Abstract
Cervical cancer (CC) with early metastasis of the primary tumor results in poor prognosis and poor therapeutic outcomes. MicroRNAs (miRNAs) are small, noncoding RNA molecules that play a substantial role in regulating gene expression post-transcriptionally and influence the development and progression of tumors. Numerous studies have discovered that miRNAs play significant roles in the invasion and metastasis of CC by affecting specific pathways, including Notch, Wnt/β-catenin, and phosphoinositide-3 kinase (PI3K)-Akt pathways. miRNAs also effectively modulate the process of epithelial–mesenchymal transition. Many studies provide new insights into the role of miRNAs and the pathogenesis of metastatic CC. In this review, we will offer an overview and update of our present understanding of the potential roles of miRNAs in metastatic CC.
Keywords: cervical cancer, invasion, MicroRNAs, metastasis
Introduction
Cervical cancer (CC) is one of the most commonly diagnosed cancers and is the third leading cause of cancer mortality amongst females [1]. An increasing amount of research has revealed that long-lasting infections of high-risk human papillomavirus (HPV), such as HPV-16 and HPV-18, mainly compose the majority of CC cases [2,3]. In fact, not all metastatic CC patients are diagnosed with HPV infection, which indicates that a number of indeterminate factors might contribute to the initiation and progression of CC [4–6]. Despite advances in surgery combined with radiotherapy and/or chemotherapy, some CC patients undergo early metastases of the primary tumor, especially lymph node metastasis (LNM), that lead to poor prognosis and poor therapeutic outcomes [7–10]. Hence, it is important to elucidate the molecular mechanisms underlying the metastasis of CC.
Based on sufficient research, numerous signaling pathways and molecules are involved in the metastasis of CC. For instance, the phosphatidylinositol 3-kinase/protein kinase-B (PI3K/AKT) signaling pathway, known as a key driver in carcinogenesis, plays an important role in the migration and invasion of CC [11,12]. Wnt/β-catenin, p38/MAPK, p53, and hedgehog signaling pathways were also reported to be related to carcinogenesis and progression in CC [13–17]. In addition, emerging molecules such as circular RNAs (circRNAs), long noncoding RNAs (lncRNAs), and exosomes were also shown to be related to the tumorigenesis of CC [18–20]. In recent decades, molecule-targetted therapy of CC has been developed. According to many studies, microRNAs (miRNAs) function to modulate the pathophysiologic mechanism in CC partly through the signaling pathways mentioned above, which might offer a new therapeutic method in the future and bring CC patients a hope for treatment [4,21,22].
miRNAs are a class of endogenous, highly conserved, noncoding RNAs (18–25 nts in length) that adjust gene expression both transcriptionally and post-transcriptionally [23,24]. They are involved in various physiological and pathological processes via binding to mRNAs at their 3′-UTRs [25,26]. Dysregulated miRNAs can be loosely divided into two groups: oncogenic miRNAs and oncosuppressor miRNAs. Both groups of miRNAs correlate with numerous biological processes such as invasion and metastasis in CC, thereby suggesting that miRNAs might serve as a set of novel biomarkers for the diagnosis and molecule-targetted therapy of metastatic CC.
Herein, we conclude that existing studies focus on the identification of miRNAs as diagnostic and prognostic markers for metastatic CC. Furthermore, we provide insight into the strategies for using miRNAs in metastatic CC therapy based on their putative functions.
Dysregulated miRNAs in CC invasion and metastasis
A previous review summarized that miRNAs with altered expression patterns were related to oncogenic or tumor-suppressing functions in CC, and the differential miRNA expression pattern was closely contacted with complex CC progression [27]. In detail, the existence of oncogenic miRNAs or oncosuppressor miRNAs indicated that miRNAs played a promotive or suppressive role in the development of tumors. However, more detailed information about the role of miRNAs in the invasion and metastasis of CC is lacking. In the following section, we will further discuss the specific mechanism in which dysregulated miRNAs modulate the invasion and metastasis of CC through targetting genes.
Oncogenic miRNAs in the metastasis of CC
Plentiful findings revealed that the autophagy-related protein (ATG) family plays crucial roles in autophagosome formation through communication between members of the ATG family [28,29]. For instance, ATG7 has been implicated in metastasis as one of the master regulators of the autophagy process and is responsible for autophagosome formation and vesicle progression [30]. Afterward, Zhao et al. [31] published that miR-20a functions as a promoter of metastasis via ATG7. Migration and invasion of CC were also found to be enhanced by miR-378 through ATG12 [32].
Tissue inhibitors of metalloproteinases and matrix metalloproteinases
In 2017, Wei et al. [33] alleged that miR-21 participated in promoting LNM of CC, but they did not discuss the pathways of metastasis. In fact, during the process of invasion and metastasis, tissue inhibitors of metalloproteinases (TIMPs), particularly TIMP2 and TIMP3, were equipped to reverse the degradation of collagenous substrates in the surrounding extracellular matrix (ECM) by matrix metalloproteinases (MMPs) [34]. For example, miR-21 showed its ability to advance invasion of CC through suppressing TIMP3 [35]. In addition, miR-20a [31] as well as miR-106a [36] suppressed the migration and invasion of CC cells by targetting TIMP2.
Other genes
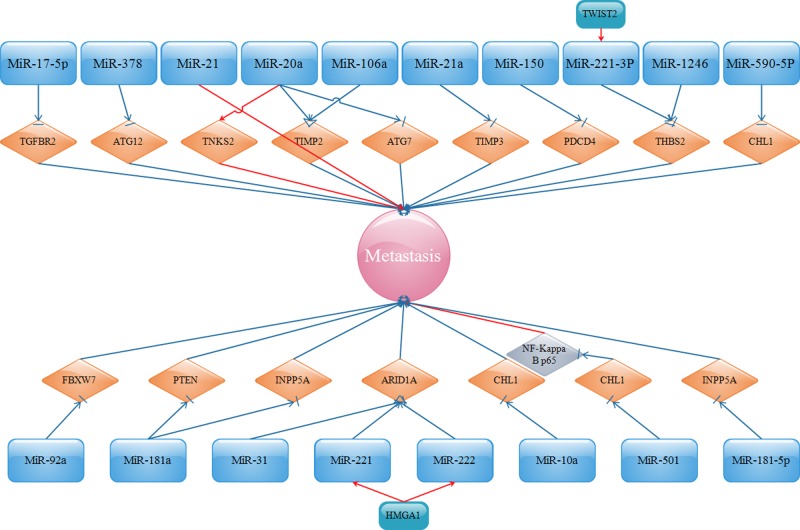
It was reported by Chen et al. [37] and Wei et al. [38] that miR-1246 and miR-221-3p facilitated the metastasis of CC via targetting thrombospondin-2 (THBS2, TSP2). THBS2 is a member of the thrombospondin family that regulates cell migration and inhibits tumor metastasis [39,40]. In addition, tankyrase 2 (TNKS2), which belongs to the human telomere-associated poly (ADP-ribose) polymerase (PARP) family, was claimed to increase telomere length, thus enhancing tumor progression [41,42]. miR-20a was announced to directly up-regulate TNKS2 and correspondingly strengthen the migration and invasion of CC [43]. Moreover, programmed cell death protein 4 (PDCD4), inhibited by miR-150, was attributed to the suppression of cancer cell migration and invasion [44]. miR-31 [45] and miR-221 [46], along with miR-222 [46], were verified as upstream of the AT-rich interactive domain-containing protein 1A (ARID1A), which is involved in the SWI/SNF family and recognized as a tumor suppressor in cancer through multiple kinds of pathways, such as p53 and PI3K/AKT pathways [47]. Furthermore, the positive effect of miR-221/222 on the metastasis of CC could also be exacerbated by high-mobility group AT-hook1 (HMGA1), an architectural transcription factor that directly binds to AT-rich regions in the minor groove of DNA4 [48]. Furthermore, miR-10a [49] and miR-590-5p [50] inversely correlated with the expression of a close homolog of l1 (CHL1), a putative tumor suppressor and a member of cell adhesion molecules (CAMs), that results in increased migration and invasion of CC. The migratory and invasive potentials of CC cells could also be activated by miR-501 by lowering the expression of cylindromatosis (CYLD) and subsequently stimulating NF-κB/p65 activation [51]. miR-92a functions as an onco-miRNA by targetting the F-box and WD repeat domain-containing 7 (FBXW7), thereby elevating the metastasis of CC [52]. The expression of miR-181a-5p was positively associated with the progression of CC through adversely targetting inositol polyphosphate-5-phosphatase A (INPP5A) [53]. In addition, the suppression of miR-181a, which was up-regulated in CC cell lines, evidently regulates metastasis of CC by regulating the PTEN/AKT/FOXO1 pathway [54]. MiR-19a/b were noted to be up-regulated in CC and promoted CC cell invasion through direct and negative regulation of Cullin-5 (CUL5) expression, termed as vasopressin-activated calcium mobilizing receptor (VACM-1) [55]. In general, according to the miRNAs mentioned above, detailed signaling pathways are shown in Figure 1.
Figure 1. Oncogenic miRNAs and their targets in promoting metastasis.
Oncosuppressor miRNAs in the metastasis of CC
Mitogen-activated protein kinases
Mitogen-activated protein kinases (MAPKs) are crucial in modulating cancer cell invasion and metastasis and have been implicated in a wide range of physiological processes such as cell growth, differentiation, and apoptosis. HOX transcript antisense RNA (HOTAIR) attributes to the migration and invasion of CC via targetting the miR-23b/MAPK1 axis [56]. As a result, miR-23b plays an inhibitory role in the metastasis of CC. In addition, miR-329-3p [57] and miR-200c [58] suppress cell migration and invasion by targetting MAPK1 or MAPK4.
MMPs
Additionally, the protective roles of miR-132 [59] and miR-183 [60] in the invasion of CC are related to the decreased production of MMP2 and/or MMP9. miR-454-3p restrained CC cell migration and invasion partly due to targetting c-Met, correspondingly leading to the down-regulation of p-AKT, MMP-2, and MMP-9, the downstream proteins of c-Met [61]. miR-486-3p is a significantly down-regulated miRNA in CC and functions to repress CC cell metastasis via down-regulating ECM1 [62]. The metastasis and invasion of CC can also be inhibited by miR-7 through targetting focal adhesion kinase (FAK), an important adhesion kinase that is related to ECM integrin signaling, cell motility, and proliferation [63].
Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF), a well-known tumor metastasis-driving factor, plays a crucial role not only in angiogenesis and vascular permeability but also in the function and trafficking of growth factor receptors and integrins [64]. The down-regulation of LncRNA UCA1 suspends VEGF expression with the introduction of miR-206 [65]. miR-144 exerts a suppressive impact on the migration and invasion of CC cells by targetting VEGFA and VEGFC, which belong to the VEGF family [66].
Up-regulated miR-375, miR-337, and miR-296 lessen CC cell malignant behaviors by targetting transcription factor specificity protein 1 (SP1), exerting many biological functions and contributing to the metastasis of CC [67–69]. SP1 is a member of the specificity protein/Krüppel-like factor (Sp/KLF) transcription factor family and plays a substantial role in the migration, invasion, and metastasis of various tumors.
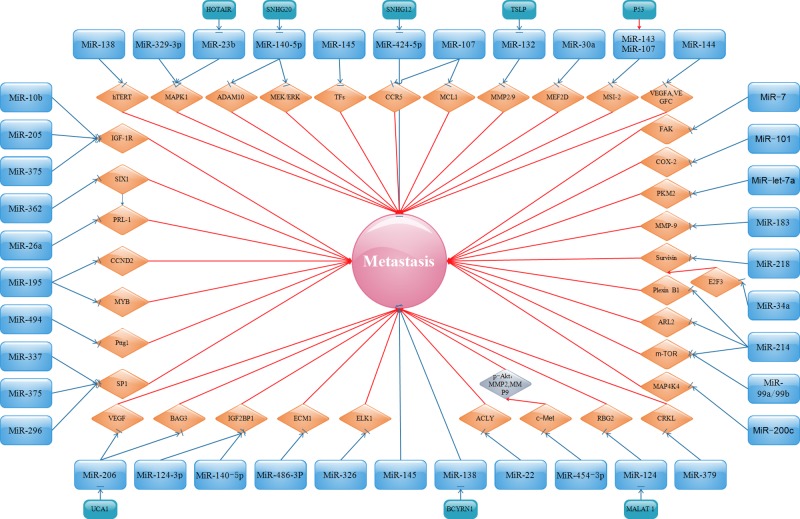
MiR-424-5p, modulated by small nucleolar RNA host gene 12 (SNHG12), serves as a suppressor in metastatic CC [70]. In detail, a disintegrin and metalloproteinase 10 (ADAM10), an important mediator of cell signaling events, is commonly known as a contributor to the metastasis of oral squamous cell carcinoma (OSCC) [71] and osteosarcoma [72]. In fact, miR-140-5p plays a major part in the SNHG20-miR-140-5p-ADAM10-MEK/ERK axis, thus lessening the invasion of CC [73]. MiR-139-3p was recognized as a repressor of CC cell migration and invasion by reducing the expression of NIN1/RPNI2 binding protein 1 homolog (NOB1), a subunit of the 26S proteasome, and acting as an oncogene and inducing metastasis [74]. miR-138 significantly slows HeLa cell (a human CC cell line) migration by targetting human telomerase reverse transcriptase (hTERT), a catalytic subunit of telomerase involved in modulating telomerase activity [75]. Recently, Peng et al. [76] announced that brain cytoplasmic RNA 1 (BCYRN1), clearly up-regulated in CC, can increase invasion via adjusting the expression of miR-138 both in vitro and in vivo. It was demonstrated by Che et al. [77] that miR-107 also plays a suppressive role in the invasion of CC through targetting C–C chemokine receptor type 5 (CCR5), which is recognized as a mediator of chemotaxis and cellular homing and is involved in various biological processes such as the development of tumors. miR-107 also exhibits its inhibitory function in CC metastasis through another target, myeloid cell leukemia-1 (MCL1), an anti-apoptotic member of the Bcl-2 protein family and the activated ATR/Chk1 pathway [78]. Zhou et al. [79] found an adverse relationship between miR-145 levels and core transcription factors (TFs) such as Sox2, Nanog, and Oct4 and determined that high expression of miR-145 was able to inhibit invasion of tumor cells in CC. Thymic stromal lymphopoietin (TSLP), aberrantly highly expressed in CC cells, down-regulates the expression of miR-132 in CC cells and further induces the invasion of HeLa and SiHa cells, which are typical CC cells. The effects of miR-30a on the invasion of CC due to its inverse correlation with myocyte enhancer factor 2D (MEF2D), one member of the MEF2 family that is involved in the progression of various cancers [80]. ETS domain-containing protein Elk-1 (ELK1), which belongs to the ETS oncogene family and mediates transcriptional regulation, might rescue the inhibitory effects on migration and invasion activated by miR-326 [81]. Wang and Tian [82] also published that the overexpression of miR-206 inhibited B-cell lymphoma 2-associated athanogene 3 (BAG3), which is implicated in cell growth and metastasis and correspondingly reduces metastasis. miR-22 reduces CC cell invasion by targetting ATP citrate lyase (ACLY), which is effective in increasing metabolic capacity [83]. Overexpression of insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1) alters the suppressive role of both miR-124-3p [84] and miR-140-5p [85] on the malignant phenotypes of CC cells.
In addition, miR-124 is involved in the inhibition of CC cell invasion partly through the metastasis-associated lung adenocarcinoma transcript 1 (MALAT 1)-miR-124-RBG2 axis [86]. Insulin-like growth factor-1 receptor (IGF-1R), a transmembrane receptor that can enhance cell proliferation and differentiation through the PI3K/AKT and RAS/RAF/MEK/ERK signaling pathways, is involved in miR-10b-, miR-205-, or miR-375-mediated migration and invasion of CC cells [87–89]. The invasion of CC can be clearly suppressed by the up-regulation of miR-99a/b or miR-214 via modulating the mTOR signaling pathway [90,91]. Plexin-B1, as well as ADP ribosylation factors such as 2 (ARL2), negatively correlate with miR-214, and is shown to promote the invasion of HeLa cells [92,93]. The suppression of migration and invasion of CC, resulting from the overexpression of miR-362, was at least partly through the repression of the sineoculis homeobox homolog 1 (SIX1), a member of the homeodomain of the SIX families and related to development and progression of multiple tumors [94]. miR-494 suppresses CC invasiveness by targetting Pttg1, which is shown to induce a cell to enter the active cell cycle and promote cancer cell growth and metastasis [95]. Kogo et al. [96] and Geng et al. [97] declared that the miR-218/survivin or miR-34a/E2F3/survivin axis is pivotal in regulating migration and invasion of CC. E2F3 is a well-known transcription factor that regulates the cell cycle and cell differentiation and modulates the expression of survivin. MiR-let-7a inhibits CC cell migration and invasion via down-regulating pyruvate kinase muscle isozyme M2 (PKM2) [98]. The expression of phosphatase type IVA 1 (PRL-1) is inversely associated with miR-26a and can reverse the inhibitory effect of miR-26a on metastasis in CC [99]. miR-195 represses the expression of cyclin D2 (CCND2) and v-myb avian myeloblastosis viral oncogene homolog (MYB), thereby suppressing metastasis in CC [100]. CCND2 can regulate the cell cycle G1/S transition by communicating with cyclin-dependent kinases (CDKs). MYB is characterized as a cellular homolog of v-myb and a transforming oncogene in certain kinds of cancer. miR-101 negatively regulates cell migration and invasion in CC through inhibition of the target gene cyclooxygenase-2 (COX-2), which is positively involved in tumor development and progression [101,102]. miR-143/miR-107 elevated by p53 directly reduces the expression of Musashi-2 (MSI-2), resulting in the suppression of CC cell invasion [103]. miR-379 might act as a tumor suppressor in CC via negatively modulating V-crk avian sarcoma virus CT10 oncogene homolog-like (CRKL) [104]. miR-485, which is inversely associated with metastasis associated in colon cancer 1 (MACC1), was proven by Wang et al. [105] to inhibit the invasion of CC cells. Finally, based on the miRNAs mentioned above, related signaling pathways can be seen in Figure 2.
Figure 2. Oncosuppressor miRNAs and their targets in inhibiting metastasis.
The role of EMT-related miRNAs in the metastasis of CC
Epithelial–mesenchymal transition (EMT) is widely perceived as a phenotypic switch and allows the tumor to adopt a metastatic and invasive behavior with the down-regulation of E-cadherin and the up-regulation of N-cadherin, vimentin, and other EMT markers [106]. Many factors are extensively known to trigger the process of EMT, such as zinc finger E-box binding homeobox 1 (ZEB1), ZEB2, Snail1, and Snail2 [107,108]. According to Zaravinos [109], numerous signaling pathways play a substantial part in inducing or restraining EMT such as the TGF-β, Wnt, Hedgehog (Hh), Notch, integrin-linked kinase (ILK), and urokinase-type plasminogen activator receptor (uPAR) signaling pathways. Moreover, miRNAs such as miR-34a [110] and miR-200b [111] arise as regulators of EMT and thus modulate metastasis via certain signaling pathways. As discussed above, we have entered a new exciting era and have further explored the present research about the role of EMT-related miRNAs in the metastasis of CC.
Oncogenic EMT-related miRNAs in the metastasis of CC
First, we discuss the promoting role of EMT-related miRNAs in the metastasis of CC.
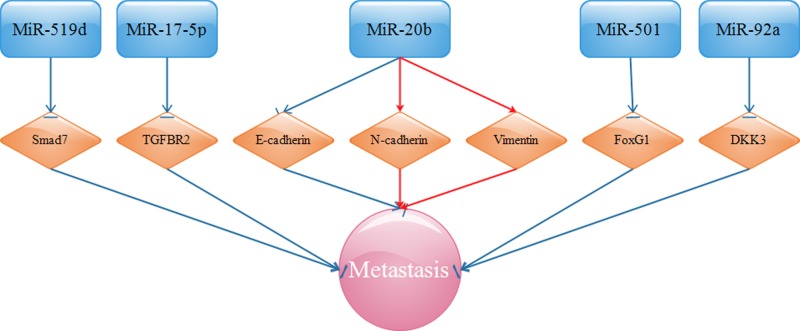
TGF-β is known to play a complex and dichotomous role during tumorigenesis, functioning as a tumor suppressor in normal and early-stage cancers and as a tumor promoter in their late-stage counterparts. The switch in TGF-β function is known as the ‘TGF-β Paradox’, whose manifestations are linked to the initiation of EMT [112]. For instance, miR-17-5p advances the metastasis of CC cells by suppressing transforming growth factor-β receptor 2 (TGF-β R2), a member of the TGF-β signaling pathway [113]. Based on the same signaling pathway, miR-519d facilitates the metastasis of CC by down-regulating Smad7 [114], a member of the Smads family, is documented to play a pivotal role in co-ordinating tumor metastasis via the TGF-β/Smads signaling pathway [115].
Overexpressed miR-20b critically reinforces EMT by decreasing E-cadherin but increasing N-cadherin and vimentin and promoting metastasis [116]. The enrichment of miR-506, inversely modulated by circRNA 000284, prevents metastasis by inhibiting Snail2, an oncogene related to EMT [117]. The augmented expression of miR-92a was shown to eliminate the inhibitory effects of Dickkopf-related protein 3 (DKK3) on CC metastasis [118]. DDK3 acts as a vital tumor suppressor by interacting with the EMT-related Wnt signaling pathway and participating in many biological processes [119]. Up-regulated miR-200b is proposed to positively regulate the metastasis of CC via its definitely validated target, FoxG1 [120]. FoxG1 is perceived as a negative regulator of the TGF-β signaling pathway, thereby showing its oncogenic potential. Given the miRNAs mentioned above, we present these signaling pathways in Figure 3.
Figure 3. EMT-related miRNAs target TGF-β R2, Smad7, E-cadherin, N-cadherin, vimentin, Snail2, and FoxG1, and advance CC migration, invasion, and metastasis.
Oncosuppressor EMT-related miRNAs in the metastasis of CC
We next explore the inhibitory role of EMT-related miRNAs in the metastasis of CC.
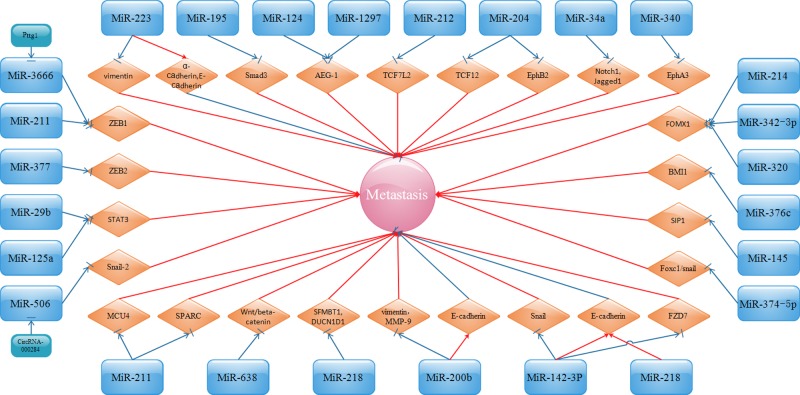
miR-142-3p functions in a tumor suppressive role in the invasion and EMT of CC cells through inhibition of the Frizzled 7 receptor (FZD7), vimentin, and Snail with up-regulation of E-cadherin [121]. FZD7, a member of Wnt receptors, is recognized as pivotal for the activation of both canonical and noncanonical Wnt pathways. Zhou et al. [122] noted that miR-212 plays its suppressive role in the metastasis of CC via inhibiting transcription factor 7-like 2 (TCF7 L2) expression. TCF7 L2 was affirmed as a new transcriptional factor and the critical factor in the Wnt signaling pathway, thus promoting EMT in tumor cells. miR-638 functions as a tumor suppressor in CC metastasis via modulation of the Wnt/β-catenin signaling pathway [123].
The metastasis of CC was clearly inhibited by miR-3666 and miR-211 through the pituitary tumor-transforming gene 1 (Pttg1)/miR-3666/ZEB1 and the miR-211/ZEB1 [124,125] pathways, respectively. Additionally, miR-377 decreases the invasion of CC cells by inversely modulating the expression of ZEB2 [126]. miR-195, which is markedly down-regulated in CC and negatively correlates with Smad3, plays an apparently inhibitory role in CC cell migration and invasion [127]. Smad3 belongs to the Smad family and participates in TGF signaling. TGFβ/Smad3 is shown to induce EMT and the migration and invasion of CC cells. Research by Zhang et al. [128] showed that miR-124 is particularly down-regulated in CC and regarded as anti-miRNA and is involved in the inhibition of EMT and metastasis through directly targetting astrocyte-elevated gene-1 (AEG-1). AEG-1, also known as metadherin (MTDH), and lysine-rich CEACAM1 co-isolate protein (LYRIC) greatly participate in carcinogenesis and tumor progression in several malignancies. Fortunately, it was also discovered by Wang et al. [129] that alteration of AEG-1 eliminates the suppressive effects of miR-1297 on the metastasis of CC and EMT. The down-regulation of miR-145 might generate EMT and the metastasis of CC in conjunction with up-regulated expression of SMAD-interacting protein 1 (SIP1, also known as ZEB2) [130]. SIP1 is accepted as a strong suppressor of E-cadherin and is known to inhibit kinds of junctional complex genes, thus activating invasion and metastasis. miR-211 appears to be anti-miRNA due to its suppressive effect on Mucin 4 (MUC4) [131] and protein acidic and rich in cysteine (SPARC) [132], thus inhibiting CC cell invasion and reversing EMT properties. SPARC, which belongs to the matricellular family of secreted proteins, is related to cell matrix interactions and affects cell progression and might serve as an important factor in the EMT of CC. miR-218 inhibits EMT, migration, and invasion by targetting the 3′-UTRs of Scm-like with four MBT domains 1 (SFMFBT1) and defective in cullin neddylation 1 domain containing 1 (DCUN1D1) in CC [133]. Induced expression of miR-34a suppresses not only Notch1 and Jagged1 but also Notch signaling, thereby inhibiting the invasion capacity of CC cells [134]. miR-204 was verified by Shu et al. [135] as a metastasis-associated gene and might lead to the metastasis of CC via regulating transcription factor 12 (TCF12), a transcriptional repressor of E-cadherin. Moreover, miR-204 acts as a tumor suppressor in the metastasis of CC by directly targetting Ephrin type B receptor 2 (EphB2), which might promote the progression of tumors by inducing EMT and affecting its major downstream signaling pathway, PI3K/AKT [136]. The overexpression of miR-200b in CC cells decreases their migratory potential and EMT as shown by up-regulated E-cadherin and down-regulated vimentin and MMP-9 [137]. Li et al. [138] and Fan et al. [139] found that miR-29b, as well as miR-12 expression, participates in the inhibition of metastasis and EMT procedure of CC cells via targetting the signal transducer and activator of transcription 3 (STAT3) pathway, which performs a vital role in the cellular signaling pathway. The overexpression of Forkhead box M1 (FOXM1) can counteract the inhibitory influence of miR-214, miR-342-3p, and miR-320 on the metastasis of CC [140–142]. Highly expressed FOXM1 is positively associated with tumor metastasis and EMT. miR-374c-5p effectively inhibits the invasion and migration of CC cells and the process of EMT by targetting FOXC1, which belongs to the FOX transcription factor superfamily and greatly participates in EMT and tumor metastasis [143]. miR-376c affects CC metastasis by directly targetting B cell-specific Moloney murine leukemia virus insertion site 1 (BMI1), which might greatly affect EMT [144]. miR-340 is verified to slow the process of tumor metastasis by suppressing Ephrin receptor A3 (EphA3) [145,146]. This kind of regulation is dependent on the EMT pathway, since when miR-340 is overexpressed, the expression of E-cadherin increases and that of vimentin and α-SMA decreases. miR-223 up-regulates the epithelial markers E-cadherin and α-cadherin and down-regulates the mesenchymal marker vimentin, thus suppressing the metastasis of CC [147]. miR-218 overexpression inhibits cell migration partly due to the down-regulation of Bcl-2 and NF-κB and the up-regulation of Bax and E-cadherin [148]. Regarding the miRNAs mentioned above, associated signaling pathways are shown in Figure 4.
Figure 4. EMT-related miRNAs inhibit CC migration, invasion, and metastasis by targetting ZEB1/2, Smad3, AEG-1, TCF7 L2, MUC4, SPARC, Wnt/β-catenin, SIP1, SFMBT, Bcl-2, Bax, NF-κB, E-cadherin, α-cadherin, Notch1, Jagged1, TCF-12, EphB2, Vimentin, MMP-9, STAT3, FZD7, Snail, FOMX1, BMI1, and EphA3.
The role of HPV-related miRNAs in the metastasis of CC
Persistent infection with HPV is acknowledged as one of the greatest risk factors for CC [149]. It is probable that the recent breakthroughs with respect to CC have come from the cognition that HPV silences tumor suppressor genes through HPV-encoded oncoproteins E6 and E7 (HPV E6 and HPV E7). Nonetheless, single HPV infection is not sufficient for the metastasis of CC, and some other HPV-related risk factors are emerging [150]. A number of studies have confirmed that the expression of miRNAs is closely related to HPV, mainly through HPV E6 and HPV E7 [151].
Given that multiple oncosuppressor miRNAs such as miR-99a/b [90], miR-214 [91], and miR-21 [152] are suggested to communicate with the mTOR pathway, we further discuss the interaction between HPV and mTOR. It is recognized that mTOR plays pleiotropic pathogenetic roles not only in different types of cancer including breast cancer [153] and in the development of chemoresistance [154] but also in autoimmune diseases [155,156] and viral diseases such as HIV [157,158]. In 2010, Spangle and Munger [159] showed that HPV16 E6-mediated activation of mTORC1 signaling might result in the promotion of protein synthesis. In fact, as early as 2012, mTOR has become a potential therapeutic target in HPV-associated oral and cervical squamous carcinomas [160]. In addition, mTOR downstream effectors 4EBP1 and eIF4E, which control protein synthesis initiation, are closely correlated with oncogenic HPV types [161]. In an inducible HPV-16 E6/E7 mouse model, mTOR inhibition via rapamycin protected HPV-E6/E7-expressing tissues from carcinogen-induced malignancies [162].
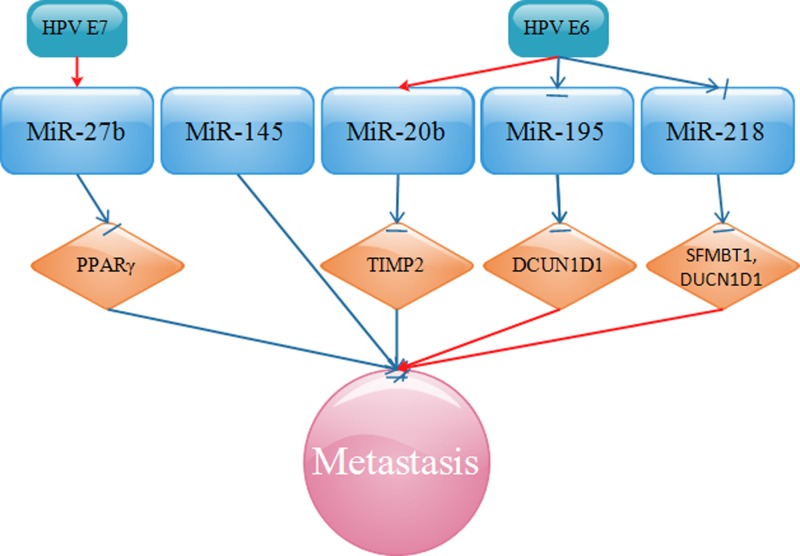
Next, we discuss the role of HPV-related miRNAs in the metastasis of CC. miR-27b, up-regulated by HPV E7, functions to inhibit the expression of peroxisome proliferator-activated receptor γ (PPARγ), a tumor suppressor [163], and to promote invasion of CC cells [164]. miR-20b, up-regulated by HPV E6, acts to restrain TIMP2, thus advancing invasion of CC cells [116]. In addition, HPV E6 promotes CC metastasis by modulating miR-218, thus targetting SFMFBT1 and DCUN1D1 [133]. SFMBT1, a member of the malignant brain tumor (MBT) domain-containing protein family, participates in multiple cellular processes including cell metastasis. DCUN1D1 is recognized as an oncogene and is overexpressed in many types of malignant tumors that leads to a series of diseases including cancers.
In contrast with the miRNAs mentioned above, we next summarized miRNAs that are inversely associated with metastasis of CC. Shi et al. [165] published that a novel HPV-E6-p53-miR-145 pathway plays an important part in the modulation of CC cell invasion. miR-195, targetted by oncogenic HPV E6, negatively mediates CC cell migration and invasion partly through defects in cullin neddylation 1 domain containing 1 (DCUN1D1), which is significantly up-regulated in CC [166]. All the miRNAs related to HPV in the metastasis of CC are shown in Figure 5.
Figure 5. HPV-related miRNAs regulate CC migration, invasion, and metastasis by targetting PPARγ, TIMP2, DCUN1D1, and SFMBT1.
The role of miRNAs in vivo and the diagnosis and treatment of metastatic CC
To date, many studies have been carried out to verify whether miRNAs could play biological functions in in vivo models of CC. Luckily, it was verified that induced expression of miR-let-7a [98], miR-17-5p [113], miR-26a [99], miR-138 [75], miR-145 [79], and miR-206 [82] indeed inhibit the growth of in vivo tumor xenografts of CC. Furthermore, both miR-22 [83] and miR-140-5p [85] significantly suppress not only tumor growth but also metastasis in nude mice. However, silencing miR-200b notably inhibits in vivo tumor growth of CC [120]. In addition, overexpressed miR-21 results in an increase not only in the size of tumors but also in the frequency of lymph node metastasis [33].
With regard to the diagnosis and treatment of metastatic CC, researchers have studied cervical tissues and found a relationship between miRNAs and the diagnosis and treatment of metastatic CC. It was of interest to find that decreased miR-99a/b [90], miR-125a [139], miR-138 [75], miR-140-5p [85], miR-144 [66], miR-195 [127], miR-205 [88], miR-214 [91], miR-218 [96,133,148,167], miR-329-3p [57], miR-337 [68], miR-362 [94], miR-374c-5p [143], miR-375 [67], miR-377 [126], miR-379 [104], miR-485 [105], miR-486-3p [62], miR-638 [123], and miR-1297 [129] expression strongly correlate with tumor size, TNM stage, tissue pathology grade, International Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis, or distant metastasis in patients with CC. In addition, overexpressed miR-20a [31], miR-21 [168], miR-92a [118], miR-145 [79], miR-195 [166], miR-199b-5p [169], and miR-501 [51] closely correlate with histological grade, tumor diameter, overall survival (OS), progression-free survival (PFS), late FIGO stages, lymph node metastasis, or preoperative metastasis. Based on the above discussion, we considered that miRNAs might function as effective tools or potential markers with utility in advances in the diagnosis and treatment of metastatic CC.
Conclusion
miRNA-based cancer therapy is a relatively new concept, and emerging studies are starting to show the potential roles of miRNAs in the possible clinical therapy for human malignancies. miRNAs have been found to play an important role in the metastasis of cancers such as breast cancer [170,171]. Accompanied with the above studies, a preliminary understanding demonstrates the intrinsic features and biological functions of miRNAs during the metastasis of CC. From Figures 1 to 5, it is easy for us to distinguish miRNAs between those communicating with oncogenes or tumor suppressor genes and those affecting invasion and metastasis. miRNAs have a vital role in all stages of CC progression from cell invasion and migration to eventual tumor metastasis. Because miRNAs are comprehensively associated with the metastasis of CC, intensive research on the roles of miRNAs is urgently needed, which will provide novel probable targets for the development of therapies for CC.
In recent years, the rapid development of miRNA profiling microarray chips and high-throughput sequencing have shown a great advantage in accelerating the study of the relationship between CC and miRNAs. Secreted miRNAs in serum could be detected for cancer diagnosis, including early metastasis of CC based on alterations in various miRNA serum levels. Furthermore, according to advances in the depth of sequencing and the recognition of tumor metastasis, miRNAs interact with other molecules previously unknown to us such as extracellular vesicles (EVs), circRNAs, and lncRNAs. These molecules, along with miRNAs, have been found to function together to modulate the progression of cancers [172–174].
Thus, miRNA-based therapy may be possible, as there are many approaches to miRNA-specific personalized treatment and molecular targetted therapy. In the meantime, it might be a potential future anticancer therapy by regulating the expression of oncogenic miRNAs.
Abbreviations
- 3′-UTR
3′-Untranslated region
- AEG-1
Astrocyte-elevated gene-1
- ACLY
ATP citrate lyase
- ADAM10
A disintegrin and metalloproteinase 10
- ARF
ADP-ribosylation factor
- ARID1A
AT-rich interactive domain-containing protein 1A
- ARL2
ADP-ribosylation factor like 2
- ATG
Autophagy-related protein
- ATR/Chk1
ATM- and RAD2-related/Chk1
- BAG3
B-cell lymphoma 2-associated athanogene 3
- Bcl-2
B-cell lymphoma-2
- BCYRN1
Brain cytoplasmic RNA 1
- BIRC5
Survivin
- BMI1
B-cell-specific moloney murine leukemia virus insertion site 1
- CAM
Cell adhesion molecule
- CC
cervical cancer
- CircRNA
Circular RNA
- CCND2
Cyclin D2
- CCR5
C–C chemokine receptor type 5
- CDK
Cyclin-dependent kinase
- CHL1
Close homolog of l1
- circRNA
Circular RNA
- CYLD
Cylindromatosis
- COX-2
Cyclooxygenase-2
- CRKL
V-crk avian sarcoma virus CT10 oncogene homolog-like
- CUL5
Cullin-5
- DCUN1D1
Defective in cullin neddylation 1, domain containing 1
- DDK3
recombinant human dickkopf-related protein 3
- DKK3
Dickkopf-related protein 3
- E2F3
E2F transcription factor 3
- ECM
Extracellular matrix
- eIF4E
eukaryotic translation initiation factor 4E
- ELK1
ETS domain-containing protein Elk-1
- EMT
Epithelial–mesenchymal transition
- EphA3
Ephrin receptor A3
- EphB2
Ephrin type B receptor 2
- EphA3
Ephrin receptor A3
- EV
Extracellular vesicle
- FAK
Focal adhesion kinase
- FBXW7
F-box and WD repeat domain-containing 7
- FIGO
International Federation of Gynecology and Obstetrics
- FoxG1
forkhead box G1
- FOXM1
Forkhead box M1
- FZD7
Frizzled7 receptor
- Hh
Hedgehog
- HMGA1
High-mobility group AT-hook1
- HOTAIR
HOX transcript antisense RNA
- HOX
homeobox
- HPV
Human papillomavirus
- hTERT
ATCC human telomerase reverse transcriptase
- hTERT
Human telomerase reverse transcriptase
- IGF2BP1
Insulin-like growth factor 2 mRNA binding protein 1
- IGF-1R
Insulin-like growth factor-1 receptor
- ILK
Integrin-linked kinase
- INPP5A
Inositol polyphosphate-5-phosphatase A
- LNM
Lymph node metastasis
- MACC1
Metastasis associated in colon cancer1
- MALAT 1
Metastasis-associated lung adenocarcinoma transcript 1
- MAPK
Mitogen-activated protein kinase
- MAP4K4
Mitogen-activated protein kinase kinase kinase kinase 4
- MBT
Malignant brain tumor
- MCL1
Myeloid cell leukemia-1
- MEF2D
Myocyte enhancer factor 2D
- MiRNA
MicroRNA
- MMP
Matrix metalloproteinase
- mRNA
Messenger RNA
- MSI-2
Musashi-2,
- mTOR
Rapamycin
- mTOR
mechanistic target of rapamycin
- mTORC1
mammalian target of rapamycin complex-1
- MUC 4
Mucin 4
- MYB
V-myb avian myeloblastosis viral oncogene homolog
- NF-κB
nuclear factor-kappa B
- NOB1
NIN1/RPNI2 binding protein 1 homolog
- OS
Overall survival
- PARP
Human telomere-associated poly (ADP-ribose) polymerase
- PDCD4
Programmed cell death protein 4
- PEBP1
phosphatidylethanolamine binding protein 1
- PFS
Progression-free survival
- PI3K/AKT
Phophatidylinositol 3-kinase/protein kinase-B
- PKM2
Pyruvate kinase muscle isozyme M2
- PPARγ
Peroxisome proliferators-activated receptor γ
- PRL-1
Phosphatase type IVA 1
- PTEN/FOXO1
phosphatase and tensin homolog deleted on chromosome ten/forkhead box O1
- Pttg1
Pituitary tumor-transforming gene 1
- SCC
Squamous cell carcinoma
- SFMBT1
Scm-like with four MBT domains 1
- SIP1
SMAD-interacting protein 1
- SIX1
Sineoculis homeobox homolog 1
- SNHG12
Small nucleolar RNA host gene 12
- SPARC
Protein acidic and rich in cysteine
- Sp1
Specificity protein 1
- STAT3
Signal transducer and activator of transcription 3
- SWI/SNF
switch in mating type/sucrose non fermentation
- TCF7L2
Transcription factor 7-like 2
- TCF12
Transcription factor 12
- TF
Transcription factor
- TGFβR2
Transforming growth factor-β receptor 2
- THBS2, TSP2
Thrombospondin-2
- TIMP
Tissue inhibitor of metalloproteinase
- TNKS2
Tankyrase 2
- TSLP
Thymic stromal lymphopoietin
- TWIST2
Twist homolog 2
- UCA1
Urothelial cancer associated 1
- VACM-1
Vasopressin-activated calcium mobilizing receptor
- VEGF
Vascular endothelial growth factor
- VEGFA
Vascular endothelial growth factor A
- ZEB1
Zinc finger E-box binding homeobox 1
- ZEB2
Zinc finger E-box-binding homeobox 2
Author contribution
All authors are responsible for the content and writing of the paper.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81602551, 81702613]; the Key Research Program for the Social Development of Jiangsu Province, China [grant number BE2015718]; the National Program on Key Research Project [grant number 2016YFC0905900]; the Medical Training Programme Foundation for the Talents by Jiangsu Provincial Department of Health [grant number 17 [2016]]; the 333 Talent Project of Jiangsu Province, top-level [grant number 4 [2016]]; the National Key Clinical Specialist Construction Programs of China [grant number 544 [2013]]; the Major Program of Natural Science Foundation of Jiangsu Province [grant number BL2014090]; the Natural Science Foundation of Jiangsu Province [grant number BK20151579]; the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) [grant number JX10231802]; the Postgraduate Research and Practice Innovation Program of Jiangsu Province [grant number SJCX17_0387]; and the Young Talents Program of Jiangsu Cancer Hospital [grant number 2017YQL-10].
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J. and Jemal A. (2015) Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Park S., Eom K., Kim J., Bang H., Wang H.Y., Ahn S.. et al. (2017) MiR-9, miR-21, and miR-155 as potential biomarkers for HPV positive and negative cervical cancer. BMC Cancer 17, 658 10.1186/s12885-017-3642-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabelo-Santos S.H., Termini L., Boccardo E., Derchain S., Longatto-Filho A., Andreoli M.A.. et al. (2018) Strong SOD2 expression and HPV-16/18 positivity are independent events in cervical cancer. Oncotarget 9, 21630–21640 10.18632/oncotarget.24850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardini B., De Maria D., Francavilla A., Di Gaetano C., Ronco G. and Naccarati A. (2018) MicroRNAs as markers of progression in cervical cancer: a systematic review. BMC Cancer 18, 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H.H. and Li A.H. (2018) Long non-coding RNA FEZF1-AS1 is up-regulated and associated with poor prognosis in patients with cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 22, 3357–3362 [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi-Ponciano K., Marrero-Rodriguez D., Arreola-De la Cruz H., Huerta-Padilla V., Munoz N., Gomez-Ortiz L.. et al. (2018) The KISS1 gene overexpression as a potential molecular marker for cervical cancer cells. Cancer Biomark. 22, 709–719 10.3233/CBM-181215 [DOI] [PubMed] [Google Scholar]
- 7.Xu F., Li Y., Fan L., Ma J., Yu L., Yi H.. et al. (2018) Preoperative SCC-Ag and thrombocytosis as predictive markers for pelvic lymphatic metastasis of squamous cervical cancer in early FIGO stage. J. Cancer 9, 1660–1666 10.7150/jca.24049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanthamongkolkul K. and Hanprasertpong J. (2018) Predictive factors of pelvic lymph node metastasis in early-stage cervical cancer. Oncol. Res. Treat. 41, 194–198 10.1159/000485840 [DOI] [PubMed] [Google Scholar]
- 9.Guo H., Dai Y., Wang A., Wang C., Sun L. and Wang Z. (2018) Association between expression of MMP-7 and MMP-9 and pelvic lymph node and para-aortic lymph node metastasis in early cervical cancer. J. Obstet. Gynecol. Res. 44, 1274–1283 10.1111/jog.13659 [DOI] [PubMed] [Google Scholar]
- 10.Wright J.D., Huang Y., Ananth C.V., Tergas A.I., Duffy C., Deutsch I.. et al. (2015) Influence of treatment center and hospital volume on survival for locally advanced cervical cancer. Gynecol. Oncol. 139, 506–512 10.1016/j.ygyno.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi X., Ran L., Liu Y., Zhong S.H., Zhou P.P., Liao M.X.. et al. (2018) Knockdown of hnRNP A2/B1 inhibits cell proliferation, invasion and cell cycle triggering apoptosis in cervical cancer via PI3K/AKT signaling pathway. Oncol. Rep. 39, 939–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li A., Gu Y., Li X., Sun H., Zha H., Xie J.. et al. (2018) S100A6 promotes the proliferation and migration of cervical cancer cells via the PI3K/Akt signaling pathway. Oncol. Lett. 15, 5685–5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan K., Zhao Y., Fan Y., Ma B., Yang S., Liu Q.. et al. (2017) Sulfiredoxin may promote cervical cancer metastasis via Wnt/beta-Catenin signaling pathway. Int. J. Mol. Sci. 18, 10.1016/ijms18050917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L.Z., Huang L.Y., Huang A.L., Liu J.X. and Yang F. (2018) CRIP1 promotes cell migration, invasion and epithelial-mesenchymal transition of cervical cancer by activating the Wnt/betacatenin signaling pathway. Life Sci. 207, 420–427 10.1016/j.lfs.2018.05.054 [DOI] [PubMed] [Google Scholar]
- 15.Hou J., Yang H., Huang X., Leng X., Zhou F., Xie C.. et al. (2017) N-WASP promotes invasion and migration of cervical cancer cells through regulating p38 MAPKs signaling pathway. Am. J. Transl. Res. 9, 403–415 [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F., Ren C.C., Liu L., Chen Y.N. and Yang L. (2018) SHH gene silencing suppresses epithelial-mesenchymal transition, proliferation, invasion, and migration of cervical cancer cells by repressing the hedgehog signaling pathway. J Cell Biochem 119, 3829–3842 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Li L., Liu Y., Geng P., Li G., Yang Y.. et al. (2018) RECK inhibits cervical cancer cell migration and invasion by promoting p53 signaling pathway. J Cell Biochem 119, 3058–3066 [DOI] [PubMed] [Google Scholar]
- 18.Zhang J., Zhao X., Zhang J., Zheng X. and Li F. (2018) Circular RNA hsa_circ_0023404 exerts an oncogenic role in cervical cancer through regulating miR-136/TFCP2/YAP pathway. Biochem. Biophys. Res. Commun. 501, 428–433 10.1016/j.bbrc.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Shi Y., Wang W., Yang B. and Tian H. (2017) ATF1 and RAS in exosomes are potential clinical diagnostic markers for cervical cancer. Cell Biochem Funct 35, 477–483 [DOI] [PubMed] [Google Scholar]
- 20.Chen X., Xiong D., Yang H., Ye L., Mei S., Wu J.. et al. (2018) Long noncoding RNA OPA-interacting protein 5 antisense transcript 1 upregulated SMAD3 expression to contribute to metastasis of cervical cancer by sponging miR-143-3p. J Cell Physiol 234, 5264–5275 [DOI] [PubMed] [Google Scholar]
- 21.Granados Lopez A.J. and Lopez J.A. (2014) Multistep model of cervical cancer: participation of miRNAs and coding genes. Int. J. Mol. Sci. 15, 15700–15733 10.3390/ijms150915700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lajer C.B., Garnaes E., Friis-Hansen L., Norrild B., Therkildsen M.H., Glud M.. et al. (2012) The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br. J. Cancer 106, 1526–1534 10.1038/bjc.2012.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R., Zhao N., Li S., Fang J.H., Chen M.X., Yang J.. et al. (2013) MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology 58, 642–653 10.1002/hep.26373 [DOI] [PubMed] [Google Scholar]
- 24.Luo B., Kang N., Chen Y., Liu L. and Zhang Y. (2018) Oncogene miR-106a promotes proliferation and metastasis of prostate cancer cells by directly targeting PTEN in vivo and in vitro. Minerva Med. 109, 24–30 [DOI] [PubMed] [Google Scholar]
- 25.Xue Y., Xu W., Zhao W., Wang W., Zhang D. and Wu P. (2017) miR-381 inhibited breast cancer cells proliferation, epithelial-to-mesenchymal transition and metastasis by targeting CXCR4. Biomed. Pharmacother. 86, 426–433 10.1016/j.biopha.2016.12.051 [DOI] [PubMed] [Google Scholar]
- 26.Zhou L., Zhao L.C., Jiang N., Wang X.L., Zhou X.N., Luo X.L.. et al. (2017) MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2. Eur. Rev. Med. Pharmacol. Sci. 21, 87–94 [PubMed] [Google Scholar]
- 27.Gonzalez-Quintana V., Palma-Berre L., Campos-Parra A.D., Lopez-Urrutia E., Peralta-Zaragoza O., Vazquez-Romo R.. et al. (2016) MicroRNAs are involved in cervical cancer development, progression, clinical outcome and improvement treatment response (Review). J. Cell. Physiol. 35, 3–12 [DOI] [PubMed] [Google Scholar]
- 28.Haller M., Hock A.K., Giampazolias E., Oberst A., Green D.R., Debnath J.. et al. (2014) Ubiquitination and proteasomal degradation of ATG12 regulates its proapoptotic activity. Autophagy 10, 2269–2278 10.4161/15548627.2014.981914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murrow L., Malhotra R. and Debnath J. (2015) ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat. Cell Biol. 17, 300–310 10.1038/ncb3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandelbaum J., Rollins N., Shah P., Bowman D., Lee J.Y., Tayber O.. et al. (2015) Identification of a lung cancer cell line deficient in atg7-dependent autophagy. Autophagy 19, 10.1080/15548627.2015.1056966 [DOI] [PubMed] [Google Scholar]
- 31.Zhao S., Yao D., Chen J., Ding N. and Ren F. (2015) MiR-20a promotes cervical cancer proliferation and metastasis in vitro and in vivo. PLoS ONE 10, e0120905 10.1371/journal.pone.0120905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan D., Zhou C., Han S., Hou X., Kang S. and Zhang Y. (2018) MicroRNA-378 enhances migration and invasion in cervical cancer by directly targeting autophagy-related protein 12. Mol. Med. Rep. 17, 6319–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei W.F., Han L.F., Liu D., Wu L.F., Chen X.J., Yi H.Y.. et al. (2017) Orthotopic xenograft mouse model of cervical cancer for studying the role of microRNA-21 in promoting lymph node metastasis. Int. J. Gynecol. Cancer 27, 1587–1595 10.1097/IGC.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 34.Pietruszewska W., Bojanowska-Pozniak K. and Kobos J. (2016) Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: an immunohistochemical study. Otolaryngol. Pol. 70, 32–43 10.5604/00306657.1202546 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., Wang J., Wang X., Song W., Shi Y. and Zhang L. (2018) MicroRNA-21 promotes proliferation, migration, and invasion of cervical cancer through targeting TIMP3. Arch. Gynecol. Obstet. 297, 433–442 10.1007/s00404-017-4598-z [DOI] [PubMed] [Google Scholar]
- 36.Li X., Zhou Q., Tao L. and Yu C. (2017) MicroRNA-106a promotes cell migration and invasion by targeting tissue inhibitor of matrix metalloproteinase 2 in cervical cancer. Cell Oncol. 38, 1774–1782 [DOI] [PubMed] [Google Scholar]
- 37.Chen J., Yao D., Zhao S., He C., Ding N., Li L.. et al. (2014) MiR-1246 promotes SiHa cervical cancer cell proliferation, invasion, and migration through suppression of its target gene thrombospondin 2. Arch. Gynecol. Obstet. 290, 725–732 10.1007/s00404-014-3260-2 [DOI] [PubMed] [Google Scholar]
- 38.Wei W.F., Zhou C.F., Wu X.G., He L.N., Wu L.F., Chen X.J.. et al. (2017) MicroRNA-221-3p, a TWIST2 target, promotes cervical cancer metastasis by directly targeting THBS2. Cell Death Dis. 8, 3220 10.1038/s41419-017-0077-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Roudnicky F., Yoon S.Y., Poghosyan S., Schwager S., Poyet C., Vella G.. et al. (2018) Alternative transcription of a shorter, non-anti-angiogenic thrombospondin-2 variant in cancer-associated blood vessels. Oncogene 37, 2573–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacLauchlan S.C., Calabro N.E., Huang Y., Krishna M., Bancroft T., Sharma T.. et al. (2018) HIF-1alpha represses the expression of the angiogenesis inhibitor thrombospondin-2. Oncogene 65, 45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prescott J., McGrath M., Lee I.M., Buring J.E. and De Vivo I. (2010) Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer 116, 4275–4282 10.1002/cncr.25328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafnar T., Sulem P., Stacey S.N., Geller F., Gudmundsson J., Sigurdsson A.. et al. (2009) Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat. Genet. 41, 221–227 10.1038/ng.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang H.W., Wang F., Wei Q., Zhao Y.F., Liu M., Li X.. et al. (2012) miR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett. 586, 897–904 10.1016/j.febslet.2012.02.020 [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z., Wang J., Li J., Wang X. and Song W. (2018) MicroRNA-150 promotes cell proliferation, migration, and invasion of cervical cancer through targeting PDCD4. Biomed. Pharmacother. 97, 511–517 10.1016/j.biopha.2017.09.143 [DOI] [PubMed] [Google Scholar]
- 45.Wang N., Zhou Y., Zheng L. and Li H. (2014) MiR-31 is an independent prognostic factor and functions as an oncomir in cervical cancer via targeting ARID1A. Gynecol. Oncol. 134, 129–137 10.1016/j.ygyno.2014.04.047 [DOI] [PubMed] [Google Scholar]
- 46.Yang Y., Zhao X. and Li H.X. (2016) MiR-221 and miR-222 simultaneously target ARID1A and enhance proliferation and invasion of cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 20, 1509–1515 [PubMed] [Google Scholar]
- 47.Wu R.C., Wang T.L. and Shih Ie M. (2014) The emerging roles of ARID1A in tumor suppression. Cancer Biol. Ther. 15, 655–664 10.4161/cbt.28411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu F., Wang T., Wu Z., Feng Y., Wang W., Zhou S.. et al. (2018) HMGA1 exacerbates tumor growth through regulating the cell cycle and accelerates migration/invasion via targeting miR-221/222 in cervical cancer. Cell Death Dis. 9, 594 10.1038/s41419-018-0683-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long M.J., Wu F.X., Li P., Liu M., Li X. and Tang H. (2012) MicroRNA-10a targets CHL1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer Lett. 324, 186–196 10.1016/j.canlet.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 50.Chu Y., Ouyang Y., Wang F., Zheng A., Bai L., Han L.. et al. (2014) MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J. Cell. Biochem. 115, 847–853 10.1002/jcb.24726 [DOI] [PubMed] [Google Scholar]
- 51.Sanches J.G.P., Xu Y., Yabasin I.B., Li M., Lu Y., Xiu X.. et al. (2018) miR-501 is upregulated in cervical cancer and promotes cell proliferation, migration and invasion by targeting CYLD. Chem. Biol. Interact. 285, 85–95 10.1016/j.cbi.2018.02.024 [DOI] [PubMed] [Google Scholar]
- 52.Zhou C., Shen L., Mao L., Wang B., Li Y. and Yu H. (2015) miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by targeting FBXW7. Biochem. Biophys. Res. Commun. 458, 63–69 10.1016/j.bbrc.2015.01.066 [DOI] [PubMed] [Google Scholar]
- 53.Yang M., Zhai X., Ge T., Yang C. and Lou G. (2018) miR-181a-5p promotes proliferation and invasion and inhibits apoptosis of cervical cancer cells via regulating inositol polyphosphate-5-phosphatase A (INPP5A). Oncol. Res. 26, 703–712 10.3727/096504017X14982569377511 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Xu H., Zhu J., Hu C., Song H. and Li Y. (2016) Inhibition of microRNA-181a may suppress proliferation and invasion and promote apoptosis of cervical cancer cells through the PTEN/Akt/FOXO1 pathway. J. Physiol. Biochem. 72, 721–732 10.1007/s13105-016-0511-7 [DOI] [PubMed] [Google Scholar]
- 55.Xu X.M., Wang X.B., Chen M.M., Liu T., Li Y.X., Jia W.H.. et al. (2012) MicroRNA-19a and -19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 322, 148–158 10.1016/j.canlet.2012.02.038 [DOI] [PubMed] [Google Scholar]
- 56.Li Q., Feng Y., Chao X., Shi S., Liang M., Qiao Y.. et al. (2018) HOTAIR contributes to cell proliferation and metastasis of cervical cancer via targetting miR-23b/MAPK1 axis. Biosci. Rep. 38, 10.1042/BSR20171563 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Li W., Liang J., Zhang Z., Lou H., Zhao L., Xu Y.. et al. (2017) MicroRNA-329-3p targets MAPK1 to suppress cell proliferation, migration and invasion in cervical cancer. Oncol. Rep. 37, 2743–2750 10.3892/or.2017.5555 [DOI] [PubMed] [Google Scholar]
- 58.Mei J., Wang D.H., Wang L.L., Chen Q., Pan L.L. and Xia L. (2018) MicroRNA-200c suppressed cervical cancer cell metastasis and growth via targeting MAP4K4. Eur. Rev. Med. Pharmacol. Sci. 22, 623–631 [DOI] [PubMed] [Google Scholar]
- 59.Zhou W.J., Yang H.L., Chang K.K., Meng Y., Wang M.Y., Yuan M.M.. et al. (2017) Human thymic stromal lymphopoietin promotes the proliferation and invasion of cervical cancer cells by downregulating microRNA-132 expression. Oncol. Lett. 14, 7910–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan D., Wang Y., Qi P., Chen Y., Xu P., Yang X.. et al. (2016) MicroRNA-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol. Oncol. 141, 166–174 10.1016/j.ygyno.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 61.Guo Y., Tao M. and Jiang M. (2018) MicroRNA-454-3p inhibits cervical cancer cell invasion and migration by targeting c-Met. Exp. Ther. Med. 15, 2301–2306 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Ye H., Yu X., Xia J., Tang X., Tang L. and Chen F. (2016) MiR-486-3p targeting ECM1 represses cell proliferation and metastasis in cervical cancer. Biomed. Pharmacother. 80, 109–114 10.1016/j.biopha.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 63.Hao Z., Yang J., Wang C., Li Y., Zhang Y., Dong X.. et al. (2015) MicroRNA-7 inhibits metastasis and invasion through targeting focal adhesion kinase in cervical cancer. Int. J. Clin. Exp. Med. 8, 480–487 [PMC free article] [PubMed] [Google Scholar]
- 64.Goel H.L. and Mercurio A.M. (2013) VEGF targets the tumour cell. Nat. Rev. Cancer 13, 871–882 10.1038/nrc3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan Q., Tian Y. and Hao F. (2018) Downregulation of lncRNA UCA1 inhibits proliferation and invasion of cervical cancer cells through miR-206 expression. Oncol. Res. 10.3727/096504018X15185714083446 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Tao P., Wen H., Yang B., Zhang A., Wu X. and Li Q. (2018) miR-144 inhibits growth and metastasis of cervical cancer cells by targeting VEGFA and VEGFC. Exp. Ther. Med. 15, 562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F., Li Y., Zhou J., Xu J., Peng C., Ye F.. et al. (2011) miR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am. J. Pathol. 179, 2580–2588 10.1016/j.ajpath.2011.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong W., Li B., Wang J., Song Y., Zhang Z. and Fu C. (2017) MicroRNA-337 inhibits cell proliferation and invasion of cervical cancer through directly targeting specificity protein 1. Tumour Biol. 39, 10.1177/1010428317711323 [DOI] [PubMed] [Google Scholar]
- 69.Lv L. and Wang X. (2018) MicroRNA-296 targets specificity protein 1 to suppress cell proliferation and invasion in cervical cancer. Oncol. Res. 26, 775–783 10.3727/096504017X15132494420120 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Dong J., Wang Q., Li L. and Xiao-Jin Z. (2018) Upregulation of long non-coding RNA small nucleolar RNA host gene 12 contributes to cell growth and invasion in cervical cancer by acting as a sponge for MiR-424-5p. Cell. Physiol. Biochem. 45, 2086–2094 10.1159/000488045 [DOI] [PubMed] [Google Scholar]
- 71.Stasikowska-Kanicka O., Wagrowska-Danilewicz M., Kulicka P. and Danilewicz M. (2018) Overexpression of ADAM10 in oral squamous cell carcinoma with metastases. Pol. J. Pathol. 69, 67–72 10.5114/pjp.2018.75339 [DOI] [PubMed] [Google Scholar]
- 72.Zhao R., Ni D., Tian Y., Ni B. and Wang A. (2014) Aberrant ADAM10 expression correlates with osteosarcoma progression. Eur. J. Med. Res. 19, 9 10.1186/2047-783X-19-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo H., Yang S., Li S., Yan M., Li L. and Zhang H. (2018) LncRNA SNHG20 promotes cell proliferation and invasion via miR-140-5p-ADAM10 axis in cervical cancer. Biomed. Pharmacother. 102, 749–757 10.1016/j.biopha.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 74.Huang P., Xi J. and Liu S. (2016) MiR-139-3p induces cell apoptosis and inhibits metastasis of cervical cancer by targeting NOB1. Biomed. Pharmacother. 83, 850–856 10.1016/j.biopha.2016.07.050 [DOI] [PubMed] [Google Scholar]
- 75.Zhou N., Fei D., Zong S., Zhang M. and Yue Y. (2016) MicroRNA-138 inhibits proliferation, migration and invasion through targeting hTERT in cervical cancer. Oncol. Lett. 12, 3633–3639 10.3892/ol.2016.5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng J., Hou F., Feng J., Xu S.X. and Meng X.Y. (2018) Long non-coding RNA BCYRN1 promotes the proliferation and metastasis of cervical cancer via targeting microRNA-138 in vitro and in vivo. Oncol. Lett. 15, 5809–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Che L.F., Shao S.F. and Wang L.X. (2016) Downregulation of CCR5 inhibits the proliferation and invasion of cervical cancer cells and is regulated by microRNA-107. Exp. Ther. Med. 11, 503–509 10.3892/etm.2015.2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou C., Li G., Zhou J., Han N., Liu Z. and Yin J. (2014) miR-107 activates ATR/Chk1 pathway and suppress cervical cancer invasion by targeting MCL1. PLoS ONE 9, e111860 10.1371/journal.pone.0111860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou X., Yue Y., Wang R., Gong B. and Duan Z. (2017) MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer stem cells. Int. J. Oncol. 50, 853–862 10.3892/ijo.2017.3857 [DOI] [PubMed] [Google Scholar]
- 80.Zhao J., Li B., Shu C., Ma Y. and Gong Y. (2017) Downregulation of miR-30a is associated with proliferation and invasion via targeting MEF2D in cervical cancer. Oncol. Lett. 14, 7437–7442 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Zhang P., Kong F., Deng X., Yu Y., Hou C., Liang T.. et al. (2017) MicroRNA-326 suppresses the proliferation, migration and invasion of cervical cancer cells by targeting ELK1. Oncol. Lett. 13, 2949–2956 10.3892/ol.2017.5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y. and Tian Y. (2018) miR-206 inhibits cell proliferation, migration, and invasion by targeting BAG3 in human cervical cancer. Oncol. Res. 26, 923–931 10.3727/096504017X15143731031009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xin M., Qiao Z., Li J., Liu J., Song S., Zhao X.. et al. (2016) miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 7, 44252–44265 10.18632/oncotarget.10020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P., Zhang L., Zhang J. and Xu G. (2018) MicroRNA-124-3p inhibits cell growth and metastasis in cervical cancer by targeting IGF2BP1. Exp. Ther. Med. 15, 1385–1393 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Su Y., Xiong J., Hu J., Wei X., Zhang X. and Rao L. (2016) MicroRNA-140-5p targets insulin like growth factor 2 mRNA binding protein 1 (IGF2BP1) to suppress cervical cancer growth and metastasis. Oncotarget 7, 68397–68411 10.18632/oncotarget.11722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S., Song L., Zeng S. and Zhang L. (2016) MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumour Biol. 37, 633–640 10.1007/s13277-015-3732-4 [DOI] [PubMed] [Google Scholar]
- 87.Hou R., Wang D. and Lu J. (2017) MicroRNA-10b inhibits proliferation, migration and invasion in cervical cancer cells via direct targeting of insulin-like growth factor-1 receptor. Oncol. Lett. 13, 5009–5015 10.3892/ol.2017.6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pang H. and Yue X. (2017) MiR-205 serves as a prognostic factor and suppresses proliferation and invasion by targeting insulin-like growth factor receptor 1 in human cervical cancer. Tumour Biol. 39, 1010428317701308 10.1177/1010428317701308 [DOI] [PubMed] [Google Scholar]
- 89.Yu X., Zhao W., Yang X., Wang Z. and Hao M. (2016) miR-375 affects the proliferation, invasion, and apoptosis of HPV16-positive human cervical cancer cells by targeting IGF-1R. Int. J. Gynecol. Cancer 26, 851–858 10.1097/IGC.0000000000000711 [DOI] [PubMed] [Google Scholar]
- 90.Wang L., Chang L., Li Z., Gao Q., Cai D., Tian Y.. et al. (2014) miR-99a and -99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med. Oncol. 31, 934 10.1007/s12032-014-0934-3 [DOI] [PubMed] [Google Scholar]
- 91.Wang F., Tan W.H., Liu W., Jin Y.X., Dong D.D., Zhao X.J.. et al. (2018) Effects of miR-214 on cervical cancer cell proliferation, apoptosis and invasion via modulating PI3K/AKT/mTOR signal pathway. Eur. Rev. Med. Pharmacol. Sci. 22, 1891–1898 [DOI] [PubMed] [Google Scholar]
- 92.Qiang R., Wang F., Shi L.Y., Liu M., Chen S., Wan H.Y.. et al. (2011) Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int. J. Biochem. Cell Biol. 43, 632–641 10.1016/j.biocel.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 93.Peng R., Men J., Ma R., Wang Q., Wang Y., Sun Y.. et al. (2017) miR-214 down-regulates ARL2 and suppresses growth and invasion of cervical cancer cells. Biochem. Biophys. Res. Commun. 484, 623–630 10.1016/j.bbrc.2017.01.152 [DOI] [PubMed] [Google Scholar]
- 94.Shi C. and Zhang Z. (2017) MicroRNA-362 is downregulated in cervical cancer and inhibits cell proliferation, migration and invasion by directly targeting SIX1. Oncol. Rep. 37, 501–509 10.3892/or.2016.5242 [DOI] [PubMed] [Google Scholar]
- 95.Chen B., Hou Z., Li C. and Tong Y. (2015) MiRNA-494 inhibits metastasis of cervical cancer through Pttg1. Tumour Biol. 36, 7143–7149 10.1007/s13277-015-3440-0 [DOI] [PubMed] [Google Scholar]
- 96.Kogo R., How C., Chaudary N., Bruce J., Shi W., Hill R.P.. et al. (2015) The microRNA-218∼Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget 6, 1090–1100 10.18632/oncotarget.2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geng D., Song X., Ning F., Song Q. and Yin H. (2015) MiR-34a inhibits viability and invasion of human papillomavirus-positive cervical cancer cells by targeting E2F3 and regulating survivin. Int. J. Gynecol. Cancer 25, 707–713 10.1097/IGC.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 98.Guo M., Zhao X., Yuan X., Jiang J. and Li P. (2017) MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget 8, 28226–28236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong J., Sui L., Wang Q., Chen M. and Sun H. (2014) MicroRNA-26a inhibits cell proliferation and invasion of cervical cancer cells by targeting protein tyrosine phosphatase type IVA 1. Mol. Med. Rep. 10, 1426–1432 10.3892/mmr.2014.2335 [DOI] [PubMed] [Google Scholar]
- 100.Du X., Lin L.I., Zhang L. and Jiang J. (2015) microRNA-195 inhibits the proliferation, migration and invasion of cervical cancer cells via the inhibition of CCND2 and MYB expression. Oncol. Lett. 10, 2639–2643 10.3892/ol.2015.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang F., Lin C., Shi Y.H. and Kuerban G. (2013) MicroRNA-101 inhibits cell proliferation, invasion, and promotes apoptosis by regulating cyclooxygenase-2 in Hela cervical carcinoma cells. Asian Pac. J. Cancer Prev. 14, 5915–5920 10.7314/APJCP.2013.14.10.5915 [DOI] [PubMed] [Google Scholar]
- 102.Lin C., Huang F., Shen G. and Yiming A. (2015) MicroRNA-101 regulates the viability and invasion of cervical cancer cells. Int. J. Clin. Exp. Pathol. 8, 10148–10155 [PMC free article] [PubMed] [Google Scholar]
- 103.Dong P., Xiong Y., Hanley S.J.B., Yue J. and Watari H. (2017) Musashi-2, a novel oncoprotein promoting cervical cancer cell growth and invasion, is negatively regulated by p53-induced miR-143 and miR-107 activation. J. Exp. Clin. Cancer Res. 36, 150 10.1186/s13046-017-0617-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi X., Yuan N., Zhang S., Yuan F. and Wang X. (2018) MicroRNA-379 suppresses cervical cancer cell proliferation and invasion by directly targeting V-crk avian sarcoma virus CT10 oncogene homolog-like. Oncol. Res. 26, 987–996 10.3727/096504017X15140534417184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S., Zhang Y., Yuan S. and Ji X. (2018) MicroRNA485 targets MACC1 and inhibits cervical cancer cell proliferation and invasion. Mol. Med. Rep. 18, 2407–2416 [DOI] [PubMed] [Google Scholar]
- 106.Nieto M.A., Huang R.Y., Jackson R.A. and Thiery J.P. (2016) EMT: 2016. Cell 166, 21–45 10.1016/j.cell.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 107.Heerboth S., Housman G., Leary M., Longacre M., Byler S., Lapinska K.. et al. (2015) EMT and tumor metastasis. Clin. Transl. Med. 4, 6 10.1186/s40169-015-0048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin Y., Wang J., Han J., Luo D. and Sun Z. (2017) MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing WNT/beta-cadherin signaling pathway. Exp. Cell Res. 360, 210–217 10.1016/j.yexcr.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 109.Zaravinos A. (2015) The regulatory role of microRNAs in EMT and cancer. J. Oncol. 2015, 865816 10.1155/2015/865816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rokavec M., Oner M.G., Li H., Jackstadt R., Jiang L., Lodygin D.. et al. (2014) IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 124, 1853–1867 10.1172/JCI73531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams L.V., Veliceasa D., Vinokour E. and Volpert O.V. (2013) miR-200b inhibits prostate cancer EMT, growth and metastasis. PLoS ONE 8, e83991 10.1371/journal.pone.0083991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morrison C.D., Parvani J.G. and Schiemann W.P. (2013) The relevance of the TGF-beta Paradox to EMT-MET programs. Cancer Lett. 341, 30–40 10.1016/j.canlet.2013.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai N., Hu L., Xie Y., Gao J.H., Zhai W., Wang L.. et al. (2018) MiR-17-5p promotes cervical cancer cell proliferation and metastasis by targeting transforming growth factor-beta receptor 2. Eur. Rev. Med. Pharmacol. Sci. 22, 1899–1906 [DOI] [PubMed] [Google Scholar]
- 114.Zhou J.Y., Zheng S.R., Liu J., Shi R., Yu H.L. and Wei M. (2016) MiR-519d facilitates the progression and metastasis of cervical cancer through direct targeting Smad7. Cancer Cell Int. 16, 21 10.1186/s12935-016-0298-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao W., Wang Y.S., Hwang E., Lin P., Bae J., Seo S.A.. et al. (2018) Rubus idaeus L. (red raspberry) blocks UVB-induced MMP production and promotes type I procollagen synthesis via inhibition of MAPK/AP-1, NF-kappabeta and stimulation of TGF-beta/Smad, Nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B 185, 241–253 10.1016/j.jphotobiol.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 116.Cheng Y., Geng L., Zhao L., Zuo P. and Wang J. (2017) Human papillomavirus E6-regulated microRNA-20b promotes invasion in cervical cancer by targeting tissue inhibitor of metalloproteinase 2. Mol. Med. Rep. 16, 5464–5470 10.3892/mmr.2017.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma H.B., Yao Y.N., Yu J.J., Chen X.X. and Li H.F. (2018) Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am. J. Transl. Res. 10, 592–604 [PMC free article] [PubMed] [Google Scholar]
- 118.Luo S., Li N., Yu S., Chen L., Liu C. and Rong J. (2017) MicroRNA-92a promotes cell viability and invasion in cervical cancer via directly targeting Dickkopf-related protein 3. Exp. Ther. Med. 14, 1227–1234 10.3892/etm.2017.4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fukusumi Y. and Meier F. (2015) Dickkopf 3 promotes the differentiation of a rostrolateral midbrain dopaminergic neuronal subset in vivo and from pluripotent stem cells in vitro in the mouse. J Neurosci 35, 13385–13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeng F., Xue M., Xiao T., Li Y., Xiao S., Jiang B.. et al. (2016) MiR-200b promotes the cell proliferation and metastasis of cervical cancer by inhibiting FOXG1. Biomed. Pharmacother. 79, 294–301 10.1016/j.biopha.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 121.Deng B., Zhang Y., Zhang S., Wen F., Miao Y. and Guo K. (2015) MicroRNA-142-3p inhibits cell proliferation and invasion of cervical cancer cells by targeting FZD7. Tumour Biol. 36, 8065–8073 10.1007/s13277-015-3483-2 [DOI] [PubMed] [Google Scholar]
- 122.Zhou C., Tan D.M., Chen L., Xu X.Y., Sun C.C., Zong L.J.. et al. (2017) Effect of miR-212 targeting TCF7L2 on the proliferation and metastasis of cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 21, 219–226 [PubMed] [Google Scholar]
- 123.Wei H., Zhang J.J. and Tang Q.L. (2017) MiR-638 inhibits cervical cancer metastasis through Wnt/beta-catenin signaling pathway and correlates with prognosis of cervical cancer patients. Eur. Rev. Med. Pharmacol. Sci. 21, 5587–5593 [DOI] [PubMed] [Google Scholar]
- 124.Li L., Han L.Y., Yu M., Zhou Q., Xu J.C. and Li P. (2015) Pituitary tumor-transforming gene 1 enhances metastases of cervical cancer cells through miR-3666-regulated ZEB1. Tumour Biol. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 125.Chen G., Huang P., Xie J. and Li R. (2018) microRNA211 suppresses the growth and metastasis of cervical cancer by directly targeting ZEB1. Mol. Med. Rep. 17, 1275–1282 [DOI] [PubMed] [Google Scholar]
- 126.Ye C., Hu Y. and Wang J. (2018) MicroRNA-377 targets zinc finger E-box-binding homeobox 2 to inhibit cell proliferation and invasion of cervical cancer. Oncol. Res., 27, 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Q., Han L.R., Zhou Y.X. and Li Y. (2016) MiR-195 suppresses cervical cancer migration and invasion through targeting Smad3. Int. J. Gynecol. Cancer 26, 817–824 10.1097/IGC.0000000000000686 [DOI] [PubMed] [Google Scholar]
- 128.Zhang X., Cai D., Meng L. and Wang B. (2016) MicroRNA-124 inhibits proliferation, invasion, migration and epithelial-mesenchymal transition of cervical carcinoma cells by targeting astrocyte-elevated gene-1. Oncol. Rep. 36, 2321–2328 10.3892/or.2016.5025 [DOI] [PubMed] [Google Scholar]
- 129.Wang Z., He S., Guo P., Guo X. and Zheng J. (2017) MicroRNA-1297 inhibits metastasis and epithelial-mesenchymal transition by targeting AEG-1 in cervical cancer. Oncol. Rep. 38, 3121–3129 10.3892/or.2017.5979 [DOI] [PubMed] [Google Scholar]
- 130.Sathyanarayanan A., Chandrasekaran K.S. and Karunagaran D. (2017) microRNA-145 modulates epithelial-mesenchymal transition and suppresses proliferation, migration and invasion by targeting SIP1 in human cervical cancer cells. Cell Oncol 40, 119–131 [DOI] [PubMed] [Google Scholar]
- 131.Xu D., Liu S., Zhang L. and Song L. (2017) MiR-211 inhibits invasion and epithelial-to-mesenchymal transition (EMT) of cervical cancer cells via targeting MUC4. Biochem. Biophys. Res. Commun. 485, 556–562 10.1016/j.bbrc.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 132.Qu X., Gao D., Ren Q., Jiang X., Bai J. and Sheng L. (2018) miR-211 inhibits proliferation, invasion and migration of cervical cancer via targeting SPARC. Oncol. Lett. 16, 853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang Z., Song Q., Zeng R., Li J., Li J., Lin X.. et al. (2016) MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Oncotarget 7, 45622–45636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pang R.T., Leung C.O., Ye T.M., Liu W., Chiu P.C., Lam K.K.. et al. (2010) MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 31, 1037–1044 10.1093/carcin/bgq066 [DOI] [PubMed] [Google Scholar]
- 135.Shu L., Zhang Z. and Cai Y. (2018) MicroRNA-204 inhibits cell migration and invasion in human cervical cancer by regulating transcription factor 12. Oncol. Lett. 15, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duan S., Wu A., Chen Z., Yang Y., Liu L. and Shu Q. (2018) miR-204 regulates cell proliferation and invasion by targeting EphB2 in human cervical cancer. Oncol. Res. 26, 713–723 10.3727/096504017X15016337254641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng Y.X., Zhang Q.F., Hong L., Pan F., Huang J.L., Li B.S.. et al. (2016) MicroRNA-200b suppresses cell invasion and metastasis by inhibiting the epithelial-mesenchymal transition in cervical carcinoma. Mol. Med. Rep. 13, 3155–3160 10.3892/mmr.2016.4911 [DOI] [PubMed] [Google Scholar]
- 138.Li Y., Zhang Z., Xiao Z., Lin Y., Luo T., Zhou Q.. et al. (2017) Chemotherapy-mediated miR-29b expression inhibits the invasion and angiogenesis of cervical cancer. Oncotarget 8, 14655–14665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan Z., Cui H., Xu X., Lin Z., Zhang X., Kang L.. et al. (2015) MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 6, 25266–25280 10.18632/oncotarget.4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang J.M., Ju B.H., Pan C.J., Gu Y., Li M.Q., Sun L.. et al. (2017) MiR-214 inhibits cell migration, invasion and promotes the drug sensitivity in human cervical cancer by targeting FOXM1. Am. J. Transl. Res. 9, 3541–3557 [PMC free article] [PubMed] [Google Scholar]
- 141.Li X.R., Chu H.J., Lv T., Wang L., Kong S.F. and Dai S.Z. (2014) miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 588, 3298–3307 10.1016/j.febslet.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 142.Shi C. and Zhang Z. (2017) MicroRNA-320 suppresses cervical cancer cell viability, migration and invasion via directly targeting FOXM1. Oncol. Lett. 14, 3809–3816 10.3892/ol.2017.6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang Y., Huang H., Li M., Zhang X., Liu Y. and Wang Y. (2017) MicroRNA-374c-5p regulates the invasion and migration of cervical cancer by acting on the Foxc1/snail pathway. Biomed. Pharmacother. 94, 1038–1047 10.1016/j.biopha.2017.07.150 [DOI] [PubMed] [Google Scholar]
- 144.Deng Y., Xiong Y. and Liu Y. (2016) miR-376c inhibits cervical cancer cell proliferation and invasion by targeting BMI1. Int. J. Exp. Pathol. 97, 257–265 10.1111/iep.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xia C., Liang S., He Z., Zhu X., Chen R. and Chen J. (2018) Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur. J. Pharmacol. 830, 59–67 10.1016/j.ejphar.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 146.Xiao H., Yu L., Li F., Wang H., Li W. and He X. (2018) MiR-340 suppresses the metastasis by targeting EphA3 in cervical cancer. Cell Biol. Int. 42, 1115–1123 10.1002/cbin.10974 [DOI] [PubMed] [Google Scholar]
- 147.Tang Y., Wang Y., Chen Q., Qiu N., Zhao Y. and You X. (2015) MiR-223 inhibited cell metastasis of human cervical cancer by modulating epithelial-mesenchymal transition. Int. J. Clin. Exp. Pathol. 8, 11224–11229 [PMC free article] [PubMed] [Google Scholar]
- 148.Shi Y.R., Liu J., He W. and Yang Y. (2016) Expression of micro-RNA 218 in cervical cancer and its effect on proliferation, apoptosis and invasion of HeLa cells. Sichuan Da Xue Xue Bao Yi Xue Ban 47, 697–702 [PubMed] [Google Scholar]
- 149.Mofolo N., Sello M., Leselo M., Chabanku N., Ndlovu S., Naidoo Q.. et al. (2018) Knowledge of cervical cancer, human papillomavirus and prevention among first-year female students in residences at the University of the Free State. Afr. J. Prim. Healthcare Fam. Med. 10, e1–e5 10.4102/phcfm.v10i1.1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mendes de Oliveira C. and Levi J.E. (2016) The biological impact of genomic diversity in cervical cancer development. Acta Cytol. 60, 513–517 10.1159/000449401 [DOI] [PubMed] [Google Scholar]
- 151.Gomez-Gomez Y., Organista-Nava J. and Gariglio P. (2013) Deregulation of the miRNAs expression in cervical cancer: human papillomavirus implications. Biomed. Res. Int. 2013, 407052 10.1155/2013/407052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang X., Song L., Liu S., Zhang L., Yao H., Gao F.. et al. (2016) MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1alpha feedback loop and the Akt-mTOR signaling pathway. J. Cell. Biochem. 37, 12161–12168 [DOI] [PubMed] [Google Scholar]
- 153.Steelman L.S., Martelli A.M., Cocco L., Libra M., Nicoletti F., Abrams S.L.. et al. (2016) The therapeutic potential of mTOR inhibitors in breast cancer. Br. J. Clin. Pharmacol. 82, 1189–1212 10.1111/bcp.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Franklin R.A., Montalto G.. et al. (2012) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 3, 1068–1111 10.18632/oncotarget.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Donia M., Mangano K., Amoroso A., Mazzarino M.C., Imbesi R., Castrogiovanni P.. et al. (2009) Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing experimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J. Autoimmun. 33, 135–140 10.1016/j.jaut.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 156.Fernandez D., Bonilla E., Mirza N., Niland B. and Perl A. (2006) Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 54, 2983–2988 10.1002/art.22085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nicoletti F., Lapenta C., Donati S., Spada M., Ranazzi A., Cacopardo B.. et al. (2009) Inhibition of human immunodeficiency virus (HIV-1) infection in human peripheral blood leucocytes-SCID reconstituted mice by rapamycin. Clin. Exp. Immunol. 155, 28–34 10.1111/j.1365-2249.2008.03780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nicoletti F., Fagone P., Meroni P., McCubrey J. and Bendtzen K. (2011) mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov. Today 16, 715–721 10.1016/j.drudis.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 159.Spangle J.M. and Munger K. (2010) The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J. Virol. 84, 9398–9407 10.1128/JVI.00974-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Molinolo A.A., Marsh C., El Dinali M., Gangane N., Jennison K., Hewitt S.. et al. (2012) mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin. Cancer Res. 18, 2558–2568 10.1158/1078-0432.CCR-11-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Asimomytis A., Karanikou M., Rodolakis A., Vaiopoulou A., Tsetsa P., Creatsas G.. et al. (2016) mTOR downstream effectors, 4EBP1 and eIF4E, are overexpressed and associated with HPV status in precancerous lesions and carcinomas of the uterine cervix. Oncol. Lett. 12, 3234–3240 10.3892/ol.2016.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Callejas-Valera J.L., Iglesias-Bartolome R., Amornphimoltham P., Palacios-Garcia J., Martin D., Califano J.A.. et al. (2016) mTOR inhibition prevents rapid-onset of carcinogen-induced malignancies in a novel inducible HPV-16 E6/E7 mouse model. Carcinogenesis 37, 1014–1025 10.1093/carcin/bgw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim J.H., Song J. and Park K.W. (2015) The multifaceted factor peroxisome proliferator-activated receptor gamma (PPARgamma) in metabolism, immunity, and cancer. Arch. Pharm. Res. 38, 302–312 10.1007/s12272-015-0559-x [DOI] [PubMed] [Google Scholar]
- 164.Zhang S., Liu F., Mao X., Huang J., Yang J., Yin X.. et al. (2015) Elevation of miR-27b by HPV16 E7 inhibits PPARgamma expression and promotes proliferation and invasion in cervical carcinoma cells. Int. J. Oncol. 47, 1759–1766 10.3892/ijo.2015.3162 [DOI] [PubMed] [Google Scholar]
- 165.Shi M., Du L., Liu D., Qian L., Hu M., Yu M.. et al. (2012) Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J. Pathol. 228, 148–157 10.1002/path.3997 [DOI] [PubMed] [Google Scholar]
- 166.Zhong J., Yuan H., Xu X. and Kong S. (2018) MicroRNA195 inhibits cell proliferation, migration and invasion by targeting defective in cullin neddylation 1 domain containing 1 in cervical cancer. Int. J. Mol. Med. 42, 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Yu J., Wang Y., Dong R., Huang X., Ding S. and Qiu H. (2012) Circulating microRNA-218 was reduced in cervical cancer and correlated with tumor invasion. J. Cancer Res. Clin. Oncol. 138, 671–674 10.1007/s00432-012-1147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang L., Zhan X., Yan D. and Wang Z. (2016) Circulating microRNA-21 is involved in lymph node metastasis in cervical cancer by targeting RASA1. Int. J. Gynecol. Cancer 26, 810–816 10.1097/IGC.0000000000000694 [DOI] [PubMed] [Google Scholar]
- 169.Xu L.J., Duan Y., Wang P. and Yin H.Q. (2018) MiR-199b-5p promotes tumor growth and metastasis in cervical cancer by down-regulating KLK10. Biochem. Biophys. Res. Commun. 503, 556–563 10.1016/j.bbrc.2018.05.165 [DOI] [PubMed] [Google Scholar]
- 170.Zare M., Bastami M., Solali S. and Alivand M.R. (2018) Aberrant miRNA promoter methylation and EMT-involving miRNAs in breast cancer metastasis: diagnosis and therapeutic implications. J Cell Physiol 233, 3729–3744 [DOI] [PubMed] [Google Scholar]
- 171.Jafri M.A., Al-Qahtani M.H. and Shay J.W. (2017) Role of miRNAs in human cancer metastasis: Implications for therapeutic intervention. J. Cell. Physiol. 44, 117–131 [DOI] [PubMed] [Google Scholar]
- 172.Wang F., Hongwei T., Li L., Piontek K., Sakaguchi M. and Selaru F.M. (2017) Exosome - miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 67, 940–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang J., Liu H., Hou L., Wang G., Zhang R., Huang Y.. et al. (2017) Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol. Cancer 16, 151 10.1186/s12943-017-0719-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tang Y.Y., Zhao P., Zou T.N., Duan J.J., Zhi R., Yang S.Y.. et al. (2017) Circular RNA hsa_circ_0001982 promotes breast cancer cell carcinogenesis through decreasing miR-143. DNA Cell Biol. 36, 901–908 10.1089/dna.2017.3862 [DOI] [PubMed] [Google Scholar]