Abstract
Key points
Studies of urothelial cells, bladder sheets or lumens of filled bladders have suggested that mediators released from urothelium into suburothelium (SubU)/lamina propria (LP) activate mechanisms controlling detrusor excitability. None of these approaches, however, has enabled direct assessment of availability of mediators at SubU/LP during filling.
We developed an ex vivo mouse bladder preparation with intact urothelium and SubU/LP but no detrusor, which allows direct access to the SubU/LP surface of urothelium during filling.
Pressure–volume measurements during filling demonstrated that bladder compliance is governed primarily by the urothelium.
Measurements of purine mediators in this preparation demonstrated asymmetrical availability of purines in lumen and SubU/LP, suggesting that interpretations based solely on intraluminal measurements of mediators may be inaccurate.
The preparations are suitable for assessments of release, degradation and transport of mediators in SubU/LP during bladder filling, and are superior to experimental approaches previously used for urothelium research.
Abstract
The purpose of this study was to develop a decentralized (ex vivo) detrusor smooth muscle (DSM)‐denuded mouse bladder preparation, a novel model that enables studies on availability of urothelium‐derived mediators at the luminal and anti‐luminal aspects of the urothelium during filling. Urinary bladders were excised from C57BL6/J mice and the DSM was removed by fine‐scissor dissection without touching the mucosa. Morphology and cell composition of the preparation wall, pressure–volume relationships during filling, and fluorescent dye permeability of control, protamine sulfate‐ and lipopolysaccharide‐treated denuded bladders were characterized. The preparation wall contained intact urothelium and suburothelium (SubU)/lamina propria (LP) and lacked the DSM and the serosa. The utility of the model for physiological research was validated by measuring release, metabolism and transport of purine mediators at SubU/LP and in bladder lumen during filling. We determined asymmetrical availability of purines (e.g. ATP, ADP, AMP and adenosine) in lumen and at SubU/LP during filling, suggesting differential mechanisms of release, degradation and bilateral transurothelial transport of purines during filling. Some observations were validated in DSM‐denuded bladder of the cynomolgus monkey (Macaca fascicularis). The novel model was superior to current models utilized to study properties of the urothelium (e.g. cultured urothelial cells, bladder mucosa sheets mounted in Ussing chambers or isolated bladder strips in organ baths) in that it enabled direct access to the vicinity of SubU/LP during authentic bladder filling. The model is particularly suitable for understanding local mechanisms of urothelium–DSM connectivity and for broad understanding of the role of urothelium in regulating continence and voiding.
Keywords: bladder model, urothelium, purines, transurothelial, lamina propria
Key points
Studies of urothelial cells, bladder sheets or lumens of filled bladders have suggested that mediators released from urothelium into suburothelium (SubU)/lamina propria (LP) activate mechanisms controlling detrusor excitability. None of these approaches, however, has enabled direct assessment of availability of mediators at SubU/LP during filling.
We developed an ex vivo mouse bladder preparation with intact urothelium and SubU/LP but no detrusor, which allows direct access to the SubU/LP surface of urothelium during filling.
Pressure–volume measurements during filling demonstrated that bladder compliance is governed primarily by the urothelium.
Measurements of purine mediators in this preparation demonstrated asymmetrical availability of purines in lumen and SubU/LP, suggesting that interpretations based solely on intraluminal measurements of mediators may be inaccurate.
The preparations are suitable for assessments of release, degradation and transport of mediators in SubU/LP during bladder filling, and are superior to experimental approaches previously used for urothelium research.
Introduction
Extensive research for the past two decades has advanced the concept that the bladder urothelium contributes significantly to the overall regulation of bladder activity by releasing biologically active mediators affecting cells in the suburothelium (SubU)/lamina propria (LP) and possibly the detrusor smooth muscle (DSM) (Apodaca et al. 2007; Fry & Vahabi, 2016; Merrill et al. 2016). Such inferences are largely based on studies that have demonstrated release of mediators from: (i) mucosal sheets mounted in Ussing chambers that are exposed to changes in hydrostatic pressure (Ferguson et al. 1997; Wang et al. 2005), (ii) cultured urothelial cells in response to stretch (Mochizuki et al. 2009; Miyamoto et al. 2014), hypotonicity‐induced cell swelling (Miyamoto et al. 2014), or drag forces (McLatchie & Fry, 2015), (iii) isolated bladder wall strips in response to receptor or nerve activation (Birder et al. 1997, 1998, 2001, 2002; Yoshida et al. 2008), and (iv) the luminal aspect of intact bladders during infusion of fluids (Collins et al. 2013; Daly et al. 2014; Beckel et al. 2015; Durnin et al. 2016; Gonzalez et al. 2016). Release of mediators into the SubU/LP has also been assumed after detecting expression of genes or proteins (e.g. enzymes, receptors and ion channels) that are involved in biosynthesis, metabolism and action of chemical mediators released from urothelial cells or measured in cells of SubU/LP or cultured urothelial cells (De Ridder et al. 1999; Persson et al. 1999; Birder et al. 2002; Tempest et al. 2004; Studeny et al. 2005). This evidence is indirect, but it has been used to develop the hypothesis that mediators released from urothelium during bladder filling regulate the micturition cycle by activation of cells, such as afferent neurons, in SubU/LP. This is an important concept suggesting a key role for the urothelium as a sensory organ that initiates reflex regulation of micturition and continence.
The physiological significance of mediators released at the luminal surface of the urothelium is unclear with regard to sensory functions and regulation of micturition, as the urothelium, through tight junctions between umbrella cells at the luminal surface, presents a highly impermeable barrier to water‐soluble molecules (Pandita & Andersson, 2002; Nishiguchi et al. 2005; Khandelwal et al. 2009). None of the current approaches to evaluate release, availability and metabolism of biologically active mediators has been capable of measuring these substances in the SubU/LP compartment directly during bladder filling. Developing an experimental preparation with this capability would be an important advance because it would allow assessment of the amounts and dynamics of excitatory and inhibitory mediators in the SubM/LP at different stages of bladder filling, and provide novel insights into what mediators might contribute to bladder regulation during storage of urine and voiding. Unknowns about the sources, levels and polarity of mediator release make it important to develop new techniques or tissue preparations that allow direct evaluation of mediators and their metabolism that occur in the SubU/LP at different stages of bladder filling.
Mice are used increasingly for studies of bladder function because of the advantages of applying genetic approaches to understanding the role of specific genes, proteins and cell types. The current study sought to develop a murine ex vivo bladder preparation with the detrusor muscle removed that would enable evaluation of the release, formation, degradation and transurothelial transport of signalling molecules that appear at the basolateral aspect of the urothelium during bladder filling. Proof‐of‐principle studies were first performed to demonstrate that this approach can provide direct access to substances appearing in the SubU/LP at a range of intraluminal (IL) volumes and pressures that approximate bladder filling. We validated the functionality of the model by demonstrating the ability to measure release and degradation of purine mediators at the basolateral aspect of the urothelium and transurothelial transport of mediators during bladder filling. Finally, we also showed that this approach can be used for studies of bladders from larger animals, such as the cynomolgus monkey (Macaca fascicularis, a primate sharing 93% sequence homology with humans; Gibbs et al. 2007) providing the possibility of translating knowledge from studies of murine bladders to primate tissues.
Part of the material included in the paper was presented at the annual meeting of the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) in Austin, TX, USA (26 February–3 March 2018).
Methods
Animals
Animals were maintained and experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Use and Care Committee at the University of Nevada.
C57BL/6J and Pdgfrα tm11(EGFP)Sor/J (PDGFRαEGFP) heterozygote male and female mice (8–14 weeks post partum) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were euthanized by sedation with isoflurane (AErrane; Baxter, Deerfield, IL, USA) followed by cervical dislocation and exsanguination. After midline laparotomy, the bladder with both ureters attached was removed and placed in oxygenated cold (10°C) Krebs–bicarbonate solution (KBS) with the following composition (mM): 118.5 NaCl, 4.2 KCl, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11.0 dextrose and 1.8 CaCl2 (pH 7.4).
Urinary bladders of cynomolgus monkeys (Macaca fascicularis) were obtained from Charles River Preclinical Services (Reno, NV, USA). Monkeys, sedated with ketamine (10 mg kg−1) and 0.7 ml Beuthanasia‐D (Schering‐Plough AH, Kenilworth, NJ, USA) were exsanguinated (Charles River Laboratories) for reasons unrelated to this project. All experimental procedures with murine and monkey tissues were approved by the University of Nevada Institutional Animal Use and Care Committee.
Ex vivo bladder preparation with intact and removed detrusor smooth muscle
The isolated bladder was pinned through serosa to a Sylgard‐covered dissecting dish filled with cold oxygenated KBS. The intact bladder preparation was prepared by cleaning fat and connective tissue around the bladder and ureters. The denuded preparation was prepared by gently pulling portions of the serosa with the muscle attached and cutting with fine surgical scissors along the submucosal surface of the muscle layer without touching the urothelium. Denuded (i.e. DSM‐free) or intact (i.e. all layers preserved) bladder preparations with ligated ureters were catheterized via the urethra with PE‐20 tubing heat flared at one end and secured with double 6‐0 silk and 6‐0 nylon sutures. Depending on the experimental protocol, bladder preparations were filled through the IL catheter either manually via a syringe (e.g. for histology and immunohistochemistry studies) or using a syringe pump (Kent Scientific, Torrington, CT, USA) to simulate bladder filling. At the end of the experiments, bladder preparations were cut longitudinally and pinned to the dissecting dish to measure the surface area of preparations, which was followed by measuring the wet weight of blotted tissues. These measurements were used to normalize data to surface area or tissue weight when needed.
Masson's trichrome staining
Mouse bladders were fixed in 4% paraformaldehyde overnight at 4°C and then sliced into cross‐sections using a razor blade. Tissues were dehydrated with increasing concentrations of ethanol, cleared with xylene and embedded in paraffin wax before cutting into 10 μm‐thick sections. Sections were stained with Masson's trichrome according to the manufacturer's instructions (American MasterTech, Lodi, CA, USA). Briefly, paraffin wax was removed with xylene and sections were rehydrated with ethanol. Slides were immersed in Bouin's fluid at room temperature overnight, then rinsed with water. Slides were placed in Weigert's haematoxylin solution for 5 min and washed before being immersed in Biebrich scarlet‐acid fuchsin for 15 min. Preparations were then rinsed in water and placed in phosphomolybdic/phosphotungstic acid for 15 min followed by aniline blue stain for 10 min and rinsed with water. Slides were then placed in 1% acetic acid for 5 min and dehydrated by rinsing with 100% ethanol three times followed by xylene and then covered with a coverslip. Digital images of stained sections were captured using a Hamamatsu C5810 CCD camera and a Zeiss Axiovert microscope using ×10 or ×40 objective.
Haematoxylin and eosin staining
Denuded bladder preparations were fixed in 4% phosphate‐buffered paraformaldehyde overnight at room temperature, dehydrated in serial ethanol concentrations, cleared in xylene and embedded in paraffin wax. The paraffin wax blocks containing the tissues were then sliced into 5 μm‐thick sections, followed by staining with haematoxylin solution for 5 min and eosin solution for 10 s. The histological structure of bladder preparations were examined and recorded under a light microscope (Nikon Corp., Tokyo, Japan).
Biotin permeability assay
EZ‐link sulfo‐NHS‐biotin (Thermo Fisher Scientific, Waltham, MA, USA) dissolved in phosphate buffer solution to a final concentration of 1 mg ml−1 was infused in the lumen of murine denuded bladder preparations. Following incubation for 30 min, the tissue was fixed with 4% paraformaldehyde for 30 min and embedded in optimal cutting temperature (OCT) compound. Cryostat sections were labelled with Alexa‐Fluor‐594–streptavidin. Images were captured using confocal microscopy as described in ‘Immunohistochemistry’.
Immunohistochemistry
Denuded bladders from PDGFRαEGFP mice were fixed in 4% paraformaldehyde overnight at 4°C. The whole bladder was imaged using an Olympus BX50 fluorescence microscope and an Andor i Xon DU‐897D EMCCD camera. Bright cell nuclei of platelet‐derived growth factor receptor α (PDGFRα+) cells indicated the presence of suburothelium. Tissues were then dehydrated in graded sucrose solutions, embedded in Tissue Tek OTC compound (Sakura Finetek, Torrance, CA, USA) and frozen in liquid nitrogen. Cross sections were cut at a thickness of 7–14 μm using a Leica CM 3050 cryostat (Leica Microsystems, Wetzlar, Germany). Enhanced green fluorescent protein (eGFP) distribution was examined using a Keyence BZ‐X700 fluorescence microscope (Keyence, Itasca, IL, USA). Rabbit polyclonal anti‐aquaporin 3 antibody (Sigma‐Aldrich, St Louis, MO, USA, cat. no. AB3276) was used at 1:500 dilution for immunohistochemistry in cryostat sections of denuded bladder wall.
Ex vivo bladder pressure–volume relationships during filling
Isolated mouse bladders (intact and denuded) were catheterized via the urethra as described in ‘Ex vivo bladder preparation with intact and removed detrusor smooth muscle’. Bladders were placed in an organ bath and perfused continuously with oxygenated KBS (37°C). The other end of the tubing was connected via a three‐way stopcock to a syringe pump (Kent Scientific) and to a pressure transducer (ADInstruments, Colorado Springs, CO, USA). KBS was infused into the bladder at a rate of 15 μl min−1 and bladder pressure was recorded using a PowerLab data acquisition system (ADInstruments). The infusion was stopped when pressure reached 25 mmHg (maximum voiding pressure in mice; Smith & Kuchel, 2010), and the bladder was emptied by disconnecting the tubing from the syringe pump. At least four reproducible pressure–volume curves were recorded at each infusion rate per bladder preparation.
Fluorescent dye fluxes
The murine denuded bladder preparation was placed in a 3 ml water‐jacketed chamber filled with oxygenated KBS (pH 7.4, 37°C). Fluorescein (sodium salt) (Flc), fluorescein isothiocyanate–dextran (FITC‐dextran) 4000 (FD‐4) or FITC–dextran 40,000 (FD‐40), each dissolved in KBS at a final concentration of 100 μM, were infused in the bladder lumen at 15 μl min−1. Aliquots of extraluminal (EL) solution were collected at time zero and three time intervals equal to the time necessary to achieve a low‐pressure filling volume (i.e. 3.3 min for 50 μl filling volume) or a high‐pressure filling volume (e.g. 10 min for 150 μl filling volume). In some experiments, protamine sulfate (PS) dissolved in KBS at 10 mg ml−1 or lipopolysaccharide (LPS) at 1 mg ml−1 in KBS were instilled in the bladder lumen for 60 min prior to carrying out FD‐4 flux measurements (Greenwood‐Van Meerveld et al. 2015). Fluorescence signals were measured in a 96‐well GloMax microplate reader (Promega, Madison, WI, USA) at 490 nm excitation wavelength and 515 nm emission wavelength. Standard curves were prepared using defined dilutions of fluorescent dyes in KBS, and linear regression was used to determine the amount of fluorescein or FD‐4 in the samples. Fluorescent dye fluxes were expressed in picomoles per unit time and tissue area (pmol mm−2 min−1).
Release of purines from the basolateral and apical sides of urothelium during filling
Denuded bladder preparations were placed in 2 ml water‐jacketed chambers superfused with oxygenated KBS. After 45 min equilibration, bladders were filled with KBS via an infusion pump (15 μl min−1) with 150–300 μl to reach 25 mmHg IL pressure. Samples from the EL and IL fluids were collected in ice‐cold Eppendorf tubes at end of filling. Monkey denuded bladders were catheterized via the urethra with larger diameter (1.6 mm) tubing, placed in 50 ml organ baths and perfused with oxygenated KBS at 37°C. Bladders were filled with 50 ml KBS via an infusion pump (600 ml h−1) and the EL and IL fluids collected in ice‐cold tubes. A schematic diagram of the isolated whole‐bladder model used to collect EL and IL samples for analysis of urothelially released purines is shown in Fig. 6 A.
Figure 6.
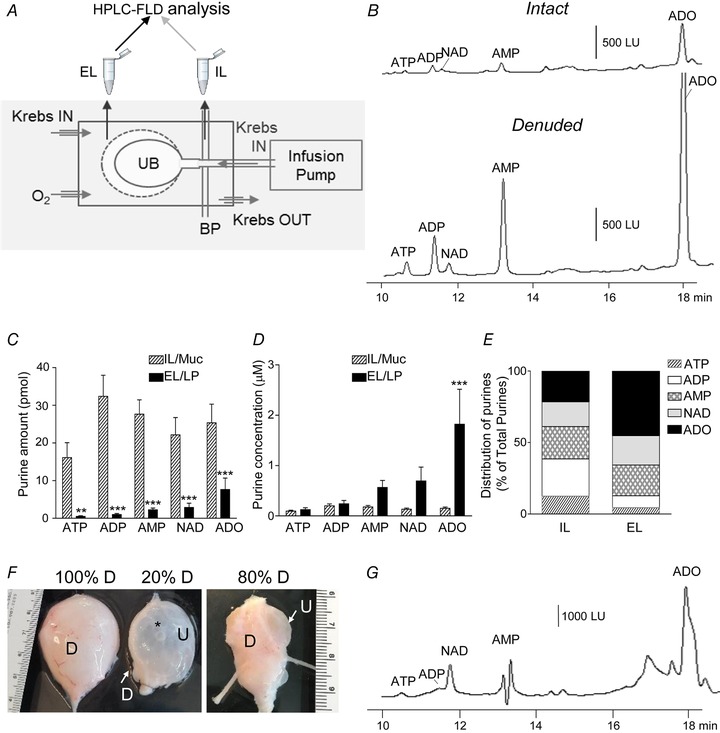
The murine and primate denuded bladder models are suitable for assessing availability of endogenous mediators (e.g. purines) at subU/LP during bladder filling
A, schematic diagram of the isolated bladder model utilized to evaluate availability of urothelially released purines at the basolateral and intraluminal (IL) aspects of the urothelium during bladder filling. Bladder preparations were placed in water‐jacketed chambers and superfused with oxygenated Krebs bicarbonate solution (KBS). KBS was infused in the bladder lumen via a catheter in the urethra connected to an infusion pump. Bladder pressure (BP) was monitored via a three‐way connector through the infusion line during bladder filling. Aliquots from extraluminal (EL, organ bath) and IL solutions were collected and processed for detection of purines by HPLC‐FLD (described in Methods). UB, urinary bladder. B, original chromatograms of EL samples collected at the end of filling with 200 μl KBS at 15 μl min−1 of murine intact and denuded bladder preparations showing that greater purine amounts were detected at the basolateral aspect of the denuded bladder preparation. Extraluminal samples were diluted 10‐fold to obtain chromatography signals within scale. LU, luminescence units. C, amounts (pmol) of purines (i.e. ATP, ADP, NAD, AMP and ADO) available at the basolateral (EL) and apical (IL) sides of urothelium at end of bladder filling to voiding pressure. Asterisks denote significant differences from IL/Muc: ** P < 0.01, *** P < 0.001, n = 10 bladder preparations, multiple t tests, paired; statistical significance determined using the Holm–Sidak method, with α = 5.000%). D, concentration (μM) of multiple purines that are available in the bladder lumen and in a 50 μm‐thick layer at the SubU/LP surface (EL) at end of denuded bladder filling. *** P < 0.001, n = 10 bladder preparations, multiple t tests, paired; statistical significance determined using the Holm–Sidak method, with α = 5.000%). E, distribution of ATP, ADP, AMP, NAD and ADO within the purine pool (Total Purines) available at SubU/LP and in lumen at the end of bladder filling; mean values of each groups are plotted. F, photographs of cynomolgus monkey bladder preparations with different degrees of SubU/LP exposure. Asterisk marks an air bubble that was introduced in the lumen to demonstrate the transparency of preparation. D, detrusor, U, urothelium. G, an original chromatogram of EL sample collected at end of filling of monkey denuded bladder with 50 ml KBS at 10 ml min−1; sample diluted 10‐fold to obtain chromatography signals in scale.
1,N 6‐Etheno‐derivatization of endogenous and exogenous purines
Samples from mouse and monkey bladders that were collected in the absence or presence of bladder filling were acidified to pH 4.0 with citric phosphate buffer and subjected to 1,N 6‐etheno‐derivatization at 80°C for 40 min for HPLC analysis (Levitt et al. 1984; Bobalova et al. 2002; Mutafova‐Yambolieva et al. 2007; Hwang et al. 2011). Detection sensitivity of 1,N 6‐etheno‐derivatized purines exceeds significantly the detection sensitivity of non‐derivatized purines (Bobalova et al. 2002). ε‐Purine standards and ε‐ATP and ε‐ADO substrates were prepared following the same procedure as described for experimental samples.
HPLC analysis of 1,N 6‐etheno‐derivatized purines
A reverse‐phase gradient Agilent Technologies 1200 liquid chromatography system equipped with a fluorescence detector (Agilent Technologies, Wilmington, DE, USA) was used to detect the 1,N 6‐etheno‐derivatized nucleotides as described previously (Mutafova‐Yambolieva et al. 2007; Hwang et al. 2011). The mobile phase consisted of 0.1 M KH2PO4 (pH 6.0) as eluent A. Eluent B consisted of 65% eluent A and 35% methanol. Gradient elution was employed according to the following linear programme: time 0, 0% eluent B; 18 min, 100% eluent B. The flow rate was 1 ml min−1 and run time was 20 min. Column and autosampler temperatures were maintained at 25°C and 4°C, respectively. The fluorescence detector was set to record 1,N 6‐etheno‐derivatized nucleotide and nucleoside signals at an excitation wavelength of 230 nm and emission wavelength of 420 nm (Bobalova et al. 2002). The amount of nucleotide/nucleoside in each sample was calculated from calibration curves of nucleotide standards run simultaneously with each set of unknown samples. Results, normalized for sample volume and tissue area, were expressed in picomoles per unit area (pmol mm−2).
Extraluminal and intraluminal degradation of purines during bladder filling
Denuded bladder preparations were placed in 3 ml chambers and bathed in oxygenated KBS.
To evaluate EL degradation of purines, εATP or εADO (each at 2 μM) was added to the external solution bathing the anti‐luminal side of the preparation. Aliquots of EL fluid were collected before adding the ε‐substrate and at 2 min intervals after starting bladder filling with KBS up to 16 min after starting the filling, at which time point the high‐pressure volume (HPV) has been reached. All EL samples were diluted 10‐ to 20‐fold in cold citric buffer (pH 4.0) to preserve purines from further degradation and to obtain chromatography signals in scale. The degradation of εATP was evaluated by measuring the decrease of εATP and the increase of the products εADP, εAMP and εADO by HPLC with fluorescence detection (HPLC‐FLD). The degradation of εADO was evaluated by the decrease of εADO at the different time intervals during bladder filling. At end of preparation filling with KBS in the presence of εATP or εADO in EL solution, the IL samples were collected and processed for purine measurements by HPLC‐FLD.
To evaluate IL degradation of ATP during bladder filling, the denuded bladder preparations were placed in 3 ml organ baths with oxygenated KBS (37°C) and filled intraluminally with εATP (2 μM) at 15 μl min−1 to voiding pressures. The IL samples were collected at the end of bladder filling. Aliquots of IL samples were diluted 20‐fold in citric phosphate buffer (pH 4.0) and processed for purine analysis by HPLC‐FLD. In addition to IL samples, aliquots of EL samples at end of filling were also processed for purine analysis and were compared with ε‐substrate in the absence of tissue.
Bilateral transurothelial transport of adenosine during bladder filling
To evaluate transport of adenosine from lumen to SubU/LP, murine denuded bladders were placed in water‐jacketed chambers and filled at 15 μl min−1 with εADO (2 μM) in oxygenated KBS (37°C). After reaching HPV, the infusion was stopped and aliquots of IL and EL samples were processed for εADO detection by HPLC‐FLD. To evaluate transport of adenosine from basolateral side to lumen, denuded bladders were placed in chambers to which εADO (2 μM) was added and the preparations were filled with KBS to voiding pressure. After reaching HPV, the infusion was stopped and EL and IL samples were processed for εADO detection. EL and IL samples were compared with εADO solution in the absence of tissues.
Reagents
Adenosine, ADP, AMP, ATP, eosin, fluorescein sodium, fluorescein isothiocyanate–dextran (FITC–dextran) 3,000‐5,000, FITC–dextran‐40,000, haematoxylin, β‐nicotinamide dinucleotide (NAD), lipopolysaccharides from Escherichia coli O111:B4 (LPS), protamine sulfate and streptavidin were purchased from Sigma‐Aldrich. EZ‐link sulfo‐NHS‐biotin was purchased from Thermo Fisher Scientific.
Data analysis
Data are presented as means ± SEM. Means were compared by a two‐tailed, paired or unpaired Student's t test or by one‐way ANOVA for comparison of more than two groups followed by a post hoc Tukey multiple comparison test (Prism, v. 6, GraphPad Software, Inc., San Diego, CA, USA). A probability value of less than 0.05 was considered statistically significant.
Results
The detrusor smooth muscle‐denuded bladder wall contains urothelium and suburothelium/lamina propria and lacks tunica muscularis and serosa
Murine ex vivo bladder preparations were cannulated through the urethra and filled with KBS (Fig. 1 A). The intact bladder contains the urothelium and detrusor smooth muscle layers whereas after removal of the detrusor, muscle‐denuded preparations contained only the urothelium and underling LP and a portion of the SubU. As demonstrated by haematoxylin–eosin and Masson's trichrome stainings, all layers of urothelium and suburothelium were preserved in the muscle‐denuded preparations (Fig. 1 B–D). Histological examination showed that cell layers at both aspects of the mucosa were intact, including the bladder uroepithelium and SubU, after removal of the detrusor. Further evidence that the integrity of the urothelium was preserved was shown by the following observations: (i) IL instillation of biotin resulted in restriction of biotin–streptavidin staining to the bladder epithelium with no penetration of biotin beyond the umbrella cell layer (Fig. 2 A–C), (ii) aquaporin 3 (AQP3)‐like immunoreactivity was expressed predominantly in basal and some intermediate cells of urothelium (Fig. 2 D) as previously described (Spector et al. 2002; Kim et al. 2012), and (iii) suburothelial PDGFRα+ cells were preserved in the wall of a denuded bladder of a PDGFRαEGFP mouse (Koh et al. 2012; Koh et al. 2018) (Fig. 2 E and F).
Figure 1.
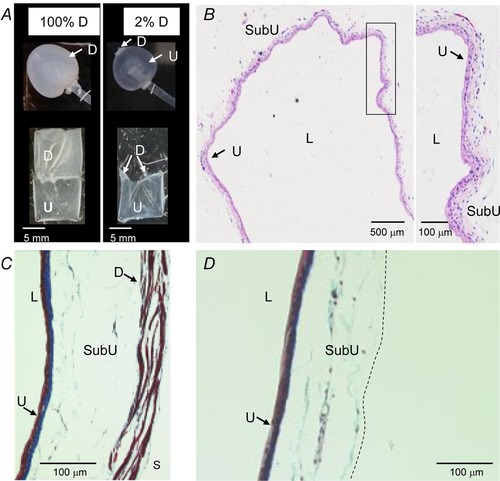
The wall of murine detrusor‐free bladder preparation is intact and contains all layers except the detrusor smooth muscle and the serosa
A, photographs of intact and denuded bladder preparations filled with 200 μl KBS (upper panels) and dissected open at end of studies (bottom panels). B, a light microscopic view of hematoxylin and eosin‐stained denuded bladder wall demonstrating intact layers of the urothelium and submucosa. The area designated with black rectangle is magnified in the right panel. C and D, Masson's trichrome staining of filled intact (left) and denuded (right) bladders. The intact preparation has urothelium, suburothelium, detrusor smooth muscle and serosa whereas the denuded preparation has intact urothelium and suburothelium. The dashed line in D illustrates the boundary of the denuded preparation. D, detrusor smooth muscle; L, lumen; SubU, suburothelium; U, urothelium.
Figure 2.
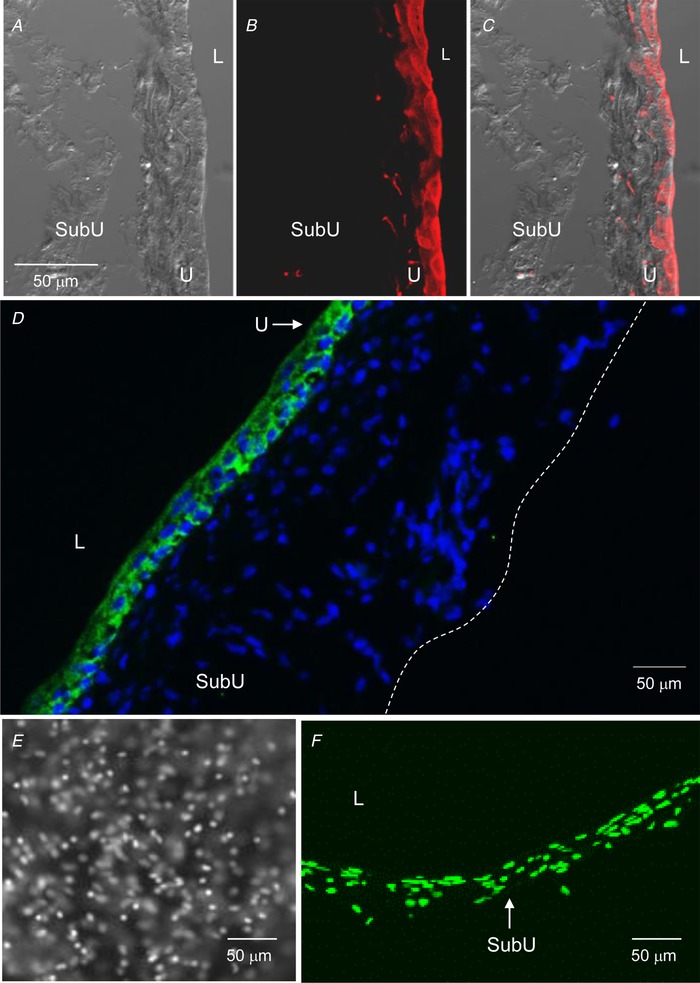
The murine detrusor‐free bladder preparation is intact and contains specific cell types that are characteristic for urothelium and suburothelium
A–C, confocal microscopy of cryostat sections of the wall of a denuded bladder that was filled with sulfo‐NHS‐biotin (1 mg ml−1 PBS) for 30 min, then fixed in 4% paraformaldehyde and labelled with Alexa Fluor 594‐streptavidin (red, B and C). A is a differential interference contrast image of the same section, revealing no fluorescence in intermediate and basal cells and suburothelium. C is a merged image of A and B. Biotin–streptavidin fluorescence is limited to epithelia. Scale bar in A applies to A–C. D, cryostat section of denuded bladder wall showing cell nuclei (4′,6‐diamidino‐2‐phenylindole, blue) throughout the width of the preparation wall and localization of AQP3‐immunoreactivity (green) that is limited to basal cells and some intermediate cells of urothelium. Dashed line represents the outer edge of preparation. E and F, front wall (left) and cross section (right) of filled denuded bladder preparation from a PDGFRαEGFP mouse containing bright nuclei of eGFP‐tagged PDGFRα+ cells localized in suburothelium. L, lumen; SubU, suburothelium; U, urothelium.
Filling of the detrusor‐denuded bladder approximates normal bladder filling
Pressure–volume recordings of ex vivo intact or denuded bladder preparations demonstrated that both preparations require a broad range of filling volumes (e.g. ∼150–600 μl) to reach the voiding pressure (Fig. 3 A). A more careful examination revealed that the ex vivo bladder preparations, either intact or muscle‐denuded, can be divided into two groups based on the volume that was accommodated to achieve voiding pressure: group 1 included bladders that accommodated <250 μl at a voiding pressure of ∼15–20 mmHg, whereas group 2 included bladder preparations that were able to accommodate >250 μl. No significant differences in the infused volumes necessary to reach voiding pressure in intact and denuded preparations were observed (Fig. 3 A). As shown in Fig. 3 B, D and F, the intact preparations commonly developed transient contractions (TCs) whereas these contractions were not recorded in denuded bladders. The pressure–volume curves were similar in intact and denuded bladders and demonstrated that both types of preparations were able to accommodate significant volumes with minimal change in IL pressure, after which the pressure rose steeply to voiding pressure when filling and recordings were terminated. Figure 4 and Videos 1 and 2 (see ‘Supporting information’) demonstrate similar patterns and rates of filling of intact and denuded bladder preparations.
Figure 3.
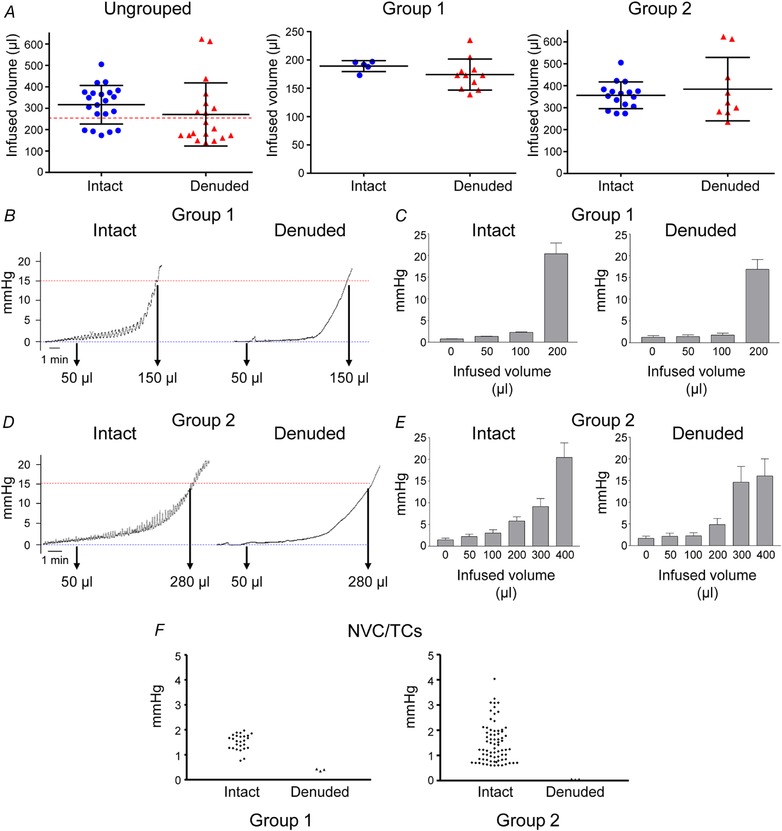
Filling pressures are similar in murine ex vivo intact and denuded bladder preparations
A, scatter plots of infused volumes required to achieve voiding pressure (e.g. 15 mmHg) in intact and denuded bladder preparation filled at 15 μl min−1. The left panel shows the ungrouped data, middle panel shows data in bladders that required <250 μl to reach 15 mmHg (Group 1), and the right panel shows data in preparations that were filled with >250 μl solution to reach voiding pressure (Group 2). Infused volumes at voiding pressures in intact and denuded bladders were similar in each group of preparations (P = 0.2522, 0.2670 and 0.4980, respectively), unpaired t test, two‐tailed. Pressure traces (B and D) and summarized data (C and E) from ex vivo bladder preparations that accommodated less (Group 1, n = 5–11) or more (Group 2, n = 6–16) of 250 μl at voiding pressures (15 μl min−1 filling rate). Unlike the denuded preparations, the intact bladders typically developed transient non‐voiding contractions (NVCs/TCs) (F). [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
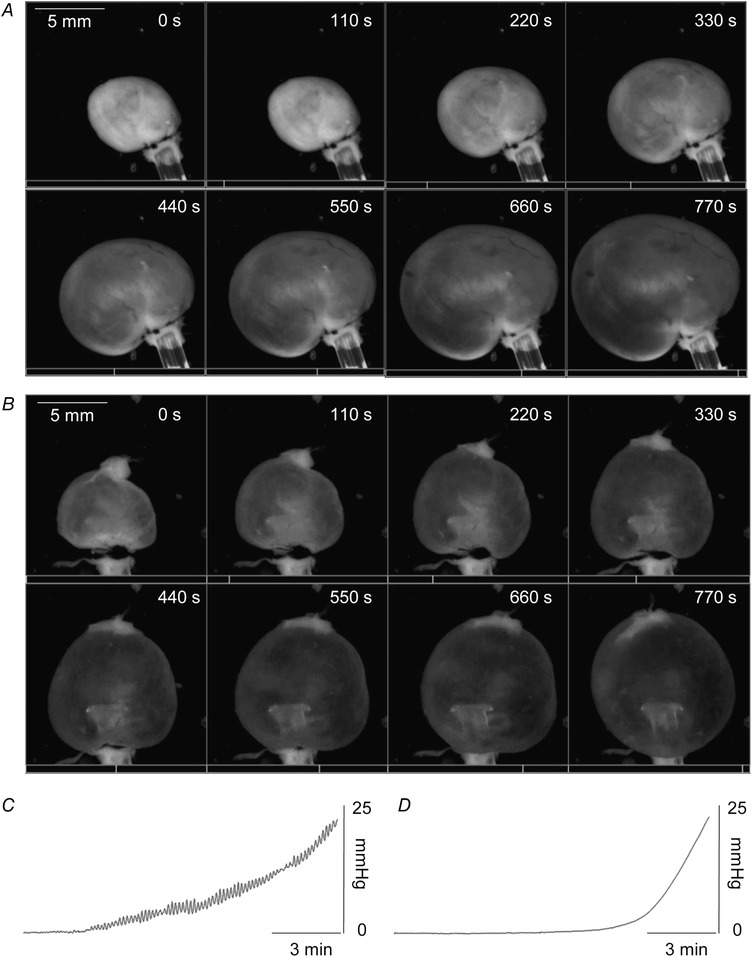
Filling of murine ex vivo bladder preparations
Time‐lapse montages showing intact (A) and denuded (B) bladder preparations during filling at 15 μl min−1. Scale bar applies to all images in a panel. Videos were recorded using a SMZ1000 zoom stereomicroscope (Nikon Instruments Inc., Melville, NY, USA) with a DMK31AF03 Monochrome Firewire 400 CCD camera (The Imaging Source, LLC, Charlotte, NC, USA) at 5 Hz using Astro IIDC software (Aupperle Services and Contracting, Calgary, Alberta, Canada), recordings were stopped upon reaching 25 mmHg. Still images were collected at 8 equal intervals between the beginning and end of the video recordings to create the still image panel. C and D, pressure recordings of the preparations shown in A and B (C, intact bladder; D, denuded bladder).
Permeability of the detrusor muscle‐denuded bladder wall for fluorescent dyes is increased after treatment with protamine sulfate or lipopolysaccharide
Intraluminal instillation of the fluorescent dyes fluorescein (FLC), FITC–dextran 4000 (FD‐4), and FITC–dextran 40000 (FD‐40) into detrusor‐denuded bladders at 15 μl min−1 resulted in the appearance of small quantities of the fluorescent dyes as reported previously (Carattino et al. 2013; Greenwood‐Van Meerveld et al. 2015). The rate of efflux of fluorescent dyes was inversely proportional to the molecular mass of the dye at both low‐pressure volume (LPV) and high‐pressure volume (HPV; Fig. 5 A). As expected, larger efflux was observed at larger filling volumes. The rate of efflux of fluorescent dyes evaluated at time intervals equal to filling time was relatively constant (e.g. Fig. 5 B, FD‐4 control). Intraluminal pretreatment of the denuded bladder preparation with either LPS or PS resulted in an increased efflux of FD‐4 immediately after the HPV was reached (Interval 1, Fig. 5 B). The rate of efflux of FD‐4 was constant in non‐treated bladders, but declined in the LPS‐ or PS‐treated bladders at later time intervals (Fig. 5 B).
Figure 5.
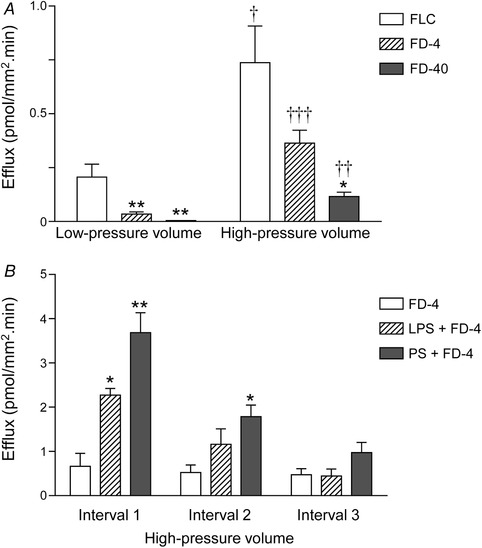
Rate of efflux of fluorescent dyes from bladder lumen during filling
A, efflux of fluorescein (FLC, mw 376), FITC–dextran 4000 (average mw 3000–5000, FD‐4) and FITC–dextran 40,000 (average mw 40,000, FD‐40) instilled in the lumen at 15 μl min−1 at low (i.e. 50 μl) or high (i.e. 150 μl) pressure filling volumes. Note that the rate of efflux is inversely proportional to the molecular weight of fluorescent dye and increases with increased duration of filling. Asterisks denote significant differences from FLC fluxes (* P < 0.05, ** P < 0.01; n = 3–9 bladder preparations, one‐way ANOVA with Tukey's multiple comparisons test). Crosses denote significant differences between fluorescent dye fluxes at high‐pressure volume vs. fluxes at low pressure volume († P < 0.05, †† P < 0.01, ††† P < 0.001; n = 3–9 bladder preparations, unpaired t test, two‐tailed). B, pretreatment of denuded bladder preparations with lipopolysaccharide (LPS, 1 mg ml−1 for 60 min) or protamine sulfate (PS, 10 mg ml−1 for 60 min) increased the efflux of FD‐4 at high‐pressure filling volume. Interval 1 = Interval 2 = Interval 3; each interval corresponds to the time required to fill the preparation to voiding pressure. Asterisks denote significant differences from FD‐4 efflux in untreated bladder preparations (* P < 0.05, ** P < 0.01; n = 3–4 bladder preparations, one‐way ANOVA with Tukey's multiple comparisons test). Note that the efflux of FD‐4 was significantly increased by LPS and PS in the first time interval, whereas the difference between fluxes was reduced or insignificant at later time intervals.
Filling of detrusor muscle‐denuded bladder preparation elicits release of purine mediators at the basolateral surface of urothelium
A schematic diagram of the experimental set‐up for collecting EL or IL samples during bladder filling is shown in Fig. 6 A. Negligible amounts of purines were observed in EL samples from intact bladder at the end of filling, but greater amounts of ATP, ADP, NAD, AMP and adenosine (ADO) were observed in EL samples from denuded bladder (Fig. 6 B). Purines measured in luminal samples exceeded the amounts of purines at SubU/LP (Fig. 6 C). However, the concentration of some purines at the vicinity of SubU/LP taken as ∼50 μm thick (Winder et al. 2014) appeared to be greater than the concentration of purines in the lumen (Fig. 6 D). Surprisingly, the concentrations of AMP, NAD and ADO at SubU/LP exceeded the concentrations of ATP and ADP at the end of filling (Fig. 6 D). Interestingly, the distribution of endogenous purines available in the lumen and at SubU/LP was different at the end of filling (Fig. 6 E). Similar to the murine denuded bladder, denuded bladder preparations from cynomolgus monkey (Fig. 6 F and G) also showed small amounts of ATP and ADP in the EL space and significant amounts of β‐NAD, AMP and ADO.
Bladder filling is associated with differential ATP degradation patterns at the luminal and anti‐luminal surfaces of urothelium
We next sought to determine if detrusor‐denuded bladders can be utilized to evaluate biotransformation of mediators at the SubU/LP surface of urothelium during filling. Therefore, we examined degradation εATP (2 μM) added to the EL solution bathing the bladder preparation after filling the preparation with KBS at 15 μl min−1. The substrate concentration was selected based on pilot studies showing that at this concentration V max reached a plateau. As shown in Fig. 7 Aa, Ab and Ca, bladder filling was associated with a decrease in εATP substrate and increase in εATP products, εADP, εAMP and εADO. Surprisingly, in the IL solution collected at the end of bladder filling no εATP, very small amounts of εADP, and greater amounts of εAMP and εADO were detected (Fig. 7 Ac and Cb). The distribution of individual purines as a percentage of the purine pool at the location where the εATP substrate was added (EL, Location 1) was significantly different from the distribution of purine at the opposite site of the urothelium (e.g. bladder lumen, Location 2). Thus, εATP was the dominant substance at Location 1, whereas εADO was the dominant substance at Location 2 (Fig. 7 Cc).
Figure 7.
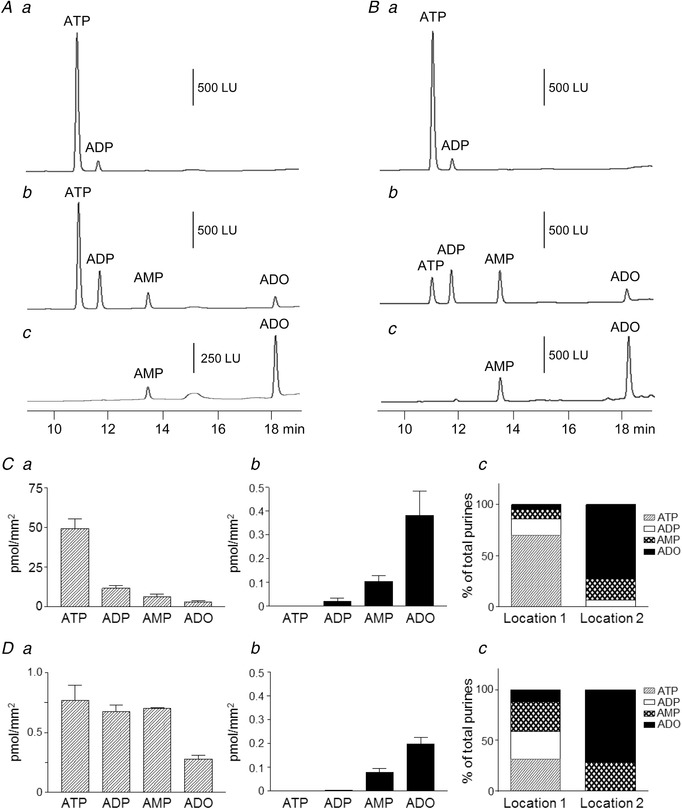
The denuded bladder model can be used to examine asymmetrical availability of mediators at SubU/LP and in lumen during bladder filling that is caused by unequal metabolism and transurothelial transport of mediators and metabolites
A, original chromatograms showing εATP added in bath before bladder filling (a), extraluminal (EL) sample collected at the end of bladder filling with KBS (15 μl min−1) to voiding pressure (b), and intraluminal (IL) sample collected at the end of bladder filling. B, original chromatograms showing εATP substrate before bladder filling (a), IL sample at the end of bladder filling with εATP in KBS (15 μl min−1) to voiding pressure (b), and EL sample at end of bladder filling. C, summed data showing EL (a) and IL (b) purines at end of bladder filling with KBS after adding εATP substrate to bath. c shows distribution of individual purines in total purine pool at end of filling with KBS. Location 1 is the site of εATP application (i.e. SubU/LP); Location 2 is the opposite site of preparation wall (i.e. preparation lumen). n = 4 bladder preparations. D, summed data showing IL (a) and EL (b) purines at end of bladder filling with εATP substrate to voiding pressure. c shows distribution of individual purines in total purine pool at end of filling. Location 1 is the site of εATP application (i.e. lumen); Location 2 is the opposite site of preparation wall (i.e. SubU/LP); n = 4.
When the εATP substrate (2 μM) was added to the bladder lumen, εATP was reduced and the εATP products εADP, εAMP and εADO were increased at end of filling, suggesting that εATP was degraded in the bladder lumen during filling (Fig. 7 Ba, Bb and Da). No εATP or εADP was observed in the EL solution, but εAMP and εADO were both detected in the EL solution at end of filling (Fig. 7 Bc and Db). The distribution of purines at Location 1 (bladder lumen) and Location 2 (SubU/LP) after infusion of εATP in the bladder lumen differed significantly at end of filling (Fig. 7 Dc). The results from these experiments suggest that (i) ATP is differentially metabolized on both sides of urothelium and (ii) transurothelial transport of purines might occur during bladder filling.
Bilateral transurothelial transport of adenosine occurs during bladder filling
We next evaluated whether εADO applied in the EL solution before bladder filling appears in the bladder lumen at the end of filling (ADO influx) to evaluate whether transurothelial transport of mediators can occur during filling. Conversely, we also evaluated whether εADO infused into the bladder lumen appears in the EL solution at the end of filling (ADO efflux). εADO efflux and εADO influx were observed during bladder filling (Fig. 8). Treatment of the bladder lumen with LPS for 1 h increased εADO efflux (Fig. 8 A and B) and increased εADO influx (Fig. 8 C and D) in the detrusor‐denuded bladder preparation.
Figure 8.
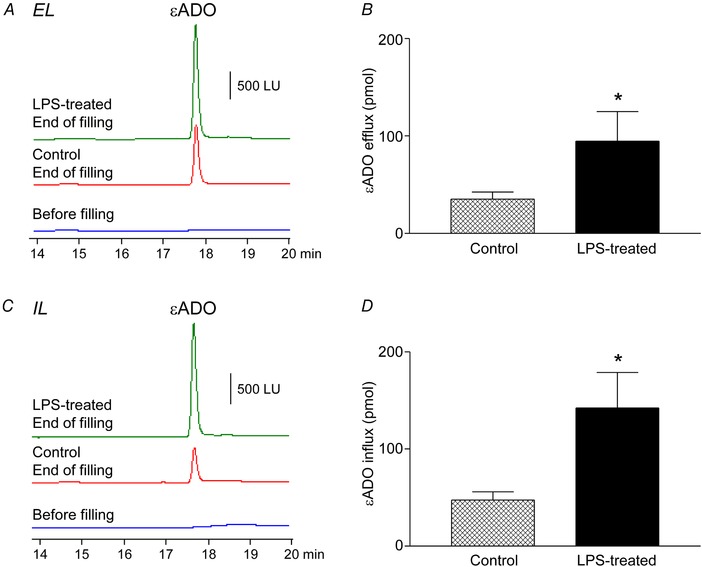
The detrusor muscle‐denuded bladder model can be used to determine bilateral transurothelial transport of mediators during bladder filling
A and B, εADO efflux. A, original chromatograms of εADO in extraluminal (EL) compartment before filling of murine denuded bladder preparation with εADO (2 μM) at 15 μl min−1 (before filling), after filling with εADO of untreated preparation (Control), and after filling with εADO of LPS‐treated bladder preparation (LPS‐treated). B, εADO amounts (pmol) detected in EL solution of untreated (Control) and LPS‐treated bladder preparations. * P < 0.05 vs. control, n = 4–6 bladder preparations, unpaired t test, two‐tailed. C and D, εADO influx. C, original chromatograms of εADO in intraluminal (IL) compartment after exposure of bladder preparation to εADO (2 μM) in the bath: before filling of murine denuded bladder preparation with KBS at 15 μl min−1, after filling of untreated preparation (Control), and after filling of LPS‐treated bladder preparation (LPS‐treated). D, εADO amounts (pmol) detected in IL solution of untreated (Control) and LPS‐treated bladder preparations. * P < 0.05 vs. control, n = 4 bladder preparations, unpaired t test, two‐tailed. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
We developed and validated a novel ex vivo mouse bladder preparation to measure the availability of biologically active mediators at SubU/LP and in the bladder lumen during bladder filling. This preparation allows direct access to the basolateral aspect of the urothelium at different filling volumes and intravesical pressures, and it is suitable for investigating urothelium‐derived mediators that might be involved in activation of sensory afferent neurons or other mechanisms for regulation of bladder excitability during filling.
It is widely believed that the bladder urothelium actively participates in regulation of bladder excitability by releasing biologically active mediators such as ATP, acetylcholine, nitric oxide, prostaglandins and other substances that can bind to receptors and regulate the behaviours of cells deeper within the bladder wall (e.g. afferent neurons in SubU/LP) during bladder filling (Birder et al. 1997, 1998; Apodaca et al. 2007; Fry & Vahabi, 2016; Merrill et al. 2016). An important drawback of most experimental approaches utilized to investigate urothelial release of mediators is the lack of ability to perform measurements at different IL volumes and pressures that directly simulate bladder filling. For example, studies in cultured urothelial cells demonstrate that mechanical stimulation causes release of substances, but it is difficult to establish physiological relevance of the stimuli used (e.g. cell swelling, undefined drag forces or non‐physiological stretch). Possible phenotypic changes of cells in culture may also misrepresent physiological mechanisms, and this caveat is frequently overlooked.
Bladder sheets mounted in Ussing chambers have also been used for studies examining release of mediators from urothelium. For instance, this preparation occasioned the discovery of ATP release from the bladder mucosa upon changes in hydrostatic pressure (Ferguson et al. 1997). A significant drawback of this approach, however, is that the volumes of fluid on either side of the bladder sheet forming a diaphragm between the serosal and mucosal chambers of the apparatus are extremely large in comparison with the volumes in the bladder lumen or in the vicinity of SubU/LP during bladder filling. In addition, in most cases hydrostatic pressure changes are generated in a rather unphysiological way by removing fluid on either side of the bladder sheet. Removing fluid from the serosal chamber technically mimics the direction of hydrostatic pressure changes during bladder filling, but changes of mechanical forces on flat sheets do not reproduce the changes that develop during filling of the hollow bladder. On the other hand, removing fluids from the mucosal chamber as used in some studies does not reproduce bladder filling. Such concerns apply to both the use of the entire bladder wall and of isolated bladder mucosa as flat sheets.
Some studies have measured release of mediators into the bladder lumen during filling and assumed that luminal release of mediators reflects the profile of mediators in subU/LP where afferent nerves and other cells with receptors for urothelial mediators might reside (Daly et al. 2014; Beckel et al. 2015; Gonzalez et al. 2016; Grundy et al. 2018). Such assumptions, however, overlook possible differential regulation of release and/or metabolism of mediators at the apical and basolateral sides of urothelium that other studies have suggested (Yu et al. 2006; Yu, 2015). Whether such asymmetry/polarization of mediator availability at subU/LP and in bladder lumen exists during filling and whether and how these differences might be affected by IL volumes and pressures during filling is currently unknown. Studies in Ussing chambers have also argued that stretch and not hydrostatic pressure induces urothelial ATP release (Yu, 2015) emphasizing the importance of studying changes during physiological filling in which the volume of fluid in the bladder increases dramatically with minimal increase in intravesical pressure until critical volume and pressure are achieved to trigger voiding. Along these lines, it is important to understand how the urothelium contributes to stabilizing bladder excitability during the storage phase of bladder filling and whether altered availability of inhibitory and excitatory mediators at SubU/LP (in close proximity to DSM) participates in the switch from storage to voiding. It is also unclear whether substances present in the bladder lumen are transported into the suburothelium during filling as suggested indirectly by studies that report stimulation of afferent nerves deeper in the bladder wall after IL application of mediators (Collins et al. 2013; Daly et al. 2014; Gonzalez et al. 2016). These are all important questions prompting the development of novel experimental approaches, such as the one described in this study, that enable direct access to both sides of the urothelium during authentic bladder filling.
In the present study, we used bladder preparation devoid of the detrusor smooth muscle layer. Immunohistochemistry confirmed the integrity of the urothelium and the presence of intact umbrella cells (e.g. IL biotin was restricted to bladder epithelium), intermediate and basal urothelial cells (e.g. AQP3‐immunoreactivity was limited to basal and some intermediate cells but was absent in umbrella cells). The subU/LP also appeared to be intact as demonstrated by Masson's trichrome staining, haematoxylin and eosin staining, and differential interference contrast imaging. Fluorescence microscopy of denuded bladders from PDGFRαEGFP mice demonstrated the presence of eGFP‐tagged PDGFRα+ cells, a novel population of interstitial cells that occur in high density in the SubU/LP in the bladder (Koh et al. 2012; Koh et al. 2018). Fluorescent dye permeability assays during filling of the denuded bladder preparation demonstrated efflux of fluorescent dyes, as documented previously (Carattino et al. 2013; Greenwood‐Van Meerveld et al. 2015). As expected, the efflux of fluorescent dyes applied intraluminally was directly proportional to the time for bladder filling and inversely proportional to the size of the dye molecules. The rate of efflux was constant after completion of bladder filling. Pretreatment of the DSM‐denuded bladder preparation with IL PS or LPS resulted in increased efflux of FD‐4, suggesting that the model can also be used to study urothelial mechanisms in inflammation. Due to disrupted integrity of the bladder urothelium in response to PS or LPS treatment, the rate of efflux of FD‐4 was reduced progressively at later time intervals, likely because the concentration gradient had diminished due to the enhanced efflux early in the filling.
One might assume that the lack of detrusor smooth muscle would result in a modified pressure–volume relationship. Our studies demonstrated, however, that the patterns of filling of bladder preparations with and without the detrusor layer of muscle were quite similar (Figs. 3 and 4): infusion of fluid into intact bladders typically leads to the development of transient contractions (TCs) that are superimposed upon slowly developing intraluminal pressure, until a critical pressure is reached at which voiding is initiated. TCs of the detrusor cause small, rapid increases in intravesical pressure and are due to localized contractions (maybe contractions of single muscle bundles) (Heppner et al. 2016). When the detrusor is removed, TCs cannot occur, but like the intact bladders, the denuded preparations accommodated significant volumes with negligible changes in pressure. Notably, the pressure–volume relationships were comparable in intact and detrusor‐denuded bladders, validating the suitability of denuded bladders to investigate mechano‐sensitive mechanisms occurring during authentic bladder filling. It should be emphasized that the filling volumes that were required to reach voiding pressure (e.g. 15–20 mmHg) varied broadly in both decentralized intact and denuded bladder preparations. The mechanisms responsible for the variability of filling volumes are currently unknown, but differences in bladder sizes, collagen content within the urothelium, and elasticity and elongation properties of urothelium components may contribute. Further studies on the biomechanical properties of denuded bladders will be necessary to investigate this question. The differences in the time required for bladder filling are likely to have an impact on the profiles of mediators available at either side of the urothelium because the release and metabolism of mediators is time‐dependent.
We also investigated the suitability of the denuded bladder to measure the availability of biologically active mediators at the luminal and anti‐luminal surfaces of the urothelium. The present study focused on adenine purines as mediators, but this preparation could be used to collect and measure other mediators as well. In accordance with previous observations using intact murine and monkey bladders (Durnin et al. 2016), ATP and other purines (e.g. ADP, NAD, AMP and adenosine) were detected in the lumen of denuded bladder preparations during filling. Of particular importance in the present study was the observation that the same purines, but in different proportions, were available at the basolateral side of murine and monkey denuded bladder preparations. Our results provide the first direct measurement of purines from the SubU/LP during bladder filling. It is also important to point out that the distribution of individual purines differed significantly in the lumen and the EL (i.e. SubU/LP) spaces, suggesting that differential mechanisms of release and/or purine metabolism occur at opposite sides of the urothelium.
These findings also indicate that there is no direct correlation between IL and submucosal availability of mediators during filling, and assumptions about types and amounts of mediators available in LP cannot be ascertained from measuring mediators in the bladder lumen. In our study the amounts of purines released into the lumen far exceeded the amounts of purines released into the SubU/LP, but the concentrations of purines released into the SubU/LP may exceed the concentration of purines in the lumen due to the small interstitial space between the urothelium and the detrusor layer, particularly at end of filling. These differences are important because receptor binding is a function of agonist concentration, and thus the balance between the concentrations of different mediators will define the receptors and downstream signalling pathways that are important in the regulation of bladder function. Once released, many mediators are degraded to active or inactive metabolites. This is particularly true for purines. Thus, if enzymes that degrade nucleotides and nucleosides are available on either side of the urothelium, ATP will be degraded to ADP, which in turn will be degraded to AMP and then to adenosine. Previous studies have documented that ATP and ADP cause DSM contractions via stimulation of P2X1 and P2Y12 receptors on smooth muscle cells, respectively (Burnstock, 2014; Yu et al. 2014), whereas NAD (Breen et al. 2006) and ADO (Burnstock, 2014) cause DSM relaxation. Therefore, having the ability to measure simultaneously excitatory and inhibitory mediators during different stages of bladder filling will provide new insights into how the urothelium contributes to regulation of storage of urine during filling and initiation of voiding.
We also performed experiments in which εATP was added to either the SubU/LP or into the lumen to characterize the pattern and time course of ATP metabolism. Formation of εADP, εAMP and εADO was assessed at the end of filling. Use of 1,N 6‐etheno‐nucleotides as substrates grants high sensitivity of measurements of nucleotide catabolism (Secrist et al. 1972; Jamal et al. 1988; Todorov et al. 1997; Bobalova et al. 2002; Durnin et al. 2012), and small changes in substrate or product amounts can be detected. Furthermore, use of ε‐purines as substrates eliminates interference with endogenous purines that might have been released, as they remain under the detection levels because the samples do not undergo additional etheno‐derivatization. We found that εATP is degraded within minutes to εADP, εAMP and εADO on both the basolateral and apical sides of urothelium. However, the profile of the purine pool and ratios between individual purines differed significantly at SubU/LP and in the bladder lumen during filling period, supporting again the conclusion that the availability of mediators at SubU/LP cannot be deduced by measuring levels of mediators in the lumen.
A surprising finding was that small amounts of εAMP and larger amounts εADO were observed in the opposite side of the εATP application, suggesting transurothelial transport of nucleosides during bladder filling. Indeed, we observed bilateral transport of εADO during filling, and this transurothelial flux was increased significantly after IL application of LPS. Transurothelial transport of mediators during filling is a novel mechanism that may influence the availability of mediators at the luminal and anti‐luminal surfaces of urothelium during filling. The fact that transurothelial flux is augmented by an inflammatory mediator could provide one explanation for why bladder functions are altered during inflammation. Other pathophysiological conditions, such as spinal‐cord injury, where a breakdown in urothelial barrier function occurs (Kullmann et al. 2017), might also have significant consequences on the balance of mediators in the luminal and SubU/LP compartments. These findings once again validate the usefulness of the novel detrusor‐denuded bladder preparation for studies of local mechanisms of urothelial control of bladder function and connectivity of the urothelium with sensory afferent neurons and detrusor contractile activity during filling. Mediators released in the SubU/LP might affect a variety of cell types in the bladder wall, including SMC, interstitial cells (Koh et al. 2012; Koh et al. 2018) and afferent nerves in both urothelium and detrusor muscle (Spencer et al. 2018). In fact, recent studies highlight the role of detrusor muscle TCs in stimulating afferent neurons within the muscle to trigger micturition (Heppner et al. 2016). The preparation described in this study will likely be instrumental in understanding what chemical mediators (e.g. purines) are available at the submucosal aspect of urothelium and might affect detrusor muscle excitability and activation of afferent neurons.
Limitations of the denuded bladder preparation include the fact that it is excised from the body and has no connectivity to the central nervous system or circulation. These limitations are shared, however, with bladder wall preparations mounted in Ussing chambers, urothelial cell cultures, in situ bladders in the presence of ganglionic blockers, and isolated bladder wall strips stimulated with receptor agonists or ion channel activators. Nonetheless, the ex vivo nature of this preparation is of benefit for studies of local regulatory mechanisms that are not dependent upon spinal or central reflexes. This model allows detailed investigation of local mechanisms of mucosa–detrusor connectivity during bladder filling, and its use will enhance understanding of mechanisms involving mechanotransduction mechanisms that are initiated in the urothelium during filling. One might argue that the absence of the detrusor would alter the mechanosensitive properties of the urothelium in response to filling, but as we observed, the characteristics of the pressure–volume relationship during filling are very similar in intact and in detrusor‐denuded bladders.
In summary, we have developed a novel ex vivo bladder preparation, in which the detrusor muscle is removed. We demonstrated the usefulness of this model for studies of biologically active mediators that appear in the luminal or extra‐luminal compartments during filling. The preparations showed excellent experimental reproducibility and pressure–volume characteristics that were very similar to intact bladders. Thus, mechanosensitive mechanisms in the urothelium are likely to be similar in intact and denuded bladders. Particular advantages of this preparation are that samples of bathing solutions can be sampled from either aspect of the urothelium, at different times and different volumes and pressures, and at different filling rates. This preparation was used to determine profiles of purine release, metabolism and transepithelial transport during filling. Our data show that purine profiles and time course differ in the luminal and suburothelial compartments and make the point that measurement of luminal purines alone is not an adequate representation of the purines that might activate afferent neurons or influence the contractility of the detrusor. Understanding the levels and types of purines and mechanisms for the differences in luminal and suburothelial purine availability during filling will likely shift paradigms about purinergic regulation of the bladder. Measurements of other putative mediators will also be facilitated using the preparation developed, and it will also be useful for evaluating the effects of inflammatory or other pathophysiological conditions in which the urothelial barrier is disrupted. Potential drawbacks of the preparation include detachment from the CNS and circulation. However, even though the detrusor muscle is physically removed, the model offers the potential to advance knowledge about local mechanisms of urothelium–detrusor connectivity by measuring urothelium‐derived mediators that could affect functions of the detrusor muscle during filling. The model will also likely facilitate studies aimed at understanding the role of urothelial mediators and specific cell types in urothelium in regulation of bladder excitability.
Additional information
Competing interests
No conflicts of interest, financial or otherwise, are declared by the authors.
Author contributions
V.M.Y. and K.M.S. conceived and designed the research; L.D., B.K., P.K., R.M., R.C., Y.Z., Q.C., S.M.W. and V.M.Y. performed the research; L.D., B.K., P.K., R.M., S.M.W., S.D.K. and V.M.Y. analysed the data; L.D., Y.Z., Q.C., S.M.W., S.D.K., K.M.S., V.M.Y. interpreted results of experiments; L.D., R.C., B.K., P.K., S.M.W., Y.Z., Q.C. and V.M.Y. prepared figures; V.M.Y. and K.M.S. wrote the paper. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK41315.
Supporting information
Video 1. Recording of intact bladder filling at 15 μl min−1 corresponding to data presented in Fig. 4A
Video was recorded using a SMZ1000 zoom stereomicroscope (Nikon Instruments Inc., Melville, NY, USA) with a DMK31AF03 Monochrome Firewire 400 CCD camera (The Imaging Source, LLC, Charlotte, NC, USA) at 5 Hz using Astro IIDC software (Aupperle Services and Contracting, Calgary, Alberta, Canada); recording was stopped upon reaching 25 mmHg intraluminal pressure. The full duration of the image is 64 times real‐time.
Video 2. Recording of detrusor muscle‐denuded bladder filling at 15 μl min−1 corresponding to data presented in Fig. 4B
Video was recorded using a SMZ1000 zoom stereomicroscope (Nikon Instruments Inc.) with a DMK31AF03 Monochrome Firewire 400 CCD camera (The Imaging Source) at 5 Hz using Astro IIDC software (Aupperle Services and Contracting, Calgary, Alberta, Canada); recording was stopped upon reaching 25 mmHg intraluminal pressure. The full duration of the image is 64 times real‐time.
Biography
Leonie Durnin obtained a BSc degree in Biology from the University of Ulster, Coleraine, UK (2007) and a PhD in Physiology and Cell Biology from the University of Nevada Reno (2013) in the group of Prof. Violeta Mutafova‐Yambolieva where she received extensive training in mechanisms of purinergic neurotransmission. She then undertook postdoctoral training in the same group expanding her expertise to novel approaches of studying enteric neurotransmitters in the large intestine and purine‐mediated regulation of bladder physiology. She is currently involved in preclinical research studies assessing the safety and efficacy of novel drug candidates to treat human pathologies.

Edited by: Kim Barrett & David Grundy
Linked articles: This article is highlighted in a Perspectives article by McCloskey. To read this article, visit https://doi.org/10.1113/JP277268.
References
- Apodaca G, Balestreire E & Birder LA (2007). The uroepithelial‐associated sensory web. Kidney Int 72, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Daugherty SL, Tyagi P, Wolf‐Johnston AS, Birder LA, Mitchell CH & de Groat WC (2015). Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Apodaca G, de Groat WC & Kanai AJ (1998). Adrenergic‐ and capsaicin‐evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol 275, F226–F229. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ & de Groat WC (1997). DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol 158, 1989–1995. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ & Caterina MJ (2001). Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A 98, 13396–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G & Kanai AJ (2002). β‐Adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22, 8063–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobalova J, Bobal P & Mutafova‐Yambolieva VN (2002). High‐performance liquid chromatographic technique for detection of a fluorescent analogue of ADP‐ribose in isolated blood vessel preparations. Anal Biochem 305, 269–276. [DOI] [PubMed] [Google Scholar]
- Breen LT, Smyth LM, Yamboliev IA & Mutafova‐Yambolieva VN (2006). β‐NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol 290, F486–F495. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2014). Purinergic signalling in the urinary tract in health and disease. Purinergic Signal 10, 103–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattino MD, Prakasam HS, Ruiz WG, Clayton DR, McGuire M, Gallo LI & Apodaca G (2013). Bladder filling and voiding affect umbrella cell tight junction organization and function. Am J Physiol Renal Physiol 305, F1158–F1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins VM, Daly DM, Liaskos M, McKay NG, Sellers D, Chapple C & Grundy D (2013). Onabotulinumtoxin A significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int 112, 1018–1026. [DOI] [PubMed] [Google Scholar]
- Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C & Grundy D (2014). Age‐related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol 592, 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Roskams T, Van PH & Baert L (1999). Nitric oxide synthase expression in neurogenic bladder disease: a pilot study. Acta Neurol Belg 99, 57–60. [PubMed] [Google Scholar]
- Durnin L, Hayoz S, Corrigan RD, Yanez A, Koh SD & Mutafova‐Yambolieva VN (2016). Urothelial purine release during filling of murine and primate bladders. Am J Physiol Renal Physiol 311, F708–F716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin L, Hwang SJ, Ward SM, Sanders KM & Mutafova‐Yambolieva VN (2012). Adenosine 5’‐diphosphate‐ribose (ADPR) is a neural regulator in primate and murine large intestine along with β‐NAD+ . J Physiol 590, 1921–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I & Burton TJ (1997). ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol 505, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH & Vahabi B (2016). The role of the mucosa in normal and abnormal bladder function. Basic Clin Pharmacol Toxicol 119 (Suppl 3), 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan‐Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour‐Fard A, Kim YH, Pollack JR, Vinar T, Addo‐Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer‐Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O'brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE & Zwieg AS (2007). Evolutionary and biomedical insights from the rhesus macaque genome. Science 316, 222–234. [DOI] [PubMed] [Google Scholar]
- Gonzalez EJ, Heppner TJ, Nelson MT & Vizzard MA (2016). Purinergic signalling underlies transforming growth factor‐beta‐mediated bladder afferent nerve hyperexcitability. J Physiol 594, 3575–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood‐Van Meerveld MB, Mohammadi E, Tyler K, Van GS, Parker A, Towner R & Hurst R (2015). Mechanisms of visceral organ crosstalk: importance of alterations in permeability in rodent models. J Urol 194, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy L, Chess‐Williams R, Brierley SM, Mills K, Moore KH, Mansfield K, Rose'Meyer R, Sellers DJ & Grundy D (2018). NKA enhances bladder afferent mechanosensitivity via urothelial and detrusor activation. Am J Physiol Renal Physiol 315, F1174–F1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Tykocki NR, Hill‐Eubanks D & Nelson MT (2016). Transient contractions of urinary bladder smooth muscle are drivers of afferent nerve activity during filling. J Gen Physiol 147, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM & Mutafova‐Yambolieva VN (2011). β‐Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Z, Afkham‐Ebrahimi A & Saggerson ED (1988). A novel assay for 5X′‐nucleotidase using 1,N 6‐etheno‐AMP as substrate, and comments on the properties of the reaction product, ethenoadenosine. Biochem J 250, 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal P, Abraham SN & Apodaca G (2009). Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297, F1477–F1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SO, Song SH, Hwang EC, Oh KJ, Ahn K, Jung SI, Kang TW, Kwon D, Park K & Ryu SB (2012). Changes in aquaporin (AQP)2 and AQP3 expression in ovariectomized rat urinary bladder: potential implication of water permeability in urinary bladder. World J Urol 30, 207–212. [DOI] [PubMed] [Google Scholar]
- Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM & Koh SD (2012). Platelet‐derived growth factor receptor‐α cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med 16, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Lee H, Ward SM & Sanders KM (2018). The mystery of the interstitial cells in the urinary bladder. Annu Rev Pharmacol Toxicol 58, 603–623. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Clayton DR, Ruiz WG, Wolf‐Johnston A, Gauthier C, Kanai A, Birder LA & Apodaca G (2017). Urothelial proliferation and regeneration after spinal cord injury. Am J Physiol Renal Physiol 313, F85–F102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt B, Head RJ & Westfall DP (1984). High‐pressure liquid chromatographic‐fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem 137, 93–100. [DOI] [PubMed] [Google Scholar]
- McLatchie LM & Fry CH (2015). ATP release from freshly isolated guinea‐pig bladder urothelial cells: a quantification and study of the mechanisms involved. BJU Int 115, 987–993. [DOI] [PubMed] [Google Scholar]
- Merrill L, Gonzalez EJ, Girard BM & Vizzard MA (2016). Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M & Tominaga M (2014). Functional role for Piezo1 in stretch‐evoked Ca2+ influx and ATP release in urothelial cell cultures. J Biol Chem 289, 16565–16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M & Tominaga M (2009). The TRPV4 cation channel mediates stretch‐evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284, 21257–21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova‐Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM & Sanders KM (2007). β‐Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A 104, 16359–16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H & Yoshimura N (2005). Detrusor overactivity induced by intravesical application of adenosine 5′‐triphosphate under different delivery conditions in rats. Urology 66, 1332–1337. [DOI] [PubMed] [Google Scholar]
- Pandita RK & Andersson KE (2002). Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168, 1230–1234. [DOI] [PubMed] [Google Scholar]
- Persson K, Poljakovic M, Johansson K & Larsson B (1999). Morphological and biochemical investigation of nitric oxide synthase and related enzymes in the rat and pig urothelium. J Histochem Cytochem 47, 739–750. [DOI] [PubMed] [Google Scholar]
- Secrist JA, Barrio JR, Leonard NJ & Weber G (1972). Fluorescent modification of adenosine‐containing coenzymes. Biological activities and spectroscopic properties. Biochemistry 11, 3499–3506. [DOI] [PubMed] [Google Scholar]
- Smith PP & Kuchel GA (2010). Continuous uroflow cystometry in the urethane‐anesthetized mouse. Neurourol Urodyn 29, 1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector DA, Wade JB, Dillow R, Steplock DA & Weinman EJ (2002). Expression, localization, and regulation of aquaporin‐1 to ‐3 in rat urothelia. Am J Physiol Renal Physiol 282, F1034–F1042. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Greenheigh S, Kyloh M, Hibberd TJ, Sharma H, Grundy L, Brierley SM, Harrington AM, Beckett EA, Brookes SJ & Zagorodnyuk VP (2018). Identifying unique subtypes of spinal afferent nerve endings within the urinary bladder of mice. J Comp Neurol 526, 707–720. [DOI] [PubMed] [Google Scholar]
- Studeny S, Torabi A & Vizzard MA (2005). P2X2 and P2X3 receptor expression in postnatal and adult rat urinary bladder and lumbosacral spinal cord. Am J Physiol Regul Integr Comp Physiol 289, R1155–R1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA & Ferguson DR (2004). P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93, 1344–1348. [DOI] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova‐Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA & Westfall DP (1997). Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387, 76–79. [DOI] [PubMed] [Google Scholar]
- Wang EC, Lee JM, Ruiz WG, Balestreire EM, von BM, Barrick S, Cockayne DA, Birder LA & Apodaca G (2005). ATP and purinergic receptor‐dependent membrane traffic in bladder umbrella cells. J Clin Invest 115, 2412–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder M, Tobin G, Zupancic D & Romih R (2014). Signalling molecules in the urothelium. Biomed Res Int 2014, 297295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T & Inadome A (2008). Basic and clinical aspects of non‐neuronal acetylcholine: expression of non‐neuronal acetylcholine in urothelium and its clinical significance. J Pharmacol Sci 106, 193–198. [DOI] [PubMed] [Google Scholar]
- Yu W (2015). Polarized ATP distribution in urothelial mucosal and serosal space is differentially regulated by stretch and ectonucleotidases. Am J Physiol Renal Physiol 309, F864–F872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sun X, Robson SC & Hill WG (2014). ADP‐induced bladder contractility is mediated by P2Y12 receptor and temporally regulated by ectonucleotidases and adenosine signaling. FASEB J 28, 5288–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Zacharia LC, Jackson EK & Apodaca G (2006). Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol 291, C254–C265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Recording of intact bladder filling at 15 μl min−1 corresponding to data presented in Fig. 4A
Video was recorded using a SMZ1000 zoom stereomicroscope (Nikon Instruments Inc., Melville, NY, USA) with a DMK31AF03 Monochrome Firewire 400 CCD camera (The Imaging Source, LLC, Charlotte, NC, USA) at 5 Hz using Astro IIDC software (Aupperle Services and Contracting, Calgary, Alberta, Canada); recording was stopped upon reaching 25 mmHg intraluminal pressure. The full duration of the image is 64 times real‐time.
Video 2. Recording of detrusor muscle‐denuded bladder filling at 15 μl min−1 corresponding to data presented in Fig. 4B
Video was recorded using a SMZ1000 zoom stereomicroscope (Nikon Instruments Inc.) with a DMK31AF03 Monochrome Firewire 400 CCD camera (The Imaging Source) at 5 Hz using Astro IIDC software (Aupperle Services and Contracting, Calgary, Alberta, Canada); recording was stopped upon reaching 25 mmHg intraluminal pressure. The full duration of the image is 64 times real‐time.


