Abstract
The retinal protonated Schiff-base (RPSB) in its all-trans form is found in bacterial rhodopsins, whereas visual rhodopsin proteins host 11-cis RPSB. In both cases, photoexcitation initiates fast isomerization of the retinal chromophore, leading to proton transport, storage of chemical energy or signaling. It is an unsolved problem, to which degree this is due to protein interactions or intrinsic RPSB quantum properties. Here, we report on time-resolved action-spectroscopy studies, which show, that upon photoexcitation, cis isomers of RPSB have an almost barrierless fast 400 fs decay, whereas all-trans isomers exhibit a barrier-controlled slow 3 ps decay. Moreover, formation of the 11-cis isomer is greatly favored for all-trans RPSB when isolated. The very fast photoresponse of visual photoreceptors is thus directly related to intrinsic retinal properties, whereas bacterial rhodopsins tune the excited state potential-energy surface to lower the barrier for particular double-bond isomerization, thus changing both the timescale and specificity of the photoisomerization.
The primary photoresponse of protonated Schiff-base retinal in visual and bacterial rhodopsins is fast sub-ps isomerisation. Here, the authors show that the fast photoisomerization of rhodopsin is related to an intrinsic retinal property, whereas bacterial rhodopsins tune the excited-state potential-energy surface and improve the isomerization timescale and specificity.
Introduction
Bacteriorhodopsin (bR) is a seven helix membrane protein employed by halobacteria as a proton pump to convert light into chemical energy by transporting protons across the cell membrane1,2. It contains the all-trans retinal chromophore in the protonated Schiff-base form (RPSB), which isomerizes upon photoexcitation from its native all-trans to the 13-cis form with high specificity (64%) and speed (500 fs)3–5. According to the current belief, interactions within the protein facilitate isomerization that takes place with a speed about ten times faster than encountered without such interactions since isomerization takes about 2–10 ps in solutions4,6,7. In solutions, both 11-cis and 13-cis products are formed, with total quantum yields on the order of 10–30%4,6,8,9, and hence high specificity is obtained in proteins and not in solutions. It is also known that the retinal chromophore isomerizes upon photoexcitation in the gas phase; however, the efficiency as well as speed are unknown10,11.
Another class of retinal proteins is rhodopsins, responsible for the primary event in vision12, and having the 11-cis RPSB chromophore. Experimental13–18 and theoretical19–21 studies have shown that the 11-cis to all-trans isomerization in rhodopsins is extremely fast and likely caused by dynamics on an almost barrierless potential energy surface of the first (S1) excited state22,23. The progression from the initially populated Franck–Condon point to a fluorescent state in S1 takes, for example, <100 fs16, and full isomerization through a conical intersection is completed within 200 fs13,14,19. Rhodopsins also show high 65% quantum yields for isomerization to all-trans. The cis isomer is found to behave much faster than all-trans in MeOH at early times, but showed the same slow (4 ps) behavior on the longer timescale24–26. To account for both fast and slow dynamics, as well as the low quantum yield of only 0.22 in solution, it was proposed that a primary role of the protein is to adjust the ratio between ground-state chromophore conformations that lead to reactive and nonreactive decay channels27.
The two classes of proteins with all-trans and 11-cis RPSB chromophores both exhibit ultrafast sub-ps excited-state dynamics in their host proteins, yet their dynamics in solutions is multi-exponential and shows a long ps component. Interestingly, the lifetime of all-trans RPSB in solutions may be shortened by an order of magnitude from ~4 to ~400 fs upon chemical modifications of the retinal backbone with a wide spread in quantum yields28,29. The most likely explanation points towards tuning of the excited-state potential-energy surface around one or more conical intersections. These are notoriously hard to characterize theoretically, and electron correlation as well as nuclear dynamics must be treated accurately30. Based on QM/MM calculations of an RPSB model chromophore, it was proposed that the excited-state lifetime difference obtained in isolation and in MeOH may be traced to isomerization barriers primarily due to electrostatic interactions in the solution phase23.
Years of studies have been concerned with the related subject of spectral tuning (opsin shift) in proteins, where the band maximum traditionally has been compared to that obtained in MeOH solution (440 nm)8. This value is far from the intrinsic transition wavelength of about 610 nm obtained in the gas phase for both all-trans and 11-cis RPSB chromophores31–33, as well as those found in retinal-containing proteins. Interactions inside proteins, as well as in solutions, hence significantly change the energy gap between the ground and excited electronic states in the Franck–Condon region, and may also change the excited-state potential-energy surface around conical intersections. This may cause optimum conditions for fast isomerization and high quantum yields in proteins. Alternatively, the difference may be ascribed to significant perturbations taking place primarily in solutions, which slow down the isomerization and change the quantum yield significantly.
Despite numerous and advanced time-resolved studies of retinal proteins and RPSB chromophores in solutions, reference measurements obtained in the gas phase without solvent effects (H-bonding, steric interactions, electric fields, counterions, etc.) have not been obtained. Such measurements are needed to access the exact role of a hosting medium with respect to key properties such as quantum yields and excited-state lifetimes, which in turn are also related to fluorescence properties25,34,35. The reason for this situation is the tremendous experimental challenge encountered when working with extremely dilute clouds of ions in the gas phase, where the chromophore density is orders of magnitude smaller than in solutions. Despite the challenges, such data may provide a stringent test of ab initio theories, which must be able to satisfactorily deal with the chromophore in isolation before any predictions of transition energies, lifetimes, and quantum yields in environments may be considered fully reliable.
Here, we report on an experimental excited-state dynamics study of the RPSB chromophores in isolation (gas phase). A new experimental approach, combining time-resolved action spectroscopy36 with femtosecond pump-probe techniques37 in an ion-storage ring, is used. The new technique is applicable to anions36 as well as cations, such as the retinal protonated Schiff-base chromophore. The studies include variations of key parameters, such as the pump and probe wavelengths and the chromophore-ion temperature. High-level ab initio calculations are performed to support our conclusions.
Results
Pump-probe measurements
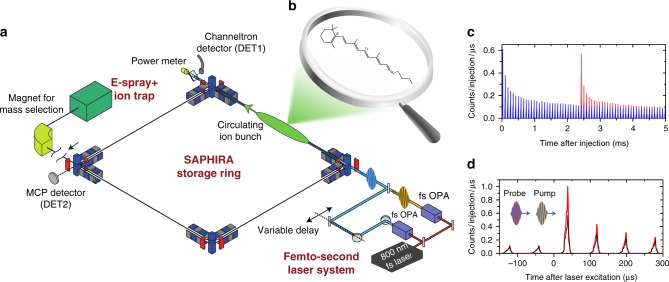
The experiments are performed at the ion-storage ring SAPHIRA38 (Fig. 1a), where RPSB chromophore ions (Fig. 1b) are stored in vacuo for several milliseconds. The absorption of photons is registered by the induced fragmentation and subsequent detection of neutral photofragments (Fig. 1c, d). The rate of statistical fragmentation (action) depends on the total internal energy of the ions, and therefore on the number of photons absorbed39. From the fragmentation rate, we are able to deduce when, after initial S0 → S1 excitation, a second probe photon of given energy and delay may be absorbed. This has been utilized in two different pump-probe schemes to obtain the excited-state decay as well as the ground-state recovery.
Fig. 1.
Experimental technique. a The experimental setup at the electrostatic ion-storage ring SAPHIRA38. Ions are produced by electrospraying retinal chromphores dissolved in methanol. The chromophore ions are stored in SAPHIRA before being irradiated by femtosecond laser pulses. b The all-trans protonated Schiff-base retinal chromophore. c Circulating ions produce neutral fragments from collisions with the residual gas (blue curve). Absorption of photons (after 2.4 ms of ion storage) increases the internal energy and causes statistical fragmentation after internal conversion to the ground state, as measured by the micro-channel plate (MCP) detector (red curve)39. d At each pump-probe delay laser-induced fragmentation is registered as a function of storage time in SAPHIRA. The pump-probe signal is obtained by subtracting background contributions (collisional background as well as single-pulse events) from the total two-pulse signal (see Supplementary Figure 1)
The present new approach of two-dimensional time-resolved action spectroscopy works as follows. A pump pulse first excites the RPSB chromophore in the broad S0 → S1 absorption band, which in the gas phase is between 550 and 650 nm31–33. To follow the evolution in the excited state, a probe wavelength of either 800 or 900 nm was used. At these wavelengths, subsequent S1 → Sn excitation may take place33 causing fast statistical fragmentation of the chromophore ions after sequential absorption of two photons (pump and probe) and internal conversion to the S0 ground state. The ground state is not re-excited at these probe wavelengths, and hence the fast two-photon fragmentation signal disappears as soon as the system has left the S1 excited state and returned to S0 (Fig. 2a, b). In contrast, when a probe wavelength, resonant with the S0 → S1 electronic transition is used, one retrieves the ground-state recovery after internal conversion by the growing fast, two-photon action signal with increasing pump-probe delay (Fig. 2c, d). Identification of the absorption of two consecutive photons is provided by the time at which the molecular dissociation takes place, hence time in two dimensions: the statistical fragmentation time and the pump-probe delay.
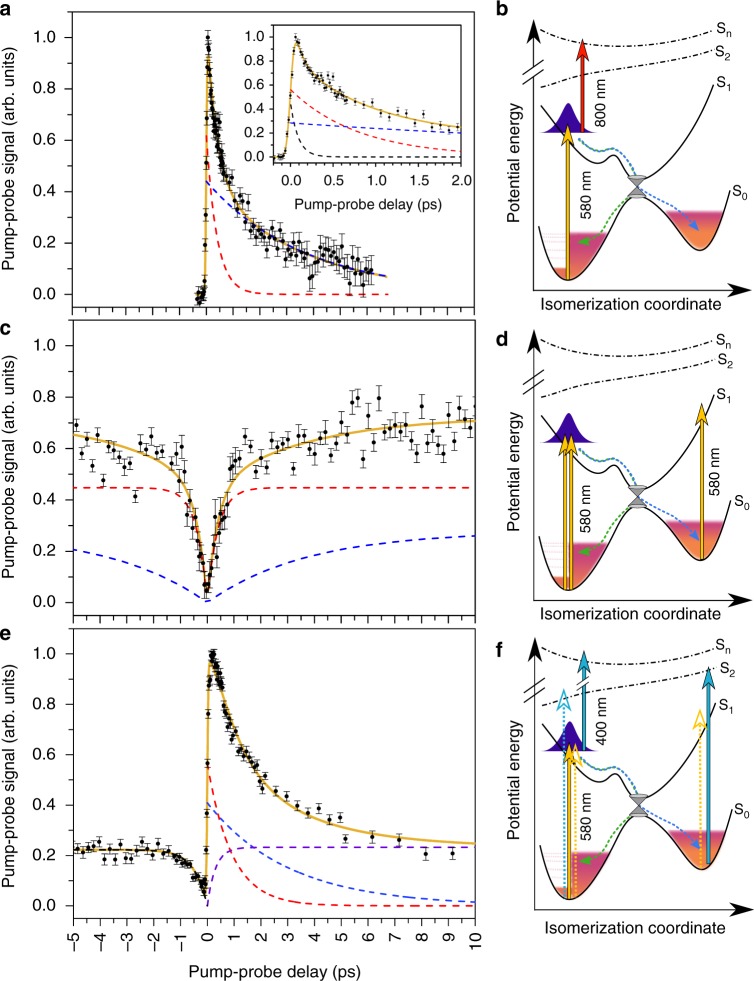
Fig. 2.
Pump-probe measurements of retinal protonated Schiff-base (RPSB) in vacuo. a, b Excited-state decay seen as the two-photon, pump-probe signal as a function of delay between pump (580 nm) and probe (800 nm) pulses. The full curve is from a fit with two exponential functions folded with the 80 fs cross-correlation in the experiment. The dashed lines represent the two decay components in the fit (395±42 fs and 3.7±0.4 ps). The insert shows a fit with three lifetime components, which specifically takes into account the separate early sub ~100 fs dynamics (fitted lifetimes 70±20 fs, 785±185 fs, and 5.6±1.8 ps). Data with 900 nm probe and other pump wavelengths within the S0→ S1 absorption band may be found in the Supplementary Table 1. c, d Ground-state recovery revealed by the two-photon, pump-probe signal as a function of delay between pump (580 nm) and probe (580 nm) pulses. The full curve is a fit with the two lifetimes found from the decay of the excited state (shown in a). e, f Dynamics revealed by the two-photon, pump-probe signal as a function of delay between pump (580 nm) and probe (400 nm) pulses. The chromophore may here absorb two photons (pump and probe) at all times, except right after excitation to S2 by 400 nm at small negative pump-probe delays. The fast recovery on the negative time axis is associated with the return to S0 after initial excitation (400 nm) to S2. All data shown here are recorded at room temperature. All error bars presented are one standard deviation (SD)
The time-resolved pump-probe fragmentation signal with 580 nm pump pulses and 800 nm probe pulses is shown in Fig. 2a. A fast decay is observed when the 580 nm pump comes prior to the 800 nm probe (positive delay). Here the RPSB chromophore is first excited to S1 by the pump pulse, and further excited to S3 by the 800 nm probe pulse33. No fast, two-photon, action is observed when the pulses arrive in the reversed order (negative delay) due to the lack of ground-state excitation with 800 nm pulses. The signal also disappears at late times when the ground state is repopulated after internal conversion. The data show two lifetimes of approximately 400 fs and 3 ps, with an ultrafast sub-100fs component, revealed from a three-exponential fit (Fig. 2a inset), ascribed to wavepacket dynamics in the Franck–Condon region. Similarly, when the probe-pulse wavelength is set to probe the same S0 to S1 transition as the pump pulse (580 nm, Fig. 2c), the two-photon absorption signal appears when the S0 ground state becomes repopulated with the same set of lifetimes. The two lifetimes were found to be insensitive to the choice of wavelength (see Supplementary Figures 2 and 3).
In Fig. 2e, we consider the case with 580 and 400 nm pulses. Both are capable of exciting the ground state (to S1 and S2, respectively), though with different efficiency. This gives a signal at asymptotic negative as well as positive delay times. The S0 → S2 transition has a low oscillator strength, and the 400 nm probe pulse is absorbed from the S1 state more efficiently than from S0 after internal conversion33, resulting in the overall decay of the signal at positive delay times. Notably, when the RPSB is excited to S2 by 400 nm excitation, it returns to the ground state in ca. 500 fs, as evidenced from the dynamics at negative delay times. Such fast relaxation of RPSB in S2 was also seen in fluorescence studies of RPSB ions in solvents35. However, more detailed gas-phase studies are required to fully resolve the relaxation dynamics of RPSB following its excitation to S2.
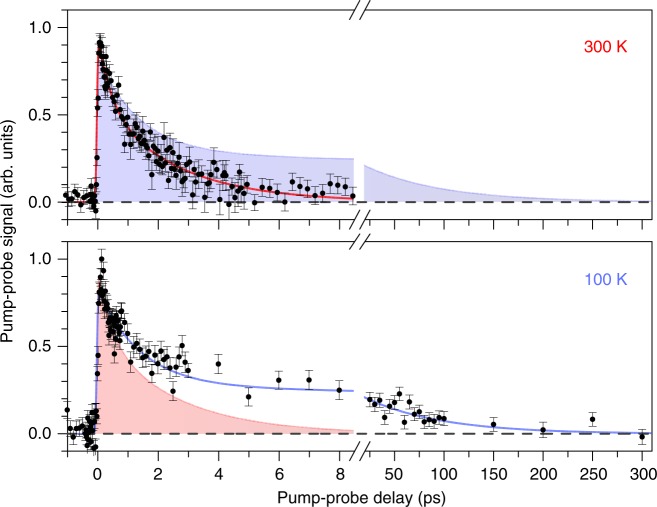
The temperature of the chromophore ions prior to injection into the ion-storage ring may be controlled by cooling the ion trap inside the ion source with liquid nitrogen. At 100 K, the lifetimes are found to increase significantly to 1.4 and 77 ps, as shown in Fig. 3 (compared to 400 fs and 3 ps at 300 K). This indicates that both lifetime components are controlled by one or more barriers in the excited state.
Fig. 3.
Pump-probe signal as a function of the pump-probe delay at 300 and 100 K. The excited-state decay of retinal protonated Schiff-base (RPSB) at two temperatures. Upper graph: Two-photon signal as a function of delay between pump (600 nm) and probe (800 nm) pulses, recorded at 300 K. Lifetimes of about 400 fs and 3 ps are here deduced. Lower graph: Same as upper graph, but recorded at 100 K where the lifetimes are 1.4 and 77 ps. The shaded areas are shown to provide a cross-comparison with the data obtained at the other temperature. All error bars presented are one standard deviation (SD)
It is in general challenging to completely isolate all-trans from single-cis isomers of RPSB in the gas phase as well as in solutions, where exposure to light generates isomerized forms that are non-statistically populated32,40,41. Ion mobility measurements of the RPSB chromophore have shown an almost equal content of all-trans versus single cis isomers after electrospraying the all-trans isomer41. Based on these results, we might expect a significant fraction of the ions populated in single cis isomers, separated from the all-trans configuration by relatively high barriers. We therefore consider both all-trans and single cis isomers as relevant RPSB forms in the following discussion.
High-level ab initio calculations
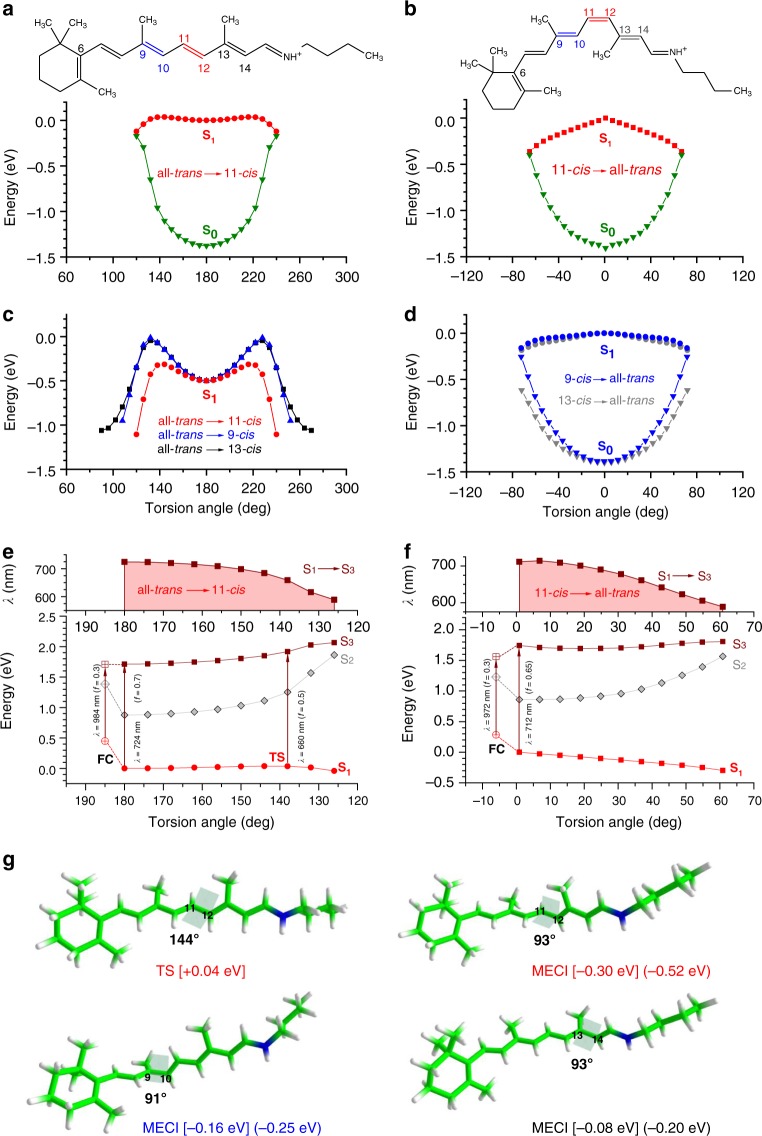
To interpret the experimental results, we have performed multi-state multi-reference perturbation theory excited-state calculations for the all-trans and single cis isomers of RPSB, using the XMCQDPT242/SA(2)-CASSCF(12/12)/cc-pVDZ method. In Fig. 4a–d, S1 relaxed geometry scans for rotation about three central double bonds in the all-trans and single cis chromophores are shown. We found that all-trans RPSB has small barriers that hinder rotation about the C9 = C10, C11 = C12, and C13 = C14 bonds and specifically that the barrier is the smallest (by a factor of 2.5) for rotation about the C11 = C12 bond. For all-trans → 11-cis isomerization, the barrier is 0.04 eV and the corresponding minimum-energy conical intersection (MECI) lies 0.3 eV below the minimum in S1, thus providing a direct route to the ground state after crossing the barrier in S1. The associated excited-state lifetime of all-trans RPSB is calculated to be 4 ps at 300 K and as long as 92 ps at 100 K. These values are in agreement with the long time component observed in the present measurements, 3 ps at 300 K and 77 ps at 100 K, which is thus assigned to the decay of S1 in all-trans RPSB.
Fig. 4.
Calculated potential-energy curves along the isomerization coordinates in all-trans, 11-cis, 9-cis, and 13-cis retinal protonated Schiff-base (RPSB). a–d Excited-state relaxed geometry scans for rotation about double bonds are shown in red (∠C10C11 = C12C13), blue (∠C8C9 = C10C11), and black (∠C12C13 = C14C15). Ground-state energies calculated at the S1 optimized geometries along the lowest-energy pathway are shown in green. The planar structures in S1 are obtained through the unconstrained optimization for all isomers, except for the 11-cis isomer, where the torsion angle is kept fixed. Note that the 6s-cis and 6s-trans rotomers are found to have the same barrier heights that hinder isomerization in S1 in all-trans RPSB (see Supplementary Figure 4). e, f Transient absorption calculated as vertical excitation energies S1–S3 in all-trans and 11-cis RPSB. Oscillator strengths f along the reaction pathways are shown in brackets. Note that the S1–S2 transition has a lower oscillator strength of <0.1 and gains some intensity (f = 0.2) through mixing with the S3 state near the Franck–Condon (FC) point and closer to the S1/S0 conical intersections. g Optimized structures of the lowest-energy transition state in S1 in all-trans RPSB (TS) and the minimum-energy conical intersections S1/S0 (MECI). Carbon atoms are depicted in green, hydrogen atoms in white, and the nitrogen atom in blue. Energies in square and round brackets are shown with respect to the all-trans and the corresponding single cis RPSB planar structures in S1, respectively
According to our calculations, the 9-cis, 11-cis, and 13-cis isomers exhibit essentially flat potential-energy surfaces around the corresponding minima in S1 and hence undergo ultrafast, almost barrierless internal conversion from S1 to S0. The steepest pathway is found for the 11-cis isomer (Fig. 4b). This is consistent with our measured fast sub-ps component. However, this component also shows a temperature dependence and increases from 400 fs at 300 K to 1.4 ps at 100 K (Fig. 3). Using the experimentally determined lifetime at the low temperature, we can estimate a barrier height for the cis isomers in S1. This gives a barrier which is approximately one order of magnitude lower than that found in all-trans RPSB. Such a small barrier height is consistent with the calculated flat potential-energy surface of the S1 state for the cis isomers, in particular for 9-cis and 13-cis RPSB. The almost barrierless photoisomerization in the gas phase documented here is indeed in agreement with CASSCF theory for the 11-cis isomer21.
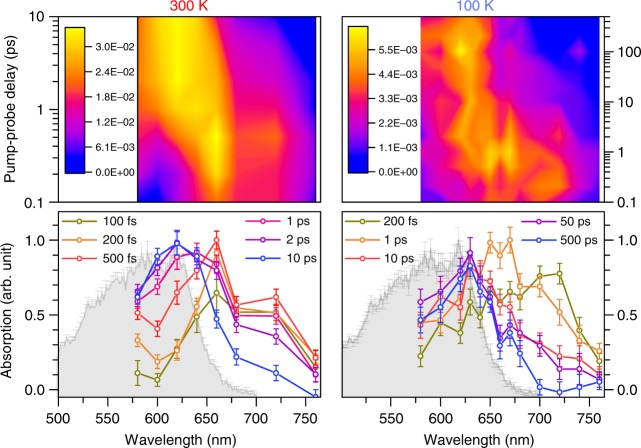
The calculated S1–Sn vertical excitation energies along the C11 = C12 isomerization coordinate of all-trans and 11-cis RPSB (Fig. 4e, f) show that the transient absorption (TA) corresponds to the S1–S3 excitation, which is the brightest transition along the entire reaction pathway. It shifts to shorter wavelengths as a vibrational wavepacket travels in the S1 state towards the conical intersection (Fig. 4g). Indeed, the enhanced experimental TA signals, seen at 720 and 660 nm, match the bright S1–S3 vertical transitions at the optimized geometries of the S1 minimum and transition state, respectively (see Fig. 5).
Fig. 5.
Transient absorption (TA) measurements of retinal protonated Schiff-base (RPSB) in the gas phase at 300 and 100 K. The TA data are shown as a function of variable probe wavelength (fixed 600 nm pump). The grey area shows the ground-state absorption profile at 300 K (one-photon spectrum). The transient absorption spectra at asymptotic long times (10–500 ps) correspond to absorption from a hot ground state, peaking at 620 nm. The excited-state absorption in the red part disappears with time concomitantly with a rise of the ground-state absorption with a lifetime, which strongly depends on temperature. Three major peaks at 720, 660, and 620 nm are seen at both temperatures. See discussion in the text
The S2 state does not appreciably mix with S1 along the reaction pathway as also shown by other methods like CASSCF21. The smallest energy gap is 0.9 eV and steeply rising as the chromophore twists (see Fig. 4e, f). The mechanism of internal conversion is thus defined solely by the interplay between the ground and first excited states in RPSB, where the latter is dominated by a charge-transfer character, as also demonstrated recently for RPSB in the gas phase43.
The absence of a potential-energy barrier in the single cis isomers can be attributed to sterical hindrance imposed by the repulsive interaction of the methyl groups with the hydrogen atoms of the conjugated tail of the chromophore, resulting in destabilization of the planar equilibrium structures in S1 and almost barrierless internal conversion in cis isomers. The equilibrium structures of 9-cis and 13-cis RPSB lie 0.09 and 0.12 eV higher in energy compared to that of all-trans RPSB, respectively. In the 11-cis isomer, the S1 planar structure lies 0.22 eV higher in energy compared to that of all-trans RPSB and is not stable with respect to rotation about the C11 = C12 bond at the XMCQDPT2/SA(2)-CASSCF(12,12)/cc-pVDZ level of theory.
The fraction of ions in the short-lived component increases from 40 to 57% in going from 300 to 100 K, thus indicating that the cis isomers are most likely formed through light exposure or collisions. The thermal backward cis → trans reaction in the ground state is strongly influenced by the temperature of the ions, hence resulting in a larger fraction of the cis isomers trapped in the ground state at low temperature. These fractions are also consistent with ion mobility spectrometry (IMS) measurements32,41. Furthermore, the all-trans → cis photoreaction shows specificity in the gas phase, as evidenced by our calculations, thus predominantly resulting in the 11-cis isomer.
Starting with an 11-cis sample, the decay is also described by two components, a short 650 ± 160 fs component and a long 4.7 ± 3 ps component at 300 K (see Fig. 6), similar to the dynamics of the all-trans sample. Here, the fraction of ions in the short-lived component is 78% (300 K), supporting that the fast component is connected with the 11-cis isomer.
Fig. 6.
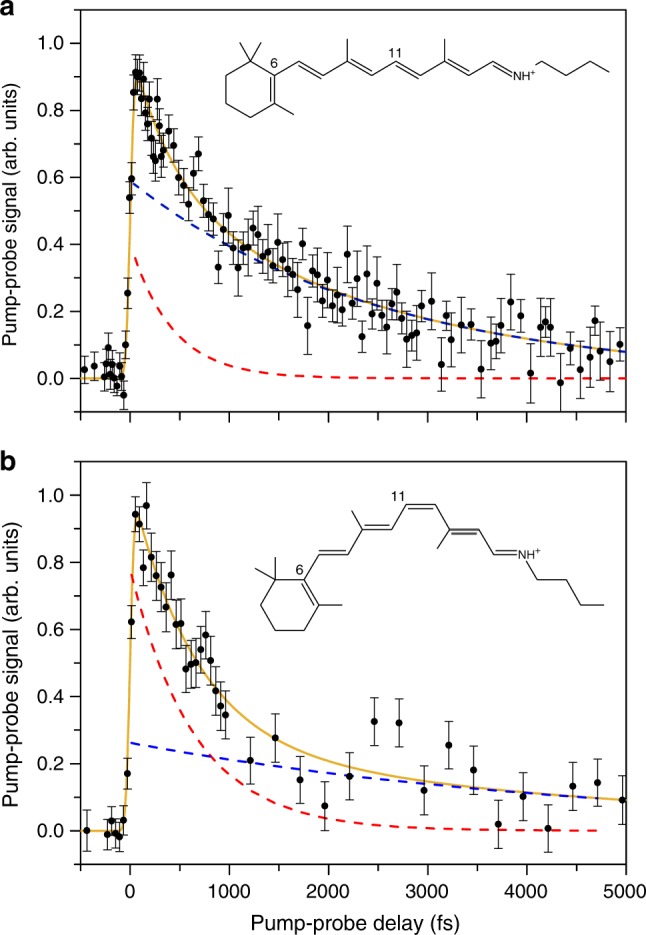
Pump-probe signal for all-trans and 11-cis retinal protonated Schiff-base (RPSB) samples in the gas phase. Pump-probe signal for all-trans (top) and 11-cis (bottom) samples obtained with 600 nm pump and 800 nm probe at room temperature. The full curve is from a fit with two exponential functions folded with the 80 fs cross-correlation in the experiment. The dashed lines represent the two decay components in the fit 424 ± 121 fs and 2.5 ± 0.3 ps for all-trans and 650 ± 160 fs and 4.7 ± 3 ps for 11-cis RPSB. All error bars presented are one standard deviation (SD)
The excited-state relaxation dynamics of a recently discovered bacterial rhodopsin, KR2, which functions as a light-driven sodium-ion pump and contains all-trans RPSB shows several time constants44. A major 180 fs component is attributed to the reactive decay of S1, while two minor components of 3 and 30 ps are assigned to nonreactive S1 states. The decay by isomerization of S1 occurs three times faster in KR2 than in bR, and hence the protein environment can indeed change the timescale of photoisomerization.
The mechanism that produces nonreactive S1 states, also reported for other retinal-containing proteins44, is not clear. However, in light of our findings, it is tempting to assign the slow picosecond components observed in KR2 to the dynamics of all-trans RPSB in its native, planar conformation inside the protein, assuming that active-site structural heterogeneity involves different conformations of all-trans RPSB inside the chromophore-binding pocket. The protein may facilitate photoisomerization by changing the native conformation of all-trans RPSB through pre-twisting it in S0, thus reducing the barrier height for double-bond rotation in S1 and decreasing the timescale considerably. The slow 3 ps component found in KR2 is remarkably similar to that observed in the present study for the bare all-trans RPSB. Moreover, our calculated lifetimes for isomerization about C11 = C12 and C13 = C14 (or C9 = C10) bonds are ~5 and ~40 ps, respectively. These values are to be compared with the slow 3 and 30 ps time constants of KR2. In both cases, the planar all-trans PSBR chromophore is slow and most likely not operational in the photocycle of the protein. Hence, the native planar conformation of the chromophore might be responsible for slow nonreactive dynamics in retinal-containing proteins.
Discussion
The excited-state dynamics of RPSB-containing proteins as well as of RPSB in solutions shows a variety of time constants, typically in the sub-ps and few-ps regime. We have studied, for the first time, the dynamics of the bare RPSB chromophore in vacuo and found timescales of the very same order. When excited to the first electronically excited state, the decay is found to be tri-exponential, where the two short lifetimes together are represented by a ~400 fs decay. This decay time is independent of the excitation wavelength, but temperature dependent, raising by a factor of about three when the RPSB chromophore is cooled to 100 K. It gives evidence that a barrier as small as 30 cm−1 is operative in internal conversion for the bare chromophore. A longer, several picosecond component is also involved. This component reaches a decay time of almost 100 ps when cooled to 100 K. According to our calculations this is consistent with a 0.04 eV (~300 cm−1) energy barrier in S1 for all-trans RPSB. Experimentally and theoretically the long picosecond decay is thus assigned to RPSB in the all-trans form. Theoretically, we find the isomerization of RPSB in the 11-cis form to be essentially barrierless in S1 and the short sub-picosecond component may therefore be ascribed to 11-cis RPSB. Based on our findings, we conclude that the visual photoreceptors rely on the ultrafast intrinsic photoresponse of their 11-cis chromophore, whereas bacterial rhodopsins tune the photoresponse of the all-trans RPSB chromophore by particularly reducing the barrier height about the C13 = C14 double bond, thus changing both the timescale and specificity of photoisomerization of RPSB compared to those found in the intrinsic photoresponse, where the lowest barrier is for rotation about the C11 = C12 double bond.
Methods
Experimental setup
The experiments were performed at the SAPHIRA electrostatic ion-storage ring38,45, where an electrospray ion source with a multipole ion trap was used to produce bunches of bio-chromophores in vacuo.
Ion source and sample preparation
All-trans as well as 11-cis Schiff-base retinal chromophores were dissolved in methanol and electrosprayed as protonated ions from a steel needle. Solvent molecules were evaporated off in the capillary, which was heated to about 100 °C, resulting in bare, singly charged molecules, which were transported through an octopole ion guide to a 16-pole radio frequency ion trap. Helium buffer-gas collisions reduced the translational kinetic energy causing the ions to be trapped. The ions were thermalized to the buffer-gas temperature (100 or 300 K) while trapped. At 100 Hz repetition rate, ions were extracted as 20 μs bunches. The ion source was located on a 4 kV high-voltage platform, which provided extraction from the source and subsequent acceleration to 4 keV. The retinal ions were mass-to-charge selected through a dipole magnet prior to injection into SAPHIRA.
Ion storage and detection
SAPHIRA has four quadrupole 90° bending electrodes, mounted with quadrupole triplet lenses at the entrance and exit of each corner to keep the ion beam collimated in the ring. Two particle detectors mounted at the end of corner 3 and 4 detected neutrals generated by residual gas collisions (background pressure 2.0E−8 mbar and from photo-induced dissociation, see Supplementary Figure 1).
Laser system and optical setup
Laser pulses were generated by a Ti:Sapphire-based Libra HE-UFS oscillator and regenerative amplifier system, delivering 4 mJ, 50 fs pulses with 1 kHz repetition rate. The pulses were split 3:1 to separately pump two TOPAS-C optical parametric amplifiers, which generated the pump and probe pulses. One of the beams was delayed by a motorized delay stage. The two beams were collinearly overlapped using a beam splitter or dichroic mirror, depending on the pump and probe wavelength. The cross-correlation of two 580 nm pulses was measured to be 80 fs in a β-barium borate crystal by sum-frequency generation. The pulse energies were 100 μJ at 580 nm, 60 μJ at 610 nm, and 200 μJ at 400 nm.
Typical experimental cycle
The ion bunches circulated for about 2 ms in SAPHIRA prior to laser excitation. The first laser pulse excited the retinal chromophores from the S0 ground state to the S1 excited state. Internal conversion brought the ions back into the ground state, now with significantly increased internal energy, leading to fragmentation on the millisecond timescale. The total pump-probe signal appears from the increased fragmentation of both pump and probe photon absorption. For the final data analysis, we consider only the counts of the first pump-probe signal peak (as a function of the pump-probe delay), to limit statistical fluctuations from background subtraction. Error bars are estimated considering only the statistics of the neutral counts.
Computational methods
All energy and gradient calculations in both excited and ground states are performed using the extended multiconfiguration quasi-degenerate perturbation theory, XMCQDPT242. Structures of the stationary points in S1 and the S1/S0 MECIs, as well as relaxed geometry potential-energy scans along isomerization coordinates in S1 are obtained at the XMCQDPT22/SA(2)-CASSCF(12,12)/cc-pVDZ level of theory. Vertical excitation energies S1–Sn along reaction pathways are calculated at the XMCQDPT2[7]/SA(7)-CASSCF(12,12)/cc-pVDZ level of theory. The Firefly package46, version 8.2.0, is used for all electronic structure calculations. Excited-state lifetimes of all-trans RPSB are calculated using transition state theory as an inverse of a sum of rate constants that refer to rotation about three double bonds. We assume the same thermal population of vibrational levels in S1 as in S0, following excitation that is close to the adiabatic transition in RPSB. An excess of vibrational energy gained upon excitation is therefore considered to be small compared to an average vibrational energy of the chromophore ions equilibrated in the ground state prior to excitation. Partition functions for the reaction modes are calculated based on the exact eigenvalues found as numerical solutions to the one-dimensional Schrödinger equation using the calculated XMCQDPT2 potentials that hinder rotation about double bonds in S1.
Supplementary information
Acknowledgements
This work was supported by the Villum foundation as a Villum Experiment, project no. 17512 and the Carlsberg Foundation. A.V.B. and P.A.K. acknowledge support from the Russian Science Foundation (17-13-01276). The research is carried out using the equipment of the shared research facilities of HPC computing resources at Lomonosov Moscow State University. We thank Oriol Vendrell, Stefan Haacke, and Mudi Sheves for discussions and Henrik B. Pedersen for support with the SAPHIRA ion-storage ring.
Author contributions
L.H.A. conceived the 2D action-spectroscopy technique, and L.H.A. and H.V.K. designed the experiment together. E.G., H.V.K., J.L. and L.H.A. performed the measurements. E.G. and H.V.K. analyzed the experimental data. A.V.B. and P.A.K. performed ab initio calculations. L.H.A. and A.V.B. wrote the paper after discussions with all authors.
Data availability
The authors declare that the data supporting the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09225-7.
References
- 1.Stoeckenius W, Bogomolni RA. Bacteriorhodopsin and related pigments of halobacteria. Annu. Rev. Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC, et al. Environmental genome shotgun sequencing of the sargasso sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 3.Mathies R, Brito Cruz C, Pollard W, Shank C. Direct observation of the femtosecond excited-state cis–trans isomerization in bacteriorhodopsin. Science. 1988;240:777–779. doi: 10.1126/science.3363359. [DOI] [PubMed] [Google Scholar]
- 4.Hamm P, et al. Femtosecond spectroscopy of the photoisomerisation of the protonated Schiff base of all-trans retinal. Chem. Phys. Lett. 1996;263:613–621. doi: 10.1016/S0009-2614(96)01269-9. [DOI] [Google Scholar]
- 5.Logunov SL, El-Sayed MA. Redetermination of the quantum yield of photoisomerization and energy content in the k-intermediate of bacteriorhodopsin photocycle and its mutants by the photoacoustic technique. J. Phys. Chem. B. 1997;101:6629–6633. doi: 10.1021/jp970955c. [DOI] [Google Scholar]
- 6.Logunov SL, Song L, El-Sayed MA. Excited-state dynamics of a protonated retinal Schiff base in solution. J. Phys. Chem. 1996;100:18586–18591. doi: 10.1021/jp962046d. [DOI] [Google Scholar]
- 7.Kandori H, Sasabe H. Excited-state dynamics of a protonated Schiff base of all-trans retinal in methanol probed by femtosecond fluorescence measurement. Chem. Phys. Lett. 1993;216:126–172. doi: 10.1016/0009-2614(93)E1262-F. [DOI] [Google Scholar]
- 8.Freedman KA, Becker RS. Comparative investigation of the photoisomerization of the protonated and unprotonated n-butylamine Schiff bases of 9-cis-, 11-cis-, 13-cis-, and all-trans-retinals. J. Am. Chem. Soc. 1986;108:1245–1251. doi: 10.1021/ja00266a020. [DOI] [Google Scholar]
- 9.Koyama Y, Kubo K, Komori M, Yasuda H, Mukai Y. Effect of protonation on the isomerization properties of n-butylamine Schiff base of isomeric retinal as revealed by direct hplc analyses: selection of isomerization pathways by retinal proteins. Photochem. Photobiol. 1991;54:433–443. doi: 10.1111/j.1751-1097.1991.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 10.Toker Y, et al. Photoresponse of the protonated Schiff-base retinal chromophore in the gas phase. Phys. Chem. Chem. Phys. 2013;15:19566–19569. doi: 10.1039/c3cp51759d. [DOI] [PubMed] [Google Scholar]
- 11.Coughlan NJA, Catani KJ, Adamson BD, Wille U, Bieske EJ. Photoisomerization action spectrum of retinal protonated Schiff base in the gas phase. J. Chem. Phys. 2014;140:164307. doi: 10.1063/1.4871883. [DOI] [PubMed] [Google Scholar]
- 12.Fernald RD. Casting a genetic light on the evolution of eyes. Science. 2006;313:1914–1918. doi: 10.1126/science.1127889. [DOI] [PubMed] [Google Scholar]
- 13.Schoenlein R, Peteanu L, Mathies R, Shank C. The first step in vision: femtosecond isomerization of rhodopsin. Science. 1991;254:412–415. doi: 10.1126/science.1925597. [DOI] [PubMed] [Google Scholar]
- 14.Peteanu LA, Schoenlein RW, Wang Q, Mathies RA, Shank CV. The first step in vision occurs in femtoseconds: complete blue and red spectral studies. Proc. Natl. Acad. Sci. USA. 1993;90:11762–11766. doi: 10.1073/pnas.90.24.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haran G, Morlino EA, Matthes J, Callender RH, Hochstrasser RM. Femtosecond polarized pump-probe and stimulated emission spectroscopy of the isomerization reaction of rhodopsin. J. Phys. Chem. A. 1999;103:2202–2207. doi: 10.1021/jp9832847. [DOI] [Google Scholar]
- 16.Kandori H, et al. Excited-state dynamics of rhodopsin probed by femtosecond fluorescence spectroscopy. Chem. Phys. Lett. 2001;334:271–276. doi: 10.1016/S0009-2614(00)01457-3. [DOI] [Google Scholar]
- 17.Wand A, Gdor I, Zhu J, Sheves M, Ruhman S. Shedding new light on retinal protein photochemistry. Annu. Rev. Phys. Chem. 2013;64:437–458. doi: 10.1146/annurev-physchem-040412-110148. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PJM, et al. Local vibrational coherences drive the primary photochemistry of vision. Nat. Chem. 2015;7:980. doi: 10.1038/nchem.2398. [DOI] [PubMed] [Google Scholar]
- 19.Polli D, et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature. 2010;467:440–443. doi: 10.1038/nature09346. [DOI] [PubMed] [Google Scholar]
- 20.Schapiro I, et al. The ultrafast photoisomerizations of rhodopsin and bathorhodopsin are modulated by bond length alternation and HOOP driven electronic effects. J. Am. Chem. Soc. 2011;133:3354–3364. doi: 10.1021/ja1056196. [DOI] [PubMed] [Google Scholar]
- 21.Rivalta I, Nenov A, Garavelli M. Modelling retinal chromophores photoisomerization: from minimal models in vacuo to ultimate bidimensional spectroscopy in rhodopsins. Phys. Chem. Chem. Phys. 2014;16:16865–16879. doi: 10.1039/C3CP55211J. [DOI] [PubMed] [Google Scholar]
- 22.Garavelli M, Negri F, Olivucci M. Initial excited-state relaxation of the isolated 11-cis protonated Schiff base of retinal: evidence for in-plane motion from ab initio quantum chemical simulation of the resonance raman spectrum. J. Am. Chem. Soc. 1999;121:1023–1029. doi: 10.1021/ja981719y. [DOI] [Google Scholar]
- 23.Punwong C, Owens J, Martínez TJ. Direct QM/MM excited-state dynamics of retinal protonated Schiff base in isolation and methanol solution. J. Phys. Chem. B. 2014;119:704–714. doi: 10.1021/jp5038798. [DOI] [PubMed] [Google Scholar]
- 24.Becker RS, Freedman K. A comprehensive investigation of the mechanism and photophysics of isomerization of a protonated and unprotonated Schiff base of 11-cis-retinal. J. Am. Chem. Soc. 1985;107:1477–1485. doi: 10.1021/ja00292a005. [DOI] [Google Scholar]
- 25.Kandori H, Katsuta Y, Ito M, Sasabe H. Femtosecond fluorescence study of the rhodopsin chromophore in solution. J. Am. Chem. Soc. 1995;117:2669–2670. doi: 10.1021/ja00114a040. [DOI] [Google Scholar]
- 26.Bassolino G, et al. Barrierless photoisomerization of 11-cis retinal protonated Schiff base in solution. J. Am. Chem. Soc. 2015;137:12434–12437. doi: 10.1021/jacs.5b06492. [DOI] [PubMed] [Google Scholar]
- 27.Zgrablić G, Novello AM, Parmigiani F. Population branching in the conical intersection of the retinal chromophore revealed by multipulse ultrafast optical spectroscopy. J. Am. Chem. Soc. 2011;134:955–961. doi: 10.1021/ja205763x. [DOI] [PubMed] [Google Scholar]
- 28.Sovdat T, et al. Backbone modification of retinal induces protein-like excited state dynamics in solution. J. Am. Chem. Soc. 2012;134:8318–8320. doi: 10.1021/ja3007929. [DOI] [PubMed] [Google Scholar]
- 29.Bassolino G, et al. Synthetic control of retinal photochemistry and photophysics in solution. J. Am. Chem. Soc. 2014;136:2650–2658. doi: 10.1021/ja4121814. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Liu J, Martinez TJ. Dynamical correlation effects on photoisomerization: Ab initio multiple spawning dynamics with MS-CASPT2 for a model trans-protonated Schiff base. J. Phys. Chem. B. 2016;120:1940–1949. doi: 10.1021/acs.jpcb.5b09838. [DOI] [PubMed] [Google Scholar]
- 31.Andersen LH, et al. Absorption of Schiff-base retinal chromophores in vacuo. J. Am. Chem. Soc. 2005;127:12347–12350. doi: 10.1021/ja051638j. [DOI] [PubMed] [Google Scholar]
- 32.Coughlan NJA, Adamson BD, Gamon L, Catani K, Bieske EJ. Retinal shows its true colours: photoisomerization action spectra of mobility-selected isomers of the retinal protonated schiff base. Phys. Chem. Chem. Phys. 2015;17:22623–22631. doi: 10.1039/C5CP03611A. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen JL, Kluge A, Bochenkova AV, Kiefer HV, Andersen LH. The UV–visible action-absorption spectrum of all-trans and 11-cis protonated Schiff base retinal in the gas phase. Phys. Chem. Chem. Phys. 2018;20:7190–7194. doi: 10.1039/C7CP07512J. [DOI] [PubMed] [Google Scholar]
- 34.Delori FC, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995;36:718. [PubMed] [Google Scholar]
- 35.Zgrablić G, Voïtchovsky K, Kindermann M, Haacke S, Chergui M. Ultrafast excited state dynamics of the protonated Schiff base of all-trans retinal in solvents. Biophys. J. 2005;88:2779–2788. doi: 10.1529/biophysj.104.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svendsen A, Kiefer HV, Pedersen HB, Bochenkova AV, Andersen LH. Origin of the intrinsic fluorescence of the green fluorescent protein. J. Am. Chem. Soc. 2017;139:8766–8771. doi: 10.1021/jacs.7b04987. [DOI] [PubMed] [Google Scholar]
- 37.Kiefer HV, Pedersen HB, Bochenkova AV, Andersen LH. Decoupling electronic versus nuclear photoresponse of isolated green fluorescent protein chromophores using short laser pulses. Phys. Rev. Lett. 2016;117:243004. doi: 10.1103/PhysRevLett.117.243004. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen HB, et al. Characterization of a new electrostatic storage ring for photofragmentation experiments. Rev. Sci. Instrum. 2015;86:063107. doi: 10.1063/1.4922826. [DOI] [PubMed] [Google Scholar]
- 39.Andersen LH, et al. Experimental studies of the photophysics of gas-phase fluorescent protein chromophores. Phys. Chem. Chem. Phys. 2004;6:2617–2627. doi: 10.1039/B315763F. [DOI] [Google Scholar]
- 40.Goran Z, Haacke S, Chergui M. Heterogeneity and relaxation dynamics of the photoexcited retinal Schiff base cation in solution. J. Phys. Chem. B. 2009;113:4384–4393. doi: 10.1021/jp8077216. [DOI] [PubMed] [Google Scholar]
- 41.Dilger J, et al. Direct measurement of the isomerization barrier of the isolated retinal chromophore. Angew. Chem. Int. Ed. 2015;54:4748–4752. doi: 10.1002/anie.201411894. [DOI] [PubMed] [Google Scholar]
- 42.Granovsky AA. Extended multi-configuration quasi-degenerate perturbation theory: the new approach to multi-state multi-reference perturbation theory. J. Chem. Phys. 2011;134:214113. doi: 10.1063/1.3596699. [DOI] [PubMed] [Google Scholar]
- 43.Toker Y, et al. Counterion-controlled spectral tuning of the protonated Schiff-base retinal. Phys. Rev. A. 2018;98:043428. doi: 10.1103/PhysRevA.98.043428. [DOI] [Google Scholar]
- 44.Tahara S, et al. Ultrafast photoreaction dynamics of a light-driven sodium-ion-pumping retinal protein from krokinobacter eikastus revealed by femtosecond time-resolved absorption spectroscopy. J. Phys. Chem. Lett. 2015;6:4481–4486. doi: 10.1021/acs.jpclett.5b01994. [DOI] [PubMed] [Google Scholar]
- 45.Svendsen A, Teiwes R, Kiefer HV, Andersen LH, Pedersen HB. Analysis of ionic photofragments stored in an electrostatic storage ring. Rev. Sci. Instrum. 2016;87:013111. doi: 10.1063/1.4940423. [DOI] [PubMed] [Google Scholar]
- 46.Granovsky, A. A. Firefly. http://classic.chem.msu.su/gran/firefly/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available from the corresponding author upon reasonable request.