Abstract
Reactive oxygen and nitrogen species (RONS) are formed as byproducts of many endogenous cellular processes, in response to infections, and upon exposure to various environmental factors. An increase in RONS can saturate the antioxidation system and leads to oxidative stress. Consequently, macromolecules are targeted for oxidative modifications, including DNA and protein. The oxidation of DNA, which leads to base modification and formation of abasic sites along with single and double strand breaks, has been extensively investigated. Protein oxidation is often neglected and is only recently being recognized as an important regulatory mechanism of various DNA repair proteins. This is a review of the current state of research on the regulation of DNA repair by protein oxidation with emphasis on the correlation between inflammation and cancer.
Keywords: Oxidative stress, DNA damage, oxidation of DNA repair proteins, double strand break repair, base excision repair
1. Introduction
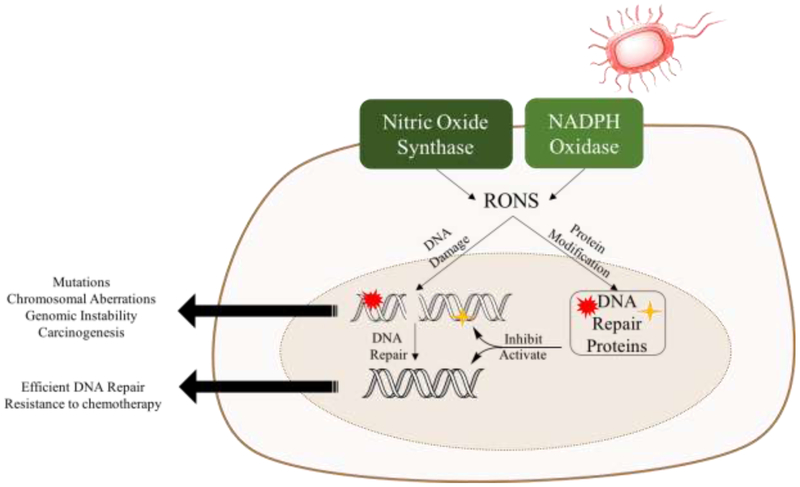
Balanced reduction and oxidation reactions (redox), which are mediated by reactive oxygen and nitrogen species (RONS), are important to maintain cellular homeostasis as they play a key role in cell signaling [1-3]. About 109 RONS are produced per cell per hour as a result of oxidative phosphorylation, NADPH oxidation, histone demethylation, along with other reactions [4-7]. In addition, environmental factors, such as ultraviolet light (UV) and pesticides, along with other toxins, lead to RONS production [8, 9]. RONS act as secondary messengers in various physiological processes including vasodilation, thrombosis, neuronal activity, transcription activation, and also in response to pathogens (e.g. macrophages release nitric oxide for the defense against pathogens) [10-15]. It is essential to tightly regulate RONS levels in order to maintain cellular integrity and prevent macromolecular damage. This is achieved by a number of functionally redundant radical scavengers such as peroxiredoxins, glutathione and peroxidases [16-18]. Glutathione is the most abundant oxygen radical scavenger in the cell (0.1-10 mM), localized mainly in the cytosol (90%), with a small percentage in the nucleus [19, 20]. Additionally, superoxide dismutase converts superoxide into hydrogen peroxide, which is converted into water by catalase [21]. RONS scavengers are reversibly oxidized to maintain cells under reducing conditions, which prevents nonspecific protein modifications [22]. Elevated levels of RONS can saturate the antioxidant machinery, leading to oxidative stress [23, 24]. For example, as a result of the inflammatory response to pathogens, RONS are produced by NADPH oxidase and nitric oxide synthase, leading to oxidative stress [25-30]. Inflammation and oxidative stress are both hallmarks for cancer, which is a disease of genomic instability [31-34]. Thus, the relationship between genomic instability, inflammation, and oxidative stress in cancer merits a deeper appreciation [35]. Specifically, inflammation is correlated with cancer progression and has been shown to increase the incidence of cancer, contributing to 25% of cancer cases [34-36]. Moreover, in response to pathogens, macrophages and neutrophils release cytokines and RONS, leading to damage of both DNA and the repair proteins, which results in genomic instability (Figure 1) [37]. Additionally, the cytokine TNFα is a driver of inflammation and is produced at high levels in tumors, leading to oxidative stress and subsequently genomic instability [38-40]. Therefore, activation of the inflammatory response increases the risk of oxidation of DNA and proteins, which increases the risk for cancer (Figure 1) [25, 34, 35, 41].
Figure 1:
Exposure to pathogens triggers the production of RONS by nitric oxide synthase and NADPH oxidase. Consequently, DNA and proteins are oxidized. Depending on the effect of oxidative modification on protein function, DNA repair can be inhibited, which results in the accumulation of damage and leading to carcinogenesis. Alternatively, protein modification may activate DNA repair, which may result in resistance to chemotherapy.
DNA is an unstable molecule, making it susceptible to damage with about 20,000-70,000 lesions forming per cell per day under physiological conditions [42-44]. Oxidation is the most predominant type of DNA damage with more than 100 different oxidized lesions identified [44-46]. The most abundant type of oxidized lesion is 8-oxoguanine [47]. Cancer cells accumulate 8-oxoguanine at a frequency of 105 per cell per day [44, 48]. To prevent the accumulation of damaged DNA, various repair pathways are utilized in the cell to target specific lesion types [43, 49-53]. Thus, a deficiency in DNA repair leads to a mutator phenotype and to genomic instability [33, 54].
In addition to damaging DNA, oxidative stress can target proteins, specifically DNA repair proteins, which may further regulate levels of DNA damage [55-58]. Under oxidative stress, cysteine residues (Cys) are targeted for modification because of the oxidizable sulfhydryl group [57, 59]. Protein oxidation has been shown to act as a post-translational modification, which regulates protein structure and function, allowing cells to adapt to the environment by activating or inhibiting different cellular functions [60, 61]. Oxidation has also been proposed to diversify many protein functions by two orders of magnitude [62-64]. Alternatively, oxidative modification can cause nonspecific and irreversible damage to the protein’s function [65]. Though protein oxidation is an important regulatory mechanism, it is often overlooked that proteins involved in the recognition and repair of DNA damage can also be modified. Oxidation of DNA repair proteins by RONS can act as a specific regulatory mechanism of protein structure and function (activation vs inhibition) [66, 67]. Oxidative modification can also act to select a DNA repair pathway [5, 68, 69]. Alternatively, oxidation can lead to nonspecific inactivation of DNA repair [70]. Thus, modulating DNA repair by oxidative modification can amplify or attenuate the effect of RONS on DNA damage. As a consequence of DNA repair inhibition, DNA lesions accumulate, leading to genomic instability and cancer [44]. Alternatively, activating repair proteins leads to efficient DNA repair, and potentially resistance to chemo- and radiotherapy (Figure 1) [71, 72].
This review focuses on the effect of oxidation on the activity of DNA repair proteins involved in the repair of double strand breaks (DSBs) and base damage (Table 1) and how oxidation may contribute to genomic instability and lead to cancer. It is important to understand the effect of oxidative stress on DNA repair in order to design therapeutic targets for cancer treatment and to understand the resistance of certain cancers to chemo- and radiotherapy.
Table 1.
DNA repair proteins targeted for oxidation regulation.
| Name of protein |
Pathway | Potential target site |
Effect on activity | Reference |
|---|---|---|---|---|
| ATM | DSB repair | C-terminal Kinase domain | Activate alternate function | [66, 73-75] |
| XRCC3 | DSB repair, HDR | C86, C328 | Inhibit DNA synthesis | [76] |
| Ku | DSB repair, NHEJ | C-terminus | Reduced DNA binding, inhibit repair | [67] |
| DNA-PKcs | DSB repair, NHEJ | Unknown | Activation or Inhibition, depending on exposure | [77] |
| MGMT | Direct reversal, MGMT | Cys145 | Inhibition of activity and protein degradation | [78, 79] |
| AAG | BER | Cys167 | Decrease specificity, increase activity | [80] |
| OGG1 | BER | Cys, unknown | Inhibition of activity | [70, 81] |
| APE1 | BER | Cys65, Cys99 | Alter localization; reduced DNA binding | [82, 83] |
| PARP1 | BER | Zinc finger domain | Reduced DNA binding | [84, 85] |
| XRCC1 | BER | Cys12, Cys20 | Increased affinity to polβ | [86] |
2. Repair of double strand breaks
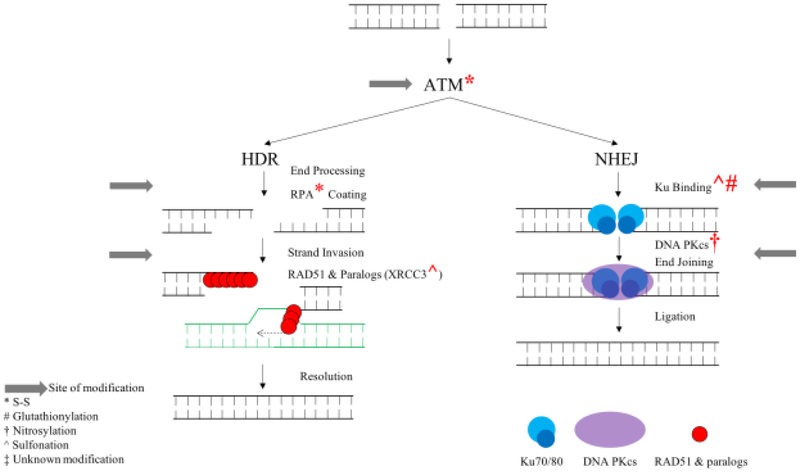
DSBs occur from exposure to ionizing radiation and also from collapsed replication forks that encounter damage [87]. The inability of cells to repair DSBs induces cell death [88-90]. Additionally, inaccurate repair leads to the formation of chromosomal translocations, deletions, and mutations, which can result in genomic instability [33, 91]. DSBs are mainly repaired by two pathways, homology directed repair (HDR) or nonhomologous end joining (NHEJ) [92]. HDR occurs during the G2 and S phases of the cell cycle and requires a sister chromatid for accurate repair [93]. On the other hand, NHEJ occurs during the G1 phase of the cell cycle and allows the rejoining of DNA ends, which can produce deletions and insertions [94]. Various members of these repair pathways are prone to oxidation (Figure 2, Table 1), which results in either regulating specific activities, inhibiting repair, or coordinating the selection of DNA repair pathway. For example, in the presence of hydrogen peroxide and DNA breaks, NHEJ is reduced and HDR is induced [68]. Therefore, it is important to understand the effect of oxidative stress on proteins involved in the repair of DSBs (Figure 2).
Figure 2:
The effect of RONS on the repair of double strand breaks by HDR and NHEJ.
2.1. ATM
Ataxia-telangiectasia mutated protein kinase (ATM) is a member of the PI3K-like protein kinase family [95]. It is a 350 kDa protein with 3,056 amino acids (89 Cys). ATM is activated in the presence of damage by the Mre11-Rad50-Nbs1 (MRN) complex, which further activates the repair of DSBs [96]. In the presence of DSBs, ATM is converted into the active monomer from the inactive homodimer in an MRN-dependent manner [97]. The kinase domain of ATM is located in the C-terminus and functions as a serine/threonine kinase [98]. Autophosphorylation of ATM triggers the recruitment and activation of a myriad of DNA repair proteins including p53 and Chk2, which are important for regulating the cell cycle [95, 97, 99-102]. Loss of a functional ATM is correlated with genomic instability and with ataxia-telangiectasia (A-T) syndrome [103]. Patients with this syndrome also exhibit high levels of oxidative stress [104].
It has been recently shown that ATM is regulated by the oxidative modification of Cys, which sensitizes ATM to cellular redox state [66, 95]. Oxidation is proposed to act as a regulatory mechanism along with phosphorylation and acetylation [66, 105]. In the presence of oxidative stress, ATM has been shown to increase its activity by phosphorylating downstream proteins [106]. Additionally, under oxidative stress, ATM can be activated, as indicated by autophosphorylation and by phosphorylation of p53 and Chk2, independent of MRN and in the absence of DNA damage (as indicated by lack of γH2AX staining) [66, 73]. The affinity of ATM to its substrates, p53 and ATP, is also increased in the presence of hydrogen peroxide independent of MRN, suggesting that the oxidized ATM undergoes conformational changes that allow it to bind tightly to its substrate [66, 73]. Furthermore, phosphorylation of p53 by ATM (independent of MRN) was inhibited in the presence of the antioxidant N-acetyl-cysteine (NAC), indicating that Cys modification may be responsible for the redox sensitivity of ATM [66, 73]. It has been shown that the active form of ATM is a dimer in the presence of hydrogen peroxide, as opposed to a monomer in the case of MRN activation [66, 73, 97]. Further analysis identified that the active dimer is linked by a disulfide bond using a Cys located near the kinase domain (Cys2991). A mutation at this Cys prevents the redox sensitivity of ATM but retains its ability to be activated by DNA damage and MRN [66, 74]. These results indicate that the oxidation state of this Cys allows for a separation of function between the two activation pathways of ATM (through MRN or by oxidative stress).
Furthermore, in vitro analysis of an ATM truncation mutation present in A-T patients, where the last 10 amino acids of ATM are deleted (R3047X), suggests that the R3047X variant is insensitive to oxidative stress, similar to the Cys mutant [66, 73]. This truncation mutation is located near the redox-sensitive Cys2991. Both the Cys and truncation mutants had similar responses to that of the wild-type in the presence of camptothecin (CPT), which is a non-oxidative stress DNA-damage-inducing agent [66, 73]. Thus, the Cys and truncation mutants can be activated by DNA damage but not by oxidative stress [66, 73]. These findings led to the conclusions that ATM acts to sense oxidative stress in cells and this may be important for a novel function of ATM, which is independent of DNA damage [66, 74, 75, 107]. The ability of ATM to respond to oxidative stress by forming disulfide bonds may activate an alternate cellular response pathway that is necessary to prevent DNA damage by oxidative stress. Therefore, this activity is specifically important under oxidative stress conditions, when DNA is targeted for damage.
2.2. XRCC3
X-ray repair cross complementing 3 (XRCC3) is a Rad51 paralog important for DNA repair by HDR [108, 109]. It contains 346 amino acids, eight of which are Cys, and has a molecular weight of 37 kDa. XRCC3 deficiency has been shown to impair RAD51 foci formation and inhibit HDR, which results in genomic instability [110, 111].
Upon exposure of cells to UVA, which produces singlet oxygen in the presence of photosensitizers (6-thioguanine, 6TG), DNA synthesis is inhibited, although HDR is stimulated [8, 76, 112]. This indicates that HDR does not contribute to the effect of UVA on DNA synthesis [76]. This phenomenon prompted a study on the sensitivity of XRCC3 to oxidative stress caused by UVA. When cells were exposed to UVA, a change in the electrophoretic mobility of XRCC3 was observed, suggesting a potential conformational change as a result of oxidation [76]. Additionally, a reduction in the ability to detect XRCC3 with a C-terminal-specific antibody confirmed that the C-terminus undergoes a conformational change that occludes the antibody detection region [76]. The C-terminus of XRCC3 contains Cys328, which can be a target site for oxidation. The oxidative sensitivity was reversible in the presence of a reducing agent, confirming the contribution of Cys oxidation [76]. Additionally, this effect was abolished when all Cys residues were mutated to Ser.
The specificity of XRCC3 oxidation to singlet oxygen was confirmed because in the presence of singlet oxygen scavengers (NaN3, L-Histidine) oxidation of XRCC3 was prevented [76]. Molecular modeling studies suggest that two Cys residues (Cys86 and Cys328) can form an intermolecular disulfide bond in the presence of oxidants [76]. Cells expressing XRCC3 with either the Cys86Ser or Cys328Ser mutation have similar sensitivity to CPT as the wild-type, however, when all Cys residues were mutated into Ser, the cells were more sensitive to CPT treatment. This provides evidence that Cys residues, other than Cys86 and Cys328, are important for HDR [76]. Furthermore, Cys221 modification, which is located near a phosphorylation site (Ser225), may affect XRCC3 phosphorylation by ATM and ATR upon the induction of DNA damage [113]. This indicates that Cys residues in XRCC3 are not only important for the redox sensitivity effect, but also for the response to DNA damage [76]. Therefore, the oxidation state of XRCC3 may serve to differentiate two functions of XRCC3 in response to oxidative stress or DNA damage.
2.3. Ku
Ku is a heterodimer protein composed of two subunits with molecular weights 70 and 80 kDa (Ku70 and Ku80). This complex is important in the repair of DSBs as part of the NHEJ pathway [114]. It has been shown that Ku forms a ring at the DNA ends and functions to rejoin DNA ends [67, 114]. This complex binds DNA with high affinity and translocates along the DNA, though the mechanism of dissociation is unknown [115]. Previous work suggests that Ku is prone to oxidation by UVA, which inhibits NHEJ [116]. Specifically, it has been shown that Ku binding to DNA is inhibited in the presence of oxidative stress and that this inhibition is reversible in the presence of a reducing agent [67]. Upon oxidation, Ku undergoes a conformational change that leads to higher rates of dissociation from DNA, which reduces its binding affinity [117]. Furthermore, limited tryptic digestion suggests that Ku80 undergoes conformational changes dependent on the redox state of the protein. Ku is more prone to limited tryptic digestion under reduced conditions as compared to the oxidized form [67]. As part of the complex, Ku80 is a 732 amino acid protein with ten Cys residues. Cys493 and Cys638 of Ku80 were identified as being susceptible to oxidative modification [67, 118]. Both of these residues are located in the C-terminus, which is important for the recruitment of the downstream binding partner, DNA-PKcs [114]. Furthermore, it has been shown that binding of Ku to DNA-PKcs is sensitive to the redox state of Ku [118]. This suggests that a reduced state of Ku is required for DNA binding and for recruiting downstream proteins. The inhibitory effect of oxidative stress on Ku suggests that oxidation may regulate the selection of the DNA repair pathway at an early stage in the repair process, which may help the cell decide on the specific repair pathway.
2.4. DNA-PKcs
DNA dependent protein kinase catalytic subunit (DNA-PKcs) is important for the repair of DSBs by NHEJ [119]. It is important for the early stage detection of DNA damage and for DNA repair [119]. This protein has 4128 amino acids (87 Cys) and has a molecular weight of 469 kDa. DNA PKcs has been shown to be sensitive to oxidative stress and to be targeted for nitrosylation [120, 121]. However, the effect of nitrosylation on activity has not been identified. Nitrosylation has also been shown to have an indirect effect on DNA-PKcs. In the presence of nitric oxide (NO), the expression levels of DNA-PKcs increase 5-fold due to higher binding of the transcription factor, SP1, to the promoter region of DNA PKcs [122]. This also results in a 4-fold increase in activity, which may provide a protective role against DNA damage [122]. NO is produced as a result of an inflammatory response by nitric oxide synthase [123]. Therefore, nitrosylation of DNA repair proteins may define the relationship between inflammation and cancer. It may also help guide future studies into the resistance of certain cancers to chemo- and radiotherapy.
Additional studies suggest that sustained production of RONS sensitizes cells to genotoxic drugs by reducing the kinase activity of DNA-PKcs [77]. As a result, DNA repair is inhibited and toxic DSBs accumulate in the cell [77]. Therefore, the reactivity and specificity of DNA-PKcs towards RONS may determine the effect on its activity.
3. Base Excision Repair
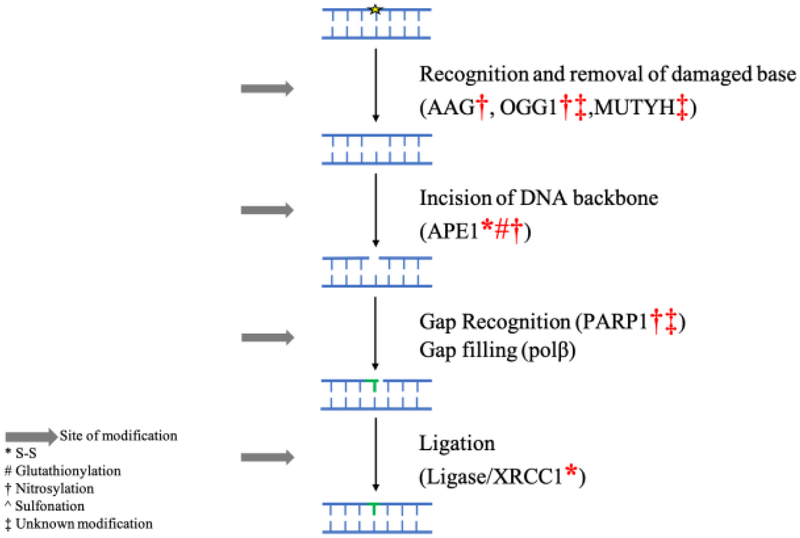
Base excision repair (BER), along with direct removal of damage by specialized enzymes, is responsible for the repair of a large percentage of base lesions [124]. This includes the repair of oxidative and methylated lesions, along with abasic sites and single strand breaks, in order to maintain genomic stability [124]. The inability to repair DNA damage results in propagation of errors, DNA breaks, and in genomic instability [33]. Various members of the BER pathway have been shown to be modified and inhibited under oxidative stress. This inactivation may fully account for the accumulation of DNA damage in the presence of oxidative stress and under inflammatory conditions. Therefore, inhibition of BER by oxidative stress helps with defining the relationship between oxidative stress and cancer (Figure 3). Furthermore, the inactivation of BER may act as a stress signal to trigger the activation of alternative pathways in order to conserve energy and invest it into essential functions that promote cell survival. Though this area needs to be investigated. The current state of literature regarding the oxidative modification of BER proteins is presented (Table 1).
Figure 3:
The effect of RONS on the capacity of BER to repair small base lesions and single strand breaks.
3.1. AAG
Alkyl-adenine DNA glycosylase (AAG) is a monofunctional glycosylase important for the recognition and removal of methylated DNA bases as part of the BER pathway [125]. It is composed of 298 amino acids, seven of which are Cys, and has a molecular weight of 33 kDa. Treatment of cells with methyl methanesulfonate (MMS) results in the production of methylated DNA lesions, which are recognized and removed by AAG [126]. MMS treatment results in the accumulation of BER DNA intermediates but to a greater extent in the presence of GSNO [80]. Levels of BER intermediates formed immediately after MMS treatment were similar in the presence and absence of GSNO. However, when cells were allowed to recover from MMS treatment, cells treated with GSNO had higher levels of BER intermediates as compared to no GSNO treatment. Additionally, in the absence of AAG, GSNO treatment did not increase levels of alkylated bases. This indicates that GSNO treatment induces cells to be susceptible to damage from MMS in an AAG dependent manner [80].
Furthermore, studies have shown that GSNO can transfer an NO group onto AAG, which enhances the activity of AAG [80]. Therefore, GSNO leads to the activation of BER by AAG and results in increased production of abasic sites. In the presence of GSNO, AAG has been shown to be nitrosylated at the active site Cys167 leading to increased activity [127]. Though a mutation at this site completely inhibits AAG activity, modification of Cys167 is proposed to decrease substrate specificity of AAG [80]. High levels of GSNO also inhibit the downstream protein APE1 (see below), which leads to the accumulation of abasic sites [80]. Abasic sites are toxic to the cells. An adenine can be misincorporated opposite the abasic site, which leads to increased mutations [128]. Alternatively, abasic sites are prone to hydrolysis, leading to single strand breaks and ultimately DSBs [129]. Therefore, accumulation of abasic sites, resulting from increased activity of AAG in the presence of GSNO, can be detrimental to the cells.
3.2. OGG1
8-oxoguanine glycosylase is a bifunctional glycosylase important for the recognition and removal of oxidized DNA bases, creating abasic sites [130]. Additionally, OGG1 has a slow lyase activity, which cleaves the DNA backbone, creating single strand breaks [130]. Studies performed with OGG1 null mice suggest that OGG1 is solely responsible for removal of 8-oxoguanine lesions [124]. It is a 39 kDa protein with 345 amino acids, eight of which are Cys. OGG1 has been proposed to be sensitive to oxidative stress by various oxidants. When the cellular redox state is altered from exposure to cadmium, OGG1 activity is inhibited [70]. Cadmium is an environmental toxin that exerts an effect on glutathione, leading to its depletion and accumulation of RONS [131]. It has been shown that cadmium treatment results in OGG1 inhibition in vitro independent of chelating agents but dependent on antioxidants (such as NAC) [70]. Cadmium treatment also results in a shift in the electrophoretic mobility of OGG1, which is reversible in the presence of reducing agents, indicating that cadmium changes the oxidation state of OGG1 [70]. Therefore, it is suggested that the oxidation state of Cys residues in OGG1 is modified in the presence of cadmium, which alters the activity of OGG1 [70]. Additionally, OGG1 has been shown to be regulated by nitrosylation [81]. Cells exhibit sensitivity to NO in a manner dependent on OGG1, which suggests that nitrosylation inhibits OGG1 [81]. These studies were initiated because NO release causes an accumulation of oxidized DNA bases even though it does not itself oxidize DNA directly [132]. Lastly, singlet oxygen, which is produced from UVA exposure, has also been shown to modify and inhibit OGG1, resulting in the accumulation of oxidative DNA damage [8, 116].
The sensitivity of OGG1 to oxidation state has also been observed in the presence of a common OGG1 cancer-associated variant, Ser326Cys, which is associated with various cancers (lung [133]. gastric [134] along with others). This variant has been shown to be redox sensitive as a result of the introduced Cys residue [135-137]. Ser326Cys exhibits minimal catalytic alterations (2-fold reduction in glycosylase rates), however, under oxidative stress, it is inactivated [138]. Specifically, the glycosylase activity of Ser326Cys OGG1 is abolished upon exposing cells to oxidative stress inducing agents (TNFα or hydrogen peroxide) [138]. Though these agents can generate DNA damage directly, they also inhibit the enzyme responsible for the repair, which amplifies the effect of oxidative stress on DNA. Ser326Cys has been shown to form disulfide bonds in the presence of oxidative stress, which results in the dimerization of OGG1 [138, 139]. The dimer is proposed to bind DNA in a nonproductive manner, preventing activity [138]. The inhibition of Ser326Cys is reversed in the presence of NAC, which suggests that the Cys in Ser326Cys is responsible for the inhibition by oxidants [138]. Along with sensitivity to TNFα, cells expressing this variant are sensitive to NO [81]. The variant loses the ability to bind DNA tightly and to coordinate with additional BER steps [81]. As a result, DNA damage accumulates, which results in genomic instability [81]. The effect of this variant on genomic instability is more pronounced with inflammation. TNFα is a cytokine produced as part of an inflammatory response and causes high oxidative stress [140]. Additionally, NO is also released as part of an inflammatory response [123]. Therefore, the studies discussed above, which suggest that OGG1 is inhibited in the presence of TNFα and NO, highlight the relationship between inflammation and cancer risk.
In addition to repair by OGG1, oxidative DNA damage can also be repaired by MUTYH, a glycosylase with a complementary function to OGG1 [48]. MUTYH removes the adenine misincorporated opposite 8-oxoG by polymerases [48]. It is composed of 546 amino acids, twelve of which are Cys, with a molecular weight of 60 kDa. Mutations in MUTYH are associated with increased mutation frequency in the adenomatous polyposis coli gene (APC), which is a highly mutated gene in familial adenomatous polyposis [141, 142]. Therefore, individuals carrying mutations in MUTYH are at higher risk of developing colorectal cancer [143]. As a result, dysregulation of MUTYH activity is an important risk factor for developing cancer. MUTYH contains an iron sulfur cluster, which makes the enzyme susceptible to redox modification [144]. Consequently, MUTYH has been shown to be inhibited in the presence of oxidative stress by UVA [116]. This suggests that RONS produced from exposure to UVA can inhibit two of the enzymes responsible for detecting and removing oxidized DNA damage [116].
3.3. APE1
Apurinic/apyrimidinic endonuclease 1 (APE1) is composed of 318 amino acids (seven Cys) with a molecular weight of 35.5 kDa. The overexpression of APE1 is associated with resistance to chemotherapy [145]. APE1 is important in BER as an endonuclease that cleaves the DNA backbone 5’ of abasic sites to create DNA ends with 3’OH and 5’ deoxyribose phosphate [146, 147]. In addition to its function in BER, APE1 acts as a nuclear redox factor that regulates the redox state of various transcription factors, including AP-1, NFκB, HIF1α, and p53 [148]. The two APE1 functions are shown to be independent of each other [149]. The N-terminus of APE1 (35-127) is responsible for protein-protein binding and for the redox function of APE1, and the C-terminus is responsible for the endonuclease activity [149, 150]. The redox domain contains three Cys residues. Though no disulfide bond is observed in the crystal structure, many have proposed that APE1 exhibits redox sensitivity by forming a disulfide bond [151]. Specifically, Cys65 has been shown to be important for the redox activity of APE1 [151]. A Cys65Ser mutation inhibits the redox function of APE1 by affecting folding and localization [82]. When Cys65 is oxidatively modified, APE1 is proposed to undergo conformational changes, which alters the subcellular localization of APE1 by exposing a nuclear export signal [82]. Additionally, nitrosylation at Cys93 and Cys310 allows APE1 to translocate from the nucleus to the cytoplasm, leading to accumulation of abasic sites [152]. APE1 is mainly localized in the nucleus, however, various cancers have high levels of cytoplasmic APE1, such as colon, breast and hepatocellular cancers [153]. Therefore, nitrosylation of APE1 and its effect on localization may act as a driving force for carcinogenesis. It has also been suggested that a known APE1 inhibitor, E3330, triggers the formation of a disulfide bond between Cys65 and Cys138, which is proposed to be the mechanism of action of this inhibitor [154].
The effect of oxidative modification on the endonuclease activity has also been documented [83]. In the presence of oxidative stress, APE1 has been shown to be modified by glutathionylation at Cys99 in a reversible manner in the presence of reducing agents. Glutathionylation of APE1 leads to reduced DNA binding capacity, which inhibits the endonuclease activity [83].
3.4. PARP1
Poly(ADP-ribose) polymerase 1 (PARP1) is a nuclear protein involved in the repair of DNA breaks through the BER pathway [155, 156]. It is a 113 kDa protein with 1,014 amino acids, fourteen of which are Cys. PARP1 binds DNA breaks and catalyzes autoribosylation using NAD+ as a substrate, this in turn signals for additional repair proteins to be recruited to the break site [156, 157]. PARP1 contains 3 zinc finger domains, which are important for DNA-protein binding [158]. In these domains, a zinc is coordinated by either 4 Cys residues (C4), 3 Cys and 1 Histidine (C3H1) or C2H2 [159]. The presence of zinc is important structurally and also to protect Cys residues from oxidative modification [84]. In the presence of heavy metals, arsenic for example, zinc is released, which sensitizes the Cys residues to oxidation and results in inhibition of DNA binding [84, 85]. Specifically, arsenic has been shown to replace zinc in two zinc motifs C3H1 and C4, which inhibits PARP1 and allows the coordinating Cys residues to become more sensitive to cellular RONS [84].
In addition to being prone to inhibition by heavy metals, it has been suggested that nitrosylation acts as a regulatory mechanism of PARP1 activity [160]. Upon nitrosylation, the coordination of zinc is disrupted, which inhibits PARP1 activity and results in accumulation of DNA damage. NO is a released by macrophages in the presence of pathogens as a defense mechanism [123]. Thus, the correlation between inflammation and cancer can be deduced from inhibition of DNA repair factors.
3.5. XRCC1
XRCC1 is composed of 633 amino acids (six Cys) with a molecular weight of 69.5 kDa. It is a scaffolding protein involved in recruiting proteins as part of the BER pathway and is shown to interact with PARP1, ligase III, APE1, and DNA polymerase beta (polβ) [161]. The N-terminal domain of XRCC1, which contains two redox sensitive Cys residues, has been shown to interact with polβ [162]. These Cys residues, Cys12 and Cys20, form a disulfide bond in the presence of an oxidant, leading to conformational changes at the N-terminus [86]. Oxidation of XRCC1 results in a 6.4 Å shift between Cys12 and Cys20, from 8.5 Å in the reduced to 2.1 Å in the oxidized form [86]. This shift changes the binding affinity of XRCC1 to polβ. When oxidized, XRCC1 binds 25-fold more tightly to polβ as compared to the reduced state [86]. This change in affinity occurs because XRCC1 oxidation makes the interaction interface with polβ more accessible. Cys12 is important for recruiting polβ; expressing the XRCC1 with Cys12Ala in cells leads to slower recruitment of polβ and faster rate of release [86]. Additionally, a proline carbamate adduct has been shown to form at position Pro2 from exposure to CO2. This adduct results in stabilizing the oxidized state of XRCC1 [163]. Since XRCC1 is important for recruiting various members of the BER pathway, the effect of oxidation on conformational changes will have a direct effect on BER efficiency and DNA repair.
4. Direct Reversal Pathway, MGMT
O6 methylguanine methyl transferase (MGMT) is important for the repair of O6 methyl guanine [125]. It contains 207 amino acids, five of which are Cys, and has a molecular weight of 21 kDa. O6 methylguanine is a mutagenic and toxic DNA lesion because DNA polymerases can misincorporate thymine opposite O6 methylguanine, resulting in GC to AT mutations [164]. MGMT functions by transferring a methyl group from a methylated DNA base into an active site Cys (Cys145) [165]. MGMT is a suicide protein; it is targeted for degradation upon transferring the methyl group onto the active site Cys [165, 166]. This Cys is located near charged amino acids, which makes the sulfhydryl group reactive and prone to modification. Specifically, Cys145 has been shown to be targeted for nitrosylation by nitrosoglutathione (GSNO) [78, 79, 167]. The nitrosylation of MGMT inactivates the enzyme and leads to its degradation as it becomes ubiquitinated and targeted by proteasomes [168]. Nitrosylated MGMT has a reduced half-life, from 24 h to 1.3 h, though this sensitivity is abolished upon mutating Cys145 into Ala [78]. Furthermore, MGMT degradation results in the accumulation of DNA damage. The inability of cells to repair O6 methylguanine as a result of MGMT nitrosylation has been proposed to lead to hepatocarcinogenesis [168]. In support of this, it is reported that in many hepatocellular tumors levels of NO synthase (which produces NO) are high, while levels of GSNOR (GSNO reductase, which degrades NO) are low [169].
5. Conclusions
Redox-dependent Cys modifications enhance the diversity of protein structure and function by two orders of magnitude [62]. Though most proteins are found in a reduced state, there are various examples of proteins that exist in the oxidized form in the cell [170, 171]. These proteins have reactive Cys residues that sense small changes in the redox potential. The sulfhydryl group in Cys is an ionizable group that is sensitive to changes in nearby environment (pH and redox potential) [172]. The protonation state of Cys is dependent on the location and secondary structure. At the N-terminal end of α helices, the pKa of the sulfhydryl decreases due to the positive dipole moment; this renders the group more reactive, providing a basis for selectivity. The reactivity of Cys is also increased when positioned near a basic amino acid in the three dimensional space. Moreover, the redox potential of the cell has been documented to change depending on the cellular function including proliferation and apoptosis [173].Therefore, the potential for protein oxidation not only depends on oxidative stress induced in a disease state, it also depends on normal cellular functions.
In the context of DNA repair, protein modifications are important under conditions of oxidative stress, when there is an imbalance between oxidants and reductants and when DNA is more prone for damage. It is important to ensure that the activation of signaling proteins, such as ATM, occurs only under conditions when DNA is damaged, otherwise, if it is constitutively active, it will result in premature cell cycle arrest. The activation of ATM under oxidative stress may occur as a stress response and can act as a sensor to transmit a stress signal into a DNA repair response. Alternatively, when ATM is mutated at sites that prevent it from responding to oxidative stress, the cell will not be able to respond to oxidative stress and that may be associated with various diseases such as the A-T syndrome. This indicates that the oxidation state of ATM is important for homeostatic cellular functions.
The levels of ROS are correlated with aging and various neurodegenerative diseases and cancer. Though, the correlation between oxidative stress and these diseases is still not fully understood. Oxidative stress and the consequential oxidative modification of DNA repair proteins may result in their activation or inhibition, which will directly affect DNA repair and the accumulation of DNA damage. Consequently, these protein modifications may correlate with the incidence of various diseases, including cancer. Therefore, it is important to understand the full extent to which DNA repair proteins respond to oxidative stress. Modulating the response of proteins to oxidative stress by using small molecule inhibitors may provide a better handle for various oxidative stress-induced diseases such as neurodegenerative and autoimmune diseases and cancer. Therefore, it is important to identify residues within the DNA repair proteins that are sensitive to oxidative modification. In addition to directly oxidizing DNA repair proteins, other redox sensitive proteins which regulate the expression/localization/folding of DNA repair proteins may also indirectly affect DNA repair [174]. Thus, these regulatory proteins may act as novel therapeutic targets for cancer and other diseases.
In addition to the proteins discussed in this review, more proteins that are directly and indirectly involved in DNA repair have been shown to be targeted for oxidation: T4 ligase [175], p53 [176], HMGB1 [177], XPA [178], PCNA [179], RPA [116, 180-182]. Therefore, additional studies are necessary to perform in order to understand the comprehensive effect of oxidative stress on DNA damage and repair and to fully understand the relationship between inflammation and cancer. Additionally, various DNA repair proteins have cancer-associated variants where a Cys residue is introduced. These variants may alter the sensitivity of DNA repair to oxidation. Therefore, more studies are necessary in order to understand the effect of a Cys on the sensitivity of the protein to oxidative stress.
Acknowledgments
This work was supported by the National Cancer Institute [CA080830].
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- [1].D'Autreaux B, Toledano MB, ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis, Nat Rev Mol Cell Biol, 8 (2007) 813–824. [DOI] [PubMed] [Google Scholar]
- [2].Rhee SG, Cell signaling. H2O2, a necessary evil for cell signaling, Science, 312 (2006) 1882–1883. [DOI] [PubMed] [Google Scholar]
- [3].Hancock JT, Desikan R, Neill SJ, Role of reactive oxygen species in cell signalling pathways, Biochemical Society Transactions, 29 (2001) 345–349. [DOI] [PubMed] [Google Scholar]
- [4].Lieber MR, The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway, Annu Rev Biochem, 79 (2010) 181–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C, Avvedimento EV, DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression, Science, 319 (2008) 202–206. [DOI] [PubMed] [Google Scholar]
- [6].Meitzler JL, Antony S, Wu Y, Juhasz A, Liu H, Jiang G, Lu J, Roy K, Doroshow JH, NADPH oxidases: a perspective on reactive oxygen species production in tumor biology, Antioxid Redox Signal, 20 (2014) 2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bae YS, Oh H, Rhee SG, Yoo YD, Regulation of reactive oxygen species generation in cell signaling, Mol Cells, 32 (2011) 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baier J, Maisch T, Maier M, Engel E, Landthaler M, Baumler W, Singlet oxygen generation by UVA light exposure of endogenous photosensitizers, Biophys J, 91 (2006) 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Al-Gubory KH, Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development, Reprod Biomed Online, 29 (2014) 17–31. [DOI] [PubMed] [Google Scholar]
- [10].Sena LA, Chandel NS, Physiological roles of mitochondrial reactive oxygen species, Mol Cell, 48 (2012) 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bogdan C, Nitric oxide and the immune response, Nature Immunology, 2 (2001) 907. [DOI] [PubMed] [Google Scholar]
- [12].Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K, Regulation of platelet activation and thrombus formation by reactive oxygen species, Redox Biol, 14 (2018) 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T, Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling, Proc Natl Acad Sci U S A, 97 (2000) 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dalton TP, Shertzer HG, Puga A, Regulation of gene expression by reactive oxygen, Annu Rev Pharmacol Toxicol, 39 (1999) 67–101. [DOI] [PubMed] [Google Scholar]
- [15].Di Meo S, Reed TT, Venditti P, Victor VM, Role of ROS and RNS Sources in Physiological and Pathological Conditions, Oxid Med Cell Longev, 2016 (2016) 1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He L, He T, Farrar S, Ji L, Liu T, Ma X, Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species, Cell Physiol Biochem, 44 (2017) 532–553. [DOI] [PubMed] [Google Scholar]
- [17].Sies H, Glutathione and its role in cellular functions, Free Radic Biol Med, 27 (1999) 916–921. [DOI] [PubMed] [Google Scholar]
- [18].Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA, Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling, Trends Biochem Sci, 40 (2015) 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meister A, Glutathione metabolism, Methods Enzymol, 251 (1995) 3–7. [DOI] [PubMed] [Google Scholar]
- [20].Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R, Molecular mechanisms and potential clinical significance of S-glutathionylation, Antioxid Redox Signal, 10 (2008) 445–473. [DOI] [PubMed] [Google Scholar]
- [21].Buettner GR, Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide, Anticancer Agents Med Chem, 11 (2011) 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Finkel T, Holbrook NJ, Oxidants, oxidative stress and the biology of ageing, Nature, 408 (2000) 239–247. [DOI] [PubMed] [Google Scholar]
- [23].Trachootham D, Alexandre J, Huang P, Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?, Nat Rev Drug Discov, 8 (2009) 579–591. [DOI] [PubMed] [Google Scholar]
- [24].Schieber M, Chandel NS, ROS function in redox signaling and oxidative stress, Curr Biol, 24 (2014) R453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kohchi C, Inagawa H, Nishizawa T, Soma G, ROS and innate immunity, Anticancer Res, 29 (2009) 817–821. [PubMed] [Google Scholar]
- [26].Nathan C, Cunningham-Bussel A, Beyond oxidative stress: an immunologist's guide to reactive oxygen species, Nat Rev Immunol, 13 (2013) 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H, ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity, Nat Immunol, 6 (2005) 587–592. [DOI] [PubMed] [Google Scholar]
- [28].Paiva CN, Bozza MT, Are reactive oxygen species always detrimental to pathogens?, Antioxid Redox Signal, 20 (2014) 1000–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Espey MG, Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota, Free Radic Biol Med, 55 (2013) 130–140. [DOI] [PubMed] [Google Scholar]
- [30].Liaudet L, Vassalli G, Pacher P, Role of peroxynitrite in the redox regulation of cell signal transduction pathways, Front Biosci (Landmark Ed), 14 (2009) 4809–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Safford SE, Oberley TD, Urano M, St Clair DK, Suppression of fibrosarcoma metastasis by elevated expression of manganese superoxide dismutase, Cancer Res, 54 (1994) 4261–4265. [PubMed] [Google Scholar]
- [32].Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C, Chronic inflammation and oxidative stress in human carcinogenesis, Int J Cancer, 121 (2007) 2381–2386. [DOI] [PubMed] [Google Scholar]
- [33].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell, 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [34].Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB, Oxidative stress, inflammation, and cancer: how are they linked?, Free Radic Biol Med, 49 (2010) 1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Coussens LM, Werb Z, Inflammation and cancer, Nature, 420 (2002) 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Eiro N, Vizoso FJ, Inflammation and cancer, World J Gastrointest Surg, 4 (2012) 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Forman HJ, Torres M, Redox signaling in macrophages, Mol Aspects Med, 22 (2001) 189–216. [DOI] [PubMed] [Google Scholar]
- [38].Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR, TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway, Cell Signal, 19 (2007) 1419–1433. [DOI] [PubMed] [Google Scholar]
- [39].Blaser H, Dostert C, Mak TW, Brenner D, TNF and ROS Crosstalk in Inflammation, Trends Cell Biol, 26 (2016) 249–261. [DOI] [PubMed] [Google Scholar]
- [40].Komori A, Yatsunami J, Suganuma M, Okabe S, Abe S, Sakai A, Fujiki H, Tumor necrosis factor acts as a tumor promoter in BALB/3T3 cell transformation, Cancer Res, 53 (1993) 1982–1985. [PubMed] [Google Scholar]
- [41].Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT, Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer?, Gut Microbes, 4 (2013) 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lindahl T, Instability and decay of the primary structure of DNA, Nature, 362 (1993) 709–715. [DOI] [PubMed] [Google Scholar]
- [43].Lindahl T, Barnes DE, Repair of endogenous DNA damage, Cold Spring Harb Symp Quant Biol, 65 (2000) 127–133. [DOI] [PubMed] [Google Scholar]
- [44].Tubbs A, Nussenzweig A, Endogenous DNA Damage as a Source of Genomic Instability in Cancer, Cell, 168 (2017) 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cadet J, Douki T, Ravanat JL, Oxidatively generated base damage to cellular DNA, Free Radic Biol Med, 49 (2010) 9–21. [DOI] [PubMed] [Google Scholar]
- [46].Cadet J, Wagner JR, DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation, Cold Spring Harb Perspect Biol, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA, Base excision repair of oxidative DNA damage and association with cancer and aging, Carcinogenesis, 30 (2009) 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].David SS, O'Shea VL, Kundu S, Base-excision repair of oxidative DNA damage, Nature, 447 (2007) 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiricny J, The multifaceted mismatch-repair system, Nat Rev Mol Cell Biol, 7 (2006) 335–346. [DOI] [PubMed] [Google Scholar]
- [50].Hoeijmakers JH, DNA damage, aging, and cancer, N Engl J Med, 361 (2009) 1475–1485. [DOI] [PubMed] [Google Scholar]
- [51].Moldovan GL, D'Andrea AD, How the fanconi anemia pathway guards the genome, Annu Rev Genet, 43 (2009) 223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Caldecott KW, Single-strand break repair and genetic disease, Nat Rev Genet, 9 (2008) 619–631. [DOI] [PubMed] [Google Scholar]
- [53].West SC, Molecular views of recombination proteins and their control, Nat Rev Mol Cell Biol, 4 (2003) 435–445. [DOI] [PubMed] [Google Scholar]
- [54].Loeb LAS, C. F., Battula N, Errors in DNA replication as a basis of malignant changes, Cancer Res, 34 (1974) 2311–2321. [PubMed] [Google Scholar]
- [55].Wall SB, Oh JY, Diers AR, Landar A, Oxidative modification of proteins: an emerging mechanism of cell signaling, Front Physiol, 3 (2012) 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang J, Gupta V, Carroll KS, Liebler DC, Site-specific mapping and quantification of protein S-sulphenylation in cells, Nat Commun, 5 (2014) 4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Poole LB, The basics of thiols and cysteines in redox biology and chemistry, Free Radic Biol Med, 80 (2015) 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weerapana E, Wang C, Simon GM, Richter F, Khare S, Dillon MB, Bachovchin DA, Mowen K, Baker D, Cravatt BF, Quantitative reactivity profiling predicts functional cysteines in proteomes, Nature, 468 (2010) 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA, Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications, Free Radic Biol Med, 43 (2007) 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gould N, Doulias PT, Tenopoulou M, Raju K, Ischiropoulos H, Regulation of protein function and signaling by reversible cysteine S-nitrosylation, J Biol Chem, 288 (2013) 26473–26479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Roos G, Messens J, Protein sulfenic acid formation: from cellular damage to redox regulation, Free Radic Biol Med, 51 (2011) 314–326. [DOI] [PubMed] [Google Scholar]
- [62].Spicer CD, Davis BG, Selective chemical protein modification, Nat Commun, 5 (2014) 4740. [DOI] [PubMed] [Google Scholar]
- [63].Go YM, Chandler JD, Jones DP, The cysteine proteome, Free Radic Biol Med, 84 (2015) 227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Reddie KG, Carroll KS, Expanding the functional diversity of proteins through cysteine oxidation, Curr Opin Chem Biol, 12 (2008) 746–754. [DOI] [PubMed] [Google Scholar]
- [65].Berlett BS, Stadtman ER, Protein oxidation in aging, disease, and oxidative stress, J Biol Chem, 272 (1997) 20313–20316. [DOI] [PubMed] [Google Scholar]
- [66].Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT, ATM activation by oxidative stress, Science, 330 (2010) 517–521. [DOI] [PubMed] [Google Scholar]
- [67].Bennett SM, Neher TM, Shatilla A, Turchi JJ, Molecular analysis of Ku redox regulation, BMC Mol Biol, 10 (2009) 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Duquette ML, Kim J, Shi LZ, Berns MW, LSD1 mediated changes in the local redox environment during the DNA damage response, PLoS One, 13 (2018) e0201907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Strobel T, Madlener S, Tuna S, Vose S, Lagerweij T, Wurdinger T, Vierlinger K, Wohrer A, Price BD, Demple B, Saydam O, Saydam N, Ape1 guides DNA repair pathway choice that is associated with drug tolerance in glioblastoma, Sci Rep, 7 (2017) 9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bravard A, Vacher M, Gouget B, Coutant A, de Boisferon FH, Marsin S, Chevillard S, Radicella JP, Redox regulation of human OGG1 activity in response to cellular oxidative stress, Mol Cell Biol, 26 (2006) 7430–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Begg AC, Stewart FA, Vens C, Strategies to improve radiotherapy with targeted drugs, Nat Rev Cancer, 11 (2011) 239–253. [DOI] [PubMed] [Google Scholar]
- [72].Kaur G, Cholia RP, Mantha AK, Kumar R, DNA repair and redox activities and inhibitors of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1): a comparative analysis and their scope and limitations toward anticancer drug development, J Med Chem, 57 (2014) 10241–10256. [DOI] [PubMed] [Google Scholar]
- [73].Guo Z, Deshpande R, Pauli TT, ATM activation in the presence of oxidative stress, Cell Cycle, 9 (2010) 4805–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lee JH, Mand MR, Kao CH, Zhou Y, Ryu SW, Richards AL, Coon JJ, Pauli TT, ATM directs DNA damage responses and proteostasis via genetically separable pathways, Sci Signal, 11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang Y, Lee JH, Pauli TT, Gehrke S, D'Alessandro A, Dou Q, Gladyshev VN, Schroeder EA, Steyl SK, Christian BE, Shadel GS, Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity, Sci Signal, 11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Girard PM, Graindorge D, Smirnova V, Rigolet P, Francesconi S, Scanlon S, Sage E, Oxidative stress in mammalian cells impinges on the cysteines redox state of human XRCC3 protein and on its cellular localization, PLoS One, 8 (2013) e75751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boldogh I, Roy G, Lee M-S, Bacsi A, Hazra TK, Bhakat KK, Das GC, Mitra S, Reduced DNA double strand breaks in chlorambucil resistant cells are related to high DNA-PKcs activity and low oxidative stress, Toxicology, 193 (2003) 137–152. [DOI] [PubMed] [Google Scholar]
- [78].Liu L, Xu-Welliver M, Kanugula S, Pegg AE, Inactivation and degradation of O(6)-alkylguanine-DNA alkyltransferase after reaction with nitric oxide, Cancer Res, 62 (2002) 3037–3043. [PubMed] [Google Scholar]
- [79].Laval F, Wink DA, Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase, Carcinogenesis, 15 (1994) 443–447. [DOI] [PubMed] [Google Scholar]
- [80].Parrish MC, Chaim IA, Nagel ZD, Tannenbaum SR, Samson LD, Engelward BP, Nitric oxide induced S-nitrosation causes base excision repair imbalance, DNA Repair (Amst), 68 (2018) 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ, Nitric oxide–mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes, Gastroenterology, 120 (2001) 190–199. [DOI] [PubMed] [Google Scholar]
- [82].Vascotto C, Bisetto E, Li M, Zeef LA, D'Ambrosio C, Domenis R, Comelli M, Delneri D, Scaloni A, Altieri F, Mavelli I, Quadrifoglio F, Kelley MR, Tell G, Knock-in reconstitution studies reveal an unexpected role of Cys-65 in regulating APE1/Ref-1 subcellular trafficking and function, Mol Biol Cell, 22 (2011) 3887–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kim YJ, Kim D, Illuzzi JL, Delaplane S, Su D, Bernier M, Gross ML, Georgiadis MM, Wilson DM 3rd, S-glutathionylation of cysteine 99 in the APE1 protein impairs abasic endonuclease activity, J Mol Biol, 414 (2011) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhou X, Cooper KL, Sun X, Liu KJ, Hudson LG, Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding, J Biol Chem, 290 (2015) 18361–18369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kroncke KD, Klotz LO, Zinc fingers as biologic redox switches?, Antioxid Redox Signal, 11 (2009) 1015–1027. [DOI] [PubMed] [Google Scholar]
- [86].Cuneo MJ, London RE, Oxidation state of the XRCC1 N-terminal domain regulates DNA polymerase beta binding affinity, Proc Natl Acad Sci U S A, 107 (2010) 6805–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cortez D, Preventing replication fork collapse to maintain genome integrity, DNA Repair (Amst), 32 (2015) 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nowsheen S, Yang ES, The intersection betwen DNA damage response and cell death pathways, Exp Oncol, 34 (2012) 243–254. [PMC free article] [PubMed] [Google Scholar]
- [89].De Zio D, Cianfanelli V, Cecconi F, New insights into the link between DNA damage and apoptosis, Antioxid Redox Signal, 19 (2013) 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Branzei D, Foiani M, Regulation of DNA repair throughout the cell cycle, Nat Rev Mol Cell Biol, 9 (2008) 297–308. [DOI] [PubMed] [Google Scholar]
- [91].Jackson SP, Sensing and repairing DNA double-strand breaks, Carcinogenesis, 23 (2002) 687–696. [DOI] [PubMed] [Google Scholar]
- [92].Ceccaldi R, Rondinelli B, D'Andrea AD, Repair Pathway Choices and Consequences at the Double-Strand Break, Trends Cell Biol, 26 (2016) 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wright WD, Shah SS, Heyer WD, Homologous recombination and the repair of DNA Double-Strand Breaks, J Biol Chem, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chang HHY, Pannunzio NR, Adachi N, Lieber MR, Non-homologous DNA end joining and alternative pathways to double-strand break repair, Nat Rev Mol Cell Biol, 18 (2017) 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Pauli TT, Mechanisms of ATM Activation, Annu Rev Biochem, 84 (2015) 711–738. [DOI] [PubMed] [Google Scholar]
- [96].Lee JH, Pauli TT, ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex, Science, 308 (2005) 551–554. [DOI] [PubMed] [Google Scholar]
- [97].Bakkenist CJ, Kastan MB, DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation, Nature, 421 (2003) 499. [DOI] [PubMed] [Google Scholar]
- [98].Wang X, Chu H, Lv M, Zhang Z, Qiu S, Liu H, Shen X, Wang W, Cai G, Structure of the intact ATM/Tel1 kinase, Nat Commun, 7 (2016) 11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kastan MB, Lim DS, The many substrates and functions of ATM, Nat Rev Mol Cell Biol, 1 (2000) 179–186. [DOI] [PubMed] [Google Scholar]
- [100].Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD, Activation of the ATM kinase by ionizing radiation and phosphorylation of p53, Science, 281 (1998) 1677–1679. [DOI] [PubMed] [Google Scholar]
- [101].Sullivan KD, Galbraith MD, Andrysik Z, Espinosa JM, Mechanisms of transcriptional regulation by p53, Cell Death And Differentiation, 25 (2017) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bartek J, Falck J, Lukas J, Chk2 kinase — a busy messenger, Nature Reviews Molecular Cell Biology, 2 (2001) 877. [DOI] [PubMed] [Google Scholar]
- [103].Lavin MF, Shiloh Y, The genetic defect in ataxia-telangiectasia, Annu Rev Immunol, 15 (1997) 177–202. [DOI] [PubMed] [Google Scholar]
- [104].Barzilai A, Rotman G, Shiloh Y, ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage, DNA Repair (Amst), 1 (2002) 3–25. [DOI] [PubMed] [Google Scholar]
- [105].Sun Y, Xu Y, Roy K, Price BD, DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity, Mol Cell Biol, 27 (2007) 8502–8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Shackelford RE, Innes CL, Sieber SO, Heinloth AN, Leadon SA, Paules RS, The Ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts, J Biol Chem, 276 (2001) 21951–21959. [DOI] [PubMed] [Google Scholar]
- [107].Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, Gao J, Socci ND, Solit DB, Olshen AB, Schultz N, Taylor BS, Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity, Nat Biotechnol, 34 (2016) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sung P, Klein H, Mechanism of homologous recombination: mediators and helicases take on regulatory functions, Nature Reviews Molecular Cell Biology, 7 (2006) 739–750. [DOI] [PubMed] [Google Scholar]
- [109].Kurumizaka H, Ikawa S, Nakada M, Eda K, Kagawa W, Takata M, Takeda S, Yokoyama S, Shibata T, Homologous-pairing activity of the human DNA-repair proteins Xrcc3.Rad51C, Proc Natl Acad Sci U S A, 98 (2001) 5538–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bishop DK, Ear U, Bhattacharyya A, Calderone C, Beckett M, Weichselbaum RR, Shinohara A, Xrcc3 is required for assembly of Rad51 complexes in vivo, J Biol Chem, 273 (1998) 21482–21488. [DOI] [PubMed] [Google Scholar]
- [111].Tebbs RS, Zhao Y, Tucker JD, Scheerer JB, Siciliano MJ, Hwang M, Liu N, Legerski RJ, Thompson LH, Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene, Proc Natl Acad Sci U S A, 92 (1995) 6354–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Brem R, Karran P, Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine, Photochem Photobiol, 88 (2012) 5–13. [DOI] [PubMed] [Google Scholar]
- [113].Somyajit K, Basavaraju S, Scully R, Nagaraju G, ATM- and ATR-mediated phosphorylation of XRCC3 regulates DNA double-strand break-induced checkpoint activation and repair, Mol Cell Biol, 33 (2013) 1830–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Downs JA, Jackson SP, A means to a DNA end: the many roles of Ku, Nat Rev Mol Cell Biol, 5 (2004) 367–378. [DOI] [PubMed] [Google Scholar]
- [115].Turchi JJ, Henkels KM, Zhou Y, Cisplatin-DNA adducts inhibit translocation of the Ku subunits of DNA-PK, Nucleic Acids Res, 28 (2000) 4634–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gueranger Q, Li F, Peacock M, Larnicol-Fery A, Brem R, Macpherson P, Egly JM, Karran P, Protein oxidation and DNA repair inhibition by 6-thioguanine and UVA radiation, J Invest Dermatol, 134 (2014) 1408–1417. [DOI] [PubMed] [Google Scholar]
- [117].Zhang WW, Yaneva M, Reduced sulphydryl groups are required for DNA binding of Ku protein, Biochem J, 293 ( Pt 3) (1993) 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Andrews BJ, Lehman JA, Turchi JJ, Kinetic analysis of the Ku-DNA binding activity reveals a redox-dependent alteration in protein structure that stimulates dissociation of the Ku-DNA complex, J Biol Chem, 281 (2006) 13596–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kurimasa A, Kumano S, Boubnov NV, Story MD, Tung CS, Peterson SR, Chen DJ, Requirement for the kinase activity of human DNA-dependent protein kinase catalytic subunit in DNA strand break rejoining, Mol Cell Biol, 19 (1999) 3877–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, Stamler JS, Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture, Nat Biotechnol, 27 (2009) 557–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD, Snyder SH, GAPDH mediates nitrosylation of nuclear proteins, Nat Cell Biol, 12 (2010) 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Xu W, Liu L, Smith GC, Charles l G, Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents, Nat Cell Biol, 2 (2000) 339–345. [DOI] [PubMed] [Google Scholar]
- [123].Bogdan C, Nitric oxide synthase in innate and adaptive immunity: an update, Trends Immunol, 36 (2015) 161–178. [DOI] [PubMed] [Google Scholar]
- [124].Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE, Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage, Proc Natl Acad Sci U S A, 96 (1999) 13300–13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fu D, Calvo JA, Samson LD, Balancing repair and tolerance of DNA damage caused by alkylating agents, Nature Reviews Cancer, 12 (2012) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wyatt MD, Pittman DL, Methylating agents and DNA repair responses: Methylated bases and sources of strand breaks, Chem Res Toxicol, 19 (2006) 1580–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jones LE Jr., Ying L, Hofseth AB, Jelezcova E, Sobol RW, Ambs S, Harris CC, Espey MG, Hofseth LJ, Wyatt MD, Differential effects of reactive nitrogen species on DNA base excision repair initiated by the alkyladenine DNA glycosylase, Carcinogenesis, 30 (2009) 2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Chan K, Resnick MA, Gordenin DA, The choice of nucleotide inserted opposite abasic sites formed within chromosomal DNA reveals the polymerase activities participating in translesion DNA synthesis, DNA Repair (Amst), 12 (2013) 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Clauson CL, Oestreich KJ, Austin JW, Doetsch PW, Abasic sites and strand breaks in DNA cause transcriptional mutagenesis in Escherichia coli, Proc Natl Acad Sci U S A, 107 (2010) 3657–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Faucher F, Doublie S, Jia Z, 8-oxoguanine DNA glycosylases: one lesion, three subfamilies, Int J Mol Sci, 13 (2012) 6711–6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chrestensen CA, Starke DW, Mieyal JJ, Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis, J Biol Chem, 275 (2000) 26556–26565. [DOI] [PubMed] [Google Scholar]
- [132].Caulfield JL, Wishnok JS, Tannenbaum SR, Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides, J Biol Chem, 273 (1998) 12689–12695. [DOI] [PubMed] [Google Scholar]
- [133].Hung RJ, Hall J, Brennan P, Boffetta P, Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review, Am J Epidemiol, 162 (2005) 925–942. [DOI] [PubMed] [Google Scholar]
- [134].Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P, The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk, Carcinogenesis, 23 (2002) 1229–1234. [DOI] [PubMed] [Google Scholar]
- [135].Bravard A, Vacher M, Moritz E, Vaslin L, Hall J, Epe B, Radicella JP, Oxidation status of human OGG1-S326C polymorphic variant determines cellular DNA repair capacity, Cancer Res, 69 (2009) 3642–3649. [DOI] [PubMed] [Google Scholar]
- [136].Smart DJ, Chipman JK, Hodges NJ, Activity of OGG1 variants in the repair of pro-oxidant-induced 8-oxo-2'-deoxyguanosine, DNA Repair (Amst), 5 (2006) 1337–1345. [DOI] [PubMed] [Google Scholar]
- [137].Kershaw RM, Hodges NJ, Repair of oxidative DNA damage is delayed in the Ser326Cys polymorphic variant of the base excision repair protein OGG1, Mutagenesis, 27 (2012) 501–510. [DOI] [PubMed] [Google Scholar]
- [138].Morreall J, Limpose K, Sheppard C, Kow YW, Werner E, Doetsch PW, Inactivation of a common OGG1 variant by TNF-alpha in mammalian cells, DNA Repair (Amst), 26 (2015) 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hill JW, Evans MK, Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase, Nucleic Acids Res, 34 (2006) 1620–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Semenzato G, Tumour necrosis factor: a cytokine with multiple biological activities, Br J Cancer, 61 (1990) 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP, Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors, Nature Genetics, 30 (2002) 227. [DOI] [PubMed] [Google Scholar]
- [142].Fearnhead NS, Britton MP, Bodmer WF, The ABC of APC, Human Molecular Genetics, 10 (2001) 721–733. [DOI] [PubMed] [Google Scholar]
- [143].Win AK, Dowty JG, Cleary SP, Kim H, Buchanan DD, Young JP, Clendenning M, Rosty C, MacInnis RJ, Giles GG, Boussioutas A, Macrae FA, Parry S, Goldblatt J, Baron JA, Burnett T, Le Marchand L, Newcomb PA, Haile RW, Hopper JL, Cotterchio M, Gallinger S, Lindor NM, Tucker KM, Winship IM, Jenkins MA, Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer, Gastroenterology, 146 (2014) 1208–1211 e1201-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Luncsford PJ, Chang DY, Shi G, Bernstein J, Madabushi A, Patterson DN, Lu AL, Toth EA, A structural hinge in eukaryotic MutY homologues mediates catalytic activity and Rad9-Rad1-Hus1 checkpoint complex interactions, J Mol Biol, 403 (2010) 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wang D, Xiang DB, Yang XQ, Chen LS, Li MX, Zhong ZY, Zhang YS, APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells, Lung Cancer, 66 (2009) 298–304. [DOI] [PubMed] [Google Scholar]
- [146].Chou KM, Cheng YC, An exonucleolytic activity of human apurinic/apyrimidinic endonuclease on 3' mispaired DNA, Nature, 415 (2002) 655–659. [DOI] [PubMed] [Google Scholar]
- [147].Wong D, DeMott MS, Demple B, Modulation of the 3'-->5'-exonuclease activity of human apurinic endonuclease (Ape1) by its 5'-incised Abasic DNA product, J Biol Chem, 278 (2003) 36242–36249. [DOI] [PubMed] [Google Scholar]
- [148].Bhakat KK, Mantha AK, Mitra S, Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein, Antioxid Redox Signal, 11 (2009) 621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Xanthoudakis S, Miao GG, Curran T, The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains, Proc Natl Acad Sci U S A, 91 (1994) 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T, Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme, EMBO J, 11 (1992) 3323–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Walker LJ, Robson CN, Black E, Gillespie D, Hickson ID, Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding, Mol Cell Biol, 13 (1993) 5370–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Qu J, Liu GH, Huang B, Chen C, Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of cysteines 93 and 310, Nucleic Acids Res, 35 (2007) 2522–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Tell G, Damante G, Caldwell D, Kelley MR, The intracellular localization of APE1/Ref-1: more than a passive phenomenon?, Antioxid Redox Signal, 7 (2005) 367–384. [DOI] [PubMed] [Google Scholar]
- [154].Luo M, Zhang J, He H, Su D, Chen Q, Gross ML, Kelley MR, Georgiadis MM, Characterization of the redox activity and disulfide bond formation in apurinic/apyrimidinic endonuclease, Biochemistry, 51 (2012) 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Krishnakumar R, Kraus WL, The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets, Mol Cell, 39 (2010) 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].D'Amours D, Desnoyers S, D'Silva I, Poirier GG, Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions, Biochem J, 342 ( Pt 2) (1999) 249–268. [PMC free article] [PubMed] [Google Scholar]
- [157].Schreiber V, Dantzer F, Ame JC, de Murcia G, Poly(ADP-ribose): novel functions for an old molecule, Nat Rev Mol Cell Biol, 7 (2006) 517–528. [DOI] [PubMed] [Google Scholar]
- [158].Ali AA, Timinszky G, Arribas-Bosacoma R, Kozlowski M, Hassa PO, Hassler M, Ladurner AG, Pearl LH, Oliver AW, The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks, Nat Struct Mol Biol, 19 (2012) 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Langelier MF, Planck JL, Roy S, Pascal JM, Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1, Science, 336 (2012) 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sidorkina O, Espey MG, Miranda KM, Wink DA, Laval J, Inhibition of poly(ADP-RIBOSE) polymerase (PARP) by nitric oxide and reactive nitrogen oxide species, Free Radic Biol Med, 35 (2003) 1431–1438. [DOI] [PubMed] [Google Scholar]
- [161].London RE, The structural basis of XRCC1-mediated DNA repair, DNA Repair (Amst), 30 (2015) 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kubota Y, Nash RA, Klungland A, Schar P, Barnes DE, Lindahl T, Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein, EMBO J, 15 (1996) 6662–6670. [PMC free article] [PubMed] [Google Scholar]
- [163].Gabel SA, Smith CE, Cuneo MJ, Mueller GA, Kirby TW, DeRose EF, Krahn JM, London RE, Characterization of the redox transition of the XRCC1 N-terminal domain, Structure, 22 (2014) 1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kaina B, Christmann M, Naumann S, Roos WP, MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents, DNA Repair (Amst), 6 (2007) 1079–1099. [DOI] [PubMed] [Google Scholar]
- [165].Rasimas JJ, Dalessio PA, Ropson IJ, Pegg AE, Fried MG, Active-site alkylation destabilizes human O6-alkylguanine DNA alkyltransferase, Protein Sci, 13 (2004) 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Pegg AE, Repair of O6-alkylguanine by alkyltransferases, Mutation Research/Reviews in Mutation Research, 462 (2000) 83–100. [DOI] [PubMed] [Google Scholar]
- [167].Pegg AE, Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools, Chem Res Toxicol, 24 (2011) 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wei W, Yang Z, Tang CH, Liu L, Targeted deletion of GSNOR in hepatocytes of mice causes nitrosative inactivation of O6-alkylguanine-DNA alkyltransferase and increased sensitivity to genotoxic diethylnitrosamine, Carcinogenesis, 32 (2011) 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, Reich M, Chan JA, Glickman JN, Ikeda K, Hashimoto M, Watanabe G, Daidone MG, Roayaie S, Schwartz M, Thung S, Salvesen HB, Gabriel S, Mazzaferro V, Bruix J, Friedman SL, Kumada H, Llovet JM, Golub TR, Gene expression in fixed tissues and outcome in hepatocellular carcinoma, N Engl J Med, 359 (2008) 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Linke K, Jakob U, Not every disulfide lasts forever: disulfide bond formation as a redox switch, Antioxid Redox Signal, 5 (2003) 425–434. [DOI] [PubMed] [Google Scholar]
- [171].Tu BP, Weissman JS, Oxidative protein folding in eukaryotes: mechanisms and consequences, J Cell Biol, 164 (2004) 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Poole LB, Karplus PA, Claiborne A, Protein sulfenic acids in redox signaling, Annu Rev Pharmacol Toxicol, 44 (2004) 325–347. [DOI] [PubMed] [Google Scholar]
- [173].Go YM, Jones DP, Redox compartmentalization in eukaryotic cells, Biochim Biophys Acta, 1780 (2008) 1273–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Azam S, Jouvet N, Jilani A, Vongsamphanh R, Yang X, Yang S, Ramotar D, Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1, J Biol Chem, 283 (2008) 30632–30641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Graziewicz M, Wink DA, Laval F, Nitric oxide inhibits DNA ligase activity: potential mechanisms for NO-mediated DNA damage, Carcinogenesis, 17 (1996) 2501–2505. [DOI] [PubMed] [Google Scholar]
- [176].Kim D-H, Kundu JK, Surh Y-J, Redox modulation of p53: Mechanisms and functional significance, Molecular Carcinogenesis, 50 (2011) 222–234. [DOI] [PubMed] [Google Scholar]
- [177].Janko C, Filipovic M, Munoz LE, Schorn C, Schett G, Ivanovic-Burmazovic I, Herrmann M, Redox modulation of HMGB1-related signaling, Antioxid Redox Signal, 20 (2014) 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Smirnova J, Zhukova L, Witkiewicz-Kucharczyk A, Kopera E, Oledzki J, Wyslouch-Cieszynska A, Palumaa P, Hartwig A, Bal W, Reaction of the XPA zinc finger with S-nitrosoglutathione, Chem Res Toxicol, 21 (2008) 386–392. [DOI] [PubMed] [Google Scholar]
- [179].Montaner B, O'Donovan P, Reelfs O, Perrett CM, Zhang X, Xu YZ, Ren X, Macpherson P, Frith D, Karran P, Reactive oxygen-mediated damage to a human DNA replication and repair protein, EMBO Rep, 8 (2007) 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Peacock M, Brem R, Macpherson P, Karran P, DNA repair inhibition by UVA photoactivated fluoroquinolones and vemurafenib, Nucleic Acids Res, 42 (2014) 13714–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Guven M, Brem R, Macpherson P, Peacock M, Karran P, Oxidative Damage to RPA Limits the Nucleotide Excision Repair Capacity of Human Cells, J Invest Dermatol, 135 (2015) 2834–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Men L, Roginskaya M, Zou Y, Wang Y, Redox-dependent formation of disulfide bonds in human replication protein A, Rapid Commun Mass Spectrom, 21 (2007) 2743–2749. [DOI] [PubMed] [Google Scholar]