Abstract
Low-frequency oscillations (LFOs) in hemodynamics assessed by fMRI reflect synchronized neuronal activities and are the basis for mapping brain function and its disruption by drugs and disease. Here we assess if cocaine disrupts coupling between neuronal and vascular LFOs by simultaneously measuring cortical field potentials (FP) and cerebral blood flow (CBF) regarding their LFOs (0–1 Hz) spectral bandwidths in the somatosensory cortex of naïve and chronic cocaine-exposed rats at baseline and during cocaine intoxication. While across all conditions the dominant oscillation frequencies for FP and CBF LFOs were ~0.1 Hz, the bandwidth of FP LFOs was about 4.8 ± 0.67 times broader than that of CBF LFOs. Acute cocaine depressed high-frequency FP events but increased the relative intensity of neuronal and hemodynamic LFOs, an effect that was markedly accentuated in magnitude and duration in chronic cocaine-exposed animals. Neuronal LFOs were correlated with CBF LFOs in control animals but not in chronically cocaine-exposed animals, which suggests neurovascular uncoupling. The marked increases in neuronal LFOs with chronic cocaine, which we interpret to reflect increases in neuronal synchronization in the LFOs, and the uncoupling of hemodynamics with resting neuronal activities could contribute to brain dysfunction in cocaine abusers and confound the interpretation of fMRI studies.
Keywords: cocaine, cortical hemodynamic oscillation, low-frequency oscillation, neurovascular coupling, spontaneous neuronal activity
Introduction
Low-frequency oscillations (LFOs) in spontaneous neuronal activity and cerebral hemodynamic fluctuations have been widely observed by different imaging modalities, including Blood Oxygen Level Dependent (BOLD) signals using fMRI (Fransson 2005), oxy- and deoxyhemoglobin levels (HbO2 and HbR) using optical intrinsic signal imaging (OISI) (Du et al. 2014; Rayshubskiy et al. 2014), intracellular calcium (Ca2+) oscillations using optical fluorescence imaging (OFI) (Pasti et al. 1997; Du et al. 2014), and local field potentials (FP) using electrophysiological recording (e.g., EEG) (Obrig et al. 2000; Buzsáki and Draguhn 2004; Rayshubskiy et al. 2014). Generally, it is hypothesized that hemodynamic LFOs reflect neuronal activity through neurovascular coupling, which underlies the basis for fMRI studies that correlate LFOs in BOLD signals between brain regions to map resting functional connectivity (Biswal et al. 1995; Fair et al. 2007; Raichle and Snyder 2007). A very recent study by single electrode recording and OISI showed that resting-state hemodynamic oscillations were regionally coupled to the multiunit activity (MUA) of a neuron population (Ma et al. 2016). However, neurovascular coupling might be modified by psychoactive drugs and disrupted in substance use disorders, such as cocaine use disorders (Li et al. 2000; Chen, Liu et al. 2016).
Cocaine, which is one of the most commonly abused drugs, impairs neuronal function (Goldstein and Volkow 2011) and disrupts the cerebral circulation (Volkow et al. 1988). The dual effects of cocaine on neuronal and vascular systems are likely to contribute to its neurotoxicity (You et al. 2017) and are a confound when interpreting functional brain imaging results that are based on cerebral hemodynamics, such as BOLD and optical intrinsic signals. For example, fMRI showed that acute cocaine in cocaine abusers decreased functional connectivity in primary visual and motor cortices (Li et al. 2000). However, due to the complexity of the BOLD signal, that is, a mixture of multiple hemodynamic components (e.g., CBF, cerebral blood volume, HbO2, and HbR) (Biswal et al. 1997; Elwell et al. 1999), the effects of cocaine on neuronal oscillations are difficult to discriminate from those on hemodynamic fluctuations. Some studies have shown that acute cocaine changes neuronal firing rates and patterns (Ruskin et al. 2001; Shi et al. 2004), which could underlie the observed changes in hemodynamic fluctuations. However, other studies have observed disparate neuronal and hemodynamic responses to external sensory stimulation during cocaine intoxication (Gollub et al. 1998; Chen, Liu et al. 2016) that suggested decoupling of neuronal and vascular reactivity after acute cocaine exposure. To assess if cocaine disrupts neurovascular coupling we simultaneously measured LFOs of neuronal activity and hemodynamics in the somatosensory cortex of naïve and chronic cocaine-exposed (2 weeks) rats at baseline and after acute cocaine and compared them with those in control animals after saline administration.
Cortical FP was measured using a single electrode placed on the somatosensory cortex to track the synchronized activity from a localized neuronal population (Uhlhaas and Singer 2006) and CBF was measured using laser Doppler flowmetry (LDF) in the same cortical region (Fredriksson et al. 2009). In this study, the neuronal activity and CBF fluctuations were simultaneously recorded prior to and after acute cocaine (naïve and chronic cocaine rats) or after saline challenge (control rats) in 3 groups of animals (control, naïve, and chronic cocaine exposed). We hypothesized that in: (1) naïve animals, acute cocaine would increase the relative intensity of the neuronal LFOs, which would be coupled with increases in the relative intensity of hemodynamic LFOs; and that in (2) chronic cocaine-exposed animals, acute cocaine would increase neuronal but not hemodynamic LFOs reflecting a disruption of neurovascular coupling.
Methods
Animal Preparation
Male Sprague-Dawley rats (250–350 g, n = 15) were used for this study and divided into 3 groups: controls (pretreated with saline 0.1mL/100 g/day, i.p.), naïve (pretreated with saline 0.1mL/100 g/day, i.p.), and chronic cocaine (pretreated with cocaine 30 mg/kg/day, i.p.) (details in Table 1). Animals were intubated and ventilated at ~45 times/min (i.e., 0.75 Hz) with 1.8–2% isoflurane in 70% O2/air mixture to perform the surgical procedures. The femoral artery was catheterized for continuous arterial blood pressure monitoring. After the surgery, animals were then positioned in a stereotaxic frame (Kopf 900) and the anesthesia was switched to α-chloralose using a bolus of 50 mg/kg followed by continuous infusion of 25 mg/kg/h. The skull over the somatosensory cortex (4 mm×4 mm, AP: +1 to −3 mm; LR: +2 to +6 mm) was thinned to ~90–100 μm using a microdental drill (0.8 mm drill bit, Ideal micro drill; Roboz) and a small hole was created on the thinned skull (AP: −1 mm, LR: +4 mm) for electrocorticography (ECoG) measurements. During the experiments, a bolus of saline (0.1mL/100 g, i.v.) for the controls or cocaine (1 mg/kg, i.v.) for naïve and chronic cocaine groups, was injected via the femoral vein after 10 min of baseline recording. Physiological parameters, including pCO2, heart beat rate (HBR), mean arterial blood pressure (MABP), respiratory rate, and body temperature, were continuously monitored throughout the experiment to ensure physiological conditions within the normal range (e.g., pCO2 30–45 mmHg). The physiological status of animals is provided in Supplemental Table S1. The experimental procedures were approved by the Institutional Animal Care and Use Committees of Stony Brook University.
Table 1.
Animal groups and treatments
| Group | Pretreatment (2 weeks daily) | Drug challenge (during experiment) |
|---|---|---|
| Control (n = 5) | Saline (0.9% NaCl, 0.1 mL/100 g, i.p.) | Saline (0.9% NaCl, 0.1 mL/100 g, i.v.) |
| Naïve (n = 5) | Saline (0.9% NaCl, 0.1 mL/100 g, i.p.) | Cocaine hydrochloride (1 mg/kg, i.v.) |
| Chronic (n = 5) | Cocaine hydrochloride (30 mg/kg, i.p.) | Cocaine hydrochloride (1 mg/kg, i.v.) |
Protocol for Resting-State FP/CBF Recordings
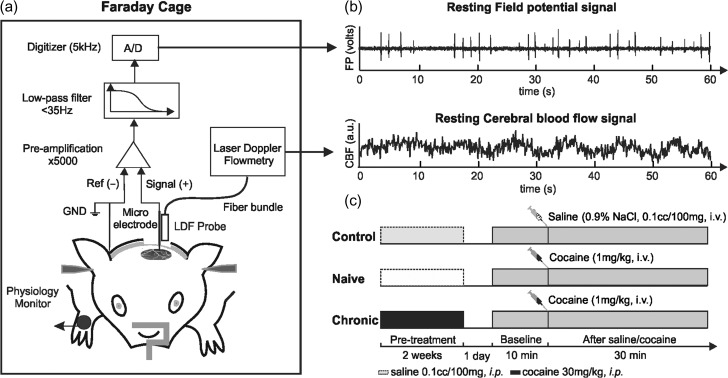
The system setup is shown in Figure 1a. The measurements were performed in a grounded Faraday cage to minimize background noise. For FP measurement, a signal electrode (ϕ = 0.3 mm, EL450, Biopac) was implanted through the skull hole (AP: −1 mm, LR: +4 mm) to reach the cortex (~0.1 mm under the dura) using a stereotaxic frame. A ground electrode (Biopac, EL452) was inserted under the skin in the neck. A differential amplifier (Biopac, MP150/EEG100C) was used to amplify (×5000), low-pass filter (<35 Hz) and digitize the FP signal at 5 kHz. For CBF measurements, a fiberoptic LDF probe (ϕ = 0.8 mm, Moor Instr.) was precisely positioned at the same location (AP: −1 mm LR: +4 mm) and suspended at ~0.1 mm above the cortical surface using a stereotaxic frame. A droplet of mineral oil was applied to optimize light coupling. CBF was sampled at 20 Hz using LDF. Both FP and CBF signals were synchronized and displayed in real-time during the experiment and the data were streamed into a workstation for post processing (Fig. 1b). LDF measures CBF within a ~0.56 mm3 cortical volume (Fredriksson et al. 2009); allowing for CBF fluctuations and neuronal activity to be simultaneously tracked in the same region. Figure 1c illustrates the timeline of pretreatments for the 3 groups. After pretreatments, animals were withdrawn from cocaine/saline administration for 24 h before measurements. During the measurements, the resting FP and CBF were simultaneously measured for 40 min, including a 10 min baseline and a 30 min post cocaine bolus/saline administration measurements.
Figure 1.
System setup for simultaneous measurement of field potential (FP) and cerebral blood flow (CBF) fluctuations in the rat somatosensory cortex in the resting state. (a) Resting-state ECoG/LDF recording performed in the Faraday cage. ECoG, electrocochleography; LDF, laser Doppler flowmetry; A/D, analog to digital converter. (b) Simultaneous measures of FP and CBF signals to assess spontaneous neuronal activities and hemodynamic fluctuations. (c) Pretreatment timeline and FP/CBF measurement protocol.
Extraction of LFOs From FP Events and CBF Fluctuations
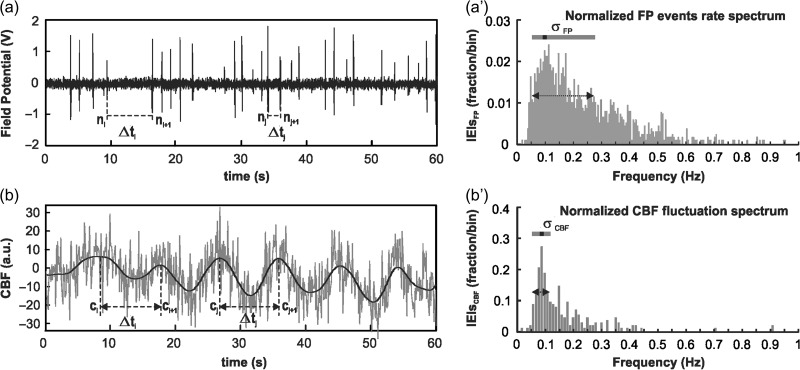
The FP events rate spectrum was calculated from the FP signals, in which each FP event was treated as a synchronized event from a group of neurons (Buzsáki et al. 2012). Based on the events rate spectrum, LFOs in the resting-state can be tracked as a time-variant signal with high spectral and temporal resolutions. As shown in Figure 2a, population neuronal activities (FP events) in the resting state were localized in time per the predefined amplitude threshold (greater than 4 times the standard deviation) and the temporal duration of 100±40ms. Based on the FP event episodes, the interevent-intervals (IEIs), , can be calculated from consecutive events (i.e., the time between and or and ), and the instant FP events rate at the time point is calculated as . The FP events counts were obtained by counting the FP events within a 2 min-wide moving window, which was slid across the FP temporal trace with a 30 s step size.
Figure 2.
Demonstration of extracting LFOs of neuronal activity and CBF fluctuations from ECoG and LDF signals at resting-state. (a) Detection of instant FP events rates by calculating the interevent-intervals between spontaneous FP spikes. (a′) The corresponding FP events rate spectrum of the instant FP events rate normalized by the total FP events number. (b) Detection of instant CBF fluctuations by calculating the time intervals between consecutive CBF fluctuations. (b′) The corresponding CBF fluctuation rate spectrum of the instant CBF fluctuation.
Simultaneously measured CBF fluctuations were similarly analyzed for consistency. The CBF LFO changes were first demonstrated using short-time Fourier transform and then analyzed based on the frequency distribution of instantaneous CBF oscillations, which provides higher sensitivity in the ultralow frequency band. A MATLAB program was developed and optimized to identify the instantaneous CBF oscillation periods and thus their frequencies. The quantification The CBF raw data (gray curve in Fig. 2b) was band-pass-filtered (0.03–1 Hz, zero-phase 3rd order Butterworth filter) first to remove high-frequency noise components and other noninterest frequency components (e.g., respiration at 0.75 Hz, heartbeats at around 5 Hz) without introducing extra phase delays. Supplemental Figure S1 compares Fourier transform spectra of the original CBF signal and filtered CBF signal. A customized peak-detection algorithm in Matlab was applied to find all time intervals () between 2 consecutive oscillations (e.g., between and or and ), and to allow the instant CBF oscillation frequency to be calculated as .
Considering that the change in absolute intensity of LFOs can be different from the change in the relative LFOs intensity (Zou et al. 2008), 2 aspects of LFOs spectra were analyzed: the absolute LFOs spectrum (ALFS) and the normalized LFOs spectrum (NLFS). While the ALFS method presents the absolute intensity change of LFOs in the spectrum, NLFS is able to show the relative LFOs change. Mathematically, the 2 methods can be described as follows:
-
Absolute low frequency–oscillation spectrum (ALFS)
(1) denotes the total counts of the instant FP events or CBF fluctuations within the local frequency range, which is defined by , , where, is the frequency resolution.
Normalized low frequency–oscillation spectrum (NLFS):
| (2) |
By normalizing the intensity of instant FP events rate or CBF fluctuations rate at each frequency to the total intensity through the entire band, the relative change of LFOs can be obtained.
Furthermore, the distribution of LFOs in the spectrum is quantified by its bandwidth, σ, which is obtained by fitting the spectrum to a skewed-Gaussian function (Buzsáki and Mizuseki 2014), as follows:
| (3) |
where is a Gaussian function and depicts the skew property. In the expression, defines the bandwidth. Therefore, by fitting the LFOs spectrum to a skewed-normal distribution, the bandwidth, , can be quantified.
Statistics
The statistical significance of cocaine’s effects in neuronal and hemodynamic LFOs was determined by comparing measurements across experimental groups. Student’s t-test was used for comparison of mean values unless specified. For comparison within a group at different time points, one-way ANOVA was used to analyze the repeated measures and pair-wise comparisons were made to determine if any difference was detected. The numbers of sampling points were also specified in this case. For comparisons of the data distribution between 2 groups, F-test was used to test the change in standard deviation. In all cases, P < 0.05 (two tailed) was considered significant. All numerical data are presented as the mean ± standard error of mean (SEM).
Results
Acute Cocaine Depressed FP Activity More Significantly in Chronically Exposed than in Naïve Animals
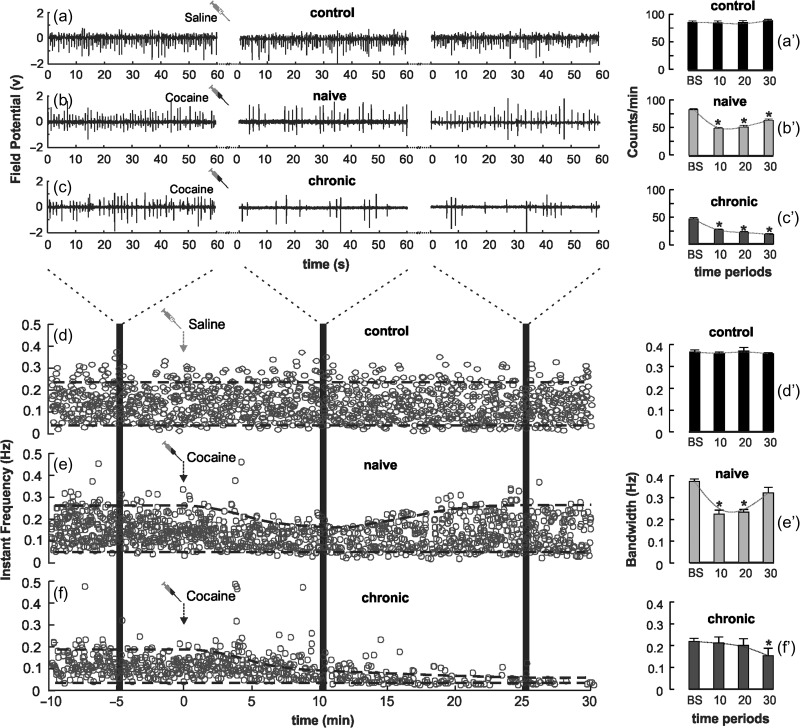
Figure 3 demonstrates typical FP signal changes before and after saline (0.1mL/100 g, i.v.) and cocaine (1 mg/kg, i.v.) for control, naïve, and chronic animals. The 60 s snapshots from different time periods (e.g., 10 min prior and 30 min post cocaine) are presented to show the details of the FP events (Fig. 3a–c). To monitor the dynamic changes in neuronal activity we plotted the instantaneous FP events rate () as a function of time in spot diagrams for the 3 groups in Figure 3d–f.
Figure 3.
(a–c) Demonstration of FP signals for control (a), naïve (b) and chronic cocaine-exposed (c) groups from 5–6 min before (baseline period) to 10 and 25 min after saline or acute cocaine administration; (a′–c′) Bar diagrams of FP events per minute for control (a′), naïve (b′), and chronic (c′) groups. (d–f) Spot diagrams to show FP events rate within 0.01–0.5 Hz for control (d), naïve (e′), and chronic (f) groups before and after saline or cocaine administration. Shaded areas indicate the corresponding time traces in (a–c). Dashed lines delineate the distribution profile of the FP events rate. (d′–f′) Statistic analysis of FP LFOs distribution bandwidth change before and after cocaine for the naïve and chronic groups. *P < 0.05 compared with baseline.
Statistical results are summarized in Figure 3a′–c′. In controls, neither the FP events rate nor the FP events pattern changed after saline injection, that is, 85.8 ± 1.28 counts/min from baseline versus 84.6 ± 1.87 counts/min at 10–20 min after saline (P = 0.859) (Fig. 3a′). In the naïve group, the FP events rate decreased from 82.0 ± 1.61 counts/min at baseline to 48.1 ± 3.26 counts/min at 10–20 min after acute cocaine (P < 0.001), which partially recovered to 63.0 ± 2.15 counts/min (P = 0.042) at 20–30 min (Fig. 3b′). In the chronic group, the baseline FP events rate was 46.1 ± 1.49 counts/min, which was significantly lower to that in control and naïve groups (P < 0.001) and it was further decreased to 21.2 ± 0.93 counts/min (P < 0.001) at 10–20 min after acute cocaine and to 17.6 ± 0.91 counts/min (P < 0.001) at 30 min (Fig. 3c′).
By subtracting between the upper frequency limit () to the lower frequency limit (), the spectral distribution of FP events rate () also revealed significant changes in FP LFOs after acute cocaine between the groups. In controls, there were no changes in FP events rate distribution before ( and after ( saline injection, as shown in Figure 3d′. In the naïve group, the LFOs distribution was narrowed post cocaine injection from ( at baseline to ( at 10 min and ( at 20 min, and gradually restored to ( at 30 min (Fig. 3e,e′). In the chronic group, the baseline LFOs distribution ( was already significantly narrower than the baseline of controls or naïve animals (P < 0.001), and was further decreased after acute cocaine to ( and remained decreased throughout the 30 min post cocaine measurement (Fig. 3f,f′).
Cocaine Reduced High-Frequency FP Events and Increased Low-Frequency FP Oscillations (LFOs at 0.03–0.15 Hz)
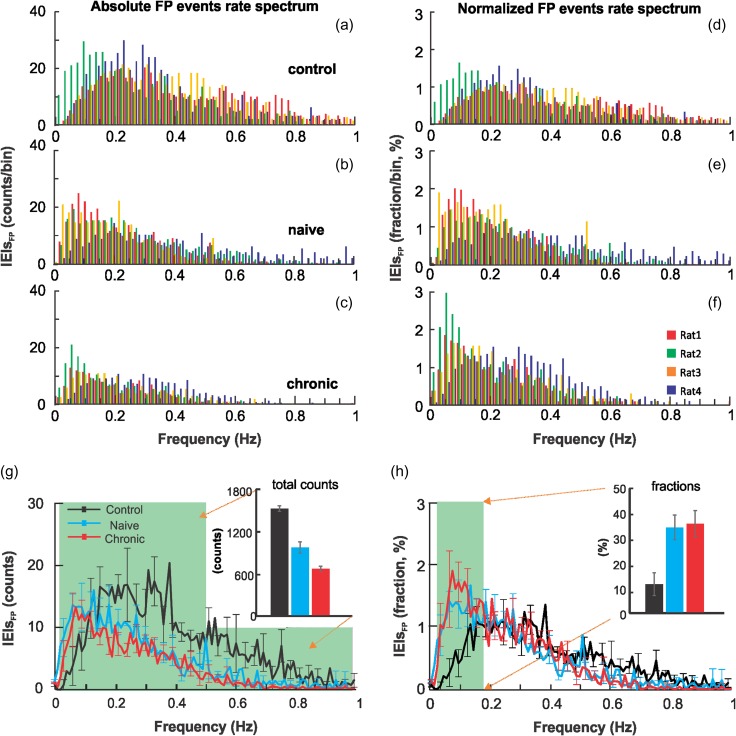
Cocaine’s effects on the spectral properties of neuronal LFOs were quantified as FP events rate spectra based on 30 min FP recordings after saline or cocaine injection with 0.01 Hz frequency resolution. Figure 4a–c shows the absolute FP events rate spectra with frequency obtained from individual animals (superposed in different colors). The normalized FP events spectrum for each animal was calculated by dividing the FP events at each frequency unit by the total events, and expressed as a percentage of total FP events, as shown in Figure 4(d–f). Supplemental Figure S2 presents the spectra for each individual animal (N = 15, n = 5 per group) separately. In the naïve group, the absolute FP events after acute cocaine (982 ± 78.6) were significantly lower than in controls after saline (1510 ± 35.59; P < 0.001), corresponding to a 34.96 ± 5.71% decrease (Fig. 4g). In the chronic cocaine group, the absolute FP events after acute cocaine (676 ± 35.4) were significantly lower than in controls after saline (P < 0.001), corresponding to a 44.76 ± 3.32% decrease (Fig. 4g) and lower than in the naïve group after acute cocaine (P < 0.001).
Figure 4.
(a–c) Absolute FP events rate spectra for animals in the controls after acute saline and in naïve and chronic cocaine groups after acute cocaine. Within each group, the FP events rate spectrum from individual animals (n = 4) are identified using 4 different colors. (d–f) Relative FP events rate spectra for the control, naïve and chronic groups. Relative FP events rate spectra were normalized by the total FP events and presented as the percentage of total events. (g) Bar diagram to compare the total FP events <1 Hz (shaded area) for the 3 groups in unit of absolute FP events counts, that is, 1510 ± 35.59, 982 ± 78.6, and 676 ± 35.4 events for the control, naïve, and chronic groups, respectively. (h) Bar diagram to compare the normalized FP events within the low frequency 0.03–0.15 Hz range (shaded area) in percentage, that is, 13.1 ± 4.1% (control), 35.6 ± 4.8% (naïve), and 36.3 ± 5.1% (chronic). Data from individual animals is illustrated in Supplemental Figure S2.
The normalized FP events within the lower-frequency range of 0.03–0.15 Hz was increased by acute cocaine in the naïve (35.6 ± 4.8%, P = 0.010) and chronic (36.3 ± 5.1%, P = 0.013) groups compared with controls after saline (13.1 ± 4.1%; Fig. 4h). There was no difference between the effects of acute cocaine on the naïve and chronic groups (P = 0.914). These results indicate that acute and chronic cocaine depressed FP events rate, and the decreases were greater for the higher-frequency bands (>0.15 Hz), which accounted for the increases in the relative FP LFOs at 0.03–0.15 Hz spectra.
Cocaine Abolished Higher-Frequency CBF Oscillations and Increased Synchronization of Low-Frequency Oscillatory Activity
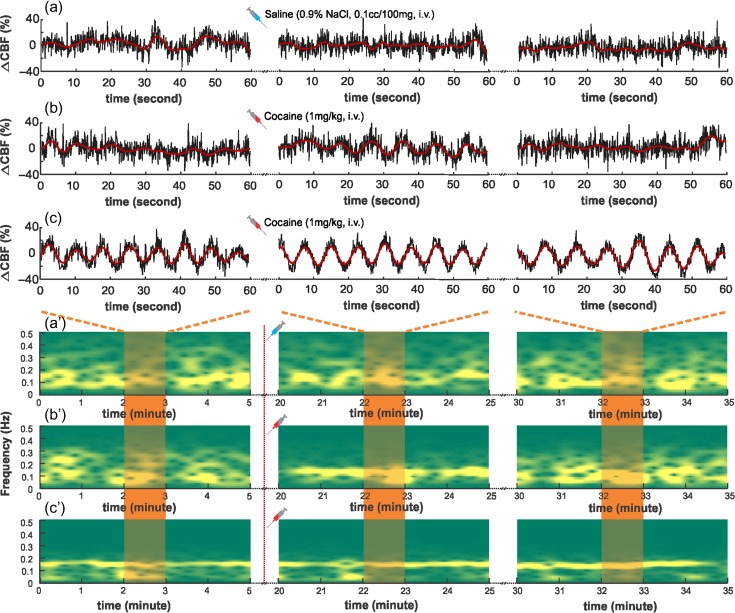
Figure 5 shows typical CBF fluctuations simultaneously recorded with FP signals. CBF fluctuations are presented as the relative CBF change (∆CBF) by removing mean CBF, so that only the oscillatory pattern is taken into consideration. The 60 s snapshots of CBF fluctuations are from the baseline and 10 and 25 min after cocaine (or saline for controls). Raw CBF data are presented in black curves overlaid with band-pass filtered (0.03–1 Hz) oscillations in red curves. Spectral analysis of CBF fluctuations was performed using STFT and presented as spectrograms to demonstrate the evolution of CBF LFOs before and after saline or cocaine injection.
Figure 5.
(a–c) Time-lapse CBF fluctuations (∆CBF) obtained from control, naïve, and chronic cocaine animals before and after saline/cocaine administration. The time traces were snapshots from baseline (4–5 min), 24–25, and 34–35 min, respectively (saline or cocaine administration at 10 min). (a′–c′) Short-time Fourier transform (STFT) to show the CBF oscillations as a function of time and the distribution involution in the frequency domain. Red shaded areas indicate the corresponding time traces in (a–c).
In controls, ∆CBF showed spontaneous and random fluctuations; its spectrogram exhibited a broad distribution ( and did not change after saline (Fig. 5a′). In the naïve group, ∆CBF had a similar baseline LFOs pattern as that of controls ((Fig. 5b), but after acute cocaine, it showed increased coherence in its oscillatory patterns and its spectrogram became narrower banded at 10 min post cocaine ( though at 20 min it no longer differed from baseline (. In the chronic group, ∆CBF showed strong coherent oscillations even at baseline (, which did not further change after acute cocaine, for example, at 10 min and at 20 min (Fig. 5c). These results suggest that acute cocaine temporarily increases the synchronization of CBF LFOs in naïve animals but in the chronic cocaine-exposed animals these increases persist for at least 24h after last cocaine exposure as evidenced by the increased regularity of their baseline CBF measurements. These findings are consistent with cocaine synchronizing CBF LFOs, such that this synchronization is transient after an acute exposure, but long-lasting after chronic cocaine exposures.
Cocaine Reduced High-Frequency CBF Oscillations and Increased Low-Frequency CBF Oscillations (LFOs Synchronization at 0.08–0.15 Hz)
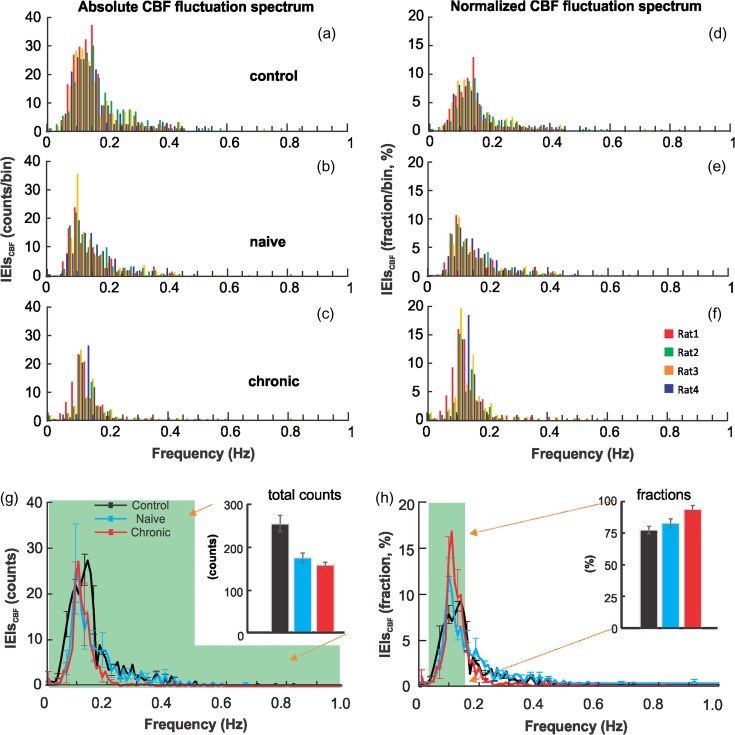
After saline or cocaine injection, CBF fluctuations were measured for 30 min and their fluctuation spectra were quantified. Figure 6a–c shows the superposed CBF LFOs distributions (absolute CBF fluctuation spectra) from individual animals for each group, and Figure 6d–f shows the corresponding normalized CBF fluctuation spectra by dividing the CBF fluctuation intensity at each frequency by the total fluctuation intensity from 0.03 to 1 Hz. The CBF LFOs spectra for each individual animal is summarized in Supplemental Figure S3.
Figure 6.
(a–c) Absolute CBF fluctuation spectra quantified as oscillations counts for the control, naïve and chronic groups. In each group, the CBF fluctuation spectra from individual animals (n = 4) were overlapped in 4 different colors. (d–f) Relative CBF fluctuation spectra for control, naïve and chronic groups, which were normalized by the total CBF oscillations counts and presented in percentage. (g) Bar diagram compares the total CBF oscillations counts <1 Hz (shaded area) for the 3 groups, 259.0 ± 17.1 (control), 177.2 ± 10.72 (naïve), and 164.6 ± 4.92 (chronic). (h) Bar diagram compares the normalized CBF fluctuation in the unit of percentage within 0.03–0.15 Hz (shaded area) for the 3 groups, 75.30 ± 2.9% (control), 83.4 ± 2.1% (naïve), and 94.4 ± 1.3% (chronic). Data from individual animals is illustrated in Supplemental Figure S3.
The total CBF fluctuation intensity within 0.03–1 Hz decreased after acute cocaine in the naïve (177.2 ± 10.72, P < 0.001) and chronic (164.6 ± 4.92, P < 0.001) groups compared with the controls after saline (259.0 ± 17.1) (Fig. 6g). The difference between naïve and chronic groups was not significant (P = 0.317). In contrast, the relative CBF fluctuation intensity within 0.08–0.13 Hz (Du et al. 2014) in naïve animals after acute cocaine was significant higher (83.4 ± 2.1%, P = 0.016) than that of controls after saline (75.30 ± 2.9%). Further increase in the relative intensity of CBF LFOs was observed in chronic animals after acute cocaine (94.4 ± 1.3%), which was significantly higher than that in the control (P < 0.001) and naïve groups (P = 0.014).
These results indicate that acute and chronic cocaine depressed CBF fluctuations predominantly for the higher-frequency bands (>0.15 Hz), resulting in the increases in relative CBF fluctuations observed in both the chronic and the naïve animals in the low frequency bands. The results are consistent with an increase in the synchronization of CBF LFOs (0.08–0.13 Hz) with chronic cocaine exposure.
Neuronal LFOs Correlate With CBF Fluctations in Control and Naïve Animals but not in Chronic Cocaine Animals
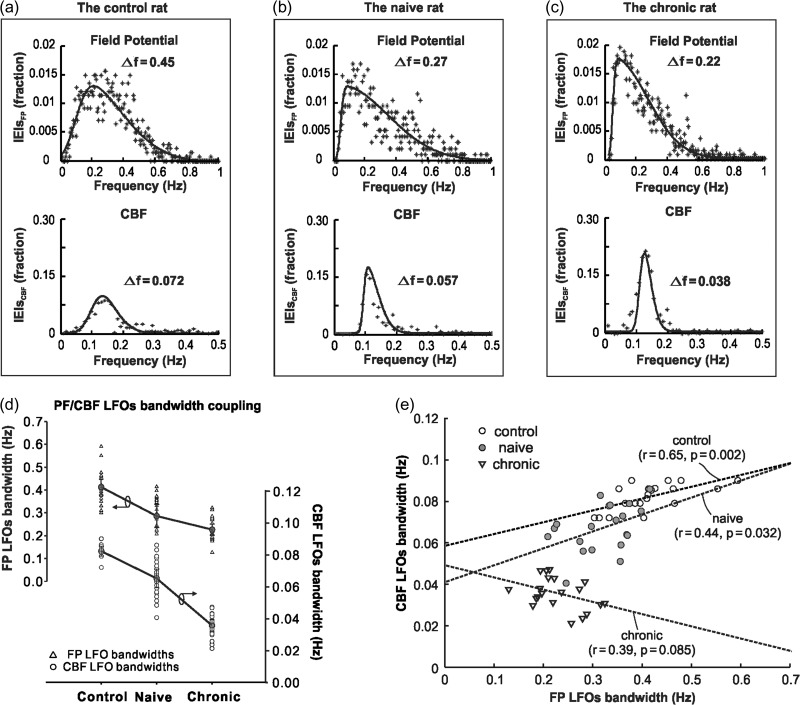
To analyze the relationship between LFOs in FP activity and LFOs in CBF fluctuations, we mathematically fitted the normalized LFOs distribution of FP and CBF in the frequency domain to a skewed-Gaussian function, and quantified the bandwidth change of LFOs between CBF and neuronal oscillations.
Figure 7a–c shows representative FP and CBF LFOs distributions from individual rats for different animal groups. Raw spectra are plotted in scattered spots (crosses) and overlapped by their skewed-Gaussian fitted profiles (solid lines), from which the LFOs bandwidths (Δf) were obtained. Compared with controls, acute cocaine decreased FP LFOs bandwidth in the naïve and chronic groups from ΔfFP = 0.413 ± 0.035 Hz to ΔfFP = 0.286 ± 0.050 Hz (P < 0.001) and to ΔfFP = 0.227 ± 0.024 Hz (P < 0.001), respectively. Similarly, compared with controls, acute cocaine decreased the CBF LFOs bandwidth in the naïve and chronic groups from ΔfCBF = 0.082 ± 0.003 Hz to ΔfCBF = 0.065 ± 0.005 Hz (P < 0.001) and to ΔfCBF = 0.036 ± 0.003 Hz (P < 0.001), respectively. These results show that both FP and CBF LFOs bandwidths decreased and that FP and CBF LFOs synchronization increased after acute cocaine and these effects were larger in animals exposed chronically to cocaine.
Figure 7.
(a–c) Representative FP and CBF LFOs distributions from individual rats grouped by different experiment groups. Raw spectra are plotted in scattered spots (crosses) and overlapped by their skewed-Gaussian fitted profiles (solid lines). (d) The FP LFOs bandwidths and CBF LFOs bandwidths from each animal (control, naïve and chronic) at different time segments (baseline: −10 to 0, 10–20, 20–30, and 30–40 min). (e) Linear regression between the FP LFO bandwidths and the CBF LFOs bandwidths for each group. The FP-CBF bandwidth pairs (m = 20 for each group) were sampled at baseline (−10 to 0 min) and at 10–20, 20–30, and 30–40 min following saline or cocaine for each animal (n = 5) within each group.
Figure 7e shows the correlations between the LFOs bandwidth for FP (ΔfFP) and for CBF (ΔfCBF). Linear regression analyses between ΔfFP and ΔfCBF were performed at multiple time periods (baseline: −10 to 0 min; saline or cocaine injection: 10–20, 20–30, and 30–40 min), and showed that FP LFOs were correlated with CBF LFOs in the control (r = 0.65, P = 0.002) and naïve groups (r = 0.44, P = 0.032) but not in the chronic cocaine group in which the patterns of FP LFOs were not associated with those in CBF LFOs (r = 0.39, P = 0.085). This indicates that while the relationship between neuronal and CBF LFOs was preserved after acute cocaine in naïve animals, this association was lost with chronic cocaine exposure. Note also that in controls and naïve animals there is an association of LFOs bandwidth between higher-frequency FP (ΔfFP = 0.25–0.7 Hz) and CBF (ΔfCBF = 0.05–0.1 Hz); whereas in the chronic animals the neuronal range is mostly located in ΔfFP = 0.2–0.35 Hz and the CBF is around ΔfCBF = 0.02–0.05 Hz.
Discussion
Brain imaging studies in cocaine abusers have reported decreased functional cortical connectivity (Li et al. 2000; Gu et al. 2010; Kelly et al. 2011; Hu et al. 2015), which was interpreted to reflect cocaine’s effects on neuronal activity (Goldstein and Volkow 2011; Hanlon et al. 2015). Similarly, animal studies showed that cocaine altered both the neuronal firing pattern and influenced low-frequency oscillations in neuronal activity in the Globus pallidus (Ruskin et al. 2001; Wei-Xing et al. 2004). However, in addition to its neuronal effects, cocaine also has direct vascular effects (Koob and Volkow 2010; Ren et al. 2012), so the interpretation of findings on cocaine effects assessed with fMRI that depend on hemodynamic measures is confounded. Though studies have investigated the effects of psychostimulants in neuronal and vascular response by simultaneously measuring EEG and fMRI, these studies have mostly focused on stimulation evoked states (Berwick et al. 2005; Musso et al. 2011; Parvaz et al. 2011). Instead in our study, we focused on cocaine’s effect in spontaneous neuronal and CBF low-frequency oscillations as assessed in the resting state. This is relevant since increasing studies are relying on resting-state fMRI measures to assess disruption in brain activity associated with brain diseases including addiction. Our results showed that acute and chronic cocaine depressed spontaneous FP events and CBF fluctuations predominantly for high-frequency ranges (>0.15 Hz), while increasing the synchronization of FP and CBF in the low-frequency bandwidth (0.03–0.15 Hz). We also showed that these effects were accentuated in chronic cocaine-exposed animals in whom these changes were already present at baseline and in whom we also observed a dissociation between the changes in FP and CBF, suggestive of neurovascular uncoupling. By simultaneously measuring FP and CBF fluctuations we also note that regardless of drug condition the range of spontaneous FP LFOs was much broader than that of CBF oscillations, which were mostly restricted to a 0.01–0.3 Hz bandwidth. Overall the spectral bandwidth of FP LFOs was 4.8 ± 0.67 times higher for that in CBF either for baseline in the control and naïve animals or during cocaine intoxication. The relationship between FP events and CBF LFOs in the resting state is not properly understood but there is some evidence to indicate that the low-frequency FP events but not high-frequency FP events might underlie BOLD LFOs at rest in the somatosensory cortex of the rat (Lu et al. 2014).
Acute and Chronic Cocaine Reduced Spontaneous-Synchronized Neuronal Population Firing Rates and Enhanced the Relative Contribution of LFOs at 0.03–0.15HZ
In this study, we observed that both acute and chronic cocaine decreased spontaneously synchronized FP events in the somatosensory cortex. We interpret the cocaine-induced decreases in spontaneously FP events to reflect reduced spontaneous neuronal firing, which is consistent with our prior studies showing that cocaine depressed spontaneous neuronal activity in somatosensory cortex (Chen, Liu et al. 2016) and that of others reporting reduced activity in prefrontal cortex (PFC) after acute cocaine (Bekavac and Waterhouse 1995). The reduction in spontaneous neuronal activity has been interpreted to reflect cocaine’s dopamine enhancing effects (perhaps also its noradrenergic actions) (Bunney et al. 1973; Ritz et al. 1987; Einhorn et al. 1988). In the PFC, DA suppresses spontaneous neuronal firing in pyramidal neurons and this effect is further enhanced by cocaine (Kroener et al. 2009). Moreover, these effects have been linked to the ability of stimulant drugs to enhance attention since under physiological conditions, optimal levels of DA (and norepinephrine) suppress background neuronal activity, enhancing the signal-to-noise ratio for salient stimuli, thus helping to focus attention (Volkow et al. 2008; Arnsten 2009). With chronic cocaine exposure the suppression of spontaneous neuronal activity in the PFC could contribute to addiction (Volkow et al. 2011). Specifically, an enhanced signal-to-noise ratio to a conditioned stimulus could produce an accentuated response to it while reducing the influence of weaker competitive stimuli. This would further strengthen conditioning while reducing the salience of nonrelated drug stimuli accounting in part for the behavioral inflexibility observed in addiction (Wise and Kiyatkin 2011; Volkow et al. 2013).
Acute cocaine, in addition to depressing spontaneous synchronized neuronal spiking activity, rendered the relative FP events oscillations in the LFOs band (0.03–0.15 Hz) more synchronized. This was observed as a narrowing of the FP events rate spectrum distribution, and as an increase in the relative neuronal oscillations at 0.03–0.15 Hz. This reflected in part the fact that acute cocaine decreased the FP events to a greater extent for the higher frequency bands (>0.15 Hz), thus increasing the relative contribution of LFOs (0.03–0.15 Hz). This effect of acute cocaine was observed in both naïve and chronic cocaine animals. However, in naïve animals it was transitory and recovered about 20 min post cocaine; whereas in chronic animals, it was more pronounced and longer lasting, persisting throughout the 30 min measurement period. Moreover, in chronic animals the contribution of LFOs in neuronal activity was already accentuated at baseline indicating that they persisted for 24 h after the last chronic cocaine injection.
Our findings are consistent with those previously reported showing an increase in low-frequency oscillations (0.017–0.1 Hz) in neuronal activity in the Globus pallidus in the presence of cocaine and other dopamine agonists (Ruskin et al. 1999). Moreover, subsequent studies interpreted the increase in the low-frequency oscillations triggered by cocaine to reflect the predominance of dopamine D2 receptor mediated inhibition following cocaine-induced increases in dopamine (Zhou et al. 2006).
Chronic Cocaine Reduced Resting CBF Fluctuations and Increased the Synchronization of LFOs
As for FP, acute cocaine reduced resting CBF fluctuations predominantly in the higher-frequency range (>0.15 Hz) increasing the predominance of low-frequency CBF fluctuations (<0.15 Hz). Though in the naive animals the effects were temporary and recovered after 30 min of cocaine exposure, in the chronic cocaine animals these CBF fluctuations were observed at baseline (24 h after last cocaine exposure) and did not change further with cocaine challenge. This change in CBF fluctuations was also reflected in the CBF fluctuation spectra, as shown by a narrower distribution of CBF fluctuations in naïve and chronic cocaine animals. Though acute cocaine’s effects on CBF fluctuation spectra were similar to those on neuronal activity in naïve animals, they differed in the chronic animals. Specifically, while acute cocaine further narrowed the neuronal LFOs distribution in the chronic cocaine animals, it did not further change the hemodynamic LFOs. This might reflect the direct effects of cocaine in the cerebral vasculature (Chen, You et al. 2016; Yin et al. 2017) that with chronic exposure might compromise the ability of CBF to respond to further changes in neuronal activity.
Coupling Between Neuronal and CBF LFOs in Control and Naïve Animals but not in Chronic Animals
The correspondent changes in resting LFOs between FP events and CBF signals indicate that the spontaneous low-frequency fluctuations in CBF reflect oscillatory neuronal activity. Correlation of the resting LFOs changes in FP and CBF in control animals before and after saline and in naïve animals before and after cocaine showed that the neurovascular coupling was preserved in the resting state. We had previously shown that acute cocaine in naïve rats did not reduce the neuronal response to sensory stimulation (e.g., forepaw stimulation), but that it did reduce the CBF response to this stimulation, which suggested that cocaine disrupted neurovascular coupling during stimulation (Chen et al. 2016). Here, we expand these findings to show that while the correlation between FP LFOs and CBF LFOs in the resting state was preserved after acute cocaine in naïve animals, it was not present in the chronic cocaine animals. Although acute cocaine administration reduced the bandwidths of FP and CBF LFOs for both naïve and chronic cocaine groups, the correlation between the FP and CBF LFO bandwidths deviated from the linear relationship in the chronic group but not in the naïve group. This indicates that while acute cocaine might not initially disrupt resting-state neurovascular coupling, chronic cocaine exposure could result in neurovascular uncoupling during resting state conditions. This uncoupling might reflect the marked and persistent vasoconstriction associated with chronic cocaine exposure (Yin et al. 2017), which might limit the hemodynamic responses associated with neuronal oscillations.
Cocaine’s Effects in the Somatosensory Cortex
In this study, we focused on cocaine’s effect in the somatosensory cortex and not in the prefrontal cortex (PFC), which is the main cortical region implicated in addiction (Volkow et al. 2016). However, cocaine has been shown to affect other cortical regions including primary visual cortex (Li et al. 2000), which as for the somatosensory cortex is not a target of dopamine neurons. Though we had initially hypothesized that cocaine’s effects in the somatosensory cortex would reflect its vascular effects our findings documenting significant effects in neuronal activity (as assessed by FP events) indicate that was not the case. The mechanisms underlying these neuronal effects are unclear and might reflect cocaine’s noradrenergic effects (Morrison and Foote 1986) and or its local anesthetic properties (sodium channel blocker). Though typically changes in neuronal activity are believed to underlie the changes in CBF, in our study we cannot rule out the possibility that cocaine-induced vasoconstriction might have affected neuronal activity. Our preliminary results using Ca2+ imaging (GCaMP6f) to measure neuronal activity show that cocaine also appears to increase neuronal Ca2+ LFO at ~0.1 Hz in the rat’s PFC (Supplemental Fig. S5), suggesting that the increase of LFOs after cocaine are not restricted to the somatosensory cortex.
Study Limitations
We interpret FP events to reflect synchronized neuronal activity from a neuronal population but technical limitations precluded us from corroborating that indeed FP events were associated with synchronized activity from neuronal spikes recorded in individual neurons. Nonetheless, temporal duration of FP events, which we observed using a low pass filter, was ~100ms both for those detected during the resting state and during forepaw stimulation (Supplemental Fig. S4), consistent with FP events reflecting synchronized activity from a neuron population (Buzsáki et al. 2012). Also, when we tracked individual neurons in the somatosensory cortex with Ca2+ imaging (using genetically encoded Ca indicator, GCaM6f) we observed that as we showed with FP, cocaine also increased neuronal oscillations at ~0.1 Hz (Supplemental Fig. S5). Moreover, since this study focuses on neuronal oscillations, which is a neuronal population property, FP events are appropriate for measuring changes in neuronal oscillations. Although other analysis methods, such as cross-frequency-coupling, could be adapted to investigate the temporal correlation between neuronal oscillations and CBF fluctuations across different frequency bands (Shmuel and Leopold 2008; Magri et al. 2012), the events rate spectrum method applied in this study offers better spectral resolution and higher sensitivity to detect changes in LFOs around 0.1 Hz compared with conventional Fourier-based spectral analysis methods, as shown in Supplemental Figure S6. Another limitation was that the experiments were conducted under anesthesia (e.g., α-chloralose), so we cannot rule out the possibility that the anesthetic drug might have attenuated resting oscillatory neuronal activity as compared with that in awake rats (Ruskin et al. 1999). To minimize the anesthetic effects we chose α-chloralose because it has been shown to preserve neurovascular coupling (Bonvento et al. 1994) and provides a CBF baseline similar to that measured in the awake state (Masamoto et al. 2006). Though it has been shown that large decreases in blood pressure (reduction from 110 to 60 mmHg) increased LFOs fluctuations in CBF (Hudetz et al. 1992; Kannurpatti et al. 2008), this is not a confound in our study since MABP, which was slightly increased by cocaine (not decreased) was maintained within a range of 102–120 mmHg.
In summary, acute and chronic cocaine administration, depressed neuronal activity in the somatosensory cortex but increased LFP oscillations in neuronal activity and CBF fluctuations. The changes of neuronal LFOs were spectral-temporally correlated with the changes of CBF LFOs in resting-state in naïve animals, demonstrating preserved neurovascular coupling in response to an acute cocaine Challenge. In contrast, the LFOs changes in neuronal activity and CBF fluctuations deviated from a linear relationship in chronic cocaine-exposed animals, suggestive of hemodynamic uncoupling from neuronal activity under resting state conditions.
Supplementary Material
Notes
We specially thank to Peng Liu, MD assisting animal and experimental procedures, and Craig Allen, PhD, for partially assisting with discussions. Conflict of Interest: The authors declare no conflict of interest.
Funding
National Institutes of Health (NIH) grants (1R01DA029718 to C.D. and Y.P.), (R21DA042597 to Y.P. and C.D.), and NIH’s Intramural Program of NIAAA (N.D.V.).
References
- Arnsten AF. 2009. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 23:33–41. [DOI] [PubMed] [Google Scholar]
- Bekavac I, Waterhouse BD. 1995. Systemically administered cocaine selectively enhances long-latency responses of rat barrel field cortical neurons to vibrissae stimulation. J Pharmacol Exp Ther. 272:333–342. [PubMed] [Google Scholar]
- Berwick J, Devonshire IM, Martindale AJ, Johnston D, Zheng Y, Kennerley AJ, Overton PG, Mayhew JEW. 2005. Cocaine administration produces a protracted decoupling of neural and haemodynamic responses to intense sensory stimuli. Neuroscience. 132:361–374. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kylen JV, Hyde JS. 1997. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10:165–170. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med. 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Charbonné R, Corrèze J-L, Borredon J, Seylaz J, Lacombe P. 1994. Is α-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain Res. 665:213–221. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. 1973. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 185:560–571. [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13:407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. 2004. Neuronal oscillations in cortical networks. Science. 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Mizuseki K. 2014. The log-dynamic brain: how skewed distributions affect network operations. Nat Rev Neurosci. 15:264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu P, Volkow ND, Pan Y, Du C. 2016. Cocaine attenuates blood flow but not neuronal responses to stimulation while preserving neurovascular coupling for resting brain activity. Mol Psychiatry. 21:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Park K, Volkow N, Pan Y, Du C. 2016. Cocaine-induced abnormal cerebral hemodynamic responses to forepaw stimulation assessed by integrated multi-wavelength spectroimaging and laser speckle contrast imaging. IEEE J Sel Top Quantum Electron. 22:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, You J, Gu X, Du C, Pan Y. 2016. High-speed swept source optical coherence Doppler tomography for deep brain microvascular imaging. Sci Rep. 6:38786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Volkow ND, Koretsky AP, Pan Y. 2014. Low-frequency calcium oscillations accompany deoxyhemoglobin oscillations in rat somatosensory cortex. Proc Natl Acad Sci. 111:E4677–E4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. 1988. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 8:100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell CE, Springett R, Hillman E, Delpy DT. 1999. Oscillations in cerebral haemodynamics. In: Eke A, Delpy DT, editors Oxygen transport to tissue XXI. Boston, MA: Springer US; p. 57–65. [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. 2007. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 35:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. 2005. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson I, Larsson M, Stromberg T. 2009. Measurement depth and volume in laser Doppler flowmetry. Microvas Res. 78:4–13. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T. 1998. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 18:724–734. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 53:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, DeVries W, Dowdle LT, West JA, Siekman B, Li X, George MS. 2015. A comprehensive study of sensorimotor cortex excitability in chronic cocaine users: integrating TMS and functional MRI data. Drug Alcohol Depend. 157:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron B, Gu H, Stein EA, Yang Y. 2015. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 72:584–592. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Roman RJ, Harder DR. 1992. Spontaneous flow oscillations in the cerebral cortex during acute changes in mean arterial pressure. J Cereb Blood Flow Metabol. 12:491–499. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB, Kim YR, Rosen BR. 2008. Spatio-temporal characteristics of low-frequency BOLD signal fluctuations in isoflurane-anesthetized rat brain. NeuroImage. 40:1738–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo X-N, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, et al. . 2011. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 69:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. 2010. Neurocircuitry of addiction. Neuropsychopharmacology. 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Chandler LJ, Phillips PE, Seamans JK. 2009. Dopamine modulates persistent synaptic activity and enhances the signal-to-noise ratio in the prefrontal cortex. PLoS One. 4:e6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. 2000. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 43:45–51. [DOI] [PubMed] [Google Scholar]
- Lu H, Wang L, Rea WW, Brynildsen JK, Jaime S, Zuo Y, Stein EA, Yang Y. 2014. Low-but not high-frequency LFP correlates with spontaneous BOLD fluctuations in rat whisker barrel cortex. Cereb Cortex. 26:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Shaik MA, Kozberg MG, Kim SH, Portes JP, Timerman D, Hillman EM. 2016. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proc Natl Acad Sci USA. 113:E8463–E8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. 2012. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci. 32:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim S-G. 2006. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 17:942–950. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL. 1986. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 243:117–138. [DOI] [PubMed] [Google Scholar]
- Musso F, Brinkmeyer J, Ecker D, London MK, Thieme G, Warbrick T, Wittsack H-J, Saleh A, Greb W, de Boer P, et al. . 2011. Ketamine effects on brain function—simultaneous fMRI/EEG during a visual oddball task. NeuroImage. 58:508–525. [DOI] [PubMed] [Google Scholar]
- Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhäupl K, Villringer A. 2000. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. NeuroImage. 12:623–639. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ. 2011. Neuroimaging for drug addiction and related behaviors. Rev Neurosci. 22:609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. 1997. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 17:7817–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. 2007. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 37:1083–1090. [DOI] [PubMed] [Google Scholar]
- Rayshubskiy A, Wojtasiewicz TJ, Mikell CB, Bouchard MB, Timerman D, Youngerman BE, McGovern RA, Otten ML, Canoll P, McKhann GM, et al. . 2014. Direct, intraoperative observation of similar to 0.1 Hz hemodynamic oscillations in awake human cortex: implications for fMRI. NeuroImage. 87:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Du C, Yuan Z, Park K, Volkow ND, Pan Y. 2012. Cocaine induced cortical microischemia in the rodent brain: clinical implications. Mol Psychiatry. 17:1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb R, Goldberg SR, Kuhar MJ. 1987. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 237:1219–1224. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Baek D, Freeman LE, Walters JR. 2001. Cocaine or selective block of dopamine transporters influences multisecond oscillations in firing rate in the globus pallidus. Neuropsychopharmacology. 25:28–40. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Kaneoke Y, Patel BN, Twery MJ, Walters JR. 1999. Multisecond oscillations in firing rate in the basal ganglia: robust modulation by dopamine receptor activation and anesthesia. J Neurophysiol. 81:2046–2055. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. 2004. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 29:2160–2167. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. 2008. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp. 29:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2006. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 52:155–168. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. 2008. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One. 3:e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. 2016. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 374:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. 1988. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 152:641–648. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. 2011. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci. 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Tomasi D, Baler RD. 2013. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol. 23:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei-Xing S, Chen-Lun P, Zhou Y. 2004. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 29:2160. [DOI] [PubMed] [Google Scholar]
- Wise RA, Kiyatkin EA. 2011. Differentiating the rapid actions of cocaine. Nat Rev Neurosci. 12:479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Clare K, Zhang Q, Volkow ND, Du C. 2017. Chronic cocaine induces HIF-VEGF pathway activation along with angiogenesis in the brain. PLoS One. 12:e0175499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Volkow ND, Park K, Zhang Q, Clare K, Du C, Pan Y. 2017. Cerebrovascular adaptations to cocaine-induced transient ischemic attacks in the rodent brain. JCI insight. 2:e90809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bunney BS, Shi W-X. 2006. Differential effects of cocaine on firing rate and pattern of dopamine neurons: role of α1 receptors and comparison with L-dopa and apomorphine. J Pharmacol Exp Ther. 317:196–201. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. 2008. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 172:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.