Abstract
Background/Aim
Nobiletin, a major polymethoxyflavones (PMFs) from citri reticulatae pericarpium (CRP), can inhibit several forms of cancer proliferation. However, the effects of nobiletin on nasopharyngeal carcinoma (NPC) C666‐1 cells remain largely unknown.
Materials and Methods
Cell counting kit 8 (CCK8) assay was used to measure cell vitality. Flow cytometry was performed to measure the apoptosis rate. Quantitative real‐time polymerase chain reaction (qRT‐PCR) and Western blot analysis were applied to determine the expression of mRNA and protein, respectively.
Results
We showed that the proliferation rate of C666‐1 cells was inhibited and the apoptosis rate was raised after treating with nobiletin. Moreover, nobiletin inhibited the expression of poly(ADP‐ribose)polymerase‐2 (PARP‐2), and the tumor suppression effect of nobiletin on C666‐1 is associated with PARP‐2‐dependent pathway.
Conclusion
We demonstrated for the first time that nobiletin inhibited the growth of C666‐1 cells, which may be relative to its regulation on PARP‐2/SIRT1/AMPK signaling pathway. Our result implied that nobiletin may serve as a strategy to treat nasopharyngeal carcinoma.
Keywords: AMPK, apoptosis, C666‐1, nobiletin, PARP‐2
1. INTRODUCTION
Nasopharyngeal carcinoma (NPC), a distinctive vicious tumor derives from the epithelium of nasopharynx, is the cancer of the highest prevalence in South‐East Asia, especially in southern China (Chen et al., 2010; Kwok et al., 2014). NPC is closely related to Epstein‐Barr virus, which has been regarded as one of the major etiologic factors of NPC (Xiao et al., 2016). In its early stages, NPC is likely to be undetected because it is usually asymptomatic or only presents with clinically insignificant symptoms, which leads to late detection and therapy (Voon et al., 2015). Although radiotherapy and/or chemotherapy have been shown to be effective therapeutic methods for NPC and are able to improve the survival rate, adverse effects and recurrence were also not uncommon (Chan et al., 2015).
Nasopharyngeal carcinoma (NPC), a distinctive vicious tumor derives from the epithelium of nasopharynx, is the cancer of the highest prevalence in South‐East Asia, especially in southern China (Chen et al., 2010; Kwok et al., 2014). NPC is closely related to Epstein‐Barr virus, which has been regarded as one of the major etiologic factors of NPC (Xiao et al., 2016). In its early stages, NPC is likely to be undetected because it is usually asymptomatic or only presents with clinically insignificant symptoms, which leads to late detection and therapy (Voon et al., 2015). Although radiotherapy and/or chemotherapy have been shown to be effective therapeutic methods for NPC and are able to improve the survival rate, adverse effects and recurrence were also not uncommon (Chan et al., 2015).
Nasopharyngeal carcinoma (NPC), a distinctive vicious tumor derives from the epithelium of nasopharynx, is the cancer of the highest prevalence in South‐East Asia, especially in southern China (Chen et al., 2010; Kwok et al., 2014). NPC is closely related to Epstein‐Barr virus, which has been regarded as one of the major etiologic factors of NPC (Xiao et al., 2016). In its early stages, NPC is likely to be undetected because it is usually asymptomatic or only presents with clinically insignificant symptoms, which leads to late detection and therapy (Voon et al., 2015). Although radiotherapy and/or chemotherapy have been shown to be effective therapeutic methods for NPC and are able to improve the survival rate, adverse effects and recurrence were also not uncommon (Chan et al., 2015).
Poly(ADP‐ribose)polymerases (PARPs) are a family of enzymes that catalyze poly(ADP‐ribosyl)ation (PARylation) conserving widespread and highly post‐translational modification (Ali, Khan, Galindo‐Campos, & Yélamos, 2016). Among the 18 members identified so far, poly(ADP‐ribose)polymerase‐1 (PARP‐1) and poly(ADP‐ribose)polymerase‐2 (PARP‐2) are the only proteins stimulated by DNA strand breaks and implicated in the repair of DNA injury (Mégnin‐Chanet, Bollet, & Hall, 2010). Sirtuin 1 (SIRT1), a NAD+‐dependent histone deacetylase, is the important downstream target of PARP (Vida, Márton, Mikó, & Bai, 2017). During the last decades, PARP/SIRT1 signaling pathway was extensively studied in metabolic disorders and evidence has suggested its implication in cancer cell biology (Mégnin‐Chanet et al., 2010). Moreover, PARP‐1 inhibition has been reported to reduce the proliferation and promote radiation sensitization in CNE‐2 human NPC cells, which suggested that PARP may be an attractive target for the cancer therapy including NPC (Chen, Zhao, et al., 2015).
Many natural products are the potential antitumor drugs because of its low toxicity and few side effects (Mohamed, Jantan, & Haque, 2017; Park et al., 2017). Citri reticulatae pericarpium (CRP), which is prepared from sundried citrus peel, possesses the functions of anti‐inflammation, anticancer, and cardiovascular protection as a traditional Chinese medicine (Fu et al., 2017; Yi, Ma, & Ren, 2017). Apart from essential oil, flavonoids are another primary biological active constituents of CRP and are categorized into flavonoid glycosides and polymethoxyflavones (PMFs) (Luo et al., 2018; Zheng et al., 2013). PMFs consist of nobiletin, tangeretin, 3,5,6,7,8,3′,4′‐heptamethoxyflavone, 5‐hydroxy‐6,7,8,3′,4′‐pentamethoxyflavone (Luo et al., 2018). Nobiletin (Figure 1A), with the highest abundance in PMFs, has attracted extensive attention due to its bioactive effects. Through the last decade, multiple antidisease effects of nobiletin have been discovered, especially its antitumor capability. Nobiletin can be used to treat glioblastoma due to its ability to inhibit the proliferation and migration of glioma cells (Lien et al., 2016). It can also be used as prevention for triple‐negative breast cancer since it can blockade the cell cycle at G0/G1 phase and induce cell apoptosis (Chen, Ono, Takeshima, & Nakano, 2014). Moreover, nobiletin‐induced apoptosis in SNU‐16 cells is regulated via intracellular endoplasmic reticulum stress‐mediated protective autophagy (Moon & Cho, 2016). It had been reported that nobiletin can inhibit the invasion and migration of HONE‐1 and NPC‐BM, the human NPC cell lines (Chien, Hsieh, Chen, Yang, & Chen, 2015). Other than the rest of NPC‐derived cell lines, C666‐1 is the exclusive one that still retains the natural EBV. Thus, it has become an important and representative tool to evaluate the antitumor activity of NPC (Chan et al., 2015). Nevertheless, the effect of nobiletin on human NPC C666‐1 cells and its molecular mechanism are still unclear. The aim of this study is to determine whether nobiletin induces C666‐1 cell apoptosis by regulating PARP‐dependent signaling pathways.
Figure 1.
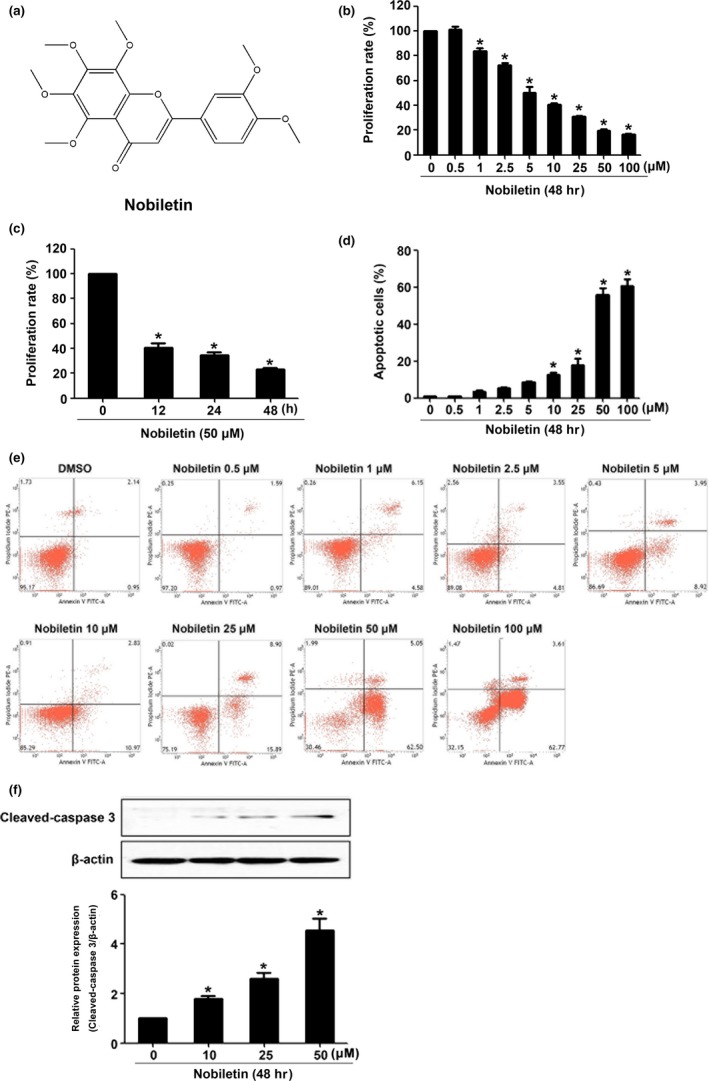
Nobiletin inhibited the cell viability and induced apoptosis in C666‐1 cells. The structure of nobiletin is shown in (A). C666‐1 cells were treated with gradient concentrations of nobiletin for 48 hr or with 50 μM nobiletin for indicated time periods. The cell proliferation rate was detected using CCK8 assay (B and C). The apoptosis rate was detected using flow cytometry (D and E). The expression of cleaved‐caspase 3 was measured with Western blot (F). *p < 0.05 vs. the group without treatment, n = 3
2. MATERIALS AND METHODS
2.1. Materials and reagents
Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco (Logan, UT, USA). Fetal bovine serum (FBS) was purchased from Yeasen Biotechnology Co., Ltd. Cell counting kit 8 (CCK8) was purchased from DOJINDO (Japan). Antibodies against cleaved‐caspase 3, p‐AMPK, p‐S6, p‐P70S6K, and SIRT1 were purchased from Cell Signaling Technology (USA); anti‐AMPK and β‐actin were purchased from Santa Cruz Biotech (USA). Antibody against PARP‐2 was purchased from Abcam (UK). Nobiletin was purchased from Chengdu Must Bio‐Technology Co., Ltd (Sichuan, China) and dissolved in dimethyl sulfoxide and stored at −20°C until diluted upon use. Recombinant adenovirus vectors expressing green fluorescent protein (Ad‐Flag) and Flag‐tagged PARP‐2 (Ad‐PARP‐2) were purchased from Genechem Co., Ltd. (Shanghai, China). The viruses were extended in HEK293A cells and purified by virus purification kit (Biomiga, USA), then dialyzed in dilution buffer and stored at −80°C.
2.2. Cell culture
C666‐1 cells were cultured in DMEM supplemented with appropriate proportion of FBS at 37°C in humid incubator contains 95% air and 5% CO2. Medium was changed every other day. Cells were digested with trypsin (0.25%) and then subcultured when reached 80%–90% confluence. Before treated with nobiletin, cells were cultured in FBS free for 12 hr.
C666‐1 cells were cultured in DMEM supplemented with appropriate proportion of FBS at 37°C in humid incubator contains 95% air and 5% CO2. Medium was changed every other day. Cells were digested with trypsin (0.25%) and then subcultured when reached 80%–90% confluence. Before treated with nobiletin, cells were cultured in FBS free for 12 hr.
2.3. Cell viability assay
CCK8 was used to detect the cell viability with method described in previous study (Cai, Hong, Zhao, Yue‐Peng, & Qin, 2015). C666‐1 cells were seeded in 96‐well plates with a density of 1 × 104 per well, and circumjacent wells were filled with aseptic phosphate‐buffered saline (PBS). After being cultured in complete medium for 24 hr, the cells changed to FBS‐free medium for another 12 hr at 37°C. Then, cells were treated with nobiletin at the indicated concentration (0, 0.5, 1, 2.5, 5, 10, 25, 50, and 100 μM) or with 50 μM after overexpression of PARP‐2 for 48 hr. CCK8 solution was added 10 μl per well and incubated for 1 hr, followed by reading the absorbance at 450 nm using a Multi‐Volume Spectrophotometer System (BioTek Instruments, Inc., USA).
2.4. Flow cytometry
To observe the cell apoptosis, an Annexin V‐FITC Apoptosis Detection Kit (BioVision, USA) and flow cytometry were applied simultaneously with the same protocol as of the previous research (Cai, Zhao, Qin, & He, 2015). Briefly, after 5 × 105 cells reached about 60% confluence, the cells were treated with different concentrations of nobiletin (0, 0.5, 1, 2.5, 5, 10, 25, 50, 100 μM) or treated with 50 μM after overexpression of PARP‐2 for 48 hr. Then, both suspending and adherent cells were collected and resuspended in binding buffer. After the addition of Annexin V‐FITC and PI, the mixture was incubated in the dark at room temperature for 5 min and analyzed immediately with flow cytometer (BD FACSVerse™) and BD FACSuite software.
2.5. Western blot analysis
As described previously (Cai, Zhao, Qin, Zhang, & He, 2015), C666‐1 cells (5 × 105 per dish) were seeded into 60‐mm dishes. After incubating for 24 hr, cells were treated nobiletin in different concentrations (0, 10, 25, 50 μM) or treated with 50 μM after overexpression of PARP‐2 for 48 hr. Cells were harvested with detergent‐containing lysis buffer, and protein was separated after 30 min of incubation on ice. Protein concentration was determined with BCA Protein Assay Kit (Thermo Fisher Scientific). Same amount of protein (20 μg per lane) was separated using 12% SDS‐PAGE gel and then transferred to PVDF membrane. The membrane was blocked with 5% nonfat milk in 1× PBST (maybe TBST) for 1 hr at room temperature, then incubated with primary antibodies overnight at 4°C. All primary antibodies were diluted into 1:1,000 upon use. Next, membranes were incubated in anti‐mouse/rabbit secondary antibodies for 1 hr at room temperature. At last, the blotted membranes were visualized by enhanced chemiluminescent (ECL) method and films were then developed.
2.6. Quantitative real‐time polymerase chain reaction
Total RNA from C666‐1 cells was extracted with TRIzol, and PrimeScript RT Reagent Kit (Takara Bio Inc., Japan) was used for reverse transcription. PCR primers were designed using the sequences shown in Table 1. mRNA concentrations were measured using Quantitative PCR Kit (Takara Biotechnology) by iCycler iQ system (Bio‐Rad). GAPDH was used as endogenous control. All PCRs were performed in triplicate.
Table 1.
Primer sequences for qRT‐PCR
| Primer | Sequences |
|---|---|
| PARP‐1 |
Forward: 5′‐CTAAAGGCTCAGAACGACC‐3′ Reverse: 5′‐GAAGGAGGGCACCGAACA‐3′ |
| PARP‐2 |
Forward: 5′‐ACAGTGGCACAAATCAAG‐3′ Reverse: 5′‐TACGGAGTCCAAAGTCAT‐3′ |
| SIRT1 |
Forward: 5′‐CTTGTACGACGAAGACGA‐3′ Reverse: 5′‐TCACCGAACAGAAGGTTAT‐3′ |
| GAPDH |
Forward: 5′‐AGGAGTAAGAAACCCTGGAC‐3′ Reverse: 5′‐CTGGGATGGAATTGTGAG‐3′ |
2.7. Statistical analysis
Each result was repeated at least for three times. All data were expressed as means ± SD. Unpaired Student's t test was performed for the statistical analyses between two groups. The analyses among different groups were carried out with one‐way analysis of variance (ANOVA). In general, statistically significant was defined as p < 0.05.
3. RESULTS
3.1. Nobiletin suppressed the proliferation of C666‐1 cells
The cytotoxicity of nobiletin to C666‐1 cells was detected. C666‐1 cells were treated with nobiletin of gradient concentrations (0, 0.5, 1, 2.5, 5, 10, 25, 50, or 100 μM) for 48 hr, or 50 μM nobiletin was used to treat C666‐1 cells for different time periods (0, 12, 24, or 48 hr), then cell viability was evaluated with CCK8 assay. As shown in Figure 1B,C, the number of survival cells decreased in a dose‐ and time‐dependent manner.
3.2. Nobiletin‐induced apoptosis in C666‐1 cells
To clarify whether nobiletin affects cell apoptosis, changes in C666‐1 cells were observed through flow cytometry analysis. After being treated with different doses of nobiletin (0, 0.5, 1, 2.5, 5, 10, 25, 50, 100 μM), the apoptosis rates of C666‐1 cells were detected with flow cytometry. As shown in Figure 1D,E, the percentage of remaining C666‐1 cells was obviously decreased as the concentration of nobiletin increased. Therefore, we concluded that nobiletin induces the apoptosis in C666‐1 cells in a dose‐dependent manner. In addition, cleaved‐caspase 3 was also decreased by nobiletin treatment in a dose‐dependent manner (Figure 1F).
3.3. Nobiletin inhibited the expression of PARP‐2
As shown in Figure 1, the nobiletin‐induced apoptosis had obvious variation, while the dose was larger than 10 μM. We also noticed that the effect of nobiletin in 50 and 100 μM is indistinctive. Thus, 0, 10, 25, and 50 μM were selected to perform the following experiments. The results in Figure 2A indicated that mRNA expression of PARP‐2 was decreased after being treated with nobiletin in gradient concentration while the mRNA expression of PARP‐1 remained the same. In addition, as shown in Figure 2B, the expression of PARP‐2 was downregulated by nobiletin in a dose‐dependent manner.
Figure 2.
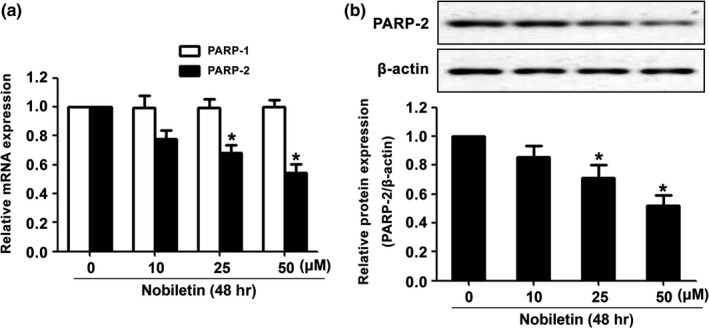
The expression of PARP‐1 and PARP‐2 mRNA and the expression of PARP‐2 protein after being treated with different concentrations of nobiletin. C666‐1 cells were treated with increasing concentrations of nobiletin for 48 hr. The expression of PARP‐1 and PARP‐2 mRNA was measured by qRT‐PCR (A). The protein expression of PARP‐2 protein was detected by Western blot (B). β‐Actin was used as control. *p < 0.05 vs. the group without treatment, n = 3
As shown in Figure 1, the nobiletin‐induced apoptosis had obvious variation, while the dose was larger than 10 μM. We also noticed that the effect of nobiletin in 50 and 100 μM is indistinctive. Thus, 0, 10, 25, and 50 μM were selected to perform the following experiments. The results in Figure 2A indicated that mRNA expression of PARP‐2 was decreased after being treated with nobiletin in gradient concentration while the mRNA expression of PARP‐1 remained the same. In addition, as shown in Figure 2B, the expression of PARP‐2 was downregulated by nobiletin in a dose‐dependent manner.
3.4. PARP‐2 overexpression attenuated the nobiletin‐induced apoptosis
To explore the function of PARP‐2 in nobiletin‐induced apoptosis, C666‐1 cells were transfected with PARP‐2. Overexpression of PARP‐2 significantly increased the mRNA and protein level of PARP‐2 (Supporting Information Figure S1A,B). Next, we measured the apoptosis rate and apoptosis‐related protein expression. As shown in Figure 3A,B, nobiletin treatment led to significant increase in cell apoptosis, which could be attenuated by preincubation with PARP‐2 overexpression for 24 hr and subsequently treated with nobiletin for 24 hr. Moreover, PARP‐2 overexpression also increased the proliferation rate of C666‐1, which was inhibited by nobiletin (Figure 3C). Western blotting analysis also showed that PARP‐2 could inhibit the protein level of cleaved‐caspase 3 induced by nobiletin (Figure 3D).
Figure 3.
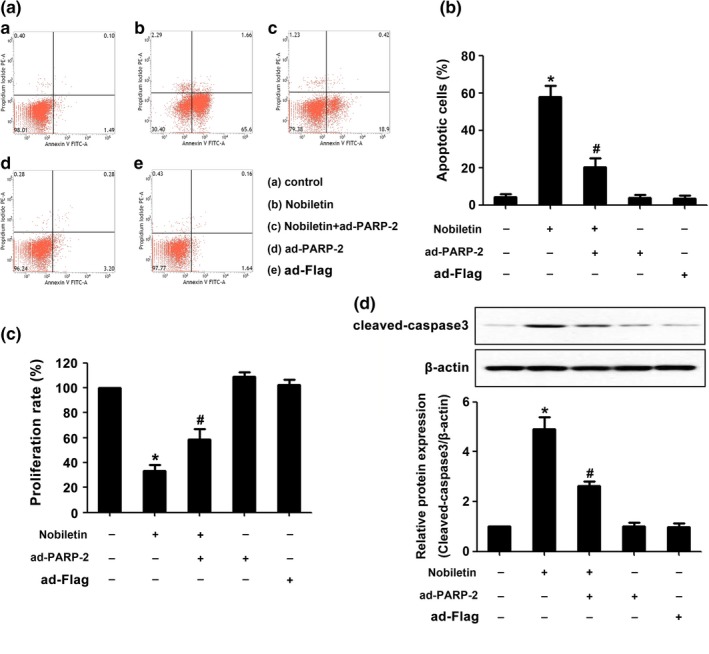
The overexpression of PARP‐2 attenuated the effect of nobiletin on C666‐1 cells. Before being treated with 50 μM nobiletin, C666‐1 cells have been pretreated with PARP‐2 for 1 hr. The groups treated with nobiletin or PARP‐2 only are the positive controls, and the group treated with DMSO is the negative control. After being treated for 48 hr, the cells were applied to perform flow cytometry (A–C) or protein extraction for Western blot of cleaved‐caspase 3 and β‐actin (D). *p < 0.05 vs. the group without treatment, n = 3. # p < 0.05 vs. the group treating with nobiletin, n = 3
3.5. PARP‐2 inhibited expression of SIRT1 induced by nobiletin
The mechanism of how PARP‐2 inhibits nobiletin‐induced apoptosis is unclear. SIRT1 is the important downstream target of PARP. So, we examined the mRNA and protein level of SIRT1. As shown in Figure 4A–D, PARP‐2 overexpression could inhibit the mRNA and protein expression of SIRT1 induced by nobiletin.
Figure 4.
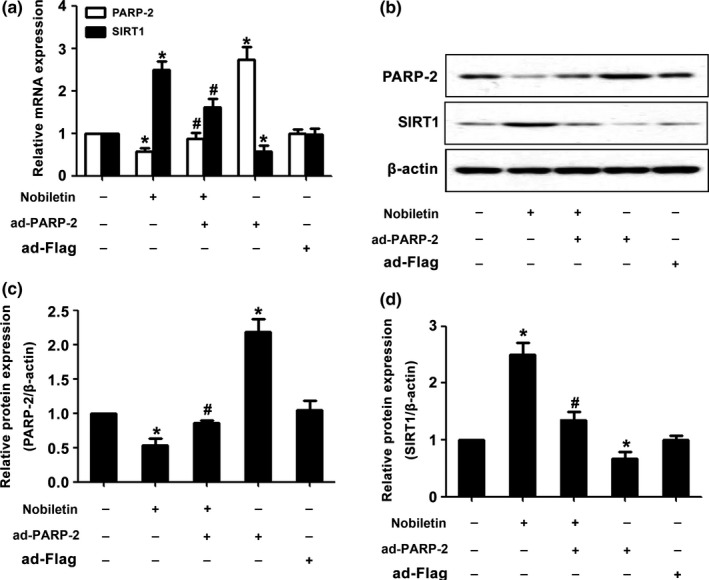
PARP‐2 overexpression reduced the level of SIRT1 induced by nobiletin. Before being treated with 50 μM nobiletin, C666‐1 cells have been pretreated with PARP‐2 for 1 hr. The groups treated with nobiletin or PARP‐2 only are the positive controls, and the group treated with DMSO is the negative control. After being treated for 48 hr, mRNA and protein were extracted to measure the expression of PARP‐2 and SIRT1 (A–D). *p < 0.05 vs. the group without treatment, n = 3. # p < 0.05 vs. the group treating with nobiletin, n = 3
3.6. Nobiletin‐activated SIRT1/AMPK signaling pathways
We then detected the expression of SIRT1 and its downstream AMP‐activated protein kinase (AMPK)‐dependent signaling pathways. As shown in Figure 5A–E, the SIRT1 expression level and the phosphorylation of AMPK (p‐AMPK) were upregulated. Moreover, the expression of p‐p70S6K and p‐S6 was both downregulated along with AMPK.
Figure 5.
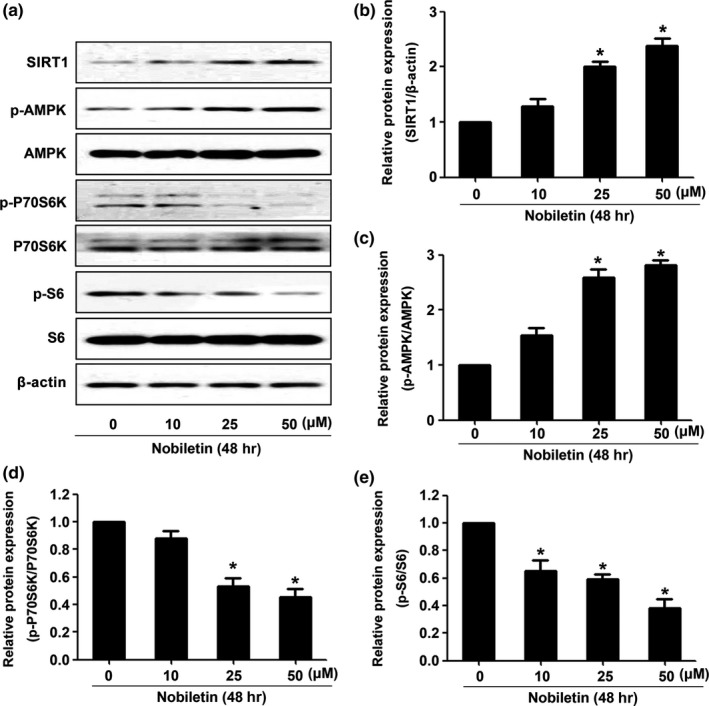
Nobiletin regulated SIRT1/AMPK/mTOR signaling pathways. After being treated with different concentrations of nobiletin, protein was extracted to examine the expression of SIRT1, p‐AMPK, AMPK, p‐P70S6K, P70S6K, p‐S6, and S6 (A–E). *p < 0.05 vs. the group without treatment, n = 3
4. DISCUSSION
In recent years, it has been discovered that nobiletin could inhibit the adhesion, epithelial–mesenchymal transition, metastasis, and invasion of lung cancer (Da, Liu, Zhan, Liu, & Wang, 2016). Moreover, the effect of nobiletin on phosphorylated Akt is the potential method to selectively inhibit ovarian cancer cell proliferation (Chen, Chen, et al., 2015). However, there is little evidence to support the use of nobiletin against NPC. In the present study, we focused on the antiproliferative effect of nobiletin on C666‐1 cells and our results indicated that nobiletin significantly inhibited the viability of NPC C666‐1 cells. Apoptosis, as a programmed cell death, has been known to play a crucial role in obliterating the abnormally proliferated cells (Fan, Yang, & Bi, 2015). Caspase‐3, an enzyme that could cleave most of the caspase substrates in apoptosis pathway, is essential for apoptosis. Activated caspase 3 was regarded as the pivotal slayer to induce cell death in apoptosis (Wu et al., 2016). In the present study, the upregulation of cleaved‐caspase 3 indicated that nobiletin could induce apoptosis in C666‐1 cells. This is also consistent with the result of flow cytometry.
The mechanism why nobiletin mediates its anticancer effects in C666‐1 cells remains unclear. PARPs catalyze a reaction in which the ADP‐ribose moiety of NAD+ is transferred to a receptor amino acid, building poly(ADP‐ribose) (PAR) polymers (Mégnin‐Chanet et al., 2010). PARP‐1, the founding member of the PARP superfamily, has been demonstrated to regulate the growth of various tumor cells, such as breast cancer, ovarian cancer, and NPC (Chen, Zhao, et al., 2015; Franzese et al., 2019; Nur Husna, Tan, Mohamud, Dyhl‐Polk, & Wong, 2018). PARP‐2 possesses a catalytic domain structurally similar to PARP‐1, whereas it is less active than PARP‐1 (Ali et al., 2016). Recently, a growing body of evidence also showed that PARP‐2 inhibition served to resist tumor growth via induction of chromosome mis‐segregation, exacerbation of replication stress, and dysregulation of cancer epigenetics, which suggested that PARPs, especially PARP‐1 and PARP‐2, may be an attractive target for cancer therapy (Mégnin‐Chanet et al., 2010). In this study, we showed that nobiletin inhibited the mRNA and protein level of PARP‐2. Moreover, we found that PARP‐2 overexpression could abolish partly the effect of nobiletin on C666‐1 cells, which suggested PARP‐2 may participate in the growth inhibition and apoptosis induced by nobiletin in C666‐1 cells.
SIRT1, a NAD+‐dependent histone deacetylase, is the important downstream target of PARP (Vida et al., 2017). PARP‐1 and PARP‐2 regulate SIRT1 via different mechanisms. PARP‐1 increase SIRT1 activity indirectly through the modulation of NAD+ levels (Pinton et al., 2013). Instead, PARP‐2 is found to bind directly to the SIRT1 proximal promoter where it acts to negatively regulate SIRT1 expression (Chung & Joe, 2014). SIRT1 involves in cancer progression already obtaining cumulative evidence, but its exact role in carcinogenesis remains controversial. Studies showed that silencing SIRT1 inhibited cell proliferation and tumor formation in some human cancer cell lines such as non‐small‐cell lung cancer and breast cancer (Abdolvahabi et al., 2018; Xu et al., 2018). But another studies claimed that SIRT1 inhibits tumor progression and invasion in human gastric cancer cell lines (Dong et al., 2018). Thus, it remains controversial whether SIRT1 acts as a tumor promoter or suppressor. In this present study, we found that nobiletin could increase the protein level of SIRT1, which suggested that SIRT1 activation may inhibit the growth of C666‐1 cells.
AMPK, a key regulator of energy metabolism, is one of the substrates of SIRT1 and activated by phosphorylation (Cheng et al., 2018; You, Cheng, Yu, Duan, & Peng, 2018). Besides, AMPK is reported to be related to cell cycle and apoptosis (Shrestha et al., 2016). Moreover, a large number of studies have shown that multiple anticancer agents could activate AMPK‐dependent cell death pathways (An et al., 2014; Gao, Ge, & Sun, 2019). AMPK may induce cancer cell death via regulating multiple downstream signal targets, including in‐activating cancer‐promoting mammalian target of rapamycin (mTOR) signaling (Chen, Zhao, et al., 2015). Recent study indicated that 4‐O‐methyl‐ascochlorin induced the human leukemia cells by suppressing c‐Myc protein synthesis via an AMPK/mTOR‐dependent mechanism (Shin et al., 2016). In addition, AMPK knocking down prevents capsaicin‐induced cell death in hepatocellular carcinoma cells (Bort, Spínola, Rodríguez‐Henche, & Díaz‐Laviada, 2017). Our previous study also demonstrated that resveratrol could inhibit the growth of NPC cell line C666‐1 through AMPK activation, which means AMPK could be a therapeutic target in NPC (Cai, Zhao, Qin, Zhang, et al., 2015). Consistent with our previous studies, we also showed that nobiletin could activate AMPK and inhibit mTOR signaling, as manifested by dephosphorylation of P70S6K and S6 in C666‐1 cells.
In conclusion, this study revealed that nobiletin has the ability to significantly inhibit the proliferation of and induce the apoptosis in C666‐1 cells. PARP‐2/SIRT1/AMPK signaling pathway might be the potential molecular mechanism underlying nobiletin‐induced apoptosis (Figure 6). Therefore, we suggest nobiletin as a strategy to treat NPC.
Figure 6.
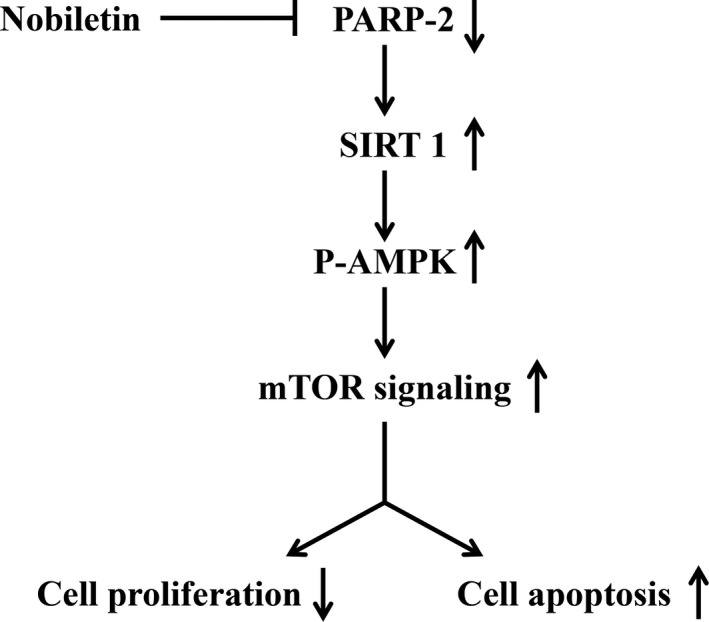
Model for nobiletin inhibiting proliferation and inducing apoptosis. Nobiletin inhibits PARP‐2 level, which activates SIRT1, regulates the downstream AMPK‐mTOR signaling, and suppresses the growth of C666‐1 cell
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
This study does not involve any human or animal testing.
Supporting information
ACKNOWLEDGMENTS
This study was supported by National Key R&D Program of China (NO. 2017YFC1701103), National Modern Agricultural Industrial Park of China (NO. njf [2017] 110), Natural Science Foundation of Guangdong Province (NO. 2017A030313571), National Natural Science Foundation of China (NO. 31401613), Cultivation Plan for High‐level University Academic Backbone of Guangzhou Medical University in 2017 (NO. gydf [2017] 210), and the Major Science and Technology Programs of Guangdong Province (NO. 2015B020225006). This work had been obtained help from Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat‐Sen Memorial Hospital, Sun Yat‐Sen University.
Zheng GD, Hu PJ, Chao YX, et al. Nobiletin induces growth inhibition and apoptosis in human nasopharyngeal carcinoma C666‐1 cells through regulating PARP‐2/SIRT1/AMPK signaling pathway. Food Sci Nutr. 2019;7:1104–1112. 10.1002/fsn3.953
Contributor Information
Xi Yong Yu, Email: yuxycn@aliyun.com.
Yi Cai, Email: yicaisysu@163.com.
REFERENCES
- Abdolvahabi, Z. , Nourbakhsh, M. , Hosseinkhani, S. , Hesari, Z. , Alipour, M. , Jafarzadeh, M. , … Golpour, P. (2018). MicroRNA‐590‐3P suppresses cell survival and triggers breast cancer cell apoptosis via targeting sirtuin‐1 and deacetylation of p53. Journal of Cellular Biochemistry. 10.1002/jcb.28211 [DOI] [PubMed] [Google Scholar]
- Ali, S. O. , Khan, F. A. , Galindo‐Campos, M. A. , & Yélamos, J. (2016). Understanding specific functions of PARP‐2: New lessons for cancer therapy. American Journal of Cancer Research, 6, 1842–1863. [PMC free article] [PubMed] [Google Scholar]
- An, H. , Kim, K. , Lee, J. , Park, M. , Moon, H. , Park, S. , … Lee, Y. (2014). Mimulone‐induced autophagy through p53‐mediated AMPK/mTOR pathway increases caspase‐mediated apoptotic cell death in A549 human lung cancer cells. PLoS ONE, 9, e114607 10.1371/journal.pone.0114607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort, A. , Spínola, E. , Rodríguez‐Henche, N. , & Díaz‐Laviada, I. (2017). Capsaicin exerts synergistic antitumor effect with sorafenib in hepatocellular carcinoma cells through AMPK activation. Oncotarget, 8, 87684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. , Hong, H. , Zhao, L. , Yue‐Peng, C. , & Qin, Y. (2015). (‐)‐Epicatechin‐3‐gallate (a polyphenol from green tea) potentiates doxorubicin‐induced apoptosis in H9C2 cardiomyocytes. Biotechnology Letters, 37, 1937–1943. 10.1007/s10529-015-1879-0 [DOI] [PubMed] [Google Scholar]
- Cai, Y. , Zhao, L. , Qin, Y. , & He, Y. (2015). High dose of epigallocatechin‐3‐gallate inhibits proliferation and induces apoptosis of H9C2 cardiomyocytes through down‐regulation of SIRT1. Die Pharmazie, 70, 12–16. 10.1691/ph.2015.4717 [DOI] [PubMed] [Google Scholar]
- Cai, Y. , Zhao, L. , Qin, Y. , Zhang, M. , & He, Y. (2015). Resveratrol inhibits proliferation and induces apoptosis of nasopharyngeal carcinoma cell line C666‐1 through AMPK activation. Die Pharmazie, 70, 399–403. 10.1691/ph.2015.4815 [DOI] [PubMed] [Google Scholar]
- Chan, K. C. , Chan, L. S. , Ip, J. C. Y. , Lo, C. , Yip, T. T. C. , Ngan, R. K. C. , … Mak, N. K. (2015). Therapeutic targeting of CBP/β‐catenin signaling reduces cancer stem‐like population and synergistically suppresses growth of EBV‐positive nasopharyngeal carcinoma cells with cisplatin. Scientific Reports, 5, 9979 10.1038/srep09979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Chen, A. Y. , Huang, H. , Ye, X. , Rollyson, W. D. , Perry, H. E. , … Chen, Y. C. (2015). The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. International Journal of Oncology, 46, 2629–2638. 10.3892/ijo.2015.2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Hu, C. , Hou, J. , Shao, Q. , Yan, L. , Zhu, X. , … Shao, J. (2010). Epstein‐Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. Journal of Translational Medicine, 8, 30 10.1186/1479-5876-8-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Ono, M. , Takeshima, M. , & Nakano, S. (2014). Antiproliferative and apoptosis‐inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Research, 34, 1785–1792. [PubMed] [Google Scholar]
- Chen, Z. , Zhao, W. , Qu, S. , Li, L. , Lu, X. , Su, F. , … Zhu, X. (2015). PARP‐1 promotes autophagy via the AMPK/mTOR pathway in CNE‐2 human nasopharyngeal carcinoma cells following ionizing radiation, while inhibition of autophagy contributes to the radiation sensitization of CNE‐2 cells. Molecular Medicine Reports, 12, 1868–1876. 10.3892/mmr.2015.3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, G. , Li, B. , Sun, X. , Cao, Z. , Zhang, G. , Zhao, Z. , … Liu, W. (2018). LncRNA LOC730101 promotes osteosarcoma cell survival under energy stress. Biochemical and Biophysical Research Communications, 496, 1–6. 10.1016/j.bbrc.2017.12.074 [DOI] [PubMed] [Google Scholar]
- Chien, S. , Hsieh, M. , Chen, C. , Yang, S. , & Chen, M. (2015). Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP‐2. Expert Opinion on Therapeutic Targets, 19, 307–320. 10.1517/14728222.2014.992875 [DOI] [PubMed] [Google Scholar]
- Chung, H. T. , & Joe, Y. (2014). Antagonistic crosstalk between SIRT1, PARP‐1, and ‐2 in the regulation of chronic inflammation associated with aging and metabolic diseases. Integrative Medicine Research, 3, 198–203. 10.1016/j.imr.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da, C. , Liu, Y. , Zhan, Y. , Liu, K. , & Wang, R. (2016). Nobiletin inhibits epithelial‐mesenchymal transition of human non‐small cell lung cancer cells by antagonizing the TGF‐ β 1/Smad3 signaling pathway. Oncology Reports, 35, 2767–2774. 10.3892/or.2016.4661 [DOI] [PubMed] [Google Scholar]
- Dong, G. , Wang, B. , An, Y. , Li, J. , Wang, X. , Jia, J. , & Yang, Q. (2018). SIRT1 suppresses the migration and invasion of gastric cancer by regulating ARHGAP5 expression. Cell Death & Disease, 9, 977 10.1038/s41419-018-1033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , Yang, X. , & Bi, Z. (2015). 6‐Gingerol inhibits osteosarcoma cell proliferation through apoptosis and AMPK activation. Tumour Biology, 36, 1135–1141. 10.1007/s13277-014-2723-1 [DOI] [PubMed] [Google Scholar]
- Franzese, E. , Centonze, S. , Diana, A. , Carlino, F. , Guerrera, L. P. , Di Napoli, M. , … Orditura, M. (2019). PARP inhibitors in ovarian cancer. Cancer Treatment Reviews, 73, 1–9. 10.1016/j.ctrv.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Fu, M. , Xu, Y. , Chen, Y. , Wu, J. , Yu, Y. , Zou, B. , … Xiao, G. (2017). Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chemistry, 230, 649–656. 10.1016/j.foodchem.2017.03.098 [DOI] [PubMed] [Google Scholar]
- Gao, W. , Ge, S. , & Sun, J. (2019). Ailanthone exerts anticancer effect by up‐regulating miR‐148a expression in MDA‐MB‐231 breast cancer cells and inhibiting proliferation, migration and invasion. Biomedicine & Pharmacotherapy, 109, 1062–1069. 10.1016/j.biopha.2018.10.114 [DOI] [PubMed] [Google Scholar]
- Kwok, H. , Wu, C. W. , Palser, A. L. , Kellam, P. , Sham, P. C. , Kwong, D. L. W. , & Chiang, A. K. S. (2014). Genomic diversity of Epstein‐Barr virus genomes isolated from primary nasopharyngeal carcinoma biopsy samples. Journal of Virology, 88, 10662–10672. 10.1128/JVI.01665-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien, L. , Wang, M. , Chen, R. , Chiu, H. , Wu, J. , Shen, M. , … Lu, W. (2016). Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytotherapy Research, 30, 214–221. 10.1002/ptr.5517 [DOI] [PubMed] [Google Scholar]
- Luo, M. , Luo, H. , Hu, P. , Yang, Y. , Wu, B. , & Zheng, G. (2018). Evaluation of chemical components in citri reticulatae pericarpium of different cultivars collected from different regions by GC‐MS and HPLC. Food Science & Nutrition, 6, 400–416. 10.1002/fsn3.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégnin‐Chanet, F. , Bollet, M. A. , & Hall, J. (2010). Targeting poly(ADP‐ribose) polymerase activity for cancer therapy. Cellular and Molecular Life Sciences, 67, 3649–3662. 10.1007/s00018-010-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, S. I. A. , Jantan, I. , & Haque, M. A. (2017). Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. International Immunopharmacology, 50, 291–304. 10.1016/j.intimp.2017.07.010 [DOI] [PubMed] [Google Scholar]
- Moon, J. , & Cho, S. (2016). Nobiletin induces protective autophagy accompanied by ER‐stress mediated apoptosis in human gastric cancer SNU‐16 cells. Molecules, 21, 914 10.3390/molecules21070914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur Husna, S. M. , Tan, H. T. , Mohamud, R. , Dyhl‐Polk, A. , & Wong, K. K. (2018). Inhibitors targeting CDK4/6, PARP and PI3K in breast cancer: A review. Therapeutic Advances in Medical Oncology, 10, 175883591880850 10.1177/1758835918808509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. N. , Song, H. S. , Kim, M. , Lee, M. , Cho, W. , Lee, H. , … Kim, B. (2017). Review of natural product‐derived compounds as potent antiglioblastoma drugs. BioMed Research International, 2017, 1–24. 10.1155/2017/8139848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton, G. , Manente, A. G. , Murer, B. , De Marino, E. , Mutti, L. , & Moro, L. (2013). PARP1 inhibition affects pleural mesothelioma cell viability and uncouples AKT/mTOR axis via SIRT1. Journal of Cellular and Molecular Medicine, 17, 233–241. 10.1111/jcmm.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J. , Jeong, Y. , Cho, H. , Magae, J. , Bae, Y. , & Chang, Y. (2016). Suppression of c‐Myc induces apoptosis via an AMPK/mTOR‐dependent pathway by 4‐O‐methyl‐ascochlorin in leukemia cells. Apoptosis, 21, 657–668. 10.1007/s10495-016-1228-3 [DOI] [PubMed] [Google Scholar]
- Shrestha, A. , Nepal, S. , Kim, M. J. , Chang, J. H. , Kim, S. , Jeong, G. , … Park, P. (2016). Critical role of AMPK/FoxO3A axis in globular adiponectin‐induced cell cycle arrest and apoptosis in cancer cells. Journal of Cellular Physiology, 231, 357–369. 10.1002/jcp.25080 [DOI] [PubMed] [Google Scholar]
- Vida, A. , Márton, J. , Mikó, E. , & Bai, P. (2017). Metabolic roles of poly(ADP‐ribose) polymerases. Seminars in Cell & Developmental Biology, 63, 135–143. 10.1016/j.semcdb.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Voon, Y. , Ahmad, M. , Wong, P. , Husaini, R. , Ng, W. T. , Leong, C. , … Khoo, A. S. (2015). Nutlin‐3 sensitizes nasopharyngeal carcinoma cells to cisplatin‐induced cytotoxicity. Oncology Reports, 34, 1692–1700. 10.3892/or.2015.4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Qi, Y. , Liu, H. , Wang, X. , Zhu, H. , & Wang, Z. (2016). AMPK activator AICAR promotes 5‐FU‐induced apoptosis in gastric cancer cells. Molecular and Cellular Biochemistry, 411, 299–305. 10.1007/s11010-015-2592-y [DOI] [PubMed] [Google Scholar]
- Xiao, K. , Yu, Z. , Li, X. , Li, X. , Tang, K. , Tu, C. , … Xiong, W. (2016). Genome‐wide analysis of Epstein‐Barr virus (EBV) integration and strain in C666‐1 and Raji cells. Journal of Cancer, 7, 214–224. 10.7150/jca.13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G. , Cai, J. , Wang, L. , Jiang, L. , Huang, J. , Hu, R. , & Ding, F. (2018). MicroRNA‐30e‐5p suppresses non‐small cell lung cancer tumorigenesis by regulating USP22‐mediated Sirt1/JAK/STAT3 signaling. Experimental Cell Research, 362, 268–278. 10.1016/j.yexcr.2017.11.027 [DOI] [PubMed] [Google Scholar]
- Yi, L. , Ma, S. , & Ren, D. (2017). Phytochemistry and bioactivity of citrus flavonoids: A focus on antioxidant, anti‐inflammatory, anticancer and cardiovascular protection activities. Phytochemistry Reviews, 16, 479–511. 10.1007/s11101-017-9497-1 [DOI] [Google Scholar]
- You, J. , Cheng, J. , Yu, B. , Duan, C. , & Peng, J. (2018). Baicalin, a Chinese herbal medicine, inhibits the proliferation and migration of human non‐small cell lung carcinoma (NSCLC) cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Medical Science Monitor, 24, 2126–2133. 10.12659/msm.909627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, G. , Zhou, P. , Yang, H. , Li, Y. , Li, P. , & Liu, E. H. (2013). Rapid resolution liquid chromatography–electrospray ionisation tandem mass spectrometry method for identification of chemical constituents in Citri Reticulatae Pericarpium. Food Chemistry, 136, 604–611. 10.1016/j.foodchem.2012.08.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


