Abstract
Purpose: Wilson’s disease (WD) is a genetic disorder of copper metabolism with pathological copper accumulation in the brain. The purpose of the present study was to evaluate the relationship between the damaged white matter and the impaired cognitive function in WD patients. Materials and methods: Thirty WD adolescents and thirty age- and sex-matched healthy controls (HC) were enrolled. All subjects had received brain MRI, including conventional and diffusion-tensor imaging (DTI) scans. The DTI parameter of fractional anisotropy (FA) was calculated by diffusion kurtosis estimator software. The t test was used to compare the differences between two groups. The correlation between cognitive function and whiter matter disorders were analyzed by linear regression. The results of FA parameter and MD parameter intergroup analysis were both corrected with False Discovery Rate (FDR) simulations by SPSS. Results: WD adolescents showed significantly lower scores of time-based prospective memory (TBPM) and verbal fluency test (VFT) compared with HC. We found significantly higher FA in the right thalamus, right lentiform nucleus, left thalamus, left lentiform nucleus, and brain stem in WD adolescents. Besides, WD adolescents exhibited significantly lower FA in right cerebellum and cingulum and left middle frontal lobe compared with controls (P<0.05). There were significantly negative correlations between FA in bilateral lentiform and thalamus and cognitive impairment in WD adolescents (P<0.05). Conclusion: The whiter matter of WD adolescents was impaired and mainly distributed in subcortical brain regions. The impaired cognitive function was affected by the damaged whiter matter. The present study may be helpful for recognition and understanding of WD.
Keywords: adolescent, cognitive impairment, fractional anisotropy, Wilson’s disease
Introduction
Wilson’s disease (WD), known as progressive hepatolenticular degeneration, is a genetic disorder commonly supposed due to a mutation of gene ATP7B responsible for copper metabolism, and usually occurs in children. WD may lead to severe disability and death [1,2]. Routine MRI is usually used in its diagnosis, that exhibits symmetrical T2 hyperintensity or mixed intensity in caudate nuclei, thalami, pons putamina, or globi pallida [3–6]. However, some types of WD do not have significant signs in the MRI method, and thus more accurate quantitative measurement could be applied, such as diffusion-weighted imaging (DWI) and diffusion-tensor imaging (DTI) [7,8]. Fractional anisotropy (FA) is the main parameter of DTI, which is usually applied to identify the microstructural abnormalities in whiter matter. In previous studies, DTI has been applied to assess the microstructure of thalamus and evaluate diffusion abnormalities in the white matter regions in WD patients [9].
Cognitive impairment of WD patients was known to be associated with the low educational level and MRI hyperintensity in the basal ganglia nucleus [10,11]. The correlation between decreased FA in white matter and cognitive impairment was also reported in different brain diseases [12–14]. But little is known about the relationship(s) between the abnormal FA and cognitive impairment in WD patients. Therefore, the current study was to evaluate the microstructural abnormities in whiter matter with DTI technique and further assess how such abnormities affect cognitive function in WD patients by exploring the correlation between cognitive function and brain FA changes.
Materials and methods
Patients
The present study was approved by the First Affiliated Hospital of Anhui University of Chinese Medicine (AUCH) Ethics Committee and was conducted in accordance with the Declaration of Helsinki. The informed consents were obtained from all patients before enrollment.
WD adolescents who were hospitalized in the First Affiliated Hospital of ACUM from April 2014 to December 2016 were recruited in the present study. The diagnostic criteria of the WD group were as follows: (i) with neurological symptoms and psychiatric symptoms; (ii) with hepatic symptoms; (iii) with corneal Kayser–Fleischer ring; and (iv) other findings from microscopic examination or lab examination: serum copper oxidase level < 0.2 mg/ml and/or serum copper-blue protein level < 200 mg/l, 24-h urinary copper excretion > 100 μg (1.56 μmol), hepatic copper dry weight ≥ 250 μg/gm, hematuria, microalbuminuria, renal tubular acidosis, osteoarthrosis etc. [15,16].
The inclusion criteria were: (i) between 14 and 40 years and with ≥5 years of education; (ii) without intelligence deficits (IQ > 80 score); (iii) one or two grades of Modified Goldstein’s Degree [17]; (iv) the Chinese version of Unified WD Rating Scale for neurological function < 35 score [18,19]; (v) right-handedness; (vi) without color blindness, blindness, or deafness; (vii) without cognition impairment caused by other diseases or drugs; (viii) no drug abuse history; and (ix) Mini-Mental State Examination (MMSE) score > 23.
For healthy control (HC) group, the inclusion criteria were: (i) between 14 and 40 years and with ≥5 years of education; (ii) without intelligence deficits (IQ > 80 score); (iii) right-handedness; (iv) no color blindness, blindness, or deafness; (v) no cognitive impairment caused by other diseases or drugs; (vi) no history of neurological and mental disease; (vii) no drug abuse history; (viii) no family history of mental illness; and (ix) MMSE score > 23.
MRI protocol
All these included patients had received brain MRI, including conventional and DTI scan. Healthy volunteers received only DTI scan. A Signa VH/i 3.0 T MR imaging system (General Electric Medical Systems, Milwaukee, WI) was used with 8-channel high-resolution radio-frequency head coil. For conventional MRI, the sequences included T2 Flair (repetition time = 9000 ms, echo time = 124 ms, flip angle = 111°, matrix size = 256 × 256, field of view = 250 × 250 mm, layer thickness = 5 mm, no spacing and scanning 20 layers), T1-3D BRAVO (repetition time = 8.2 ms, echo time = 3.2 ms, flip angle = 12°, matrix size = 256 × 256, field of view = 256 × 256 mm, layer thickness = 1 mm, no spacing and scanning 166 layers). DTI was performed using the echo-planar imaging (EPI) sequence (repetition time = 6000 ms, echo time = minimum, matrix size = 128 × 128, field of view = 256 × 256 mm, layer thickness = 3 mm, no spacing and scanning 50 layers, diffusion sensitivity coefficient b = 0 s/mm2 and 1000 s/mm2, 64 direction).
Data analysis
A voxel-based TBSS approach was used for the group analysis of DTI data. DTI datasets were processed with the Functional MRI of the brain (FMRIB) Software Library (FSL) software package (www.fmrib.ox.ac.uk/fsl). Preprocessing included Eddy current and motion correction and brain-tissue extraction. After preprocessing, DTI images were averaged and concatenated, and a diffusion tensor model was fitted at each voxel to generate FA maps. [20,21]. Images were warped to the Montreal Neurological Institute (MNI) 152 template, available as a standard T1 dataset in the FSL software package. TBSS was run with FA maps to create the ‘skeleton’, which represented the center of all fiber bundles common to all subjects. Intergroup analysis was performed with test to investigate variation of microstructure in the brain between the patients of WD and control group. The results of FA parameter and MD parameter intergroup analysis were both corrected with False Discovery Rate (FDR) simulations.
Evaluation of cognitive function
The MMSE, the time-based (TBPM) and event-based prospective memory (EBPM) tests, as well as digit span (DS) and verbal fluency test (VFT), were performed as previous studies mentioned [22–25].
Correlation analysis
Categorical variables were expressed as number and percentage. All continuous variables were expressed as mean ± S.D. (normal distribution) or median and interquartile range (non-normal distribution) based on the results of the Kolmogorov–Smirnoff test. The ttest (normal distribution) or Mann–Whitney U test (non-normal distribution) was used to compare WD and control individuals. The Spearman coefficient was used to assess the correlation between FA and cognitive function amongst WD adolescents. A P-value less than 0.05 was considered statistically significant in all analyses.
Results
Patient characteristics
A total of 30 WD adolescents and 30 HC were enrolled in the present study. Compared with controls, WD patients showed longer T1, longer T2 in bilateral basal ganglia and brain stem. There were no significant differences between WD and HC groups in age, gender, and education levels (P>0.05, Table 1). The mean WD duration of the patients was 2.64 ± 0.88 years (Table 1).
Table 1. Characteristics of two groups of participants.
| Control (n=30) | WD (n=30) | P | |
|---|---|---|---|
| Age (years) | 16.97 ± 1.16 | 16.90 ± 2.04 | 0.877 |
| Male/Female | 15 (50%)/15 (50%) | 15 (50%)/15 (50%) | 1.000 |
| Education (years) | 8 (8–8) | 8 (8–8) | 1.000 |
| Duration of disease (years) | - | 2.64 ± 0.88 | - |
FA changes in WD patients
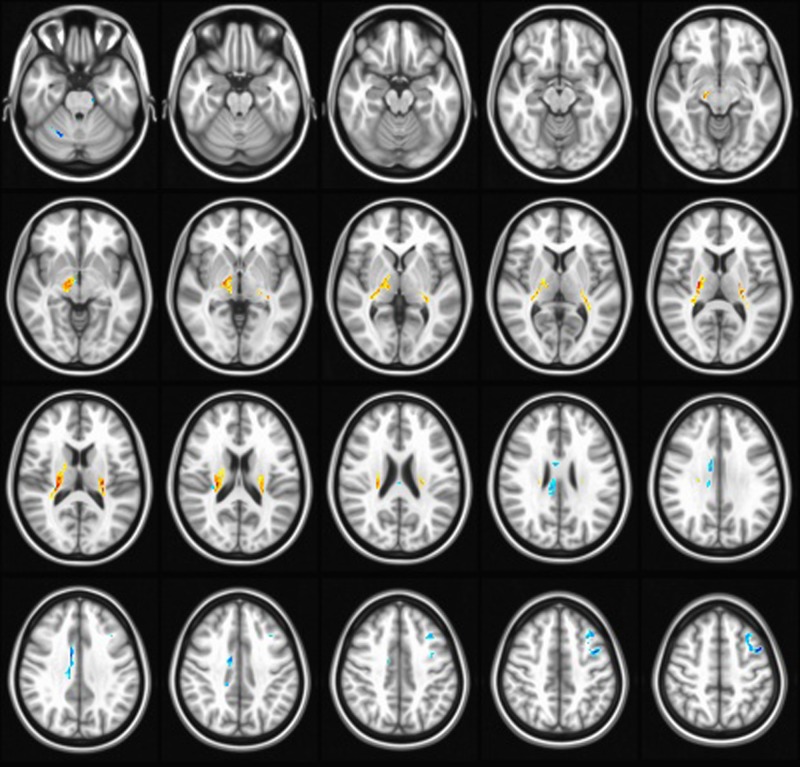
For cognitive function evaluation (Table 2), the WD patients had scores of EBPM, MMSE, and DS similar to controls (P>0.05), while they exhibited significantly decreased TBPM (P<0.001) and VFT (P=0.019) scores. For MRI evaluation (Table 3 and Figure 1), WD patients showed increased FA values in bilateral thalamus, bilateral lentiform nucleus, compared with HC (Figure 1). Simultaneously, FA in right cerebellum and cingulum, and left middle frontal lobe were significantly lower in WD patients compared with the HC (Figure 1).
Table 2. Comparison of cognitive function between WD and HC groups.
| Control (n=30) | WD (n=30) | P | |
|---|---|---|---|
| EBPM | 6 (5–6) | 5 (5–6) | 0.350 |
| TBPM | 6 (5.75–6) | 3 (2–4) | <0.001 |
| MMSE | 28 (28–29) | 28 (27–28) | 0.069 |
| DS | 7 (6.75–8) | 7 (5.75–8) | 0.195 |
| VFT | 9 (8–9) | 8 (7–9) | 0.019 |
Table 3. Comparison of the FA in different brain areas between WD and HC groups.
| Areas | Control (n=30) | WD (n=30) | P |
|---|---|---|---|
| Right thalamus | 0.137 ± 0.020 | 0.200 ± 0.048 | <0.001 |
| Right lentiform nucleus | 0.132 ± 0.011 | 0.194 ± 0.044 | <0.001 |
| Left thalamus | 0.132 ± 0.014 | 0.199 ± 0.055 | <0.001 |
| Left lentiform nucleus | 0.130 ± 0.011 | 0.194 ± 0.047 | <0.001 |
| Right head of caudate nucleus | 0.200 ± 0.037 | 0.126 ± 0.024 | <0.001 |
| Brain stem | 0.292 ± 0.018 | 0.352 ± 0.053 | <0.001 |
| White matter | 0.282 ± 0.060 | 0.210 ± 0.042 | <0.001 |
Figure 1. FA parameter differences of brain regions between patients and controls (FDR simulation, P=0.001, α = 0.05, cluster size = 326).
Compared with the HC, patients showed increased FA in in bilateral thalamus, bilateral lentiform nucleus, and decreased FA in right cerebellum and cingulum, and left middle frontal lobe.
Correlation between FA and cognitive function
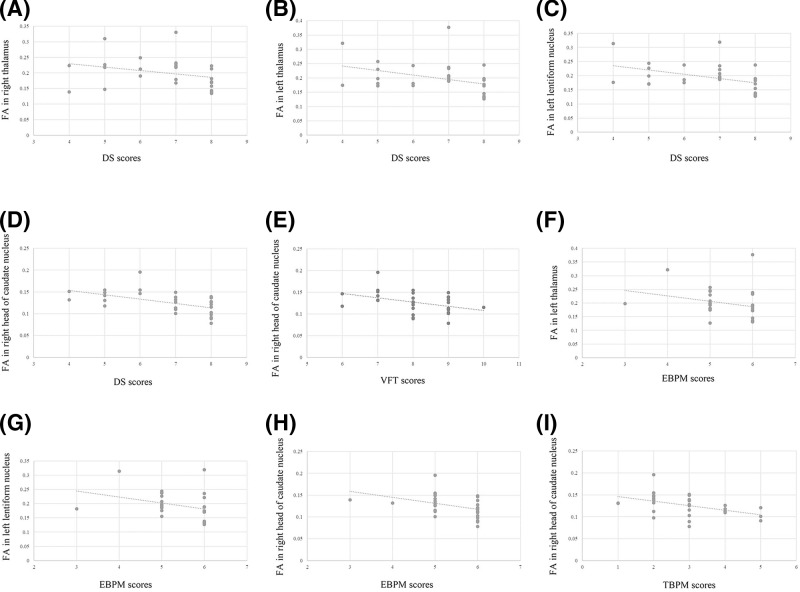
As shown in Table 4, no significant correlation between FA and cognitive function was found in the control group, based on EBPM, TBPM, MMSE, DS, and VFT (P>0.05 or/and Pearson r < 0.4). However, a significant correlation between FA and cognitive function was found in WD patients, based on EBPM, TBPM, DS, and VFT (Table 5 and Figure 2). We observed significantly negative correlation between the EBPM score and FA in left thalamus (r = −0.424, P=0.020), left lentiform nucleus (r = −0.447, P=0.013), and between the DS score and FA in right thalamus (r = −0.424, P=0.020), left thalamus (r = −0.421, P=0.021), left lentiform nucleus (r = −0.435, P=0.016).
Table 4. Correlation of FA and cognitive function in HC group.
| EBPM | TBPM | MMSE | DS | VFT | |
|---|---|---|---|---|---|
| Right thalamus | 0.134 | −0.168 | −0.079 | −0.328 | 0.073 |
| Right lentiform nucleus | 0.079 | −0.077 | 0.163 | −0.183 | 0.099 |
| Left thalamus | −0.399* | −0.141 | 0.283 | −0.146 | 0.236 |
| Left lentiform nucleus | −0.291 | 0.023 | 0.287 | −0.083 | 0.138 |
| Right head of caudate nucleus | -0.166 | −0.241 | −0.045 | −0.213 | 0.247 |
| Brain stem | 0.312 | 0.059 | 0.144 | 0.106 | 0.000 |
| White matter | 0.083 | −0.159 | −0.005 | 0.207 | 0.059 |
P=0.029.
Table 5. Correlation of FA and cognitive function in WD group.
| EBPM | TBPM | MMSE | DS | VFT | |
|---|---|---|---|---|---|
| Right thalamus | −0.211 | −0.102 | −0.213 | −0.424* | −0.111 |
| Right lentiform nucleus | −0.163 | −0.122 | 0.173 | −0.376* | −0.040 |
| Left thalamus | −0.424* | −0.285 | 0.220 | −0.421* | 0.103 |
| Left lentiform nucleus | −0.447* | −0.296 | 0.172 | −0.435* | 0.052 |
| Right head of caudate nucleus | −0.490† | −0.510† | 0.081 | −0.590† | −0.408* |
| Brain stem | −0.100 | −0.072 | −0.008 | −0.354 | −0.218 |
| White matter | 0.067 | 0.223 | 0.003 | 0.110 | 0.116 |
*P<0.05.
†P<0.01.
Figure 2. The correlation of FA and cognitive function in WD patients.
The DS score and FA in the right thalamus (A), left thalamus (B), left lentiform nucleus (C) and right head of caudate nucleus (D); the FT score and FA in right head of caudate nucleus (E); the EBPM score and FA in left thalamus (F), left lentiform nucleus (G) and right head of caudate nucleus (H); the TBPM score and FA in right head of caudate nucleus (I).
Discussion
WD is an autosomal recessive disorder of copper metabolism. The recognized mechanism is the dysfunction of a copper-transporting ATP7B, which causes aberrant copper accumulation. However, the changes in diffusion images and the cognition damage have been seldom analyzed. Theoretically, WD involves pathological changes in a cerebello–thalamo–cortical network. In the present study, we found verbal intelligence ability and memory speed in WD adolescents was markedly deficient, in comparison with heathy controls based on the tests including TBPM and VFT. MRI results showed significantly enhanced FA in right thalamus, bilateral lentiform nucleus, bilateral thalamus, as well as reduced FA in right cerebellum and cingulum and left middle frontal lobe in WD adolescents. FA is the main parameter of DTI, which is usually applied to identify the microstructural abnormalities in whiter matter. FA variation strongly implied the microstructural changes in whiter matter [26–29]. Interestingly, we found a correlation between FA and EBPM and DS in WD adolescents, but WD and healthy individuals had similar EBPM and DS scores. This outcome suggests that the indicators like EBPM and DS scores could not sufficiently reflect the pathological characteristics, and may be impacted much later during WD development, compared with TBPM and VFT scores, in despite that they may be truly influenced by WD and enhanced FA. The negative correlation between FA in right head of caudate nucleus and TBPM/VFT score suggested the relationship of cognitive impairment (in the aspects of verbal intelligence ability and memory speed) and FA in right head of caudate nucleus. This is the first report noticing the FA variation features in WD and the its correlation with cognitive impairment in WD. Our observation innovatively suggests that the impaired cognitive function was affected by the damaged whiter matter, and the conclusion may be helpful for recognition and understanding of WD.
There exists some indirect supportive evidences. For example, WD adolescents had decreased FA in white matter [9]. In caudate nucleus, decreased signal intensity in WD patients by MRI was reported [30], which was consistent with our findings. Currently, it has been seldom reported about the MR spectroscopy diffusion MRI in WD patients. Although no indirect evidence in previous studies support the results of increased FA in right thalamus, right lentiform nucleus, left thalamus, left lentiform nucleus, and brain stem, the lesions in thalamus, lentiform nucleus, and brain stem have been seen repeatedly [30–32]. We here provided new evidences using the MRI signs. In consistency, other advanced MRI application except DWI and DTI, such as susceptibility-weighted imaging (SWI), have indicated decreased corrected phase values in WD patients [33].
There have been limited studies that applied DTI to assess the FA variation in WD patients. Taly et al. observed decreased FA in the frontal and occipital white matter, bilateral internal capsules, midbrain, and pons in WD patients [34]. Chen et al. reported significantly different FA values in thalamus between WD and healthy population [35]. They and Zhang et al. found increased FA in the bilateral head of the caudate nucleus, lenticular nucleus, ventral thalamus, substantia nigra, red nucleus, right dentate nucleus, and decreased in the mediodorsal thalamus and extensive white matter [36]. Some of their findings were proved by our study, e.g. the changes in caudate nucleus and white matter.
Previous studies have noticed significant correlation between FA in white matter and cognitive impairment [12–14], but this was not found in WD adolescents in our study. Many studies have showed the correlation of cognitive impairment with white matter damage based on the diffusion MRI [37–39]. We found no association between FA in white matter and verbal intelligence ability or memory speed at present. However, the negative correlation of FA in right head of caudate nucleus with cognitive impairment was determined in the present study. Cognitive impairment is known to be associated with the caudate nucleus lesion in published articles [40,41]. Moreover, the decreased FA in right head of caudate nucleus may be associated with deficient verbal intelligence ability and memory speed in WD adolescents, resulting in the significantly negative correlation between FA in right head of caudate nucleus with TBPM and VFT scores in the present study. A previous study reported that the caudate nucleus volume was associated with the dopamine receptor D2 (DRD2) Taq I genetic polymorphism in the memory impaired subjects [42]. DRD2 plays an important role in memory processes [43] and is associated with verbal intelligence quotient [44]. Thus, the abnormal FA in right head of caudate nucleus may be associated with cognitive function of WD adolescents via the mechanism of DRD2. The cognitive function of WD adolescents should be further evaluated by additional measurements in order to verify these above speculations.
Also, cognitive decline might be associated with the atrophy of cortices. The voxel-based morphometry study showed that the structural alterations in gray matter and white matter may implied a cognitive decline [45,46]. Further studies are needed to investigate the association between cognitive impairment with voxel-based morphometry, MD, or other factors in WD adolescents.
Conclusion
WD adolescents had cognitive impairment, indicated by TBPM and VFT, and abnormal FA in several brain regions. There exists significantly negative correlation between FA in bilateral lentiform and thalamus and cognitive impairment in WD adolescents. This indicates that the impaired cognitive function was affected by the damaged whiter matter. The present study may be helpful for recognition and understanding of WD.
Abbreviations
- DRD2
dopamine receptor D2
- DS
digit span
- DTI
diffusion-tensor imaging
- DWI
diffusion-weighted imaging
- EBPM
event-based prospective memory
- FA
fractional anisotropy
- FSL
Functional MRI of the brain (FMRIB) Software Library
- HC
healthy control
- MMSE
Mini-Mental State Examination
- TBPM
time-based prospective memory
- VFT
verbal fluency test
- WD
Wilson’s disease
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81373599, 81774299, 81872504]; the Natural Science Foundation of Anhui Province [grant number 1808085MH263]; and the Anhui Provincial Education Research Project [grant number 2017jyxm1389].
Author contribution
Conceived and designed the experiments: T.D., W.-m.Y., and M.-c.W. Performed the experiments: T.D., P.H., and C.-j.K. Analyzed the data: T.D., A.-q.W., C.-s.X., and Z.-l.G. Contributed reagents/materials/analysis tools: T.D. and J.Z. Wrote the paper: T.D.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Ala A., Walker A.P., Ashkan K., Dooley J.S. and Schilsky M.L. (2007) Wilson’s disease. Lancet 369, 397–408 10.1016/S0140-6736(07)60196-2 [DOI] [PubMed] [Google Scholar]
- 2.Litwin T., Gromadzka G. and Czlonkowska A. (2012) Gender differences in Wilson’s disease. J. Neurol. Sci. 312, 31–35 10.1016/j.jns.2011.08.028 [DOI] [PubMed] [Google Scholar]
- 3.Da C.M.D., Spitz M., Bacheschi L.A., Leite C.C., Lucato L.T. and Barbosa E.R. (2009) Wilson’s disease: two treatment modalities. Correlations to pretreatment and posttreatment brain MRI. Neuroradiology 51, 627–633 10.1007/s00234-009-0536-5 [DOI] [PubMed] [Google Scholar]
- 4.Prashanth L.K., Taly A.B., Sinha S., Ravishankar S., Arunodaya G.R., Vasudev M.K.. et al. (2005) Prognostic factors in patients presenting with severe neurological forms of Wilson’s disease. QJM 98, 557–563 10.1093/qjmed/hci095 [DOI] [PubMed] [Google Scholar]
- 5.Pulai S., Biswas A., Roy A., Guin D.S., Pandit A., Gangopadhyay G.. et al. (2014) Clinical features, MRI brain, and MRS abnormalities of drug-naive neurologic Wilson’s disease. Neurol. India 62, 153–158 10.4103/0028-3886.132349 [DOI] [PubMed] [Google Scholar]
- 6.Litwin T., Gromadzka G., Czlonkowska A., Golebiowski M. and Poniatowska R. (2013) The effect of gender on brain MRI pathology in Wilson’s disease. Metab. Brain Dis. 28, 69–75 10.1007/s11011-013-9378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sener R.N. (2003) Diffusion MR imaging changes associated with Wilson disease. AJNR Am. J. Neuroradiol. 24, 965–967 [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X.X., Li X.H., Qin H., Li G.D., Huang H.W., Liang Y.Y.. et al. (2016) Diffusion tensor imaging of the extracorticospinal network in the brains of patients with Wilson disease. J. Neurol. Sci. 362, 292–298 10.1016/j.jns.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Jadav R., Saini J., Sinha S., Bagepally B., Rao S. and Taly A.B. (2013) Diffusion tensor imaging (DTI) and its clinical correlates in drug naive Wilson’s disease. Metab. Brain Dis. 28, 455–462 10.1007/s11011-013-9407-1 [DOI] [PubMed] [Google Scholar]
- 10.Frota N.A.F., Barbosa E.R., Porto C.S., Lucato L.T., Ono C.R., Buchpiguel C.A.. et al. (2016) Which factors are associated with global cognitive impairment in Wilson’s disease. Dement. Neuropsychol. 10, 320–326 10.1590/s1980-5764-2016dn1004011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde S., Sinha S., Rao S.L., Taly A.B. and Vasudev M.K. (2010) Cognitive profile and structural findings in Wilson’s disease: a neuropsychological and MRI-based study. Neurol. India 58, 708–713 10.4103/0028-3886.72172 [DOI] [PubMed] [Google Scholar]
- 12.Wada T., Asano Y. and Shinoda J. (2012) Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR Am. J. Neuroradiol. 33, 2117–2122 10.3174/ajnr.A3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rueckriegel S.M., Bruhn H., Thomale U.W. and Hernaiz D.P. (2015) Cerebral white matter fractional anisotropy and tract volume as measured by MR imaging are associated with impaired cognitive and motor function in pediatric posterior fossa tumor survivors. Pediatr. Blood Cancer 62, 1252–1258 10.1002/pbc.25485 [DOI] [PubMed] [Google Scholar]
- 14.Sato Y., Ito K., Ogasawara K., Sasaki M., Kudo K., Murakami T.. et al. (2013) Postoperative increase in cerebral white matter fractional anisotropy on diffusion tensor magnetic resonance imaging is associated with cognitive improvement after uncomplicated carotid endarterectomy: Tract-based spatial statistics analysis. Neurosurgery 73, 592–599, 10.1227/NEU.0000000000000013 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Xie C.L., Fu D.L., Lu L., Lin Y., Dong Q.Q.. et al. (2012) Clinical efficacy and safety of Chinese herbal medicine for Wilson’s disease: a systematic review of 9 randomized controlled trials. Complement. Ther. Med. 20, 143–154 10.1016/j.ctim.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Faa G. (1996) The role of the pathologist in the diagnosis and monitoring of Wilson’s disease. Pathologica 88, 102–110 [PubMed] [Google Scholar]
- 17.Goldstein N.P., Randall R.V., Gross J.B., Rosevear J.W. and McGuckin W.F. (1962) Treatment of Wilson’s disease (hepatolenticular degeneration) with DL-penicillamine. Neurology 12, 231–244 10.1212/WNL.12.4.231 [DOI] [PubMed] [Google Scholar]
- 18.Czlonkowska A., Tarnacka B., Moller J.C., Leinweber B., Bandmann O., Woimant F.. et al. (2007) Unified Wilson’s Disease Rating Scale - a proposal for the neurological scoring of Wilson’s disease patients. Neurol. Neurochir. Pol. 41, 1–12 [PubMed] [Google Scholar]
- 19.Han Y.S., Wang X., Han Y.Z., Chen L., Xu Y., Wang W.. et al. (2013) Study of reliability and validity of Chinese version of united Wilson’s disease rating scale. J. Clin. Neurol. 26, 241–243 [Google Scholar]
- 20.Zhu J.J., Zhuo C.J., Qin W., Wang D., Ma X.M., Zhou Y.J.. et al. (2015) Performances of diffusion kurtosis imaging and diffusion tensor imaging in detecting white matter abnormality in schizophrenia. Neuroimage Clin. 7, 170–176 10.1016/j.nicl.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander A.L., Lee J.E., Lazar M. and Field A.S. (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein M.F., Robins L.N. and Helzer J.E. (1983) The Mini-Mental state examination. Arch. Gen. Psychiatry 40, 812 10.1001/archpsyc.1983.01790060110016 [DOI] [PubMed] [Google Scholar]
- 23.Cockrell J.R. and Folstein M.F. (2002) Mini-mental state examination. In Principles and Practice of Geriatric Psychiatry, pp. 140–141, 10.1002/0470846410.CH27(II) [DOI] [Google Scholar]
- 24.Gonneaud J., Rauchs G., Groussard M., Landeau B., Mezenge F., de La Sayette V.. et al. (2014) How do we process event-based and time-based intentions in the brain? An fMRI study of prospective memory in healthy individuals. Hum. Brain Mapp. 35, 3066–3082 10.1002/hbm.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quesnel C., Savard J. and Ivers H. (2009) Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res. Treat. 116, 113–123 10.1007/s10549-008-0114-2 [DOI] [PubMed] [Google Scholar]
- 26.Xia W., Zhou R., Zhao G., Wang F., Mao R., Peng D.. et al. (2018) Abnormal white matter integrity in Chinese young adults with first-episode medication-free anxious depression:a possible neurological biomarker of subtype major depressive disorder. Neuropsych. Dis. Treat 14, 2017–2026 10.2147/NDT.S169583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao S., Liu P., Guo J., Zju Y., Liu P., Sun J.. et al. , (2017) White matter microstructure within the superior longitudinal fasciculus modulates the degree of response conflictindexed by N2 in healthy adults. Brain Res 1676, 1–8 10.1016/j.brainres.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 28.Mo Y., Chao F., Song M., Liu C.R., Liu H.L., Qian X.Y. (2014) Parameter comparison of white matter diffusion tensor imaging(DTI) in rhesus macaques (Macaca mulatta). Dongwuxue Yanjiu 35, 182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F., Tang Y., Xu K., Kong L., Sun W., Wang F.. et al. (2011) Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res 191, 80–83 10.1016/j.pscychresns.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saatci I., Topcu M., Baltaoglu F.F., Kose G., Yalaz K., Renda Y.. et al. (1997) Cranial MR findings in Wilson’s disease. Acta Radiol. 38, 250–258 10.1080/02841859709172059 [DOI] [PubMed] [Google Scholar]
- 31.Huang C.C. and Chu N.S. (1998) Acute dystonia with thalamic and brainstem lesions after initial penicillamine treatment in Wilson’s disease. Eur. Neurol. 39, 32–37 10.1159/000007894 [DOI] [PubMed] [Google Scholar]
- 32.Hawkins R.A., Mazziotta J.C. and Phelps M.E. (1987) Wilson’s disease studied with FDG and positron emission tomography. Neurology 37, 1707–1711 10.1212/WNL.37.11.1707 [DOI] [PubMed] [Google Scholar]
- 33.Zhou X.X., Qin H.L., Li X.H., Huang H.W., Liang Y.Y., Liang X.L.. et al. (2014) Characterizing brain mineral deposition in patients with Wilson disease using susceptibility-weighted imaging. Neurol. India 62, 362–366 10.4103/0028-3886.141221 [DOI] [PubMed] [Google Scholar]
- 34.Jadav R., Saini J., Sinha S., Bagepally B., Rao S. and Taly A.B. (2013) Diffusion tensor imaging (DTI) and its clinical correlates in drug naive Wilson’s disease. Metab. Brain Dis. 28, 455–462 10.1007/s11011-013-9407-1 [DOI] [PubMed] [Google Scholar]
- 35.Li G., Zhou X., Xu P., Pan X. and Chen Y. (2014) Microstructure assessment of the thalamus in Wilson’s disease using diffusion tensor imaging. Clin. Radiol. 69, 294–298 10.1016/j.crad.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 36.Wang A., Wu H., Xu C., Tang L., Lee J., Wang M.. et al. (2017) Study on lesion assessment of cerebello-thalamo-cortical network in Wilson’s disease with diffusion tensor imaging. Neural Plast. 2017, 7323121 10.1155/2017/7323121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.E., Park H.J., Park B., Song S.K., Sohn Y.H. and Lee J.D. (2010) A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson’s disease dementia and dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 81, 320–326 10.1136/jnnp.2009.184747 [DOI] [PubMed] [Google Scholar]
- 38.Pasi M., Salvadori E., Poggesi A., Ciolli L., Del Bene A., Marini S.. et al. (2015) White matter microstructural damage in small vessel disease is associated with Montreal cognitive assessment but not with mini mental state examination performances: vascular mild cognitive impairment Tuscany study. Stroke 46, 262–264 10.1161/STROKEAHA.114.007553 [DOI] [PubMed] [Google Scholar]
- 39.Reijmer Y.D., Brundel M., de Bresser J., Kappelle L.J., Leemans A. and Biessels G.J. (2013) Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care 36, 137–144 10.2337/dc12-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen S.K., Ho A.J., Hua X., Saharan P.S., Toga A.W., Jack C.J.. et al. (2010) 3D maps localize caudate nucleus atrophy in 400 Alzheimer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiol. Aging 31, 1312–1325 10.1016/j.neurobiolaging.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohnen N.I., Albin R.L., Muller M.L., Petrou M., Kotagal V., Koeppe R.A.. et al. (2015) Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol. 72, 194–200 10.1001/jamaneurol.2014.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartres-Faz D., Junque C., Serra-Grabulosa J.M., Lopez-Alomar A., Moya A., Bargallo N.. et al. (2002) Dopamine DRD2 Taq I polymorphism associates with caudate nucleus volume and cognitive performance in memory impaired subjects. Neuroreport 13, 1121–1125 10.1097/00001756-200207020-00010 [DOI] [PubMed] [Google Scholar]
- 43.El-Ghundi M., O’Dowd B.F. and George S.R. (2007) Insights into the role of dopamine receptor systems in learning and memory. Rev. Neurosci. 18, 37–66 10.1515/REVNEURO.2007.18.1.37 [DOI] [PubMed] [Google Scholar]
- 44.Guo J.F., Yang Y.K., Chiu N.T., Yeh T.L., Chen P.S., Lee I.H.. et al. (2006) The correlation between striatal dopamine D2/D3 receptor availability and verbal intelligence quotient in healthy volunteers. Psychol. Med. 36, 547–554 10.1017/S0033291705006732 [DOI] [PubMed] [Google Scholar]
- 45.Antonova E., Kumari V., Morris R., Halari R., Anilkumar A., Mehrotra R.. et al. (2005) The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol. Psychiatry 58, 457–467 10.1016/j.biopsych.2005.04.036 [DOI] [PubMed] [Google Scholar]
- 46.Demey I., Ventrice F., Rojas G., Ricardo A., Somsle V., Zubiri V. (2015) Hippocampal mean diffusivity is a biomarker of neuronal injury in patients with mild cognitive impairment and Alzheimer’s disease dementia. Alzheimers Dementia 11, 802 10.1016/j.jalz.2015.06.1787 [DOI] [Google Scholar]