Abstract
Extracellular vesicles (EVs) are important mediators of intercellular communication in cancer and in normal tissues. EVs transfer biologically active molecules from the cell of origin to recipient cells. This review summarizes the studies on EVs derived from renal cell carcinoma and from a subpopulation of CD105-positive renal cancer stem cells. While EVs from renal cell carcinoma show mild biological activity, EVs from renal cancer stem cells enhance tumor angiogenesis and metastasis formation. The effect is probably due to the transfer of proangiogenic RNA cargo to endothelial cells, which acquire an activated angiogenic phenotype. In vivo, treatment with EVs favors the formation of a premetastatic niche in the lungs. Moreover, EVs derived from renal cancer stem cells modify gene expression in mesenchymal stromal cells, enhancing the expression of genes involved in matrix remodeling, cell migration, and tumor growth. Mesenchymal stromal cells preconditioned with tumor EVs and then coinjected in vivo with renal cancer cells support tumor growth and vessel formation. Finally, tumor EVs promote tumor immune escape by inhibiting the differentiation process of dendritic cells and the activation of T cells. Thus, tumor-derived EVs act on the microenvironment favoring tumor aggressiveness, may contribute to angiogenesis through both direct and indirect mechanisms and are involved in tumor immune escape.
Subject terms: Kidney
Kidney cancer: Packages that promote aggression
Membrane-bound packages called extracellular vesicles (EVs) released by kidney cancer stem cells can make tumors more aggressive, promote the onset of cancer at other sites, and help tumors escape the anti-cancer immune response. Giovanni Camussi and colleagues at the University of Turin, Italy, review understanding of EVs from kidney cancer cells. EVs from cancer stem cells are especially effective in promoting cancer, unlike those from mature cancer cells. This is partly due to their ability to promote the formation of new blood vessels to sustain tumor growth. Some of the vesicles’ effects are mediated by transferring small molecules of ribonucleic acid (RNA) into other cells. These RNAs can regulate the activity of specific genes, promoting cancer. Studying patients’ EVs may assist cancer diagnosis and help predict the likely progression of the disease.
Introduction
Cancer cells, as well as all other cells, are capable of releasing extracellular vesicles (EVs) into the extracellular space. EVs are vesicles surrounded by a lipid bilayer containing protein and nucleic acid cargo. EVs are shed in physiological and pathological circumstances. After release, EVs can reach close or distant sites by entering the circulation and can be found in all biofluids. The term “extracellular vesicles”, suggested by the International Society of Extracellular Vesicles (ISEV), designates a mixed population of vesicles with overlapping dimensions released by cells and typically distinguished into exosomes and microvesicles, ectosomes and shed vesicles based on their biogenesis1. Exosomes come from the membrane invagination of multivesicular bodies (MVBs); exosomes are vesicles of 30–150 nm in diameter secreted into the extracellular space after fusion of MVBs with the plasma membrane. The formation of exosomes partially relies on the endosomal sorting complex required for transport (ESCRT) complex2,3, but it may also take place independently from ESCRT, with the participation of tetraspanins in protein sorting4 or of ceramide5. The RAB proteins are other players involved in exosome biogenesis4,6. At variance, microvesicles are 100–1000 nm in diameter and directly bud from the plasma membrane. Vesicles shed from the plasma membrane may include vesicles released by normal cells, such as stem cells, which are in the nano-range (100–200 nm in diameter), and larger preapoptotic vesicles, which are released by injured cells. Apoptotic bodies are vesicles of 1000–5000 nm in diameter secreted by cells undergoing programmed death and containing nuclear fragments7.
In recent years, EVs have been profusely studied, and their roles in cell-to-cell communication, as well as their involvement in cell microenvironment homeostasis, have been recognized. In fact, EVs can exchange specific bioactive molecules, such as proteins and nucleic acids, among cells, influencing the phenotype and functions of recipient cells7.
EVs show different proportions of membrane lipid molecules, such as cholesterol, sphingomyelin, and ceramide, with respect to the cell of origin6 and carry various proteins involved in EV biogenesis. For example, EVs carry proteins involved in the formation of MVBs, such as TSG101, ALIX3,8 and clathrin, and proteins contributing to membrane transport and fusion, such as flotillins, annexins, and GTPases6. RAB proteins, involved in docking and fusion of EVs with recipient cells, and heat shock proteins, such as HSP90 and HSP70, are also present in EVs4,6,7. Of interest, tumor EVs convey mediators of oncogenesis, such as growth factors, oncoproteins, and immunomodulatory molecules, that may affect the tumor microenvironment and metastatic niche9–11.
The tumorigenic activity of EVs relies on their luminal cargo and on the assortment of transmembrane proteins involved in EV tropism, such as integrins interacting with the extracellular matrix. CD63, CD9, and CD81 tetraspanins are the most frequently mentioned exosome markers, but not all exosomes express these proteins; in addition, these tetraspanins may also be present in microvesicles and apoptotic bodies12. In addition to proteins, EVs may contain fragments of DNA of genomic and mitochondrial origin, single or double-stranded, carried on the surface or inside the EVs13–16. Moreover, they contain numerous classes of RNA, such as mRNA, microRNA, long noncoding RNA, mitochondrial RNA, transfer RNA, and ribosomal RNA6,17–19.
Cancer-derived EVs
Evidence demonstrates that cancer cells release higher amounts of EVs with functional alterations compared to normal cells, probably due to biogenesis and cargo sorting deregulation. Different mechanisms possibly involved in increased EV production have been described, including the overexpression of syntenin8,20, RAB proteins10, ESCRT components21,22, and heparinase23. In addition, EV production can also be induced by a hypoxic microenvironment24, as well as by the activation of oncogenic signaling pathways, such as EGFRvIII25, h-RAS26, and proto-oncogene SRC27. The altered secretion and function of tumor EVs critically affects the cross-talk between cancer and surrounding tissues, engendering a favorable microenvironment for tumor development and invasiveness. In particular, tumor EVs influence normal and cancer cell behavior, promoting tumor growth through the stimulation of angiogenesis, the migration and invasion of cells, the development of premetastatic niche, the reduction of cell-to-cell adhesion, and the modulation of an immune response11,28–30 (Table 1).
Table 1.
Role of cancer-derived EVs in tumor progression
| Biological effect | Mechanism | Tumor | Refences |
|---|---|---|---|
| Stimulation of angiogenesis | Transfer of proangiogenic mRNAs/miRNAs | Renal carcinoma | 11 |
| Transfer of miR-23a and upregulation of HIF-1α | Lung cancer | 31 | |
| VEGF upregulation | Renal carcinoma | 32 | |
| Induction of c-Kit, the receptor tyrosine kinase Tie2 and Met in bone marrow progenitors | Melanoma | 10 | |
| Transfer of sphingomyelin, MMPs and plasminogen activator | Fibrosarcoma | 30 | |
| Activation of SRC signaling | Chronic myeloid leukemia | 33 | |
| Transfer of EDIL-3 and activation of epidermal growth factor receptor signaling | Bladder cancer | 35 | |
| Decrease in cell-to-cell adhesion | Reduction of E-cadherin and β-catenin expression | Bladder cancer | 36 |
| Downregulation of tight junction protein ZO-1 mediated by miR-23a and miR-105 | Lung and breast cancer | 8, 31 | |
| Increase in cell migration/invasion | Transfer of KIT | Gastrointestinal stromal tumor (GIST) | 41 |
| Transfer of mRNAs/miRNAs | Renal carcinoma | 11 | |
| Development of premetastatic niche |
Recruitment and reprograming of bone marrow progenitors, inducing the transforming growth factor β secretion and upregulating fibronectin production in surrounding hepatic cells Mediation of cancer stem cells stimulation of the premetastatic niche formation in the lungs |
Pancreatic ductal adenocarcinomas (PDACs) Renal carcinoma |
42 11 |
| Transfer of RNAs that activated Toll-like receptor 3, promoting neutrophil recruitment in the lungs | Lung cancer | 43 | |
| Induction of a prometastatic phenotype in bone marrow progenitors mediated by the expression of c-Kit, the receptor tyrosine kinase Tie2 and Met | Melanoma | 10 | |
| Immune-modulation | Inhibition of dendritic cell and T-cell functions | Renal and nasopharyngeal carcinoma, pancreatic, lung and breast cancer | 29, 44 |
| Promotion of tumor-supportive inflammation through the stimulation of cytokine secretion by macrophages | Gastric, breast, and prostate cancer | 44 |
Indeed, tumor EVs have been reported to promote angiogenesis via modulation of different pathways. They shuttle proangiogenic mRNAs and microRNAs implicated in tumor progression and metastasis11. A recent study by Hsu et al.31 demonstrated that EVs released by cancer cells under hypoxic conditions overexpressed miR-23a, which induced enhanced angiogenesis by leading to an accumulation of HIF-1α in endothelial cells. In another study, tumor EVs were able to upregulate VEGF expression in endothelial cells, possibly via the downregulation of the hepatocyte cell adhesion molecule hepaCAM32. In melanoma, tumor EVs were shown to reprogram bone marrow progenitors toward a provasculogenic and prometastatic phenotype, inducing the expression of c-Kit, Tie2 and Met10. In chronic myeloid leukemia, instead, cancer EVs stimulated tube formation in vitro and in vivo through SRC signaling activation33. In renal cell carcinoma, EVs were enriched with azurocidin protein, which is involved in vascular permeabilization. Thus, tumor EVs were shown to modify the endothelial cell phenotype, disrupting vascular morphology34. In addition, EVs released by bladder-cancer patients were shown to be enriched with EDIL-3, which activated epidermal growth factor receptor signaling in cancer and endothelial cells, promoting their angiogenesis and migration35. In addition to their proangiogenic activity, EVs released by cancer cells have been recognized as important mediators of the epithelial-to-mesenchymal transition, favoring target-cell migration and invasion. Indeed, tumor EVs can target adherent junction molecules in tumor cells and reduce the expression of E-cadherin and β-catenin epithelial markers36–38. Moreover, vesicular miR-23a and miR-105 were shown to inhibit the tight junction protein ZO-1 (TJP1), thereby increasing vascular permeability and cancer migration of lung and metastatic breast cancers31,39. Indeed, by analyzing breast cancer cells, Harris et al.40 demonstrated that cells with higher metastatic potentials released EVs characterized by a unique protein signature and by an enhanced promigration activity in comparison to normal EVs40. In gastrointestinal stromal tumors, tumor EVs contained the oncogenic protein tyrosine kinase KIT and were shown to promote the invasion of the interstitial stroma, inducing a tumor phenotype in progenitor smooth muscle cells41. The increased migration capacity of cancer cells leads to metastasis, and tumor EVs were reported to be critically involved in premetastatic niche formation. In the liver, EVs released by pancreatic ductal adenocarcinoma cells were able to promote the recruitment of bone marrow-derived macrophages, inducing the transforming growth factor β secretion by Kupffer cells and the upregulation of fibronectin production by hepatic stellate cells42. For the lung metastasis niche, tumor EVs contained RNAs that activated Toll-like receptor 3 in lung epithelial cells, inducing chemokine secretion and promoting neutrophil recruitment in the lungs43. Finally, tumor-derived EVs mediate the promotion of tumor-supportive inflammation, stimulating the secretion of cytokines from macrophages44. Another tumor-promoting function that has been associated with EVs is the chemo-resistance. In fact, Qu et al.45 showed that EVs transport sunitinib outside the cell. They discovered that the EV-associated lncRNA competitively binds miR-34/miR-449 and promotes AXL and MET expression in RCC cells. Thus, they proposed EV-associated lncRNA as a biomarker and therapeutic target for sunitinib resistance.
Renal cancer stem cells
Kidney malignancies represent the ninth most common cancer in men and the 14th most common cancer in women worldwide. More than 90% of kidney cancers can be classified as renal cell carcinoma (RCC), which arises from renal tubular epithelial cells and includes clear cell (70%), papillary (10–15%), and chromophobe (5%) carcinoma histologic subtypes. RCC is characterized by poor prognosis due to a high metastasis rate and resistance to both radiotherapy and chemotherapy46. Treatment limitations are mainly represented by the incomplete eradication of tumor cells due to cellular heterogeneity. In particular, the presence of a small subpopulation of cancer cells with stem cell features, called cancer stem cells (CSCs), is raising interest in the field as the major cause of tumor recurrence and resistance to therapy47,48. These cells can differentiate into all tumor cell types and drive tumor development, tumor growth and metastasis formation47. CSCs have been isolated from several cancers, including RCC47–51. Renal CSCs (rCSCs) are capable of self-renewal and contribute to tumor vasculogenesis, metastasis development and resistance to therapy52. To date, CSCs have been isolated from several renal tumors using different isolation techniques. Despite experimental heterogeneity, all renal CSC populations share important stem cell features and characteristics, such as tumor-initiating ability, which helps to define rCSC identity. In 2008, Bussolati et al. identified rCSCs as a cell population of less than 10% of the tumor mass positive for the mesenchymal marker CD10549. These cells also express other mesenchymal stem cell markers, such as CD73, CD90, CD44, CD29, CD146, and vimentin; embryonic renal marker Pax2; embryonic stem cells markers, such as OCT4, NANOG, Nestin, and Musashi; but lack differentiative epithelial markers, such as cytokeratin and the adult renal progenitor markers CD13349. The authors hypothesized that the rCSCs might derive from resident renal stem cells with mesenchymal characteristics or from an embryonic dedifferentiated progenitor cell52. In fact, rCSCs display several features, including clonogenicity, sphere generation and tumor-initiating ability. Moreover, rCSCs generate serially transplantable tumors and differentiate toward several lineages, such as epithelial and endothelial cells. A very low number of rCSCs implanted in vivo in SCID mice were able to recapitulate the tumor of origin. Of interest, several endothelial cells present within the tumor expressed the HLA class 1 antigen, suggesting that rCSCs not only originate from the epithelial compartment of the tumor but also contribute to the intratumor vessel structures49. Previous studies have shown that in normal kidneys there are cells with multipotent properties expressing the CD133 stem cell marker. Bruno et al.53 investigated whether the CD133-positive population isolated from cancer had tumor-initiating ability and whether it could be defined as a CSC population. The results showed that these cells were able to enhance tumor engraftment and growth when implanted together with renal cancer cells, but they were not tumorigenic per se. Similarly, Galleggiante et al.54 characterized RCC cells that stained positive for CD133, CD24, and copper transport protein 2, a membrane marker important in RCC cisplatin-based resistance and that lacked mesenchymal markers. These cells displayed self-maintenance and differentiating capabilities in vitro and promoted angiogenesis in vivo. In another study, CXCR4 + cells derived from a highly tumorigenic RCC cell line expressed embryonic stem cell makers (NANOG, SOX2 and OCT3/4), displayed sphere-forming ability, increased resistance to tyrosine kinase inhibitors and caused tumor growth in vivo55. Moreover, rCSCs have been isolated using functional approaches. Zhong et al.56 selected rCSCs from a renal cancer cell line based on their ability to grow in suspension and to form spheres. These cells were CD105-positive and expressed several stem cell genes (OCT4, β-catenin, BMI, and NANOG). Moreover, these cells had self-renewal ability and were resistant to irradiation and chemotherapeutic agents. Cells able to form spheres contained a CD44 + /CD24- subpopulation with enhanced aldehyde dehydrogenase activity. These cells showed CSC features, such as the expression of stem cell survival signaling pathways, mesenchymal and prometastatic genes, and resistance to radiation57. Addla et al.58, instead, identified rCSCs from human renal tissues using a dye-exclusion assay to select cells that could rapidly extrude dyes due to specific membrane transporters typically associated with stemness. These cells displayed a high proliferative potential and were able to produce differentiated spheroids.
rCSC-EV characterization
After the discovery of rCSCs, Grange and coworkers11 isolated and characterized EVs from CD105-positive rCSCs (rCSC-EVs) and compared them to EVs from non-stem, CD105-negative renal cancer cells. To identify any differences, the two EV types were analyzed by zeta-potential and size and by electron microscopy. These two EV types showed similar size (10–100 nm) and zeta potential (22.4 ± 3.5 mV). On the other hand, FACS analysis revealed that both kinds of EVs expressed CD44 and adhesion molecules (α5- and α6-integrins), which were characteristic of the cells of origin, but only rCSC-EVs expressed CD105. Both EV types did not express HLA class I or CD73. The authors analyzed the RNA content of EVs by bioanalyzer, showing that both EVs carried RNA of different sizes, mostly small RNAs (below 80 nucleotides in length) but also longer RNAs. The ribosomal subunits 28 S and 18 S were nearly absent, as this usually occur in EVs59–61. Then, the miRNA content of EVs was screened by qRT-PCR, profiling 365 human miRNAs. Both EVs carried approximately 80–90 miRNAs, but with a statistically significant difference; the rCSC-EVs contained 24 upregulated and 33 downregulated miRNAs compared to EVs derived from a non-stem renal cancer cell population. The upregulation of several miRNAs carried by rCSC-EVs (miR-200c, miR-92, and miR-141) has been detected in other cancers, such as ovarian62,63, colorectal64, and prostate cancers65. Moreover, miR-29a, miR-650, and miR-151 detected in rCSC-EVs have been previously associated with tumor invasion and metastasis66–68. A previous comparison of renal carcinomas with normal renal tissue showed the upregulation of miR-19b, miR-29c, and miR-151 which are enriched in rCSC-EVs69. To better understand the role of miRNAs carried by rCSC-EVs, the authors performed gene ontology (GO) enrichment analysis on the 24 miRNAs upregulated in rCSC-EVs. They observed that these miRNAs were strongly related to biological processes, such as metabolic processes, transcription, cell adhesion molecules, and regulation of cell proliferation.
Moreover, rCSC-EVs, but not those derived from non-stem renal cancer cells, were shown to carry several mRNAs of proangiogenic genes, such as VEGF, fibroblast growth factor 2 (FGF2), angiopoietin 1, ephrin A3, MMP2, and MMP911.
rCSC-EV proangiogenic role
Following the observation that rCSC-EVs carry proangiogenic miRNAs, Grange et al.11 decided to investigate whether rCSC-EVs exert a proangiogenic effect. Both rCSC-EVs and EVs derived from non-stem renal cancer cell populations were equally incorporated by endothelial cells; however, only rCSC-EVs affected angiogenetic processes. In fact, rCSC-EVs significantly enhanced the formation of capillary-like structures in Matrigel, promoted cell invasion through Matrigel-coated transwells and promoted apoptosis resistance after doxorubicin treatment, whereas EVs derived from non-stem renal cancer cells were ineffective. Moreover, endothelial cells pretreated with rCSC-EVs favored the adhesion of renal tumor cells to the monolayer of endothelial cells. Interestingly, EVs derived from unsorted tumor cells showed a modest biological effect, which was greater than that of EVs derived from renal cancer cells deprived of the stem cell population or vehicle alone. This result was probably due to the presence of rCSCs in the total population derived from the primary tumor. Moreover, endothelial cells prestimulated with rCSC-EVs were embedded in Matrigel and injected subcutaneously in SCID mice. The pretreatment with EVs significantly increased the formation of capillary structures expressing human markers von Willebrand Factor and HLA class I, which are connected with the murine vasculature. Furthermore, repeated rCSC-EV injections for 5 days followed by the administration of renal tumor cells significantly enhanced the incidence of lung metastasis, compared to EVs derived from non-stem renal cancer cells, vehicle or RNase-treated rCSC-EVs. An enhanced expression of VEGFR1 protein and VEGF and MMP2 genes in lung endothelial cells, and MMP9 gene in lung tissue was observed after treatment with rCSC-EVs, but not with EVs derived from non-stem renal cancer cells.
Overall, these results indicate that EVs released specifically by rCSCs, and not by all tumor populations, were able to promote tumor angiogenesis and invasion. These vesicles may coordinate angiogenesis within the tumor microenvironment and promote tumor growth. In addition, they condition lung tissues, creating a premetastatic niche and a favorable environment for cancer cell adhesion. Finally, the RNA cargo analysis of rCSC-EVs and EVs derived from non-stem renal cancer cells support different biological effects of EVs. In fact, rCSC-EVs were enriched in protumorigenic miRNAs and proangiogenic mRNAs11.
rCSC-EVs protumorigenic role and cross-talk with MSC
The biological effect of rCSC-EVs was further investigated by Lindoso and colleagues28 to study the possible cross-talk between rCSCs and MSCs. In fact, some studies reported that MSCs are recruited within the tumor and promoted tumor growth and angiogenesis70–73; however the mechanism is still unclear. Thus, the authors evaluated whether rCSC-EVs could promote MSC recruitment. MSCs preconditioned with rCSC-EVs showed significantly increased migration toward CSC-conditioned medium. It was observed that MMP1, MMP2, MMP3, COL4A3, CXCR4, and CXCR7 were significantly upregulated compared with unstimulated control MSCs after 2 weeks of stimulation. CXCR4 is involved in MSC migration74,75, and CXCR7 is involved in survival and paracrine actions of MSCs76,77, angiogenesis, modulation of the immune system and tumor invasion78,79,. MMPs are known to modulate matrix remodeling and are increased in many human cancers, where they modulate invasion, metastasis, growth, and angiogenesis80. Moreover, the COL4A3 gene can regulate cell adhesion, migration and metastasis in various tumors81–83. Indeed, the upregulation was maintained for another 2 weeks in MSCs cultured after the removal of EVs from conditioned medium, confirming the persistence of phenotypic changes observed in stimulated MSCs. Moreover, MSCs stimulated for 2 weeks with rCSC-EVs induced angiogenesis in endothelial cells and migration in renal carcinoma cell when plated on the opposite side of a transwell. By analyzing cytokines secreted by MSCs stimulated or not with rCSC-EVs, Lindoso et al.28 observed that the secretion pattern was different. A significant increase in IL-8, myeloperoxidase (MPO) and osteopontin (OPN) gene transcription was observed by real-time PCR, and the corresponding protein release was determined by ELISA. MPO participates in oxidative stress response in tumors84 and may support tumor development. IL-8 can mimic VEGF enhancing endothelial cells proliferation and survival85 and is associated with several signaling pathways involved in tumor cell proliferation86. OPN is known to mediate the cross-talk between MSCs and cancer cells and to promote MSC migration87. This molecule is highly expressed in the tumor stroma and is involved in signaling regulation processes linked to angiogenesis, metastasis and tumor growth in different tumors and in apoptosis resistance in RCC88. Furthermore, the authors studied the behavior of rCSC-EV-stimulated MSCs in vivo and their effects on tumor growth. Tumors coinjected with stimulated MSCs showed an increased size and weight, a higher number of vessels and a proliferative rate compared to tumors alone or tumors coinjected with unstimulated MSCs. In conclusion, Lindoso et al. provided new insights into the putative involvement of rCSC-EVs in tumor communication with stromal surrounding cells, such as MSCs. Moreover, rCSC-EVs induce epigenetic reprogramming of MSCs and favor tumor vascularization and growth.
Role of rCSC-EVs in immune-modulation
Another important mechanism promoting tumor development is tumor immune escape. The tumor microenvironment inhibits the maturation and activation of dendritic cell (DCs), limits their role as antigen-presenting cells and reduces their ability to activate naïve T lymphocytes89. Grange et al.29 analyzed the ability of rCSC-EVs to modulate the behavior and differentiation of monocyte-derived DCs. At first, the authors evaluated the ability of rCSC-EVs and EVs derived from non-stem renal cancer cells to inhibit DC differentiation by culturing monocytes in the presence of EVs. rCSC-EVs interfered with the phenotype of monocyte-derived cells, with a reduced expression of activation markers CD83 and CD40, costimulatory molecules CD80 and CD86, antigen-presenting molecule HLA-DR, and adhesion molecules involved in T-cell contact. DCs that differentiated in the presence of rCSC-EVs had a significantly reduced ability to stimulate CD3 + lymphocyte proliferation and released a significant amount of IL-10 compared with control DCs. Moreover, the soluble nonclassical human leukocyte antigen G (sHLA-G) was significantly increased in the supernatant of monocyte-derived cells cocultured with rCSC-EVs. HLA-G is known to suppress the function of natural killer (NK) cells, T cells, and DCs90 and is associated with cancer immune escape91,92. Moreover, 50% of clear cell RCCs (ccRCCs) have shown HLA-G upregulation and the presence of sHLA-G in patients’ plasma93,94. Western blot analysis demonstrated the presence of sHLA-G within rCSC-EVs at a higher level compared to EVs derived from renal cancer cells deprived of the stem cell population. The addition of the sHLA-G blocking antibody to monocyte-derived cells incubated with rCSC-EVs induced a partial reversion of the inhibitory effect and increased the expression of CD86, HLA-DR, CD1a, and α5 integrin on monocyte-derived cells.
In conclusion, the results demonstrated that rCSC-EVs impair the maturation of DCs and inhibit the T-cell immune response, partially through EV-associated sHLA-G.
Conclusions
Taken together, these studies show that EVs released by rCSCs but not those derived from the more differentiated tumor cell population may influence the tumor microenvironment by acting on interstitial and endothelial cells and favor the formation of premetastatic niches. Moreover, rCSC-EVs may induce a protumorigenic phenotype in primed MSCs, and, by affecting the DC maturation and function of T cells, rCSC-EVs may favor tumor immune escape (Fig. 1). This is an outstanding example of how cancer stem cell-derived EVs are active players in the cancer microenvironment and can promote tumor growth and metastasis formation. Due to the emerging role of cancer EVs in tumor biology, several researchers are working to collect EVs from patients’ biofluids to identify new diagnostic, or even prognostic, biomarkers. As an example, De Palma et al.95 isolated urinary EVs, screened the RNA cargo and identified three mRNAs (GSTA1, CEBPA, and PCBD1) that were significantly reduced in ccRCC patients. This mRNA signature may be used for diagnostic screening. Moreover, the association between miRNAs and cancer was confirmed by the restoration of a gene expression level comparable to that of healthy controls after cancer removal. Two studies analyzed the expression of some miRNAs in sera EVs of RCC patients and identified miR-22496, miR-210, miR-1233, and miR-15a97 as being overexpressed in serum EVs; these miRNAs may eventually be suitable as diagnostic markers for the disease. This line of research is particularly important because the identification of sensible and specific serum or urinary EV biomarkers would be useful for the development of reliable, noninvasive and less expensive screening techniques for RCC.
Fig. 1. Role of rCSC-EVs in RCC. Renal carcinoma contains tumoral cells (TC) that are CD105-negative and cancer stem cells (rCSCs) that are CD105-positive. Renal cancer stem cells that are CD105-positive release EVs (rCSC-EVs) that are able to promote tumor growth.
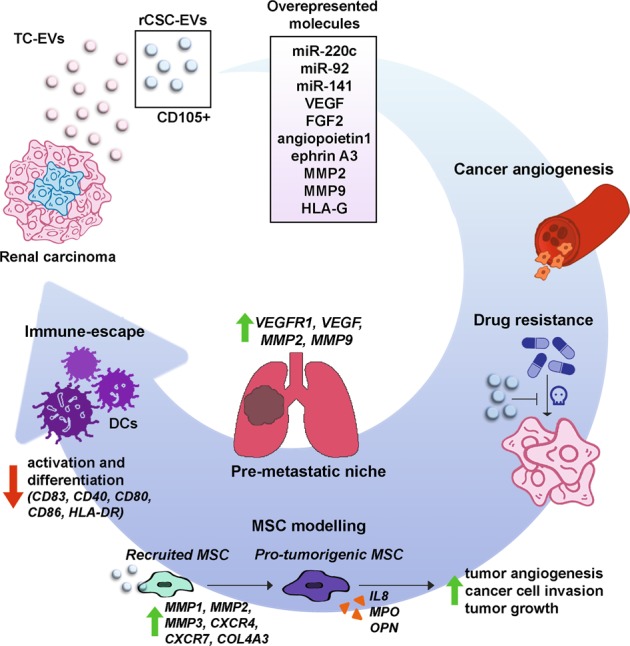
There are more than 24 upregulated miRNAs, including miR-200c miR-92 and miR-141, in CSC-EVs compared to tumoral cell-derived EVs (TC-EVs). Unlike TC-EVs, CSC-EVs carry several mRNAs of proangiogenic genes, such as VEGF, fibroblast growth factor 2 (FGF2), angiopoietin 1, ephrin A3, MMP2 and MMP9. Additionally, HLA-G protein was enriched in rCSC-EVs compared to that in TC-EVs. Moreover, rCSC-EVs support cancer development by several mechanisms. These vesicles were shown to promote tumor angiogenesis in vitro and in vivo and to promote apoptosis resistance following treatment with anticancer drug doxorubicin. Moreover, rCSC-EVs were proven to favor lung premetastatic niche formation through the upregulation of VEGFR1, VEGF, MMP2 and MMP9 in target tissue. In addition, rCSC-EVs can promote MSC migration inducing the upregulation of genes, such as MMP1, MMP3, CXCR4, MMP2, COL4A3 and CXCR7. MSCs stimulated with rCSC-EVs released IL-8, myeloperoxidase (MPO) and osteopontin (OPN) at higher concentrations, promoted cancer angiogenesis and tumor cell migration and increased tumor development in vivo. Finally, rCSC-EVs were shown to mediate cancer immunosuppression by reducing dendritic cell (DC) differentiation and activation. In particular, rCSC-EVs contained higher levels of HLA-G compared to TC-EVs and decreased the expression of activation markers CD83 and CD40, costimulatory molecules CD80 and CD86, and the antigen-presenting molecule HLA-DR in DCs
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gould Stephen J., Raposo Graça. As we wait: coping with an imperfect nomenclature for extracellular vesicles. Journal of Extracellular Vesicles. 2013;2(1):20389. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurley JH. ESCRTs are everywhere. EMBO J. 2015;34:2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iavello A, et al. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int. J. Mol. Med. 2016;37:958–966. doi: 10.3892/ijmm.2016.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Niel G, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 6.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 7.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baietti MF, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 9.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 10.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grange C, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 12.Kowal J, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA. 2016;113:e968–e977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaj L, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lázaro-Ibáñez E, et al. Different gdna content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74:1379–1390. doi: 10.1002/pros.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010;117:1. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 16.Sansone P, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl Acad. Sci. USA. 2017;114:e9066–e9075. doi: 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratajczak J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 18.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 19.Fatima, F. & Nawaz, M. Vesiculated long non-coding RNAs: offshore packages deciphering trans-regulation between cells, cancer progression and resistance to therapies. Noncoding RNA. 3, E10 (2017). [DOI] [PMC free article] [PubMed]
- 20.Fares J, Kashyap R, Zimmermann P. Syntenin: key player in cancer exosome biogenesis and uptake? Cell Adh. Migr. 2017;11:124–126. doi: 10.1080/19336918.2016.1225632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma XR, Edmund Sim UH, Pauline B, Patricia L, Rahman J. Overexpression of WNT2 and TSG101 genes in colorectal carcinoma. Trop. Biomed. 2008;25:46–57. [PubMed] [Google Scholar]
- 22.Liu RT, et al. Overexpression of tumor susceptibility gene TSG101 in human papillary thyroid carcinomas. Oncogene. 2002;21:4830–4837. doi: 10.1038/sj.onc.1205612. [DOI] [PubMed] [Google Scholar]
- 23.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl Acad. Sci. USA. 2014;111:e3234–e3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, et al. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem. Biophys. Res. Commun. 2014;451:295–301. doi: 10.1016/j.bbrc.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 27.Imjeti NS, et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl Acad. Sci. USA. 2017;114:12495–12500. doi: 10.1073/pnas.1713433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindoso RS, Collino F, Camussi G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget. 2015;6:7959–7969. doi: 10.18632/oncotarget.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grange C, et al. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer. 2015;15:1009. doi: 10.1186/s12885-015-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim CW, et al. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 31.Hsu YL, et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, et al. The 786-0 renal cancer cell-derived exosomes promote angiogenesis by downregulating the expression of hepatocyte cell adhesion molecule. Mol. Med. Rep. 2013;8:272–276. doi: 10.3892/mmr.2013.1458. [DOI] [PubMed] [Google Scholar]
- 33.Mineo M, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 2012;15:33–45. doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jingushi K, et al. Extracellular vesicles isolated from humanrenal cell carcinoma tissues disrupt vascular endothelial cell morphology viaazurocidin. Int. J. Cancer. 2018;142:607–617. doi: 10.1002/ijc.31080. [DOI] [PubMed] [Google Scholar]
- 35.Beckham CJ, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J. Urol. 2014;192:583–592. doi: 10.1016/j.juro.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 36.Franzen CA, et al. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. 2015;4:e163. doi: 10.1038/oncsis.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman MA, et al. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget. 2016;7:54852–54866. doi: 10.18632/oncotarget.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramteke A, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015;54:554–565. doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris DA, et al. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS ONE. 2015;10:e0117495. doi: 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atay S, et al. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc. Natl Acad. Sci. USA. 2014;111:711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Jabalee, J., Towle, R. & Garnis, C. The role of extracellular vesicles in cancer: cargo, function, and therapeutic implications. Cells7, E93 (2018). [DOI] [PMC free article] [PubMed]
- 45.Qu L, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Corrò C, Moch H. Biomarker discovery for renal cancer stem cells. J. Pathol. Clin. Res. 2018;4:3–18. doi: 10.1002/cjp2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int. J. Biochem. Cell. Biol. 2012;44:2144–2151. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bussolati B, et al. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696–3705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 50.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology. 2014;28:1101–1107. [PubMed] [Google Scholar]
- 51.Jin Xiong, Jin Xun, Kim Hyunggee. Cancer stem cells and differentiation therapy. Tumor Biology. 2017;39(10):101042831772993. doi: 10.1177/1010428317729933. [DOI] [PubMed] [Google Scholar]
- 52.Bussolati B, et al. Human renal cancer stem cells. Cancer Lett. 2013;338:141–146. doi: 10.1016/j.canlet.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Bruno S, et al. CD133+renal progenitor cells contribute to tumor angiogenesis. Am. J. Pathol. 2006;169:2223–2235. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galleggiante V, et al. CTR2 identifies a population of cancer cells with stem cell-like features in patients with clear cell renal cell carcinoma. J. Urol. 2014;192:1831–1841. doi: 10.1016/j.juro.2014.06.070. [DOI] [PubMed] [Google Scholar]
- 55.Gassenmaier M, et al. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma initiating cells and predicts metastasis. Stem Cells. 2013;31:1467–1476. doi: 10.1002/stem.1407. [DOI] [PubMed] [Google Scholar]
- 56.Zhong Y, et al. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett. 2010;299:150–160. doi: 10.1016/j.canlet.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Debeb BG, et al. Characterizing cancer cells with cancer stem cell-like features in 293T human embryonic kidney cells. Mol. Cancer. 2010;9:180. doi: 10.1186/1476-4598-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Addla SK, Brown MD, Hart CA, Ramani VA, Clarke NW. Characterization of the Hoechst 33342 side population from normal and malignant human renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2008;295:e680–e687. doi: 10.1152/ajprenal.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lässer C, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J. Transl. Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willms E, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016;6:22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santonocito M, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014;102:e1751–e1761. doi: 10.1016/j.fertnstert.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 63.Iorio MV, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 64.Motoyama K, et al. Over- and under-expressed microRNAs in human colorectal cancer. Int. J. Oncol. 2009;34:1069–1075. doi: 10.3892/ijo_00000233. [DOI] [PubMed] [Google Scholar]
- 65.Brase JC, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer. 2011;128:608–616. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 66.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, et al. MicroRNA-650 targets ING4 to promote gastric cancer tumorigenicity. Biochem. Biophys. Res. Commun. 2010;395:275–280. doi: 10.1016/j.bbrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Luedde T. MicroRNA-151 and its hosting gene FAK (focal adhesion kinase) regulate tumor cell migration and spreading of hepatocellular carcinoma. Hepatology. 2010;52:1164–1166. doi: 10.1002/hep.23854. [DOI] [PubMed] [Google Scholar]
- 69.Chow TF, et al. Differential expression profiling of microRNAs and their potential involvement in renal cell carcinoma pathogenesis. Clin. Biochem. 2010;43:150–158. doi: 10.1016/j.clinbiochem.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Bonuccelli G, et al. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget. 2014;5:7575–7588. doi: 10.18632/oncotarget.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu S, et al. Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells. 2012;30:266–279. doi: 10.1002/stem.787. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park SA, et al. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int. J. Oncol. 2011;38:97–103. [PubMed] [Google Scholar]
- 75.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 76.Liu H, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE. 2012;7:e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J. Biol. Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 79.Miao Z, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl Acad. Sci. USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 81.Nie XC, et al. COL4A3 expression correlates with pathogenesis, pathologic behaviors, and prognosis of gastric carcinomas. Hum. Pathol. 2013;44:77–86. doi: 10.1016/j.humpath.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 82.Ando H, et al. Prognostic significance of the expression of MUC1 and collagen type IV in advanced gastric carcinoma. Br. J. Surg. 2009;96:901–909. doi: 10.1002/bjs.6635. [DOI] [PubMed] [Google Scholar]
- 83.Smrkolj S, Erzen M, Rakar S. Prognostic significance of topoisomerase II alpha and collagen IV immunoexpression in cervical cancer. Eur. J. Gynaecol. Oncol. 2010;31:380–385. [PubMed] [Google Scholar]
- 84.Ortiz de Montellano PR. Catalytic sites of hemoprotein peroxidases. Annu. Rev. Pharmacol. Toxicol. 1992;32:89–107. doi: 10.1146/annurev.pa.32.040192.000513. [DOI] [PubMed] [Google Scholar]
- 85.Zhu YM, Woll PJ. Mitogenic effects of interleukin-8/CXCL8 on cancer cells. Future Oncol. 2005;1:699–704. doi: 10.2217/14796694.1.5.699. [DOI] [PubMed] [Google Scholar]
- 86.MacManus CF, et al. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol. Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 87.Zou C, et al. Osteopontin promotes mesenchymal stem cell migration and lessens cell stiffness via integrin β1, FAK, and ERK pathways. Cell Biochem. Biophys. 2013;65:455–462. doi: 10.1007/s12013-012-9449-8. [DOI] [PubMed] [Google Scholar]
- 88.Bandopadhyay M, et al. Osteopontin as a therapeutic target for cancer. Expert. Opin. Ther. Targets. 2014;18:883–895. doi: 10.1517/14728222.2014.925447. [DOI] [PubMed] [Google Scholar]
- 89.Hivroz C, Chemin K, Tourret M, Bohineust A. Crosstalk between T lymphocytes and dendritic cells. Crit. Rev. Immunol. 2012;32:139–155. doi: 10.1615/critrevimmunol.v32.i2.30. [DOI] [PubMed] [Google Scholar]
- 90.Banas R, Miller C, Guzik L, Zeevi A. Amnion-derived multipotent progenitor cells inhibit blood monocyte differentiation into mature dendritic cells. Cell Transplant. 2014;23:1111–1125. doi: 10.3727/096368913X670165. [DOI] [PubMed] [Google Scholar]
- 91.Kochan G, Escors D, Breckpot K, Guerrero-Setas D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology. 2013;2:e26491. doi: 10.4161/onci.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.González A, et al. The immunosuppressive molecule HLA-G and its clinical implications. Crit. Rev. Clin. Lab. Sci. 2012;49:63–84. doi: 10.3109/10408363.2012.677947. [DOI] [PubMed] [Google Scholar]
- 93.Dunker K, et al. Expression and regulation of non-classical HLA-G in renal cell carcinoma. Tissue Antigens. 2008;72:137–148. doi: 10.1111/j.1399-0039.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 94.Li BL, et al. Characterization of HLA-G expression in renal cell carcinoma. Tissue Antigens. 2009;74:213–221. doi: 10.1111/j.1399-0039.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 95.De Palma G, et al. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J. Cancer. 2016;7:1960–1967. doi: 10.7150/jca.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujii N, et al. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget. 2017;8:e109877–e109888. doi: 10.18632/oncotarget.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang W, et al. MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur. Urol. Focus. 2016;4:412–419. doi: 10.1016/j.euf.2016.09.007. [DOI] [PubMed] [Google Scholar]


