Summary
Nutritional zinc deficiency leads to immune dysfunction and aggravates inflammation. However, the underlying mechanism remains unknown. In this study, the relationship between macrophage subtypes (M1 and M2) and helper T lymphocytes (Th1 and Th2) was investigated using the spleen from rats fed zinc‐deficient or standard diet. In experiment I, 5‐week‐old male Sprague‐Dawley rats were fed a zinc‐deficient diet (without zinc additives) or a standard diet (containing 0·01% zinc) for 6 weeks. In experiment II, the rats were divided into four groups: one group was fed a standard diet for 6 weeks; two groups were fed zinc‐deficient diets and were injected three times a week with either saline or interleukin‐4 (IL‐4) (zinc‐deficient/IL‐4 i.p.); a fourth group (zinc‐deficient/standard) was fed a zinc‐deficient diet for 6 weeks followed by a standard diet for 4 weeks. In experiment I; GATA‐binding protein 3 (GATA‐3) protein level, M2 macrophage, CD3+ CD8+ cells, and IL‐4/IL‐13‐positive cells significantly decreased in the spleens of the zinc‐deficient group. Additionally, IL‐1β and macrophage inflammatory protein‐1α (MIP‐1α) mRNA levels significantly increased in the splenic macrophages of the zinc‐deficient group. In experiment II; M2 macrophages, CD3+ CD8+ cells, IL‐4/IL‐13‐positive cells, and GATA‐3 protein levels significantly increased in the spleens of the zinc‐deficient/IL‐4 i.p. and zinc‐deficient/standard groups. Furthermore, IL‐1β and MIP‐1α mRNA levels decreased in the splenic macrophages of the zinc‐deficient/IL‐4 i.p. and zinc‐deficient/standard groups. Zinc deficiency‐induced aggravated inflammation is related to Th2 lymphocytes and followed by the association with loss of GATA‐3, IL‐4 and anti‐inflammatory M2 macrophages. Importantly, IL‐4 injection or zinc supplementation can reverse the effects of zinc deficiency on immune function.
Keywords: helper T lymphocyte (Th1 and Th2), inflammation, interleukin‐4, macrophage subtype (M1 and M2), zinc deficiency
Abbreviations
- APC
allophycocyanin
- Cu
copper
- CD3+ CD4+
helper T lymphocytes
- CD3+ CD8+
cytotoxic T lymphocytes
- FBS
fetal bovine serum
- FITC
fluorescein isothiocayanate
- GATA‐3
GATA‐binding protein 3
- HRP
horseradish peroxidase
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- MCP‐1
monocyte chemoattractant protein
- MDA
malondialdehyde
- MIP‐1α
macrophage inflammatory protein 1α
- PBS
phosphate‐buffered saline
- PE
phycoerythrin
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBS
Tris‐buffered saline
- Th1
type 1 T helper lymphocyte
- Th2
type 2 T helper lymphocyte
- TNF
tumor necrosis factor
- Zn
zinc
Introduction
Zinc is an essential trace element in humans and animals.1, 2, 3 Indeed, zinc is required by > 300 zinc finger transcription factors and enzymes that regulate cell activation and gene expression.4 Accordingly, nutritional zinc deficiency leads to growth retardation, taste abnormalities, dermatitis, depilation, immune dysfunction,5 and higher risk of inflammatory diseases.6, 7 Inadequate zinc intake was recently reported in children and adults in the USA,8, 9 Mexico, Colombia,10 and Japan.11 The effects of zinc deficiency on immunity are related to the lower production of T lymphocytes and cytokines.12 In addition, overexpression of the inflammatory proteins inducible nitric oxide synthase and interleukin‐1β (IL‐1β) aggravated lung inflammation in rats with zinc deficiency.13 In vitro studies showed that the levels of inflammatory cytokines [IL‐1β, IL‐6, or tumor necrosis factor‐α (TNF‐α)] increased due to the formation of reactive oxygen species (ROS) in immune cells under conditions of zinc deficiency.14, 15 However, other mechanisms may also be involved, which have not yet been elucidated.
All T lymphocytes express CD3 on their surfaces and play important roles in pathogen defense, tumor development, and inflammation. T lymphocytes include various subsets, which are produced via the differentiation of naive T lymphocytes (CD4+ CD8+) into helper T lymphocytes (CD3+ CD4+) or cytotoxic T lymphocytes (CD3+ CD8+) in the thymus.16 Helper T lymphocytes also exhibit various subtypes, such as T helper type 1 (Th1), Th2, Th17, and regulatory T cells. All helper T lymphocyte subtypes are associated with various inflammatory diseases.17 In particular, the balance between Th1 and Th2 lymphocytes, and pro‐inflammatory M1 and anti‐inflammatory M2 macrophages determines the macrophage subtype‐based inflammatory response. Interferon‐γ (IFN‐γ) or IL‐12 induce the differentiation of naive CD4 cells into Th1 lymphocytes through the transcription factor T‐bet. Subsequently, Th1 lymphocytes produce TNF‐α and IL‐6, which promote the differentiation of immature macrophages into M1 macrophages.18 However, Th2 lymphocytes generated from naive CD4 cells by IL‐4 or IL‐13 through the transcription factor GATA‐binding protein 3 (GATA‐3) also produce IL‐4 and IL‐13, which induce the differentiation of immature macrophages into M2 macrophages.19 GATA‐3 is a zinc finger transcription factor that requires zinc for its function and stability.20 Reports show that zinc deficiency induces oxidative stress‐induced DNA damage,21 which underscores the importance of zinc finger proteins in cellular processes.20 Zinc deficiency and increased oxidative stress could be possibly related to loss of zinc finger structure. Zinc deficiency inhibits zinc finger proteins, poly (ADP‐ribose) polymerase‐1, and hepatocyte nuclear factor‐4α, an effect that was reversed by zinc supplementation.22, 23 Similarly, zinc deficiency may affect Th2 lymphocyte differentiation and/or their ability to produce IL‐4 and IL‐13 because of their GATA‐3 requirement. Therefore, zinc deficiency‐induced aggravation of inflammation may be caused by imbalances in Th1/Th2 and M1/M2 macrophage ratios. Although zinc deficiency reduces IL‐4 mRNA levels in vitro,24 its association with Th1/Th2 levels has not been demonstrated in vivo.
Therefore, we hypothesized that zinc deficiency leads to immune dysfunction and exacerbation of inflammatory responses because of loss of GATA‐3 function. This inhibits Th2 lymphocyte formation and decreases IL‐4 production, which subsequently decreases IL‐4‐induced M2 macrophage numbers and inhibits anti‐inflammatory reactions.
To elucidate this hypothesis, we analyzed spleens from rats fed a standard diet or a zinc‐deficient diet and determined the mRNA levels of macrophage and T lymphocyte cytokines (IL‐1β, IL‐4, IL‐6, IL‐13, TNF‐α, and IFN‐γ) and chemokines [monocyte chemoattractant protein 1 (MCP‐1) and macrophage inflammatory protein‐1α (MIP‐1α)]. Furthermore, we performed immunohistochemical staining of M1/M2 macrophages and Th1/Th2 lymphocytes, determined the population of CD3+ CD8+ and CD3+ CD4+ cells, and quantified GATA‐3 protein expression. Finally, we tested whether the effects of zinc deficiency on inflammatory markers are inhibited by IL‐4 administration or recovered by zinc supplementation.
Methods
Experimental design
Five‐week‐old male Sprague‐Dawley rats weighing 180–200 g were obtained from Charles River (Tokyo, Japan). As illustrated in Fig. 1, in the first experiment (experiment I), the rats were divided into two groups (n = 13/group) and fed 17 g/day of either a zinc‐deficient diet (without zinc additives) or standard diet (containing 0·01% zinc) for 6 weeks. Standard and zinc‐deficient diets were specially made by Oriental Yeast Co. Ltd., Tokyo, Japan (see Supplementary material, Table S1). In the second experiment, (experiment II), the rats were divided into four groups (n = 7/group), one of which was fed 17 g/day of a standard diet (containing 0·01% zinc) for 6 weeks (n = 7); two groups were fed a zinc‐deficient diet (n = 7/each) and were injected intraperitoneally (i.p.) three times a week25 with either saline or 100 ng IL‐4/rat (Wako, Tokyo, Japan) dissolved in saline (zinc‐deficient/IL‐4 i.p.); a fourth group (zinc‐deficient/standard) was fed a zinc‐deficient diet for 6 weeks followed by a standard diet for 4 weeks. The animals were housed in separate cages at 22° under a 12‐hr light/dark cycle, in accordance with the protocols and guidelines approved by the Animal Experimentation and Ethics Committee of The Jikei University School of Medicine. After dietary manipulation, blood samples were collected from the abdominal aorta under isoflurane anesthesia, and the spleens were harvested in 10% formalin neutral buffer solution or RPMI‐1640 buffer.
Figure 1.
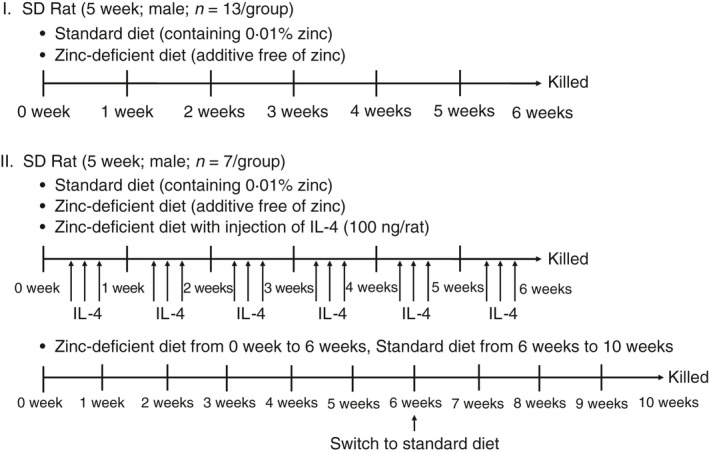
Experimental design.
Serum zinc, copper, and malondialdehyde levels, and superoxide dismutase activity
Blood was collected in a 15‐ml centrifuge tube and centrifuged at 1600 g for 10 min. Serum zinc and copper were quantified using ACCURASAUTO Zn and QUICKAUTO NEO Cu kits (Shino‐test, Kanagawa, Japan), as described previously.26, 27 Malondialdehyde (MDA) was quantified using an MDA/thiobarbituric acid reactive substances assay kit (Japan Institute for the Control of Aging, Shizuoka, Japan), following the manufacturer's protocol.28 Total superoxide dismutase (SOD) activity was measured using the SOD assay kit‐WST (Dojindo, Rockville, MD) according to the manufacturer's instructions. Mn‐SOD activity was calculated by adding 1·5 mm diethyldithiocarbamate to the serum and incubating the mixture for 30 min at 37°. Subsequently, Cu/Zn‐SOD activity was calculated as total SOD activity – Mn‐SOD activity.
Histology and immunohistochemistry
Spleen sections (3 μm thick) were deparaffinized by serial incubation for 3 min each in xylene (×3), 99·5% ethanol (×2), 80% ethanol (×1), and 70% ethanol (×1). The specimens were then washed in gently running tap water for 5 min (×1), immersed in distilled water for 1 min (×2), and stained with hematoxylin & eosin (H&E).
The spleen sections were analyzed by immunohistochemistry as described previously.29, 30, 31 Spleen sections (6 μm thick) were deparaffinized, washed in gently running tap water for 5 min (×1), and immersed in distilled water for 1 min (×2). Endogenous peroxidase activity was inactivated using 0·3% hydrogen peroxide, and the spleen sections were blocked for 50 min in Blocking One (Nacalai Tesque, Tokyo, Japan), washed twice for 5 min each in Tris‐buffered saline (TBS), and probed overnight with antibodies against ED1 (CD68, a marker of immature/general macrophages),32, 33 ED2 (CD163, a marker of M2 macrophages),34, 35, 36 ED3 (CD169, a marker of M1 macrophages),37 IL‐4, IL‐13, or GATA‐3 (markers of Th2 lymphocyte‐related factors), and IFN‐γ or T‐bet (markers of Th1 lymphocyte‐related factors). The antibodies and immune cell markers used in this study are listed in Table 1. Antibodies were diluted 1 : 50 in TBS containing 1% bovine serum albumin. The sections were then washed with TBS for 5 min (×2), incubated for 50 min with Envision™ + polymer (Dako, Tokyo, Japan), thoroughly washed with TBS, reacted with streptavidin conjugated to horseradish peroxidase (HRP), and stained with 0·3% diaminobenzidine. Following staining of the nuclei with hematoxylin, the sections were mounted with Entellan Neu (Merck‐Millipore, Tokyo, Japan). For computer‐assisted imaging analysis of CD68, CD163, CD169, IL‐4, IL‐13, and IFN‐γ immunohistochemical staining, positive cells were counted in the area of the spleen. Twenty randomly selected fields from the same spleen section were photographed at a magnification of 200× using a fluorescence microscope (BZ‐9000; Keyence, Tokyo, Japan). The CD68‐, CD163‐, CD169‐, IL‐4‐, IL‐13‐, GATA‐3‐, IFN‐γ‐, and T‐bet‐positive cells were counted and averaged (cells/20 fields). To objectively confirm the results of histological scoring, a computer‐assisted analysis was conducted using the winroof2013 image processing software (Mitani, Tokyo, Japan).
Table 1.
Antibodies and immune cell markers for rats
| Immune cells | Immature macrophage | M1 macrophage | M2 macrophage | T helper lymphocyte | Cytotoxic lymphocyte | T helper 1 lymphocyte | T helper 2 lymphocyte |
|---|---|---|---|---|---|---|---|
| Markers | ED1 (CD68) | ED3 (CD169) | ED2 (CD163) | FITC‐CD3 | FITC‐CD3 | INF‐γ | IL‐4 |
| APC‐CD4 | PE‐CD8 | IL‐13 | |||||
| Using methods | Immuno histochemistry | Immuno histochemistry | Immuno histochemistry | FACS analysis | FACS analysis | Immuno histochemistry | Immuno histochemistry |
| a | a | a | b | b | c | d, e |
Sampling of splenic macrophages and lymphocytes, and RNA extraction
Splenic cell cultures and total mRNA were obtained as described previously.38, 39, 40 Splenic cell suspensions were prepared using a cell strainer (BD Falcon, Tokyo, Japan). Red blood cells were removed by adding 5 ml erythrocyte lysis buffer (0·15 m NH4Cl, 1 mm KHCO3, and 0·1 mm ethylenediaminetetraacetic acid), incubating on ice for 1 min, and centrifuging at 140 g for 5 min at 4°. After discarding the supernatant, the cells were resuspended in 10 ml RPMI‐1640 medium containing 100 U/ml penicillin, 100 μg/ml streptomycin (Nikken, Kyoto, Japan), and 5% heat‐inactivated fetal bovine serum (FBS; Equitech‐Bio, Kerrville, TX), and were incubated for 50 min at 37°. The medium was collected to obtain non‐adherent cells, whereas the adherent cells were harvested in 1 ml medium. Adherent and non‐adherent cells were counted using a hemocytometer, diluted to 2·5 × 106 cells/ml in RPMI‐1640, and seeded in a 24‐well culture plate at 5 × 106 cells/well. The adherent cells were activated for 6 hr at 37° in 2 ml medium containing 100 ng/ml lipopolysaccharide type 0111:B4 from Escherichia coli (Sigma‐Aldrich, Tokyo, Japan), a macrophage‐activating factor, and total RNA was extracted using 1 ml TRIzol reagent (Thermo Fisher Scientific, Tokyo, Japan) according to the manufacturer's protocol. Similarly, non‐adherent cells were activated for 24 hr in 2 ml medium containing 1·25 μg/ml concanavalin A (Wako, Tokyo, Japan), a T‐lymphocyte mitogen, and centrifuged at 140 g for 5 min. RNA was extracted using TRIzol reagent.
Quantitative reverse transcription–polymerase chain reaction
Complementary DNA was synthesized from mRNA using a Transcriptor first‐strand cDNA synthesis kit (Roche Diagnostics, Tokyo, Japan). The mRNA levels of TNF‐α, IL‐1β, IL‐6, MCP‐1, and MIP‐1α (Universal Probe Library; Roche Applied Science, Basel, Schweiz) in macrophages and those of IFN‐γ, IL‐4, and IL‐13 (Universal Probe Library) in T lymphocytes were assessed by quantitative polymerase chain reaction and normalized to that of 18S rRNA. Primers and annealing temperatures are listed in Table 2. Targets were amplified on a LightCycler Nano (Roche Diagnostics) using LightCycler FastStart DNA MasterPLUS SYBR Green I (Roche Diagnostics) over 40 cycles of denaturation at 94°, annealing at temperatures indicated in Table 2, and extension at 72°. Calibrator‐normalized cytokine to 18S rRNA relative ratio was calculated as the index of mRNA expression of each cytokine, following the manufacturer's protocols (Roche).40, 41, 42
Table 2.
Sequences and annealing temperatures of primers used in this study
| Primers | Base sequence | Annealing temperatures (°C) |
|---|---|---|
| 18S rRNA | sense: 5′‐CCTGGAAAGGGCTCAACAC‐3′ | 48a |
| anti‐sense: 5′‐GCTCTAGAATTACCACAGTTATCCAA‐3′ | ||
| IL‐1β | sense: 5′‐ACAAGTGRTATTCTCCATGAGC‐3′ (R is a mixture of A and G) | 55b |
| anti‐sense: 5′‐CCACTTTGSTCTTGACTTCTAT‐3′ (S is a mixture of C and G) | ||
| IL‐4 | sense: 5′‐ACCAGACGTCCTTACGGC‐3′ | 48a |
| anti‐sense: 5′‐GCGTGGACTCATTCACGG‐3′ | ||
| IL‐6 | sense: 5′‐GTTGCCTTCTTGGGACTGATGT‐3′ | 511 |
| anti‐sense: 5′‐GGTCTGTTGTGGGTGGTATCCT‐3′ | ||
| IL‐13 | sense: 5′‐GCTGTTGCACAGGGAAGTCT‐3′ | 48a |
| anti‐sense: 5′‐GCTGTTGCACAGGGAAGTCT‐3′ | ||
| TNF‐α | sense: 5′‐CTTATCTACTCCCAGGTTCTCTTCAA‐3′ | 54c |
| anti‐sense: 5′‐GAGACTCCTCCCAGGTACATGG‐3′ | ||
| MCP‐1 | sense: 5′‐CGTGCTGTCTCAGCCAGAT‐3′ | 48c |
| anti‐sense: 5′‐GGATCATCTTGCCAGTGAATG‐3′ | ||
| MIP‐1α | sense: 5′‐GCGCTCTGGAACGAAGTCT‐3′ | 48c |
| anti‐sense: 5′‐GAATTTGCCGTCCATAGGAG‐3′ | ||
| IFN‐γ | sense: 5′‐AGGACGGTAACACGAAA‐3′ | 48a |
| anti‐sense: 5′‐CTGTGGGTTGTTCACCTC‐3′ |
Roche universal probe library.
Tsunoda et al., 2006.
Kido et al., 2014.
Flow cytometry
Splenic cell cultures were prepared following the method used for RNA extraction. Isolated macrophages and lymphocytes were diluted to 1 × 106 cells/ml in phosphate‐buffered saline (PBS) with 5% FBS, blocked with goat serum (Jackson ImmunoResearch, Baltimore, PA) at 4° for 10 min, washed with PBS containing 5% FBS, and probed with fluorescein isothiocyanate‐conjugated (FITC‐) CD3, phycoerythrin‐conjugated (PE‐) CD8a, and allophycocyanin‐conjugated (APC‐) CD4 antibodies diluted in PBS with 5% FBS at 4° for 30 min. The antibodies and immune cell markers are listed in Table 1. Background fluorescence was quantified using FITC mouse IgM к isotype control, APC mouse IgG1 к isotype control, and PE mouse IgG1 к isotype control (BioLegend, San Diego, CA). Data were collected on a MACSQuant analyzer and processed using MACSQuantify (Miltenyi Biotec, Bergisch Gladbach, Germany).
Immunomagnetic separation
Splenic cell cultures were prepared following the method used for RNA extraction, diluted to 5 × 107 cells/ml in RPMI‐1640 with 5% FBS, and probed with CD4 antibodies (BioLegend) diluted in the same medium at 4° for 40 min. Then, the cells were washed with 5 ml PBS, centrifuged at 140 g and 4° for 5 min, and incubated with immunomagnetic mouse secondary antibodies (Miltenyi Biotec) at 4° for 30 min. Finally, CD4+ cells were isolated on an OctoMACS™ separator (Miltenyi Biotec) using MS columns. CD4+ cells stimulated with concanavalin A were collected in the same manner after stimulation for 24 hr and centrifugation at 140 g for 5 min.
Western blotting
Whole spleen samples and T lymphocytes were homogenized by sonication on ice in tissue protein extraction reagent (Thermo Fisher Scientific), 5 mm 2‐mercaptoethanol, and a protease inhibitor followed by centrifugation. The total protein content in the lysates was measured using the Bradford assay, and 60 μg protein from whole spleen and 30 μg from CD4+ cells were mixed with sample buffer, boiled at 95° for 10 min, separated on a 10% polyacrylamide gel, and transferred to polyvinylidene fluoride membranes (Merck‐Millipore) using a semi‐dry blot transfer system (Nihon Eido, Tokyo, Japan). The membranes were blocked in 5% skim milk for 2 hr and probed overnight with 1 : 100 GATA‐3 (Santa Cruz Biotechnology, Dallas, TX) and 1 : 5000 β‐actin (Sigma‐Aldrich, St. Louis, MO) antibodies. After washing thrice with skim milk for 5 min each, the membranes were incubated with 1 : 5000 dilution of goat anti‐mouse IgG conjugated to HRP (Sigma‐Aldrich) for 1 hr. The immunocomplexes were visualized using Lumina™ Forte Western HRP substrate (Merck‐Millipore). GATA‐3 and β‐actin were quantified using multi gauge version 3.0 (Fujifilm, Tokyo, Japan).
Statistical analysis
Groups in experiment I were compared using the Mann–Whitney U‐test, whereas groups in experiment II were compared using analysis of varaince, followed by the Student–Newman–Keuls test. Data were analyzed using statview J‐5.0 software (SAS Institute, Cary, NC), and differences were considered significant at P < 0·05.
Results
Experiment I
Physiological effects of zinc deficiency in rats
Zinc deficiency leads to growth retardation, thymus atrophy,43 accumulation of ROS, dermatitis, and depilation.44 In this study, we examined rats fed a zinc‐deficient or standard diet for 6 weeks.2 The mean body weight, relative spleen weight, serum zinc, copper, and MDA levels, and Cu/Zn‐SOD activity of these rats were determined using the procedure in experiment I (Fig. 1) and are listed in Table 3. The final body weight, serum zinc, and total SOD and Cu/Zn‐SOD activities were significantly lower, whereas relative spleen weight and serum copper, copper : zinc ratio, and MDA levels were significantly higher in rats fed a zinc‐deficient diet than in those on a standard diet. These results suggested that zinc deficiency affects body weight, relative spleen weight, and serum antioxidant enzyme activity, and increases oxygen radical levels.
Table 3.
Body weight, relative spleen weight, and serum indices in rats on standard or zinc‐deficient diets
| Standard | Zinc‐deficient | P‐value | |
|---|---|---|---|
| Body weight (g) | 338·9 ± 8·36 | 232·0 ± 3·85 | 0·0001*** |
| Spleen weight (g) | 0·578 ± 0·02 | 0·528 ± 0·06 | 0·4347 |
| Relative spleen weight (mg/g body weight) | 1·683 ± 0·05 | 2·323 ± 0·24 | 0·0015*** |
| Serum zinc (μg/dl) | 150·5 ± 3·59 | 39·13 ± 11·9 | 0·0001*** |
| Serum copper (μg/dl) | 126·3 ± 9·28 | 170·2 ± 15·9 | 0·0375* |
| Copper : zinc ratio | 0·756 ± 0·08 | 5·116 ± 0·94 | 0·0036** |
| MDA assay (μmol/l) | 28·39 ± 3·53 | 46·52 ± 4·19 | 0·0051** |
| Serum Total SOD (U/ml) | 203·3 ± 4·58 | 183·2 ± 6·15 | 0·0256* |
| Serum Mn‐SOD (U/ml) | 95·60 ± 2·70 | 93·27 ± 7·30 | 0·7713 |
| Serum Cu/Zn‐SOD (U/ml) | 107·7 ± 3·17 | 89·95 ± 2·08 | 0·0009*** |
Zn, zinc; Cu, Copper; MDA, malondialdehyde; SOD, superoxide dismutase.
Mean values and standard errors are indicated (n = 5 per group). The mean values were compared to the standard and zinc‐deficient groups. *P < 0·05, **P < 0·01, ***P < 0·001 vs. standard by Mann‐Whitney U test, respectively. After dietary manipulation, the spleen was harvested from rats. Relative spleen weight was calculated using the following formula: Relative spleen weight (mg/g) = spleen weight/body weight. Serum was collected from rats after dietary manipulation. Cu/Zn‐SOD was calculated using the following formula: Cu/Zn‐SOD activity = Total SOD activity–Mn‐SOD activity.
Zinc deficiency affected cytokine mRNA expression
Zinc deficiency reportedly increases inflammatory cytokine expression45 and suppresses T lymphocyte and cytokine production.12 The adherent (macrophages) and non‐adherent (lymphocytes) cells were separated from the spleen using a cell culture plate. In addition, macrophages and lymphocytes were also obtained from lymphocytes stimulated with the T lymphocyte mitogen concanavalin A and macrophages stimulated with lipopolysaccharide. We determined the mRNA levels of inflammatory cytokines or chemokines (TNF‐α, IL‐1β, IL‐6, MIP‐1α, and MCP‐1) in lipopolysaccharide‐stimulated splenic macrophages, as well as those of IFN‐γ, IL‐4, and IL‐13 in concanavalin A‐stimulated lymphocytes in rats fed standard or a zinc‐deficient diet (Fig. 2). Macrophage IL‐1β and MIP‐1α levels were significantly higher, whereas T lymphocyte IL‐4 and IL‐13 mRNA levels were significantly lower in rats on a zinc‐deficient diet. Macrophage TNF‐α, IL‐6, and MCP‐1 mRNA levels were also higher, albeit not significantly, in rats on a zinc‐deficient diet. However, T lymphocyte IFN‐γ mRNA level was comparable between the two groups. These results suggested that zinc deficiency increases the macrophage‐mediated inflammatory reaction and decreases IL‐4 and IL‐13 production by T lymphocytes in rat spleen.
Figure 2.
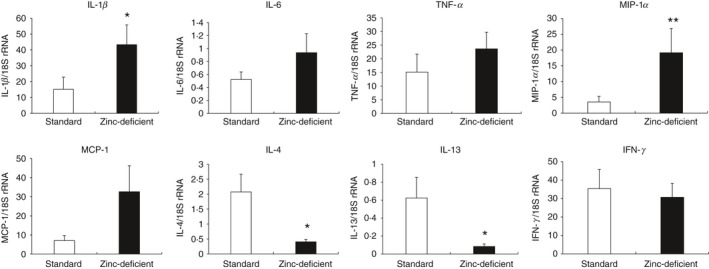
Relative mRNA expression of cytokines and chemokines in splenic macrophages and T lymphocytes from rats on standard or zinc‐deficient diet. Quantitative RT‐PCR was used to determine macrophage mRNA levels of TNF‐α, IL‐1β, IL‐6, MCP‐1, and MIP‐1α, and T lymphocyte mRNA levels of IFN‐γ, IL‐4, and IL‐13. Messenger RNA expression was normalized to that of the housekeeping 18S rRNA gene in each sample. Data represent the mean ± standard error (n = 7 per group). P = 0·037 for IL‐1β, P = 0·245 for IL‐6, P = 0·846 for TNF‐α, P = 0·005 for MIP‐1α, P = 0·093 for MCP‐1, P = 0·010 for IL‐4, P = 0·015 for IL‐13, and P = 0·716 for IFN‐γ versus Standard using the Mann–Whitney U‐test. *P < 0·05. RT‐PCR, reverse transcription polymerase chain reaction; TNF‐α, tumor necrosis factor‐α; IL, interleukin; MCP‐1, monocyte chemotactic protein‐1; MIP‐1α, macrophage inflammatory protein‐1α; IFN‐γ, interferon‐γ.
Zinc deficiency affected Th2 cytokines and M2 macrophages in the spleen
Based on the above results, we performed morphological analysis and immunohistochemistry to evaluate the effects of zinc deficiency on the number of macrophage subtypes (M1 and M2) and Th1 (IFN‐γ)/Th2 (IL‐4 or IL‐13) cytokine‐producing lymphocytes in the spleens of rats fed a zinc‐deficient diet. Histologically, the spleen was comparable between rats provided standard and zinc‐deficient diets, as assessed on sections stained with H&E (Fig. 3). Macrophages and T lymphocytes were also observed by immunohistochemistry in the red pulp area (Fig. 4a, 5a). The number of CD163‐positive cells was significantly decreased in rats that were on a zinc‐deficient diet, whereas the numbers of CD68‐ and CD169‐positive cells were comparable between the two groups (Fig. 4b). Similarly, the numbers of IL‐4‐ and IL‐13‐positive cells were significantly decreased in rats on a zinc‐deficient diet than in rats on a standard diet, whereas the number of IFN‐γ‐positive cells was comparable between the two groups (Fig. 5b). Hence, zinc deficiency affects the number of Th2 (IL‐4 or IL‐13) cytokine‐producing lymphocytes and M2 macrophages in the spleen.
Figure 3.
Representative photomicrographs of spleen sections obtained from rats on standard or zinc‐deficient diet. Sections were stained with hematoxylin and eosin. Magnification, 100×.
Figure 4.
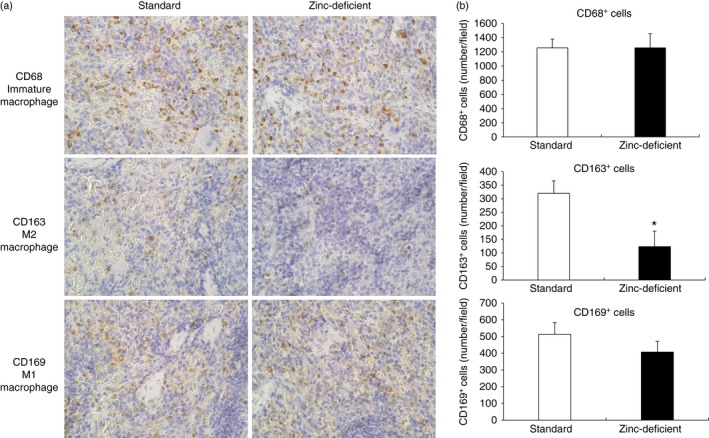
Immunohistochemistry for immature, M1, and M2 macrophages in spleens of rats on standard or zinc‐deficient diet. (a) Representative photomicrographs. Brown spots indicate immunohistochemical staining for CD68 (a marker of immature macrophages), CD163 (a marker of M2 macrophages), and CD169 (a marker of M1 macrophages). Magnification, 400×. (b) Number of positive cells per field. Data represent the mean ± standard error (n = 5 per group). *P < 0·05 versus standard using the Mann–Whitney U‐test.
Figure 5.
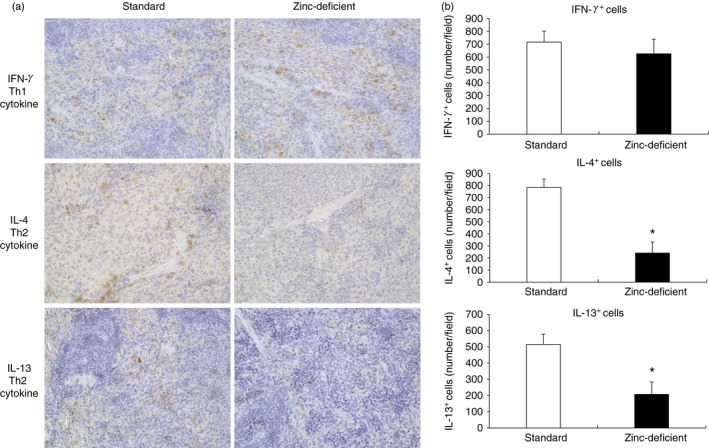
Immunohistochemistry for IL‐4, IL‐13, and IFN‐γ in spleens of rats on standard or zinc‐deficient diet. (a) Representative photomicrographs. Brown spots indicate immunohistochemical staining for IFN‐γ (a marker of Th1 cytokine), or IL‐4 and IL‐13 (markers of Th2 cytokines). Magnification, 400×. (b) Number of positive cells per field. Data represent the mean ± standard error (n = 5 per group). *P < 0·05 versus standard using the Mann–Whitney U‐test. IL, interleukin; IFN‐γ, interferon‐γ; Th1, T helper type 1.
Zinc deficiency affected cytotoxic T lymphocytes, but not helper T lymphocytes, in the spleen
Zinc deficiency can lead to thymus atrophy.44 Therefore, we assessed the effect of zinc deficiency on the numbers of CD3+, CD4+, and CD8+ cells in the spleen. Flow cytometry was used to determine the percentages of CD3+, CD4+, and CD8+ cells in splenocytes obtained from rats fed a standard or zinc‐deficient diet (Fig. 6a). The population of CD3+ CD8+ cells (cytotoxic T lymphocytes) was significantly decreased in rats that were on zinc‐deficient diet than in rats on a standard diet (Fig. 6b), whereas the population of CD3+ CD4+ cells (helper T lymphocytes) was comparable between the two groups. Hence, zinc deficiency affects the percentages of cytotoxic T lymphocytes, but not those of helper T lymphocytes, in the spleen. In addition, the decrease in the number of cytotoxic T lymphocytes may be related to the inflammatory response because of zinc deficiency.
Figure 6.
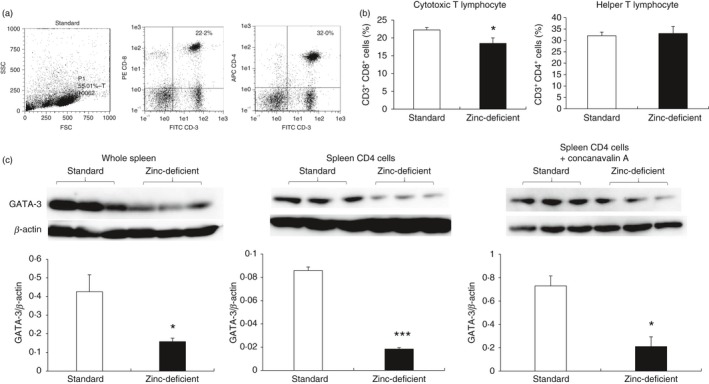
Populations of CD3+, CD4+, and CD8+ cells, and relative GATA‐3 expression in spleens of rats on a standard or zinc‐deficient diet. (a) Flow cytometry of cells stained with FITC‐CD3, APC‐CD4, and PE‐CD8a antibodies, and gated on SSC/FSC. (b) Percentages of CD3+ CD8+ and CD3+ CD4+ cells. Data represent mean ± standard error (n = 4 per group). *P < 0·05 versus standard using Mann–Whitney U‐test. (c) Western blotting showing the expression of the transcription factor GATA‐3 in whole spleen (60 μg/lane), unstimulated CD4+ cells (30 μg/lane), and CD4+ cells stimulated with concanavalin A (28 μg/lane). CD4+ cells were isolated immunomagnetically, GATA‐3 level was quantified by densitometry and normalized to β‐actin level (n = 3 per group). *P < 0·05 versus standard using the Mann–Whitney U‐test. SSC/FSC, side scatter/forward scatter; GATA‐3, GATA‐binding protein 3.
GATA‐3 expression was lost upon zinc deficiency
GATA‐3 is a zinc finger transcription factor essential for the differentiation of Th2 lymphocytes.20 Therefore, zinc deficiency may affect Th2 lymphocyte differentiation. GATA‐3 expression was analyzed using Western blotting of whole spleen samples from rats fed standard or zinc‐deficient diet, as well as of CD4+ cells unstimulated or stimulated with concanavalin A (Fig. 6c). GATA‐3 expression was significantly lower in all samples collected from rats on a zinc‐deficient diet but was unaffected by stimulation with concanavalin A in CD4+ cells. These results indicate that loss of GATA‐3 in the whole spleen and in isolated splenic CD4+ cells unstimulated or stimulated with concanavalin A is possibly because of zinc deficiency.
Experiment II
Physiological indices of rats fed a zinc‐deficient diet and the effects of IL‐4 injection or zinc supplementation
Based on the above observations, we investigated whether the effects of zinc deficiency can be inhibited by IL‐4 administration or recovered by zinc supplementation. The mean body weight, relative spleen weight, serum zinc, copper, and MDA levels, and SOD activity in rats treated according to the experiment II protocol were determined (Fig. 1) and are listed in Table 4. The final measured body weight was significantly higher in rats fed a standard diet than in rats on the zinc‐deficient diet and additional treatments. Similarly, the final measured body weight was significantly higher in rats that were returned to a standard diet after 6 weeks on a zinc‐deficient diet than in rats that were on a zinc‐deficient diet only or were injected with IL‐4 while on a zinc‐deficient diet. In addition, the spleen weight was significantly lower in rats that were in the zinc‐deficient/IL‐4 i.p. group, whereas the relative spleen weight was significantly higher in rats fed zinc‐deficient diet alone than in rats of other groups. In contrast, the serum zinc level, and total SOD, and Cu/Zn‐SOD activities were significantly lower, whereas copper : zinc ratio and MDA levels were significantly higher in rats that were on a zinc‐deficient diet and were injected with IL‐4 than in rats fed a standard diet or those returned to standard diet after being on a zinc‐deficient diet. Serum copper level was significantly higher only in rats on a zinc‐deficient diet compared with rats in other groups. This showed that return to standard diet recovered the effects of zinc deficiency on body weight and serum indices. In contrast, IL‐4 injection while on the zinc‐deficient diet did not inhibit these parameters, as there was no zinc supplementation.
Table 4.
Body weight, relative spleen weight, and serum indices in rats on a standard diet and zinc‐deficient diet and injected with either saline (zinc‐deficient) or IL‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard)
| Standard | Zinc‐deficient | Zinc‐deficient/IL‐4 i.p. | Zinc‐deficient/Standard | P‐value | |
|---|---|---|---|---|---|
| Body weight (g) | 358·1 ± 9·12 | 221·7 ± 3·71***,+++ | 223·6 ± 2·98***,+++ | 312·3 ± 5·43*** | 0·0001 |
| Spleen weight (g) | 0·590 ± 0·09 | 0·497 ± 0·03 | 0·395 ± 0·02**,§§,++ | 0·531 ± 0·03 | 0·0005 |
| Relative spleen weight (mg/g body weight) | 1·649 ± 0·07 | 2·189 ± 0·14***,#,++ | 1·804 ± 0·08 | 1·703 ± 0·10 | 0·003 |
| Serum zince (μg/dl) | 109·0 ± 3·59 | 34·22 ± 5·46***,+++ | 28·40 ± 3·28***,+++ | 104·5 ± 2·78 | 0·0001 |
| Serum copper (μg/dl) | 139·7 ± 5·89 | 277·9 ± 52·4*,++ | 194·8 ± 21·8 | 125·7 ± 12·3 | 0·006 |
| copper : zinc ratio | 1·288 ± 0·07 | 8·008 ± 0·39***,+++ | 6·984 ± 0·58***,+++ | 1·206 ± 0·12 | 0·0001 |
| MDA assay (μmol/l) | 23·87 ± 0·59 | 29·70 ± 2·01**,+ | 29·29 ± 0·80*+ | 24·33 ± 0·55 | 0·0012 |
| Serum total SOD (U/ml) | 200·3 ± 7·00 | 163·7 ± 5·32***,+++ | 151·9 ± 8·71***,+++ | 206·1 ± 5·15 | 0·0001 |
| Serum Mn‐SOD (U/ml) | 99·04 ± 3·71 | 93·25 ± 5·55 | 88·57 ± 4·32 | 98·49 ± 5·90 | 0·4226 |
| Serum Cu/Zn‐SOD (U/ml) | 101·2 ± 5·47 | 70·50 ± 7·96*+ | 63·32 ± 10·15*,++ | 107·6 ± 5·70 | 0·0013 |
i.p., intraperitoneal; IL, interleukin; Zn, zinc; Cu, Copper; MDA, malondialdehyde; SOD, superoxide dismutase.
Mean values and standard errors are indicated (n = 5 per group). The mean values were compared to the standard, zinc‐deficient, zinc‐deficient/IL‐4 i.p., and zinc‐deficient/standard groups.
*P < 0·05, **P < 0·01, ***P < 0·001 vs. standard; §§ P < 0·01 vs. zinc‐deficient; # P < 0·05 vs. zinc‐deficient/IL‐4 i.p.; + P < 0·05, ++ P < 0·01, +++ P < 0·001 vs. zinc‐deficient/standard by ANOVA using the Student‐Newman‐Keuls test, respectively. After dietary manipulation, the spleen was harvested from rats. Relative spleen weight was calculated using the following formula: Relative spleen weight (mg/g) = spleen weight/body weight. After dietary manipulation, serum was collected from rats. Cu/Zn‐SOD was calculated using the following formula: Cu/Zn‐SOD activity = Total SOD activity – Mn‐SOD activity.
Interleukin‐4 injection and zinc supplementation inhibited the increase in inflammatory cytokine expression induced by zinc deficiency
Next, we evaluated whether the effects of zinc deficiency on inflammatory cytokine expression are inhibited by IL‐4 administration or recovered by zinc supplementation. The mRNA levels of inflammatory cytokines and chemokines (IL‐1β and MIP‐1α) in lipopolysaccharide‐stimulated splenic macrophages, as well as IFN‐γ and IL‐4 mRNA levels in concanavalin A‐stimulated lymphocytes, in all groups fed according to experiment II are shown in Fig. 7. Macrophage IL‐1β and MIP‐1α levels were significantly higher in rats on a zinc‐deficient diet than in all other groups. In contrast, T lymphocyte IL‐4 level was decreased in rats on a zinc‐deficient diet, whereas IFN‐γ mRNA level was comparable among all groups. These results indicated that the effects of zinc deficiency on inflammatory cytokines are inhibited by IL‐4 administration or recovered by zinc supplementation.
Figure 7.
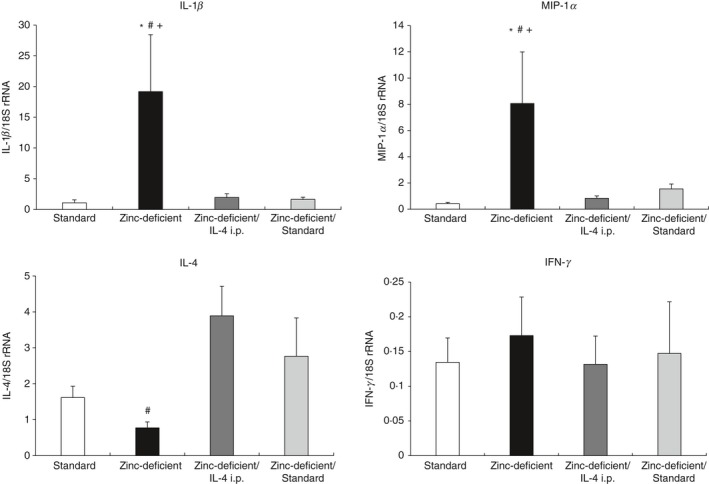
Relative mRNA expression of cytokines and chemokines in splenic macrophages and T lymphocytes in rats on a standard diet or zinc‐deficient diet and injected with either saline (zinc‐deficient) or IL‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard). Quantitative RT‐PCR was used to determine macrophage mRNA levels of IL‐1β and MIP‐1α, and T lymphocyte mRNA levels of IFN‐γ and IL‐4. Messenger RNA expression was normalized to that of the housekeeping 18S rRNA gene in each sample. Data represent the mean ± standard error (n = 7 per group). P = 0·026 for IL‐1β, P = 0·036 for MIP‐1α, P = 0·027 for IL‐4, and P = 0·937 for IFN‐γ. *P < 0·05 versus standard; # P < 0·05 versus zinc‐deficient/IL‐4 i.p.; + P < 0·05 versus zinc‐deficient/standard using anova, followed by the Student–Newman–Keuls test. i.p., intraperitoneal; RT‐PCR, reverse transcription‐polymerase chain reaction; IL, interleukin; MIP‐1α, macrophage inflammatory protein ‐1α; IFN‐γ, interferon‐γ; anova, analysis of variance.
Interleukin‐4 injection and zinc supplementation reversed the zinc deficiency‐induced decrease in the number of positive cells for Th2 lymphocyte‐related factors and M2 macrophages
Zinc deficiency decreased the number of M2 macrophages and Th2 cytokine levels in experiment I, indicating that the inflammatory response was not inhibited. Therefore, we evaluated whether the effect of zinc deficiency on the number of positive cells for Th2 lymphocyte‐related factors (IL‐4, IL‐13, and GATA‐3) and M2 macrophages could be inhibited by IL‐4 administration or recovered by zinc supplementation. Histologically, the spleen did not change in rats treated according to experiment II as assessed on spleen sections stained with H&E (see Supplementary material, Fig. S1). The spleen sections were also analyzed by immunohistochemistry for CD163, IL‐4, GATA‐3, IFN‐γ, and T‐bet, the representative results of which are shown in Fig. 8(a). The number of CD163‐, IL‐4‐, IL‐13‐, and GATA‐3‐positive cells was significantly decreased in rats that were on a zinc‐deficient diet only compared with all other groups (Fig. 8b; see Supplementary material, Fig. S2), whereas the number of IFN‐γ‐ and T‐bet‐positive cells was comparable among all groups. In both experiments (see previous paragraph), IL‐4 injection or zinc supplementation increased the number of positive cells for Th2 lymphocyte‐related factors (IL‐4, IL‐13, and GATA‐3) and anti‐inflammatory M2 macrophage levels, which inhibited the increase in zinc deficiency‐induced inflammatory cytokine levels.
Figure 8.
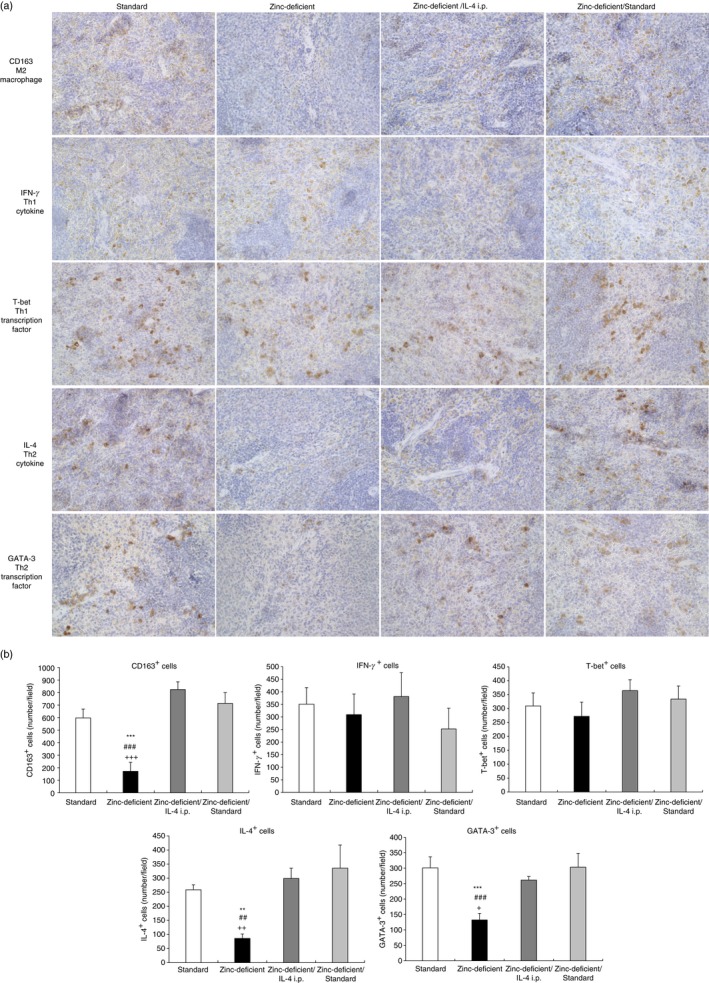
Immunohistochemistry for M2 macrophages, IL‐4, GATA‐3, IFN‐γ, and T‐bet in spleens of rats on a standard diet and zinc‐deficient diet and injected with either saline (zinc‐deficient) or IL‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard). (a) Representative photomicrographs. Brown spots indicate immunohistochemical staining for CD163 (a marker of M2 macrophages), IFN‐γ or T‐bet (a marker of Th1 lymphocyte‐related factors), and IL‐4 or GATA‐3 (a marker of Th2 lymphocyte‐related factors). Magnification, 400×. (b) Number of positive cells per field. Data represent the mean ± standard error (n = 5 per group). *P < 0·05 versus standard; # P < 0·05 vs. zinc‐deficient/IL‐4 i.p.; + P < 0·05 versus zinc‐deficient/standard by ANOVA, followed by the Student–Newman–Keuls test. i.p., intraperitoneal; IL, interleukin; IFN‐γ, interferon‐γ; ANOVA analysis of variance.
Interleukin‐4 injection and zinc supplementation inhibited the reduction in the number of cytotoxic cells due to zinc deficiency
Flow cytometry analyses in experiment I indicated that zinc deficiency decreased CD3+ CD8+ cell numbers (cytotoxic T lymphocytes), whereas it did not affect the total percentages of helper T lymphocytes (CD3+ CD4+ cells) in the spleen. Figure 9(a,b) shows the percentages of CD3+, CD4+, and CD8+ cells in splenocytes, as assessed by flow cytometry, in rats fed a zinc‐deficient diet and subjected to additional treatments (experiment II). The population of CD3+ CD8+ cells (cytotoxic T lymphocytes) was significantly decreased in rats fed a zinc‐deficient diet only compared with all other groups. In contrast, there was no significant difference in the population of CD3+ CD4+ cells (helper T lymphocytes). Interleukin‐4 injection and zinc supplementation inhibited inflammation due to zinc deficiency and thereby prevented the reduction in the number of cytotoxic T lymphocytes.
Figure 9.
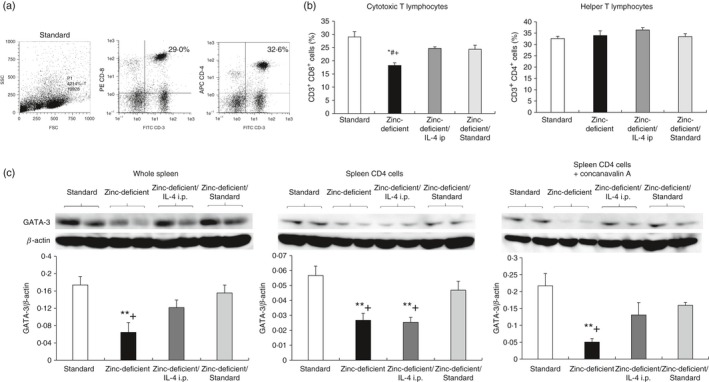
Populations of CD3+, CD4+, and CD8+ cells, and relative GATA‐3 expression in spleens of rats on a standard diet and zinc‐deficient diet and injected with either saline (zinc‐deficient) or IL‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard). (a) Flow cytometry of cells stained with FITC‐CD3, APC‐CD4, and PE‐CD8a antibodies, and gated on SSC/FSC. (b) Percentages of CD3+ CD8+, and CD3+ CD4+ cells. Data represent the mean ± standard error (n = 4 per group). (c) Western blot analysis showing the expression of the transcription factor GATA‐3 in the whole spleen (50 μg/lane), unstimulated CD4+ cells (28 μg/lane), and CD4+ cells stimulated with concanavalin A (28 μg/lane). CD4+ cells were isolated immunomagnetically, and the GATA‐3 level was quantified by densitometry and normalized to β‐actin level (n = 4 per group). *P < 0·05 versus standard; # P < 0·05 versus zinc‐deficient/IL‐4 i.p.; + P < 0·05 versus zinc‐deficient/standard by anova, followed by the Student–Newman–Keuls test. i.p., intraperitoneal; IL, interleukin; SSC/FSC, side scatter/forward scatter; GATA‐3, GATA‐binding protein 3; anova, analysis of variance.
Zinc supplementation, and not IL‐4 injection, recovered the zinc deficiency‐induced reduction in GATA‐3 expression
GATA‐3 expression in whole spleen and CD4+ cells isolated from the spleen unstimulated or stimulated with concanavalin A was assessed using Western blotting (Fig. 9c) in rats fed a zinc‐deficient diet with additional treatments. GATA‐3 expression was significantly lower in all samples from rats that were on a zinc‐deficient diet only compared with rats fed a standard diet or those returned to a standard diet after the zinc‐deficient diet. Furthermore, GATA‐3 expression in the unstimulated CD4+ cells was significantly lower in rats on a zinc‐deficient diet and injected with IL‐4 than in rats fed a standard diet or those returned to standard diet following the zinc‐deficient diet. As serum zinc concentrations remained low due to lack of dietary zinc (Table 4), IL‐4 injections might not increase GATA‐3 expression. In contrast, GATA‐3 expression was recovered by the increase in serum zinc level in rats that were reverted to standard diet following the zinc‐deficient diet. Moreover, the CD4+ cells after concanavalin A stimulation did not differ significantly between rats of the zinc‐deficient diet/IL‐4 i.p. group and those of the ‘standard diet after zinc‐deficient diet’ group.
Dermatitis/depilation and inflammatory response recovery after zinc deficiency
Previous reports show that zinc deficiency leads to dermatitis and depilation.46 Our results suggest that dietary zinc intake for 4 weeks effectively resolves dermatitis, depilation, and inflammatory response (Fig. 10). Strong dermatitis and depilation were observed in rats fed a zinc‐deficient diet for 6 weeks or those returned to a standard diet after 6 weeks of the zinc‐deficient diet. Recovery from dermatitis was observed in rats 7 weeks after they were returned to standard diet after a zinc‐deficient diet. Furthermore, recovery from depilation was observed 8, 9, and 10 weeks after return to standard diet from a zinc‐deficient diet.
Figure 10.
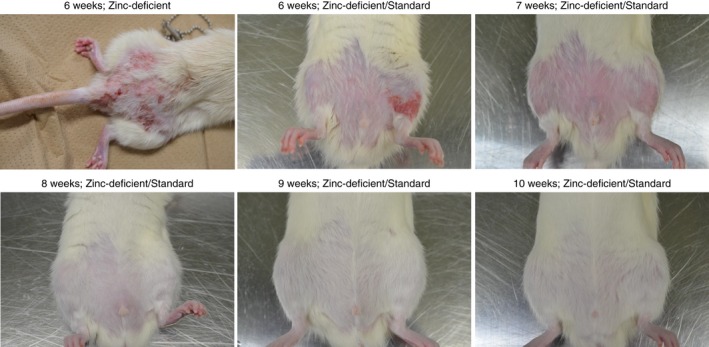
Recovery from dermatitis and depilation in rats returned to standard diet following consumption of the zinc‐deficient diet. Dermatitis and depilation were evident at 6 weeks. Dermatitis was resolved at 7 weeks, whereas depilation was resolved at 8, 9, and 10 weeks.
Discussion
Zinc deficiency induces immune dysfunction and aggravates inflammation, although the mechanism underlying these effects has not yet been completely elucidated. For this reason, we analyzed the balance between M1 and M2 macrophages and Th1 and Th2 lymphocyte‐related factors in the spleens of rats on a zinc‐deficient diet. We observed that these rats gained significantly lower body weight than rats on a standard diet (Table 3), confirming that zinc deficiency delays growth, probably because of inefficient DNA/RNA synthesis and cell division.47 A significant increase in the relative weight of spleen without any morphological changes (Fig. 3) was also observed, suggesting that this increase was probably because of less body weight gain and inflammation‐induced spleen swelling.48 Zinc deficiency also altered the ratio of zinc to copper in blood, resulting in reduced Cu/Zn‐SOD activity,49 and consequent accumulation of ROS in the blood, as indicated by the increase in MDA level (Table 3). Hence, zinc deficiency may aggravate inflammatory reactions by promoting ROS production.2
Based on mRNA expression and immunohistochemical staining for macrophage subtypes and lymphocyte‐associated cytokines in the spleen, zinc deficiency was found to enhance the production of inflammatory cytokines (IL‐1β, TNF‐α, and IL‐6) and chemokines (MCP‐1 and MIP‐1α) by macrophages, suggesting that the associated inflammatory response is related to splenic macrophages (Fig. 2). This result is consistent with the observations of Wong et al., who demonstrated a relationship between zinc deficiency, macrophages, and inflammatory cytokines in human monocytes.15 Nevertheless, zinc deficiency did not affect the number of immature and M1 macrophages in the spleen (Fig. 4), suggesting that the increase in the expression of inflammatory cytokines is not due to the formation of additional M1 macrophages. Therefore, it is possible that zinc deficiency activates resident M1 macrophages in response to dermatitis or ROS.45 In contrast, zinc deficiency decreased the number of M2 macrophages (Fig. 4), indicating that a decrease in the anti‐inflammatory activity of M2 macrophages may indirectly contribute to inflammation. Furthermore, the number of M2 macrophage inducers, i.e. IL‐4‐producing and IL‐13‐producing cells, was also low (Fig. 5), suggesting that zinc deficiency promotes inflammation by suppressing the anti‐inflammatory Th2 cytokine–M2 macrophage pathway. Indeed, zinc deficiency suppresses IL‐4 expression in T lymphocytes in vitro.24 Notably, zinc deficiency did not affect the number of IFN‐γ‐ and T‐bet‐positive cells (Th1 lymphocyte‐related factors), as well as IFN‐γ mRNA levels. Reports show that Th1/Th2 cytokine production and IFN‐γ expression are lowered during zinc deficiency.50, 51 Prasad et al. demonstrated that Th1 cytokine levels are reduced by daily intake of 2–3 mg low zinc50 and Lu et al. showed that IFN‐γ production is low during zinc deficiency.51 In contrast, we observed that IFN‐γ expression and the number of IFN‐γ‐producing cells did not decrease significantly in zinc‐deficient rats; furthermore, rats on 6 weeks of zinc‐deficient diet were more susceptible to strong dermatitis‐associated infections (Fig. 10). Therefore, IFN‐γ expression and the number of IFN‐γ‐producing cells may increase in response to viral and bacterial infections associated with strong dermatitis, which is induced by the 6‐week‐long zinc‐deficient diet.
Zinc deficiency also significantly lowered the percentages of cytotoxic T lymphocytes (CD3+ CD8+ cells) (Fig. 6b), which are required to exclude cells infected with viruses and bacteria.52 As mentioned above, local dermatitis was observed in rats on a zinc‐deficient diet (Fig. 10), indicating that the reduction in cytotoxic T lymphocytes may also contribute to aggravation of the inflammatory response.53 In contrast, the percentages of helper T lymphocytes (CD3+ CD4+ cells) (Fig. 6b) in the spleen were not affected, suggesting that zinc deficiency reduces the number of Th2 lymphocyte‐related factors (IL‐4‐, IL‐13‐, and GATA‐3‐positive cells) without affecting the total number of helper T lymphocytes.
Zinc deficiency reduced the level of GATA‐3, a master transcription factor involved in Th2 lymphocyte differentiation and function, in whole spleen, as well as in isolated splenic CD4+ cells unstimulated or stimulated with concanavalin A. Muzzioli et al. show that zinc supplementation of natural killer cells increases the expression of the zinc finger transcription factor GATA‐3.54 In addition, the loss of GATA‐3 may possibly be directly attributed to zinc deficiency, as the protein contains a C‐terminal zinc finger domain. Indeed, Sun et al., demonstrated that zinc deficiency inhibited the function of poly (ADP‐ribose) polymerase‐1, a zinc finger protein.23 Therefore, the zinc finger structure of GATA‐3 may possibly be lost due to zinc deficiency, which affects differentiation to Th2 lymphocytes and subsequently decreases IL‐4 and IL‐13 expression. Alternatively, zinc deficiency led to low activity of GATA‐3, resulting in an early decrease in IL‐4 expression, consequently causing low IL‐4 signaling20 and inhibiting the induction of Th2 differentiation.
Based on these results, we propose that zinc deficiency destabilizes GATA‐3, a master transcription factor, and zinc finger protein. Consequently, naive CD4 cells do not differentiate into Th2 lymphocytes or produce IL‐4 and IL‐13, which in turn inhibit immature macrophages from differentiating into M2 macrophages. As M2 macrophages are anti‐inflammatory in function, the inflammatory response to ROS or infection may persist (see Supplementary material, Fig. S3).
In additional experiments, we investigated whether the effects of zinc deficiency could be inhibited by IL‐4 administration or recovered by zinc supplementation.
Rats injected with IL‐4 while on the zinc‐deficient diet did not show a reversal of the lack of weight gain. However, the relative spleen weight was comparable between rats that were on a standard diet and those that were returned to standard diet after a zinc‐deficient diet as well as those that were administered with IL‐4. This is possibly because IL‐4 administration inhibited the inflammatory response‐induced spleen swelling.48 Similar to the observations made in rats on only zinc‐deficient diet, injection of IL‐4 while on the zinc‐deficient diet also altered the balance between the concentrations of zinc and copper, as well as their ratio in the blood, so suppressing total SOD, and Cu/Zn‐SOD activities. Reductions in the activities of these antioxidant enzymes ultimately resulted in ROS accumulation, as indicated by the increase in MDA level (Table 4). In rats injected with IL‐4 while on a zinc‐deficient diet, the IL‐4 mRNA level and the number of M2 macrophages and IL‐4‐ or IL‐13‐positive cells increased to levels higher than those in rats on standard diet (Figs 7 and 8, and see Supplementary material, Fig. S2), although these values decreased significantly in rats on only zinc‐deficient diet (Figs 7 and 8, and see Supplementary material, Fig. S2). This is probably due to the stabilizing autocrine effect of IL‐4 injections (thrice a week) on the resident Th2 lymphocyte population.55, 56 Importantly, IL‐4 injections also induced the M2 macrophages, thereby decreasing the mRNA levels of inflammatory cytokines (IL‐1β) and chemokines (MIP‐1α) to levels lower than those in rats only on the zinc‐deficient diet (Fig. 7). Furthermore, compared with rats on a zinc‐deficient diet alone, IL‐4 injections increased the percentages of cytotoxic T lymphocytes (CD3+ CD8+ cells) in the spleen (Fig. 9). It is possible that IL‐4 inhibits local inflammation and dermatitis (see Supplementary material, Fig. S4) caused by zinc deficiency, and thereby prevents cytotoxic T lymphocytes from migrating out of the spleen, which usually occurs following viral or bacterial infection‐associated inflammation.53 Hence, IL‐4 injections inhibited inflammation induced by zinc deficiency without affecting the cytotoxic T lymphocytes in the spleen. Alternatively, zinc deficiency has been reported to cause thymus atrophy,43 and IL‐4 injections may inhibit thymus atrophy. Therefore, this finding calls for the need to investigate the detailed relationship between cytotoxic lymphocytes and thymus atrophy.
In addition, immunohistochemistry for GATA‐3‐positive cells (Fig. 8) and western blotting for GATA‐3 expression in whole spleen (Fig. 9c: right image) showed a similar number of GATA‐3‐positive cells and protein levels. Reports show that GATA‐3 is expressed in lipocytes, endothelial cells, and the nervous system.57 Therefore, these results can be explained by the stabilizing effect of the IL‐4 injections on the entire resident Th2 lymphocyte population of the spleen55, 56 and the detection of adipocytes or endothelial cells. However, we isolated the unstimulated CD4+ cells using high‐quality immunomagnetic separation for determining the GATA‐3 levels (Fig. 9c: central image); the separated cells were helper T lymphocytes that showed low GATA‐3 expression. This may be because serum zinc concentration remained low owing to the lack of dietary zinc, and IL‐4 injections alone did not stabilize GATA‐3, which requires zinc for its expression. In contrast, GATA‐3 expression in the concanavalin A‐stimulated CD4+ cells was slightly increased. Possibly, GATA‐3 was slightly stabilized and its expression was induced when concanavalin A was added to the IL‐4 administration group and incubated for 24 hours. However, this observation warrants detailed investigation in the future.
Based on the above discussion, we propose that the reduction in GATA‐3 level during zinc deficiency is possibly because of structural changes in the protein that render it unstable, which subsequently reduces IL‐4 expression. However, after IL‐4 administration, Th2 lymphocytes or other IL‐4‐responding cells are stabilized at an early stage and may inhibit the decrease in IL‐4 expression in the whole spleen via autocrine effects of IL‐4. Taken together, these observations suggest that IL‐4 reduces inflammation in zinc‐deficient rats not through GATA‐3 or any antioxidant enzyme, but through anti‐inflammatory M2 macrophages.
Rats returned to a standard diet following a zinc‐deficient diet started gaining weight, suggesting that the former rescues DNA/RNA synthesis and cell division.47 In addition, rats recovered their zinc and copper balance in the blood, and so had higher antioxidant enzyme activity and lower levels of ROS (Table 4). Interleukin‐4 mRNA level, the number of M2 macrophages, IL‐4‐ and IL‐13‐positive cells, cytotoxic T lymphocytes (CD3+ CD8+ cells), and helper T lymphocytes (CD3+ CD4+ cells) (Figs 7,8, and 9, and see Supplementary material, Fig. S2), and GATA‐3 expression in the whole spleen and isolated CD4+ cells (unstimulated or stimulated with concanavalin A) were comparable with or higher than those in rats on standard diet. In addition, inflammatory cytokine (IL‐1β) and chemokine (MIP‐1α) levels decreased upon return to a standard diet (Fig. 7). Collectively, these results suggest that dietary zinc intake for 4 weeks effectively increased serum zinc level, and resolved dermatitis, depilation (Fig. 10), and inflammation. Zinc intake also stabilized GATA‐3 and thereby increased the number of Th2 lymphocytes, IL‐4 production, and the number of anti‐inflammatory M2 macrophages.
In conclusion, the results of this study suggest that zinc deficiency leads to immune dysfunction and aggravates inflammation. It may be due at least in part to the loss of the zinc finger transcription factor GATA‐3, which inhibits the formation of Th2 lymphocytes. This decreases the production of the cytokine IL‐4, which is required by immature macrophages to differentiate into M2 macrophages. The consequent loss of M2 macrophages inhibits anti‐inflammatory reactions and aggravates inflammatory reactions. Importantly, inflammation induced by zinc deficiency can be inhibited by IL‐4 administration or recovered by zinc supplementation.
Authors contribution
The authors’ responsibilities were as follows: TK, KI, MS, and HY designed the study and wrote the paper; TK analyzed data and performed statistical analysis; TK and HY have primary responsibility for the final content; all authors have read and approved the final manuscript.
Disclosure
The authors declare that they have no conflicts of interest regarding the contents of this article.
Supporting information
Fig. S1. Representative spleen sections obtained from rats on standard diet and zinc‐deficient diet and injected intraperitoneally with either saline (zinc‐deficient) or interleukin‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard).
Fig. S2. Immunohistochemistry for interleukin‐13 (IL‐13) in spleens of rats on standard diet and zinc‐deficient diet and injected intraperitoneally with either saline (zinc‐deficient) or IL‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard).
Fig. S3. Mechanism underlying aggravation of inflammatory response by zinc deficiency via the T helper type 2 lymphocyte–M2 macrophage pathway.
Fig. S4. Representative photograph of rats injected with interleukin‐4 while on zinc‐deficient diet for 6 weeks.
Table S1. Components of special diet used this study.
Acknowledgments
We thank Dr. Masashi Tsunoda (National Defense Medical College), Dr. Watatu Yoshioka and Dr. Shingo Yogosawa (The Jikei University School of Medicine), and the Department of Pathology, The Jikei University School of Medicine, for technical support. This work was supported by the Research Fund of The Uehara Memorial Foundation and JSPS KAKENHI grant number JP18K173590001.
References
- 1. Wirth JJ, Fraker PJ, Kierszenbaum F. Zinc requirement for macrophage function: effect of zinc deficiency on uptake and killing of a protozoan parasite. Immunology 1989; 68:114–9. [PMC free article] [PubMed] [Google Scholar]
- 2. Yanagisawa H, Moridaira K, Wada O. Zinc deficiency further increases the enhanced expression of endothelin‐1 in glomeruli of the obstructed kidney. Kidney Int 2000; 58:575–86. [DOI] [PubMed] [Google Scholar]
- 3. Yanagisawa H. Zinc deficiency and clinical practice – validity of zinc preparations. Yakugaku Zasshi 2008; 128:333–9. [DOI] [PubMed] [Google Scholar]
- 4. Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol 2012; 26:66–9. [DOI] [PubMed] [Google Scholar]
- 5. Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J Immunol Res 2016;2016: 6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD et al A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr 2011; 93:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Natchu UC, Fataki MR, Fawzi WW. Zinc as an adjunct for childhood pneumonia – interpreting early results. Nutr Rev 2008; 66:398–405. [DOI] [PubMed] [Google Scholar]
- 8. Jordi MP, David TB, Joy L, Greg LM, Glynis LK, Jason AP et al Protein‐ and zinc‐deficient diets modulate the murine microbiome and metabolic phenotype. Am J Clin Nutr 2016; 104:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruktanonchai D, Lowe M, Norton SA, Garret T, Soghier L, Weiss E et al Zinc deficiency‐associated dermatitis in infants during a nationwide shortage of injectable zinc – Washington, DC, and Houston, Texas, 2012‐2013. Morb Mortal Wkly Rep 2014; 63:35–7. [PMC free article] [PubMed] [Google Scholar]
- 10. Cediel G, Olivares M, Brito A, Cori H, López de Romaña D. Zinc deficiency in Latin America and the Caribbean. Food Nutr Bull 2015; 36:S129–38. [DOI] [PubMed] [Google Scholar]
- 11. Kogirima M, Kurasawa R, Kubori S, Sarukura N, Nakamori M, Okada S et al Ratio of low serum zinc levels in elderly Japanese people living in the central part of Japan. Eur J Clin Nutr 2007; 61:375–81. [DOI] [PubMed] [Google Scholar]
- 12. Bao B, Prasad AS, Beck FW, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab 2003; 285:1095–102. [DOI] [PubMed] [Google Scholar]
- 13. Miyazaki T, Takenaka T, Inoue T, Sato M, Miyajima Y, Nodera M et al Lipopolysaccharide‐induced overproduction of nitric oxide and overexpression of iNOS and interleukin‐1β proteins in zinc‐deficient rats. Biol Trace Elem Res 2012; 145:375–81. [DOI] [PubMed] [Google Scholar]
- 14. Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL‐1β and TNFα in promyeloid cells via epigenetic and redox‐dependent mechanisms. J Nutr Biochem 2013; 24:289–97. [DOI] [PubMed] [Google Scholar]
- 15. Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res 2015; 59:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bedoui S, Heath WR, Mueller SN. CD4+ T‐cell help amplifies innate signals for primary CD8+ T‐cell immunity. Immunol Rev 2016; 272:52–64. [DOI] [PubMed] [Google Scholar]
- 17. Naufel AO, Aguiar MCF, Madeira FM, Abreu LG. Treg and Th17 cells in inflammatory periapical disease: a systematic review. Braz Oral Res 2017; 31:e103. [DOI] [PubMed] [Google Scholar]
- 18. Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 2010; 298:R1173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015; 2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho IC, Tai TS, Pai SY. GATA3 and the T‐cell lineage: essential functions before and after T‐helper‐2‐cell differentiation. Nat Rev Immunol 2009; 9:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 2004; 15:572–8. [DOI] [PubMed] [Google Scholar]
- 22. Sun X, Zhou X, Du L, Liu W, Liu Y, Hudson LG et al Arsenite binding‐induced zinc loss from PARP‐1 is equivalent to zinc deficiency in reducing PARP‐1 activity, leading to inhibition of DNA repair. Toxicol Appl Pharmacol 2014; 274:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor‐4α mediates alcohol‐induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol 2010; 24:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gruber K, Maywald M, Rosenkranz E, Haase H, Plumakers B, Rink L. Zinc deficiency adversely influences interleukin‐4 and interleukin‐6 signaling. J Biol Regul Homeost Agents 2013; 27:661–71. [PubMed] [Google Scholar]
- 25. Oh IS, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW. Central administration of interleukin‐4 exacerbates hypothalamic inflammation and weight gain during high‐fat feeding. Am J Physiol Endocrinol Metab 2010; 299:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Makino T, Saito M, Horiguchi D, Kina K. A highly sensitive colorimetric determination of serum zinc using water‐soluble pyridylazo dye. Clin Chim Acta 1982; 120:127–35. [DOI] [PubMed] [Google Scholar]
- 27. Higurashi K. ‘‘ACCURAS AUTO Zn’’, colorimetric reagent for zinc. Biomed Res Trace Elem 2015; 26:7–9. (in Japanese). [Google Scholar]
- 28. Shahin D, Toraby EE, Abdel‐Malek H, Boshra V, Elsamanoudy AZ, Shaheen D. Effect of peroxisome proliferator‐activated receptor γ agonist (pioglitazone) and methotrexate on disease activity in rheumatoid arthritis (experimental and clinical study). Clin Med Insights Arthritis Musculoskelet Disord 2011; 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yanagisawa H, Nodera M, Wada O. Zinc deficiency aggravates tubulointerstitial nephropathy caused by ureteral obstruction. Biol Trace Elem Res 1998; 65:1–6. [DOI] [PubMed] [Google Scholar]
- 30. Yanagisawa H, Yamazaki N, Sato G, Wada O. L‐arginine treatment may prevent tubulointerstitial nephropathy caused by germanium dioxide. Kidney Int 2000; 57:2275–84. [DOI] [PubMed] [Google Scholar]
- 31. Moridaira K, Yanagisawa H, Nodera M, Tamura J, Tsuchiya J, Naruse T et al Enhanced expression of vsmNOS mRNA in glomeruli from rats with unilateral ureteral obstruction. Kidney Int 2000; 57:1502–11. [DOI] [PubMed] [Google Scholar]
- 32. Alves AM, Diel LF, Lamers ML. Macrophages and prognosis of oral squamous cell carcinoma: a systematic review. J Oral Pathol Med 2018; 47:460–7. [DOI] [PubMed] [Google Scholar]
- 33. Bösmüller H, Nann D, Horger M, Fend F. Erdheim–Chester disease and Rosai–Dorfman disease: pathological, radiological and clinical features of adult non‐Langerhans cell histiocytosis. Pathologe 2015; 36:458–66. [DOI] [PubMed] [Google Scholar]
- 34. Yamashita M, Saito R, Yasuhira S, Fukuda Y, Sasamo H, Sugai T et al Distinct profiles of CD163‐positive macrophages in idiopathic interstitial pneumonias. J Immunol Res 2018; 2018:1436236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shao X, Wu B, Cheng L, Li F, Zhan Y, Liu C et al Distinct alterations of CD68+CD163+ M2‐like macrophages and myeloid‐derived suppressor cells in newly diagnosed primary immune thrombocytopenia with or without CR after high‐dose dexamethasone treatment. J Transl Med 2018; 16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kovaleva OV, Samoilova DV, Shitova MS, Gratchev A. Tumor associated macrophages in kidney cancer. Anal Cell Pathol (Amst) 2016; 2016:9307549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albiero M, Poncina N, Ciciliot S, Cappellari R, Menegazzo L, Ferraro F et al Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes 2015; 64:2957–68. [DOI] [PubMed] [Google Scholar]
- 38. Tsunoda M, Yamamoto K, Ito K, Inoue Y, Miki T, Kudo Y et al Dibutyltin (DBT) dichloride inhibits cytokine productions in murine macrophage cell line, J774.1. Biomed Res Trace Elem 2006; 17:417–22. [Google Scholar]
- 39. ‐Tsunoda M, Yoshida T, Tsuji M, Zhang Y, Sugaya C, Inoue Y et al The effects of dibutyltin (DBT) dichloride on the viability and the productions of tumor necrosis factor α and interleukin‐12 in murine macrophage cell line, J774.1. Biomed.Res Trace Elem 2008; 19:67–71. [Google Scholar]
- 40. Kido T, Tsunoda M, Kasai T, Sasaki T, Umeda Y, Senoh H et al The increases in relative mRNA expressions of inflammatory cytokines and chemokines in splenic macrophages from rats exposed to multi‐walled carbon nanotubes by whole‐body inhalation for 13 weeks. Inhal Toxicol 2014; 26:750–8. [DOI] [PubMed] [Google Scholar]
- 41. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 2001; 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kido T, Sugaya C, Ikeuchi R, Kudo Y, Tsunoda M, Aizawa Y. The increases in mRNA expressions of inflammatory cytokines by adding cleaning solvent or tetrachloroethylene in the murine macrophage cell line J774.1 evaluated by real‐time PCR. Ind Health 2013; 51:319–25. [DOI] [PubMed] [Google Scholar]
- 43. Nodera M, Yanagisawa H, Wada O. Increased apoptosis in a variety of tissues of zinc‐deficient rats. Life Sci 2001; 69:1639–49. [DOI] [PubMed] [Google Scholar]
- 44. Yanagisawa H, Kido T, Yogosawa S, Sato O, Sakae K, Suka M. Inadequate intake of zinc exacerbates blood pressure and renal function via superoxide radical‐induced oxidative stress. Biomed Res Trace Elem 2015; 26:117–23. [Google Scholar]
- 45. Dierichs L, Kloubert V, Rink L. Cellular zinc homeostasis modulates polarization of THP‐1‐derived macrophages. Eur J Nutr 2018; 57:2161–9. [DOI] [PubMed] [Google Scholar]
- 46. Kawamura T, Ogawa Y, Nakamura Y, Nakamizo S, Ohta Y, Nakano H et al Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J Clin Invest 2012; 122:722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ploysangam A, Falciglia GA, Brehm BJ. Effect of marginal zinc deficiency on human growth and development. J Trop Pediatr 1997; 43:192–8. [DOI] [PubMed] [Google Scholar]
- 48. Thomsen KL, Møller HJ, Graversen JH, Magnusson NE, Moestrup SK, Vilstrup H et al Anti‐CD163‐dexamethasone conjugate inhibits the acute phase response to lipopolysaccharide in rats. World J Hepatol 2016; 8:726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osredkar J, Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinical Toxicol 2011; S3:001. [Google Scholar]
- 50. Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis 2000; 182(Suppl 1):S62–8. [DOI] [PubMed] [Google Scholar]
- 51. Lu H, Xin Y, Tang Y, Shao G. Zinc suppressed the airway inflammation in asthmatic rats: effects of zinc on generation of eotaxin, MCP‐1, IL‐8, IL‐4, and IFN‐γ . Biol Trace Elem Res 2012; 150:314–21. [DOI] [PubMed] [Google Scholar]
- 52. Oykhman P, Mody CH. Direct microbicidal activity of cytotoxic T‐lymphocytes. J Biomed Biotechnol 2010; 2010:249482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang N, Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity 2011; 35:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muzzioli M, Stecconi R, Donnini A, Re F, Provinciali M. Zinc improves the development of human CD34+ cell progenitors towards Natural Killer cells and induces the expression of GATA‐3 transcription factor. Int J Biochem Cell Biol 2007; 39:955–65. [DOI] [PubMed] [Google Scholar]
- 55. Paludan SR. Interleukin‐4 and interferon‐γ: the quintessence of a mutual antagonistic relationship. Scand J Immunol 1998; 48:459–68. [DOI] [PubMed] [Google Scholar]
- 56. Nakamura T, Lee RK, Nam SY, Podack ER, Bottomly K, Flavell RA. Roles of IL‐4 and IFN‐γ in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol 1997; 158:2648–53. [PubMed] [Google Scholar]
- 57. Lagarkova MA, Volchkov PY, Philonenko ES, Kiselev SL. Efficient differentiation of hESCs into endothelial cells in vitro is secured by epigenetic changes. Cell Cycle 2008; 7:2929–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative spleen sections obtained from rats on standard diet and zinc‐deficient diet and injected intraperitoneally with either saline (zinc‐deficient) or interleukin‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard).
Fig. S2. Immunohistochemistry for interleukin‐13 (IL‐13) in spleens of rats on standard diet and zinc‐deficient diet and injected intraperitoneally with either saline (zinc‐deficient) or IL‐4 (zinc‐deficient/IL‐4 i.p.), or subsequently switched to a standard diet (zinc‐deficient/standard).
Fig. S3. Mechanism underlying aggravation of inflammatory response by zinc deficiency via the T helper type 2 lymphocyte–M2 macrophage pathway.
Fig. S4. Representative photograph of rats injected with interleukin‐4 while on zinc‐deficient diet for 6 weeks.
Table S1. Components of special diet used this study.


