Key Points
Liver-associated tissue factor drives rapid intrahepatic coagulation after PHx.
Intrahepatic fibrin(ogen) deposition, but not thrombin-mediated platelet activation, promotes liver regeneration after PHx.
Abstract
Platelets play a pivotal role in stimulating liver regeneration after partial hepatectomy in rodents and humans. Liver regeneration in rodents is delayed when platelets are inhibited. However, the exact mechanisms whereby platelets accumulate and promote liver regeneration remain uncertain. Thrombin-dependent intrahepatic fibrin(ogen) deposition was recently reported after partial hepatectomy (PHx) in mice, but the role of fibrin(ogen) deposits in liver regeneration has not been investigated. We tested the hypothesis that fibrin(ogen) contributes to liver regeneration by promoting intrahepatic platelet accumulation and identified the trigger of rapid intrahepatic coagulation after PHx. PHx in wild-type mice triggered rapid intrahepatic coagulation, evidenced by intrahepatic fibrin(ogen) deposition. Intrahepatic fibrin(ogen) deposition was abolished in mice with liver-specific tissue factor deficiency, pinpointing the trigger of coagulation after PHx. Direct thrombin activation of platelets through protease-activated receptor-4 did not contribute to hepatocyte proliferation after PHx, indicating that thrombin contributes to liver regeneration primarily by driving intrahepatic fibrin(ogen) deposition. Fibrinogen depletion with ancrod reduced both intrahepatic platelet accumulation and hepatocyte proliferation after PHx, indicating that fibrin(ogen) contributes to liver regeneration after PHx by promoting intrahepatic platelet accumulation. Consistent with the protective function of fibrin(ogen) in mice, low postoperative plasma fibrinogen levels were associated with liver dysfunction and mortality in patients undergoing liver resection. Moreover, increased intrahepatic fibrin(ogen) deposition was evident in livers of patients after liver resection but was remarkably absent in patients displaying hepatic dysfunction postresection. The results suggest a novel mechanism whereby coagulation-dependent intrahepatic fibrin(ogen) deposition drives platelet accumulation and liver regeneration after PHx.
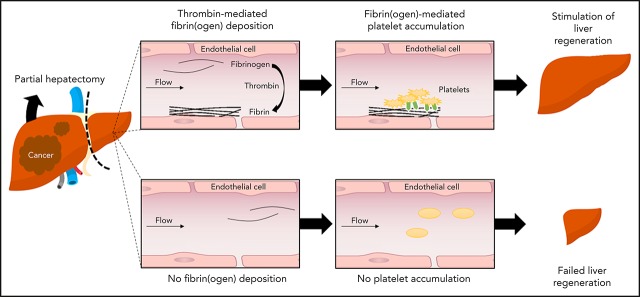
Visual Abstract
Introduction
The liver has a unique regenerative capacity. Following a liver resection, in which up to 70% of liver tissue can be safely removed, the liver remnant rapidly regenerates to its original size.1 Successful regeneration is essential for the functioning of the liver remnant. In some patients, however, liver regeneration is insufficient or not initiated at all due to poorly understood mechanisms. In fact, failure of regeneration is frequent in patients with acute liver failure and in patients after extensive liver resection.2,3 Postresection liver failure remains one of the most serious complications of liver resection, and represents a significant source of morbidity and mortality.3 Despite this, no effective treatment options are available to improve liver regeneration in patients undergoing liver resection. Patients who suffer from failed regeneration may require a liver transplantation or may die of liver insufficiency.4 A better understanding of the mechanisms involved in liver regeneration could identify new therapeutic targets to improve postoperative organ function, which would benefit patients with liver failure caused by failed regeneration.
Experimental and clinical evidence suggests a central role for platelets and platelet-derived factors in the regeneration of the liver remnant after partial hepatectomy. Platelets rapidly accumulate in the liver remnant following a partial hepatectomy (PHx) in mice5 and liver resection in humans,6 and liver regeneration is significantly delayed when platelets are depleted or functionally impaired.5,7 Conversely, an elevated platelet count, for example, as induced by thrombopoietin, stimulates regeneration of the liver after PHx.5,8,9 In humans, platelet transfusion seems to improve regeneration in living donor transplant recipients10 and liver function in patients with cirrhosis.11 A low platelet count, measured immediately after a liver resection, is associated with liver dysfunction and postoperative mortality.12,13 Using a more direct measurement of liver regeneration (by cross-sectional imaging volumetry), Margonis et al13 found that the relative increase in liver volume was significantly lower in patients with a low platelet count. Collectively, these studies indicate that platelets play a pivotal role in stimulating regeneration of the liver. One proposed mechanism whereby platelets stimulate liver regeneration relates to secretion of growth factors from activated platelets in the liver microvasculature.6,14 However, other mechanisms whereby platelets could stimulate regeneration, including the transfer of RNA from the platelets to hepatocytes, and platelet-mediated recruitment of inflammatory cells, have been reported as well (reviewed elsewhere by Lisman and Luyendyk15). The exact mechanism(s) whereby platelets accumulate and promote liver regeneration thus remains uncertain.
Platelets can become activated by several triggers, including thrombin, which leads to activation of the platelet receptor αIIbβ3. Binding of fibrinogen and fibrin, the end product of the coagulation cascade, to αIIbβ3 mediates platelet–platelet interactions, resulting in platelet aggregation.16 Activated platelets can amplify coagulation through exposure of a procoagulant surface, which facilitates thrombin generation, and by excretion of coagulation proteins such as fibrinogen.17 Recently, Beier et al18 documented fibrin(ogen) deposits in the liver after PHx in mice and reported that inhibition of thrombin reduced intrahepatic fibrin(ogen) deposits and hepatocyte proliferation after PHx. Although intrahepatic fibrin(ogen) deposition was reduced by the thrombin inhibitor hirudin, fibrin(ogen) was not definitively identified as the thrombin target driving the regeneration. Moreover, the mechanisms responsible for increased thrombin activity after PHx are unknown, and, at present, it is unclear whether fibrin(ogen) has a direct role in liver regeneration after PHx. We sought to define the mechanism driving fibrin(ogen) deposition in the liver remnant after PHx and to determine whether fibrin(ogen) and “platelet–coagulation cross talk” contributes to liver regeneration after PHx.
Patients, materials, and methods
Animals
Male mice with selective deletion of liver tissue factor (TF) in hepatocytes (TFflox/flox/albumin Cre mice, abbreviated to HPCΔTF mice here) backcrossed 8 generations onto the C57BL/6J background were generated as previously described.19 Male littermate TFflox/flox mice were used as controls. Protease-activated receptor-4–deficient (PAR-4−/−) male mice backcrossed 8 generations onto the C57BL/6J background were maintained by homozygous breeding, and male wild-type (WT) mice on an identical background bred in the same colony were used as control mice.20 Polymerase chain reaction was routinely used to confirm genotypes of the mice. Congenic C57BL/6 male WT mice (Charles River Laboratories, Leiden, The Netherlands) were used for fibrinogen and platelet depletion studies performed at the University of Groningen. Mice were housed under a 12-hour light/dark cycle. Standard rodent chow and drinking water were provided ad libitum. Procedures were approved by the Institutional Animal Care and Use Committees of the University of Groningen (Groningen, The Netherlands) and Michigan State University (East Lansing, MI).
PHx in mice
Age-matched cohorts of mice between the ages of 11 and 17 weeks underwent a two-thirds partial liver resection according to published protocols with some modifications.21,22 Briefly, PHx was performed in unfasted mice during the light cycle by resection of the left lateral lobe, the right portion of the median lobe, and the left portion of the median lobe using 3 separate ligatures. This method preserves the gallbladder to prevent obstruction of the extrahepatic biliary tree. Sham surgeries involved gentle manipulation of the liver lobes without removal of liver tissue. Surgical procedures were performed under deep surgical anesthesia induced by isoflurane (Abbott, Chicago, IL). Mice were subcutaneously injected before surgery with 5 mg/kg carprofen (Rimadyl; Pfizer, New York, NY) for analgesia. For fibrinogen depletion studies, 2.5 U ancrod per mouse (National Institute for Biological Standards and Control, South Mimms, UK) or vehicle (sterile saline) was administered by subcutaneous injection 2 hours before or 2 hours after PHx. For platelet depletion studies, a rat monoclonal antibody directed against mouse glycoprotein Ibα (GPIbα; 4 μg/g body weight; R300, Emfret Analytics, Würzburg, Germany) or vehicle (sterile saline) was administered by IV injection 24 hours before the surgery. Platelet depletion was confirmed by measuring platelet count before and 24 hours after injection in 4 mice. Blood and liver samples were collected either 30 minutes or 3 days after PHx or sham surgery. Blood was collected under deep surgical anesthesia, by exsanguination from the inferior vena cava immediately after injection of 150 μL 3.4% sodium citrate (Merck, Darmstadt, Germany) diluted in saline in the spleen. Blood samples were centrifuged at 1400g for 10 minutes (without brake) to obtain plasma and were stored at −80°C. Plasma thrombin-antithrombin (TAT) levels were determined by using a commercially available enzyme-linked immunosorbent assay kit (Siemens Health Care Diagnostics, Deerfield, IL). Livers were flushed with saline and fixed in either 4% formaldehyde or snap-frozen in liquid nitrogen for immunohistochemical analyses.
Patient population and quantification of plasma fibrinogen levels
Plasma fibrinogen levels were assessed in patients undergoing liver resection at the Medical University of Vienna (Vienna, Austria). In total, 312 patients undergoing liver resection between 2001 and 2014 were included. Measurement was performed in citrated blood taken before liver resection, as well as on the first and fifth postoperative day (POD) according to the Clauss method.23,24 In addition, liver biopsy specimens were obtained from 11 patients, both at the beginning of surgery (before) and from the regenerating liver lobe 2 hours after ligation of the portal vein (postresection). After surgery, patients were followed up for 90 days, and postoperative outcome was prospectively documented. In particular, liver dysfunction (LD) was defined following the International Study Group of Liver Surgery criteria. Briefly, patients with bilirubin levels >1.2 mg/dL and a prothrombin time <70% on POD 5 were classified as having LD.25 In cases of abnormal bilirubin level or prothrombin time before the operation, patients had to show an aggravation in both parameters on 2 consecutive days after POD 5 to be considered positive for LD according to the International Study Group of Liver Surgery criteria. In addition, patients with normal serum bilirubin or prothrombin time values before POD 5 were discharged early due to good clinical performance and hence had no further blood collection and were therefore considered as no LD. Postoperative mortality was defined as death within 90 days after surgery.26 The study was approved by the ethics committee of the Medical University of Vienna, and all patients gave written informed consent.
Immunohistochemical staining and intravital microscopy
Mouse liver sections were stained for Ki-67 by the Investigative Histopathology Laboratory at Michigan State University. In brief, sections were incubated for 10 minutes with ready-to-use Background Punisher (BioCare Medical, Pacheco, CA) to reduce nonspecific background staining. Slides were then incubated with a polyclonal rabbit anti-mouse Ki-67 antibody (1.5 µg/mL final concentration; MilliporeSigma, Burlington, MA) for 30 minutes at room temperature. Sections were subsequently incubated with ready-to-use Rabbit-on-Rodent HRP-Polymer (BioCare Medical) for 1 hour, followed by Romulin AEC Chromogen (BioCare Medical) incubation for 5 minutes. In selective studies, sections were stained for Ki-67 as previously described.27 The extent of fibrin(ogen) deposition was assessed in paraffin sections of mouse and human livers as described elsewhere.28 In brief, acetone-fixed sections were incubated with Avidin/Biotin Blocking Kit (Dako, Glostrup, Denmark). Sections were then incubated with a polyclonal rabbit anti-human fibrinogen (1:750, A0080; Dako) for 1 hour at room temperature. After washing, sections were incubated with a biotinylated goat anti-rabbit immunoglobulin G (1:100, BA-1000; Vector Labs, Brunschwig, Burlingame, CA) for 45 minutes. After washing, sections were incubated with the Vectastain ABC-AP reagent (SK-5100 Vector Red, Vector Labs) for 30 minutes, washed, and incubated for 20 minutes with the Vector Red Alkaline Phosphatase reagent (AK-5000; Vector Labs). All sections were counterstained with hematoxylin, mounted with Kaiser’s glycerol gelatin, and scanned by using the Nano-Zoomer digital slide scanner (Hamamatsu Photonics K.K., Hamamatsu, Japan). Ki-67–positive hepatocytes were counted in at least 5 high-power fields per mouse by using ImageJ software (version 1.51w; National Institutes of Health, Bethesda, MD) and expressed as percentage of all hepatocytes.
Quantification of fibrin deposits was performed by using ImageJ software. Five high-power fields per tissue were randomly selected for analysis. The area of positive fibrin(ogen) staining in each image was determined in an unbiased fashion by using a batch macro and the color deconvolution tool in ImageJ. The positive signal was expressed as positive pixel count. In addition, for human livers, the percentage of positive fibrin staining was also expressed as a fold change relative to values from the liver biopsy specimen taken before the surgery. Platelet accumulation was assessed in living mice shortly after PHx by intravital microscopy as previously described.27,29 In brief, platelets were labeled in vivo just before imaging by IV injection of 1.6 µg phycoerythrin-conjugated hamster anti-mouse CD49b (clone HMα2; BioLegend, San Diego, CA). Image acquisition was started within 5 minutes after PHx and performed for 1 hour by using an inverted Zeiss LSM 780 NLO microscope (Axio Observer.Z1; Carl Zeiss, Oberkochen, Germany). Images were captured by using a 488 nm argon laser and a gallium arsenide phosphide spectral detector at 508 to 561 nm for autofluorescence detection of the liver and at 569 to 655 nm for phycoerythrin detection. Platelet aggregate analysis was performed as previously described.27
Statistical analyses
Statistical analyses were performed with Prism version 5 (GraphPad Software, La Jolla, CA) software package or SPSS software (IBM SPSS Statistics, IBM Corporation, Armonk, NY). Continuous variables are presented as mean + SEM or median and range, as appropriate. Comparison of 2 groups for studies in experimental mice was performed by using the Student t test. Comparison of 3 or more groups was performed by using one-way analysis of variance with the Bonferroni post hoc test. Similarly, differences in circulating levels of fibrinogen between patients with and without postoperative LD or mortality were assessed by using the Student t test. Receiver-operating characteristic analysis was applied to assess the discriminatory potential of plasma fibrinogen levels between patients with and without postoperative LD. Youden’s J statistic (J = sensitivity + specificity − 1) was used to compute the optimal cutoff value. P values <.05 were considered statistically significant.
Results
Hepatic tissue factor activates coagulation and drives intrahepatic fibrin(ogen) deposition after PHx
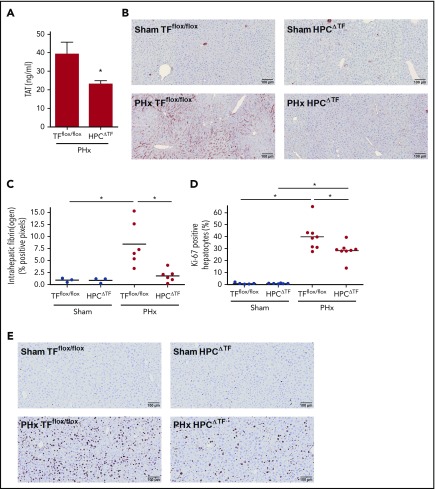
Previous studies showed that liver TF drives coagulation in mice with acute liver injury.19,30 Plasma TAT levels increase after PHx in mice, suggesting that PHx activates the coagulation cascade.18 To determine if rapid coagulation activation after PHx is driven by hepatic TF, mice with liver-specific TF deficiency (HPCΔTF mice) and control mice (TFflox/flox) underwent a two-thirds PHx, and TAT levels were examined 30 minutes later. HPCΔTF mice had significantly reduced TAT levels 30 minutes after PHx compared with control mice, suggesting that hepatic TF is an important source of TF required for the generation of thrombin following PHx (Figure 1A). We next investigated whether this decrease in coagulation activation affected intrahepatic fibrin(ogen) deposition after PHx. There was no effect of genotype on intrahepatic fibrin(ogen) deposition in the liver of sham-operated mice (Figure 1B-C). Extensive intrahepatic fibrin(ogen) deposits were observed in livers of TFflox/flox mice after PHx (8.4 ± 4.6% positive pixels). In contrast, deletion of liver TF led to significant attenuation of intrahepatic fibrin(ogen) staining in HPCΔTF mice after PHx (1.8 ± 1.3% positive pixels). To determine whether this early reduction in intrahepatic fibrin(ogen) deposition was linked to impaired regeneration, we assessed hepatocyte proliferation 3 days after PHx in HPCΔTF and TFflox/flox mice. There was no difference in the amount of Ki-67–positive hepatocytes between TFflox/flox mice and HPCΔTF mice undergoing sham surgeries (Figure 1D-E). Abundant proliferating hepatocytes were present in livers of TFflox/flox mice after PHx, and this was significantly reduced in the HPCΔTF mice (40 ± 12% vs 28 ± 7% Ki-67–positive hepatocytes).
Figure 1.
Effect of liver TF deficiency on coagulation activation, fibrin(ogen) deposition, and liver regeneration after PHx. TFflox/flox mice and TFflox/flox/albumin Cre (HPCΔTF) mice were euthanized 30 minutes (n = 3 for sham, n = 6 for PHx) or 3 days (n = 6 for sham, n = 8 for PHx) after sham or PHx. (A) TAT plasma levels 30 minutes after PHx for TFflox/flox mice and HPCΔTF mice. (B) Representative images of fibrin(ogen) immunohistochemical staining 30 minutes after sham (upper panels) or PHx (lower panels). (C) Quantification of fibrin(ogen) deposition in TFflox/flox and HPCΔTF mice, expressed as percent positive pixel count. (D) Quantification of Ki-67–positive hepatocytes, expressed as the percentage of the total number of hepatocytes. (E) Representative images of Ki-67–stained livers for sham (upper panels) or PHx (lower panels) 3 days after surgery. Bars represent mean + SEM. Horizontal lines represent the mean, closed circles are individual mice. *P < .05.
Intrahepatic fibrin(ogen), rather than PAR-4–mediated platelet activation, drives coagulation-mediated stimulation of liver regeneration
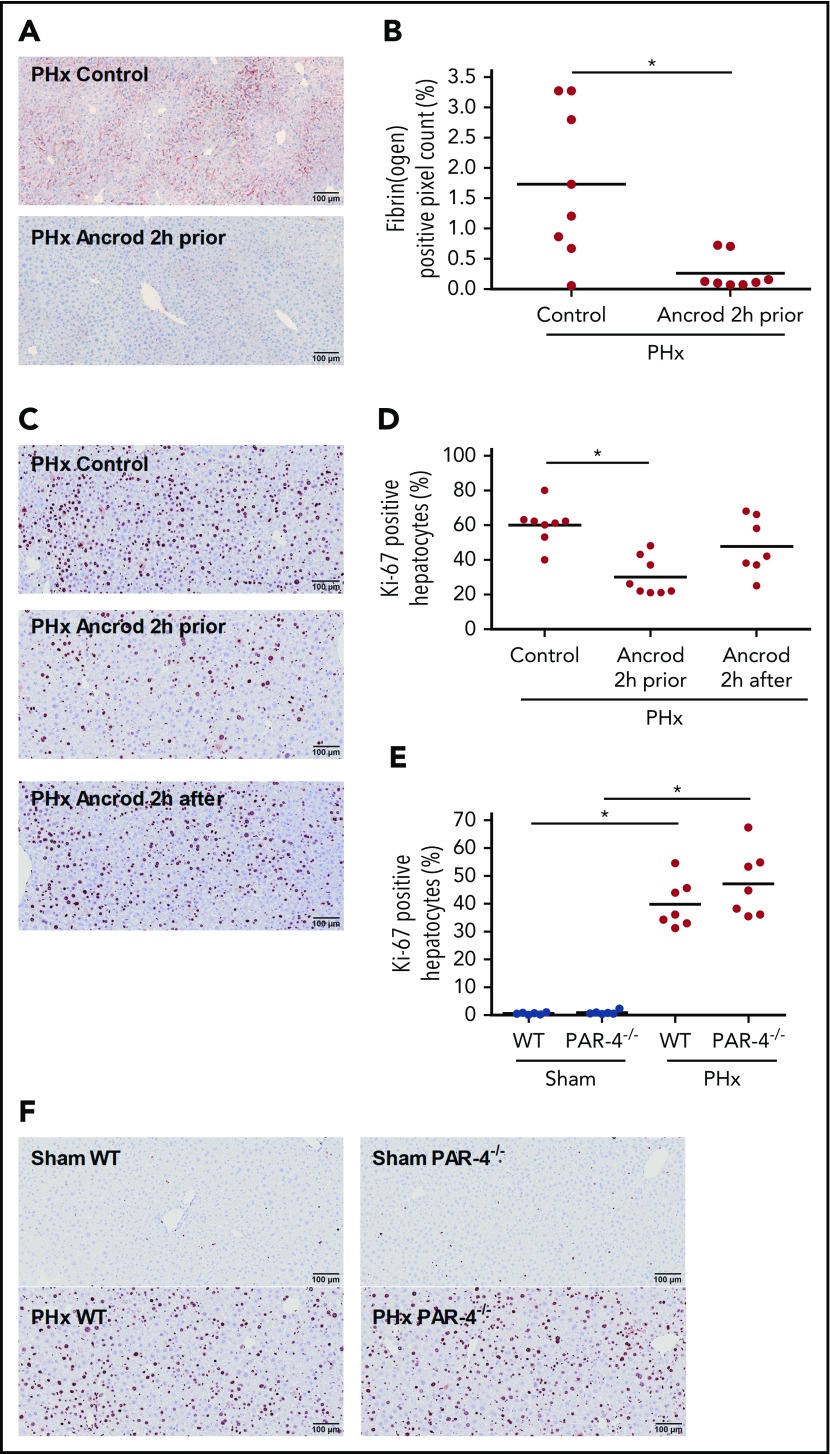
A previous study showed that inhibition of thrombin activity delays liver regeneration after PHx.18 To investigate whether intrahepatic fibrin(ogen) and/or platelet activation are the thrombin targets driving regeneration after PHx, regeneration was examined in fibrinogen-depleted mice and mice lacking the major thrombin receptor on mouse platelets (PAR-4−/− mice). The defibrinating agent ancrod was given 2 hours before or 2 hours after PHx to assess if early intrahepatic fibrin(ogen) deposition (ie, within the first 2 hours after PHx) is required for sufficient liver regeneration, as was previously observed for platelets.27 As anticipated, intrahepatic fibrin(ogen) deposition was dramatically attenuated 30 minutes after PHx in mice that had received ancrod 2 hours before the surgery (Figure 2A-B). Hepatocyte proliferation 3 days after PHx was significantly impaired in mice that received ancrod 2 hours before PHx compared with vehicle-treated mice (30 ± 11% vs 60 ± 11% Ki-67–positive hepatocytes) (Figure 2C-D). In contrast, depleting fibrinogen 2 hours after PHx had no significant effect on hepatocyte proliferation 3 days after PHx compared with vehicle-treated mice (48 ± 16% vs 60 ± 11% Ki-67–positive hepatocytes). To determine whether platelet activation by thrombin plays a role in coagulation-mediated stimulation of liver regeneration, we performed PHx in PAR-4−/− mice. There was no effect of genotype on hepatocyte proliferation in the livers of sham-operated mice (Figure 2E-F). In addition, there was also no difference in hepatocyte proliferation between PAR-4−/− mice and WT mice 3 days after PHx (47 ± 12% vs 40 ± 8% Ki-67–positive hepatocytes).
Figure 2.
Effect of fibrinogen depletion and PAR-4 deficiency on liver regeneration after PHx. Mice were euthanized 30 minutes or 3 days after sham or PHx. (A) Representative images of fibrin(ogen) immunohistochemical staining 30 minutes after PHx in vehicle-treated (control, upper panel) and ancrod-treated (lower panel) mice. (B) Quantification of fibrin(ogen) deposition after PHx, expressed as positive pixel count (n = 8). (C) Representative images of Ki-67–stained livers 3 days after PHx for vehicle-treated mice (upper panel), mice receiving ancrod 2 hours before PHx (middle panel), and mice receiving ancrod 2 hours after PHx (lower panel). (D) Quantification of Ki-67–positive hepatocytes, expressed as percentage of total number of hepatocytes for control mice (n = 8), and mice treated with ancrod 2 hours prior (n = 8) or after (n = 7) PHx. (E) Quantification of Ki-67–positive hepatocytes, expressed as percentage of total number of hepatocytes for WT and PAR-4−/− mice (n = 6 for sham, n = 7 for PHx). (F) Representative images of Ki-67–stained livers for sham (upper panels) or PHx (lower panels) 3 days after surgery for WT and PAR-4−/− mice. Horizontal lines represent the mean; closed circles are individual mice. *P < .05.
Intrahepatic fibrin(ogen) deposition drives platelet accumulation in the liver remnant
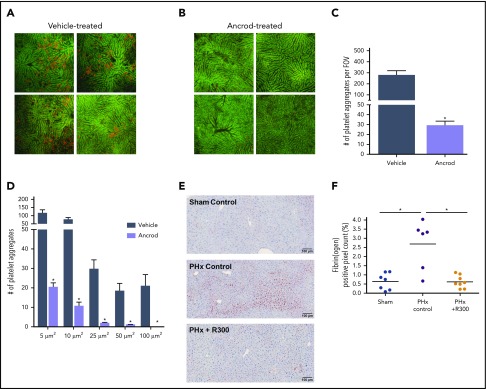
To assess whether intrahepatic fibrin(ogen) deposition drives platelet accumulation after PHx, we measured platelet accumulation within the liver remnant in vehicle-treated mice and fibrinogen-depleted mice by using intravital microscopy. Platelets rapidly accumulated in the liver remnant after PHx in the vehicle-treated mice but not in the fibrinogen-depleted mice (Figure 3A-B). Fibrin(ogen) depletion significantly attenuated both the number and size of platelet aggregates in the liver remnant after PHx (Figure 3C-D), suggesting that intrahepatic fibrin(ogen) deposition indeed drives platelet accumulation. We next evaluated the effect of platelet depletion, by injection of a platelet-depleting antibody, on intrahepatic fibrin(ogen) deposition after PHx. Injection of the platelet-depleting antibody 24 hours before PHx decreased circulating platelets >98% (from 640 ± 81 × 109/L before injection to 4.5 ± 2.6 × 109/L 24 hours after injection of the platelet-depleted antibody; n = 4). In thrombocytopenic mice, intrahepatic fibrin(ogen) deposition 30 minutes after PHx was dramatically attenuated compared with control mice (0.62 ± 0.34% positive pixel count vs 2.7 ± 1.3% positive pixels) (Figure 3E-F). Thus, intrahepatic fibrin(ogen) deposition and platelet accumulation after PHx seem to be connected mechanisms.
Figure 3.
Coagulation–platelet cross talk during liver regeneration after PHx. Platelets were visualized in the liver remnant using intravital microscopy. Platelets were labeled by IV injection of phycoerythrin-conjugated anti-mouse CD49b (red) immediately after PHx. Imaging was performed for 1 hour. Autofluorescent signal of the liver is shown in green to visualize liver anatomy. Representative images at 30 minutes after PHx in (A) vehicle-treated mice (n = 4) and (B) ancrod-treated mice (n = 4); images are from 4 individual mice per treatment group. Original magnification, 100×. Quantification of: (C) the number of platelet aggregates per field of view (FOV) and (D) the size equal to or larger than the indicated sizes. (E) Representative images of fibrin(ogen)-stained livers 30 minutes after sham (upper left panel) or PHx (upper right panel) for control mice, and 30 minutes after PHx for mice receiving R300 to deplete platelets (lower left panel). (F) Quantification of fibrin(ogen) deposition, expressed as percentage of positive pixel count, in livers of vehicle-treated mice (n = 7) or mice receiving R300 (n = 8) to deplete platelets. (F) Data are expressed as mean + SEM. *P < .05.
Lack of increase in intrahepatic fibrin(ogen) deposition is associated with postresection LD in humans
We next investigated whether intrahepatic fibrin(ogen) deposition also increases in patients undergoing liver resection. A total of 312 patients undergoing liver resection were included in this study. Patient characteristics of this cohort are shown in Table 1. Liver biopsy specimens were obtained from 11 patients before resection (before) and 2 hours after (post-resection) ligation of the portal vein and consequent initiation of liver regeneration. Consistent with the experimental data obtained in mice, intrahepatic fibrin(ogen) deposition increased significantly in human livers after completion of resection compared with livers before resection (4.6 ± 3.22% vs 1.8 ± 0.75% positive pixels) (Figure 4A). Interestingly, not all patients undergoing liver resection exhibited an increase in intrahepatic fibrin(ogen) deposition in their liver remnant (Figure 4B). In particular, patients with normal postoperative development collectively showed an increase in intrahepatic fibrin(ogen) deposition (Figure 4B-C). Remarkably, minimal to no increase in intrahepatic fibrin(ogen) deposition was observed in livers of patients who developed postresection LD. Indeed, the magnitude of increase in intrahepatic fibrin(ogen) deposition was significantly higher in patients without LD (4.7 ± 2.6-fold vs 1.5 ± 0.9-fold) (Figure 4D).
Table 1.
Patient demographic characteristics
| Parameter | Entire cohort (n = 312) | Intraoperative cohort (n = 11) |
|---|---|---|
| Sex | ||
| Male | 199 (63.8%) | 6 (54.5%) |
| Female | 113 (36.2%) | 5 (45.5%) |
| Age, y | 62 (28-84) | 63 (35-76) |
| Hepatic resection | ||
| Minor (<3 segments) | 187 (59.9%) | 0 (0.0%) |
| Major (≥3 segments) | 125 (40.1%) | 11 (100.0%) |
| Cofactors | ||
| Neoadjuvant CTx | 274 (87.8%) | 7 (63.6%) |
| Portal vein embolization | 6 (1.9%) | 0 (0.0%) |
| Intraoperative RBC count | 44 (14.1%) | 2 (18.2%) |
| Steatosis, % | 10 (0-95) | 3 (0-20) |
| Preoperative parameters | ||
| PDR, % | 19.4 (3.5-36.0) | 22.0 (17.0-32.0) |
| R15, % | 5.8 (0.4-59.2) | 4.0 (1.0-17.0) |
| Platelets, ×103/µL | 157 (49-503) | 252 (172-335) |
| Serum bilirubin, mg/dL | 0.62 (0.19-2.87) | 0.84 (0.31-1.18) |
| Prothrombin time, % | 106 (45-150) | 105 (68-124) |
| AP, U/L | 103 (42-1111) | 102 (65-707) |
| GGT, U/L | 46 (9-968) | 77 (36-968) |
| AST, U/L | 28 (5-496) | 29 (22-58) |
| ALT, U/L | 23 (2-410) | 39 (17-372) |
| Albumin, g/L | 41.0 (21.0-50.0) | 40.0 (35.5-44.5) |
| Postoperative outcome | ||
| Liver dysfunction (ISGLS) | 32 (10.3%) | 6 (54.5%) |
| Mortality (within POD 90) | 8 (2.6%) | 1 (9.1%) |
| Hepatic failure | 5 (62.5%) | |
| Infection | 2 (25%) | |
| Pulmonary embolism | 1 (12.5%) |
Data are presented as median (range) unless otherwise indicated. ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CTx, chemotherapy; GGT, gamma-glutamyl transpeptidase; ISGLS, International Study Group of Liver Surgery; PDR, plasma disappearance rate; R15 = retention rate at 15 minutes; RBC, red blood cell.
Figure 4.
Lack of increase in intrahepatic fibrin(ogen) deposition after resection corresponds with LD following liver resection in humans. Liver biopsy specimens were obtained from 11 patients before resection (before) and 2 hours after ligation of the portal vein (postresection) and stained for fibrin(ogen). (A) Changes in intrahepatic fibrin(ogen) deposition before the surgery and postresection. (B) Individual changes in intrahepatic fibrin(ogen) deposition before (open circles) and post-resection (open squares) between patients who did (LD, n = 6) or did not (no LD, n = 5) suffer from postoperative LD. (C) Representative images of intrahepatic fibrin(ogen) immunohistochemical staining in liver biopsy specimens obtained before surgery (upper panels) or postresection (lower panels). Left panels show images of liver biopsy specimens taken from a patient who did not develop LD. Right panels show images of liver biopsy specimens taken from a patient who developed postresection LD. (D) The fold change (percent positive pixels postresection divided by percent positive pixels before) in intrahepatic fibrin(ogen) deposition in patients who did not develop LD (no LD) following PHx and patients who did (LD). Intrahepatic fibrin(ogen) deposition is expressed as percent positive pixels, and individual patients are plotted in panels A, B, and D. Dashed lines connect individual changes in intrahepatic fibrin(ogen) deposition before and after resection. *P < .05.
Low postoperative plasma fibrinogen levels are associated with LD and poor outcome after liver resection in humans
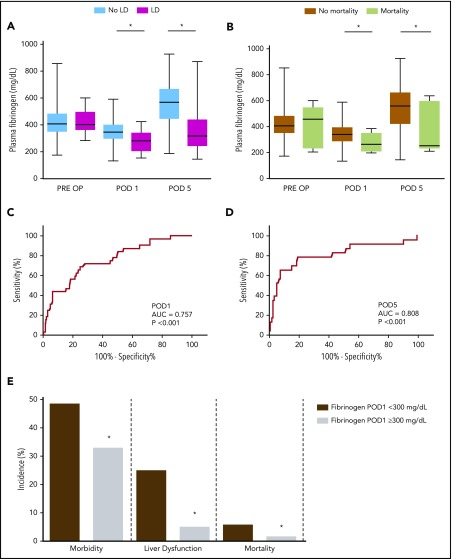
Because we observed no increase in intrahepatic fibrin(ogen) deposition in patients who suffered from postoperative LD, we evaluated whether plasma fibrinogen levels are associated with poor recovery of liver function after liver resection. Blood samples were obtained from 312 patients, 1 day before surgery and on POD 1 and POD 5. Of the 312 patients included, 32 (10%) developed postresection LD. Preoperative plasma fibrinogen levels did not differ between patients regardless of whether they suffered from LD after the resection (421 ± 102 mg/dL vs 429 ± 91 mg/dL) (Figure 5A). However, patients who suffered from postoperative LD had significantly lower plasma fibrinogen levels on POD 1 compared with patients who did not (349 ± 82 mg/dL vs 272 ± 73 mg/dL). This difference in plasma fibrinogen levels was also observed on POD 5 (565 ± 147 mg/dL vs 376 ± 182 mg/dL). We further assessed whether plasma fibrinogen levels were associated with postoperative mortality, defined as death within 90 days after surgery. Preoperative plasma fibrinogen levels did not differ between patients who died and patients who survived (421 ± 97 mg/dL vs 419 ± 158 mg/dL) (Figure 5B). However, patients who suffered from postoperative mortality had significantly lower plasma fibrinogen levels on both POD 1 (343 ± 83 mg/dL vs 267 ± 73 mg/dL) and POD 5 (548 ± 160 mg/dL vs 363 ± 192 mg/dL) compared with patients who did not suffer from postoperative mortality.
Figure 5.
Low postoperative plasma fibrinogen levels are associated with LD and mortality in patients undergoing liver resection. Plasma fibrinogen levels were measured 1 day before resection (PRE OP) and on POD 1 and POD 5 in 312 patients. (A) Plasma fibrinogen levels in patients who did (magenta boxplots, n = 32) or did not (light blue boxplots, n = 280) develop LD after resection. (B) Plasma fibrinogen levels in patients who did (lime green boxplots, n = 8) or did not (mustard boxplots, n = 304) suffer from postoperative mortality within 90 days after resection. Receiver-operating characteristic curve analysis of plasma fibrinogen levels on (C) POD 1 and (D) POD 5 to predict postoperative liver dysfunction. (E) Incidence of morbidity, postoperative LD, and mortality according to the defined cutoff value for plasma fibrinogen levels on POD 1 (300 mg/dL). Panels A and B show boxplots with median, 25% to 75% interquartile ranges, and minimum and maximum fibrinogen levels. Panel E shows incidence (in percentage) of morbidity, LD, and mortality. *P < .05. AUC, area under the curve.
We further aimed to characterize the potential of postoperative plasma fibrinogen levels to predict postoperative clinical outcome. Receiver-operating characteristic curve analysis revealed a significant association between postoperative plasma fibrinogen levels and LD at both POD 1 (area under the curve, 0.757) (Figure 5C) and POD 5 (area under the curve, 0.808) (Figure 5D). Because early prediction of postoperative LD is critical for clinical decision-making, a cutoff level for plasma fibrinogen level on POD 1 was identified by using Youden’s J statistic. Indeed, patients with plasma fibrinogen levels below the cutoff of 300 mg/dL on POD 1 (Figure 5E) exhibited a higher incidence of morbidity (48.3% vs 32.7%), postoperative LD (24.7% vs 4.8%), and mortality within 90 days after surgery (5.6% vs 1.4%).
Discussion
It has been previously observed that a low platelet count is an independent predictor of delayed postoperative liver function recovery and is associated with an increased risk of postoperative mortality after liver resection in humans.12 In mice, liver regeneration is also significantly delayed when platelets are depleted or functionally impaired.5,7 Within the present study, we conclusively identified intrahepatic fibrin(ogen) deposition as a central molecule for early intrahepatic platelet accumulation driving liver regeneration after PHx. Previous studies also indicated that other hemostatic factors, including von Willebrand factor (VWF), contributed to intrahepatic platelet accumulation and regeneration after PHx.27,31 Indeed, we found that early and selective removal of individual components of hemostasis (ie, platelets, TF, VWF,27 fibrin(ogen)) similarly delays liver regeneration after liver resection. Of note, the reciprocal interactions between these various factors (eg, platelets promote coagulation and vice versa) complicate identification of a strictly linear mechanism whereby these mediators contribute to regeneration. Still, this complex interplay highlights the importance of the hemostatic system in liver regeneration, which is reflected by the fact that deficiency in any one of these hemostatic components can have a profound negative impact on liver regeneration after PHx.
Fibrin(ogen) deposits were recently observed in livers of mice after PHx,18 but the mechanism triggering intrahepatic fibrin(ogen) deposition was unknown. We discovered that liver-associated TF triggers the rapid intrahepatic procoagulant response (ie, within 30 min) after PHx. As a consequence of typical vascular injury, subendothelial TF is exposed to its ligand factor VIIa (FVIIa), triggering coagulation.32 However, in the liver, hepatocytes express a TF:FVIIa complex in an encrypted form that has minimal to no procoagulant activity and requires activation via decryption to activate coagulation.19 The triggers for hepatocyte TF activation after PHx are not known. Among the primary stimuli for the activation of TF is the loss of phospholipid asymmetry on the outer cell membrane and interaction of the TF:FVIIa complex with externalized phosphatidylserine. In models of acute and chronic liver injury, apoptotic cell death is one potential trigger for phosphatidylserine externalization and coagulation33-35; however, cell death is minimal after PHx due to strong activation of the antiapoptotic Akt pathway.36 Nonetheless, this finding does not exclude nonapoptotic mechanisms of phosphatidylserine externalization as a driver for coagulation after PHx. Coagulation activation may also occur as a consequence of hemodynamic alterations within the liver remnant. Indeed, increased intrahepatic shear stress has been proposed as a start signal for regeneration, and it has been shown to increase TF expression and activity in other experimental settings.37,38 Although the mechanism whereby hepatic TF becomes activated after PHx is not known, our studies are the first to identify hepatic TF as an early and critical trigger of coagulation-mediated liver regeneration.
A previous study showed that inhibition of thrombin activity delayed liver regeneration in mice after PHx, suggesting a potential role for fibrin(ogen) in liver regeneration.18 Moreover, lack of plasminogen activator inhibitor-1, which increases intrahepatic fibrin(ogen) deposition, improved regeneration after PHx. Although both of these studies indirectly implicated fibrin(ogen), neither definitively identified fibrin(ogen) as a proregenerative stimulus after PHx. Here, we show for the first time that fibrin(ogen) contributes directly to liver regeneration after PHx in mice. Notably, rapid intrahepatic fibrin(ogen) deposition is critical to support later events in regeneration, which is depicted by the reduced hepatocyte proliferation in mice where fibrinogen was depleted before PHx. In contrast, depletion of fibrinogen 2 hours after PHx had no effect on later hepatocyte proliferation. Strikingly, this observation could also be translated into the clinical setting of liver resection, where the lack of intrahepatic fibrin(ogen) deposition in the regenerating liver was directly linked to the development of postoperative LD. Furthermore, we found that low postoperative plasma fibrinogen levels were associated with LD and mortality after liver resection in humans and that plasma fibrinogen levels on POD 1 have the potential to predict poor postoperative outcome in patients undergoing resection. Taken together, the parallels between experimental data and human validation with respect to fibrin(ogen) deposition underline its relevance during regenerative processes after liver resection. The basis for failed hepatic fibrin(ogen) deposition in some patients is not known but could be a consequence of decreased potential to generate thrombin during liver regeneration or a failure of TF decryption in the liver. Future investigations should consider the calibrated automated thrombin generation test or markers of coagulation activation such as prothrombin fragment 1+2 to determine a relative hypocoagulable state and to measure thrombin generation in patients undergoing resection. However, the amount of thrombin generated in the liver might be too small to produce detectable increases in prothrombin fragment 1+2 in the systemic circulation, and there are clear challenges in measuring hepatic TF decryption in patients.
A previous study suggested that thrombin contributes to regeneration after PHx.18 Our studies uncovered that the mechanism whereby thrombin drives liver regeneration is not mediated by platelet activation, as hepatocyte proliferation was unaffected in PAR-4−/− mice after PHx. Although platelet activation by thrombin is not required for regeneration, additional platelet activation pathways may explain the stimulatory effects of platelets on liver regeneration. Indeed, mice given the P2Y12 inhibitor clopidogrel have impaired liver regeneration after PHx.7 Here, we document for the first time that thrombin-driven intrahepatic fibrin(ogen) deposition drives liver regeneration. Our results indicate that fibrin(ogen) supports the localization and aggregation of platelets in the liver after PHx, as elimination of fibrinogen in mice reduced intrahepatic platelet accumulation. Indeed, fibrin(ogen) is known to drive platelet aggregation directly by binding to αIIbβ3 integrin on platelets.16 Intrahepatic fibrin(ogen) deposition may thus directly stimulate regeneration by functioning as a matrix to localize and promote aggregation of platelets in the liver after PHx. Indeed, in a small study, we found that platelet accumulation after PHx was dramatically reduced in mice lacking integrin αIIb (CD41) on their platelets (supplemental Figure 1A-C, available on the Blood Web site). However, it is possible that intrahepatic fibrin(ogen) also promotes the recruitment and activation of other cell types essential for liver regeneration in addition to platelet recruitment. For example, fibrin(ogen) engagement of the integrin αMβ2 is a catalyst for leukocyte-driven repair of the liver after acetaminophen overdose.39 Leukocytes are recruited to the liver remnant in mice undergoing PHx, and liver regeneration is delayed in leukocytopenic mice.40 Thus, it is conceivable that fibrin(ogen)–leukocyte interactions promote regeneration after PHx, as in toxic liver injury.39 Alternatively, fibrin(ogen) may be required to promote leukocyte-directed platelet recruitment, or vice versa, after PHx. Although the interaction between platelets and leukocytes in the setting of PHx has not been studied extensively, in other models, platelets form a surface for leukocytes adhesion, and the interaction between these 2 cell types can facilitate liver repair.41
Major liver resections have become increasingly safe due to refinement of operative techniques. Nevertheless, impairment of liver regeneration occurs in a meaningful number of patients.4 This outcome is of specific interest, as there are currently no strategies available to stimulate liver regeneration. Importantly, the hemostatic system may offer biomarkers capable of predicting successful regeneration in patients and could provide novel putative therapeutic targets to recover regeneration after liver resection in humans. For example, some clinical evidence suggests that administration of platelet concentrates improves outcome after liver surgery in humans.10,11 However, increased risk for (portal vein) thrombosis,42 mortality,43 and cancer recurrence44 create a high risk/benefit ratio. In this context, an initial burst in VWF plasma levels was shown to be relevant for adequate platelet accumulation and liver regeneration in patients undergoing liver resection.31 Hence, infusion of 1-deamino-8-d-arginine vasopressin (DDAVP), a drug that stimulates VWF secretion, or VWF supplementation seem attractive alternatives to platelet concentrates. However, DDAVP will most likely only be effective in patients with relatively low baseline VWF levels,31,45 and VWF supplementation might increase the risk for thrombosis.46-48 Our experimental and clinical data show that intrahepatic fibrin(ogen) deposition directly after liver regeneration needs to be achieved to support liver regeneration. Supplementation with fibrinogen concentrate before or during the resection procedure might therefore be a novel clinical approach to target failing regenerative responses with a much better risk/benefit ratio than platelet or VWF supplementation. The thrombogenic potential of fibrinogen concentrates seems to be low,49 and fibrinogen supplementation has been shown to be safe in the context of liver transplantation.50 Based on both our experimental and clinical data, additional studies exploring the pro-regenerative effects of fibrinogen in experimental settings are warranted, as these studies could lead to novel strategies to improve liver regeneration.
In conclusion, we identified rapid intrahepatic fibrin(ogen) deposition as a hepatic TF-driven process in the liver remnant after PHx in mice. This study is the first to definitively identify fibrin(ogen) deposition as a direct stimulus for liver regeneration after PHx. In addition, our data underline the relevance of this process for human postoperative liver regeneration. The results suggest that coagulation- and platelet-stimulated liver regeneration pathways are closely connected, and a failure of either of these pathways in the early phase after liver resection might reduce the capacity of the remnant liver to regenerate. Hence, therapies assuring normal hemostatic function during the earliest phase of regeneration after liver resection might offer a new opportunity to improve liver regeneration. More research on this topic, as well as clinical examination, needs to be performed to validate this hypothesis.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors gratefully acknowledge the excellent technical intravital microscopy assistance and provision of the CD41−/− mice from C. N. Jenne from the Department of Microbiology, Immunology & Infectious Diseases (University of Calgary, Calgary, AB, Canada).
This work was supported in part by a grant from The Netherlands Organisation for Scientific Research to T.L. (VIDI, 917.11.304), a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases to J.P.L. (R01 DK105099), an EHA Research Grant from the European Hematology Association to D.G., and the Michigan State University College of Veterinary Medicine Visiting Scholars Program.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors designed and conducted experiments; D.G., D.P., P.S., T.L., and J.P.L. analyzed the data; and D.G., D.P., A.K.K., P.S., T.L., and J.P.L. drafted initial sections of the manuscript. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.G. is Michigan State University, East Lansing, MI.
Correspondence: Ton Lisman, Surgical Research Laboratory, University Medical Center Groningen, BA44, Hanzeplein 1, 9713 GZ, Groningen, The Netherlands; e-mail: j.a.lisman@umcg.nl; James P. Luyendyk, Pathobiology & Diagnostic Investigation, Michigan State University, 253 Food Safety & Toxicology Building, 1129 Farm Ln, East Lansing, MI 48824; e-mail: luyendyk@msu.edu; and Patrick Starlinger, Department of Surgery, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; e-mail: patrick.starlinger@meduniwien.ac.at.
REFERENCES
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 suppl 1):S45-S53. [DOI] [PubMed] [Google Scholar]
- 2.Lisman T, Porte RJ. Mechanisms of platelet-mediated liver regeneration. Blood. 2016;128(5):625-629. [DOI] [PubMed] [Google Scholar]
- 3.Rahnemai-Azar AA, Cloyd JM, Weber SM, et al. Update on liver failure following hepatic resection: strategies for prediction and avoidance of post-operative liver insufficiency. J Clin Transl Hepatol. 2018;6(1):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendizabal M, Silva MO. Liver transplantation in acute liver failure: a challenging scenario. World J Gastroenterol. 2016;22(4):1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31(4):808-816. [DOI] [PubMed] [Google Scholar]
- 6.Starlinger P, Haegele S, Offensperger F, et al. The profile of platelet α-granule released molecules affects postoperative liver regeneration. Hepatology. 2016;63(5):1675-1688. [DOI] [PubMed] [Google Scholar]
- 7.Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312(5770):104-107. [DOI] [PubMed] [Google Scholar]
- 8.Shimabukuro R, Kawanaka H, Tomikawa M, et al. Effect of thrombopoietin on platelet counts and liver regeneration after partial hepatectomy in a rat model. Surg Today. 2009;39(12):1054-1059. [DOI] [PubMed] [Google Scholar]
- 9.Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248(5):821-828. [DOI] [PubMed] [Google Scholar]
- 10.Han S, Park HW, Song JH, et al. Association between intraoperative platelet transfusion and early graft regeneration in living donor liver transplantation. Ann Surg. 2016;264(6):1065-1072. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama T, Murata S, Takahashi K, et al. Platelet transfusion improves liver function in patients with chronic liver disease and cirrhosis. Tohoku J Exp Med. 2013;229(3):213-220. [DOI] [PubMed] [Google Scholar]
- 12.Alkozai EM, Nijsten MW, de Jong KP, et al. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251(2):300-306. [DOI] [PubMed] [Google Scholar]
- 13.Margonis GA, Amini N, Buettner S, et al. Impact of early postoperative platelet count on volumetric liver gain and perioperative outcomes after major liver resection. Br J Surg. 2016;103(7):899-907. [DOI] [PubMed] [Google Scholar]
- 14.Starlinger P, Assinger A, Haegele S, et al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60(1):257-266. [DOI] [PubMed] [Google Scholar]
- 15.Lisman T, Luyendyk JP. Platelets as modulators of liver diseases. Semin Thromb Hemost. 2018;44(2):114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett JS. Platelet-fibrinogen interactions. Ann N Y Acad Sci. 2001;936(1):340-354. [DOI] [PubMed] [Google Scholar]
- 17.Lisman T, Weeterings C, de Groot PG. Platelet aggregation: involvement of thrombin and fibrin(ogen). Front Biosci. 2005;10(1-3):2504-2517. [DOI] [PubMed] [Google Scholar]
- 18.Beier JI, Guo L, Ritzenthaler JD, Joshi-Barve S, Roman J, Arteel GE. Fibrin-mediated integrin signaling plays a critical role in hepatic regeneration after partial hepatectomy in mice. Ann Hepatol. 2016;15(5):762-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan BP, Kopec AK, Joshi N, et al. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood. 2013;121(10):1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi N, Kopec AK, O’Brien KM, et al. Coagulation-driven platelet activation reduces cholestatic liver injury and fibrosis in mice. J Thromb Haemost. 2015;13(1):57-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell C, Willenbring H. Addendum: a reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2014;9(6):1532. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3(7):1167-1170. [DOI] [PubMed] [Google Scholar]
- 23.Winter WE, Flax SD, Harris NS. Coagulation testing in the core laboratory. Lab Med. 2017;48(4):295-313. [DOI] [PubMed] [Google Scholar]
- 24.Clauss A. Rapid physiological coagulation method in determination of fibrinogen [in German]. Acta Haematol. 1957;17(4):237-246. [DOI] [PubMed] [Google Scholar]
- 25.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713-724. [DOI] [PubMed] [Google Scholar]
- 26.Schiergens TS, Dörsch M, Mittermeier L, et al. Thirty-day mortality leads to underestimation of postoperative death after liver resection: a novel method to define the acute postoperative period. Surgery. 2015;158(6):1530-1537. [DOI] [PubMed] [Google Scholar]
- 27.Kirschbaum M, Jenne CN, Veldhuis ZJ, et al. Transient von Willebrand factor-mediated platelet influx stimulates liver regeneration after partial hepatectomy in mice. Liver Int. 2017;37(11):1731-1737. [DOI] [PubMed] [Google Scholar]
- 28.Hugenholtz GC, Meijers JC, Adelmeijer J, Porte RJ, Lisman T. TAFI deficiency promotes liver damage in murine models of liver failure through defective down-regulation of hepatic inflammation. Thromb Haemost. 2013;109(5):948-955. [DOI] [PubMed] [Google Scholar]
- 29.Jenne CN, Wong CH, Petri B, Kubes P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS One. 2011;6(9):e25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rautou PE, Tatsumi K, Antoniak S, et al. Hepatocyte tissue factor contributes to the hypercoagulable state in a mouse model of chronic liver injury. J Hepatol. 2016;64(1):53-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starlinger P, Pereyra D, Haegele S, et al. Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology. 2018;67(4):1516-1530. [DOI] [PubMed] [Google Scholar]
- 32.Rao LV, Kothari H, Pendurthi UR. Tissue factor: mechanisms of decryption. Front Biosci (Elite Ed). 2012;4(4):1513-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez M, Kopec AK, Joshi N, et al. Fas-induced apoptosis increases hepatocyte tissue factor procoagulant activity in vitro and in vivo. Toxicol Sci. 2014;141(2):453-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weerasinghe SV, Moons DS, Altshuler PJ, Shah YM, Omary MB. Fibrinogen-γ proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage. Hepatology. 2011;53(4):1323-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopec AK, Spada AP, Contreras PC, Mackman N, Luyendyk JP. Caspase inhibition reduces hepatic tissue factor-driven coagulation in vitro and in vivo. Toxicol Sci. 2018;162(2):396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong F, Nguyen VA, Shen X, Kunos G, Gao B. Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochem Biophys Res Commun. 2000;279(3):974-979. [DOI] [PubMed] [Google Scholar]
- 37.Lin MC, Almus-Jacobs F, Chen HH, et al. Shear stress induction of the tissue factor gene. J Clin Invest. 1997;99(4):737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houston P, Dickson MC, Ludbrook V, et al. Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. Arterioscler Thromb Vasc Biol. 1999;19(2):281-289. [DOI] [PubMed] [Google Scholar]
- 39.Kopec AK, Joshi N, Cline-Fedewa H, et al. Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte αMβ2 integrin-dependent upregulation of Mmp12. J Hepatol. 2017;66(4):787-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124(3):692-700. [DOI] [PubMed] [Google Scholar]
- 41.Slaba I, Wang J, Kolaczkowska E, McDonald B, Lee WY, Kubes P. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology. 2015;62(5):1593-1605. [DOI] [PubMed] [Google Scholar]
- 42.Afdhal NH, Giannini EG, Tayyab G, et al. ; ELEVATE Study Group. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367(8):716-724. [DOI] [PubMed] [Google Scholar]
- 43.de Boer MT, Christensen MC, Asmussen M, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106(1):32-44. [DOI] [PubMed] [Google Scholar]
- 44.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109(32):E2165-E2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arshad F, Stoof SC, Leebeek FW, et al. Infusion of DDAVP does not improve primary hemostasis in patients with cirrhosis. Liver Int. 2015;35(7):1809-1815. [DOI] [PubMed] [Google Scholar]
- 46.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonneveld MA, de Maat MP, Leebeek FW. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis [published correction appears in Blood Rev. 2014;28(6):281-282]. Blood Rev. 2014;28(4):167-178. [DOI] [PubMed] [Google Scholar]
- 48.Groeneveld DJ, Alkozai EM, Adelmeijer J, Porte RJ, Lisman T. Balance between von Willebrand factor and ADAMTS13 following major partial hepatectomy. Br J Surg. 2016;103(6):735-743. [DOI] [PubMed] [Google Scholar]
- 49.Dickneite G, Pragst I, Joch C, Bergman GE. Animal model and clinical evidence indicating low thrombogenic potential of fibrinogen concentrate (Haemocomplettan P). Blood Coagul Fibrinolysis. 2009;20(7):535-540. [DOI] [PubMed] [Google Scholar]
- 50.Donohue CI, Mallett SV. Reducing transfusion requirements in liver transplantation. World J Transplant. 2015;5(4):165-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.