Abstract
Shallow, tropical marine ecosystems provide essential ecosystem goods and services, but it is unknown how these ecosystems will respond to the increased exposure to the temperature extremes that are likely to become more common as climate change progresses. To address this issue, we tracked the fitness and productivity of a key zooplankton species, the copepod Pseudodiaptomus annandalei, acclimated at two temperatures (30 and 34 °C) over three generations. 30 °C is the mean temperature in the shallow water of the coastal regions in Southeast Asia, while 34 °C simulated a temperature extreme that occurs frequently during the summer period. For each generation, we measured the size at maturity and reproductive success of individuals. In all three generations, we found strong negative effects of warming on all measured fitness-related parameters, including prolonged development time, reduced size at maturity, smaller clutch sizes, lower hatching success, and reduced naupliar production. Our results suggest that P. annandalei are already exposed to temperatures that exceed their upper thermal optimum. Increased exposure to extreme temperatures may reduce the abundance of these tropical marine copepods, and thus reduce the availability of resources to higher trophic levels.
Introduction
Temperature is one of the most important eco-physiological variables affecting the survival, abundance and performance of ectothermic organisms1–3. Increased sea surface temperature4,5 has become one of the major drivers of the change in structure and functioning of coastal marine ecosystems worldwide6–9. In tropical marine ecosystems, sea surface temperature increased more than 1 °C over the past 100 years and the rate of warming is expected to triple or quadruple by the end of the 21 century4,10. Shallow, coastal waters in the tropics are particularly prone to experiencing temperature extremes in a warming climate11. Such extreme temperatures are expected to negatively impact thermally specialised species occurring in these zones12,13 and many species already approach their upper thermal limits14,15.
Species can respond to warming by shifting distribution16,17, shifting phenology18–20 or changing morphological traits such as reducing body size21,22. A reduction of body size has been documented with warming temperatures in various taxa from marine phytoplankton23, zooplankton21,24,25, benthic invertebrates26, and fish27. This also aligns with the predictions of Bergmann’s rule, whereby tropical species tend to be smaller than related taxa at higher latitudes27. Warming has also been shown to impact fitness parameters in many taxa, either positively or negatively, depending on the taxa. For example, elevated temperature increased fitness over multiple generations in high latitude zooplankton28, but reduced the reproductive success of a coral reef damselfish Acanthochromis polyacanthus29. However, it remains relatively unknown how tropical coastal marine organisms, especially those at the base of the marine food web such as copepods, will be affected by extreme warming, particularly across generations.
In the tropical coastal ecosystems in the Indo-Pacific region the calanoid copepod Pseudodiaptomus annandalei is highly abundant30,31. They are also important grazers on small plankton32 and prey for fish larvae31. Any effects on fitness and productivity of this species are expected to have bottom-up controls that reduce food resources for small fish31 and top-down controls that reduce grazing rate on phytoplankton33, with ecological consequences. Here, we experimentally tested the effect of extreme temperature on the copepod P. annandalei by exposing them to the extreme temperature for three consecutive generations: F1, F2 and F3. The objective of this study is to assess the ecological consequences of the extreme temperature over the course of three generations. We documented how warming affects key fitness-related traits of copepods as the secondary producers including the development time, size at maturity of males and females, the clutch size, the hatching success and the naupliar production.
Results
Development of P. annandalei
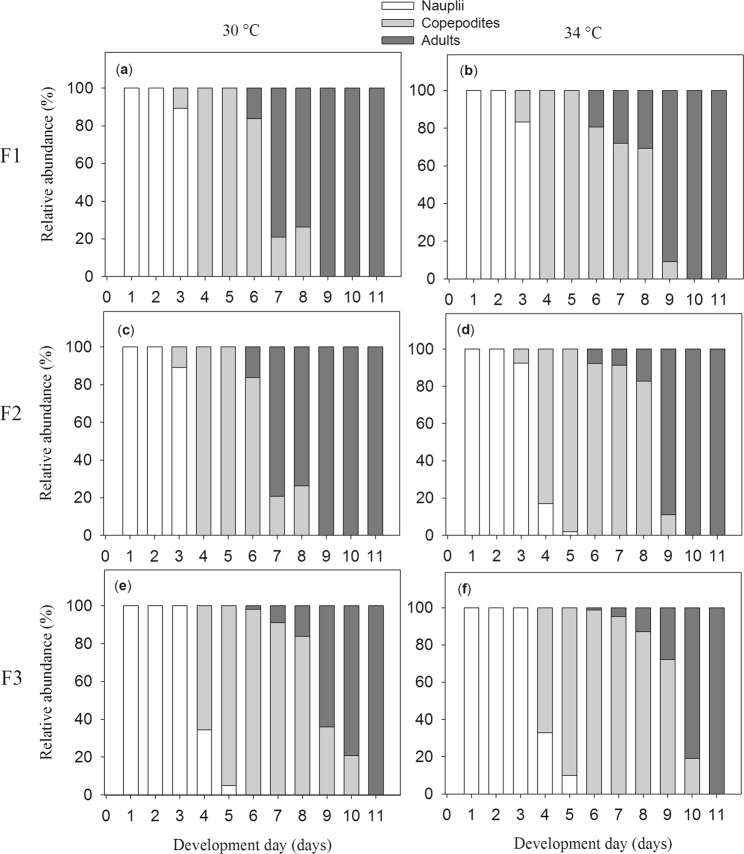
In the F1 and F2 generations, P. annandalei showed no difference in naupliar development time between 30 °C and 34 °C (Fig. 1a–d). The naupliar stages lasted from day 1 to day 3 after hatching. In contrast, the copepodite stages lasted approximately 2 days longer at 34 °C than at 30 °C (Fig. 1a–d). The adult stage started on day 7 at 30 °C and on day 9 at 34 °C (Fig. 1a–d). The nauplii development time of the F3 generation was similar to the F1 and F2 generations, but the copepodite stages lasted longer, and they reached the maturity on day 9 at 30 °C and day 10 at 34 °C (Fig. 1e,f).
Figure 1.
The development of Pseudodiaptomus annandalei in response to temperatures across three generations F1, F2 and F3. Data are the relative percentage of nauplii, copepodites and adults.
Size at maturity
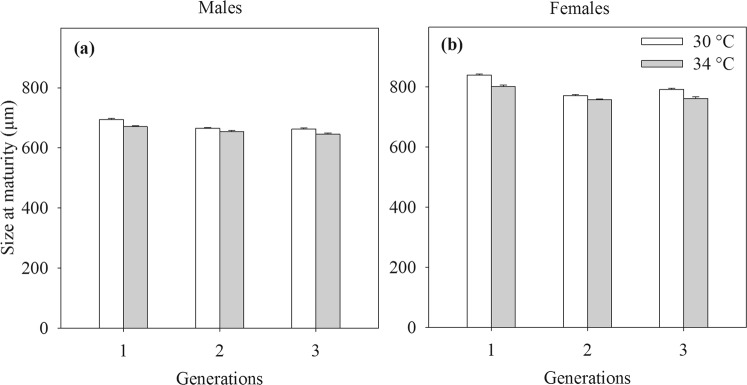
In both sexes, the exposure to extreme temperature resulted in smaller size at maturity (main effect Temperature). For males, the difference in size at maturity between 30 and 34 °C was detectable all three generations as the interaction of Temperature × Generation was not significant. Females showed a more pronounced size reduction in F1 than in F2 and F3 generations (interaction of Temperature × Generation, Table 1, Fig. 2). In both sexes, the size at maturity of F1 was larger than of F2 and F3 (for both males and females, F1 > F2, P < 0.001, F1 > F3, P < 0.001, Turkey posthoc tests). For males and females (at 34 °C), there was no difference between the two subsequent generations (Males: P = 0.99 at 30 °C, and P = 0.72 at 34 °C; and Females: P = 0.98 at 34 °C, Tukey posthoc test). At 30 °C, the size at maturity of F3 females was larger than of F2 females (P = 0.0015, Turkey posthoc test). Overall, the size at maturity of males and females of F1, F2 and F3 generations was smaller than of F0 generation (P values < 0.001, Tukey posthoc tests).
Table 1.
The results of generalised mixed models, using bottle as a random effect, testing for the effects of the temperature on the size at maturity of males and females of the copepod Pseudodiaptomus annandalei across three generations.
| Effects | Males | Females | ||||
|---|---|---|---|---|---|---|
| df1,df2 | F | P | df1,df2 | F | P | |
| Temperature | 1, 540 | 32.45 | <0.001 | 1, 500 | 65.48 | <0.001 |
| Generation | 2, 539 | 35.29 | <0.001 | 2, 500 | 137.17 | <0.001 |
| Temperature × Generation | 2, 539 | 1.64 | 0.19 | 2, 500 | 5.82 | 0.0032 |
Significant P values (P < 0.05) are indicated in bold.
Figure 2.
Size at maturity of males (a) and females (b) of the copepod Pseudodiaptomus annandalei as a function of temperature and generation. Data are means + 1 SE.
Clutch size
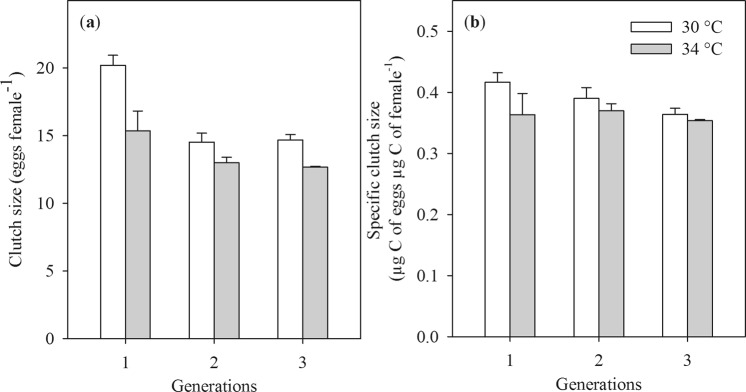
The number of eggs per clutch (two egg sacs) was lower in females reared at 34 °C than that of 30 °C (Table 2, Fig. 3). The clutch size was larger in the F1 generation than in F2 and F3, but there was no difference of clutch size between the two later generations (main effect Generation, and the interaction of Temperature × Generation, Table 1, Fig. 3). The effects of temperature and generation on the clutch size were still significant when the clutch size was normalized for the size of females (specific clutch size, Table 2, Fig. 3b). At 30 °C, the specific clutch size of females decreased after each generation (main effect of Generation, Table 2, Fig. 3b). The interaction between temperature and generation on specific clutch size was not significant (Table 2).
Table 2.
The results of 5 responsive variables as a function of temperature for the copepod Pseudodiaptomus annandalei across three generations.
| Effects | Clutch size | Specific clutch size | Hatching success | Nauplii production | Specific nauplii production | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df1,df2 | F | P | df1,df2 | F | P | df1,df2 | F | P | df1,df2 | F | P | df1,df2 | F | P | |
| Temperature | 1, 191 | 61.45 | <0.001 | 1, 191 | 9.98 | 0.0019 | 1, 210 | 9.90 | 0.0019 | 1, 24 | 52.79 | <0.001 | 1, 24 | 29.21 | <0.001 |
| Generation | 2, 191 | 54.17 | <0.001 | 2, 191 | 3.81 | 0.024 | 2, 210 | 0.02 | 0.99 | 2, 24 | 10.85 | <0.001 | 2, 24 | 1.72 | 0.20 |
| Temperature × Generation | 2, 191 | 8.80 | <0.001 | 2, 191 | 2.22 | 0.11 | 2, 210 | 0.02 | 0.99 | 2, 24 | 4.97 | 0.016 | 2, 24 | 2.17 | 0.14 |
The first three response variables (clutch size, specific clutch size, hatching success) were modelled with GLMMIX using bottle as a random effect. The remaining two response variables (nauplii production and specific nauplii production) were modelled using linear model. Significant P values (P < 0.05) are indicated in bold.
Figure 3.
The clutch size (a) and specific clutch size (b) of the copepod Pseudodiaptomus annandalei as a function of the temperature and the generation. Data are means + 1 SE.
Hatching success
The hatching success was 70% at 34 °C (thus approximately 20% lower than that at 30 °C) and this pattern was independent of generations (Table 2, Fig. 4).
Figure 4.
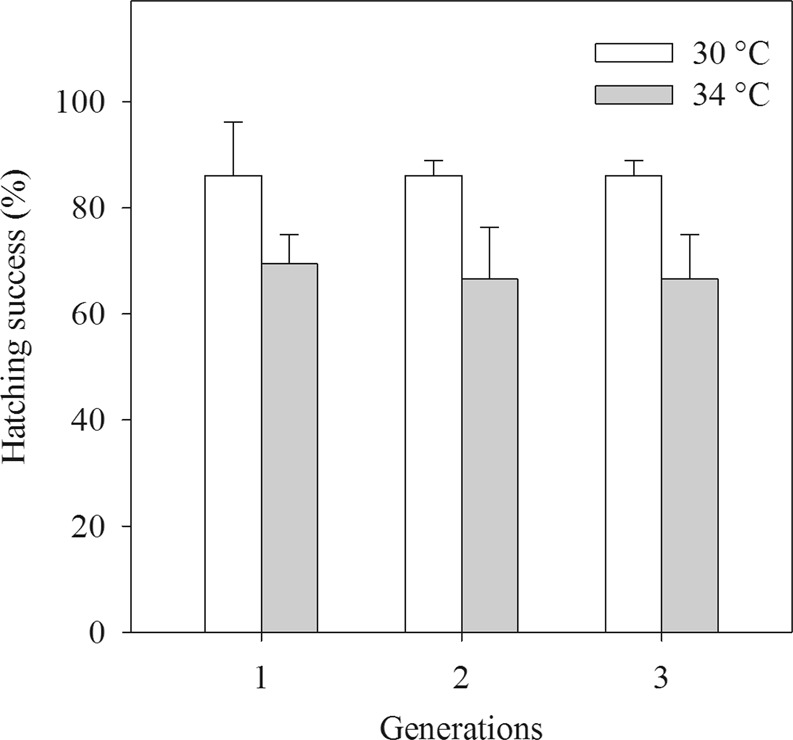
The hatching success of the copepod Pseudodiaptomus annandalei as a function of temperature and generation. Data are means + 1 SE.
Naupliar production
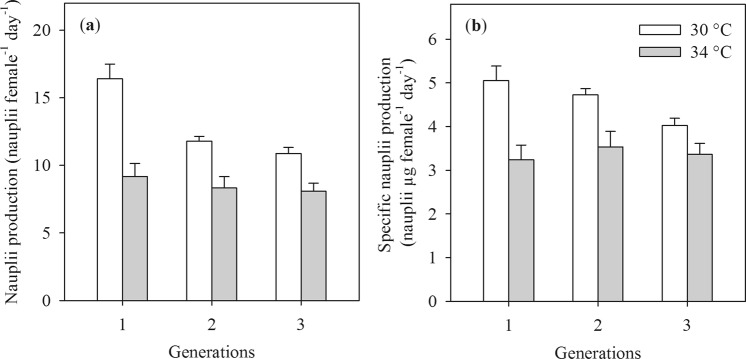
Nauplii and specific nauplii production were considerably lower at 34 °C than at 30 °C (main effect Temperature, Table 2, Fig. 5). The difference in nauplii production between temperatures was more pronounced in F1 generation than in the F2 and F3 generations (Temperature × Generation interaction, Table 2, Fig. 5a,b). Nauplii production of the F1 generation was higher than in the F2 and F3 generations while there was no difference between two later generations. The effect of generation and its interaction with temperature on nauplii production were lost after correcting nauplii production for the biomass of the females (Table 2, Fig. 5a,b).
Figure 5.
Nauplii production (a) and specific nauplii production (b) of the copepod Pseudodiaptomus annandalei as a function of the temperature and the generation. Data are means + 1 SE.
Discussion
In general higher temperature reduced all measured fitness-related traits of the tropical coastal copepod P. annandalei such as the size at maturity and reproductive success: clutch size, hatching success and nauplii production. A reduction in body size induced by warming is a widespread phenomenon in aquatic ecosystems21–24,26,34 but this is the first time this pattern has been documented for P. annandalei, a key zooplankton species in the tropical coastal environment. An accelerated development that outpaces growth rate in response to temperature increase is thought to explain the reduced size at maturity of marine copepod species35,36. However, this cannot explain the pattern in P. annandalei as the development time of this species was longer at 34 °C than at 30 °C. The higher, suboptimal temperature may result in a higher energy expenditure on basal metabolism (indicated by a higher respiration37). It may also intensify energy expenditure on additional biological functions such as the upregulation of heat shock proteins38, a universal mechanism used by many animals to cope with the thermal stress39 by diverting energy away from somatic growth and development. Furthermore, the energy from food prey may also be reduced under elevated temperature as the grazing rate of P. annandalei strongly decreased at 35 °C compared to 30 °C33.
Interestingly, the overall size at maturity of F1 was larger than those of F2 and F3 generations, irrespective of temperatures. Nauplii used to start F1 generation were hatched from wild collected females with a short-term acclimation period to the experimental temperatures. It was likely that the acclimation period of three days (approximately one-third of the generation time) may not fully eliminate the field conditions on F1 generation as size of F0 > control F1 > control F2. The culture conditions in the laboratory may also contribute to the changes in size at maturity of P. annandalei in the control treatment across generations. The control F3 females showed an increased size at maturity compared to control F2 females, indicating an adaptation to culture conditions other than temperature. However, this would not change our general finding that extreme temperature reduced the size of P. annandalei across three generations. The reduced size at maturity may have ecological consequences as adult size is positively associated with the grazing rate40, and reproductive output24. Our results supported this as they showed that smaller females exhibited lower nauplii production at 34 °C (see further below).
The clutch size was smaller at the temperature of 34 °C compared to 30 °C. Similar patterns have been observed in other copepod species e.g. Pseudodiaptomus pelagicus41 and Tigriopus californicus42. It has likewise been shown that the clutch size and egg production of marine copepods43,44 may be positively associated with the size of females. Although the smaller size of female P. annandalei at 34 °C may partly contribute to the smaller clutch size, the effect of extreme temperature on clutch size of P. annandalei was still significant after the clutch size was normalized for the biomass of females (specific clutch size). This suggests that egg formation and development may have been physiologically impaired by extreme temperature.
The hatching success of P. annandalei in the control temperature was high (80–90%), and was similar to that observed in previous studies45,46, while hatching success of P. annandalei was 70% at 34 °C. Previous work has shown that increased temperature can decrease hatching success47. For example, although C. finmarchicus hatching success was not affected at the range of the temperature from 6 to 19 °C, it was reduced to 25% at 22 °C and none of the eggs hatched at temperature of 25 °C47, possibly due to poor maternal provisioning48. Poor maternal provisioning may explain the lower hatching success in P. annandalei as they showed a reduced grazing rate at 34 °C compared to 30 °C33. Additionally, exposure to the extreme temperature of 34 °C may have negatively affected on the development of embryos, thereby contributing to the lower hatching success. Previous studies have shown a direct negative effect of extreme temperature on the embryonic survival of some spiders49 and marine fish50.
As a consequence of the smaller clutch size and lower hatching success, nauplii production was lower at 34 °C than at 30 °C. The reduced clutch size, hatching success and nauplii production at 34 °C were similar across all three generations and independent of generation, indicating no thermal acclimation and/or adaptation developed after three generations. Interestingly, the specific nauplii production of the control females decreased after each generation, similar to the pattern observed for specific clutch size. Some studies have shown that some transgenerational effects of e.g. antidepressants51 and cyanobacterial toxin52 on parental conditions may last at least three generations. This may be the case of P. ananndalei outside the laboratory.
While a number of studies have shown a rapid acclimation and/or adaptation of tropical marine species such as the coral damselfish Acanthochromis polyacanthus53 to global warming, this may not be the case for the tropical copepod P. annandalei. It is unlikely that this species could evolve the capacity to adapt to extreme temperature after only three generations. A previous study carried out in the shallow coastal waters of the Southeast Asian region showed that warming, together with other anthropogenic disturbance, promotes the dominance of opportunistic small copepod species such as Oithona simplex, Hemicyclops sp., Pseudomacrochiron sp. and Microsetella norvegica and a decrease in large-body copepod species such as Pseudodiaptomus bowmani, a congeneric species to P. annandalei54. While this pattern will be complicated by a number of other factors, particularly food quantity and quality, extreme warming may drastically change the structure, function and dynamics of coastal food webs as have been observed in a global biodiversity hotspot in the subtropical region7.
Copepods account for approximately 80% of the biomass of mesozooplankton and play a key role as major secondary producers, transferring energy from photosynthesis organisms to higher trophic levels31,55. Understanding the responses of tropical copepods such as P. annandalei to extreme warming is crucial for understanding the transfer of energy in coastal food webs31. Our study shows that extreme warming may negatively affect the secondary production by lowering copepod biomass and reproductive success. We found a reduction in P. annandalei reproductive success with exposure to extreme temperatures. Increased exposure to extreme temperatures in coastal areas could have population consequences for the species, especially as other studies have shown that longevity of copepods also declines at higher temperatures56,57.
The negative effect of warming on fitness of the tropical copepod P. annandalei supports the concern that these tropical organisms occur close to their upper thermal threshold7,12,14. Copepods, especially, P. annandalei are the major food source of young and small nekton species31. Our results suggest that with increased exposure to the extreme temperature predicted under warmer climate scenarios may yield bottom-up consequences for marine food webs by lowering the resources for higher trophic levels. Sea surface temperatures of 34 °C are often measured in the tropical coastal regions in the South East Asia33,38,58 and such extreme temperatures are predicted to occur more frequently and to last longer in the coming years4,59. The temperature-induced reduction in body size of P. annandalei we observed may also have an influence on its grazing rate as copepods (e.g. Centropages typicus60, Acartia omorii61, A. tonsa46) typically show a positive correlation between body size and grazing rate. The South China Sea is one of the most polluted regions on Earth62 and the majority of its coastal marine ecosystems are impacted by multiple stressors from anthropogenic activities (e.g. pollutions, overfishing, climate change)63,64. The combined effects of reduced body size and lower reproductive success of a key secondary producer under warming may be compounded by the presence of additional stressors (fisheries, pollution)65 and with consequences for ecosystem function and the provision of ecosystem services.
Methods
Study species
Adult copepods Pseudodiaptomus annandalei were collected from a coastal aquaculture pond in the Cam Ranh Centre for Tropical Marine Research and Aquaculture in January 2018. The mean prosome length and SE of F0 males and F0 females was 755.6 ± 2.71 and 927.1 ± 3.6 µm, respectively (n = 100 individuals per sex). The mean clutch size and SE of F0 females was 16 ± 1 eggs (n = 25). During sampling, the temperature in the pond varied between 28 and 29 °C, and the salinity was 21 ppt. The copepods were gradually acclimated to the laboratory condition at 30 and 34 °C over three days (equivalent to one-third of the generation time66) before introducing them to the experimental temperatures for producing nauplii to start the F1 generation. During the acclimation period, copepods were fed ad libitum on the haptophyte Isochrisys galbana (30.000–35.000 cells L−1 33). They were kept under a photoperiod of 12L: 12D (light: dark cycle). Salinity was kept stable at 20 ppt and dissolved oxygen was 5–7 mg L−1.
Experimental design
To test for the effect of warming on the fitness of the tropical copepod Pseudodiaptomus annandalei across three generations, we conducted a multiple generational exposure experiment in which copepods were reared at 30 and 34 °C. Each treatment had three replicates that were 5-L bottles, containing approximately 1,500 individuals (300 ind. L−1) similar to the copepod density observed in the pond. We chose the temperature of 30 °C as the control as it is close to the temperature measured in the field where P. annandalei is abundant33,38,58, and 34 °C simulated a temperature extreme that occurs frequently during the summer period.
The cultures of F1, F2 and F3 generations
To start the F1 generation, 600 F0 females carrying two egg sacs were isolated and assigned randomly to 6 1-L bottles (100 females per bottle and three bottles per temperature), each filled with 900 ml filtered seawater. They were fed ad libitum on Isochrisys galbana (30,000–35,000 cell mL−1, equivalent to 800–900 µC L−1 33,67). After incubating at 30 and 34 °C for 30 h, it was expected that 90% of eggs would hatch (Grønning, Doan, Dinh, Dinh and Nielsen, under review). We collected nauplii (approximately 1,500 individuals per 1-L bottle) to start a culture in one 5-L glass bottle (three cultures per temperature). The rearing conditions such as temperature, light regime, salinity and dissolved oxygen were similar to the acclimation period. The rearing water and algal food were renewed every two days. When F1 reached adult stage, we collected all of the adults through a filter (mesh size = 200 µm) and allowed them to produce nauplii for 30 h to start the F2 generation. Nauplii were collected, and the total number of nauplii in each bottle was determined. We used approximately 1,500 nauplii per experimental bottle to start the F2 generation. Adults were used to measure the prosomal length, the clutch size, the hatching success and the nauplii production (see details further below). The F3 generation was initiated from F2 in the same way.
To identify the development stages of P. annandalei, we daily took a subsample (100–300 ml) from one additional 5-L bottle per temperature treatment that was set up in parallel with three 5-L experimental bottles at 30 and 34 °C. Samples were filtered onto a filter (25 µm) and the content was poured on a petri dish and fixed by Lugol (4%). Developmental stages were identified based on Golez et al.66. The relative abundance (%) of nauplii, copepodites and adults in the samples was determined daily until all individuals moulted into adults.
Response variables
Adult males and females (10–34 individuals per sex depending on the abundance) were randomly selected from each of the six 5-L bottles for measuring the size (prosome length) at maturity. The prosomal length was measured using a microscope (SZ51, Olympus, Japan). The biomass of copepods was calculated using the equation of Rayner et al.68.
| 1 |
where C = biomass in carbon unit (μg C) and L = prosomal length (µm).
Clutch size was defined as the number of eggs in the two egg sacs per female. To determine the clutch size, 9 to 13 females per experimental bottle were collected and fixed in Lugol (4%). The egg sacs were opened with a needle under a stereo-microscope (Olympus) and the number of eggs in two sacs was counted. The specific clutch size was also determined by normalizing the clutch size (0.067 µg C egg−1, Grønning, Doan, Dinh, Dinh and Nielsen, under review) for the biomass of the females (µg C of eggs per µg C of the female).
To investigate the effect of temperature on the hatching success of F1, F2 and F3, in each generation we collected 12 females carrying two egg sacs from each of six 5-L experimental bottle, and assigned them individually into a 12-well plate (Thermo Fisher Scientific). Each well was filled with 3 mL of clean sea water (temperatures of 30 or 34 °C, salinity = 20 ppt) and the food I. galbana at the concentration of 30,000–35,000 cell mL−1. Plates were placed in water baths at their corresponding temperatures. After 30 h, we checked for the hatching in each well under a stereo microscope (SZ51, Olympus, Japan). The hatching success was determined as 1 for a female with hatched eggs and 0 for a female with unhatched eggs.
Nauplii production
We randomly collected 25 females (each carrying two egg sacs) from each temperature, and assigned them to five 125-ml glass bottles (5 females per bottle) at each temperature. They were fed on I. galbana (30,000–35,000 cell mL−1) for 30 h. The content was then filtered (mesh size = 25 m) and fixed in Lugol (4%). The total number of nauplii in each sample was counted using a stereo microscope (SZ51, Olympus, Japan).
Statistical analyses
We tested for the effects of temperature, generation, and their interaction, on the size of maturity for females and males using generalised mixed models. As observations were nested within bottle, bottle was treated a as a random effect (Gaussian link, GLMMIX function). To test for the effects of the experimental treatments on the fitness parameters, we modelled the three response variables: clutch size, specific clutch size, and hatching success, as a function of the fixed effects (temperature, generation and their interaction). Observations were nested within the random effect ‘bottle’, and we used the GLMMIX function within SAS9.4. Clutch size and specific clutch size were modelled with the Poisson function, while hatching success was modelled with a binomial error structure and the logit link function. We modelled nauplii production and specific nauplii production as a function of temperature, generation and their interaction with linear models. Statistical differences were considered significant if P < 0.05. All statistical analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, United States). Data are presented in the figures as means + SEs.
Data deposition
Data for this study are available at the Dryad Digital Repository when the manuscript is accepted for publication.
Supplementary information
Acknowledgements
This research was supported by the British Ecological Society to Khuong Van Dinh (Grant LRB17\1003). We thank Huynh Chi Thien for providing laboratory assistance.
Author Contributions
N.X.D. and M.T.T.Vu contributed equally to this work. K.V.D., N.X.D., M.T.T.V., M.S.W., T.G.N. and H.Q.P. designed the experiment; N.X.D. conducted the experiment. K.V.D., N.X.D. and M.T.T.V. performed statistical analyses and wrote the first draft of the manuscript. M.S.W., T.G.N. and H.Q.P. contributed to the later version of the manuscript; all authors read and approved the manuscript for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nam X. Doan and Minh T. T. Vu contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40996-7.
References
- 1.Sagarin RD, Barry JP, Gilman SE, Baxter CH. Climate-related change in an intertidal community over short and long time scales. Ecological Monographs. 1999;69:465–490. doi: 10.1890/0012-9615(1999)069[0465:CRCIAI]2.0.CO;2. [DOI] [Google Scholar]
- 2.Angilletta MJ, Niewiarowski PH, Navas CA. The evolution of thermal physiology in ectotherms. J. Therm. Biol. 2002;27:249–268. doi: 10.1016/s0306-4565(01)00094-8. [DOI] [Google Scholar]
- 3.Angilletta, M. J. Thermal adaptation: a theoretical and empirical synthesis. (Oxford University Press, 2009).
- 4.IPCC. Climate change 2013: The physical science basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, 2013).
- 5.Lima, F. P. & Wethey, D. S. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nature Communications3, 10.1038/ncomms1713 (2012). [DOI] [PubMed]
- 6.Hoegh-Guldberg O, et al. Coral reefs under rapidclimate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 7.Wernberg T, et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 2013;3:78–82. doi: 10.1038/nclimate1627. [DOI] [Google Scholar]
- 8.Poloczanska ES, et al. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013;3:919–925. doi: 10.1038/nclimate1958. [DOI] [Google Scholar]
- 9.Pecl, G. T. et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science355, 10.1126/science.aai9214 (2017). [DOI] [PubMed]
- 10.United Nations Framework Convention on Climate Change Conference of Parties (COP). Paris Agreement FCCC/CP/2015/L.9/Rev.1 (2015).
- 11.Stuart-Smith RD, Edgar GJ, Bates AE. Thermal limits to the geographic distributions of shallow-water marine species. Nature Ecology & Evolution. 2017;1:1846–+. doi: 10.1038/s41559-017-0353-x. [DOI] [PubMed] [Google Scholar]
- 12.Tewksbury JJ, Huey RB, Deutsch CA. Putting the heat on tropical animals. Science. 2008;320:1296–1297. doi: 10.1126/science.1159328. [DOI] [PubMed] [Google Scholar]
- 13.Lough, J. M. Small change, big difference: Sea surface temperature distributions for tropical coral reef ecosystems, 1950-2011. J. Geophys. Res.-Oceans117, 10.1029/2012jc008199 (2012).
- 14.Wernberg T, Smale DA, Thomsen MS. A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Chang. Biol. 2012;18:1491–1498. doi: 10.1111/j.1365-2486.2012.02656.x. [DOI] [Google Scholar]
- 15.Nguyen, K. D. T. et al. Upper temperature limits of tropical marine ectotherms: global warming implications. Plos One6, 10.1371/journal.pone.0029340 (2011). [DOI] [PMC free article] [PubMed]
- 16.Reygondeau G, Beaugrand G. Future climate-driven shifts in distribution of Calanus finmarchicus. Glob. Chang. Biol. 2011;17:756–766. doi: 10.1111/j.1365-2486.2010.02310.x. [DOI] [Google Scholar]
- 17.Chust G, et al. Are Calanus spp. shifting poleward in the North Atlantic? A habitat modelling approach. ICES J. Mar. Sci. 2014;71:241–253. doi: 10.1093/icesjms/fst147. [DOI] [Google Scholar]
- 18.Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 19.Asch RG. Climate change and decadal shifts in the phenology of larval fishes in the California Current ecosystem. Proc. Natl. Acad. Sci. USA. 2015;112:E4065–E4074. doi: 10.1073/pnas.1421946112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown CJ, et al. Ecological and methodological drivers of species’ distribution and phenology responses to climate change. Glob. Chang. Biol. 2016;22:1548–1560. doi: 10.1111/gcb.13184. [DOI] [PubMed] [Google Scholar]
- 21.Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA. 2009;106:12788–12793. doi: 10.1073/pnas.0902080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlberger J. Climate warming and ectotherm body size - from individual physiology to community ecology. Funct. Ecol. 2013;27:991–1001. doi: 10.1111/1365-2435.12098. [DOI] [Google Scholar]
- 23.Sommer U, Peter KH, Genitsaris S, Moustaka-Gouni M. Do marine phytoplankton follow Bergmann’s rule sensu lato? Biol. Rev. 2017;92:1011–1026. doi: 10.1111/brv.12266. [DOI] [PubMed] [Google Scholar]
- 24.Horne CR, Hirst AG, Atkinson D, Neves A, Kiorboe T. A global synthesis of seasonal temperature-size responses in copepods. Glob. Ecol. Biogeogr. 2016;25:988–999. doi: 10.1111/geb.12460. [DOI] [Google Scholar]
- 25.Pan YJ, et al. Effects of cold selective breeding on the body length, fatty acid content, and productivity of the tropical copepod Apocyclops royi (Cyclopoida, Copepoda) J. Plankton Res. 2017;39:994–1003. doi: 10.1093/plankt/fbx041. [DOI] [Google Scholar]
- 26.Gibbin EM, et al. Can multi-generational exposure to ocean warming and acidification lead to the adaptation of life history and physiology in a marine metazoan? J. Exp. Biol. 2017;220:551–563. doi: 10.1242/jeb.149989. [DOI] [PubMed] [Google Scholar]
- 27.Cheung WWL, et al. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Chang. 2013;3:254–258. doi: 10.1038/nclimate1691. [DOI] [Google Scholar]
- 28.Bouquet, J. M. et al. Increased fitness of a key appendicularian zooplankton species under warmer, acidified seawater conditions. Plos One13, 10.1371/journal.pone.0190625 (2018). [DOI] [PMC free article] [PubMed]
- 29.Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010;401:233–243. doi: 10.3354/meps08366. [DOI] [Google Scholar]
- 30.Hwang JS, et al. Patterns of zooplankton distribution along the marine, estuarine, and riverine portions of the Danshuei ecosystem in northern Taiwan. Zool. Stud. 2010;49:335–352. [Google Scholar]
- 31.Chew LL, Chong VC, Tanaka K, Sasekumar A. Phytoplankton fuel the energy flow from zooplankton to small nekton in turbid mangrove waters. Mar. Ecol. Prog. Ser. 2012;469:7–24. doi: 10.3354/meps09997. [DOI] [Google Scholar]
- 32.Dhanker R, Kumar R, Hwang JS. Predation by Pseudodiaptomus annandalei (Copepoda: Calanoida) on rotifer prey: Size selection, egg predation and effect of algal diet. J. Exp. Mar. Biol. Ecol. 2012;414:44–53. doi: 10.1016/j.jembe.2012.01.011. [DOI] [Google Scholar]
- 33.Doan NX, et al. Temperature- and sex-specific grazing rate of a tropical copepod Pseudodiaptomus annandalei to food availability: implications for live feed in aquaculture. Aquac. Res. 2018;49:3864–3873. doi: 10.1111/are.13854. [DOI] [Google Scholar]
- 34.Messmer V, et al. Global warming may disproportionately affect larger adults in a predatory coral reef fish. Glob. Chang. Biol. 2017;23:2230–2240. doi: 10.1111/gcb.13552. [DOI] [PubMed] [Google Scholar]
- 35.Forster J, Hirst AG, Woodward G. Growth and development rates have different thermal responses (vol 178, pg 668, 2011) American Naturalist. 2012;180:404–405. doi: 10.1086/667359. [DOI] [PubMed] [Google Scholar]
- 36.Horne, C. R., Hirst, A. G., Atkinson, D., Almeda, R. & Kiørboe, T. Rapid shifts in the thermal sensitivity of growth but not development rate causes temperature–size response variability during ontogeny in arthropods. Oikos0, 10.1111/oik.06016.
- 37.Lehette P, Ting SM, Chew LL, Chong VC. Respiration rates of the copepod Pseudodiaptomus annandalei in tropical waters: beyond the thermal optimum. J. Plankton Res. 2016;38:456–467. doi: 10.1093/plankt/fbv119. [DOI] [Google Scholar]
- 38.Low JSY, et al. Heat shock response and metabolic stress in the tropical estuarine copepod Pseudodiaptomus annandalei converge at its upper thermal optimum. J. Therm. Biol. 2018 doi: 10.1016/j.jtherbio.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- 40.Kiørboe T. How zooplankton feed: mechanisms, traits and trade-offs. Biol. Rev. 2011;86:311–339. doi: 10.1111/j.1469-185X.2010.00148.x. [DOI] [PubMed] [Google Scholar]
- 41.Rhyne AL, Ohs CL, Stenn E. Effects of temperature on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture. 2009;292:53–59. doi: 10.1016/j.aquaculture.2009.03.041. [DOI] [Google Scholar]
- 42.Kelly MW, DeBiasse MB, Villela VA, Hope L, Cecola CF. Adaptation to climate change: trade-offs among responses to multiple stressors in an intertidal crustacean. Evol. Appl. 2016;9:1147–1155. doi: 10.1111/eva.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sichlau MH, Kiørboe T. Age- and size-dependent mating performance and fertility in a pelagic copepod, Temora longicornis. Mar. Ecol. Prog. Ser. 2011;442:123–132. doi: 10.3354/meps09402. [DOI] [Google Scholar]
- 44.Kiørboe T, Sabatini M. Scaling of fecundity, growth and development in marine planktonic copepods. Mar. Ecol. Prog. Ser. 1995;120:285–298. doi: 10.3354/meps120285. [DOI] [Google Scholar]
- 45.Tang KW, Dam HG. Phytoplankton inhibition of copepod egg hatching: test of an exudate hypothesis. Mar. Ecol. Prog. Ser. 2001;209:197–202. doi: 10.3354/meps209197. [DOI] [Google Scholar]
- 46.Krause KE, Dinh KV, Nielsen TG. Increased tolerance to oil exposure by the cosmopolitan marine copepod Acartia tonsa. Sci. Total Environ. 2017;607–608:87–94. doi: 10.1016/j.scitotenv.2017.06.139. [DOI] [PubMed] [Google Scholar]
- 47.Preziosi BM, Runge JA. The effect of warm temperatures on hatching success of the marine planktonic copepod, Calanus finmarchicus. J. Plankton Res. 2014;36:1381–1384. doi: 10.1093/plankt/fbu056. [DOI] [Google Scholar]
- 48.Preziosi, B. M., Runge, J. A., Christensen, J. P. & Jones, R. J. Effects of pH and temperature on egg hatching success of the marine planktonic copepod, Calanus finmarchicus. Mar. Biol. 164, 10.1007/s00227-017-3243-5 (2017).
- 49.Napiorkowska T, Kobak J, Napiorkowski P, Templin J. The effect of temperature and light on embryogenesis and post-embryogenesis of the spider Eratigena atrica (Araneae, Agelenidae) J. Therm. Biol. 2018;72:26–32. doi: 10.1016/j.jtherbio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Pepe-Victoriano R, Araya M, Faundez V. Temperature effect on embryonic survival and early larval stages of Psetta maxima. International Journal of Morphology. 2012;30:1551–1557. doi: 10.4067/s0717-95022012000400046. [DOI] [Google Scholar]
- 51.Vera-Chang MN, et al. Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proc. Natl. Acad. Sci. USA. 2018;115:E12435–E12442. doi: 10.1073/pnas.1811695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dao T-S, Vo T-M-C, Wiegand C, Bui B-T, Dinh KV. Transgenerational effects of cyanobacterial toxins on a tropical micro-crustacean Daphnia lumholtzi across three generations. Environ. Pollut. 2018;243:791–799. doi: 10.1016/j.envpol.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 53.Donelson JM, Munday PL, McCormick MI, Pitcher CR. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Chang. 2012;2:30–32. doi: 10.1038/nclimate1323. [DOI] [Google Scholar]
- 54.Chew, L. L. & Chong, V. C. Response of marine copepods to a changing tropical environment: winners, losers and implications. Peerj4, 10.7717/peerj.2052 (2016). [DOI] [PMC free article] [PubMed]
- 55.Kiørboe T. Population regulation and role of mesozooplankton in shaping marine pelagic food webs. Hydrobiologia. 1998;363:13–27. doi: 10.1023/A:1003173721751. [DOI] [Google Scholar]
- 56.Jimenez-Melero R, Parra G, Guerrero F. Effect of temperature, food and individual variability on the embryonic development time and fecundity of Arctodiaptomus salinus (Copepoda: Calanoida) from a shallow saline pond. Hydrobiologia. 2012;686:241–256. doi: 10.1007/s10750-012-1014-3. [DOI] [Google Scholar]
- 57.Kiørboe T, Ceballos S, Thygesen UH. Interrelations between senescence, life-history traits, and behavior in planktonic copepods. Ecology. 2015;96:2225–2235. doi: 10.1890/14-2205.1. [DOI] [PubMed] [Google Scholar]
- 58.Yong YL, Chew LL, Lee CW, Chong VC. Monsoonal and lunar variability in microzooplankton abundance and community structure in the Terusan mangrove creek (Malaysia) Marine Biology Research. 2016;12:278–293. doi: 10.1080/17451000.2016.1143107. [DOI] [Google Scholar]
- 59.Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 60.Carlotti F, Rey C, Javanshir A, Nival S. Laboratory studies on egg and faecal pellet production of Centropages typicus: Effect of age, effect of temperature, individual variability. J. Plankton Res. 1997;19:1143–1165. doi: 10.1093/plankt/19.8.1143. [DOI] [Google Scholar]
- 61.Shin K, et al. Influence of food quality on egg production and viability of the marine planktonic copepod Acartia omorii. Prog Oceanogr. 2003;57:265–277. doi: 10.1016/s0079-6611(03)00101-0. [DOI] [Google Scholar]
- 62.Jambeck JR, et al. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 63.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 64.Barlow J, et al. The future of hyperdiverse tropical ecosystems. Nature. 2018;559:517–526. doi: 10.1038/s41586-018-0301-1. [DOI] [PubMed] [Google Scholar]
- 65.Crain CM, Kroeker K, Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008;11:1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 66.Golez MSN, Takahashi T, Ishimaru T, Ohno A. Post-embryonic development and reproduction of Pseudodiaptomus annandalei (Copepoda: Calanoida) Plankton Biol. and Ecol. 2004;51:15–25. [Google Scholar]
- 67.Berggreen U, Hansen B, Kiørboe T. Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for the determination of copepod production. Mar. Biol. 1988;99:341–352. doi: 10.1007/bf02112126. [DOI] [Google Scholar]
- 68.Rayner TA, et al. Biochemical composition of the promising live feed tropical calanoid copepod Pseudodiaptomus annandalei (Sewell 1919) cultured in Taiwanese outdoor aquaculture ponds. Aquaculture. 2015;441:25–34. doi: 10.1016/j.aquaculture.2015.01.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.