Summary
Murine γδ T cells display diverse responses to pathogens and tumours through early provision of pro‐inflammatory cytokines such as interleukin‐17A (IL‐17) and interferon‐γ (IFN‐γ). Although it is now clear that acquisition of these cytokine‐secreting effector fates is to a great extent developmentally pre‐programmed in the thymus, the stages through which γδ progenitor cells transition, and the underlying mechanistic processes that govern these commitment events, are still largely unclear. Here, we review recent progress in the field, with particular consideration of how TCR‐γδ signalling impacts on developmental programmes initiated before TCR‐γδ expression.
Keywords: T‐cell development, thymus, γδ T cells, IL‐17, IFN‐γ, TCR‐γδ
Thymocyte commitment to a γδ T‐cell fate
Consensus holds that αβ and γδ T cells originate from a common thymic progenitor.1 Expression of a pre‐T‐cell receptor (TCR‐β paired with the invariant pre‐TCR‐α chain) directs CD4− CD8− double‐negative (DN) cells to the αβ lineage and promotes their progression to a CD4+ CD8+ double‐positive (DP) stage that serves as a precursor pool for conventional CD4+ and CD8+ T cells.2 By contrast, DN cells that express TCR‐γδ commit to the γδ lineage and become functionally mature without progression through the DP stage. Two basic models were proposed to explain αβ/γδ lineage commitment; a pre‐commitment‐selection model and a signal strength model.3
The pre‐commitment‐selection model suggested that lineage fate was determined before TCR‐chain rearrangement. Subsequent TCR‐γδ expression in γδ precursors or pre‐TCR expression in αβ precursors then served to confirm lineage specification and promote further development of γδ lineage and αβ lineage cells, respectively. Cells with mismatched lineage specification/TCR were thought to die. In support of this, higher expression in early DN cells of both interleukin‐7 receptor α (IL‐7Rα),4 and Sox13,5 was shown to identify TCR− thymocyte precursors with greater propensity to develop as γδ T cells. Despite this, the pre‐commitment‐selection model never really took hold. Indeed, recent reports suggest that only IL‐17‐secreting T γδ cells that use TCR‐γ chain variable region‐4 (Vγ4) (using Tonegawa nomenclature6) were absent in mice that lacked Sox13.7, 8
By contrast, the signal strength model of αβ/γδ lineage choice has gained widespread support.9, 10 It proposes that TCR signal strength, rather than TCR identity, dictates αβ/γδ lineage fate. Hence, DN cells receiving a weak TCR signal commit to the αβ lineage, whereas DN cells perceiving a strong TCR signal develop as γδ T cells. This could also be considered an instructional model, as under normal conditions the pre‐TCR provides a weak signal (presumably due to its very low surface expression), whereas TCR‐γδ signals more strongly.
Although compelling evidence for the signal strength model now exists, it is still unclear how DN cells discriminate between different signal strengths. Activation of the extracellular signal‐regulated kinase (ERK) pathway appears to be important, which can induce early growth response (Egr) family transcription factors and inhibitor of DNA binding 3 (Id3).11 More recently, non‐canonical targeting of docking site for ERK, FXF (DEF) domain containing ERK substrates through the ERK DEF‐binding pocket has also been implicated in γδ T‐cell fate commitment.12 Hence, strong TCR signalling delivered mainly by TCR‐γδ complexes drives DN cell commitment to the γδ T‐cell lineage.
Waves of thymic γδ T‐cell development
It is long established that γδ T cells develop in waves that are associated with the usage of certain Vγ‐regions and eventual tissue location.13 Murine γδ T cells begin to appear during the mid‐to‐late fetal period (~E13.5). Vγ5+ precursors of skin‐resident dendritic epidermal T cells develop first, closely followed by Vγ6+ cells that locate to various epithelial sites such as the reproductive tract. Vγ7+ precursors of ileal intraepithelial lymphocytes appear next, around birth, followed by Vγ1+, Vγ2+ and Vγ4+ cells that are the dominant subsets in tissues such as the dermis, lung, colon and liver, and in peripheral lymphoid organs. Interestingly, the sequential appearance of Vγ5+, Vγ6+, Vγ7+ and then Vγ4+ cells reflects the order of these Vγ regions at the Cγ1‐TCR‐γ locus.14
Thymic γδ T‐cell development can also be correlated to acquisition of distinct effector potentials.15 The initial skin‐homing Vγ5+ cells are reported to secrete interferon‐γ (IFN‐γ).16 There then follows a perinatal period dominated by generation of IL‐17‐secreting γδ T cells that include initially all Vγ6+ cells and then later a majority of Vγ4+ cells. Nonetheless, Vγ1+ and Vγ2+ cells also develop during this period (alongside Vγ4+ cells) with IL‐17‐secreting potential.17 The perinatal period also sees the development of Vγ7+ precursors of ileal intraepithelial lymphocytes, and a natural killer T (NKT) ‐like γδ T‐cell subset that uses a Vγ1Vδ6.3/6.4+ invariant TCR γδ, can secrete IFN‐γ, IL‐4 and IL‐15, and that homes preferentially to the liver.18 Interferon‐γ‐secreting γδ T (γδIFN) cells without IL‐4 secretion also begin to appear in the neonatal period and continue to be produced well into adulthood.19 Finally, a subset of seemingly uncommitted γδ T cells is also generated from the neonatal period onwards. These appear to possess ‘naive’‐like qualities and may retain the potential to adopt multiple effector fates in the periphery.20, 21, 22
Development of IFN‐γ‐secreting γδ T‐cell subsets
TCR‐γδ signalling above a certain threshold is clearly required to commit DN cells to the γδ lineage. However, beyond this commitment step, TCR‐γδ signalling also plays a key role in thymic acquisition of γδ T‐cell effector fate.16, 17, 23, 24, 25 Thymic TCR‐agonist selection events are associated with development of Vγ5Vδ1+ dendritic epidermal T cells,16, 26 Vγ1Vδ6.3/6.4+ NKT‐like γδ T cells27, 28 and Vγ4Vδ5+ T10/T22‐specific γδ T cells23, 25 that all acquire the potential to secrete IFN‐γ. Conversely, attenuation of TCR‐γδ signal strength adversely affects the generation of γδIFN cells.16, 17, 23, 24
Up‐regulation of several surface proteins appears to mark TCR‐γδ‐mediated progression of CD24+ γδ precursors toward a general IFN‐γ‐secreting phenotype (Fig. 1).16, 17, 19, 23, 29, 30 However, an understanding of how these markers relate to ligand‐independent (akin to tonic signalling) versus ligand‐induced TCR‐γδ signalling (which are both presumably of sufficient strength for commitment to the γδ lineage) is challenging. Recent evidence from adult mice suggests that ligand‐independent TCR‐γδ signalling may be sufficient to generate naive‐like γδ T cells that progress from a CD25+ CD371+ CD24+ stage by down‐regulating first CD25 and then CD371 to generate CD25− CD371− CD24+ cells that can exit the thymus to seed peripheral lymphoid organs.30 By contrast, increased TCR signal strength (as induced for example by anti‐TCR‐δ antibody engagement) up‐regulates CD200, closely followed by CD73 and CD45RB.16, 17, 30 However, it is not yet clear whether these events are a consequence of only TCR γδ/ligand interactions, or whether stronger ligand‐independent signalling is also sufficient.31 Nonetheless, it is more certain that bona fide cell‐bound agonist/TCR engagement (e.g. for selection of Vγ1Vδ6.3/6.4+ NKT‐like γδ T cells28) drives up‐regulation of additional markers such as CD44, NK1.1 and CD122 that identify various terminally differentiated γδIFN cell subsets.27 Many of these also become CD24−, especially when developing during the neonatal period.30
Figure 1.
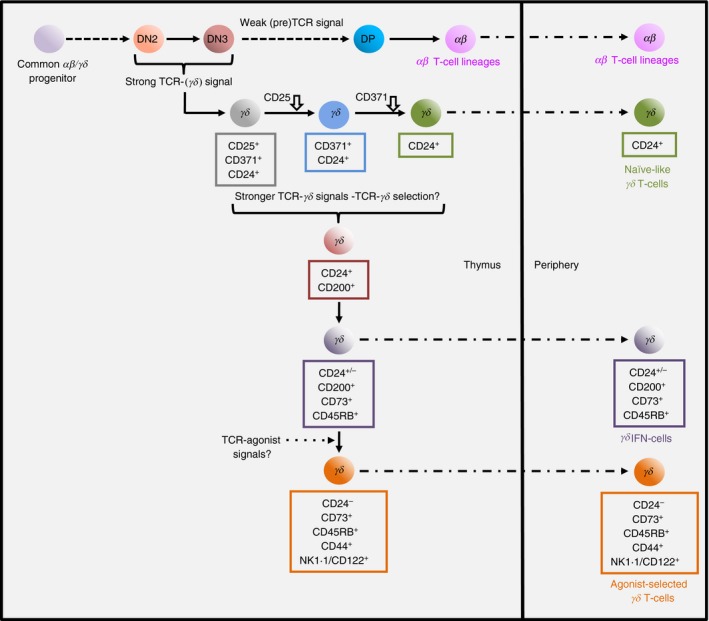
Murine thymic γδ T‐cell development from a common αβ/γδ progenitor. αβ/γδ progenitors are diverted into the γδ lineage as a result of strong T‐cell receptor (TCR) signals. Stronger TCR γδ signal strength (ligand‐independent or cell‐bound agonist‐driven) appears to drive γδ thymocytes to interferon‐γ (IFN‐γ) ‐committed (γδ IFN) or agonist‐selected γδ T‐cell fates (the latter possibly aided by co‐stimulatory signals). Phenotypes of thymic γδ subsets and their peripheral counterparts are indicated. Downward arrow indicates marker down‐regulation. ‘?’ denotes ongoing uncertainty.
IL‐17‐secreting γδ T‐cell development
There is presently no convincing model that adequately explains the distinctive characteristics of thymic γδ17 cell development. Such a model would need to describe the restricted perinatal generation of γδ17 cells, which is generally attributed to largely undefined features of the perinatal thymic environment that are different to the adult thymus.15 Nonetheless, adoptive transfer of adult bone marrow into IL‐17‐deficient recipients fails to reconstitute the γδ17 cell compartment.32 In addition, adult thymocyte precursors also fail to generate γδ17 cells in a grafted embryonic thymus, suggesting that capacity to initiate an IL‐17‐specific γδ T‐cell programme is intrinsic to embryonic progenitors.32 However, substantial evidence to support this idea is still largely lacking.
Particularly perplexing for the development of γδ17 cells is the role of TCR‐γδ signal strength.17 A recent report that observed CD73 expression (purported to indicate TCR γδ engagement) on adult thymic γδ17 cells suggested a requirement for strong TCR‐γδ signals.33 As too did absence of γδ17 cells in mice with defective Zap‐70 function.34 However, this Zap‐70 dependence was not obviously correlated with strong TCR‐γδ signalling, and the role of Zap‐70 has since been disputed by claims that γδ17 cells instead require Syk.35 Furthermore, CD73 and CD45RB (that also marks TCR‐γδ engagement16, 33) are both absent on thymic γδ17 cells that develop in the perinatal window.17
By contrast, a body of evidence instead suggests that γδ17 cell development requires weak (or even absent) TCR γδ signalling.16, 17, 19, 23, 25 For example, generation of γδ17 cells is favoured by the reduction of ERK activity.12, 17 In addition, Vγ5Vδ1+ γδ T cells developing in the absence of Skint1 adopt IL‐17‐secreting characteristics.16 Also, engagement of TCR‐γδ with even very low concentrations of activating anti‐TCR‐δ antibody reduces the generation of all IL‐17‐committed γδ T cells dramatically.17 Whether weak TCR‐γδ signalling equates to ligand‐independent tonic signalling (as has been suggested23) remains unclear. Indeed, it is even possible that weak TCR‐γδ signalling actually translates as ‘alternative’ TCR‐γδ signalling in which the TCR acts simply as a scaffold to enable certain downstream pathways to operate. Such ‘adapter‐like’ signalling has been described for the B‐cell receptor that is suggested (at certain stages of B‐cell development) to act as an adaptor protein that brings Syk to the BAFF receptor.36 Nonetheless, a conclusion that only weak or alternative/absent TCR γδ signalling is compatible with the generation of γδ17 cells is difficult to reconcile with the requirement for strong TCR‐γδ signalling to commit DN progenitors to the γδ lineage. Unfortunately, this consistently ignored paradox has yet to be adequately resolved.
A further defining feature of γδ17 cell development is involvement of distinct signalling modalities that are not required for the generation of other γδ T‐cell subsets. For example, thymic γδ17 cells are absent or significantly reduced in mice deficient for the following: Hes‐1, which is downstream of Notch‐1 signalling;37 HEB, an E protein that functions alongside the E2A proteins (E12 and E47);38 Syk, which is possibly linked in γδ17 cells to phosphoinositide 3‐kinase;35 Blk, a B‐cell kinase;39 and transforming growth factor‐β and Smad3.40 At present, the interconnection of these pathways is unclear. As too is their relationship to the weak/absent TCR‐γδ signalling that is permissive for γδ17 cell development.
Transcription factor networks
Much recent effort has been directed at understanding the transcriptional networks that underpin the thymic development of γδ T cells. Strong TCR‐γδ signalling, for example in Vγ5Vδ1+ progenitors that engage Skint1+ cells,16 up‐regulates Egr3 and Id3 in an ERK‐dependent manner.11 Egr3 induces T‐bet (and CD45RB), while suppressing Sox13 and RORγt, that appear to specify a γδ17 fate (Sox13 also down‐regulates the γδIFN markers NK1.1 and CD27).16 Moreover, Id3 antagonizes the function of the E protein transcription factor HEB, which directs γδ17 development by up‐regulation of Sox4 and Sox13.38 Sox4−/− and Sox13−/− mice specifically lack Vγ4+ γδ17 cells (although the Vγ6+ γδ17 pool is still largely intact).7 Indeed, Sox4 and Sox13 deficiency dramatically decreased RORγt and Blk (in Vγ4+ γδ17 cells). This study also demonstrated a dramatic increase in the ratio of γδ17 : γδIFN cells in both the Vγ1+ and Vγ4+ compartments of TCF1−/− mice, and suggested that antagonism between TCF1 (which is induced by Notch‐1 signalling) and Sox13 may control downstream regulators such as TCF1‐induced LEF1 to influence γδ17 versus γδIFN fates.7 Notch‐1 signalling via RBP‐Jκ can also increase IL‐7Rα expression.41 IL‐7 was previously shown to expand γδ17 cells during fetal thymic development, largely through activation of signal transducer and activator of transcription 3.42 Moreover, IL‐7 in combination with IL‐1β, IL‐21 and IL‐23 was suggested to ‘dampen’ strong TCR‐γδ signals by reducing Id3 expression to allow γδ precursors to adopt a γδ17 cell fate.43 Finally, evidence also suggests possible roles for nuclear factor of activated T cells and nuclear factor‐κB in γδIFN development,16 whereas generation of Vγ1Vδ6.3/6.4+ NKT‐like γδ T cells that can secrete both IFN‐γ and IL‐4 also involves the TCR‐signalling‐induced transcription factors promyelocytic leukaemia zinc finger (PLZF) and T‐helper inducing POZ‐kruppel like factor (ThPOK).27, 44, 45
Re‐thinking γδ T‐cell development
Weight of evidence now suggests that an alternative view of γδ T‐cell development should be considered. Consensus has long held that all γδ T‐cell subsets (i.e. γδIFN and γδ17) stem from a common progenitor that can also generate conventional αβ T cells.1 Certainly, development of naive‐like γδ T cells, γδIFN cells, and the known agonist‐selected γδ subsets fits with this idea, with strong (compared with the pre‐TCR) TCR‐γδ signalling first committing a common αβ/γδ progenitor to the γδ lineage, before then determining effector fate (i.e. naive‐like versus γδIFN) based on levels of TCR signal strength with perhaps additional factors directing specific fates (e.g. SLAM signalling for NKT‐like γδ T cells). Importantly, such a model that excludes the development of γδ17 cells does not generate the paradox of requiring weak or absent TCR‐γδ signalling for acquisition of (IL‐17‐secreting) γδ T‐cell effector function, while at the same time requiring those cells to have experienced strong TCR‐γδ signalling for entry into the γδ lineage.
But what about γδ17 cells; how then do they develop? The answer may lie with a re‐imagining of the pre‐commitment‐selection model (Fig. 2). Such a model would propose that a γδ17 programme be established in a distinct thymic γδ17‐progenitor before the expression of TCR‐γδ. Subsequent to this specification, appropriate alternative/weak signalling from certain TCR‐γδ would allow full manifestation of the pre‐committed γδ17 transcriptional programme. By contrast, stronger (e.g. ligand‐dependent) TCR‐γδ signalling may be incompatible for further development or survival.17 Importantly, this γδ17‐progenitor may have different developmental potentials when compared with the conventional common αβ/γδ progenitor (which gives rise to other γδ subsets). Hence, a ‘committing’ TCR‐γδ signal may not be required to divert the γδ17‐progenitor from an alternative (e.g. αβ T‐cell) fate. Some evidence for such a model already exists; DN cells (i.e. TCR−) with IL‐17‐secreting potential and with an open TCR‐δ locus have been observed,32 and fetal or adult c‐kit+ CD44+ CD25− ‘early thymic progenitors’, but not DN3 cells, were shown to favour development of Vγ4+ CCR6+ CD27− γδ T cells on OP9‐DL1 cultures.7 These observations were further extended to show that fetal c‐kit++ DN2 cells but not c‐kit− DN3 cells could generate γδ17 cells, possibly in a Bcl11b‐dependent manner.46 Moreover, expression in all DN cells of a Vγ4+ transgenic TCR‐γδ did not markedly increase generation of γδ17 cells, implying that a restricted IL‐17‐progenitor pool exists.7 Certainly, more evidence is required to confirm and extend these initial observations, and to assess whether all γδ17 cells could develop from such an alternative source.
Figure 2.
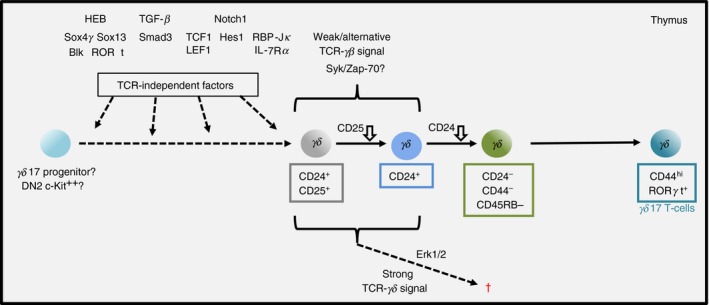
Model for the development of murine interleukin‐17 (IL‐17) ‐secreting (γδ17) γδ T cells. IL‐17‐secreting γδ T cells may develop from a separate pool of progenitors with a pre‐determined ‘IL‐17 programme’ that is established by expression of transcription factors (depicted) before expression of the T‐cell receptor (TCR). Subsequently, the programme is reinforced by weak or alternative signalling mediated by the expression of specific TCR‐γδ. In the event of increased signal strength, TCR‐γδ‐expressing thymocytes may undergo programmed cell death (†). Downward arrow indicates marker down‐regulation. ‘?’ denotes ongoing uncertainty.
Concluding remarks
An alternative developmental origin for IL‐17‐secreting immune cells may hark back to very early evolutionary times as IL‐17‐like genes are found in a variety of invertebrates that possess only innate‐like immune systems.47 Moreover, γδ17‐like cells may be a modern‐day example of one of the first types of lymphocyte. Support for this has come from recent work in jawless vertebrates (e.g. lampreys), whose lymphocytes express antigen receptors (variable lymphocyte receptors) that contain activation‐induced cytidine deaminase‐dependent somatically rearranged leucine‐rich repeat modules.48 Three divisions of lymphocytes can be identified in these animals, two of which resemble conventional T cells, and B cells. Interestingly, the third lymphocyte lineage largely occupies epithelial sites such as the skin and gut, can respond rapidly to challenge, and expresses both Sox13 and IL‐17.49 These γδ17‐like cells that pre‐date RAG and vertebrate T‐cell receptors appear to have an evolutionarily ancient history.50 It may therefore not be a surprise that their developmental origins are segregated from the developmental pathways of more recently evolved lymphocyte subsets (e.g. γδIFN cells). Obviously, such innate‐like origins invoke a relationship with the recently described family of innate lymphoid cells.51 The transcriptional programmes of γδ17 cells and certain subsets of innate lymphoid cell type 3 show clear similarities (e.g. expression of RORγt, CD127, CD25, CCR6).52 Further investigation of the links between these two cell types may therefore provide further insight into the developmental peculiarities of these highly enigmatic γδ T cells.
Disclosure
The authors have no conflicting financial interests.
Acknowledgements
This work was funded by the Wellcome Trust (092973/Z/10/Z) and BBSRC (BB/R017808/1) to DJP.
References
- 1. Kreslavsky T, Gleimer M, von Boehmer H. αβ versus γδ lineage choice at the first TCR‐controlled checkpoint. Curr Opin Immunol 2010; 22:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fehling HJ, Krotkova A, Saint‐Ruf C, von Boehmer H. Crucial role of the pre‐T‐Cell receptor‐α gene in development of αβ but not γδ T‐cells (Vol 375, Pg 795, 1995). Nature 1995; 378:419. [DOI] [PubMed] [Google Scholar]
- 3. Pennington DJ, Silva‐Santos B, Hayday AC. γδ T cell development – having the strength to get there. Curr Opin Immunol 2005; 17:108–15. [DOI] [PubMed] [Google Scholar]
- 4. Kang J, Volkmann A, Raulet DH. Evidence that γδ versus αβ T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med 2001; 193:689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G et al Regulation of γδ versus αβ T lymphocyte differentiation by the transcription factor SOX13. Science 2007; 315:230–3. [DOI] [PubMed] [Google Scholar]
- 6. Heilig JS, Tonegawa S. Diversity of murine γ genes and expression in fetal and adult T lymphocytes. Nature 1986; 322:836–40. [DOI] [PubMed] [Google Scholar]
- 7. Malhotra N, Narayan K, Cho OH, Sylvia KE, Yin C, Melichar H et al A network of high‐mobility group box transcription factors programs innate interleukin‐17 production. Immunity 2013; 38:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray EE, Ramírez‐Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE et al Deficiency in IL‐17‐committed Vγ4+ γδ T cells in a spontaneous Sox13‐mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat Immunol 2013; 14:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T et al Attenuation of γδTCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity 2005; 22:595–606. [DOI] [PubMed] [Google Scholar]
- 10. Hayes SM, Li L, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity 2005; 22:583–93. [DOI] [PubMed] [Google Scholar]
- 11. Lauritsen JPH, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M et al Marked induction of the Helix‐Loop‐Helix protein Id3 promotes the γδ T cell fate and renders their functional maturation notch independent. Immunity 2009; 31:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SY, Coffey F, Fahl SP, Peri S, Rhodes M, Cai KQ et al Noncanonical mode of ERK action controls alternative αβ and γδ T cell lineage fates. Immunity 2014; 41:934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2002; 2:336–45. [DOI] [PubMed] [Google Scholar]
- 14. Xiong N, Baker JE, Kang C, Raulet DH. The genomic arrangement of T cell receptor variable genes is a determinant of the developmental rearrangement pattern. Proc Natl Acad Sci USA 2004; 101:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prinz I, Silva‐Santos B, Pennington DJ. Functional development of γδ T cells. Eur J Immunol 2013; 43:1988–94. [DOI] [PubMed] [Google Scholar]
- 16. Turchinovich G, Hayday AC. Skint‐1 identifies a common molecular mechanism for the development of interferon‐γ‐secreting versus interleukin‐17‐secreting γδ T cells. Immunity 2011; 35:59–68. [DOI] [PubMed] [Google Scholar]
- 17. Sumaria N, Grandjean CL, Silva‐Santos B, Pennington DJ. Strong TCR γδ signaling prohibits thymic development of IL‐17A‐secreting γδ T cells. Cell Rep 2017; 19:2469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grigoriadou K, Boucontet L, Pereira P. Most IL‐4‐producing γδ thymocytes of adult mice originate from fetal precursors. J Immunol 2003; 171:2413–20. [DOI] [PubMed] [Google Scholar]
- 19. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ et al CD27 is a thymic determinant of the balance between interferon‐γ‐ and interleukin 17‐producing γδ T cell subsets. Nat Immunol 2009; 10:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lombes A, Durand A, Charvet C, Rivière M, Bonilla N, Auffray C et al Adaptive immune‐like γδ T lymphocytes share many common features with their αβ T cell counterparts. J Immunol 2015; 195:1449–58. [DOI] [PubMed] [Google Scholar]
- 21. Papotto PH, Gonçalves‐Sousa N, Schmolka N, Iseppon A, Mensurado S, Stockinger B et al IL‐23 drives the differentiation of peripheral γδ17 T cells from adult bone marrow‐derived precursors. EMBO Rep 2017; 18:1957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA et al γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen‐specific interleukin‐17 response. Immunity 2012; 37:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S et al Thymic selection determines γδ T cell effector fate: antigen‐naive cells make interleukin‐17 and antigen‐experienced cells make interferon γ . Immunity 2008; 29:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Munoz‐Ruiz M, Ribot JC, Grosso AR, Gonçalves‐Sousa N, Pamplona A, Pennington DJ et al TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets. Nat Immunol 2016; 17:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fahl SP, Coffey F, Kain L, Zarin P, Dunbrack RL Jr, Teyton L et al Role of a selecting ligand in shaping the murine γδ‐TCR repertoire. Proc Natl Acad Sci USA 2018; 115:1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM et al Skint‐1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci USA 2011; 108:3330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP et al Development of promyelocytic zinc finger and ThPOK‐expressing innate γδ T cells is controlled by strength of TCR signaling and Id3. J Immunol 2010; 184:1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pereira P, Berthault C, Burlen‐Defranoux O, Boucontet L. Critical role of TCR specificity in the development of Vγ1Vδ6.3+ innate NKTγδ cells. J Immunol 2013; 191:1716–23. [DOI] [PubMed] [Google Scholar]
- 29. Haas JD, González FH, Schmitz S, Chennupati V, Föhse L, Kremmer E et al CCR6 and NK1.1 distinguish between IL‐17A and IFN‐γ‐producing γδ effector T cells. Eur J Immunol 2009; 39:3488–97. [DOI] [PubMed] [Google Scholar]
- 30. Buus TB, Ødum N, Geisler C, Lauritsen JPH. Three distinct developmental pathways for adaptive and two IFN‐γ‐producing γδ T subsets in adult thymus. Nat Commun 2017; 8:1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahtani‐Patching J, Neves JF, Pang DJ, Stoenchev KV, Aguirre‐Blanco AM, Silva‐Santos B et al PreTCR and TCRγδ signal initiation in thymocyte progenitors does not require domains implicated in receptor oligomerization. Sci Signal 2011; 4:ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E et al Development of interleukin‐17‐producing γδ T cells is restricted to a functional embryonic wave. Immunity 2012; 37:48–59. [DOI] [PubMed] [Google Scholar]
- 33. Coffey F, Lee SY, Buus TB, Lauritsen JP, Wong GW, Joachims ML et al The TCR ligand‐inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J Exp Med 2014; 211:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A et al Innate‐like T cells straddle innate and adaptive immunity by altering antigen‐receptor responsiveness. Nat Immunol 2014; 15:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muro R, Nitta T, Nakano K, Okamura T, Takayanagi H, Suzuki H. γδ TCR recruits the Syk/PI3K axis to drive proinflammatory differentiation program. J Clin Invest 2018; 128: 415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N et al The BAFF receptor transduces survival signals by co‐opting the B cell receptor signaling pathway. Immunity 2013; 38:475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T et al Notch‐Hes1 pathway is required for the development of IL‐17‐producing γδ T cells. Blood 2011; 118:586–93. [DOI] [PubMed] [Google Scholar]
- 38. In TSH, Trotman‐Grant A, Fahl S, Chen ELY, Zarin P, Moore AJ et al HEB is required for the specification of fetal IL‐17‐producing γδ T cells. Nat Commun 2017; 8:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laird RM, Laky K, Hayes SM. Unexpected role for the B cell‐specific Src family kinase B lymphoid kinase in the development of IL‐17‐producing γδ T cells. J Immunol 2010; 185:6518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ et al Cutting edge: spontaneous development of IL‐17‐producing γδ T cells in the thymus occurs via a TGF‐β1‐dependent mechanism. J Immunol 2010; 184:1675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamura M, Shibata K, Hatano S, Sato T, Ohkawa Y, Yamada H et al A genome‐wide analysis identifies a notch‐RBP‐Jκ‐IL‐7Rα axis that controls IL‐17‐producing γδ T cell homeostasis in mice. J Immunol 2014; 194:243–51. [DOI] [PubMed] [Google Scholar]
- 42. Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL‐7) selectively promotes mouse and human IL‐17‐producing γδ cells. Proc Natl Acad Sci USA 2012; 109:17549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zarin P, In TS, Chen EL, Singh J, Wong GW, Mohtashami M et al Integration of T‐cell receptor, Notch and cytokine signals programs mouse γδ T‐cell effector differentiation. Immunol Cell Biol 2018; 96:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P et al TCR‐inducible PLZF transcription factor required for innate phenotype of a subset of γδ T cells with restricted TCR diversity. Proc Natl Acad Sci USA 2009; 106:12453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park K, He X, Lee HO, Hua X, Li Y, Wiest D et al TCR‐mediated ThPOK induction promotes development of mature (CD24‐) γδ thymocytes. EMBO J 2010; 29:2329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shibata K, Yamada H, Nakamura M, Hatano S, Katsuragi Y, Kominami R et al IFN‐γ‐producing and IL‐17‐producing γδ T cells differentiate at distinct developmental stages in murine fetal thymus. J Immunol 2014; 192:2210–8. [DOI] [PubMed] [Google Scholar]
- 47. Hibino T, Loza‐Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP et al The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol 2006; 300:349–65. [DOI] [PubMed] [Google Scholar]
- 48. Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI et al Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID‐APOBEC family cytosine deaminase. Nat Immunol 2007; 8:647–56. [DOI] [PubMed] [Google Scholar]
- 49. Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T et al Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 2013; 501:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol 2006; 24:497–518. [DOI] [PubMed] [Google Scholar]
- 51. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells: a new paradigm in immunology. Science 2015; 348:aaaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kang J, Malhotra N. Transcription factor networks directing the development, function, and evolution of innate lymphoid effectors. Annu Rev Immunol 2015; 33:505–38. [DOI] [PMC free article] [PubMed] [Google Scholar]


