 30–36 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.73 (0.50–1.06) | 0.94 (0.86–1.02)b | 0.66 (0.48–0.92) | |
| Breastmilk only | 0.75 (0.54–1.03) | 0.75 (0.70–0.81) | 0.73 (0.56–0.96) | |
| 42–48 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.82 (0.56–1.21) | 0.97 (0.89–1.05)b | 0.69 (0.50–0.97) | |
| Breastmilk only | 0.92 (0.66–1.27) | 0.85 (0.79–0.91) | 0.76 (0.58–1.00) | |
| 54–60 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.93 (0.63–1.37) | 0.92 (0.84–1.00) | 0.75 (0.54–1.03) | |
| Breastmilk only | 1.19 (0.86–1.64)b | 0.87 (0.81–0.93) | 0.69 (0.53–0.90) | |
| 66–72 | ||||
| Formula milk | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| Mixed | 0.68 (0.49–0.96) | 0.95 (0.88–1.04) | 1.02 (0.72–1.45)b | |
| Breastmilk only | 0.79 (0.60–1.03) | 0.87 (0.81–0.93) | 1.24 (0.92–1.66)b | |
BW = birth weight, OR = odds ratio, CI = confidence interval, LBW = low birth weight, NBW = normal birth weight, HBW = high birth weight.
aORs and its associated 95% CIs; bP > 0.05.
DISCUSSION
Our study found out that HBW infants are more likely to be overweight/obese compared to NBW infants, and LBW infants were more likely to become underweight compared to NBW infants until 6 years of age. And exclusive breastfeeding for 6 months was associated with a lower risk of childhood overweight and obesity mostly in HBW group.
Extensive epidemiological studies have demonstrated a linear positive relationship between BW and BMI in adulthood.15,16A population-based cohort study from Denmark indicated an increased risk of overweight for children 6–13 years of age with BWs > 4.0 kg compared to those with a BW between 3.0 and 3.49 kg.17 A meta-analysis of 66 studies from 26 countries demonstrated that HBW (> 4 kg) was positively associated with increased odds of childhood overweight (OR, 1.66; 99% CI, 1.55–1.77) compared to NBW (2.5–4 kg).18 A multinational, cross-sectional study of 5,141 children aged 9–11 years in 12 countries found that the ORs of childhood obesity were significantly higher among children whose BW was 3,500–3,999 g (OR, 1.45; 95% CI, 1.10–1.92) or > 4,000 g (OR, 2.08; 95% CI, 1.47–2.93) compared to the control group (2,500–2,999 g).7 The present study showed similar results as previous ones. Our study demonstrated that the mean BMI of the HBW group was higher than that of the NBW group until 6 years of age. HBW (≥ 4,000 g) infants were likely to be overweight/obese compared to NBW infants at 3 years (OR, 2.25; 95% CI, 1.99–2.54) and at 5 years (OR, 2.35; 95% CI, 2.09–2.65), and there was higher OR between HBW (≥ 4,000 g) and childhood overweight/obesity compared to other previous studies.
The risk factors for higher BW including gestation over-nutrition, maternal diabetes mellitus, maternal obesity, maternal smoking, excessive maternal weight gain, and prolonged gestation increased the risk of later fatness.19,20 In addition, genetic predisposition, nutritional status, SES, lifestyle, low level of education, intrauterine growth retardation, maternal and parental body size, and short sleep duration may all have an impact on BW and the risk of overweight in adulthood. More recent evidence also suggests that the effect of maternal nutrition on fetal growth can be programmed during the periconceptional period, thus contributing to an intergenerational cycle of overweight and obesity.21 Unfortunately, we did not have information on maternal factors (esp. gestational age), but these may also be directly associated with subsequent obesity in the offspring.
Evidence supporting the relationship between breastfeeding and childhood obesity development is controversial and less conclusive. According to the World Health Organization recommendations, infants should be exclusively breastfed for the first 6 months, and breastfeeding should be supplemented with additional foods for the first 2 years.22 Systematic review and meta-analysis demonstrated that breastfeeding significantly reduces the incidence of overweight and obesity and that exclusive long term breastfeeding has been strongly correlated with a reduction in LDL cholesterol, blood pressure-related disorders, type 2 diabetes mellitus, and cardiovascular dysfunction.23 Bergman et al.24 conducted a longitudinal birth cohort study that tested whether exclusive breastfeeding was correlated with BMI at six years of age. By 3 months, bottle-fed had significantly higher BMIs and thicker skin folds than breast-fed. From the age 4 years to 5 years and 6 years in bottle-fed, the prevalence of obesity nearly doubled and tripled, respectively. Early bottle feeding brings forward the obesity rebound, predictive of obesity in later life. Harder et al.25 reported a dose-dependent association between a longer duration of breastfeeding and a decrease in the risk of overweight. Breastfeeding for < 3 months provided a minor protective effect for childhood obesity, while breastfeeding for > 7 months showed high protection. Our study showed that exclusive breastfeeding for 6 months in HBW and NBW infants was associated with a lower risk of childhood overweight and obesity. The protective effect was higher in the HBW group compared to the NBW group.
In Table 3, some data showed discordant results, such as the 95% CI in the 54–60 months check-up period in LBW group is below 1 and the 4–6 months check-up period in NBW and HBW groups are over 1. These discrepancies can be explained because the physical and environmental conditions of the subjects during the check-up period could change, affecting weight, and this could bias the results.
There are several possible explanations for breast feeding on protection against obesity. First, cow's milk provides higher levels of fat and protein than breast milk. Higher protein and fat levels found in bovine based formula leads to increased secretion of insulin growth factor (IGF-1) and stimulates the overproduction of adipocytes, which are associated with overweight and obesity.26 Second, breast milk contains more bioactive substances, such as leptin and ghrelin, which can influence the proliferation and differentiation of infant adipocytes.27 Third, the different composition of gut microflora in formula-fed infants from breastfeeding could be linked to an increased risk of obesity. Fourth, concerning the feeding behavior hypothesis characterized by differential appetite regulation, one study noted that breastfed infants display a greater influence over their food intake, thereby developing self-control of caloric ingestion.28 Thus, breast milk is rich in effective ingredients with higher nutritional value.
Prevention strategies for childhood obesity to date have usually been unsuccessful and typically focus on changes in lifestyle during childhood or adolescence. Our study demonstrated that exclusive breastfeeding for the first 6 months is a significant protective factor against overweight/obesity in children with HBW. Future interventions might focus on environmental changes targeted at relatively short periods in early life, attempting to modify factors in uterus, in infancy, or in early childhood, which are independently related to the later risk of obesity.
The strengths of our study are large sample size from longitudinal birth cohort and high attendance rate, which minimize selection bias. However, there are some limitations as well. The information on maternal gestational age, maternal body weight, and BMI were not available in our study. We sub-analyzed our LBW survey item and identified that the portion of preterm babies was 3.58% in our data, which was lower than Korea statistics birth data. However, we admit that we could not check corrected age in LBW groups. Finally, we were not able to fully control for the effect of these variables in the association between BW and the risk of childhood obesity. A further study can be expected to require large-scale prospective long-term follow up on the correlation between breastfeeding and overweight/obesity, along with maternal information.
Footnotes
Funding: The research was supported by the Ministry of Health & Welfare of the Republic of Korea (grant No. HI15C2059) through the Korea Health Industry Development Institute.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee JW, Lee M, Lee J, Kim YJ, Ha E, Kim HS.
- Formal analysis: Lee M, Lee J, Ha E.
- Supervision: Ha E, Kim HS.
- Writing - original draft: Lee JW.
- Writing - review & editing: Kim HS.
References
- 1.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149(10):1085–1091. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening and interventions for overweight in children and adolescents: recommendation statement. Pediatrics. 2005;116(1):205–209. doi: 10.1542/peds.2005-0302. [DOI] [PubMed] [Google Scholar]
- 3.National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160(7):898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 4.Nader PR, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118(3):e594–e601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RW, Grant AM, Goulding A, Williams SM. Early adiposity rebound: review of papers linking this to subsequent obesity in children and adults. Curr Opin Clin Nutr Metab Care. 2005;8(6):607–612. doi: 10.1097/01.mco.0000168391.60884.93. [DOI] [PubMed] [Google Scholar]
- 6.Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity--a vicious circle across generations. Int J Obes. 2012;36(10):1320–1324. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Y, Ma J, Wang Y, Li W, Katzmarzyk PT, Chaput JP, et al. Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl. 2015;5(Suppl 2):S74–S79. doi: 10.1038/ijosup.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell EA, Stewart AW, Braithwaite I, Hancox RJ, Murphy R, Wall C, et al. Birth weight and subsequent body mass index in children: an international cross-sectional study. Pediatr Obes. 2017;12(4):280–285. doi: 10.1111/ijpo.12138. [DOI] [PubMed] [Google Scholar]
- 9.Taal HR, Vd Heijden AJ, Steegers EA, Hofman A, Jaddoe VW. Small and large size for gestational age at birth, infant growth, and childhood overweight. Obesity (Silver Spring) 2013;21(6):1261–1268. doi: 10.1002/oby.20116. [DOI] [PubMed] [Google Scholar]
- 10.Binkin NJ, Yip R, Fleshood L, Trowbridge FL. Birth weight and childhood growth. Pediatrics. 1988;82(6):828–834. [PubMed] [Google Scholar]
- 11.Chivers P, Hands B, Parker H, Bulsara M, Beilin LJ, Kendall GE, et al. Body mass index, adiposity rebound and early feeding in a longitudinal cohort (Raine Study) Int J Obes. 2010;34(7):1169–1176. doi: 10.1038/ijo.2010.61. [DOI] [PubMed] [Google Scholar]
- 12.Metzger MW, McDade TW. Breastfeeding as obesity prevention in the United States: a sibling difference model. Am J Hum Biol. 2010;22(3):291–296. doi: 10.1002/ajhb.20982. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Bowes WA., Jr Birth-weight-for-gestational-age patterns by race, sex, and parity in the United States population. Obstet Gynecol. 1995;86(2):200–208. doi: 10.1016/0029-7844(95)00142-e. [DOI] [PubMed] [Google Scholar]
- 14.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51(1):1–25. [Google Scholar]
- 15.Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr. 1998;132(5):768–776. doi: 10.1016/s0022-3476(98)70302-6. [DOI] [PubMed] [Google Scholar]
- 16.Martins EB, Carvalho MS. Birth weight and overweight in childhood: a systematic review. Cad Saude Publica. 2006;22(11):2281–2300. doi: 10.1590/s0102-311x2006001100003. [DOI] [PubMed] [Google Scholar]
- 17.Rugholm S, Baker JL, Olsen LW, Schack-Nielsen L, Bua J, Sørensen TI. Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obes Res. 2005;13(12):2187–2194. doi: 10.1038/oby.2005.271. [DOI] [PubMed] [Google Scholar]
- 18.Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7(10):e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers I EURO-BLCS Study Group. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27(7):755–777. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 20.Monasta L, Batty GD, Cattaneo A, Lutje V, Ronfani L, Van Lenthe FJ, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11(10):695–708. doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 21.McMillen IC, MacLaughlin SM, Muhlhausler BS, Gentili S, Duffield JL, Morrison JL. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin Pharmacol Toxicol. 2008;102(2):82–89. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Evidence on The Long-term Effects of Breastfeeding: Systematic Reviews and Meta-analyses. Geneva: World Health Organization; 2007. pp. 1–52. [Google Scholar]
- 23.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 24.Bergmann KE, Bergmann RL, Von Kries R, Böhm O, Richter R, Dudenhausen JW, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord. 2003;27(2):162–172. doi: 10.1038/sj.ijo.802200. [DOI] [PubMed] [Google Scholar]
- 25.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 26.Dewey KG. Is breastfeeding protective against child obesity? J Hum Lact. 2003;19(1):9–18. doi: 10.1177/0890334402239730. [DOI] [PubMed] [Google Scholar]
- 27.McCrory C, Layte R. Breastfeeding and risk of overweight and obesity at nine-years of age. Soc Sci Med. 2012;75(2):323–330. doi: 10.1016/j.socscimed.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Redsell SA, Edmonds B, Swift JA, Siriwardena AN, Weng S, Nathan D, et al. Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern Child Nutr. 2016;12(1):24–38. doi: 10.1111/mcn.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]