Abstract
Correlating cultural, technological and ecological aspects of both Upper Pleistocene modern humans (UPMHs) and Neandertals provides a useful approach for achieving robust predictions about what makes us human. Here we present ecological information for a period of special relevance in human evolution, the time of replacement of Neandertals by modern humans during the Late Pleistocene in Europe. Using the stable isotopic approach, we shed light on aspects of diet and mobility of the late Neandertals and UPMHs from the cave sites of the Troisième caverne of Goyet and Spy in Belgium. We demonstrate that their diet was essentially similar, relying on the same terrestrial herbivores, whereas mobility strategies indicate considerable differences between Neandertal groups, as well as in comparison to UPMHs. Our results indicate that UPMHs exploited their environment to a greater extent than Neandertals and support the hypothesis that UPMHs had a substantial impact not only on the population dynamics of large mammals but also on the whole structure of the ecosystem since their initial arrival in Europe.
Introduction
Nowadays modern humans (Homo sapiens) are the only species of humans left on Earth. This was different during the Late Pleistocene when Neandertals and Upper Pleistocene modern humans (UPMH) coexisted in Europe. Relatively soon after the arrival of UPMHs in this region about 45–43,000 years ago, the Neandertals became extinct1–4. Differences in the ecological niches of UPMHs and Neandertals while coexisting in the same ecosystems are regularly suggested as being the possible cause for the demise of Neandertals. Emphasis is placed on late Neandertals being ecologically less flexible than UPMHs (but see5–7) and therefore giving an advantage to UPMHs. According to this hypothesis, UPMHs had a broader dietary ecological spectrum, especially having possibly included more aquatic resources than that of Neandertals. This was suggested based on higher nitrogen isotopic ratios (δ15N) of bone collagen for UPMHs in comparison with late Neandertals8–10, with the latter supposedly having had a diet whose protein part consisted purely of terrestrial herbivorous mammal meat11–14. These conclusions are extremely relevant when considering overall human evolution, since they present potential causes for the extinction of Neandertals and the rise of UPMHs. Unfortunately, they are essentially drawn from only a few UPMH remains8,15–17 from locations where late Neandertals have not been discovered and therefore direct comparisons of the two human groups in the same ecological conditions are missing18–22. In addition, most of these studies are missing the environmental context of the isotopic data they present (see discussion in12,14). So far, a direct comparison of isotopic values of Neandertals and UPMHs through time and space has been only tentative and does not lead to reliable conclusions about potential differences in the ecological niches of the two types of humans. It is indeed crucial to contextualize human isotopic data using coeval herbivorous prey, as well as carnivorous species, since shifts of the isotopic baseline occurred for δ15N through time and space during the Late Pleistocene in Europe23–25. As a consequence, consuming the same diet at different time periods might lead to different nitrogen isotopic values, which would result in incorrect and misleading conclusions about the dietary strategies of humans23,26. The Troisième caverne of Goyet site (Belgium) provides a unique opportunity to fill the gap because skeletal remains of both types of humans representing several individuals have been recovered, directly radiocarbon dated, and their taxonomic attribution confirmed by palaeogenetic analysis27,28 (Fig. 1 and Supplementary Data 1).
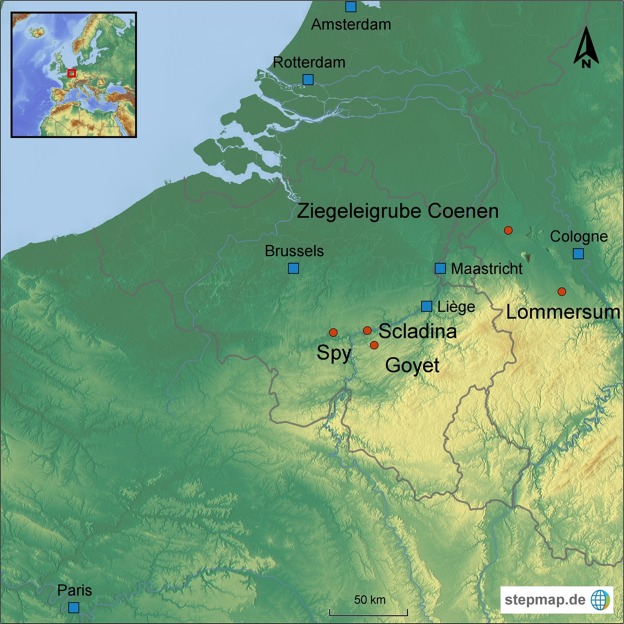
Figure 1.
Current map of Europe with the site locations (red dots). Map produced through the website stepmap.de.
The Goyet human remains were associated with a rich faunal assemblage, and both the human and faunal skeletal specimens are biochemically well preserved. These circumstances make this site a key location for the understanding of the ecological behavior of the last Neandertals and UPMHs close to the time of replacement between 45,000 and 35,000 years BP in Europe1,29. Here we applied a Bayesian mixing model (SIAR)30 using carbon and nitrogen stable isotopic data of bone collagen to determine the relative proportions of different prey species in the diet of the UPMHs (this study) and Neandertals from the Troisième caverne of Goyet14. Additionally, we used the same approach with the already published isotopic data of the late Neandertals from the nearby site of Spy31 in order to make regional comparisons between late Neandertal groups.
Through insights into dietary and mobility aspects of late Neandertals and UPMHs, we aim to reveal ecological aspects in the context of the transition phase from the Neandertal to UPMH occupation of Northern Europe32. Finally, a key assertion resulting from this study is an estimation of the ecological impact that each type of human had on the ecosystem. For this we considered faunal remains from the sites of Troisème caverne of Goyet, Spy and Scladina, contemporaneous with the Neandertals occupation of Belgium approximately 39,000 to 47,000 years ago (Supplementary Data 1, 2 3 and 4) as well as some Upper Palaeolithic (UP) sites resulting from the presence of UPMHs in Germany. We present new isotopic data from the Aurignacian open air site of Lommersum (Supplementary Data 5), which is dated to 33,250 and 35,100 years BP33, and which is in the vicinity of the Belgian sites (Fig. 1). This site is of high relevance in terms of providing adequate quantities of well-preserved faunal remains dating to the early UP in this region and therefore gives an insight into the fauna exploited by UPMHs. The oldest site considered here, Ziegeleigrube Coenen (ZC) in Germany (Fig. 1), is contemporary with late Neandertals and reflects the niche partitioning of the mammoth steppe fauna during a cold spell between a minimum age of ~40,000 BP to a finite age of ~47,000 BP24,34.
Given that mobility is a key variable, especially in relation to how interactions within the ecosystem occurred in hunter-gatherer societies35–39, we also investigate the mobility history using sulphur isotopic composition in bone collagen within and across two Neandertal groups, one from Spy and the other from Goyet, as well as from the UPMHs from Goyet (Tables 1, S1). In the context of evaluating potential group mobility aspects, it is of special interest that the Goyet Neandertals show features of intensive cannibalism on their highly fragmented bones, this being in contrast to the Spy Neandertals27. In addition to the humans, broad selections of mammal species from the Belgian sites as well as from Lommersum in Germany (Tables S2 and S3) were investigated for their sulphur stable isotopic signal. The sulphur stable isotopic composition has been found to reflect underlying geology and therefore can be considered as an indicator of geographic location with the potential to track aspects of mobility, and thus of the behavioral patterns across the landscape40–43.
Table 1.
List of the stable isotopic data and related 14C dates from Late Pleistocene Neandertal and UPMH remains from the Troisième caverne of Goyet and Spy.
| ID | Species | Lab# | 14C age (BP) | δ13C | δ15N | δ34S | Reference for 14C | Reference for δ13C and δ 15N |
|---|---|---|---|---|---|---|---|---|
| Site: Goyet | ||||||||
| Q116-1 | Homo sapiens | GrA-46175 | 30,880 + 170, −160 | −19.1 | 10.9 | 8.6 | 28 | * |
| Q376-3 | Homo sapiens | GrA-60034 | 29,370 + 180, −170 | −18.8 | 11.4 | 4.4 | 28 | * |
| C5-1 | Homo neanderthalensis | −19.7 | 12.1 | 10.3 | * | |||
| Q48-1 | Homo neanderthalensis | −19.6 | 11.3 | 11.5 | * | |||
| Q53-4 | Homo neanderthalensis | GrA-54022 | 39,870 + 400, −350 | −19.0 | 11.7 | 9.7 | 27 | 14 |
| Q55-1 | Homo neanderthalensis | GrA-54257 | 37,860 + 350 −310 | −19.2 | 11.3 | 9.8 | 27 | 14 |
| Q55-4 | Homo neanderthalensis | −19.2 | 11.6 | 11.4 | 14 | |||
| Q56-1 | Homo neanderthalensis | GrA-46170 | 38,440 + 340, −300 | −19.5 | 11.5 | 9.2 | 27 | 14 |
| Q57-1 | Homo neanderthalensis | GrA-46173 | 41,200 + 500, −410 | −19.2 | 11.8 | 10.9 | 27 | 14 |
| Q57-2 | Homo neanderthalensis | GrA-54024 | 36,590 + 300, −270 | −19.1 | 11.9 | 10.8 | 4 | 14 |
| Q57-3 | Homo neanderthalensis | GrA-60019 | 38,260 + 350, −310 | −19.6 | 11.2 | 10.9 | 27 | 14 |
| Q 119-2 | Homo neanderthalensis | — | — | −19.3 | 11.5 | 11.9 | * | |
| Q305-4 | Homo neanderthalensis | GrA-46176 | 40,690 + 480, −400 | −19.4 | 10.7 | 7.5 | 27 | 14 |
| Q305-7 | Homo neanderthalensis | — | — | −19.0 | 11.3 | 11.3 | 14 | |
| Q374a-1 | Homo neanderthalensis | — | — | −19.1 | 11.8 | 10.2 | 14 | |
| Q376-1 | Homo neanderthalensis | GrA-46178 | 39,140 + 390, −340 | −19.2 | 10.9 | 27 | 14 | |
| Q376-20 | Homo neanderthalensis | GrA-60018 | 37,250 + 320, −280 | −19.4 | 11.8 | 11.6 | 27 | 14 |
| Q376-9 | Homo neanderthalensis | — | — | −19.2 | 11.8 | 12.9 | * | |
| Q376-25 | Homo neanderthalensis | — | — | −19.0 | 11.5 | 11.4 | * | |
| 2878-2D | Homo neanderthalensis | GrA-54028 | 32,190 + 200, −190 | −19.0 | 12.5 | 27 | * | |
| Site: Spy | ||||||||
| Spy 94a (Spy II?) | Homo neanderthalensis | GrA-32623 | 35,810 + 260, −240 | −19.4 | 11.4 | 3.6 | 79 | 31 |
| Spy 430a (Spy II?) | Homo neanderthalensis | GrA-32630 | 33,940 + 220, −210 | −20.3 | 10.8 | 79 | 31 | |
| Spy 92b (Spy I?) | Homo neanderthalensis | GrA-32626 | 36,350 + 310,−280 | −19.8 | 10.9 | 79 | 31 | |
| Spy 572a (Spy I/II?) | Homo neanderthalensis | GrA-21546 | 31,810 + 250,−250 | −19.8 | 11.0 | 79 | 31 | |
| Spy 646a Spy (VI) | Homo neanderthalensis | GrA-32627 | 32,970 + 200,−190 | −19.8 | 12.5 | 2.6 | 78 | * |
Thanks to the rich archeological and human fossil records of the Troisième caverne of Goyet27,44, our study using stable isotopes has the potential to contribute essential new information and to provide an extensive view on ecological issues of both Neandertals and UPMHs during a particularly important time in European prehistory.
Results
Isotopic results
For all considered samples the collagen preservation fulfilled the conditions for reliable biogenic stable carbon, nitrogen and sulphur isotopic values45–47 (Table 1, Tables S1, S2, S3 and Supplementary Data 6).
Carbon and nitrogen stable isotope values
For the study we analysed collagen from two UPMHs and 18 Neandertal specimens, including six new samples from the Troisième caverne of Goyet as well as one Neandertal individual from Spy (Spy 646a) for δ13C and δ15N (Table 1, Table S1). The UPMHs yielded δ13C values of −19.1‰ for the individual represented by Q116-1 and −18.8‰ for the second individual represented by Q376-3. The δ15N values were 10.9‰ for Q116-1 and 11.4‰ for Q376-3. The Goyet Neandertals (n = 18) from this study yielded δ15N values ranging from 10.9‰ to 12.5‰ (av. 11.8‰; s.d. 0.43‰) and δ13C ranging from −19.7‰ to −19.0‰ (av. 19.2‰; s.d. 0.22‰). This study presents one Neandertal immature individual Spy 646a (Spy VI) in addition to the already published specimens representing the adults Spy I and II. The Spy Neandertal (Spy 646a) produced values of 12.5‰ for δ15N and −19.8‰ for δ13C. These additional values match those previously presented14.
From the site of Lommersum in Germany we analysed δ13C and δ15N ratios of collagen for horse (Equus ferus, n = 9), reindeer (Rangifer tarandus, n = 10), mammoth (Mammuthus primigenius, n = 1), wolf (Canis lupus, n = 1) and cave lion (Panthera spelaea, n = 1) (Table S3). Here the δ13C values obtained ranged from −20.9‰ for a horse (Lom-20) to −18.3‰ for a reindeer (Lom-8) (av. −19.7‰; s.d. 0.9‰). The δ15N values ranged from 2.0‰ for a reindeer (Lom-7) to 8.5‰ for the cave lion (Lom-15) (av. 4.8‰; s.d. 2.0‰).
Sulphur stable isotopes
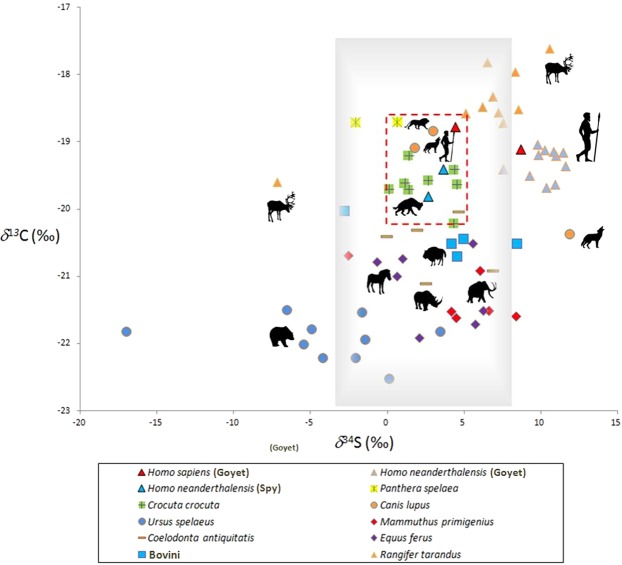
The δ34S values for the UPMHs were 8.6‰ (Q116-1) and 4.4‰ (Q376-3). The values for the Goyet Neandertals (n = 11) range from 7.5‰ (Q305-4) to 11.6‰ (Q376-20) with a mean of 10.2‰. The δ34S values for the Spy Neandertals were 3.6‰ for the adult Spy 94a and 2.6‰ for Spy 646a (Spy VI). The faunal δ34S values from Goyet (n = 27) range from −7.2 to 8.4‰ (mean 1.2‰; s.d. 4.1‰) and those from Scladina (n = 23) from −17.0‰ to 11.8‰ (mean 2.4‰; s.d. 5.8‰). The δ34S analysis of the Spy horse provided a value of 5.5‰ (sample IV2A 4207) (Table S2). For the Belgian sites, δ34S values were obtained from the same collagen samples as for the δ13C and δ15N data that were processed by Bocherens et al. and Wißing et al.14,31,48,49 or in this study. The faunal remains from Lommersum (n = 7) provided δ34S values between 2.0 and 4.7‰ (mean 3.4‰; s.d. 1.5‰) (Table S3). For Lommersum, all stable isotopes were measured on the same collagen samples. Figure 2 shows a bivariate plot of the δ34S and δ13C values from the Late Pleistocene in Belgium. The faunal remains have a mean δ34S value of 2.61‰ and the grey background in Fig. 2 represents the mean ±2 s.d. range in δ34S values of between −2.6‰ and 7.8‰.
Figure 2.
Bivariate plot of δ34S and δ13C stable isotopic values of bone collagen representing the Late Pleistocene ecosystem in Belgium. The plot includes Neandertals as well as UPMHs from Goyet and Neandertals from Spy. Faunal remains are from Scladina, Spy and Goyet. Different species are associated with different symbols and colors. Individual specimens are plotted. The shaded grey rectangle represents the local sulphur signal, the red dashed line essentially encompasses most of the carnivorous species. Carnivores reflect the average δ34S values of their prey75.
Reconstruction of isospace
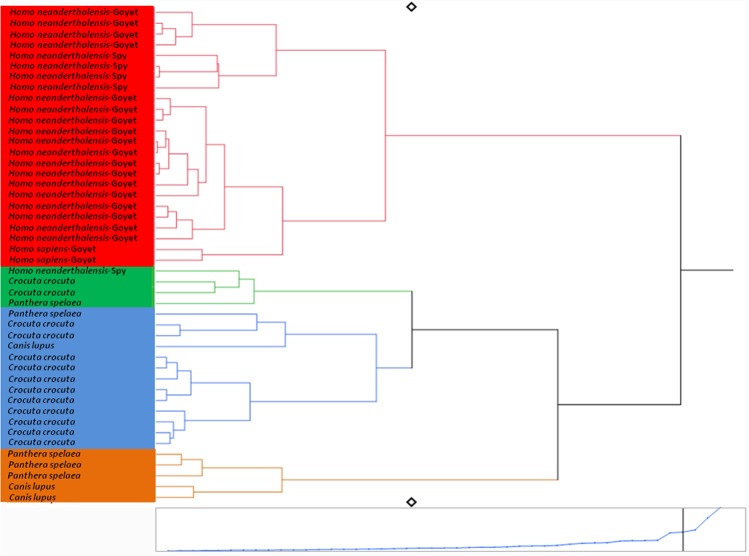
We define the isospace as the range of isotopic values typical of a given species and representing the specific ecological niche of this species in terms of the δ13C and δ15N values of its bone collagen. It is not limited to defining only the physical space, although the place of habitation impacts the isotopic signal. In our concept, the most paramount aspect is the diet, more precisely the protein part of the diet of a particular species. Isospaces can be described in several ways and we used two approaches: on the one hand a cluster analysis for the carnivorous species (Fig. 3) and the core niche concept (implemented as a classical bivariate scatter plot of δ13C and δ15N values) for the herbivorous species (Fig. 4). The cluster analysis provides information at the individual level, whereas for the herbivores the core niche concept presents the isospace at a species level (Figs. 3 and 4). The cluster analysis includes carnivorous and omnivorous species alongside the three groups of humans: the Neandertals from Spy and from the Troisième caverne of Goyet, as well as the UPMHs from Goyet. It demonstrates that the humans are the predators that isotopically overlap the least with other potential animal competitors (Fig. 3).
Figure 3.
Cluster analysis showing Late Pleistocene carnivorous and omnivorous mammal species (incl. humans) from the sites of the Troisième caverne of Goyet, Scladina and Spy based on δ15N and δ13C bone collagen values. Cluster analysis using the Ward’s minimum variance method with the software SAS JMP version 10.0.
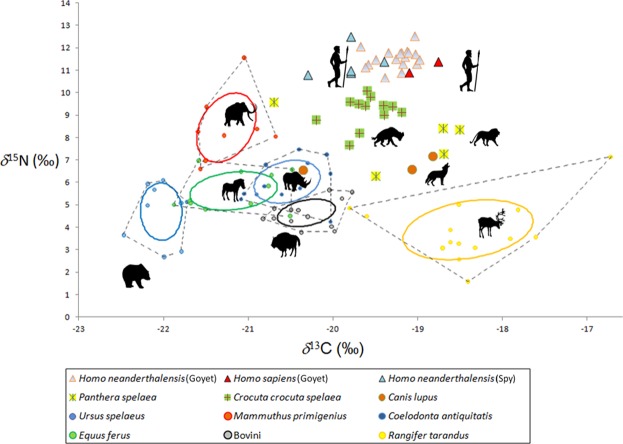
Figure 4.
Bivariate plot of δ13C and δ15N stable isotopic values of bone collagen, representing the Late Pleistocene ecosystem from Scladina, Spy and Goyet in Belgium. The plot includes Neandertals and UPMHs from Goyet as well as Neandertals from Spy. Herbivores’ core niches (= standard ellipse areas) in total niche (dashed convex hulls) were calculated using SIBER86 (Stable Isotope Bayesian Ellipses in R). Omnivores and carnivores are displayed as individual specimens. Silhouettes represent species attribution. The same silhouette is used to represent Neandertals and UPMHs.
It is particularly interesting that among the carnivores and omnivores, the first branch-off divides the humans (except one from Spy) from all other species. All other carnivorous species group closer to each other than to Neandertals and UPMHs. This early branch-off of the humans demonstrates rather that Neandertals and UPMHs ate more similar protein sources compared to the other carnivores and omnivores. This isotopic pattern further indicates that both types of humans occupied the most distinct ecological niche among the carnivores and omnivores.
Most observable is that the different herbivorous species occupied distinct isospaces similar to the data already published in the existing literature23,50–52.
Dietary Protein Reconstruction of the Goyet UPMHs
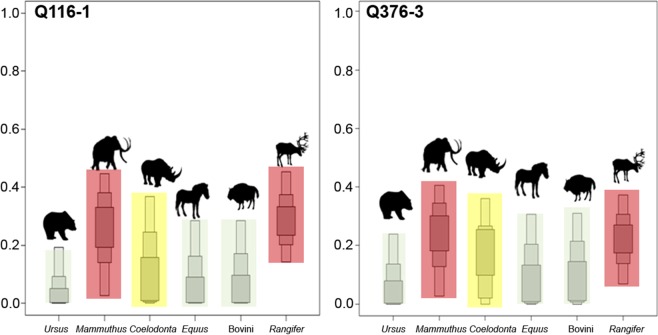
In this study, the relative contributions of different prey species as a dietary protein source have been reconstructed. Protein reconstruction for the Goyet Neandertals was previously presented in another study14. The additional Neandertal remains that are analysed here (Table 1) are very similar to those of the former study and will not be detailed here, but it is worth mentioning that the most important prey species are mammoth and reindeer for all of the analysed Neandertal remains14. For the UPMHs, a reconstruction of the relative contributions of the most important prey species is presented in Fig. 5 (Supplementary Figs. 2, 3 and Data 7 ).
Figure 5.
Box plots of the relative contributions (in %) of different prey species to the protein portion of the diet of the UPMHs from Goyet. Calculations were performed through the application of a Bayesian method (SIAR V4, Stable Isotope Analysis-package in R)87,88. Within the proportion box plots, three shades of grey are shown. Light grey represents a probability of 95%, medium grey 75% and dark grey 25%. Background colors highlight relative importance with red: most important, yellow: second most important and bright green: least important for a single species. The relative contributions of each species are similar for both individuals (Q116-1 and Q376-3). Here, faunal remains from the sites of Goyet, Scladina and Spy are included Table S1).
For both UPMH individuals, the two most relevant prey species are the mammoth and the reindeer. Each species comprised roughly 25–30% of the meat protein source. The rhinoceros contributed ca. 15 to 20%, the bovines and horses around 10% of the dietary proteins. Cave bears played the least important role, with a maximum contribution of around 5% of the total protein intake. These results are similar to those of Neandertals, which indicates that both UPMHs and Neandertals had a similar prey choice (Supplementary Data 7) with preference for mammoth and reindeer.
Discussion
The isotopic signatures of the Goyet UPMHs and of the Neandertals are similar (Figs. 3 and 4), both indicating a purely terrestrial diet and a similar preference for particular terrestrial herbivores. The notion that UPMHs had a broader dietary spectrum cannot be supported in this study, more specifically there was no indication of intake of aquatic resources, as suggested in some studies based on relatively higher δ15N values for UPMHs5,6,12. The present study did not find high δ15N values, which may have indicated a substantial intake of aquatic resources. In contrast, the focus on preying upon terrestrial herbivores by UPMHs53 as well as by late Middle Palaeolithic humans is well documented11,14,18,31 and is well confirmed here for both the Goyet UPMHs and late Neandertals from Goyet and Spy (Figs. 5 and 6). The zooarchaeological records from Goyet and Spy fully support mammoth hunting episodes with a special preference for younger individuals and possibly their mothers (Supplementary Fig. 10, Supplementary Data 2, Tables S4, S5, S6,)54. Interestingly, based on stable isotopes, the mammoth seems to contribute the major part of the dietary protein of humans in a time range between 50,000 and 30,000 years ago and across wide areas spanning from SW France11 to the Crimean Peninsula53 (Fig. 6, Supplementary Fig. 5–8).
Figure 6.
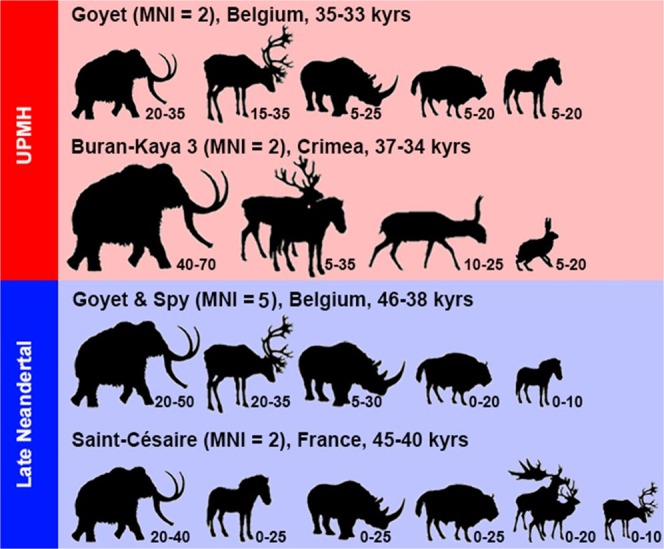
Relative proportions (in %) of different prey species to the protein intake of UPMHs and late Neandertals. Calculations based on δ13C and δ15N in percentage, using the SIAR Bayesian model (SIAR V4, Stable Isotope Analysis-package in R)87,88. Note that mammoth is the most important prey species, contributing systematically a minimum of ca. 20% of the dietary protein in both types of humans.
Analysing the bulk collagen fraction underestimates the plant protein contribution to the diet12, but another approach more sensitive to plant food intake using δ15N values of specific amino acids of bone collagen from Neandertals from Spy in Belgium indicates a substantial amount of plant protein in the diet of the Spy Neandertals55,56. This supports rather broader subsistence strategies for late Neandertals than previously considered in a palaeoecological context typical of the MIS 3. It has been argued that Neandertals altered their diets in response to changing palaeoecological conditions, while the diets of UPMHs were more associated to changes in their technological complexes, possibly having given them advantages over Neandertals57,58. Despite higher numbers of individuals and of analysed bone specimens for the Belgian Neandertals, we could not identify a more restricted dietary strategy in comparison to the UPMHs from Goyet. More data from other sites and areas would help to see if this is a generalized pattern or a more localized phenomenon.
We are aware that even if the relative proportions of dietary components were similar for late Neandertals and UPMHs, it is still possible that differences existed in hunting impact via exploitation intensity. This translates into a different impact on the surrounding environment, especially on the prey abundance caused by a potentially substantial human population increase during the Neandertal to UPMHs transition in Europe around 40,000 years ago43,59,60. Changes in isotopic niche partitioning among herbivorous prey species have been attributed to a decline of the mammoth population and the spread of the horse in the under-occupied niche43. Based on the finding that both UPMHs and Neandertals had a preference for mammoth meat, as demonstrated in this study and elsewhere61,62, a more detailed investigation into human-ecosystem interactions should offer crucial knowledge about the ecological role Neandertals and UPMHs played. Consequently, we investigated the ecological setting on a chronological scale spanning from around 45,000 to 25,000 years ago in the broader region of Western Europe, representing sites contemporaneous with late Neandertals and UPMHs (Supplementary Fig. 9). The ecological niche partitioning and especially deviations from the expected niche partitioning can reflect ecological stress, which may be recorded in the stable isotopic composition50,63. The mammoth was a key prey species (Fig. 6) so the potential ecological stress on it shall be investigated.
The oldest site considered, Ziegeleigrube Coenen (ZC) in Germany (Fig. 1, Supplementary Fig. 9), reflects the niche partitioning of the mammoth steppe fauna during a cold spell between a minimum age of ~40,000 years BP and a finite age of ~47,000 years BP24,34,50,51,63 which makes the site contemporaneous with late Neandertals. In this region, there was obviously a coherent food web structure in which all species maintained their niche, without any signs of ecological stress.
During all the following chronological phases (Supplementary Fig. 9) represented by the last Neandertal sites from Belgium and the early UP site of Lommersum as well as the Swabian Jura UP sites in Germany43,64, the situation changed. We observe a trend for single horse individuals to overlap in their isotopic values with those of the mammoth. During the UP in the Swabian Jura, we can even detect that individual horses interfered with the core niche of the mammoth (Supplementary Fig. 9). This trend is reinforced with the early UP which can also be seen in the total reduction of the isotopic distance of the horse and mammoth core niches (Supplementary Fig. 9, Data 8).
Not all horses during the final MP and early UP in the region studied (Fig. 1) occupied their traditional ecological niche, as observed in the context of earlier phases of mammoth steppe in Europe and other areas in Northern Eurasia and Beringia during the Late Pleistocene14,48,49,51,52. While considering the partial overlap of the isotopic signatures of the mammoth and horse, the SIAR model cannot create a sufficient distinction between the relative proportions of dietary protein for the UPMHs from the Troisième caverne of Goyet. An underestimation of the contribution of the horse can affect the results provided by SIAR. However, the UPMHs relied on one of the two species or perhaps both occupying this specific ecological niche. Consequently, the ecological reliance of both types of humans as predators on this niche was comparable from a qualitative point of view. We hypothesize that with the arrival of UPMHs in the study area, the predation pressure on the mammoth population increased relative to the time before. An UPMH population density up to ten times higher than during the Neandertal occupation60,65–69, in combination with different spatial and information exchange systems70–73, supports the phenomenon of the partially occupied ecological mammoth niche and its partial invasion by horses. In terms of dietary ecology, Neandertals and UPMHs behaved very similarly with respect to their prey choice, with the difference appearing to be that UPMHs exploited their resources more intensively than Neandertals and thereby causing ecological stress on the mammoth populations in the research area.
In addition to dietary ecology, facets of individual and group mobility (more precisely land use procurement strategies) are necessary to consider to be able to discuss the possible differences between late Neandertal and UPMH ecological niches39,74,75. Sulphur isotopic data can provide information about these aspects76. Figure 2 shows δ34S vs. δ13C of Late Pleistocene faunal and human remains from the Troisième caverne of Goyet, Scladina and Spy sites. To provide a representation of the local region (grey background), we used the mean values ±2 s.d. from the fauna. In comparison to all available studies presenting sulphur isotopic data from a Late Pleistocene archeological context24,41–43 the indicated range of around 10.0‰ in this study is the widest. However, since the geological bedrock in this area is quite diverse77, a wider range of sulphur isotopic compositions for the (semi-) local terrestrial ecosystem is to be expected. The defined δ34S range in this area is especially substantiated by the central positioning of most of the carnivores (the red dashed rectangle), which reflects the average δ34S values of their prey. Two carnivores, one cave lion and one canid, are outside the central area, which indicates a regular intake of protein sources with a sulphur isotopic signal different from the rest of the carnivores. Potential prey species represented by a comparable sulphur signal are the cave bear (for the cave lion) as well as the reindeer (for the canid). In Fig. 2, the Spy Neandertals plot centrally within the local signal, in between the local carnivores. Consequently, we find that the adult individual represented by Spy 94a had its main foraging area in the surrounding ecosystem, or at least in the same regions as the carnivores of the three Belgian sites. Spy 646a (Spy VI) is a child of around 1.5 years78, who was probably raised in this region. The sulphur values of both individuals are very close. On the other hand, the Neandertals from the Troisième caverne of Goyet yielded high δ34S values. No other species except the already mentioned canid and some reindeer yielded similar values. The δ34S values for the Goyet Neandertals clearly indicate an origin (for the main part of their diet) outside of the local ecosystem as reflected by the animal remains deposited in the Belgian caves of Spy, Scladina and Goyet. It seems unlikely that the δ34S values of the Goyet Neandertals were affected by a possible regular intake of aquatic resources, since the carbon and nitrogen isotopic values are very homogeneous for all three groups of humans. In the context of potential intake of aquatic resources, it becomes evident that the occurrence of individual faunal specimens with higher δ34S than the main group demonstrates the presence of one or more isotopically different terrestrial ecosystems that were accessible to the Goyet Neandertals and UPMHs. Unfortunately, at this stage of research we have been unable to locate the Goyet Neandertals’ catchment area but we are able to at least exclude some regions where δ34S values for Late Pleistocene faunal remains are available from. The values for Lommersum are substantially lower (Table S2), the same being true for Ziegeleigrube Coenen24, the Swabian Jura (SW Germany)43 farther south, as well as areas farther east, such as Kraków Spadzista (Southern Poland)42 and Predmostí I in the Moravian Plains in the Czech Republic41. The only known Late Pleistocene ecosystem with very similar δ34S values has been observed in SW France43. We therefore conclude that the Neandertals from the Troisième caverne of Goyet were not local in respect to their foraging area based on sulphur isotopic values, which is in contrast to the Neandertals from Spy whose δ34S values are consistent with the local sulphur isotopic signal.
Interestingly, the non-local Neandertals from the Troisième caverne of Goyet show evidence of intensive cannibalism27, which is not the case for the local Spy Neandertals79. These new isotopic results encourage hypothesizing that the Neandertal group from the Troisième caverne of Goyet was of foreign origin and may have been slaughtered perhaps by local inhabitants (exocannibalism), either Neandertals or another so far unknown group of older UPMHs. We can also envisage the scenario that the Goyet Neandertal group was killed somewhere else and their remains brought into the site. It is also crucial to emphasize the occurrence of early UPMH sites with ages in the range of the Goyet Neandertals in the broader region such as in Italy3, Germany71, and Austria4, but no Upper Palaeolithic stone tool industry with a similar age to the Neandertals from Goyet has been identified in Belgium so far. No more hypotheses may be formulated at this point, since the Goyet site has yielded the victims of cannibalistic activity and not the initiators.
The Goyet UPMHs are represented by two individuals28. Individual Q376-3 gave values centered in the area defined as the local δ34S signal, whereas individual Q116-1 is outside of the local δ34S signal and within the variation of the Goyet Neandertals (Fig. 2). The argument, that UPMHs had a higher intake of aquatic resources in their diet as a the reason for their δ34S values to be different (at least for one individual) to the Goyet Neandertals could only hold true if this was in combination with higher δ15N and/or δ13C values (compared to the Goyet Neandertals). The two UPMH individuals cover the total range of sulphur values (around 4‰) of all the Goyet Neandertal specimens studied (n = 11). Unfortunately, given the limited UPMH fossil record, we cannot determine if this range represents the endpoints of this chronogroup of UPMH nor if individual mobility history among UPMHs is actually more diverse than seems to be the case for the Goyet Neandertals. If the latter is true, more variable, broader and probably stronger interconnections with (trans-) regional networks, not only for land use procurement and exploitation strategies but also for the exchange of ideas and even people (see e.g. in80), would be supported for UPMHs. Regardless of this hypothesis, the application of a sulphur isotopic approach appears to be an adequate tool for retrieving information of spatial aspects among humans in Late Pleistocene archeological contexts. A broader dietary spectrum for UPMHs cannot be stated as being a substantial reason for the success of one human type over the other; instead, it seems necessary to investigate further the possibility of different concepts of landscape utilization that could have given UPMHs the edge over Neandertals.
Methods
Collagen preparation and isotopic analysis
Bone sampling followed standard procedure and a protocol modified from Longin81 was implemented48. A preliminary determination of the potential collagen preservation (nitrogen content in whole bone) was conducted82–84. These measurements were performed with a Vario EL III elemental analyser (Elementar) (mean standard error 0.02%, 0.05%, and 0.03% for %C, %N and %S, respectively). Isotopic measurements were performed at the Geochemical Unit of the Department of Geosciences at the University of Tübingen (Germany), using an elemental analyser NC 2500 connected to a Thermo Quest Delta + XL mass spectrometer. Collagen preservation was determined and general criteria were considered for the chemical integrity of this protein45. The isotopic ratios are expressed using the “δ” (delta) value as follows:
and
The standard for δ13C is the internationally defined marine carbonate V-PDB. For δ15N the standard atmospheric nitrogen (AIR) is used. Samples are calibrated to δ13C values of USGS 24 (δ13C = −16.00‰, relative to V-PDB) and to δ15N values of IAEA-N-2 (δ15N = 20.30‰, relative to ATM).
The standard for δ34S is the internationally defined Vienna-Canyon Diablo Troilite (VCDT). δ34S values are calibrated to δ34S values of NBS 123 (δ34S = 17.10‰, relative to CDT), NBS 127 (δ34S = 20.31‰, relative to VCDT) and IAEA-S-1 (δ34S = −0.30‰, relative to CDT) and IAEA-S-3 (δ34S = 21.70‰, relative to CDT).
Analytical error based on laboratory standards is ±0.1‰ for δ13C values, ±0.2‰ for δ15N results and ±0.4‰ for δ34S measurements.
δ34S values with the atomic C/Scoll and N/Scoll ratios in the range of 300 to 900 and 100 to 300, respectively, were considered to be valid for our purpose47,76,85. Modern day mammal collagen contains sulphur from 0.14 to 0.33%85, which fits with the theoretical range of 0.14 to 0.29% based on DNA and amino acid sequencing47. Only samples with sulphur content in collagen between 0.13 and 0.24% were considered in this study.
Cluster analysis and calculation of core niches (SIBER)
To identify patterns for individual distribution of single herbivorous and carnivorous specimens regardless of species attribution, we performed a cluster analysis using the Ward’s minimum variance method with the software SAS JMP version 10.0 (Fig. 3 and Supplementary Fig. 1). The reconstruction of the ecological niches for the herbivorous species (Fig. 4 and Supplementary Fig. 9) were done through SIBER (Stable Isotope Bayesian Ellipses in R)86. Through this R package, we reconstructed the complete niches (=convex hulls) and the core niches (=standard ellipse areas). The complete niche includes all individuals of a niche, but is quite sensitive to sample size. However, the core niche depicts the centre of a niche that is calculated by using most likelihood estimation and Bayesian statistics, and is less sensitive to the sample size86.
Here, the relative distances between species and the relative size and potential overlapping of convex hull and core niches are of special interest.
Protein source reconstruction with the Bayesian mixing model (SIAR)
With SIAR87,88, a package of the software R, it is possible to estimate quantitative and qualitative aspects of the animal proteins consumed by a specific carnivore or omnivore species. For the reconstruction of a hominin diet, it is of importance to realize that the importance of plant proteins is in general underestimated. The reason for this is the existence of a non-linear isotopic correlation between the most extreme end points of a pure vegetarian and a pure carnivorous feeding behavior. Even a very small amount of meat consumption substantially increases the δ15N values of bone collagen. For example, an amount of ~50% of plant intake results in collagen δ15N values that are not even 1 standard deviation lower than the δ15N values of a pure carnivore12. To consider this adequately, we provide data relative to the protein source of the different prey species but not absolute values. The applied SIAR package87,88 in the R software version 3.3.088 has the capability to cope with multiple dietary sources and incorporates uncertainties (standard deviations) in the input data. The tool provides not only a probability range for a specific protein source proportion but also the relative probability distribution at different amounts (Supplementary Figs. 2 and 7). Within the box plot generated (Fig. 5) the light grey represents a probability range of 95%, medium grey 75% and dark grey 25% for different prey proportions. The software provides diagnostic matrix plots in which the statistical dependencies between different protein sources are summarized (Supplementary Figs. 3 and 6). Dependencies have either a negative or positive character. This means, that a given protein source, e.g. A, can be partly replaced by source B if we included source C. The mixing of two or more protein sources can end up in the isotopic range of the third source. The SIAR software takes into account these correlations among different sources (prey species), which end up in an increasing probability range. In correspondence to previous works89–92, we applied a Trophic Enrichment Factor (TEF) of +1.1 ± 0.2‰ for δ13C and +3.8 ± 1.1‰ for δ15N values. The only precondition here is that we assumed that the prey species are the herbivorous ones we included in our study.
Supplementary information
Acknowledgements
Parts of our research were funded by the Wenner-Gren Foundation (Gr. 7837 to HR), the College of Social and Behavioral Sciences of CSUN, the CSUN Probationary Faculty Support Program, the CSUN Competition for Research, Scholarship and Creative Activity Awards, and the RBINS. We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen. The laboratory work was conducted with the assistance of Alex Bertacchi, Dobereiner Chaala Aldana, Sophie Habinger and Sara Rhodes (University of Tübingen). We are very thankful to Peter Tung for proofreading the manuscript and improving the language. We are also grateful to the two anonymous reviewers of the journal, whose comments significantly improved the manuscript.
Author Contributions
C.W. and H.B. designed the research. All authors performed the research. C.W., H.R., C.B., A.C., I.C., D.-G.D., S.G.-W., M.G., A.G.-O., J.K., T.M., Y.I.N., C.P., P.S., M.S. and H.B. analysed the data. C.W., H.R., C.B., I.C., D.-G.D., S.G.-W., M.G., A.G.-O., Y.I.N., C.P., M.S. and H.B. wrote the paper with input from all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41033-3.
References
- 1.Higham T, et al. The timing and spatiotemporal patterning of Neanderthal disappearance. Nature. 2014;512:306–309. doi: 10.1038/nature13621. [DOI] [PubMed] [Google Scholar]
- 2.Hublin J-J. The modern human colonization of western Eurasia: when and where? Quaternary Science Reviews. 2015;118:194–210. doi: 10.1016/j.quascirev.2014.08.011. [DOI] [Google Scholar]
- 3.Benazzi S, et al. The makers of the Protoaurignacian and implications for Neandertal extinction. Science. 2015;348:793–796. doi: 10.1126/science.aaa2773. [DOI] [PubMed] [Google Scholar]
- 4.Nigst PR, et al. Early modern human settlement of Europe north of the Alps occurred 43,500 years ago in a cold steppe-type environment. PloS one. 2014;111:14394–14399. doi: 10.1073/pnas.1412201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Olivencia, A. et al. First data of Neandertal bird and carnivore exploitation in the Cantabrian Region (Axlor; Barandiaran excavations; Dima, Biscay, Northern Iberian Peninsula). Scientific reports8 (2018). [DOI] [PMC free article] [PubMed]
- 6.Ramos-Muñoz J, et al. Early use of marine resources by Middle/Upper Pleistocene human societies: The case of Benzú rockshelter (northern Africa) Quaternary International. 2016;407:6–15. doi: 10.1016/j.quaint.2015.12.092. [DOI] [Google Scholar]
- 7.Cortés-Sánchez M, et al. Earliest Known Use of Marine Resources by Neanderthals. PloS one. 2011;6:e24026. doi: 10.1371/journal.pone.0024026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards MP, Pettitt PB, Stiner MC, Trinkaus E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6528–6532. doi: 10.1073/pnas.111155298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards MP, Trinkaus E. Out of Africa: modern human origins special feature: isotopic evidence for the diets of European Neanderthals and early modern humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16034–16039. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettitt PB, Richards M, Maggi R, Formicola V. The Gravettian burial known as the Prince (Il Principe): new evidence for his age and diet. Antiquity. 2003;77:15–19. doi: 10.1017/S0003598X00061305. [DOI] [Google Scholar]
- 11.Bocherens H, Drucker DG, Billiou D, Patou-Mathis M, Vandermeersch B. Isotopic evidence for diet and subsistence pattern of the Saint-Césaire I Neanderthal: review and use of a multi-source mixing model. Journal of Human Evolution. 2005;49:71–87. doi: 10.1016/j.jhevol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Bocherens, H. Neanderthal dietary habits: review of the isotopic evidence. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence., (eds Hublin, J.-J. & Richards, M. P.) 241–250 (2009).
- 13.Fizet M, Mariotti A, Bocherens H. Effect of Diet, Physiology and Climate on Carbon and Nitrogen Stable Isotopes of Collagen in a Late Pleistocene Anthropic Palaeoecosystem: Marillac, Charente, France. Journal of Archaeological Science. 1995;22:67–79. doi: 10.1016/S0305-4403(95)80163-4. [DOI] [Google Scholar]
- 14.Wißing C, et al. Isotopic evidence for dietary ecology of late Neandertals in North-Western Europe. Quaternary International. 2016;411:327–345. doi: 10.1016/j.quaint.2015.09.091. [DOI] [Google Scholar]
- 15.Trinkaus E, et al. Stable Isotope Evidence for Early Modern Human Diet in Southeastern Europe: Peştera cu Oase, Peştera Muierii and Peştera Cioclovina Uscată. MATERIALE ŞI CERCETĂRI ARHEOLOGICE. 2009;5:5–14. [Google Scholar]
- 16.Jacobi RM, Higham TF. The “Red Lady” ages gracefully: new ultrafiltration AMS determinations from Paviland. Journal of Human Evolution. 2008;55:898–907. doi: 10.1016/j.jhevol.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Prat S, et al. The Oldest Anatomically Modern Humans from Far Southeast Europe: Direct Dating, Culture and Behavior. PloS one. 2011;6:1–13. doi: 10.1371/journal.pone.0020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocherens H, et al. New isotopic evidence for dietary habits of Neandertals from Belgium. Journal of Human Evolution. 2001;40:497–505. doi: 10.1006/jhev.2000.0452. [DOI] [PubMed] [Google Scholar]
- 19.Richards MP, Schmitz RW. Isotope evidence for the diet of the Neanderthal type specimen. Antitquity. 2008;82:553–559. doi: 10.1017/S0003598X00097210. [DOI] [Google Scholar]
- 20.Schmitz, R. W. et al. The Neandertal type site revisited: interdisciplinary investigations of skeletal remains from the Neander Valley, Germany. Proceedings of the National Academy of Sciences of the United States of America99,13342–13347 (2002). [DOI] [PMC free article] [PubMed]
- 21.Krause J, et al. Neanderthals in central Asia and Siberia. Nature. 2007;449:902–904. doi: 10.1038/nature06193. [DOI] [PubMed] [Google Scholar]
- 22.Beauval C, Lacrampe-Cuyaubere F, Maureille B, Trinkaus E. Direct radiocarbon dating and stable isotopes of the neandertal femur from Les Rochers-de-Villeneuve (Lussac-les-Châteaux, Vienne) Bulletins et mémoires de la Société d’Anthropologie de Paris. 2006;18:35–42. [Google Scholar]
- 23.Bocherens H, Drucker DG, Madelaine S. Evidence for a (15)N positive excursion in terrestrial foodwebs at the Middle to Upper Palaeolithic transition in south-western France: Implications for early modern human palaeodiet and palaeoenvironment. Journal of Human Evolution. 2014;69:31–43. doi: 10.1016/j.jhevol.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Wißing C, Matzerath S, Turner E, Bocherens H. Paleoecological and climatic implications of stable isotope results from late Pleistocene bone collagen, Ziegeleigrube Coenen, Germany. Quaternary Research. 2015;84:96–105. doi: 10.1016/j.yqres.2015.05.005. [DOI] [Google Scholar]
- 25.Stevens RE, et al. Nitrogen isotope analyses of reindeer (Rangifer tarandus), 45,000 BP to 9,000 BP: Palaeoenvironmental reconstructions. Palaeogeography, Palaeoclimatology, Palaeoecology. 2008;262:32–45. doi: 10.1016/j.palaeo.2008.01.019. [DOI] [Google Scholar]
- 26.Drucker D, Bocherens H. Carbon and nitrogen stable isotopes as tracers of change in diet breadth during Middle and Upper Palaeolithic in Europe. International Journal of Osteoarchaeology. 2004;14:162–177. doi: 10.1002/oa.753. [DOI] [Google Scholar]
- 27.Rougier H, et al. Neandertal cannibalism and Neandertal bones used as tools in Northern Europe. Scientific reports. 2016;6:29005. doi: 10.1038/srep29005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posth C, et al. Pleistocene Mitochondrial Genomes Suggest a Single Major Dispersal of Non-Africans and a Late Glacial Population Turnover in Europe. Current biology: CB. 2016;26:827–833. doi: 10.1016/j.cub.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Higham T, et al. The earliest evidence for anatomically modern humans in northwestern Europe. Nature. 2011;479:521–524. doi: 10.1038/nature10484. [DOI] [PubMed] [Google Scholar]
- 30.Parnell AC, et al. Bayesian stable isotope mixing models. Environmetrics. 2013;24:387–399. [Google Scholar]
- 31.Bocherens H, Germonpré M, Toussaint M, Semal P. Stable isotopes. In: Rougier, H. & Semal, P. (Eds), Spy cave. 125 years of multidisciplinary research at the Betche aux Rotches (Jemeppe-sur-Sambre, Province of Namur, Belgium), Volume 1. Anthropologia et Praehistorica, 123/2012. Brussels, Royal Belgian Institute of Natural Sciences, Royal Belgian Society of Anthropology and Praehistory & NESPOS Society. 2013;1:357–370. [Google Scholar]
- 32.Bocherens, H. & Drucker, D. Dietary competition between Neanderthals and Modern humans: insights from stable isotopes. In When Neanderthals and Modern Humans Met (ed. Conard, N. J.), 129–143 (Kerns Verlag, 2006).
- 33.Matthies, T. Subsistence strategies during the Early Upper Palaeolithic of northern Central Europe: A re-analysis of the faunal remains from Lommersum (Germany). Proceedings of the European Society for the study of Human Evolution,122 (2012).
- 34.Huels M, van der Plicht J, Brock F, Matzerath S, Chivall D. Laboratory Intercomparison of Pleistocene Bone Radiocarbon Dating Protocols. Radiocarbon. 2017;59:1543–1552. doi: 10.1017/RDC.2017.23. [DOI] [Google Scholar]
- 35.Binford L. Willow smoke and dog’s tails: hunter-gatherer settlement systems and archaeological site formation. American Antiquity. 1980;45:4–20. doi: 10.2307/279653. [DOI] [Google Scholar]
- 36.Binford L. Human Ancestors: Changing Views of Their Behavior. Journal of Anthropological Archeology. 1985;4:292–327. doi: 10.1016/0278-4165(85)90009-1. [DOI] [Google Scholar]
- 37.Britton K, Gaudzinski-Windheuser S, Roebroeks W, Kindler L, Richards MP. Stable isotope analysis of well-preserved 120,000-year-old herbivore bone collagen from the Middle Palaeolithic site of Neumark-Nord 2, Germany reveals niche separation between bovids and equids. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;333–334:168–177. doi: 10.1016/j.palaeo.2012.03.028. [DOI] [Google Scholar]
- 38.Bocherens H, et al. Direct isotopic evidence for subsistence variability in Middle Pleistocene Neanderthals (Payre, southeastern France) Quaternary Science Reviews. 2016;154:226–236. doi: 10.1016/j.quascirev.2016.11.004. [DOI] [Google Scholar]
- 39.Richards M, et al. Strontium isotope evidence of Neanderthal mobility at the site of Lakonis, Greece using laser-ablation PIMMS. Journal of Archaeological Science. 2008;35:1251–1256. doi: 10.1016/j.jas.2007.08.018. [DOI] [Google Scholar]
- 40.Drucker DG, Bridault A, Cupillard C. Environmental context of the Magdalenian settlement in the Jura Mountains using stable isotope tracking (13C, 15N, 34S) of bone collagen from reindeer (Rangifer tarandus) Quaternary International. 2012;272–273:322–332. doi: 10.1016/j.quaint.2012.05.040. [DOI] [Google Scholar]
- 41.Bocherens H, et al. Reconstruction of the Gravettian food-web at Předmostí I using multi-isotopic tracking (13C, 15N, 34S) of bone collagen. Quaternary International. 2015;359–360:211–228. doi: 10.1016/j.quaint.2014.09.044. [DOI] [Google Scholar]
- 42.Drucker, D., Rivals, F., Münzel, S. & Bocherens, H. Stable isotope and microwear investigation on the mammoth (Mammuthus primigenius) of Kraków Spadzista: insights into diet and environment. A Gravettian Site in Southern Poland: Kraków Spadzista: Piotr Wojtal, Jaroslaw Wilczyñski, Gary Haynes (Eds), 189–202 (2015).
- 43.Drucker DG, et al. Tracking possible decline of woolly mammoth during the Gravettian in Dordogne (France) and the Ach Valley (Germany) using multi-isotope tracking (13C, 14C, 15N, 34S, 18O) Quaternary International. 2015;359–360:304–317. doi: 10.1016/j.quaint.2014.11.028. [DOI] [Google Scholar]
- 44.Rougier H, et al. The First Upper Paleolithic Human Remains from Belgium: Aurignacian, Gravettian and Magdalenian Fossils at the “Troisième caverne” of Goyet. PaleoAnthropology. 2013;2013 A:33. [Google Scholar]
- 45.DeNiro MJ. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature. 1985;317:806–809. doi: 10.1038/317806a0. [DOI] [Google Scholar]
- 46.Ambrose SH. Preparation and Characterization of Bone and Tooth Collagen for Isotopic Analysis. Journal of Archaeological Science. 1990;17:431–451. doi: 10.1016/0305-4403(90)90007-R. [DOI] [Google Scholar]
- 47.Nehlich O, Richards MP. Establishing collagen quality criteria for sulphur isotope analysis of archaeological bone collagen. Archaeological and Anthropological Sciences. 2009;1:59–75. doi: 10.1007/s12520-009-0003-6. [DOI] [Google Scholar]
- 48.Bocherens H, et al. Paleobiological Implications of the Isotopic Signatures (13C, 15N) of Fossil Mammal Collagen in Scladina Cave (Sclayn, Belgium) Quaternary Research. 1997;48:370–380. doi: 10.1006/qres.1997.1927. [DOI] [Google Scholar]
- 49.Bocherens H, et al. Isotopic evidence for dietary ecology of cave lion (Panthera spelaea) in North-Western Europe: Prey choice, competition and implications for extinction. Quaternary International. 2011;245:249–261. doi: 10.1016/j.quaint.2011.02.023. [DOI] [Google Scholar]
- 50.Yeakel JD, Guimaraes PR, Jr., Bocherens H, Koch PL. The impact of climate change on the structure of Pleistocene food webs across the mammoth steppe. Proceedings. Biological sciences/The Royal Society. 2013;280:20130239. doi: 10.1098/rspb.2013.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bocherens H. Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quaternary Science Reviews. 2015;117:42–71. doi: 10.1016/j.quascirev.2015.03.018. [DOI] [Google Scholar]
- 52.Fox-Dobbs K, Leonard JA, Koch PL. Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records. Palaeogeography, Palaeoclimatology, Palaeoecology. 2008;261:30–46. doi: 10.1016/j.palaeo.2007.12.011. [DOI] [Google Scholar]
- 53.Drucker DG, et al. Isotopic analyses suggest mammoth and plant in the diet of the oldest anatomically modern humans from far southeast Europe. Scientific reports. 2017;7:6833. doi: 10.1038/s41598-017-07065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Germonpré M, Udrescu M, Fiers E. Possible evidence of mammoth hunting at the Neanderthal site of Spy (Belgium) Quaternary International. 2014;337:28–42. doi: 10.1016/j.quaint.2012.10.035. [DOI] [Google Scholar]
- 55.Naito YI, et al. Reply to “Comment on “Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids incollagen.” [J. Hum. Evol. 93 (2016) 82e90]” [J. Hum. Evol. 117 (2018) 53e55] Journal of Human Evolution. 2018;117:56–60. doi: 10.1016/j.jhevol.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Naito YI, et al. Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen. Journal of Human Evolution. 2016;93:82–90. doi: 10.1016/j.jhevol.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 57.El Zaatari S, Grine FE, Ungar PS, Hublin JJ. Neandertal versus Modern Human Dietary Responses to Climatic Fluctuations. PloS one. 2016;11:e0153277. doi: 10.1371/journal.pone.0153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estalrrich A, El Zaatari S, Rosas A. Dietary reconstruction of the El Sidrón Neandertal familial group (Spain) in the context of other Neandertal and modern hunter-gatherer groups. A molar microwear texture analysis. Journal of Human Evolution. 2017;104:13–22. doi: 10.1016/j.jhevol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Nikolskiy P, Pitulko V. Evidence from the Yana Palaeolithic site, Arctic Siberia, yields clues to the riddle of mammoth hunting. Journal of Archaeological Science. 2013;40:4189–4197. doi: 10.1016/j.jas.2013.05.020. [DOI] [Google Scholar]
- 60.Mellars P, French CJ. Tenfold Population Increase in Western Europe at the Neandertal–to–Modern Human Transition. Science. 2011;333:623–627. doi: 10.1126/science.1206930. [DOI] [PubMed] [Google Scholar]
- 61.Agam A, Barkai R. Elephant and Mammoth Hunting during the Paleolithic: A Review of the Relevant Archaeological, Ethnographic and Ethno-Historical Records. Quaternary. 2018;1:3. doi: 10.3390/quat1010003. [DOI] [Google Scholar]
- 62.Reshef H, Barkai R. A taste of an elephant: The probable role of elephant meat in Paleolithic diet preferences. Quaternary International. 2015;379:28–34. doi: 10.1016/j.quaint.2015.06.002. [DOI] [Google Scholar]
- 63.Yeakel, J. D. The structure of mammalian food-webs: interpreting, predicting, and informing estimates of species interactions in paleontological and modern communities. PhD Dissertation 1–200 (2012).
- 64.Fellows Yates, J. A. et al. Central European Woolly Mammoth Population Dynamics: Insights from Late Pleistocene Mitochondrial Genomes. Scientific reports7, 17714 (2017). [DOI] [PMC free article] [PubMed]
- 65.Bocquet-Appel J-P, Demars P-Y. Population Kinetics in the Upper Palaeolithic in Western Europe. Journal of Archaeological Science. 2000;27:551–570. doi: 10.1006/jasc.1999.0471. [DOI] [Google Scholar]
- 66.Bocquet-Appel J-P, Demars P-Y, Noiret L, Dobrowsky D. Estimates of Upper Palaeolithic meta-population size in Europe from archaeological data. Journal of Archaeological Science. 2005;32:1656–1668. doi: 10.1016/j.jas.2005.05.006. [DOI] [Google Scholar]
- 67.Bocquet-Appel, J.-P. & Degioanni, A. Neanderthal Demographic Estimates. Current Anthropology54, 202–213 (2013).
- 68.Starkovich BM, Ntinou M. Climate change, human population growth, or both? Upper Paleolithic subsistence shifts in southern Greece. Quaternary International. 2017;428:17–3. doi: 10.1016/j.quaint.2015.03.044. [DOI] [Google Scholar]
- 69.Davies W, White D, Lewis M, Stringer C. Evaluating the transitional mosaic: frameworks of change from Neanderthals to Homo sapiens in eastern Europe. Quaternary Science Reviews. 2015;118:211–242. doi: 10.1016/j.quascirev.2014.12.003. [DOI] [Google Scholar]
- 70.Münzel SC, Conard NJ. Change and continuity in subsistence during the Middle and Upper Palaeolithic in the Ach Valley of Swabia (south-west Germany) International Journal of Osteoarchaeology. 2004;14:225–243. doi: 10.1002/oa.758. [DOI] [Google Scholar]
- 71.Higham T, et al. Testing models for the beginnings of the Aurignacian and the advent of figurative art and music: the radiocarbon chronology of Geißenklösterle. Journal of Human Evolution. 2012;62:664–676. doi: 10.1016/j.jhevol.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Conard NJ, Bolus M. Radiocarbon dating the appearance of modern humans and timing of cultural innovations in Europe: new results and new challenges. Journal of Human Evolution. 2003;44:331–371. doi: 10.1016/S0047-2484(02)00202-6. [DOI] [PubMed] [Google Scholar]
- 73.Conard NJ, Malina M, Münzel SC. New flutes document the earliest musical tradition in southwestern Germany. Nature. 2009;460:737–740. doi: 10.1038/nature08169. [DOI] [PubMed] [Google Scholar]
- 74.Niven L, et al. Neandertal mobility and large-game hunting: the exploitation of reindeer during the Quina Mousterian at Chez-Pinaud Jonzac (Charente-Maritime, France) Journal of Human Evolution. 2012;63:624–635. doi: 10.1016/j.jhevol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Delagnes A, Rendu W. Shifts in Neandertal mobility, technology and subsistence strategies in western France. Journal of Archaeological Science. 2011;38:1771–1783. doi: 10.1016/j.jas.2011.04.007. [DOI] [Google Scholar]
- 76.Nehlich O. The application of sulphur isotope analyses in archaeological research: A review. Earth-Science Reviews. 2015;142:1–17. doi: 10.1016/j.earscirev.2014.12.002. [DOI] [Google Scholar]
- 77.Azmy K, Poty E, Brand U. High-resolution isotope stratigraphy of the Devonian–Carboniferous boundary in the Namur–Dinant Basin, Belgium. Sedimentary Geology. 2009;216:117–124. doi: 10.1016/j.sedgeo.2009.03.002. [DOI] [Google Scholar]
- 78.Crevecoeur I, et al. The Spy VI child: a newly discovered Neandertal infant. Journal of Human Evolution. 2010;59:641–656. doi: 10.1016/j.jhevol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 79.Semal P, et al. New data on the late Neandertals: direct dating of the Belgian Spy fossils. American journal of physical anthropology. 2009;138:421–428. doi: 10.1002/ajpa.20954. [DOI] [PubMed] [Google Scholar]
- 80.Sikora, M. et al. Ancient genomes show social and reproductive behavior of early Upper Paleolithic foragers. Science 358, 659-662 (2017). [DOI] [PubMed]
- 81.Longin R. New method of collagen extraction for radiocarbon dating. Nature. 1971;230:241–242. doi: 10.1038/230241a0. [DOI] [PubMed] [Google Scholar]
- 82.Iacumin P, Bocherens H, Mariotti A, Longinelli A. An isotopic palaeoenvironmental study of human skeletal remains from the Nile Valley. Palaeogeography, Palaeoclimatology, Palaeoecology. 1996;126:15–30. doi: 10.1016/S0031-0182(96)00067-3. [DOI] [Google Scholar]
- 83.Iacumin P, Bocherens H, Huertas D, Mariotti A, Longinelli A. A stable isotope study of fossil mammal remains from the Paglicci cave, Southern Italy. N and C as palaeoenvironmental indicators. Earth and Planetary Science Letters. 1997;148:349–357. doi: 10.1016/S0012-821X(97)00015-0. [DOI] [Google Scholar]
- 84.Bocherens H, Drucker D, Billiou D, Moussa I. Une nouvelle approche pour évaluer l'état de conservation de l’os et du collagène pour les mesures isotopiques (datation au radiocarbone, isotopes stables du carbone et de l’azote) L’Anthropologie. 2005;109:557–567. doi: 10.1016/j.anthro.2005.06.005. [DOI] [Google Scholar]
- 85.Bocherens H, Drucker DG, Taubald H. Preservation of bone collagen sulphur isotopic compositions in an early Holocene river-bank archaeological site. Palaeogeography, Palaeoclimatology, Palaeoecology. 2011;310:32–38. doi: 10.1016/j.palaeo.2011.05.016. [DOI] [Google Scholar]
- 86.Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. The Journal of Animal Ecology. 2011;80:595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- 87.Parnell AC, Inger R, Bearhop S, Jackson AL. Source partitioning using stable isotopes: coping with too much variation. PloS one. 2010;5:e9672. doi: 10.1371/journal.pone.0009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.The-R-CoreTeam. R: A language and environment for statistical computing (2013).
- 89.Bocherens H, Drucker D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: case studies from recent and ancient terrestrial ecosystems. International Journal of Osteoarchaeology. 2003;13:46–53. doi: 10.1002/oa.662. [DOI] [Google Scholar]
- 90.Fox-Dobbs K, Bump JK, Peterson RO, Fox DL, Koch PL. Carnivore-specific stable isotope variables and variation in the foraging ecology of modern and ancient wolf populations: case studies from Isle Royale, Minnesota, and La Brea. Canadian Journal of Zoology. 2007;85:458–471. doi: 10.1139/Z07-018. [DOI] [Google Scholar]
- 91.Bocherens, H. et al. Reconstruction of the Gravettian food-web at Předmostí I using multiisotopic tracking (13C, 15N, 34S) of bone collagen. Quaternary International359–360 (2015).
- 92.Krajcarz MT, Krajcarz M, Bocherens H. Collagen-to-collagen prey-predator isotopic enrichment (Δ 13 C, Δ 15 N) in terrestrial mammals - a case study of a subfossil red fox den. Palaeogeography, Palaeoclimatology, Palaeoecology. 2018;490:563–570. doi: 10.1016/j.palaeo.2017.11.044. [DOI] [Google Scholar]
- 93.Semal, P. et al. Radiocarbon dating of human remains and associated archaeological material. Spy cave. 125 years of multidisciplinary research at the Betche aux Rotches (Jemeppe-sur-Sambre, Province of Namur, Belgium), Volume 1, 331–356 (Anthropologica et Praehistorica, 123/2012, Royal Belgian Institute of Natural Sciences, Royal Belgian Society of Anthropology and Praehistory & NESPOS Society, 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.