Abstract
Although consumers often rely on chemical information to optimize their foraging strategies, it is poorly understood how top carnivores above the third trophic level find resources in heterogeneous environments. Hyperparasitoids are a common group of organisms in the fourth trophic level that lay their eggs in or on the body of other parasitoid hosts. Such top carnivores use herbivore-induced plant volatiles (HIPVs) to find caterpillars containing parasitoid host larvae. Hyperparasitoids forage in complex environments where hosts of different quality may be present alongside non-host parasitoid species, each of which can develop in multiple herbivore species. Because both the identity of the herbivore species and its parasitization status can affect the composition of HIPV emission, hyperparasitoids encounter considerable variation in HIPVs during host location. Here, we combined laboratory and field experiments to investigate the role of HIPVs in host selection of hyperparasitoids that search for hosts in a multi-parasitoid multi-herbivore context. In a wild Brassica oleracea-based food web, the hyperparasitoid Lysibia nana preferred HIPVs emitted in response to caterpillars parasitized by the gregarious host Cotesia glomerata over the non-host Hyposoter ebeninus. However, no plant-mediated discrimination occurred between the solitary host C. rubecula and the non-host H. ebeninus. Under both laboratory and field conditions, hyperparasitoid responses were not affected by the herbivore species (Pieris brassicae or P. rapae) in which the three primary parasitoid species developed. Our study shows that HIPVs are an important source of information within multitrophic interaction networks allowing hyperparasitoids to find their preferred hosts in heterogeneous environments.
Electronic supplementary material
The online version of this article (10.1007/s00442-019-04352-w) contains supplementary material, which is available to authorized users.
Keywords: Hyperparasitoid foraging behavior, Non-host parasitoid species, Fourth trophic level organisms, Multitrophic interactions, Plant-based food web
Introduction
Consumers often forage in heterogeneous environments in which resources of different quality are interspersed among other resources that are nutritionally unsuitable. The efficiency in finding and exploiting nutritionally suitable resources is crucial for maximizing the consumers’ fitness (Charnov 1976; Pyke 1984). The problem of foraging in heterogeneous environments is widespread among consumers structured within food webs; for example, herbivorous and carnivorous insects that are part of plant-based food webs have to find resources which are commonly embedded within larger patches of non-resources (Aartsma et al. 2017, 2019). Herbivores need to find their food plants among a diverse array of non-food plants, whereas carnivores such as parasitoids have to find their herbivore hosts and discriminate between non-infested and infested plants (Bruce and Pickett 2011; De Rijk et al. 2013). To orient towards suitable resources in structurally complex vegetation, herbivorous and carnivorous insects often rely on chemical sources of information among which plant volatiles play a key role (Bruce et al. 2005; Clavijo McCormick et al. 2012; Webster and Cardé 2017). Herbivores have been shown to exploit specific ratios of ubiquitous plant volatiles to locate host–plant species (Bruce et al. 2005; Webster et al. 2010) whereas parasitoids use plant volatiles induced by herbivore attack (HIPVs) as cues for host location (Mumm and Dicke 2010; Clavijo McCormick et al. 2012; Turlings and Erb 2018).
Plant-based food webs usually go beyond the third trophic level (Bukovinszky et al. 2008; Harvey et al. 2009; Frago 2016; Sanders et al. 2016; Seibold et al. 2018). Obligate hyperparasitoids are a common group of insects in the fourth trophic level which lay their eggs in or on the body of other parasitoid hosts. Primary hyperparasitoids develop on parasitoid host larvae whereas secondary hyperparasitoids attack parasitoid prepupae or pupae (Sullivan 1987; Sullivan and Volkl 1999). The foraging behavior of hyperparasitoids has received little attention compared to insects in lower trophic levels, such as herbivores and primary parasitoids. Despite this paucity of information, hyperparasitoids clearly must also deal with several constraints when foraging. For one thing, their primary parasitoid hosts are more scarce (and thus presumably harder to find) than herbivore hosts of primary parasitoids. Moreover, parasitoid host larvae do not feed on plants and, therefore, they are inconspicuous and often concealed within the body of their herbivore hosts (Sullivan and Volkl 1999; Brodeur 2000). Additional challenges faced by hyperparasitoids include the fact that the same herbivore species may be attacked by several parasitoid species which differ in host quality and some of them may not even be suitable for hyperparasitoid offspring development (Harvey 2005). Furthermore, the same parasitoid host may develop on/in different herbivore species. Because of all these challenges, hyperparasitoids clearly need to make the best use of all available information when searching for hosts to optimize their foraging efficiency.
Previous studies show that the hyperparasitoid Lysibia nana can use plant volatiles emitted in response to feeding by parasitized caterpillars to locate their parasitoid hosts and discriminate HIPVs according to the parasitization status of the caterpillar feeding on the plant (Poelman et al. 2012; Zhu et al. 2015). The changes in HIPV composition that allow hyperparasitoids to find their hosts are mainly driven by an alteration in the composition of oral secretions of caterpillar hosts as a result of being parasitized (Poelman et al. 2011; Shikano et al. 2017; Tan et al. 2018; Zhu et al. 2018), which in turn play a key role in herbivore recognition and plant defense signaling (Bonaventure et al. 2011; Bonaventure 2012; Rivera-Vega et al. 2017). Interestingly, the specificity of the parasitoid signature is reflected in the physiology of the caterpillar in such a way that each parasitoid species induces a specific effect on herbivore oral secretions and plant responses to herbivory (Poelman et al. 2011; Zhu et al. 2015; Kaplan et al. 2016; Ode et al. 2016). As a consequence, HIPVs may convey valuable information that hyperparasitoids can use to assess the identity of the parasitoid host and, possibly, even parasitoid host quality and suitability (Poelman and Kos 2016). Yet, how hyperparasitoids forage in complex environments containing hosts and non-hosts has not been explored.
In this study, we used a food web based on wild Brassica oleracea to investigate the foraging behavior of hyperparasitoids in a scenario in which hosts of different quality and non-host parasitoid species develop in different herbivore species feeding on neighboring plants (Fig. 1). As focal hyperparasitoid species we used L. nana which is a specialist attacking pupae of parasitoids in the genus Cotesia. In cabbage fields of The Netherlands, L. nana mainly attacks the gregarious parasitoid Cotesia glomerata and the solitary parasitoid C. rubecula; both parasitoid species parasitize the co-occurring herbivorous caterpillars Pieris brassicae and P. rapae (Geervliet et al. 1998, 2000). However, the solitary parasitoid Hyposoter ebeninus can also attack both P. brassicae and P. rapae and is locally sympatric with C. glomerata and C. rubecula (Feltwell 1982; Poelman et al. 2014). As H. ebeninus is not a suitable resource for L. nana offspring development, it can potentially disrupt HIPV exploitation and limit the hyperparasitoid’s foraging efficiency if no discrimination between hosts and non-host parasitoids is displayed. Furthermore, the two Cotesia species represent hosts of different quality for L. nana in terms of maternal fitness investments. In fact, the gregarious C. glomerata is a high-quality resource because a L. nana female will often parasitize most of the gregarious brood in sequence during a single foraging bout. By contrast, the solitary C. rubecula represents a host of lower quality as it allows only a single reproductive opportunity with the consequence that an L. nana female has to disperse after attacking a parasitized caterpillar.
Fig. 1.
Overview of the four-trophic-level food web used in this study. The hyperparasitoid Lysibia nana attacks cocoons of Cotesia glomerata (CG) and C. rubecula (CR) but it cannot develop in Hyposoter ebeninus (HE) which represents a non-host species for the hyperparasitoid. In turn, each primary parasitoid species can develop in both Pieris brassicae (PB) and P. rape (PR) caterpillars which feed on the wild Brassica oleracea “Kimmeridge” population
Plant-mediated discrimination between hosts of different quality and non-host parasitoid species may also be affected by the herbivore in which parasitoid larvae are developing: because the two Pieris species display different feeding behavior (P. brassicae caterpillars feed gregariously on the plants whereas P. rapae caterpillars feed individually), the way the parasitized herbivore species interact with the plant may differ with consequences for variation in HIPV blends and hyperparasitoid foraging behavior (Poelman et al. 2012).
As previous studies have shown that L. nana exploits HIPVs during host location (Poelman et al. 2012; Zhu et al. 2015), here we tested the hypothesis that HIPVs allow hyperparasitoids to orient themselves in complex environments by conveying information about the identity of the parasitoid species developing in different herbivore species. We combined laboratory and field investigations to specifically address the following questions: (1) whether L. nana hyperparasitoids discriminate between hosts (C. glomerata, C. rubecula) and non-hosts (H. ebeninus) based on HIPVs emitted in response to feeding by parasitized caterpillars; (2) whether the herbivore species (P. brassicae, P. rapae) in which hosts and non-host parasitoid larvae develop induce variation in HIPVs that affect the foraging behavior of L. nana hyperparasitoids.
Materials and methods
Plants and insects
Seeds of the wild Brassica oleracea population ‘Kimmeridge’ (Dorset, UK, 50°360N, 2°070W) were grown in a glasshouse compartment (22 ± 3 °C, 50–70% relative humidity and 16:8 h L:D photoperiod). In the field, the Kimmeridge population is attacked by different herbivores and colonization by Pieris brassicae and P. rapae is frequent (Newton et al. 2010). The Kimmeridge population was selected for this study due to its strong induced responses to Pieris herbivory compared to other B. oleracea populations (Gols et al. 2008).
The herbivores (P. brassicae and P. rapae) and parasitoids (C. glomerata and C. rubecula) were originally collected from field sites near Wageningen University, The Netherlands whereas the colony of H. ebeninus was originally collected as cocoons from cabbage fields near the University of Rennes, France (Harvey et al. 2010). We confirmed the non-host status of H. ebeninus by offering H. ebeninus cocoons to L. nana females. We observed that the hyperparasitoids made only occasional visits to these cocoons and never attempted to oviposit in the cocoons. None of the H. ebeninus cocoons exposed for several days in a cage with L. nana yielded a new generation of L. nana emerging from the cocoons. Unparasitized Pieris species were reared in glasshouse compartments (22 ± 1 °C, 50–70% relative humidity and 16:8 h L:D photoperiod) on cabbage plants (B. oleracea var gemmifera cv. Cyrus). To prepare parasitized caterpillars for the induction treatments, individual first instar P. brassicae or P. rapae caterpillars were exposed to a single female parasitoid (C. glomerata, C. rubecula or H. ebeninus) which was allowed to parasitize the caterpillars in a glass vial. The Pieris caterpillar was considered to be parasitized when the wasp had inserted her ovipositor in the herbivore for at least 5 s in the case of the gregarious C. glomerata (which lays about 15–40 eggs per caterpillar) or for 1 s in the case of the solitary C. rubecula and H. ebeninus (Poelman et al. 2011, 2014). No more than ten caterpillars were offered to a single female parasitoid to avoid possible negative effects caused by depletion of the parasitoid’s egg load. Parasitized caterpillars were reared on cabbage plants until used for induction treatments in laboratory and field experiments.
The hyperparasitoid Lysibia nana was originally recovered from C. glomerata cocoons collected from field sites near Wageningen University, The Netherlands, and was reared on C. glomerata cocoons in the absence of plant- and herbivore-derived cues. When possible, all insect colonies were annually refreshed with field collected material.
Y-tube olfactometer bioassays
Wild B. oleracea plants used for the olfactometer bioassays were 5-weeks-old and treated for 24 h as described by Poelman et al. (2012) before the tests were carried out. Briefly, plants were left undamaged (UD) or infested with either two unparasitized fourth instar P. brassicae (PB) or P. rapae (PR) caterpillars or two-fourth instar Pieris caterpillars that contained fully grown parasitoid larvae of either C. glomerata (CG), C. rubecula (CR) or H. ebeninus (HE) as a result of parasitism of the caterpillar in their first instar. We carried out two parallel groups of olfactometer bioassays according to herbivore identity (i.e., all pairwise combinations using plants induced only with P. brassicae or with P. rapae caterpillars, respectively). The same replicates per experimental day were tested with the different herbivore species.
In a first set of bioassays, we investigated L. nana preferences for plant odors emitted in response to damage by unparasitized caterpillars (PB or PR) or caterpillars parasitized by non-host parasitoids (PB-HE or PR-HE) vs. odor of undamaged control plants (UD). In a second set of bioassays, we investigated whether hyperparasitoids discriminate between plant volatiles induced by caterpillars carrying parasitoid hosts (PB-CG, PB-CR or PR-CG, PR-CR) or non-hosts (PB-HE or PR-HE) over plants damaged by unparasitized caterpillars (PB or PR). In a third set of bioassays, we used only plants damaged by parasitized herbivores to investigate whether L. nana females discriminate the identity of the parasitoid species growing inside the caterpillars based on HIPV composition. Each treatment combination was replicated with seven plant pairs and ten hyperparasitoids per plant pair (n = 70 hyperparasitoid females per treatment).
Shortly before L. nana females were tested for their behavioral response to plant volatiles in Y-tube olfactometer bioassays, we removed caterpillars and their feces from the plants and placed the plants in one of two glass jars (30 l each) that were connected to the two olfactometer arms. A charcoal-filtered airflow (4 l/min) was led through each arm of the Y-tube olfactometer, and a single wasp was released at the base of the stem Section (3.5 cm diameter, 22 cm length) in each test. The Y-tube olfactometer setup was illuminated with four fluorescent tubes (FTD 32 W/84 HF, Pope, The Netherlands). Wasps that passed a set line at the end of one of the olfactometer arms within 10 min and stayed there for at least 15 s were considered to have made a choice. To compensate for unforeseen asymmetry in the setup, we swapped the jars containing the plants after testing five wasps and replaced the set of plants by a new set of plants after testing ten wasps. Each wasp was only used once.
Common garden experiment
Four-week-old plants were transplanted into the field with 1 × 1 m spacing between plants and allowed to adjust to field conditions for 1 week. Thereafter, the plants were subjected to one of the nine following induction treatments: (1) not treated with herbivory (i.e., undamaged controls, UD); (2) infested individually with either two unparasitized first instar P. brassicae (PB) or (3) P. rapae caterpillars (PR); (4) two C. glomerata–parasitized P. brassicae (PB-CG) or (5) P. rapae caterpillars (PR-CG); (6) two C. rubecula–parasitized P. brassicae (PB-CR) or (7) P. rapae caterpillars (PR-CR); (8) two H. ebeninus–parasitized P. brassicae (PB-HE) or (9) P. rapae caterpillars (PR-HE). Unparasitized and parasitized caterpillars were allowed to feed on plants for 10 days, which was approximately the whole development period of the koinobiont endoparasitoid larvae used in this study. Each plant was covered with a fine-mesh net to avoid other herbivore infestations on the plant and to prevent the herbivores used for induction to wander off the plant.
To test the effects of plant induction by different types of herbivory on hyperparasitism, we attached C. glomerata cocoon clutches each consisting of about 20–30 cocoons onto the plants in the field (Poelman et al. 2012). Individual cocoon clutches of C. glomerata were first attached to a piece of cardboard (3 × 3 cm) with a small droplet of glue (HEMA, The Netherlands). We removed nets and caterpillars just before attaching the cardboards carrying the parasitoid pupae with a pin. We attached five cocoon clutches onto each plant. To increase the abundance of L. nana in the field, 100 laboratory-reared females were released at the four cardinal points 3 m away from the edges of the experimental field immediately after the cocoon clutches were attached to the plants. Cocoon clutches were recollected after 5 days of exposure to the hyperparasitoid communities in the field. Subsequently, they were individually kept in the laboratory in 2-mL Eppendorf vials that were closed with cotton wool. The Eppendorf vials were checked daily for emergence of C. glomerata parasitoids and hyperparasitoids. The large majority of the hyperparasitoids were identified to the species level.
A completely randomized design was applied to the field assays. We repeated the experiment five times from May until October 2016, each replicate including 100 plants that included 10 replicates of each treatment (except the UD treatment which had 20 replicates).
Statistical analyses
Hyperparasitoid preferences for herbivore-induced plant volatiles, as tested in two-choice Y-tube olfactometer assays, were analyzed with a Generalized Linear Model (GLM) with a binomial distribution and a logit link function. When overdispersion in the model was detected, a quasi-binomial distribution was fitted. To determine whether there was a significant preference for one of the offered plants within the pairwise combination, we tested H0: logit = 0.
Hyperparasitoid preferences for plant volatiles induced by unparasitized and parasitized caterpillars under field conditions were analyzed using two additional GLMs. In the first model, we tested the caterpillar induction treatment as a factor with 9 levels (UD, PR, PB, PR-CG, PB-CG, PR-CR, PB-CR, PR-HE, PB-HE) including in the GLM also the effect of the replicate (five replicates). In the second model, we included the factors herbivore species (P. rapae or P. brassicae), parasitism effect (C. glomerata, C. rubecula, H. ebeninus, none) and replicate (five replicates) to evaluate the overall effect of parasitism and herbivore identity on hyperparasitism rates. We tested both models, using as response variables the hyperparasitism rates achieved by all species (total hyperparasitism) or by L. nana only. For both GLMs, we analyzed the effects at plant level, by modeling the response variable as a binomial occurrence of hyperparasitism per plant and scored presence of hyperparasitoids in cocoon clutches as “1” and absence as “0”. Additionally, to test the effects at cocoon clutch level, we modeled the response variable as the number of clutches yielding hyperparasitoids out of the fixed totals of five cocoon clutches attached to the plant. Analyses at the plant level provide insight into whether a given treatment is visited more frequently by hyperparasitoids (i.e., attraction preference). Analyses at the cocoon clutch level may provide additional indication of the number of hyperparasitoids that visited an individual plant, or whether in some treatments hyperparasitoids stay longer on the plant to parasitize multiple cocoons (i.e., arrestment preference). Data were analyzed with R statistical software (R Development Core Team 2013).
Results
Y-tube olfactometer bioassays
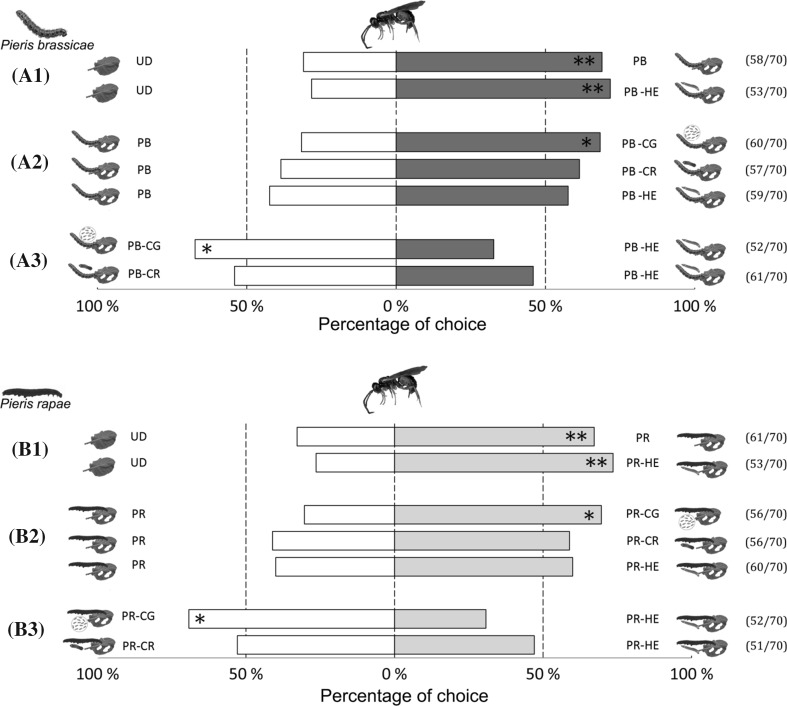
Hyperparasitoids preferred plant volatiles induced by unparasitized Pieris caterpillars over undamaged control plants (GLM, PB: χ2 = 8.34, n = 7, P = 0.0039; PR: χ2 = 7.23, n = 7, P = 0.0072) (Fig. 2a1, b1). Similarly, L. nana females were attracted to HIPVs emitted in response to attack by both P. brassicae and P. rapae caterpillars carrying non-host parasitoid larvae when tested against undamaged control plants (PB: χ2 = 9.98, n = 7, P = 0.0016; PR: χ2 = 11.79, n = 7, P = 0.0006).
Fig. 2.
Preference of Lysibia nana females for herbivore-induced plant volatiles (HIPVs) in two-choice olfactometer tests. Above: olfactometer tests using Pieris brassicae as herbivore species comparing undamaged control plants (UD), P. brassicae-damaged plants (PB), plants damaged by Hyposoter ebeninus-parasitized P. brassicae caterpillars (PB-HE), plants damaged by Cotesia glomerata-parasitized P. brassicae caterpillars (PB-CG), plants damaged by Cotesia rubecula-parasitized P. brassicae caterpillars (PB-CR). Below: olfactometer tests using Pieris rapae as herbivore species comparing undamaged control plants (UD), P. rapae-damaged plants (PR), plants damaged by Hyposoter ebeninus-parasitized P. rapae caterpillars (PR-HE), plants damaged by Cotesia glomerata-parasitized P. rapae caterpillars (PR-CG), plants damaged by Cotesia rubecula-parasitized P. rapae caterpillars (PR-CR). Asterisks indicate a preference which is significantly different from a 50:50 distribution within a choice test (GLM, *P < 0.05, **P < 0.01). Numbers between brackets indicate the number of responding wasps vs. the total number of wasps tested
When hyperparasitoids were offered plant odors induced by parasitized caterpillars over unparasitized caterpillars, discrimination based on the parasitism status of the attacking herbivore only occurred in the case of C. glomerata, regardless of the Pieris species (PB: χ2 = 8.34, n = 7, P = 0.0045; PR: χ2 = 8.64, n = 7, P = 0.0033) (Fig. 2a2, b2). In fact, hyperparasitoids did not discriminate between HIPVs induced by unparasitized Pieris caterpillars vs. HIPVs induced by caterpillars parasitized by the host C. rubecula (PB: χ2 = 2.96, n = 7, P = 0.0851; PR: χ2 = 1.79, n = 7, P = 0.1814) or by the non-host H. ebeninus (PB: χ2 = 1.37, n = 7, P = 0.2413; PR: χ2 = 2.40, n = 7, P = 0.1213).
The hyperparasitoids preferred HIPVs emitted upon herbivory by Pieris caterpillars parasitized by the gregarious host C. glomerata over the non-host H. ebeninus (PB: χ2 = 6.23, n = 7, P = 0.0125; PR: χ2 = 7.69, n = 7, P = 0.0055) (Fig. 2a3, b3). However, L. nana did not discriminate between HIPVs induced by caterpillars carrying the solitary host C. rubecula or the solitary non-host H. ebeninus (PB: χ2 = 0.41, n = 7, P = 0.5221; PR: χ2 = 0.17, n = 7, P = 0.6744).
Common garden experiments
In our field experiment, we recovered a total of five hyperparasitoid species from C. glomerata cocoons among which L. nana was by far the most abundant (recorded in 89.1% of the cases) as shown in the Online Resource 1 of the Electronic Supplementary Material (ESM). The plant induction treatment significantly affected total hyperparasitism rates both at the plant level (Table 1) and at the cocoon clutch level (Online Resource 2, ESM).
Table 1.
The effect of plant induction treatment on the overall hyperparasitism rates achieved on Cotesia glomerata cocoons at the plant level
| Model factor | Deviance | Degrees of freedom | P value |
|---|---|---|---|
| Overall | 494.81 | 499 | |
| Factor | |||
| Induction treatment (1) | 37.889 | 8 | < 0.001 |
| Replicate (2) | 48.207 | 4 | < 0.001 |
| (1) × (2) | 16.863 | 32 | 0.9871 |
Hyperparasitism was modeled as a binomial occurrence of hyperparasitoids (presence = 1, absence = 0) emerging from the five cocoon clutches attached per plant
Parasitism status of the herbivores that induced the plants strongly affected the proportion of hyperparasitized cocoons both at the plant level (Table 2) and at the cocoon clutch level (Online Resource 3, ESM). In contrast, the identity of the Pieris caterpillar feeding on the plant did not have a statistically significant effect. Cocoon clutches that were attached to plants previously induced by C. glomerata–parasitized Pieris caterpillars (CG) were more frequently hyperparasitized than cocoon clutches attached to plants previously damaged by Pieris caterpillars carrying C. rubecula (CR) or H. ebeninus (HE) larvae (Fig. 3). No significant differences between hyperparasitism rates were found for plants previously damaged by C. rubecula-parasitized caterpillars or H. ebeninus-parasitized caterpillars.
Table 2.
The effect of herbivore (Pieris brassicae, P. rapae) and parasitism (Cotesia glomerata, C. rubecula, Hyposoter ebeninus, none) on the overall hyperparasitism rates achieved on C. glomerata cocoons at the plant level
| Model factor | Deviance | Degrees of freedom | P value |
|---|---|---|---|
| Overall | 431.42 | 399 | |
| Factor | |||
| Herbivore species (1) | 0.056 | 1 | 0.8122 |
| Parasitism (2) | 19.01 | 3 | < 0.001 |
| Replicate (3) | 43.471 | 4 | < 0.001 |
| (1) × (2) | 0.899 | 3 | 0.8257 |
| (1) × (3) | 0.543 | 4 | 0.9692 |
| (2) × (3) | 5.355 | 12 | 0.9450 |
| (1) × (2) × (3) | 9.243 | 12 | 0.6820 |
Hyperparasitism was modeled as a binomial occurrence of hyperparasitoids (presence = 1, absence = 0) emerging from the five cocoon clutches attached per plant
Fig. 3.
Percentage of Cotesia glomerata cocoon clutches that contained hyperparasitoids in the field trials either at the plant level (i.e., at least one cocoon clutch out of the five clutches attached to the plant yielded hyperparasitoids) (left) or at the individual clutch level (right). The cocoons were collected from plants that were either left untreated (UD), infested with unparasitized Pieris caterpillars (UNPAR) or parasitized by C. glomerata (CG), C. rubecula (CR) or Hyposoter ebeninus (HE). Dark green bars indicate plant treatments with P. brassicae caterpillars; light green bars indicate treatments with P. rapae caterpillars, white bars indicate undamaged plants. Letters indicate significant differences between treatment groups (GLM, P < 0.05)
Similar results were found when hyperparasitism rates, calculated either at the plant level or at the cocoon clutch level, were restricted only to L. nana (Online Resource 4–8, ESM).
Discussion
To maximize fitness, consumers need to efficiently find suitable resources which in natural environments are often embedded among non-resources. Although foraging strategies of consumers have received considerable attention in plant-based food webs, it is still poorly understood how top carnivores beyond the third trophic level exploit chemical information to locate their resources (Dicke 2009; Poelman and Kos 2016; Aartsma et al. 2019). We found that hyperparasitoids in the fourth trophic level rely on HIPVs to orient themselves in environments containing hosts of different quality as well as non-host parasitoid species which can develop in different herbivore species.
In this study, we investigated a possible multi-parasitoid species scenario that the hyperparasitoid L. nana may experience in brassicaecous fields in The Netherlands. When foraging for hosts, L. nana may encounter Pieris caterpillars parasitized by the gregarious host C. glomerata, the solitary host C. rubecula or the solitary non-host H. ebeninus (Geervliet et al. 2000; Poelman et al. 2014). In laboratory olfactometer bioassays, L. nana females preferred HIPVs induced by C. glomerata-parasitized caterpillars over those emitted by caterpillars parasitized by H. ebeninus, whereas no plant-mediated discrimination occurred between C. rubecula and H. ebeninus. Thus, L. nana females appear to be partially capable of discriminating between hosts and non-hosts based on plant volatile blends released in response to feeding by parasitized caterpillars. The amount of feeding damage inflicted on plants (and thus the quantity of HIPVs released) depends on the parasitism status as well as by parasitoid identity of the attacking caterpillars (Poelman et al. 2011; Cusumano et al. 2018). Yet, bioassays using mechanically treated plants to standardize the amount of damage across treatments have shown the key role played by caterpillar oral secretions on foraging behavior of L. nana (Poelman et al. 2012). Thus, hyperparasitoid responses towards HIPVs observed in our study are likely mediated by changes induced by parasitism on composition of caterpillar oral secretions rather than quantitative effects due to differential feeding damage. Chemical analyses of HIPVs induced by parasitized and unparasitized caterpillars in previous studies identified that indeed the composition of HIPVs differs for the parasitism status of the caterpillars and includes variation induced by the identity of the parasitoid species developing inside the caterpillar (Poelman et al. 2012). Future studies should be carried out to identify how non-host parasitoids induce changes in HIPVs to provide a better understanding on the mechanisms behind L. nana foraging behavior. The preference for HIPVs induced by caterpillars carrying C. glomerata larvae may suggest that host gregarious development is an important trait affecting hyperparasitoid foraging behavior in environments where hosts of different quality co-occur with non-hosts. As hyperparasitoids often face many constraints during the host location process, finding enough resources to sustain the next generation can be challenging: in these situations, adopting a foraging strategy that is finely tuned to target hosts that maximize maternal fitness investments can be adaptive. In the field, complete exploitation of C. glomerata broods by L. nana females is common and the egg load of L. nana closely matches the brood size of C. glomerata (15–40 parasitoid larvae/caterpillar) indicating that hyperparasitoid egg load may have evolved to exploit gregarious hosts such as C. glomerata (Harvey 2005; Poelman et al. 2012). However, because the host C. glomerata and the non-host H. ebeninus differ in many traits including developmental lifestyles, how host gregariousness affects L. nana discrimination between parasitoid hosts and non-hosts needs to be further investigated. Unfortunately, in the Brassica-based food web where this study has been carried out, we were not able to find a hymenopteran parasitoid species which is a non-host for L. nana and develops gregariously in Pieris caterpillars.
Lysibia nana females did not discriminate between the solitary host C. rubecula and solitary non-host H. ebeninus based on HIPVs induced by parasitized caterpillars. While no other study has yet focused on plant-mediated discrimination between hosts and non-hosts in hyperparasitoids, similar findings have been found for insect parasitoids at the third trophic level. For example, de Rijk et al. (2013) reviewed 26 studies on this topic and found that in 50% of the studies, parasitoids did not discriminate between plants infested with host herbivores or non-host herbivores; thus, non-hosts have potential to reduce foraging efficiency for both parasitoids at the third trophic level and hyperparasitoids at the fourth trophic level. Nonetheless, the similar responses displayed by L. nana towards HIPVs emitted by C. rubecula-parasitized caterpillars and H. ebeninus-parasitized caterpillars may be due to different and non-mutually exclusive, ecological effects. First, olfactometer bioassays and field hyperparasitism rates both hint that C. rubecula may be inconspicuous to L. nana. In our laboratory bioassays, hyperparasitoid responses to HIPVs emitted by C. rubecula-parasitized caterpillars are similar to unparasitized caterpillars; furthermore, overall hyperparasitism rates as well as mortality inflicted by L. nana on C. rubecula are low compared with C. glomerata, at least in cabbage fields located in The Netherlands (Poelman et al. 2012). In addition, it is also possible that L. nana can discriminate between C. rubecula-parasitized caterpillars and H. ebeninus-parasitized caterpillars after landing on the plant. Hyperparasitoids may use body odors of parasitized caterpillars as well as waste products associated with parasitized herbivores (such as honeydew of parasitized aphids) for host localization at the short-range distance (Buitenhuis et al. 2004, 2005; Zhu et al. 2014).
In the field, the pattern of hyperparasitism was similar at the plant and cocoon clutch levels as the highest hyperparasitism rates were consistently found on plants previously induced by C. glomerata-parasitized caterpillars. Since plant and cocoon results show a very similar picture, they likely indicate an effect in terms of hyperparasitoid attraction to plant odors, rather than parasitoid arrestment with more frequent ovipositions.
Interestingly, the Pieris species in which the different parasitoid species were developing neither affected hyperparasitoid responses to HIPVs, nor field hyperparasitism rates (i.e., cocoon visits, plant visits). It is known that koinobiont endoparasitoids (such as C. glomerata, C. rubecula and H. ebeninus) affect physiology and metabolism of their herbivore hosts in ways that benefit the parasitoid offspring (Pennacchio and Strand 2006). Evidence is accumulating that host regulation not only affects the caterpillar host but also extends to the herbivore food plant via alterations of the oral secretions of parasitized caterpillars (Poelman et al. 2011; Kaplan et al. 2016; Shikano et al. 2017; Mason et al. 2018). As a conclusion, parasitism can override herbivore identity in terms of plant defense responses and HIPV emission. Indeed, Zhu et al. (2015) showed that L. nana females locate C. glomerata larvae equally well when developing in P. brassicae and in P. rapae caterpillars. Our study extends these findings to other host and non-host species that may be encountered under natural conditions by L. nana, suggesting that different parasitoid species may rely on similar mechanisms to override herbivore identity at the plant–insect interface. Remarkably, the parasitoid species used in this study do not only inject eggs into their caterpillar hosts but also species-specific polydnaviruses (CgBV, CrBV, or HeIV) which, in addition to regulating host growth, also suppress the immune responses of the herbivores allowing parasitoid offspring to develop (Strand and Burke 2013; Doremus et al. 2014; Drezen et al. 2014). Recent findings have shown that polydnaviruses and not the parasitoid larvae developing within the herbivore body are the major drivers of specific changes induced by parasitism in plant responses including HIPV emission (Cusumano et al. 2018; Tan et al. 2018; Zhu et al. 2018). Future studies should test the hypothesis that the polydnaviruses associated with the parasitoid species studied here are indeed major hidden players that allow L. nana to exploit HIPVs in a multi-parasitoid multi-herbivore scenario.
The preferences displayed by L. nana in olfactometer bioassays matched hyperparasitism rates (cocoon visits and plant visits) found in common garden experiments. On the one hand, this finding suggests that hyperparasitoid foraging responses obtained in laboratory settings are ecologically meaningful in more complex conditions which better approximate the natural environments where multiple trophic level interactions obviously evolved. On the other hand, this finding hints at a possible direct link between HIPV response and host exploitation efficiency in hyperparasitoid species. The differences in behavioral responses displayed by L. nana in the olfactometer towards plant volatiles emitted by C. glomerata-parasitized caterpillars and C. rubecula-parasitized caterpillars may explain why, in The Netherlands at least, L. nana is the main hyperparasitoid species attacking pupae of C. glomerata while it is much less frequently associated with C. rubecula (Poelman et al. 2012). Nonetheless, L. nana offspring perform better on C. rubecula than on C. glomerata as the higher pupal mass of the solitary host provides more resources for the hyperparasitoid development suggesting a trade-off between cumulative maternal fitness and per capita offspring fitness. Yet, the main hyperparasitoids recorded on C. rubecula are primary species that attack parasitoid larvae such as Mesochorus gemellus and Baryscapus galactopus whereas secondary species that oviposit in parasitoid pupae such as L. nana are less common (Poelman et al. 2012); this finding could be related to the fact that primary species attack hosts earlier than secondary species and this headstart in resource exploitation is known to often confer a competitive advantage (Harvey et al. 2013; Cusumano et al. 2016). However, whether HIPVs play a role in interspecific competition and resource partition among hyperparasitoid species is unknown.
In terrestrial food webs, plant volatiles are an important source of information which help foraging insects to navigate among complex environments to find suitable resources (Stam et al. 2014). Many studies have shown how plant volatiles are exploited by herbivorous and carnivorous insects to locate hosts, avoid unsuitable resources and detect the presence of competitors (Turlings and Wäckers 2004; Fatouros et al. 2005, 2012). Here, we have shown that plant volatiles are also an important source of information that organisms at the fourth trophic level can use to forage in complex environments where hosts of different quality may be present alongside with non-host resources. Thus, our study contributes to a better understanding of the ecological role that plant volatiles play in structuring species interaction towards the end of the trophic webs.
Hyperparasitoid impacts in basic and applied ecology are likely to be underestimated because these top carnivores are understudied. Increasing our knowledge on hyperparasitoids’ foraging behavior, including the cues they use when foraging in complex environments, is crucial to understand the conditions under which hyperparasitoids can disrupt top-down regulation leading to herbivore outbreaks (Nenzén et al. 2018) and failure of biological pest control programs (Tougeron and Tena 2019).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Léon Westerd, André Gidding and Frans van Aggelen for culturing insects and the experimental farm of Wageningen University (Unifarm) for rearing plants. Field assistance by Gabriel Joachim was appreciated. AC acknowledges funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie Grant agreement No. 655178. EHP was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 677139).
Author contribution statement
AC MD and EHP conceived and designed the experiments. AC performed the experiments. AC analyzed the data. AC, JAH, MD and EHP wrote the manuscript.
References
- Aartsma Y, Bianchi F, van der Werf W, Poelman EH, Dicke M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017;216:1054–1063. doi: 10.1111/nph.14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma Y, Cusumano A, Fernández de Bobadilla M, Rusman Q, Vosteen I, Poelman EH. Understanding insect foraging in complex habitats by comparing trophic levels: insights from specialist host-parasitoid-hyperparasitoid systems. Curr Opin Insect Sci. 2019;32:54–60. doi: 10.1016/j.cois.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Bonaventure G. Perception of insect feeding by plants. Plant Biol. 2012;14:872–880. doi: 10.1111/j.1438-8677.2012.00650.x. [DOI] [PubMed] [Google Scholar]
- Bonaventure G, van Doorn A, Baldwin IT. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011;16:294–299. doi: 10.1016/j.tplants.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Brodeur J. Host specificity and trophic relationships of hyperparasitoids. In: Hochberg ME, Ives AR, editors. Parasitoid population biology. New Jersey: Princeton University Press; 2000. pp. 163–183. [Google Scholar]
- Bruce TJA, Pickett JA. Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochemistry. 2011;72:1605–1611. doi: 10.1016/j.phytochem.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Bruce TJA, Wadhams LJ, Woodcock CM. Insect host location: a volatile situation. Trends Plant Sci. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Buitenhuis R, McNeil J, Boivin G, Brodeur J. The role of honeydew in host searching of aphid hyperparasitoids. J Chem Ecol. 2004;30:273–285. doi: 10.1023/B:JOEC.0000017977.39957.97. [DOI] [PubMed] [Google Scholar]
- Buitenhuis R, Vet LEM, Boivin G, Brodeur J. Foraging behaviour at the fourth trophic level: a comparative study of host location in aphid hyperparasitoids. Entomol Exp Appl. 2005;114:107–117. doi: 10.1111/j.1570-7458.2005.00234.x. [DOI] [Google Scholar]
- Bukovinszky T, van Veen FF, Jongema Y, Dicke M. Direct and indirect effects of resource quality on food web structure. Science. 2008;319:804–807. doi: 10.1126/science.1148310. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Optimal foraging: the marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-X. [DOI] [PubMed] [Google Scholar]
- Clavijo McCormick A, Unsicker SB, Gershenzon J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012;17:303–310. doi: 10.1016/j.tplants.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Cusumano A, Peri E, Colazza S. Interspecific competition/facilitation among insect parasitoids. Curr Opin Insect Sci. 2016;14:12–16. doi: 10.1016/j.cois.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Cusumano A, Zhu F, Volkoff AN, Verbaarschot P, Bloem J, Vogel H, Dicke M, Poelman EH. Parasitic wasp-associated symbiont affects plant-mediated species interactions between herbivores. Ecol Lett. 2018;21:957–967. doi: 10.1111/ele.12952. [DOI] [PubMed] [Google Scholar]
- de Rijk M, Dicke M, Poelman EH. Foraging behaviour by parasitoids in multiherbivore communities. Anim Behav. 2013;85:1517–1528. doi: 10.1016/j.anbehav.2013.03.034. [DOI] [Google Scholar]
- Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- Doremus T, Darboux I, Cusson M, Ravallec M, Jouan V, Frayssinet M, Stoltz D, Webb B, Volkoff AN. Specificities of ichnoviruses associated with campoplegine wasps: genome, genes and role in host–parasitoid interaction. Curr Opin Insect Sci. 2014;6:44–51. doi: 10.1016/j.cois.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Drezen JM, Chevignon G, Louis F, Huguet E. Origin and evolution of symbiotic viruses associated with parasitoid wasps. Curr Opin Insect Sci. 2014;6:35–43. doi: 10.1016/j.cois.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Fatouros NE, van Loon JJA, Hordijk KA, Smid HM, Dicke M. Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J Chem Ecol. 2005;31:2033–2047. doi: 10.1007/s10886-005-6076-5. [DOI] [PubMed] [Google Scholar]
- Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJ, Dicke M, Harvey JA, Gols R, Huigens ME. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One. 2012;7:e43607. doi: 10.1371/journal.pone.0043607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltwell J. Large white butterfly: the biology, biochemistry, and physiology of Pieris brassicae (Linnaeus) London: Dr W. Junk publishers; 1982. [Google Scholar]
- Frago E. Interactions between parasitoids and higher order natural enemies: intraguild predation and hyperparasitoids. Curr Opin Insect Sci. 2016;14:81–86. doi: 10.1016/j.cois.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Geervliet JBF, Ariens S, Dicke M, Vet LEM. Long-distance assessment of patch profitability through volatile infochemicals by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae) Biol Control. 1998;11:113–121. doi: 10.1006/bcon.1997.0585. [DOI] [Google Scholar]
- Geervliet JBF, Verdel MSW, Snellen H, Schaub J, Dicke M, Vet LEM. Coexistence and niche segregation by field populations of the parasitoid Cotesia glomerata and C. rubecula in the Netherlands: predicting field performance from laboratory data. Oecologia. 2000;124:155–163. doi: 10.1007/s004420050024. [DOI] [PubMed] [Google Scholar]
- Gols R, Wagenaar R, Bukovinszky T, Dam NM, Dicke M, Bullock JM, Harvey JA. Genetic variation in defense chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology. 2008;89:1616–1626. doi: 10.1890/07-0873.1. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol Exp Appl. 2005;117:1–13. doi: 10.1111/j.1570-7458.2005.00348.x. [DOI] [Google Scholar]
- Harvey JA, Wagenaar R, Bezemer TM. Interactions to the fifth trophic level: secondary and tertiary parasitoid wasps show extraordinary efficiency in utilizing host resources. J Anim Ecol. 2009;78:686–692. doi: 10.1111/j.1365-2656.2008.01516.x. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Poelman EH, Gols R. Development and host utilization in Hyposoter ebeninus (Hymenoptera: Ichneumonidae), a solitary endoparasitoid of Pieris rapae and P. brassicae caterpillars (Lepidoptera: Pieridae) Biol Control. 2010;53:312–318. doi: 10.1016/j.biocontrol.2010.02.004. [DOI] [Google Scholar]
- Harvey JA, Poelman EH, Tanaka T. Intrinsic inter-and intraspecific competition in parasitoid wasps. Annu Rev Entomol. 2013;58:333–351. doi: 10.1146/annurev-ento-120811-153622. [DOI] [PubMed] [Google Scholar]
- Kaplan I, Carrillo J, Garvey M, Ode PJ. Indirect plant–parasitoid interactions mediated by changes in herbivore physiology. Curr Opin Insect Sci. 2016;14:112–119. doi: 10.1016/j.cois.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Mason CJ, Jones AG, Felton GW. Co-option of microbial associates by insects and their impact on plant-folivore interactions. Plant Cell Environ. 2018 doi: 10.1111/pce.13430. [DOI] [PubMed] [Google Scholar]
- Mumm R, Dicke M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool. 2010;88:628–667. doi: 10.1139/Z10-032. [DOI] [Google Scholar]
- Nenzén HK, Martel V, Gravel D. Can hyperparasitoids cause large-scale outbreaks of insect herbivores? Oikos. 2018;127:1344–1354. doi: 10.1111/oik.05112. [DOI] [Google Scholar]
- Newton E, Bullock JM, Hodgson D. Temporal consistency in herbivore responses to glucosinolate polymorphism in populations of wild cabbage (Brassica oleracea) Oecologia. 2010;164:689–699. doi: 10.1007/s00442-010-1702-5. [DOI] [PubMed] [Google Scholar]
- Ode PJ, Harvey JA, Reichelt M, Gershenzon J, Gols R. Differential induction of plant chemical defenses by parasitized and unparasitized herbivores: consequences for reciprocal, multitrophic interactions. Oikos. 2016;125:1398–1407. doi: 10.1111/oik.03076. [DOI] [Google Scholar]
- Pennacchio F, Strand MR. Evolution of developmental strategies in parasitic Hymenoptera. Annu Rev Entomol. 2006;51:233–258. doi: 10.1146/annurev.ento.51.110104.151029. [DOI] [PubMed] [Google Scholar]
- Poelman EH, Kos M. Complexity of plant volatile-mediated interactions beyond the third trophic level. In: Blande JD, Glinwood R, editors. Deciphering chemical language of plant communication. Switzerland: Springer; 2016. pp. 211–225. [Google Scholar]
- Poelman EH, Zheng SJ, Zhang Z, Heemskerk NM, Cortesero AM, Dicke M. Parasitoid-specific induction of plant responses to parasitized herbivores affects colonization by subsequent herbivores. Proc Natl Acad Sci USA. 2011;108:19647–19652. doi: 10.1073/pnas.1110748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelman EH, Bruinsma M, Zhu F, Weldegergis BT, Boursault AE, Jongema Y, van Loon JJ, Vet LE, Harvey JA, Dicke M. Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol. 2012;10:e1001435. doi: 10.1371/journal.pbio.1001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelman EH, Gols R, Gumovsky AV, Cortesero AM, Dicke M, Harvey JA. Food plant and herbivore host species affect the outcome of intrinsic competition among parasitoid larvae. Ecol Entomol. 2014;39:693–702. doi: 10.1111/een.12150. [DOI] [Google Scholar]
- Pyke GH. Optimal foraging theory: a critical review. Annu Rev Ecol Syst. 1984;15:523–575. doi: 10.1146/annurev.es.15.110184.002515. [DOI] [Google Scholar]
- R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 8 Sept 2018
- Rivera-Vega LJ, Acevedo FE, Felton GW. Genomics of lepidoptera saliva reveals function in herbivory. Curr Opin Insect Sci. 2017;19:61–69. doi: 10.1016/j.cois.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Sanders D, Moser A, Newton J, van Veen FF. Trophic assimilation efficiency markedly increases at higher trophic levels in four-level host–parasitoid food chain. Proc R Soc B. 2016;283:20153043. doi: 10.1098/rspb.2015.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold S, Cadotte MW, MacIvor JS, Thorn S, Müller J. The necessity of multitropic approaches in community ecology. Trends Ecol Evol. 2018;33:754–764. doi: 10.1016/j.tree.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Shikano I, Rosa C, Tan CW, Felton GW. Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annu Rev Phytopathol. 2017;55:313–331. doi: 10.1146/annurev-phyto-080516-035319. [DOI] [PubMed] [Google Scholar]
- Stam JM, Kroes A, Li Y, Gols R, van Loon JJ, Poelman EH, Dicke M. Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol. 2014;65:689–713. doi: 10.1146/annurev-arplant-050213-035937. [DOI] [PubMed] [Google Scholar]
- Strand MR, Burke GR. Polydnavirus-wasp associations: evolution, genome organization, and function. Curr Opin Virol. 2013;3:587–594. doi: 10.1016/j.coviro.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Sullivan DJ. Insect hyperparasitism. Annu Rev Entomol. 1987;32:49–70. doi: 10.1146/annurev.en.32.010187.000405. [DOI] [Google Scholar]
- Sullivan DJ, Volkl W. Hyperparasitism: multitrophic ecology and behavior. Annu Rev Entomol. 1999;44:291–315. doi: 10.1146/annurev.ento.44.1.291. [DOI] [PubMed] [Google Scholar]
- Tan CW, Peiffer M, Hoover K, Rosa C, Acevedo FE, Felton GW. Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. Proc Natl Acad Sci USA. 2018;115:5199–5204. doi: 10.1073/pnas.1717934115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tougeron K, Tena A. Hyperparasitoids as new targets in biological control in a global change context. Biol Control. 2019 [Google Scholar]
- Turlings TCJ, Erb M. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol. 2018;63:433–452. doi: 10.1146/annurev-ento-020117-043507. [DOI] [PubMed] [Google Scholar]
- Turlings T, Wäckers F. Recruitment of predators and parasitoids by herbivore-damaged plants. In: Cardé RT, Millar J, editors. Advances in insect chemical ecology. Cambridge: Cambridge University Press; 2004. pp. 21–75. [Google Scholar]
- Webster B, Cardé RT. Use of habitat odour by host-seeking insects. Biol Rev. 2017;92:1241–1249. doi: 10.1111/brv.12281. [DOI] [PubMed] [Google Scholar]
- Webster B, Bruce T, Pickett J, Hardie J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim Behav. 2010;79:451–457. doi: 10.1016/j.anbehav.2009.11.028. [DOI] [Google Scholar]
- Zhu F, Weldegergis BT, Lhie B, Harvey JA, Dicke M, Poelman EH. Body odors of parasitized caterpillars give away the presence of parasitoid larvae to their primary hyperparasitoid enemies. J Chem Ecol. 2014;40:986–995. doi: 10.1007/s10886-014-0500-7. [DOI] [PubMed] [Google Scholar]
- Zhu F, Broekgaarden C, Weldegergis BT, Harvey JA, Vosman B, Dicke M, Poelman EH. Parasitism overrides herbivore identity allowing hyperparasitoids to locate their parasitoid host by using herbivoreinduced plant volatiles. Mol Ecol. 2015;24:2886–2899. doi: 10.1111/mec.13164. [DOI] [PubMed] [Google Scholar]
- Zhu F, Cusumano A, Bloem J, Weldegergis BT, Villela A, Fatouros NE, van Loon JJA, Dicke M, Harvey JA, Vogel H, Poelman EH. Symbiotic polydnavirus and venom reveal parasitoid to its hyperparasitoids. Proc Natl Acad Sci USA. 2018;115:5205–5210. doi: 10.1073/pnas.1717904115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.