Abstract
Background and purpose
Patients with stroke can experience neurological deterioration in the prehospital setting. We evaluated patients with stroke to determine factors associated with prehospital neurological deterioration (PND).
Methods
Among the Greater Cincinnati/Northern Kentucky region (population ~1.3 million), we screenedall 15 local hospitals’ admissions from 2010 for acute stroke and included patients aged ≥20. The GCS was compared between emergency medical services (EMS) arrival and hospital arrival, with decrease ≥2 points considered PND. Data obtained retrospectively included demographics, medical history and medication use, stroke subtype (eg, ischaemic stroke (IS), intracerebral haemorrhage (ICH), subarachnoid haemorrhage(SAH)) and IS subtype (eg, small vessel, large vessel, cardioembolic), seizure at onset, time intervals between symptom onset, EMS arrival and hospital arrival, EMS level of training, and blood pressure and serum glucose on EMS arrival.
Results
Of 2708 total patients who had a stroke, 1092 patients (median (IQR) age 74 (61–83) years;56% women; 21% black) were analysed. PND occurredin 129 cases (12%), including 9% of IS, 24% of ICHand 16% of SAH. In multivariable analysis, black race, atrial fibrillation, haemorrhagic subtype and ALS level of transport were associated with PND.
Conclusion
Haemorrhage and atrial fibrillation is associated with PND in stroke, and further investigationis needed to establish whether PND can be predicted. Further studies are also needed to assess whether preferential transport of patients with deterioration to hospitals equipped with higher levels of care is beneficial, identify why race is associated with deterioration and to test therapies targeting PND.
INTRODUCTION
Neurological deterioration, which may occur soon after the onset of stroke, has been associated with worse outcomes.1,2 Previous studies have shown early deterioration in the presence of intracerebral haemorrhage,3–5 but the frequency and importance of acute worsening in ischaemic stroke and subarachnoid haemorrhage is unclear. There have been no large, population-based studies to evaluate prehospital neurological deterioration (PND) in both ischaemic and haemorrhagic cases of stroke. While current prehospital stroke severity scales have shown potential benefit in triage,6,7 these do not include clinical change in their assessments. Acute deterioration may be another useful marker for patients requiring comprehensive stroke centres or other higher levels of care.
We evaluated patients with ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage to determine the frequency of PND in patients with acute haemorrhagic and ischaemic stroke presenting to regional Emergency Departments via emergency medical services (EMS), and we explore the association of PND with demographic and clinical variables.
METHODS
Greater Cincinnati/Northern Kentucky Strokestudy
The methods of the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS) have been previously described.8,9 Briefly, the National Institutes of Health (NIH)–funded GCNKSS systematically ascertained the population-based stroke incidence in calendar year 2010 from a 5-county, 15-hospital region with a population of about 1.3 million. During 2010, there was no specified triage of patients with stroke, and EMS was advised to transport to the nearest appropriate hospital. The medical records of all patients in the region with a hospital diagnosis of stroke or transient ischaemic attack using discharge International Classification of Diseases, Ninth Revision codes of 430–436 are screened by research nurses, with further adjudication by study physicians to an epistry. The epistry contains detailed sociodemographic and clinical data, including medical history, medication use, imaging/laboratory results and outcomes, inclusive of the prehospital, ED and inpatient phases of care. The GCNKSS was approved by the institutional review board (IRB) or equivalent at all 15 hospitals. Written informed consent was waived by the IRB.
Subject selection
The present analysis included patients with acute ischaemic stroke (IS), intracranial haemorrhage (ICH) or subarachnoid haemorrhage (SAH) who were at least 20 years of age, presented to an ED via EMS, and had GCS data from both EMS and ED records.
Primary outcome
PND was defined as a decrease in GCS score of at least two points between the initial EMS and ED evaluations, as has been defined in previous literature.10
Variables of interest
Variables were chosen for analysis based on previous reports that identified possible factors that influence early neurological change.3,4 Demographic and clinical variables included age, sex, race (black, white, other), medical history (hypertension, diabetes, atrial fibrillation and prior stroke), previous antiplatelet or anticoagulation use, stroke type (IS, ICH or SAH) and IS subtype (small vessel, large vessel, cardioembolic, other known cause, cryptogenic), seizure at onset, time from symptom onset to EMS arrival, time from EMS to ED arrival, EMS level of training as ALS or basic life support, and initial blood pressure and serum glucose on EMS arrival.
In cases where the onset of symptoms was not witnessed, the time last seen normal was used to calculate the elapsed time from symptom onset to EMS arrival. In cases where EMS arrival time was not documented, time of the first set of vital signs documented by EMS was used to estimate EMS arrival time. Seizure at onset was derived from patient-reported symptoms that led to calling EMS.
Statistical analysis
Demographic and clinical variables were compared between cohorts with and without PND using χ2 tests for categorical data and Wilcoxon rank-sum tests for continuous variables. Age, glucose on EMS arrival and blood pressure on EMS arrival were treated as continuous variables. Elapsed time between onset and EMS arrival was treated as a categorical variable (in hours) as 0–4.5, 4.5–24 and ‘>24 or unknown’. Time was categorised as such due to the significance of the cut-offs of 4.5 hours after symptom onset and 24 hours after symptom onset in acute stroke medical and endovascular treatment, respectively.
Multivariable logistic regression was used to identify potential risk factors with PND as the dependent variable and potential risk factors determined by those variables with p values ≤0.20 in the univariate analysis. In regards to race, ‘white’ and ‘other’ groups were combined due to the lack of a significant percentage in the ‘other’ category in our population. OR with 95% CIs and p values were reported, with p values less than 0.05 considered statistically significant. SAS V9.4 (SAS Institute, Cary, North Carolina, USA) was used for all data analyses.
RESULTS
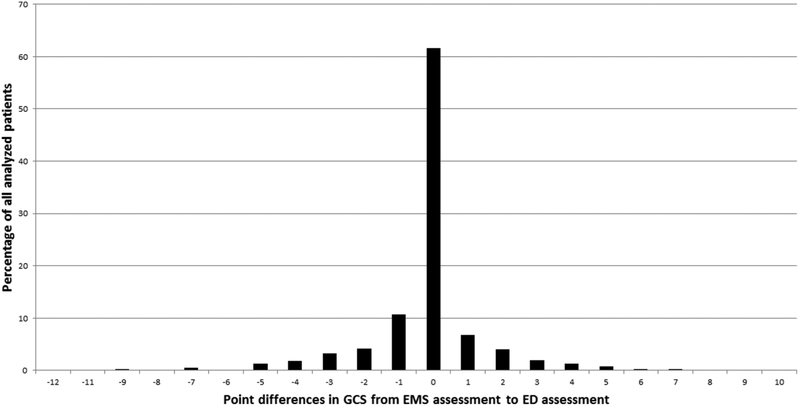
Among 2708 patients ≥20 years of age who had an IS, ICH or SAH event in 2010, 1092 used EMS, presented to an ED, and had GCS data for both EMS and the ED. These subjects had a median age of 74 years (IQR 61–83), 55% were women and 21% were black. Ischaemic stroke was diagnosed in 866, 175 had an ICH and 51 had a SAH. Time from witnessed onset or last known well to EMS arrival was ≤4.5 hours for 504 (46%), and median time from EMS arrival on-scene to ED arrival was 26 min. PND between EMS and ED occurred in 129 cases overall (12%), including 9% of IS, 24% of ICH and 16% of SAH cases. Figure 1 depicts the distribution of changes in GCS.
Figure 1.
Distribution of changes in GCS score between EMS assessment and ED assessment in all analysed patients.
Factors associated with PND in the univariate analysis are listed in table 1, showing that female gender, history of atrial fibrillation, previous use of antiplatelet medication, haemorrhagic and subarachnoid stroke subtype, cardioembolic subtype of ischaemic stroke, lower baseline and hospital GCS score, ALS level of transport and reported symptom of seizure were all significantly associated with PND. Multivariable analysis completed on all variables with p values ≤0.20 in the univariate analysis found that black race, atrial fibrillation, haemorrhagic subtype of stroke and ALS transport were independently associated with PND (table 2).
Table 1.
Univariate analysis of factors associated with PND, n=1092
| PND (n=129) | No PND (n=963) | P values | |
|---|---|---|---|
| Age, median (IQR) | 76 (63–85) | 73 (60–83) | 0.18 |
| Female gender | 82 (64%) | 524 (54%) | 0.049 |
| Race | 0.07 | ||
| White | 91 (71%) | 763 (79%) | |
| Black | 37 (29%) | 191 (20%) | |
| Other | 1 (1%) | 9 (1%) | |
| Medical history | |||
| Hypertension | 110 (85%) | 783 (81 %) | 0.27 |
| Diabetes | 36 (28%) | 311 (32%) | 0.31 |
| Atrial fibrillation | 49 (38%) | 233 (24%) | <0.01 |
| Previous stroke | 28 (22%) | 253 (26%) | 0.27 |
| Medication prior to onset | |||
| Antiplatelet | 50 (39%) | 462 (48%) | 0.049 |
| Anticoagulation | 22(17%) | 125 (13%) | 0.20 |
| Stroke type | <0.01 | ||
| Ischaemic | 79(61%) | 787 (82%) | |
| Haemorrhagic | 42 (33%) | 133 (14%) | |
| Subarachnoid | 8 (6%) | 43 (4%) | |
| Ischaemic stroke subtype | n=79 | n=787 | <0.01 |
| Large vessel | 7 (9%) | 114 (14%) | |
| Cardioembolic | 44 (56%) | 264 (34%) | |
| Small vessel | 1 (1%) | 100 (13%) | |
| Cryptogenic—undetermined aetiology | 25 (32%) | 276 (35%) | |
| Other | 2 (3%) | 33 (4%) | |
| LKN to EMS arrival at scene | 0.31 | ||
| 0–4.5 hours | 66 (51%) | 438 (45%) | |
| 4.5–24hours | 42 (33%) | 317 (33%) | |
| >24 hours | 21 (16%) | 208 (22%) | |
| EMS arrival to hospital in minutes, median (IQR) | n=127 26 (21–33) | n=907 26 (20–34) | 0.99 |
| GCS EMS, median (IQR) | 13 (11–15) | 15 (12–15) | <0.01 |
| GCS hospital, median (IQR) | 10 (6–11) | 15 (13–15) | <0.01 |
| Glucose EMS in mg/dL, median (IQR) | n=96 130 (106–151) | n=631 134 (109–171) | 0.26 |
| BP EMS, mm Hg | |||
| Systolic, median (IQR) | n=122 157 (138,180) | n=923 150 (132,177) | 0.44 |
| Diastolic, median (IQR) | n=121 80 (62–100) | n=919 82 (68–98) | 0.95 |
| EMS level of transport | 0.04 | ||
| ALS | 105 (83%) | 698 (74%) | |
| BLS | 22 (17%) | 240 (26%) | |
| Symptom of seizure | 11 (9%) | 42 (4%) | 0.04 |
BLS, basic life support; EMS, emergency medical services; LKN, last known normal; PND, prehospital neurological deterioration.
Table 2.
Multivariable logistic regression of predictors of prehospital neurological deterioration for variables with p values ≤0.20 in univariate analysis
| OR(95%CI) | P values | |
|---|---|---|
| Age | 1.01 (0.99 to 1.02) | 0.33 |
| Female gender | 1.37 (0.92 to 2.05) | 0.12 |
| Race | ||
| White/other | Reference | |
| Black | 2.32 (1.42 to 3.77) | <0.01 |
| Medical history | ||
| Atrial fibrillation | 2.52 (1.54 to 4.11) | <0.01 |
| Medication prior to onset | ||
| Antiplatelet | 0.71 (0.47 to 1.06) | 0.09 |
| Anticoagulation | 0.86 (0.48 to 1.54) | 0.61 |
| Stroke type | ||
| Ischaemic | Reference | |
| Haemorrhagic/subarachnoid | 3.14 (2.03 to 4.86) | <0.01 |
| GCS EMS | 1.02 (0.96 to 1.08) | 0.47 |
| EMS level of training | ||
| BLS | Reference | |
| ALS | 1.84 (1.06 to 3.18) | 0.03 |
| Symptom of seizure | 1.35 (0.64 to 2.85) | 0.43 |
BLS, basic life support: EMS, emergency medical services.
In univariate analysis of IS cases, age, female sex, medical history of atrial fibrillation, cardioembolic subtype, higher initial EMS GCS score and reported symptom of seizure were associated with PND. In multivariable analysis of IS cases, female sex (OR 1.93, p = 0.01), black race (OR 1.84, p = 0.03) and stroke subtype (cardioembolic vs cryptogenic (OR 1.73) and cardioem- bolic vs large vessel/small vessel/other (OR 3.38), p<0.01) were significant predictors of PND.
In the ICH subgroup, only EMS glucose on arrival was significantly associated with PND in the univariate analysis, which remained significant in the multivariable model (OR 0.99, p = 0.03). SAH subgroup analysis was not performed because data were limited due to small number of events (51 cases and eight deterioration events).
DISCUSSION
In this analysis of a large population-based study of patients with stroke, we found that PND occurs in 12% of subjects overall, is more common with black race and in patients with intracranial haemorrhage, and is associated with atrial fibrillation and cardioembolic ischaemic strokes. Our study was based out of a metropolitan area, and it is possible that there may be even more significant proportions of PND in rural areas due to longer times of transport. To our knowledge, this is the first population-based estimate of PND in patients with stroke transported by EMS. Our study provides evidence that deterioration may be used in initial triage and management of patients in the prehospital setting. Future studies also need to be conducted to assess whether therapies to target PND are beneficial.
Guidelines have already established preferential triage of patients with suspected stroke to primary or comprehensive stroke centres,11 and there has been some evidence supporting more specified levels of triage to endovascular capable centres or comprehensive stroke centres based on clinical scales for large vessel occlusion.6,7 The significant association of haemor- rhagic stroke with PND may help providers by guiding hospital prenotification and justify preferential triage of patients with acute worsening to centres capable of managing haemorrhagic stroke with on-call neurosurgical services. Also, there is evidence that large-vessel occlusions related to cardioembolic aetiology may lead to a longer time during the mechanical thrombectomy procedure until recanalisation is achieved,12 which may support triage of patients with atrial fibrillation to an endovascular capable centre initially in the prehospital setting, based on the association between PND and atrial fibrillation. Furthermore, the occurrence of PND in a significant amount of patients with stroke supports including deterioration in the formulation of future prehospital clinical scales that are used in triage, which all currently only include a single assessment.6
Previous studies on PND in stroke have involved only patients with ICH, and these showed higher proportions of deterioration than our study.3,4 Haemorrhagic stroke has previously been described to lead to more cases of early neurological deterioration than ischaemic stroke, with 22% of haemorrhage cases experiencing deterioration versus 7% of ischaemic cases in one study,1 which are similar rates as our results. Our study likely had a lower proportion of deterioration also because its population-based design does not have the severity bias common in single-centre cohorts. Including all subtypes of stroke in the analysis is appropriate, given that EMS is unable to differentiate between subtypes of stroke with certainty in the prehospital setting.
In contrast to the previous studies on PND in patients with ICH,3–5 EMS blood pressure, prior antiplatelet use and time of transport were not significantly associated with PND in our study, even with stratified analysis on patients with IS and ICH separately. A previous study of patients with acute stroke treated with intravenous tissue plasminogen activator found that early neurological deterioration was significantly associated with atrial fibrillation.13 In our study, atrial fibrillation was significantly associated with PND and cardioembolic subtype was significantly associated with PND in IS cases in the multivariable analysis.
We found that black race was an independent predictor of PND. One other single-centre study in patients with ICH included race as a covariate but did not find race to be associated with deterioration.3 Further studies are needed to evaluate how race is linked to PND. We also found that ALS transport was associated with PND. This finding could reflect appropriate initial triage of patients by the EMS dispatcher and by EMS providers in the field, as patients who were identified as having more severe conditions or those deemed likely of undergoing rapid change in status would be transported preferentially by providers with a higher level of training. It is unclear why more acute stroke cases were not more likely to have further deterioration, reflected by the lack of significant association between PND and time interval of LKN to EMS arrival, even when haem- orrhagic stroke cases were analysed separately. However, the subgroup analysis on haemorrhagic cases does show a non-significant trend with more cases exhibiting PND in the 0–4.5 hour subgroup (52% of all ICH cases with PND) than the 4.5–24 hour subgroup (29% of all ICH cases with PND) or the greater than 24 hours subgroup (19% of all ICH cases with PND). It remains possible that the number of haemorrhagic cases was too few to show a significant association.
A limitation of this study is the use of ‘decrease in GCS between EMS and ED arrival’ as a surrogate for deterioration of neurological function while en route, which has been shown to have only moderate inter-rater reliability.14 In the absence of widespread standardised use of other measures of neurological function in EMS setting, GCS is the best measure of neurological status and has been used previously in stroke.10 GCS score has also been shown to be associated with outcome in stroke.15,16 Variability in full NIH stroke scale would be a better determinant of prehospital clinical changes for a smaller prospective study, but the full scale is not performed uniformly by EMS, if at all. Also, possibly due to our urban setting, there was lack of large variances in transport times in our population, and EMS arrival to ED arrival time was not found to be significantly associated with PND. There are further limitations inherent to a retrospective study, such as limitation to what is documented in chart review, errors with classification of stroke subtype or IS subtype, and errors with the estimation methods used for EMS arrival.
CONCLUSION
PND is not uncommon in patients with stroke transported by EMS, and deterioration is associated with race, intracranial haemorrhage and cardioembolic subtype of ischaemic strokes. Further studies are needed to assess whether the occurrence of deterioration can help aid in triage or intervention in the prehospital setting. The additive value of PND to existing prehospital stroke triage criteria also requires further evaluation.
Key messages.
What is already known on the subject
Early neurological deterioration has been associated with worse outcomes.
In cases of intracerebral haemorrhage, deterioration can occur in the prehospital and early hospital settings.
There have been no large, population-based studies to evaluate prehospital neurological deterioration and its association with predictive factors in all cases of stroke.
What this study adds
In a large population sample, the occurrence of prehospital neurological deterioration is independently associated with race, haemorrhagic type of stroke and cardioembolic subtype of ischaemic stroke.
Funding
This study has been funded by NIH grant RO1NS30678.
Footnotes
Competing interests None declared.
Patient consent Not required.
Ethics approval Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM 2006;99:625–33. [DOI] [PubMed] [Google Scholar]
- 2.Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 2004;63:461–7. [DOI] [PubMed] [Google Scholar]
- 3.Moon JS, Janjua N, Ahmed S, et al. Prehospital neurologic deterioration in patients with intracerebral hemorrhage. Crit Care Med 2008;36:172–5. [DOI] [PubMed] [Google Scholar]
- 4.Fan JS, Chen YC, Huang HH, et al. The association between on-scene blood pressure and early neurological deterioration in patients with spontaneous intracerebral haemorrhage. Emerg Med J 2015;32:239–43. [DOI] [PubMed] [Google Scholar]
- 5.Fan JS, Huang HH, Chen YC, et al. Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: incidence, predictors, and prognostic significance. Acad Emerg Med 2012;19:133–8. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Coote S, Pesavento L, et al. Large vessel occlusion scales increase delivery to endovascular centers without excessive harm from misclassifications. Stroke 2017;48:568–73. [DOI] [PubMed] [Google Scholar]
- 7.McMullan JT, Katz B, Broderick J, et al. Prospective prehospital evaluation of the cincinnati stroke triage assessment tool. Prehosp Emerg Care 2017;21:481–8. [DOI] [PubMed] [Google Scholar]
- 8.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010;41:1326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke 1998;29:415–21. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Mohammad YM, Yahia AM, et al. A prospective multicenter study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracerebral hemorrhage. J Intensive Care Med 2005;20:34–42. [DOI] [PubMed] [Google Scholar]
- 11.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 12.Giray S, Ozdemir O, Baş DF, et al. Does stroke etiology play a role in predicting outcome of acute stroke patients who underwent endovascular treatment with stent retrievers? J Neurol Sci 2017;372:104–9. [DOI] [PubMed] [Google Scholar]
- 13.Awadh M, MacDougall N, Santosh C, et al. Early recurrent ischemic stroke complicating intravenous thrombolysis for stroke: incidence and association with atrial fibrillation. Stroke 2010;41:1990–5. [DOI] [PubMed] [Google Scholar]
- 14.Gill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med 2004;43:215–23. [DOI] [PubMed] [Google Scholar]
- 15.Diringer MN, Edwards DF. Does modification of the Innsbruck and the Glasgow Coma Scales improve their ability to predict functional outcome? Arch Neurol 1997;54:606–11. [DOI] [PubMed] [Google Scholar]
- 16.Weir CJ, Bradford AP, Lees KR. The prognostic value of the components of the Glasgow Coma Scale following acute stroke. QJM 2003;96:67–74. [DOI] [PubMed] [Google Scholar]