Abstract
Background
Multiple‐micronutrient (MMN) deficiencies often coexist among women of reproductive age in low‐ and middle‐income countries. They are exacerbated in pregnancy due to the increased demands of the developing fetus, leading to potentially adverse effects on the mother and baby. A consensus is yet to be reached regarding the replacement of iron and folic acid supplementation with MMNs. Since the last update of this Cochrane Review in 2017, evidence from several trials has become available. The findings of this review will be critical to inform policy on micronutrient supplementation in pregnancy.
Objectives
To evaluate the benefits of oral multiple‐micronutrient supplementation during pregnancy on maternal, fetal and infant health outcomes.
Search methods
For this 2018 update, on 23 February 2018 we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP), and reference lists of retrieved studies. We also contacted experts in the field for additional and ongoing trials.
Selection criteria
All prospective randomised controlled trials evaluating MMN supplementation with iron and folic acid during pregnancy and its effects on pregnancy outcomes were eligible, irrespective of language or the publication status of the trials. We included cluster‐randomised trials, but excluded quasi‐randomised trials. Trial reports that were published as abstracts were eligible.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed the quality of the evidence using the GRADE approach.
Main results
We identified 21 trials (involving 142,496 women) as eligible for inclusion in this review, but only 20 trials (involving 141,849 women) contributed data. Of these 20 trials, 19 were conducted in low‐ and middle‐income countries and compared MMN supplements with iron and folic acid to iron, with or without folic acid. One trial conducted in the UK compared MMN supplementation with placebo. In total, eight trials were cluster‐randomised.
MMN with iron and folic acid versus iron, with or without folic acid (19 trials) MMN supplementation probably led to a slight reduction in preterm births (average risk ratio (RR) 0.95, 95% confidence interval (CI) 0.90 to 1.01; 18 trials, 91,425 participants; moderate‐quality evidence), and babies considered small‐for‐gestational age (SGA) (average RR 0.92, 95% CI 0.88 to 0.97; 17 trials; 57,348 participants; moderate‐quality evidence), though the CI for the pooled effect for preterm births just crossed the line of no effect. MMN reduced the number of newborn infants identified as low birthweight (LBW) (average RR 0.88, 95% CI 0.85 to 0.91; 18 trials, 68,801 participants; high‐quality evidence). We did not observe any differences between groups for perinatal mortality (average RR 1.00, 95% CI 0.90 to 1.11; 15 trials, 63,922 participants; high‐quality evidence). MMN supplementation led to slightly fewer stillbirths (average RR 0.95, 95% CI 0.86 to 1.04; 17 trials, 97,927 participants; high‐quality evidence) but, again, the CI for the pooled effect just crossed the line of no effect. MMN supplementation did not have an important effect on neonatal mortality (average RR 1.00, 95% CI 0.89 to 1.12; 14 trials, 80,964 participants; high‐quality evidence). We observed little or no difference between groups for the other maternal and pregnancy outcomes: maternal anaemia in the third trimester (average RR 1.04, 95% CI 0.94 to 1.15; 9 trials, 5912 participants), maternal mortality (average RR 1.06, 95% CI 0.72 to 1.54; 6 trials, 106,275 participants), miscarriage (average RR 0.99, 95% CI 0.94 to 1.04; 12 trials, 100,565 participants), delivery via a caesarean section (average RR 1.13, 95% CI 0.99 to 1.29; 5 trials, 12,836 participants), and congenital anomalies (average RR 1.34, 95% CI 0.25 to 7.12; 2 trials, 1958 participants). However, MMN supplementation probably led to a reduction in very preterm births (average RR 0.81, 95% CI 0.71 to 0.93; 4 trials, 37,701 participants). We were unable to assess a number of prespecified, clinically important outcomes due to insufficient or non‐available data.
When we assessed primary outcomes according to GRADE criteria, the quality of evidence for the review overall was moderate to high. We graded the following outcomes as high quality: LBW, perinatal mortality, stillbirth, and neonatal mortality. The outcomes of preterm birth and SGA we graded as moderate quality; both were downgraded for funnel plot asymmetry, indicating possible publication bias.
We carried out sensitivity analyses excluding trials with high levels of sample attrition (> 20%). We found that results were consistent with the main analyses for all outcomes. We explored heterogeneity through subgroup analyses by maternal height, maternal body mass index (BMI), timing of supplementation, dose of iron, and MMN supplement formulation (UNIMMAP versus non‐UNIMMAP). There was a greater reduction in preterm births for women with low BMI and among those who took non‐UNIMMAP supplements. We also observed subgroup differences for maternal BMI and maternal height for SGA, indicating greater impact among women with greater BMI and height. Though we found that MMN supplementation made little or no difference to perinatal mortality, the analysis demonstrated substantial statistical heterogeneity. We explored this heterogeneity using subgroup analysis and found differences for timing of supplementation, whereby higher impact was observed with later initiation of supplementation. For all other subgroup analyses, the findings were inconclusive.
MMN versus placebo (1 trial) A single trial in the UK found little or no important effect of MMN supplementation on preterm births, SGA, or LBW but did find a reduction in maternal anaemia in the third trimester (RR 0.66, 95% CI 0.51 to 0.85), when compared to placebo. This trial did not measure our other outcomes.
Authors' conclusions
Our findings suggest a positive impact of MMN supplementation with iron and folic acid on several birth outcomes. MMN supplementation in pregnancy led to a reduction in babies considered LBW, and probably led to a reduction in babies considered SGA. In addition, MMN probably reduced preterm births. No important benefits or harms of MMN supplementation were found for mortality outcomes (stillbirths, perinatal and neonatal mortality). These findings may provide some basis to guide the replacement of iron and folic acid supplements with MMN supplements for pregnant women residing in low‐ and middle‐income countries.
Plain language summary
Vitamin and mineral supplements for women during pregnancy
What is the issue?
In low‐ and middle‐income countries, many women have poor diets and are deficient in nutrients and micronutrients that are required for good health. Micronutrients are vitamins and minerals that are needed by the body in very small quantities, but are important for normal functioning, growth and development. During pregnancy, these women often become more deficient because of the need to provide nutrition for the baby too, and this can negatively affect their health, along with the health of the baby.
Why is this important?
Combining multiple micronutrients into one supplement has been suggested as a cost‐effective way to achieve multiple benefits for women during pregnancy. Micronutrient deficiencies are known to interact, and a greater effect may be achieved by multiple supplementation rather than single‐nutrient supplementation. However, interactions could also lead to poor absorption of some of the nutrients. High doses of some nutrients may also cause harm to the mother or her baby.
What evidence did we find?
We searched Cochrane Pregnancy and Childbirth's Trials Register (23 February 2018). This systematic review included 21 trials (involving 142,496 women), but only 20 trials (involving 141,849 women) contributed data. The included trials compared pregnant women who supplemented their diets with multiple micronutrients (including iron and folic acid) with pregnant women who received iron (with or without folic acid) or a placebo. Overall, we found that pregnant women who received multiple‐micronutrient supplementation had fewer babies that were born too small (weighing less than 2500 g), fewer babies who were smaller in size than normal for their gestational age, and fewer births that occurred before week 37 of pregnancy. The evidence for the main outcomes of low birthweight and small‐for‐gestational age was found to be of high quality and moderate quality, respectively.
What does this mean?
These findings, which have been observed elsewhere, may provide a basis to guide the replacement of iron and folic acid supplements with multiple‐micronutrient supplements for pregnant women in low‐ and middle‐income countries.
Summary of findings
Summary of findings for the main comparison. Multiple micronutrients compared to control (iron with or without folic acid) for women during pregnancy.
| Multiple micronutrients compared to control (iron with or without folic acid) for women during pregnancy | ||||||
| Patient or population: women during pregnancy Setting: low‐ and middle‐income countries Intervention: multiple micronutrients Comparison: control (iron with or without folic acid) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Risk with control (iron with or without folic acid) | Risk with multiple micronutrients | |||||
| Preterm births | Study population | RR 0.95 (0.90 to 1.01) | 91,425 (18 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 197 per 1000 | 188 per 1000 (178 to 199) | |||||
| Small‐for‐gestational age | Study population | RR 0.92 (0.88 to 0.97) | 57,348 (17 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 337 per 1000 | 310 per 1000 (296 to 327) | |||||
| Low birthweight | Study population | RR 0.88 (0.85 to 0.91) | 68,801 (18 RCTs) | ⊕⊕⊕⊕ High | ||
| 212 per 1000 | 187 per 1000 (181 to 193) | |||||
| Perinatal mortality | Study population | RR 1.00 (0.90 to 1.11) | 63,922 (15 RCTs) | ⊕⊕⊕⊕ High | Raw event and participant data were unavailable for Ramakrishnan 2003 and West 2014, so have not been included in No of participants column. | |
| 39 per 1000 | 39 per 1000 (35 to 43) | |||||
| Stillbirths | Study population | RR 0.95 (0.86 to 1.04) | 97,927 (17 RCTs) | ⊕⊕⊕⊕ High | ||
| 30 per 1000 | 28 per 1000 (26 to 31) | |||||
| Neonatal mortality | Study population | RR 1.00 (0.89 to 1.12) | 80,964 (14 RCTs) | ⊕⊕⊕⊕ High | Raw event and participant data were unavailable for Bhutta 2009a and Fawzi 2007 so have not been included in No of participants column. | |
| 29 per 1000 | 29 per 1000 (26 to 32) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aStrong evidence of funnel plot asymmetry, indicating possible publication bias.
Background
Description of the condition
Micronutrient deficiencies are common among women of reproductive age (15 to 49 years of age), especially those residing in low‐ and middle‐income countries where diets often lack diversity and fortified foods are less available (Black 2013; FAO/WHO 2004). Infections and chronic illness can also contribute to micronutrient deficiencies by directly inhibiting nutrient absorption (Bailey 2015). Micronutrient deficiencies are exacerbated during pregnancy due to increased requirements of the growing fetus, placenta and maternal tissues. An inability to fulfil the increased demands results in potentially adverse effects on the mother and the fetus (Berti 2011). Additionally, there can be sustained intergenerational effects. Maternal malnutrition has been shown to affect both short‐term and long‐term outcomes for the offspring, including growth, neurodevelopment and cognition, and cardiometabolic, pulmonary, and immune function (Gernand 2016).
Up‐to‐date population‐level estimates are largely lacking for individual micronutrients due to measurement and cost challenges associated with collecting these indicators (Gernand 2016). In addition, there are few data that have been disaggregated by age, parity, wealth status and other factors that can influence nutrition throughout pregnancy (Gernand 2016). However, we do know that anaemia due to iron deficiency is one of the most prevalent micronutrient deficiencies globally. According to 2013 estimates, the worldwide prevalence of prenatal iron‐deficiency anaemia was 19.2% (95% confidence interval (CI) 17.1% to 21.5%; Black 2013). Anaemia during pregnancy has been found to be associated with increased risk of maternal mortality, perinatal mortality, and infants with low birthweight (LBW) (Allen 2001; Christian 2010; Haider 2013; Murray‐Kolb 2013). Vitamin A is another important nutrient that, when deficient, can lead to night blindness. According to global estimates for the time period between 1995 and 2005, vitamin A deficiency, measured using night blindness and low serum retinol levels, affected 9.8 million (95% CI 8.7 to 10.8 million), and 19.1 million pregnant women (95% CI 9.30 to 29.0 million), respectively. This corresponds to 15.3% (95% CI 6.0% to 24.6%), of pregnant women being deficient in vitamin A (Black 2013). Deficiency of vitamin A has been associated with poor birth and mortality outcomes; however, supplementation with vitamin A during pregnancy has demonstrated no beneficial effect on these outcomes (McCauley 2015), but has been shown to reduce the risk of maternal anaemia, infection, and night blindness (McCauley 2015).
In the past decade, deficiency of vitamin D has also emerged as an important nutritional problem with high prevalence being reported in high‐income, as well as low‐income, populations (Datta 2002; Ginde 2010; Sachan 2005). Iodine deficiency, often measured by urinary iodine, is also common among pregnant women. The median urinary iodine level in a nationally representative sample of pregnant women in Nepal was reported to be 134 mcg/L (Benoist 2008), indicating insufficient iodine intake (Andersson 2007). Severe iodine deficiency during pregnancy results in pregnancy loss, mental retardation and cretinism (Dunn 1993). Although severe deficiency is now rare, mild to moderate deficiency continues to be a problem (Andersson 2007).
Deficiencies of other micronutrients are also common among pregnant women. According to the 2012 estimates, around 17% of the world’s population have reduced dietary intake of zinc (Wessells 2012). Zinc deficiency has been associated with complications of pregnancy and delivery such as pre‐eclampsia, premature rupture of membranes, and congenital abnormalities (Black 2001; Caulfield 1998). However, a review of trials of zinc supplementation showed a reduction in the risk of preterm birth only (Hess 2009; Ota 2015). Folic acid deficiency can lead to haematological consequences and congenital malformations; however, association with other birth outcomes is equivocal (Black 2001; De‐Regil 2010). Concurrent deficiencies that could include vitamins A, D, E, riboflavin, B6, B12, folic acid, iron, and zinc have also been reported in studies conducted among pregnant women (Jiang 2005; Pathak 2004). Deficiencies in other minerals such as magnesium, selenium, copper and calcium may also potentially be associated with complications of pregnancy, childbirth or fetal development (Black 2001).
Description of the intervention
The World Health Organization (WHO) currently recommends iron and folic acid supplementation for women during pregnancy as part of routine antenatal care (WHO 2012). The recommended dose of iron ranges from 30 mg to 60 mg. In areas where anaemia is a severe public health problem, defined as a prevalence of 40% or higher, a daily dose of 60 mg of iron is preferred. The standard dose of 60 mg of iron was first recommended in 1959 and was based on maternal requirements during pregnancy (WHO 1959). Despite its provision as part of national antenatal care programmes for the last few decades in most low‐ and middle‐income countries, compliance with the supplement is low. Gastrointestinal side‐effects including constipation, nausea, vomiting, and diarrhoea are the most common complaints among women consuming high doses of iron (Oriji 2011; Seck 2008).
Supplementation with iron and folic acid during pregnancy has been found to be associated with reduction in the risk of maternal anaemia and infants with LBW (Haider 2013; Pena‐Rosas 2015). To overcome other possible maternal micronutrient deficiencies, the United Nations Children's Fund (UNICEF), United Nations University (UNU) and the WHO, in 1999, agreed on the composition of a proposed multiple‐micronutrient (MMN) tablet (UNICEF 1999). This UNIMMAP tablet provides one recommended daily allowance of vitamin A, vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12, folic acid, vitamin C, vitamin D, vitamin E, copper, selenium and iodine with 30 mg of iron and 15 mg of zinc for pregnant women. In contrast to the WHO recommendation, a lower dose of iron was recommended as the absorption of iron was expected to be enhanced due to vitamin C, vitamin A, and riboflavin, and given that the majority of pregnant women suffer from mild anaemia and the potential side‐effects associated with higher doses of iron.
How the intervention might work
Vitamins and minerals play critical roles in cellular metabolism, growth and maintenance of normal functioning of the human body. They are also important in many enzymatic processes, signal transduction and transcription pathways (McArdle 1999; WHO 2004). Recent studies have suggested a possible benefit of multiple micronutrient supplementation for improving pregnancy outcomes through placental function, including modulation of inflammation and oxidative stress and vascular function (Owens 2015; Richard 2017). Deficiencies of these micronutrients rarely exist in isolation. Additionally, because of their role at various levels in the biological pathways, it is difficult to assign a clinical or pre‐clinical condition to the deficiency of a single micronutrient (McArdle 1999). Micronutrient deficiencies are also known to interact. Combining MMN in a single delivery mechanism has been suggested as a cost‐effective way to achieve multiple benefits.
Why it is important to do this review
The interest of the global research community in eliminating micronutrient deficiencies stems from their significant negative impact on the health of women and infants. The health effects during the fetal life may also have consequences later as an adult. Several trials have demonstrated that supplementation with MMN during pregnancy reduces the risk of micronutrient deficiencies (Haider 2012). Findings from individual trials regarding the benefit on other maternal and pregnancy outcomes are inconsistent, as individual studies may not have statistical power to evaluate effects on these outcomes. Several meta‐analyses have systematically reviewed and synthesised the evidence of the effect of supplementation with multiple micronutrients, with the first such synthesis of evidence being an earlier version of this Cochrane Review (Bhutta 2012; Haider 2006; Haider 2011; Haider 2012; Kawai 2011; Ramakrishnan 2012). On the basis of the evidence, supplementation with MMN during pregnancy has been recommended (Bhutta 2008; Bhutta 2013). However, a consensus is yet to be reached regarding the replacement of iron and folic acid supplementation with MMN. Since the last update of this Cochrane Review (Haider 2017), evidence from a few large trials has recently been made available, inclusion of which is critical to inform global policy.
This review updates a previously published Cochrane Review on MMN supplementation during pregnancy that had demonstrated positive effect of supplementation on birth outcomes (Haider 2017). The effects of supplementation with individual micronutrients during pregnancy have been evaluated in other Cochrane Reviews. The effect of MMN supplementation in HIV‐infected pregnant women has been evaluated in another Cochrane Review (Siegfried 2012).
Objectives
To evaluate the benefits of oral multiple‐micronutrient supplementation during pregnancy on maternal, fetal and infant health outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All prospective randomised controlled trials evaluating multiple micronutrient (MMN) supplementation during pregnancy and its effects on pregnancy outcomes were eligible, irrespective of language or publication status of the trials. Trial reports that were published as abstracts were eligible for inclusion. We included cluster‐randomised trials, but excluded quasi‐randomised trials.
Types of participants
Pregnant women. There was no limit on the length of gestation at the time of enrolment in the study. We excluded HIV‐infected pregnant women from the review, as this population is at a greater risk of nutritional disorders compared to uninfected women. We also excluded studies recruiting women at high risk of nutritional disorders for other reasons. Another Cochrane Review has evaluated the effect of MMN supplementation in HIV‐infected pregnant women (Siegfried 2012).
Types of interventions
Since WHO recommends use of iron and folic acid supplementation in women during pregnancy as a part of routine antenatal care, we evaluated the effect of MMN supplementation with iron and folic acid in pregnant women versus supplementation with iron, with or without folic acid. We also included trials comparing the outcomes of providing pregnant women with MMN supplements with iron and folic acid compared to placebo. The composition of MMN supplement by trial can be found in Table 5.
1. Micronutrients given to women in the intervention group.
| Study ID | Iron | Folic acid | Vit A | Beta‐carotene | Vit C | Vit D | Vit E | Vit B1 (thiamine) | Vit B2 (riboflavin) | Vit B3 (niacin) | Vit B5 (pantothenic acid) | Vit B6 | Vit B12 | Vit K | Copper | Selenium | Zinc | Iodine | Magnesium | Calcium | Phosphorus | Biotin | Potassium | Manganese |
| Ashorn 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Bhutta 2009a | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Biggs 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Brough 2010 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Christian 2003 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Dewey 2009 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Fawzi 2007 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Friis 2004 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Kaestel 2005 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lui 2013 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Moore 2009 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Osrin 2005 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Ramakrishnan 2003 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Roberfroid 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Sood 1975 | ✓ | ✓ | ✓ | |||||||||||||||||||||
| SUMMIT 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Sunawang 2009 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Tofail 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| West 2014 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Zagre 2007 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Zeng 2008 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
We excluded trials that used fewer than three micronutrients in the intervention group, regardless of their outcomes. There were no limits on the duration of supplementation.
We included the following specific comparisons in the review.
Multiple micronutrients with iron and folic acid versus control (iron with or without folic acid)
Multiple micronutrients with iron and folic acid versus control (placebo)
The review focuses on daily oral supplements. One trial (Biggs 2010), provided MMN twice weekly; we excluded data from this trial from the main results, but included them in the subgroup analysis for dose of iron to examine whether there are any notable differences when women are provided with a lower dose of iron throughout pregnancy (Biggs 2010 provided women with 120 mg per week compared to 210 mg or 420 mg per week in all other trials). We excluded trials examining parenteral MMN or food fortification with MMN.
Types of outcome measures
Primary outcomes
Preterm births (births before 37 weeks of gestation)
Small‐for‐gestational age (SGA) (as defined by the authors of the trials)
Low birthweight (LBW) (birthweight less than 2500 g)
Perinatal mortality
Stillbirths
Neonatal mortality
Secondary outcomes
Maternal anaemia (third trimester haemoglobin (Hb) < 110 g/L)
Maternal mortality
Miscarriage (loss of pregnancy before 28 weeks of gestation)
Premature rupture of membranes
Pre‐eclampsia
Mode of delivery: caesarean section (not prespecified)
Macrosomia (not prespecified)
Placental abruption
Very preterm births (births before 34 weeks of gestation)
Neurodevelopmental delay (assessed using Bayley Scale of Infant Development (BSID) at six and 12 months of age)
Nutritional status of children (stunting, wasting and underweight at six, 12 and 24 months of age)
Cost of supplementation
Side‐effects of MMN supplements
Congenital anomalies (including neural tube defects)
Maternal well‐being or satisfaction
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (23 February 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review section (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (24 February 2018) using the search methods detailed in Appendix 1.
Searching other resources
We searched reference lists of retrieved articles and key reviews. We contacted experts in the field for additional and ongoing trials.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Haider 2017.
For this update, we used the following methods for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager 5 software (Review Manager 2014), and checked them for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Given some of the changes and data edits in the previous versions of this review, for this update, we re‐extracted data for all primary and secondary outcomes for all included studies from the outset, not just those found from the most recent search.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered its likelihood to impact the findings. For cluster‐randomised trials, we carefully considered recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials (Higgins 2011a). We explored the impact of attrition bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook (Schünemann 2013). We assessed the quality of the body of evidence relating to the following outcomes for the comparison of MMN versus iron and folic acid supplements:
Preterm births
SGA
LBW
Perinatal mortality
Stillbirths
Neonatal mortality
We used the GRADEpro Guideline Development Tool (GRADEpro GDT 2015), to import data from Review Manager 5.3 (Review Manager 2014), in order to create a 'Summary of findings' table. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious limitations (or by two levels for very serious), depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. We did not downgrade evidence for heterogeneity with an I² statistic value of less than 50% (Deeks 2017; Higgins 2003).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. In future updates as appropriate, we plan to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
If both adjusted and unadjusted effect estimates were provided in the primary report, we utilised the adjusted estimate. If only event and participant raw data were provided, we used these to calculate a relevant effect estimate (RR or MD) using the Review Manager 5 calculator function (Review Manager 2014). Where there were significant discrepancies between our re‐extracted data and what had been reported in previous versions of this review, we contacted trial investigators for clarification.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials (Christian 2003; Bhutta 2009a; Biggs 2010; SUMMIT 2008; Sunawang 2009; West 2014; Zagre 2007; Zeng 2008), in the analyses along with individually‐randomised trials. We extracted cluster‐adjusted effect estimates with their confidence intervals, which we analysed along with individually‐randomised trials using the generic inverse variance method. If effect estimates were not cluster‐adjusted, or if number of events was used to calculate the effect estimate for a given outcome, then we used the reported intracluster correlation coefficient (ICC) and average cluster size (M) to determine the design effect for a trial (1+(M‐1)*ICC) (Higgins 2011a). Some trials reported the design effect, which we then applied to the number of events and sample size for dichotomous outcomes, and sample size only for continuous outcomes, to reduce cluster‐randomised trial data to their effective sample size.
We had to adjust all reported estimates from each cluster‐randomised trial, with the exception of Bhutta 2009a (all outcomes), Christian 2003 (all outcomes), SUMMIT 2008 (SGA, LBW, perinatal mortality, neonatal mortality, stillbirth, and maternal mortality), and West 2014 (preterm birth, SGA, LBW, perinatal mortality, neonatal mortality, and stillbirth). Details on reported ICCs and design effect by trial can be found in Table 6.
2. Details on adjustments made for cluster‐randomised controlled trials.
| Trial | Outcome(s) | Reported design effect | Reported ICC | Calculated M | Calculated design effecta |
| SUMMIT 2008 | Preterm birth, miscarriage | 1.2 | ‐ | ‐ | ‐ |
| Sunawang 2009 | All | 1.2 | ‐ | ‐ | ‐ |
| West 2014 | Maternal anaemia, miscarriage, maternal mortality, very preterm birth | 1.15 | ‐ | ‐ | ‐ |
| Zagre 2007 | All | 1.2 | ‐ | ‐ | ‐ |
| Zeng 2008 | Preterm birth, very preterm birth | ‐ | 0.02 | 11 | 1.2 |
| Zeng 2008 | LBW | ‐ | 0.03 | 11 | 1.3 |
| Zeng 2008 | All other outcomes | ‐ | 0.03b | 11 | 1.3 |
| Biggs 2010 | Maternal anaemia | ‐ | 0.03 | 12.1 | 1.3 |
| Biggs 2010 | All other outcomes | ‐ | 0 | 12.1 | 1.0 |
| ICC: intracluster correlation coefficient; M: average cluster size | |||||
aDesign effect = 1 + (M‐1)*ICC. bZeng 2008 reported ICCs specific to gestational age and birthweight. For all other outcomes, we used the more conservative of the two ICCs (0.03) to calculate the design effect.
Trials with multiple intervention groups
For the majority of trials with multiple intervention groups, we selected one pair of interventions and excluded the others. This is one approach recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We included trials with more than two intervention groups in the analysis after selecting the comparison groups (intervention and control groups) that satisfied the 'types of intervention' criterion and were relevant to the review. For Christian 2003, we included data from group 4 (MMN group with iron and folic acid) versus group 2 (control group iron with or without folic acid). We did not include groups 1 (folic acid with vitamin A) and 5 (vitamin A only), since they did not satisfy the inclusion criterion of the review. Further, group 3 (iron, folic acid, vitamin A, and zinc) did not include the majority of micronutrients being considered for inclusion in a MMN supplement for pregnant women and was also not comparable to the UNIMMAP formulation proposed by UNICEF, UNU, and WHO. For Kaestel 2005, we included group 1 (MMN with iron and folic acid) and group 3 (iron and folic acid) in the review. We selected the group with one recommended daily allowance, since the MMN supplement in group 1 was comparable to the UNIMMAP formulation. For Lui 2013, data from group 3 (MMN with iron and folic acid) versus group 2 (iron and folic acid) fitted the types of intervention criterion of the review and we included them in the analyses. Similarly, we included data for the comparison of groups 3 (MMN with iron and folic acid) versus 2 (iron and folic acid) for Zeng 2008. Group 1 in both Lui 2013 and Zeng 2008 had received folic acid only and did not satisfy the control definition of the review.
For Moore 2009, we included data from group 1 (iron and folic acid) versus group 2 (MMN with iron and folic acid). We excluded data from groups 3 (protein‐energy plus iron and folic acid) and 4 (protein‐energy plus MMN with iron and folic acid) due to the provision of a food‐based supplement in both groups. For Biggs 2010, we included data from groups 1 (daily iron and folic acid) versus 3 (twice weekly MMN with iron and folic acid), though only for the purpose of the subgroup analysis looking at dose of iron. We excluded group 2 because they provided iron and folic acid twice weekly as opposed to daily.
If more than two intervention groups had met the eligibility criteria, we would have combined groups to create a single pair‐wise comparison, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We used this approach for Tofail 2008, where we combined early invitation and usual invitation to food groups to compare MMN with iron and folic acid to iron (30 mg) and folic acid.
Dealing with missing data
For included studies, we noted levels of attrition. We assessed the impact of including studies with high levels of missing data (> 20%) in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003), and Chi² statistics. We regarded heterogeneity as substantial if an I² statistic was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we explored it by pre‐specified subgroup analysis (Deeks 2017).
Assessment of reporting biases
Where there are 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually (Sterne 2017).
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). We pooled together data from individually randomised and cluster‐randomised trials during meta‐analysis, using the generic inverse variance method, after appropriate adjustment of estimates from cluster‐randomised trials, as noted above. In response to feedback received for the previous version of this review, we conducted all analyses using a random‐effects model, given the clinical heterogeneity amongst the included trials. We treated the random‐effects summary as the average of the range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. We have presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I² statistic.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity at the outcome‐level, we investigated it using subgroup analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Timing of supplementation (categorised as either before or after 20 weeks of gestation)
Dose of iron in the MMN and control supplements (*15 to 20 mg versus 30 mg versus 60 mg of iron)
Baseline nutritional status of the mother, including body mass index (BMI < 20 kg/m2 versus ≥ 20 kg/m2) and height (< 154.9 cm versus ≥ 154.9 cm)
UNIMMAP versus **non‐UNIMMAP MMN supplement formulation
*We used the 15 to 20 mg dosage range to allow us to incorporate the trial that provided 60 mg of iron to women twice weekly, which equates to about 17 mg iron daily.
**Variations on the UNIMMAP formulation (e.g. varying concentrations of micronutrients) would be included in the non‐UNIMMAP group.
We assessed subgroup differences by interaction tests available within Review Manager 5 (Review Manager 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value (Deeks 2017; Higgins 2003).
Sensitivity analysis
We carried out sensitivity analyses for all outcomes to explore the effect of risk of bias, as assessed by high attrition rates. We excluded trials at high risk of attrition bias (> 20% loss to follow up) from each analysis in order to assess whether this exclusion affected the overall result.
Results
Description of studies
Results of the search
See: Figure 1
1.
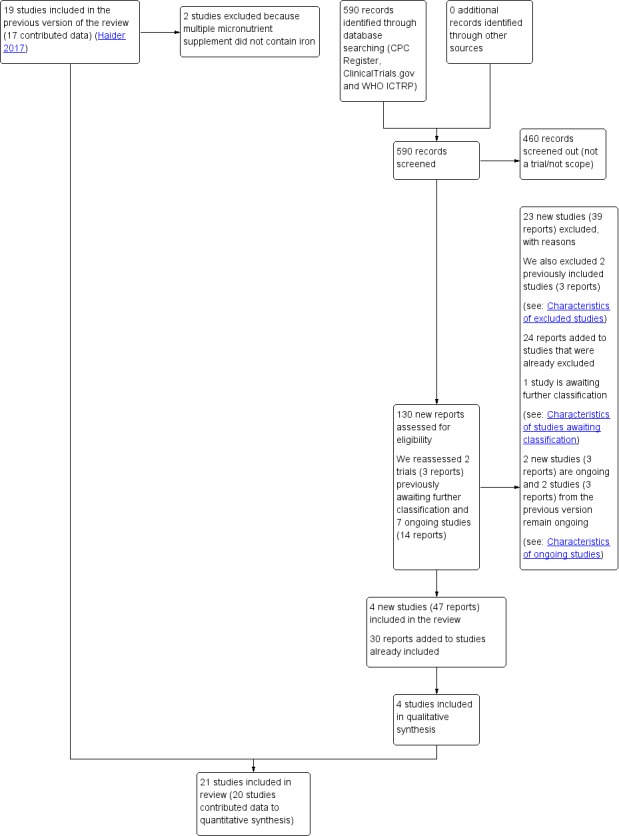
Study flow diagram
For this 2018 updated search, we retrieved and assessed 130 trial reports. We also reassessed the two studies (three reports) awaiting classification in the previous version of the review, and we checked the progress of the seven ongoing studies (14 reports).
We included four new trials in this update (Ashorn 2010; Biggs 2010; Dewey 2009; Moore 2009). We also added 30 new reports to trials already included.
We identified a total of 21 trials (involving 142,496 women) as eligible, of which 20 trials (involving 141,849 number of women) contributed data to the review.
We excluded 23 new studies (39 reports) and added 24 reports to studies that we had previously excluded. We also excluded two trials that we had included in the previous update (Hininger 2004; Theobald 1937), because the MMN supplement did not contain iron.
One study is awaiting classification (Gathwala 2012), due to missing group denominators. Two new studies (three reports) are ongoing (NCT03287882; Sumarmi 2015), in addition to NCT02190565 and Tu 2013 that have remained ongoing since the previous update. See Characteristics of ongoing studies for more information.
Included studies
We identified a total of 21 trials (involving 142,496 women) as eligible for inclusion in this review. Of these, one study (Sood 1975), presented data in a format that precluded its inclusion. Hence, this study did not contribute data to the analyses. A total of 141,849 women participated in the remaining 20 included trials (Ashorn 2010; Bhutta 2009a; Biggs 2010; Brough 2010; Christian 2003; Dewey 2009; Fawzi 2007; Friis 2004; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; Sunawang 2009; Tofail 2008; West 2014; Zagre 2007; Zeng 2008), of which eight were cluster‐randomised (Bhutta 2009a; Biggs 2010; Christian 2003; SUMMIT 2008; Sunawang 2009; West 2014; Zagre 2007; Zeng 2008). One trial was conducted in a high‐income country (Brough 2010). Most of the outcomes were defined in the same way across different trials except for one trial that used different cut‐offs for stillbirth and very preterm birth (West 2014), and three trials that used different cut‐offs for miscarriage (Osrin 2005; West 2014; Zagre 2007). See Characteristics of included studies table for further details of included studies.
All trials reported sources of funding, with the exception of Sood 1975.
Seventeen trials included a statement of disclosure as to whether or not there was a potential conflict of interest related to the study (Ashorn 2010; Biggs 2010; Brough 2010; Christian 2003; Dewey 2009; Fawzi 2007; Friis 2004; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; Tofail 2008; West 2014; Zeng 2008). Of these, there were 12 trials where all authors declared no conflict of interest (Biggs 2010; Brough 2010; Christian 2003; Fawzi 2007; Friis 2004; Kaestel 2005; Lui 2013; Moore 2009; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; Tofail 2008), and five studies where an author or several authors had indicated a conflict of interest (Ashorn 2010; Dewey 2009; Osrin 2005; West 2014; Zeng 2008). The remaining four studies did not provide a statement of disclosure, and therefore we could not assess potential conflicts of interest (Bhutta 2009a; Sood 1975; Sunawang 2009; Zagre 2007).
Participants
The 20 trials contributing data to the analyses included 141,849 pregnant women at varying gestational stages, ranging from early pregnancy to 36 weeks of gestation. Pregnant women with a haemoglobin (Hb) concentration of less than 80 g/L, with a serious medical condition or a complication of pregnancy such as cardiac disease, pneumonia and threatened abortion were not eligible for inclusion in the trials. Two trials (Ashorn 2010; Friis 2004), included a subgroup of pregnant women who were HIV‐1‐infected, but the data for these subgroups were excluded from the review. Baseline characteristics of the participants in the intervention and the control groups were comparable in the included trials except for minor differences in five trials (Christian 2003; Friis 2004; Ramakrishnan 2003; Roberfroid 2008; Zagre 2007). In Friis 2004, a higher proportion of primigravidae was found in the placebo group. In Ramakrishnan 2003, there was a higher proportion of single mothers and a lower mean BMI in the intervention group. In Christian 2003, more participants in the control group belonged to a specific ethnic background and owned land. In Roberfroid 2008, the Hb level was lower in the intervention group and the BMI was lower in the control group. In Zagre 2007, the intervention group had more households and preventive measures against malaria, whereas the placebo group had less education and more poverty.
Intervention
Seventeen trials assessed MMN supplementation versus control; iron with or without folic acid (Ashorn 2010; Bhutta 2009a; Biggs 2010; Christian 2003; Dewey 2009; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; Sunawang 2009; Tofail 2008; West 2014; Zagre 2007; Zeng 2008). Two trials had a component of nutritional education along with MMN supplementation (Bhutta 2009a; Zagre 2007), and one trial had a food supplement co‐intervention along with MMN supplementation (Tofail 2008). Three trials assessed MMN supplementation against a placebo (Brough 2010; Fawzi 2007; Friis 2004); however, in Fawzi 2007 and Friis 2004, all participants received iron and folic acid supplements. In Brough 2010, participants not taking folic acid received recommendations to take it daily.
The composition of the MMN supplement was different in all included trials. Eighteen trials included iron and folic acid in the MMN supplement (Ashorn 2010; Bhutta 2009a; Biggs 2010; Brough 2010; Christian 2003; Dewey 2009; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; Sunawang 2009; Tofail 2008; West 2014; Zagre 2007; Zeng 2008). All supplements were given orally to the pregnant women throughout pregnancy from the time of enrolment, except for one trial where supplementation was started when the participants reached 14 weeks of gestation (Tofail 2008). The duration of supplementation varied because the time of enrolment differed across the trials. Six trials enrolled participants in the first trimester of pregnancy (Brough 2010; Christian 2003; Ramakrishnan 2003; Tofail 2008; West 2014; Zagre 2007). Two trials enrolled participants with a gestation of less than 16 weeks (Bhutta 2009a; Biggs 2010), four trials less than 20 weeks (Ashorn 2010; Dewey 2009; Lui 2013; Moore 2009), and one trial less than 28 weeks (Zeng 2008). Two trials enrolled participants in the second trimester (Osrin 2005; Sunawang 2009), one trial enrolled women in both the second and third trimester (Friis 2004), and two trials enrolled women who were less than 37 weeks of gestation (Fawzi 2007; Kaestel 2005). Two trials enrolled pregnant women irrespective of gestational age (Roberfroid 2008; SUMMIT 2008). Supplementation was given until delivery in 11 of the included trials (Bhutta 2009a; Brough 2010; Friis 2004; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Tofail 2008; Zagre 2007; Zeng 2008). Supplementation continued until four weeks after delivery in Sunawang 2009, six weeks after delivery in Fawzi 2007, 12 weeks after delivery in five trials (Biggs 2010; Christian 2003; Roberfroid 2008; SUMMIT 2008; West 2014), and for five weeks after a stillbirth or miscarriage in Christian 2003, and 24 weeks after delivery in two trials (Ashorn 2010; Biggs 2010). The frequency of MMN supplementation in all included trials was once daily, except for one trial where supplementation was provided six days a week (Ramakrishnan 2003), and another trial where supplementation was twice weekly (Biggs 2010).
Excluded studies
We excluded 117 trials from the review. Briefly, 32 trials evaluated the effects of a single or two micronutrients or compounds (Beazley 2002; Bergmann 2006; Carrasco 1962; Caulfield 1999; Chames 2002; Goldenberg 1995; Gopalan 2004; Hillman 1963; Holly 1955; Hossain 2014; Hunt 1983; Hunt 1985; Iannotti 2008; Lucia 2007; Ma 2008; Malvasi 2014; Marya 1987; Mathan 1979; Merialdi 1999; Muslimatun 2001; NCT01795131; Ochoa‐Brust 2007; Robertson 1991; Sachdeva 1993; Sagaonkar 2009; Salzano 2001; Schmidt 2001; Semba 2000; Suharno 1993; Suprapto 2002; Tanumihardjo 2002; Zavaleta 2000). Fourteen trials did not satisfy the trial design criteria (ACTRN12616001449426; Aguayo 2005; Biswas 1984; Kubik 2004; Kynast 1986; Itam 2003; Menon 1962; Patimah 2013; Park 1999; People's League 1946; Pezzack 2014; Sun 2010; Thauvin 1992; Wijaya‐Erhardt 2014), and six trials studied HIV‐positive women (Arsenault 2010; Fawzi 1998; Khavari 2014; Merchant 2005; Olofin 2014; Webb 2009), and hence we excluded them from the review. Six trials gave MMN supplements to both groups of participants (Ahn 2006; Asemi 2014; Dawson 1987; Dawson 1998; Magon 2014; Taghizadeh 2014). Czeizel 1996, ICMR 2000, Cooper 2012, Gunaratna 2015, Khulan 2012 and Otoluwa 2017 evaluated supplementation in the periconceptional period, and Nguyen 2012 evaluated the effect of pre‐conception supplementation. An 2001, Guldholt 1991, Graham 2007, Fleming 1986, and Wibowo 2012 assessed different doses of micronutrients; Agarwal 2012 evaluated different durations of the same micronutrients, while Feyi‐Waboso 2005 and Nwagha 2010 evaluated parenteral infusion or injection. Ramirez‐Velez 2011 did not contain an adequate comparison arm (they provided calcium in addition to ferrous sulphate and folic acid). Godfrey 2017 evaluated a drink enriched with micronutrients, probiotics and myo‐inositol, Callaghan‐Gillespie 2017 evaluated a food supplement (corn‐soy blend) enriched with micronutrients, Fall 2006 evaluated a micronutrient‐rich snack, Huang 2017 evaluated different maternal milk preparations, Nakano 2010 assessed the effect of chlorella tablets and Ling 1996 evaluated a herbal tonic. Li 2014 evaluated the effect of supplementation with folic acid and milk. Six excluded trials assessed the effect of fortification with MMN (Dieckmann 1944; Janmohamed 2016; Jarvenpaa 2007; Kureishy 2017; Tatala 2002; Vadillo‐Ortega 2011). Twelve trials included high‐risk women (Asemi 2015; Azami 2016; Christian 2003; Devi 2017; Gupta 2007; IRCT2015041321736N1; IRCT201704225623N109; ISRCTN83599025; NCT02802566; NCT02959125; Nossier 2015; Rumiris 2006). We excluded eight trials because they evaluated different forms of supplementation such as powder, tablet or spread (Choudhury 2012; Hambidge 2014; Huynh 2017; Lanou 2014; Young 2010); balanced energy protein supplementation (Huybregts 2009); weekly food provision (Wijaya‐Erhardt 2011); or polyunsaturated fatty acids fortification in milk fortified with MMN (Mardones 2007). The cohort of an included trial (Tofail 2008), was later randomised to breastfeeding counselling or standard care groups measuring the impact on postnatal growth in children (Kabir 2009), and hence we excluded it. We excluded Leroy 2010 because it compared a traditional food‐assisted maternal and child health and nutrition (MCHN) programme versus a newly designed approach to prevent malnutrition in children; Nguyen 2017 because it compared a nutrition‐focused MNCH programme with a standard MNCH (antenatal care with standard nutrition counselling) programme. We excluded one abstract of a trial because it was a trial in women with alcohol consumption during pregnancy (Kable 2012). We excluded Coles 2015 because of the trial's quasi‐randomised method of allocation to intervention and control arms. We excluded Dewey 2012 and Fernald 2016 because they evaluated the effects of lipid‐based nutrient supplements.
We reclassified Hininger 2004 and Theobald 1937 from included to excluded for the 2018 update because the MMN supplement did not contain iron.
See the Characteristics of excluded studies table for more details.
One report comparing MMN supplementation versus iron folic acid remains awaiting assessment due to missing group denominators (Gathwala 2012).
Risk of bias in included studies
The risk of bias of the included studies was generally low with at least 50% of the judgements at 'low risk' for two domains (allocation concealment and incomplete outcome data) and at least 75% of judgements at 'low risk' for the remaining five domains. The domain with the highest risk of bias was incomplete outcome data (attrition bias), for which we have conducted a sensitivity analysis. It is unlikely that the evidence presented in this review is affected by the biases evaluated.
See Figure 2; Figure 3 and Characteristics of included studies table for further details on the risk of bias of the included studies.
2.
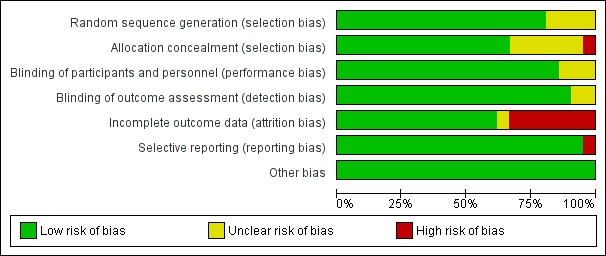
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
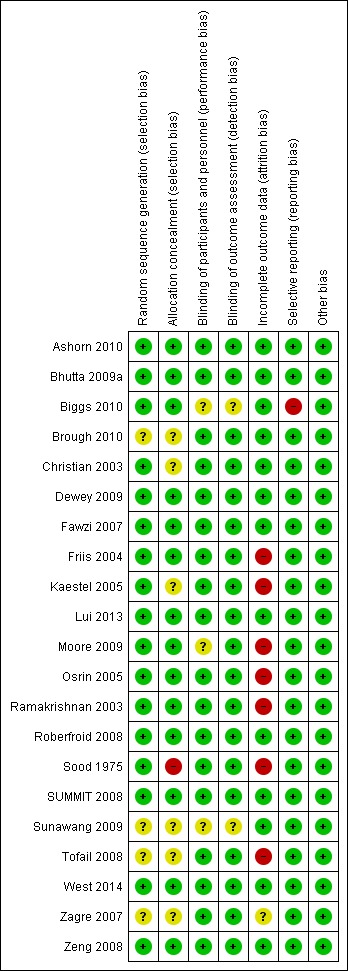
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
The included trials were of variable risk of bias. Seventeen trials adequately randomised participants to the treatment groups (Ashorn 2010; Bhutta 2009a; Biggs 2010; Christian 2003; Dewey 2009; Fawzi 2007; Friis 2004; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; Sood 1975; SUMMIT 2008; West 2014; Zeng 2008), whereas the remaining trials did not describe the method they used for generating the randomisation sequence in sufficient detail to permit judgement.
Fourteen trials concealed the allocation of participants to the intervention and control groups (Ashorn 2010; Bhutta 2009a; Biggs 2010; Dewey 2009; Fawzi 2007; Friis 2004; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; West 2014; Zeng 2008); it was unclear in six trials (Brough 2010; Christian 2003; Kaestel 2005; Sunawang 2009; Tofail 2008; Zagre 2007); whereas the remaining one trial probably did not conceal allocation (Sood 1975).
Blinding
Eighteen trials blinded the participants, personnel and outcome assessors to the treatment allocation (Ashorn 2010; Bhutta 2009a; Brough 2010; Christian 2003; Dewey 2009; Fawzi 2007; Friis 2004; Kaestel 2005; Lui 2013; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; Sood 1975; SUMMIT 2008; Tofail 2008; West 2014; Zagre 2007; Zeng 2008). However, Sunawang 2009 showed blinding of participants only and Moore 2009 indicated blinding of outcome assessors only. In Biggs 2010, it was not possible to blind the participants and personnel to the daily supplementation arm.
Incomplete outcome data
Loss to follow‐up was less than 5% in four trials (Dewey 2009; Moore 2009; West 2014; Zeng 2008); between 5% to 9.9% in eight trials (Ashorn 2010; Biggs 2010; Christian 2003; Fawzi 2007; Lui 2013; Osrin 2005; Roberfroid 2008; SUMMIT 2008); and between 10% to 19.9% in four trials (Bhutta 2009a; Brough 2010; Sunawang 2009; Zagre 2007). In one trial (Zagre 2007), although attrition was less than 20% and they reported the reasons for attrition, the proportion of women who dropped out was significantly higher in the MMN versus the iron‐folic acid group. In addition, they did not report exclusion data, so we have deemed this trial as 'unclear risk' of bias. Loss to follow‐up was more than 20% in five trials (Friis 2004; Kaestel 2005; Ramakrishnan 2003; Sood 1975; Tofail 2008). In Osrin 2005, although attrition was 5% and they reported reasons for it, exclusion was 39.5% and without reported reasons, and so we assessed it as being at 'high risk'. Similarly, we assessed Moore 2009 to be 'high risk' because, although attrition was 4.8% and reasons for it were not reported, exclusion was 25.6% and reasons were reported. All of the trials used intention‐to‐treat analysis except for two trials that used modified intention‐to‐treat analysis (Ashorn 2010; Biggs 2010).
Selective reporting
There was no indication of selective reporting in most of the included trials. One trial, however, did not present growth outcomes mentioned in the methods section in the results section of the paper, including weight‐for‐age and weight‐for‐length (Biggs 2010).
Other potential sources of bias
We did not identify any other potential sources of bias, including those related specifically to cluster design, in the included trials.
Effects of interventions
See: Table 1
Comparison 1: multiple micronutrients (MMN) versus control (all trials)
Twenty trials contributed data to this comparison. Nineteen out of 20 of these trials were carried out in low‐ and middle‐income countries and compared MMN supplements containing iron and folic acid to iron, with or without folic acid. One trial carried out in the UK compared MMN with placebo and contributed data to only four outcomes. In view of the differences in the settings where trials were conducted, and considering the control group conditions, we have presented results separately in the forest plots and in the text below.
Multiple micronutrients (MMN) with iron and folic acid versus iron, with or without folic acid
In this comparison, we included 19 trials conducted in low‐ and middle‐income countries that evaluated UNIMMAP or similar formulations of MMN supplement (Ashorn 2010; Bhutta 2009a; Biggs 2010; Christian 2003; Dewey 2009; Fawzi 2007; Friis 2004; Kaestel 2005; Lui 2013; Moore 2009; Osrin 2005; Ramakrishnan 2003; Roberfroid 2008; SUMMIT 2008; Sunawang 2009; Tofail 2008; West 2014; Zagre 2007; Zeng 2008). In two trials (Fawzi 2007; Friis 2004), women received iron and folic acid as separate supplements, and in one trial (Ramakrishnan 2003), women in the control group received iron only.
Primary outcomes
When we compared MMN supplementation against supplementation with iron with or without folic acid, there was probably a slight reduction in preterm births (average risk ratio (RR) 0.95, 95% confidence interval (CI) 0.90 to 1.01; studies = 18; random‐effects, Tau² = 0.00, I² = 49%; moderate‐quality evidence; Analysis 1.1), although the confidence interval for the pooled effect estimate just crossed the line of no effect. MMN supplementation also probably reduced births that were considered small‐for‐gestational age (SGA) (average RR 0.92, 95% CI 0.88 to 0.97; studies = 17; random‐effects, Tau² = 0.00, I² = 39%; moderate‐quality evidence; Analysis 1.2). MMN reduced births that were considered low birthweight (LBW) (average RR 0.88, 95% CI 0.85 to 0.91; studies = 18; random‐effects, Tau² = 0.00, I² = 0%; high‐quality evidence; Analysis 1.3), and made little or no difference to perinatal mortality (average RR 1.00, 95% CI 0.90 to 1.11; studies = 15; random‐effects, Tau² = 0.01, I² = 42%; high‐quality evidence; Analysis 1.4). Similar to preterm births, there was a slight reduction in stillbirths (average RR 0.95, 95% CI 0.86 to 1.04; studies = 17; random‐effects, Tau² = 0.00, I² = 12%; high‐quality evidence; Analysis 1.5), though the confidence interval for the pooled effect estimate just crossed the line of no effect. MMN supplementation did not have an important effect on neonatal mortality (average RR 1.00, 95% CI 0.89 to 1.12; studies = 14; random‐effects, Tau² = 0.01, I² = 22%; high‐quality evidence; Analysis 1.6). Visual inspection of funnel plots for all primary outcomes revealed no obvious funnel plot asymmetry (Figure 4; Figure 5; Figure 6; Figure 7), with the exception of preterm births (Figure 8), and SGA (Figure 9), where smaller studies appeared to report slightly more pronounced treatment effects.
1.1. Analysis.
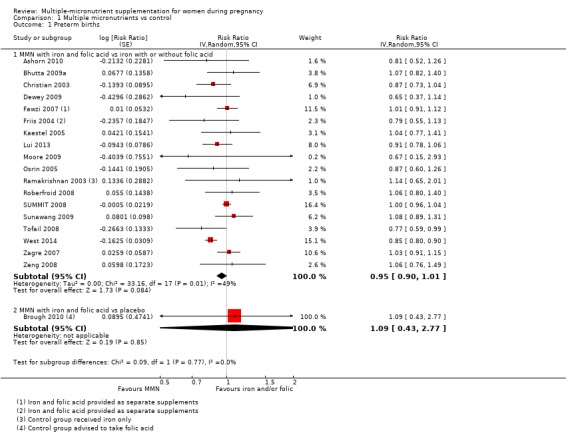
Comparison 1 Multiple micronutrients vs control, Outcome 1 Preterm births.
1.2. Analysis.
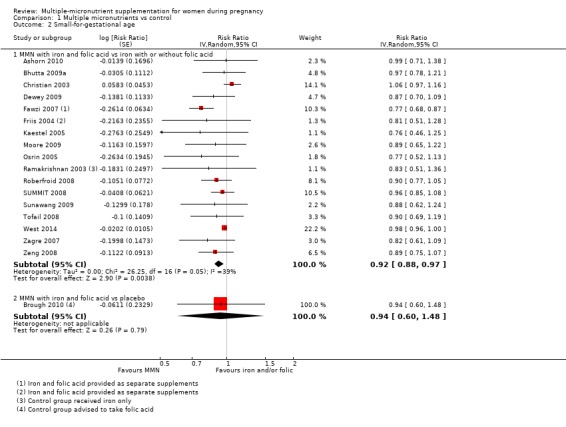
Comparison 1 Multiple micronutrients vs control, Outcome 2 Small‐for‐gestational age.
1.3. Analysis.
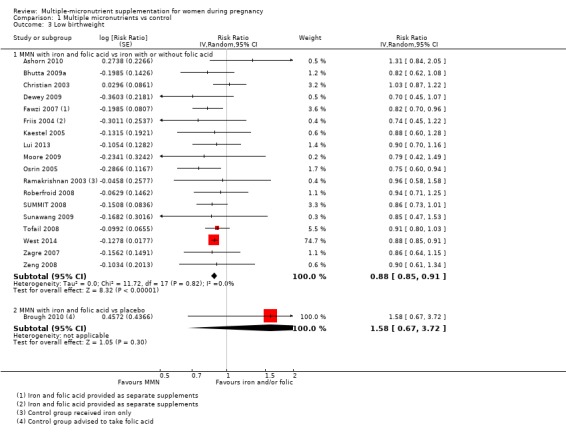
Comparison 1 Multiple micronutrients vs control, Outcome 3 Low birthweight.
1.4. Analysis.
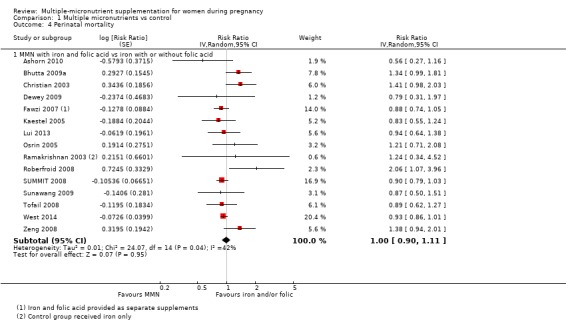
Comparison 1 Multiple micronutrients vs control, Outcome 4 Perinatal mortality.
1.5. Analysis.
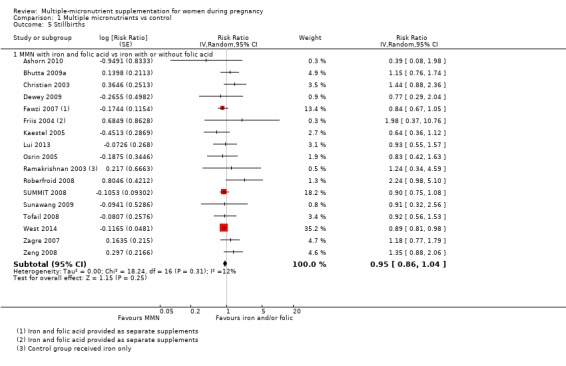
Comparison 1 Multiple micronutrients vs control, Outcome 5 Stillbirths.
1.6. Analysis.
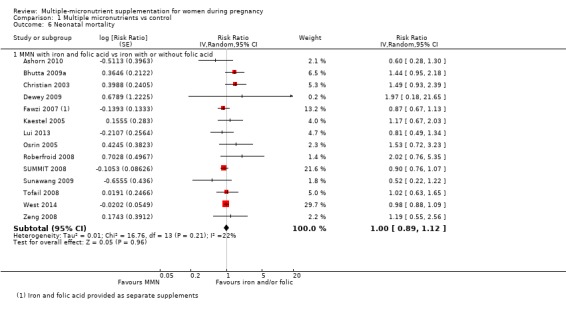
Comparison 1 Multiple micronutrients vs control, Outcome 6 Neonatal mortality.
4.
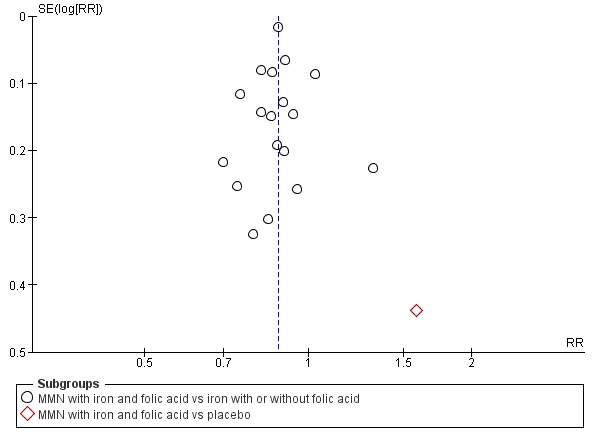
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.3 Low birthweight
5.
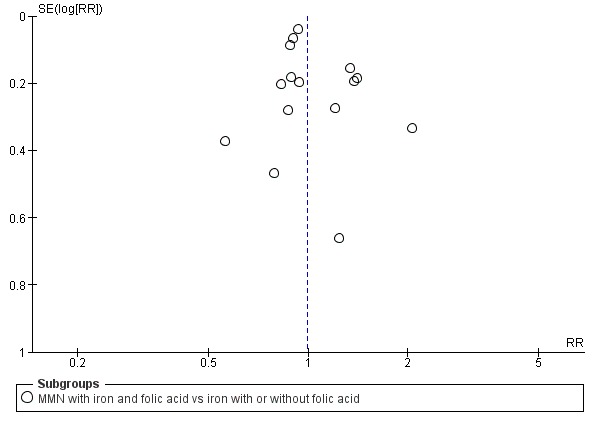
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.4 Perinatal mortality
6.
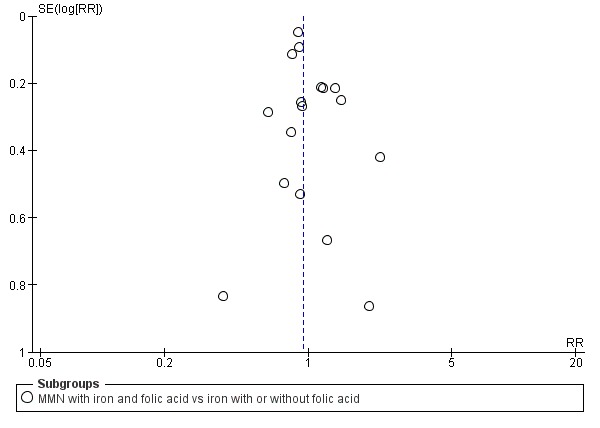
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.5 Stillbirths
7.
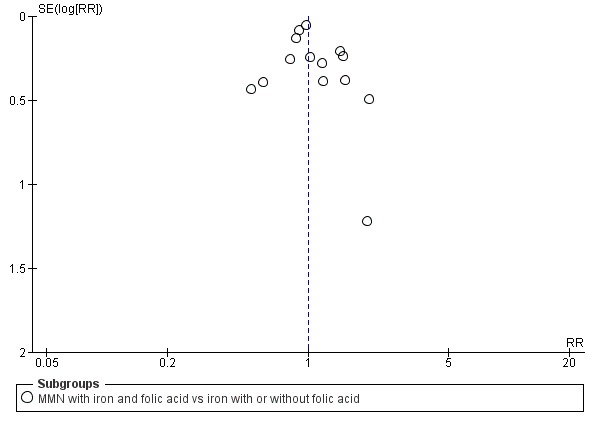
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.6 Neonatal mortality
8.
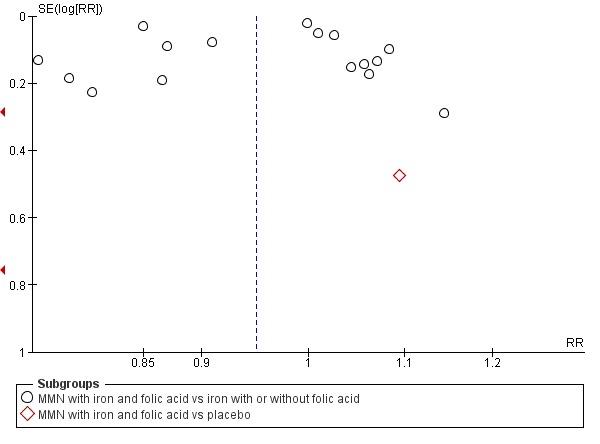
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.1 Preterm births
9.
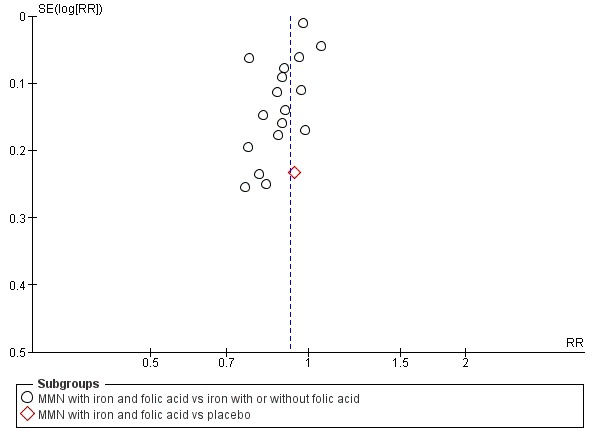
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.2 Small‐for‐gestational age
It should be noted that where none of the individual trial reports reported an outcome, then we obtained data from a separate supplement (Food and Nutrition Bulletin 2009), where possible. We took data for SGA estimates for the following trials from the Food and Nutrition Bulletin: Friis 2004; Kaestel 2005; Osrin 2005; Ramakrishnan 2003; Sunawang 2009; Tofail 2008; Zagre 2007. Similarly, we took data for preterm birth for the following trials: Kaestel 2005; Sunawang 2009; Tofail 2008; Zagre 2007; data for LBW for Tofail 2008; data for perinatal mortality for Sunawang 2009; and data for stillbirth for Kaestel 2005, from the same report.
Secondary outcomes
When we compared MMN supplementation to iron supplementation with or without folic acid, there was little or no difference between groups in: maternal anaemia in the third trimester (average RR 1.04, 95% CI 0.94 to 1.15; studies = 9; random‐effects, Tau² = 0.01, I² = 50%; Analysis 1.7); maternal mortality (average RR 1.06, 95% CI 0.72 to 1.54; studies = 6; random‐effects, Tau² = 0.00, I² = 0%; Analysis 1.8); miscarriage (average RR 0.99, 95% CI 0.94 to 1.04; studies = 12; random‐effects, Tau² = 0.00, I² = 0%; Analysis 1.9); delivery via a caesarean section (average RR 1.13, 95% CI 0.99 to 1.29; studies = 5; random‐effects, Tau² = 0.00, I² = 0%; Analysis 1.10); and congenital anomalies (average RR 1.34, 95% CI 0.25 to 7.12; studies = 2; random‐effects, Tau² = 0.00, I² = 0%; Analysis 1.11). However, there was probably a reduction in very preterm births (average RR 0.81, 95% CI 0.71 to 0.93; studies = 4; random‐effects, Tau² = 0.00, I² = 10%; Analysis 1.12). Of the secondary outcomes examined, only miscarriage had a sufficient amount of studies to create a funnel plot. Visual inspection of the funnel plot for this outcome (Figure 10), suggested that smaller studies reported more substantial treatment effects.
1.7. Analysis.
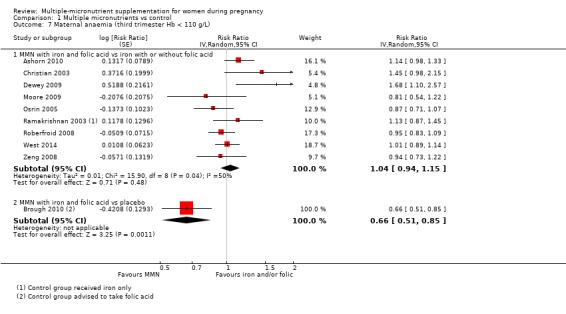
Comparison 1 Multiple micronutrients vs control, Outcome 7 Maternal anaemia (third trimester Hb < 110 g/L).
1.8. Analysis.
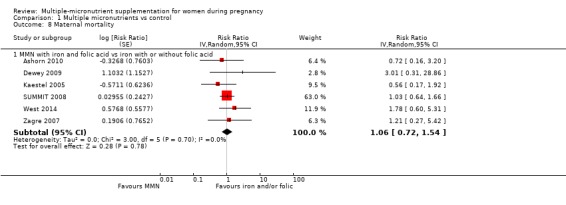
Comparison 1 Multiple micronutrients vs control, Outcome 8 Maternal mortality.
1.9. Analysis.
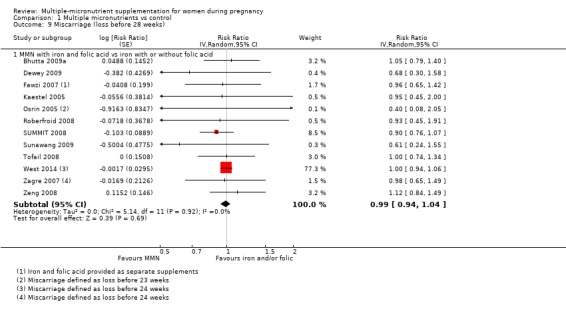
Comparison 1 Multiple micronutrients vs control, Outcome 9 Miscarriage (loss before 28 weeks).
1.10. Analysis.
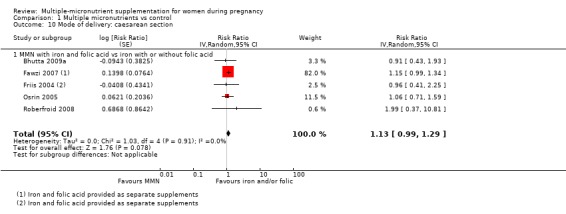
Comparison 1 Multiple micronutrients vs control, Outcome 10 Mode of delivery: caesarean section.
1.11. Analysis.
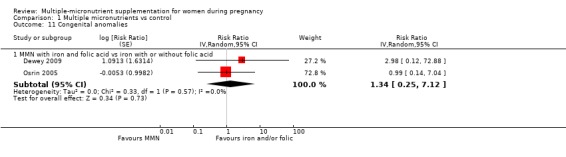
Comparison 1 Multiple micronutrients vs control, Outcome 11 Congenital anomalies.
1.12. Analysis.
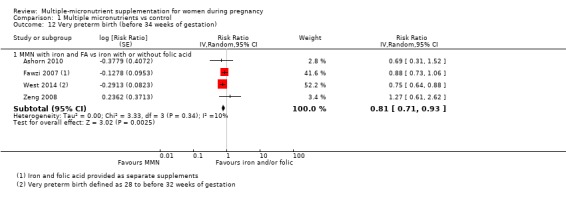
Comparison 1 Multiple micronutrients vs control, Outcome 12 Very preterm birth (before 34 weeks of gestation).
10.
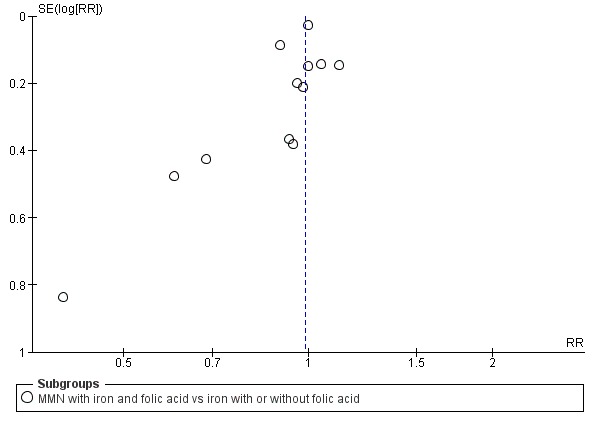
Funnel plot of comparison 1. Multiple micronutrients vs control, outcome: 1.9 Miscarriage (loss before 28 weeks)
There were a number of prespecified clinically important outcomes that we could not assess due to insufficient data from the included trials. These included the following outcomes, which only one or no trials measured, or which the trials presented in a format that precluded their inclusion in the analysis: premature rupture of membranes, pre‐eclampsia, macrosomia (Roberfroid 2008), placental abruption, neurodevelopmental delay of infants (Zeng 2008), nutritional status of the children (Dewey 2009; Roberfroid 2008), cost of supplementation, side‐effects of MMN supplementation (Lui 2013; Tofail 2008), and maternal well‐being or satisfaction.
Multiple micronutrients (MMN) versus placebo
One trial conducted in the UK (Brough 2010), contributed data to this analysis. In this trial, women in the control group were advised to take folic acid. Brough 2010 randomised 402 women. Women receiving supplements were at reduced risk of anaemia in the third trimester (average RR 0.66, 95% CI 0.51 to 0.85; Analysis 1.7), but there were little or no differences between women receiving supplements and those in the placebo group for any of the other outcomes reported: preterm birth (average RR 1.09 95% CI 0.43 to 2.77; Analysis 1.1); SGA (average RR 0.94, 95% CI 0.60 to 1.48; Analysis 1.2); or LBW (average RR 1.58, 95% CI 0.67 to 3.72; Analysis 1.3).
Subgroup analysis (data shown in comparison 2) multiple micronutrients (MMN) with iron and folic acid versus iron with or without folic acid)
For the trials comparing MMN with iron and folic acid versus iron with or without folic acid (19 trials), we found substantial heterogeneity in the analyses for preterm birth, SGA, and perinatal mortality, and explored its presence through subgroup analyses.
For the outcome preterm birth, MMN supplementation probably led to fewer preterm births for women in a subgroup of trials with mean maternal BMI of less than 20 kg/m² (average RR 0.85, 95% CI 0.81 to 0.90; studies = 3), compared to women with a BMI of at least 20 kg/m² (average RR 0.99, 95% CI 0.96 to 1.03; studies = 15; the test for subgroup differences P < 0.00001, I² = 95.2%; Analysis 2.1). There were little or no differences among subgroups based on mean maternal height (Analysis 2.2), the timing of supplementation (Analysis 2.3), or the dose of iron (Analysis 2.4) (all P > 0.05). However, MMN supplementation probably led to fewer preterm births among women who took non‐UNIMMAP supplements (average RR 0.89, 95% CI 0.82 to 0.98; studies = 8) compared to those who took UNIMMAP supplements (average RR 1.00, 95% CI 0.96 to 1.03; studies = 10; the test for subgroup differences P = 0.02, I2 = 80.4%; Analysis 2.5).
2.1. Analysis.
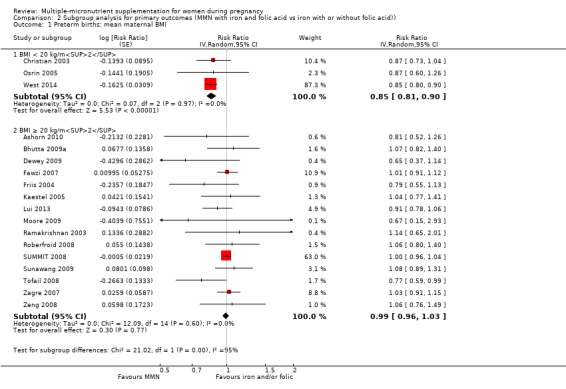
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 1 Preterm births: mean maternal BMI.
2.2. Analysis.
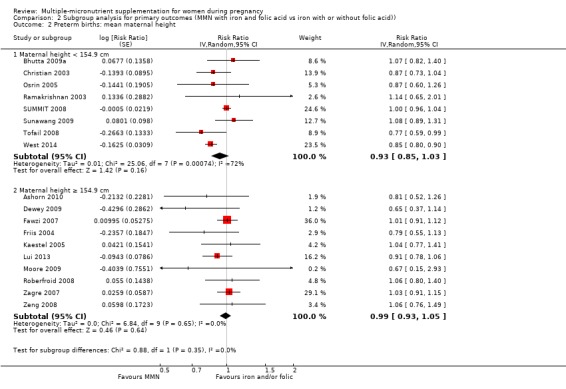
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 2 Preterm births: mean maternal height.
2.3. Analysis.
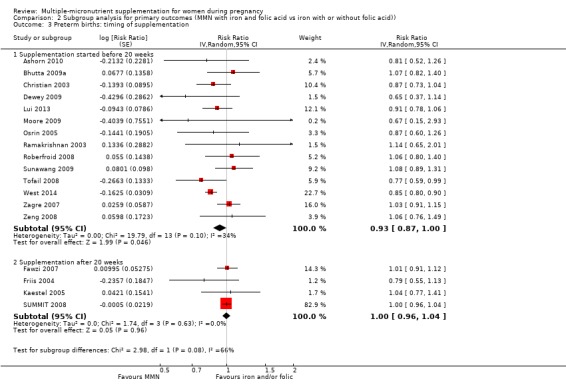
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 3 Preterm births: timing of supplementation.
2.4. Analysis.
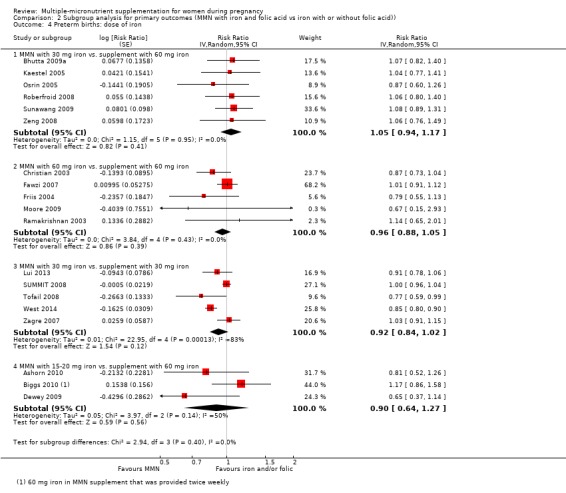
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 4 Preterm births: dose of iron.
2.5. Analysis.
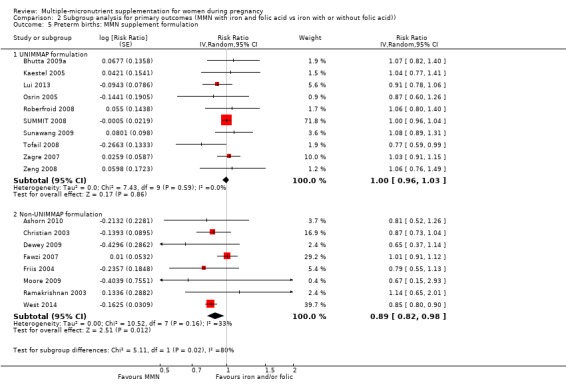
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 5 Preterm births: MMN supplement formulation.
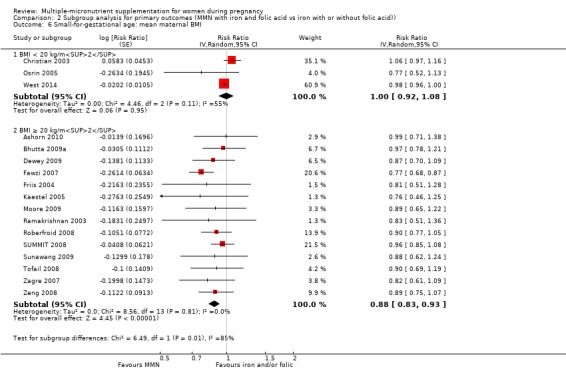
For the outcome SGA, MMN supplementation probably led to fewer SGA births for women in a subgroup of trials with mean maternal BMI of at least 20 kg/m² (average RR 0.88, 95% CI 0.83 to 0.93; studies = 14), but not for women with a mean maternal BMI of less than 20 kg/m² (average RR 1.00, 95% CI 0.92 to 1.08; studies = 3; test for subgroup differences P = 0.01, I² = 84.6%; Analysis 2.6). Similarly, we observed some differences between the subgroups of studies based on maternal height (Analysis 2.7). MMN supplementation probably reduced SGA births for women with a mean maternal height of at least 154.9 cm (average RR 0.85, 95% CI 0.79 to 0.91; studies = 9), and probably slightly reduced SGA births for women with a mean maternal height of less than 154.9 cm (average RR 0.98, 95% CI 0.96 to 1.00; studies = 8; test for subgroup differences P < 0.0001, I² = 93.7%; Analysis 2.7). There were little or no differences in SGA among subgroups based on timing of supplementation (Analysis 2.8), dose of iron (Analysis 2.9), or MMN supplement formulation (Analysis 2.10), when comparing MMN supplementation to iron with or without folic acid.
2.6. Analysis.
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 6 Small‐for‐gestational age: mean maternal BMI.
2.7. Analysis.
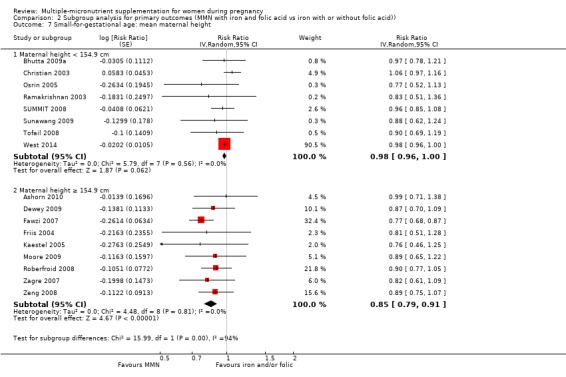
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 7 Small‐for‐gestational age: mean maternal height.
2.8. Analysis.
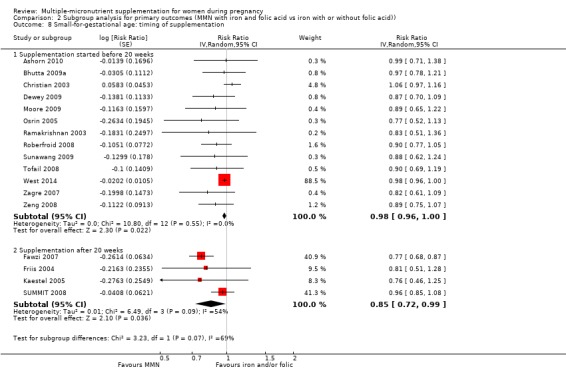
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 8 Small‐for‐gestational age: timing of supplementation.
2.9. Analysis.
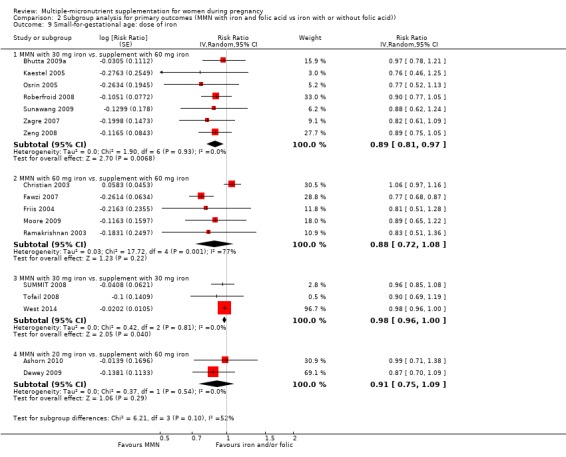
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 9 Small‐for‐gestational age: dose of iron.
2.10. Analysis.
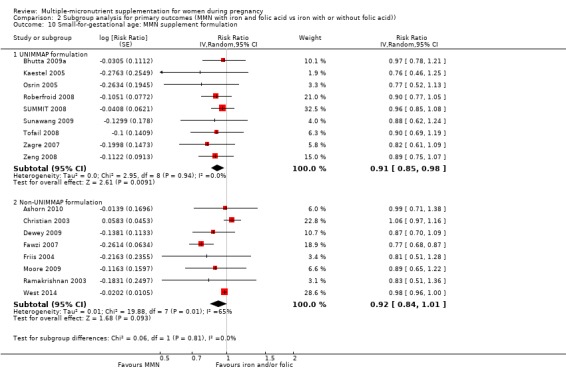
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 10 Small‐for‐gestational age: MMN supplement formulation.
While we found that MMN supplementation made no difference to perinatal mortality as an outcome, the analysis demonstrated substantial statistical heterogeneity. However, subgroup analyses did not show clear differences based on mean maternal BMI (Analysis 2.11), mean maternal height (Analysis 2.12), dose of iron (Analysis 2.14) and MMN supplement formulation (Analysis 2.15) (all P > 0.05). However, we observed differences between subgroups based on the timing of supplementation. The reduction in perinatal mortality was probably higher in the subgroup with supplementation after 20 weeks (average RR 0.89, 95% CI 0.80 to 0.98; studies = 3) compared to the subgroup where women began supplementation before 20 weeks (average RR 1.09, 95% CI 0.92, 1.27; studies = 12; test for subgroup differences P = 0.04, I² = 76.5%; Analysis 2.13).
2.11. Analysis.
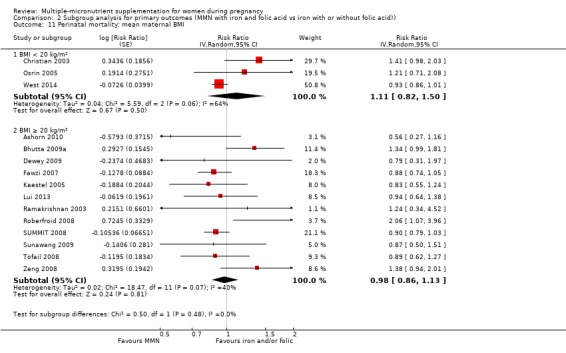
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 11 Perinatal mortality: mean maternal BMI.
2.12. Analysis.
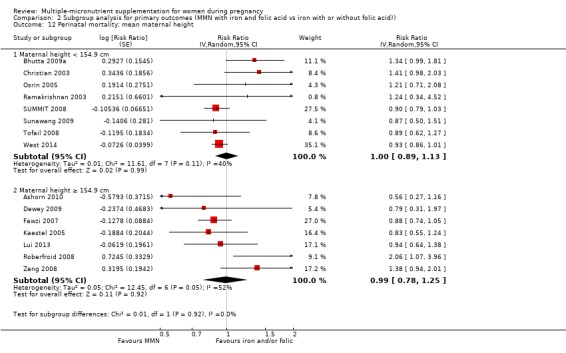
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 12 Perinatal mortality: mean maternal height.
2.14. Analysis.
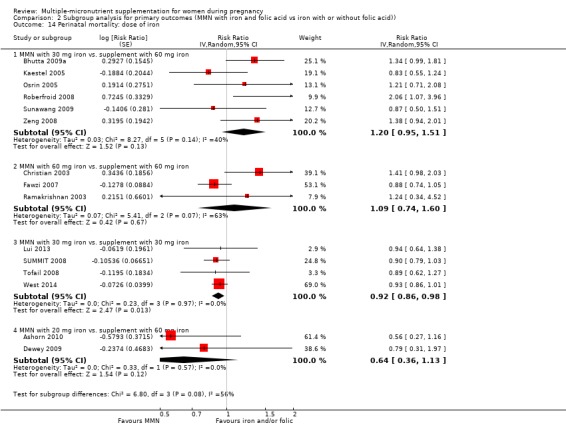
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 14 Perinatal mortality: dose of iron.
2.15. Analysis.
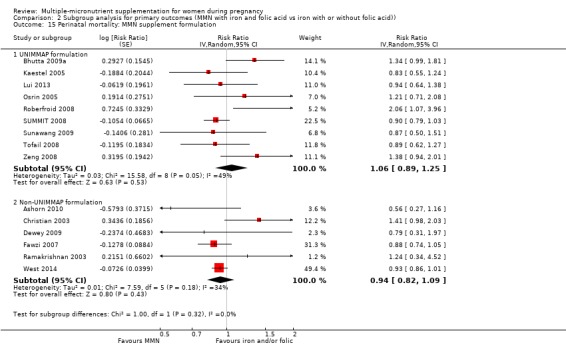
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 15 Perinatal mortality: MMN supplement formulation.
2.13. Analysis.
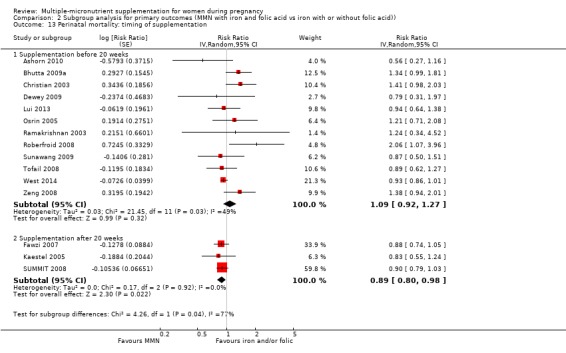
Comparison 2 Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)), Outcome 13 Perinatal mortality: timing of supplementation.
Sensitivity analysis (data shown in comparison 3) multiple micronutrients (MMN) with iron and folic acid versus iron, with or without folic acid
We undertook sensitivity analysis to study the effect of MMN supplementation on each outcome by excluding trials with loss to follow‐up of more than 20% from the analyses (Friis 2004; Kaestel 2005; Ramakrishnan 2003; Tofail 2008). This exclusion did not significantly affect the findings for the outcomes.
Discussion
Summary of main results
We identified 21 trials (involving 142,496 women) as eligible for inclusion in this review but only 20 trials (involving 141,849 women) contributed data to the review. This updated Cochrane Review summarises the current evidence on the effect of MMN supplementation during pregnancy on fetal, infant, and maternal outcomes. Overall, MMN supplementation with iron and folic acid versus supplementation with iron (with or without folic acid) showed an 8% reduction in the risk of SGA births (average risk ratio (RR) 0.92, 95% CI 0.88 to 0.97; moderate‐quality evidence), and a 12% reduction in the risk of LBW (average RR 0.88, 95% CI 0.85 to 0.91; high‐quality evidence). MMN supplementation slightly reduced the risk of both preterm births (average RR 0.95, 95% CI 0.90 to 1.01; 18 trials, moderate‐quality evidence), and stillbirths (average RR 0.95, 95% CI 0.86 to 1.04; high‐quality evidence), with the caveat that the confidence intervals for the pooled effects for both of these outcomes just crossed the line of no effect. We found that MMN supplementation did not have an important effect on perinatal mortality (average RR 1.00, 95% CI 0.90 to 1.11; high‐quality evidence), and neonatal mortality (average RR 1.00, 95% CI 0.89 to 1.12; high‐quality evidence). Of the secondary outcomes examined, we found that MMN supplementation reduced the risk of very preterm births by 19% (average RR 0.81, 95% CI 0.71 to 0.93). A summary of the main findings for trials comparing MMN with iron and folic acid versus iron with or without folic acid is presented in Table 1.
Overall completeness and applicability of evidence
This review included a total of 21 trials that evaluated the impact of MMN supplementation. The earliest trial was published in 1975 (Sood 1975), though this trial did not contribute data to the review because it reported its outcomes in a format that we could not use in the meta‐analyses. An additional trial (Biggs 2010), did not contribute data to the main analyses because women received supplements twice‐weekly as opposed to daily. All trials evaluating the UNIMMAP supplement (Bhutta 2009a; Kaestel 2005; Lui 2013; Osrin 2005; Roberfroid 2008; SUMMIT 2008; Sunawang 2009; Tofail 2008; Zagre 2007; Zeng 2008), proposed in 1999 by UNICEF, UNU, and WHO, started recruitment of participants as early as 2001 and we included them in the analysis along with trials that evaluated adapted‐UNIMMAP formulations. Inclusion of these and older trials, identified as a result of an extensive search of literature published over the last several decades, represents overall completeness of evidence.
As iron/folic acid is recommended for pregnant women in low‐ and middle‐income countries, we primarily evaluated the effect of adding additional micronutrients to the iron and folic acid supplement. The vast majority (95%) of the included trials were conducted in low‐ and middle‐income countries, and included pregnant women at varying gestational stages, ranging from early pregnancy to 36 weeks of gestation.
In many low‐ and middle‐income countries, fertility rates are high, alongside a high prevalence of maternal iron‐deficiency anaemia and subclinical micronutrient deficiencies (Bhutta 2008). Studies have shown that a significant proportion of pregnant women suffer from multiple concurrent micronutrient deficiencies, especially throughout pregnancy when nutritional demands are increased. These deficiencies have been associated with a host of poor pregnancy outcomes including LBW, preterm, SGA, perinatal mortality, and maternal mortality (Allen 2001; Allen 2005; Christian 2010; De‐Regil 2016; Dror 2011; Haider 2013; Keen 2003; MacKay 2001; Ota 2015). Increased risk of infection has also been postulated to be associated with anaemia, especially as a result of iron deficiency (Oppenheimer 2001). Our findings of improved LBW, SGA, and very preterm births, then, could be reflective of improved nutritional status of mothers and hence better resistance to maternal infections following MMN supplementation.
Maternal anthropometry pre‐pregnancy and weight gain during pregnancy have also been associated with various neonatal and child outcomes. Maternal height is a relatively stable and easily measurable variable in the setting of low‐ and middle‐income countries. Reviews have identified short maternal stature (short height) as an important determinant of intrauterine growth retardation and LBW (Kramer 2003; WHO 1995). Short maternal stature has been associated with an increased risk of child mortality, underweight infants, and stunting (Ozaltin 2010; Voigt 2010). Our subgroup analyses indicated that MMN supplementation did not reduce SGA in women with poor nutritional status at baseline, defined as maternal height less than 154.9 cm and BMI less than 20 kg/m². However, MMN supplementation did reduce the risk of SGA babies among women with a mean maternal height of at least 154.9 cm. Similarly, MMN supplementation reduced the risk of SGA babies among women with a mean BMI of at least 20 kg/m². These findings should be interpreted with caution, but suggest a possible role of MMN in preventing SGA only among women with good nutritional status at baseline, and underscore an absence of similar effect in women with poor nutritional status at the time of conception. On the contrary, the findings for the subgroup analysis for preterm birth indicate a reduction among women with low BMI, but not among those with higher BMI. These differences further highlight the complex contribution of maternal malnutrition to fetal anthropometry and infant growth that relates to the intergenerational transfer of malnutrition. Though micronutrient supplementation has been implicated in improved child growth and survival, there is currently no evidence to suggest that maternal MMN supplementation during pregnancy improves child growth or survival when compared with iron/folic acid supplementation. Additional studies with long‐term follow‐up are required.
Quality of the evidence
We evaluated the quality of the available evidence by using the GRADE methodology as outlined in the GRADE Handbook. We created a 'Summary of findings' table for the primary outcomes of preterm birth, SGA, LBW, stillbirths, perinatal and neonatal mortality for Comparison 1: MMN with iron and folic acid versus iron with or without folic acid supplement.
When assessed according to GRADE criteria, the quality of evidence for the review's primary outcomes overall was moderate to high. We based pooled results for all primary outcomes on multiple randomised controlled trials with large sample sizes and precise estimates. For the comparison of MMN versus control (iron with or without folic acid) we graded the following outcomes as high quality: LBW, stillbirth, neonatal mortality, and perinatal mortality. The outcomes of preterm birth and SGA we graded as moderate quality; we downgraded both by one for funnel plot asymmetry, indicating possible publication bias.
Potential biases in the review process
This update of the review includes additional data published since the last update (2017). We conducted an extensive literature search to identify any additional studies since the last search. Two review authors independently conducted the screening of the updated search results, selection of eligible studies and data extraction. Two review authors also assessed the risk of bias. Given the application of the above Cochrane methodology, it is unlikely that the findings of this review are affected by biases in the review process.
Agreements and disagreements with other studies or reviews
The findings of reduction in SGA, LBW and very preterm risk as a result of MMN supplementation in the current review corroborate those of other systematic reviews and meta‐analyses conducted since the first version of this Cochrane Review (Bhutta 2012; Kawai 2011; Margetts 2009; Ramakrishnan 2012). Another systematic review and meta‐analysis (Christian 2015), also reports reduction in the risk of preterm births, LBW, and SGA; however, the effect on SGA is reported to be marginal (RR 0.91, 95% CI 0.84 to 1.00). This review included a smaller number of studies in the SGA analysis (studies = 7), as opposed to the current review (studies = 17), thereby explaining the difference between the estimates reported in the two reviews.
Another recent exercise (Smith 2017), used individual participant data from 17 randomised trials in low‐ and middle‐income countries to perform a two‐stage meta‐analysis comparing MMN and iron‐folic acid supplementation among pregnant women and identify potential modifiers of effect. Smith 2017 also found a reduction in LBW (random‐effects RR 0.86, 95% CI 0.81 to 0.92), a slight reduction in SGA (random‐effects RR 0.94, 95% CI 0.90 to 0.98), and some reduction in preterm births (random‐effects RR 0.93, 95% CI 0.87 to 0.98). Similar to this review, there were no important survival benefits (or harms) noted. Smith 2017 also demonstrated a greater effect of MMN supplementation on preterm births among underweight women when compared to normal weight women and, although tests for heterogeneity were not significant, there appeared to be a trend towards higher impact on SGA for women with greater BMI and height, as we have also shown. Though we did not stratify data by anaemia status, authors found that MMN supplementation caused greater reductions in LBW, SGA, and six‐month mortality among anaemic pregnant women when compared to those who were not anaemic. Taken together with the results of our subgroup analyses, this points to maternal nutritional status at baseline as a modifier of the effect of MMN supplementation for some important outcomes, and should instigate further studies to better elucidate these effects and the mechanisms behind them.
We found that MMN supplementation did not have an important effect on perinatal mortality, stillbirths, and neonatal mortality, which are similar to findings from earlier versions of this Cochrane Review and other systematic reviews and meta‐analyses (Christian 2005; Haider 2011; Ronsmans 2009; Smith 2017). With the additional studies included in this 2018 update, RR estimates for perinatal mortality, stillbirths, and neonatal mortality declined from 1.01 (0.91 to 1.13), 0.97 (0.87 to 1.09), and 1.06 (0.92 to 1.22) to 1.00 (0.90 to 1.11), 0.95 (0.86 to 1.04), and 1.00 (0.89 to 1.12), respectively. Previously, concerns were raised regarding the possibly harmful effects of MMN supplements relating to risk of perinatal and neonatal mortality through increased birth asphyxia in heavier babies (Christian 2005). Indeed, the Smith 2017 analysis found a slightly increased risk of large‐for‐gestational‐age births (defined by the Intergrowth standard) following MNN supplementation, but no indication of heightened risk of stillbirth or mortality. Two earlier trials conducted in Nepal (Christian 2003; Osrin 2005), both found no important increase in the risk of neonatal and perinatal mortality, though their pooled effect estimate showed an increase in the risk of these outcomes. This concern was questioned by other researchers in the field and has not been observed in other studies (Bhutta 2009b; Huffman 2005; Shrimpton 2005). Importantly, our current findings of no impact on neonatal mortality are supported by those of two MMN supplementation trials that were individually powered to evaluate an effect on early infant mortality (SUMMIT 2008; West 2014). The recent large trial in Bangladesh did not show an increase in neonatal or early infant mortality risk in the MMN supplementation group verus iron and folic acid (West 2014). The trial authors, however, in post‐hoc analysis, report higher neonatal mortality among boys due to birth asphyxia (West 2014). This finding should be interpreted with caution as the cause of death was ascertained by verbal autopsy with parents, which may result in misclassification of the underlying cause of death. Moreover, the trial was not powered to detect a statistically significant difference in cause‐specific mortality by gender. Interestingly, Smith 2017 found about a 15% reduction in neonatal mortality for females babies but no effect for male babies, indicating that there may be a biological difference in the impact of MMN supplementation relating to sex. Previous work has suggested that this may be a function of birth complications relating to size, with greater length, weight, and head circumference typically being true of male babies (West 2014). However, Smith 2017 noted no sex‐specific differences in stillbirths, indicating that other, potentially context‐specific, mechanisms may be responsible for these differences. These findings warrants further research.
Authors' conclusions
Implications for practice.
Our findings suggest a benefit in the use of multiple‐micronutrient (MMN) supplements with iron and folic acid in low‐ and middle‐income settings to improve low birthweight, small‐for‐gestational age, and possibly preterm births. We have also demonstrated that MMN supplementation does not have an important effect (neither beneficial nor harmful), on mortality outcomes, including stillbirths, perinatal, and neonatal mortality. These findings have been consistently observed in other systematic evaluations of evidence.
Implications for research.
Based on results from our subgroup analyses, further research could be conducted to better understand how baseline nutritional status (maternal BMI and height) may affect pregnancy and birth outcomes following supplementation with MMN. Additionally, determining the optimal formulation for MMN supplements could have beneficial effects in practice. A greater understanding of the biological mechanisms through which MMN supplementation acts would be beneficial.
Feedback
Professor Caroline Fall, 31 May 2016
Summary
Our comments relate mainly to the stillbirth analysis. We believe a fixed‐effect model is inappropriate for this analysis. While there was no statistical heterogeneity, the Cochrane handbook and the methods section of the review state that clinical (contextual) heterogeneity should be the main driver of the choice of model, rather than statistical heterogeneity. Contextual heterogeneity was evident in a number of aspects of these trials, including their design (individual or cluster randomized), interventions (composition of multiple micronutrient supplements and duration of supplementation), co‐interventions (for example early or late food supplementation), comparison groups (iron alone, IFA, and different doses of these, especially iron), participants (geographic location, phenotype, and gestational age at randomization) and outcome definition (definition of stillbirths varied from ≥24 weeks to ≥28 weeks gestation, including or excluding multiple births). There is inconsistency in the use of fixed or random‐effects models within the review; for example, for perinatal mortality (of which stillbirths form a sub‐set, Analysis 1.4), including 12 of the 15 trials contributing to the stillbirth analysis, a random effects meta‐analysis was used.
We think that three eligible trials have been omitted from the review (Ashorn 20151; Hanieh 20132 [protocol of this trial is cited as Biggs 2011a]; and Adu‐Afarwuah 20153 [protocol cited as Dewey 2011a). These are all listed in the review as ‘ongoing trials’, but they were published before the stated search date of March 2015. Inclusion of these trials makes little difference to the overall results, but a Cochrane review is expected to be complete.
The Methods section under “Unit of analysis issues” does not clearly define the strategy for comparisons in trials with factorial designs. Further, the comparisons made are unclear in places and inconsistent across studies. This issue potentially applies to 4 studies (Christian 2003; Kaestel 2005; Lui 2013; Zeng 2008), all of which had more than one eligible intervention and/or control group.
A reduction in stillbirths is the only direct benefit to health that has been reported as a result of multiple micronutrient supplements in pregnancy (as opposed to ‘metric’ outcomes like birth weight and rates of preterm birth), and is currently the only evidence sufficient to justify changing routine supplementation from IFA to MMN. A significant increase in birth weight might be expected to lead to significant health benefits (e.g.. reduced infant mortality) but until this review, there was no apparent effect of MMN supplementation in pregnancy on mortality. The soundness of the stillbirth evidence is therefore crucial to the policy review, and we believe that the evidence remains inconclusive.
Finally, we are curious to know how the review authors presented data for smallness for gestational age (SGA) as an outcome (Analysis 1.2) because data for SGA were not published for some of the trials.
(Summary of feedback from Harshpal Singh Sachdev, Delanjathan Devakumar, Caroline Fall, Clive Osmond, David Osrin, Jonathan Broad, Barrie Margetts, May 2016).
References
Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, et al. The impact of lipid‐based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. The American Journal of Clinical Nutrition 2015; 101: 387‐97.
Hanieh S, Ha TT, Simpson JA, Casey GJ, Khuong NC, Thoang DD, et al. The effect of intermittent antenatal iron supplementation on maternal and infant outcomes in rural Viet Nam: a cluster randomised trial. PLoS Med 2013; 10: e1001470.
Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, et al. Lipid‐based nutrient supplement increases the birth size of infants of primiparous women in Ghana. The American Journal of Clinical Nutrition 2015; 101: 835‐46.
Reply
We would like to thank you and your colleagues for the detailed comments and queries on our review. We have made edits to the review to address the specific queries. We also plan to update this important review over the coming year, since the search will become out of date in March 2017.
1. Random‐effects versus fixed‐effect model – stillbirth analysis
Regarding the comment about the inconsistent use of fixed or random effects model in the review, please note we had not used these inconsistently. While we agree regarding the presence of contextual differences between the included trials, the decision to select fixed or random effects model for stillbirth analysis was based on the values of I², Tau² and/or p value, as per the methodological guidelines of Cochrane Pregnancy and Childbirth reviews: “We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.” We have taken on board your comments and changed the stillbirth results to RR 0.97, 95% CI 0.87 to 1.09, using a random effects model. However, as our group statistician has advised, this is a very conservative approach and an interpretation of a possible reduction in stillbirth would also be valid.
2. Omission of trials
Regarding the omission of three eligible trials, namely Hanieh 2013, Ashorn 2015, and Adu‐Afarwuah 2015, we consulted the Information Specialist of Cochrane Pregnancy and Childbirth. The Information Specialist has informed us that one of the trials, Adu‐Afarwuah 2015 was not identified in the literature search conducted on 11th March 2015 – and had not been added to the Cochrane Pregnancy and Childbirth group trials register at that time. Regarding Ashorn 2015, this was in Ongoing studies, but has now been moved to Characteristics of studies awaiting classification. The review authors have requested additional data from the trial authors for Ashorn 2015, as stated in the notes section of the Characteristics of studies awaiting classification table. This trial included HIV+ patients: “This trial included women with a + HIV test: IFA = 15.6%; MMN 11.1% and LNS 14.4%. We have contacted authors to see if separate analyses for HIV‐ women are available.” Hanieh 2013 had been assigned to another review on intermittent iron, but will be re‐assessed at next update. The review authors will assess all three of these studies in the next update of this review.
3. Unit of analysis issues ‐ Trials with multiple intervention groups
We appreciate that our methods section had not detailed clearly how unit of analysis issues for trials with multiple intervention groups had been dealt with. We have made edits to address this issue, as detailed in the Unit of analysis issues section for Christian 2003; Kaestel 2005; Lui 2013; Zeng 2008. For trials with multiple intervention groups, we selected one pair of interventions and excluded the others. This is one approach recommended by the Cochrane Handbook [16.5.4]. If more than two intervention groups had met the eligibility criteria, we would have combined groups to create a single pair‐wise comparison as per [16.5.4] of the Cochrane Handbook.
4. Source of data for Small for gestational age (SGA) Unit of analysis issues ‐ Trials with multiple intervention groups
We have spoken to the Research Associate, Cochrane Pregnancy and Childbirth, who helped with the last update. The SGA data for all but three trials, came from a separate report (Food and Nutrition Bulletin 2009). Only in three trials were we able to extract SGA data directly from the trial reports (Brough 2010; Fawzi 2007; West 2014).
(Reply from Batool A Haider, Zulfiqar A Bhutta, Philippa Middleton, March 2017).
Contributors
Harshpal Singh Sachdev MD, Senior Consultant in Pediatrics and Clinical Epidemiology, Sitaram Bhartia Institute of Science and Research, New Delhi, India Delanjathan Devakumar PhD, NIHR Clinical Lecturer in Public Health, Institute for Global Health, Faculty of Population Health Sciences, University College London, London, UK Caroline Fall DM, Professor of International Paediatric Epidemiology, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK Clive Osmond PhD, Professor of Biostatistics, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK David Osrin PhD, Professor of Global Health, Institute for Global Health, Faculty of Population Health Sciences, University College London, London, UK Jonathan Broad MBChB, Honorary research fellow, University of Bristol, Academic Foundation Doctor, Peninsula Deanery Barrie Margetts PhD, Emeritus Professor, Population Health Sciences Research Group, Faculty of Medicine, University of Southampton, Southampton, UK
Batool A Haider, Researcher, Department of Global Health and Population, Harvard School of Public Health, Boston, MA, USA
Zulfiqar A Bhutta, Robert Harding Chair in Global Child Health & Policy, Centre for Global Child Health, Hospital for Sick Children, Toronto, Canada
Philippa Middleton, Editor, Australian Satellite of Cochrane Pregnancy and Childbirth/Principal Research Fellow, Healthy Mothers, Babies and Children, South Australian Health and Medical Research Institute, Women's and Children's Hospital, Adelaide, Australia
What's new
| Date | Event | Description |
|---|---|---|
| 19 July 2019 | Amended | To clarify, for this 2019 update, we re‐extracted data for all primary and secondary outcomes for all included studies from the outset, not just those found from the most recent search. Minor recalculations of cluster parameters resulted in slight differences in results in studies between this version and the previously published version (Haider 2017) of this review. This has made very little difference to the overall results. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 23 February 2018 | New citation required but conclusions have not changed | Along with the previous conclusions (which have not changed significantly), we found that multiple‐micronutrient supplementation reduced the risk of very preterm birth when compared to iron, with or without folic acid. |
| 23 February 2018 | New search has been performed | Search updated and four additional studies added (Ashorn 2010; Biggs 2010; Dewey 2009; Moore 2009). Two previously included studies, Hininger 2004 and Theobald 1937, were reclassified from included to excluded for the 2018 update because the multiple‐micronutrient supplement did not contain iron. |
| 7 April 2017 | Amended | Summary of amendments: 10 November 2015 We have corrected I² values for footnotes 1 and 2 in the Summary of findings table 1. 22 March 2016 We have corrected the stillbirth data for Friis 2004 and corrected the data for preterm birth, SGA, LBW, stillbirth, perinatal mortality, neonatal mortality, maternal anaemia, caesarian section, and miscarriage for Bhutta 2009a. 16 March 2017 Estimates for Christian 2003 trial were updated. In response to feedback received from Professor Caroline Fall, all analyses have now been changed to random‐effects models, given the clinical heterogeneity amongst the included trials. The Unit of Analysis section has been updated to describe the inclusion of data from trials with more than two intervention groups. All feedback has been incorporated and addressed. |
| 7 April 2017 | New citation required but conclusions have not changed | There have been a number of cumulative amendments since the last published version in 2015. The overall conclusions remain unchanged. |
| 22 March 2016 | Amended | We have corrected the stillbirth data for Friis 2004 and corrected the data for preterm birth, SGA, LBW, stillbirth, perinatal mortality, neonatal mortality, maternal anaemia, caesarian section, and miscarriage for Bhutta 2009a. |
| 10 November 2015 | Amended | We have corrected I² values for footnotes 1 and 2 in the Summary of findings table 1. |
| 11 March 2015 | New citation required and conclusions have changed | It is now explicit that the review focuses on oral supplements and trials examining parenteral provision of multiple micronutrients (MMN) or MMN via food fortification are now not included. The updated review includes 19 studies. There is now evidence to suggest that women who receive MMN are at lower risk of having a stillbirth. |
| 11 March 2015 | New search has been performed | Search updated and two new trials included (Lui 2013 and West 2014) and 51 new studies excluded. Six trials included in previous versions of the review have now been excluded: four trials assessed the effect of fortification with multiple micronutrients (MMN) (Dieckmann 1944; Jarvenpaa 2007; Tatala 2002; Vadillo‐Ortega 2011) and two trials included high‐risk women (Gupta 2007; Rumiris 2006). A 'Summary of findings' table has been added. Two new outcomes, mode of delivery and macrosomia, have been added to the review. The list of primary outcomes has been modified. |
| 17 February 2012 | New citation required but conclusions have not changed | Review updated. Conclusions not changed. |
| 17 February 2012 | New search has been performed | Search updated. For this update we have added 17 new included studies (Bhutta 2009a; Brough 2010; Fawzi 2007; Gupta 2007a; Hininger 2004a; Jarvenpaa 2007; Kaestel 2005; Roberfroid 2008; Rumiris 2006a; Sood 1975; SUMMIT 2008; Sunawang 2009; Theobald 1937a; Tofail 2008; Vadillo‐Ortega 2011; Zagre 2007; Zeng 2008) and 15 new excluded studies. We have also identified six ongoing studies (Biggs 2011a; Cogswell 2006a; Dewey 2011a; Fall 2007a; Moore 2011a; West 2011a). This review is now comprised of 23 included studies; 64 excluded studies and six ongoing studies. The methods have been updated. Conclusions have not changed. |
| 20 September 2008 | Amended | Converted to new review format. |
Notes
For this update, we re‐extracted data for all primary and secondary outcomes for all included studies from the outset, not just those found from the most recent search. Minor recalculations of cluster parameters resulted in slight differences in results in studies between this version and the previously published version (Haider 2017). This has made very little difference to the overall results.
Acknowledgements
The original review was prepared in part during the Fellowship Programme organised by Cochrane Infectious Diseases in July 2003 and March 2005. The Department for International Development (UK) supports this programme through the Effective Health Care Research Programme Consortium at the Liverpool Tropical School of Medicine. The views expressed are not necessarily those of the Department for International Development.
This update (2018) was made possible through an unrestricted subgrant under a Program Cooperative Agreement between UNICEF (Headquarters) and the Centre for Global Child Health, The Hospital for Sick Children, Toronto, Canada.
We would like to thank Ms Lynn Hampson for her assistance with the literature search, and Professor James Neilson who provided support and guidance for previous versions of the review. We would also like to thank Nabila Hossain, Anoosh Moin, Nancy Medley, and Nasreen Aflaifel for their assistance with the screening of search results and data extraction, and Roland Kupka from UNICEF for comments on the review. Nancy Medley created the 'Summary of findings' table for the 2017 review. We would also like to thank Therese Dowswell for making edits in response to feedback from the Editor.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We thank Arjumand Rizvi who helped with data extraction for the Bhutta 2009a trial and re‐confirmed estimates for the 2018 update.
Appendices
Appendix 1. ICTRP and ClinicalTrials.gov ‐ search methods
ICTRP
(We ran each line separately)
micronutrients AND pregnancy
vitamins AND pregnancy
multivitamins AND pregnancy
supplements AND pregnancy
supplementation AND pregnancy
multimicronutrients AND pregnancy
multi‐micronutrients AND pregnancy
nutrients AND pregnancy
ClinicalTrials.gov
Advanced search
Interventional studies| pregnancy | micronutrients
Interventional studies| pregnancy | supplements
Data and analyses
Comparison 1. Multiple micronutrients vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm births | 19 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 MMN with iron and folic acid vs iron with or without folic acid | 18 | Risk Ratio (Random, 95% CI) | 0.95 [0.90, 1.01] | |
| 1.2 MMN with iron and folic acid vs placebo | 1 | Risk Ratio (Random, 95% CI) | 1.09 [0.43, 2.77] | |
| 2 Small‐for‐gestational age | 18 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 MMN with iron and folic acid vs iron with or without folic acid | 17 | Risk Ratio (Random, 95% CI) | 0.92 [0.88, 0.97] | |
| 2.2 MMN with iron and folic acid vs placebo | 1 | Risk Ratio (Random, 95% CI) | 0.94 [0.60, 1.48] | |
| 3 Low birthweight | 19 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 MMN with iron and folic acid vs iron with or without folic acid | 18 | Risk Ratio (Random, 95% CI) | 0.88 [0.85, 0.91] | |
| 3.2 MMN with iron and folic acid vs placebo | 1 | Risk Ratio (Random, 95% CI) | 1.58 [0.67, 3.72] | |
| 4 Perinatal mortality | 15 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 MMN with iron and folic acid vs iron with or without folic acid | 15 | Risk Ratio (Random, 95% CI) | 1.00 [0.90, 1.11] | |
| 5 Stillbirths | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 MMN with iron and folic acid vs iron with or without folic acid | 17 | Risk Ratio (Random, 95% CI) | 0.95 [0.86, 1.04] | |
| 6 Neonatal mortality | 14 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 MMN with iron and folic acid vs iron with or without folic acid | 14 | Risk Ratio (Random, 95% CI) | 1.00 [0.89, 1.12] | |
| 7 Maternal anaemia (third trimester Hb < 110 g/L) | 10 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 MMN with iron and folic acid vs iron with or without folic acid | 9 | Risk Ratio (Random, 95% CI) | 1.04 [0.94, 1.15] | |
| 7.2 MMN with iron and folic acid vs placebo | 1 | Risk Ratio (Random, 95% CI) | 0.66 [0.51, 0.85] | |
| 8 Maternal mortality | 6 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 8.1 MMN with iron and folic acid vs iron with or without folic acid | 6 | Risk Ratio (Random, 95% CI) | 1.06 [0.72, 1.54] | |
| 9 Miscarriage (loss before 28 weeks) | 12 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 9.1 MMN with iron and folic acid vs iron with or without folic acid | 12 | Risk Ratio (Random, 95% CI) | 0.99 [0.94, 1.04] | |
| 10 Mode of delivery: caesarean section | 5 | Risk Ratio (Random, 95% CI) | 1.13 [0.99, 1.29] | |
| 10.1 MMN with iron and folic acid vs iron with or without folic acid | 5 | Risk Ratio (Random, 95% CI) | 1.13 [0.99, 1.29] | |
| 11 Congenital anomalies | 2 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 11.1 MMN with iron and folic acid vs iron with or without folic acid | 2 | Risk Ratio (Random, 95% CI) | 1.34 [0.25, 7.12] | |
| 12 Very preterm birth (before 34 weeks of gestation) | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 12.1 MMN with iron and FA vs iron with or without folic acid | 4 | Risk Ratio (Random, 95% CI) | 0.81 [0.71, 0.93] |
Comparison 2. Subgroup analysis for primary outcomes (MMN with iron and folic acid vs iron with or without folic acid)).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm births: mean maternal BMI | 18 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 BMI < 20 kg/m2 | 3 | Risk Ratio (Random, 95% CI) | 0.85 [0.81, 0.90] | |
| 1.2 BMI ≥ 20 kg/m2 | 15 | Risk Ratio (Random, 95% CI) | 0.99 [0.96, 1.03] | |
| 2 Preterm births: mean maternal height | 18 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Maternal height < 154.9 cm | 8 | Risk Ratio (Random, 95% CI) | 0.93 [0.85, 1.03] | |
| 2.2 Maternal height ≥ 154.9 cm | 10 | Risk Ratio (Random, 95% CI) | 0.99 [0.93, 1.05] | |
| 3 Preterm births: timing of supplementation | 18 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Supplementation started before 20 weeks | 14 | Risk Ratio (Random, 95% CI) | 0.93 [0.87, 1.00] | |
| 3.2 Supplementation after 20 weeks | 4 | Risk Ratio (Random, 95% CI) | 1.00 [0.96, 1.04] | |
| 4 Preterm births: dose of iron | 19 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 MMN with 30 mg iron vs. supplement with 60 mg iron | 6 | Risk Ratio (Random, 95% CI) | 1.05 [0.94, 1.17] | |
| 4.2 MMN with 60 mg iron vs. supplement with 60 mg iron | 5 | Risk Ratio (Random, 95% CI) | 0.96 [0.88, 1.05] | |
| 4.3 MMN with 30 mg iron vs. supplement with 30 mg iron | 5 | Risk Ratio (Random, 95% CI) | 0.92 [0.84, 1.02] | |
| 4.4 MMN with 15‐20 mg iron vs. supplement with 60 mg iron | 3 | Risk Ratio (Random, 95% CI) | 0.90 [0.64, 1.27] | |
| 5 Preterm births: MMN supplement formulation | 18 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 UNIMMAP formulation | 10 | Risk Ratio (Random, 95% CI) | 1.00 [0.96, 1.03] | |
| 5.2 Non‐UNIMMAP formulation | 8 | Risk Ratio (Random, 95% CI) | 0.89 [0.82, 0.98] | |
| 6 Small‐for‐gestational age: mean maternal BMI | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 BMI < 20 kg/m2 | 3 | Risk Ratio (Random, 95% CI) | 1.00 [0.92, 1.08] | |
| 6.2 BMI ≥ 20 kg/m2 | 14 | Risk Ratio (Random, 95% CI) | 0.88 [0.83, 0.93] | |
| 7 Small‐for‐gestational age: mean maternal height | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Maternal height < 154.9 cm | 8 | Risk Ratio (Random, 95% CI) | 0.98 [0.96, 1.00] | |
| 7.2 Maternal height ≥ 154.9 cm | 9 | Risk Ratio (Random, 95% CI) | 0.85 [0.79, 0.91] | |
| 8 Small‐for‐gestational age: timing of supplementation | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 8.1 Supplementation started before 20 weeks | 13 | Risk Ratio (Random, 95% CI) | 0.98 [0.96, 1.00] | |
| 8.2 Supplementation after 20 weeks | 4 | Risk Ratio (Random, 95% CI) | 0.85 [0.72, 0.99] | |
| 9 Small‐for‐gestational age: dose of iron | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 9.1 MMN with 30 mg iron vs. supplement with 60 mg iron | 7 | Risk Ratio (Random, 95% CI) | 0.89 [0.81, 0.97] | |
| 9.2 MMN with 60 mg iron vs. supplement with 60 mg iron | 5 | Risk Ratio (Random, 95% CI) | 0.88 [0.72, 1.08] | |
| 9.3 MMN with 30 mg iron vs. supplement with 30 mg iron | 3 | Risk Ratio (Random, 95% CI) | 0.98 [0.96, 1.00] | |
| 9.4 MMN with 20 mg iron vs. supplement with 60 mg iron | 2 | Risk Ratio (Random, 95% CI) | 0.91 [0.75, 1.09] | |
| 10 Small‐for‐gestational age: MMN supplement formulation | 17 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 10.1 UNIMMAP formulation | 9 | Risk Ratio (Random, 95% CI) | 0.91 [0.85, 0.98] | |
| 10.2 Non‐UNIMMAP formulation | 8 | Risk Ratio (Random, 95% CI) | 0.92 [0.84, 1.01] | |
| 11 Perinatal mortality: mean maternal BMI | 15 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 11.1 BMI < 20 kg/m² | 3 | Risk Ratio (Random, 95% CI) | 1.11 [0.82, 1.50] | |
| 11.2 BMI ≥ 20 kg/m² | 12 | Risk Ratio (Random, 95% CI) | 0.98 [0.86, 1.13] | |
| 12 Perinatal mortality: mean maternal height | 15 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 12.1 Maternal height < 154.9 cm | 8 | Risk Ratio (Random, 95% CI) | 1.00 [0.89, 1.13] | |
| 12.2 Maternal height ≥ 154.9 cm | 7 | Risk Ratio (Random, 95% CI) | 0.99 [0.78, 1.25] | |
| 13 Perinatal mortality: timing of supplementation | 15 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 13.1 Supplementation before 20 weeks | 12 | Risk Ratio (Random, 95% CI) | 1.09 [0.92, 1.27] | |
| 13.2 Supplementation after 20 weeks | 3 | Risk Ratio (Random, 95% CI) | 0.89 [0.80, 0.98] | |
| 14 Perinatal mortality: dose of iron | 15 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 14.1 MMN with 30 mg iron vs. supplement with 60 mg iron | 6 | Risk Ratio (Random, 95% CI) | 1.20 [0.95, 1.51] | |
| 14.2 MMN with 60 mg iron vs. supplement with 60 mg iron | 3 | Risk Ratio (Random, 95% CI) | 1.09 [0.74, 1.60] | |
| 14.3 MMN with 30 mg iron vs. supplement with 30 mg iron | 4 | Risk Ratio (Random, 95% CI) | 0.92 [0.86, 0.98] | |
| 14.4 MMN with 20 mg iron vs. supplement with 60 mg iron | 2 | Risk Ratio (Random, 95% CI) | 0.64 [0.36, 1.13] | |
| 15 Perinatal mortality: MMN supplement formulation | 15 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 15.1 UNIMMAP formulation | 9 | Risk Ratio (Random, 95% CI) | 1.06 [0.89, 1.25] | |
| 15.2 Non‐UNIMMAP formulation | 6 | Risk Ratio (Random, 95% CI) | 0.94 [0.82, 1.09] |
Comparison 3. Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm births | 14 | Risk Ratio (Random, 95% CI) | 0.96 [0.90, 1.02] | |
| 2 Small‐for‐gestational age | 10 | Risk Ratio (Random, 95% CI) | 0.92 [0.85, 0.99] | |
| 3 Low birthweight | 14 | Risk Ratio (Random, 95% CI) | 0.88 [0.85, 0.91] | |
| 4 Perinatal mortality | 12 | Risk Ratio (Random, 95% CI) | 1.02 [0.91, 1.15] | |
| 5 Stillbirths | 13 | Risk Ratio (Random, 95% CI) | 0.97 [0.86, 1.08] | |
| 6 Neonatal mortality | 11 | Risk Ratio (Random, 95% CI) | 1.03 [0.88, 1.20] | |
| 7 Maternal anaemia (third trimester Hb < 110 g/L) | 8 | Risk Ratio (Random, 95% CI) | 1.03 [0.92, 1.15] | |
| 8 Miscarriage (loss before 28 weeks) | 9 | Risk Ratio (Random, 95% CI) | 0.99 [0.94, 1.04] | |
| 9 Maternal mortality | 5 | Risk Ratio (Random, 95% CI) | 1.13 [0.76, 1.68] | |
| 10 Very preterm birth (before 34 weeks of gestation) | 4 | Risk Ratio (Random, 95% CI) | 0.81 [0.71, 0.93] | |
| 11 Congenital anomalies | 2 | Risk Ratio (Random, 95% CI) | 1.34 [0.25, 7.12] | |
| 12 Neurodevelopmental outcome: BSID scores | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Mental development scores at 6 months of age: new subgroup | 1 | 592 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐6.75, 6.71] |
| 12.2 Mental development scores at 12 months of age | 1 | 572 | Mean Difference (IV, Random, 95% CI) | 1.21 [‐5.04, 7.46] |
| 12.3 Psychomotor development scores ar 6 months of age | 1 | 592 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐3.89, 3.57] |
| 12.4 Psychomotor development scores at 12 months of age | 1 | 572 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐2.72, 3.40] |
| 13 Mode of delivery: caesarean section | 4 | Risk Ratio (Random, 95% CI) | 1.13 [0.99, 1.30] |
3.1. Analysis.
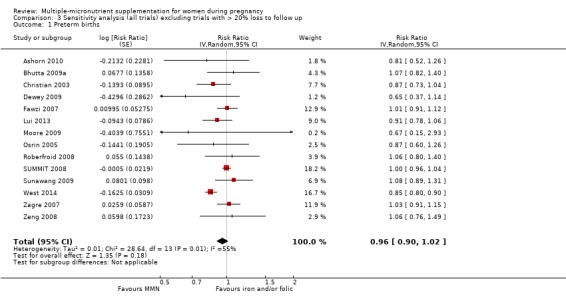
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 1 Preterm births.
3.2. Analysis.
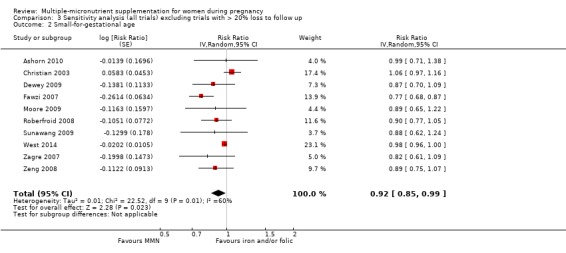
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 2 Small‐for‐gestational age.
3.3. Analysis.
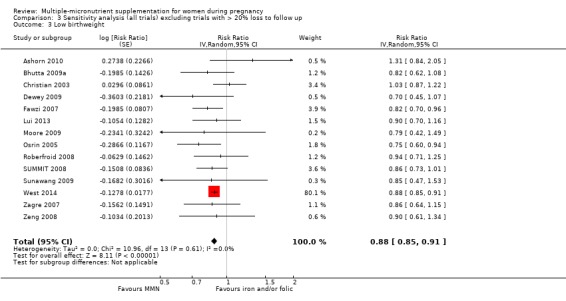
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 3 Low birthweight.
3.4. Analysis.
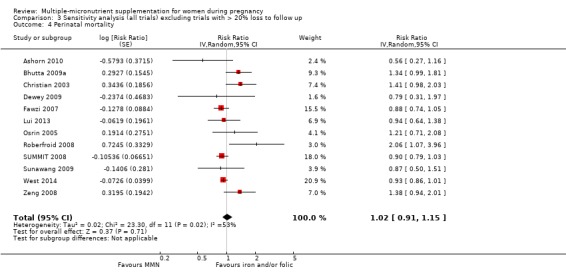
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 4 Perinatal mortality.
3.5. Analysis.
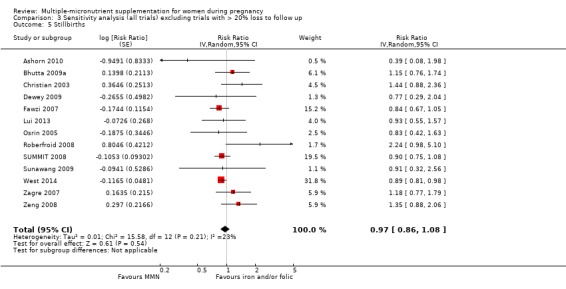
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 5 Stillbirths.
3.6. Analysis.
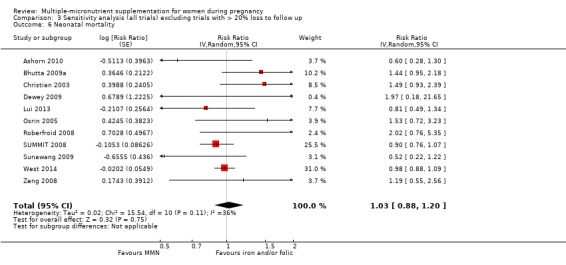
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 6 Neonatal mortality.
3.7. Analysis.
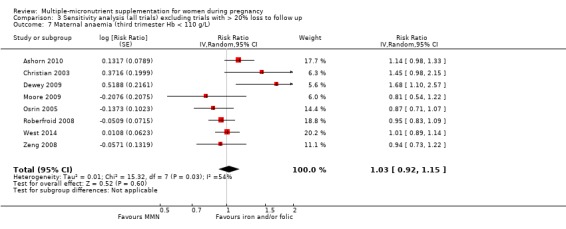
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 7 Maternal anaemia (third trimester Hb < 110 g/L).
3.8. Analysis.
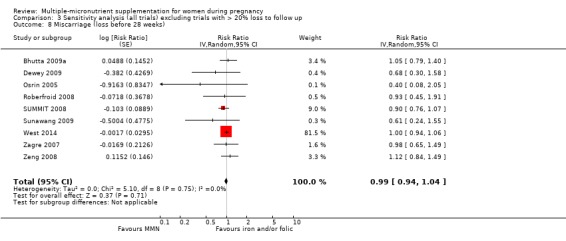
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 8 Miscarriage (loss before 28 weeks).
3.9. Analysis.
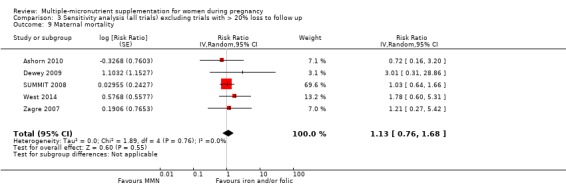
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 9 Maternal mortality.
3.10. Analysis.
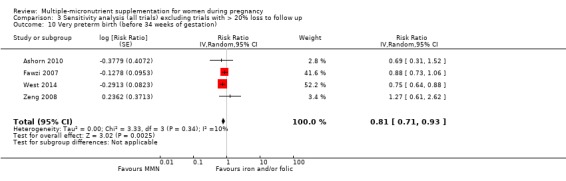
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 10 Very preterm birth (before 34 weeks of gestation).
3.11. Analysis.
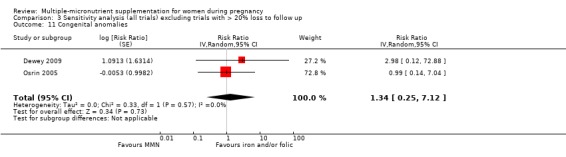
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 11 Congenital anomalies.
3.12. Analysis.
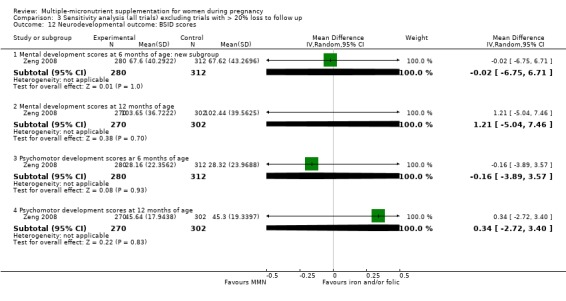
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 12 Neurodevelopmental outcome: BSID scores.
3.13. Analysis.
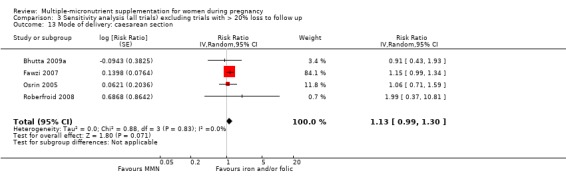
Comparison 3 Sensitivity analysis (all trials) excluding trials with > 20% loss to follow up, Outcome 13 Mode of delivery: caesarean section.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashorn 2010.
| Methods | This was a randomised trial with 3 intervention groups conducted at 4 sites: a public district hospital (Mangochi), a semi private hospital (Malindi), and 2 public health centres (Lugwena and Namwera), in Mangochi District in southern Malawi. Dates of study: February 2011‐December 2015 |
|
| Participants | Participants (n = 1391) were pregnant women who were < 20 weeks of gestation confirmed by ultrasound, resided in the defined catchment area, were available during the study period, and signed or thumb‐printed an informed consent form. Women who were < 15 years of age, needed frequent medical attention due to a chronic health condition, diagnosed with asthma treated with regular medication, had an illness warranting hospital referral, had a history of peanut allergy, anaphylaxis or serious allergic reaction to any substance, required emergency medical care, had pregnancy complications at enrolment (moderate‐severe oedema, blood Hb concentration < 50 g/L, systolic BP > 160 mmHg or diastolic BP > 100 mmHg), participated in the iLiNS‐DYAD‐M trial during a previous pregnancy, or were concurrently participating in other clinical trials were excluded. | |
| Interventions |
|
|
| Outcomes | Birthweight, newborn length, newborn weight, head and arm circumference, pregnancy duration, maternal and newborn SAEs | |
| Notes | All participants also received 2 doses of intermittent preventative malaria treatment with sulfadoxine‐pyrimethamine (3 tablets of 500 mg sulfadoxine and 25 mg pyrimethamine orally). 1 dose was given at enrolment and the other between 28 and 34 weeks of gestation. In this review, the MMN group was used as the intervention group and the IFA group was used as the comparison group. HIV‐ve data included in this review was obtained from personal correspondence with trial investigators. Declarations of interest: MZ worked as a director of research for Nutriset S.A.S., a company that produces and sells lipid‐based nutrient supplements and also prepared the LNS supplements purchased for the current trial. The other authors declared no conflict of interest. Funding sources: supported in part by a grant from the Bill & Melinda Gates Foundation, with additional funding from the Office of Health, Infectious Diseases, and Nutrition, Bureau for Global Health, US Agency for International Development (USAID) under terms of Cooperative Agreement No. AID‐OAA‐A‐12‐00005, through the Food and Nutrition Technical Assistance III Project (FANTA), managed by FHI360. For data management and statistical analysis, the team received additional support in grants from the Academy of Finland (grant 252075) and the Medical Research Fund of Tampere University Hospital (grant 9M004). YBC was supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist Award. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A study statistician not involved in data collection generated 4 randomization code lists in blocks of 9 (one list for each of the 4 enrolment sites)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "a researcher not involved with the trial created individual randomisation slips (in blocks of 9) and packed them in sealed, numbered, opaque randomisation envelopes that were stored in numerical order. Eligible pregnant women were requested to choose 1 of the top 6 envelopes in the stack, and the contents of the envelope indicated her participant number and group allocation." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The IFA and MMN interventions were provided by using double‐masked procedures—that is, the capsules looked identical, and neither the participants nor the research team members were aware of the nutrient contents of the supplement capsules." Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The data collectors who performed the anthropometric measurements or assessed other outcomes were not aware of group allocation. Researchers responsible for the data cleaning remained blind to the trial code until the database was fully cleaned." Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (until delivery) was 6.0% (and was balanced between treatment arms); reasons were not reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes presented in the methods section were reported in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Bhutta 2009a.
| Methods | This cluster‐randomised trial was conducted in urban and rural areas in Pakistan. Dates of study: not reported |
|
| Participants | Pregnant women with gestational age < 16 weeks were eligible for enrolment. MMN group (n = 1148), IFA group (n = 1230) | |
| Interventions |
|
|
| Outcomes | Size at birth, gestational age at birth, perinatal mortality, maternal anaemia (Hb < 11 g/dl), mode of delivery (caesarean section) It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | MMN and MMN + nutritional education groups were compared with IFA and IFA + nutritional education group. IFA given to all participants. Maternal malnutrition, vitamin A deficiency, anaemia and iron deficiency were common. 2 methods of community outreach were implemented that is, basic nutrition along with antenatal care messages and quarterly community‐based group sessions conducted by CHWs and social scientist. There was no significant difference in baseline characteristics between 2 groups. Data for caesarean section were presented (intervention 18/743; control 22/832). Data were not included in the analysis as this was a cluster‐RCT. Declarations of interest: not reported Funding sources: United Nations Children's Fund (UNICEF) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a cluster‐based allocation strategy of supplements (either IF or MMN supplementation) by respective CHWs was implemented". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Comment: "allocated to either the IF or MMN supplements according to their respective location and allocation by the AKU Pharmacy". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both tablets were identical in colour, shape and packaging" and "field staff (medical officers, CHWs, social scientists and data collection team) remained completely blinded as to the supplements allocation. All pregnant women were allocated a unique code and allocated a uniquely labelled and numerically coded specific supplement supply". Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both tablets were identical in colour, shape and packaging" and "field staff (medical officers, CHWs, social scientists and data collection team) remained completely blinded as to the supplements allocation". Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (15.8%) and exclusion (around 1%) along with their reasons were reported. Attrition and exclusions were balanced across the treatment arms. |
| Selective reporting (reporting bias) | Low risk | Comment: results of all outcomes mentioned in methods section were presented in the paper |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design specific biases (recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials) |
Biggs 2010.
| Methods | This was a cluster‐randomised trial comparing the impact of daily IFA, twice weekly IFA and twice weekly MMN for pregnant women on birthweight in Ha Nam province, Vietnam Dates of study: September 2010‐2012 |
|
| Participants | Participants (n = 1258) were pregnant women who were confirmed to be < 16 weeks of gestation, resided in trial communes (n = 104), were > 16 years of age, and were registered with the commune health station. Women with a high‐risk pregnancy including those with a multi‐fetal pregnancy (confirmed by palpation or ultrasound), a significant medical condition or severe anaemia (Hb concentration < 80 g/L) at enrolment were excluded. | |
| Interventions |
All groups were provided supplements for the duration of the pregnancy until 3 months postpartum |
|
| Outcomes | Birthweight, maternal Hb and ferritin at 32 weeks, and infant length‐for‐age z‐scores, Hb, ferritin, and infant cognitive development at 6 months of age | |
| Notes | In the review, the twice‐weekly MMN group was used as the intervention group and the daily IFA group was used as the comparison group. Since this review focuses on daily supplementation, outcomes from this study were omitted from the main analyses, however, they were included in the subgroup analyses. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: National Health and Medical Research Coucil of Australia (Grant number 628751) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed by an independent statistician not involved in the study and blinded to the identity of the communes, using ‘ralloc’ in Stata (StataCorp)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Supplements were received from the manufacturing company in blister packs, with a code A, B, or C embossed on each blister pack. The intermittent IFA and MMN capsules were identical in both appearance and packaging. The manufacturing company confidentially notified the chairperson of the Data Monitoring and Safety Committee at the University of Melbourne of the allocation code, and the code was kept in a locked file cabinet at the University of Melbourne, Australia." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The investigators, field staff, and participants were blinded to the codes of the intermittent supplement groups throughout the study and during data analysis. Laboratory staff were unaware of the intervention groups. It was not possible to blind the field team to the daily supplementation arm, but participants were not informed about the dosing frequency of the intervention being given in other communes." Comment: participants and caregivers were probably not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The investigators, field staff, and participants were blinded to the codes of the intermittent supplement groups throughout the study and during data analysis. Laboratory staff were unaware of the intervention groups. It was not possible to blind the field team to the daily supplementation arm" and "The allocation code was broken at the completion of data analysis. An independent team undertook the BSID III assessments, and were blinded to the intervention arms." Comment: it is unclear whether outcome assessors were blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (until delivery) was 7.2% and (and was balanced between treatment arms); reasons were reported |
| Selective reporting (reporting bias) | High risk | Comment: growths outcomes including weight‐for‐age, underweight, weight‐for‐length and wasted were mentioned in the methods section, however, results were not presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design‐specific biases (recruitment bias, baseline imbalance, loss of clusters, and comparability with individually randomised trials). Any incorrect analysis was corrected by adjustment for clustering within data reported in this review. |
Brough 2010.
| Methods | This randomised trial was conducted in a socially deprived, multi‐ethnic population in east London, United Kingdom. Dates of study: June 2002‐May 2004 |
|
| Participants | Participants included women aged ≥ 16 years with a singleton pregnancy. Exclusion criteria included a gestation of > 13 weeks of gestation, chronic disease or use of micronutrient supplements (excluding IFA). MMN group n = 207 and placebo n = 195 | |
| Interventions | Participants were randomised to receive either MMN supplements, known as Pregnacare, or a placebo comprising starch with an iron oxide coating. MMN supplement contained beta‐carotene 3 mg, thiamin (as thiamin mononitrate, 3·6 mg) 3 mg, riboflavin 2 mg, niacin (as nicotinamide) 20 mg, vitamin B6 (as pyridoxine HCl) 10 mg, vitamin B12 (as cyanocobalamin) 6 mcg, folic acid 400 mcg, vitamin C (as ascorbic acid, 73 mg) 70 mg, vitamin D (as cholecalciferol, 200 IU) 5 mcg, vitamin E (as D‐a‐tocopheryl acid succinate, 21 mg) 20 mg, vitamin K 70 mcg, Fe (as ferrous fumarate, 63·3 mg) 20 mg, zinc (as zinc sulphate H2O, 41 mg) 15 mg, Mg (as magnesium hydroxide, 372 mg) 150 mg, Iodine (as potassium iodide, 183 mg) 140 mcg and copper (as copper sulphate H2O, 2·8 mg) 1 mg | |
| Outcomes | Birthweight, preterm birth, SGA, head circumference, Hb | |
| Notes | Women not using folic acid were also given 400 mcg folic acid to take daily until 12 weeks of gestation. There were no significant differences in age, height, weight, BMI or parity regarding treatment group allocation. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: the Mother and Child Foundation, and Nutricia Research Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a randomised, double‐blind, placebo‐controlled trial" and "Participants were randomised to receive either multiple‐micronutrient supplements, known as Pregnacare, or a visually identical placebo". Comment: insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "a randomised, double‐blind, placebo‐controlled trial" Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were randomised to receive either multiple micronutrient supplements, known as Pregnacare, or visually identical placebo comprising starch with an iron oxide coating. All tablets were provided by Vitabiotics (London, UK) and packaged to allow double blinding. Only Vitabiotics knew the code and it was not broken until statistical analysis had been completed". Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All tablets were provided by Vitabiotics (London, UK) and packaged to allow double blinding. Only Vitabiotics knew the code and it was not broken until statistical analysis had been completed". Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (8.7%) and attrition (12.2%) were reported along with their reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: results of all outcomes mentioned in methods were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Christian 2003.
| Methods | This was a double‐blind cluster‐randomised trial, carried out in rural Nepal. Dates of study: December 1998‐April 2001 |
|
| Participants | A total of 4926 pregnant women were enrolled in the study. The women were randomised into 5 groups as follows: group 1 (n = 941), group 2 (n = 957), group 3 (n = 999), group 4 (n = 1050) and group 5 (n = 1051) Women who were currently pregnant or those who were breastfeeding an infant < 9 months old were excluded from the study. Also excluded were menopausal, sterilised or widowed women | |
| Interventions |
All supplements were given orally from the time of pregnancy detection until 12 weeks after a live birth or 5 weeks after a still birth or a miscarriage. |
|
| Outcomes | Preterm births, SGA (weight < 10 percentile of gestational age), LBW (< 2500 g), side‐effects, fetal loss, perinatal mortality, neonatal mortality, 3‐month infant mortality It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | All women were offered 2 x 400 mg single‐dose albendazole in the second and third trimester of pregnancy because of the high prevalence of hookworm infestation in this population. Hookworm infestation and vitamin A deficiency are one of the major causes of anaemia in this population. Due to this reason, vitamin A was given to all the participants including the control group. Baseline characteristics did not differ significantly among the various randomisation groups except for ethnicity and land holding. In this review, we used the group 4 data for the MMN group and group 2 data for the control group. All the estimates were adjusted for the cluster design and provided by the trial authors. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: US Agency for International Development (USAID) and additional support from the Unicef Country Office, Kathmandu, Nepal, and the Bill and Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done in blocks of five within each village development community by the senior study investigators, who drew numbered chips from a hat" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomisation was done in blocks of five within each village development community by the senior study investigators, who drew numbered chips from a hat" Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants, investigators, field staff and statisticians did not know supplement codes", "supplements, which were of identical shape, size, and color" and "code allocation was kept locked at the Johns Hopkins University, Baltimore". Comment: participants and caregivers were blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "participants, investigators, field staff and statisticians did not know supplement codes" Comment: outcome assessors were blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (1.43%) and attrition (6.9%) were reported along with their reasons |
| Selective reporting (reporting bias) | Low risk | Comment: results of all outcomes mentioned in methods were presented in the various publications of this trial. |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design specific biases (recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials). |
Dewey 2009.
| Methods | This was a randomised trial with 3 intervention groups conducted in the Somanya‐Kpong area of the Yilo Krobo and Lower Manya Krobo districts in southern Ghana. Date of study: December 2009‐March 2014 |
|
| Participants | Participants (n = 1320) were pregnant women who were ≥ 18 years of age and < 20 weeks of gestation confirmed by ultrasound. Women who were not residing in the target area, who intended to move within the next 2 years, had a known milk or peanut allergy, were unwilling to receive field workers or take the study supplement, were participating in another trial, or had an antenatal card indicating HIV infection, asthma, epilepsy, tuberculosis, or any malignancy were excluded. | |
| Interventions |
|
|
| Outcomes | Primary outcomes were child length at birth and length‐for‐age Z‐score (LAZ, based on WHO 2006 growth standards) at 18 months of age. Secondary outcomes included the following:
|
|
| Notes | Temporary mislabelling of IFA and MMN capsules resulted in some women in the IFA (n = 92) and MMN (n = 85) groups receiving both IFA and MMN supplements during pregnancy. These women were excluded in the analysis of pregnancy outcomes. In this review, we used the MMN group as the intervention group and the IFA group as the comparison group. Declarations of interest: MZ was an employee of Nutriset S.A.S., which is a commercial producer of LNS products. The other trial authors declared no conflict of interest. Funding sources: Bill & Melinda Gates Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "women were randomly allocated into one of 3 groups by using a computer‐generated scheme (SAS version 9.3; SAS Institute) in blocks of 9." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Sheets bearing supplement allocations represented by 6 different color codes (3 for IFA and 3 for MMN) and an inscription “LNS” (for the LNS group) and numbered 1–1320 were placed in opaque envelopes and stacked in increasing order. At each enrolment, the study nurse shuffled the 9 topmost envelopes in the stack, and the woman picked one to reveal allocation. Allocation information was kept by the field supervisor (HO) in a password‐protected file, which was shared with the study statistician (JMP) at UC Davis, who designed the randomisation scheme." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Two individuals in Ghana who were independent of the research team color‐coded the capsules by placing color stickers (which also included the letter P or L to indicate pregnancy or lactation) on the blister packs of IFA and MMN, so that no investigator, study worker, or participant knew the identities of the capsules except by the colors." Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "none of the maternal or newborn anthropometrists was aware of the code allocations. Likewise, data analysts remained blinded until all preliminary analyses had been completed, and the allocation codes were broken." Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (until delivery) was 20% (and was balanced between treatment arms); the reason was reported. Attrition (until delivery) was 4.4% and reasons were reported |
| Selective reporting (reporting bias) | Low risk | Comment: results of all outcomes mentioned in methods section were presented in the paper |
| Other bias | Low risk | Comment: no other bias was identified |
Fawzi 2007.
| Methods | This was a double‐blind trial in Dar es Salam, Tanzania. Pregnant women who attended antenatal clinics were included. Dates of study: August 2001‐July 2004 |
|
| Participants | Pregnant women who attended antenatal clinics, had a negative test for HIV infection, planned to stay in the city until delivery and for 1 year thereafter with gestational age between 12 and 27 weeks according to LMP were included. The study groups were similar with respect to baseline characteristics. | |
| Interventions |
All women irrespective of group received daily iron (60 mg) and folic acid (0.25 mg). Women were randomly assigned to receive either MM or control from the time of enrolment until 6 weeks after delivery. |
|
| Outcomes | LBW (< 2500 g), preterm delivery (< 37 weeks of gestation), very LBW (< 2000 g), extremely preterm delivery (< 34 weeks of gestation), SGA (< 10th percentile for gestational age), fetal death, death in first 6 weeks, length, head circumference, placental weight, risk of caesarean section, maternal mortality, haematological status (Hb < 11 g/dL and < 8.5 g/dL, immune status (CD4 count < 775 per cubic mm, CD8 count < 480 per cubic mm and CD3 count < 1350 per cubic mm) | |
| Notes | Malaria prophylaxis (sulphadoxine‐pyrimethamine tablets) at 20 and 30 weeks of gestation was given to all. The study groups were similar with respect to baseline characteristics. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: National Institute of Child Health and Human Development (NICHD R01 37701) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A list was prepared according to a randomisation sequence in blocks of 20; at enrolment, each eligible women was assigned to the next numbered bottle" and computerised random number generator was used (personal communication) Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Each eligible women was assigned to the next numbered bottle" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Active tablets and placebo were similar in shape, size and color and were packaged in identical coded bottles" and "Each eligible women was assigned to the next numbered bottle" Comment: participants and caregivers were blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "research assistants who assessed the study outcome were unaware of the intervention group" Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (0.5%) and attrition (5.4%) were reported with reasons in each arm. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Friis 2004.
| Methods | This trial was carried out in Zimbabwe. Dates of study: 1996‐1997 |
|
| Participants | Pregnant women who were between 22 and 36 weeks of gestation were eligible for enrolment. Participants (n =1669) were randomised into 2 groups, MMN group n = 837 and placebo n = 832. Out of the 1106 women that were followed, 725 were HIV‐ve and 360 were HIV+ve. | |
| Interventions |
An IFA supplement was given separately as part of the routine antenatal care and was not part of the MMN tablet. Tablets were given from the day of enrolment until delivery. |
|
| Outcomes | Gestational age, birthweight, birth length, head circumference, preterm delivery (< 37 weeks of gestation), LBW (< 2500 g), IUGR‐LBW (> 37 weeks' gestational age and < 2500 g birthweight). It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Study intervention was approximately the RDA for pregnant or lactating women, except for vitamin A for which a higher amount was given.
Out of 1106 women who were followed, 725 were HIV‐ve whereas 360 were HIV+ve and HIV status of 21 was indeterminate. We have used data of HIV‐ve women only in this review. The intervention and the placebo groups were comparable at baseline except for the higher proportion of primigravida in the placebo group. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: Council for Development Research, Danish International Development Assistance (to HF), the Danish Council for Medical Research (to HF), Southampton Insurance, Zimbabwe, the Foundation of 1870, BASF Health and Nutrition, the Hørslev Foundation, the Dagmar Marshall Foundation, the Sophus Jacobsens Foundation, and the Lily Benthine Lunds Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation to daily supplementation with multimicronutrient or identical‐looking placebo tablets was based on simple blocked randomisation. The digits 0–5 in a computer‐generated random sequence were replaced by 6 preassigned permuted blocks of 4: AABB, ABAB, ABBA, BABA, BBAA, and BAAB; the digits 6–9 were deleted". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Containers with 110 multimicronutrient or placebo tablets, which were coded A or B, respectively, were delivered by the manufacturer together with the code in 2 sealed envelopes. Duplicate containers, which corresponded to the random sequence, were consecutively numbered from 1 to 1800. The study participants were numbered consecutively at recruitment". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind", "multimicronutrient or identical‐looking placebo tablets" Comment: study participants and care providers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind", "multimicronutrient or identical‐looking placebo tablets" Comment: investigators were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was > 20% and reasons for it were reported. Exclusions were not reported in the trial |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Kaestel 2005.
| Methods | This trial was conducted in Guinea‐Bissau Dates of study: January 2001‐October 2002 |
|
| Participants | Pregnant women with < 37 weeks of gestation were eligible for enrolment. A total of 2100 women were randomised into 3 groups, MMN RDA group, MMN 2 RDA group and 60 mg iron 400 mcg folic acid group | |
| Interventions | 15 micronutrients were included in the supplement at RDA level, except for folic acid that was included at 400 mcg level. Supplement consisted of vitamin A 800 mcg, D 200 IU, E 10 mg, C 70 mg, B1 1.4 mg, B2 1.4 mg, niacin 18 mg, B6 1.9 mg, B12 2.6 mg, folic acid 400 mcg, iron 30 mg, zinc 15 mg, copper 2 mg, selenium 65 mcg and iodine 150 mcg
|
|
| Outcomes | Size at birth, gestational age at birth, preterm birth (< 37 weeks of gestation), LBW (< 2500 g), miscarriage (fetal loss < 28 completed weeks of gestation), perinatal mortality (fetal loss between 28 weeks of gestation and first 7 days of life), neonatal mortality (deaths within the first 28 days of life), maternal Hb, anaemia (Hb < 100 g/L) and maternal death (death during pregnancy or within 42 days after termination of pregnancy), childhood mortality. It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Malaria is endemic but HIV prevalence is relatively low.
IFA given to all participants. There was no significant difference in baseline characteristics between randomisation groups. We used the 1 RDA and control groups in this review. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: the Royal Veterinary and Agricultural University, Denmark’s Development Assistance (Danida), the Novo Nordisk Foundation, UNICEF, the Foundation of 1870, and Jakob & Olga Madsen’s Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple block randomisation with a block size of 150 was managed as follows: at entry, the project midwife randomly drew 1 piece of coloured paper corresponding to the colour code on the tablet containers from envelopes with initially 50 pieces of each of the three colours" Comment: probably done. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "at entry, the project midwife randomly drew one piece of coloured paper corresponding to the colour code on the tablet containers from envelopes with initially 50 pieces of each of the three colours" Comment: insufficient evidence to determine whether allocation was concealed following generation of the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "three identical‐looking micronutrient supplements", "code was kept secret from study participants, study personnel, and data analysts until data cleaning and preliminary data analysis had been carried out." and "the health workers who collected outcome data after delivery did not have any knowledge of intervention group of the women" Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "three identical‐looking micronutrient supplements", "code was kept secret from study participants, study personnel, and data analysts until data cleaning and preliminary data analysis had been carried out." and "the health workers who collected outcome data after delivery did not have any knowledge of intervention group of the women" Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion (3.1%) and attrition (20.4%) data were reported along with their reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Lui 2013.
| Methods | This was a double‐blind RCT conducted in 5 rural counties in Hebei Provinve, China Dates of study: May 2006‐April 2009 |
|
| Participants | Pregnant women who recorded dates of their menstruation for ≥ 2 months before they became pregnant, were nulliparous, ≥ 20 years old, < 20 weeks' gestation, legally competent, had not consumed micronutrient supplements other than folic acid in the prior 6 months, had a Hb level > 10.0 g/dL, resided in and received prenatal care in 1 of 5 counties, and consented to participate were eligible. 18,775 pregnant women with singleton pregnancies were randomised to group A (n = 6261), group B (n = 6252), and group C (n = 6262). | |
| Interventions | The study had 3 arms.
Supplements were take from enrolment until delivery. |
|
| Outcomes | Perinatal mortality, neonatal mortality, infant mortality, maternal Hb and anaemia at 24‐28 weeks of gestation, gestational age at birth, preterm birth, LBW, birthweight, low weight for height, low weight for age, low height for age, infant anaemia, gastrointestinal side‐effects (nausea, vomiting, or other mild gastrointestinal discomfort) at monthly visits | |
| Notes | There were no significant differences at baseline between the groups Data for side‐effects: 6% (n = 355) in the MMN group while 3.6% (n = 212) in the IFA group We used the estimates for the comparison of MMN vs. IFA groups in this review. Declarations of interest: the trial authors declared no conflict of interest Funding sources: supported by a co‐operative agreement between Peking University Health Science Center and the Centers for Disease Control and Prevention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician external to the study randomly assigned ten 4‐digit lot numbers to each of the 3 supplement types (masked to the formulation and allocation) and generated the assignment list for each county proportional to the expected number of participants; within each county and block, lot numbers were randomly assigned using RANUNI in SAS statistics software (SAS Institute Inc)" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "A statistician external to the study randomly assigned ten 4‐digit lot numbers to each of the 3 supplement types (masked to the formulation and allocation)" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Aside from a pharmaceutical engineer who ensured allocation of lot numbers to the correct supplement formulations, all others (i.e., participants, local physicians, study personnel, and investigators) were masked to the identity of the supplements" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment codes were broken after completion of the study and main analyses.", " double blind" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate (6.2%) was < 20% and reasons for attrition and exclusions were provided |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned were reported. |
| Other bias | Low risk | Comment: no other bias was identified. |
Moore 2009.
| Methods | This was a randomised trial with 4 intervention groups investigating the effects of prenatal and infancy nutritional supplementation on infant immune development in the West Kiang region of the Gambia Dates of study: October 2009‐September 2013 |
|
| Participants | Participants (n = 875) were pregnant women who were 10‐20 weeks of gestation confirmed by ultrasound. Women who were pregnant > 20 weeks of gestation on ultrasound assessment, enrolled in another Medical Research Council study, severely anaemic at booking (Hb < 7 g/dL), or reported the onset of menopause were excluded. | |
| Interventions | The study had 4 pregnancy interventions given daily to participants from 12 weeks of gestation until delivery:
From 6 months of age, infants were further randomised to receive either a lipid‐based nutritional supplement, with or without additional MMN, or placebo from 6‐12 months of age |
|
| Outcomes | Thymic index at 1, 8, 24 and 52 weeks of age, antibody response to EPI vaccines (diphtheria, tetanus toxoid, HiB, measles) and cellular markers of immunity in a randomly selected subcohort of infants assessed at 12, 24 and 52 weeks of age. Subsidiary studies to the main trial will additionally assess the impact of supplementation on infant growth and development to 24 months of age. | |
| Notes | In the review, the MMN group was used as the intervention group and the FeFol group was used as the comparison group. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: UK Medical Research Council (MRC) (MC‐A760‐5QX00) and the UK Department for International Develop‐ ment (DFID) under the MRC/DFID Concordat agreement. WJ and SEM are funded by the UK MRC programme MC_UP_1005/1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization into the trial is in blocks of 8, using an automated system, with the 8 groups reflecting the 8 combinations of prenatal and infancy supplements." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of each supplement combination to a number between 1 and 8 was performed by Dr Mathilde Savy (IRD, France), with this information passed directly to the supplement manufacturers. Each box of supplement is then distinguished by a number between 1 and 8. An additional hard copy of the code assignment is held in the safe in Keneba, accessible by the field station senior administrator and only at the request of the trial monitors." and "Using the automated allocation system, a member of the data office at MRC Keneba, independent to the trial analysis, allocates mother‐infant pairs to their supplement codes and then generates printed labels for the supplement pots (including subject ID, name, and date of supplement period). Four members of the MRC Keneba field staff working on a different study then label the supplements using lists, by supplement allocation number (e.g. Group 1, women a, b, c etc.) provided by the data office." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The antenatal arm of the trial is partly open, since it is not be possible to blind the field assistants or the women to the supplement type (tablet vs. LNS); all other investigators however will not know to which group the women belong." Comment: it is unclear whether participants and personnel were blinded to IFA and MMN groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all other investigators however will not know to which group the women belong." Comment: outcome assessors were probably blinded to the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion (until delivery) was 25.6% and reasons were reported. Attrition (until delivery) was 4.8% and reasons were not reported. Whether exclusion and attrition rates were balanced between treatment arms were not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Osrin 2005.
| Methods | This study was undertaken in Nepal. All women attending a designated antenatal clinic at Janakpur zonal hospital were considered for enrolment. Dates of study: August 2002‐July 2004 |
|
| Participants | Women were eligible for enrolment if an ultrasound examination confirmed a singleton pregnancy, a gestational age between 12‐20 completed weeks, no notable fetal abnormality, no existing maternal illness of a severity that could compromise the outcome of pregnancy; and the participant lived in an area of Dhanusha or the adjoining district of Mohattari accessible for home visits. Participants received supplements throughout pregnancy until delivery. | |
| Interventions |
There were 2 prespecified deviations from the protocol: if a participant's enrolment blood Hb concentration was < 70 g/L, she was given an extra 60 mg of iron daily, anthelmintic treatment, and her Hb was rechecked after 1 month; and if a participant described night blindness at any time, she was given 2000 ug of vitamin A daily and referred for medical follow up. |
|
| Outcomes | Birthweight, LBW (< 2500 g), gestational duration, preterm delivery (< 37 weeks of gestation), miscarriage, stillbirth, early and late neonatal death, infant length, head circumference. It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Infants were followed up to 3 months. Both groups of participants were comparable at baseline. There is a discrepancy in the number of neonatal deaths reported. Figure: 'Study profile' in the Devakumar 2014 Lancet publication (p e655) reports 12 neonatal deaths in the control group and Osrin 2005 reports 11 neonatal deaths in the control group. Declarations of interest: in the planning phase of the study, DO, SF, and AT attended an international principal investigators’ meeting funded by the Micronutrient Initiative. After study completion, but before the paper was written, AV, DSM, and AT attended a second meeting funded by UNICEF. The other trial authors declared no conflict of interest. Funding sources: Wellcome Trust, UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly allocated 1200 participant identification numbers by computer into two groups in permuted blocks of 50". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "We did randomisation in advance of recruitment", "The allocation code was kept on file in Kathmandu and London. We allocated every identification number a supplement container to last throughout the trial. Containers were filled with either intervention or control tablets in Kathmandu by a team member who was otherwise uninvolved in the trial; these containers were then marked only with identification numbers and transported to the study centre in Janakpur" and "After screening, consent, and enrolment, one of us (YS) allocated participants sequential identification numbers and the corresponding supplement containers". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The allocation code was kept on file in Kathmandu and London" and "Containers were filled with either intervention or control tablets in Kathmandu by a team member who was otherwise uninvolved in the trial; these containers were then marked only with identification numbers and transported to the study centre in Janakpur. Intervention and control supplements were manufactured to look, smell, and taste identical" Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The allocation code was kept on file in Kathmandu and London" and "Containers were filled with either intervention or control tablets in Kathmandu by a team member who was otherwise uninvolved in the trial; these containers were then marked only with identification numbers and transported to the study centre in Janakpur. Intervention and control supplements were manufactured to look, smell, and taste identical" Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was 5% and reasons for it were reported. Exclusion was 39.5% and reasons were not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Ramakrishnan 2003.
| Methods | This RCT was carried out in Mexico Dates of study: 1997‐2000 |
|
| Participants | Pregnant women who were < 13 weeks' pregnant, were not receiving MMN supplementation and who agreed to participate were included in the study. A total of 873 women were randomised into the MMN group (n = 435, mean age 23.09 ± 5.48) and the iron‐only group (n = 438, mean age 23.00 ± 5.08). | |
| Interventions |
All were given orally, from recruitment 6 days a week until delivery. |
|
| Outcomes | Preterm births (< 37 weeks of gestation), SGA (below the 10th percentile for birthweight‐for‐gestational age), LBW (< 2500 g), perinatal mortality, mean Hb concentration, mean serum ferritin It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Data on birth outcomes were only available for 656 pregnancies (MMN group n = 328 and control group, iron only n = 326). The 2 groups did not differ significantly in most of the characteristics at recruitment, except for marital status (more single mothers in MMN supplementation group) and mean BMI (significantly lower in the MMN supplementation group). Declarations of interest: the trial authors declared no conflict of interest. Funding sources: Thrasher Research Fund, United Nations Children's Fund (UNICEF) New York, Conacyt, and the Instituto Nacional de Salud Pública, Mexico |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by using 4 color‐coded groups (2 per treatment) that were assigned a priori with the use of a computer‐generated list". Comment: probably done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Four colors were used to ensure masking and were assigned at random before the study began to a list of serial numbers from 1 to 1000" and "pregnant women were allocated to the pre‐assigned color code as they were added to this list at the time of recruitment". Comment: probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study personnel and investigators were blinded to the group assignment, the details of which were kept at Emory University and the INSP in sealed envelopes that were opened only after preliminary data analysis was completed". Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All study personnel and investigators were blinded to the group assignment, the details of which were kept at Emory University and the INSP in sealed envelopes that were opened only after preliminary data analysis was completed". Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion was 5.2% but reasons for it were not reported. Attrition (26.2%) along with their reasons were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the various publications of this trial. |
| Other bias | Low risk | Comment: no other bias was identified. |
Roberfroid 2008.
| Methods | This was a factorial, double‐blind, RCT conducted in the Hounde health district of Burkina Faso. Dates of study: March 2004‐October 2006 |
|
| Participants | Pregnant women irrespective of gestational age. Exclusion criterion was if women planned to leave area within 2 years | |
| Interventions |
|
|
| Outcomes | Stillbirths (fetal death between 28 weeks of gestation till birth), neonatal deaths, perinatal death, gestation age, preterm births (< 37 weeks of gestation), birthweight, LBW (< 2500 g), SGA (birthweight < 10 percentile of a reference population), LGA, birth length, Rohrer index, arm circumference, chest circumference, head circumference, Hb in cord blood, soluble serum transferrin receptor, stunting, wasting, underweight, and infant mortality during the first year of life. It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Supplement intake was observed directly and given till 3 months after delivery. Participants were also randomly assigned to receive either malaria chemoprophylaxis (300 mg chloroquine/week) or intermittent preventive treatment (1500 mg sulfadoxine and 75 mg pyrimethamine once in the second and third trimester). All participants received albendazole 400 mg during second and third trimester. Severely anaemic women received ferrous sulphate 200 mg and folic acid 0.25 mg twice daily for 3 months regardless of their allocation groups. The study groups were similar with respect to baseline characteristics except for small difference in Hb (lower in intervention group) and BMI (lower in control group). Stunting, wasting, underweight, and infant mortality during the first year of life were presented as hazard ratios and could not be included in the analysis of outcome using risk ratios. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: Nutrition Third World and the Belgian Ministry of Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation scheme was generated by a computer program in permuted blocks of 4". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization numbers were sealed in opaque envelopes. At each inclusion, the consulting physician opened the next sealed envelope and transmitted the randomisation number to a pharmacist managing the allocation sequence and the packaging of drugs in Center Muraz. The pharmacist was also blinded to the intervention. Individual plastic zip bags contained 31 tablets each and were labelled with the participant’s name, address, and identification numbers only" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind", "Intervention and control micronutrient tablets were identical in appearance" and "code was kept secret from study participants and staff until completion of preliminary data analysis" and "Pharmacist was also blinded to the intervention". Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind", "Intervention and control micronutrient tablets were identical in appearance" and "code was kept secret from study participants and staff until completion of preliminary data analysis" and "Pharmacist was also blinded to the intervention". Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 7.5% and reason for it was provided. Only 1 woman was excluded because of therapeutic abortion. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
Sood 1975.
| Methods | Trial conducted in New Dehli and Tamil Nadu, India. Dates of study: not reported |
|
| Participants | Pregnant women with gestational age 22 ± 2 were eligible to participate in the trial. A total of 647 pregnant women participated in the trial. Women with chronic diseases like heart diseases, tuberculosis, leprosy, chronic diarrhoea and a Hb < 5 g/100 mL were excluded from the study. | |
| Interventions | There were total of 7 study groups. 2 in the control group and 5 in the intervention group.
Supplementation was given for 10‐12 weeks. |
|
| Outcomes | Outcomes were improvement in maternal Hb/haematocrit, iron absorption from maternal gut, fetal birthweight, maternal and fetal Hb 3 months postpartum, hookworm infestation in mother and side‐effects of supplementation | |
| Notes | None of the outcomes were reported in a format that allowed inclusion of the data in this review. Declarations of interest: not reported Funding sources: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By reference to previously prepared random tables the women were allocated to one of the two streams A or B" and "within each stratum subjects were allotted to final treatment groups according to a set of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "By reference to previously prepared random tables the women were allocated to one of the two streams A or B" and "within each stratum subjects were allotted to final treatment groups according to a, set of random numbers" Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All the tablets had the same appearance and had the daily folic acid and iron dose divided into two tablets". Comment: participants, caregivers probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the tablets had the same appearance and had the daily folic acid and iron dose divided into two tablets". Comment: outcome assessors probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was 30% and reasons for it were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper |
| Other bias | Low risk | Comment: no other bias was identified |
SUMMIT 2008.
| Methods | A double‐blind cluster‐randomised trial conducted at Lombok island of Indonesia. Dates of study: July 2001‐April 2004 |
|
| Participants | Pregnant women of any gestational age assessed by physical exam and reported LMP | |
| Interventions |
|
|
| Outcomes | Early infant mortality (death within 12 weeks of birth), neonatal mortality (death within 28 days of birth), early neonatal mortality (death within 7 days of birth), late neonatal mortality (death between 7 and 28 days of birth), postneonatal mortality (death between 28 days and 12 weeks of birth), fetal loss, abortions (fetal loss before 28 weeks of gestation), still births (death between 28 weeks and before delivery), perinatal mortality (still birth or death within 7 days of birth), maternal mortality related to pregnancy up to 12 weeks postpartum, maternal cognition and mood, and child cognition (motor, cognitive and socioeconomic abilities) at 42 months of age. It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Women in both groups received supplements throughout pregnancy until 90 days postpartum. Intervention and placebo groups were comparable in terms of baseline characteristics. Study was stopped early due to insufficient funds Declarations of interest: the trial authors declared no conflict of interest. Funding sources: Turner Foundation, UNICEF, the Centre for Health and Human Development, and the United States Agency for International Development‐Indonesia (grant no 497‐G‐00‐01‐00001‐00) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before enrolment, midwife identification numbers were sequentially allocated to computer‐generated, randomly permuted blocks of groups numbered one to eight, stratified by community health centre or village health clinic". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "midwives at village health centres and community health centres were assigned midwife identification numbers" and "Before enrolment, midwife identification numbers were sequentially allocated to computer‐generated, randomly permuted blocks of groups numbered one to eight, stratified by community health centre or village health clinic" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study scientists and personnel, government staff and enrolees were unaware of the allocation." and "The code to indicate which strip was IFA or MMN was known only by the manufacturing production manager and a quality control officer from UNICEF, Copenhagen, neither of whom had any connection to the study or its personnel". Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All study scientists and personnel, government staff and enrolees were unaware of the allocation." and "The code to indicate which strip was IFA or MMN was known only by the manufacturing production manager and a quality control officer from UNICEF, Copenhagen, neither of whom had any connection to the study or its personnel". Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (25.2%) and attrition (5%) were reported along with their reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design specific biases (recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials). |
Sunawang 2009.
| Methods | A cluster‐randomised trial conducted in 2 subdistricts of Indramayu district of west Java province of Indonesia. Dates of study: May 2000‐August 2003 |
|
| Participants | Pregnant women irrespective of gestational age. Women suffering from diabetes mellitus, coronary heart disease and tuberculosis were excluded. | |
| Interventions |
|
|
| Outcomes | Birthweight, birth length, head and chest circumference, Hb, serum ferritin, serum zinc, serum retinol and urinary Iodine, miscarriage, stillbirths, neonatal mortality It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Study groups were similar with respect to baseline characteristics. Supplements were given from the time of enrolment at 12‐20 weeks' gestation and continued up to 30 days postpartum. Declarations of interest: not reported Funding sources: UNICEF Indonesia Office, Jakarta |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We restructured the 157 hamlets into 160 dwelling clusters.", "these 160 clusters (and the pregnant women living within them) were randomly assigned into 4 blocks of 40 clusters each". Comment: method used for generating the randomisation sequence is not described in sufficient detail to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment:method used for allocation concealment is not described in sufficient detail to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This study had a single‐blind design, since the supplement for the treatment and control group looked different physically. However, participants residing in each cluster received the same supplement, so they were not aware that other participants from other clusters received a different supplement" Comment: study participants were blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: " This study had a single‐blind design" Comment: blinding of outcome assessors probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (< 1%) and attrition (10.4%) were reported along with their reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: when considering cluster‐design‐specific biases, we found low risk of baseline imbalance, loss of clusters, and comparability with individually randomised trials. While participants may have been recruited after assignment of clusters (843 pregnant women enrolled after intensive surveillance), we found that this likely would not have led to recruitment bias because supplements were provided to both groups and pregnant women were similar (including mean gestational age at baseline). Any incorrect analysis was corrected by adjustment for clustering within data reported in this review. |
Tofail 2008.
| Methods | The study was conducted in Matlab, a rural subdistrict in the east central plain of Bangladesh. Dates of study: November 2001‐December 2003 |
|
| Participants | Pregnant women with gestational age 6‐8 weeks, Hb > equal to 80 g/L and no serious disease were eligible for enrolment. | |
| Interventions |
|
|
| Outcomes | Size at birth, gestational age at birth, perinatal mortality, maternal Hb at 30 weeks, birthweight, spontaneous abortions, infant mortality, motor development and behavioural development, infant micronutrient status, under 5 child mortality, BP and kidney function in children, child growth outcomes, and adverse effects It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Women were divided into 2 groups, that is, early food group and usual food group. Each food group was then divided into 3 subgroups of MMN and IFA groups.
IFA given to all participants. There was no significant difference in baseline characteristics between randomisation groups. Maternal malnutrition was prevalent. Control group with 30 mg iron is included in this review. Data for child growth outcomes presented in a manner that precluded its inclusion in the analysis. Adverse effects included nausea (MMN supplementation 154/786, 30FeFA 126/763), vomiting (MMN 91/787, 30FeFA 54/762), loose motions (MMN 19/786, 30FeFA 18/763), heartburn (MMN 86/786, 30FeFA 83/763), and constipation (MMN 219/788, 30FeFA 227/762). Other trial (Gupta 2007) measuring this outcome presents data for all side‐effects using a composite measure. Analyses for individual side‐effects will be presented once additional trials are available. Cost data are published in Shaheen 2013 (see Tofail 2008 secondary reference). Stunting data relevant to this review are presented in Kahn 2013 (see Tofail 2008 secondary reference). The data for the Fe30F group are presented in Figure 1, a line graph, and we will contact the authors to clarify the exact numbers for inclusion in the next update. Declarations of interest: the trial authors declared no conflict of interest. Funding sources: The International Centre for Diarrheal Disease Research Bangladesh, the UK Medical Research Council, the Swedish Research Council, the UK Department for International Development, the Global Health Research Fund Japan, the Child Health and Nutrition Research Initiative, Uppsala University, the US Agency for International Development under the Cooperative Agreement #388‐G‐00‐02‐00125‐00, the Australian International Development Agency, the Government of Bangladesh, the Canadian International Development Agency, The Kingdom of Saudi Arabia, the Government of the Netherlands, the Government of Sri Lanka, the Swedish International Development Cooperative Agency, and the Swiss Agency for Development and Cooperation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "individual randomisation was done in blocks of 12" and "After enrolment, women were randomly assigned to 6 intervention groups". Comment: method used for generating the randomisation sequence was not described in sufficient detail to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method used for allocation concealment was not described in sufficient detail to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "pills were identical in appearance, and monthly supplies were provided in identical bottles", " the mothers were unaware of their micronutrient supplement" and "double masking was practiced" Comment: study participants and caregivers were blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "pills were identical in appearance, and monthly supplies were provided in identical bottles", "the testers were unaware of children’s groups" and "double masking was practiced" Comment: outcome assessors were blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was (26%), reported along with their reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified. |
West 2014.
| Methods | Community‐based, cluster‐randomised, double‐blind trial to examine whether a daily antenatal and postnatal MMN supplement given to women will enhance newborn and infant survival and health and other birth outcomes in a rural setting in northwestern Bangladesh. Dates of study: January 2008‐August 2012 |
|
| Participants | Pregnant women, aged 12‐45 years, consenting to participate were recruited (n = 45,000). Women not interviewed for consent within 12 consecutive weeks after being ascertained as pregnant by urine testing were excluded. | |
| Interventions |
Mothers instructed to take 1 tablet per day, from the 1st trimester through 12 weeks postpartum. |
|
| Outcomes | Infant mortality through 6 months of age, perinatal mortality, neonatal mortality, birth size (weight, length, circumferences), gestational age at birth, infant health outcomes, maternal morbidity, obstetric complications, body composition, nutritional status. Christian 2014, an additional report of this trial, reports length‐for‐age Z score and stunting at 1 and 3 months in an abstract. | |
| Notes | The composition of the MMN supplement followed the UN MMN preparation, except with higher amounts as needed to meet the RDA from the Institute of Medicine. The substudy area was selected to be representative of the parent trial across a range of factors, including sociodemographic and geographic variations, which were evaluated during an earlier trial in the same area. Declarations of interest: Dr. West reported a grant from DSM awarded to the Program in Human Nutrition at Johns Hopkins Bloomberg School of Public Health and having given 2 scientific presentations in 2 consecutive years at DSM in Basel, Switzerland, with accommodations provided. Dr. Christian reported giving a presentation at DSM in Basel with accommodations provided. The other trial authors declared no conflict of interest. Funding sources: grant OPP614 (Global Controlof Micronutrient Deficiency) from the Bill and Melinda Gates Foundation and additional assistance was received from the Sight and Life Global Nutrition Research Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used an in‐house program (VBScript, Microsoft) that recognized 70 possible permutations for n=8 sectors and k=2 supplement allocations and 6 for the last block of n=4 sectors. Using this program, we randomized sectors within blocks to 1 of 2 codes such that each permutation had an equal probability of being chosen." |
| Allocation concealment (selection bias) | Low risk | Quote: "The resulting 2 lists of sectors were securely transmitted to field headquarters. One envelope with the code key was securely transmitted to the supplement producer and the other sealed in an envelope and secured at Johns Hopkins. At no time during the trial did study investigators or field or data management staff have access to the key." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐masked", "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)", "received daily supplementation, so treatment effect (still blinded due to the ongoing trial)" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐masked", "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)", "received daily supplementation, so treatment effect (still blinded due to the ongoing trial)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete information was not available as the main trial has not been published; however, attrition is reported to be < 20% (trial presentations). |
| Selective reporting (reporting bias) | Low risk | Comment: reports from the study are still being published |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design specific biases (recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials) |
Zagre 2007.
| Methods | This study was a cluster‐randomised, double‐blind controlled programmatic study in rural Niger aiming to compare MMN supplementation versus iron and folic acid. Dates of study: not reported |
|
| Participants | Women residing in target villages and experiencing amenorrhoea for < 12 weeks were eligible for recruitment. All villages within the coverage of the 17 health centres of Mayahi district were included. Women with night blindness and/or signs of severe anaemia were excluded. | |
| Interventions |
|
|
| Outcomes | Birthweight and incidence of LBW, miscarriage, stillbirth, maternal mortality It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | Study participants received reproductive health services including malaria chemoprophylaxis, behaviour‐change communication activities to increase awareness and adoption of better lifestyles (feeding and rest during pregnancy). Outreach prenatal care sessions were also conducted throughout intervention villages. Randomisation resulted in comparable groups for most baseline characteristics except for households and more preventive measures against malaria (more in MMN group) and less education and more poverty in IFA group. Declarations of interest: not reported Funding sources: UNICEF |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Villages ‐ not individuals were randomly assigned to one treatment group or the other" Comment: method used for generating the randomisation sequence was not described in sufficient detail to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method used for allocation concealment was not described to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Because the two supplements did not look identical and may have been recognizable, a coding system was put in place by the SONIPHAR pharmaceutical company in Niger. Six codes were assigned to the treatments: three for iron/folic acid and three for multimicronutrient supplements. SONIPHAR packaged the supplements in boxes with identical labelling except for the supplement code. Health workers, traditional midwives, and data collectors were informed that each supplement came in two sizes and colors, so that the code letter did not distinguish which supplement was used". Comment: participants and caregivers were probably blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Because the two supplements did not look identical and may have been recognizable, a coding system was put in place by the SONIPHAR pharmaceutical company in Niger. six codes were assigned to the treatments: three for iron/folic acid and three for multimicronutrient supplements. SONIPHAR packaged the supplements in boxes with identical labelling except for the supplement code. Health workers, traditional midwives, and data collectors were informed that each supplement came in two sizes and colors, so that the code letter did not distinguish which supplement was used". Comment: outcome assessors were probably blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was 18%. Reasons for attrition were reported, and dropout was significantly higher in the MMN (25/1893 (1.3%)) versus IFA (8/1777 (0.5%)) group. Exclusion data were not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper. |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design‐specific biases (recruitment bias, baseline imbalance, loss of clusters, and comparability with individually randomised trials). Any incorrect analysis was corrected by adjustment for clustering within data reported in this review. |
Zeng 2008.
| Methods | Community‐based cluster‐randomised trial conducted in 2 poor rural counties in Shaanxi province of north west China. Dates of study: August 2002‐Feburary 2006 |
|
| Participants | Pregnant women of < 28 weeks' gestation between August 2002 and January 2006. Pregnancy was confirmed using LMP and urine pregnancy test | |
| Interventions |
|
|
| Outcomes | Birthweight, LBW (< 2500 g), SGA (weight < 10 percentile for gestational age), preterm birth (< 37 weeks of gestation), very preterm birth (< 34 weeks of gestation), gestational age, birth length, head circumference, Hb, anaemia (Hb < 110 g/L in third trimester), neonatal deaths (death within 28 days of delivery), early neonatal deaths (death within 7 days of delivery), perinatal deaths (fetal death after 28 weeks of gestation plus early neonatal deaths); and mental and psychomotor development outcomes until 1 year of age by using the Bayley Scales of Infant Development, and growth outcomes (stunting, underweight, and wasting) in children in the first 30 months of life. It should be noted that the data for SGA were obtained from a separate report (Food and Nutrition Bulletin 2009) and not from the individual trial report. |
|
| Notes | For review purposes, we used the MMN and IFA groups. Intervention was administered until 6 weeks postpartum. Baseline characteristics at enrolment, and both cluster‐ and individual‐level baseline characteristics were balanced by treatment groups. Stunting, underweight, and wasting data presented as odds ratio and could not be included in the analysis. Declarations of interest: MJD was consultant for UNICEF China UNICEF Pyongyang during the conduct of the trial. SC was nutrition consultant for UNICEF China from 2001‐2002, and is now the liaison officer for UNICEF with the Ministry of Health. Funding sources: United Nations Children's Fund (grant No YH101‐H12/03) through a co‐operative agreement between UNICEF and the Centers for Disease Control and Prevention, Atlanta, US, and the National Natural Science of Foundation of China (grant No 30271131), Beijing, China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was generated off site with a pseudo‐random number generator in SAS". Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation schedule was generated off site with a pseudo‐random number generator in SAS version 6 (SAS Institute, Cary, NC). A treatment colour code was assigned to each village based on the treatment allocation schedule". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind", "treatment colour code was assigned to each village based on the treatment allocation schedule. The treatment codes were opened only once all data had been collected and blinded analysis of the primary hypothesis was completed" and "were of identical appearance and packaged in blister packs" Comment: participants and caregivers were blinded to the treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind", "treatment colour code was assigned to each village based on the treatment allocation schedule. The treatment codes were opened only once all data had been collected and blinded analysis of the primary hypothesis was completed" Comment: outcome assessors were blinded to the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion (4.8%) and attrition (2.3%) were reported along with their reasons |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes mentioned in the methods section were presented in the paper |
| Other bias | Low risk | Comment: no other bias was identified, including cluster‐design‐specific biases (recruitment bias, baseline imbalance, loss of clusters, and comparability with individually randomised trials). Investigators did not adjust for the cluster‐randomised design in their sample size or outcome estimations, but this was corrected. |
BMI: body mass index; BP: blood pressure; CHW: community health worker; EPI: Expanded Programme on Immunization; FeFol: iron folate; Hb: haemoglobin; HIV‐ve: HIV‐negative; HIV+ve: HIV‐positive; IF(A): iron folic acid; IU: international unit; IUGR: intrauterine growth retardation; LBW: low birthweight; LGA: large‐for‐gestational age; LMP: last menstrual period; LNS: lipid‐based nutrient supplement; mcg: microgram; mg: milligram; Mg: magnesium; MMN: multiple micronutrient; PE: protein energy; RCT: randomised controlled trial; RDA: recommended daily allowance; SAE: serious adverse effects; SGA: small‐for‐gestational age; UNICEF: United Nations International Children's Emergency Fund; UNU: United Nations University; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12616001449426 | Protocol of a study that is not a RCT. |
| Agarwal 2012 | Abstract only; similar micronutrients given for different durations. |
| Aguayo 2005 | Not a RCT. Assessing acceptability of MMN supplements by pregnant and lactating women. |
| Ahn 2006 | Comparing 2 MMN supplements. |
| An 2001 | Compares different doses of iron (35 mg vs 60 mg). |
| Arsenault 2010 | Includes HIV‐1 positive women. |
| Asemi 2014 | Single‐blind trial comparing multivitamin vs multivitamin‐mineral. Supplements differed in composition and dose. |
| Asemi 2015 | Study population is women at high risk of pre‐eclampsia. |
| Azami 2016 | Study population is women with at least 1 risk factor for pre‐eclampsia. |
| Beazley 2002 | Assesses vitamin C and E supplementation only. |
| Bergmann 2006 | Assesses docosahexaenoic acid and fructo‐oligosaccharide. |
| Biswas 1984 | Cross‐over design, measuring only serum iron levels after single doses of different vitamin formulations. |
| Callaghan‐Gillespie 2017 | MMN is provided in the form of food supplements (fortified corn‐soy blend). |
| Carrasco 1962 | Study has assessed the impact of D‐sorbitol on the absorption of MMNs in pregnant women. |
| Caulfield 1999 | Only assesses zinc supplementation. |
| Chames 2002 | Only assesses calcium supplementation. |
| Choudhury 2012 | Comparing micronutrient powder (home fortification) containing iron, folic acid, vitamin C and zinc vs iron folic acid tablet to study impact on anaemia during pregnancy. |
| Christian 2009 | Assesses the effectiveness of the standard of care (iron folic acid and single‐dose mebendazole) for the treatment of severe anaemia (haemoglobin < 70 g/L) along with enhanced regimens. |
| Coles 2015 | Investigators utilized a quasi‐randomised method of allocation to intervention and control groups. |
| Cooper 2012 | Evaluates periconceptional MMN supplementation only (no MMNs given during pregnancy) (same as Khulan 2012). |
| Czeizel 1996 | Assesses periconceptional supplementation of 18 micronutrients against 4 micronutrients. |
| Dawson 1987 | Assesses supplementation of 14 micronutrients against 11 micronutrients. |
| Dawson 1998 | Assesses supplementation of different doses of 12 to 17 micronutrients. |
| Devi 2017 | The study investigates the effect of supplemental high‐quality protein (milk) and vitamin B12 on third‐trimester methionine kinetics in pregnant women with low vitamin B12. |
| Dewey 2012 | The study asesses the effect of LNS and child only micronutrient powder (MNP). |
| Dieckmann 1944 | Fortification trial. |
| Fall 2006 | This trial evaluates a micronutrient‐rich snack containing vegetables, fruit, and milk. |
| Fawzi 1998 | Includes pregnant women who are HIV‐1 positive. |
| Fernald 2016 | The study investigates the effect of various combined interventions including lipid‐based suppplementation on growth and development during pregnancy and early childhood. Does not study MMN supplements. |
| Feyi‐Waboso 2005 | Parenteral preparation. |
| Fleming 1986 | Only assesses iron, folate and vitamin B in different combinations. |
| Godfrey 2017 | Protocol of a study that investigates the effect of a micronutrient‐enriched nutritional drink with probiotics and myo‐inositol vs. a standard supplementation drink on the maintenance of healthy glucose metabolism in mothers and promotion of offspring health. |
| Goldenberg 1995 | Only assesses zinc supplementation. |
| Gopalan 2004 | Evaluates effect of soya oil. |
| Graham 2007 | Study has looked at the impact of vitamin A fortified rice with and without iron and riboflavin supplementation in night‐blinded women. |
| Guldholt 1991 | Only assesses high‐dose vs low‐dose iron supplementation. |
| Gunaratna 2015 | The study investigates the efficacy of pre‐pregnancy multivitamins with IFA to reduce the prevalence of anemia during the periconceptional period. |
| Gupta 2007 | Women with BMI < 18.5 and/or haemoglobin level of 7‐9 g/dL were included. |
| Hambidge 2014 | Protocol of a study comparing nutrition intervention (MMN fortified lipid‐based supplement) in pre‐conceptional and peri‐conceptional stage. |
| Hillman 1963 | Only assesses pyridoxine supplementation. |
| Hininger 2004 | MMN supplement did not contain iron. |
| Holly 1955 | Only assesses iron and cobalt supplementation. |
| Hossain 2014 | Trial evaluates the effect of vitamin D supplementation. Both groups received iron and calcium. |
| Huang 2017 | Protocol of a study that investigates at the effect of complex milk lipids and different maternal milk preparations during pregnancy. |
| Hunt 1983 | Only assesses zinc supplementation. |
| Hunt 1985 | Only assesses zinc supplementation. |
| Huybregts 2009 | Assesses impact of balanced energy, protein dietary supplement. |
| Huynh 2017 | The study compares maternal nutrient supplementation as a powder and breastfeeding support vs usual care (IFA). |
| Iannotti 2008 | Only assesses zinc supplementation. |
| ICMR 2000 | Assesses periconceptional supplementation of folic acid containing vitamins. |
| IRCT2015041321736N1 | Protocol of a study that compares vitamin D injections every 2 weeks plus multi prenatal tablet including 400 units of vitamin D daily vs multi prenatal tablet only (control). Study population is women with vitamin D deficiency. |
| IRCT201704225623N109 | Protocol of a study in which the study population is women with gestational diabetes. |
| ISRCTN83599025 | Protocol of a study in which the intervention arm of the study includes pregnant women with nutrient deficiencies. |
| Itam 2003 | Not a randomised trial. |
| Janmohamed 2016 | This study assesses the effect of prenatal corn soya blend (CSB+) vs normal diet (control) on pregnancy outcomes. |
| Jarvenpaa 2007 | Fortification trial. |
| Kabir 2009 | This is the same cohort as Tofail 2008. However, all pregnant women were again randomised to breastfeeding counselling or a control (standard health message) group. Effect was evaluated on anthropometric outcomes in children. |
| Kable 2012 | Trial in women consuming alcohol during pregnancy evaluating effect of MMNs in ameliorating the impact of prenatal alcohol exposure in infants. |
| Khavari 2014 | The trial recruited HIV positive women. |
| Khulan 2012 | Evaluates periconceptional MMN supplementation only (no MMNs given during pregnancy). |
| Kubik 2004 | Original papers in Polish. Translated versions of the papers show that this study is not a randomised trial. |
| Kureishy 2017 | Protocol of a study that assesses the effectiveness of food‐based interventions: Wheat Soya Blend (WSB) for women during pregnancy and lactation, Wawa Mum for children 6‐23 months, Micronutrient Powders (MNP) for children 24‐59 months and Behaviour Change Communication vs. routine public health services (control) in preventing stunting in children under 5 years. |
| Kynast 1986 | Study presented at a conference. Abstract does not indicate that it as a randomised trial. |
| Lanou 2014 | Evaluated the effect of a lipid‐based nutrient supplement LNS. |
| Leroy 2010 | The study compares a traditional food assisted MCHN program vs a newly designed approach to prevent malnutrition in children. |
| Li 2014 | The study evaluates the effect of supplementation with folic acid and milk. |
| Lindström 2011 | Not an RCT of MMN versus iron and or folic acid. Describes prevalence of micronutrient deficiencies at baseline and its determinants. |
| Ling 1996 | Evaluating the impact of traditional Chinese tonics with nutrients. |
| Lucia 2007 | Evaluating impact of docosahexaenoic acid and fructo‐oligosaccharide. |
| Ma 2008 | Evaluating retinol and riboflavin supplementation. |
| Magon 2014 | The trial evaluating the effect of use of fortified snacks during pregnancy. Both groups received the same micronutrients (i.e. iron, folic acid, beta‐carotene, and calcium), however, the dose of micronutrients in the intervention group was higher than the control group. |
| Malvasi 2014 | Study evaluating the effect of myoinositol, d‐chiro inositol, folic acid and manganese during pregnancy on maternal blood pressure, glycaemic and cholesterol parameters. Inositol is not an essential nutrient. |
| Mardones 2007 | Impact of fortification of fortified dairy product with polyunsaturated fatty acids. |
| Marya 1987 | Only assesses calcium and vitamin D supplementation. |
| Mathan 1979 | Assesses supplementation of vitamin C and protein. |
| Menon 1962 | Not a RCT. |
| Merchant 2005 | Includes pregnant women who are HIV‐1 positive. |
| Merialdi 1999 | Only assesses zinc supplementation. |
| Muslimatun 2001 | Only assesses vitamin A supplementation. |
| Nakano 2010 | The study assesses the effect of Chlorella tablets vs control (no supplements) on pregnancy anemia and pregnancy‐induced hypertension. Does not study MMN supplements. |
| NCT01795131 | Evaluated the effect of vitamin B12 only. |
| NCT02802566 | Protocol of a study that investigates the efficacy of a BMI‐based prenatal vitamin in decreasing markers of inflammation and oxidative stress in pregnancies complicated by obesity. Study population is obese (BMI > 30) women. |
| NCT02959125 | Study population is women with anemia, mid upper arm circumference of less than 23.5 cm, or no weight gain |
| Nguyen 2012 | Protocol of a study evaluating pre‐conceptional MMN vs IFA supplements. |
| Nguyen 2017 | The study compares a nutrition‐focused Maternal, Neonatal, and Child Health (MNCH) program with a standard MNCH program. |
| Nossier 2015 | Study population is women with low serum zinc level below the estimated median for gestational age. |
| Nwagha 2010 | Micronutrients given via injection. |
| Ochoa‐Brust 2007 | Assesses impact of vitamin C only. |
| Olofin 2014 | The trial included HIV‐positive women. |
| Otoluwa 2017 | Conference abstract; the study evaluates the effect MMN supplementation vs IFA in periconception period. |
| Park 1999 | Semi‐randomized study design does not satisfy the eligibility criteria of the review. |
| Patimah 2013 | Not a randomised trial. |
| People's League 1946 | Quasi‐randomised trial. Women were divided into 2 groups by placing them alternately on separate lists. |
| Pezzack 2014 | The study is a randomised cross‐over trial that compares the fractional calcium absorption (FCA) from enteric coated (EC) calcium carbonate granules with non‐EC granules in pregnant women. |
| Ramirez‐Velez 2011 | The comparator was not eligible. The control group received calcium in addition to ferrous sulphate and folic acid. |
| Robertson 1991 | Only assesses zinc supplementation. |
| Rumiris 2006 | Pregnant women with superoxidedismutase (SOD) levels below 1102 U/gHb included in the study. |
| Sachdeva 1993 | Evaluated calcium supplementation. |
| Sagaonkar 2009 | Comparison of 4 micronutrients with 3. |
| Salzano 2001 | Evaluated supplementation with calcium and fatty acids (linoleic acid, mono and poly unsaturated fatty acids) vs control. |
| Schmidt 2001 | Only assesses vitamin A supplementation. |
| Semba 2000 | A trial of vitamin A supplementation in HIV‐infected women. |
| Suharno 1993 | Only assesses vitamin A supplementation. |
| Sun 2010 | Quasi‐randomised trial. Women were allocated to 4 groups in the order of enrolment. |
| Suprapto 2002 | Only assesses vitamin A and riboflavin supplementation. |
| Taghizadeh 2014 | Comparing 2 different MMN supplements (13 micronutrients vs 10 micronutrients). |
| Tanumihardjo 2002 | Only assesses vitamin A and iron supplementation. |
| Tatala 2002 | Fortification trial. |
| Thauvin 1992 | Not a randomised trial. |
| Theobald 1937 | MMN supplement did not contain iron. |
| Vadillo‐Ortega 2011 | Fortification trial |
| Webb 2009 | Participants include HIV‐positive women. |
| Wibowo 2012 | Evaluating effect of milk fortified with different doses of minerals and vitamins. |
| Wijaya‐Erhardt 2011 | Evaluated weekly food provision (optimised diet) vs the control. |
| Wijaya‐Erhardt 2014 | Evaluated educational intervention using a pre‐post design. |
| Young 2010 | Study assessed the acceptability of a micronutrient powder (Sprinkles), a fortified food (Nutrivida), and tablets by the participants. All supplements has similar composition of micronutrients. |
| Zavaleta 2000 | Only assesses zinc supplementation. |
BMI: body mass index IFA: iron and folic acid LNS: lipid‐based nutrient supplement MMN: multiple micronutrient RCT: randomised controlled trial vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
Gathwala 2012.
| Methods | RCT |
| Participants | Pregnant women 12‐14 weeks' gestation. Fetal malformation excluded. Total number randomised 560. Group denominators not stated |
| Interventions | MMN vs iron 100 mg and folic acid 500 mcg. MMN not described |
| Outcomes | Mean birthweight, LBW |
| Notes | No usable data due to missing group denominators. Trial authors contacted (g_gathwala@hotmail.com) in hopes of adding data in next update. |
LBW: low birthweight; MMN: multiple micronutrient; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02190565.
| Trial name or title | Protocol identifier: NCT02190565 Supplementation with WellnessPack mama during pregnancy and lactation ‐ a randomised double‐blind, placebo‐controlled study |
| Methods | Parallel, randomised, double‐blind trial investigating food supplementation for the primary prevention of anaemia |
| Participants | Inclusion criteria:
Exclusion criteria:
Upon miscarriage or transfer to prenatal care specialist, the participant is excluded from the study. |
| Interventions | Placebo comparator: placebo Placebo consisting of 2 sham multivitamin and mineral tablets and 2 capsules of oil Active comparator: food supplement Food supplement consisting of fish oil (omega 3 fatty acids DHA and EPA) and multivitamin and mineral tablets with extra IFA Intervention: dietary supplement: food supplement |
| Outcomes | Primary outcome: prevalence of anaemia in active and placebo groups (time frame: pregnancy weeks 28‐30) (Designated as safety issue: no) Blood will be analysed for Hb and ferritin values. We will compare how many women in each group (active vs placebo) are ordinated iron supplementation due to anaemia by mid‐pregnancy. Secondary outcome: levels of nutritional biomarkers in maternal blood and breast milk (time frame: 6‐10 weeks after delivery) (Designated as safety issue: no) Levels of nutritional biomarkers such as DHA and vitamin D in maternal blood and breast milk will be measured in active and placebo groups. |
| Starting date | October 2014‐January 2016 |
| Contact information | Professor Angelica Lindén Hirschberg: Angelica.Hirschberg.Linden@ki.se |
| Notes | Sudy sponsor: Oriflame Cosmetics AB. Collaborators: Karonlinska Institutet |
NCT03287882.
| Trial name or title | Protocol identifier: NCT03287882 Prospective, cluster randomised effectiveness trial of multiple micronutrient supplementation and life skills education provided from preconception on health and nutrition outcomes of young, reproductive‐age Pakistani women (15‐24 years) |
| Methods | Parallel, randomised, double‐blind trial investigating
|
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | No Intervention: standard of care
Experimental: MMN supplementation (UNIMMAP composition) and life skills education
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 30 June 2017‐30 April 2021 |
| Contact information | Zulfiqar A Bhutta: zulfiqar.bhutta@aku.edu |
| Notes |
Sumarmi 2015.
| Trial name or title | Protocol identifier: TCTR20150614001 Preconceptional supplementation of multi micronutrients to improve maternal iron status and pregnancy outcomes: a randomized double blind community‐based trial (Laduni) |
| Methods | Parallel, randomised, double‐blind trial investigating the efficacy of MMN supplementation during preconception period on maternal iron status and pregnancy outcomes |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: MMN group; participants received multinutrient capsule every 2 days, for 2‐6 months before pregnancy and continued to receive multinutrient capsule daily during pregnancy (38 weeks). Comparator: placebo‐IFA group; participants received placebo capsule every 2 days, for 2‐6 months before pregnancy and received IFA daily during pregnancy (38 weeks) |
| Outcomes | Primary outcomes: birthweight, maternal iron status Secondary outcomes: maternal interleukin‐12, maternal human placental lactogen, umbilical cord insulin‐like growth factor‐1 |
| Starting date | 21 March 2011‐1 December 2014 |
| Contact information | Sri Sumarmi: msrisumarmi@gmail.com |
| Notes | Asoociated conference abstract: Sumarmi 2017: Prolonging micronutrients supplementation 2‐6 months prior to pregnancy significantly improves birthweight by increasing hPL production and controlling il‐12 concentration: a randomised double blind controlled study |
Tu 2013.
| Trial name or title | Effect of animal‐source food supplement prior to and during pregnancy on birthweight and prematurity in rural Vietnam |
| Methods | Cluster‐randomised trial recruiting women from 29 communes in Vietnam |
| Participants | Women recruited when registering for marriage |
| Interventions |
|
| Outcomes | The primary outcome is birthweight and the secondary outcome is the prevalence of prematurity. Other outcomes include maternal micronutrient status (iron, zinc, folic acid, vitamins A and B12), the incidence of infections; infant growth and infections from 0‐6 months of age are also assessed. Maternal data and information are measured at recruitment, 16, and 34 weeks' gestation. Infant anthropometric status is measured at birth, 1, 3, and 6 months. Infant gestational age is assessed at birth to determine the prevalence of pre‐term deliveries, and the mother’s activity or physical work during pregnancy is also determined. |
| Starting date | Not stated |
| Contact information | N. Tu, Vietnam Nutrition Association, Hanoi, Vietnam. C. King, Children's Hospital Oakland Research Institute, Oakland, CA, USA |
| Notes |
BMI: body mass index; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; Hb: haemoglobin; IFA: iron and folic acid; ITT: intention‐to‐treat; LBW: low birthweight; LNS: lipid‐based nutrient supplement; mcg: microgram; MMN: multiple micronutrient; RDA: recommended daily allowance; SAE: serious adverse event; SGA: small‐for‐gestational‐age
Differences between protocol and review
We have updated the methods to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We merged the prespecified subgroup analysis 'duration of treatment' with another prespecified subgroup analysis 'gestational age at which supplementation was started' because it uses the same information. We also deleted a subgroup 'micronutrient interactions'. We have undertaken an additional subgroup analysis to look at UNIMMAP versus non‐UNIMMAP formulation for the multiple micronutrient (MMN) supplement.
We have included two new secondary outcomes; these are macrosomia and mode of delivery. We have modified the list of primary outcomes so that it now includes some outcomes that were earlier included as secondary outcomes. We changed the primary and secondary outcomes to address issues identified in the recent literature, by experts in the field and given their importance from the policy perspective.
It is now explicit that the review focuses on oral supplements and trials examining parenteral provision of MMN or MMN via food fortification are now not included.
We added a 'Summary of findings' table.
For the 2018 update, we added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
For this update, we re‐extracted data for all primary and secondary outcomes for all included studies from the outset, not just those found from the most recent search. Minor recalculations of cluster parameters resulted in slight differences in results in studies between this version and the previously published version (Haider 2017). This has made very little difference to the overall results. The data files corresponding to the changes are available from the authors on request.
Contributions of authors
Emily C Keats (ECK), Emily Tam (ET), Batool A Haider (BAH) and Zulfiqar A Bhutta (ZAB) undertook the current 2018 update of the 2017 Cochrane Review. ECK and ET undertook the revised analysis with input from BAH and ZAB. All authors approved the final version of this review.
ZAB was the principal investigator of Bhutta 2009a, and data extraction was undertaken by BAH for this trial. BAH created the comparisons, did the analysis and wrote the text of the review. ZAB provided guidance and approved the review.
Sources of support
Internal sources
The Aga Khan University Hospital, Pakistan.
External sources
Department for International Development, UK.
United Nations Children's Fund (UNICEF), USA.
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Emily Keats: none
Batool A Haider: none
Emily Tam: none
Zulfiqar A Bhutta was the principal investigator of the UNIMAPP trial conducted in Pakistan (Bhutta 2009a). He was not involved in the screening and data extraction for this paper, which was conducted by other review authors acknowledged above. Dr Bhutta is also the recipient of a grant from the Bill & Melinda Gates Foundation to undertake an individual participant data analysis of nutrition interventions in adolescents and women during pregnancy.
Edited (no change to conclusions)
References
References to studies included in this review
Ashorn 2010 {published data only}
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Gondwe A, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small‐quantity lipid‐based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. Journal of Nutrition 2015;145(6):1345‐53. [DOI] [PubMed] [Google Scholar]
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, et al. The impact of lipid‐based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. American Journal of Clinical Nutrition 2015;101(2):387‐97. [DOI] [PubMed] [Google Scholar]
- Barua P, Chandrasiri UP, Beeson JG, Dewey KG, Maleta K, Ashorn P, et al. Effect of nutrient supplementation on the acquisition of humoral immunity to plasmodium falciparum in young Malawian children. Malaria Journal 2018;17(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasiri UP, Fowkes FJ, Richards JS, Langer C, Fan YM, Taylor SM, et al. The impact of lipid‐based nutrient supplementation on anti‐malarial antibodies in pregnant women in a randomized controlled trial. Malaria Journal 2015;14(1):193. [NCT01239693] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM, Arnold C, Ashorn P, Ashorn U, Chaima D, Cheung YB, et al. Lipid‐based nutrient supplements during pregnancy and lactation did not affect human milk oligosaccharides and bioactive proteins in a randomized trial. Journal of Nutrition 2017 [epub ahead of print]. [DOI] [PMC free article] [PubMed]
- NCT01239693. Supplementing maternal and infant diet with high‐energy, micronutrient fortified lipid‐based nutrient supplements (LNS) (iLiNS‐DYAD‐M). clinicaltrials.gov/show/NCT01239693 (first received 11 November 2010).
- Nkhoma M, Ashorn P, Ashorn U, Dewey KG, Gondwe A, Mbotwa J, et al. Providing lipid‐based nutrient supplement during pregnancy does not reduce the risk of maternal P falciparum parasitaemia and reproductive tract infections: a randomised controlled trial. BMC Pregnancy and Childbirth 2017;17(1):35. [NCT01239693] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, et al. Effects of maternal and child lipid‐based nutrient supplements on infant development: a randomized trial in Malawi. American Journal of Clinical Nutrition 2016;103(3):784–93. [DOI] [PubMed] [Google Scholar]
- Pulakka A, Cheung YB, Maleta K, Dewey KG, Kumwenda C, Bendabenda J, et al. Effect of 12‐month intervention with lipid‐based nutrient supplement on the physical activity of Malawian toddlers: a randomised, controlled trial. British Journal of Nutrition 2017;117(4):511‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Oaks BM, Laugero KD, Ashorn U, Harjunmaa U, Kumwenda C, et al. Maternal cortisol and stress are associated with birth outcomes, but are not affected by lipid‐based nutrient supplements during pregnancy: an analysis of data from a randomized controlled trial in rural Malawi. BMC Pregnancy and Childbirth 2015;15(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RC, Ashorn P, Umar E, Dewey KG, Ashorn U, Creed F, et al. The impact of maternal diet fortification with lipid‐based nutrient supplements on postpartum depression in rural Malawi: a randomised‐controlled trial. Maternal & Child Nutrition 2017;13(2):e12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2009a {published and unpublished data}
- Bhutta ZA, Rizvi A, Raza F, Hotwani S, Zaidi S, Soofi S, et al. A comparative evaluation of multiple micronutrient and iron‐folate supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food and Nutrition Bulletin 2009;30(4):S496‐S505. [DOI] [PubMed] [Google Scholar]
- Persson LA, Eneroth H, Ekstrom EC. Multiple micronutrient supplementation during pregnancy: a review of effects on birth size, maternal haemoglobin and perinatal mortality demonstrated in trials in Bangladesh, Guinea‐Bissau and Pakistan. Report for UNICEF/UNU/WHO 2004.
Biggs 2010 {published data only}
- ACTRN12610000944033. A randomised controlled trial to compare the impact on birth weight of daily iron‐folic acid, twice weekly iron‐folic acid and twice weekly multiple micronutrient supplementation for pregnant women in Ha Nam province, Vietnam. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000944033 (first received 28 October 2010).
- Hanieh S, Ha TT, Simpson JA, Casey GC, Thuy T, Khuong NC, et al. The effect of intermittent antenatal iron supplementation on infant outcomes in rural Vietnam: a cluster randomised trial. Annals of Nutrition and Metabolism 2013;63(Suppl 1):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanieh S, Ha TT, Simpson JA, Casey GJ, Khuong NC, Thoang DD, et al. The effect of intermittent antenatal iron supplementation on maternal and infant outcomes in rural Vietnam: a cluster randomised trial. Plos Medicine 2013;10(6):e1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Fisher J, Hanieh S, Tran T, Simpson JA, Tran H, et al. Antenatal iron supplementation regimens for pregnant women in rural Vietnam and subsequent haemoglobin concentration and anaemia among their infants. Plos One 2015;10(4):e0125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brough 2010 {published data only}
- Brough L, Rees GA, Crawford MA, Morton RH, Dorman EK. Effect of multiple‐micronutrient supplementation on maternal nutrient status, infant birth weight and gestational age at birth in a low‐income, multi‐ethnic population. British Journal of Nutrition 2010;104(3):437‐45. [DOI] [PubMed] [Google Scholar]
Christian 2003 {published data only}
- Bahnfleth C, Murray‐Kolb L, Schaefer B, Cole P, Khatry S, LeClerq S, et al. Effects of prenatal micronutrient supplementation on school age child behavior. FASEB Journal 2014;28(1 Suppl 1):Abstract no. 619.6. [Google Scholar]
- Christian P, Darmstadt GL, Wu L, Khatry SK, LeClerq SC, Katz J, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Archives of Disease in Childhood 2008;93(8):660‐4. [DOI] [PubMed] [Google Scholar]
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83:788‐94. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ 2003;326:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Khatry SK, LeClerq SC, Dali SM. Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. International Journal of Gynaecology and Obstetrics 2009;106(1):3‐7. [DOI] [PubMed] [Google Scholar]
- Christian P, Murray‐Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school‐aged children in Nepal. JAMA 2010;304(24):2716‐23. [DOI] [PubMed] [Google Scholar]
- Christian P, Nanayakkara‐Bind A, Schulze K, Wu L, LeClerq SC, Khatry SK. Antenatal micronutrient supplementation and third trimester cortisol and erythropoietin concentrations. Maternal & Child Nutrition 2016;12(1):64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Shrestha J, LeClerq SC, Khatry SK, Jiang T, Wagner T, et al. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. Journal of Nutrition 2003;133:3492‐8. [DOI] [PubMed] [Google Scholar]
- Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow‐up in a randomized, controlled community trial. American Journal of Epidemiology 2009;170(9):1127‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, West KP Jr, Khatry SK, LeClerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomised trial in Nepal. American Journal of Clinical Nutrition 2003;78:1194‐202. [DOI] [PubMed] [Google Scholar]
- Katz J, Christian P, Dominici F, Zeger SL. Treatment effects of maternal micronutrient supplementation vary by percentiles of the birth weight distribution in rural Nepal. Journal of Nutrition 2006;136:1389‐94. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Christian P, LeClerq SC, Khatry SK. Determinants of compliance to antenatal micronutrient supplementation and women's perceptions of supplement use in rural Nepal. Public Health Nutrition 2010;13(1):82‐90. [DOI] [PubMed] [Google Scholar]
- Murray‐Kolb L, Bahnfleth C, Hurley K, Cole P, Khatry S, LeClerq S, et al. Micronutrient supplementation during pregnancy and the preschool years and child behavior at 7‐9 years of age. FASEB Journal 2014;28(1 Suppl):Abstract no: 619.3. [Google Scholar]
- Nanayakkara‐Bind A, Schulze K, Wu L, Le SC, Khatry SK, Christian P. Effects of antenatal micronutrient supplementation on cortisol and erythropoietin in pregnant Nepalese women. FASEB Journal 2011;25:Abstract no: 779.15. [Google Scholar]
- Stewart CP, Christian P, LeClerq SC, West KP Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school‐age children in rural Nepal. American Journal of Clinical Nutrition 2009;90(1):132‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Arguello M, Leclerq SC, Khatry SK, et al. Low maternal vitamin B‐12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. Journal of Nutrition 2011;141(10):1912‐7. [DOI] [PubMed] [Google Scholar]
- Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6‐ to 8‐year‐old children in rural Nepal. Journal of Nutrition 2009;139(8):1575‐81. [DOI] [PubMed] [Google Scholar]
Dewey 2009 {published data only}
- Adams KP, Vosti SA, Ayifah E, Phiri TE, Adu‐Afarwuah S, Maleta K, et al. Willingness to pay for small‐quantity lipid‐based nutrient supplements for women and children: evidence from Ghana and Malawi. Maternal & Child Nutrition 2018;14(2):e12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Dewey KG, Lartey A, Okronipa H, Ashorn P, Arimond M. Prenatal supplementation with small‐quantity lipid‐based nutrient supplements or multiple micronutrients increases urinary iodine concentration in semi‐urban Ghana: a randomized controlled trial. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:309.
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Ashorn U, Zeilani M, et al. Maternal supplementation with small‐quantity lipid‐based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi‐urban Ghana: a randomized controlled trial. Journal of Nutrition 2017;147(4):697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, et al. Small‐quantity, lipid‐based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18‐mo‐old children in semi‐urban Ghana: a randomized controlled trial. American Journal of Clinical Nutrition 2016;104:797‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Baldiviez LM, et al. Impact of small‐quantity lipid‐based nutrient supplement on hemoglobin, iron status and biomarkers of inflammation in pregnant Ghanaian women. Maternal & Child Nutrition 2017;13(2):e12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Ashorn P, Zeilani M, Peerson JM, et al. Lipid‐based nutrient supplement increases the birth size of infants of primiparous women in Ghana. American Journal of Clinical Nutrition 2015;101(4):835‐46. [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Maleta K, Ashorn P, Ashorn U, et al. Efficacy of LNS products for pregnant and lactating women: pregnancy outcomes from the iLiNS‐dyad studies in Ghana and Malawi. Annals of Nutrition and Metabolism 2013;63:24‐5. [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Peerson J, Ashorn P, Dewey K. Effects of small‐quantity lipid‐based nutrient supplement on hemoglobin and iron status of pregnant Ghanaian women. FASEB Journal 2015;29(1 Suppl):39.5. [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Okronipa H, Peerson J, Vosti S, Ashorn P, et al. Lipid‐based nutrient supplement for pregnant women improve birth outcomes among primiparous but not multiparous women in Ghana. FASEB Journal 2014;28(1 Suppl 1):Abstract no: 256.7. [Google Scholar]
- Adu‐Afarwuah S, Young RT, Lartey A, Okronipa H, Ashorn P, Ashorn U, et al. Supplementation during pregnancy with small‐quantity lipid‐based nutrient supplements or multiple micronutrients, compared with iron and folic acid, increases women's urinary iodine concentration in semiurban Ghana: a randomized controlled trial. Maternal & Child Nutrition 2018;14(2):e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey KG, Arimond M, Harding K, Matias S, Adu‐Afarwuah S, Ashorn P, et al. T2_001. Effects of maternal lipid‐based nutrient supplementation during pregnancy and the first six months postpartum on lactation outcomes. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:47.
- Klevor M, Haskell M, Lartey A, Adu‐Afarwuah S, Zeilani M, Dewey K. Effect of small‐quantity lipid‐based nutrient supplement (SQLNS) on breast milk vitamin a concentration among Ghanaian women. FASEB Journal 2015;29(1 Suppl):[898.17]. [Google Scholar]
- Klevor MK, Adu‐Afarwuah S, Ashorn P, Arimond M, Dewey KG, Lartey A, et al. A mixed method study exploring adherence to and acceptability of small quantity lipid‐based nutrient supplements (SQ‐LNS) among pregnant and lactating women in Ghana and Malawi. BMC Pregnancy and Childbirth 2016;16:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevor MK, Haskell MJ, Lartey A, Adu‐Afarwuah S, Zeilani M, Dewey KG. Lipid‐based nutrient supplements providing approximately the recommended daily intake of vitamin a do not increase breast milk retinol concentrations among Ghanaian women. Journal of Nutrition 2016;146:335‐42. [DOI] [PubMed] [Google Scholar]
- Kumordzie S, Arimond M, Young RR, Ocansey ME, Okronipa H, Prado E, et al. The long‐term effect of maternal and early childhood supplementation on growth and body composition at 4‐6 years of age in Ghanaian children. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:554‐5.
- NCT00970866. Efficacy of lipid‐based nutrient supplements (LNS) for pregnant and lactating women and their infants. clinicaltrials.gov/ct2/show/NCT00970866 (first received 3 September 2009).
- Oaks B, Adu‐Afarwuah S, Lartey A, Stewart C, Ashorn P, Vosti S, et al. Lipid‐based nutrient supplementation during pregnancy decreases maternal cortisol in younger women. FASEB Journal 2014;28(1 Suppl 1):Abstract no. 389.6. [Google Scholar]
- Oaks BM, Adu‐Afarwuah S, Lartey A, Stewart C, Ashorn P, Vosti S, et al. Lipid‐based nutrient supplementation during pregnancy decreases maternal cortisol in younger women (389.6). FASEB Journal 2014;28(1 Suppl):Abstract Number: 389.6. [Google Scholar]
- Oaks BM, Laugero KD, Stewart CP, Adu‐Afarwuah S, Lartey A, Ashorn P, et al. Late‐pregnancy salivary cortisol concentrations of Ghanaian women participating in a randomized controlled trial of prenatal lipid‐based nutrient supplements. Journal of Nutrition 2016;146(2):343‐52. [DOI] [PubMed] [Google Scholar]
- Oaks BM, Young RR, Okronipa H, Dewey KG, Adu‐Afarwuah S, Lartey A, et al. Effects of a lipid‐based nutrient supplement during pregnancy and lactation on maternal plasma fatty acid status and lipid profile: results of two randomized controlled trials. Prostaglandins Leukotrienes and Essential Fatty Acids 2017;117:28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocansey ME, Prado E, Young RR, Kumordzie S, Okronipa H, Oaks B, et al. Pre‐and post‐natal lipid‐based nutrient supplements and cognitive, socioemotional and motor function in preschool‐aged children in Ghana. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:304.
- Okronipa H, Adu‐Afarwuah S, Lartey A, Ashorn P, Vosti SA, Young RR, et al. Maternal supplementation with small‐quantity lipid‐based nutrient supplements during pregnancy and lactation does not reduce depressive symptoms at 6 months postpartum in Ghanaian women: a randomized controlled trial. Archives of Women's Mental Health 2018; Vol. 21, issue 1:55‐63. [DOI] [PMC free article] [PubMed]
- Okronipa H, Arimond M, Arnold CD, Young RR, Ocansey ME, Kumordzie S, et al. Impact of exposure to lipid‐based nutrient supplements in early life on sweet taste preference of Ghanaian children aged 4‐6 years: a non‐inferiority study. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:547.
- Okronipa HET, Adu‐Afarwuah S, Lartey A, Ashorn P, Vosti SA, Young RT, et al. The impact of lipid‐based nutrient supplements on maternal depression at 6 mo postpartum in Ghana: a randomized‐controlled trial. FASEB Journal 2016;30(Suppl 1):1172.11. [Google Scholar]
- Prado E, Abbeddou S, Adu‐Afarwuah S, Arimond M, Ashorn P, Ashorn U, et al. Associations between linear growth and language development in Ghana, Malawi, and Burkina Faso. FASEB Journal 2015; Vol. 29, issue 1 Suppl.
- Prado EL, Adu‐Afarwuah S, Lartey A, Ocansey M, Ashorn P, Vosti SA, et al. Effects of pre and post‐natal lipid‐based nutrient supplements on infant development in a randomized trial in Ghana. Early Human Development 2016;99:43‐51. [DOI] [PubMed] [Google Scholar]
Fawzi 2007 {published data only}
- Changamire FT, Mwiru RS, Peterson KE, Msamanga GI, Spiegelman D, Petraro P, et al. Effect of multivitamin supplements on weight gain during pregnancy among HIV‐negative women in Tanzania. Maternal & Child Nutrition 2015;11(3):297‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, et al. Vitamins and perinatal outcomes among HIV‐negative women in Tanzania. New England Journal of Medicine 2007;356(14):1423‐31. [DOI] [PubMed] [Google Scholar]
Friis 2004 {published data only}
- Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Kaestel P, et al. Effect of micronutrient supplementation on gestational length and birth size: a randomized, placebo‐controlled, double‐blind effectiveness trial in Zimbabwe. American Journal of Clinical Nutrition 2004;80:178‐84. [DOI] [PubMed] [Google Scholar]
Kaestel 2005 {published data only}
- Andersen GS, Friis H, Michaelsen KF, Rodrigues A, Benn CS, Aaby P, et al. Effects of maternal micronutrient supplementation on fetal loss and under‐2‐years child mortality: long‐term follow‐up of a randomised controlled trial from Guinea‐Bissau. African Journal of Reproductive Health 2010;14(2):17‐26. [PubMed] [Google Scholar]
- Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea‐Bissau. European Journal of Clinical Nutrition 2005;59(9):1081‐9. [DOI] [PubMed] [Google Scholar]
Lui 2013 {published data only}
- Chen S, Li N, Mei Z, Ye R, Li Z, Liu J, et al. Micronutrient supplementation during pregnancy and the risk of pregnancy‐induced hypertension: a randomized clinical trial. Clinical Nutrition (Edinburgh, Scotland) 2018 [epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Li HT, Mei Z, Ren A, Serdula M, Cogswell M, Liu JM. Impact of iron‐containing micronutrient supplementation on high hemoglobin concentration during pregnancy. FASEB Journal 2012;26:Abstract no. 1021.2. [Google Scholar]
- Li Z, Mei Z, Zhang L, Li H, Zhang Y, Li N, et al. Effects of prenatal micronutrients supplementation on spontaneous preterm birth: double‐blind randomized controlled trial in China. American Journal of Epidemiology 2017;186(3):318‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JM, Mei Z, Ye R, Serdula M, Li HT, Cogswell M. Impact of iron‐contained micronutrient supplementation on macrosomia and large for gestational age births. FASEB Journal 2012;26:1021.2. [Google Scholar]
- Liu JM, Mei Z, Ye R, Serdula MK, Ren A, Cogswell ME. Micronutrient supplementation and pregnancy outcomes: Double‐blind randomized controlled trial in China. JAMA Internal Medicine 2013;173(4):276‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Serdula MK, Liu JM, Flores‐Ayala RC, Wang L, Ye R, et al. Iron‐containing micronutrient supplementation of Chinese women with no or mild anemia during pregnancy improved iron status but did not affect perinatal anemia. Journal of Nutrition 2014;144(6):943‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00133744. Impact of prenatal vitamin/mineral supplements on perinatal mortality (planned trial). clinicaltrials.gov/ct2/show/NCT00133744 (first received 24 August 2005).
- Wang L, Mei Z, Li H, Zhang Y, Liu J, Serdula MK. Modifying effects of maternal Hb concentration on infant birth weight in women receiving prenatal iron‐containing supplements: a randomised controlled trial. British Journal of Nutrition 2016;115(4):644‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jin L, Liu JM, Ye R, Ren A. Maternal hemoglobin concentration during gestation and risk of anemia in infancy: secondary analysis of a randomized controlled trial. Journal of Pediatrics 2016;175:106‐10.e2. [NCT00133744] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Z, Li H, Jin L, Zhang Y, Zhang L, et al. Maternal haemoglobin concentration and risk of preterm birth in a Chinese population. Journal of Obstetrics and Gynaecology 2018;38(1):32‐7. [DOI] [PubMed] [Google Scholar]
Moore 2009 {published data only}
- Eriksen K, Allen LH, Shahab‐Ferdows S, Hampel D, Moore SE. Maternal, breast milk and infant B12 status in rural Gambia. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:541.
- ISRCTN49285450. Investigating the effects of pre‐natal and infancy nutritional supplementation on infant immune development in The Gambia: the Early Nutrition and Immune Development (ENID) trial. isrctn.com/ISRCTN49285450?q=&filters=recruitmentCountry:Gambia&sort=&offset=14&totalResults=34&page=1&pageSize=20&searchType=basic‐search (first received 24 August 2009).
- Jobarteh ML, McArdle HJ, Holtrop G, Sise EA, Prentice AM, Moore SE. mRNA levels of placental iron and zinc transporter genes are upregulated in Gambian women with low iron and zinc status. Journal of Nutrition 2017;147(7):1401‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobarteh ML, Moore S, Kennedy C, Gambling L, McArdle HJ. The effect of nutritional supplementation during pregnancy on placental transporter expression in placentas from Gambian women. Placenta 2013;34(9):A81‐2. [Google Scholar]
- Johnson W, Darboe MK, Sosseh F, Nshe P, Prentice AM, Moore SE. Association of prenatal lipid‐based nutritional supplementation with fetal growth in rural Gambia. Maternal & Child Nutrition 2017;13(2):e12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SE, Fulford AJC, Darboe MK, Jobarteh ML, Jarjou LM, Prentice AM. A randomized trial to investigate the effects of pre‐natal and infant nutritional supplementation on infant immune development in rural Gambia: The ENID trial: Early Nutrition and Immune Development. BMC Pregnancy and Childbirth 2012;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osrin 2005 {published data only}
- Devakumar D, Chaube SS, Wells JC, Saville NM, Ayres JG, Manandhar DS, et al. Effect of antenatal multiple micronutrient supplementation on anthropometry and blood pressure in mid‐childhood in Nepal: follow‐up of a double‐blind randomised controlled trial. Lancet Global Health 2014;2(11):e654‐e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devakumar D, Stocks J, Ayres JG, Kirkby J, Yadav SK, Saville NM, et al. Effects of antenatal multiple micronutrient supplementation on lung function in mid‐childhood: follow‐up of a double‐blind randomised controlled trial in Nepal. European Respiratory Journal 2015;45(6):1566‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devakumar D, Wells JC, Chaube SS, Saville NM, Manandhar DS, Costello A, et al. The phenotypic effects of antenatal multiple micronutrient supplementation in Nepalese children. Archives of Disease in Childhood 2014;99(Suppl 1):A2, Abstract no: P04. [Google Scholar]
- Hindle LJ, Gitau R, Filteau SM, Newens KJ, Osrin D, Costello AM, et al. Effect of multiple micronutrient supplementation during pregnancy on inflammatory markers in Nepalese women. American Journal of Clinical Nutrition 2006;84:1086‐92. [DOI] [PubMed] [Google Scholar]
- MIRA (Mother Infant Research Unit). MIRA Janakpur. Multiple micronutrient supplementation study. The effects of multiple micronutrient supplementation on birthweight, gestation and infection: a double blind, randomised controlled trial conducted in Nepal. Personal communication 2003:1‐18.
- Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, et al. Effects of antenatal micronutrient supplementation on birthweight and gestational duration in Nepal: double‐blind, randomised controlled trial. Lancet 2005;365:955‐62. [DOI] [PubMed] [Google Scholar]
- Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow‐up of a double‐blind randomised controlled trial. Lancet 2008;371(9611):492‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramakrishnan 2003 {published data only}
- Garcia‐Guerra A, Neufeld LM, Hernandez‐Cordero S, Rivera J, Martorell R, Ramakrishnan U. Prenatal multiple micronutrient supplementation impact on biochemical indicators during pregnancy and postpartum. Salud Publica de Mexico 2009;51(4):327‐35. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Cossio TG, Neufeld LM, Rivera J, Martorell R. Effect of prenatal multiple micronutrient supplements on maternal weight and skinfold changes: a randomized double‐blind clinical trial in Mexico. Food and Nutrition Bulletin 2005;26(3):273‐80. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Gonzalez‐Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron‐only supplementation: a randomized controlled trial in a semirural community in Mexico. American Journal of Clinical Nutrition 2003;77:720‐5. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Neufeld LM, Gonzalez‐Cossio T, Villalpando S, Garcia‐Guerrra A, Juan R, et al. Multiple micronutrient supplements during pregnancy do not reduce anemia or improve iron status compared to iron‐only supplements in semirural Mexico. Journal of Nutrition 2004;134:898‐903. [DOI] [PubMed] [Google Scholar]
- Lourdes Flores M, Neufeld LM, Gonzalez‐Cossio T, Rivera J, Martorell R, Ramakrishnan U. Multiple micronutrient supplementation and dietary energy intake in pregnant women. Salud Publica de Mexico 2007;49(3):190‐8. [DOI] [PubMed] [Google Scholar]
Roberfroid 2008 {published data only}
- Huybregts L, Roberfroid D, Lanou H, Kolsteren P, Camp J. Prenatal lipid‐based nutrient supplements increase cord leptin concentration in pregnant women from rural Burkina Faso. Annals of Nutrition & Metabolism 2013;63(Suppl 1):799, Abstract no: PO1134. [DOI] [PubMed] [Google Scholar]
- Lanou H, Huybregts L, Roberfroid D, Kolsteren P. Effect of prenatal lipid‐based nutrient supplementation on gestational weight gain. Annals of Nutrition & Metabolism 2013;63(Suppl 1):783, Abstract no: PO1099. [Google Scholar]
- Lanou HB, Kouanda S, Bouckaert K, Kolsteren P, Roberfroid D, Huybregts L. Effect of multiple micronutrient supplementation in lactating women on infant growth and morbidity: a double‐ blind randomized controlled trial in rural Burkina Faso. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:308‐9.
- Roberfroid D, Huybregts L, Habicht JP, Lanou H, Henry MC, Meda N, et al. Randomized controlled trial of 2 prenatal iron supplements: is there a dose‐response relation with maternal hemoglobin?. American Journal of Clinical Nutrition 2011;93:1012‐8. [DOI] [PubMed] [Google Scholar]
- Roberfroid D, Huybregts L, Lanou H, Habicht JP, Henry MC, Meda N, et al. Prenatal micronutrient supplements cumulatively increase fetal growth. Journal of Nutrition 2012;142(3):548‐54. [DOI] [PubMed] [Google Scholar]
- Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Kolsteren FP, et al. Effect of maternal multiple micronutrient supplements on cord blood hormones: a randomized controlled trial. American Journal of Clinical Nutrition 2010;91(6):1649‐58. [DOI] [PubMed] [Google Scholar]
- Roberfroid D, Huybregts L, Lanou H, Henry MC, Meda N, Menten J, et al. Effects of maternal multiple micronutrient supplementation on fetal growth: a double‐blind randomized controlled trial in rural Burkina Faso. American Journal of Clinical Nutrition 2008;88:1330‐40. [DOI] [PubMed] [Google Scholar]
- Roberfroid D, Huybregts L, Lanou H, Ouedraogo L, Henry MC, Meda N, et al. Impact of prenatal multiple micronutrients on survival and growth during infancy: a randomized controlled trial. American Journal of Clinical Nutrition 2012;95(4):916‐24. [DOI] [PubMed] [Google Scholar]
Sood 1975 {published data only}
- Sood SK, Ramachandran K, Mathur M, Gupta K, Ramalingaswami V, Swarnbai C, et al. WHO sponsored collaborative study on nutritional anemia in India. Quarterly Journal of Medicine, New Series XLIV 1975;174:241‐58. [PubMed] [Google Scholar]
SUMMIT 2008 {published data only}
- Iskandar W, Smith E, Aditiawarman A, Apriatni M, Tjiong R, Shankar A. Effect of maternal multiple micronutrients supplementation on the APGAR score of Indonesian neonates. FASEB Journal 2015;29(1 Suppl):741.13. [Google Scholar]
- Prado E, Sebayang S, Apriatni M, Hidayati N, Adawiyah S, Islamiyah A, et al. Maternal multiple micronutrient supplementation and children's cognition at age 9‐12 years in Indonesia. FASEB Journal 2015;29(1 Suppl):28.7. [DOI] [PubMed] [Google Scholar]
- Prado EL, Alcock KJ, Muadz H, Ullman MT, Shankar AH, for the SUMMIT Study Group. Maternal multiple micronutrient supplements and child cognition: a randomized trial in Indonesia. Pediatrics 2012;130(3):e536‐46. [DOI] [PubMed] [Google Scholar]
- Prado EL, Sebayang SK, Apriatni M, Adawiyah SR, Hidayati N, Islamiyah A, et al. Maternal multiple micronutrient supplementation and other biomedical and socioenvironmental influences on children's cognition at age 9‐12 years in Indonesia: follow‐up of the SUMMIT randomised trial. Lancet Global Health 2017;5(2):e217‐28. [DOI] [PubMed] [Google Scholar]
- Prado EL, Ullman MT, Muadz H, Alcock KJ, Shankar AH. The effect of maternal multiple micronutrient supplementation on cognition and mood during pregnancy and postpartum in Indonesia: a randomized trial. PLoS ONE 2012;7(3):e32519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebayang SK, Dibley MJ, Kelly P, Shankar AV, Shankar AH. Modifying effect of maternal nutritional status on the impact of maternal multiple micronutrient supplementation on birthweight in Indonesia. European Journal of Clinical Nutrition 2011;65(10):1110‐7. [DOI] [PubMed] [Google Scholar]
- Shankar AV, Asrilla Z, Kadha JK, Sebayang S, Apriatni M, Sulastri A, et al. Programmatic effects of a large‐scale multiple‐micronutrient supplementation trial in Indonesia: using community facilitators as intermediaries for behavior change. Food and Nutrition Bulletin 2009;30(2 Suppl):S207‐S214. [DOI] [PubMed] [Google Scholar]
- Siddiq S, Apriatni M, Prado E, Santyowibowo S, Lestari Y, Sitorus R, et al. The long term impact of maternal multiple micronutrient supplementation, infant nutrition and health on visual acuity of 9‐12 year old children in Indonesia. FASEB Journal 2015;29(1 Suppl):729.21. [Google Scholar]
- The Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group, Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double‐blind cluster‐randomised trial. Lancet 2008;371(9608):215‐27. [DOI] [PubMed] [Google Scholar]
Sunawang 2009 {published data only}
- Sunawang, Utomo B, Hidayat A, Kusharisupeni, Subarkah. Preventing low birth weight through maternal multiple micronutrient supplementation: a cluster‐randomized controlled trial in Indramayu, West Java. Food and Nutrition Bulletin 2009;30(4):S488‐S495. [DOI] [PubMed] [Google Scholar]
Tofail 2008 {published and unpublished data}
- Ekstrom EC, Eneroth H, Arifeen SE, Persson L. Efficacy of micronutrient supplement intake in increasing hemoglobin in pregnancy: dose‐effect comparisons with multiple micronutrient in the MINIMat trial in rural Bangladesh. FASEB Journal 2013;27 Suppl:Abstract no: 845.25. [Google Scholar]
- Ekstrom EC, Lindstrom E, Raqib R, Arifeen S, Basu S, Brismar K, et al. Effects of prenatal micronutrient and early food supplementation on metabolic status of the offspring at 4.5 years of age. The MINIMat randomized trial in rural Bangladesh. International Journal of Epidemiology 2016;45(5):1656‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eneroth H, Arifeen S, Persson LA, Lonnerdal B, Hossain MB, Stephensen CB, et al. Maternal multiple micronutrient supplementation has limited impact on micronutrient status of Bangladeshi infants compared with standard iron and folic acid supplementation. Journal of Nutrition 2010;140(3):618‐24. [DOI] [PubMed] [Google Scholar]
- Eneroth H, Persson LA, Arifeen S, Ekstrom EC. Infant anaemia is associated with infection, low birthweight and iron deficiency in rural Bangladesh. Acta Paediatrica 2011;100(2):220‐5. [DOI] [PubMed] [Google Scholar]
- Frith AL, Naved RT, Persson LA, Frongillo EA. Early prenatal food supplementation ameliorates the negative association of maternal stress with birth size in a randomised trial. Maternal & Child Nutrition 2015;11(4):537‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith AL, Naved RT, Persson LA, Rasmussen KM, Frongillo EA. Early participation in a prenatal food supplementation program ameliorates the negative association of food insecurity with quality of maternal‐infant interaction. Journal of Nutrition 2012;142(6):1095‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesworth S, Wagatsuma Y, Kahn AI, Hawlader MD, Fulford AJ, Arifeen SE, et al. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. Journal of Nutrition 2013;143(5):728‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioinen M, Ekstrom EC, Khan AI, Lindstrom E, Persson LA, Rahman A, et al. Prenatal early food and multiple micronutrient supplementation trial reduced infant mortality in Bangladesh but did not influence morbidity. Acta Paediatrica 2017;106(12):1979‐86. [DOI] [PubMed] [Google Scholar]
- Khan A, Kabir I, Ekstrom EC, Asling‐Monemi K, Alam D, Frongillo EA, et al. Effects of prenatal food and micronutrient supplementation on child growth from birth to 54 months of age: a randomized trial in Bangladesh. Nutrition Journal 2011;10(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AI. Effects of pre‐ and postnatal nutrition interventions on child growth and body composition: the MINIMat trial in rural Bangladesh. Global Health Action 2013;6:22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AI, Hawkesworth S, Ekstrom EC, Arifeen S, Moore SE, Frongillo EA, et al. Effects of exclusive breastfeeding intervention on child growth and body composition: The MINIMat trial, Bangladesh. Acta Paediatrica 2013;102(8):815‐23. [DOI] [PubMed] [Google Scholar]
- Khan AI, Kabir I, Eneroth H, Arifeen S, Ekstrom EC, Frongilo EA, et al. Effect of a randomised exclusive breastfeeding counselling intervention nested into the MINIMat prenatal nutrition trial in Bangladesh. Acta Paediatrica 2017;106(1):49‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AI, Kabir I, Hawkesworth S, Ekstrom EC, Arifeen S, Frongillo EA, et al. Early invitation to food and/or multiple micronutrient supplementation in pregnancy does not affect body composition in offspring at 54 months: follow‐up of the MINIMat randomised trial, Bangladesh. Maternal & Child Nutrition 2015;11(3):385‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SE, Fulford AJ, Wagatsuma Y, Persson LA, Arifeen SE, Prentice AM. Thymus development and infant and child mortality in rural Bangladesh. International Journal of Epidemiology 2014;43:216‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson LA, Arifeen S, Ashfaful K, Rahman A, Ekstrom EC. Effects of early prenatal food supplementation and multiple micronutrients on under‐five survival, linear growth, metabolic markers and blood pressure up to 10 years of age. The MINIMat trial in rural Bangladesh. FASEB Journal 2017;31(1 Suppl):Abstract Number: 786.35. [Google Scholar]
- Persson LA, Arifeen S, Ekstrom EC, Rasmussen KM, Frongillo EA, Yunus M. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: The MINIMat randomized trial. JAMA 2012;307(19):2050‐9. [DOI] [PubMed] [Google Scholar]
- Persson LA, Eneroth H, Ekstrom EC. Multiple micronutrient supplementation during pregnancy: a review of effects on birth size, maternal haemoglobin and perinatal mortality demonstrated in trials in Bangladesh, Guinea‐Bissau and Pakistan. Report for UNICEF/UNU/WHO 2004.
- Shaheen R, Persson LA, Ahmed S, Lindholm L. Early invitation to prenatal food combined with multiple micronutrients is cost‐effective compared to iron‐folic acid supplementations: results from MINIMat trial. Tropical Medicine and International Health 2013;18(Suppl 1):88. [Google Scholar]
- Shaheen R, Persson LA, Ahmed S, Streatfield PK, Lindholm L. Cost‐effectiveness of invitation to food supplementation early in pregnancy combined with multiple micronutrients on infant survival: analysis of data from MINIMat randomized trial, Bangladesh. BMC Pregnancy and Childbirth 2015;15(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Streatfield PK, Naved RT, Lindholm L, Persson LA. Equity in adherence to and effect of prenatal food and micronutrient supplementation on child mortality: results from the MINIMat randomized trial, Bangladesh. BMC Public Health 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svefors P, Rahman A, Ekstrom EC, Khan AI, Lindstrom E, Persson LA, et al. Stunted at 10 years. Linear growth trajectories and stunting from birth to pre‐adolescence in a rural Bangladeshi cohort. PLoS ONE 2016;11(3):e0149700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svefors P, Selling KE, Shaheen R, Khan AI, Persson LA, Lindholm L. Cost‐effectiveness of prenatal food and micronutrient interventions on under‐five mortality and stunting: analysis of data from the minimat randomized trial, Bangladesh. Plos One 2018;13(2):e0191260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svefors P, Selling KE, Shaheen R, Khan AI, Persson LA, Lindholm L. Prenatal food and micronutrient interventions in rural Bangladesh remain cost‐effective when assessing both favorable and unfavorable outcomes: cost‐effectiveness analysis of the MINIMat trial on under five‐mortality and stunting. FASEB Journal 2017;31(Suppl 1):Abstract Number: 786.33. [Google Scholar]
- Tofail F, Persson LA, Arifeen SE, Hamadani JD, Mehrin F, Ridout D, et al. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from maternal and infant nutrition intervention, Matlab (MINIMat) study. American Journal of Clinical Nutrition 2008;87:704‐11. [DOI] [PubMed] [Google Scholar]
- Ziaei S, Ekstrom EC, Rahman A, Raqib R, Lonnerdal B. A prenatal multiple micronutrient supplement produces higher maternal vitamin B‐12 concentrations and similar folate, ferritin, and zinc concentrations as the standard 60‐mg iron plus 400‐mg folic acid supplement in rural Bangladeshi women. Journal of Nutrition 2016;146(12):2520‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2014 {published data only}
- Christian P, Kim J, Mehra S, Shaikh S, Ali H, Shamim AA, et al. Effects of prenatal multiple micronutrient supplementation on growth and cognition through 2 years of age in rural Bangladesh: the JiVitA‐3 trial. American Journal of Clinical Nutrition 2016;104(4):1175‐82. [DOI] [PubMed] [Google Scholar]
- Christian P, Shamim A, Shaikh S, Ali H, Mehra S, Lee W, et al. Antenatal multiple micronutrient supplementation and growth in the first two years of life and cognitive function at 24 months in rural Bangladesh. FASEB Journal 2014;28(1 Suppl 1):Abstract no. 256.5. [Google Scholar]
- Gernand A, Schulze K, Shaikh S, Shamim A, Ali H, Wu L, et al. The effect of prenatal multiple micronutrient supplementation on biomarkers of placental angiogenesis in rural Bangladesh. FASEB Journal 2014;28(1 Suppl 1):Abstract no: 804.2. [Google Scholar]
- Gernand AD, Christian P, Paul RR, Shaikh S, Labrique AB, Schulze KJ, et al. Maternal weight and body composition during pregnancy are associated with placental and birth weight in rural Bangladesh. Journal of Nutrition 2012;142(11):2010‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Christian P, Schulze KJ, Shaikh S, Labrique AB, Shamim AA, et al. Maternal nutritional status in early pregnancy is associated with body water and plasma volume changes in a pregnancy cohort in rural Bangladesh. Journal of Nutrition 2012;142(6):1109‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Paul RR, Shaikh S, Shamim AA, Schulze K, Labrique AB, et al. Maternal gestational weight and body composition are associated with placental weight in rural Bangladesh. FASEB Journal 2011;25:Abstract no: 780.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Schulze KJ, Nanayakkara‐Bind A, Arguello M, Shamim AA, Ali H, et al. Effects of prenatal multiple micronutrient supplementation on fetal growth factors: a cluster‐randomized, controlled trial in rural Bangladesh. Plos One 2015;10(10):e0137269. [NCT00860470] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra S, Schulze KJ, Merrill RD, Shaikh S, Ali H, Shamim AA, et al. Exploration of non‐nutritional factors associated with stunting in 24 month old children of rural Bangladesh. FASEB Journal 2013;27 Suppl:Abstract no: 618.8. [Google Scholar]
- NCT00860470. Antenatal micronutrient supplementation and infant survival (JiVitA‐3). clinicaltrials.gov/ct2/show/NCT00860470 (first received 12 March 2009).
- Shamim AA, Christian P, Labrique AB, Ali H, Shaikh S, Mehra S, et al. Antenatal multiple micronutrients compared to iron‐folic acid lengthens gestation, increases birth size and reduces risk of LDW in rural Bangladesh. Annals of Nutrition & Metabolism 2013;63(Suppl 1):226, Abstract no: O138. [Google Scholar]
- West KP Jr, Shamim AA, Mehra S, Labrique AB, Ali H, Shaikh S, et al. Effect of maternal multiple micronutrient vs iron–folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA‐3 randomized trial. JAMA 2014;312(24):2649‐58. [DOI] [PubMed] [Google Scholar]
- West KP, Shamim AA, Labrique AB, Ali H, Shaikh S, Mehra S, et al. Efficacy of antenatal multiple micronutrient (MM) vs iron‐folic acid (IFA) supplementation in improving gestational and postnatal viability in rural Bangladesh: the JiVitA‐3 trial. FASEB Journal 2013;27 Suppl:Abstract no: 358.6. [Google Scholar]
Zagre 2007 {published data only}
- Zagre NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster‐randomized, double‐blind, controlled programmatic study in rural Niger. Food and Nutrition Bulletin 2007;28(3):317‐27. [DOI] [PubMed] [Google Scholar]
Zeng 2008 {published data only}
- Cao Y, Wang T, Zeng L. Risk of childhood malnutrition at 24 months related to small for gestational age and low birth weight in rural Western China. Annals of Nutrition & Metabolism 2013;63(Suppl 1):556, Abstract no: PO619. [Google Scholar]
- Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics 2013;131(3):e755‐63. [DOI] [PubMed] [Google Scholar]
- ISRCTN08850194. Impact of iron/folate versus multi‐micronutrient supplementation during pregnancy on birth weight: a randomised controlled trial in rural Western China. isrctn.com/ISRCTN08850194 (first received 7 November 2006).
- Li C, Zeng L, Wang D, Yang W, Dang S, Zhou J, et al. Prenatal micronutrient supplementation is not associated with intellectual development of young school‐aged children. Journal of Nutrition 2015;145(8):1844‐9. [DOI] [PubMed] [Google Scholar]
- Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, et al. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow‐up evaluation of a double‐blind, randomized, controlled trial. Pediatrics 2009;123(4):e685‐e692. [DOI] [PubMed] [Google Scholar]
- Wang W, Yan H, Zeng L, Cheng Y, Wang D, Li Q. No effect of maternal micronutrient supplementation on early childhood growth in rural western China: 30 month follow‐up evaluation of a double blind, cluster randomized controlled trial. European Journal of Clinical Nutrition 2012;66(2):261‐8. [DOI] [PubMed] [Google Scholar]
- Zeng L, Cheng Y, Dang S, Yan H, Dibley MJ, Chang S, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Yan H, Cheng Y, Dang S, Dibley MJ. Adherence and costs of micronutrient supplementation in pregnancy in a double‐blind, randomized, controlled trial in rural western China. Food and Nutrition Bulletin 2009;30(4):S480‐S487. [DOI] [PubMed] [Google Scholar]
- Zeng L, Yan H, Cheng Y, Dibley M. Modifying effects of maternal nutrition status on the response to multiple micronutrients supplementation on preterm and neonatal mortality in China. Annals of Nutrition & Metabolism 2013;63(Suppl 1):859‐60, Abstract no: PO1241. [Google Scholar]
- Zeng L, Yan H, Cheng Y, Dibley MJ. Modifying effects of wealth on the response to nutrient supplementation in pregnancy on birth weight, duration of gestation and perinatal mortality in rural western China: double‐blind cluster randomized controlled trial. International Journal of Epidemiology 2011;40(2):350‐62. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zeng L, Dang S, Pei L, Gao W, Li C, et al. Maternal prenatal nutrition and birth outcomes on malnutrition among 7‐ to 10‐year‐old children: A 10‐year follow‐up. Journal of Pediatrics 2016;178:40‐6. [ISRCTN08850194] [DOI] [PubMed] [Google Scholar]
- Zhu Z, Elhoumed M, He G, Li W, Zhang M, Li D, et al. Effect of maternal micronutrient supplementation in pregnancy on the intellectual development of adolescents: longterm follow‐up evaluation based on a randomized controlled trial in rural China. Annals of Nutrition and Metabolism 2017; Vol. 71:281.
References to studies excluded from this review
ACTRN12616001449426 {published data only}
- ACTRN12616001449426. Effect of multi aspect interventions in reduction of low birth weight incidence and maternal anemia during pregnancy. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001449426 (first received 17 October 2010).
Agarwal 2012 {published data only}
- Agarwal N, Dora S, Kriplani A, Garg P, Vivekanandhan S, Kulshrestha V. Response of therapy with vitamin B6, B12 and folic acid on homocysteine level and pregnancy outcome in hyperhomocysteinemia with unexplained recurrent abortions. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S759. [Google Scholar]
Aguayo 2005 {published data only}
- Aguayo VM, Kone D, Bamba SI, Diallo B, Sidibe Y, Traore D, et al. Acceptability of multiple micronutrient supplements by pregnant and lactating women in Mali. Public Health Nutrition 2005;8(1):33‐7. [DOI] [PubMed] [Google Scholar]
Ahn 2006 {published data only}
- Ahn E, Pairaudeau N, Pairaudeau N, Cerat Y, Couturier B, Fortier A, et al. A randomized cross over trial of tolerability and compliance of a micronutrient supplement with low iron separated from calcium vs high iron combined with calcium in pregnant women. BMC Pregnancy and Childbirth 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
An 2001 {published data only}
- An H, Yin S, Xu Q. Effects of supplementing calcium, iron and zinc on the fetus development and growth during pregnancy. Chinese Journal of Preventive Medicine 2001;35(6):370‐3. [PubMed] [Google Scholar]
Arsenault 2010 {published data only}
- Arsenault JE, Aboud S, Manji KP, Fawzi WW, Villamor E. Vitamin supplementation increases risk of subclinical mastitis in HIV‐infected women. Journal of Nutrition 2010;140(10):1788‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asemi 2014 {published data only}
- Asemi Z, Samimi M, Tabassi Z, Ahmad E. Multivitamin versus multivitamin‐mineral supplementation and pregnancy outcomes: a single‐blind randomized clinical trial. International Journal of Preventive Medicine 2014;5(4):439‐46. [PMC free article] [PubMed] [Google Scholar]
- IRCT201212105623N3. Comparison of effectiveness of multivitamin, multivitamin‐mineral supplements, probiotic and normal Gaz on pregnancy outcomes in pregnant women. en.search.irct.ir/view/11730 (first received 26 December 2012). [IRCT201212105623N3]
Asemi 2015 {published data only}
- Asemi Z, Esmaillzadeh A. The effect of multi mineral‐vitamin D supplementation on pregnancy outcomes in pregnant women at risk for pre‐eclampsia. International Journal of Preventive Medicine 2015;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azami 2016 {published data only}
- Azami M, Azadi T, Farhang S, Rahmati S, Pourtaghi K. The effects of multi mineral‐vitamin D and vitamins (C+E) supplementation in the prevention of preeclampsia: an RCT. International Journal of Reproductive Biomedicine (Yazd, Iran) 2017;15(5):273‐8. [PMC free article] [PubMed] [Google Scholar]
- IRCT2016062528617N1. The effects of multi minerals (Zn, Mg and Ca) and vitamins (C and E) supplementation in the prevention of preeclampsia. en.search.irct.ir/view/31125 2016.
Beazley 2002 {published data only}
- Beazley D, Ahokas R, Livingston J, Griggs M, Scroggs M, Sibai B. Effects of vitamin C and E supplementation on total antioxidant status (TAS) and 8‐isoprostane (IP) levels in women at high risk for preeclampsia. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S76. [DOI] [PubMed] [Google Scholar]
- Beazley D, Livingston J, Kao L, Sibai L. Vitamin C and E supplementation in women at high risk for preeclampsia: a double‐blind placebo controlled trial. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S216. [DOI] [PubMed] [Google Scholar]
Bergmann 2006 {published data only}
- Bergmann RL, Haschke‐Becher, Bergmann KE, Dudenhausen JW, Haschke F. Low dose docosahexaenoic acid supplementation improves the DHA status of pregnant women. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, CA, USA 2006.
Biswas 1984 {published data only}
- Biswas MK, Pernoll MJ, Mabie WC. A placebo controlled comparative trial of various prenatal vitamin formulations in pregnant women. Clinical Therapeutics 1984;6(6):763‐9. [PubMed] [Google Scholar]
Callaghan‐Gillespie 2017 {published data only}
- Callaghan‐Gillespie M, Schaffner AA, Garcia P, Fry J, Eckert R, Malek S, et al. Trial of ready‐to‐use supplemental food and corn‐soy blend in pregnant Malawian women with moderate malnutrition: a randomized controlled clinical trial. American Journal of Clinical Nutrition 2017;106(4):1062‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrasco 1962 {published data only}
- Carrasco EO, Jose FR, Samson GD, Germar E, Padilla B. Effect of D‐sorbitol on the absorption and transfer of nutrients from mother to fetus. American Journal of Clinical Nutrition 1962;11:533‐6. [DOI] [PubMed] [Google Scholar]
Caulfield 1999 {published data only}
- Caulfield LE, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. American Journal of Clinical Nutrition 1999;69:1257‐63. [DOI] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Figueroa A, Leon Z. Maternal zinc supplementation does not affect size at birth or pregnancy duration in Peru. Journal of Nutrition 1999;129(8):1563‐8. [DOI] [PubMed] [Google Scholar]
Chames 2002 {published data only}
- Chames M, Liu H, Bendich A, Bogden J, Sibai B, Prada J. A randomized trial of calcium supplementation effects on blood lead levels in pregnancy. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S137. [Google Scholar]
Choudhury 2012 {published data only}
- Choudhury N, Aimone A, Hyder SM, Zlotkin SH. Relative efficacy of micronutrient powders versus iron‐folic acid tablets in controlling anemia in women in the second trimester of pregnancy. Food & Nutrition Bulletin 2012;33(2):142‐9. [DOI] [PubMed] [Google Scholar]
Christian 2009 {published data only}
- Christian P, Shahid F, Rizvi A, Klemm RDW, Bhutta ZA. Treatment response to standard of care for severe anemia in pregnant women and effect of multivitamins and enhanced anthelminthics. American Journal of Clinical Nutrition 2009;89:853‐61. [DOI] [PubMed] [Google Scholar]
- NCT00116493. Severe anemia treatment trials, Pakistan. clinicaltrials.gov/ct2/show/NCT00116493 (first received 30 June 2005).
Coles 2015 {published data only}
- Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, et al. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6‐month‐old infants. Maternal and Child Health Journal 2015;19(12):2605‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2012 {published data only}
- Cooper WN, Khulan B, Owens S, Elks CE, Seidel V, Prentice AM, et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB Journal 2012;26(5):1782‐90. [DOI] [PubMed] [Google Scholar]
Czeizel 1996 {published data only}
- Czeizel AE. Controlled studies of multivitamin supplementation on pregnancy outcomes. Annals of New York Academy of Sciences 1993;678:266‐75. [DOI] [PubMed] [Google Scholar]
- Czeizel AE. Prevention of congenital abnormalities by periconceptional multivitamin supplementation. BMJ 1993;306:1645‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. American Journal of Medical Genetics 1996;62:179‐83. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural tube defects by periconceptional vitamin supplementation. New England Journal of Medicine 1992;327:1832‐5. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I, Fritz G, Tecsoi A, Hanck A, Kunovits G. The effect of periconceptional multivitamin ‐mineral supplementation on vertigo, nausea and vomiting in the first trimester of pregnancy. Archives of Gynecology and Obstetrics 1992;251:181‐5. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I, Metneki J. Pregnancy outcomes in a randomized controlled trial of periconceptional multivitamin supplementation. Archives of Gynecology and Obstetrics 1994;255:131‐9. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Metneki J, Dudas I. The effect of preconceptional multivitamin supplementation on fertility. International Journal of Vitamin and Nutrition Research 1996;66:55‐8. [PubMed] [Google Scholar]
Dawson 1987 {published data only}
- Dawson EB, McGanity WJ. Protection of maternal iron stores in pregnancy. Journal of Reproductive Medicine 1987;32(6):478‐87. [PubMed] [Google Scholar]
Dawson 1998 {published data only}
- Dawson EB, Dawson R, Behrens J, DeVora M, McGanity WJ. Iron in prenatal multivitamin/multimineral supplements. Journal of Reproductive Medicine 1998;43:133‐40. [PubMed] [Google Scholar]
Devi 2017 {published data only}
- Devi S, Mukhopadhyay A, Dwarkanath P, Thomas T, Crasta J, Thomas A, et al. Combined vitamin B‐12 and balanced protein‐energy supplementation affect homocysteine remethylation in the methionine cycle in pregnant South Indian women of low vitamin B‐12 status. Journal of Nutrition 2017;147(6):1094‐103. [DOI] [PubMed] [Google Scholar]
Dewey 2012 {published data only}
- Dewey KG, Mridha MK, Matias SL, Arnold CD, Cummins JR, Khan MS, et al. Lipid‐based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):944‐57. [DOI] [PubMed] [Google Scholar]
- Harding KL, Matias SL, Moniruzzaman M, Stewart CP, Mridha MK, Vosti SA, et al. Rang‐Din Nutrition Study: assessment of participant adherence to lipid‐based nutrient and iron‐folic acid supplements among pregnant and lactating women in the context of a study on the effectiveness of supplements in Bangladesh. FHI 360/FANTA. Washington, DC: US Agency for International Development (USAID), 2014. [Google Scholar]
- Harding KL, Matias SL, Mridha MK, Vosti SA, Dewey KG, Stewart CP, et al. Adherence to recommendations on lipid‐based nutrient supplement and iron and folic acid tablet consumption among pregnant and lactating women participating in a community health programme in northwest Bangladesh. Maternal and Child Nutrition 2017;13(1):e12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias S, Harding K, Mridha M, Vosti S, Paul R, Hussain S, et al. T3_005. Breastfeeding and postpartum weight change among rural Bangladeshi women. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:55‐6.
- Matias S, Harding K, Mridha M, Vosti S, Paul R, Hussain S, et al. TS_024. Breastfeeding and postpartum weight change among rural Bangladeshi women. 17th Conference of the International Society for Research in Human Milk and Lactation (ISRHML); 2014 Oct 23‐27; Kiawah Island, South Carolina, USA. 2014:189.
- Matias SL, Mridha MK, Paul RR, Hussain S, Vosti SA, Arnold CD, et al. Prenatal lipid‐based nutrient supplements affect maternal anthropometric indicators only in certain subgroups of rural Bangladeshi women. Journal of Nutrition 2016;146(9):1775‐82. [DOI] [PubMed] [Google Scholar]
- Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MS, Siddiqui Z, et al. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):958‐69. [NCT01715038] [DOI] [PubMed] [Google Scholar]
- Mridha M, Chaparro C, Paul R, Hussain S, Vosti S, Matias S, et al. Lipid‐based nutrient supplements for pregnant women reduce newborn stunting in Bangladesh. FASEB Journal 2014;28(1 Suppl):Abstract no: 256.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, et al. Lipid‐based nutrient supplements for pregnant women reduce newborn stunting in a cluster‐randomized controlled effectiveness trial in Bangladesh. American Journal of Clinical Nutrition 2016;103(1):236‐50. [NCT01715038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mridha MK, Matias SL, Paul RR, Hussain S, Khan MSA, Siddiqui Z, et al. Daily consumption of lipid‐based nutrient supplements containing 250 mug iodine does not increase urinary iodine concentrations in pregnant and postpartum women in Bangladesh. Journal of Nutrition 2017 [epub ahead of print]. [DOI] [PubMed]
- Mridha MK, Matias SL, Paul RR, Hussain S, Sarker M, Hossain M, et al. Prenatal lipid‐based nutrient supplements do not affect pregnancy or childbirth complications or cesarean delivery in Bangladesh: a cluster‐randomized controlled effectiveness trial. Journal of Nutrition 2017;147(9):1776‐84. [DOI] [PubMed] [Google Scholar]
- NCT01715038. Effectiveness of LNS and MNP supplements to prevent malnutrition in women and their children in Bangladesh (RDNS). clinicaltrials.gov/ct2/show/NCT01715038 (first received 26 October 2012).
- Ullah B, Mridha M, Matias S, Arnold CD, Khan S, Siddiki Z, et al. Effect of pre‐and postnatal nutritional supplements on childhood illnesses in Bangladesh: a cluster‐randomized effectiveness trial. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:542‐3.
Dieckmann 1944 {published data only}
- Dieckmann WJ, Adair FL, Michel H, Kramer S, Dunkle F, Arthur B, et al. Calcium, phosphorus, iron and nitrogen balances in pregnant women. American Journal of Obstetrics and Gynecology 1944;47:357‐68. [Google Scholar]
Fall 2006 {published data only}
- ISRCTN62811278. Mumbai maternal nutrition project. isrctn.com/ISRCTN62811278 (first received 11 January 2006).
- Lawande A, Gravio C, Potdar RD, Sahariah SA, Gandhi M, Chopra H, et al. Effect of a micronutrient‐rich snack taken preconceptionally and throughout pregnancy on ultrasound measures of fetal growth: the Mumbai Maternal Nutrition Project (MMNP). Maternal & Child Nutrition 2018; Vol. 14, issue 1. [DOI] [PMC free article] [PubMed]
- Potdar RD, Sahariah SA, Gandhi M, Kehoe SH, Brown N, Sane H, et al. Improving women's diet quality preconceptionally and during gestation: effects on birth weight and prevalence of low birth weight‐a randomized controlled efficacy trial in India (Mumbai Maternal Nutrition Project). American Journal of Clinical Nutrition 2014;100(5):1257‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potdar RD, Sahariah SA, Gandhi M, Sane H, Brown NB, Fall C, et al. Preconceptual nutrition and weight gain in a) women during pregnancy and b) in babies at birth in Mumbai Maternal Nutrition Project (a RCT). The Power of Programming 2014: International Conference on Developmental Origins of Adiposity and Long‐Term Health; 2014 March 13‐15; Munich, Germany. 2014:61.
- Sahariah SA, Zagar EM, Potdar R, Gandhi M, Chopra H, Sane H, et al. Interaction of pre‐conceptional food supplementation with maternal BMI: reduces gestational diabetes in undernourished women and increases birthweight in well‐nourished women. The Power of Programming 2014: International Conference on Developmental Origins of Adiposity and Long‐Term Health; 2014 March 13‐15; Munich, Germany. 2014:59.
Fawzi 1998 {published data only}
- Fawzi W, Msamanga G, Antelman G, Xu C, Hertzmark E, Spiegelman D, et al. Effect of prenatal vitamin supplementation on lower genital levels of HIV type 1 and interleukin type 1 beta at 36 weeks of gestation. Clinical Infectious Diseases 2004;38(5):716‐22. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga G, Hunter D, Urassa E, Renjifo B, Mwakagile D, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV‐1 in Tanzania. Journal of Acquired Immune Deficiency Syndromes 2000;23(3):246‐54. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga G, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomised trial of multivitamin supplements and HIV disease progression and mortality. New England Journal of Medicine 2004;351(1):23‐32. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, et al. Multivitamin supplementation improves hematologic status in HIV‐infected women and their children in Tanzania. American Journal of Clinical Nutrition 2007;85(5):1335‐43. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGarth N, Mwakagile D, et al. Randomized controlled trial of the effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV ‐1 infected women in Tanzania. Lancet 1998;351:1477‐82. [DOI] [PubMed] [Google Scholar]
- Kawai K, Kupka R, Mugusi F, Aboud S, Okuma J, Villamor E, et al. A randomized trial to determine the optimal dosage of multivitamin supplements to reduce adverse pregnancy outcomes among HIV‐infected women in Tanzania. American Journal of Clinical Nutrition 2010;91(2):391‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Msamanga G, Manji K, Villamor E, Bosch RJ, Hertzmark E, et al. Sex differences in the effects of maternal vitamin supplements on mortality and morbidity among children born to HIV‐infected women in Tanzania. British Journal of Nutrition 2010;103(12):1784‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Koulinska IN, Aboud S, Murrin C, Bosch RJ, Manji KP, et al. Effect of vitamin supplements on HIV shedding in breast milk. American Journal of Clinical Nutrition 2010;92(4):881‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Msamanga G, Saathoff E, Manji K, Fawzi WW. Effect of vitamin supplements on the incidence of malaria among children born to HIV‐infected women. FASEB Journal 2006;20(4 Pt 1):A125. [PubMed] [Google Scholar]
- Villamor E, Msamanga G, Spiegelman D, Antelman G, Peterson KE, Hunter DJ, et al. Effect of multivitamin and vitamin A supplements on weight gain during pregnancy among HIV‐1 infected women. American Journal of Clinical Nutrition 2002;76:1082‐90. [DOI] [PubMed] [Google Scholar]
- Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, et al. Vitamin supplementation of HIV‐infected women improves postnatal child growth. American Journal of Clinial Nutrition 2005;81(4):880‐8. [DOI] [PubMed] [Google Scholar]
Fernald 2016 {published data only}
- Fernald LC, Galasso E, Qamruddin J, Ranaivoson C, Ratsifandrihamanana L, Stewart CP, et al. A cluster‐randomized, controlled trial of nutritional supplementation and promotion of responsive parenting in Madagascar: the MAHAY study design and rationale. BMC Public Health 2016;16(1):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feyi‐Waboso 2005 {published data only}
- Feyi‐Waboso PA, Chris A, Nwaogu GC, Archibong EI, Ejikem EC. The role of parenteral multivitamin preparation (Eldervit‐12) in the prevention of anaemia in pregnancy. Tropical Journal of Obstetrics and Gynaecology 2005;22(2):159‐63. [Google Scholar]
Fleming 1986 {published data only}
- Fleming AF, Ghatoura GBS, Harrison KA, Briggs ND, Dunn DT. The prevention of anemia in pregnancy in primigravidae in the Guinea Savana of Nigeria. Annals of Tropical Medicine and Parasitology 1986;80(2):211‐33. [DOI] [PubMed] [Google Scholar]
Godfrey 2017 {published data only}
- Godfrey KM, Cutfield W, Chan SY, Baker PN, Chong YS. Nutritional intervention preconception and during pregnancy to maintain healthy glucose metabolism and offspring health ("NiPPeR"): study protocol for a randomised controlled trial. Trials 2017;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldenberg 1995 {published data only}
- Goldenberg R, Tamura T, Neggers Y, Copper R, Johnston K, DuBard M, et al. Maternal zinc supplementation increases birthweight and head circumference. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):368. [Google Scholar]
Gopalan 2004 {published data only}
- Gopalan S, Patnaik R, Ganesh K. Feasible strategies to combat low birth weight and intra‐uterine growth retardation. Journal of Pediatric Gastroenterology and Nutrition 2004;39 Suppl 1:S37. [Google Scholar]
Graham 2007 {published data only}
- Graham JM, Haskell MJ, Pandey P, Shrestha RK, Brown KH, Allen LH. Supplementation with iron and riboflavin enhances dark adaptation response to vitamin A‐fortified rice in iron‐deficient, pregnant, nightblind Nepali women. American Journal of Clinical Nutrition 2007;85(5):1375‐84. [DOI] [PubMed] [Google Scholar]
Guldholt 1991 {published data only}
- Guldholt IS, Trolle BG, Hvidman LE. Iron supplementation during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1991;70:9‐12. [DOI] [PubMed] [Google Scholar]
Gunaratna 2015 {published data only}
- Gunaratna NS, Masanja H, Mrema S, Levira F, Spiegelman D, Hertzmark E, et al. Multivitamin and iron supplementation to prevent periconceptional anemia in rural Tanzanian women: a randomized, controlled trial. PLoS One 2015;10(4):e0121552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2007 {published data only}
- Gupta P, Ray M, Dua T, Radhakrishnan G, Kumar R, Sachdev HP. Multimicronutrient supplementation for undernourished pregnant women and the birth size of their offspring: a double‐blind, randomized, placebo‐controlled trial. Archives of Pediatrics and Adolescent Medicine 2007;161(1):58‐64. [DOI] [PubMed] [Google Scholar]
Hambidge 2014 {published data only}
- Borengasser S, Kerns M, Palacios A, Baker P, Kemp J, Morrison S, et al. Preconceptional lipid‐based micronutrient supplementation reduced circulating branched chain amino acids in Guatemalan women who are overweight or obese at 12 weeks gestation: a pilot study. FASEB Journal 2017;31(1 Suppl):Abstract Number: 639.50. [Google Scholar]
- Hambidge KM, Krebs NF, Westcott JE, Garces A, Goudar SS, Kodkany BS, et al. Preconception maternal nutrition: a multi‐site randomized controlled trial. BMC Pregnancy and Childbirth 2014;14(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hillman 1963 {published data only}
- Hillman RW, Cabaud PE, Nilsson DE, Arpin PD, Tufano RJ. Pyridoxine supplementation during pregnancy. American Journal of Clinical Nutrition 1963;12:427‐30. [DOI] [PubMed] [Google Scholar]
Hininger 2004 {published data only}
- Hininger I, Favier M, Arnaud J, Faure H, Thoulon JM, Hariveau E, et al. Beneficial effects of a combined micronutrient supplementation on maternal oxidative stress and newborn anthropometric measurements: a randomised double blind, placebo‐controlled trial in healthy pregnant women. 21st Conference on Priorities in Perinatal Care in South Africa; 2002 March 5‐8; Eastern Cape, South Africa. 2002.
- Hininger I, Favier M, Arnaud J, Faure H, Thoulon JM, Hariveau E, et al. Effects of a combined micronutrient supplementation on maternal biological status and newborn anthropometric measurements: a randomized double‐blind, placebo‐controlled trial in apparently healthy pregnant women. European Journal of Clinical Nutrition 2004;58:52‐9. [DOI] [PubMed] [Google Scholar]
Holly 1955 {published data only}
- Holly RG. Anemia in pregnancy. Obstetrics and Gynecology 1955;5:562‐9. [PubMed] [Google Scholar]
Hossain 2014 {published data only}
- Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open label randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. Journal of Clinical Endocrinology and Metabolism 2014;99(7):2448‐55. [DOI] [PubMed] [Google Scholar]
Huang 2017 {published data only}
- Huang S, Mo TT, Norris T, Sun S, Zhang T, Han TL, et al. The CLIMB (Complex Lipids In Mothers and Babies) study: protocol for a multicentre, three‐group, parallel randomised controlled trial to investigate the effect of supplementation of complex lipids in pregnancy, on maternal ganglioside status and subsequent cognitive outcomes in the offspring. BMJ Open 2017; Vol. 7, issue 10:e016637. [DOI] [PMC free article] [PubMed]
Hunt 1983 {published data only}
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AH, et al. Zinc supplementation during pregnancy: effects on selected blood constituents and on progress and outcome of pregnancy in low‐income women of Mexican descent. American Journal of Clinical Nutrition 1984;40:508‐21. [DOI] [PubMed] [Google Scholar]
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Coulson AH, et al. Zinc supplementation during pregnancy: zinc concentration of serum and hair from low‐income women of Mexican descent. American Journal of Clinical Nutrition 1983;37:572‐82. [DOI] [PubMed] [Google Scholar]
Hunt 1985 {published data only}
- Hunt IF, Murphy NJ, Cleaver AE, Faraji B, Swendseid ME, Browdy BL, et al. Zinc supplementation during pregnancy in low‐income teenagers of Mexican descent: effects on selected blood constituents and on progress and outcome of pregnancy. American Journal of Clinical Nutrition 1985;42:815‐28. [DOI] [PubMed] [Google Scholar]
Huybregts 2009 {published data only}
- Huybregts L, Roberfroid D, Lanou H, Meda N, Taes Y, Valea I, et al. Prenatal lipid‐based nutrient supplements increase cord leptin concentration in pregnant women from rural Burkina Faso. Journal of Nutrition 2013;143(5):576‐83. [DOI] [PubMed] [Google Scholar]
- Huybregts L, Roberfroid D, Lanou H, Menten J, Meda N, Camp J, et al. Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. American Journal of Clinical Nutrition 2009;90(6):1593‐600. [DOI] [PubMed] [Google Scholar]
- Toe LC, Bouckaert KP, Beuf K, Roberfroid D, Meda N, Thas O, et al. Seasonality modifies the effect of a lipid‐based nutrient supplement for pregnant rural women on birth length. Journal of Nutrition 2015;145(3):634‐9. [DOI] [PubMed] [Google Scholar]
Huynh 2017 {published data only}
- Huynh DT, Low YL, Tey SL, Tran NT, Nguyen LT, Berde Y. Maternal nutritional adequacy and gestational weight gain in Vietnamese pregnant women. Annals of Nutrition and Metabolism 2017;71(Suppl 2):629. [Google Scholar]
- Huynh DT, Tran NT, Nguyen LT, Berde Y, Low YL. Impact of maternal nutritional supplementation in conjunction with a breastfeeding support program on breastfeeding performance, birth and growth outcomes in a Vietnamese population. Journal of Maternal‐Fetal & Neonatal Medicine 2018;31(12):1586‐94. [DOI] [PubMed] [Google Scholar]
Iannotti 2008 {published data only}
- Iannotti LL, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. American Journal of Clinical Nutrition 2008;88(1):154‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICMR 2000 {published data only}
- Indian Council of Medical Research (ICMR) Collaborating Centers, Central Technical Co‐ordinating Unit. Multicentric study of efficacy of periconceptional folic acid containing vitamin supplementation in prevention of open neural tube defects in India. Indian Journal of Medical Research 2000;112:206‐11. [PubMed] [Google Scholar]
IRCT2015041321736N1 {published data only}
- IRCT2015041321736N1. Clinical trial on the evaluation of calcium and vitamin D in the cord serum of neonates, whose mothers were under vitamin D treatment during their pregnancy. en.search.irct.ir/view/22976 (first received 25 January 2016). [IRCT2015041321736N1]
IRCT201704225623N109 {published data only}
- IRCT201704225623N109. Clinical trial of the effect of multi mineral‐vitamin D supplementation compared with the placebo on pregnancy outcomes in women with gestational diabetes. en.search.irct.ir/view/37291 2017.
ISRCTN83599025 {published data only}
- ISRCTN83599025. GIFTS: mother and child health study. isrctn.com/ISRCTN83599025 (first received 14 July 2014). [ISRCTN83599025]
Itam 2003 {published data only}
- Itam IH. The effect of "Chemiron" on haematological parameters and ferritin levels in pregnant Nigerian women in Calabar. Mary Slessor Journal of Medicine 2003;3(2):17‐24. [Google Scholar]
Janmohamed 2016 {published data only}
- Janmohamed A, Karakochuk CD, Boungnasiri S, Chapman GE, Janssen PA, Brant R, et al. Prenatal supplementation with corn soya blend plus reduces the risk of maternal anemia in late gestation and lowers the rate of preterm birth but does not significantly improve maternal weight gain and birth anthropometric measurements in rural Cambodian women: a randomized trial. American Journal of Clinical Nutrition 2016;103:559‐66. [DOI] [PubMed] [Google Scholar]
Jarvenpaa 2007 {published data only}
- Jarvenpaa J, Schwab U, Lappalainen T, Pakkila M, Niskanen L, Punnonen K, et al. Fortified mineral water improves folate status and decreases plasma homocysteine concentration in pregnant women. Journal of Perinatal Medicine 2007;35(2):108‐14. [DOI] [PubMed] [Google Scholar]
- Jarvenpaa J, Schwab U, Lappalainen T, Pakkila M, Niskanen L, Punnonen K, et al. Mineral water fortified with folic acid and vitamins B6, B12, D and calcium improves folate status and decreases plasma homocysteine concentration in pregnant women. 35th Nordic Congress of Obstetrics and Gynecology; 2006 May 23‐25; Goteburg, Sweden. 2008:55.
Kabir 2009 {published data only}
- Kabir I, Khan AI, Arifeen S, Alam DS, Persson LA. Effects of prenatal food and micronutrient supplementation and breastfeeding counseling on postnatal growth of rural Bangladeshi children. Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, USA. 2009.
Kable 2012 {published data only}
- Chambers C, Yevtushok L, Zymak‐Zakutnya N, Wertelecki W, Jones KL, Keen CL, et al. Maternal alcohol consumption during pregnancy, nutritional status and impact on infant outcomes. Alcohol and Alcoholism 2013;48 Suppl:i21‐i22. [Google Scholar]
- Coles C, Yevtushok L, Zymak‐Zakutnya N, Wertelecki W, Keen CL, Y YJ, et al. Micronutrient supplements can mitigate the teratogenic effects of prenatal alcohol exposure on Ukrainian infants at 6 months. Alcohol and Alcoholism 2013;48 Suppl:i22. [Google Scholar]
- Coles CD, Chambers CD, Yevtushok L, Zymak‐Zakutnya N, Wertelecki W, Keen CL, et al. Micronutrient supplements can mitigate the teratogenic effects of prenatal alcohol exposure on Ukrainian infants at six months. Birth Defects Research Part A ‐ Clinical and Molecular Teratology 2012;94(5):361. [Google Scholar]
- Kable J, Coles C, Chambers C, Keen C, Uriu‐Adams J, Jones K, et al. Preliminary analysis of the impact of micronutrient supplements on neurophysiological encoding and memory in Ukranian infants 12‐18 months postpartum. Alcoholism: Clinical and Experimental Research 2012;36(S1):212A. [Google Scholar]
- Kable J, Coles C, Chambers C, Keen C, Uriu‐Adams J, Jones K, et al. The impact of micronutrient supplementation in alcohol‐exposed pregnancies on information processing skills in Ukrainian infants. Alcohol and Alcoholism 2013;48 Suppl:i22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JA, Coles CD, Keen CL, Uriu‐Adams JY, Jones KL, Yevtushok L, et al. The impact of micronutrient supplementation in alcohol‐exposed pregnancies on information processing skills in Ukrainian infants. Alcohol 2015;49(7):647‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khavari 2014 {published data only}
- Khavari N, Jiang H, Manji K, Msamanga G, Spiegelman D, Fawzi W, et al. Maternal multivitamin supplementation reduces the risk of diarrhoea among HIV‐exposed children through age 5 years. International Health 2014;6:298‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khulan 2012 {published data only}
- Khulan B, Cooper WN, Skinner BM, Bauer J, Owens S, Prentice AM, et al. Periconceptional maternal micronutrient supplementation is associated with widespread gender related changes in the epigenome: a study of a unique resource in the Gambia. Human Molecular Genetics 2012;21(9):2086‐101. [DOI] [PubMed] [Google Scholar]
- Owens S, Gulati R, Fulford AJ, Sosseh F, Denison FC, Brabin BJ, et al. Periconceptional multiple‐micronutrient supplementation and placental function in rural Gambian women: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2015;102:1450‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubik 2004 {published data only}
- Chelchowska M, Laskowska‐Klita T, Kubik P, Leibschang J. The effect of vitamin‐mineral supplementation on the level of MDA and activity of glutathione peroxidase and superoxide dismutase in blood of matched maternal‐cord pairs [Wplyw suplementacji witaminowo‐mineralnej na poziom MDA oraz aktywnosc peroksydazy glutationowej i dysmutazy ponadtlenkowej w krwi kobiet ciezarnych i krwi pepowinowej ich dzieci]. Przeglad Lekarski 2004;61(7):760‐3. [PubMed] [Google Scholar]
- Kubik P, Kowalska B, Laskowska‐Klita T, Chelchowska M, Leibschang J. Effect of vitamin‐mineral supplementation on the status of some microelements in pregnant women. Przeglad Lekarski 2004;61(7):764‐8. [PubMed] [Google Scholar]
- Laskowska‐Klita T, Chelchowska M, Ambroszkiewicz J, Kubik P, Leibschang J. The effect of vitamin‐mineral supplementation on vitamins D, A (beta‐carotene) and E concentration in blood of matched maternal‐cord pairs [Wplyw suplementacji witaminowo‐mineralnej na stezenie witamin D, A (beta‐karoten) i E w krwi kobiet ciezarnych i krwi pqpowinowej ich dzieci.]. Przeglad Lekarski 2004;61(7):755‐9. [PubMed] [Google Scholar]
Kureishy 2017 {published data only}
- Kureishy S, Khan GN, Arrif S, Ashraf K, Cespedes A, Habib MA, et al. A mixed methods study to assess the effectiveness of food‐based interventions to prevent stunting among children under‐five years in districts Thatta and Sujawal, Sindh Province, Pakistan: study protocol. BMC Public Health 2017;17(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02422953. Effectiveness of food/nutrient based interventions to prevent stunting among children under five in Thatta and Sajawal districts, Sindh Province, Pakistan. clinicaltrials.gov/ct2/show/record/NCT02422953 (first received 22 April 2015).
Kynast 1986 {published data only}
- Kynast G, Langner K, Saling E. Clinical results of oral application of trace elements in risk pregnancies. Proceedings of 10th European Congress of Perinatal Medicine; 1986 Aug 12‐16; Leipzig, Germany. 1986.
Lanou 2014 {published data only}
- Lanou H, Huybregts L, Roberfroid D, Nikiema L, Kouanda S, Camp J, et al. Prenatal nutrient supplementation and postnatal growth in a developing nation: an RCT. Pediatrics 2014;133(4):e1001‐e1008. [DOI] [PubMed] [Google Scholar]
Leroy 2010 {published data only}
- Heckert J, Leroy J, Olney D, Ruel M, Iruhiriye E, Richter S. Cost and cost‐effectiveness of food‐assisted maternal and child health and nutrition programs in Burundi and Guatemala. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:719‐20.
- Leroy J, Olney D, Ruel M. The impact of tubaramure, a food‐assisted integrated health and nutrition program in Burundi, on maternal and child anemia. FASEB Journal 2015;29(1 Suppl):898.26. [Google Scholar]
- NCT01072279. Preventing malnutrition in children under two years of age approach. clinicaltrials.gov/ct2/show/NCT01072279 (first received 22 February 2010).
- Olney D, Leroy J, Bliznashka L, Ruel M. The impact of tubaramure, a food‐assisted integrated health and nutrition program in Burundi, on children's development. FASEB Journal 2015;29(1 Suppl):898.16. [Google Scholar]
- Richter SM, Leroy JL, Olney D, Quinones E, Ruel M. Strengthening and Evaluating the Preventing Malnutrition in Children under 2 Approach in Guatemala: Report of the Enrollment Survey. Washington DC: FHI 360, 2013. [Google Scholar]
Li 2014 {published data only}
- Li YF, Hu NS, Tian XB, Li L, Wang SM, Xu XB, et al. Effect of daily milk supplementation on serum and umbilical cord blood folic acid concentrations in pregnant Han and Mongolian women and birth characteristics in China. Asia Pacific Journal of Clinical Nutrition 2014;23(4):567‐74. [DOI] [PubMed] [Google Scholar]
Lindström 2011 {published data only}
- Lindström E, Hossain MB, Lönnerdal B, Raqib R, Arifeen S, Ekström EC. Prevalence of anemia and micronutrient deficiencies in early pregnancy in rural Bangladesh, the MINIMat trial. Acta Obstetricia et Gynecologica Scandinavica 2011;90(1):47‐56. [CENTRAL: 2879403] [DOI] [PubMed] [Google Scholar]
Ling 1996 {published data only}
- Ling CD, Zhang ZJ, Chen ZL. Studies on nutritional effects of traditional Chinese tonics with strengthened nutrients on pregnant women and rats. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1996;16:270‐3. [PubMed] [Google Scholar]
Lucia 2007 {published data only}
- Lucia Bergmann RL, Bergmann KE, Haschke‐Becher E, Richter R, Dudenhausen JW, Barclay D, et al. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy?. Journal of Perinatal Medicine 2007;35(4):295‐300. [DOI] [PubMed] [Google Scholar]
Ma 2008 {published data only}
- Ma AG, Schouten EG, Zhang FZ, Kok FJ, Yang F, Jiang DC, et al. Retinol and riboflavin supplementation decreases the prevalence of anemia in Chinese pregnant women taking iron and folic acid supplements. Journal of Nutrition 2008;138(10):1946‐50. [DOI] [PubMed] [Google Scholar]
Magon 2014 {published data only}
- Magon A, Collin SM, Joshi P, Davys Late G, Attlee A, Mathur B. Leaf concentrate fortification of antenatal protein‐calorie snacks improves pregnancy outcomes. Journal of Health, Population and Nutrition 2014;32(3):430‐40. [PMC free article] [PubMed] [Google Scholar]
Malvasi 2014 {published data only}
- Malvasi A, Casciaro F, Minervini MM, Kosmas I, Mynbaev OA, Pacella E, et al. Myo‐inositol, D‐chiro‐inositol, folic acid and manganese in second trimester of pregnancy: a preliminary investigation. European Review for Medical and Pharmacological Sciences 2014;18(2):270‐4. [PubMed] [Google Scholar]
Mardones 2007 {published data only}
- Mardones F, Urrutia MT, Villarroel L, Rioseco A, Castillo O, Rozowski J, et al. Effects of a dairy product fortified with multiple micronutrients and omega‐3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutrition 2007;11(1):30‐40. [DOI] [PubMed] [Google Scholar]
Marya 1987 {published data only}
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecologic and Obstetric Investigation 1987;24:38‐42. [DOI] [PubMed] [Google Scholar]
Mathan 1979 {published data only}
- Mathan BVI, Baker SH, Sood SK, Ramachandran K, Ramalingaswami V. WHO sponsored collaborative studies on nutritional anaemia in India. The effects of ascorbic acid and protein supplementation on the response of pregnant women to iron, pteroyglutamic acid and cyanocobalamin therapy. British Journal of Nutrition 1979;42:391‐8. [DOI] [PubMed] [Google Scholar]
Menon 1962 {published data only}
- Menon MK, Rajan L. Prophylaxis of anaemia in pregnancy. British Journal of Obstetrics and Gynaecology of the British Commonwealth 1962;12:382‐9. [Google Scholar]
Merchant 2005 {published data only}
- Merchant AT, Msamanga G, Villamor E, Saathoff E, O'Brien M, Hertzmark E, et al. Multivitamin supplementation of HIV‐ positive women during pregnancy reduces hypertension. Journal of Nutrition 2005;135(7):1776‐81. [DOI] [PubMed] [Google Scholar]
Merialdi 1999 {published data only}
- Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, DiPietro JA. Adding zinc to prenatal iron and folate tablets improves fetal neurobehavioral development. American Journal of Obstetrics and Gynecology 1999;180(2 Pt 1):483‐90. [DOI] [PubMed] [Google Scholar]
Muslimatun 2001 {published data only}
- Muslimatun S, Schmidt MK, Schultink W, West CE, Hautvast JG, Gross R, et al. Weekly supplementation with iron and vitamin A during pregnancy increases hemoglobin concentration but decreases serum ferritin concentration in Indonesian pregnant women. Journal of Nutrition 2001;131(1):85‐90. [DOI] [PubMed] [Google Scholar]
- Muslimatun S, Schmidt MK, West CE, Schultink W, Hautvast JG, Karyadi D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. Journal of Nutrition 2001;131(10):2664‐9. [DOI] [PubMed] [Google Scholar]
Nakano 2010 {published data only}
- Nakano S, Takekoshi H, Nakano M. Chlorella pyrenoidosa supplementation reduces the risk of anemia, proteinuria and edema in pregnant women. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 2010;65(1):25‐30. [DOI] [PubMed] [Google Scholar]
NCT01795131 {published data only}
- NCT01795131. Role of vitamin B12 supplementation during pregnancy and postpartum to reduce nutritional anemia and improve immunity in Bangladeshi women and their infants. clinicaltrials.gov/ct2/show/NCT01795131 (first received 20 February 2013).
NCT02802566 {published data only}
- NCT02802566. BMI‐based prenatal vitamins to ameliorate oxidative stress in obese pregnancy. clinicaltrials.gov/ct2/show/record/NCT02802566 (first received 16 June 2016). [NCT02802566]
NCT02959125 {published data only}
- NCT02959125. NutFish and nutrient supplementation in pregnancy class to improve maternal and birth outcomes. clinicaltrials.gov/show/NCT02959125 (first received 8 October 2016). [NCT02959125]
Nguyen 2012 {published data only}
- Gonzalez‐Casanova I, Nguyen PH, Young MF, Harding KB, Reinhart G, Nguyen H, et al. Predictors of adherence to micronutrient supplementation before and during pregnancy in Vietnam. BMC Public Health 2017;17(1):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01665378. Impact of pre‐pregnancy micronutrient supplementation on maternal and child outcomes. clinicaltrials.gov/ct2/show/NCT01665378 (first received 15 August 2012).
- Nechitilo M, Webb‐Girard A, Gonzalez‐Casanova I, Martorell R, Ramakrishnan U, Nguyen P, et al. A qualitative study of factors influencing initiation and adherence to micronutrient supplementation among women of reproductive age in Vietnam. Food and Nutrition Bulletin 2016;37(4):461‐74. [DOI] [PubMed] [Google Scholar]
- Nguyen P, Young M, Gonzalez‐Casanova I, Truong TV, Nguyen S, Martorell R, et al. Effects of preconceptional weekly micronutrient supplements on maternal and child anemia during the first 2 years of life. FASEB Journal 2017, issue 1 Suppl 1.
- Nguyen PH, DiGirolamo AM, Gonzalez‐Casanova I, Pham H, Hao W, Nguyen H, et al. Impact of preconceptional micronutrient supplementation on maternal mental health during pregnancy and postpartum: results from a randomized controlled trial in Vietnam. BMC Women's Health 2017;17(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PH, Gonzalez‐Casanova I, Young MF, Truong TV, Hoang H, Nguyen H, et al. Preconception micronutrient supplementation with iron and folic acid compared with folic acid alone affects linear growth and fine motor development at 2 years of age: a randomized controlled trial in Vietnam. Journal of Nutrition 2017;147(8):1593‐601. [DOI] [PubMed] [Google Scholar]
- Nguyen PH, Lowe AE, Martorell R, Nguyen H, Pham H, Nguyen S, et al. Rationale, design, methodology and sample characteristics for the Vietnam pre‐conceptual micronutrient supplementation trial (PRECONCEPT): a randomized controlled study. BMC Public Health 2012;12:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PH, Young M, Gonzalez‐Casanova I, Pham HQ, Nguyen H, Truong TV, et al. Impact of preconception micronutrient supplementation on anemia and iron status during pregnancy and postpartum: a randomized controlled trial in rural Vietnam. Plos One 2016;11(12):e0167416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan U, DiGirolamo A, Nguyen P, Gonzalez‐Casanova I, Nguyen H, Pham H, et al. Effects of preconceptual micronutrient supplementation on maternal depression in Vietnamese women. FASEB Journal 2015;29(1 Suppl):28.1. [Google Scholar]
- Ramakrishnan U, Nguyen PH, Gonzalez‐Casanova I, Pham H, Hao W, Nguyen H, et al. Neither preconceptional weekly multiple micronutrient nor iron‐folic acid supplements affect birth size and gestational age compared with a folic acid supplement alone in rural Vietnamese women: a randomized controlled trial. Journal of Nutrition 2016;146:1445S‐52S. [DOI] [PubMed] [Google Scholar]
- Young M, Gonzalez Canaova I, Addo Y, Martorell R, Ramakrishnan U, Hong Nguyen P, et al. Role of preconception nutrition in offspring growth and risk of stunting across the first 1000 days in Vietnam. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:538.
- Young MF, Nguyen PH, Addo OY, Hao W, Nguyen H, Pham H, et al. The relative influence of maternal nutritional status before and during pregnancy on birth outcomes in Vietnam. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2015;194:223‐7. [DOI] [PubMed] [Google Scholar]
Nguyen 2017 {published data only}
- Nguyen PH, Kim SS, Sanghvi T, Mahmud Z, Tran LM, Shabnam S, et al. Integrating nutrition interventions into an existing maternal, neonatal, and child health program increased maternal dietary diversity, micronutrient intake, and exclusive breastfeeding practices in Bangladesh: results of a cluster‐randomized program evaluation. Journal of Nutrition 2017; Vol. 147, issue 12:2326‐37. [DOI] [PMC free article] [PubMed]
Nossier 2015 {published data only}
- Nossier SA, Naeim NE, El‐Sayed NA, Abu Zeid AA. The effect of zinc supplementation on pregnancy outcomes: a double‐blind, randomised controlled trial, Egypt. British Journal of Nutrition 2015;114(2):274‐85. [DOI] [PubMed] [Google Scholar]
Nwagha 2010 {published data only}
- Nwagha UI, Okeji N, Clems‐Anunwa O, Ejezie FE, Nwagha TU, Iyare E. The role of some micronutrients (Eldervit) in the management of the anaemic pregnant Nigerian women: a preliminary report. Tropical Journal of Obstetrics & Gynaecology 2010;27(2):34‐9. [Google Scholar]
Ochoa‐Brust 2007 {published data only}
- Ochoa‐Brust GJ, Fernandez AR, Villanueva‐Ruiz GJ, Velasco R, Trujillo‐Hernandez B, Vasquez C. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2007;86(7):783‐7. [DOI] [PubMed] [Google Scholar]
Olofin 2014 {published data only}
- Olofin IO, Spiegelman D, Aboud S, Duggan C, Danaei G, Fawzi WW. Supplementation with multivitamins and vitamin A and incidence of malaria among HIV‐infected Tanzanian women. Journal of Acquired Immune Deficiency Syndromes (1999) 2014;67(Suppl 4):S173‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Otoluwa 2017 {published data only}
- Otoluwa AS, Hadju V, Thaha AR, As'ad S, Monoarfa Y, Yatim H. Effect of periconceptional multi micronutrient supplementation on the level of total antioxidant status. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:502‐3.
Park 1999 {published data only}
- Park E, Wagenbichler P, Elmadfa I. Effects of multivitamin/mineral supplementation, at nutritional doses, on plasma antioxidant status and DNA damage estimated by sister chromatid exchanges in lymphocytes in pregnant women. International Journal for Vitamin and Nutrition Research 1999;69:396‐402. [DOI] [PubMed] [Google Scholar]
Patimah 2013 {published data only}
- Patimah S, As'ad S, Jusoff K, Hadju V, Razak A, Bahar B. The influence of multiple micronutrient supplementations on hemoglobin and serum ferritin levels of pregnant women. World Journal of Medical Sciences 2013;8(3):177‐85. [Google Scholar]
People's League 1946 {published data only}
- People's League of Health. Nutrition of expectant and nursing mothers: interim report. Lancet 1942;2:10‐2. [Google Scholar]
- People's League of Health. The nutrition of expectant and nursing mothers in relation to maternal and infant mortality and morbidity. Journal of Obstetrics and Gynaecology of the British Empire 1946;53:498‐509. [DOI] [PubMed] [Google Scholar]
Pezzack 2014 {published data only}
- Pezzack B, Zlotkin S, Abrams S, Hawthorne K, Mahmud AA, Baxter JA, et al. Bioavailability of enteric‐coated microencapsulated calcium during pregnancy: a randomized crossover trial in Bangladesh. FASEB Journal 2014;28(1 Suppl):abstract no: 804.4. [DOI] [PubMed] [Google Scholar]
Ramirez‐Velez 2011 {published data only}
- Ramirez‐Velez R, Romero M, Echeverri I, Ortega JG, Mosquera M, Salazar B, et al. A factorial randomized controlled trial to evaluate the effect of micronutrients supplementation and regular aerobic exercise on maternal endothelium‐dependent vasodilatation and oxidative stress of the newborn. Trials 2011;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 1991 {published data only}
- Robertson JS, Heywood B, Atkinson SM. Zinc supplementation during pregnancy. Journal of Public Health Medicine 1991;13:227‐9. [DOI] [PubMed] [Google Scholar]
Rumiris 2006 {published data only}
- Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertension in Pregnancy 2006;25:241‐53. [DOI] [PubMed] [Google Scholar]
Sachdeva 1993 {published data only}
- Sachdeva R, Mann SK. Impact of nutrition education and medical supervision on pregnancy outcome. Indian Pediatrics 1993;30(11):1309‐14. [PubMed] [Google Scholar]
Sagaonkar 2009 {published data only}
- Sagaonkar S, Sukhija S, Tayal R, Sagaonkar PD. Pregnancy induced iron deficiency and the evaluation and comparison of the efficacy and safety of ferrous fumarate and carbonyl iron in its treatment ‐ PERFECT trial. Journal of Obstetrics and Gynecology of India 2009;59(6):552‐62. [Google Scholar]
Salzano 2001 {published data only}
- Salzano P, Felicetti M, Laboccetta A, Borrelli P, Domenico A, Borrelli A. Prevention of gestational hypertension with calcium, linoleic acid, mono and polyunsaturated fatty acid supplements. Minerva Ginecologica 2001;53(4):235‐8. [PubMed] [Google Scholar]
Schmidt 2001 {published data only}
- Schmidt MK, Muslimatun S, Schultink W, West CE, Hautvast JG. Randomised double‐blind trial of the effect of vitamin A supplementation of Indonesian pregnant women on morbidity and growth of their infants during the first year of life. European Journal of Clinical Nutrition 2002;56(4):338‐46. [DOI] [PubMed] [Google Scholar]
- Schmidt MK, Muslimatun S, West CE, Schultink W, Hautvast JG. Vitamin A and iron supplementation of Indonesian pregnant women benefits vitamin A status of their infants. British Journal of Nutrition 2001;86(5):607‐15. [DOI] [PubMed] [Google Scholar]
Semba 2000 {published data only}
- Semba RD, Kumwenda N, Taha TE, Mtimavalye L, Broadhead R, Garrett E, et al. Impact of vitamin A supplementation on anaemia and plasma erythropoietin concentrations in pregnant women: a controlled clinical trial. European Journal of Haematology 2001;66(6):389‐95. [DOI] [PubMed] [Google Scholar]
- Semba RD, Kumwenda N, Taha TE, Mtimavalye L, Broadhead R, Miotti PG, et al. Plasma and breast milk vitamin A as indicators of vitamin A status in pregnant women. International Journal for Vitamin and Nutrition Research 2000;70(6):271‐7. [DOI] [PubMed] [Google Scholar]
Suharno 1993 {published data only}
- Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet 1993;342:1325‐8. [DOI] [PubMed] [Google Scholar]
Sun 2010 {published data only}
- Sun YY, Ma AG, Yang F, Zhang FZ, Luo YB, Jiang DC, et al. A combination of iron and retinol supplementation benefits iron status, IL‐2 level and lymphocyte proliferation in anemic pregnant women. Asia Pacific Journal of Clinical Nutrition 2010;19(4):513‐9. [PubMed] [Google Scholar]
Suprapto 2002 {published data only}
- Suprapto B, Widardo, Suhanantyo. Effect of low‐dosage vitamin A and riboflavin on iron‐folate supplementation in anaemic pregnant women. Asia Pacific Journal of Clinical Nutrition 2002;11(4):263‐7. [DOI] [PubMed] [Google Scholar]
Taghizadeh 2014 {published data only}
- Taghizadeh M, Samimi M, Kolahdooz F, Tabassi Z, Jamilian M, Asemi Z. Effect of multivitamin versus multivitamin‐mineral supplementation on metabolic profiles and biomarkers of oxidative stress in pregnant women: a double‐blind randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(11):1336‐42. [DOI] [PubMed] [Google Scholar]
- Taghizadeh M, Samimi M, Tabassi Z, Heidarzadeh Z, Asemi Z. Effect of multivitamin‐mineral versus multivitamin supplementation on maternal, newborns' biochemical indicators and birth size: a double‐blind randomized clinical trial. Oman Medical Journal 2014;29(2):123‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanumihardjo 2002 {published data only}
- Tanumihardjo SA. Vitamin A and iron status are improved by vitamin A and iron supplementation in pregnant Indonesian women. Journal of Nutrition 2002;132:1909‐12. [DOI] [PubMed] [Google Scholar]
Tatala 2002 {published data only}
- Makola D, Ask DM, Tatala SR, Latham MC, Ndossi G, Mehansho H. A micronutrient‐fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration in pregnant Tanzanian women. Journal of Nutrition 2003;133:1339‐46. [DOI] [PubMed] [Google Scholar]
- Tatala SR, Ash D, Makola D, Latham M, Ndosi G, Grohn Y. Effect of micronutrient fortified beverage on nutritional anaemia during pregnancy. East African Medical Journal 2002;79(11):598‐603. [DOI] [PubMed] [Google Scholar]
Thauvin 1992 {published data only}
- Thauvin E, Fusselier M, Arnaud J, Faure H, Favier M, Coudray C, et al. Effects of multivitamin mineral supplement on zinc and copper status during pregnancy. Biological Trace Element Research 1992;32:405‐14. [DOI] [PubMed] [Google Scholar]
Theobald 1937 {published data only}
- Theobald GW, Camb MD. Effect of calcium and vitamins A and D on incidence of pregnancy toxaemia. Lancet 1937;2:1397‐9. [Google Scholar]
Vadillo‐Ortega 2011 {published data only}
- Vadillo‐Ortega F, Perichart‐Perera O, Espino S, Avila‐Vergara MA, Ibarra I, Ahued R, et al. Effect of supplementation during pregnancy with L‐arginine and antioxidant vitamins in medical food on pre‐eclampsia in high risk population: randomised controlled trial. BMJ 2011;342:d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb 2009 {published data only}
- Webb AL, Aboud S, Furtado J, Murrin C, Campos H, Fawzi WW, et al. Effect of vitamin supplementation on breast milk concentrations of retinol, carotenoids and tocopherols in HIV‐infected Tanzanian women. European Journal of Clinical Nutrition 2009;63(3):332‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wibowo 2012 {published data only}
- Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Idriansyah L, Fitriana I. Antioxidant supplementation in pregnant women with low antioxidant status. Journal of Obstetrics & Gynaecology Research 2012;38(9):1152‐61. [DOI] [PubMed] [Google Scholar]
Wijaya‐Erhardt 2011 {published data only}
- Wijaya‐Erhardt M, Muslimatun S, Erhardt JG. Fermented soyabean and vitamin C‐rich fruit: a possibility to circumvent the further decrease of iron status among iron‐deficient pregnant women in Indonesia. Public Health Nutrition 2011;14(12):2185‐96. [DOI] [PubMed] [Google Scholar]
Wijaya‐Erhardt 2014 {published data only}
- Wijaya‐Erhardt M, Muslimatun S, Erhardt G. Effect of an educational intervention related to health and nutrition on pregnant women in the villages of Central Java Province, Indonesia. Health Education Journal 2014;73(4):370‐81. [Google Scholar]
Young 2010 {published data only}
- Hernandez A, Garcia‐Guerra A, Dominguez CP, Garcia‐Feregrino R, Neufeld LM. Effect of three supplements with identical micronutrient content on anemia in pregnant Mexican women. FASEB Journal 2008;22:Abstract no: 677.8. [Google Scholar]
- Young SL, Blanco I, Hernandez‐Cordero S, Pelto GH, Neufeld LM. Organoleptic properties, ease of use, and perceived health effects are determinants of acceptability of micronutrient supplements among poor Mexican women. Journal of Nutrition 2010;140(3):605‐11. [DOI] [PubMed] [Google Scholar]
Zavaleta 2000 {published data only}
- Zavaleta N, Caulfield LE, Garcia T. Changes in iron status during pregnancy in Peruvian women receiving prenatal iron and folic acid supplements with or without zinc. American Journal of Clinical Nutrition 2000;71(4):956‐61. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gathwala 2012 {published data only}
- Gathwala G. Effect of antenatal multiple micronutrient supplementation of mothers on the birthweight of their infants. Journal of Paediatrics and Child Health 2012;48(Suppl 1):47‐8. [Google Scholar]
References to ongoing studies
NCT02190565 {published data only}
- NCT02190565. Supplementation with WellnessPack mama during pregnancy and lactation‐ a randomized double‐blind, placebo‐controlled study. clinicaltrials.gov/ct2/show/NCT02190565 (first received 15 July 2014).
NCT03287882 {published data only}
- NCT03287882. Prospective, cluster randomized effectiveness trial of multiple micronutrient supplementation and life skills education provided from preconception on health and nutrition outcomes of young, reproductive‐age Pakistani women (15‐24 years). clinicaltrials.gov/ct2/show/NCT03287882 (first received 19 September 2017).
Sumarmi 2015 {published data only}
- Sumarmi S, Wirjatmadi B, Melaniani S, Kuntoro K, Dachlan EG, Thaha AR, et al. Prolonging micronutrients supplementation 2‐6 months prior to pregnancy significantly improves birth weight by increasing hPl production and controlling il‐12 concentration: a randomized double blind controlled study. Annals of Nutrition and Metabolism 2017; Vol. 71, issue Suppl 2:554.
- TCTR20150614001. Preconceptional supplementation of multi micronutrients to improve maternal iron status and pregnancy outcomes: a randomized double blind community‐based trial (Laduni). clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1424 (first received 11 June 2015).
Tu 2013 {published data only}
- Tu N, King JC, Dean D, Dirren H. Effect of food‐based supplement prior to and during pregnancy on birth weight and prematurity in rural Vietnam (VINAVAC study). Annals of Nutrition & Metabolism 2013;63(Suppl 1):806, Abstract no: PO3314. [Google Scholar]
- Tu N, King JC, Dirren H, Thu HN, Ngoc QP, Diep AN. Effect of animal‐source food supplement prior to and during pregnancy on birthweight and prematurity in rural Vietnam: a brief study description. Food and Nutrition Bulletin 2014;35(4 Suppl):S205‐S208. [DOI] [PubMed] [Google Scholar]
Additional references
Allen 2001
- Allen LH. Biological mechanisms that might underlie iron's effects on fetal growth and preterm birth. Journal of Nutrition 2001;131(2S‐2):581S‐589S. [DOI] [PubMed] [Google Scholar]
Allen 2005
- Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. American Journal of Clinical Nutrition 2005;81(5):1206S–1212S. [DOI] [PubMed] [Google Scholar]
Andersson 2007
- WHO Secretariat, Andersson M, Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2‐years‐old: conclusions and recommendations of the technical consultation. Public Health Nutrition 2007;10(12A):1606‐11. [DOI] [PubMed] [Google Scholar]
Bailey 2015
- Bailey RL, West KP, Black RE. The epidemiology of global micronutrient deficiencies. Annals of Nutrition & Metabolism 2015;66(Suppl 2):22‐33. [DOI] [PubMed] [Google Scholar]
Benoist 2008
- Benoist B, McLean E, Andersson M, Rogers L. Iodine deficiency in 2007: global progress since 2003. Food and Nutrition Bulletin 2008;29(3):195‐202. [DOI] [PubMed] [Google Scholar]
Berti 2011
- Berti C, Biesalski HK, Gärtner R, Lapillonne A, Pietrzik K, Poston L, et al. Micronutrients in pregnancy: current knowledge and unresolved questions. Clinical Nutrition (Edinburgh, Scotland) 2011;30(6):689‐701. [DOI] [PubMed] [Google Scholar]
Bhutta 2008
- Bhutta ZA, Haider BA. Maternal micronutrient deficiencies in developing countries. Lancet 2008;371(9608):186‐7. [DOI] [PubMed] [Google Scholar]
Bhutta 2009b
- Bhutta ZA, Haider BA. Prenatal micronutrient supplementation: are we there yet?. CMAJ : Canadian Medical Association Journal 2009;180(12):1188‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2012
- Bhutta ZA, Imdad A, Ramakrishnan U, Martorell R. Is it time to replace iron folate supplements in pregnancy with multiple micronutrients?. Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:27‐35. [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet 2013;382(9890):452‐77. [DOI] [PubMed] [Google Scholar]
Black 2001
- Black RE. Micronutrients in pregnancy. British Journal of Nutrition 2001;85 Suppl 2:S193‐S197. [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382:427‐51. [DOI] [PubMed] [Google Scholar]
Caulfield 1998
- Caulfield L, Zavaleta N, Shankur AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. American Journal of Clinical Nutrition 1998;65:S499‐S508. [DOI] [PubMed] [Google Scholar]
Christian 2005
- Christian P, Osrin D, Manandhar DS, Khatry SK, L Costello AM, West KP Jr. Antenatal micronutrient supplements in Nepal. Lancet 2005;366:711‐2. [DOI] [PubMed] [Google Scholar]
Christian 2010
- Christian P. Micronutrients, birth weight, and survival. Annual Review of Nutrition 2010;30:83‐104. [DOI] [PubMed] [Google Scholar]
Christian 2015
- Christian P. Evidence of multiple micronutrient supplementation (MMS) in pregnancy. Sight and Life 2015;29(1):28‐34. [Google Scholar]
Datta 2002
- Datta S, Alfaham M, Davies DP, Dunstan F, Woodhead S, Evans J, et al. Vitamin D deficiency in pregnant women from a non‐European ethnic minority population‐‐an interventional study. BJOG 2002;109(8):905‐8. [DOI] [PubMed] [Google Scholar]
De‐Regil 2010
- De‐Regil LM, Fernandez‐Gaxiola AC, Dowswell T, Pena‐Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007950.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2016
- De‐Regil L, Palacios C, Lombardo LK, Pena‐Rosas J. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD008873.pub3] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dror 2011
- Dror DK. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Current Opinion in Obstetrics & Gynecology 2011;23(6):422‐6. [DOI] [PubMed] [Google Scholar]
Dunn 1993
- Dunn JT. Iodine supplementation and the prevention of cretinism. Annals of New York Academy of Sciences 1993;678:158‐68. [DOI] [PubMed] [Google Scholar]
FAO/WHO 2004
- FAO/WHO. Vitamin and mineral requirements in human nutrition. 2nd Edition. World Health Organization and Food and Agriculture Organization of the United Nations, 2004. [Google Scholar]
Food and Nutrition Bulletin 2009
Gernand 2016
- Gernand AD, Schulze KJ, Steward CP, West KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nature Reviews. Endocrinology 2016;12(5):274‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginde 2010
- Ginde AA, Sullivan AF, Mansbach JM, Camargo CA Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. American Journal of Obstetrics and Gynecology 2010;202(5):436.e1‐436.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed September 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haider 2011
- Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health 2011;11 Suppl 3:S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2013
- Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta‐analysis. BMJ 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2009
- Hess SY, King JC. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food and Nutrition Bulletin 2009;30(1):S60‐S78. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Huffman 2005
- Huffman SL, Habicht JP, Scrimshaw N. Micronutrient supplementation in pregnancy. Lancet 2005;366:2001. [DOI] [PubMed] [Google Scholar]
Jiang 2005
- Jiang T, Christian P, Khatry SK, Wu L, West KP Jr. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. Journal of Nutrition 2005;135(5):1106‐12. [DOI] [PubMed] [Google Scholar]
Kawai 2011
- Kawai K, Spiegelman D, Shankar AH, Fawzi WW. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: meta‐analysis and meta‐regression. Bulletin of the World Health Organization 2011;89(6):402‐411B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keen 2003
- Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, et al. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. Journal of Nutrition 2003;133:1597S–1605S. [DOI] [PubMed] [Google Scholar]
Kramer 2003
- Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. Journal of Nutrition 2003;133:1592S–1596S. [DOI] [PubMed] [Google Scholar]
MacKay 2001
- MacKay AP, Berg CJ, Atrash HK. Pregnancy‐related mortality from preeclampsia and eclampsia. Obstetrics and Gynecology 2001;97(4):533‐8. [DOI] [PubMed] [Google Scholar]
Margetts 2009
- Margetts BM, Fall CH, Ronsmans C, Allen LH, Fisher DJ, Maternal Micronutrient Supplementation Study Group. Multiple micronutrient supplementation during pregnancy in low‐income countries: review of methods and characteristics of studies included in the meta‐analyses. Food and Nutrition Bulletin 2009;30(4 Suppl):S517‐S526. [DOI] [PubMed] [Google Scholar]
McArdle 1999
- McArdle HJ, Ashworth CJ. Micronutrients in fetal growth and development. British Medical Bulletin 1999;55:499‐510. [DOI] [PubMed] [Google Scholar]
McCauley 2015
- McCauley ME, Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD008666.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray‐Kolb 2013
- Murray‐Kolb LE, Chen L, Chen P, Shapiro M, Caulfield L. CHERG iron report: maternal mortality, child mortality, perinatal mortality, child cognition, and estimates of prevalence of anemia due to iron deficiency. CHERG Iron Report (cherg.org/publications/iron‐report.pdf) (accessed 3 February 2015).
Oppenheimer 2001
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131:616S–635S. [DOI] [PubMed] [Google Scholar]
Oriji 2011
- Oriji VK, Enyindah CE, Nyeche S. Factors determining compliance to routine iron supplementation in pregnancy at the University of Portharcout Teaching Hospital. Nigerian Journal of Medicine 2011;20(1):131‐4. [PubMed] [Google Scholar]
Ota 2015
- Ota E, Mori R, Middleton P, Tobe‐Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD000230.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Owens 2015
- Owens S, Gulati R, Fulford AJ, Sosseh F, Denison FC, Brabin BJ, et al. Periconceptional multiple‐micronutrient supplementation and placental function in rural Gambian women: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2015;102(6):1450‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozaltin 2010
- Ozaltin E, Hill K, Subramanian SV. Association of maternal stature with offspring mortality, underweight, and stunting in low‐ to middle‐income countries. JAMA 2010;303(15):1507‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pathak 2004
- Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian Journal of Pediatrics 2004;71(11):1007‐14. [DOI] [PubMed] [Google Scholar]
Pena‐Rosas 2015
- Peña‐Rosas JP, De‐Regil LM, Garcia‐Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramakrishnan 2012
- Ramakrishnan U, Grant FK, Goldenberg T, Bui V, Imdad A, Bhutta ZA. Effect of multiple micronutrient supplementation on pregnancy and infant outcomes: a systematic review. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):153‐67. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richard 2017
- Richard K, Holland O, Landers K, Vanderlelie JJ, Hofstee P, Cuffe JS, et al. Review: Effects of maternal micronutrient supplementation on placental function. Placenta 2017;54:38‐44. [DOI] [PubMed] [Google Scholar]
Ronsmans 2009
- Ronsmans C, Fisher DJ, Osmond C, Margetts BM, Fall CH, Maternal Micronutrient Supplementation Study Group. Multiple micronutrient supplementation during pregnancy in low‐income countries: a meta‐analysis of effects on stillbirths and on early and late neonatal mortality. Food and Nutrition Bulletin 2009;30(4 Suppl):S547‐S555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachan 2005
- Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. American Journal of Clinical Nutrition 2005;81(5):1060‐4. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Seck 2008
- Seck BC, Jackson RT. Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutrition 2008;11(6):596‐605. [DOI] [PubMed] [Google Scholar]
Shrimpton 2005
- Shrimpton R, Dalmiya N, Danton‐Hill I, Gross R. Micronutrient supplementation in pregnancy. Lancet 2005;366:2001‐2. [DOI] [PubMed] [Google Scholar]
Siegfried 2012
- Siegfried N, Irlam JH, Visser ME, Rollins NN. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009755] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2017
- Smith ER, Shankar AH, Wu LS, Aboud S, Adu‐Afarwuah S, Ali H, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta‐analysis of individual patient data from 17 randomised trials in low‐income and middle‐income countries. Lancet Global Health 2017;5(11):e1090‐e1100. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
UNICEF 1999
- UNICEF. Composition of a multi‐micronutrient supplement to be used in pilot programmes among pregnant women in developing countries 1999. UNICEF (www.UNICEF.org) (accessed 25 April 2003).
Voigt 2010
- Voigt M, Rochow N, Jährig K, Straube S, Hufnagel S, Jorch G. Dependence of neonatal small and large for gestational age rates on maternal height and weight ‐ an analysis of the German Perinatal Survey. Journal of Perinatal Medicine 2010;38(4):425‐30. [DOI] [PubMed] [Google Scholar]
Wessells 2012
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS One 2012;7:e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1959
- WHO. Iron deficiency anaemias: report of a WHO study group. WHO Technical Report Series (whqlibdoc.who.int/trs/WHO_TRS_182.pdf) (accessed 1 February 2014); Vol. 182.
WHO 1995
- Kelly A, Kevanya J, Onis M, Shah PM. A WHO collaborative study of maternal anthropometry and pregnancy outcomes. International Journal of Gynecology & Obstetrics 1996;53:219‐33. [DOI] [PubMed] [Google Scholar]
WHO 2004
- FAO/WHO. Vitamin and Mineral Requirements in Human Nutrition: Joint FAO/WHO Expert Consultation. 2nd Edition. WHO/FAO, 2004. [Google Scholar]
WHO 2012
- WHO. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. Geneva: World Health Organization, 2012. [PubMed] [Google Scholar]
References to other published versions of this review
Haider 2006
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004905.pub2] [DOI] [PubMed] [Google Scholar]
Haider 2012
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD004905.pub3] [DOI] [PubMed] [Google Scholar]
Haider 2017
- Haider BA, Bhutta ZA. Multiple‐micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD004905.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


