Abstract
Disruption of circadian rhythmicity induced by prolonged light exposure, altered sleep patterns and shift work is associated with the development of obesity and related metabolic disorders, including type 2 diabetes and cardiovascular diseases. White and brown adipose tissue activity shows circadian rhythmicity, with daily variations in the regulation of metabolic processes such as lipolysis, glucose and lipid uptake, and adipokine secretion. The role of the circadian clock in the regulation of energy homeostasis has raised interest in clock‐related strategies to mitigate metabolic disturbances associated with type 2 diabetes, including ‘resynchronizing’ metabolism through diet or targeting a particular time of a day to potentiate the effect of a pharmacological or physiological treatment. Exercise is an effective intervention to prevent insulin resistance and type 2 diabetes. Beyond its effect on skeletal muscle, exercise training also has a profound effect on adipose tissue. Adipose tissue partly mediates the beneficial effect of exercise on glucose and energy homeostasis, via its metabolic and endocrine function. The interaction between zeitgeber time and diet or exercise is likely to influence the metabolic response of adipose tissue and therefore impact the whole‐body phenotype. Understanding the impact of circadian clock systems on human physiology and how this is regulated by exercise in a tissue‐specific manner will yield new insights for the management of metabolic disorders.
Keywords: Adipose tissue, Exercise, Nutrition, Circadian Rhythm, Type 2 Diabetes, Energy Homeostasis, Adipokine
Introduction
The circadian clock is a key homeostatic regulator that is synchronized by photic and non‐photic stimuli and controls many genomic and physiological responses (Eckel‐Mahan & Sassone‐Corsi, 2013; Gerhart‐Hines & Lazar, 2015). The circadian programme is regulated at both a central and a peripheral level, with the suprachiasmatic nucleus (SCN) in the hypothalamus acting as a master clock that synchronizes peripheral oscillators in most cells of the body. On a molecular level, the circadian clock is composed of an auto‐regulatory transcription–translation feedback loop with the transcriptional activators CLOCK and BMAL1 and their target genes that encode Period (PER) and cryptochrome (CRY), which form a repressor complex, and reverse‐erb α (REV‐ERBα) that together inhibit CLOCK and BMAL1 transcription activity (Fig. 1). A variety of stimuli including the timing of food intake, dietary composition, temperature and the level of physical activity can alter the activity of circadian clocks in peripheral tissues, highlighting the interplay between circadian rhythm and metabolism (Gabriel & Zierath, 2019). Disrupted circadian rhythms lead to insulin resistance and increased risk for the development of type 2 diabetes (Eckel‐Mahan & Sassone‐Corsi, 2013; Gerhart‐Hines & Lazar, 2015; Bescos et al. 2018; Vetter et al. 2018). Thus, synchronizing exercise or nutrient strategies to the molecular circadian clock may maximize the health‐promoting benefits of these interventions to enhance insulin sensitivity and prevent type 2 diabetes.
Figure 1.
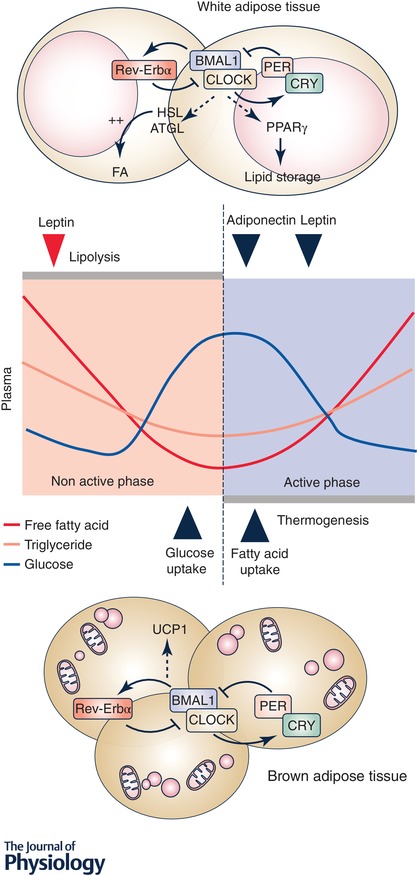
Circadian regulation of metabolic and endocrine function of adipose tissue and the contribution to glucose and lipid oscillations in plasma
White adipocyte tissue lipolysis and lipid storage undergo diurnal oscillations. Adipokines such as adiponectin and leptin are released into circulation in a circadian pattern and peak at different times of the day. Brown adipose tissue displays a circadian rhythm of glucose and fatty acid uptake, with higher thermogenesis activity in the active phase. On the molecular level, the intrinsic clock influences adipocyte function via direct regulation of the expression of several genes, including the lipolytic enzymes ATGL and HSL, the fat storage factor PPARγ and the thermogenic protein UCP1. This schematic representation is mainly derived from experimental evidence from mouse models and therefore it requires additional corroboration in humans. The peak in leptin level in humans is identified by the red triangle.
White and brown adipose tissue play an important role in the regulation of lipid metabolism, whole‐body energy homeostasis and thermogenesis. Adipose tissue integrates several external stimuli, including nutrients and hormones, which modulate its function in a diurnal pattern. Accordingly, adipose tissue dysfunction is central to the development of obesity and type 2 diabetes, and other related metabolic disorders. Regular physical exercise is a potent modulator of glucose and energy homeostasis by affecting cellular processes in a variety of peripheral tissues including skeletal muscle, liver and adipose tissue (Hawley et al. 2014). While skeletal muscle is one of the main tissues directly affected by physical activity, exercising skeletal muscle ‘communicates’ with adipose tissue to improve whole‐body glucose and energy homeostasis (Townsend & Wright, 2019). The role of adipose tissue in mediating the health‐promoting effects of exercise is a topic of emerging interest. Intimate links between gene regulation and the circadian clock are likely to contribute to the plasticity of a variety of insulin‐sensitive organs in response to exercise and nutritional interventions. This review focuses on the circadian regulation of adipose tissue metabolism, and the interplay between diet, exercise and the circadian clock in orchestrating the adipose tissue adaptation and, in a more integrative way, the consequences of these factors for the whole‐body metabolism.
White and brown adipose tissues contribute to the circadian regulation of energy homeostasis
White adipose tissue activity shows circadian rhythmicity, with daily variations in the expression of genes involved in metabolic processes such as lipid metabolism and energy expenditure (Zvonic et al. 2006; Shostak et al. 2013a). The integration of several stimuli, including nutrients and hormones, regulate the balance between substrate storage and mobilization in adipose tissue throughout the day, consistent with the oscillation of the circulating levels of free fatty acids (FFAs) and triglycerides (TGs). However, growing evidence suggests that the endogenous adipose clock plays an important role in lipid metabolism. Indeed, white and brown adipose tissue gene expression of the components of the core clock machinery is cyclic, with a significant percentage of genes displaying associated coordinated circadian expression profiles (Zvonic et al. 2006). The key enzymes of lipolysis, including adipose triglyceride lipase (ATGL) and hormone‐sensitive lipase (HSL), are under the transcriptional control of BMAL1–CLOCK and the modulation of these transcriptional activators ultimately affects the plasma FFA level in transgenic mouse models (Shostak et al. 2013b). Of note, the increase in mRNA level of ATGL and HSL precedes the elevation of the plasma FFA concentration by several hours, suggesting that in vivo, the clock is likely responsible for the fine‐tuning of the regulation of lipolysis, while acute induction of lipolysis is activated by phosphorylation events under the control of hormonal cues (Duncan et al. 2007). PER2 directly inhibits the activity of peroxisome proliferator‐activated receptor γ (PPARγ), a master regulator of adipogenesis and promotor of lipid storage (Grimaldi et al. 2010). Metabolomic studies in humans reveal that many circulating metabolites, with the majority being lipid products, show circadian oscillations, pointing to a specific role of the endogenous circadian clocks in the regulation of the plasma lipid profile (Dallmann et al. 2012; Skene et al. 2018). In addition to a metabolic role, white adipose tissue contributes to energy homeostasis through the production of adipokines. For example, leptin, an adipokine that regulates food intake and energy expenditure, is released in a pulsatile pattern with a peak in the early non‐active phase in humans (Heptulla et al. 2001). Accordingly, decreased leptin pulsatility has been suggested to contribute to leptin resistance and the development of obesity (Arble et al. 2011). Conversely, adiponectin, an adipokine implicated in the regulation of insulin sensitivity and fatty acid (FA) oxidation, peaks in the early active phase. The diurnal pattern of adipokine release may contribute to the withdrawal of circulating FAs in the active phase, and the control of glucose and energy homeostasis (Gomez‐Abellan et al. 2010; Froy & Garaulet, 2018). Adipokine levels also vary in response to environmental factors such as light–dark and feeding–fasting cycles that typically occur in time‐of‐day‐dependent patterns (Shea et al. 2005). Thus, adipokine regulation can provide a feedback loop between the systemic environment and adipose tissue to regulate whole‐body metabolism.
In the last decade, brown adipose tissue has re‐emerged as an important organ for the regulation of glucose and energy homeostasis. Brown adipose tissue activity is rapidly modulated throughout the course of a day, thereby affecting thermogenic plasticity and influencing whole‐body metabolism. Fluorodeoxyglucose positron emission tomography (PET) imaging analysis of anaesthetized C57Bl/6 male and female mice over the course of the light–dark cycle reveals a diurnal pattern of glucose uptake in brown adipose tissue (van der Veen et al. 2012), possibly driven by glucocorticoids (Ramage et al. 2016). In humans, brown adipose tissue explants display circadian rhythmicity of the gene expression of insulin‐sensitive glucose transporter protein 4 (GLUT4) and the mitochondrial uncoupling protein 1 (UCP1), with increased glucose uptake in brown adipose tissue in the early morning as assessed in vivo using PET–computed tomography scanning (Lee et al. 2016). Although the diurnal variation in brown adipose tissue activity did not influence blood glucose oscillations, a modest correlation was detected with the supraclavicular temperature response and glucose excursions among individuals with high brown adipose tissue abundance (Lee et al. 2016). In male C57Bl/6J mice, brown adipose tissue also displays a diurnal rhythm in FA uptake, with increased TG‐derived FA uptake at the onset of the active period (van den Berg et al. 2018). These results indicate that photic cues and modulation of the light exposure duration influence brown adipose tissue activity in part via the central circadian clock machinery. Indeed, TG‐derived FA uptake in brown adipose tissue and thermogenic activity has been previously shown to be under the direct control of the SCN via the nuclear factor REV‐ERBα (Gerhart‐Hines et al. 2013; Moran‐Ramos et al. 2017). While the circadian brown adipose tissue activity predicts the variation in plasma TG and FA level, and determines the postprandial handling of TGs in mice, this finding has not been corroborated in humans (van den Berg et al. 2018).
Disruption of the clock alters adipose tissue metabolism
Disruption of circadian rhythmicity induced by prolonged light exposure, altered sleep patterns and shift working is associated with the development of obesity and related disorders, including type 2 diabetes and cardiovascular diseases (Karlsson et al. 2001). Considering the important role of adipose tissue in the regulation of glucose and lipid metabolism, misalignment or perturbation of the internal adipose clock is a plausible mechanism for the development of metabolic disorders. In rodents, advance phase shift or time‐restricted sleeping increases the expression of lipogenic genes in white adipose tissue (Husse et al. 2012; Herrero et al. 2015). Moreover, prolonged light exposure in mice leads to obesity by decreasing brown adipose tissue activity via impaired adrenergic signalling (Kooijman et al. 2015). In humans, forced dissynchrony of the clock by altered eating and sleeping patterns to mimic conditions of acute jet lag or chronic shift work decreases plasma leptin levels, increases glucose and insulin levels, and disrupts the cortisol rhythm (Scheer et al. 2009). These changes in endocrine function indicate that circadian misalignment can affect adipose tissue metabolism, which if maintained, could lead to the development of obesity.
A role for the clock machinery in adipose tissue function is directly supported from studies of animal models in which components of the core clock machinery have been mutated or ablated. Clock‐mutant ClockΔ19 mice exhibit a loss of rhythmicity in mRNA expression of adipose tissue lipogenic and lipolytic genes, leading to decreased lipolysis, increased adipose tissue mass and adipocyte hypertrophy (Shostak et al. 2013b). Studies of Rev‐Erbα knockout mice link circadian and thermogenic networks together by regulating brown adipose tissue function. Indeed, UCP1 rhythmicity was abolished and its protein abundance was persistently upregulated in Rev‐Erbα −/− mice, concomitant with increased glucose uptake and thermogenic activity (Gerhart‐Hines et al. 2013). Further compelling evidence supporting a role of the clock machinery in adipose function has emerged from the generation of tissue‐specific mouse models. Adipose‐specific deletion of BMAL1 (Ad‐Bmal1 −/− mice) decreased the concentration of adipose tissue polyunsaturated FA‐containing TGs, which increased saturated versus unsaturated FAs both in circulation and in hypothalamic neurons, and in turn altered feeding behaviour to drive obesity in mice challenged with high‐fat diet (Paschos et al. 2012). Thus, a well‐functioning circadian clock in adipose tissue is critical for whole‐body energy homeostasis and body weight balance.
Cycling of the intracellular clock is not intrinsically altered in white adipose tissue of obese healthy or type 2 diabetic subjects, at least in the early stages of the disease, indicating that clock disruption in white adipose tissue may not be a primary cause linked to the development of insulin resistance (Otway et al. 2011). Consistently, acute sleep loss alters the circadian clock in skeletal muscle, but not in the subcutaneous adipose tissue (Cedernaes et al. 2018). Nevertheless, in isolated adipocytes from obese patients transduced in vitro with a circadian reporter construct, the increased age of the donor correlates with a shortened circadian period in BMAL1 expression. This suggests that ageing‐related development of metabolic disorders could be associated with adipose tissue clock perturbation (Kolbe et al. 2019).
Synchronizing nutrient interventions to the molecular circadian clock to alter adipose tissue metabolism
Adipose tissue is characterized by its exquisite ability to adapt to an energy surplus through processes of adipocyte hypertrophy and/or hyperplasia, concomitant with remodelling of the vascular and immune environment. In adipocytes, activation of nutrient‐sensing nuclear receptors such as PPAR or G protein‐coupled receptor (GPCR) family members enable the adipose tissue to adapt to metabolic stimuli and induce the appropriate pathways (Goto et al. 2011; Quesada‐Lopez et al. 2016). Under a physiological setting, altered dietary patterns have also been shown to interfere with the molecular clock. For example, oscillations of clock gene expression are diminished in adipose tissue of mice fed a high‐fat diet, with decreased amplitude of the mRNA expression of Pparγ and its target lipogenic genes (Kohsaka et al. 2007). Thus, activation of the nuclear receptor PPARγ by fat‐soluble hormones or lipid compounds may also serve as a mechanism to integrate metabolic output and feedback to the molecular clock in adipose tissue (Yang et al. 2006; Chen & Yang, 2014). While high‐fat diet‐induced obesity may alter the molecular clock and impair metabolism, weight loss may have beneficial effects. For example, hypocaloric diet‐induced weight loss increases clock gene expression in adipose tissue in overweight humans (Pivovarova et al. 2016). Strikingly, the weight loss‐induced changes of clock gene expression were tightly correlated with the mRNA level of genes involved in fat metabolism, energy metabolism, autophagy and inflammatory responses (Pivovarova et al. 2016). However, whether these changes can impact the adipocyte proteome and improve its metabolic function remains to be determined.
The role of the circadian clock in the regulation of the energy homeostasis has raised interest in the notion of ‘chrono‐nutrition’ whereby dietary challenges such as altering the macronutrient content or timing of meals can reprogramme the clock and affect whole‐body physiology and metabolism (Asher & Sassone‐Corsi, 2015). Strategies to mitigate metabolic disturbances associated with type 2 diabetes include ‘resynchronizing’ metabolism through diet or targeting the time of day to potentiate the effect of a physiological or pharmacological treatment. As an example, time‐restricted feeding, without reducing calorie intake, improves circadian rhythms, increases brown adipose tissue thermogenesis, and prevents obesity and associated metabolic disorders in mice fed a high‐fat diet (Hatori et al. 2012). Interestingly, time‐restricted feeding in circadian mutant mice also improves the adipose tissue profile, by decreasing adipocyte hypertrophy and inflammation and increasing adiponectin secretion (Chaix et al. 2019). While the effects of time‐restricted feeding in humans are not fully characterized, some preliminary studies suggest that restoring feeding–fasting cycles with diurnal rhythms may have beneficial effects on metabolism. Indeed, time‐restricted feeding, with feeding time restricted to less than 8 h a day, sustains robust diurnal rhythms and has beneficial effects on insulin sensitivity in people with increased risk for type 2 diabetes (Panda, 2016; Sutton et al. 2018). Moreover, varying the calorie consumption throughout the day may influence body weight balance and adiposity. Greater caloric intake at breakfast versus dinner promotes weight loss, and improves fasting glucose levels, insulin sensitivity, and the blood lipid profiles in obese women fed an isocaloric diet (Jakubowicz et al. 2013). Thus, time‐targeted interventions as ‘chrono‐nutrition’ may represent a therapeutic strategy to improve adipose tissue function and manage metabolic diseases such as obesity and diabetes. However, in addition to nutrition, exercise is also a potent lifestyle intervention to combat metabolic dysfunction. The subsequent sections will focus on the role of exercise in adipocyte physiology and whether training at a specific time of day favours the adaptive response to exercise to increase insulin sensitivity and prevent metabolic diseases.
Exercise training in the fight against metabolic disorders: muscling in on adipose tissue
Regular physical activity is a major public health recommendation for the prevention and the management of type 2 diabetes and associated cardiovascular risks (Egan & Zierath, 2013). Myriad data demonstrate that exercise training improves glycaemic control, blood pressure and TG level in people with type 2 diabetes (Chudyk & Petrella, 2011). The beneficial effects of exercise on glucose and lipid metabolism are commonly explained by the improvement of skeletal muscle function (Gabriel & Zierath, 2017), but exercise also has a profound effect on insulin sensitivity in adipose tissue (Wallberg‐Henriksson & Zierath, 2015).
Adipose tissue has long been appreciated as a source of substrates for skeletal muscle, by releasing FAs in response to increased energetic demands associated with each exercise bout (Martin, 1996). Adipose tissue undergoes metabolic and genomic changes in response to acute exercise and training (Fig. 2). For example, exercise training decreases size and increases insulin sensitivity of isolated rat adipocytes (Craig et al. 1981), which may partly contribute to reductions in body fat and improvements in glucose metabolism (Weiss & Holloszy, 2007; Mavros et al. 2013; Drenowatz et al. 2015). The effects of exercise training on the metabolic and morphological properties of adipocytes are rapidly diminished within days after the last training session (Craig et al. 1983), indicating that genomic processes are involved. In mice, the exercise training‐induced remodelling of white adipose tissue, including reduced adipocyte size and increased FA metabolism, is associated with profound modifications of the transcriptional profile (Gollisch et al. 2009; Stanford et al. 2015b). Remarkably, exercise training induces a ‘browning’ of white adipose tissue, to generate a ‘brown adipose tissue‐like’ phenotype characterized by the presence of small multilocular lipid droplets and increased expression of thermogenic genes including Ucp1, Prdm16, Pgc1α and Cox8β (Stanford et al. 2015a). Several stimuli have been proposed to account for the browning of white adipose tissue including increased sympathetic innervation, or tissue cross‐talk between the working skeletal muscle and adipose tissue via secreted ‘myokines’ including irisin (Bostrom et al. 2012) and meteorin‐like 1 (Rao et al. 2014), or metabolites such as β‐aminoisobutyric acid (Roberts et al. 2014) and lactate (Carriere et al. 2014). Collectively, this has highlighted the importance of skeletal muscle–adipose tissue crosstalk to improve whole‐body glucose and energy homeostasis in response to exercise (Stanford & Goodyear, 2018). While the physiological relevance of browning as an adaptive response to exercise seems tenuous, this phenomenon could contribute to increased whole‐body energy expenditure and partly mediate the beneficial effect of exercise on glucose and lipid homeostasis (Seale et al. 2011).
Figure 2.
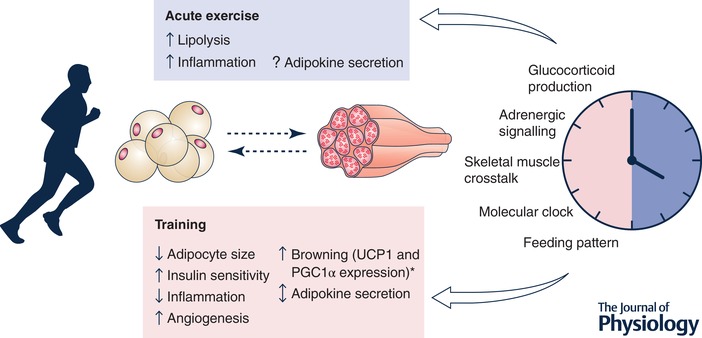
Exercise adaptation of adipose tissue and regulation by circadian factors
Evidence in mouse models and humans suggests that adipose tissue responds to exercise training by metabolic and genomic remodelling. Acute exercise induces lipolysis and inflammation. Exercise training leads to a profound remodelling of the metabolic and endocrine profile of adipose tissue. Skeletal muscle and adipose tissue communicate during exercise and play a role in adaptation to exercise training. Several physiological and metabolic processes are regulated in a circadian pattern, in response to hormonal, neuronal or feeding patterns or the molecular clock machinery. Changes in the molecular clock function can influence adipose tissue physiology, which in turn could potentiate the adaptive response to exercise. *Evidence from mouse models. PGC‐1α, peroxisome proliferator‐activated receptor γ coactivator 1α.
Adipose tissue can also play an endocrine role to mediate exercise‐induced improvements in glucose homeostasis. Transplantation of subcutaneous adipose tissue from mice trained for 11 days decreased fasting blood glucose and insulin level in a host sedentary mouse, likely via increased glucose uptake into oxidative skeletal muscle and brown adipose tissue (Stanford et al. 2015b). Although adipokines were proposed as the causative mechanism, the precise molecular mechanism remains elusive (Stanford et al. 2015b). However, recent studies suggested the existence of an adipokine secreted from brown adipose tissue, namely the lipokine 12,13‐dihydroxy‐9Z‐octadecenoic acid (12,13‐diHOME), which is released in response to cold exposure or acute exercise to potentially activate skeletal muscle and brown adipose tissue FA uptake and oxidation (Lynes et al. 2017; Stanford et al. 2018).
Much of the earlier work to understand the role of exercise on adipocyte biology was performed in rodents. However, exercise also affects adipocyte metabolism in humans. A recent study of healthy young men indicates the transcriptomic and epigenetic profile of abdominal subcutaneous adipose biopsies is altered after acute exercise, further highlighting the plasticity of adipose tissue (Fabre et al. 2018). While evidence for adipose tissue browning in humans in response to exercise training is lacking (Tsiloulis et al. 2018), profound adipocyte remodelling occurs, with increased angiogenesis and modification of the endocrine profile (Walton et al. 2015). Moreover, an exercise intervention (6‐months’ supervised training) altered the subcutaneous adipose tissue transcriptome, with an enrichment of oxidative phosphorylation pathways (Ronn et al. 2014). These training‐induced genomic changes might provide a mechanism for the beneficial effect of exercise in human adipocytes. Additionally, systemic changes in secreted factors may play a role. For example, in healthy individuals, the plasma level of the lipokine 12,13‐diHOME is acutely increased after 40 min of aerobic exercise, indicating a possible endocrine response of adipose tissue (Stanford et al. 2018). Nevertheless, emerging data suggests that the adipose tissue‐specific adaptation to exercise may be partly defective in obese insulin‐resistant individuals. For example, the angiogenic response to aerobic exercise is blunted, concomitant with reduced adrenergically induced lipolysis in subcutaneous adipose tissue (Walton et al. 2015; Verboven et al. 2018), while the beneficial effect on inflammation is maintained (Khadir et al. 2018).
While great strides have been made in identifying tissue‐specific secreted factors that communicate information regarding the metabolic or energetic stressors induced by exercise, the identification of specific adipokines and metabolites that signal from the adipocyte to the peripheral tissues during exercise is not a trivial undertaking, particularly in humans. Model systems to identify exercise‐induced adipokines are challenging given that exercise may not be fully recapitulated in isolated cellular systems, and whole‐body approaches may be impractical or imprecise for adequate sampling directly from adipocytes. An area of fertile research going forward is the identification of systemic factors that integrate the individual organ response to exercise with the entire body.
Interaction between circadian rhythms and the metabolic response of adipose tissue to exercise
Many of the pathways and genes identified in the control of whole‐body and cellular glucose and energy homeostasis are under circadian control (Eckel‐Mahan & Sassone‐Corsi, 2013). Furthermore, many of these circadian controlled genes have been described as being altered in response to exercise (Schroder & Esser, 2013) or nutrient stress (high‐fat diet) (Dyar et al. 2018). In mice, gene expression and metabolic adaptations to exercise vary according to zeitgeber time (Peek et al. 2017). Mitochondrial function also displays a circadian rhythm with oxidative metabolism peaking in the late afternoon (Deschenes et al. 1998; van Moorsel et al. 2016). Thus, optimizing the timing of a training bout to coincide with the greatest genomic or physiological response throughout the day may augment the potency of exercise as a therapeutic tool in regards to health outcomes.
The interaction between zeitgeber time and the metabolic response of the adipocyte is likely to be influenced by exercise, given the recent paradigm underscoring how intra‐ and inter‐tissue relationships coordinate the organismal response to nutrient stressors (Dyar et al. 2018). Therefore, the field is ripe to address several questions related to exercise and nutrient timing. For example, can exercise reset the adipose tissue intrinsic clock? While this question has not yet been experimentally addressed, acute exercise does affect the SCN circadian pattern, which may signal to the adipose tissue clock (Maywood et al. 1999). Conversely, the deleterious effect of high‐fat diet on the circadian clock and the concomitant increase in FA synthase gene expression in white adipose tissue is prevented by treatment of mice with the insulin‐sensitizer drug rosiglitazone (Ribas‐Latre et al. 2019). Therefore, it is plausible that as a physiological ‘insulin sensitizer’, exercise may reset the clock in adipose tissue, thereby improving whole‐body glucose metabolism and reducing adiposity.
Another question of interest is whether exercise timing can augment the metabolic effects on adipose tissue? The adaptive response of adipose tissue to exercise is likely dependent on nutritional status (fed or fasted) and time of day (day or night), with nutritional or hormonal status putatively potentiating or inhibiting the induction of specific molecular pathways that will contribute to adipose remodelling. Indeed, in overweight men, acute exercise performed after a meal attenuates the increase in mRNA expression of several genes involved in lipolysis and glucose metabolism such as ATGL, HSL, PDK4 and IRS2, compared to the same exercise performed in the fasting state (Chen et al. 2017). Although only the change in IRS2 was recapitulated at the protein level, it was found that the acute nutritional status affects the magnitude of the exercise response, and could therefore alter the adaptive response of adipose tissue to exercise training. As another example, in mice, browning of white adipose tissue is linked to vascular endothelial growth factor (VEGF) abundance and adrenergic signalling (During et al. 2015; Aldiss et al. 2018). Several studies indicate that the molecular clock interacts with hypoxia‐inducible factor (HIF) signalling and regulates the skeletal muscle glycolytic response and Vegfα expression during exercise in mice (Peek et al. 2017; Wu et al. 2017). Exercise‐induced catecholamine release also depends on the time of the day, while receptor expression displays a circadian pattern in adipose tissue (Yang et al. 2006; Scheer et al. 2010). Therefore, ‘browning’ of white adipose tissue and induction of metabolic programmes may be more effective if the exercise bout is performed when the adrenergic or hypoxia signalling is amplified. Moreover, in human myotubes, the secretion of myokines such as interleukin 6 and VEGF is under the control of the circadian clock (Perrin et al. 2015), suggesting that the exercise‐induced myokines profile can vary over the time of the day and therefore contribute to a different response in adipose tissue (Fig. 2).
The metabolic properties of adipose tissue are influenced in a distinct depot‐ and sex‐dependent manner (Karastergiou & Fried, 2017). Thus, adipose tissues from distinct depots may respond differently to exercise partly due to the sensitivity or rhythmicity of the response to various hormones, especially glucocorticoids, but also due to their intrinsic circadian gene expression profiles (Garaulet et al. 2011; Lee et al. 2014). In particular, the circadian rhythm of adipokine expression is depot‐specific, with substantial differences noted between subcutaneous and visceral human adipose tissue explants (Garaulet et al. 2011). Therefore, exercise‐induced adipokine secretion may be regulated differentially in men versus women based on sex‐dependent hormone and adipose tissue depot distribution profiles. Accordingly, subcutaneous adipose tissue seems to drive the metabolic response to exercise in mouse models (Stanford et al. 2015b). Other factors, such as the circadian oscillation of circulating immune cells, may influence the adipose tissue status and therefore the metabolic response (Zhao et al. 2017).
Concluding remarks
Type 2 diabetes is one of the fastest‐growing diseases world‐wide. For many patients, pharmacological intervention is required to manage this disease, yet effective insulin sensitizers are lacking from the current diabetes pharmacopeia. Exercise is an effective intervention to prevent insulin resistance and type 2 diabetes, and should be considered a first line treatment. While exercise training mainly targets skeletal muscle, it is a physiological ‘insulin sensitizer’ that also has a profound effect on adipose tissue. Several lines of evidence show that adipose tissue partly mediates the beneficial effect of exercise on glucose tolerance, via its metabolic and endocrine function. Diet and exercise have an additive effect on insulin sensitivity, involving metabolic and genomic adaptations at the level of skeletal muscle and adipose tissue, which are influenced by the time of day. Given the circadian role of white and brown adipose tissues in the regulation of metabolism, we speculate that time‐targeted diet and exercise strategies may potentiate adipose tissue metabolism and therefore contribute to the management of metabolic disorders. Understanding the relationship between nutrient state and circadian homeostasis, as well as the impact of circadian clock systems on human physiology and how this is regulated by exercise and nutrition in a tissue‐specific manner, will be a challenge for the field. Ultimately, a greater understanding of these relationships may yield insight into the connection between genes, behaviour and the development of metabolic diseases.
Additional information
Competing interests
We confirm that the authors do not have any conflicts of interests regarding the material in this review.
Author contributions
Both authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The authors are supported by grants from the Novo Nordisk Foundation (NNF14OC0011493, NNF14OC0009941, NNF18CC0034900), Swedish Research Council (2015‐00165) to J.R.Z., and L.D. is supported by a Novo Nordisk postdoctoral fellowship run in partnership with Karolinska Institutet.
Glossary.
Chrono nutrition: the concept of adapting the macronutrient content or timing of a meal to the circadian rhythm to affect whole‐body physiology and metabolism.
Genomic response: a response depending on gene transcription and protein synthesis events.
Time restricted feeding: daily eating pattern in which all nutrient intake occurs within a few hours.
Zeitgeber time: time as defined after the synchronization of the circadian system by a zeitgeber, an external cue that can reset the time‐keeping system of organisms.
Biographies
Lucile Dollet is currently a Post‐Doctoral fellow in the Department of Physiology and Pharmacology at Karolinska Institutet where she is studying mechanisms by which exercise training affects adipocyte metabolism and adipose–skeletal muscle cross talk in diabetes.

Juleen R. Zierath is Professor of Physiology at Karolinska Institutet and University of Copenhagen. She delivered The Physiological Society's Annual Review Prize Lecture in 2018. As an exercise physiologist, she has a long‐standing interest in the health‐promoting benefits of physical exercise. The ultimate goal of her work is to identify and validate molecular candidates to prevent or treat insulin resistance in type 2 diabetes. She is President of the European Association for the Study of Diabetes. She is a member of the Royal Swedish Academy of Sciences and the Nobel Assembly. She was Chair of the Nobel Committee at Karolinska Institutet between 2013 and 2015 and is currently a member of this Committee.
Edited by: Ole Petersen & Javier Gonzalez
This is an Editor's Choice article from the 15 March 2019 issue.
References
- Aldiss P, Betts J, Sale C, Pope M, Budge H & Symonds ME (2018). Exercise‐induced ‘browning’ of adipose tissues. Metabolism 81, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Vitaterna MH & Turek FW (2011). Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS One 6, e25079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G & Sassone‐Corsi P (2015). Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92. [DOI] [PubMed] [Google Scholar]
- Bescos R, Boden MJ, Jackson ML, Trewin AJ, Marin EC, Levinger I, Garnham A, Hiam DS, Falcao‐Tebas F, Conte F, Owens JA, Kennaway DJ & McConell GK (2018). Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol (Oxf) 223, e13039. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP & Spiegelman BM (2012). A PGC1‐α‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature 481, 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A, Jeanson Y, Berger‐Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F & Casteilla L (2014). Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, 3253–3265. [DOI] [PubMed] [Google Scholar]
- Cedernaes J, Schonke M, Westholm JO, Mi J, Chibalin A, Voisin S, Osler M, Vogel H, Hornaeus K, Dickson SL, Lind SB, Bergquist J, Schioth HB, Zierath JR & Benedict C (2018). Acute sleep loss results in tissue‐specific alterations in genome‐wide DNA methylation state and metabolic fuel utilization in humans. Sci Adv 4, eaar8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Lin T, Le HD, Chang MW & Panda S (2019). Time‐restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29, P303–319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L & Yang G (2014). PPARs integrate the mammalian clock and energy metabolism. PPAR Res 2014, 653017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Travers RL, Walhin JP, Gonzalez JT, Koumanov F, Betts JA & Thompson D (2017). Feeding influences adipose tissue responses to exercise in overweight men. Am J Physiol Endocrinol Metab 313, E84–E93. [DOI] [PubMed] [Google Scholar]
- Chudyk A & Petrella RJ (2011). Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta‐analysis. Diabetes Care 34, 1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig BW, Hammons GT, Garthwaite SM, Jarett L & Holloszy JO (1981). Adaptation of fat cells to exercise: response of glucose uptake and oxidation to insulin. J Appl Physiol Respir Environ Exerc Physiol 51, 1500–1506. [DOI] [PubMed] [Google Scholar]
- Craig BW, Thompson K & Holloszy JO (1983). Effects of stopping training on size and response to insulin of fat cells in female rats. J Appl Physiol Respir Environ Exerc Physiol 54, 571–575. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Viola AU, Tarokh L, Cajochen C & Brown SA (2012). The human circadian metabolome. Proc Natl Acad Sci U S A 109, 2625–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Kraemer WJ, Bush JA, Doughty TA, Kim D, Mullen KM & Ramsey K (1998). Biorhythmic influences on functional capacity of human muscle and physiological responses. Med Sci Sports Exerc 30, 1399–1407. [DOI] [PubMed] [Google Scholar]
- Drenowatz C, Hand GA, Sagner M, Shook RP, Burgess S & Blair SN (2015). The prospective association between different types of exercise and body composition. Med Sci Sports Exerc 47, 2535–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi‐Nagy E & Sul HS (2007). Regulation of lipolysis in adipocytes. Annu Rev Nutr 27, 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C & Cao L (2015). Adipose VEGF links the white‐to‐brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology 156, 2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, Abbondante S, Tognini P, Orozco‐Solis R, Kinouchi K, Wang C, Swerdloff R, Nadeef S, Masri S, Magistretti P, Orlando V, Borrelli E, Uhlenhaut NH, Baldi P, Adamski J, Tschop MH, Eckel‐Mahan K & Sassone‐Corsi P (2018). Atlas of circadian metabolism reveals system‐wide coordination and communication between clocks. Cell 174, 1571–1585.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel‐Mahan K & Sassone‐Corsi P (2013). Metabolism and the circadian clock converge. Physiol Rev 93, 107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B & Zierath JR (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17, 162–184. [DOI] [PubMed] [Google Scholar]
- Fabre O, Ingerslev LR, Garde C, Donkin I, Simar D & Barres R (2018). Exercise training alters the genomic response to acute exercise in human adipose tissue. Epigenomics 10, 1033–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O & Garaulet M (2018). The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocr Rev 39, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel BM & Zierath JR (2017). The limits of exercise physiology: From performance to health. Cell Metab 25, 1000–1011. [DOI] [PubMed] [Google Scholar]
- Gabriel BM & Zierath JR (2019). Circadian rhythm and exercise: re‐setting the clock in metabolic disease. Nat Rev Endocrinol, 10.1038/s41574-018-0150-x [DOI] [PubMed] [Google Scholar]
- Garaulet M, Ordovas JM, Gomez‐Abellan P, Martinez JA & Madrid JA (2011). An approximation to the temporal order in endogenous circadian rhythms of genes implicated in human adipose tissue metabolism. J Cell Physiol 226, 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart‐Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, Pryma DA, Khurana TS & Lazar MA (2013). The nuclear receptor Rev‐erbα controls circadian thermogenic plasticity. Nature 503, 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart‐Hines Z & Lazar MA (2015). Circadian metabolism in the light of evolution. Endocr Rev 36, 289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF & Goodyear LJ (2009). Effects of exercise training on subcutaneous and visceral adipose tissue in normal‐ and high‐fat diet‐fed rats. Am J Physiol Endocrinol Metab 297, E495–E504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Abellan P, Gomez‐Santos C, Madrid JA, Milagro FI, Campion J, Martinez JA, Ordovas JM & Garaulet M (2010). Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology 151, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Lee JY, Teraminami A, Kim YI, Hirai S, Uemura T, Inoue H, Takahashi N & Kawada T (2011). Activation of peroxisome proliferator‐activated receptor‐α stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res 52, 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T & Sassone‐Corsi P (2010). PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab 12, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH & Panda S (2012). Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab 15, 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ & Zierath JR (2014). Integrative biology of exercise. Cell 159, 738–749. [DOI] [PubMed] [Google Scholar]
- Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ & Caprio S (2001). Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab 86, 90–96. [DOI] [PubMed] [Google Scholar]
- Herrero L, Valcarcel L, da Silva CA, Albert N, Diez‐Noguera A, Cambras T & Serra D (2015). Altered circadian rhythm and metabolic gene profile in rats subjected to advanced light phase shifts. PLoS One 10, e0122570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husse J, Hintze SC, Eichele G, Lehnert H & Oster H (2012). Circadian clock genes Per1 and Per2 regulate the response of metabolism‐associated transcripts to sleep disruption. PLoS One 7, e52983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz D, Barnea M, Wainstein J & Froy O (2013). High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 21, 2504–2512. [DOI] [PubMed] [Google Scholar]
- Karastergiou K & Fried SK (2017). Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. Adv Exp Med Biol 1043, 29–51. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Knutsson A & Lindahl B (2001). Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadir A, Kavalakatt S, Cherian P, Warsame S, Abubaker JA, Dehbi M & Tiss A (2018). Physical exercise enhanced heat shock protein 60 expression and attenuated inflammation in the adipose tissue of human diabetic obese. Front Endocrinol (Lausanne) 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW & Bass J (2007). High‐fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–421. [DOI] [PubMed] [Google Scholar]
- Kolbe I, Carrasco‐Benso MP, Lopez‐Minguez J, Lujan J, Scheer F, Oster H & Garaulet M (2019). Circadian period of luciferase expression shortens with age in human mature adipocytes from obese patients. FASEB J 33, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TC, Lucassen EA, Sips HC, Chatzispyrou IA, Houtkooper RH, Meijer JH, Coomans CP, Biermasz NR & Rensen PC (2015). Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci U S A 112, 6748–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Pramyothin P, Karastergiou K & Fried SK (2014). Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta 1842, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A, Govendir MA, Emmett L & Greenfield JR (2016). Brown adipose tissue exhibits a glucose‐responsive thermogenic biorhythm in humans. Cell Metab 23, 602–609. [DOI] [PubMed] [Google Scholar]
- Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, Baer LA, May FJ, Gao F, Narain NR, Chen EY, Kiebish MA, Cypess AM, Bluher M, Goodyear LJ, Hotamisligil GS, Stanford KI & Tseng YH (2017). The cold‐induced lipokine 12,13‐diHOME promotes fatty acid transport into brown adipose tissue. Nat Med 23, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WH 3rd (1996). Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Rev 24, 203–231. [PubMed] [Google Scholar]
- Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R, Meiklejohn J, Climstein M, O'Sullivan A, de Vos N, Baune BT, Blair SN, Simar D, Rooney K, Singh N & Fiatarone Singh MA (2013). Changes in insulin resistance and HbA1c are related to exercise‐mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care 36, 2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N, Field MD & Hastings MH (1999). Rapid down‐regulation of mammalian Period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci U S A 96, 15211–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran‐Ramos S, Guerrero‐Vargas NN, Mendez‐Hernandez R, Basualdo MDC, Escobar C & Buijs RM (2017). The suprachiasmatic nucleus drives day‐night variations in postprandial triglyceride uptake into skeletal muscle and brown adipose tissue. Exp Physiol 102, 1584–1595. [DOI] [PubMed] [Google Scholar]
- Otway DT, Mantele S, Bretschneider S, Wright J, Trayhurn P, Skene DJ, Robertson MD & Johnston JD (2011). Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 60, 1577–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S ( 2016). Circadian physiology of metabolism. Science 354, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA & Fitzgerald GA (2012). Obesity in mice with adipocyte‐specific deletion of clock component Arntl. Nat Med 18, 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM & Bass J (2017). Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 25, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L, Loizides‐Mangold U, Skarupelova S, Pulimeno P, Chanon S, Robert M, Bouzakri K, Modoux C, Roux‐Lombard P, Vidal H, Lefai E & Dibner C (2015). Human skeletal myotubes display a cell‐autonomous circadian clock implicated in basal myokine secretion. Mol Metab 4, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovarova O, Gogebakan O, Sucher S, Groth J, Murahovschi V, Kessler K, Osterhoff M, Rudovich N, Kramer A & Pfeiffer AF (2016). Regulation of the clock gene expression in human adipose tissue by weight loss. Int J Obes (Lond) 40, 899–906. [DOI] [PubMed] [Google Scholar]
- Quesada‐Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda‐Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL & Villarroya F (2016). The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 7, 13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L, Akyol M, Fletcher A, Forsythe J, Nixon M, Carter R, van Beek E, Morton N, Walker B & Stimson R (2016). Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species‐specific differences in UCP‐1 regulation. Cell Metab 24, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA & Spiegelman BM (2014). Meteorin‐like is a hormone that regulates immune‐adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas‐Latre A, Fekry B, Kwok C, Baumgartner C, Shivshankar S, Sun K, Chen Z & Eckel‐Mahan K (2019). Rosiglitazone reverses high fat diet‐induced changes in BMAL1 function in muscle, fat, and liver tissue in mice. Int J Obes (Lond) 43, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM & Gerszten RE (2014). β‐Aminoisobutyric acid induces browning of white fat and hepatic beta‐oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 19, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronn T, Volkov P, Tornberg A, Elgzyri T, Hansson O, Eriksson KF, Groop L & Ling C (2014). Extensive changes in the transcriptional profile of human adipose tissue including genes involved in oxidative phosphorylation after a 6‐month exercise intervention. Acta Physiol (Oxf) 211, 188–200. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS & Shea SA (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 106, 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF & Shea SA (2010). Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 107, 20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder EA & Esser KA (2013). Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc Sport Sci Rev 41, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S & Spiegelman BM (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Orlova C, Ayers RT & Mantzoros CS (2005). Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab 90, 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak A, Husse J & Oster H (2013a). Circadian regulation of adipose function. Adipocyte 2, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shostak A, Meyer‐Kovac J & Oster H (2013b). Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 62, 2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene DJ, Skornyakov E, Chowdhury NR, Gajula RP, Middleton B, Satterfield BC, Porter KI, Van Dongen HPA & Gaddameedhi S (2018). Separation of circadian‐ and behavior‐driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc Natl Acad Sci U S A 115, 7825–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI & Goodyear LJ (2018). Muscle‐adipose tissue cross talk. Cold Spring Harb Perspect Med 8, a029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, Chen EY, Gao F, Narain NR, Distefano G, Shettigar VK, Hirshman MF, Ziolo MT, Kiebish MA, Tseng YH, Coen PM & Goodyear LJ (2018). 12,13‐diHOME: An exercise‐induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27, 1111–1120.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ & Goodyear LJ (2015a). Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes 64, 2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH & Goodyear LJ (2015b). A novel role for subcutaneous adipose tissue in exercise‐induced improvements in glucose homeostasis. Diabetes 64, 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E & Peterson CM (2018). Early time‐restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27, 1212–1221.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend LK & Wright DC (2019). Looking on the “brite” side exercise‐induced browning of white adipose tissue. Pflugers Arch (in press; 10.1007/s00424-018-2177-1). [DOI] [PubMed] [Google Scholar]
- Tsiloulis T, Carey AL, Bayliss J, Canny B, Meex RCR & Watt MJ (2018). No evidence of white adipocyte browning after endurance exercise training in obese men. Int J Obes (Lond) 42, 721–727. [DOI] [PubMed] [Google Scholar]
- van den Berg R, Kooijman S, Noordam R, Ramkisoensing A, Abreu‐Vieira G, Tambyrajah LL, Dijk W, Ruppert P, Mol IM, Kramar B, Caputo R, Puig LS, de Ruiter EM, Kroon J, Hoekstra M, van der Sluis RJ, Meijer OC, Willems van Dijk K, van Kerkhof LWM, Christodoulides C, Karpe F, Gerhart‐Hines Z, Kersten S, Meijer JH, Coomans CP, van Heemst D, Biermasz NR & Rensen PCN (2018). A diurnal rhythm in brown adipose tissue causes rapid clearance and combustion of plasma lipids at wakening. Cell Rep 22, 3521–3533. [DOI] [PubMed] [Google Scholar]
- van der Veen DR, Shao J, Chapman S, Leevy WM & Duffield GE (2012). A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET‐FDG imaging. Obesity (Silver Spring) 20, 1527–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Moorsel D, Hansen J, Havekes B, Scheer FA, Jorgensen JA, Hoeks J, Schrauwen‐Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MK, Staels B & Schrauwen P (2016). Demonstration of a day‐night rhythm in human skeletal muscle oxidative capacity. Mol Metab 5, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboven K, Stinkens R, Hansen D, Wens I, Frederix I, Eijnde BO, Jocken JWE, Goossens GH & Blaak EE (2018). Adrenergically and non‐adrenergically mediated human adipose tissue lipolysis during acute exercise and exercise training. Clin Sci (Lond) 132, 1685–1698. [DOI] [PubMed] [Google Scholar]
- Vetter C, Dashti HS, Lane JM, Anderson SG, Schernhammer ES, Rutter MK, Saxena R & Scheer F (2018). Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 41, 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg‐Henriksson H & Zierath JR (2015). Metabolism: Exercise remodels subcutaneous fat tissue and improves metabolism. Nat Rev Endocrinol 11, 198–200. [DOI] [PubMed] [Google Scholar]
- Walton RG, Finlin BS, Mula J, Long DE, Zhu B, Fry CS, Westgate PM, Lee JD, Bennett T, Kern PA & Peterson CA (2015). Insulin‐resistant subjects have normal angiogenic response to aerobic exercise training in skeletal muscle, but not in adipose tissue. Physiol Rep 3, 12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP & Holloszy JO (2007). Improvements in body composition, glucose tolerance, and insulin action induced by increasing energy expenditure or decreasing energy intake. J Nutr 137, 1087–1090. [DOI] [PubMed] [Google Scholar]
- Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, Ma Z, Zhao H, Chen P, Qi X & Zhang EE (2017). Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab 25, 73–85. [DOI] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ & Evans RM (2006). Nuclear receptor expression links the circadian clock to metabolism. Cell 126, 801–810. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liu M, Chan XY, Tan SY, Subramaniam S, Fan Y, Loh E, Chang KTE, Tan TC & Chen Q (2017). Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood 130, 1995–2005. [DOI] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL & Gimble JM (2006). Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55, 962–970. [DOI] [PubMed] [Google Scholar]


