Abstract
Mannosylerythritol lipids (MELs) are glycolipids possessing unique biosurfactant properties. However, the prices of substrates currently used for MEL formation caused its unsustainable commercial development. Waste cooking oil poses significant ecological and economical problems. Thus, the production of MELs from used waste cooking oil using the biotransformation route is one of the better alternatives to utilize it efficiently and economically. This work aims at the production of MELs using waste cooking oil instead of soybean oil and evaluating the major characteristics and compositions of MELs. The titers reached 61.50 g/L by the optimization of culture medium, higher than the counterpart (10.25 ± 0.32 g/L) of the nonoptimized medium. MELs exhibited good surface activity and better performance in contrast to MELs grown on soybean oil. The water phase behavior of MEL‐A was also evaluated. The process showed higher productivity of MELs with better surface activity and application stability than the conventional process using soybean oil. The findings of this study imply that the use of inexpensive fermentation substrates associated with straightforward downstream processing is expected to have a great impact on the economy of MEL production.
Keywords: gas chromatography–mass spectrometry, mannosylerythritol lipids, response surface methodology, surface activity, waste cooking oil
1. INTRODUCTION
Mannosylerythritol lipids (MELs) are one of the most promising nonionic biosurfactants, secreted by plant‐associated fungi of the genera Pseudozyma and Ustilago(Arutchelvi, Bhaduri, Uppara, & Doble, 2008; Yu et al., 2015). MELs are amphiphilic molecules with 4‐O‐β‐d‐mannopyranose‐erythritol as a hydrophilic moiety and a fatty acid and/or an acetyl group as the hydrophobic moiety (Arutchelvi & Doble, 2011). According to the different number and location of acetyl group at mannosyl C‐4 and C‐6, MELs are classified as MEL‐A, MEL‐B, MEL‐C, and MEL‐D (Figure 1; Gunther et al., 2015). MELs were first noted as oily compounds in the culture suspension of Ustilago maydis(Haskins, Thorn, & Booroyd, 1955). Though MELs and their properties have been documented for several decades, they gained more and more attention only in recent years because of their interesting application possibilities in the biochemical and pharmaceutical industries. Up to date, researches have shown that MELs are mainly produced by anamorphic basidiomycetous yeasts, Pseudozymaspp., as relatively high quantities, and fungi, Ustilago maydis, as relatively low quantities (Faria et al., 2014), which is with regard to the substrate, fermentation condition, and downstream processing.
Figure 1.
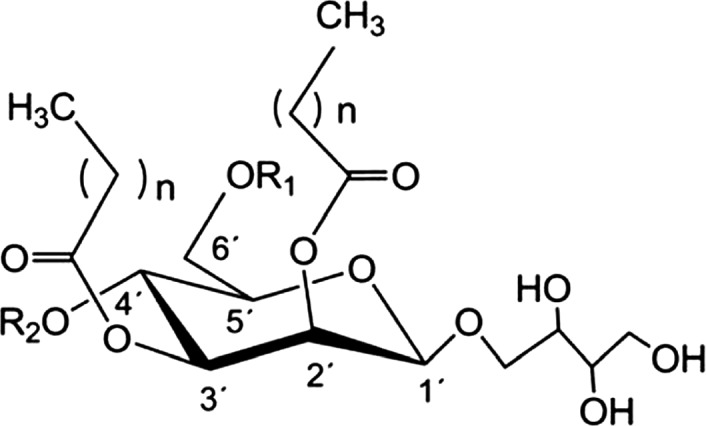
General structure of mannosylerythritol lipids (MELs). MEL‐A: R1/R2=acetyl‐; MEL‐B: R1=acetyl‐, R2=H; MEL‐C: R1=H, R2=acetyl‐; MEL‐D: R1/R2=H
In contrast to other biosurfactants, for example, rhamnolipids and sophorolipids, MELs own many excellent physicochemical properties such as good emulsifying properties, biodegradability, and lower critical micelle concentration (CMC), but also many special physiologic activities, such as inhibiting the growth of microorganisms (Kitamoto et al., 1993), inducing cell mutation (Isoda & Nakahara, 1997; Wakamatsu et al., 2001), differentiate human myeloid leukemia cell lines and melanoma cells (Fan, Li, Niu, & Chen, 2016; Zhao et al., 2000, 2001), improving the efficiency of gene transfection (Kitamoto, 2008), and a strong coordination ability with glycoproteins (Fan, Xie, Wang, Huang, & Zhou, 2018; Im, Nakane, Yanagishita, Ikegami, & Kitamoto, 2001; Im et al., 2003); thus, MELs could be used in lots of industries (Morita, Fukuoka, Imura, & Kitamoto, 2013; Noh, Suh, & Park, 2016; Safdel, Anbaz, Daryasafar, & Jamialahmadi, 2017), for example, environmental protection, food, cosmetic, and pharmaceutical.
Until now, noncommercial MEL products rarely appeared. One of the main reasons is high production costs, which is related to the expensive raw materials, low production, and tough downstream, etc. Among them, several substrates have been used for MEL production by Pseudozymaspp., including soybean oil, alkanes, glycerol, glucose, and xylose. (Arutchelvi & Doble, 2011; Morita, Fukuoka, Imura, & Kitamoto, 2013; Rau, Nguyen, Schulz et al., 2005). In addition, almost all vegetable oils (except palm oil and coconut oil) have been found to serve as a good carbon source for MELs by different Pseudozymaspp. However, the sustainability of MEL production from the above‐mentioned materials is doubtful due to high cost (Faria et al., 2014). Consequently, a positive strategy to reduce the production cost is to search for cheap substrates. So far, biosurfactant that has been shown could be produced using some renewable industrial residues such as olive oil mill effluent (Mercadé et al., 1993), oil refinery wastes (Bednarski, Adamczak, Tomasik, & Plaszczyk, 2004), distillery and whey wastes, potato process effluent (Dubey & Juwarkar, 2001), soapstock (Benincasa, Contiero, Manresa, & Moraes, 2002), and waste cooking oil (Raza, Khan, Khalid, & Rehman, 2006). Waste cooking oil is generated in large quantities during food preparation in both household and food processing industries (Hingu, Gogate, & Rathod, 2010; Zhang, Wang, & Mortimer, 2012). It has been considered a problematic waste product contributing to the environmental pollution. Currently, waste cooking oils are only allowed to produce biofuels. In general, the disposal of waste cooking oil is a growing problem that needs effective solution.
Biosurfactant production using the technique of microorganism transformation may be an effective way to utilize waste cooking oil. There were some studies on glycolipid production using waste cooking oil. Waste frying oil and the methyl ester of coco/palm oil were used to produce sophorolipids by Candida bombicola in shaking flasks (Fleurackers, 2006). Haba, Espuny, Busquets, and Manresa (2000) reported the reuse of olive and sunflower cooking oil as substrate to produce rhamnolipids at equivalent productivity of 6.75–9.25 g/L (measured as 2.7 g rhamnose/L) in shaking flasks. Waste frying oil as the sole carbon source was used to synthesize rhamnolipids by Pseudomonas aeruginosa ZJU at a concentration of 12.47 g/L, and its mutant after treatment by UV light increased this productivity to 24.61 g/L cultured in shaking flasks. Future, the productivity of fermentation which conducted in a 50‐L bioreactor reached over 20 g/L (Zhu et al., 2007).
There is no report on the MEL production from waste cooking oil, while Włodzimierz Bednarski, Adamczak, and Nawotka (2006) studied on the synthesis of MELs from glucose and products of enzymatically hydrolyzed lactose in milk permeate (after ultrafiltration) supplemented with the waste fats (fish, pork, and postrefining fatty acids), indicated that the fatty acids from the waste fats used seemed to be directly incorporated in the mannosylerythritol lipids.
To obtain higher MEL yield, the fermentation conditions have been widely studied, showing that the type and concentrations of nitrogen and inorganic salts, the amount of vegetable oil, inoculum size, and initial pH are potentially important impact factors (Luo et al., 2013). In this work, waste cooking oil was used as the sole carbon source to produce MELs by Pseudozyma aphidisZJUDM34. The aim of this work was to use response surface methodology (RSM) to optimize the amount of waste cooking oil, inoculum size, initial pH, and medium volume for MEL production. Furthermore, to demonstrate the surface activity and stability, MELs from waste cooking oil were compared to that from soybean oil via the analyses of gas chromatography–mass spectrometry (GC‐MS) and surface tension examinations.
2. METHODS
2.1. Fermentation strain
The experimental strain was P. aphidisDSM 70725, which obtained from Deutsche Sammlungvon von Mikroorganismen und Zellkulturen GmbH (DSMZ), Braunschweig, Germany. The P. aphidis ZJUDM34 mutant was evolved from wild‐type yeast using UV mutagenesis and low‐energy N+ implantation methods. It exhibited enhanced ability to produce MEL‐A. This mutant was stored in our laboratory. Stock cultures were grown for 2 days at 28°C in a liquid medium containing (g/L) yeast extract 3.0, malt extract 3.0, peptone from soybeans 5.0, and glucose 10.0 at initial pH and then mixed with an equal volume of 50% glycerol. They were stored at −80°C and renewed every half‐year.
Waste cooking oil was collected from major restaurant in Hangzhou with soybean oil in the preparation of cooked food. The crude waste oil was preliminarily prepared using the process as shown in Figure 2, prior to use as the carbon source in replace of soybean oil.
Figure 2.
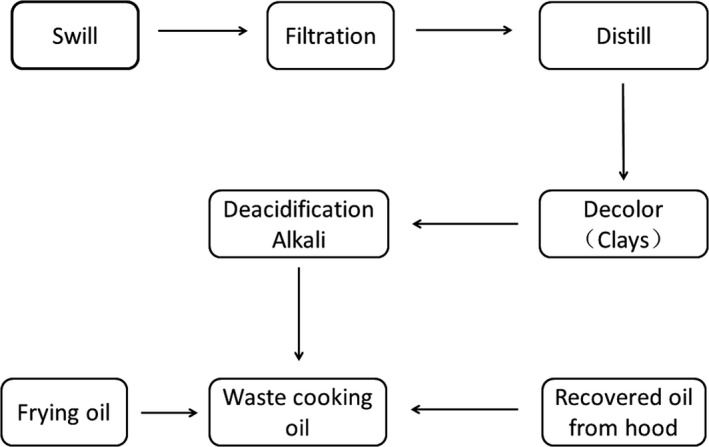
The preparation route of waste cooking oil
2.2. Inoculum preparation
At first, the yeast was activated by inoculating 1‐ml stock cultures into 50‐ml YM medium composed of (g/L): yeast extract 3.0, malt extract 3.0, peptone from soybeans 5.0, and glucose 10.0 at the natural pH in 250‐ml Erlenmeyer flasks, and was then incubated for 36 hr at 28°C and 180 rpm. Seed culture was prepared by inoculating 1 ml of the activated cells in 250‐ml Erlenmeyer flasks containing 50‐ml culture medium (g/L) (NaNO3 3, KH2PO4 0.3, MgSO4·7H2O 0.3, yeast extract 1, and glucose 40) and incubated for 2 days at 28°C and 180 rpm. Then, the inoculum cells were prepared by the following procedure: Seed was centrifuged to obtain the cells, which were washed two times with 0.9% physiologic saline, dissolved in an appropriate amount of 0.9% physiologic saline to formulate into the inoculum with the amount of 0.12 g/ml cell wet weight.
2.3. Fermentation medium and conditions
The liquid submerged fermentation (g/L) medium was comprised of NaNO3 3.0, KH2PO4 0.3, MgSO4·7H2O 0.3, yeast extract 1.0, and certain amount of waste cooking oil (ml/L) or soybean oil (ml/L) depending on experimental design. Fermentation was inoculated with the above inoculum and conducted in 250‐ml Erlenmeyer flasks for 10 days at 28°C and 180 rpm.
2.4. Experimental design
Response surface methodology is a statistical method to find the optimal process parameters to solve multivariable problems with multiple regression analysis for the quantitative data obtained from properly designed experiments. In order to obtain optimum conditions for increasing MELs’ yield, a central composite design with five coded levels was performed. In the experimental design, four factors (waste cooking oil amount, inoculum size, medium volume in 250‐ml Erlenmeyer flasks, and initial pH) that having impact on the production of MELs were identified by the optimization strategies.
All the experiments were performed in triplicate. The test factors were coded according to the following equation (Zhang, Dong, Fan, Jiao, & Chen, 2015):
| (1) |
where xi is the coded value of the ith independent variable, Xi is the natural value of the ith independent variable, X 0 is the natural value of the ith independent variable at the center point, and ΔXi is the step change value. The range and the levels of the test factors with both coded values and natural values investigated in this study are given in Table 1. The production of MELs was considered as the response.
Table 1.
Range of values for response surface methodology
| Independent variables | Variable name | Levelsa | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| X 1 (ml/L) | Waste cooking oil amount | 54.8 | 65.0 | 80.0 | 95.0 | 105.2 |
| X 2 (ml/L) | Inoculum size | 11.6 | 15.0 | 20.0 | 25.0 | 28.4 |
| X 3 (ml/250 ml) | Medium volume | 24.77 | 35.00 | 50.00 | 65.00 | 75.23 |
| X 4 | Initial pH | 3.41 | 4.43 | 5.93 | 7.43 | 8.45 |
x 1 = (X 1 − 80)/15; x 2 = (X 2 − 20)/5; x 3 = (X 3 − 50)/15; x 4 = (X 4 – 5.93)/1.5.
The quadratic model for predicting the optimal point was expressed based on the following equation:
| (2) |
where Y was the response variable, b 0, bi, bii, and bij were the regression coefficients variables, for intercept, linear, quadratic, and interaction coefficients, respectively, and xi and xj were independent variables. Results were analyzed using the response surface regression (RSREG) procedure (SAS Institute Inc, Cary, NC, USA).
Three‐dimensional surface plots were drawn to illustrate the main and interactive effects of the independent variables on MEL production. Solving the regression equation and analyzing the response surface contour plots, the optimum values of the selected variables were obtained for the further experiments (Ghorbani, Karimi, Biria, Kariminia, & Jeihanipour, 2015).
To make clear the fermentation process, according to the optimal results of 2.3.1 experiments, design experiments to conduct the assay of biomass, pH, and MEL yield at different culture times. Selected measurement time points were the 3rd, 4th, 5th, 6th, 7th, 8th, 9th, and 10th day.
2.5. Determination of biomass, isolation, and purification of MELs
After fermentation, pH was measured with pH meter. Then, thoroughly mixed culture suspension with equal volume of ethyl acetate was extracted three times. Aqueous and organic phases were separated by centrifugation, with the yeast cells between the two phases. The collected organic phases were rotary evaporated (45–60°C, 3,000 Pa) to obtain crude MELs, and the yeast cells were collected in weighed dry flats which were put in the oven to constant weight for measurement of biomass (g dry cells/L fermentation broth). Then, the crude MELs were treated three times by solving the extract in methanol–cyclohexane (v:v, 1:1). The methanol phases were collected and again rotary evaporated, obtained the purified MEL product containing minor amounts of oil (Rau, Nguyen, Roeper, Koch, & Lang, 2005).
2.6. Thin‐layer chromatography
The purified MEL extracts were analyzed by thin‐layer chromatography (TLC). As a solvent system, a chloroform–methanol–water (65:15:2) mixture was used. Location of the components on the plates was carried out by fumigating with iodine or spraying the plates with a solution of 0.1% orcinol in a 5% sulfuric acid solution and heating the plates for 5 min at 110°C.
2.7. Gas chromatography–mass spectrometry
2.7.1. Methyl ester of samples
In this study, the methyl ester derivatives were prepared by the followed steps. The purified MEL fraction or oil sample (2.0 g) was mixed with 20 ml methanol and 0.5 ml KOH (1 M in methanol) in round bottom flask, then added a few zeolite, and begun to heat reflux with shaking the beaker, until the solution was clear. Afterward, the solution was cooled in cold water, transferred to a separating funnel, washed the flask with 10 ml n‐heptane and transferred to the separating funnel, added to 20 ml distilled water, shook and still layered; the upper is ester layer, and the lower is aqueous layer. The lower layer was treated with 10 ml n‐heptane extraction and mixed with the upper. Subsequently, the n‐heptane solution was washed several times with distilled water until the water was neutral; acetate layer was obtained, dried with anhydrous sodium sulfate, filtered, and evaporated to 10 ml n‐heptane solution for analysis.
2.7.2. Determination procedures of GC‐MS
About 3 ml of the upper phase from the isolated MELs or soybean oil was injected into the GC‐MS system (Agilent 6890 Gas Chromatography with a 5973 Mass Spectrometer) using an Agilent HP‐5MS (30 m × 0.25 mm×0.25 μm) column. The oven temperature was programmed from 100°C to 300°C within 5 min. A scan range of 30–550 u was applied, using a source temperature of 250°C (Onghena et al., 2011).
2.8. Determination of surface activity and stability of MELs
2.8.1. Surface‐activity determination
Equilibrium surface (water/air) tension measurements of MEL solution were performed at 25°C with a QBZY‐1 tensiometer (Fangrui Instrument Co., Shanghai, China) using the Wilhelmy plate method (Benincasa et al., 2002). To increase the accuracy of the surface tension measurements, an average of triplicates was determined. The platinum plate and all glassware used were cleaned in ultrapure water.
2.8.2. The critical micelle concentration and stability
Critical micelle concentration is the concentration of an amphiphilic component in solution at which the formation of micelles is initiated. It is important for some biosurfactant applications. The CMC was determined by plotting the surface tension as y‐axis versus the concentration of MELs as x‐axis and was found at the break point. Aqueous solutions of MELs (in the concentration range of 1.50–100 mg/L) were obtained by successive dilutions of a concentrated sample prepared by weight in ultrapure water (Gudina, Teixeira, & Rodrigues, 2010).
The applicability of biosurfactants can be conditioned by their stability to pH changes, temperature range, and salt tolerance. Thus, we evaluated the stability of MELs under different environmental conditions. The MEL solutions with CMC were prepared for evaluating the stability. Then, the stability of the MELs was determined by measuring the surface tension with different pH values (2.00–12.00). Furthermore, the MEL solutions were treated at different temperatures (−20°C, 0°C, 50°C, 65°C, 80°C, and 95°C) for 1 or 2 hr and evaluated the stability by assaying the surface tension. Lastly, the surface tension of MEL solutions with different amounts of sodium chloride was assayed for evaluating salt tolerance. Surface tension of each sample was determined as described above, and all measurements were performed in triplicate.
2.9. Statistical analysis
The regression analysis of the obtained experimental data was performed by Design‐Expert software (Stat‐Ease Inc., Minneapolis, MN, USA). The quality degree of the fit for the polynomial model equation is expressed as the coefficient of determination R2, and its statistical significance was checked by the F test. Three independent experiments were carried out for standard error analysis of the mean (SEM), which was analyzed by Origin 9.1 software (OriginLab Corp., Northampton, MA, USA). Data were expressed as means ± SEM. The error bars in the figures represent the standard error of the mean.
3. RESULTS
3.1. Optimization under RSM‐based experimental design
Previous investigations showed that waste cooking oil, inoculum size, culture medium volume, and initial pH all affected the production of MELs by P. aphidisZJUDM34. Thus, to further improve the fermentation conditions for MEL production, RSM was employed to determine the optimal levels of four factors (waste cooking oil amount, inoculum size, medium volume in 250‐ml Erlenmeyer flasks, and initial pH) that affected MEL production (Table 2). As a result, the following regression equation was obtained:
| (3) |
Table 2.
Experimental design and the results of response surface methodology
| Run | x 1 | x 2 | x 3 | x 4 | MELs (g/L) observed | MELs (g/L) predicted |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | −1 | 55.38 | 57.35 |
| 2 | 0 | 0 | 1.68 | 0 | 13.29 | 50.65 |
| 3 | 0 | 0 | 0 | 0 | 14.00 | 3.50 |
| 4 | 0 | 0 | 0 | 0 | 10.00 | 2.08 |
| 5 | 0 | 0 | 0 | 0 | 8.00 | 16.36 |
| 6 | 1 | 1 | −1 | −1 | 51.43 | 5.04 |
| 7 | 0 | 0 | 0 | 0 | 10.00 | 5.04 |
| 8 | 1 | −1 | 1 | 1 | 1.54 | 4.94 |
| 9 | 0 | −1.68 | 0 | 0 | 12.00 | 1.16 |
| 10 | 0 | 0 | 0 | 1.68 | 10.00 | 59.16 |
| 11 | −1 | −1 | 1 | −1 | 3.08 | 11.16 |
| 12 | −1.68 | 0 | 0 | 0 | 2.00 | 39.16 |
| 13 | 0 | 0 | 0 | 0 | 10.00 | 10.50 |
| 14 | 0 | 1.68 | 0 | 0 | 40.00 | 9.19 |
| 15 | −1 | 1 | 1 | 1 | 3.08 | 5.16 |
| 16 | 0 | 0 | −1.68 | 0 | 8.07 | 9.16 |
| 17 | 0 | 0 | 0 | −1.68 | 6.00 | 17.99 |
| 18 | 1 | −1 | −1 | 1 | 17.14 | 17.99 |
| 19 | 1.68 | 0 | 0 | 0 | 60.00 | 57.99 |
| 20 | −1 | 1 | −1 | 1 | 2.86 | 17.99 |
| 21 | −1 | −1 | −1 | −1 | 5.71 | 11.99 |
MEL: mannosylerythritol lipid.
With Y, MEL production (the response); x 1, waste cooking oil amount; x 2, inoculum size; x 3, medium volume in 250‐ml Erlenmeyer flasks; and x 4, initial pH (coded values). The significance of each coefficient was determined by p‐values, which are listed in Table 3.
Table 3.
Model coefficients estimated by multiple linear regression
| Factor | Coefficient estimate | F value | p‐Value | |
|---|---|---|---|---|
| Model | 17.99 | 19.06 | 0.0004a | |
| x 1 | 17.24 | 69.75 | <0.0001a | |
| x 2 | 8.32 | 16.25 | 0.0040a | |
| x 3 | −0.38 | 0.08 | 0.7549 | |
| x 4 | 1.19 | 0.33 | 0.5416 | |
|
|
4.30 | 11.47 | 0.0016a | |
|
|
2.53 | 3.98 | 0.0080 | |
|
|
−2.88 | 5.14 | 0.4085 | |
|
|
−3.83 | 9.09 | 0.1350 | |
| x 1 x 2 | 12.56 | 21.69 | 0.0020a | |
| x 1 x 3 | −1.15 | 0.44 | 0.4831 | |
| x 1 x 4 | −2.33 | 0.75 | 0.3684 | |
| x 2 x 3 | 2.80 | 2.60 | 0.1200 | |
| x 2 x 4 | 3.40 | 1.59 | 0.2068 | |
| x 3 x 4 | −2.09 | 1.45 | 0.2255 | |
| Lack of fit | 0.92 | 0.4700 |
Statistically significant at 95% of confidence level.
The regression equation indicated the R 2 value of 0.9780. This value ensured a satisfactory adjustment of the quadratic model to the experimental data and indicated that the model could explain 97.80% of the variability in the response. As can be seen from Table 3, the p‐value and lack of fit check indicated that the model was significant for MEL production. As well, the production was significantly influenced by the variables such as waste cooking oil amount and inoculum size, independently. Moreover, there were interactions among x 1and x 2 factors (p < 0.05). However, other factors demonstrated insignificant effects on the production of MELs.
The three‐dimensional plot obtained from the calculated response surface is presented in Figure 3. Three‐dimensional response surface plots of four factors against MEL production (Y) can explain the results of statistical and mathematical analyses. The plot clearly showed that Y reached its maximum at a combination of coded level −1(x 1), 0.99(x 2), −0.09(x 3), −0.05(x 4). The predicted maximum production of MELs is 61.51 g/L, which is much higher than the yield from the control fermentation (10.25 ± 0.32 g/L).
Figure 3.
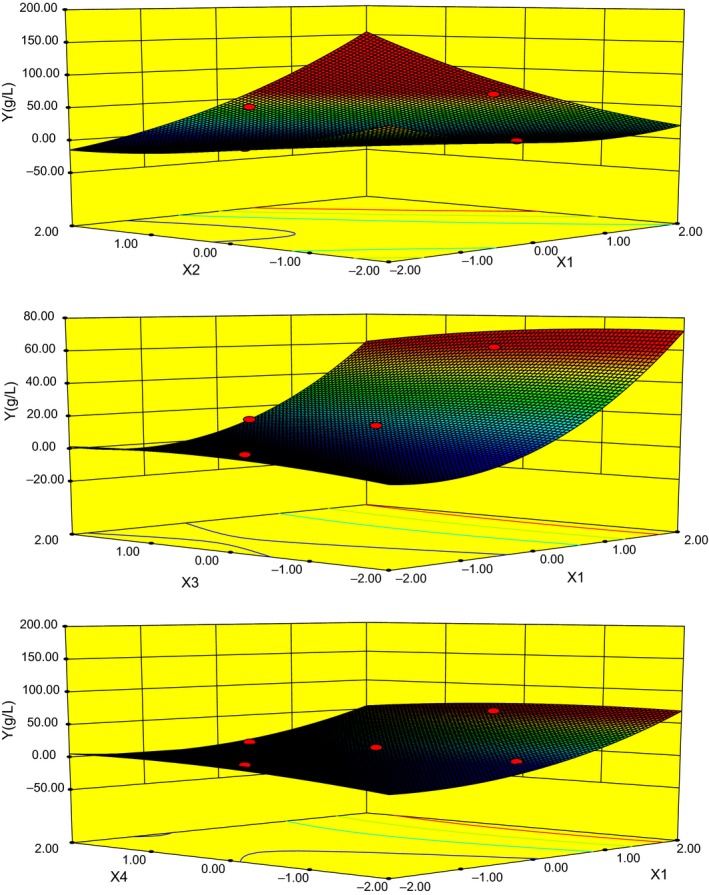
Response surface plots of x 1, x 2, x 3, and x 4 against MEL (g/L) production by Pseudozyma aphidis ZJUDM34
3.2. Experimental validation of the optimized culture conditions
The predicted optimal fermentation conditions for MEL production composed of waste cooking oil amount 95 ml/L, 24.95 inoculum size (ml/L), 48.65‐ml medium volume per 250 ml, and initial pH 5.76. The response surface plots are presented in Figure 3. In order to validate the adequacy of this optimized medium formula for MEL production, verification experiments were carried out at the predicted optimal soybean oil and waste cooking oil. The mean output of MELs from waste cooking oil was 55.00 ± 0.80, which was little lower than the predicted value (61.51 g/L) and slightly lower than 61.00 ± 0.80 g/L from soybean oil after 10 days (Figure 4a). In comparison, waste cooking oil showed lower MEL production and biomass than those of soybean oil (Figure 4b), whereas there was no obvious change of pH during the fermentation process (Figure 4c). These results suggested that the model obtained from RSM was adequate and waste cooking oil was a suitable carbon source for MEL production by P. aphidisZJUDM34.
Figure 4.
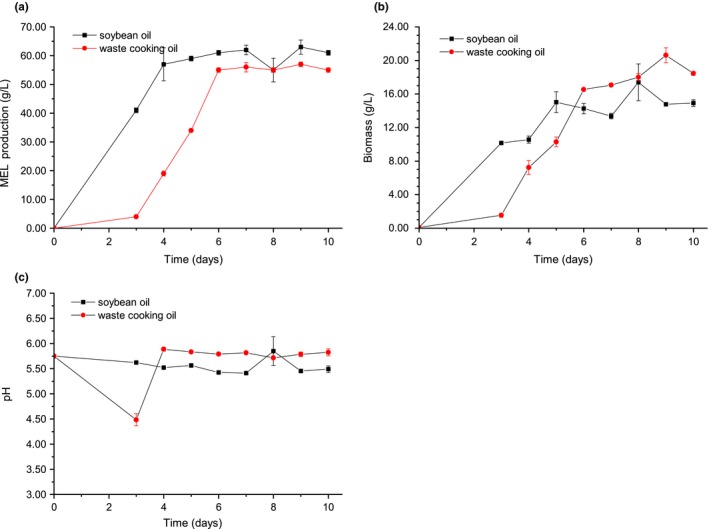
Mannosylerythritol lipid (MEL) production from soybean oil or waste cooking oil by Pseudozyma aphidis ZJUDM34. (a–c) Time course showing MEL formation yield, biomass, and pH from soybean oil (black square) or waste cooking oil (red circle) with Table 4 conditions by P. aphidis, respectively
3.3. Fatty acid profiles in substrates and MELs
Furthermore, by the use of TLC, MELs contain type‐A, type‐B, and type‐C grown on soybean oil and type‐A, type‐B, type‐C, and type‐D from waste cooking oil (Figure 5(1)). Soybean oil and waste cooking oil as the substrates were composed of a variety of fatty acids and likely influenced the properties of MELs; as a result, GC‐MS was used for detecting fatty acid contents of substrate and MEL product. To compare the fatty acid profiles of soybean oil, waste cooking oil and a mixture of MEL derived from soybean oil or waste cooking oil are presented in Table 4 and Figure 5(2). The substrate, soybean oil, included C16:0, C16:1, C17:0, C18:0, C18:0, C18:1, C18:2, C18:3, C20:0, and C22:0, and waste cooking oil had similar fatty acid composition to soybean oil except for lack of C18:3 and a little additional C14:0, C20:1, and C20:2. In contrast, there is more C18:2 (48.11%) in soybean oil than waste cooking oil (30.63%). However, these two oils showed no significant difference in the overall fatty acids. For MELs grown on soybean oil, main fatty acid compositions are C6:0 (23.19%), C10:0 (47.49%), C12:0 (5.94%), C18:1 (7.37%), and C18:2 (9.57%), and the MELs from waste cooking oil were composed of C10:0 (6.66%), C16:0 (7.92%), C18:1 (59.78), and C18:2 (13.78%). This result indicated soybean oil contributed to more short‐chain fatty acids in MEL production, while waste cooking oil showed favorable for producing more long‐chain fatty acid.
Figure 5.
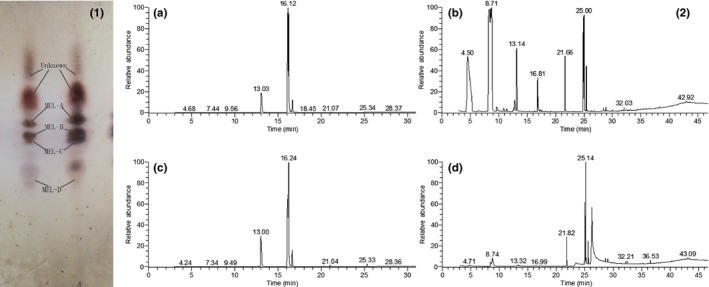
(1) Thin‐layer chromatography of mannosylerythritol lipid (MEL) obtained from liquid–liquid extraction with ethyl acetate of culture broth samples (after 10 days, left: MELs from soybean oil; right: MELs from waste cooking oil); (2) The total ion chromatogram of oil samples and MELs by means of gas chromatography–mass spectrometry. (a) Soybean oil sample; (b) waste cooking oil sample; (c) MELs derived from soybean oil; (d) MELs from waste cooking oil
Table 4.
Main fatty acid contents (percent of main fatty acid, MFA) in feedstock and fermentation products
| Fatty acid chain | Fatty acid chain in each sample composition (%) | |||
|---|---|---|---|---|
| Soybean oil | Waste cooking oil | MELs (SO) | MELs (WCO) | |
| C8:0 | — | — | 23.19 | 1.50 |
| C9:0 | — | — | 0.10 | — |
| C10:0 | — | — | 47.49 | 6.66 |
| C10:1 | — | — | 0.70 | 3.22 |
| C11:0 | — | — | 0.42 | — |
| C12:0 | — | — | 5.94 | 0.31 |
| C12:2 | — | — | 0.07 | — |
| C14:0 | — | 0.04 | 0.05 | 0.02 |
| C16:0 | 13.08 | 18.59 | 2.13 | 7.92 |
| C16:1 | 0.06 | 0.37 | — | — |
| C17:0 | 0.02 | 0.04 | — | — |
| C18:0 | 4.36 | 5.13 | 1.60 | 3.79 |
| C18:1 | 33.60 | 43.57 | 7.37 | 59.78 |
| C18:2 | 48.11 | 30.63 | 9.57 | 13.78 |
| C18:3 | 0.51 | — | — | 1.92 |
| C20:0 | 0.13 | 0.60 | 0.13 | 0.55 |
| C20:1 | — | 0.43 | 0.16 | — |
| C20:2 | — | 0.05 | — | — |
| C20:3 | — | — | — | 0.06 |
| C20:4 | — | — | 0.95 | — |
| C22:0 | 0.13 | 0.56 | 0.13 | 0.48 |
—: not detected; MEL: mannosylerythritol lipid; WCO: waste cooking oil.
3.4. Surface activity and stability
3.4.1. Critical micelle concentration
The surface (water/air) tensions of crude MEL from different substrates were comparatively measured. As shown in Figure 6a, the strongest reduction (30.63 or 32.83 mN/m) of the surface tension caused by 20.00 mg/L MEL was separately achieved with the product from soybean oil or waste cooking oil. Besides, MEL‐A was of two CMCs; thus, the surface tensions became a little higher with the concentration of MELs from 20.00 to 33.33 mg/L (Imura et al., 2006). Thus, the CMCs of MELs grown on soybean oil or waste cooking oil were both 20.00 mg/L.
Figure 6.
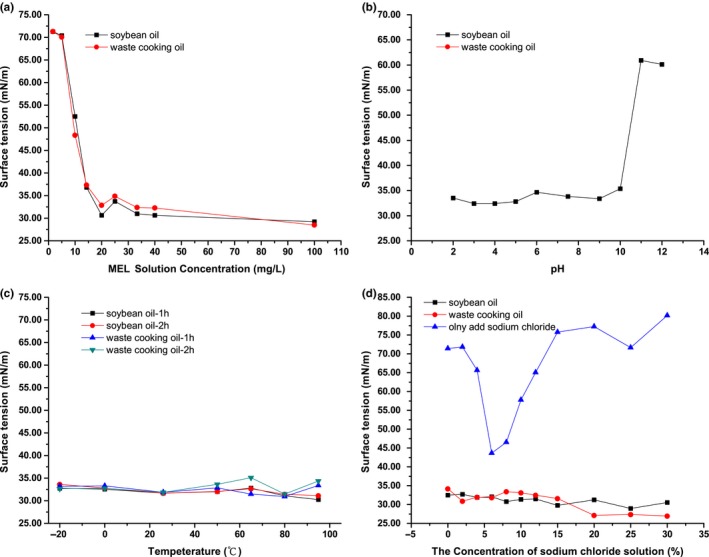
The critical micelle concentration and stability to pH, temperature, and sodium chloride of MELs from soybean oil or waste cooking oil by Pseudozyma aphidis. (a) Surface tension (mN/m) of the crude MELs obtained from soybean oil (black square) or waste cooking oil (red circle) by P. aphidisdissolved in ultrapure water at different concentrations at 25°C; (b) effect of pH (2.00–12.00) on the surface tension of crude MELs; (c) effect of temperature (−20.0°C to 95.0°C) with different treatment times (1 or 2 hr) on the surface tension of crude MELs; (d) effect of sodium chloride concentration (m/v, 0.0%–30.0%) on the surface tension of ultrapure water and crude MEL solutions. Samples were prepared with a concentration of 20.00 mg/ml, and measurements were done at 25°C. The reference surface tension value was 71.97 mN/m. Results represent the average of three independent measurements
3.4.2. The stability of MELs to pH, temperature, and sodium chloride solution
To evaluate the pH effect on stability of MELs from different substrates, the surface tension of several MEL samples prepared with a concentration of 20.00 mg/ml at different pH values (2.00–12.00) was determined. As can be seen from Figure 6b, the surface tension values were between 30.00 and 35.00 mN/m as pH less than 10.00. However, if pH is higher than 10.00, the surface tension rapidly rises up to about 60.00 mN/m, which indicated that MELs from soybean oil or waste cooking oil were unsuitable at high alkali environmental condition. As presented in Figure 6c, both MELs produced from soybean oil and waste cooking oil remain the unaltered activity after incubation for 2 hr at −20°C, 0°C, 50°C, 65°C, 80°C, 95°C, and room temperature. Therefore, it can be concluded that the surface properties of MELs are stable within −20°C to 95°C.
Further, the salt tolerance of MELs was explored, as the results presented in Figure 6d, MELs from soybean oil and waste cooking oil showed constant surface tensions and even a downward trend as the salt concentration increases, which indicated that both of MELs retained good surface activity at high salt environment.
4. DISCUSSIONS
The aim of this work was to investigate the use of waste cooking oil for MEL production by P. aphidis ZJUDM34. The technical feasibility of the transforming waste cooking oil into MELs was demonstrated. The novel fermentation strategy for MEL production was developed by optimization using RSM. The novel MELs were further characterized through the comparative analyses of its surface property to that produced from soybean oil. In term of the fermentation capability and the major properties, it is obvious that the replacement of soybean oil with waste cooking oil (WCO) is feasible for MEL production. The choice of cheap raw materials is vital to overall economics of the fermentation process since they account for nearly 50% of final production cost (Makkar & Cameotra, 1999). Moreover, in contrast to waste cooking oil, the average price of soybean oil was up to about three times (Yaakob, Mohammad, Alherbawi, Alam, & Sopian, 2013). The finding of this study showed the complete replacement of fermentation substrate with waste oil for MEL production is feasible and economic.
In consideration of the reuse of waste used oil, the industrial significance is rather high. Presently, it was reported that world production of oils and fats is above 200 million tonnes, 81% of which are derived from plants (Smith, 2004). Most of the oils and fats are used in the food industry and other consumed factories, which generates great quantities of waste oils. Naturally, the disposal of waste cooking oil is a growing problem, which explains the increasing interest in the use of waste oils for microbial transformation. Fleurackers (2006) reported the use of waste frying oils for the production of sophorolipids at 50 g/L medium by Candida bombicola, which was comparable to the yield of more commonly used carbon sources such as oleic acid. After then, rhamnolipids were successfully produced by Pseudomonas aeruginosa grown on various waste frying oils, which indicated soybean waste frying oil was the best substrate, produced 9.3 g/L under batch‐fed cultivation with the addition of rhamnolipid precursor (Raza et al., 2006). In this work, we found that P. aphidis ZJUDM34 can transform waste cooking oil into MELs, and the yield of MELs is close to that grown on soybean oil substrate at the optimized culture conditions and higher than the yields of sophorolipids and rhamnolipids.
Generally, the fatty acid compositions in MEL structure determine the characterization of MELs in actual application. The total fatty acid profile of purified MELs from WCO showed that fatty acid groups mainly composed of C18:n, followed by C16:0 (Table 4). Such profile was different from that found in MELs grown on soybean oil, preferentially C8:0 and C10:n, as also previously reported (Rau, Nguyen, Schulz et al., 2005). Meanwhile, the CMC values of MEL produced from soybean oil and WCO were both 20.00 mg/L. It implies that the use of WCO substrate represents the same characteristics as soybean oil in considering the surface property. Naturally, the replacement of major fermentation substrate with waste cooking oil remains the same chemical bioactivity.
In summary, this work shows that waste cooking oil can be used as an alternative substrate for MEL production from P. aphidis ZJUDM34, which helps to lower the production cost of MELs, facilitating its production and application in the commercial use.
ETHICAL STATEMENT
This study does not involve any human or animal testing.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ACKNOWLEDGMENTS
This study was financially supported by Public Projects of Zhejiang Province (LGF18C200003) and Nature Science Foundation of Zhejiang Province (LR13C200002), China.
Niu Y, Wu J, Wang W, Chen Q. Production and characterization of a new glycolipid, mannosylerythritol lipid, from waste cooking oil biotransformation by Pseudozyma aphidis ZJUDM34. Food Sci Nutr. 2019;7:937–948. 10.1002/fsn3.880
REFERENCES
- Arutchelvi, J. I. , Bhaduri, S. , Uppara, P. V. , & Doble, M. (2008). Mannosylerythritol lipids: A review. Journal of Industrial Microbiology & Biotechnology, 35, 1559–1570. 10.1007/s10295-008-0460-4 [DOI] [PubMed] [Google Scholar]
- Arutchelvi, J. , & Doble, M. (2011). Mannosylerythritol lipids: Microbial production and their applications In SOBER N ‐ CH VEZ, G (eds.), Biosurfactants (pp. 145–177). Berlin, Heidelberg, Germany: Springer‐Verlag. [Google Scholar]
- Bednarski, W. , Adamczak, M. , Tomasik, J. , & Plaszczyk, M. (2004). Application of oil refinery waste in the biosynthesis of glycolipids by yeast. Bioresource Technology, 95, 15–18. 10.1016/j.biortech.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Benincasa, M. , Contiero, J. , Manresa, M. A. , & Moraes, I. O. (2002). Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. Journal of Food Engineering, 54, 283–288. 10.1016/S0260-8774(01)00214-X [DOI] [Google Scholar]
- Dubey, K. , & Juwarkar, A. (2001). Distillery and curd whey wastes as viable alternative sources for biosurfactant production. World Journal of Microbiology & Biotechnology, 17, 61–69. [Google Scholar]
- Fan, L. , Li, H. , Niu, Y. , & Chen, Q. (2016). Characterization and inducing melanoma cell apoptosis activity of mannosylerythritol lipids‐A produced from Pseudozyma aphidis . PLoS ONE, 11, e0148198 10.1371/journal.pone.0148198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, L. , Xie, P. , Wang, Y. , Huang, Z. , & Zhou, J. (2018). Biosurfactant‐protein interaction: Influences of mannosylerythritol lipids‐A on beta‐glucosidase. Journal of Agriculture and Food Chemistry, 66, 238–246. [DOI] [PubMed] [Google Scholar]
- Faria, N. T. , Santos, M. , Ferreira, C. , Marques, S. , Ferreira, F. C. , & Fonseca, C. (2014). Conversion of cellulosic materials into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma spp. under SHF and SSF processes. Microbial Cell Factories, 13, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleurackers, S. J. J. (2006). On the use of waste frying oil in the synthesis of sophorolipids. European Journal of Lipid Science and Technology, 108, 5–12. 10.1002/ejlt.200500237 [DOI] [Google Scholar]
- Ghorbani, F. , Karimi, M. , Biria, D. , Kariminia, H. R. , & Jeihanipour, A. (2015). Enhancement of fungal delignification of rice straw by Trichoderma viride sp. to improve its saccharification. Biochemical Engineering Journal, 101, 77–84. 10.1016/j.bej.2015.05.005 [DOI] [Google Scholar]
- Gudina, E. J. , Teixeira, J. A. , & Rodrigues, L. R. (2010). Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei . Colloids and Surfaces B: Biointerfaces, 76, 298–304. 10.1016/j.colsurfb.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Gunther, M. , Grumaz, C. , Lorenz, S. , Stevens, P. , Lindemann, E. , Hirth, T. , … Rupp, S. (2015). The transcriptomic profile of Pseudozyma aphidis during production of mannosylerythritol lipids. Applied Microbiology and Biotechnology, 99, 1375–1388. 10.1007/s00253-014-6359-2 [DOI] [PubMed] [Google Scholar]
- Haba, E. , Espuny, M. J. , Busquets, M. , & Manresa, A. (2000). Screening and production of rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from waste frying oils. Journal of Applied Microbiology, 88, 379–387. 10.1046/j.1365-2672.2000.00961.x [DOI] [PubMed] [Google Scholar]
- Haskins, R. H. , Thorn, J. A. , & Booroyd, B. (1955). Biochemistry of the ustilaginales Xi. Metabolic products of ustilago zeae in submerged culture. Canadian Journal of Microbiology, 1, 749–756. [DOI] [PubMed] [Google Scholar]
- Hingu, S. M. , Gogate, P. R. , & Rathod, V. K. (2010). Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrasonics Sonochemistry, 17, 827–832. 10.1016/j.ultsonch.2010.02.010 [DOI] [PubMed] [Google Scholar]
- Im, J. H. , Nakane, T. , Yanagishita, H. , Ikegami, T. , & Kitamoto, D. (2001). Mannosylerythritol lipid, a yeast extracellular glycolipid, shows high binding affinity towards human immunoglobulin G. BMC Biotechnology, 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im, J. H. , Yanagishita, H. , Ikegami, T. , Takeyama, Y.‐I. , Idemoto, Y. , Koura, N. , & Kitamoto, D. (2003). Mannosylerythritol lipids, yeast glycolipid biosurfactants, are potential affinity ligand materials for human immunoglobulin G. Journal of Biomedical Materials Research Part A, 65, 379–385. 10.1002/jbm.a.10491 [DOI] [PubMed] [Google Scholar]
- Imura, T. , Ohta, N. , Inoue, K. , Yagi, N. , Negishi, H. , Yanagishita, H. , & Kitamoto, D. (2006). Naturally engineered glycolipid biosurfactants leading to distinctive self‐assembled structures. Chemistry, 12, 2434–2440. 10.1002/chem.200501199 [DOI] [PubMed] [Google Scholar]
- Isoda, H. , & Nakahara, T. (1997). Mannosylerythritol lipid induces granulocytic differentiation and inhibits the tyrosine phosphorylation of human myelogenous leukemia cell line K562. Cytotechnology, 25, 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto, D. (2008). Naturally engineered glycolipid biosurfactants leading to distinctive self‐assembling properties. Yakugaku Zasshi, 128, 695–706. 10.1248/yakushi.128.695 [DOI] [PubMed] [Google Scholar]
- Kitamoto, D. , Yanagishita, H. , Shinbo, T. , Nakane, T. , Kamisawa, C. , & Nakahara, T. (1993). Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica . Journal of Biotechnology, 29, 91–96. 10.1016/0168-1656(93)90042-L [DOI] [Google Scholar]
- Luo, Z. , Yuan, X.‐Z. , Zhong, H. , Zeng, G.‐M. , Liu, Z.‐F. , Ma, X.‐L. , & Zhu, Y.‐Y. (2013). Optimizing rhamnolipid production by Pseudomonas aeruginosa ATCC 9027 grown on waste frying oil using response surface method and batch‐fed fermentation. Journal of Central South University, 20, 1015–1021. 10.1007/s11771-013-1578-8 [DOI] [Google Scholar]
- Makkar, R. S. , & Cameotra, S. S. (1999). Biosurfactant production by microorganisms on unconventional carbon sources. Journal of Surfactants and Detergents, 2, 237–241. 10.1007/s11743-999-0078-3 [DOI] [Google Scholar]
- Mercadé, M. E. , Manresa, M. A. , Robert, M. , Espuny, M. J. , de Andrés, C. , & Guinea, J. (1993). Olive oil mill effluent (OOME). New substrate for biosurfactant production. Bioresource Technology, 43, 1–6. 10.1016/0960-8524(93)90074-L [DOI] [Google Scholar]
- Morita, T. , Fukuoka, T. , Imura, T. , & Kitamoto, D. (2013). Accumulation of cellobiose lipids under nitrogen‐limiting conditions by two ustilaginomycetous yeasts, Pseudozyma aphidis and Pseudozyma hubeiensis . FEMS Yeast Research, 13, 44–49. [DOI] [PubMed] [Google Scholar]
- Morita, T. , Fukuoka, T. , Imura, T. , & Kitamoto, D. (2013). Production of mannosylerythritol lipids and their application in cosmetics. Applied Microbiology and Biotechnology, 97, 4691–4700. [DOI] [PubMed] [Google Scholar]
- Noh, G. Y. , Suh, J. Y. , & Park, S. N. (2016). Ceramide‐based nanostructured lipid carriers for transdermal delivery of isoliquiritigenin: Development, physicochemical characterization, and in vitro skin permeation studies. Korean Journal of Chemical Engineering, 34, 400–406. [Google Scholar]
- Onghena, M. , Geens, T. , Goossens, E. , Wijnants, M. , Pico, Y. , Neels, H. , … Lemiere, F. (2011). Analytical characterization of mannosylerythritol lipid biosurfactants produced by biosynthesis based on feedstock sources from the agrofood industry. Analytical and Bioanalytical Chemistry, 400, 1263–1275. 10.1007/s00216-011-4741-9 [DOI] [PubMed] [Google Scholar]
- Rau, U. , Nguyen, L. A. , Roeper, H. , Koch, H. , & Lang, S. (2005). Downstream processing of mannosylerythritol lipids produced by Pseudozyma aphidis . European Journal of Lipid Science and Technology, 107, 373–380. [Google Scholar]
- Rau, U. , Nguyen, L. A. , Schulz, S. , Wray, V. , Nimtz, M. , Roeper, H. , … Lang, S. (2005). Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis . Applied Microbiology and Biotechnology, 66, 551–559. [DOI] [PubMed] [Google Scholar]
- Raza, Z. A. , Khan, M. S. , Khalid, Z. M. , & Rehman, A. (2006). Production kinetics and tensioactive characteristics of biosurfactant from a Pseudomonas aeruginosa mutant grown on waste frying oils. Biotechnology Letters, 28, 1623–1631. 10.1007/s10529-006-9134-3 [DOI] [PubMed] [Google Scholar]
- Safdel, M. , Anbaz, M. A. , Daryasafar, A. , & Jamialahmadi, M. (2017). Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renewable and Sustainable Energy Reviews, 74, 159–172. 10.1016/j.rser.2017.02.045 [DOI] [Google Scholar]
- Smith, G. (2004). CESIO 6th world surfactants congress. Journal of Surfactants and Detergents, 7, 337–341. [Google Scholar]
- Wakamatsu, Y. , Zhao, X. , Jin, C. , Day, N. , Shibahara, M. , Nomura, N. , … Yokoyama, K. K. (2001). Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK‐related signal cascade. European Journal of Biochemistry, 268, 374–383. 10.1046/j.1432-1033.2001.01887.x [DOI] [PubMed] [Google Scholar]
- Włodzimierz Bednarski, M. N. , Adamczak, M. , & Nawotka, R. (2006). Carbon‐source‐dependent synthesis and composition of biosurfactant synthesized by Pseudozyma antarctica . Environmental Biotechnology, 2, 31–36. [Google Scholar]
- Yaakob, Z. , Mohammad, M. , Alherbawi, M. , Alam, Z. , & Sopian, K. (2013). Overview of the production of biodiesel from waste cooking oil. Renewable and Sustainable Energy Reviews, 18, 184–193. 10.1016/j.rser.2012.10.016 [DOI] [Google Scholar]
- Yu, M. , Liu, Z. , Zeng, G. , Zhong, H. , Liu, Y. , Jiang, Y. , … He, Y. (2015). Characteristics of mannosylerythritol lipids and their environmental potential. Carbohydrate Research, 407, 63–72. 10.1016/j.carres.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Wang, Q. , & Mortimer, S. R. (2012). Waste cooking oil as an energy resource: Review of Chinese policies. Renewable and Sustainable Energy Reviews, 16, 5225–5231. 10.1016/j.rser.2012.05.008 [DOI] [Google Scholar]
- Zhang, J. , Dong, Y. C. , Fan, L. L. , Jiao, Z. H. , & Chen, Q. H. (2015). Optimization of culture medium compositions for gellan gum production by a halobacterium Sphingomonas paucimobilis . Carbohydrate Polymers, 115, 694–700. 10.1016/j.carbpol.2014.09.029 [DOI] [PubMed] [Google Scholar]
- Zhao, X. , Geltinger, C. , Kishikawa, S. , Ohshima, K. , Murata, T. , Nomura, N. , … Yokoyama, K. K. (2000). Treatment of mouse melanoma cells with phorbol 12‐myristate 13‐acetate counteracts mannosylerythritol lipid‐induced growth arrest and apoptosis. Cytotechnology, 33, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Murata, T. , Ohno, S. , Day, N. , Song, J. , Nomura, N. , … Yokoyama, K. K. (2001). Protein kinase C alpha plays a critical role in mannosylerythritol lipid‐induced differentiation of melanoma B16 cells. Journal of Biological Chemistry, 276, 39903–39910. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Gan, J.‐J. , Zhang, G.‐L. , Yao, B. , Zhu, W.‐J. , & Meng, Q. (2007). Reuse of waste frying oil for production of rhamnolipids using Pseudomonas aeruginosa zju.u1M. Journal of Zhejiang University Science A, 8, 1514–1520. 10.1631/jzus.2007.A1514 [DOI] [Google Scholar]


