Abstract
Furanodiene is a natural terpenoid isolated from Rhizoma Curcumae, a well-known Chinese medicinal herb that presents anticancer effects in various types of cancer cell lines. In this study, we have successfully established zebrafish xenografts with 5 various human cancer cell lines; and validated these models with anti-cancer drugs used clinically for treating human cancer patients. We found that Furanodiene was therapeutically effective for human JF 305 pancreatic cancer cells and MCF-7 breast cancer cells xenotranplanted into zebrafish. Furanodiene showed a markedly synergistic anti-cancer effect when used in combination with 5-FU (5-Fluorouracil) for both human breast cancer MDA-MB-231 cells and human liver cancer BEL-7402 cells xenotransplanted into zebrafish. Unexpectedly, Furanodiene reversed multiple drug resistance in the zebrafish xenotransplanted with cis-Platinum-resistant human non-small cell lung cancer cells and Adriamycin-resistant human breast cancer cells. Furanodiene played its anti-cancer effects through anti-angiogenesis and inducing ROS production, DNA strand breaks and apoptosis. Furanodiene suppresseed efflux transporter Pgp (P-glycoprotein) function and reduced Pgp protein level, but no effect on Pgp related gene (MDR1) expression. These results suggest sensitizition and synergistic anti-cancer effects of Furanodiene that is worthy of a further investigation.
Introduction
In recent years, the anti-cancer potential of natural products from medicinal plants, especially those used in traditional Chinese medicine, has been gradually recognized by the scientific community1–5. A few pure compounds isolated from traditional Chinese medicine, such as Berberine, Curcumin, Quercetin, and so on, have been studied to treat cancer in experimental and clinical investigations6–8.
Curcumae Rhizoma (R. curcumae), a commonly used traditional Chinese medicinal herb, has displayed a wide and diverse medicinal value for nearly one thousand years, including removal of blood stasis and pain alleviation. The herb has been widely prescribed to treat cardiovascular diseases and cancer in Chinese clinical practice9. Growing evidence suggests that R. curcumae extract has anti-proliferative and pro-apoptotic effects on various types of cancer cells10–12. Pharmacodynamic studies found that R. curcumae extracts, including curcumin and β-elemene, not only inhibit cancer cell growth13, but also demonstrate synergistic therapeutic effects with many chemotherapeutic agents such as 5-Fluorouracil (5-FU), Paclitaxel, Doxorubicin, and reduce the toxicity induced by anti-cancer drugs14.
Furanodiene is another sesquiterpene isolated from the essential oil of Curcuma wenyujin15, exhibiting hepatoprotective, anti-inflammatory, anti-angiogenic and anti-tumor activities16–24. In vitro studies suggested that Furanodiene enhanced the anti-cancer effects of doxorubicin on ERα-negative breast cancer cells25, presented synergistic anti-proliferative activity with paclitaxel via altering cell cycle and integrin signaling in 95-D lung cancer cells26, and combinational treatments with Furanodiene and Doxorubicin blocked the invasion and migration of MDA-MB-231 breast cancer cells in vitro27. However, the synergistic anti-cancer effects as well as probably sensitizing drugs-resistant cancer cells of Furanodiene have never been investigated in vivo in any animal cancer models.
Given the high genetic and physiological similarities with humans, zebrafish has been used as a powerful and cost-effective animal model for cancer research and cancer drug discovery28–33. Using zebrafish in cancer drug research and development provides several advantages including transparency, easy manipulation, high predictability, short testing period, and small amount of testing drugs required. Zebrafish cancer models could be established by carcinogen treatment, genetic knockout, gene overexpression or xenotransplanting human cancer cells into the zebrafish33. The translucent body of zebrafish enables researchers to visualize all processes associated with tumor formation, progression, metastasis and death29–33. The small molecule compounds or drugs can be added directly to the water environment of the zebrafish and the therapeutic effects could be assessed qualitatively and quantitatively.
In this study, we have successfully developed zebrafish xenografts with human A549 non-small-cell lung cancer cells, SGC-7901 stomach cancer cells, HepG2 liver cancer cells, JF 305 pancreatic cancer cells and MCF-7 breast cancer cells; and validated these models with anti-cancer drugs used clinically in treating cancer patients. We have also established zebrafish xenografts with 5 drug-resistant human gastric cancer cells. We found that the known anti-cancer agent Furanodiene isolated from traditional Chinese herbs are effective in treating human JF 305 pancreatic cancer cells and MCF-7 breast cancer cells xenotranplanted into the zebrafish. Furanodiene has clearly shown a synergistic anti-cancer effects when used in combination with 5-FU for both human breast cancer MDA-MB-231 cells and human liver cancer BEL-7402 cells xenotransplanted into the zebrafish. Surprisingly, we found that Furanodiene could reverse multiple drug resistance in zebrafish xenotransplanted with cis-Platinum-resistant human non-small cell lung cancer cells and Adriamycin-resistant human breast cancer cells. In the mechanistic studies, we have found that Furanodiene plays its anti-cancer roles through anti-angiogenesis and inducing ROS production, DNA strand breaks and apoptosis. We have also discovered that Furanodiene markedly suppresses efflux transporter P-glycoprotein (Pgp) function and reduces Pgp protein level, but no effect on Pgp related gene (MDR1) expression.
Results
Anti-cancer effects
To confirm whether response of zebrafish xenografts to cancer drugs is similar to the response of mammalian model systems, we treated the xenotransplanted zebrafish with each of 10 known anti-cancer drugs (Bevacizumab, cis-Platinum, Endostar, Paclitaxel, Vinorelbine, Adriamycin, 5-FU, Hydroxyurea, Irinotecan and Gemcitabine) and Furanodiene for 48 h. Three concentrations or dosages for each drug were assessed. The maximum tolerated concentration/dosages MTC/MTD of a testing drug was defined as a maximum concentration or maximum dosage that did not induce any observable adverse effect on zebrafish and was determined under a dissecting stereomicroscope by a well-trained zebrafish toxicologist. The MTC/MTD was 400 ng for Bevacizumab, 2 ng for cis-Platinum, 80 ng for Endostar, 2.56 ng for Paclitaxel, 0.5 ng for Vinorelbine, 2.5 ng for Adriamycin, 2 ng for Irinotecan, 20 ng for Gemcitabine, 260 μg/mL for 5-FU and 1000 μg/mL for Hydroxyurea, respectively and 12.2 ng for Furanodiene.
Xenotransplanted zebrafish were generated by microinjection of approximately 800 human cancer cells labeled with CM-Dil into the yolk sac of zebrafish that were at 48 hpf as we reported previously34. As expected, after a 48 h treatment, Bevacizumab, cis-Platinum, Endostar, Paclitaxel, Vinorelbine, Adriamycin, 5-FU, Hydroxyurea, Irinotecan and Gemcitabine all significantly inhibit tumor growth in the zebrafish xenotransplanted with various types of human cancer cells (Fig. 1). The cancer inhibition percentages in zebrafish xenotransplant model of A549 non-small-cell lung cancer cells were (4 ± 1.39)–(65 ± 2.54)% for Bevacizumab, (34 ± 1.78)–(55 ± 2.89)% for cis-Platinum, (11 ± 2.13)–(39 ± 3.22)% for Endostar, (24 ± 1.99)–(27 ± 0.99)% for Paclitaxel, (38 ± 1.22)–(40 ± 2.01)% for Vinorelbine, respectively; in zebrafish xenotransplant model of SGC-7901 stomach cancer cells were (29 ± 1.87)–(31 ± 1.86)% for 5-FU, (29 ± 1.96)–(46 ± 2.31)% for Hydroxyurea, (5 ± 0.77)–(24 ± 1.11)% for cis-Platinum, (4 ± 0.89)–(31 ± 1.23)% for Irinotecan, (3 ± 0.56)–(26 ± 1.99)% for Paclitaxel, respectively; in zebrafish xenotransplant model of HepG2 liver cancer cells were (17 ± 1.22)–(45 ± 2.04)% for Adriamycin, (45 ± 1.94)–(46 ± 1.25)% for Gemcitabine, (33 ± 1.79)–(56 ± 1.96)% for Hydroxyurea, (45 ± 1.91)–(64 ± 2.08)% for cis-Platinum, (15 ± 1.95)–(38 ± 2.51)% for 5-FU, respectively. The cancer inhibition percentages for Furanodiene at 3 various concentrations were (13 ± 2.95)%, (27 ± 2.78)%, (40 ± 3.30)% in zebrafish xenotransplant model of JF 305 pancreatic cancer cells, and (26 ± 2.12)%, (33 ± 1.56)%, (41 ± 1.89)% in zebrafish xenotransplant model of MCF-7 breast cancer cells, respectively. Statistically significant inhibitory effects on zebrafish xenotransplants were observed for all the tested anticancer drugs and Furanodiene (p < 0.05 or p < 0.01 or p < 0.001) (Fig. 2).
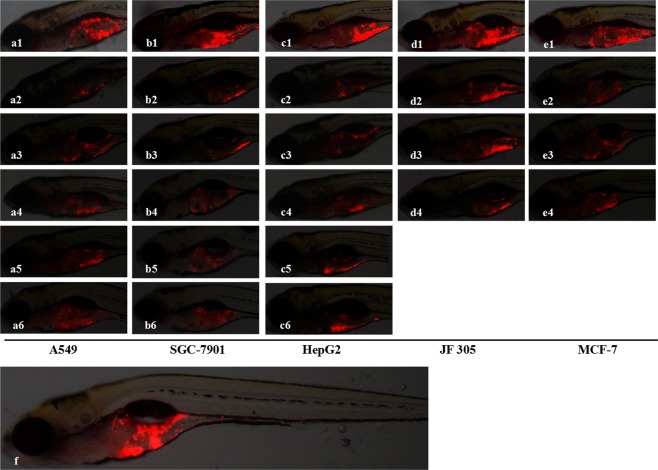
Figure 1.
Cancer cell proliferations were suppressed after 48-h drug treatment in the zebrafish xenotransplanted with human A549 non-small-cell lung cancer cells, human SGC-7901 stomach cancer cells, human HepG2 liver cancer cells, human JF 305 pancreatic cancer cells, and human MCF-7 breast cancer cells. a1, b1, c1, d1 and e1 were the control xenotransplanted zebrafish without drug treatment. a2–a6 were treated with Bevacizumab, cis-Platinum, Endostar, Paclitaxel and Vinorelbine; b2–b6 were treated with 5-FU, Hydroxyurea, cis-Platinum, Irinotecan and Paclitaxel; c2–c6 were treated with Adriamycin, Gemcitabine, Hydroxyurea, cis-Platinum and 5-FU; and d2–d4 as well as e2–e4 were treated with Furanodiene at concentrations of 1/9 MTC, 1/3 MTC and MTC, respectively. A representative entire body of the xenotransplanted zebrafish body was shown in f.
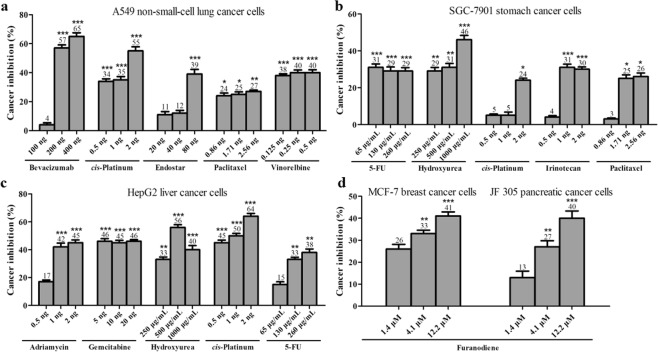
Figure 2.
Quantitative analyses of cancer cell proliferations suppression in the zebrafish xenotransplanted with human A549 non-small-cell lung cancer cells (a) human SGC-7901 stomach cancer cells (b) human HepG2 liver cancer cells (c) human JF 305 pancreatic cancer cells and human MCF-7 breast cancer cells (d) after 48-h treatment of various anti-cancer drugs. As compared with untreated control xenotransplanted zebrafish, *p < 0.05, **p < 0.01, and ***p < 0.001.
The prolonged survival period was observed in the zebrafish xenotransplanted with HepG2 hepatocellular carcinoma cells and treated with Furanodiene. The survival days were extended for 1 time, 2 times and 2.67 times when treated with Furanodiene at 1/9 MTC, 1/3 MTC and MTC, respectively, as compared with untreated xenotransplanted zebrafish. Statistical differences (p < 0.05) were found in the zebrafish xenografs treated with Furanodiene at 1/3 MTC and MTC.
Synergistic effects
To determine whether Furanodiene had a synergistic effect, zebrafish at 48 hpf was xenotransplanted with human liver cancer BEL-7402 or breast cancer MDA-MB-231 sensitive cancer cell lines labeled with CM-Dil. The results showed that Furanodiene significantly enhance 5-FU inhibition of tumor growth on BEL-7402 and MDA-MB-231 sensitive cancer cell lines (Fig. 3, Table 1). The cancer inhibition % in zebrafish xenotransplanted with BEL-7402 was (34 ± 2.31)% for 5-FU, (23 ± 2.78)% for Furanodiene, (48 ± 3.26)% for the combination respectively; in zebrafish xenotransplant model of MDA-MB-231 was (35 ± 1.98)% for 5-FU, (5 ± 0.56)% for Furanodiene, and (45 ± 3.30)% for Furanodiene cotreatment with 5-FU, respectively. Statistical differences (p < 0.05 & p < 0.01) were found in the zebrafish xenografs treated with Furanodiene cotreatment with 5-FU as compared with 5-FU and Furanodiene alone.
Figure 3.
Synergistic anti-cancer effects of Furanodiene with 5-FU in zebrafish xenotransplanted with human BEL-7402 liver cancer cells (above) or with human MDA-MB-231 breast cancer cancer cells (below).
Table 1.
Synergistic anti-cancer effects of Furanodiene in zebrafish xenotransplant models (Mean ± SE).
| Drugs | Concentration (μM) | Cancer inhibition on BEL-7402 (%) |
|---|---|---|
| 5-FU | 333 | 34 ± 2.31 |
| Furanodiene | 1.4 | 23 ± 2.78 |
| Furanodiene + 5-FU | 1.4 + 333 | 48 ± 3.26#/## |
| Drugs | Concentration (μM) | Cancer inhibition on MDA-MB-231 (%) |
| 5-FU | 333 | 35 ± 1.98 |
| Furanodiene | 1.4 | 5 ± 0.56 |
| Furanodiene + 5-FU | 1.4 + 333 | 45 ± 3.30#/## |
#p < 0.05, as compared with 5-FU; and ##p < 0.01, as compared with Furanodiene.
Anti-cancer sensitization effects
Zebrafish xenotransplanted with drug-resistance human cancer cells were used to assess anti-multidrug resistance effects of Furanodiene. We have successfully established 5 zebrafish xenotransplant models for anti-multidrug resistance assessment, including the zebrafish xenotransplanted with cis-Platinum-resistant human gastric cancer cells, cis-Platinum-resistant human non-small cell lung cancer cells, Adriamycin-resistant human breast cancer cells, paclitaxel-resistant human ovarian cancer cells, and Adriamycin-resistant human oral squamous cancer cells. To determine whether Furanodiene could reverse multidrug resistance, zebrafish at 48 hpf was xenotransplanted approximately 800 human cancer drug resistant cells (A549/cis-Platinum and MCF-7/Adriamycin) labeled with CM-Dil. The results showed that cis-Platinum or Adriamycin had no statistically effect on the zebrafish xenotransplanted with human cancer drug resistant cells (A549/cis-Platinum and MCF-7/Adriamycin); Furanodiene and Furanodiene cotreatment with cis-Platinum or Adriamycin significantly inhibit tumor growth in zebrafish xenotransplanted with human cancer drug resistant cells (A549/cis-Platinum and MCF-7/Adriamycin) (Fig. 4). The cancer inhibition percentages in zebrafish xenotransplant model of A549/cis-Platinum were (2 ± 1.68)–(28 ± 1.85)% for Furanodiene, (21 ± 2.03)–(41 ± 3.41)% for Furanodiene cotreatment with cis-Platinum, respectively; in zebrafish xenotransplant model of MCF-7/Adriamycin were (11 ± 1.20)–(40 ± 2.23)% for Furanodiene, (27 ± 2.56)–(59 ± 4.20)% for Furanodiene cotreatment with Adriamycin, respectively (Table 2). Statistically significant inhibitory effects on zebrafish xenotransplants with human cancer drug resistant cells (A549/cis-Platinum and MCF-7/Adriamycin) were observed for Furanodiene (p < 0.05 or p < 0.01 or p < 0.001), and statistical differences (p < 0.001) were found in the zebrafish xenografs treated with Furanodiene cotreatment with cis-Platinum/Adriamycin as compared with cis-Platinum/Adriamycin alone.
Figure 4.
Sensitizing effect of Furanodiene on cis-Platinum-resistant human A549 non-small-cell lung cancer cells (above) and Adriamycin-resistant MCF-7 breast cancer cells (below) xenotransplanted into zebrafish.
Table 2.
Sensitizing effect of Furanodiene on zebrafish xenografts (Mean ± SE).
| Drugs | Concentration (μM) | Cancer inhibition on A549/cis-platinum (%) |
|---|---|---|
| cis-Platinum | 100 | −2 ± 0.99 |
| Furanodiene | 1.4 | 2 ± 1.68 |
| 4.1 | 16 ± 3.26* | |
| 12.2 | 28 ± 1.85*** | |
| Furanodiene + cis-Platinum | 1.4 | 21 ± 2.03*### |
| 4.1 | 39 ± 3.26**### | |
| 12.2 | 41 ± 3.41***### | |
| Drugs | Concentration (μM) |
Cancer inhibition on
MCF-7/adriamycin (%) |
| Adriamycin | 10 µg/mL | −0.1 ± 0.39 |
| Furanodiene | 1.4 | 11 ± 1.20 |
| 4.1 | 14 ± 2.01* | |
| 12.2 | 40 ± 2.23*** | |
| Furanodiene + Adriamycin | 1.4 | 27 ± 2.56***### |
| 4.1 | 36 ± 3.01***### | |
| 12.2 | 59 ± 4.20***### |
Compared with control: *p < 0.05, **p < 0.01 & ***p < 0.001.
Furanodiene cotreat compared with a tested drug alone: #p < 0.05, ##p < 0.01, and ###p < 0.001.
Mechanisms
Antiangiogenesis
Antiangiogenic effect of Furanodiene was assessed on 2 dpf Tg (fli1a: EGFP) transgenic zebrafish with fluorescent blood vessels. In the initial pilot study, we found the growth of angiogenic SIVs were markedly inhibited in zebrafish treated with Furanodiene at a concentration of MTC for 24 h. Some of the SIVs were incompletely formed resulting in a significant reduction in the total area of SIVs, as compared with untreated control zebrafish. In the dose-response experiments, the SIVs inhibition % of Furanodiene at 1/9 MTC, 1/3 MTC and MTC on angiogenesis was (19 ± 2.19)%, (21 ± 2.33)% and (43 ± 3.55)%, respectively (Table 3). Statistically significant differences (p < 0.05 & p < 0.01) were found in zebrafish treated with Furanodiene at 1/3 MTC and MTC.
Table 3.
Mechanism study of Furanodiene in zebrafish models (Mean ± SE).
| Assays | Biomarkers | Efficacy (%) | ||
|---|---|---|---|---|
| 1.4 μM | 4.1 μM | 12.2 μM | ||
| Antiangiogenesis | SIVs inhibition | 19 ± 2.19 | 21 ± 2.33* | 43 ± 3.55** |
| Apoptosis | AO staining | 20 ± 3.29 | 51 ± 2.31*** | 65 ± 0.99*** |
| caspase-3/7 | 2 ± 1.78 | 16 ± 1.47* | 67 ± 1.66*** | |
| caspase-8 | 11 ± 1.56 | 17 ± 1.46* | 68 ± 2.17*** | |
| caspase-9 | 8 ± 1.69 | 17 ± 2.31* | 69 ± 2.85*** | |
| Comet assay | Single stranded DNA strand fracture analysis | 116 ± 3.58 | 370 ± 2.45** | 540 ± 3.97*** |
| Double stranded DNA strand fracture analysis | 118 ± 2.79 | 143 ± 3.12** | 208 ± 2.25*** | |
| ROS | ROS production | 145 ± 1.56 | 190 ± 3.18* | 521 ± 3.98*** |
| P-glycoprotein | Pgp inhibition | 21 ± 1.88* | 34 ± 2.05* | 58 ± 1.82* |
Compared with control: *p < 0.05, **p < 0.01 and ***p < 0.001.
ROS, DNA strand breaks and apoptosis
ROS levels in zebrafish treated with Furanodiene were analyzed using an oxidation sensitive probe, 5-(and 6-)-chloromethyl-20, 70-dichloro-dihydrofluoresceindiacetate (CM-H2DCFDA, Life Technologies, Carlsbad, CA). Furanodiene treatment resulted in increased ROS production. ROS levels relative to untreated control zebrafiash were (145 ± 1.56)%, (190 ± 3.18)% and (521 ± 3.98)%, respectively, in zebrafish treated with Furanodiene at concentrations of 1/9 MTC, 1/3MTC and MTC (Table 3). Statistically significant differences (p < 0.05 & p < 0.001) were found in zebrafish treated with Furanodiene at 1/3 MTC and MTC.
Single strand DNA breaks in the zebrafish cells treated with 1/9 MTC, 1/3 MTC and MTC of Furanodiene were (116 ± 3.58)%, (370 ± 2.45)% and (540 ± 3.97)% higher than in untreated control zebrafish cells. Double DNA breaks were elevated to (118 ± 2.79)%, (143 ± 3.12)% and (208 ± 2.25)% relative to untreated control zebrafish (Table 3). Noteworthy differences (p < 0.01 & p < 0.001) in both single and double DNA breaks were observed in zebrafish treated with Furanodiene at 1/3 MTC and MTC (Fig. 5, Table 3).
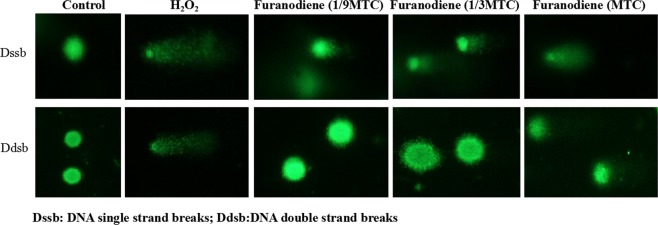
Figure 5.
Apoptosis in zebrafish treated with cis-Platinum or Adriamycin alone, and cis-Platinum or Adriamycin in combination with Furanodiene at 1/9 MTC, 1/3 MTC and MTC, respectively (a). Quantitative data of (a) were shown in (b).
The tailbud of zebrafish was chosen for the observation of apoptosis induction35. Zebrafish treated with 0.1% DMSO had a few apoptotic cells, while cis-Platinum treatment significantly increased the number of apoptotic cells. When treated with different concentrations of Furanodiene, apoptosis were increased at a dose-dependent manner. The promotion percentage on apoptosis was (14 ± 2.38)% for cis-Platinum at 100 μM, (29 ± 2.65)% for Adriamycin at 10 µg/mL, (20 ± 3.29)–(65 ± 1.99)% for Furanodiene at 1/9 MTC, 1/3 MTC and MTC, (20 ± 2.59)–(87 ± 1.49)% for Furanodiene cotreatment with cis-Platinum, and (44 ± 3.15)–(85 ± 2.49)% for Furanodiene cotreatment with Adriamycin, respectively (Fig. 6). Statistically significant (p < 0.01 & p < 0.001) promotion effects on apoptosis were observed for Furanodiene, Adriamycin, cis-Platinum, Furanodiene cotreatment with Adriamycin/cis-Platinum, Statistical differences (p < 0.05 & p < 0.01 & p < 0.001) were found in the zebrafish treated with Furanodiene cotreatment with cis-Platinum/Adriamycin as compared with cis-Platinum/Adriamycin alone. Caspase activities were measured using caspase-Glo reagents (Promega, USA) through cleavage of colorless substrates specific for caspase 3/7 caspase 8 and caspase 9. As indicated in Table 3, after treatment with Furanodiene at 1/9 MTC, 1/3 MTC and MTC, caspase-3/7, -8 and -9 activities were all notably increased by (2 ± 1.78)%, (16 ± 1.47)% and (67 ± 1.66)%; (11 ± 1.56)%, (17 ± 1.46)% and (68 ± 2.17)%; and (8 ± 1.69)%, (17 ± 2.31)% and (69 ± 2.85)%, respectively. Statistically significant differences (p < 0.05 & p < 0.001) for caspase 3/7 caspase 8 and caspase 9 were all found in zebrafish treated with Furanodiene at 1/3 MTC and MTC.
Figure 6.
DNA single strand breaks (above) and DNA double strand breaks (below) in zebrafish treated with Furanodiene at 1/9 MTC, 1/3 MTC and MTC. H2O2 was used as a positive control.
Pgp efflux regulation
Based on retention of rhodamine 123 in the whole zebrafish, we developed and validated an in vivo assay for assessing drug effects on Pgp efflux in zebrafish (our published patent: China patent No. 201110231726.2). As shown in Fig. 7, fluorescence signal was absent in control group, indicating that the dye was pumped out of zebrafish; while was retained in zebrafish after treatment with Furanodiene. The Pgp inhibition percentage of Furanodiene at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC) was (21 ± 1.88)%, (34 ± 2.05)% and (58 ± 1.82)%, respectively (Table 3). Statistically significant differences were found in zebrafish treated with Furanodiene at 1/9 MTC (p < 0.05), 1/3 MTC (p < 0.05) and MTC (p < 0.05).
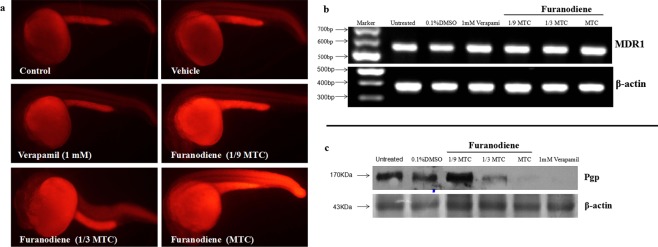
Figure 7.
Pgp efflux transporter regulation in zebrafish treated with Furanodiene. (a) Pgp function inhibition by Furanodiene at 1/9 MTC, 1/3 MTC and MTC, 1 mM Verapamil was used as a positive control; (b) Pgp gene expression analyses by PCR; and (c) Pgp protein assay by Western blot.
The mRNA levels of the Pgp related gene (MDR1) was determined in zebrafish treated with Furanodiene. The relative expression level of MDR1 gene was unchanged in zebrafish treated with Furanodiene at concentrations of 1/3 MTC and MTC as compared with control group, whereas the expression level of Pgp protein was severely decreased.
Dissussion
In this study, we have demonstrated that all the human anti-cancer drugs currently used clinically for cancer patients and selected for this study are therapeutically effective on the human cancer cells xenotranplanted into zebrafish, further validating and supporting zebrafish xenografts as a rapid, reliable, reproducible, and cost-efficient animal model for anti-cancer drug screening and assessment34–39. Our results from this investigation have confirmed that zebrafish xenotransplanted cancer models are also suitable for assessing sensitization and synergistic effects of anti-cancer drugs.
Furanodiene has been extensively studied as an anti-cancer agent in a variety of in vitro and in vivo models19–27. Here we have indicated that Furanodiene are effective for two types of human cancer cells (JF 305 pancreatic cancer cells and MCF-7 breast cancer cells) xenotranplanted into zebrafish, supporting that Furanodiene could be a potent anti-cancer drug20–27. Interestingly, in this study, Furanodiene has shown statistically significant synergistic anti-cancer effects when used in combination with 5-FU for both human breast cancer MDA-MB-231 cells and human liver cancer BEL-7402 cells xenotransplanted into the zebrafish.
In the mechanistic studies, we have found that Furanodiene plays its anti-cancer role through anti-angiogenesis, inducing ROS production, DNA strand breaks and apoptosis. Neoangiogenesis has been recognized as a hallmark of tumor progression and about a dozen of anti-angiogenetic drugs have been globally marked40–44. Elevated ROS production has been indicated to damage large biological molecules including DNA, leading to DNA strand breaks and apoptosis45–47. Caspase 8 participates in the intracellular signaling cascade leading to apoptosis while caspase 9 has been linked to the mitochondrial death pathway48–51. Caspase 3/7 is either partially or totally responsible for the proteolytic cleavage of many key proteins during apoptosis and interacts with caspase 8 and caspase 948–51. Our results showed that the activity of caspases 8, 9 and 3/7 were all notablely increased in the cancer cell xenotransplanted zebrafish treated with Furanodiene, revealing that Furanodiene-induced zebrafish apoptosis are both caspase 8 and caspase 9 dependent, leading to cancer cell death.
Multiple-drug resistance is a most common and challengable problem in cancer treatment for both cytotoxic and targeted anti-cancer drugs52 and there are no effective sensitizing drugs available in market53. Surprisingly, in this study, when cis-Platinum-resistant human non-small cell lung cancer cells and Adriamycin-resistant human breast cancer cells xenotransplanted into zebrafish are co-treated with Furanodiene, significant cancer suppression is demonstrated that are statistically more potent than either cis-Platinum/Adriamycin (no significant inhibition) or Furanodiene alone. We have also discovered that Furanodiene markedly suppresses efflux transporter Pgp function and reduces Pgp protein, but no effect on Pgp gene expression. These data suggest that Furanodiene could be a potential sensitizing agent to anti-cancer drugs probably mainly through blocking Pgp efflux transportation. These results are consistant with other reports indicating that Pgp is one of major players responsible for anti-cancer drug resistance and blocking Pgp could reverse the cancer drug resistence54–57, Further studies are under progresses to confirm Furanodiene pharmacology in mammalian models and to investigate other cellular, biochemical, and molecular mechanisms involved in Furanodiene anti-cancer pathways.
Materials and Methods
Zebrafish care and maintenance
Two lines of zebrafish were used in this study: wild-type AB line and Tg (fli1a: EGFP) y1 zebrafish. Adult zebrafish were housed and maintained in accordance with standard procedures. Zebrafish were generated by natural pair-wise mating according to the Zebrafish Handbook34. Four to five pairs of adult zebrafish were set up for each mating, and on average 200–300 zebrafish per pair were generated. Zebrafish were maintained at 28 °C in fish water (0.2% Instant Ocean Salt in deionized water, pH 6.9–7.2, conductivity 480–510 μS/cm, and hardness 53.7–71.6 mg/L CaCO3). Zebrafish were cleaned and staged at 6 and 24 hours post fertilization (hpf)34. The University Animal Care and Use Committee approved of the animal procedures described here. These procedures are consistent with the American Veterinary Medical Association’s (AVMA) Panel on Euthanasia. All the zebrafish experiments were performed and completed at Hunter Biotechnology, Inc. and its zebrafish facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International34.
Chemicals and compounds
Endostar (lot #: 201505012) was purchased from Shandong Xiansheng Madejin Bio-Pharmaceuticals, China. Paclitaxel (lot #: 45076), cis-Platinum (lot #: k1520124) and Adriamycin (lot #: 25316-40-9) were purchased from Aladdin company of Shanghai, China. Bevacizumab (lot #: H0126805) was bought from Roche, Switzerland. Vinorelbine (lot #: 140501) was purchased from Jiangsu Haosen Pharmaceuticals. CM-DiI (lot #: V22888) was bought from Molecular Probes, USA. All the other agents were bought from Sigma-Aldrich. Cancer cells were purchased from American Type Culture Collection (ATCC).
Determination of maximum tolerated concentration/dosages (MTC/MTD)
To determine MTC/MTD of a testing drug, zebrafish were treated with a testing drug and mortality and toxicity were recorded at the end of treatment. In the initial tests, five concentrations (0.1, 1, 10, 100, and 500 μg/mL for soaking drugs and 0.1, 1, 10, 100, and 500 ng for injection drugs) were used for each drug. If a MTC/MTD could not be found from the initial tests, additional concentrations within the range of 0.01–2000 μg/mL or 0.01–2000 ng were tested. The MTC/MTD of a testing drug was defined as a maximum concentration or maximum dosage that did not induce any observable adverse effect on zebrafish and was determined under a dissecting stereomicroscope by a well-trained zebrafish toxicologist.
Cell culture
Human cancer cells were purchased from American Type Culture Collection (ATCC) and were subcultured and maintained in tissue culture flasks at 37 °C in a humidified, 5% CO2 atmosphere in RPMI 1640 essential medium or in DMEM essential medium (Gibco), supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 g/ml streptomycin, and 2 mM L-glutamine (Gibco)34.
Cell labeling
Human cancer cells were collected by centrifugation and resuspended in phosphate-buffered saline (PBS). The cells were fluorescently labeled by incubating with 10 µg/ml CM-DiI (Invitrogen, Burlington, ON, Canada) containing 0.5% DMSO and were adjusted to a density of 100 × 106 cells/ml (100 cells per nL) in HBSS. Labeled cells were injected within 2 hours. Cells prepared for injection in this way routinely had fewer than 5% dead cells and were in a single cell suspension, yielding controllable and reproducible numbers of injectable cells that were highly and uniformly fluorescent. The dye was transferred from mother to daughter cell and fluorescent single cells were clearly visible after several doublings on the periphery of late arising tumors34.
Cell transplantation
The transplantation protocol was similar to that described by our previous report34. CM-DiI-labeled human cancer cells were loaded into a pulled glass micropipette (VWR blood capillaries #53508-400) that was drawn on an electrode puller and then trimmed to form a needle with a resulting internal diameter of approximately 15 micron and outer diameter of approximately 18 micron. The microneedle was attached to an air driven Cell Tram (Eppendorf). The tip of the needle was inserted into the yolk of a 48 hpf zebrafish and the pulse time controlled to deliver ~500 cells in 15 nL using positive pressure. The number of injected cells was standardized by fixing cell density and injection volume. After one hour recovery period at 28 °C, implanted zebrafish were examined under a fluorescence microscope (AZ100, Nikon, Japan) for the presence of xenotransplanted cells that reside only in the yolk and are then transferred to 35 °C for the duration of the experiment34.
Assessment of anti-cancer effects
Xenotransplanted zebrafish were generated by microinjection of approximately 800 human cancer cells labeled with CM-Dil into the yolk sac of zebrafish that were at 48 hpf as we reported previously34,36,37. At 24 h post-xenotransplantation (hpx), drugs at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC or 1/4 MTC, 1/2 MTC and MTC) was added to the treatment solution (fish water) for a drug treatment period of 72 hours (h). The xenotransplanted zebrafish treated with 0.1% DMSO was used as the cancer model group. After treatment, xenotransplanted zebrafish were photographed under a fluorescence microscope. Nikon NIS-Elements D 3.10 software was used to quantify all the cancer cell fluorescence intensity (S) in xenotransplanted zebrafish. Drug effect was calculated using the following formula: The cancer inhibition % = (1 − S(Drug)/S(Control)) × 100%.
Determination of prolonged survival period
Xenotransplanted zebrafish were used to determine effects of drugs on the survival period of xenografted zebrafish. At 24 hpx xenotransplanted zebrafish were treated with a testing drug at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC) for a treatment period of 9 days (d). The number of dead zebrafish in each group was recorded on a daily basis. Prolonged survival period was calculated based on the survival rate of xenotransplanted zebrafish treated with tested agents, as compared with untreated cancer model group. The prolonged survival period was calculated using the following formula: The prolonged survival period time = S(Drug)/S(Control).
Quanlification of synergistic anti-cancer effects
To determine whether Furanodiene had a synergistic effect, zebrafish at 48 hpf was xenotransplanted with human liver cancer BEL-7402 or breast cancer MDA-MB-231 sensitive cancer cell lines labeled with CM-Dil. At 24 hpx, 5-Fluorouracil (5-FU) at 1/3 MTC, Furanodiene at 1/9 MTC, and Furanodiene at 1/9 MTC in combination with 5-FU at 1/3 MTC, respectively, were added to the treatment solution for a treatment period of 72 h. After treatment, xenotransplanted zebrafish were photographed under a fluorescence microscope and fluorescence intensity from the xenotransplanted cancer cells was quantified as described above35,37–39.
Measurement of anti-cancer sensitization
Zebrafish xenotransplanted with drug-resistance human cancer cells were used to assess anti-multidrug resistance effects of Furanodiene. At 24 hpx, Furanodiene at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC), chemotherapeutic drugs (cis-Platinum and Adriamycin) at MTC, Furanodiene at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC) cotreated with a chemotherapeutic drug at MTC were added to the treatment solution for a treatment period of 72 h. Xenotransplanted zebrafish were photographed and fluorescence intensity was quantified.
Mechanism study
Antiangiogenesis assessment
Tg (fli1a: EGFP) zebrafish at 48 hpf were distributed into 6-well microplates (Nest, NEST Biotech), 30 zebrafish per well in 3 mL fish water. Furanodiene at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC) were added to the treatment solution for treatment period of 24 h. After treatment, zebrafish were anesthetized with 0.016% MS222 (tricaine methanesulfonate, Sigma-Aldrich, St. Louis, MO), then the number of ISVs was counted and the area of SIVs were quantified with NIS-Elements D3.1 software as we reported previously37.
Reactive oxygen species (ROS) measurement
ROS levels in zebrafish treated with Furanodiene were analyzed using an oxidation sensitive probe, 5-(and 6-)-chloromethyl-20, 70-dichloro-dihydrofluoresceindiacetate (CM-H2DCFDA, Life Technologies, Carlsbad, CA). The treated zebrafish were incubated with 0.5 mg/mL CM-H2DCFDA for 1 h in dark at 28 °C. After rinsing for 3 times using fish water, zebrafish were transferred into a 96-well microplate (1 zebrafish per well) and ROS was measured at 488 nm under a multimode microplate reader (Berthold Technologies, Mithras LB940, Germany) as described by our group39.
DNA strand break analysis
After treatment, zebrafish were lyzed into a single cell suspension. Zebrafish cells were combined with agarose at 1:10 ratio (v/v), mixed well by pipetting and immediately transferred onto the slide. The slides were carefully transferred from either neutral solution (for double DNA strand break detection) or alkaline solution (for single DNA strand break detection) to a horizontal electrophoresis chamber and electrophoresed at 28 volts for 20 min. DNA Staining was done by Vista Green DNA Dye (Cell Biolabs, San Diego, USA) and the Olive tail moment (OTM) was utilized as a biomarker to quantify DNA strand as reported by one of our co-authors58.
Apoptosis quantifications
Acridine orange staining
AB wild type zebrafish at 6 hpf were distributed into 6-well microplates, 30 zebrafish per well in 3 mL fish water. Furanodiene at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC) were added to the treatment solution for treatment period of 24 h. After treatment, zebrafish were stained with acridine orange and observed for apoptotic cells that would display yellow-green fluorescent spots under the fluorescence microscope. Nikon NIS-Elements D 3.10 Advanced image processing software was used to capture and analyze the images. The fluorescence signal from apoptotic cells was measured and the apoptotic rate was calculated as reported by us58.
Caspase activity assay
After treatment, caspase activities were measured using caspase-Glo reagents (Promega, USA) through cleavage of colorless substrates specific for caspase 3/7 caspase 8 and caspase 9 using a multifunction microplate reader (Berthold Technologies, Mithras LB940, Germany). In each assay, at least six wells per sample were measured for each dose and the results were averaged59.
Pgp assays
Functional analysis
Based on retention of rhodamine 123 in the whole zebrafish, we developed an in vivo assay for assessing drug effects on Pgp efflux in zebrafish (our published patent: China patent No. 201110231726.2). In these studies, we determined that rhodamine 123 staining for 40 min was the optimal time point for assessing Pgp efflux in untreated and drug treated animals. AB wild type zebrafish at 6 hpf were distributed into 6-well microplates (Nest, NEST Biotech), 30 zebrafish per well in 3 mL fish water. Furanodiene at 3 various concentrations (1/9 MTC, 1/3 MTC and MTC) was added to the treatment solution. After 24 h treatment, zebrafish were stained with rhodamine 123 for 40 min, then photographed under a fluorescence microscope. Nikon NIS-Elements D 3.10 software was used to quantify the whole zebrafish fluorescence intensity.
Western blot
Total protein of compound-treated zebrafish was extracted with the Tissue Protein Rapid Extraction Kit (Keygen, China) and determined by performing the bicinchoninic acid (BCA) protein assay (Pierce, Waltham, MA). The equal amounts of 100 mg lysate proteins were loaded onto SDS-polyacrylamide gels (12%) at 80 V and electrophoretically transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA) in Tris-glycine buffer at 100 V in an ice box. After blocking with 5% nonfat milk in TBST for 1 h at room temperature, the membrane was incubated with TNNT2 (1:1000, rabbit antibodies, Abcam, London, UK) overnight at 4 °C, After washed thrice with TBST, the membrane was incubated with a horseradish peroxidase-conjugated anti-rabbit Ig G secondary antibody (Abcam, Britain) for 1 h on a shaking table at room temperature, and washed thrice with TBST and the antibody-bound proteins were detected using the ECL chemiluminescence reagent (Pierce, Waltham, MA).
Quantitative RT-PCR
After treatment, total RNA of the treated zebrafish was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, SA). First-strand cDNA was reversely transcribed from total RNA using a QuantScript RT Kit (TIANGEN, Beijing, China) with Quant Reverse Transcriptase according to the manufacturer’s instruction. The primers were as follows, β-actin: 5′-CATCAGCATGGCTTCTGCTCTGTATGG-3′ and 5′-GACTTGTCAG TGTACAGAGACACCCT-3′; MDR1: 5′-CCTGGCTTCTCAATCTCAT- 3′ and 5′-TTTAC TACTCCCTTGTAACGC-3′. The expression of selected genes was analyzed based on the optical density (OD) of the corresponding bands with β-actin as the internal reference.
Statistical analysis
All data were presented as mean ± SE. Statistical analysis and graphical representation ofthe data were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). If a single concentration of a drug was assessed, the Student’s t-test was used to identify drugs that exhibit a significant effect compared to the vehicle control group. If multiple concentrations of a drug were assessed, ANOVA was first used to assess whether there were any differences in the mean among the various concentrations of each compound; if a significant difference was determined (p < 0.05), Dunnett’s test, which is appropriate for multiple pair-wise comparisons against a control, was then performed.
Acknowledgements
This work was sponsored in part by the Zhejiang Provincial Key Science & Technology Project grant (2014C03009), the National Key Science & Technology Project Grant of the 12th 5-years program of China (2011ZX09301-003). We thank Rick Li at Boston Latin School, Massachusetts, USA for editing this manuscript.
Author Contributions
Chun-Qi Li, Dian-Wu Guo, Zhao-Ke Meng, Hua Yang and Ping Li designed the research; Xiao-Yu Zhu, Qiao-Cong Lao, Yi-Qiao Xu and Bo Xia performed the research; Xiao-Yu Zhu, Qiao-Cong Lao, Yi-Qiao Xu and Bo Xia analyzed the data; Xiao-Yu Zhu and Chun-Qi Li wrote the paper.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun-Qi Li, Email: jackli@zhunter.com.
Ping Li, Email: liping2004@126.com.
References
- 1.Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional chinese medicine to rational cancer therapy. Trends. Mol. Med. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Tan W, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin. Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional chinese medicine in cancer therapy. Mol. Cancer. 2009;8:21. doi: 10.1186/1476-4598-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian G, Guo L, Gao W. Use of compound chinese medicine in the treatment of lung cancer. Curr. Drug Discov. Technol. 2010;7:32–36. doi: 10.2174/157016310791162776. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Penchala S, Prabhu S, Wang J, Huang Y. Molecular basis of traditional chinese medicine in cancer chemoprevention. Curr. Drug Discov. Technol. 2010;7:67–75. doi: 10.2174/157016310791162794. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Xun K, Wang Y, Chen X. A systematic review of the anticancer properties of berberine, a natural product from chinese herbs. Anti-Cancer Drugs. 2009;20:757–769. doi: 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- 7.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 8.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Xia Q, et al. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J. Agric. Food Chem. 2005;53:6019–6026. doi: 10.1021/jf0508495. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, et al. Anti-angiogenesis effect of essential oil from Curcuma zedoaria in vitro and in vivo. J. Ethnopharmacol. 2011;133:220–226. doi: 10.1016/j.jep.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Lim CB, Ky N, Ng HM, Hamza MS, Zhao Y. Curcuma wenyujin extract induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr. Cancer Ther. 2010;9:36–49. doi: 10.1177/1534735409359773. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Y, et al. Essential oil of Curcuma wenyujin induces apoptosis in human hepatoma cells. World J. Gastroenterol. 2008;14:4309–4318. doi: 10.3748/wjg.14.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Nautiyal J, et al. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer. 2011;128:951–961. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang FQ, et al. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2005;39:552–558. doi: 10.1016/j.jpba.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda H, Ninomiya K, Morikawa T, Yoshikawa M. Inhibitory effect and action mechanism of sesquiterpenes from zedoariae rhizoma on-galactosamine/lipopolysaccharide- induced liver injury. Bioorg. Med. Chem. Lett. 1998;8:339–344. doi: 10.1016/S0960-894X(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 17.Makabe H, Maru N, Kuwabara A, Kamo T, Hirota M. Anti-inflammatory sesquiterpenes from Curcuma zedoaria. Nat. Prod. Res. 2006;20:680–685. doi: 10.1080/14786410500462900. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y, et al. Furanodiene induces G(2)/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in human hepatocellular carcinoma cells. Cancer Biol. Ther. 2007;6:1044–1050. doi: 10.4161/cbt.6.7.4317. [DOI] [PubMed] [Google Scholar]
- 19.Zhong ZF, et al. Anti-angiogenic effect of furanodiene on HUVECs in vitro and on zebrafish in vivo. J. Ethnopharmacol. 2012;141:721–727. doi: 10.1016/j.jep.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 20.Lu JJ, et al. Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae–a review. J. Ethnopharmacol. 2012;143:406–411. doi: 10.1016/j.jep.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Xu WS, et al. Furanodiene induces endoplasmic reticulum stress and presents antiproliferative activities in lung cancer cells. Evid. Based Complement Alternat. Med. 2012;2012:426521. doi: 10.1155/2012/426521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma E, et al. Induction of apoptosis by furanodiene in HL60 leukemia cells through activation of TNFR1 signaling pathway. Cancer Lett. 2008;271:158–166. doi: 10.1016/j.canlet.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Sun XY, et al. Potential anti-cancer activities of furanodiene, a Sesquiterpene from Curcuma wenyujin. Am. J. Chin. Med. 2009;37:589–596. doi: 10.1142/S0192415X09007077. [DOI] [PubMed] [Google Scholar]
- 24.Zhong Z, et al. Furanodiene, a natural product, inhibits breast cancer growth both in vitro and in vivo. Cell Physiol. Biochem. 2012;30:778–790. doi: 10.1159/000341457. [DOI] [PubMed] [Google Scholar]
- 25.Zhong ZF, et al. Furanodiene enhances the anti-cancer effects of doxorubicin on ERα-negative breast cancer cells in vitro. Eur. J. Pharmacol. 2016;774:10–19. doi: 10.1016/j.ejphar.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Xu WS, et al. Furanodiene presents synergistic anti-proliferative activity with paclitaxel via altering cell cycle and integrin signaling in 95-D lung cancer cells. Phytother. Res. 2014;28:296–299. doi: 10.1002/ptr.4984. [DOI] [PubMed] [Google Scholar]
- 27.Zhong ZF, et al. Combined effects of furanodiene and doxorubicin on the migration and invasion of MDA-MB-231 breast cancer cells in vitro. Oncol. Rep. 2017;37:2016–2024. doi: 10.3892/or.2017.5435. [DOI] [PubMed] [Google Scholar]
- 28.Rennekamp AJ, Peterson RT. 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 2015;24:58–70. doi: 10.1016/j.cbpa.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao S, Huang J, Ye J. A fresh look at zebrafish from the perspective of cancer research. J. Exp. Clin. Cancer Res. 2015;34:80. doi: 10.1186/s13046-015-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat. Rev. Drug. Discov. 2015;14:721–731. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- 31.Zon J, Miziak P, Amrhein N, Gancarz R. Inhibitors of phenylalanine ammonia-lyase (PAL): Synthesis and biological evaluation of 5-substituted 2-aminoindane-2-phosphonic acids. Chem. Biodivers. 2005;2:1187–1194. doi: 10.1002/cbdv.200590089. [DOI] [PubMed] [Google Scholar]
- 32.Peterson RT, MacRae CA. Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 2012;52:433–453. doi: 10.1146/annurev-pharmtox-010611-134751. [DOI] [PubMed] [Google Scholar]
- 33.Drabsch Y, He S, Zhang L, Snaar-Jagalska BE, ten Dijke P. Transforming growth factor-beta signaling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013;15:R106. doi: 10.1186/bcr3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu, X. Y. et al. Closantel suppresses angiogenesis and cancer growth in zebrafish models. Assay & Drug Dev. Techn. 14 (2016). [DOI] [PubMed]
- 35.Li, C. Q., Luo, L. Q. & McGrath, P. Zebrafish xenotransplant cancer model for drug screening. In P. McGrath (Ed.), Zebrafish: Methods for assessing drug safety and toxicity. West Sussex, UK:Wiley-Balckwell, 219–232 (2011).
- 36.Zhu XY, et al. A zebrafish thrombosis model for assessing antithrombotic drugs. Zebrafish. 2016;13:335. doi: 10.1089/zeb.2016.1263. [DOI] [PubMed] [Google Scholar]
- 37.Li, C. Q., Luo, L. Q., Awerman, J. & McGrath, P. Whole zebrafish cytochrome P450 assay for assessing drug metabolism and safety. In P. McGrath (Ed.), Zebrafish: Methods for assessing drug safety and toxicity. West Sussex, UK: Wiley-Balckwell, 103–115 (2011).
- 38.Holmes AR, et al. Targeting efflux pumps to overcome antifungal drug resistance. Future Med. Chem. 2016;8:1485–1501. doi: 10.4155/fmc-2016-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duan J, et al. Low-dose exposure of silica nanoparticles induces cardiac dysfunction via neutrophil-mediated inflammation and cardiac contraction in zebrafish embryos. Nanotoxicology. 2016;10:575–585. doi: 10.3109/17435390.2015.1102981. [DOI] [PubMed] [Google Scholar]
- 40.Villa E, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. results from a prospective study. Gut. 2016;65:861–869. doi: 10.1136/gutjnl-2014-308483. [DOI] [PubMed] [Google Scholar]
- 41.Aparicio LM, Pulido EG, Gallego GA. Vinflunine: a new vision that may translate into antiangiogenic and antimetastatic activity. Anti-cancer drugs. 2012;23:1–11. doi: 10.1097/CAD.0b013e32834d237b. [DOI] [PubMed] [Google Scholar]
- 42.Furuya K, Kaku Y, Yoshida K, Joh K, Kurosaka D. Therapeutic effects of sunitinib, one of the anti-angiogenetic drugs, in a murine arthritis. Modern Rheumatology. 2014;24:487–491. doi: 10.3109/14397595.2013.844295. [DOI] [PubMed] [Google Scholar]
- 43.De MF, et al. International experts panel meeting of the italian association of thoracic oncology on antiangiogenetic drugs for non-small cell lung cancer: realities and hopes. Journal of Thoracic Oncology. 2016;11:1153–1169. doi: 10.1016/j.jtho.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Research. 2013;33:1793–1797. [PubMed] [Google Scholar]
- 45.Wright C, Milne S, Leeson H. Sperm dna damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reproductive Biomedicine Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 46.El-Amine R, et al. Hiv-1 tat protein induces dna damage in human peripheral blood b-lymphocytes via mitochondrial ros production. Redox Biology. 2018;15:97–108. doi: 10.1016/j.redox.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang E, Huang Y, Du Q, Sun Y. Silver nanoparticle induced toxicity to human sperm by increasing ros(reactive oxygen species) production and dna damage. Environmental Toxicology and Pharmacology. 2017;52:193–199. doi: 10.1016/j.etap.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Kuwana T, et al. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. Journal of Biological Chemistry. 1998;273:16589–94. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 49.Tummers B, Green DR. Caspase-8: regulating life and death. Immunological Reviews. 2017;277:76–89. doi: 10.1111/imr.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pu X, et al. Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival. Apoptosis. 2017;22:1–12. doi: 10.1007/s10495-016-1296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung H, et al. Nlrp3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death & Differentiation. 2016;23:1331–1346. doi: 10.1038/cdd.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochel TJ, Reader JC, Ma X, Kundu N, Fulton AM. Multiple drug resistance-associated protein (mrp4) exports prostaglandin e 2 (pge 2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget. 2017;8:6540–6554. doi: 10.18632/oncotarget.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmes AR, et al. Targeting efflux pumps to overcome antifungal drug resistance. Future Med. Chem. 2016;8:1485–1501. doi: 10.4155/fmc-2016-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckford PD, Sharom FJ. P-glycoprotein (abcb1) interacts directly with lipid-based anti-cancer drugs and platelet-activating factors. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2006;84:1022–33. doi: 10.1139/o06-196. [DOI] [PubMed] [Google Scholar]
- 55.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resistance Updates. 2010;13:16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Qian JQ, Sun P, Pan ZY, Fang ZZ. Annonaceous acetogenins reverses drug resistance of human hepatocellular carcinoma bel-7402/5-fu and hepg2/adm cell lines. Int J Clin Exp Pathol. 2015;8:11934–11944. [PMC free article] [PubMed] [Google Scholar]
- 57.Shaffer BC, et al. Drug resistance: still a daunting challenge to the successful treatment of aml. Drug Resistance Updates. 2012;15:62–69. doi: 10.1016/j.drup.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li CQ, et al. Threshold effects of nitric oxide-induced toxicity and cellular responses in wild-type and p53-null human lymphoblastoid cells. Chem. Res. Toxicol. 2006;19:399–406. doi: 10.1021/tx050283e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou Y, et al. Recombinant disintegrin domain of ADAM15 inhibits the proliferation and migration of Bel-7402 cells. J. Food Sci. Biotechnol. 2013;435:640–645. doi: 10.1016/j.bbrc.2013.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.