Abstract
The sorting nexin (SNX) family consists of a diverse group of cytoplasmic- and membrane-associated phosphoinositide-binding proteins that play pivotal roles in the regulation of protein trafficking. This includes the entire endocytic pathway, such as endocytosis, endosomal sorting, and endosomal signaling. Dysfunctions of SNX pathway are involved in several forms of cardiovascular disease (CVD). Moreover, SNX gene variants are associated with CVDs. In this review, we discuss the current knowledge on SNX-mediated regulatory mechanisms and their roles in the pathogenesis and treatment of CVDs.
Keywords: Cardiovascular diseases, Endosomal sorting, Pharmacogenomics, Retromer, Sorting nexin
Introduction
Cardiovascular diseases (CVDs), including hypertension, coronary heart disease, heart failure, and stroke, are the leading causes of morbidity and mortality, worldwide, accounting for substantial suffering and healthcare-related expenditures [1]. According to the American Heart Association, the duration of informal caregiving hours increases from 0.10 h/wk for patients with coronary heart disease to 6.12 h/wk for patients with stroke. This informal caregiving for patients with CVD is projected to increase total CVD costs by 11%, from $616 billion in 2015 to $1.2 trillion in 2035, in the United States [2]. Thus, increasing the efficacy in preventing and controlling CVDs worldwide is expected to have a huge potential in decreasing health costs and improving global health [2,3].
Genetic and environmental factors and their interaction determine an individual’s risk for CVDs. Recent studies have shown that diet and environmental factors, such air pollution, influence the development of CVDs [4–7]. There is also strong evidence for the roles of genetics and epigenetics in the prediction, prevention, and treatment (pharmacogenomics) of CVDs [7–10]. Although few single-gene-associated CVDs can now be precisely diagnosed and treated [11], the underlying mechanisms of most CVDs are still unclear.
The sorting nexin (SNX) family consists of a diverse group of cytoplasmic and membrane-associated proteins orchestrating the process of cargo sorting, through the membranous maze that is the endosomal network [12,13]. SNXs play pivotal roles in the whole pathway of endocytic trafficking, including endocytosis, endosomal sorting, and endosomal signaling [12,13]. The perturbation of this process and/or deficiency of SNXs may lead to receptor dysfunction, impaired homeostatic responses, and possibly disease states, including CVDs [14–17]. To our knowledge, there is no review on the association between SNXs and CVDs. In this review, we discussed the evolving understanding of the role of SNXs in CVDs and summarized the current knowledge on SNX-mediated regulatory mechanisms and their role in the pathophysiology and treatment of CVDs.
The basic characteristics of sorting nexins
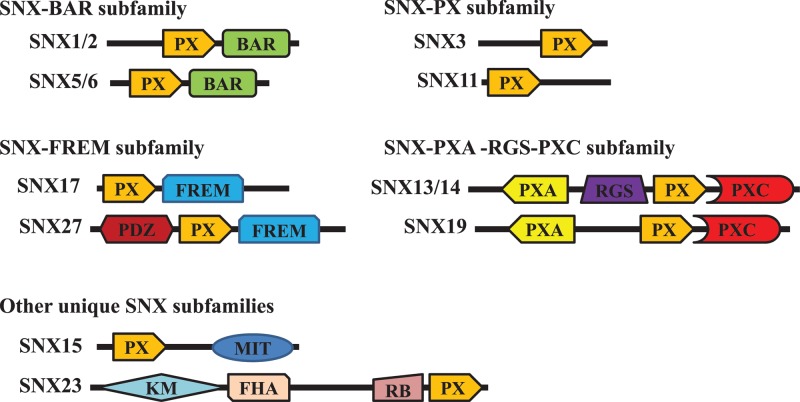
The SNX family consists of a diverse group of cytoplasmic and membrane-associated proteins involved in various aspects of cargo endocytosis and trafficking through the endosomes [12,13]. SNXs have been identified across phyla, from yeast to mammals. Currently 10 yeast, 8 fruit, and 33 mammalian SNXs have been identified [12–14]. All SNX family members contain the evolutionarily canonical 100–130-amino acid phagocyte oxidase (phox) homology (PX) domain, which facilitates the interaction of SNXs with phosphatidylinositols, most commonly phosphatidylinositol 3-phosphate (PI3P) [15,16]. Thus, most SNXs are associated with PI3P-enriched early endosomes. Based on their domain organization, the SNX family is categorized into five subfamilies: SNX-BAR (Bin/Amphiphysin/Rvs), SNX-PX, SNX-FERM (protein 4.1/ezrin/radixin/moesin), SNX-PXA-RGS-PXC, and other unique SNX subfamilies (Table 1 and Figure 1) [14–18]. In addition to these domains, certain SNXs also contain special structures. For example, SNX27 is unique within the SNX family for having a PDZ domain, a type of protein–protein interaction domain of roughly 90 amino acid residues [19].
Table 1. Summary of SNX family.
| Subfamilies | Members | Characteristics of structure | Typical examples involving in intracellular physiological functions |
|---|---|---|---|
| SNX-BAR | SNX1, SNX2, SNX4, SNX5, SNX6, SNX7, SNX8, SNX9, SNX18, SNX30, SNX32, SNX33 | In addition to the SNX-PX domain, these proteins have a C-terminal BAR domain comprising three α-helices capable of dimerizing with another BAR domain to form a rigid banana-shaped structure. | SNX1, SNX2, SNX5, and SNX6 are involved in the endosomal membrane sorting processes [25,27]. |
| Their BAR domains allow SNX-BARs to form specific homo- and hetero-dimers (e.g. SNX1 and SNX2; SNX5 and SNX6). | SNX4 prevents BACE1 trafficking to the lysosomal degradation system [40]. | ||
| SNX6, a member of the IGF1–IGF1 receptor pathway, regulates the signaling of IGF-1 stimulation [41]. | |||
| SNX9 and SNX18 are associated with clathrin-mediated and independent endocytosis [30–32]. | |||
| SNX9, SNX18, and SNX33 are required for mitosis through both endocytosis- dependent and -independent processes [103]. | |||
| SNX-PX | SNX3, SNX10, SNX11, SNX12, SNX16, SNX20, SNX21, SNX22, SNX24, SNX29 | The subfamily of proteins is a relatively poorly characterized group of molecules, which have not any conserved domains outside the defining PX domain. | SNX3-retromer is indentified as a distinct form of the mammalian retromer complex [24]. |
| These proteins are of various lengths and typically contain long extended sequences with no predicted secondary structure. | SNX11 promotes the trafficking of TRPV3 from the plasma membrane to lysosomes for degradation [35]. | ||
| SNX10 controls mTOR activation in colorectal cancer [42]. | |||
| SNX16 functions in the trafficking of proteins between the early and late endosomal compartments, and plays a role in the regulation of late endosome membrane dynamics [104,105]. | |||
| SNX-FERM | SNX17, SNX27, SNX31 | The three proteins contain a typical C-terminal FERM domain, which is comprised of approximately 300 amino acids. In addition, SNX27 possesses an N-terminal PDZ domain. The sequence homology of the PX-associated FERM domains with the canonical FERM domain is low. | SNX27-retromer is an important form of the mammalian retromer complex [26]. |
| SNX17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail [38]; SNX17 also inhibits the movement of P-selectin into lysosomes [39];. | |||
| SNX31 mediates the endocytic degradation of proteins such as uroplakins [106]. | |||
| SNX-PXA -RGS-PXC | SNX13, SNX14, SNX19, SNX25 | Each member possesses two putative N-terminal transmembrane helices. SNX13, SNX14, and SNX25 have a long C-terminal structure containing four modular domains (PXA-RGS-PX-PXC); however, SNX19 has only three (PXA-PX-PXC). | Snx13 is involved in the degradative sorting of apoptosis repressor with caspase recruitment domain [93]. |
| SNX14 is a dual endogenous negative regulator in 5-HT6R-mediated signaling pathway [107]. | |||
| SNX25 negatively regulates TGF-β signaling via enhancing the receptor degradation [108]. | |||
| Other unique SNX subfamilies (SNX15-MIT and SNX23) | SNX15 | SNX15, first identified via database searches for SNX1 homologs, includes a C-terminal MIT domain. | SNX15 is associated with the endocytic and endosomal sorting [109,110]. |
| SNX23 (also known as kinesin-family protein 16B, KIF16B) | SNX23, a member of the kinesin family, contains kinesin motor, a putative FHA domain, and coiled coil dimerization domains. | SNX23 regulates receptor recycling and degradation [111]. |
Abbreviations: BACE1, β-site amyloid precursor protein-cleaving enzyme 1; BAR, Bin/Amphiphysin/Rvs domain; FERM, protein 4.1/ezrin/radixin/moesin domain; FHA, forkhead associated domain; IGF1, insulin-like growth factor 1; MIT, microtubule interacting and trafficking domain; mTOR, mammalian target of rapamycin; PDZ, postsynaptic density 95/discs large/zonula occludens domain; PX, phagocyte oxidase (phox) homology domain; PXA, PX-associated domain A; PXC, PX-associated domain C; RGS, regulator of G-protein signaling domain; SNX, sorting nexin; TGF-β, transforming growth factor-β; TRPV3, transient receptor potential vanilloid channel 3.
Figure 1. Domain architecture of representative SNXs in different subfamilies.
All SNXs share a conserved PX domain. Other domain structures of SNXs in different subfamilies are also indicated. BAR, Bin/Amphiphysin/Rvs domain; FERM, protein 4.1/ezrin/radixin/moesin domain; FHA, forkhead associated domain; KM, kinesin motor domain; MIT, microtubule interacting and trafficking domain; PDZ, postsynaptic density 95/discs large/zonula occludens domain; PX, phagocyte oxidase (phox) homology domain; PXA, PX-associated domain A; PXC, PX-associated domain C; RB, Rab5-binding domain; RGS, regulator of G-protein signaling domain; SNX, sorting nexin.
Physiological functions of SNXs
Typical functions of SNXs
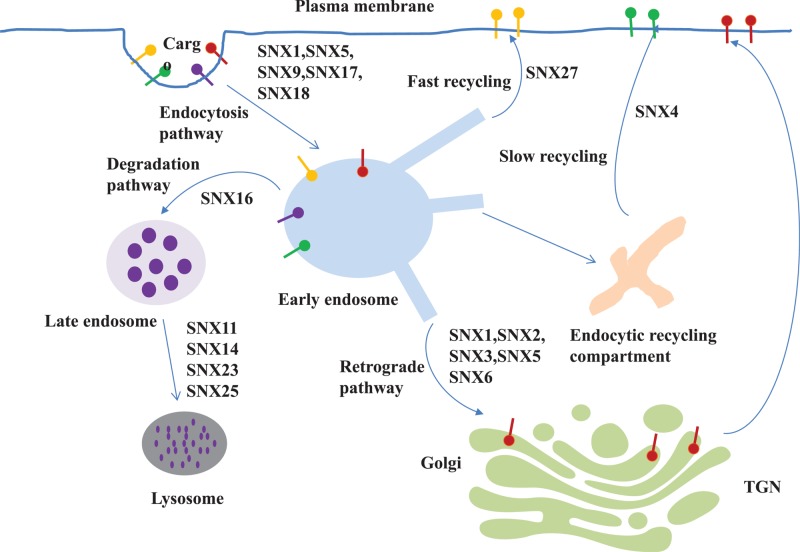
The balance of protein recycling and degradation is crucial for cellular homoeostasis; inappropriate sorting of proteins to either fate leads to cellular dysfunction. The involvement of SNXs in endocytic trafficking, includes several components [20–26] (Figure 2). First of all, the most important physiological function of SNXs is being a component of the retromer, a multi-subunit protein complex, which mediates the recycling or retrograde trafficking of transmembrane proteins, termed as cargoes, from endosomes to either the trans-Golgi network (TGN) or the plasma membrane [20–23]. These cargoes include G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs), transport carriers for enzymes and the Wnt sorting receptor, glucose and metal ion transporters, polarity proteins, among others [20–23]. The mammalian retromer complex is composed of two distinct subunits: a vacuolar protein sorting associated protein 26 (VPS26)-VPS29-VPS35 trimeric subcomplex, which engages and concentrates cargo to be transported, and a membrane-associated SNX dimer that binds to endosomal membranes and organizes the formation of tubulovesicular carriers that transport cargoes to their final destination. There are three distinct forms of the mammalian retromer complex: SNX-BAR-retromer, SNX3-retromer, and SNX27-retromer. Some SNXs have been the focus of several studies, SNX1/2/5/6 of the SNX-BAR subfamily, SNX3 of the SNX-PX subfamily, and SNX27 of the SNX-FERM subfamily [24–26]. The SNX-BAR-retromer, containing the core retromer trimer and the SNX-BAR subunits, mediates the retrograde trafficking of cargoes. For example, heterodimers of the SNX-BAR proteins, SNX1, SNX2, SNX5, and SNX6, independent of the core retromer trimer, are the cargo-selective elements required for the retrograde transport of the cation-independent mannose 6-phosphate receptor from the endosomes to the TGN [27]. The SNX3-retromer, composed of the core retromer trimer and SNX3, traffics the Wnt-binding protein, Wntless, from the endosomes to the TGN [28]. By contrast, the SNX27-retromer mediates the direct endosome-to-plasma membrane trafficking, without passing through the TGN, e.g. recycling of glucose transporter type 1 [29]. These SNXs associate with the retromer or retriever complex, which mediates endosomal trafficking pathways for the delivery of various cargo proteins to the right location for fundamental cellular processes.
Figure 2. Schematic diagram of representative SNXs involved in the trafficking pathways of cargoes.
Different transmembrane proteins, termed as cargoes, are internalized into early endosomes from the plasma membrane via the endocytosis process. Then, retromer mediates some cargo recycling to the trans-Golgi network or back to the plasma membrane. On the other side, maturation of early endosomes into late endosomes leads to cargo degradation via lysosome. Representative SNXs are shown in the different trafficking routes of cargoes. SNX, sorting nexin; trans-Golgi network, TGN.
Second, SNXs are involved in endocytosis. For example, SNX9 partially localizes to clathrin-coated pits and binds directly to both dynamin-1 and dynamin-2, which are central players in clathrin-mediated endocytosis [30]. SNX9 is required for efficient clathrin-mediated endocytosis by regulating dynamin assembly and also functions in several clathrin-independent endocytosis pathways that are driven by actin polymerization [30,31]. SNX18 heterodimerizes and colocalizes with SNX9 in tubular membrane structures. SNX18 and SNX9 can compensate for each other deficiency during clathrin-mediated transferrin endocytosis [32]. Our previous studies also found that in human renal proximal tubule (RPT) cells, SNX1 is required for the endocytosis of D5R following agonist stimulation but not for basal receptor trafficking. By contrast, SNX5 is necessary not just for the endocytosis of the agonist-activated D1R but also for its recycling and reinsertion back to the plasma membrane [33,34].
Third, SNXs are involved in protein degradation. Some SNXs are involved in the lysosomal degradation of proteins. For example, SNX11 promotes the trafficking of TRPV3 from the plasma membrane to lysosomes for degradation via protein–protein interactions [35]. SNX1 facilitates the sorting and degradation of the epidermal growth factor receptor (EGFR) to lysosomes [36]. SNX6 participates in the endolysosomal degradation of tumor suppression p27Kip1 [37]. However, other SNXs inhibit the protein degradation. SNX17 prevents the lysosomal degradation of β1 integrins by binding to the β1-integrin tail [38]. SNX17 also inhibits the movement of P-selectin into lysosomes, reducing its degradation while increasing its endocytosis from the plasma membrane [39]. SNX4 interacts with β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) and prevents BACE1 trafficking to the lysosomes, increasing the half-life of BACE1 and production of β-amyloid [40].
Fourth, SNXs play important roles in intracellular signaling. SNXs can directly participate in the intracellular signaling cascade, as a member of the pathway. For example, the knockdown of SNX6, a member of the insulin-like growth factor 1 (IGF1)–IGF1 receptor pathway, decreases IGF1-mediated ERK1/2 phosphorylation, but does not affect IGF1 receptor internalization [41]. SNX10 inhibits mTOR activation by regulating chaperone-mediated autophagy-dependent amino-acid metabolism in colorectal cancer [42]. SNX9 preferentially binds to phosphorylated Smad3, independent of Smad2 and Smad4, the primary mediators of TGF-β responses, and promotes its more rapid nuclear delivery [43].
SNXs can also indirectly regulate downstream signaling by impairing the expression or function of transmembrane proteins such as GPCRs and RTKs. For example, our studies found that SNX5 regulates insulin receptor expression, distribution, dynamics and function in human RPT cells; SNX5 knockdown leads to a decrease in insulin receptor expression, causing the decrease in downstream signaling cascade, including the abundance of phosphorylated insulin receptor substrate and phosphorylated protein kinase B [44].
Regulation of SNXs
So far, there are only a few studies on the regulation of SNXs. The expression of SNXs per se is regulated by some hormones, enzymes, and other SNX family members. Estradiol (E2) decreases SNX5 expression in the mammary gland of E2 receptor α (ERα) wild-type mice. However, in the mammary gland of ERα knockout mice, E2 increases SNX5 expression, presumably via ERβ [45]. Neuronal SNX8 expression was decreased by extreme changes in cholesterol such as treatment with mevinolin, a cholesterol-lowering statin, but unchanged in the presence of moderately high cholesterol [46]. Itch (atrophin-1 interacting protein 4), a member of the NEDD4 family of E3 ubiquitin ligases, expressed in HEK cells, increases the ubiquitilation and degradation of SNX9 [47]. In HeLa cells undergoing apoptosis, both SNX1 and SNX2 are cleaved by initiator caspases and executioner caspase-6, in the case of SNX2. Moreover, SNX1 but not SNX2 is cleaved at multiple sites, including sites that follow glutamate residues [48]. The interaction among SNXs can regulate specific SNX expression. For example, suppression of SNX5 and/or SNX6 results in the loss of SNX1 in HeLa cells, an effect that seems to result from post-translational regulation of the SNX1 level [49].
SNX function, independent of expression, can be regulated, as well. In 3T3L1 adipocytes, insulin stimulates the movement of SNX9 out of the cytosol and into the plasma membrane [50]. Another study also found in mature adipocytes that insulin stimulates the redistribution of a proportion of retromer and its associated proteins, including SNX27 but not SNX1, from intracellular compartments to the plasma membrane along with glucose transporter 4 storage vesicles [51]. Our studies found that insulin treatment increases SNX5 levels and the colocalization of SNX5 and insulin receptor in the perinuclear area of human RPT cells [44]. Stimulation with the D1-like receptor agonist, fenoldopam, promotes the internalization of SNX5 and enhances its colocalization with D1R at the brush border and cytoplasm of RPTs, demonstrating an essential role for SNX5 in regulating D1R function [33]. Treatment with fenoldopam can also markedly enhance the interaction between SNX1 and D5R and promote their internalization in HEK293 cells expressing V5-tagged D5R, showing a crucial role for SNX1 in D5R trafficking [34].
Role of SNXs in cardiovascular diseases
SNXs and hypertension
Hypertension is one of the most common and important health problems worldwide. The kidney plays an important role in the long-term control of blood pressure and is the major organ involved in sodium homeostasis. Humans with polygenic essential hypertension have increased renal sodium transport, which is not properly regulated by natriuretic and anti-natriuretic hormones and humoral factors, including dopamine [52,53]. Studies have shown that SNXs exert their physiological effects by regulating the expression and function of GPCRs, such as dopamine receptors, and RTKs, activated by hormones such as insulin, which lead to either natriuresis or anti-natriuresis to maintain a normal blood pressure [33,34,44,54].
Evidence of a role of SNXs in the regulation of blood pressure
There are a few studies showing impaired expression and function of SNXs in hypertension. The protein expression of SNX5 is decreased in the kidney of spontaneously hypertensive rats (SHRs), as well as immortalized RPT cells from hypertensive humans compared with WKY rats and normotensive subjects, respectively [55]. The pathophysiological significance of decreased SNX5 expression has been proved by down-regulating its expression, selectively in the kidney, using siRNA infusion. Depletion of renal SNX5 expression by the infusion of Snx5-specific siRNA into the kidney of SHRs caused a further increase in systolic blood pressure, a decrease in sodium excretion [33]. Moreover, renal-selective silencing of SNX5 in C57Bl/6J mice also increases blood insulin and glucose levels, decreases urinary insulin excretion, and causes insulin resistance [55].
The blood pressure can be regulated by other SNXs. Our previous study showed that Snx1−/− mice had elevated systolic and diastolic blood pressure measured under pentobarbital anesthesia, compared with wild-type littermates [56]. Further studies showed that Snx1−/− mice had elevated night time blood pressure, the level of which was not affected by dietary salt intake, indicating that the blood pressure of Snx1−/− mice is not salt-sensitive [56]. Although SNX1 is not involved in the salt-sensitive hypertension, there are evidence showing its role in hypertension, for example, SNX1 expression is decreased in RPT cells from hypertensive compared with normotensive Caucasian males [56]. Further studies also showed that both the systolic and diastolic blood pressures (under pentobarbital anesthesia) were higher by 13 and 15 mm Hg, respectively, in renal SNX1-depleted C57BL/6J mice and by 26 and 23 mm Hg in renal SNX1-depleted BALB/cJ mice compared with mock-treated controls [34]. Those evidence indicate that decreased SNX1 expression and dysfunction of SNX1 may be a potential common mechanism in several hypertensive models, including salt-sensitive- and salt-resistant hypertensive models. Our preliminary data also showed that SNX19, an SNX subtype containing a central PX domain and non-BAR domains at the C-terminal region, regulates blood pressure; depletion of renal SNX19 increases blood pressure in C57Bl/6J mice [57].
The decreased expression of SNX and increased blood pressure in SNX knockout or knockdown animal models indicate that SNX may have clinical significance as potential therapeutic targets for hypertension. Our preliminary studies also showed that the expression of endogenous SNX1 is reduced in human RPT cells from hypertensive compared with normotensive Caucasian males [56]. However, three SNX1 variants (rs34910981, rs1130604, and s1802376), and one SNX5 variant (rs6045116) are not associated with salt-sensitive hypertension in Euro-Americans [58]. It is conceivable that other gene variants could affect the expression, localization, and activity of SNX5, because SNX5 is replete with SNPs, such as rs754299876, rs758576026 and rs746381456, and structural variations, such as changes in copy number and indels (insertions and deletions) [58], which may be associated with or link to hypertension, salt sensitivity, and insulin resistance. The overexpression of SNX subtypes in the kidney may decrease blood pressure and may be another approach for the treatment of hypertension.
The mechanisms by which SNXs regulate blood pressure
Regulation of dopamine receptors by SNX
Dopamine, via 5 subtypes of receptors (D1-like receptors [D1R and D5R] and D2like receptors [D2R, D3R, and D4R]), plays an important role in the control of blood pressure. Disruption of any of the dopamine receptor genes in mice results in hypertension [52,53]. During conditions of moderate sodium balance, more than 50% of renal sodium excretion is regulated by the D1-like receptors (D1R and D5R).
SNX1 and D5R
SNX1, the first mammalian SNX, is essential for the trafficking and function of D5R. The elevated blood pressure in Snx1−/− mice or renal SNX1-depleted mice is associated with blunted natriuretic response to fenoldopam, an agonist of the two D1-like receptors [34,52,53,56]. SNX1 is required for D5R trafficking following agonist stimulation; loss of SNX1 abrogates D5R-mediated cAMP production and inhibition of renal sodium transport [34]. This is associated with increased expression of genes that increase renal sodium reabsorption, including Na+-K+-ATPase, sodium-hydrogen exchanger 3 (NHE3), and the thiazide-sensitive sodium-chloride cotransporter (NCC) [56]. The dysfunction of D5R caused by SNX1 silencing also increases oxidative stress [56]. The SNX1-mediated regulation of D5R may be related to their ability to interact directly and physically [34]. SNX1 distinguishes between D1R and D5R by binding strongly to the D5R but not to the D1R C-terminal tail [59].
SNX5 and D1R
D1R, the other D1-like receptor, is regulated by SNX5 and not SNX1 that regulates D5R as discussed above. The impaired regulation of blood pressure and renal sodium excretion caused by renal-selective depletion of SNX5, discussed above is due to impairment of D1R function [33]. Renal-selective silencing of SNX5 increases agonist-activated D1R phosphorylation, prevents D1R internalization, delays D1R recycling, and impairs of agonist-induced cAMP production [33]. The efficient recycling of the D1R and its targeting to the plasma membrane involves the C-terminus of the human and rat D1R [54,60–62]. SNX5 colocalizes with the D1R at the brush border of proximal tubules and the distal convoluted tubule in the human renal cortex [33]. Stimulation with fenoldopam, an agonist of D1-like receptors, promotes the internalization of D1R and SNX5 and enhances their colocalization at the brush border and the cytoplasm [33]. The essential role for SNX5 in the regulation of D1R function may have important implications in the diagnosis, prognosis, and treatment of essential hypertension.
SNX25, SNX19, SNX13, and D1R
Besides SNX5, other SNXs are involved in the regulation of renal D1R. The full-length SNX25 contains a typical PX domain, a PX-associated (PXA) domain, and a regulator of G protein signaling (RGS) domain. SNX25 overexpression enhances the expression levels of both D1R and D2R, causes an increase in receptor-mediated signaling, and perturbs both endocytosis and recycling of D2R, but does not affect D1R desensitization [63,64]. Depletion of endogenous SNX25 causes a decrease in D1R and D2R expression. The interaction between SNX25 and D1R in vivo remains to be determined. SNX19 also colocalizes with D1R in the human and mouse kidney. Silencing of SNX19 in human RPT cells decreases D1R, but not D5R, expression, impairs D1R mediated-cAMP response, abrogates the D1R mediated-decrease in sodium transport. Renal-selective silencing of SNX19 decreases renal D1R expression and increases blood pressure in C57Bl/6J mice (unpublished data) [57]. SNX13 may also interact with D1R in the regulation of albumin excretion [65].
Regulation of cellular sodium handling by SNXs
SNXs such as SNX27 and SNX3 can directly regulate the expression and function of genes that regulate sodium movement. SNX27 interacts with the C-terminus of NHE3 through its PDZ domain, which maintains basal NHE3 activity and surface expression in HEK-293 and polarized epithelial Caco-2BB cells [66]. It is required not only for the exocytosis of NHE3 from early endosome to plasma membrane, but also for the retention of NHE3 at the plasma membrane [66]. SNX3 increases total and plasma membrane α and γ ENaC in HEK-293 cells expressing these tagged proteins. Vasopressin increases the expression of ubiquitin-specific protease 10, which de-ubiquitinylates and stabilizes SNX3 to increase the cell surface expression of these ENaC subunits in HEK-293 and mouse renal cortical collecting duct cells [67]. SNX3 also physically interacts with αENaC [67]. However, the role of SNX27 and SNX3 in the regulation of blood pressure remains to be determined.
SNXs and insulin resistance
Insulin resistance, with or without hyperinsulinemia, participates in the pathogenesis of hypertension [68,69]. SNXs are associated with insulin resistance and hyperinsulinemia. SNXs can regulate insulin action by at least three mechanisms: insulin secretion, degradation, and signaling. SNX can regulate insulin secretion. The knockdown of SNX19 in mouse pancreatic β-cells decreases insulin secretion and the number/size of dense core vesicles (DCV) [70]. Further studies showed that the half-life of DCVs is decreased, but the number of lysosomes and the activity of lysosome enzyme cathepsin D are increased in SNX19 knockdown cells. The rescue of SNX19 in these SNX19 knockdown cells reverses the decrease in DCVs, number of lysosomes, and activity of cathepsin D [70].
Insulin degradation can be regulated by SNX5. The kidney, especially the RPT, is the major site of the clearance of insulin from the systemic circulation, removing 50% of circulating insulin [71,72]. Insulin-degrading enzyme (IDE) is the major enzyme responsible for degrading insulin in kidney [73]. We have reported that IDE and SNX5 dynamically interact in the kidney. SNX5 and IDE colocalized with IDE in the brush border membrane of proximal tubules of human, rat, and mouse kidneys. The renal-selective silencing of Snx5 in mice decreases IDE expression and activity, increases blood insulin and glucose levels, decreases urinary insulin excretion, and causes insulin resistance. The renal-selective silencing of Snx5 in normotensive Wistar-Kyoto rats also increased blood insulin and glucose levels [55]. RPT cells from hypertensive rats and humans have decreased expression of IDE and SNX5 [55].
SNX5 can also regulate insulin signaling. Our previous data have shown that SNX5 is necessary for normal insulin receptor expression and activity. SNX5 and insulin receptor colocalize in the brush border of RPTs, both of which are increased after insulin stimulation. SNX5 silencing decreases the phosphorylation of insulin receptor and its substrate, protein kinase B [44].
SNX1, SNX2, SNX4, and SNX27 can also interact with the insulin receptor [74,75]; the activation of insulin signaling increases the interaction between SNX27 and insulin receptor [74]. SNX27 has also been reported to regulate the expression of the glucose transporter isoform 1 (GLUT1) by preventing its lysosomal degradation, maintaining its cell surface levels [76]. Moreover, GLUT4, another insulin-responsive glucose transporter that mediates glucose uptake in adipose and skeletal muscle cells, is also regulated by SNX27 because SNX27 knockdown leads to decreased expression of GLUT4 [51].
SNXs can also promote insulin secretion and function by regulating other hormones, such as the glucagon-like peptide 1 (GLP-1), a 30-amino acid long peptide hormone derived from proglucagon and secreted by L-cells in small intestines [77]. For example, SNX27 and SNX1 increase insulin secretion from murine insulinoma cells mouse and human β-cell lines and human islets in response to the GLP-1 analog exendin-4. Moreover, both SNX27 and SNX1 control overall β-cell incretin responses by regulating the balance between GLP-1 receptor plasma membrane recycling and lysosomal degradation [78]. Thus, SNXs are important in the regulation of insulin function and prevention of insulin resistance.
SNXs and coronary artery disease
Population studies have shown the association between coronary artery disease and SNXs, such as SNX19. An SNX19 SNP, rs2298566, is associated with the incidence of coronary artery disease in the Atherosclerosis Risk in Communities (ARIC) study among Euro-Americans [79]. Further studies in the ARIC study showed that genetic variants of kinesin family member 6 (rs2045), myosin heavy chain 15 (rs3900940), paladin cytoskeletal-associated protein (rs7439293), vesicle-associated membrane protein 8 (rs1010), and SNX19 (rs2298566) had a 57% increased risk of coronary artery disease [80]. However, there still a lack of direct evidence to show the causal relationship between SNXs and coronary artery disease in human and animal models.
The mechanisms by which SNXs cause coronary artery disease are still unclear. However, indirect evidence suggest that SNXs may affect the pathogenesis of coronary artery disease via the regulation of lipid metabolism. First, SNX can directly regulate the serum lipid level. In animal studies, we have found that Snx1−/− mice have increased levels of triglycerides and cholesterol (Villar VA et al., unpublished data). The effects of SNXs on lipid level may be through the interaction of SNX1, SNX1A (splice variant of SNX1), SNX2, and SNX4 with the leptin receptor [75]. Another study also showed that overexpression of SNX8 exacerbates aberrant handling of neuronal cholesterol, causing redistribution of cholesterol within neurons creating a phenotype similar to the treatment of U18666a, which causes cholesterol to accumulate within the lysosome [46]. The low density lipoprotein (LDL) receptor (LDL-R) is a transmembrane protein that participates in lipid homeostasis [81]. SNX17, via its FERM-like and carboxyl-terminal domains, regulates the cellular trafficking of the LDL-R by the interaction of the NPVY motif in its (LDL-R) cytoplasmic domain and the SNX17 PX domain with subcellular membrane compartments [82]. The LDL-R-related protein (LRP), an endocytic recycling receptor with two cytoplasmic tyrosine-based basolateral sorting signals, is also regulated by SNX17. SNX17 binds to the proximal NPXY motif of the LRP tail distinct from its endocytosis signals, interacts preferentially with PtdIns(3)P through its PX domain, and promotes LRP sorting to the recycling pathway in the early endosomes [83,84]. The NPXY motif recognized by SNX17 is required for the exit of LRP1 from basolateral sorting endosome, indicating that polarized traffic of LRP1 involves SNX17 operating on Y-dependent sorting motifs [84]. Further study showed that a sorting nexin 17-binding domain, a cluster of 32 amino acid residues within the LRP1 cytoplasmic tail, mediates receptor recycling through the basolateral sorting endosome [85]. By interacting with the NPxY domain of ApoER2, another member of the LDL-R family, SNX17 facilitates its trafficking from early endosomes to recycling endosomes and to the plasma membrane, participates in the reelin-induced receptor degradation pathway, and regulates the processing of the carboxy-terminal fragment of ApoER2 [86]. Another study also suggests an essential role for endogenous SNX1 in sorting activated PAR1, a G-protein-coupled receptor activated by thrombin and contributing to the pathogenesis of atherosclerosis by impairing cholesterol efflux from cells and by recruiting leukocytes to arteries [87,88]. However, until now, the potential mechanism for the hypertriglyceridemia in the Snx1−/− mice is still unknown. Further study will be needed in the future.
Another mechanism by which SNXs can affect the development of coronary artery disease may be via the SNX-mediated regulation of inflammation. SNXs directly regulate some inflammatory factors. For example, endothelial cell injury and inflammation are known to be the main mechanisms in the development of vascular diseases. In lipopolysaccharide-treated human umbilical vein endothelial cells, SNX24 knockdown decreases the expression of some proinflammatory cytokines including IL-1β, IL-6, and IL-8, indicating its role on the regulation of endothelial inflammation [89]. Another member of SNX family, SNX17, was also identified as a P-selectin-interacting protein. P-selectin, a cell adhesion molecule found in platelet and endothelial cells, mediates the binding of leukocytes. SNX17, identified as a putative intracellular P-selectin binding protein, interacts with the C-terminus of P-selectin and promotes its endocytosis from the plasma membrane, and decreases the degradation of P-selectin in lysosomes by restricting its transport into the late endosomes, thereby affecting leucocyte recruitment [90,91].
Besides of the role of SNX in the pathogenesis of coronary artery disease, there is also evidence showing a role of SNX in the complications of coronary artery disease, especially in the ischemia-induced arrhythmia. In the ischemic myocardium, SNX17 is decreased, accompanied by cardiac electrical disturbances [92]. This is related to the finding that loss of SNX17 causes intracellular Ca2+ overload as revealed by the abnormal rise of resting [Ca2+]i and deceleration of decay of Ca2+ transient indicative of sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a) dysfunction, whereas SNX17 overexpression produced the opposite effects [92]. SNX17, via its PX domain, also facilitates the lysosomal degradation of SERCA2a. These indicate that SNX17 exerts its anti-arrhythmic effects by preserving functional SERCA2a protein in myocardial infarction.
SNXs and other cardiovascular diseases
Heart failure is also associated with abnormal SNX expression. Endogenous SNX13 is reduced in the failing hearts of humans and mice, the latter induced by transverse aortic constriction [93]. SNX13-deficient zebrafish have a marked decrease in cardiac systolic function associated with cardiomyocyte apoptosis, the inhibition of which largely rescues the cardiac dysfunction associated with SNX13 deficiency [93]. SNX13, via its N-terminal PXA domain, mediates endosomal trafficking of apoptosis repressor with caspase recruitment domain (ARC), a multifunctional inhibitor of apoptosis; loss of SNX13 facilitates the lysosomal degradation of ARC, promoting cardiomyocyte apoptosis and heart failure [93].
SNXs are also associated with some vascular diseases including coronary artery aneurysm. The SNX24 SNP rs28891 correlates with the development of coronary artery aneurysm formation in Taiwanese children of Han Chinese ethnic background with Kawasaki disease. Patients with CC + CT genotypes in SNX24 have lesser coronary artery aneurysm complications, indicating that polymorphisms in SNX24 gene may be used as genetic markers for the diagnosis and prognosis of coronary artery aneurysm formation in Kawasaki disease [89].
Potential role of SNXs in pharmacogenomics of diseases
SNXs have been shown to be valuable in the treatment for some diseases such as several types of cancer. Decreased expression of SNX1 in colorectal cancer cells is associated with overall survival. SNX1 may be a tumor suppressor; increased expression of SNX1 inhibits proliferation of colorectal cancer cells and increases their sensitivity to most commonly used chemotherapeutic drugs (oxaliplatin and 5-fluorouracil) for colorectal cancer [94]. The down-regulation of SNX2 leads to the overcoming of drug resistance to EGFR-targeted drugs in lung cancer cells harboring c-Met gene amplification [95]. The incidence of hypertension is increased in patients with cancer, including those treated with antibodies against vascular endothelial growth factor receptor [96,97].
β1-Adrenergic receptor (β1-AR) agonists and antagonists are widely used in the treatment of cardiovascular diseases, including heart failure and hypertension. SNX27 mediates the efficient recycling of the β1-AR from plasma membrane into the recycling endosome; SNX27 down-regulation increases the degradation of β1-AR in lysosomes [98]. SNXs are also associated with heart rate response to some drugs such as β-adrenergic receptor blockers (β-blockers). Shahin et al. performed a genome-wide association analyses for the decrease in heart rate in response to β1-AR blocker, as monotherapy or add-on therapy in whites and blacks separately from the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study [99]. The results showed that SNP rs2364349 in SNX9 is associated with the β1-AR-mediated decrease in heart rate [99]. SNX27 mediates the recycling of PDZ-domain binding motif-containing cargo, of β1-AR and β2-AR. The SNX27 PDZ-domain binds to the β1-AR and β2-AR tails, which is essential for PDZ-directed recycling of the β2-AR [100,102]. Indeed, knockdown of SNX27 inhibits the recycling of β2-AR [101].
Conclusions and perspectives
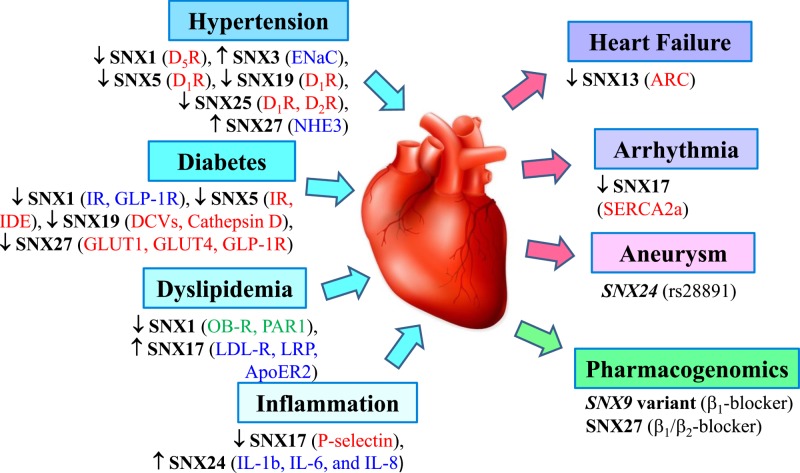
In summary, increasing evidence show that SNXs play a crucial role in various aspects of cargo endocytosis and trafficking through the endosomes, including endocytosis, endosomal sorting, and endosomal signaling. Recent studies have begun to highlight the pivotal roles of SNXs in the pathogenesis of CVDs (Figure 3), showing that dysfunction of SNXs may be involved in the pathogenesis of CVDs (Table 2). Further research using innovative silencing techniques and appropriate animal models, such as conditional and global knockout mice and renal-specific silencing, as well as gene rescure will lead to advances in our understanding of the functions of SNXs. Such studies may demonstrate not only the crucial role of SNXs in the pathogenesis of CVDs, but also their utility in the treatment of CVDs.
Figure 3. Impact of various sorting nexins (SNXs) on the etiology and sequelae of cardiovascular disease.
Several SNXs have been identified to impinge on some of the well-known underlying mechanisms, complications, and utility for pharmacogenomics for cardiovascular disease. Arrows (↑ or ↓) indicate the pathological change in the expression or activity of SNXs in the respective disease, and the corresponding change of their targets (receptor/enzyme/transporter/inflammatory cytokines) is also in brackets, either activation (in blue), inactivation (in red), or unknown (in green). rs28891 is a SNP of SNX24 that predispose to the development of coronary artery aneurysm. The PDZ domain of SNX27 is essential for PDZ-directed recycling of the β2AR, apolipoprotein E receptor 2 (ApoER2), apoptosis repressor with caspase recruitment domain (ARC), β1-adrenergic receptor blocker (β1-blocker), β2-adrenergic receptor blocker (β2-blocker), dense core vesicles (DCVs), dopamine D1 receptor (D1R); dopamine D2 receptor (D2R); dopamine D5 receptor (D5R), epithelial sodium channel (ENaC), glucagon-like peptide-1 receptor (GLP-1R), glucose transporter 1 (GLUT1) or 4 (GLUT4), insulin degrading enzyme (IDE), insulin receptor (IR), interleukin (IL), leptin receptor (OB-R), low density lipoprotein receptor (LDL-R), LDL-R-related protein (LRP), protease-activated receptor (PAR1), sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a), sodium hydrogen exchanger type 3 (NHE3), sorting nexin (SNX).
Table 2. Summary of SNX family and CVDs.
| SNX isoform | SNX modification | Effects of SNX modification on CVDs and related functions | Expression and function of SNX and SNX SNP in CVDs |
|---|---|---|---|
| SNX1 | SNX1 knockout | Elevates systolic and diastolic blood pressure, blunts natriuretic response to stimulation of D5R, increases the level of oxidative stress, and the expression of sodium transport including Na+,K+-ATPase, NHE3, and NCC [56]; increases levels of triglycerides and cholesterol (unpublished data). | Reduced SNX1 expression in human RPT cells of hypertensive Caucasian males compared with normotensive subjects [56]. |
| SNX1 knockdown | Increases the systolic and diastolic blood pressures in SNX1-depleted C57BL/6J mice and SNX1-depleted BALB/cJ mice; abrogates D5R-mediated cAMP production, GTP binding, and sodium transport inhibition [34]. | ||
| SNX3 | SNX3 overexpression | Increases ENaC levels in the total lysate and at the cell surface in mouse cortical collecting duct cells [67]. | None |
| SNX5 | SNX5 knockdown | Results in a further increase in systolic blood pressure and a decrease in sodium excretion in SHRs, and also increases blood insulin and glucose levels, decreases urinary insulin excretion, and causes insulin resistance in C57Bl/6J mice [33,55]; increases non-fasting serum insulin and glucose levels in WKY rats [55]; results in higher phosphorylation of D1R, impairment of D1R endocytosis, marked delay in receptor recycling, and failure of agonist-induced cAMP production [33]; decreases phosphorylations of insulin receptor and its substrate, protein kinase B phosphorylation [44]. | Reduced renal SNX5 expression in SHRs; decreased SNX5 expression in RPT cells of SHRs and hypertensive humans compared with WKY rats and normotensive subjects [55]. |
| SNX8 | SNX8 overexpression | Exacerbates aberrant handling of neuronal cholesterol [46]. | Reduced SNX8 expression after extreme changes in cholesterol such as treatment with mevinolin, a cholesterol-lowering statin [46]. |
| SNX9 | None | None | SNP rs2364349 in SNX9 is associated with changes in heart rate in response to β-blockers [99]. |
| SNX13 | SNX13 knockdown | Results in a markedly decrease in cardiac systolic function with striking cardiomyocyte apoptosis, facilitates the degradative sorting of apoptosis repressor with caspase recruitment domain [93]. | Reduced SNX13 expression in the failing hearts of humans and mouse model of heart failure [93]. |
| SNX17 | SNX17 overexpression | Promotes the endocytosis of P-selectin from the plasma membrane and inhibits the movement of P-selectin into lysosomes, thereby reducing its degradation in human umbilical vein endothelial cells [39]. | Decreased SNX17 expression in ischemic myocardium, accompanied with cardiac electrical disturbances [92]. |
| SNX19 | SNX19 knockdown | Increases blood pressure in C57Bl/6J mice; decreases D1R expression and its mediated-cAMP response, abrogates the D1R mediated-increased intracellular sodium in human RPT cells [57]; decreases insulin secretion and the number/size of dense core vesicles in mouse pancreatic β-cells [70]. | The SNP of SNX19 is associated with the incident of coronary artery disease in white participants of United States [79]; white participants with a high genetic risk score derived from combined multiple variants including SNX19 had a 57% increased risk of incident coronary artery disease [80]. |
| SNX24 | SNX24 knockdown | Decreases expressions of proinflammatory cytokines including IL-1β, IL-6, and IL-8 [89]. | Lesser coronary artery aneurysm complications in patients with CC + CT genotypes (rs28891) in SNX24 in Taiwanese children of Han Chinese ethnic background with Kawasaki disease [89]. |
| SNX25 | SNX25 overexpression | Enhances D1R and D2R expression and receptors-mediated signaling, perturbs both endocytosis and recycling of D2R [63]. | None |
| SNX25 knockdown | Decreases the D1R and D2R expression [64]. | ||
| SNX27 | SNX27 knockdown | Decreases GLUT4 expression [51]; inhibits recycling of β2AR [98]; decreases GLP-1R plasma membrane recycling, fails to sustain endosomal cAMP increases and reduces basal GLP-1R levels and increases exendin-4-induced receptor degradation in murine insulinoma MIN6B1 cells [78]; reduces exocytic insertion of NHE3 to the plasma membrane [66]. | None |
Abbreviations: β2AR, β2 adrenergic receptor; cAMP, cyclic adenosine monophosphate; CVD, cardiovascular disease; D1R, dopamine D1 receptor; D2R, dopamine D2 receptor; D5R, dopamine D5 receptor; ENaC, epithelial sodium channel; GLP-1R, glucagon-like peptide-1 receptor; GLUT4, glucose transporter isoform 4; GTP, guanosine triphosphate; HCTZ, hydrochlorothiazide; IL, interleukin; NCC, thiazide-sensitive sodium-chloride cotransporter; NHE3, sodium-hydrogen exchanger 3; RPT, renal proximal tubule; SHR, spontaneously hypertensive rat; SNP, single-nucleotide polymorphism; SNX, sorting nexin; WKY, Wistar-Kyoto.
Abbreviations
- ARIC
atherosclerosis risk in communities
- BACE1
β-site amyloid precursor protein-cleaving enzyme 1
- β1-AR
β1-adrenergic receptor
- β-blockers
β-adrenergic receptor blockers
- BAR
Bin/Amphiphysin/Rvs
- CVD
cardiovascular disease
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- D5R
dopamine D5 receptor
- DCV
dense core vesicles
- EGFR
epidermal growth factor receptor
- ENaC
epithelial sodium channel
- ERα
E2 receptor alpha
- GLP-1
glucagon-like peptide 1
- GLUT1
glucose transporter isoform 1
- GPCR
G protein-coupled receptor
- IDE
insulin-degrading enzyme
- IGF1
insulin-like growth factor 1
- LDL-R
low density lipoprotein receptor
- NCC
thiazide-sensitive sodium-chloride cotransporter
- NHE3
sodium-hydrogen exchanger 3
- PEAR
pharmacogenomic evaluation of antihypertensive responses
- PI3P
phosphatidylinositol 3-phosphate
- RPT
renal proximal tubule
- SERCA2a
sarcoplasmic reticulum Ca2+-ATPase2a
- SHR
spontaneously hypertensive rat
- SNX
sorting nexin
- TGN
trans-Golgi network
Funding
These studies were supported in part by grants from the National Natural Science Foundation of China [81570379, 81770425]; National Key R&D Program of China [2018YFC1312700]; Program of Innovative Research Team by National Natural Science Foundation [81721001]; the Natural Science Foundation Project of Chongqing [cstc2015jcyjA10060]; and the National Institutes of Health [5R01DK039308-31, 5R01HL092196-10, and 5P01HL074940-11].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
All authors contributed to the writing of this manuscript.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S.. et al. (2018) Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2.Dunbar S.B., Khavjou O.A., Bakas T., Hunt G., Kirch R.A., Leib A.R.. et al. (2015) Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement From the American Heart Association. Circulation 137, e558–e577 [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Benjamin E.J. and MacMahon S. (2016) Prevention and control of cardiovascular disease in the rapidly changing economy of China. Circulation 133, 2545–2560 10.1161/CIRCULATIONAHA.115.008728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks F.M., Lichtenstein A.H., Wu J.H.Y., Appel L.J., Creager M.A., Kris-Etherton P.M.. et al. (2017) Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 136, e1–e23 10.1161/CIR.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 5.Ding M., Bhupathiraju S.N., Satija A., van Dam R.M. and Hu F.B. (2014) Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129, 643–659 10.1161/CIRCULATIONAHA.113.005925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erqou S., Clougherty J.E., Olafiranye O., Magnani J.W., Aiyer A., Tripathy S.. et al. (2018) Particulate matter air pollution and racial differences in cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 38, 935–942 10.1161/ATVBAHA.117.310305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X., Ye Z., Zheng S., Ren H., Zeng J., Wang X.. et al. (2018) Long-term exposure of fine particulate matter causes hypertension by impaired renal D1 receptor-mediated sodium excretion via upregulation of G-protein-coupled receptor kinase type 4 expression in Sprague-Dawley rats. J. Am. Heart Assoc. 7, pii: e007185 10.1161/JAHA.117.007185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnett D.K., Baird A.E., Barkley R.A., Basson C.T., Boerwinkle E., Ganesh S.K.. et al. (2007) Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 115, 2878–2901 10.1161/CIRCULATIONAHA.107.183679 [DOI] [PubMed] [Google Scholar]
- 9.Ashley E.A., Hershberger R.E., Caleshu C., Ellinor P.T., Garcia J.G., Herrington D.M.. et al. (2012) Genetics and cardiovascular disease: a policy statement from the American Heart Association. Circulation 126, 142–157 10.1161/CIR.0b013e31825b07f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauersachs J. and Heymans S. (2018) Scientific updates on the interaction of genes, epigenetics, and multicellularity in cardiovascular diseases: the Working Group of Myocardial Function of the ESC. Cardiovasc. Res. 114, 1271–1272 10.1093/cvr/cvy149 [DOI] [PubMed] [Google Scholar]
- 11.Marian A.J., van Rooij E. and Roberts R. (2016) Genetics and genomics of single-gene cardiovascular diseases: common hereditary cardiomyopathies as prototypes of single-gene disorders. J. Am. Coll. Cardiol. 68, 2831–2849 10.1016/j.jacc.2016.09.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallon M. and Cullen P.J. (2015) Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 43, 33–47 10.1042/BST20140290 [DOI] [PubMed] [Google Scholar]
- 13.Cullen P.J. (2008) Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 9, 574–582 10.1038/nrm2427 [DOI] [PubMed] [Google Scholar]
- 14.Liu J.J. (2016) Retromer-mediated protein sorting and vesicular trafficking. J. Genet. Genomics 43, 165–177 10.1016/j.jgg.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 15.Teasdale R.D. and Collins B.M. (2012) Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem. J. 441, 39–59 10.1042/BJ20111226 [DOI] [PubMed] [Google Scholar]
- 16.Chandra M. and Collins B.M. (2018) The Phox Homology (PX) Domain. Adv. Exp. Med. Biol., in press 2018 Mar 23. [Epub ahead of print] 10.1007/5584_2018_185 [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., Huang T., Hong Y., Yang W., Zhang X., Luo H.. et al. (2018) The retromer complex and sorting nexins in neurodegenerative diseases. Front. Aging Neurosci. 10, 79 10.3389/fnagi.2018.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Weering J.R., Verkade P. and Cullen P.J. (2017) SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin. Cell Dev. Biol. 21, 371–380 10.1016/j.semcdb.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauffer B.E., Melero C., Temkin P., Lei C., Hong W., Kortemme T.. et al. (2010) SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 190, 565–574 10.1083/jcb.201004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elwell C. and Engel J. (2018) Emerging role of retromer in modulating pathogen growth. Trends Microbiol. 26, 769–780 10.1016/j.tim.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally K.E and Cullen P.J. (2018) Endosomal retrieval of cargo: retromer is not alone. Trends Cell Biol 28, 807–822 10.1016/j.tcb.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 22.Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J.. et al. (2013) A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 15, 461–471 10.1038/ncb2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Zhao Y., Zhang X., Badie H., Zhou Y., Mu Y.. et al. (2013) Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat. Med. 19, 473–480 10.1038/nm.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harterink M., Port F., Lorenowicz M.J., McGough I.J., Silhankova M., Betist M.C.. et al. (2011) A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 13, 914–923 10.1038/ncb2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassmer T., Attar N., Bujny M.V., Oakley J., Traer C.J. and Cullen P.J. (2007) A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J. Cell Sci. 120, 45–54 10.1242/jcs.03302 [DOI] [PubMed] [Google Scholar]
- 26.Singh V., Yang J., Yin J., Cole R., Tse M., Berman D.E.. et al. (2018) Cholera toxin inhibits SNX27-retromer-mediated delivery of cargo proteins to the plasma membrane. J. Cell Sci. 131, pii: jcs218610 10.1242/jcs.218610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvainickas A., Jimenez-Orgaz A., Nägele H., Hu Z., Dengjel J. and Steinberg F. (2017) Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J. Cell Biol. 216, 3677–3693 10.1083/jcb.201702137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harterink M., Port F., Lorenowicz M.J., McGough I.J., Silhankova M., Betist M.C.. et al. (2011) A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat. Cell Biol. 13, 914–923 10.1038/ncb2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S., Chang J. and Blackstone C. (2016) FAM21 directs SNX27-retromer cargoes to the plasma membrane by preventing transport to the Golgi apparatus. Nat. Commun. 7, 10939 10.1038/ncomms10939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soulet F., Yarar D., Leonard M. and Schmid S.L. (2005) SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol. Biol. Cell 16, 2058–2067 10.1091/mbc.e04-11-1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bendris N. and Schmid S.L. (2017) Endocytosis, metastasis and beyond: multiple facets of SNX9. Trends Cell Biol. 27, 189–200 10.1016/j.tcb.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J., Kim Y., Lee S., Park J.J., Park Z.Y., Sun W.. et al. (2010) SNX18 shares a redundant role with SNX9 and modulates endocytic trafficking at the plasma membrane. J. Cell Sci. 123, 1742–1750 10.1242/jcs.064170 [DOI] [PubMed] [Google Scholar]
- 33.Villar V.A., Armando I., Sanada H., Frazer L.C., Russo C.M., Notario P.M.. et al. (2013) Novel role of sorting nexin 5 in renal D(1) dopamine receptor trafficking and function: implications for hypertension. FASEB J. 27, 1808–1819 10.1096/fj.12-208439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villar V.A., Jones J.E., Armando I., Asico L.D., Escano C.S. Jr., Lee H.. et al. (2013) Sorting nexin 1 loss results in D5 dopamine receptor dysfunction in human renal proximal tubule cells and hypertension in mice. J. Biol. Chem. 288, 152–163 10.1074/jbc.M112.428458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C., Ma W., Yin S., Liang X., Shu X., Pei D.. et al. (2016) Sorting Nexin 11 Regulates Lysosomal Degradation of Plasma Membrane TRPV3. Traffic 17, 500–514 10.1111/tra.12379 [DOI] [PubMed] [Google Scholar]
- 36.Kurten R.C., Cadena D.L. and Gill G.N. (1996) Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272, 1008–1010 10.1126/science.272.5264.1008 [DOI] [PubMed] [Google Scholar]
- 37.Fuster J.J., González J.M., Edo M.D., Viana R., Boya P., Cervera J.. et al. , Tumor suppressor p27(Kip1) undergoes endolysosomal degradation through its interaction with sorting nexin 6. FASEB J. 24, 2998–3009 10.1096/fj.09-138255 [DOI] [PubMed] [Google Scholar]
- 38.Böttcher R.T., Stremmel C., Meves A., Meyer H., Widmaier M., Tseng H.Y.. et al. (2012) Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 14, 584–592 10.1038/ncb2501 [DOI] [PubMed] [Google Scholar]
- 39.Williams R., Schlüter T., Roberts M.S., Knauth P., Bohnensack R. and Cutler D.F. (2004) Sorting nexin 17 accelerates internalization yet retards degradation of P-selectin. Mol. Biol. Cell. 15, 3095–3105 10.1091/mbc.e04-02-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim N.Y., Cho M.H., Won S.H., Kang H.J., Yoon S.Y. and Kim D.H. (2017) Sorting nexin-4 regulates β-amyloid production by modulating β-site-activating cleavage enzyme-1. Alzheimers Res. Ther. 9, 4 10.1186/s13195-016-0232-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bareja A., Hodgkinson C.P., Soderblom E., Waitt G. and Dzau V.J. (2018) The proximity-labeling technique BioID identifies sorting nexin 6 as a member of the insulin-like growth factor 1 (IGF1)-IGF1 receptor pathway. J. Biol. Chem. 293, 6449–6459 10.1074/jbc.RA118.002406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Y., Zhang S., Ni J., You Y., Luo K., Yu Y.. et al. (2018) Sorting nexin 10 controls mTOR activation through regulating amino-acid metabolism in colorectal cancer. Cell Death Dis 9, 666 10.1038/s41419-018-0719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkes M.C., Repellin C.E., Kang J.H., Andrianifahanana M., Yin X. and Leof E.B. (2015) Sorting nexin 9 differentiates ligand-activated Smad3 from Smad2 for nuclear import and transforming growth factor β signaling. Mol. Biol. Cell 26, 3879–3891 10.1091/mbc.E15-07-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li F., Yang J., Jones J.E., Villar V.A., Yu P., Armando I.. et al. (2015) Sorting nexin 5 and dopamine d1 receptor regulate the expression of the insulin receptor in human renal proximal tubule cells. Endocrinology 156, 2211–2221 10.1210/en.2014-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aboghe D.H., Yoshioka M., Phaneuf D. and St-Amand J. (2009) Regulation of gene expression by estrogen in mammary gland of wild type and estrogen receptor alpha knockout mice. J. Steroid Biochem. Mol. Biol. 113, 116–126 10.1016/j.jsbmb.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 46.Muirhead G. and Dev K.K. (2014) The expression of neuronal sorting nexin 8 (SNX8) exacerbates abnormal cholesterol levels. J. Mol. Neurosci. 53, 125–134 10.1007/s12031-013-0209-z [DOI] [PubMed] [Google Scholar]
- 47.Baumann C., Lindholm C.K., Rimoldi D. and Lévy F. (2010) The E3 ubiquitin ligase Itch regulates sorting nexin 9 through an unconventional substrate recognition domain. FEBS J 277, 2803–2814 10.1111/j.1742-4658.2010.07698.x [DOI] [PubMed] [Google Scholar]
- 48.Duclos C.M., Champagne A., Carrier J.C., Saucier C., Lavoie C.L. and Denault J.B. (2017) Caspase-mediated proteolysis of the sorting nexin 2 disrupts retromer assembly and potentiates Met/hepatocyte growth factor receptor signaling. Cell Death Discov. 3, 16100 10.1038/cddiscovery.2016.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wassmer T., Attar N., Bujny M.V., Oakley J., Traer C.J. and Cullen P.J. (2017) A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J. Cell Sci. 120, 45–54 10.1242/jcs.03302 [DOI] [PubMed] [Google Scholar]
- 50.MaCaulay S.L., Stoichevska V., Grusovin J., Gough K.H., Castelli L.A. and Ward C.W. (2003) Insulin stimulates movement of sorting nexin 9 between cellular compartments: a putative role mediating cell surface receptor expression and insulin action. Biochem. J. 376, 123–134 10.1042/bj20030130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z., Hong L.K., Follett J., Wabitsch M., Hamilton N.A., Collins B.M.. et al. (2016) Functional characterization of retromer in GLUT4 storage vesicle formation and adipocyte differentiation. FASEB J. 30, 1037–1050 10.1096/fj.15-274704 [DOI] [PubMed] [Google Scholar]
- 52.Zhang M.Z. and Harris R.C. (2015) Antihypertensive mechanisms of intra-renal dopamine. Curr. Opin. Nephrol. Hypertens. 24, 117–122 10.1097/MNH.0000000000000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J., Villar V.A., Jones J.E., Jose P.A. and Zeng C. (2015) G protein-coupled receptor kinase 4: role in hypertension. Hypertension 65, 1148–1155 10.1161/HYPERTENSIONAHA.115.05189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Y. and Jose P.A. (2011) C-terminal di-leucine motif of dopamine D receptor plays an important role in its plasma membrane trafficking. PLoS One 6, e29204 10.1371/journal.pone.0029204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F., Yang J., Villar V.A.M., Asico L.D., Ma X., Armando I.. et al. (2018) Loss of renal SNX5 results in impaired IDE activity and insulin resistance in mice. Diabetologia 61, 727–737 10.1007/s00125-017-4482-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J., Asico L.D., Feranil J.B., Jones J.E., Armando I., Weinman E.J.. et al. (2014) Uncovering the Molecular Mechanisms Underlying the Hypertension in Snx1 Knockout Mice. Hypertension 64, A573 [Google Scholar]
- 57.Yang J., Villar V.A., Jones J.E., Yan G., Asico L.D., Armando I.. et al. (2014) Sorting nexin 19: a novel regulator of renal dopamine D1 receptor. Hypertension 64, A296 [Google Scholar]
- 58.Carey R.M., Schoeffel C.D., Gildea J.J., Jones J.E., McGrath H.E., Gordon L.N.. et al. (2012) Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension 60, 1359–1366 10.1161/HYPERTENSIONAHA.112.196071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heydorn A., Søndergaard B.P., Hadrup N., Holst B., Haft C.R. and Schwartz T.W. (2004) Distinct in vitro interaction pattern of dopamine receptor subtypes with adaptor proteins involved in post-endocytotic receptor targeting. FEBS Lett. 556, 276–280 10.1016/S0014-5793(03)01431-5 [DOI] [PubMed] [Google Scholar]
- 60.Vargas G.A. and Von Zastrow M. (2004) Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J. Biol. Chem. 279, 37461–37469 10.1074/jbc.M401034200 [DOI] [PubMed] [Google Scholar]
- 61.Lamey M., Thompson M., Varghese G., Chi H., Sawzdargo M., George S.R.. et al. (2002) Distinct residues in the carboxyl tail mediate agonist-induced desensitization and internalization of the human dopamine D1 receptor. J. Biol. Chem. 277, 9415–9421 10.1074/jbc.M111811200 [DOI] [PubMed] [Google Scholar]
- 62.Jackson A., Iwasiow R.M., Chaar Z.Y., Nantel M.F. and Tiberi M. (2002) Homologous regulation of the heptahelical D1A receptor responsiveness: specific cytoplasmic tail regions mediate dopamine-induced phosphorylation, desensitization and endocytosis. J. Neurochem. 82, 683–697 10.1046/j.1471-4159.2002.01001.x [DOI] [PubMed] [Google Scholar]
- 63.Free R.B., Hazelwood L.A., Spalding H.N., Cabrera D.M. and Sibley D.R. (2007) Sorting nexin-25, a novel member of the dopamine receptor signalplex, upregulates D1 and D2 dopamine receptor expression in HEK293 cells. FASEB J. 21, 568.9 [Google Scholar]
- 64.Free R.B., Namkung Y., Hazelwood L.A. and Sibley D.R. (2010) Sorting nexin-25 interacts with D1 and D2 dopamine receptors to regulate receptor expression and signaling. FASEB J. 771, 8 [Google Scholar]
- 65.Rao F., Wessel J., Wen G., Zhang L., Rana B.K., Kennedy B.P.. et al. (2007) Renal albumin excretion: twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension 49, 1015–1031 10.1161/HYPERTENSIONAHA.106.081679 [DOI] [PubMed] [Google Scholar]
- 66.Singh V., Yang J., Cha B., Chen T.E., Sarker R., Yin J.. et al. (2015) Sorting nexin 27 regulates basal and stimulated brush border trafficking of NHE3. Mol. Biol. Cell 26, 2030–2043 10.1091/mbc.E14-12-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boulkroun S., Ruffieux-Daidié D., Vitagliano J.J., Poirot O., Charles R.P., Lagnaz D.. et al. (2008) Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am. J. Physiol. Renal Physiol. 295, F889–F900 10.1152/ajprenal.00001.2008 [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y., Lee E.T., Howard B.V., Best L.G., Umans J.G., Yeh J.. et al. (2013) Insulin resistance, incident cardiovascular diseases, and decreased kidney function among nondiabetic American Indians: the Strong Heart Study. Diabetes Care 36, 3195–3200 10.2337/dc12-2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manucha W., Ritchie B. and Ferder L. (2015) Hypertension and insulin resistance: implications of mitochondrial dysfunction. Curr. Hypertens. Rep. 17, 504 10.1007/s11906-014-0504-2 [DOI] [PubMed] [Google Scholar]
- 70.Harashima S., Horiuchi T., Wang Y., Notkins A.L., Seino Y. and Inagaki N. (2012) Sorting nexin 19 regulates the number of dense core vesicles in pancreatic β-cells. J. Diabetes Investig. 3, 52–61 10.1111/j.2040-1124.2011.00138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valera Mora M.E., Scarfone A., Calvani M., Greco A.V. and Mingrone G. (2003) Insulin clearance in obesity. J. Am. Coll. Nutr. 22, 487–493 10.1080/07315724.2003.10719326 [DOI] [PubMed] [Google Scholar]
- 72.Hjelle J.T., Oparil S. and Peterson D.R. (1984) Subcellular sites of insulin hydrolysis in renal proximal tubules. Am. J. Physiol. 246, F409–F416 [DOI] [PubMed] [Google Scholar]
- 73.Tang W.J. (2016) Targeting insulin-degrading enzyme to treat type 2 diabetes mellitus. Trends Endocrinol. Metab. 27, 24–34 10.1016/j.tem.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghai R., Bugarcic A., Liu H., Norwood S.J., Skeldal S., Coulson E.J.. et al. (2013) Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc. Natl. Acad. Sci U.S.A. 110, E643–F652 10.1073/pnas.1216229110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haft C.R., de la Luz Sierra M., Barr V.A., Haft D.H. and Taylor S.I. (1998) Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol. Cell Biol. 18, 7278–7287 10.1128/MCB.18.12.7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinberg F., Gallon M., Winfield M., Thomas E.C., Bell A.J., Heesom K.J.. et al. (2013) A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat. Cell Biol. 15, 461–471 10.1038/ncb2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chai W., Zhang X., Barrett E.J. and Liu Z. (2014) Glucagon-like peptide 1 recruits muscle microvasculature and improves insulin’s metabolic action in the presence of insulin resistance. Diabetes 63, 2788–2799 10.2337/db13-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buenaventura T., Kanda N., Douzenis P.C., Jones B., Bloom S.R., Chabosseau P.. et al. (2018) A targeted RNAi screen identifies endocytic trafficking factors that control GLP-1 receptor signaling in pancreatic β-cells. Diabetes 67, 385–399 10.2337/db17-0639 [DOI] [PubMed] [Google Scholar]
- 79.Morrison A.C., Bare L.A., Chambless L.E., Ellis S.G., Malloy M., Kane J.P.. et al. (2007) Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 166, 28–35 10.1093/aje/kwm060 [DOI] [PubMed] [Google Scholar]
- 80.Bare L.A., Morrison A.C., Rowland C.M., Shiffman D., Luke M.M., Iakoubova O.A.. et al. (2007) Five common gene variants identify elevated genetic risk for coronary heart disease. Genet. Med. 9, 682–689 10.1097/GIM.0b013e318156fb62 [DOI] [PubMed] [Google Scholar]
- 81.Jeon H and Blacklow S.C. (2005) Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 74, 535–562 10.1146/annurev.biochem.74.082803.133354 [DOI] [PubMed] [Google Scholar]
- 82.Burden J.J., Sun X.M., García A.B. and Soutar A.K. (2004) Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J. Biol. Chem. 279, 16237–16245 10.1074/jbc.M313689200 [DOI] [PubMed] [Google Scholar]
- 83.van Kerkhof P., Lee J., McCormick L., Tetrault E., Lu W., Schoenfish M.. et al. (2005) Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J 24, 285128–285161 10.1038/sj.emboj.7600756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Donoso M., Cancino J., Lee J., van Kerkhof P., Retamal C., Bu G.. et al. (2009) Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol. Biol. Cell 20, 481–497 10.1091/mbc.e08-08-0805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farfán P., Lee J., Larios J., Sotelo P., Bu G. and Marzolo M.P. (2013) A sorting nexin 17-binding domain within the LRP1 cytoplasmic tail mediates receptor recycling through the basolateral sorting endosome. Traffic 14, 823–838 10.1111/tra.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sotelo P., Farfán P., Benitez M.L., Bu G. and Marzolo M.P. (2014) Sorting nexin 17 regulates ApoER2 recycling and reelin signaling. PLoS One 9, e93672 10.1371/journal.pone.0093672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gullapalli A., Wolfe B.L., Griffin C.T., Magnuson T. and Trejo J. (2006) An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol. Biol. Cell 17, 1228–1238 10.1091/mbc.e05-09-0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raghavan S., Singh N.K., Mani A.M. and Rao G.N. (2018) Protease-activated receptor 1 inhibits cholesterol efflux and promotes atherogenesis via cullin 3-mediated degradation of the ABCA1 transporter. J. Biol. Chem. 293, 10574–10589 10.1074/jbc.RA118.003491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin Y.J., Chang J.S., Liu X., Lin T.H., Huang S.M., Liao C.C.. et al. (2013) Sorting nexin 24 genetic variation associates with coronary artery aneurysm severity in Kawasaki disease patients. Cell Biosci 3, 44 10.1186/2045-3701-3-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Florian V., Schlüter T. and Bohnensack R. (2001) A new member of the sorting nexin family interacts with the C-terminus of P-selectin. Biochem. Biophys. Res Commun. 281, 1045–1050 10.1006/bbrc.2001.4467 [DOI] [PubMed] [Google Scholar]
- 91.Williams R., Schlüter T., Roberts M.S., Knauth P., Bohnensack R. and Cutler D.F. (2004) Sorting nexin 17 accelerates internalization yet retards degradation of P-selectin. Mol. Biol. Cell 15, 3095–3105 10.1091/mbc.e04-02-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao D., Li X., Liang H., Zheng N., Pan Z., Zhou Y.. et al. (2018) SNX17 produces anti-arrhythmic effects by preserving functional SERCA2a protein in myocardial infarction. Int. J. Cardiol. 272, 298–305 10.1016/j.ijcard.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 93.Li J., Li C., Zhang D., Shi D., Qi M., Feng J.. et al. (2014) SNX13 reduction mediates heart failure through degradative sorting of apoptosis repressor with caspase recruitment domain. Nat. Commun. 5, 5177 10.1038/ncomms6177 [DOI] [PubMed] [Google Scholar]
- 94.Bian Z., Feng Y., Xue Y., Hu Y., Wang Q., Zhou L.. et al. (2016) Down-regulation of SNX1 predicts poor prognosis and contributes to drug resistance in colorectal cancer. Tumor Biol. 37, 6619–6625 10.1007/s13277-015-3814-3 [DOI] [PubMed] [Google Scholar]
- 95.Ogi S., Fujita H., Kashihara M., Yamamoto C., Sonoda K., Okamoto I.. et al. (2013) Sorting nexin 2-mediated membrane trafficking of c-Met contributes to sensitivity of molecular-targeted drugs. Cancer Sci. 104, 573–583 10.1111/cas.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kokubo Y. and Iwashima Y. (2015) Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension 66, 254–259 10.1161/HYPERTENSIONAHA.115.03480 [DOI] [PubMed] [Google Scholar]
- 97.Qi W.X., Fu S., Zhang Q. and Guo X.M. (2016) Incidence and risk of hypertension associated with ramucirumab in cancer patients: A systematic review and meta-analysis. J. Cancer Res. Ther. 12, 775–781 10.4103/0973-1482.148700 [DOI] [PubMed] [Google Scholar]
- 98.Nakagawa T. and Asahi M. (2013) β1-adrenergic receptor recycles via a membranous organelle, recycling endosome, by binding with sorting nexin27. J. Membr. Biol. 246, 571–579 10.1007/s00232-013-9571-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shahin M.H., Conrado D.J., Gonzalez D., Gong Y., Lobmeyer M.T., Beitelshees A.L.. et al. (2018) Genome-Wide association approach identified novel genetic predictors of heart rate response to β-blockers. J. Am. Heart Assoc. 7, pii: e006463 10.1161/JAHA.117.006463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lauffer B.E., Melero C., Temkin P., Lei C., Hong W., Kortemme T.. et al. (2010) SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 190, 565–574 10.1083/jcb.201004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Temkin P., Lauffer B., Jäger S., Cimermancic P., Krogan N.J. and von Zastrow M. (2011) SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 13, 715–721 10.1038/ncb2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nooh M.M., Mancarella S. and Bahouth S.W. (2016) Identification of novel transplantable GPCR recycling motif for drug discovery. Biochem. Pharmacol. 120, 22–32 10.1016/j.bcp.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma M.P. and Chircop M. (2012) SNX9, SNX18 and SNX33 are required for progression through and completion of mitosis. J. Cell Sci. 125, 4372–4382 10.1242/jcs.105981 [DOI] [PubMed] [Google Scholar]
- 104.Hanson B.J. and Hong W. (2003) Evidence for a role of SNX16 in regulating traffic between the early and later endosomal compartments. J. Biol. Chem. 278, 34617–34630 10.1074/jbc.M300143200 [DOI] [PubMed] [Google Scholar]
- 105.Brankatschk B., Pons V., Parton R.G. and Gruenberg J. (2011) Role of SNX16 in the dynamics of tubulo-cisternal membrane domains of late endosomes. PLoS One 6, e21771 10.1371/journal.pone.0021771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vieira N., Deng F.M., Liang F.X., Liao Y., Chang J., Zhou G.. et al. (2014) SNX31: a novel sorting nexin associated with the uroplakin-degrading multivesicular bodies in terminally differentiated urothelial cells. PLoS One 9, e99644 10.1371/journal.pone.0099644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ha C.M., Park D., Kim Y., Na M., Panda S., Won S.. et al. (2015) SNX14 is a bifunctional negative regulator for neuronal 5-HT6 receptor signaling. J. Cell Sci. 128, 1848–1861 10.1242/jcs.169581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hao X., Wang Y., Ren F., Zhu S., Ren Y., Jia B.. et al. (2011) SNX25 regulates TGF-β signaling by enhancing the receptor degradation. Cell Signal. 23, 935–946 10.1016/j.cellsig.2011.01.022 [DOI] [PubMed] [Google Scholar]
- 109.Feng T., Niu M., Ji C., Gao Y., Wen J., Bu G.. et al. (2016) SNX15 regulates cell surface recycling of APP and Aβ generation. Mol. Neurobiol. 53, 3690–3701 10.1007/s12035-015-9306-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danson C., Brown E., Hemmings O.J., McGough I.J., Yarwood S., Heesom K.J.. et al. (2013) SNX15 links clathrin endocytosis to the PtdIns3P early endosome independently of the APPL1 endosome. J. Cell Sci. 126, 4885–4899 10.1242/jcs.125732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoepfner S., Severin F., Cabezas A., Habermann B., Runge A., Gillooly D.. et al. (2005) Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121, 437–450 10.1016/j.cell.2005.02.017 [DOI] [PubMed] [Google Scholar]