Abstract
Background
Anticoagulation may improve survival in patients with cancer through a speculated anti‐tumour effect, in addition to the antithrombotic effect, although may increase the risk of bleeding.
Objectives
To evaluate the efficacy and safety of parenteral anticoagulants in ambulatory patients with cancer who, typically, are undergoing chemotherapy, hormonal therapy, immunotherapy or radiotherapy, but otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
Search methods
A comprehensive search included (1) a major electronic search (February 2016) of the following databases: Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 1), MEDLINE (1946 to February 2016; accessed via OVID) and Embase (1980 to February 2016; accessed via OVID); (2) handsearching of conference proceedings; (3) checking of references of included studies; (4) use of the 'related citation' feature in PubMed and (5) a search for ongoing studies in trial registries. As part of the living systematic review approach, we are running searches continually and we will incorporate new evidence rapidly after it is identified. This update of the systematic review is based on the findings of a literature search conducted on 14 August 2017.
Selection criteria
Randomized controlled trials (RCTs) assessing the benefits and harms of parenteral anticoagulation in ambulatory patients with cancer. Typically, these patients are undergoing chemotherapy, hormonal therapy, immunotherapy or radiotherapy, but otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
Data collection and analysis
Using a standardized form we extracted data in duplicate on study design, participants, interventions outcomes of interest, and risk of bias. Outcomes of interested included all‐cause mortality, symptomatic venous thromboembolism (VTE), symptomatic deep vein thrombosis (DVT), pulmonary embolism (PE), major bleeding, minor bleeding, and quality of life. We assessed the certainty of evidence for each outcome using the GRADE approach (GRADE handbook [GRADE handbook]).
Main results
Of 6947 identified citations, 19 RCTs fulfilled the eligibility criteria. These trials enrolled 9650 participants. Trial registries' searches identified nine registered but unpublished trials, two of which were labeled as 'ongoing trials'. In all included RCTs, the intervention consisted of heparin (either unfractionated heparin or low molecular weight heparin). Overall, heparin appears to have no effect on mortality at 12 months (risk ratio (RR) 0.98; 95% confidence interval (CI) 0.93 to 1.03; risk difference (RD) 10 fewer per 1000; 95% CI 35 fewer to 15 more; moderate certainty of evidence) and mortality at 24 months (RR 0.99; 95% CI 0.96 to 1.01; RD 8 fewer per 1000; 95% CI 31 fewer to 8 more; moderate certainty of evidence). Heparin therapy reduces the risk of symptomatic VTE (RR 0.56; 95% CI 0.47 to 0.68; RD 30 fewer per 1000; 95% CI 36 fewer to 22 fewer; high certainty of evidence), while it increases in the risks of major bleeding (RR 1.30; 95% 0.94 to 1.79; RD 4 more per 1000; 95% CI 1 fewer to 11 more; moderate certainty of evidence) and minor bleeding (RR 1.70; 95% 1.13 to 2.55; RD 17 more per 1000; 95% CI 3 more to 37 more; high certainty of evidence). Results failed to confirm or to exclude a beneficial or detrimental effect of heparin on thrombocytopenia (RR 0.69; 95% CI 0.37 to 1.27; RD 33 fewer per 1000; 95% CI 66 fewer to 28 more; moderate certainty of evidence); quality of life (moderate certainty of evidence).
Authors' conclusions
Heparin appears to have no effect on mortality at 12 months and 24 months. It reduces symptomatic VTE and likely increases major and minor bleeding. Future research should further investigate the survival benefit of different types of anticoagulants in patients with different types and stages of cancer. The decision for a patient with cancer to start heparin therapy should balance the benefits and downsides, and should integrate the patient's values and preferences.
Editorial note:This is a living systematic review. Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence, as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Plain language summary
Injectable blood thinners (anticoagulants) in patients with cancer
Background Research evidence suggests that blood thinners may improve the survival of patients with cancer, by preventing life‐threatening blood clots and might also have a direct anticancer effect. However, blood thinners can also increase the risk of bleeding, which can be serious and reduce survival. It is therefore important to understand the pros and cons of treatment to allow patients and their doctors to be aware of the balance of risks and benefits.
Study characteristics We searched the scientific literature for studies of anticoagulants in people with cancer. The evidence is current to 14 August 2017. We included 19 eligible trials.
Key results We selected 19 trials including 9650 participants with cancer. Most trials included participants with various types of cancer, especially small cell lung cancer, non‐small cell lung cancer, and pancreatic cancer. All studies were conducted in the outpatient setting. The results suggest that the effect of injectable blood thinners on survival is uncertain, but if anything of small size. Also the results suggest that injectable blood thinners reduce the risk of blood clots by about half and possibly increase the risk of major bleeding and minor bleeding by 4 more per 1000 and 17 more per 1000, respectively. The effect on quality of life is uncertain.
Certainty of evidence We judged the certainty of evidence to be high for symptomatic VTE and minor bleeding, and moderate for mortality, major bleeding and quality of life.
Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence, as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Summary of findings
Summary of findings 1. Heparin prophylaxis compared with no prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy.
|
Heparin prophylaxis compared with no prophylaxis in ambulatory patients with cancer without VTE receiving systemic therapy P: Ambulatory patients with cancer without VTE receiving systemic therapy I: Heparin prophylaxis C: No prophylaxis S: Outpatient | |||||
| Outcomes | № of participants (studies) Follow‐up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with No prophylaxis | Risk difference with Heparin prophylaxis | ||||
| Mortality follow‐up: 12 months | 9575 (18 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | RR 0.98 (0.93 to 1.03) | Study population | |
| 504 per 1000 | 10 fewer per 1000 (35 fewer to 15 more) | ||||
| Mortality follow‐up: 24 months | 5229 (14 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | RR 0.99 (0.96 to 1.01) | Study population | |
| 778 per 1000 | 8 fewer per 1000 (31 fewer to 8 more) | ||||
| Symptomatic VTE follow‐up: 12 months | 9036 (16 RCTs) | ⊕⊕⊕⊕ HIGH | RR 0.56 (0.47 to 0.68) | Study population | |
| 68 per 1000 | 30 fewer per 1000 (36 fewer to 22 fewer) | ||||
| Major bleeding follow‐up: 12 months | 9592 (18 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | RR 1.30 (0.94 to 1.79) | Study population | |
| 14 per 1000 | 4 more per 1000 (1 fewer to 11 more) | ||||
| Minor bleeding follow‐up: 12 months | 9245 (16 RCTs) | ⊕⊕⊕⊕ HIGH | RR 1.70 (1.13 to 2.55) | Study population | |
| 24 per 1000 | 17 more per 1000 (3 more to 37 more) | ||||
| Thrombocytopenia | 5832 (12 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | RR 0.69 (0.37 to 1.27) | Study population | |
| 105 per 1000 | 33 fewer per 1000 (66 fewer to 28 more) | ||||
| Quality of life impairment | 2241 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 4 | ‐ | Macbeth 2016 (FRAGMATIC): " There was no difference between the two groups with respect to quality‐adjusted life years gained in the first year... No difference in overall quality of life at 6 months (P = .94) or at 12 months (P = .89)... Overall quality of life did not change significantly over the study period".Sideras 2006: "The QOL and SDS scores were similar, both at baseline and during the protocol period, in patients randomized to receive LMWH vs those not randomized to receive LMWH." | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded one level due to serious imprecision: Confidence interval includes values suggesting clinically significant benefit and values suggesting no effect.
2 Downgraded one level due to serious imprecision: Confidence interval includes values suggesting clinically significant harm and values suggesting no effect.
3 Downgraded one level due to serious imprecision: Confidence interval includes values suggesting clinically significant benefit and values suggesting harm.
4 Downgraded one level due to serious risk of bias: Both studies were open‐label studies (lack of blinding may impact the patient‐reported subjective outcomes)
Background
Please refer to the glossary for the definitions of technical terms (Table 2).
1. Glossary.
| Term | Definition |
| Adjuvant therapy | Assisting in the amelioration or cure of disease |
| Anticoagulation | The process of hindering the clotting of blood especially by treatment with an anticoagulant |
| Antithrombotic | Used against or tending to prevent thrombosis (clotting) |
| Bacteremia | The presence of bacteria in the blood |
| Central venous line | Synthetic tube that is inserted into a central (large) vein of a patient to provide temporary intravenous access for the administration of fluid, medication or nutrients |
| Coagulation | Clotting |
| Deep vein thrombosis (DVT) | A condition marked by the formation of a thrombus within a deep vein (as of the leg or pelvis) that may be asymptomatic or be accompanied by symptoms (such as swelling and pain) and that is potentially life‐threatening if dislodgment of the thrombus results in pulmonary embolism |
| Fibrin | A white insoluble fibrous protein formed from fibrinogen by the action of thrombin, especially in the clotting of blood |
| Fondaparinux | An anticoagulant medication |
| Hemostatic system | The system that shortens the clotting time of blood and stops bleeding |
| Heparin | An enzyme occurring especially in the liver and lungs that prolongs the clotting time of blood by preventing the formation of fibrin. Two forms of heparin that are used as anticoagulant medications are: unfractionated heparin (UFH) and low molecular weight heparins (LMWH) |
| Impedance plethysmography | A technique that measures the change in blood volume (venous blood volume as well as the pulsation of the arteries) for a specific body segment |
| Kappa statistics | A measure of degree of non‐random agreement between observers and/or measurements of a specific categorical variable |
| Metastasis | The spread of cancer cells from the initial or primary site of disease to another part of the body |
| Oncogene | A gene having the potential to cause a normal cell to become cancerous |
| Osteoporosis | A condition that especially affects older women and is characterized by a decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness |
| Parenteral nutrition | The practice of feeding a patient intravenously, circumventing the gut |
| Pulmonary embolism (PE) | Embolism of a pulmonary artery or one of its branches that is produced by foreign matter and most often a blood clot originating in a vein of the leg or pelvis and that is marked by labored breathing, chest pain, fainting, rapid heart rate, cyanosis, shock and sometimes death |
| Stroma | The supporting framework of an organ typically consisting of connective tissue |
| Thrombin | A proteolytic enzyme formed from prothrombin that facilitates the clotting of blood by catalyzing conversion of fibrinogen to fibrin |
| Thrombocytopenia | Persistent decrease in the number of blood platelets that is often associated with hemorrhagic conditions |
| Thrombosis | The formation or presence of a blood clot within a blood vessel |
| Vitamin K antagonists | Anticoagulant medications that are used for anticoagulation. Warfarin is a vitamin K antagonist |
| Warfarin | An anticoagulant medication that is a vitamin K antagonist, which is used for anticoagulation |
| Ximelagatran | An anticoagulant medication |
Description of the condition
Since the 1930s, scientists have been exploring the effects of anticoagulation on cancer (Smorenburg 2001). Studies have implicated the tumor‐mediated activation of the hemostatic system in both the formation of tumor stroma and in tumor metastasis (Dvorak 1986; Francis 1998; Levine 2003). There is also evidence that heparin inhibits expression of oncogenes and formation of thrombin and fibrin induced by cancer cells (Smorenburg 2001). In addition, heparin potentially inhibits intravascular arrest of cancer cells and thus metastasis (Smorenburg 2001).
Description of the intervention
Heparin and low molecular weight heparins (LMWHs), fondaparinux and danaparoid do not have intrinsic anticoagulant activity but potentiate the activity of antithrombin III in inhibiting activated coagulation factors. These agents constitute indirect anticoagulants as their activity is mediated by plasma cofactors. Recombinant hirudin, bivalirudin and argatroban directly inhibit thrombin and are classified as direct anticoagulants (Hirsh 2008). Heparin and its low molecular weight derivatives are not absorbed orally and must be administered parenterally by intravenous infusion or subcutaneous injections (Hirsh 1993).
How the intervention might work
Researchers have hypothesized that heparin may improve outcomes in patients with cancer through an anti‐tumor effect in addition to its antithrombotic effect (Thodiyil 2002). In a 1992 clinical trial comparing nadroparin, a LMWH, with unfractionated heparin in patients with proven deep vein thrombosis (DVT), nadroparin unexpectedly reduced mortality in the subgroup of patients with cancer (Prandoni 1992). At the same time, anticoagulants increase the risk for bleeding. In fact, in patients with venous thrombosis on anticoagulation, the risk of bleeding was higher if patients had cancer and correlated with the extent of cancer (Prandoni 2002). Heparins are also known to cause thrombocytopenia (reduced numbers of platelets) and heparin‐induced thrombocytopenia (HIT) syndrome (Girolami 2006).
Why it is important to do this review
We initially conducted this and other reviews on this topic and their updates to directly and better inform clinical practice guidelines. The last update of this systematic review, published in 2014 (Akl 2014 (parenteral)), identified 15 trials enrolling 7662 participants (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Altinbas 2004; Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS); Klerk 2005 (MALT); Lebeau 1994; Lecumberri 2013 (ABEL); Maraveyas 2012 (FRAGEM); Pelzer 2009 (CONKO‐2004); Perry 2010 (PRODIGE); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008). The included trials provided high‐certainty evidence for a reduction of venous thromboembolism (VTE) with heparin thromboprophylaxis compared to no heparin thromboprophylaxis. Since then, we have identified three eligible trials addressing this question (Khorana 2017 (PHACS); Macbeth 2016 (FRAGMATIC); Zwicker 2013 (MICRO TEC)) and the full‐text publication of a previously included abstract (Pelzer 2015 (CONKO‐004).
Living systematic review approach: Following the publication of this current 2017 update of the review, we will maintain it as a living systematic review. This means we will be continually running the searches and rapidly incorporating any newly identified evidence (for more information about the living systematic review approach being piloted by Cochrane, see Appendix 1. We believe a living systematic review approach is appropriate for this review for four reasons. First, the review addresses an important topic for clinical practice; patients with cancer have a relatively high rate of VTE, up to 17.7% (Ay 2010). In addition, the occurrence of VTE is associated with a 2.3 increased risk of death in patients with breast and non‐small cell lung cancer (NSCLC), 2.5 times lengthening of hospital stay among patients with lung cancer, and 50% higher total cost for patients with lung cancer (Chew 2008, Chew 2007; Connolly 2012). Second, there remains uncertainty in the existing evidence base; the 2014 update of this systematic review found a potential subgroup effect on all‐cause mortality at one year, with a possible higher reduction in mortality among patients with small cell lung cancer (SCLC) compared to other types of cancer. Third, we are aware of several recently published and ongoing trials in this area that will be important to incorporate in a timely manner. Fourth, we are planning to use this living systematic review as the basis of a living recommendation in a clinical practice guideline with the American Society of Hematology (Akl 2017). For more information about the living systematic review approach being piloted by Cochane, see Appendix 2.
Objectives
To evaluate the effectiveness and safety of parenteral anticoagulants in ambulatory patients with cancer. Typically, these patients are undergoing chemotherapy, hormonal therapy, immunotherapy or radiotherapy, but otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Participants with cancer with no standard indication for prophylactic anticoagulation (e.g. for acute illness, for central venous line placement, perioperatively) or for therapeutic anticoagulation (e.g. for the treatment of deep vein thrombosis (DVT) or pulmonary embolism (PE)). Patients could have been of any age group (including children). Typically, these participants are undergoing chemotherapy, hormonal therapy, immunotherapy or radiotherapy.
Types of interventions
Intervention arm: parenteral anticoagulants, such as unfractionated heparin or low molecular weight heparin (LMWH).
Comparator intervention: placebo or no intervention.
The trial protocol should have planned to provide all other co‐interventions (e.g. chemotherapy) similarly.
Types of outcome measures
Primary outcomes
All‐cause mortality; pre‐specified at 12 months, 24 months and over the duration of the trial.
Secondary outcomes
Symptomatic DVT: DVT events had to be suspected clinically, and diagnosed using an objective diagnostic test such as: venography, 125I‐fibrinogen‐uptake test, impedance plethysmography or compression ultrasound.
PE: PE events had to be suspected clinically, and diagnosed using an objective diagnostic test such as: pulmonary perfusion/ventilation scans, computed tomography, pulmonary angiography or autopsy.
Major bleeding: we accepted the authors' definitions of major bleeding.
Minor bleeding: we accepted the authors' definitions of minor bleeding.
Health‐related quality of life: had to be measured using a validated tool.
Thrombocytopenia.
Search methods for identification of studies
Electronic searches
The search was part of a comprehensive search for studies of anticoagulation in patients with cancer. We did not use language restrictions. We conducted comprehensive searches on 14 August 2017, following the original electronic searches in January 2007, February 2010, February 2013, and February 2016 (last major search). We electronically searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (starting 1946), and Embase (starting 1980; accessed via OVID) . The search strategies combined terms for anticoagulants, terms for cancer and a search filter for RCTs. We list the full search strategies for each of the electronic databases in Appendix 3, Appendix 4, and Appendix 5, respectively.
Living systematic review approach: Since the last major search in February 2016, we have been running searches monthly, using auto‐alerts to deliver the monthly yield by email. We will incorporate new evidence rapidly after it is identified. This update of the systematic review is based on the findings of a literature search conducted on 14 August 2017. We will review search methods and strategies approximately yearly, to ensure they reflect any terminology changes in the topic area, or in the databases.
Searching other resources
We handsearched the conference proceedings of the American Society of Clinical Oncology (ASCO, starting with its first volume, 1982 up to August 2017) and of the American Society of Hematology (ASH, starting with its 2003 issue up to August 2017).We also searched ClinicalTrials.gov and WHO International Clinical Trials Registry Platform for ongoing studies. We reviewed the reference lists of papers included in this review and of other relevant systematic reviews. We used the 'related citation' feature in PubMed to identify additional articles and 'citation tracking' of included studies in Web of Science Core Collection.In addition, experts in the field were contacted for information about unpublished and ongoing trials.
Living systematic review approach: We will search the conference proceedings of ASCO and ASH soon after their publications, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform on a monthly basis. As an additional step, we will contact corresponding authors of ongoing studies as they are identified and ask them to advise when results are available. We will continue to review the reference lists for any prospectively identified studies, with running the 'related citation' for all included studies on a monthly basis. Also, we will contact the corresponding authors of any newly included studies for advice as to other relevant studies. We will conduct citation tracking of included studies in Web of Science Core Collection on an ongoing basis, using citation alerts in Web of Science Core Collection.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of identified articles for eligibility. We retrieved the full text of articles judged as potentially eligible by at least one review author. Two review authors then independently screened the full‐text articles for eligibility using a standardized form with explicit inclusion and exclusion criteria. The two review authors resolved their disagreements by discussion or by consulting a third review author.
Living systematic review approach: For the monthly searches, we will immediately screen any new citations retrieved each month. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) available in the Cochrane Register of Studies (CSR‐Web; Wallace 2017). The classifier assigns a probability (from 0 to 100) to each citation for being a true RCT. For citations that are assigned a probability score of less than 10, the machine learning classifier currently has a specificity/recall of 99.987% (James Thomas, personal communication). For citations assigned a score from 10 to 100, we will screen them in duplicate and independently. Citations that score 9 or less will be screened by Cochrane Crowd (Cochrane Crowd). Any citations that are deemed to be potential RCTs by Cochrane Crowd will be returned to the authors for screening.
Data extraction and management
Two review authors independently extracted data from each included study and resolved their disagreements by discussion. We aimed to collect data related to the following.
Participants
Number of participants randomized to each treatment arm.
Number of participants followed up in each treatment arm.
Number of withdrawals from treatment in each treatment arm.
Population characteristics (age, gender, co‐morbidity).
History of VTE.
Type of cancer (site and histology).
Stage of cancer.
Time since cancer diagnosis.
Interventions
Type of anticoagulant: unfractionated heparin, LMWH or fondaparinux.
Dose: prophylactic versus therapeutic (Table 3).
Duration of treatment.
Control: placebo or no intervention.
Co‐interventions including chemotherapy and hormonal therapy, immunotherapy and radiotherapy (type and duration).
2. LMWH: definitions of prophylactic and therapeutic dosages.
| LMWH | Generic name | Prophylactic dose | Therapeutic dose |
| Lovenox | Enoxaparin | 40 mg once daily | 1 mg/kg twice daily |
| Fragmin | Dalteparin | 2500 to 5000 units once daily | 200 U/kg once daily or 100 U/kg twice daily |
| Innohep, Logiparin | Tinzaparin | 4500 units once daily | 90 U/kg twice daily |
| Fraxiparine | Nadroparin | 35 to 75 anti‐Xa international units/kg once daily | 175 anti‐Xa int. units/kg once daily |
| Certoparin | Sandoparin | 3000 anti‐Xa international units once daily | — |
Outcomes
We extracted both time‐to‐event data (for the survival outcome) and dichotomous data (for all outcomes). For mortality, we collected data for the pre‐specified time point of 12 months, but also for 24 months and for over the duration of follow‐up.
For time‐to‐event survival data, we abstracted the log(hazard ratio (HR)) and its variance from trial reports. If these were not reported, we digitized the published Kaplan‐Meier survival curves and estimated the log(HR) and its variance using Parmar's methods (Parmar 1998). We also noted the minimum and maximum duration of follow‐up, which are required to make these estimates. We performed these calculations in Stata 9, using a specially written program, which yielded the reported log(HR) and variance when used on the data presented in Table V of Parmar 1998.
For dichotomous data, we extracted data necessary to conduct complete case analysis as the primary analysis. We collected all‐cause mortality at one year (time point defined a priori in the protocol) and at two years (time point defined post hoc based upon results reported in the individual RCTs). When we could not obtain the number of events at the time points of interest from the paper or from the authors, two review authors calculated these numbers independently and in duplicate from survival curves, if available (Altinbas 2004; Kakkar 2004 (FAMOUS)). We used the mean of the two estimates when they differed. We assessed agreement between the two authors for each estimated value by calculating the percentage difference, which is the difference between the two estimates divided by the denominator (number of people at risk for the event) and multiplied by 100. For some studies, where VTE is not reported as a separate outcome, we added the number of events of DVT and PE.
We attempted to contact study authors for incompletely reported data. We decided a priori to consider abstracts in the main analysis only if authors supplied us with full reports of their methods and results.
Other
We extracted from each included trial any information on the following points:
source of funding;
ethical approval;
conflict of interest.
Assessment of risk of bias in included studies
We assessed risk of bias at the study level using the Cochrane 'Risk of bias' tool (Cochrane Handbook). Two review authors independently assessed the risk of bias of each included study and resolved their disagreements by discussion. 'Risk of bias' criteria included:
adequate sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
percentage of follow‐up and whether incomplete outcome data were addressed;
whether the study was free of selective reporting; and
whether the study was stopped early for benefit.
See section on Dealing with missing data about assessing risk of bias associated with participants with missing data.
Measures of treatment effect
We collected and analyzed hazard ratios (HRs) for time‐to‐event data and risk ratios (RRs) for dichotomous data. None of the outcomes of interest was meta‐analyzed as a continuous variable.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
Determining participants with missing data
It was not clear whether certain participant categories (e.g. those described as "withdrew consent" or "experienced adverse events") were actually followed up by the trial authors (versus had missing participant data) (Akl 2016). To deal with this issue, we made the following considerations:
"ineligible participants" and "did not receive the first dose" participant categories, which are defined prior to the initiation of the study intervention, most likely have missing participant data;
"withdrew consent", "lost to follow‐up" and "outcome not assessable" participant categories and other category explicitly reported as not being followed‐up, which are defined after the initiation of the study intervention, most likely have missing participant data;
"dead", "experienced adverse events", "non‐compliant", "discontinued prematurely" and similarly described participant categories are less likely to have missing participant data.
Dealing with participants with missing data in the primary meta‐analysis
In the primary meta‐analysis, we used a complete case analysis approach, i.e. we excluded participants considered to have missing data (Guyatt 2017).
For categorical data, we used the following calculations for each study arm.
Denominator: (number of participants randomized) ‐ (number of participants most likely with missing data, both pre‐ and post‐intervention initiation).
Numerator: number of participants with observed events (i.e. participants who suffered at least one event for the outcome of interest during their available follow‐up time).
For continuous data, we planned to use for each study arm the reported mean and standard deviation for participants actually followed up by the trial authors.
Assessing the risk of bias associated with participants with missing data
When the primary meta‐analysis of a specific outcome found a statistically significant effect, we conducted sensitivity meta‐analyses to assess the risk of bias associated with missing participant data. Those sensitivity meta‐analyses used a priori plausible assumptions about the outcomes of participants considered to have missing data. The assumptions we used in the sensitivity meta‐analyses were increasingly stringent in order to progressively challenge the statistical significance of the results of the primary analysis (Akl 2013; Ebrahim 2013).
For categorical data, and for RR showing a reduction in effect (RR < 1), we used the following increasingly stringent but plausible assumptions (Akl 2013):
for the control arm, relative incidence (RI) among those with missing data (lost to follow‐up (LTFU)) compared to those with available data (followed up, FU) in the same arm (RILTFU/FU) = 1; for the intervention arm, RILTFU/FU = 1.5;
for the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 2;
for the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 3;
for the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 5.
For RR showing an increase in effect (RR > 1), we switched the above assumptions between the control and interventions arms (i.e. used RILTFU/FU = 1 for the intervention arm).
Specifically, we used the following calculations for each study arm.
Denominator: (number of participants randomized) ‐ (number of participants most likely with missing data, pre‐intervention initiation).
Numerator: (number of participants with observed events) + (number of participants most likely with missing data post‐intervention initiation, with assumed events).
Assumed events are calculated by applying the a priori plausible assumptions to the participants considered most likely with missing data post‐intervention initiation.
For continuous data, we planned to use the four strategies suggested by Ebrahim and colleagues. The strategies imputed the means for participants with missing data based on the means of participants actually followed up in individual trials included in the systematic review. To impute standard deviation (SD), we used the median SD from the control arms of all included trials (Ebrahim 2013).
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, we attempted to investigate the possible reasons for this (see section on Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We assessed for selective outcome reporting by trying to identify whether the study was included in a trial registry, whether a protocol was available, and whether the methods section provided a list of outcomes. We compared the list of outcomes from those sources to the outcomes reported in the published paper. We also assessed for possible publication bias by creating an inverted funnel plot for the primary outcome of survival.
Data synthesis
For time‐to‐event data, we pooled the log(HRs) using a random‐effects model (DerSimonian 1986), and the generic inverse variance facility of RevMan 2014. For dichotomous data, we calculated the RR separately for each study. When analyzing data related to participants who were reported as not compliant, we attempted to adhere to the principles of intention‐to‐treat (ITT) analysis. We approached the issue of non‐compliance independently from that of missing data (Alshurafa 2012). We then pooled the results of the different studies using a random‐effects model. We assessed the certainty of evidence at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (GRADE handbook).
Living systematic review approach: Whenever new evidence (studies, data or information) that meets the review inclusion criteria is identified, we will immediately assess risk of bias and extract the data and incorporate it in the synthesis, as appropriate. We will not adjust the meta‐analyses to account for multiple testing given the methods related to frequent updating of meta‐analyses are under development (Simmonds 2017).
Subgroup analysis and investigation of heterogeneity
We planned to explore heterogeneity by conducting subgroup analyses based on the characteristics of participants (type and stage of cancer, and whether participants were on cancer treatment or not). In particular, we conducted subgroup analyses for patients with (1) lung cancer (either SCLC or NSCLC) versus those with non‐lung cancer; (2) patients with advanced cancer versus those with non‐advanced cancer. We included in the lung versus non lung subgroup analysis data from:
studies that recruited only patients with lung cancer (either SCLC or NSCLC) and studies that recruited only patients with non‐lung cancer;
studies that recruited both lung and non‐lung cancer if they provided data for subgroups of patients with lung cancer AND data for subgroups of patients with non‐lung cancer;
studies that recruited both lung and non‐lung cancer but did not provide subgroup data, if more than 75% of participants had lung cancer or more than 75% of participants had non‐lung cancer.
Similarly for the subgroup analysis for non‐advanced cancer. We planned to assess the credibility of subgroup effect, when statistically significant, using the criteria suggested by Sun 2010.
Sensitivity analysis
We planned for sensitivity analyses excluding trials at high risk of bias. As described above, we also planned for sensitivity meta‐analyses to assess the risk of bias associated with missing participant data when the primary meta‐analysis of a specific outcome found a statistically significant effect.
Results
Description of studies
Results of the search
Figure 1 shows the study flow diagram. As of August 2017, the search strategy identified a total of 6947 unique citations. The title and abstract screening identified 192 potentially eligible citations. The full‐text screening of the full texts of these 192 citations identified 18 eligible RCTs published as full reports (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Altinbas 2004; Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS); Khorana 2017 (PHACS); Klerk 2005 (MALT); Lebeau 1994; Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC); Maraveyas 2012 (FRAGEM); Pelzer 2015 (CONKO‐004); Perry 2010 (PRODIGE); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008; Zwicker 2013 (MICRO TEC)), and one RCT published as abstract (Vadhan‐Raj 2013) We had also identified two eligible studies published as abstracts but for which we were not able to obtain the necessary data from the authors: Salat 1990, Chazouilleres 1994,. We identified nine registered but unpublished trials: four completed (Borad 2011 (PGPC1); Germonpre 2008 (SYRINGES); Kakkar 2010 (GASTRANOX); Okuno 1999); two terminated (Chibauldel 2008 (PAM07); Pandya 2002); two ongoing (Lars 2008 (RASTEN); Meyer 2017 (PROVE)); and one withdrawn prior to enrolment (Rosovsky 2009).
1.
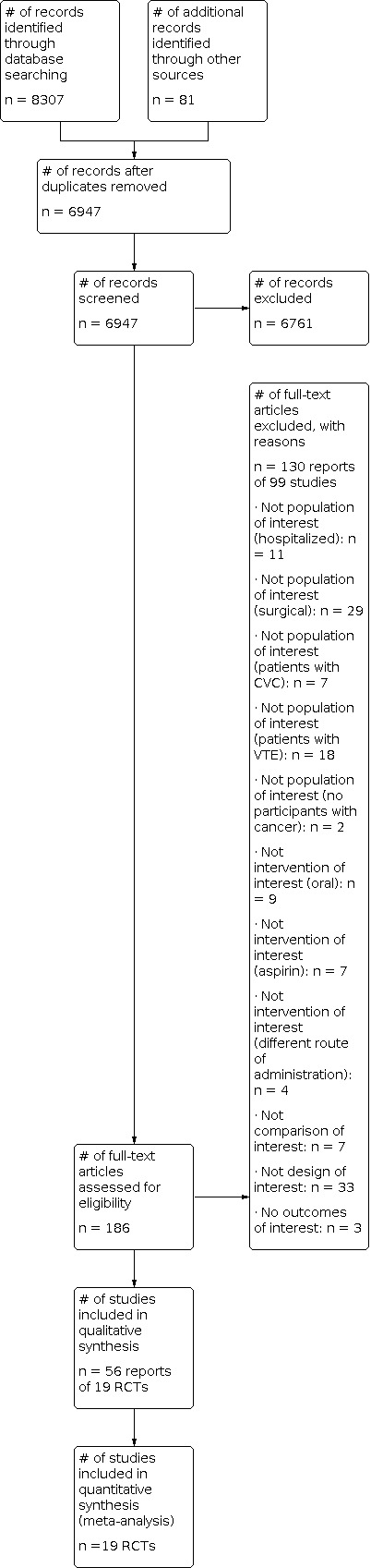
Study flow diagram.
Included studies
The 19 included studies had 9650 participants. One study used unfractionated heparin as the intervention (Lebeau 1994), another used ultra‐LMWH (Agnelli 2012 (SAVE‐ONCO)), while the other 17 used LMWH as the intervention (Agnelli 2009 (PROTECHT); Altinbas 2004; Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS); Khorana 2017 (PHACS); Klerk 2005 (MALT); Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC); Maraveyas 2012 (FRAGEM); Pelzer 2015 (CONKO‐004); Perry 2010 (PRODIGE); Sideras 2006; Vadhan‐Raj 2013; van Doormaal 2011 (INPACT); Weber 2008; Zwicker 2013 (MICRO TEC)). We did not identify any study using fondaparinux as the intervention.
Agnelli and colleagues (PROTECHT trial) recruited 1150 ambulatory participants with metastatic or locally advanced cancer (Agnelli 2009 (PROTECHT)). Participants were randomized to receive a prophylactic dose of nadroparin or placebo, each with concomitant chemotherapy. The primary efficacy outcomes were symptomatic DVT, and PE. The secondary efficacy outcomes were asymptomatic thromboembolic events incidentally diagnosed, and survival at the end of study treatment and at 12 months. Study outcomes included survival, asymptomatic VTE, and minor and major bleeding. Follow‐up was about 90% in each group.
Agnelli and colleagues (SAVE‐ONCO trial) recruited 3212 participants with advanced metastatic or locally advanced cancer. Of the participants, 91% had an ECOG performance status of zero or one and 42% had at least one risk factor for VTE (Agnelli 2012 (SAVE‐ONCO)). Participants were randomized to receive either subcutaneous injection of semuloparin or placebo for a minimum of three months. The study outcomes included mortality, PE, symptomatic DVT, major bleeding and minor bleeding. Follow‐up data were available for 99% of participants for mortality and VTE outcomes. The minimum duration of follow‐up was up to three days after last injection, with a median of 3.5 months. The maximum duration of follow‐up was 12 months.
Altinbas and colleagues recruited 84 participants with both limited and extensive SCLC and an Eastern Cooperative Oncology Group (ECOG) status < 3 (Altinbas 2004). The ECOG performance Status scale ranges from zero (fully active) to five (dead) (Oken 1982). Participants were randomized to receive either a prophylactic dose of a LMWH (dalteparin) or placebo for 18 weeks or less, in combination with chemotherapy in case of disease progression. Study outcomes included mortality (at 12 and 24 months), symptomatic DVT and bleeding. Follow‐up was complete (100%). The minimum and maximum duration of follow‐up were two and 33 months, respectively. Hazard ratios (HRs) were estimated from published survival curves.
Haas and colleagues conducted two multi‐centre double‐blind studies and recruited 900 ambulatory participants receiving chemotherapy for disseminated metastatic breast carcinoma (Haas 2012 (TOPIC 1); n = 353) or stage III/IV NSCLC carcinoma (Haas 2012 (TOPIC 2); n = 547). Participants were randomized to receive either subcutaneous certoparin or placebo for six months. The study outcomes included mortality, confirmed VTE, PE, DVT, thrombocytopenia, major bleeding and minor bleeding. A number of participants were not included in the intention‐to‐treat (ITT) analysis but it is not reported whether they were followed up. The minimum duration of follow‐up was six months.
Kakkar and colleagues recruited 385 participants with advanced stage III or IV malignant disease of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary or uterus, and a minimum life expectancy of three months (Kakkar 2004 (FAMOUS)). Participants were randomized to receive either a prophylactic dose of a LMWH (dalteparin) or placebo for 12 months, with no restriction on concomitant chemotherapy or radiotherapy. The study outcomes included mortality (at 12, 24 and 36 months), symptomatic VTE (PE, DVT), major bleeding and minor bleeding. Follow‐up data were available for 374 participants (97%). The minimum duration of follow‐up was not reported. The maximum duration of follow‐up was 81 months. HRs were estimated from published survival curves, assuming all participants were followed up for 77 months.
Khorana and colleagues conducted a multi‐center study and recruited 98 participants with cancer and a Khorana risk score of ≥ 3 (Khorana 2017 (PHACS)). Participants were randomized to subcutaneous dalteparin or observation for a period of 12 weeks. The study outcomes included symptomatic DVT and PE, and clinically significant major and non‐major bleeding. Follow‐up was complete (100%). The study was terminated early due to low accrual.
Klerk and colleagues (MALT trial) recruited 302 participants with different types of advanced solid malignant tumors and a minimum life expectancy of one month (Klerk 2005 (MALT)). Participants were randomized to receive either a LMWH or a placebo for six weeks, each with concomitant chemotherapy or radiotherapy. Study outcomes included mortality (at six, 12 and 24 months), major bleeding, non‐major bleeding and combined major and non‐major bleeding. Follow‐up was complete (100%). The minimum duration of follow‐up was not reported, whereas the maximum duration was 84 months. The HR and its standard error were reported.
Lebeau and colleagues recruited 277 participants with both limited and extensive small cell lung cancer (SCLC), 78% of which had a Karnofsky Performance Scale Index > 80 (Lebeau 1994). The Karnofsky Performance Scale Index ranges from zero (dead) to 100 (normal) (Karnofsky 1948). Participants were randomized to receive either a prophylactic dose of UFH for five weeks or no intervention, in combination with chemotherapy. The study outcomes were mortality (at 12, 24 and 36 months) and bleeding. Follow‐up was complete (100%). The minimum duration of follow‐up was not reported. The maximum duration of follow‐up was 59 months. HRs were estimated from published survival curves, assuming all participants were followed up for 59 months.
Lecumberri and colleagues recruited 38 participants diagnosed with limited SCLC in a multicenter, open‐label study (Lecumberri 2013 (ABEL)). Participants were randomized to receive standard chemoradiotherapy or the same therapy plus bemiparin for a maximum of 26 weeks. The study outcomes included all‐cause mortality, incidence of VTE, major and minor bleeding, and thrombocytopenia. All outcomes were assessed at 18 months. Follow‐up was complete (100%).
Macbeth and colleagues recruited 2202 participants diagnosed with lung cancer (Macbeth 2016 (FRAGMATIC)). Participants were on standard anticancer treatment and randomized to subcutaneous dalteparin or no anticoagulation. The study outcomes included overall survival and bleeding. The median duration of follow‐up was 23.1 months.
Maraveyas and colleagues recruited 123 participants with non‐resectable, recurrent or metastatic pancreatic adenocarcinoma, Karnofsky performance status (KPS) of 60 to 100, and estimated life expectancy of more than 12 weeks (Maraveyas 2012 (FRAGEM)). Participants were randomized to receive either subcutaneous dalteparin or placebo. The study outcomes included mortality, all‐type VTE, DVT, and PE. Data from a range of 55 to 62 participants were used for different outcome assessments. All outcomes were assessed at 12 weeks and one year follow‐up.
Pelzer and colleagues recruited 312 chemotherapy‐naive participants with advanced pancreatic cancer (Pelzer 2015 (CONKO‐004)). Participants were randomized to receive or not to receive additional LMWH (enoxaparin) starting simultaneously with palliative systemic chemotherapy. Study outcomes included overall survival, symptomatic VTE, asymptomatic subclinical DVT and major bleeding. Follow‐up for overall survival was about 95.7% in the intervention group and 93.4% in the control group. The median duration of follow‐up was 30.4 weeks.
Perry and colleagues recruited 186 participants with newly diagnosed malignant glioma (Perry 2010 (PRODIGE)). Participants were randomized to receive a prophylactic dose of LMWH (dalteparin) or placebo. Study outcomes included objectively documented symptomatic DVT or PE (primary outcome), bleeding (major and all bleeding), quality of life and death. The duration of follow‐up was 12 months.
Sideras and colleagues recruited 141 participants with different types of advanced cancer and a minimum life expectancy of 12 weeks and ECOG state zero to two (Sideras 2006). Participants were randomized either to a prophylactic dose of a LMWH (dalteparin) or to placebo or no intervention. Study outcomes included overall survival (at 12, 24 and 36 months), VTE and major bleeding. Follow‐up data were available for 138 participants (98%). The minimum duration of follow‐up was not reported, whereas the maximum duration of follow‐up was 24 months. The authors supplied us with unpublished data, giving the HR and its standard error.
Vadhan‐Raj and colleagues recruited 75 participants with metastatic or locally advanced pancreatic cancer (Vadhan‐Raj 2013). Participants were randomized to receive dalteparin 5000 U SQ daily for 16 weeks during chemotherapy or chemotherapy alone. Assessed outcomes were VTE, DVT and PE. Participants were followed‐up for 16 weeks. The study reported complete follow up.
van Doormaal and colleagues recruited 503 participants with prostate carcinoma, NSCLC, or with a locally advanced pancreatic cancer (van Doormaal 2011 (INPACT)). Participants were randomized to receive either subcutaneous nadroparin or no nadroparin. The median duration of follow‐up was 10.5 months in the nadroparin group and 10.4 months in the control group. The study outcomes included mortality (at one, two and three years versus at five, 10, 15, 20, 25, 30, 35, 40 months), PE, DVT, major bleeding and minor bleeding. The percentage of participants lost to follow‐up was 0.8% and 3.5% from the nadroparin group and the control group respectively.
Weber and colleagues recruited 20 participants with advanced cancer and an estimated life expectancy of less than six months (Weber 2008). Participants were randomized to receive either a prophylactic dose of LMWH (nadroparin) or no treatment, each with concomitant anticancer treatment. Study outcomes included mortality, VTE (including PE and DVT), minor and major bleeding, and thrombocytopenia. Follow‐up was complete (100%). The minimum duration of follow‐up was reported as three months for mortality, whereas the maximum was 18 months for all outcomes.
Zwicker and colleagues recruited 34 participants with advanced cancer and high tissue factor‐bearing microparticles (Zwicker 2013 (MICRO TEC)). Participants were randomized to subcutaneous enoxaparin or observation. The study outcomes included incidence of symptomatic VTE for a follow‐up duration of two months. The trial was originally designed as a phase III, then re‐adapted to a phase II randomized clinical trial.
Excluded studies
We excluded 99 studies (130 reports) from this review for the following reasons: not population of interest (hospitalized): n = 11; not population of interest (surgical): n = 29; not population of interest (patients with central venous catheter (CVC)): n = 7; not population of interest (patients with VTE): n = 18; not population of interest (no participants with cancer): n = 2; not intervention of interest (oral): n = 9; not intervention of interest (aspirin): n = 7; not intervention of interest (different route of administration): n=4; not comparison of interest: n = 7; not design of interest: n = 33; not outcomes of interest: n = 3.
Risk of bias in included studies
The judgments for the risk of bias are summarized in Figure 2 and Figure 3, respectively.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation to be adequately concealed in 13 of the 19 studies (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS); Klerk 2005 (MALT); Lebeau 1994; Macbeth 2016 (FRAGMATIC); Pelzer 2015 (CONKO‐004); Perry 2010 (PRODIGE); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008), and not concealed in two studies (Altinbas 2004; Lecumberri 2013 (ABEL)). Four studies did not report on allocation concealment (Khorana 2017 (PHACS); Maraveyas 2012 (FRAGEM); Vadhan‐Raj 2013Zwicker 2013 (MICRO TEC)).
Blinding
Blinding of participants and personnel (performance bias)
We judged participants and personnel to be definitely blinded in three studies (Agnelli 2009 (PROTECHT); Klerk 2005 (MALT); Perry 2010 (PRODIGE) and probably blinded in four studies (Agnelli 2012 (SAVE‐ONCO); Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS). We judged nine studies as definitely not blinded (Altinbas 2004; Khorana 2017 (PHACS); Lebeau 1994; Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC); Pelzer 2015 (CONKO‐004); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008) and three as probably not blinded (Maraveyas 2012 (FRAGEM); Vadhan‐Raj 2013Zwicker 2013 (MICRO TEC).
Blinding of outcome assessment (detection bias)
We judged outcome assessors to be definitely blinded in two studies (Klerk 2005 (MALT); Perry 2010 (PRODIGE) and probably blinded in nine studies (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS); Khorana 2017 (PHACS); Lecumberri 2013 (ABEL); Pelzer 2015 (CONKO‐004); van Doormaal 2011 (INPACT). We judged four studies as definitely not blinded due to their open‐label design (Altinbas 2004; Macbeth 2016 (FRAGMATIC); Sideras 2006; Weber 2008) and four as probably not blinded. (Lebeau 1994; Maraveyas 2012 (FRAGEM); Vadhan‐Raj 2013Zwicker 2013 (MICRO TEC). However, we judged risk of bias in relation to detection bias as low when reporting on objective outcomes (for all 19 studies) and high when reporting on patient‐reported subjective outcomes (for two studies Macbeth 2016 (FRAGMATIC); Sideras 2006).
Incomplete outcome data
Eight studies reported a complete follow‐up rate (Altinbas 2004; Khorana 2017 (PHACS); Klerk 2005 (MALT); Lebeau 1994; Lecumberri 2013 (ABEL); Weber 2008;Vadhan‐Raj 2013; Zwicker 2013 (MICRO TEC).
Agnelli and colleagues reported an approximate 90% follow‐up rate in the PROTECHT trial (Agnelli 2009 (PROTECHT)). In the SAVE‐ONCO trial, follow‐up data were reported per outcome as follows: for mortality and VTE outcomes, approximately 99% in both the intervention and control groups; for bleeding outcome, 88% in the intervention group and 95% in the control group (Agnelli 2012 (SAVE‐ONCO)).
Kakkar and colleagues reported an approximate 97% follow‐up rate in both the intervention and contro groups (Kakkar 2004 (FAMOUS)). Pelzer and colleagues reported a 95% follow‐up rate in the intervention group and 93% in the control group for the outcome overall survival (Pelzer 2015 (CONKO‐004)). Sideras and colleagues reported a 98% follow‐up rate (Sideras 2006). van Doormaal and colleagues reported a 97.85% follow‐up rate (van Doormaal 2011 (INPACT)). Macbeth and colleagues reported a 94% follow‐up rate in the intervention group and 97% in the control group (Macbeth 2016 (FRAGMATIC)).
Only one study reported follow‐up data per outcome and not per participant (Maraveyas 2012 (FRAGEM)). The follow‐up rates for the outcomes overall survival, VTE incidence, and toxicity ranged between 93% and 98%.
In both studies by Haas and colleagues, it is not reported whether participants not included in the intention‐to‐treat (ITT) analyses were followed up (Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2)). All participants in the intervention group and 99% of participants in the control group were included in the analysis for TOPIC 1, whereas 98% in the intervention group and 97% in the control group were included for TOPIC 2.
In the study by Perry and colleagues, it is not reported whether participants were followed up among those that did not receive first dose, withdrew consent, or discontinued treatment (Perry 2010 (PRODIGE)). We judged the risk of attrition bias as high since those participants represent 37% of the intervention group and 53% of the control group.
Selective reporting
The outcomes listed in the methods section were reported in the results section for 13 studies (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Kakkar 2004 (FAMOUS); Khorana 2017 (PHACS); Klerk 2005 (MALT); Lebeau 1994; Maraveyas 2012 (FRAGEM); Pelzer 2015 (CONKO‐004); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008). Seven studies are registered in ClinicalTrials.gov (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Khorana 2017 (PHACS); Lecumberri 2013 (ABEL); Maraveyas 2012 (FRAGEM); Perry 2010 (PRODIGE); van Doormaal 2011 (INPACT)). One study is registered in the ISRCTN registry (Pelzer 2015 (CONKO‐004)).
One study reported on all outcomes except for two listed in the methods section (quality of life and cognition assessment) (Perry 2010 (PRODIGE)). The outcomes of interest were all reported but were not listed in the methods section for one study (Altinbas 2004).
One study had a published protocol and reported on all outcomes listed in the protocol (Pelzer 2015 (CONKO‐004)). One study that also had a published protocol reported on all outcomes listed in the protocol except for four that will be reported elsewhere (health economics, health‐related quality of life, dyspnea and biomarker studies) (Macbeth 2016 (FRAGMATIC)).
Selective reporting bias was unclear in the study published as an abstract (Vadhan‐Raj 2013).
Other potential sources of bias
We questioned whether in the study by Agnelli and colleagues the follow‐up time "occurring between randomization and 3 days after the last injection of the study drug" could have potentially led to differential follow‐up time between the two groups (Agnelli 2012 (SAVE‐ONCO)). However, the authors report that "the duration of treatment was similar in the two study groups, with a median of approximately 3.5 months".
Klerk and colleagues reported that "chemotherapy was more frequently administered during the period of study treatment in participants receiving placebo, whereas radiotherapy was more frequently given to participants receiving nadroparin"; thus 25% of the nadroparin group and 34% of the placebo group received chemotherapy; 32% of the nadroparin group and 18% of the placebo group received radiotherapy. Having different co‐interventions between the two groups might lead to performance bias (Klerk 2005 (MALT)).
Three studies were stopped early due to insufficient accrual (Khorana 2017 (PHACS); Perry 2010 (PRODIGE); Sideras 2006).
We judged that in the study by Lebeau and colleagues participants received similar co‐interventions although brain and thoracic irradiation depended on response to treatment. In that study, 11% and 7%, respectively of participants randomized to heparin and control groups received radiotherapy (Lebeau 1994).
In the study by Pelzer and colleagues, the related abstracts published in 2005 and 2007 reported a target recruitment of 540 patients whereas 312 patients were recruited into the trial (Pelzer 2015 (CONKO‐004)).
The study by Zwicker and colleagues was originally designed as a phase III randomized clinical trial then re‐adapted to a phase II trial. Also, the trial is described as underpowered (Zwicker 2013 (MICRO TEC)).
Effects of interventions
See: Table 1
All‐cause mortality
All‐cause mortality at 12 months
Meta‐analysis of the 18 randomized controlled trials (RCTs), including 9575 participants, found that the use of heparin compared to no heparin has no effect on mortality rates at 12 months: risk ratio (RR) 0.98; 95% confidence interval (CI) 0.93 to 1.03; risk difference (RD) 10 fewer per 1000; 95% CI 35 fewer to 15 more (see Analysis 1.1). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is moderate (I2 = 31%). The inverted funnel plot for the primary outcome of mortality at one year did not suggest publication bias, but there were relatively few trials to permit an accurate assessment (Figure 4). The certainty of evidence was moderate due to imprecision (Table 1). Appendix 6 includes the GRADE Evidence Profile (a more detailed version of the Table 1).
1.1. Analysis.

Comparison 1: Heparin versus placebo, Outcome 1: Mortality at 12 months‐ Main analysis
4.

Funnel plot of comparison: 1 Heparin versus placebo, outcome: 1.1 Mortality at 12 months‐ Main analysis.
In a subgroup analysis of participants with lung cancer (either SCLC or NSCLC) (Altinbas 2004; Haas 2012 (TOPIC 2); Lebeau 1994; Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC); van Doormaal 2011 (INPACT)), versus other types of cancer (that is neither SCLC or NSCLC) (Haas 2012 (TOPIC 1); Klerk 2005 (MALT); Maraveyas 2012 (FRAGEM); Pelzer 2015 (CONKO‐004); van Doormaal 2011 (INPACT); Weber 2008), the test for subgroup difference was not statistically significant (P value = 0.47).
In a subgroup analysis of participants with advanced cancer (including participants with extensive SCLC) (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Altinbas 2004; Kakkar 2004 (FAMOUS); Klerk 2005 (MALT); Lebeau 1994; Maraveyas 2012 (FRAGEM); Pelzer 2015 (CONKO‐004); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008; Zwicker 2013 (MICRO TEC)), versus participants with non‐advanced cancer (including participants with limited SCLC) (Altinbas 2004; Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Khorana 2017 (PHACS); Lebeau 1994; Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC); Perry 2010 (PRODIGE)), the test for subgroup effect was not statistically significant (P value = 0.56).
All‐cause mortality at 24 months
In a meta‐analysis of 14 RCTs, including 5229 participants, we found that heparin compared to no heparin has no effect on mortality rates at 24 months: RR 0.99; 95% CI 0.96 to 1.01; RD 8 fewer per 1000; 95% CI 31 fewer to 8 more (see Analysis 1.4). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is moderate (I2 = 27%). The certainty of evidence was moderate due to imprecision (Table 1).
1.4. Analysis.

Comparison 1: Heparin versus placebo, Outcome 4: Mortality at 24 months‐ Main Analysis
In a subgroup analysis of participants with advanced cancer (including participants with extensive SCLC) (Kakkar 2004 (FAMOUS); Klerk 2005 (MALT); Pelzer 2015 (CONKO‐004); Sideras 2006; van Doormaal 2011 (INPACT); Weber 2008), versus participants with non‐advanced cancer (including participants with limited SCLC) (Altinbas 2004; Haas 2012 (TOPIC 1); Haas 2012 (TOPIC 2); Lebeau 1994; Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC); Maraveyas 2012 (FRAGEM); Perry 2010 (PRODIGE)), the test for subgroup effect was not statistically significant (P value = 0.97)
All‐cause mortality ‐ time‐to‐event analysis
Fifteen studies, including 8388 participants, reported data allowing their inclusion in the time‐to‐event meta‐analysis. Meta‐analysis indicated that heparin compared to no heparin has no effect on reduction in the risk of death (hazard ratio (HR) 0.93; 95% CI 0.84 to 1.03) (see Analysis 1.6). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent moderate heterogeneity (I2 = 64%).
1.6. Analysis.

Comparison 1: Heparin versus placebo, Outcome 6: Mortality over duration of study
Symptomatic venous thromboembolism (VTE)
Meta‐analysis of 16 RCTs, including 9036 participants, found that heparin reduces the risk of symptomatic VTE compared to no heparin: RR 0.56; 95% CI 0.47 to 0.68; RD 30 fewer per 1000; 36 fewer to 22 fewer (see Analysis 1.7). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is not important (I2 = 0%). Results did not change in a sensitivity analysis including the study published as abstract (Vadhan‐Raj 2013): RR 0.56, 95% CI 0.46 to 0.67. Since the primary meta‐analysis found a statistically significant effect, and in order to assess the risk of bias associated with missing participant data, we conducted sensitivity meta‐analyses using the a priori plausible assumptions detailed in the Methods section. The effect estimate remained significant across all four stringent assumptions (Appendix 7). Analysis 1.9 and Analysis 1.10 respectively show the separate analyses for PE and symptomatic DVT. The inverted funnel plot for symptomatic VTE did not suggest publication bias, but there were relatively few trials to permit an accurate assessment (Figure 5). The certainty of evidence was high (Table 1).
1.7. Analysis.

Comparison 1: Heparin versus placebo, Outcome 7: Symptomatic VTE‐ Main analysis
1.9. Analysis.

Comparison 1: Heparin versus placebo, Outcome 9: PE
1.10. Analysis.

Comparison 1: Heparin versus placebo, Outcome 10: Symptomatic DVT
5.

Funnel plot of comparison: 1 Heparin versus placebo, outcome: 1.7 Symptomatic VTE‐ Main analysis.
In a subgroup analysis of participants with lung cancer (either SCLC or NSCLC), (Agnelli 2009 (PROTECHT); Agnelli 2012 (SAVE‐ONCO); Altinbas 2004; Haas 2012 (TOPIC 2)Lecumberri 2013 (ABEL); Macbeth 2016 (FRAGMATIC)) versus participants with any type of cancer (that is neither SCLC or NSCLC), (Kakkar 2004 (FAMOUS); Khorana 2017 (PHACS); Sideras 2006; van Doormaal 2011 (INPACT); Zwicker 2013 (MICRO TEC)) the test for subgroup effect was not statistically significant (P value 0.21).
Major bleeding
Meta‐analysis of 18 RCTs, including 9592 participants, showed that heparin likely increases the risk of major bleeding compared to no heparin: RR 1.30; 95% CI 0.94 to 1.79; RD 4 more per 1000; 95% CI 1 fewer to 11 more) (see Analysis 1.11). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent no heterogeneity (I2 = 0%). The certainty of evidence was moderate due to imprecision (Table 1).
1.11. Analysis.

Comparison 1: Heparin versus placebo, Outcome 11: Major bleeding‐ Main analysis
In a subgroup analysis of participants with lung cancer (either SCLC or NSCL) ( Altinbas 2004; Haas 2012 (TOPIC 2); Lebeau 1994; Lecumberri 2013 (ABEL); ; Macbeth 2016 (FRAGMATIC)), versus participants with any type of cancer (that is neither SCLC or NSCLC) (Haas 2012 (TOPIC 1); Klerk 2005 (MALT); Pelzer 2015 (CONKO‐004); Perry 2010 (PRODIGE); Weber 2008), the test for subgroup effect was not statistically significant (P value = 0.61).
Minor bleeding
Meta‐analysis of 16 RCTs, including 9245 participants, found that heparin causes an increase in the risk of minor bleeding compared to no heparin: RR 1.70; 95% CI 1.13 to 2.55; RD 17 more per 1000; 3 more to 37 more) (see Analysis 1.13). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) may represent moderate heterogeneity (I2 = 53%). Since the primary meta‐analysis found a statistically significant effect, and in order to assess the risk of bias associated with missing participant data, we conducted sensitivity meta‐analyses using the a priori plausible assumptions detailed in the Methods section. The effect estimate did not lose significance across all four stringent assumptions (Appendix 7). The certainty of evidence was high (Table 1).
1.13. Analysis.

Comparison 1: Heparin versus placebo, Outcome 13: Minor bleeding
Thrombocytopenia
Meta‐analysis of 12 RCTs, including 5832 participants, failed to show or to exclude a beneficial or detrimental effect of heparin on the risk of thrombocytopenia compared to no heparin (RR 0.69; 95% CI 0.37 to 1.27; RD 33 fewer per 1000; 95% CI 66 fewer to 28 more) (see Analysis 1.14). The I2 value indicates that the percentage of the variability in effect estimates that is due to heterogeneity rather than chance may represent high heterogeneity (I2 = 83%). The certainty of evidence was moderate due to imprecision (Table 1).
1.14. Analysis.

Comparison 1: Heparin versus placebo, Outcome 14: Thrombocytopenia
Health‐related quality of life
Two studies assessed quality of life, one using the Uniscale and the Symptom Distress Scale (SDS) (Sideras 2006), the other using the Hospital Anxiety and Depression Score and EQ‐5D (Macbeth 2016 (FRAGMATIC)). Both studies concluded that the scores for the two scales were similar for the two study groups, both at baseline and at follow‐up. The certainty of evidence was moderate due to risk of bias (Table 1).
Sensitivity analyses
The sensitivity analysis excluding the one study at high risk of bias, Altinbas 2004, from the analyses did not change the results significantly. We have presented above the sensitivity meta‐analyses to assess the risk of bias associated with missing participant data.
Discussion
Summary of main results
Parenteral anticoagulation (with either unfractionated heparin or low molecular weight heparin (LMWH)) appears to have no effect on mortality in patients with cancer, who have no therapeutic or prophylactic indication for anticoagulation. While parenteral anticoagulation reduces venous thromboembolism (VTE), it likely increases major bleeding and minor bleeding. We did not identify any study using fondaparinux as an anticoagulant.
Overall completeness and applicability of evidence
The included studies recruited patients with a variety of cancer types and stages, which should increase the applicability of the results. The results apply best to LMWH, given that only one study evaluated unfractionated heparin. Unfortunately, not enough data were available to evaluate the impact of the intervention on bleeding outcomes or on quality of life. The latter outcome is important given the potential burden of daily subcutaneous injections.
As mentioned above, we identified three eligible studies for which we were not able to obtain the necessary data from the authors. Chazouilleres 1994 recruited 51 participants with unresectable hepatocellular carcinoma and reported a lower short‐term mortality rate with LMWH. Salat 1990 did not report on mortality outcome. Vadhan‐Raj 2013a randomized 75 participants with metastatic or locally advanced pancreatic cancer and reported a trend towards a reduction in VTE.
Quality of the evidence
Our systematic approach to searching, study selection and data extraction should have minimized the likelihood of missing relevant studies. The certainty of evidence was high for symptomatic VTE and minor bleeding, moderate for mortality, major bleeding and quality of life.
Potential biases in the review process
The inclusion of different types of cancer in the same study precluded us from conducting the subgroup analyses to explore effect modifiers such as type and stage of cancer. The interpretation of findings is also limited by not including data from the trials published as abstracts only. Also, for two studies we had to calculate the number of mortality events at 12 and 24 months from the survival curves (Altinbas 2004; Kakkar 2004 (FAMOUS)). Also, there might be potential bias associated with multiple testing in the planned meta‐analyses and currently there are no plans to adjust meta‐analyses for multiple testing.
Agreements and disagreements with other studies or reviews
A recent review by Che and colleagues assessed the effect of LMWH compared with no heparin in patients with cancer with no history of VTE (Che 2013). Similar to our findings, the review found that LMWH significantly reduced the risk of VTE and increased the risk of bleeding. Moreover, this study did not focus on the type of intervention or type of participants, for example the pooled participants included patients being started on thromboprophylaxis due the placement of a central venous catheter (CVC), or in the perioperative setting. Our review eligibility criteria focused on parenteral anticoagulation in ambulatory patients with cancer, i.e. reducing clinical heterogeneity.
Another Cochrane systematic review conducted by Di Nisio and colleagues assessed the efficacy and safety of primary thromboprophylaxis in ambulatory patients with cancer receiving chemotherapy (Di Nisio 2016). The review found that LMWH, when compared with inactive control, significantly reduced the incidence of symptomatic VTE, whereas there was no statistically significant effects on major bleeding, asymptomatic VTE, minor bleeding, one‐year mortality, symptomatic arterial thromboembolism, superficial thrombophlebitis or serious adverse events. The authors included various interventions for both prophylactic and therapeutic purposes in different populations. The interventions included parenteral anticoagulants (LMWH, unfractionated heparin), oral agents (Vitamin K antagonists (VKA), direct oral anticoagulants (DOAC), aspirin, antithrombin), and placebo. The populations included patients without VTE, with VTE, with multiple myeloma, and pediatrics. We tackled most of these comparisons in separate Cochrane reviews (Akl 2014 (initial); Akl 2014 (long‐term); Akl 2014 (oral))
Another recent publication by Phan and colleagues, studying the efficacy of heparin‐based medications for prevention of VTE, found a significant reduction in VTE with an odds ratio (OR) of 0.56 (95% confidence interval (CI) 0.45 to 0.71) (Phan 2014). However, that review had limitations in comparison to ours. That review did not include four studies we deemed to be eligible (Altinbas 2004; Pelzer 2015 (CONKO‐004); Sideras 2006; Weber 2008). The reported reason for not including two of these studies was that VTE was not assessed (Altinbas 2004; Sideras 2006). There was no reference to the two other studies (Sideras 2006; Weber 2008). Secondly, Phan 2014 included in the review the Young 2009 trial, assessing anticoagulation in patients with a CVC. This introduced increased clinical heterogeneity. We have included that trial in a separate Cochrane review evaluating prophylaxis for catheter‐related thrombosis (Akl 2014 (CVC)). Unlike the review conducted by Phan 2014, we did not include in the VTE meta‐analysis the trial conducted by Klerk and colleagues (Klerk 2005 (MALT)) because the number of VTE events reported pertains to participants who discontinued the study drug prematurely because they developed VTE; the paper does not report the total number of VTE observed in the trial. Moreover, Phan 2014 focused solely on VTE and did not assess other patient‐important outcomes, such as mortality.
Similary, another systematic review conducted by Ben Aharon and colleagues assessing the efficacy and safety of primary thromboprophylaxis with LMWH in ambulatory participants with solid malignancies (Ben‐Aharon 2014) found that primary prophylaxis with LMWH reduced symptomatic VTE (RR and the rate of PE especially in the subgroup of participants with lung and pancreatic cancers. They found no significant effect for anticoagulation on one‐year mortality or major bleeding.
Another systematic review conducted by Zhang and colleagues assessed whether anticoagulation improves survival and VTE outcomes in participants with lung cancer exclusively with no indication for anticoagulation (Zhang 2013). Anticoagulation showed a survival benefit, prolonged life expectancy, and reduced the risk of VTE in participants with lung cancer with no indication for anticoagulants, especially for those with SCLC, whereas our review included a wider range of patients with various types of cancer.
Authors' conclusions
Implications for practice.
This systematic review found no survival benefit from heparin therapy in patients with cancer patients. Heparin did decrease the number of thrombotic events with likely increases in major bleeding and minor bleeding.
The decision for a patient with cancer to start heparin therapy in the absence of a standard therapeutic or prophylactic indication should balance the benefits and downsides, and should integrate the patient's values and preferences (Haynes 2002). Patients with a high preference for a reduction in VTE and limited aversion to potential bleeding, and who do not consider heparin (both unfractionated heparin or low molecular weight heparin (LMWH)) therapy a burden, may opt to use heparin, while those with aversion to bleeding may not. Decisions at a health system level would have to consider the cost‐effectiveness of such as practice.
Implications for research.
There is a need to understand the effects of heparin (including unfractionated heparin and LMWH) and other anticoagulants in patients with different types and subtypes (small cell lung cancer versus others) and stages (advanced versus not advanced) of cancers, as well as with existing comorbidites. Similarly, there is a need to understand the differential effects of different types, dosing, schedules and duration of therapy (Alifano 2004). Some of the ongoing, or as yet unpublished studies may provide such information (Kakkar 2010 (GASTRANOX); Meyer 2017 (PROVE). Also, our forthcoming individual patient data (IPD) meta‐analysis will be useful in clarifying how the type and stage of cancer modify the effect of parenteral anticoagulation.
What's new
| Date | Event | Description |
|---|---|---|
| 21 December 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 December 2022 (new information identified but unlikely to change results/conclusions). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
History
Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 24 October 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 October 2022 (new information identified but unlikely to change results/conclusions). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 13 June 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 May 2022 (new information identified but unlikely to change results/conclusions). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 29 December 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 December 2021 (new information identified but unlikely to change results/conclusions). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 10 September 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 August 2021 (new information identified but unlikely to change results/conclusions). As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 17 February 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 February 2021 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 29 October 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 October 2020 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 17 June 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 May 2020 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 12 March 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 February 2020 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 2 January 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 November 2019 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 7 October 2019 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 August 2019 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 9 July 2019 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 June 2019 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date |
| 7 May 2019 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 24 April 2019 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date |
| 25 February 2019 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 February 2019 (new information identified but unlikely to change results/conclusions) As such, results of all available included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date |
| 29 November 2018 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 November 2018 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 2 October 2018 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 September 2018 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 9 August 2018 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 July 2018 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 28 June 2018 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 May 2018 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 28 June 2018 | Amended | Declaration of interest updated. |
| 28 June 2018 | Amended | Search date updated. |
| 23 April 2018 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 March 2018 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 25 March 2015 | Amended | Observed events of major bleeding and VTE outcomes for trial Pelzer 2015 (CONKO‐004) have been corrected. |
| 5 March 2014 | New citation required but conclusions have not changed | Data abstraction verified and detailed statistical data included as an appendix. Data reanalyzed by using a complete case analysis approach for the primary meta‐analysis. Sensitivity analysis conducted for outcomes with significant results in the primary meta‐analysis. |
| 9 February 2013 | New search has been performed | Updated search identified five new eligible studies. |
| 28 November 2012 | Amended | Author contact details amended. |
| 6 December 2010 | New search has been performed | Text revised. |
| 7 February 2010 | New citation required but conclusions have not changed | Updated search, February 2010. |
| 15 May 2007 | New citation required and conclusions have changed | Substantive amendment. We updated the classification of heterogeneity. We considered the following classification of heterogeneity based on the value of I2: 0% to 30% = low; 30% to 60% = moderate and worthy of investigation; 60% to 90% = severe and worthy of understanding; 90% to 100% = allowing aggregation only with major caution (Julian Higgins, personal communication). We rephrased the abstract conclusion as follows: "This review suggests a survival benefit of heparin in cancer patients in general, and in patients with small cell lung cancer in particular." We also added the following to the 4th paragraph of the Discussion ("Interpretation of the findings of this review is limited by the moderate heterogeneity…"): "The interpretation of findings is also limited by not including data from the seven trials published as abstracts only." |
Acknowledgements
We thank Ms. Ann Grifasi for her administrative support. We thank Dr. Loprinzi and Dr. Paul Novotny of the Mayo Clinic for supplying additional data relating to the study Sideras 2006. We also thank Dr. Lebeau, Dr. Altinbas and Dr. Pelzer for supplying additional data. We thank Rami A Ballout, Andrew Bryant, Heather Dickinson, Sameer K Gunukula, Saskia Kuipers and Saskia Middeldorp, and, Frederiek F van Doormaal for their contributions to previous versions of this review. We also thank Dr. Assem Khamis for his help with conducting the sensitivity analysis.
We thank Jo Morrison, Co‐ordinating Editor for the Cochrane Gynaecological Neuro‐oncology and Orphan Cancers (CGNOC). We also thank Gail Quinn, Managing Editor of the CGNOC for her exceptional support. We thank Joanne Platt, the Information Specialist of the CGNOC for setting up and managing the monthly alerts.
As described under “Sources of Support” this update was supported in part by the American Society of Hematology to inform ASH guidelines on the topic. We thank the ASH guideline panel for prioritizing questions previously addressed by our review and for critically reviewing our work, including Drs. Pablo Alonso, Waleed Alhazanni, Marc Carrier, Cihan Ay, Marcello DiNisio, Lisa Hicks, Alok Khorana, Andrew Leavitt, Agnes Lee, Gary Lyman, Fergus Macbeth, Rebecca Morgan, Simon Noble, and David Stenehjem and patient representatives Jackie Cook and Elizabeth Sexton. Their input was valuable in validating some of the review related decisions such as eligibility of included studies and the analytical approach.
For our update of these reviews, we followed Cochrane methods using the same eligibility criteria and outcomes used previously. The ASH guidelines group used slightly different methods that generated slightly different results. For example, the ASH guideline panel agreed to prioritize different outcomes; include unpublished data; include abstracts; use different definitions for duration of treatment; and rate certainty of evidence slightly differently for some outcomes, for instance because of imprecision or indirectness. These differences are not described in this publication. Instead, they will be described in the ASH guideline publication.
This project was supported by the National Institute for Health Research, via Cochrane Review Incentive Scheme Funding. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. Anneliese Synnot's position is funded through Cochrane's Gamechanger Grant and Australia's National Health and Medical Research Council (Partnership Project grant APP1114605).
Appendices
Appendix 1. Living systematic review protocol
The methods outlined below are specific to maintaining the review as a living systematic review in the Cochrane Library (Synnot 2017). They will be implemented immediately upon publication of this update. Core review methods, such as the criteria for considering studies in the review and assessment of risk of bias, are unchanged. As such, below we outline only those areas of the methods for which additional or different activities are planned or rules apply.
Search methods for identification of studies
We will re‐run the majority of searches monthly. For electronic databases and other electronic sources (CENTRAL, MEDLINE, Embase), we have set up auto‐alerts to deliver a monthly search yield by email. We will search the remaining resources (conference proceedings of the American Society of Clinical Oncology (ASCO); the American Society of Haematology (ASH); and clinicaltrials.gov) on a bi‐yearly basis. For that purpose, we will note when these conference proceedings are published.
As additional steps to inform the living systematic review, we will contact corresponding authors of ongoing studies as they are identified and ask them to advise when results are available, and to share early or unpublished data. We will contact the corresponding authors of any newly included studies for advice as to other relevant studies. We will conduct citation tracking of included studies in Web of Science Core Collection on an ongoing basis. For that purpose, we have set up citation alerts in Web of Science Core Collection. We will manually screen the reference list of any newly included studies, and identified relevant guidelines and systematic reviews. Also, we will use the 'related citation' feature in PubMed to identify additional articles.
We will review search methods and strategies approximately yearly, to ensure they reflect any terminology changes in the topic area, or in the databases.
Selection of studies
We will immediately screen any new citations retrieved by the monthly searches. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) available in the Cochrane Register of Studies (CSR‐Web; Wallace 2017). The classifier assigns a probability (from 0 to 100) to each citation for being a true RCT. For citations that are assigned a probability score of less than 10, the machine learning classifier currently has a specificity/recall of 99.987% (James Thomas, personal communication). For citations assigned a score from 10 to 100, we will screen them in duplicate and independently. Citations that score 9 or less will be screened by Cochrane Crowd (Cochrane Crowd). Any citations that are deemed to be potential RCTs by Cochrane Crowd will be returned to the authors for screening.
Data synthesis
Whenever new evidence (studies, data or information) that meets the review inclusion criteria is identified, we will immediately assess risk of bias and extract the data and incorporate it in the synthesis, as appropriate. We will not adjust the meta‐analyses to account for multiple testing given the methods related to frequent updating of meta‐analyses are under development (Simmonds 2017).
Other
We will review the review scope and methods approximately yearly, or more frequently if appropriate, in light of potential changes in the topic area, or the evidence being included in the review (for example, additional comparisons, interventions or outcomes, or new review methods available).
Appendix 2. Cochrane's living systematic review pilots
Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence as it becomes available (Elliott 2017). Cochrane is exploring the feasibility of preparing and publishing living systematic reviews in a series of pilots (which includes this review). For the Cochrane pilots, searching is being conducted monthly, and new relevant evidence (studies, data or other information) will be incorporated into the review in a timely manner, so that the findings of the review remain current.
For the most up to date information about the review, the results of the searches and any new evidence being incorporated, readers are encouraged to check the update status information. The update status information will be updated whenever the searches are re‐run. The review will be updated with a new citation whenever a new study is found.
Appendix 3. Full search strategies for the electronic databases ‐ Update 2010
| Database | Strategy |
| MEDLINE | #1 Heparin/
#2 Heparin.tw
#3 Heparin, Low‐Molecular‐Weight/
#4 (LMWH OR low molecular weight heparin OR nadroparin OR fraxiparin OR enoxaparin OR clexane OR lovenox OR dalteparin OR fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR innohep OR certoparin OR sandoparin OR reviparin OR clivarin OR danaproid OR orgaran).tw
#5 1 OR 2 OR 3 OR 4
#6 Coumarins/
#7 Warfarin/
#8 (warfarin OR coumadin OR acenocumarol OR phenprocumon OR 4‐hydroxicoumarins OR oral anticoagulant OR vitamin K antagonist OR VKA).tw
#9 6 OR 7 OR 8
#10 (fondaparinux OR Arixtra).tw
#11 (ximelagatran OR Exanta).tw #12 (Pradaxa or Dabigatran or rivaroxaban or Xarelto or apixaban).tw. #13 5 OR 9 OR 10 OR 11 OR 12 #14 Neoplasms/ #15 (malignan$ OR neoplasm$ OR cancer OR carcinoma$ OR adenocarcinoma OR tumour OR tumor).tw #16 14 OR 15 #17 clinical trial.pt. OR random:.tw. OR tu.xs. #18 animals/ NOT human/ #19 17 NOT 18 #20 13 AND 16 AND 19 |
| Embase | #1 Heparin/
#2 heparin.tw
#3 Low Molecular Weight Heparin/
#4 (LMWH OR low molecular weight heparin OR nadroparin OR fraxiparin OR enoxaparin OR clexane OR lovenox OR dalteparin OR fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR innohep OR certoparin OR sandoparin OR reviparin OR clivarin OR danaproid OR orgaran).tw
#5 1 OR 2 OR 3 OR 4
#6 Coumarin derivative/
#7 Warfarin/
#8 (warfarin OR coumadin OR acenocumarol OR phenprocumon OR 4‐hydroxicoumarins OR oral anticoagulant OR vitamin K antagonist OR VKA).tw
#9 6 OR 7 OR 8
#10 fondaparinux/
#11 (fondaparinux OR Arixtra).tw
#12 ximelagatran/
#13 (ximelagatran OR Exanta).tw #14 (Pradaxa OR Dabigatran OR rivaroxaban OR Xarelto OR apixaban).tw. #15 5 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 #16 Neoplasm/ #17 (malignan$ OR neoplasm$ OR cancer OR carcinoma$ OR adenocarcinoma OR tumour OR tumor).tw #18 16 OR 17 #19 Random:.tw. OR clinical trial:.mp. OR exp health care quality #20 animals/ NOT human/ #21 19 NOT 20 #22 15 AND 18 AND 21 |
| ISI (International Scientific Information) the Web of Science | #1 heparin OR low molecular weight heparin OR LMWH OR low‐molecular‐weight‐heparin OR nadroparin OR fraxiparin OR enoxaparin OR clexane OR lovenox OR dalteparin OR fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR innohep OR certoparin OR sandoparin OR reviparin OR clivarin OR danaproid OR orgaran
#2 Coumarins OR Warfarin OR coumadin OR acenocumarol OR phenprocumon OR 4‐hydroxicoumarins OR oral anticoagulant OR vitamin K antagonist OR VKA
#3 fondaparinux OR Arixtra
#4 ximelagatran OR Exanta #5 Pradaxa OR Dabigatran OR rivaroxaban OR Xarelto OR apixaban #6 1 OR 2 OR 3 OR 4 OR 5 #7 malignan$ OR neoplasm$ OR cancer OR carcinoma$ OR adenocarcinoma OR tumour OR tumor #8 random$ OR placebo$ OR versus OR vs OR double blind OR double‐blind OR compar$ OR controlled #9 6 AND 7 AND 8 |
| CENTRAL (the Cochrane Library, latest issue) | #1 heparin OR low molecular weight heparin OR LMWH OR low‐molecular‐weight‐heparin OR nadroparin OR fraxiparin OR enoxaparin OR clexane OR lovenox OR dalteparin OR fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR innohep OR certoparin OR sandoparin OR reviparin OR clivarin OR danaproid OR orgaran
#2 Coumarins OR Warfarin OR coumadin OR acenocumarol OR phenprocumon OR 4‐hydroxicoumarins OR oral anticoagulant OR vitamin K antagonist OR VKA
#3 fondaparinux OR Arixtra
#4 ximelagatran OR Exanta
#5 Pradaxa or Dabigatran or rivaroxaban or Xarelto or apixaban #6 1 OR 2 OR 3 OR 4 OR 5 #7 malignan$ OR neoplasm$ OR cancer OR carcinoma$ OR adenocarcinoma OR tumour OR tumor #8 6 AND 7 |
Appendix 4. Full search strategies for the electronic databases ‐ Update 2013
| Database | Strategy |
| MEDLINE | #1 exp Heparin/ #2 (LMWH or heparin or nadroparin or fraxiparin or enoxaparin or clexane or lovenox or dalteparin or fragmin or ardeparin or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin or danaproid or orgaran or bemiparin or hibor, badyket, semuloparin, parnaparin, fluxum).tw. #3 exp Coumarins/ #4 (warfarin or coumadin or acenocumarol or phenprocumon or 4‐hydroxicoumarins or oral anticoagulant or vitamin K antagonist or VKA).tw. #5 (fondaparinux or arixtra).tw. #6 (ximelagatran or exanta).tw. #7 (pradaxa or dabigatran or rivaroxaban or xarelto or apixaban or eliquis or edoxaban or lixiana or betrixaban or edoxaban or otamixaban).tw. #8 1 or 2 or 3 or 4 or 5 or 6 or 7 #9 exp Neoplasms/ #10 (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor*).tw. #11 9 or 10 #12 8 and 11 #13 randomized controlled trial.pt. #14 controlled clinical trial.pt. #15 randomized.ab. #16 placebo.ab. #17 drug therapy.fs. #18 randomly.ab. #19 trial.ab. #20 groups.ab. #21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 #22 12 and 21 #23 exp animals/ not humans.sh. #24 22 not 23 |
| Embase | #1 heparin/ #2 exp low molecular weight heparin/ #3 (LMWH or heparin or nadroparin or fraxiparin or enoxaparin or clexane or lovenox or dalteparin or fragmin or ardeparin or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin or danaproid or orgaran or bemiparin or hibor, badyket, semuloparin, parnaparin, fluxum).tw. #4 exp coumarin derivative/ #5 (warfarin or coumadin or acenocumarol or phenprocumon or 4‐hydroxicoumarins or oral anticoagulant or vitamin K antagonist or VKA).tw. #6 (fondaparinux or arixtra).tw. #7 (ximelagatran or exanta).tw. #8 (pradaxa or dabigatran or rivaroxaban or xarelto or apixaban or eliquis or edoxaban or lixiana or betrixaban or edoxaban or otamixaban).tw. #9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 #10 exp neoplasm/ #11 (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor*).tw. #12 10 or 11 #13 9 and 12 #14 crossover procedure/ #15 double‐blind procedure/ #16 randomized controlled trial/ #17 single‐blind procedure/ #18 random*.mp. #19 factorial*.mp. #20 (crossover* or cross over* or cross‐over*).mp. #21 placebo*.mp. #22 (double* adj blind*).mp. #23 (singl* adj blind*).mp. #24 assign*.mp. #25 allocat*.mp. #26 volunteer*.mp. #27 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 #28 13 and 27 #29 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ #30 28 not 29 |
| CENTRAL (the Cochrane Library, latest issue) | #1 MeSH descriptor: [Heparin] explode all trees #2 (LMWH or heparin or nadroparin or fraxiparin or enoxaparin or clexane or lovenox or dalteparin or fragmin or ardeparin or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin or danaproid or orgaran or bemiparin or hibor, badyket, semuloparin, parnaparin, fluxum) #3 MeSH descriptor: [Coumarins] explode all trees #4 (warfarin or coumadin or acenocumarol or phenprocumon or 4‐hydroxicoumarins or oral anticoagulant or vitamin K antagonist or VKA) #5 (fondaparinux or arixtra) #6 (ximelagatran or exanta) #7 (pradaxa or dabigatran or rivaroxaban or xarelto or apixaban or eliquis or edoxaban or lixiana or betrixaban or edoxaban or otamixaban) #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Neoplasms] explode all trees #10 (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor*) #11 #9 or #10 #12 #8 and #10 |
Appendix 5. Full search strategies for the electronic databases ‐ Update 2018
| Database | Strategy |
| MEDLINE |
RCTsearch strategy: 1. exp Anticoagulants/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp Coumarins/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. RIVAROXABAN/ 11. DABIGATRAN/ 12. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp Neoplasms/ 15. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 16. 14 or 15 17. 13 and 16 18. randomized controlled trial.pt. 19. controlled clinical trial.pt. 20. randomized.ab. 21. placebo.ab. 22. clinical trials as topic.sh. 23. randomly.ab. 24. trial.ti. 25. 18 or 19 or 20 or 21 or 22 or 23 or 24 26. (animals not (humans and animals)).sh. 27. 25 not 26 28. 17 and 27 Systematic Review search strategy: 1. exp Anticoagulants/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp Coumarins/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. RIVAROXABAN/ 11. DABIGATRAN/ 12. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp Neoplasms/ 15. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 16. 14 or 15 17. 13 and 16 18. (review or review,tutorial or review, academic).pt. 19. (medline or medlars or embase or pubmed or cochrane).tw,sh. 20. (scisearch or psychinfo or psycinfo).tw,sh. 21. (psychlit or psyclit).tw,sh. 22. cinahl.tw,sh. 23. ((hand adj2 search*) or (manual* adj2 search*)).tw,sh. 24. (electronic database* or bibliographic database* or computeri?ed database* or online database*).tw,sh. 25. (pooling or pooled or mantel haenszel).tw,sh. 26. (peto or dersimonian or der simonian or fixed effect).tw,sh. 27. (retraction of publication or retracted publication).pt. 28. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29. 18 and 28 30. meta‐analysis.pt. 31. meta‐analysis.sh. 32. (meta‐analys* or meta analys* or metaanalys*).tw,sh. 33. (systematic* adj5 review*).tw,sh. 34. (systematic* adj5 overview*).tw,sh. 35. (quantitativ* adj5 review*).tw,sh. 36. (quantitativ* adj5 overview*).tw,sh. 37. (methodologic* adj5 review*).tw,sh. 38. (methodologic* adj5 overview*).tw,sh. 39. (integrative research review* or research integration).tw. 40. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. 29 or 40 |
| Embase |
RCT search strategy: 1. exp anticoagulant agent/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp coumarin derivative/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. rivaroxaban/ 11. dabigatran/ 12. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp neoplasm/ 15. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 16. 14 or 15 17. 13 and 16 18. crossover procedure/ 19. double‐blind procedure/ 20. randomized controlled trial/ 21. single‐blind procedure/ 22. random*.mp. 23. factorial*.mp. 24. (crossover* or cross over* or cross‐over*).mp. 25. placebo*.mp. 26. (double* adj blind*).mp. 27. (singl* adj blind*).mp. 28. assign*.mp. 29. allocat*.mp. 30. volunteer*.mp. 31. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 17 and 31 Systematic Review search strategy: 1. exp anticoagulant agent/ 2. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 3. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 4. exp coumarin derivative/ 5. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl).mp. 6. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 7. (thrombin adj inhibitor*).mp. 8. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 9. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 10. rivaroxaban/ 11. dabigatran/ 12. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp neoplasm/ 15. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 16. 14 or 15 17. 13 and 16 18. exp review/ 19. (literature adj3 review*).ti,ab. 20. exp meta analysis/ 21. exp "Systematic Review"/ 22. 18 or 19 or 20 or 21 23. (medline or medlars or embase or pubmed or cinahl or amed or psychlit or psyclit or psychinfo or psycinfo or scisearch or cochrane).ti,ab. 24. RETRACTED ARTICLE/ 25. 23 or 24 26. 22 and 25 27. (systematic* adj2 (review* or overview)).ti,ab. 28. (meta?anal* or meta anal* or meta‐anal* or metaanal* or metanal*).ti,ab. 29. 26 or 27 or 28 30. 17 and 29 |
| CENTRAL (the Cochrane Library, latest issue) | #1 MeSH descriptor: [Anticoagulants] explode all trees #2 (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock) #3 FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216 #4 MeSH descriptor: [Coumarins] explode all trees #5 (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl) #6 (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix) #7 thrombin near inhibitor* #8 factor Xa inhibitor* or antithrombin* or anticoagul* #9 (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. #10 MeSH descriptor: [Rivaroxaban] this term only #11 MeSH descriptor: [Dabigatran] this term only #12 target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC* #13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Neoplasms] explode all trees #15 malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma* #16 #14 or #15 #17 #13 and #16 |
Appendix 6. GRADE Evidence Profile
| Quality assessment | № of patients | Effect | Quality | Importance | ||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Heparin prophylaxis | No prophylaxis | Relative (95% CI) | Absolute (95% CI) | ||
| Mortality (follow‐up: 12 months) | ||||||||||||
| 18 | randomised trials | not serious | not serious | not serious | serious a | none | 2469/4951 (49.9%) | 2331/4624 (50.4%) | RR 0.98 (0.93 to 1.03) | 10 fewer per 1000 (from 15 more to 35 fewer) | ⨁⨁⨁◯ MODERATE | CRITICAL |
| Mortality (follow‐up: 24 months) | ||||||||||||
| 14 | randomised trials | not serious | not serious | not serious | serious a | none | 1994/2594 (76.9%) | 2050/2635 (77.8%) | RR 0.99 (0.96 to 1.01) | 8 fewer per 1000 (from 31 fewer to 8 more) | ⨁⨁⨁◯ MODERATE | CRITICAL |
| Symptomatic VTE (follow‐up: 12 months) | ||||||||||||
| 16 | randomised trials | not serious | not serious | not serious | not serious | none | 170/4689 (3.6%) | 297/4347 (6.8%) | RR 0.56 (0.47 to 0.68) | 30 fewer per 1000 (from 36 fewer to 22 fewer) | ⨁⨁⨁⨁ HIGH | CRITICAL |
| PE (follow‐up: 12 months) | ||||||||||||
| 14 | randomised trials | not serious | not serious | not serious | not serious | none | 82/4598 (1.8%) | 136/4269 (3.2%) | RR 0.61 (0.47 to 0.80) | 12 fewer per 1000 (from 6 fewer to 17 fewer) | ⨁⨁⨁⨁ HIGH | CRITICAL |
| Symptomatic DVT (follow‐up: 12 months) | ||||||||||||
| 14 | randomised trials | not serious | not serious | not serious | not serious | none | 77/4598 (1.7%) | 163/4269 (3.8%) | RR 0.46 (0.33 to 0.63) | 21 fewer per 1000 (from 26 fewer to 14 fewer) | ⨁⨁⨁⨁ HIGH | CRITICAL |
| Major bleeding (follow up: 12 months) | ||||||||||||
| 18 | randomised trials | not serious | not serious | not serious | serious b | none | 90/4958 (1.8%) | 64/4634 (1.4%) | RR 1.30 (0.94 to 1.79) | 4 more per 1000 (from 1 fewer to 11 more) | ⨁⨁⨁◯ MODERATE | CRITICAL |
| Minor bleeding (follow‐up: 12 months) | ||||||||||||
| 16 | randomised trials | not serious | not serious | not serious | not serious | none | 219/4774 (4.6%) | 107/4471 (2.4%) | RR 1.70 (1.13 to 2.55) | 17 more per 1000 (from 3 more to 37 more) | ⨁⨁⨁⨁ HIGH | CRITICAL |
| Thrombocytopenia | ||||||||||||
| 12 | randomised trials | not serious | not serious | not serious | serious c | none | 183/2909 (6.3%) | 308/2923 (10.5%) | RR 0.69 (0.37 to 1.27) | 33 fewer per 1000 (from 66 fewer to 28 more) | ⨁⨁⨁◯ MODERATE | CRITICAL |
| Quality of life impairment | ||||||||||||
| 2 | randomised trials | serious d | not serious | not serious | not serious | none | Macbeth 2016 (FRAGMATIC): " There was no difference between the two groups with respect to quality‐adjusted life years gained in the first year... No difference in overall quality of life at 6 months (P = .94) or at 12 months (P = .89)... Overall quality of life did not change significantly over the study period". Sideras 2006: "The QOL and SDS scores were similar, both at baseline and during the protocol period, in patients randomized to receive LMWH vs those not randomized to receive LMWH." | ⨁⨁⨁◯ MODERATE | CRITICAL | |||
CI: Confidence interval; RR: Risk ratio
Explanations
a. Confidence interval includes values suggesting clinically significant benefit and values suggesting no effect.
b. Confidence interval includes values suggesting clinically significant harm and values suggesting no effect.
c. Confidence interval includes values suggesting clinically significant benefit and values suggesting harm.
d. Both studies were open‐label studies (lack of blinding may impact the patient‐reported subjective outcomes)
Appendix 7. Detailed results of sensitivity analyses
| Outcome | Symptomatic VTE |
| CCA effect estimate | RR 0.56 (95% CI 0.47 to 0.68) |
| Sensitivity analysis | |
| RI 1.5 intervention 1 control | 0.57 (95% CI 0.47 to 0.69) |
| RI 2 intervention 1 control | 0.58 (95% CI 0.47 to 0.71) |
| RI 3 intervention 1 control | 0.60 (95% CI 0.48 to 0.75) |
| RI 5 intervention 1 control | 0.63 (95% CI 0.49 to 0.80) |
| Outcome | Minor bleeding |
| CCA effect estimate | RR 1.70 (95% CI 1.13 to 2.55) |
| Sensitivity analysis | |
| RI 1 intervention 1.5 control | 1.66 (1.11 to 2.49) |
| RI 1 intervention 2 control | 1.65 (1.09 to 2.46) |
| RI 1 intervention 3 control | 1.59 (1.05 to 2.41) |
| RI 1 intervention 5 control | 1.52 (1.00 to 2.31) |
Appendix 8. Full search Strategies for the electronic databases ‐ Update 2020
Medline RCT
1. exp Anticoagulants/ 2. (anticoagulant* or anti‐coagulant*).tw. 3. (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. 4. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 5. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 6. exp Coumarins/ 7. (coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones).tw. 8. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar).mp. 9. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 10. (thrombin adj inhibitor*).mp. 11. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 12. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 13. RIVAROXABAN/ 14. DABIGATRAN/ 15. (BIBR 953 or BIBR 953 ZW or Dabigatran or HSDB 8062 or Pradaxa or UNII‐I0VM4M70GC).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 16. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp Neoplasms/ 19. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 20. 18 or 19 21. 17 and 20 22. randomized controlled trial.pt. 23. controlled clinical trial.pt. 24. randomized.ab. 25. placebo.ab. 26. drug therapy.fs. 27. randomly.ab. 28. trial.ti. 29. groups.ab. 30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. (animals not (humans and animals)).sh. 32. 30 not 31 33. 21 and 32
Embase RCT
1. exp anticoagulant agent/ 2. (anticoagulant* or anti‐coagulant*).tw. 3. (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. 4. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 5. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 6. exp coumarin derivative/ 7. (coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones).tw. 8. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar).mp. 9. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 10. (thrombin adj inhibitor*).mp. 11. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 12. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 13. rivaroxaban/ 14. dabigatran/ 15. (BIBR 953 or BIBR 953 ZW or Dabigatran or HSDB 8062 or Pradaxa or UNII‐I0VM4M70GC).mp. 16. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp neoplasm/ 19. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 20. 18 or 19 21. 17 and 20 22. crossover procedure/ 23. double‐blind procedure/ 24. randomized controlled trial/ 25. single‐blind procedure/ 26. random*.mp. 27. factorial*.mp. 28. (crossover* or cross over* or cross‐over*).mp. 29. placebo*.mp. 30. (double* adj blind*).mp. 31. (singl* adj blind*).mp. 32. assign*.mp. 33. allocat*.mp. 34. volunteer*.mp. 35. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36. 21 and 35
Central
#1 MeSH descriptor: [Anticoagulants] explode all trees #2 anticoagulant* or anti‐coagulant* #3 (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. #4 (LMWH* or Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin) #5 FR‐860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216 #6 MeSH descriptor: [Coumarins] explode all trees #7 coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones #8 (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar) #9 Dermatan Sulfate or (Chondroitin Sulfate near B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix) #10 thrombin near inhibitor* #11 factor Xa inhibitor* or antithrombin* or anti‐thrombin* or anti‐coagul* or anticoagul* #12 (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or “YM 150” or Iprivask or argatrovan or pradax* or Xarelto or “BIBR‐953” or “BIBR‐953ZW” or “BAY 59‐7939” or “BMS‐562247” or “DU‐176” or “DU‐176b”) #13 target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC* #14 MeSH descriptor: [Rivaroxaban] this term only #15 MeSH descriptor: [Dabigatran] this term only #16 "BIBR 953" or "BIBR 953 ZW" or Dabigatran or "HSDB 8062" or Pradaxa or "UNII‐I0VM4M70GC" #17 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 MeSH descriptor: [Neoplasms] explode all trees #19 malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma* #20 #18 or #19 #21 #17 and #20
Medline SR
1. exp Anticoagulants/ 2. (anticoagulant* or anti‐coagulant*).tw. 3. (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. 4. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 5. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 6. exp Coumarins/ 7. (coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones).tw. 8. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar).mp. 9. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 10. (thrombin adj inhibitor*).mp. 11. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 12. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 13. RIVAROXABAN/ 14. DABIGATRAN/ 15. (BIBR 953 or BIBR 953 ZW or Dabigatran or HSDB 8062 or Pradaxa or UNII‐I0VM4M70GC).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 16. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp Neoplasms/ 19. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 20. 18 or 19 21. 17 and 20 22. Meta‐Analysis as Topic/ 23. meta analy$.tw. 24. metaanaly$.tw. 25. Meta‐Analysis/ 26. (systematic adj (review$1 or overview$1)).tw. 27. exp Review Literature as Topic/ 28. 22 or 23 or 24 or 25 or 26 or 27 29. cochrane.ab. 30. embase.ab. 31. (psychlit or psyclit).ab. 32. (psychinfo or psycinfo).ab. 33. (cinahl or cinhal).ab. 34. science citation index.ab. 35. bids.ab. 36. cancerlit.ab. 37. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38. reference list$.ab. 39. bibliograph$.ab. 40. hand‐search$.ab. 41. relevant journals.ab. 42. manual search$.ab. 43. 38 or 39 or 40 or 41 or 42 44. selection criteria.ab. 45. data extraction.ab. 46. 44 or 45 47. Review/ 48. 46 and 47 49. Comment/ 50. Letter/ 51. Editorial/ 52. animal/ 53. human/ 54. 52 not (52 and 53) 55. 49 or 50 or 51 or 54 56. 28 or 37 or 43 or 48 57. 56 not 55 58. 21 and 57
Embase SR
1. exp anticoagulant agent/ 2. (anticoagulant* or anti‐coagulant*).tw. 3. (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. 4. (LMWH* or heparin* or nadroparin* or frixiparin* or enoxaparin* or clexane or klexane or lovenox or dalteparin or fragmin or ardeparin* or normiflo or tinzaparin or logiparin or innohep or certoparin or sandoparin or reviparin or clivarin* or danaproid or danaparoid or orgaran or antixarin or bemiparin* or hibor or zibor or ivor or badyket or semuloparin or parnaparin or tedelparin or fluxum or lohepa or lowhepa or parvoparin or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or clivarine or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or Liquaemin or Liquemin or Multiparin or Novoheparin or Eparina or Hep‐lock or Heparinate or Heparinic acid or Panheprin or Hepalean or Heparin Leo or Heparin Lock).mp. 5. (FR‐860 or FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 6. exp coumarin derivative/ 7. (coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones).tw. 8. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar).mp. 9. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 10. (thrombin adj inhibitor*).mp. 11. (factor Xa inhibitor* or antithrombin* or anticoagul*).mp. 12. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR‐953ZW or BAY 59‐7939 or BMS‐562247 or DU‐176 or DU‐176b).mp. 13. rivaroxaban/ 14. dabigatran/ 15. (BIBR 953 or BIBR 953 ZW or Dabigatran or HSDB 8062 or Pradaxa or UNII‐I0VM4M70GC).mp. 16. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp neoplasm/ 19. (malignan* or neoplasm* or cancer* or carcinoma* or adenocarcinoma* or tumour* or tumor* or glioma* or myeloma* or lymphoma* or leukemia* or leukaemia* or epithelioma* or adenoma*).tw. 20. 18 or 19 21. 17 and 20 22. exp Meta Analysis/ 23. ((meta adj analy$) or metaanalys$).tw. 24. (systematic adj (review$1 or overview$1)).tw. 25. 22 or 23 or 24 26. cancerlit.ab. 27. cochrane.ab. 28. embase.ab. 29. (psychinfo or psycinfo).ab. 30. (cinahl or cinhal).ab. 31. science citation index.ab. 32. bids.ab. 33. 26 or 27 or 28 or 29 or 30 or 31 or 32 34. reference lists.ab. 35. bibliograph$.ab. 36. hand‐search$.ab. 37. manual search$.ab. 38. relevant journals.ab. 39. 34 or 35 or 36 or 37 or 38 40. data extraction.ab. 41. selection criteria.ab. 42. 40 or 41 43. review.pt. 44. 42 and 43 45. letter.pt. 46. editorial.pt. 47. animal/ 48. human/ 49. 47 not (47 and 48) 50. 45 or 46 or 49 51. 25 or 33 or 39 or 44 52. 51 not 50 53. 21 and 52
Appendix 9. Study eligibility for subgroup analysis
I. Mortality at 12 months
a. Lung vs non‐Lung
| Name of study | Lung CA | non‐Lung CA | ||||
| Included patients only with Lung Cancer | Included patients with Lung and non‐Lung CA AND provided subgroup data for patients with Lung CA | Included patients with Lung and non‐Lung CA AND >75% of patients had Lung CA | Included patients only with non‐Lung Cancer | Included patients with Lung and non‐Lung CA AND provided subgroup data for patients with non‐Lung CA | Included patients with Lung and non‐Lung CA AND >75% of patients had non‐Lung CA | |
| Altinbas 2004 | x | |||||
| Haas 2012 (TOPIC 1) | x | |||||
| Haas 2012 (TOPIC 2) | x | |||||
| Klerk 2005 (MALT) | x | |||||
| Lebeau 1994 | x | |||||
| Lecumberri 2013 (ABEL) | x | |||||
| Macbeth 2016 (FRAGMATIC) | x | |||||
| Maraveyas 2012 (FRAGEM) | x | |||||
| Pelzer 2015 (CONKO‐004) | x | |||||
| Perry 2010 (PRODIGE) | x | |||||
| van Doormaal 2011 (INPACT) | x | x | ||||
b. Advanced vs non‐Advanced
| Name of study | Advanced CA | non‐Advanced CA | ||||
| Included patients only with Advanced Cancer | Included patients with Advanced and non‐Advanced CA AND provided subgroup data for patients with Advanced CA | Included patients with Advanced and non‐Advanced CA AND >75% of patients had Advanced CA | Included patients only with non‐Advanced Cancer | Included patients with Advanced and non‐Advanced CA AND provided subgroup data for patients with non‐Advanced CA | Included patients with Advanced and non‐Advanced CA AND >75% of patients had non‐Advanced CA | |
| Agnelli 2009 (PROTECHT) | x | |||||
| Agnelli 2012 (SAVE‐ONCO) | x | |||||
| Altinbas 2004 | x | x | ||||
| Haas 2012 (TOPIC 1) | x | |||||
| Haas 2012 (TOPIC 2) | x | |||||
| Kakkar 2004 (FAMOUS) | x | |||||
| Khorana 2017 (PHACS) | x | |||||
| Klerk 2005 (MALT) | x | |||||
| Lebeau 1994 | x | x | ||||
| Lecumberri 2013 (ABEL) | x | |||||
| Macbeth 2016 (FRAGMATIC) | x | |||||
| Maraveyas 2012 (FRAGEM) | x | |||||
| Pelzer 2015 (CONKO‐004) | x | |||||
| Perry 2010 (PRODIGE) | x | |||||
| Sideras 2006 | x | |||||
| van Doormaal 2011 (INPACT) | x | |||||
| Weber 2008 | x | |||||
| Zwicker 2013 (MICRO TEC) | x | |||||
II. Mortality at 24 months
a. Advanced vs non‐Advanced
| Name of study | Advanced CA | non‐Advanced CA | |||||
| Included patients only with Advanced Cancer | Included patients with Advanced and non‐Advanced CA AND provided subgroup data for patients with Advanced CA | Included patients with Advanced and non‐Advanced CA AND >75% of patients had Advanced CA | Included patients only with non‐Advanced Cancer | Included patients with Advanced and non‐Advanced CA AND provided subgroup data for patients with non‐Advanced CA | Included patients with Advanced and non‐Advanced CA AND >75% of patients had non‐Advanced CA | ||
| Altinbas 2004 | x | x | |||||
| Haas 2012 (TOPIC 1) | x | ||||||
| Haas 2012 (TOPIC 2) | x | ||||||
| Kakkar 2004 (FAMOUS) | x | ||||||
| Klerk 2005 (MALT) | x | ||||||
| Lebeau 1994 | x | x | |||||
| Lecumberri 2013 (ABEL) | x | ||||||
| Macbeth 2016 (FRAGMATIC) | x | ||||||
| Maraveyas 2012 (FRAGEM) | x | ||||||
| Pelzer 2015 (CONKO‐004) | x | ||||||
| Perry 2010 (PRODIGE) | x | ||||||
| Sideras 2006 | x | ||||||
| van Doormaal 2011 (INPACT) | x | ||||||
| Weber 2008 | x | ||||||
III. Symptomatic VTE
a. Lung vs non‐Lung
| Name of study | Lung CA | non‐Lung CA | ||||
| Included patients only with Lung Cancer | Included patients with Lung and non‐Lung CA AND provided subgroup data for patients with Lung CA | Included patients with Lung and non‐Lung CA AND >75% of patients had Lung CA | Included patients only with non‐Lung Cancer | Included patients with Lung and non‐Lung CA AND provided subgroup data for patients with non‐Lung CA | Included patients with Lung and non‐Lung CA AND >75% of patients had non‐Lung CA | |
| Agnelli 2009 (PROTECHT) | x | x | ||||
| Agnelli 2012 (SAVE‐ONCO) | x | x | ||||
| Altinbas 2004 | x | |||||
| Haas 2012 (TOPIC 1) | x | |||||
| Haas 2012 (TOPIC 2) | x | |||||
| Lecumberri 2013 (ABEL) | x | |||||
| Macbeth 2016 (FRAGMATIC) | x | |||||
| Maraveyas 2012 (FRAGEM) | x | |||||
| Pelzer 2015 (CONKO‐004) | x | |||||
| Perry 2010 (PRODIGE) | x | |||||
| Weber 2008 | x | |||||
IV. Major Bleeding
a. Lung vs non‐Lung
| Name of study | Lung CA | non‐Lung CA | |||||
| Included patients only with Lung Cancer | Included patients with Lung and non‐Lung CA AND provided subgroup data for patients with Lung CA | Included patients with Lung and non‐Lung CA AND >75% of patients had Lung CA | Included patients only with non‐Lung Cancer | Included patients with Lung and non‐Lung CA AND provided subgroup data for patients with non‐Lung CA | Included patients with Lung and non‐Lung CA AND >75% of patients had non‐Lung CA | ||
| Altinbas 2004 | x | ||||||
| Haas 2012 (TOPIC 1) | x | ||||||
| Haas 2012 (TOPIC 2) | x | ||||||
| Klerk 2005 (MALT) | x | ||||||
| Lebeau 1994 | x | ||||||
| Lecumberri 2013 (ABEL) | x | ||||||
| Macbeth 2016 (FRAGMATIC) | x | ||||||
| Pelzer 2015 (CONKO‐004) | x | ||||||
| Perry 2010 (PRODIGE) | x | ||||||
| Weber 2008 | x | ||||||
Data and analyses
Comparison 1. Heparin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality at 12 months‐ Main analysis | 18 | 9575 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.03] |
| 1.2 Mortality at 12 months‐ Subgroups Lung vs non‐Lung Cancer | 12 | 4768 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.86, 1.03] |
| 1.2.1 Lung Cancer (SCLC or NSCLC) | 6 | 3204 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.08] |
| 1.2.2 non‐Lung Cancer | 7 | 1564 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 1.3 Mortality at 12 months‐ Subgroups Advanced vs non‐Advanced | 18 | 9575 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.03] |
| 1.3.1 Advanced cancer | 12 | 6115 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.02] |
| 1.3.2 Non‐advanced cancer | 8 | 3460 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.12] |
| 1.4 Mortality at 24 months‐ Main Analysis | 14 | 5229 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 1.5 Mortality at 24 months‐ Subgroups Advanced vs non‐Advanced | 14 | 5229 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 1.5.1 Advanced cancer | 6 | 1554 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.03] |
| 1.5.2 Non‐advanced cancer | 8 | 3675 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.93, 1.04] |
| 1.6 Mortality over duration of study | 15 | Hazard Ratio (IV, Random, 95% CI) | 0.93 [0.84, 1.03] | |
| 1.7 Symptomatic VTE‐ Main analysis | 16 | 9036 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.47, 0.68] |
| 1.8 Symptomatic VTE‐ Subgroups Lung vs non‐Lung Cancer | 11 | 8090 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.43, 0.63] |
| 1.8.1 Lung Cancer (SCLC or NSCLC) | 6 | 4217 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.41, 0.68] |
| 1.8.2 Non‐lung cancer | 7 | 3873 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.37, 0.70] |
| 1.9 PE | 14 | 8867 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.47, 0.80] |
| 1.10 Symptomatic DVT | 14 | 8867 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.63] |
| 1.11 Major bleeding‐ Main analysis | 18 | 9592 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.94, 1.79] |
| 1.12 Major bleeding‐ Subgroups Lung vs non‐Lung Cancer | 10 | 4163 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.02, 2.56] |
| 1.12.1 Lung Cancer (SCLC or NSCLC) | 5 | 3035 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.77, 2.73] |
| 1.12.2 Non‐lung cancer | 5 | 1128 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.94, 3.58] |
| 1.13 Minor bleeding | 16 | 9245 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.13, 2.55] |
| 1.14 Thrombocytopenia | 12 | 5832 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.37, 1.27] |
1.2. Analysis.

Comparison 1: Heparin versus placebo, Outcome 2: Mortality at 12 months‐ Subgroups Lung vs non‐Lung Cancer
1.3. Analysis.

Comparison 1: Heparin versus placebo, Outcome 3: Mortality at 12 months‐ Subgroups Advanced vs non‐Advanced
1.5. Analysis.

Comparison 1: Heparin versus placebo, Outcome 5: Mortality at 24 months‐ Subgroups Advanced vs non‐Advanced
1.8. Analysis.

Comparison 1: Heparin versus placebo, Outcome 8: Symptomatic VTE‐ Subgroups Lung vs non‐Lung Cancer
1.12. Analysis.

Comparison 1: Heparin versus placebo, Outcome 12: Major bleeding‐ Subgroups Lung vs non‐Lung Cancer
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agnelli 2009 (PROTECHT).
| Study characteristics | ||
| Methods | Randomized clinical trial | |
| Participants | 1166 participants with metastatic or locally advanced lung, breast, gastrointestinal (stomach, colon‐rectum, pancreas), ovarian or head and neck cancer undergoing chemotherapy Mean age 63, males 48%, previous VTE 1.6% |
|
| Interventions | Intervention: subcutaneous LMWH nadroparin calcium 3800 IU anti‐Xa once daily for up to a maximum of 4 months Control: placebo Co‐intervention: both groups received chemotherapy Discontinued treatment: 273 of 779 participants randomized to the intervention group and 111 of 387 participants randomized to the control group |
|
| Outcomes | Follow‐up duration for the following outcomes: median of 111 and 113 days in the nadroparin and placebo groups, respectively
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was generated by an independent statistician who used a standard permuted block of six without stratification. The list was generated with SAS version 8.2." |
| Allocation concealment (selection bias) | Low risk | Quote: "The system assigned the next free number in accordance with the randomisation sequence. Participants and investigators did not know whether study drug or placebo was being given, since pre‐filled syringes were used which were identical in appearance." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatment assignments were masked from all study personnel and participants for the duration of the study." Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the study outcomes were assessed by an independent adjudication committee, whose members were unaware of the participants’ study group allocation." Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data trial report figure 1:
|
| Selective reporting (reporting bias) | Low risk | As compared to information on ClinicalTrials.gov. All outcomes listed in the methods section were reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Agnelli 2012 (SAVE‐ONCO).
| Study characteristics | ||
| Methods | Randomized. multicenter clinical trial | |
| Participants | 3212 participants with advanced metastatic or locally advanced cancer of the lung, pancreas, stomach, colon, rectum, bladder, or ovary solid tumors, planned to receive chemotherapy Mean age 60, males 60%, white 77%, 91% ECOG performance status 0 or 1, 42% with at least 1 risk factor for VTE |
|
| Interventions | Intervention: subcutaneous injection of semuloparin 20 mg once daily for a minimum of 3 months Control: placebo Co‐intervention: both groups started chemotherapy Discontinued treatment: 560 of 1608 participants randomized to the intervention group and 595 of 1604 participants randomized to the control group |
|
| Outcomes | Follow‐up duration for the following outcomes: up to 3 days after last injection, which had a median of 3.5 months
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally by means of an interactive voice‐response system. ... To balance the study groups, a minimization algorithm was used that took into account the following three factors: site of primary cancer, cancer stage (metastatic or locally advanced), and geographic region." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally by means of an interactive voice‐response system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "SAVE‐ONCO was a randomised, double‐blind, multicenter trial." Comment: probably blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Efficacy and bleeding outcomes were assessed by a central independent adjudication committee, whose members were unaware of the study treatment." Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from trial report appendix:
|
| Selective reporting (reporting bias) | Low risk | As compared to information on ClinicalTrials.gov. All outcomes listed in the methods section were reported on in the results section |
| Other bias | Low risk |
|
Altinbas 2004.
| Study characteristics | ||
| Methods | Randomized clinical trial | |
| Participants | 84 participants with histologically confirmed SCLC (both limited and extensive) undergoing combination chemotherapy Median age 58; 81% males; ECOG performance status < 3; country: Turkey |
|
| Interventions | Intervention: subcutaneous LMWH dalteparin 5000 IU once daily for up to a maximum of 18 weeks (less than 18 if disease progressed) Control: no LMWH Co‐intervention: both groups received chemotherapy Discontinued treatment: 0 participants |
|
| Outcomes | Follow‐up duration for the following outcomes: median of 10 months; range 2 to 33 months
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Open list generated by computer program" (personal communication with author) |
| Allocation concealment (selection bias) | High risk | Quote: "Open list generated by computer program" (personal communication with author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personal communication with author Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only for staging and evaluation of response to the treatment but not for the outcomes of interest (personal communication with author) Comment: definitely not blinded; however, probably low risk given that the lack of blinding may not impact the physiologic objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up (personal communication with author) |
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified. No outcomes listed in the methods section. However, all outcomes of interest were reported |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Haas 2012 (TOPIC 1).
| Study characteristics | ||
| Methods | Randomized, multicenter trial | |
| Participants | 353 ambulatory participants receiving first‐ or second‐line chemotherapy for objectively proven, disseminated metastatic breast carcinoma Mean age 55 years, postmenopausal 66% Participants were enrolled from 39 centers in Germany, Czech Republic, Ukraine, Romania and Belarus. |
|
| Interventions | Intervention: subcutaneous certoparin 3000 IU once daily for up to 6 months Control: placebo Co‐intervention: both groups received chemotherapy |
|
| Outcomes | Follow‐up duration for the following outcomes: outcomes were assessed at 6 months follow‐up
Screening test for DVT: compression ultrasound at weeks 2, 4, 8, 12, 16, 20 and 24 |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to placebo or certoparin sodium using a computer‐generated randomizations list. ... Randomization was block‐stratified according to treatment with hormone‐based chemotherapy." |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the external statistician from the Safety Committee had access to the randomizations codes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind... placebo‐controlled trial" Comment: probably blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Efficacy outcomes were validated by a blinded, independent Central Thrombosis Evaluation Team; safety end points were validated by a Data Safety Monitoring Committee consisting of 2 clinicians (blinded to treatment) and an independent statistician with access to the treatment assignments." Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A number of participants were not included in the ITT analysis but it is not reported whether they were followed up for outcome assessments:
|
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified. All outcomes listed in the methods section were reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Haas 2012 (TOPIC 2).
| Study characteristics | ||
| Methods | Randomized, multicenter trial | |
| Participants | 547 ambulatory participants receiving first‐ or second‐line chemotherapy for stage III/IV non–small cell lung carcinoma Mean age 60.5, males 83% Participants were enrolled from 39 centers in Germany, Czech Republic, Ukraine, Romania and Belarus |
|
| Interventions | Intervention: subcutaneous certoparin 3000 IU once daily for up to 6 months Control: placebo Co‐intervention: both groups received chemotherapy Discontinued treatment: 5 of 273 participants randomized to the intervention group and 9 of 274 participants randomized to the control group |
|
| Outcomes | Follow‐up duration for the following outcomes: outcomes were assessed at 6 months follow‐up
Screening test for DVT: compression ultrasound at weeks 2, 4, 8, 12, 16, 20 and 24 |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to placebo or certoparin sodium using a computer‐generated randomizations list. ... Randomization was block‐stratified according to treatment with hormone‐based chemotherapy." |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the external statistician from the Safety Committee had access to the randomizations codes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind... placebo‐controlled trial" Comment: probably blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Efficacy outcomes were validated by a blinded, independent Central Thrombosis Evaluation Team; safety end points were validated by a Data Safety Monitoring Committee consisting of 2 clinicians (blinded to treatment) and an independent statistician with access to the treatment assignments." Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A number of participants were not included in the ITT analysis but it is not reported whether they were followed up for outcome assessments:
|
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified. All outcomes listed in the methods section were reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Kakkar 2004 (FAMOUS).
| Study characteristics | ||
| Methods | Randomized, multicenter clinical trial | |
| Participants | 385 participants with histologically confirmed advanced stage III or IV (locally advanced or metastatic) malignant disease of the breast, lung, gastrointestinal tract, pancreas, liver, genitourinary tract, ovary or uterus; minimum life expectancy 3 months; median age 61 IQR (53 to 68), 43% males; 10 centers (7 in the UK, 2 in Canada and 1 in Italy) | |
| Interventions | Intervention: subcutaneous LMWH (dalteparin) 5000 IU self‐injected once daily for 12 months Control: placebo Co‐intervention: "Thirty‐four percent of the dalteparin group and 31% of the placebo group received chemotherapy while participating in the study, with 8% receiving radiotherapy in both groups … no restriction on concomitant chemotherapy or radiotherapy" Discontinued treatment: 0 of 196 participants randomized to the intervention group and 0 of 189 participants randomized to the control arm |
|
| Outcomes | Follow‐up duration for the following outcomes: maximum of 77 months
Screening test for DVT/PE: not reported Diagnostic testing for DVT/PE: "diagnosis determined according to local practices"; "not reviewed centrally" |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally by computer‐generated code" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally by computer‐generated code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind, placebo controlled study" Comment: probably blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double‐blind, placebo controlled study" Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Withdrawal of consent before commencing the study medication resulted in 11 patients (six patients in the dalteparin group and five in the placebo group) not being included in the analyses. The remaining 374 patients were analysed for both efficacy and safety" Comment:we calculated a 97% follow‐up rate in the intervention group and 97.4% follow‐up rate in the control group |
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified. All outcomes listed in the methods section are reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Khorana 2017 (PHACS).
| Study characteristics | ||
| Methods | Randomized clinical trial | |
| Participants | 98 participants from 6 sites (University of Rochester Medical Center, Duke University, Rochester General Hospital, Highland Hospital and Interlakes Oncology, Roswell Park Cancer Institute, Ottawa General Hospital, and University of California, Davis Medical Center) Males 58%, age mean 59, pancreatic cancer 37% Planned initiation of a new systemic chemotherapy regimen, Khorana risk score of ≥3 |
|
| Interventions | Intervention: either dalteparin 5000 units subcutaneously daily Control: observation for a period of 12 weeks |
|
| Outcomes | Follow‐up duration for the following outcomes: 13 weeks (12 weeks of treatment and 1 week of observation)
Screening test for DVT in lower extremities: Compression ultrasonography of lower extremities at 4, 8 and 12 weeks (at time of regularly scheduled chemotherapy cycle visits) Screening test for PE: CT chest at end of study |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled subjects were center‐stratified and block‐randomized in balanced blocks of 4 consecutively enrolled participants within each center, using a web‐based software program." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "We chose not to use placebo injections because of ethical considerations and concerns about patient acceptance of placebo injections" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Thrombotic events were adjudicated by a thrombosis adjudication committee, comprising 2 radiologists who reviewed de‐identified imaging studies and were blinded to treatment assignment" Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | As compared to information on ClinicalTrials.gov. All outcomes listed in the methods section were reported on in the results section |
| Other bias | Low risk | Quote: "The study was terminated early due to low accrual" No other bias suspected |
Klerk 2005 (MALT).
| Study characteristics | ||
| Methods | Randomized clinical trial | |
| Participants | 302 participants with different types of solid malignant tumors, "that could not be treated curatively" including: colorectal, breast, lung gastric, esophageal, liver, gallbladder, Klatskin, prostate, pancreatic, cervical, urothelial, renal, ovarian, melanoma, endometrial and other cancers; minimum life expectancy 1 month, stratified according to life expectancy (< or > 6 months); median age 64; 52% males | |
| Interventions | Intervention: subcutaneous LMWH (nadroparin) 9500 antifactor Xa U/mL for 6 weeks; 2 weeks therapeutic dose (twice daily) then 4 weeks prophylactic dose (once daily) Control: placebo Co‐intervention: both arms started concomitant antineoplastic therapy (chemotherapy, radiotherapy, hormonal therapy, other antineoplastic treatment) |
|
| Outcomes | Follow‐up duration for the following outcomes: mean of 12 months
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sequentially numbered boxes of syringes with nadroparin or placebo were prepared using a central computer‐generated randomizations schedule" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered boxes of syringes with nadroparin or placebo were prepared using a central computer‐generated randomizations schedule" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind, placebo controlled study". Personal communication with authors: "Patients, healthcare providers, data collectors and outcome adjudicators were blinded." Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double‐blind, placebo controlled study". Personal communication with authors: "Patients, healthcare providers, data collectors and outcome adjudicators were blinded." Comment: definitely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients were observed until death or until the end of the study". "No patients were lost to follow‐up" Comment: complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified. All outcomes listed in methods section are reported on in the results section. However study does not report number of thrombotic events. Personal communication with authors: "VTE was not an endpoint of the study and it was not standardly registered per protocol" |
| Other bias | High risk |
|
Lebeau 1994.
| Study characteristics | ||
| Methods | Randomized clinical trial | |
| Participants | 277 participants with histologically diagnosed SCLC both limited and extensive; 78% had Karnofsky Performance Scale index > 80; 85% older than 50; 91% males | |
| Interventions | Intervention: 2 or 3 daily subcutaneous injections of heparin adjusted initially by weight (500 IU/kg/day) then adjusted by clotting times for 5 weeks Control: no heparin Co‐intervention: participants initially were randomized between two chemotherapy regimens (sequential or alternating). "Those who did not respond received only two courses of chemotherapy. Complete responders received eight courses of chemotherapy and then were randomized either to receive or not receive thoracic radiotherapy. Partial responders either pursued chemotherapy until a relapse occurred or received thoracic irradiation if their disease remained apparently limited." |
|
| Outcomes | Follow‐up duration for the following outcomes: maximum of 84 months
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized through a centralized blind telephone assignment procedure" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomized through a centralized blind telephone assignment procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "No blinding procedure for patients and physicians was used because overall survival was chosen as the major endpoint" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "No blinding procedure for patients and physicians was used because overall survival was chosen as the major endpoint" Comment: probably not blinded; however, probably low risk given that the lack of blinding may not impact the physiologic objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient was lost to follow‐up" Comment: complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified. The outcomes listed in the methods section are reported on in the results section |
| Other bias | High risk | Study not reported as stopped early for benefit Comment: randomization may have been affected due to differential co‐intervention (radiotherapy) |
Lecumberri 2013 (ABEL).
| Study characteristics | ||
| Methods | Multicenter, randomized, open‐label study (ABEL study) | |
| Participants | 38 participants diagnosed with limited SCLC | |
| Interventions | Intervention: bemiparin (3500 IU/day) for 26 weeks, starting on the first day of chemotherapy Control: no bemiparin Co‐intervention: homogeneous standard treatment with platinum‐based chemotherapy + radiotherapy for 6 cycles |
|
| Outcomes | Follow‐up duration for the following outcomes: 18 months
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed through an automatic central randomizations system, with stratification according to center, sex, age and Eastern Cooperative Oncology Group (ECOG) performance status" |
| Allocation concealment (selection bias) | High risk | Quote: "Designed as an open‐label study, the control group did not receive a matched placebo and both, investigators and patients, were aware of the result of the randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Designed as an open‐label study, the control group did not receive a matched placebo and both, investigators and patients, were aware of the result of the randomization" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "There was no central adjudication committee. In any case, radiologists at all sites were not aware of the treatment arms and clinical records were carefully monitored by an independent CRO. ... Complementary tests for the evaluation of the response were performed by radiologists unaware of the treatment arm in which patients had been allocated" Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient who had been enrolled was subsequently found to be ineligible after review of the records" Comment: complete follow‐up |
| Selective reporting (reporting bias) | High risk | As compared to information on ClinicalTrials.gov. Different statistical data reported in the abstract from the 5th International Conference on Thrombosis and Hemostasis Issues in Cancer: Oral Communications/Thrombosis Research 125 (2010) S161–5 |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Macbeth 2016 (FRAGMATIC).
| Study characteristics | ||
| Methods | Randomized phase III trial | |
| Participants | 2202 participants with newly diagnosed newly diagnosed lung cancer of any stage and histology | |
| Interventions | Intervention: LMWH (Dalteparin) given daily at 5,000 IU, 0.2 mL, subcutaneously for 24 weeks Control: No anticoagulation Co‐intervention: standard anticancer treatment |
|
| Outcomes | Follow‐up duration for the following outcomes at 3‐ to 4‐week intervals up to week 24, then at 9 months and 1 year, and then every 6 months until death:
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned to receive either LMWH or no LMWH, by use of a computer algorithm using the method of minimization and a random element." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was by research nurses (who recruited patients) telephoning the Wales Cancer Trials Unit, where randomization and treatment allocation was done by a trial/data manager using a computerized system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study had an open‐label design" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study had an open‐label design" Comment: definitely not blinded; probably low risk for physiologic objective outcomes given that the lack of blinding may not impact the latter and probably high risk for patient‐reported subjective outcomes given that the lack of blinding may impact the latter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram:
Comment: we calculated a 94% follow‐up rate in the intervention group and 97% follow‐up rate in the control group (participants that withdrew completely were lost to follow‐up, based on personal communication with the author) |
| Selective reporting (reporting bias) | Low risk | Quote: "The trial protocol was approved by the UK Medicines and Healthcare Products Regulatory Agency and a multicenter research ethics committee. The full trial protocol is accessible online." Comment: outcomes listed in the protocol and methods section in the manuscript are reported on in the results section except for: "Detailed results from health economics, health‐related quality of life, dyspnea, and biomarker studies will be reported elsewhere." |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Maraveyas 2012 (FRAGEM).
| Study characteristics | ||
| Methods | Randomized controlled phase IIb trial | |
| Participants | 123 participants with histopathological or cytological diagnosis of non‐resectable, recurrent or metastatic pancreatic adenocarcinoma Mean age 63 years, males 59%, locally advanced disease 46%, metastatic disease 54%, KPS > 80 75%, any prior treatment 59%, estimated life expectancy > 12 weeks |
|
| Interventions | Intervention: LMWH (weight‐adjusted dalteparin) given subcutaneously at 200 IU/kg once daily for 4 weeks followed by a stepdown to 150 IU/kg for a further 8 weeks. Control: No anticoagulation Co‐intervention: both arms received chemotherapy |
|
| Outcomes | Follow‐up duration for the following outcomes: all outcomes were assessed at 12 weeks and 1‐year follow‐up
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in the facilities of the Postgraduate Medical Institute in Hull with software developed by York University. The block randomisation method was followed and patients were stratified for stage (locally advanced versus metastatic) and performance status (KPS 90–100 versus 60–80)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: probably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "There was no VTE adjudication committee" Comment: probably not blinded; however, probably low risk given that the lack of blinding may not impact the physiologic objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six patients did not complete the 12‐week WAD; three due to death (cholangitis, pneumonia and progressive disease), two due to hemorrhage (ISTH ‘severe’) and one due to patient preference." "Two patients, one from each arm withdrew consent soon after randomisation and were excluded from all analyses." Data from trial report figure 1:
|
| Selective reporting (reporting bias) | Low risk | As compared to information on ClinicalTrials.gov. The outcomes listed in the protocol and methods section in the manuscript are reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Pelzer 2015 (CONKO‐004).
| Study characteristics | ||
| Methods | Prospective, open‐label, randomized, multicenter and group‐sequential trial | |
| Participants | 312 participants with advanced pancreatic cancer who were treated with first‐line chemotherapy in an outpatient setting with or without enoxaparin | |
| Interventions | Intervention: subcutaneous LMWH (enoxaparin) intermediate dose ‐ 1 mg/kg daily for the first 3 months followed by 40 mg daily until disease progression Control: no LMWH Co‐intervention: ambulant first‐line chemotherapy (randomized to either intensified GFFC therapy (gemcitabine, 5‐FU, folinic acid, cisplatin) or to GEM therapy (gemcitabine only)) |
|
| Outcomes | Follow‐up duration for the following outcomes up to 3 months
Screening and diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated random numbers generated at the study coordination center at the Charité–Universitätsmedizin Berlin" |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated random numbers generated at the study coordination center at the Charité–Universitätsmedizin Berlin" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Prospective, open‐label, randomized, multicenter and group‐sequential trial" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All symptomatic VTEs and major hemorrhages were documented using the serious adverse event form, centrally reviewed and evaluated by an independent, blinded event review board (ERB)." Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We calculated a 95.7% follow‐up rate in the intervention group and 93.4% follow‐up rate in the control group, for the outcome OS. |
| Selective reporting (reporting bias) | Low risk | Study registered in the ISRCTN registry and published protocol identified. The outcomes listed in the protocol and methods section in the manuscript are reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit. However, 312 participants were recruited into the trial whereas the earlier published abstracts (Pelzer 2005; Pelzer 2007) reported a target recruitment of 540 participants |
Perry 2010 (PRODIGE).
| Study characteristics | ||
| Methods | Randomized, placebo‐controlled trial | |
| Participants | 186 adults with newly diagnosed malignant glioma Mean age 56 years, males 60%, perioperative DVT prophylaxis 55%, KPS 90 40%, mean time (days) from surgery to randomization 22 |
|
| Interventions | Intervention: subcutaneous LMWH (dalteparin sodium) 5000 IU once daily for 6 months; treatment beyond 6 months was optional Control: placebo Co‐intervention: the use of concurrent therapy with ASA, NSAID and dextran was permitted but discouraged |
|
| Outcomes | Follow‐up duration for the following outcomes: all participants were followed in clinic monthly for the first 6 months post‐randomization and then at 9 and 12 months
Screening test for DVT/PE: not reported Diagnostic testing for DVT/PE: for DVT with venography or compression ultrasound; for PE with autopsy, a high probability ventilation‐perfusion lung scan, conventional pulmonary angiogram, CT pulmonary angiogram, or objectively demonstrated DVT in participants with a clinical suspicion of PE and a non‐high probability lung scan |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocations were pre‐determined using a computer‐generated randomizations list with random size permuted blocks" |
| Allocation concealment (selection bias) | Low risk | Quote: "Consenting patients were randomised by contacting the Ontario Clinical Oncology Group (OCOG) Coordinating and Methods Centre at the Henderson Research Centre, Hamilton, Ontario." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Investigators, patients and outcome assessors were blinded to treatment allocation." Comment: definitely blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators, patients and outcome assessors were blinded to treatment allocation." Comment: definitely blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is not reported whether the following participants were followed up for outcome assessments:
|
| Selective reporting (reporting bias) | Low risk | As compared to information on ClinicalTrials.gov and the methods section in the manuscript: VTE, bleeding, and mortality outcomes were reported whereas quality of life and cognition assessments outcomes were not reported in the results section |
| Other bias | Low risk | Stopped early but for slow accrual No other bias suspected |
Sideras 2006.
| Study characteristics | ||
| Methods | Blinded, placebo‐controlled, randomized clinical trial changed to open‐labeled clinical trial | |
| Participants | 141 participants with advanced breast, prostate, lung or colorectal cancer Mean age 68 years, males 60%, minimum life expectancy 12 weeks; ECOG performance status 0 to 2 |
|
| Interventions | Subcutaneous LMWH (dalteparin) 5000 U once daily versus placebo for unclear duration; then changed to LMWH (dalteparin) 5000 U once daily versus no intervention; duration not specified; with concomitant chemotherapy and/or radiotherapy | |
| Outcomes | Follow‐up duration for the following outcomes:
Screening test for DVT/PE: not reported Diagnostic testing for DVT/PE: decided by the primary clinician |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization handled through the North Central Cancer Treatment Group (NCGTG) Randomization Office using a dynamic allocation method" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization handled through the North Central Cancer Treatment Group (NCGTG) Randomization Office using a dynamic allocation method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment:
|
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment:
|
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 98% follow‐up Quote: "Three patients, 1 randomised to blinded LMWH and 2 to unblinded LMWH, dropped out before receiving any protocol therapy." |
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified |
| Other bias | Low risk | Quote: "The protocol accrual was stopped before reaching the prestudy planned accrual goal by the NCCTG Data Monitoring Committee because of a slower than predicted protocol accrual rate, with the knowledge (provided by an interim analysis report) that the patient survival rates were numerically worse on one arm of the blinded study." Comment: study was stopped early for insufficient accrual but not for benefit |
Vadhan‐Raj 2013.
| Study characteristics | ||
| Methods | Randomized‐controlled trial | |
| Participants | 87 patients with metastatic or locally advanced pancreatic cancer planned to start chemotherapy | |
| Interventions | Intervention: LMWH (Dalteparin) given at 5000 units subcutaneously, daily for 16 weeks Control: no dalteparin, only chemotherapy |
|
| Outcomes | Follow‐up duration for the following outcomes: 16 weeks (study duration)
Screening testing for DVT/PE: not reported Diagnostic testing for DVT/PE: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients with metastatic or locally advanced pancreatic cancer planned to start chemotherapy were randomized 1:1 to dalteparin and control arms, stratified for the presence of metastasis and central venous catheter (CVC)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably an open‐label trial Comment: Probably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Probably an open‐label trial Comment: probably not blinded; however, probably low risk given that the lack of blinding may not impact the physiologic objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quoting "All 75 patients were evaluable for response in an intent‐to‐treat analysis." Comment: Complete follow up |
| Selective reporting (reporting bias) | Unclear risk | No pertinent details available from the abstract |
| Other bias | Low risk | Probably, not stopped early for benefit. No further details available from the abstract |
van Doormaal 2011 (INPACT).
| Study characteristics | ||
| Methods | Randomized, multicenter study | |
| Participants | 503 participants with histologically or cytologically documented prostate carcinoma within 6 months after diagnosis of hormone‐refractory state, NSCLC without clinically significant pleural effusion within 3 months after diagnosis of stage IIIB, or with a locally advanced pancreatic cancer within 3 months after diagnosis with a minimum life expectancy of less than 3 months at entry; and a KPS of fewer than 60 points Mean age 65 years, males 80%, prostate cancer 40%, NSCLC 33%, pancreatic cancer 27%, 80% KPS < or equal 80 |
|
| Interventions | Intervention: LMWH (nadroparin) given subcutaneously at body weight‐adjusted therapeutic doses for 2 weeks followed by half‐therapeutic doses for an additional 4 weeks. After these initial 6 weeks, participants were eligible to receive additional cycles of nadroparin (2 weeks at therapeutic dose, and 4 weeks of washout period). The total duration of study drug administration was 46 weeks, including the washout periods, which was also the minimum duration of follow‐up Control: no anticoagulation Co‐intervention: standard anticancer treatment |
|
| Outcomes | Follow‐up duration for the following outcomes: median of 10.5 months in the nadroparin group and 10.4 months in the control group (a minimum of 46 weeks)
Screening test for DVT/PE: not reported Diagnostic testing for DVT/PE: echo‐doppler for DVT and spiral CT scan for PE |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation of treatment proceeded centrally by using an interactive‐voice response system" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of treatment proceeded centrally by using an interactive‐voice response system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Open‐label study" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All potential outcome events were reviewed by an independent adjudication committee blinded to treatment assignment." Comment: probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A number of participants were described as having discontinued treatment but it is not reported whether they were followed up for outcome assessments:
|
| Selective reporting (reporting bias) | Low risk | As compared to information on ClinicalTrials.gov. The outcomes listed in the protocol and methods section in the manuscript are reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Weber 2008.
| Study characteristics | ||
| Methods | Prospective, open, randomized study | |
| Participants | 20 participants with advanced cancer with a minimum life expectancy of 6 months Mean age 70 years, males 45% FIM score 123, WHO performance status 2.5 |
|
| Interventions | Intervention: subcutaneous LMWH (nadroparin) 2850/3800 U (< 70/> 70 kg) once daily for unclear duration Control: no LMWH Co‐intervention: both arms received concomitant anticancer treatment |
|
| Outcomes | Follow‐up duration for the following outcomes: 18 months
Screening test for DVT/PE: not reported Diagnostic testing for DVT/PE: echo‐doppler for DVT and spiral CT scan for PE |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequence of treatments was randomly assigned in blocks of constant size (n = 20)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sets of 20 sealed envelopes (10 Yes and 10 No) were numbered consecutively. The sequence of treatments was randomly assigned in blocks of constant size." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Prospective open randomised study" Comment: definitely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Prospective open randomised study" Comment: definitely not blinded; however, probably low risk given that the lack of blinding may not impact the physiologic objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient was lost to follow‐up" Comment: complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Study not registered and no published protocol identified.. The outcomes listed in the methods section are reported on in the results section |
| Other bias | Low risk | Study not reported as stopped early for benefit No other bias suspected |
Zwicker 2013 (MICRO TEC).
| Study characteristics | ||
| Methods | Randomized phase II trial | |
| Participants | 34 participants with locally advanced or metastatic cancer and highTFMP. The number of participants randomized was 23 to the intervention group and 11 to the observation group. Moreover, 32 participants with low TFMP were placed into the observation group | |
| Interventions | Intervention: subcutaneous LMWH (Enoxaparin) given at 40 mg once daily Control: observation |
|
| Outcomes | Follow‐up duration for the following outcomes: 2 months
Screening test for DVT/PE: baseline lower extremity ultrasound evaluations for DVT; not reported for PE Diagnostic testing for DVT/PE: compression ultrasound for DVT; pulmonary angiography, ventilation/perfusion lung scan, spiral CT for PE |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized phase II trial" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: probably not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported Comment: probably low risk given that the lack of blinding may not impact the physiologic objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Apparently there are complete follow‐up data for all the participants randomized |
| Selective reporting (reporting bias) | Low risk | Study not registered in ClinicalTrials.gov and no published protocol identified. The outcomes listed in the methods section are reported on in the results section |
| Other bias | Low risk | Quote: "Although the study was not formally powered to compare the cumulative incidence of patients with higher levels of tissue factor‐bearing microparticles randomized to enoxaparin or observation, the use of enoxaparin resulted in an 80% risk reduction compared to observation." Comment: This trial was originally designed as a phase III, then re‐adapted to a phase II randomized clinical trial. The trial is described as underpowered |
5‐FU: fluorouracil; ASA: acetylsalicylic acid; CRO: contract research organization; CT: computed tomography; DVT: deep vein thrombosis; ECOG: Eastern Co‐operative Oncology Group; FDA: (US) Food and Drug Administration;FIM: functional impedance score; HR: hazard ratio; ISRCTN: International Standard Randomised Controlled Trial Number; ITT: intention‐to‐treat;IV: intravenous; IQR: interquartile range; ISTH: International Society on Thrombosis and Haemostasis; IU: international units; kg: kilogram; KPS: Karnofsky Performance Status; LMWH: low molecular weight heparin; mg: milligram; mg/m2: milligram/square meter; mL: milliliter; NEJM: New England Journal of Medicine; NSAID: non‐steroidal anti‐inflammatory drugs; NSCLC: non‐small cell lung cancer; OS: overall survival; PE: pulmonary embolism; TFMP: tissue factor bearing microparticles; SCLC: small cell lung cancer;U: units; UK: United Kingdom; VTE: venous thromboembolism; WAD: weight‐adjusted dalteparin; WHO: World Health Organization;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agnelli 1998 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Agnelli 2005 | Not population of interest (surgical setting) |
| Agnelli 2015 (AMPLIFY) | Not the population of interest (patients with cancer with VTE); includes 2 reports |
| Alifano 2005 | Not a study of interest (letter to the editor) |
| Alikhan 2003 (MEDENOX) | Not the population of interest (hospitalized patients with cancer); includes 2 reports |
| Arbit 2005 | Not a study of interest (letter to the editor) |
| Auer 2011 | Not the population of interest (patients with cancer without VTE who had a surgical procedure) |
| Barberi‐Heyob 1995 | Not a study of interest (letter to the editor) |
| Barkagan 1997 | Not the comparison of interest (LMWH versus vitamin K antagonists versus UFH) |
| Bigg 1992 | Not population of interest (surgical setting) |
| Bitsch 1990 | Not the intervention of interest (topical heparin) |
| Blaszczyk 1970 | Not a study of interest (not randomized) |
| Buckman 2005 | Not the comparison of interest (no control group) |
| Cahan 2000 | Not intervention of interest (oral AC) |
| Cavallo 2010 | Not the comparison of interest (LMWH versus aspirin) |
| Chojnowski 2002 | Not the outcome of interest (no survival outcome) |
| Cicco 2009 | Not the intervention of interest |
| Clarke‐Pearson 1993 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Cohen 1997 | Not population of interest (surgical setting) |
| Cohen 2003 | Not population of interest (hospitalitzed) |
| Cohen 2006 | Not population of interest (hospitalitzed) |
| Cohen 2007 (PREVENT) | Not the population of interest (hospitalized patients with cancer); includes 3 reports |
| Couban 2005 | Not the population of interest (patients with cancer with CVC without VTE); includes 3 reports |
| Craven 2001 | Not a study of interest (letter to the editor) |
| Crossno 2009 | Not a study of interest (letter to the editor) |
| Demir 2006 | Not the intervention of interest (oral anticoagulant) |
| Demir 2007 | Not the intervention of interest (oral anticoagulant) |
| Dickinson 1998 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Di Nisio 2005 | Not a study of interest (review) |
| Edlis 1976 | Not the comparison of interest (no control group) |
| Eichinger 2008 | Not the comparison of interest (different doses of LMWH) |
| Elias 1972 | Not a study of interest (case series) |
| Elias 1973a | Not a study of interest (not randomized) |
| Elias 1973b | Not a study of interest (case series) |
| Elias 1973c | Not a study of interest (not randomized) |
| Elias 1974 | Not a study of interest (case series) |
| Elias 1975 | Not a study of interest (not randomized) |
| Elit 2012 | Not the comparison of interest (comparing 3 different doses of LMWH) |
| Fielding 1992 | Not the intervention of interest (intraportal infusion with heparin) |
| Goldhaber 2002 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Graf 1994 | Not the comparison of interest (LMWH versus vitamin K antagonists) |
| Graf 1996 | Not the comparison of interest (LMWH versus vitamin K antagonists) |
| Green 1992 | Not a study of interest (letter to the editor) |
| Guimbretiere 1982 | Not a study of interest (not randomized) |
| Haas 2011 | Not the population of interest (hospitalized patients with cancer); includes 3 reports |
| Harenberg 1996 | Not the population of interest (hospitalized patients with cancer); includes 2 reports |
| Hata 2016 | Not population of interest (surgical setting) |
| Hoppensteadt 2011 | Not the intervention of interest (oral anticoagulant) |
| Kakkar 2010 (CANBESURE) | Not the population of interest (patients with cancer who had a surgical procedure); includes 2 reports |
| Kakkar 2014 (SAVE‐ABDO) | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure); includes 2 reports |
| Kohanna 1983 | Not a study of interest (retrospective study) |
| Koppenhagen 1992 | Not population of interest (surgical setting) |
| Larocca 2012 | Not the comparison of interest (LMWH versus aspirin) |
| Lecumberri 2005 | Not a study of interest (review) |
| Lee 2015 (CATCH) | Not the population of interest (patients with cancer with VTE); includes 9 reports |
| Lemoine 2005 | Not a study of interest (editorial) |
| Levine 1994 | Not the intervention of interest (oral anticoagulant) |
| Levine 2005 | Not a study of interest (letter to the editor) |
| Levine 2012 | Not the intervention of interest (oral anticoagulant) |
| Liebman 2009 | Not the intervention of interest (oral anticoagulant) |
| Loprinzi 1999 | Not the intervention of interest (oral anticoagulant) |
| Loynes 2002 | Not the intervention of interest (case report) |
| Lykke 2003 | Not a study of interest (review) |
| Mammen 2004 | Not a study of interest (preface) |
| Maraveyas 2010 | Not the outcome of interest (no survival outcome) |
| Maxwell 2001 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Mazilu 2014 (OVIDIUS) | Not the population of interest (patients with cancer with VTE) |
| Meyer 2007 | Not a study of interest (letter to the editor) |
| Mousa 2001 | Not the population of interest (patients without cancer) |
| Munstedt 1996 | Not the intervention of interest (consisted of only 2 doses of LMWH) |
| Murakami 2002 | Not population of interest (surgical setting) |
| Nagata 2015 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Nash 2000 | Not a study of interest (letter to the editor) |
| Nishioka 2007 | Not a study of interest (review) |
| Nitti 1997 | Not the intervention of interest (intraportal infusion with heparin) |
| Nurmohamed 1996 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Palumbo 2011 | Not the comparison of interest (aspirin versus warfarin); includes 6 reports |
| Prins 2014 (EINSTEIN) | Not the population of interest (patients with cancer with VTE) |
| Raskob 2016 (HOKUSAI) | Not the population of interest (patients with cancer with VTE) |
| Retik 1962 | Not the population of interest (patients without cancer) |
| Rohwedder 1977 | Not the comparison of interest (no control group) |
| Sakon 2010 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Schulman 2003 | Not population of interest (patients with VTE) |
| Schulman 2013 (RE‐MEDY) | Not the population of interest (patients with cancer with VTE) |
| Schulman 2015 (RECOVER) | Not the population of interest (patients with cancer with VTE) |
| Siragusa 1999 | Not a study of interest (letter to the editor) |
| Song 2014 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
| Spigel 2005 | Not a study of interest (review) |
| Stanford 1979 | Not the intervention of interest (oral anticoagulant) |
| Tethi 2011 | Not the intervention of interest (oral anticoagulant) |
| Traby 2010 | Not the comparison of interest (different dosages of enoxaparin) |
| Vedovati 2014 | Not the population of interest (patients with cancer who had a surgical procedure); includes 5 reports |
| Verso 2008 | Not the population of interest (patients with cancer with CVC without VTE); includes 4 reports |
| Von Hugo 1981 | Not the outcome of interest (no survival outcome) |
| Ward 1998 | Not population of interest (surgical setting) |
| Wester 1996 | Not the population of interest (patients with cancer with VTE); includes 2 reports |
| Wojtukiewicz 2003 | Not the comparison of interest (no control group) |
| Zacharski 2003 | Not a study of interest (editorial) |
| Zheng 2014 | Not the population of interest (patients with cancer without VTE undergoing a surgical procedure) |
AC: anticoagulant; CVC: central venous catheter; LMWH: low molecular weight heparin; UFH: unfractionated heparin; VTE: venous thromboembolism
Characteristics of ongoing studies [ordered by study ID]
Borad 2011 (PGPC1).
| Study name | A randomized phase II open‐label study to assess the efficacy & safety of gemcitabine + Abraxane® with or without ODSH (2‐0, 3‐0 desulfated heparin) as first line treatment of metastatic pancreatic cancer |
| Methods | Type: interventional Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | 60 participants with histologically confirmed metastatic adenocarcinoma of the pancreas for which potential curative measures, such as resection of an isolated metastasis, are not available and no prior radiotherapy or chemotherapy. Male or non‐pregnant and non‐lactating female and ≥ 18 to ≤ 75 years of age with acceptable coagulation studies and ECOG performance status ≤ 1 |
| Interventions | Intervention: ODSH (IV bolus at 4 mg/kg will be administered in 5 minutes immediately after completion of gemcitabine administration. ODSH 48‐hour IV continuous infusion at 0.375 mg/kg/hour should be started immediately after the ODSH IV bolus has been administered) Control: no ODSH Co‐intervention: gemcitabine + nab‐paclitaxel |
| Outcomes | Progression‐free survival Incidence of adverse events & toxicity Overall survival Objective tumor response |
| Starting date | November 2011 |
| Contact information | Jocelyn Harmon, BS, CCRC (602) 358 8385 jharmon@tgen.org Amy Stoll, MS, CCRP (602) 358‐8319 astoll@tgen.org |
| Notes | Status as of August 2017: Completed (not published yet) |
Chibauldel 2008 (PAM07).
| Study name | Chemotherapy with or without preventive anticoagulation for metastatic cancer of the pancreas |
| Methods | Study type: interventional Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: supportive care |
| Participants | Patients with a histologically confirmed metastatic adenocarcinoma of the pancreas |
| Interventions | Intervention: dalteparin: 5000 IU subcutaneous injection, from day 1 to day 28 Control: no dalteparin Co‐intervention: chemotherapy at investigator's discretion |
| Outcomes | Thromboembolic events Progression‐free survival Overall survival Tolerance of regimens |
| Starting date | October 2007 |
| Contact information | Benoist Chibauldel, MD Hopital Saint Antoine |
| Notes | Sponsor: Groupe Cooperateur Multidisciplinaire en Oncologie (GERCOR) Status as of August 2017: Terminated (not published yet) |
Germonpre 2008 (SYRINGES).
| Study name | Low molecular weight heparin in advanced non small cell lung cancer (NSCLC): a randomized open label phase III study evaluating the effect of enoxaparin (Clexane) on survival and symptom control in patients with stage IIIB and IV NSCLC undergoing a cisplatin based first line chemotherapy: the SYRINGES Trial |
| Methods | Study type: interventional Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | Locally advanced or metastatic NSCLC (stage IIIB or IV) |
| Interventions | Intervention: enoxaparin daily 1 mg/kg/day sc Control: no enoxaparin Co‐intervention: cisplatin 75 mg/m2 d1 and docetaxel 75 mg/m2 d1 (every 3 weeks for 4 cycles) |
| Outcomes | Progression‐free survival Incidence of total documented thromboembolic and hemorrhagic events |
| Starting date | June 2008 |
| Contact information | Paul R Germonpre, MD PhD Universiteit Antwerpen |
| Notes | Sponsor: University Hospital, Antwerp Universiteit Antwerpen Status as of August 2017: Completed (not published yet) |
Kakkar 2010 (GASTRANOX).
| Study name | Overall survival of inoperable gastric/gastrooesophageal cancer subjects on treating with LMWH + chemotherapy(CT) vs standard CT (GASTRANOX) |
| Methods | Study type: interventional Allocation: randomized Control: active control Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label |
| Participants | Patients with inoperable gastric and gastro‐esophageal cancer |
| Interventions | Enoxaparin (once daily dose of 1 mg/kg of body weight for 6 months) given concomitantly with chemotherapy versus chemotherapy alone |
| Outcomes | Primary outcome measures: event‐free survival (EFS) ‐ composite endpoint of overall survival plus free of symptomatic VTE (time frame: up to 1 year from start of treatment) Secondary outcome measures: incidence of symptomatic VTE, overall survival, major and minor hemorrhages during chemotherapy and/or up to 30 days after last dose is provided. Serious adverse events, all reported adverse events, heparin induced thrombocytopenia (time frame: up to 1 year from the start of treatment) |
| Starting date | July 2008 |
| Contact information | Janice M Maganji, MBBS. +00 44 7824836535; mmaganji@tri‐london.ac.uk [mailto:mmaganji%40tri‐london.ac.uk?subject=NCT00718354, TRI0702, Overall Survival of Inoperable Gastric/GastroOesophageal Cancer Subjects on Treating With LMWH + Chemotherapy(CT) vs Standard CT] |
| Notes | Principal Investigator: Ajay K Kakkar, PhD Thrombosis Research Institute Sponsors and Collaborators Thrombosis Research Institute http://clinicaltrials.gov/ct2/show/NCT00718354 Status as of August 2017: Completed (not published yet) |
Lars 2008 (RASTEN).
| Study name | A randomized phase III study of standard treatment +/‐ enoxaparin in small cell lung cancer |
| Methods | Study type: interventional Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: single‐group assignment Masking: open‐label Primary purpose: treatment |
| Participants | Histologically or cytologically verified SCLC, all stages |
| Interventions | Intervention: enoxaparin Control: no enoxaparin Co‐intervention: cisplatinum or carboplatin |
| Outcomes | Overall survival Toxicity |
| Starting date | June 2008 |
| Contact information | Lars EK, MD +46 46 17 73 40 lars.ek@skane.se Jan Sundberg, RN |
| Notes | Sponsor: Lund University Hospital Status as of August 2017: Ongoing but not recruiting participants |
Meyer 2017 (PROVE).
| Study name | Long‐term Prophylaxis of Venous Thromboembolism with low‐molecular‐weight heparin in patients with metastatic lung cancer |
| Methods | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Outcomes Assessor Primary Purpose: Prevention |
| Participants | Adult patients aged ≥ 18 years with stage IV lung cancer and elevated D‐dimer |
| Interventions | Intervention: Tinzaparin sodium subcutaneous tinzaparin 4,500 IU once daily for six months. Control: No intervention‐ usual care |
| Outcomes | All VTE events (symptomatic and asymptomatic PE and DVT) Major bleeding Death |
| Starting date | April 2017 |
| Contact information | Guy Meyer, MD +31 156 093461 guy.meyer@aphp.fr |
| Notes | Status as of August 2017: This study is not yet open for participant recruitment. |
Okuno 1999.
| Study name | Phase III double‐blind trial comparing low‐molecular weight heparin (LMWH) versus placebo in patients with advanced cancer |
| Methods | Study type: interventional Allocation: randomized Primary purpose: treatment |
| Participants | Patients with a histologically or cytologically proven breast, lung, colorectal or prostate cancer that has failed prior chemotherapy or hormone therapy. No active CNS metastases. Hormone receptor status: not specified |
| Interventions | Intervention: low molecular weight heparin (dalteparin) Control: no dalteparin Co‐intervention: standard therapy |
| Outcomes | Quality of life |
| Starting date | December 1998 |
| Contact information | Scott Okuno, MD Mayo Clinic |
| Notes | Sponsor: North Central Cancer Treatment Group National Cancer Institute (NCI) Status as of August 2017: Completed (not published yet) |
Pandya 2002.
| Study name | A prospective randomized controlled multicenter study of the effect of dalteparin on quality of life in unresectable pancreatic cancer |
| Methods | Study type: interventional Allocation: randomized Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | Patients with histologically or cytologically confirmed pancreatic adenocarcinoma or poorly differentiated carcinoma of the pancreas that is considered ineligible for curative resection |
| Interventions | Intervention: 5000 anti‐Xa units of dalteparin subcutaneously once daily for 6 months Control: no dalteparin Co‐intervention: gemcitabine IV over 30 minutes once weekly on weeks 1 to 7 for the first course only |
| Outcomes | Quality of life Survival Frequency of symptomatic venous thromboembolic complications Safety as measured by the occurrence of bleeding complications |
| Starting date | October 2002 |
| Contact information | Gary Morrow National Cancer Institute (NCI) |
| Notes | Kishan J. Pandya, MD University of Rochester Status as of August 2017: Terminated (not published yet) |
Rosovsky 2009.
| Study name | A randomized phase II study to evaluate the effect of two different doses of enoxaparin sodium in combination with standard chemotherapy (cisplatin plus etoposide) with respect to time to tumor progression (TTP) in patients with newly diagnosed extensive stage small cell lung cancer (SCLC) without underlying venous thromboembolism |
| Methods | Study type: interventional Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label |
| Participants | Patients with newly diagnosed extensive stage SCLC without underlying venous thromboembolism |
| Interventions | Group A: active comparator; cisplatin and etoposide Group B: experimental; cisplatin and etoposide, plus low‐dose enoxaparin sodium Group C: experimental; cisplatin and etoposide, plus high‐dose enoxaparin sodium |
| Outcomes | Primary outcome measures: to evaluate the prophylactic and treatment doses of enoxaparin sodium given in combination with standard chemotherapy compared to standard chemotherapy alone with respect to time to tumor progression in this patient population (time frame: 2 years) Secondary outcome measures: to determine the effect of 2 different doses of enoxaparin sodium in combination with chemotherapy and chemotherapy alone on biomarkers of angiogenesis and to identify if these markers correlate with overall survival and progression‐free survival (time frame: 2 years) (designated as safety issue: no) To evaluate toxicity and determine the rates of bleeding complications in this patient population (time frame: 2 years) |
| Starting date | July 2008 |
| Contact information | Rachel Rosovsky, MD, MPH |
| Notes | Principal Investigator: Rachel Rosovsky, MD, MPH; Massachusetts General Hospital Sponsors and Collaborators: Massachusetts General Hospital; Dana‐Farber Cancer Institute; Beth Israel Deaconess Medical Center; North Shore Medical Center; Sanofi‐Aventis http://clinicaltrials.gov/ct2/show/NCT00916669 Status as of August 2017: This study has been withdrawn prior to enrolment. |
CNS: central nervous system; CT: computerized tomography; DVT: deep vein thrombosis; ECOG: Eastern Co‐operative Oncology Group; EFS: event‐free survival; IU: international unit; IV: intravenous; LMWH: low molecular weight heparin; OD: once daily; PE: pulmonary embolism; SC: subcutaneous; SCLC: small cell lung cancer; VTE: venous thromboembolism
Differences between protocol and review
This update includes new section relevant to living systematic reviews, which are included in the Methods and also described in Appendix 1.
Contributions of authors
EAA: protocol development, data analysis, manuscript drafting, methodological expertise, review co‐ordination. LAK: searching for trials, screening, data extraction, data analysis, manuscript drafting, review co‐ordination. MH: screening, full‐text retrieval, data extraction, manuscript drafting. CM: screening, full‐text retrieval, data extraction. MB: screening, full‐text retrieval, data extraction. FS: screening, full‐text retrieval, data extraction. IT: screening, full‐text retrieval, data extraction.VY: screening, full‐text retrieval, data extraction. AS: methodological expertise related to the living systematic review approach. HJS: protocol development, data interpretation, methodological expertise.
Sources of support
Internal sources
No sources of support provided
External sources
-
NIHR Cochrane Review Incentive Scheme 2016. Award reference Number 16/72/24, UK
This project was supported by the National Institute for Health Research, via Cochrane Review Incentive Scheme Funding. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Cochrane Gamechanger Grant, UK
Anneliese Synnot's position is supported by funding through Cochrane's Gamechanger grant
-
National Health and Medical Research Council (Partnership Project grant APP1114605), Australia
Anneliese Synnot's position is supported by funding through Australia's National Health and Medical Research Council.
-
American Society of Hematology, USA
This project was supported by the American Society of Hematology
Declarations of interest
HJS: panel member of the ASH VTE in cancer patients, Vice‐Chair of the ASH VTE guidelines and played various leadership roles from 1999 until 2014 with ACCP VTE guidelines. EAA served on the executive committee the ACCP Antithrombotic Therapy Guidelines published in 2016. All other co‐authors declare no conflicts of interests.
Edited (no change to conclusions)
References
References to studies included in this review
Agnelli 2009 (PROTECHT) {published data only}
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L et al, PROTECHT Investigators. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncology 2009;10(10):943-9. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Gussoni G, Bianchini C, Verso M, Tonato M. A randomized double-blind placebo-controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: The PROTECHT Study. Blood 2008;112:6. [Google Scholar]
- Agnelli G, Tonato M. Nadroparin for prevention of thromboembolic events in cancer patients receiving chemotherapy. a randomized placebo-controlled double-blind study. Support Care Cancer 2009;17:857-1039. [Google Scholar]
- Barni S, Labianca R, Agnelli G, Bonizzoni E, Mandalà M, Verso M, et al. Thromboembolic risk related to type of chemotherapy and efficacy of nadroparin in cancer outpatients with metastatic or locally advanced cancer. Support Care Cancer 2011;19(Suppl 2):S206, 415. [Google Scholar]
- Barni S, Labianca R, Agnelli G, Bonizzoni E, Verso M, Mandalà M, et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: results of a retrospective analysis of the PROTECHT study. Journal of Translational Medicine 2011;9(179):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agnelli 2012 (SAVE‐ONCO) {published data only}
- Agnelli G, George D, Fisher WD, Kakkar AK, Lassen MR, Mismetti P, et al. Ultra-low-molecular-weight heparin (ULMWH) semuloparin for prevention of venous thromboembolism (VTE) in cancer patients receiving chemotherapy: consistent beneficial effect across cancer stage and location subgroups. European Journal of Cancer 2011;47:S222. [Google Scholar]
- Agnelli G, George DJ, Fisher W, Kakkar AK, Lassen MR, Mismetti P, et al. The ultra-low molecular weight heparin (ULMWH) semuloparin for prevention of venous thromboembolism (VTE) in patients with cancer receiving chemotherapy: SAVE ONCO study. ASCO Annual Meeting. Journal of Clinical Oncology 2011;29:LBA9014. [Google Scholar]
- Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. New England Journal of Medicine 2012;366(7):601-9. [DOI] [PubMed] [Google Scholar]
- George D, Agnelli G, Fisher W, Kakkar A, Lassen MR, Mismetti P, et al. Venous thromboembolism (VTE) prevention with semuloparin in cancer patients initiating chemotherapy: benefit-risk assessment by VTE risk in SAVE-ONCO. 53rd Annual Meeting of the American Society of Hematology. Blood 2011;118(21):96. [Google Scholar]
Altinbas 2004 {published data only}
- Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, et al. Prospective randomized study of epirubicine cyclophosphamide and vincristine combination chemotherapy (CEV): low molecular weight heparin (LMWH) in small cell lung cancer (SCLC). In: Proceedings of the American Society of Clinical Oncology. Vol. 20. 2001:1280.
- Altinbas M, Coskun HS, Ozkan M, Eser B, Unal A, Cetin M, et al. A randomized clinical trial of combination chemotherapy with and without low molecular weight heparin in small cell lung cancer. Journal of Thrombosis and Haemostasis 2004;2:1266-71. [DOI] [PubMed] [Google Scholar]
- Altinbas M, Ozkan M, Coskun HS, Er O, Eser B, Unal A, et al. Efficiency of cyclophosphamide, epirubicin, vincristine (CEV) +/- low molecular weight heparin (LMWH) in small cell lung cancer (SCLC): preliminary results. Annals of Oncology 2000;11:117. [Google Scholar]
Haas 2012 (TOPIC 1) {published data only}
- Freund M, Kakkar AK, Haas S, Heilmann L, Tempelhoff GF, Brom J, et al. A randomized trial of the low molecular weight heparin certoparin against placebo in the long-term prevention of venous thromboembolism in patients with metastatic breast cancer. Blood 2003;102(11):210A. [Google Scholar]
- Gatzemeier U, Freund M, Haas S, Kakkar A, Zatloukai P, Kelbel C, et al. Prevention of thromboembolic complications with the low-molecular-weight heparin certoparin in non-small-cell lung carcinoma (TOPIC-2). Lung Cancer 2005;49:S56. [Google Scholar]
- Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, Tempelhoff GF, et al. Low- molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer-TOPIC 1. Clinical and Applied Thrombosis/Hemostasis 2012;18(2):159-65. [DOI] [PubMed] [Google Scholar]
- Haas SK, Kakkar AK, Kemkes-Matthes B, Freund M, Gatzemeier U, Heilmann L, et al. Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer - results of the TOPIC studies. Journal of Thrombosis & Haemostasis 2005;3(1):OR059. [Google Scholar]
Haas 2012 (TOPIC 2) {published data only}
- Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, Tempelhoff GF, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer- TOPIC 2. Clinical and Applied Thrombosis/Hemostasis 2012;18(2):159-65. [DOI] [PubMed] [Google Scholar]
Kakkar 2004 (FAMOUS) {published data only}
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). Journal of Clinical Oncology 2004;22(10):1944-8. [DOI] [PubMed] [Google Scholar]
Khorana 2017 (PHACS) {published data only}
- Khorana AA, Francis CW, Kuderer N, Carrier M, Ortel TL, Wu, T, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thrombosis Research, http://dx.doi.org/10.1016/j.thromres.2017.01.009 2017. [DOI: ] [DOI] [PubMed]
- Khorana AA, Francis CW, Kuderer N, Carrier M, Ortel TL, Wun T, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Blood 2015;126(23):427. [DOI] [PubMed] [Google Scholar]
Klerk 2005 (MALT) {published data only}
- Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. Journal of Clinical Oncology 2005;23(10):2130-5. [DOI] [PubMed] [Google Scholar]
- Klerk CPW, Smorenburg S, Otten HM, Richel D, Tienhoven G, Lensing A, et al. Low-molecular-weight heparin and the survival of patients with advanced malignancy [abstract]. Journal of Clinical Oncology : ASCO annual meeting proceedings 2004;22:729. [Google Scholar]
- Klerk CPW, Smorenburg SM, Otten JMMB, Buller HR. Malignancy and low-molecular weight-heparin therapy: the MALT trial. Abstracts from XIX International ISTH Congress. Journal of Thrombosis & Haemostasis 2003;1(Suppl 1):OC195. [Google Scholar]
- Klerk CPW, Smorenburg SM, Otten JMMB, Buller HR. Malignancy and low molecular weight-heparin therapy: the MALT trial. Pathophysiology of Haemostasis and Thrombosis 2003;33(Suppl 1):75. [Google Scholar]
Lebeau 1994 {published data only}
- Lebeau B, Chastang C, Brechot JM, Capron F, Dautzenberg B, Delaisements C, et al. Subcutaneous heparin treatment increases survival in small cell lung cancer. "Petites Cellules" Group. Cancer 1994;74(1):38-45. [DOI] [PubMed] [Google Scholar]
Lecumberri 2013 (ABEL) {published data only}
- Lecumberri R, López Vivanco G, Font A, González Billalabeitia E, Gúrpide A, Gómez Codina J, et al. Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thrombosis Research 2013;132(6):666-70. [DOI] [PubMed] [Google Scholar]
- Lecumberri R, Massuti B, López Vivanco G, Font A, González Billalabeitia E, Rocha E, on behalf of the ABEL Investigators. Adjuvant bemiparin in small cell lung cancer: results from the ABEL study. In: 5th ICTHIC Abstracts: Oral Communications/Thrombosis Research. Vol. 125. 2010:S161–5.
- Massuti B, Lecumberri R, Lopez Vivanco GM, Font A, Gonzalez-Billalabeitia E, Marti-Ciriquian JL, et al. ABEL trial: A phase II randomized trial adding bemiparin (B) to chemo-radiotherapy (CT-RT) in limited-stage small cell lung cancer (SCLC)--Final results. ASCO Annual Meeting Proceedings 2012;30(15):7095. [Google Scholar]
- Massuti B, Lopez-Vivanco G, Font A, Lecumberri R, Gonzalez Billalabeitia E, Marti, et al. Multicenter randomized phase ii trial of adding Bemiparin to chemo-radio therapy in small cell Lung cancer with limited disease. Annals of Oncology 2010;21:159. [Google Scholar]
Macbeth 2016 (FRAGMATIC) {published data only}
- Griffiths GO, Burns S, Noble SI, Macbeth FR, Cohen D, Maughan TS. FRAGMATIC: A randomised phase III clinical trial investigating the effect of fragmin® added to standard therapy in patients with lung cancer. BMC cancer 2009;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth F, Noble S, Evans J, Ahmed S, Cohen D, Hood K, et al. Randomized phase III trial of standard therapy plus low molecular weight heparin in patients with lung cancer: FRAGMATIC trial. Journal of Clinical Oncology 2016;34(5):488-94. [DOI] [PubMed] [Google Scholar]
- Macbeth F, Noble S, Griffiths G, Chowdhury R, Rolfe C, Hood K, et al. Preliminary results from the fragmatic trial: a randomised phase iii clinical trial investigating the effect of fragmin (r) added to standard therapy in patients with lung cancer. Journal Of Thoracic Oncology 2013;8:S243. [Google Scholar]
- Noble S, Robbins A, Alikhan R, Hood K, Macbeth F. Prediction of venous thromboembolism in lung cancer patients receiving chemotherapy. Journal of Thrombosis and Haemostasis 2015;13:143. [Google Scholar]
Maraveyas 2012 (FRAGEM) {published data only}
- Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. European Journal of Cancer 2012;48(9):1283-92. [DOI] [PubMed] [Google Scholar]
- Maraveyas A, Waters J, Roy R, Propper D, Fyfe D, Lofts F, et al. OC-02 Gemcitabine with or without prophylactic weight-adjusted dalteparin (WAD) in patients with advanced or metastatic pancreatic cancer (APC): a multicentre, randomised phase IIB trial (the UK FRAGEM study). Thrombosis Research 2010;125:S161. [Google Scholar]
- Maraveyas AJ, Waters R, Roy D, Propper D, Fyfe F, Lofts E, et al. Gemcitabine with or without prophylactic weight-adjusted dalteparin in patients with advanced or metastatic pancreatic cancer (APC): a multicentre, randomised phase IIB trial (the UK FRAGEM study). European Journal of Cancer 2009;Supplements 7(2):362. [Google Scholar]
Pelzer 2015 (CONKO‐004) {published data only}02140505
- Pelzer U, Deutschinoff G, Opitz B, Stauch M, Reitzig P, Hahnfeld S, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy - first results of the CONKO 004 trial. In: Onkologie - DGHO meeting. Vol. 580. October 2009:Abstract.
- Pelzer U, Hilbig A, Stieler J, Roll L, Riess H, Dorken B, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy (PROSPECT - CONKO 004). Onkologie 2005;28(Suppl 3):54 (Abstract 151). [Google Scholar]
- Pelzer U, Hilbig A, Stieler J, Roll L, Stauch M, Opitz B, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy (PROSPECT-CONKO 004). ASCO Annual Meeting Proceedings 2006;24(18):4110. [Google Scholar]
- Pelzer U, Hilbig A, Stieler JM, Bahra M, Sinn M, Gebauer B, et al. Intensified chemotherapy and simultaneous treatment with heparin in outpatients with pancreatic cancer - the CONKO 004 pilot trial. BMC Cancer 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer U, Oettle H, Stauch M, Opitz B, Stieler J, Scholten T, Riess T. Prospective, randomized open trial of enoxaparin in patients with advanced pancreatic cancer undergoing first-line chemotherapy. In: XXIst Congress of the International Society on Thrombosis and Haemostasis 2007 July 6-12; Geneva. 2007:P-T-488.
- Pelzer U, Opitz B, Deutschinoff G, Stauch M, Reitzig PC, Hahnfeld S, et al. Efficacy of prophylactic low–molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. Journal of Clinical Oncology 2015;33(18):2028-34. [DOI] [PubMed] [Google Scholar]
- Riess H, Pelzer U, Deutschinoff G, Opitz B, Stauch M, Reitzig P, et al. A prospective, randomized trial of chemotherapy with or without the low molecular weight heparin (LMWH) enoxaparin in patients (pts) with advanced pancreatic cancer (APC): Results of the CONKO 004 trial. ASCO Annual Meeting Proceedings 2009;27(18S):LBA4506. [Google Scholar]
- Riess H, Pelzer U, Hilbig A, Stieler J, Opitz B, Scholten T, et al. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy). BMC Cancer 2008;8:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess H, Pelzer U, Opitz B, Stauch M, Reitzig P, Hahnfeld S, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy: Final results of the CONKO-004 trial. Journal of Clinical Oncology Conference 2010;28(15 suppl):4033. [Google Scholar]
- Riess HB, Pelzer U, Opitz B, Hilbig A, Strauch M, Hahnfeld S, et al. Late breaking clinical trial: a prospective, randomized trial of chemotherapy with and without the low molecular weight heparin (LMWH) enoxaparin in advanced pancreatic cancer patients. International Society on Thrombosis and Haemostasis 2009;7(Suppl. 2):1–1204. [Google Scholar]
Perry 2010 (PRODIGE) {published data only}
- Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low molecular weight heparin (LMWH) thromboprophylaxis in patients with newly diagnosed malignant glioma. Journal of Thrombosis and Hemostasis 2010;8(9):1959–65. [DOI] [PubMed] [Google Scholar]
- Perry JR, Rogers L, Laperriere N, Julian J, Geerts W, Agnelli G, et al, for the Ontario Clinical Oncology Group and PRODIGE Investigators. PRODIGE: a phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma. Journal of Clinical Oncology 2007;25:77s (Abstract 2011). [Google Scholar]
Sideras 2006 {published data only}
- Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Dakhil SR, et al. Phase III clinical trial evaluating low-molecular weight heparin (LMWH) in patients with advanced cancer: a North Central Cancer Treatment Group study. Journal of Clinical Oncology 2005;23(16):775S. [Google Scholar]
- Sideras K, Schaefer PL, Okuno SH, Sloan JA, Kutteh L, Fitch TR, et al. Low-molecular-weight-heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clinic Proceedings 2006;81(6):758-67. [DOI] [PubMed] [Google Scholar]
Vadhan‐Raj 2013 {published data only}
- Vadhan-Raj S, Zhou X, Varadhachary GR, Milind J, Fogelman D, Shroff R, et al. Randomized controlled trial of dalteparin for primary thromboprophylaxis for venous thromboembolism (VTE) in patients with advanced pancreatic cancer (APC): Risk factors predictive of VTE. Blood 2013;122(21):580. [Google Scholar]
van Doormaal 2011 (INPACT) {published data only}
- Buller HR, Prins MH. Late breaking clinical trial: the effect of the low molecular-weight heparin nadroparin on the survival in patients with cancer: a randomized trial (for the inpact investigators). Journal of Thrombosis and Hemostasis 2009;7:1203. [Google Scholar]
- Doormaal FF, Di Nisio M, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. Journal of Clinical Oncology 2011;29:2071-6. [DOI] [PubMed] [Google Scholar]
Weber 2008 {published data only}
- Weber C, Merminod T, Herrmann FR, Zulian GB. Prophylactic anti-coagulation in cancer palliative care: a prospective randomised study. Support Care Cancer 2008;16(7):847-52. [DOI] [PubMed] [Google Scholar]
Zwicker 2013 (MICRO TEC) {published data only}
- Zwicker, J, H A Liebman, K A Bauer, T Caughey, R Rosovsky, S Mantha, C M Kessler et al. A randomized-controlled phase II trial of primary thromboprophylaxis with enoxaparin in cancer patients with elevated tissue factor bearing microparticles (the microtec study). Journal Of Thrombosis And Haemostasis 2013;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker JI, Liebman HA, Bauer KA, Caughey T, Campigotto F, Rosovsky R, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor‐bearing microparticles: a randomized‐controlled phase II trial (the Microtec study). British Jjournal of Haematology 2013;160(4):530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Agnelli 1998 {published data only}
- Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, D'Angelo A, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. New England Jjournal of Medicine 1998;339(2):80-5. [DOI] [PubMed] [Google Scholar]
Agnelli 2005 {published data only}
- Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high‐risk abdominal surgery. British Journal of Surgery 2005;92(10):1212-20. [DOI] [PubMed] [Google Scholar]
Agnelli 2015 (AMPLIFY) {published data only}
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Pak R et al. Apixaban for the treatment of venous thromboembolism in cancer patients: Data from the amplify trial. Canadian Journal of Cardiology 2014;30:S278. [Google Scholar]
- Agnelli G, Buller HR, Cohen A, Gallus AS, Lee TC, Pak R, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. Journal of Thrombosis and Haemostasis 2015;13(12):2187191. [DOI] [PubMed] [Google Scholar]
Alifano 2005 {published data only}
- Alifano M, Maggiore O, Benedetti G, Trisolini R. Can low-molecular-weight heparin improve the outcome of patients with operable non-small cell lung cancer? An urgent call for research. Chest 2004;126:601-7. [DOI] [PubMed] [Google Scholar]
Alikhan 2003 (MEDENOX) {published data only}
- Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, et al. Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagulation & Fibrinolysis 2003;14(4):341-6. [DOI] [PubMed] [Google Scholar]
- Samama, MM, Cohen, AT, Darmon, JY, Desjardins, L, Eldor, A, Janbon, C, Leizorovicz, A, Nguyen, H, Olsson, CG, Turpie, AG and Weisslinger, N. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients.. New England Journal of Medicine 1999;341(11):pp.793-800. [DOI] [PubMed] [Google Scholar]
Arbit 2005 {published data only}
- Arbit E, Alifano M, Maggiore O, Benedetti G, Trisolini R. Low-molecular-weight heparin and outcomes. Chest 2005;128(1):471-2. [DOI] [PubMed] [Google Scholar]
Auer 2011 {published data only}
- Auer R, Scheer A, Wells PS, Boushey R, Asmis T, Jonker D, et al. The use of extended perioperative low molecular weight heparin (tinzaparin) to improve disease-free survival following surgical resection of colon cancer: a pilot randomized controlled trial.. Blood Coagulation & Fibrinolysis 2011;22(8):760-2. [DOI] [PubMed] [Google Scholar]
Barberi‐Heyob 1995 {published data only}
- Barberi-Heyob M, Merlin JL, Vigneron M, Conroy T. Addition of heparin in 5-fluorouracil solution for portal vein infusion has no influence on its stability under clinically relevant conditions. Anti-Cancer Drugs 1995;6(1):163-4. [DOI] [PubMed] [Google Scholar]
Barkagan 1997 {published data only}
- Barkagan ZS. The results of the use of low molecular weight heparin (LMWH) for prevention and treatment of thrombosis in cancer patients. Thrombosis and Haemostasis 1997;-:772. [Google Scholar]
Bigg 1992 {published data only}
- Bigg SW, Catalona WJ. Prophylactic mini-dose heparin in patients undergoing radical retropubic prostatectomy. A prospective trial. Urology 1992;39(4):309-13. [DOI] [PubMed] [Google Scholar]
Bitsch 1990 {published data only}
- Bitsch M, Hermann GG, Andersen JP, Steven K. Low dose intravesical heparin as prophylaxis against recurrent noninvasive (stage Ta) bladder cancer. Journal of Urology 1990;144(3):635-6. [DOI] [PubMed] [Google Scholar]
Blaszczyk 1970 {published data only}
- Blaszczyk M, Ursyn-Niemcewicz W, Pawlak F. Heparin precipitable fraction (HPF) in malignant tumors of the respiratory tract. Gruzlica i Choroby Pluc 1970;38(4):321-8. [PubMed] [Google Scholar]
Buckman 2005 {published data only}
- Buckman RA, Wong NS, Clemons M, Verma S, Trudeau ME, Roche K, et al. Phase I-II study of DaICM-P [daily dalteparin (Dal), cyclophosphamide (C) and prednisone (P) and bi-weekly methotrexate (M)] as therapy for metastatic breast cancer (MBC). Journal of Clinical Oncology 2005;23(16):52S. [DOI] [PubMed] [Google Scholar]
Cahan 2000 {published data only}
- Cahan, MA, Hanna, DJ, Wiley, LA, Cox, DK and Killewich, LA. External pneumatic compression and fibrinolysis in abdominal surgery. Journal of vascular surgery 2000;32(3):537-543. [DOI] [PubMed] [Google Scholar]
Cavallo 2010 {published data only}
- Cavallo F, Di Raimondo F, Harda I, Lupo B, Romano A, Catalano L et al. A phase III study of enoxaparin vs aspirin as thromboprophylaxis for newly diagnosed myeloma patients treated with lenalidomide-based regimen. In: American Society of Hematology Annual Meeting 1092. 2010.
Chojnowski 2002 {published data only}
- Chojnowski K, Trelinski J, Wawrzyniak E, Robak T. The influence of low molecular weight heparin on the intravascular activation of the coagulation system in patients with acute leukemia during induction chemotherapy - report of prospective randomized study. Leukemia and Lymphoma 2002;43(5):1021-8. [DOI] [PubMed] [Google Scholar]
Cicco 2009 {published data only}
- Cicco MD, Matovic M, Balestreri L, Steffan A, Pacenzia R, Malafronte M et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: a randomized controlled study based on serial venographies. Annals of Oncology 2009;20:1936–42. [DOI] [PubMed] [Google Scholar]
Clarke‐Pearson 1993 {published data only}
- Clarke-Pearson, Daniel L, Ingrid S Synan, Richard Dodge, John T Soper, Andrew Berchuck, and R Edward Coleman. "A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery.". American journal of obstetrics and gynecology 1993;168(4):1146-1154. [DOI] [PubMed] [Google Scholar]
Cohen 1997 {published data only}
- Cohen AT, Wagner MB, Mohamed MS. Risk factors for bleeding in major abdominal surgery using heparin thromboprophylaxis. The American journal of surgery 1997;174(1):1-5. [DOI] [PubMed] [Google Scholar]
- Kakkar VV, Cohen AT, Edmonson RA, Phillips MJ, Das SK, Maher KT, Sanderson RM, Kakkar S, Cooper DJ. Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. The Lancet 1993 Jan 30;341(8840):259-65. [DOI] [PubMed] [Google Scholar]
Cohen 2003 {published data only}
- Cohen, Alexander T, Bruce L Davidson, Alexander S Gallus, Michael R Lassen, Martin H Prins, Witold Tomkowski, Alexander GG Turpie, Jan FM Egberts, and Anthonie WA Lensing. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2003;332(7537):325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2006 {published data only}
- Cohen, Alexander T, Bruce L Davidson, Alexander S Gallus, Michael R Lassen, Martin H Prins, Witold Tomkowski, Alexander GG Turpie, Jan FM Egberts, and Anthonie WA Lensing. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006;332(7537):325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2007 (PREVENT) {published data only}
- Cohen, AT, Davidson, BL, Gallus, AS, Lassen, MR, Prins, MH, Tomkowski, W, Turpie, AG, Egberts, JF and Lensing, AW. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006;332(7537):325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, AT, Turpie, AG, Leizorovicz, A, Olsson, CG, Vaitkus, PT and Goldhaber, SZ. Thromboprophylaxis with dalteparin in medical patients: which patients benefit? Vascular Medicine 2007;12(2):123-127. [DOI] [PubMed] [Google Scholar]
- Leizorovicz, A, Cohen, AT, Turpie, AG, Olsson, CG, Vaitkus, PT, Goldhaber, SZ and PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004;110(7):874-879. [DOI] [PubMed] [Google Scholar]
Couban 2005 {published data only}
- Anderson, D, et al. A randomized double-blind placebo controlled study of low dose warfarin for the prevention of symptomatic central venous catheter-associated thrombosis in patients with cancer.. Journal of thrombosis and haemostasis 2003;JTH 1:Abstract no: P198. [DOI] [PubMed] [Google Scholar]
- Couban, S, Goodyear, M, Burnell, M, Dolan, S, Wasi, P, Barnes, D, MacLeod, D, Burton, E, Andreou, P and Anderson, DR. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter–associated thrombosis in patients with cancer. Journal of Clinical Oncology 2005;23(18):4063-4069. [DOI] [PubMed] [Google Scholar]
- Couban, S, M Goodyear, M Burnell, S Dolan, P Wasi, D Macleod, E Burton, P Andreou, and D Anderson. A randomized double-blind placebo-controlled study of low dose warfarin for the prevention of symptomatic central venous catheter-associated thrombosis in patients with cancer.. Blood, American Society of Hematology 44th Annual Meeting, Abstract No. 2769 2002;100(11):703A. [DOI] [PubMed] [Google Scholar]
Craven 2001 {published data only}
- Craven R. Heparin and cancer revisited. Trends in Pharmacological Sciences 2001;22(5):1. [DOI] [PubMed] [Google Scholar]
Crossno 2009 {published data only}
- Crossno RJ. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Journal of Pain and Palliative Care Pharmacotherapy 2009;23(1):65-6. [Google Scholar]
Demir 2006 {published data only}
- DeMir M, Hoppensteadt DA, Cunanan J, Iqbal O, Fareed J. Increased levels of inflammatory mediators in lung cancer and their modulation by oral anticoagulant treatment. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings 2006;108(11):892. [Google Scholar]
Demir 2007 {published data only}
- Demir M, Ciftci A, Hoppensteadt D, Altiay G, Tobu M, Iqbal O et al. Protein chip array profiling and markers of inflammation and thrombin generation in plasma samples from lung cancer patients and their modulation by chemotherapy with or without warfarin anticoagulation. In: Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2007.
Dickinson 1998 {published data only}
- Dickinson LD, Miller LD, Patel CP, Gupta SK, et al. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery 1998;43(5):1074-81. [DOI] [PubMed] [Google Scholar]
Di Nisio 2005 {published data only}
- Di Nisio M, Buller HR, Porreca E. Do low-molecular-weight heparins improve the survival of cancer patients? Nature Clinical Practice Oncology 2005;2(12):612-3. [DOI] [PubMed] [Google Scholar]
Edlis 1976 {published data only}
- Edlis HE, Goudsmit A, Brindley C, Niemetz J. Trial of heparin and cyclophosphamide (NSC-26271) in the treatment of lung cancer. Cancer Treatment Report 1976;60(5):575-8. [PubMed] [Google Scholar]
Eichinger 2008 {published data only}
- Eichinger S, Traby L, Kaider A, Quehenberger P, Kyrle PA. Prevention of venous thrombosis in cancer patients: a randomized, double-blind study comparing two different dosages of low-molecular weight heparin. In: Journal of Clinical Oncology, ASCO Annual Meeting Proceedings. 2008.
Elias 1972 {published data only}
- Elias EG, Brugarol A. Role of heparin in chemotherapy of solid tumors - preliminary clinical trial in carcinoma of lung. Cancer Chemotherapy Reports Part 1 1972;56(6):783-5. [PubMed] [Google Scholar]
Elias 1973a {published data only}
- Elias EG. Heparin as an adjuvant to chemotherapy In lung carcinoma. In: Proceedings of the American Association for Cancer Research. Vol. 14. 1973:26.
Elias 1973b {published data only}
- Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: "clinical study". Journal of Surgical Oncology 1973;5(2):189-93. [DOI] [PubMed] [Google Scholar]
Elias 1973c {published data only}
- Elias EG. Heparin therapy combined with chemotherapy in metastatic cancer. Cancer Bulletin 1973;25(6):116-9. [Google Scholar]
Elias 1974 {published data only}
- Elias EG. Heparin anticoagulation as adjuvant to chemotherapy in carcinoma of the lung. Journal of Medicine 1974;5(1):114-32. [PubMed] [Google Scholar]
Elias 1975 {published data only}
- Elias EG, Shukla SK, Mink IB. Heparin and chemotherapy in the management of inoperable lung carcinoma. Cancer 1975;36(1):129-36. [DOI] [PubMed] [Google Scholar]
Elit 2012 {published data only}
- Elit LM, Lee AY, Parpia S, Swystun LL, Liaw PC, Hoskins P et al. Dalteparin low molecular weight heparin (LMWH) in ovarian cancer: a phase II randomized study. Thrombosis Research 2012;130:894–900. [DOI] [PubMed] [Google Scholar]
Fielding 1992 {published data only}
- Fielding LP, Hittinger R, Grace RH, Fry JS. Randomized controlled trial of adjuvant chemotherapy by portal-vein perfusion after curative resection for colorectal adenocarcinoma. Lancet 1992;340(8818):502-6. [DOI] [PubMed] [Google Scholar]
Goldhaber 2002 {published data only}
- Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis.. Chest Journal 2002;122(6):1933-7. [DOI] [PubMed] [Google Scholar]
Graf 1994 {published data only}
- Graf B, Graf AH, Traun H, Forstner K, Rettenbacher L, Sailer S, et al. Prophylaxis of thromboembolism in radiotherapy for gynecologic malignancies: low molecular weight (LMW) heparin (fragmin (R)) vs coumarin (sintrom(R)). Haemostasis 1994;24(Suppl 1):315 (Abstract 6). [Google Scholar]
Graf 1996 {published data only}
- Graf AH, Graf B, Traun H, Staudach A. Risk and prevention of thromboembolism complications in gynecologic malignancies. Gynakologisch-Geburtshilfliche Rundschau 1996;36(1):37-9. [DOI] [PubMed] [Google Scholar]
Green 1992 {published data only}
- Green D, Hull RD, Brant R, Pineo GF. Lower mortality in cancer patients treated with low-molecular-weight versus standard heparin. Lancet 1992;339(8807):1476. [DOI] [PubMed] [Google Scholar]
Guimbretiere 1982 {published data only}
- Guimbretiere J, Raffi F, Dabouis G. Heparin therapy in hypercoagulable state of lung cancer patients. Haemostasis 1982;12(1-2):139. [Google Scholar]
Haas 2011 {published data only}
- Bauersachs R, Schellong SM, Haas S, Tebbe U, Gerlach HE, Abletshauser C, et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thrombosis and Haemostasis 2011;105(6):981-8. [DOI] [PubMed] [Google Scholar]
- Haas S, Schellong SM, Tebbe U, Gerlach HE, Bauersachs R, Abletshauser C, et al. CERTIFY: Certoparin versus UFH to prevent venous thromboembolicevents in the patients with cancer. Hämostaseologie 2011;31(1):A10. [Google Scholar]
- Haas S, Schellong SM, Tebbe U, Gerlach HE, Bauersachs R, Melzer N, et alAbletshauser C, et al. Heparin based prophylaxis to prevent venous thromboembolic events and death in patients with cancer-a subgroup analysis of CERTIFY. BMC Cancer 2011;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harenberg 1996 {published data only}
- Harenberg J, Roebruck P, Heene DL. Subcutaneous low-molecular-weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. Pathophysiology of Haemostasis and Thrombosis 1996;26(3):127-39. [DOI] [PubMed] [Google Scholar]
- Harenberg J, Roebruck P, Stehle G, Habscheid W, Biegholdt M, Heene DL. Heparin Study in Internal Medicine (HESIM): design and preliminary results. Thrombosis Research 1992;68(1):33-43. [DOI] [PubMed] [Google Scholar]
Hata 2016 {published data only}
- Hata K, Kimura T, Tsuzuki S, Ishii G, Kido M, Yamamoto T, et al. Safety of fondaparinux for prevention of postoperative venous thromboembolism in urological malignancy: A prospective randomized clinical trial [Hata K, Kimura T, Tsuzuki S, Ishii G, Kido M, Yamamoto T, et al]. International Journal of Urology 2016;23(11):923-8. [DOI] [PubMed] [Google Scholar]
Hoppensteadt 2011 {published data only}
- Hoppensteadt D, Khan H, Thethi I, Demir M, Adiguzel C, Rahman S, et al. Inflammatory and thrombotic mediators in small cell lung carcinoma: potential role in thromboembolic complications. In: American Society of Hematology, Annual Meetings and Exposition 2294. 2011.
Kakkar 2010 (CANBESURE) {published data only}
- Kakkar, VV, Balibrea JL, Martinez‐Gonzalez J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. Journal of Thrombosis and Haemostasis 2010;8(6):1223-9. [DOI] [PubMed] [Google Scholar]
- Kakkar VV, Balibrea J, Martinez-Gonzalez J, Prandoni P. Late breaking clinical trial: a randomised double blind trial to evaluate the efficacy and safety of prolonging the thromboprophylaxis with bemiparin in patients undergoing cancer abdominal or pelvic surgery (theCANBESURE study). International Society on Thrombosis and Haemostasis 2009;7(suppl. 2):1202, LB-MO-002. [Google Scholar]
Kakkar 2014 (SAVE‐ABDO) {published data only}
- Kakkar AK, Agnelli G, Fisher W, George D, Lassen MR, Mismetti P, et al, and SAVE-ABDO Investigators. Preoperative enoxaparin versus postoperative semuloparin thromboprophylaxis in major abdominal surgery: a randomized controlled trial. Annals of Surgery 2014;259(6):1073-9. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Agnelli G, Fisher WD, George D, Mouret P, Lassen MR, et al. The ultra-low-molecular-weight heparin semuloparin for prevention of venous thromboembolism In patients undergoing major abdominal surgery.. Blood 2010;116(21):188. [Google Scholar]
Kohanna 1983 {published data only}
- Kohanna FH, Sweeney J, Hussey S. Effect of perioperative low-dose heparin administration on the course of colon cancer. Surgery 1983;93(3):433-8. [PubMed] [Google Scholar]
Koppenhagen 1992 {published data only}
- Koppenhagen K, Adolf J, Matthes M, Tröster E, Roder JD, Hass S, et al. Low molecular weight heparin and prevention of postoperative thrombosis in abdominal surgery. Thrombosis and Haemostasis 1992;67(6):627-30. [PubMed] [Google Scholar]
Larocca 2012 {published data only}
- Larocca A, Cavallo F, Bringhen S, Raimondo FD, Falanga A, Evangelista A, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119:933-9. [DOI] [PubMed] [Google Scholar]
Lecumberri 2005 {published data only}
- Lecumberri R, Paramo JA, Rocha E. Anticoagulant treatment and survival in cancer patients. The evidence from clinical studies. Haematologica 2005;90(9):1258-66. [PubMed] [Google Scholar]
Lee 2015 (CATCH) {published data only}
- Bauersachs R, Lee AYY, Kamphuisen PW, Meyer G, Janas MS, Jarner MF, et al. Long-term tinzaparin versus warfarin for treatment of venous thromboembolism (VTE) in cancer patients-analysis of renal impairment (RI) in the catch study. Journal of Thrombosis and Haemostasis 2015;13:76. [Google Scholar]
- Bauersachs R. Catch-a randomised clinical trial comparing long-term tinzaparin versus warfarin for treatment of acute venous thromboembolism in cancer patients.. Hematology Reports 2011;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuisen PW, Lee AYY, Meyer G, Bauersachs R, Janas MS, Jarner MF, et al. Characteristics and risk factors of major and clinically relevant non-major bleeding in cancer patients receiving anticoagulant treatment for acute venous thromboembolism-the CATCH study. Journal of Thrombosis and Haemostasis 2015;13:182-3. [Google Scholar]
- Khorana AA, Bauersachs R, Kamphuisen PW, Meyer G, Janas MS, Jarner MF, et al. Clinical predictors of recurrent venous thromboembolism (VTE) in cancer patients from a randomized trial of long-term tinzaparin versus warfarin for treatment: The CATCH study. Journal of Clinical Oncology Conference 2015;33(15 suppl 1):9621. [Google Scholar]
- Lee AY, Bauersachs R, Janas MS, Jarner MF, Kamphuisen PW, Meyer G, et al. CATCH: a randomised clinical trial comparing long-term tinzaparin versus warfarin for treatment of acute venous thromboembolism in cancer patients. BMC Cancer 2013;13:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Bauersachs R, Janas MS, Jarner MF, Kamphuisen PW, Meyer G, et al. CATCH: a randomised clinical trial comparing long-term tinzaparin versus warfarin for treatment of acute venous thromboembolism in cancer patients. BMC Cancer 2013;13(1):284. [NCT01130025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Bauersachs R, Janas MS, Jarner MF, Kamphuisen PW, Meyer G, et al. CATCH: a randomized trial comparing tinzaparin versus warfarin for treatment of acute venous thromboembolism (VTE) in cancer patients. ASCO Annual Meeting. Journal of Clinical Oncology 2012;Suppl:TPS9149. [Google Scholar]
- Lee AY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA: Journal of the American Medical Association 2015;314:677. [DOI] [PubMed] [Google Scholar]
- Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, et al. A randomized trial of long-term tinzaparin, a Low Molecular Weight Heparin (LMWH), versus warfarin for treatment of acute venous thromboembolism (VTE) in cancer patients-the CATCH study. Blood Conference: 56th Annual Meeting of the American Society of Hematology 2014;124:21. [Google Scholar]
Lemoine 2005 {published data only}
- Lemoine NR. Antithrombotic therapy in cancer. Journal of Clinical Oncology 2005;23(10):2119-20. [DOI] [PubMed] [Google Scholar]
Levine 1994 {published data only}
- Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, et al. Double-blind randomised trial of very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 1994;343(8902):886-9. [DOI] [PubMed] [Google Scholar]
Levine 2005 {published data only}
- Levine MN, Lee AYY, Kakkar AK. Low-molecular-weight heparin versus oral anticoagulant therapy for the long-term treatment of symptomatic venous thromboembolism: is there any difference in cancer-related mortality? Reply. Journal of Clinical Oncology 2005;23(28):7250. [DOI] [PubMed] [Google Scholar]
Levine 2012 {published data only}
- Levine M, Gu C, Liebman HA, Escalante P, Solymoss S, Deitchman D, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. Journal of Thrombosis and Haemostasis 2012;10:807-14. [DOI] [PubMed] [Google Scholar]
Liebman 2009 {published data only}
- Liebman H, Levine MN, Deitchman D, Julian J, Escalante CP, O'Brien MC, et al. Apixaban in patients with metastatic cancer: a randomized phase II feasibility study. In: International Society on Thrombosis and Haemostasis. Vol. 7 (Suppl 2). 2009:792.
Loprinzi 1999 {published data only}
- Loprinzi C, Kugler J, Sloan J, Rooke T, Quella S, Novotny P, et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. New England Journal of Medicine 1999;340:346-50. [DOI] [PubMed] [Google Scholar]
Loynes 2002 {published data only}
- Loynes JT, Zacharski LR, Rigas JR. Regression of metastatic non-small cell lung cancer with low molecular weight heparin. Thrombosis and Haemostasis 2002;88(4):686. [PubMed] [Google Scholar]
Lykke 2003 {published data only}
- Lykke J, Rasmussen HM, Nielsen HJ. Heparin as adjuvant in the treatment of colorectal cancer? Ugeskrift for Laeger 2003;165(18):1866-7. [PubMed] [Google Scholar]
Mammen 2004 {published data only}
- Mammen EF. Expanded role of low-molecular-weight heparins in hematologic and oncologic indications. Seminars in Thrombosis and Hemostasis 2004;30(Suppl 1):1-2. [DOI] [PubMed] [Google Scholar]
Maraveyas 2010 {published data only}
- Maraveyas A, Ettelaie C, Echrish H, Li C, Gardiner E, Greenman H, et al. Weight-adjusted dalteparin for prevention of vascular thromboembolism in advanced pancreatic cancer patients decreases serum tissue factor and serum-mediated induction of cancer cell invasion. Blood Coagulation Fibrinolysis 2010;21:452-8. [DOI] [PubMed] [Google Scholar]
Maxwell 2001 {published data only}
- Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DL. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. American College of Obstetricians and Gynecologists 2001;98(6):989-95. [DOI] [PubMed] [Google Scholar]
Mazilu 2014 (OVIDIUS) {published data only}
- Mazilu L, Parepa IR, Suceveanu AI, Suceveanu A, Baz R, Catrinoiu D. Venous thromboembolism: secondary prevention with dabigatran vs. acenocumarol in patients with paraneoplastic deep vein thrombosis. Results from a small prospective study in Romania. Cardiovascular Research 2014;103(suppl 1):S39, P221. [Google Scholar]
Meyer 2007 {published data only}
- Meyer G. Does low-molecular-weight heparin influence cancer-related mortality. Annals of Oncology 2007;18(3):609-10. [DOI] [PubMed] [Google Scholar]
Mousa 2001 {published data only}
- Mousa SA, Mohammed S. Anti-angiogenesis mechanisms and efficacy of the low molecular weight heparin, tinzaparin: anti-cancer efficacy beyond its anticoagulants. Blood 2001;98(11):181B. [Google Scholar]
Munstedt 1996 {published data only}
- Munstedt K, Kemkes-Matthes B, Matthes KJ, Vahrson H. The behavior of the activation parameters of plasma coagulation under HDR-afterloading therapy in patients with endometrial carcinoma (German). Strahlentherapie und Onkologie 1996;172(1):39-42. [PubMed] [Google Scholar]
Murakami 2002 {published data only}
- Murakami M, Wiley LA, Cindrick-Pounds L, Hunter GC, Uchida T, Killewich LA. External pneumatic compression does not increase urokinase plasminogen activator after abdominal surgery. Journal of Vascular Surgery 2002;36(5):917-921. [DOI] [PubMed] [Google Scholar]
Nagata 2015 {published data only}
- Nagata C, Tanabe H, Takakura S, Narui C, Saito M, Yanaihara N, et al. Randomized controlled trial of enoxaparin versus intermittent pneumatic compression for venous thromboembolism prevention in Japanese surgical patients with gynecologic malignancy. Journal of Obstetrics and Gynaecology Research. 2015;41(9):1440-8. [DOI] [PubMed] [Google Scholar]
Nash 2000 {published data only}
- Nash G. Heparin for colorectal cancer. Journal of the Royal Society of Medicine 2000;93(10):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishioka 2007 {published data only}
- Nishioka J, Goodin S. Low-molecular-weight heparin in cancer-associated thrombosis: treatment, secondary prevention, and survival. Journal of Oncology Pharmacy Practice 2007;13(2):85-97. [DOI] [PubMed] [Google Scholar]
Nitti 1997 {published data only}
- Nitti D, Wils J, Sahmoud T, Curran D, Couvreur ML, Lise M, et al. Final results of a phase III clinical trial on adjuvant intraportal infusion with heparin and 5-fluorouracil (5-FU) in resectable colon cancer (EORTC GITCCG 1983-1987). European Organization for Research and Treatment of Cancer 1997;33(8):1209-15. [DOI] [PubMed] [Google Scholar]
Nurmohamed 1996 {published data only}
- Nurmohamed MT, Riel AM, Henkens CM, Koopman MM, Que GT, d'Azemar P, et al. Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thrombosis Haemostasis 1996;75:233–8. [PubMed] [Google Scholar]
Palumbo 2011 {published data only}
- Cavo M, Palumbo A, Bringhen S, Di Raimondo F, Patriarca F, Rossi D, et al. Phase III Study of enoxaparin versus aspirin versus low-dose warfarin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated upfront with thalidomide-containing regimens. Haematologica 2010;95:391. [Google Scholar]
- Cavo M, Palumbo A, Bringhen S, Falcone A, Musto P, Ciceri F, et al. A phase III study of enoxaparin versus low-dose warfarin versus aspirin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide-containing regimens.. Blood 2008;112(11):3017. [Google Scholar]
- Cavo M, Palumbo A, Bringhen S, Falcone A, Musto P, Ciceri F, et al. A phase III study of enoxaparin versus low-dose warfarin versus aspirin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide-containing regimens. Haematologica 2009;94:s4. [Google Scholar]
- Magarotto V, Brioli A, Patriarca F, Rossi D, Petrucci MT, Nozzoli C, et al. Enoxaparin, aspirin, or warfarin for the thromboprophylaxis in newly diagnosed myeloma patients receiving thalidomide: a randomized controlled trial.. XI Congress Of The Italian Society Of Experimental Hematology 2010;95:S1-S162. [Google Scholar]
- Palumbo A, Cavo M, Bringhen S, Zaccaria A, Spadano A, Palmieri S, et al. Enoxaparin versus aspirin versus low-fixed-dose of warfarin in newly diagnosed myeloma patients treated with thalidomide-containing regimens: a randomized, controlled trial [Abstract No. 0910]. Haematologica 2008;93:362. [Google Scholar]
- Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial.. Journal of Clinical Oncology 2011;29:986-993. [DOI] [PubMed] [Google Scholar]
Prins 2014 (EINSTEIN) {published data only}
- Prins MH, Lensing AWA, Brighton TA, Lyons RM, Rehm J, Trajanovic M, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematology 2014;1(1):e37-e46. [DOI] [PubMed] [Google Scholar]
Raskob 2016 (HOKUSAI) {published data only}
- Raskob GE, Es N, Segers A, Angchaisuksiri P, Oh D, Boda Z, et al. Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematology 2016;3(8):e379-e387. [DOI] [PubMed] [Google Scholar]
Retik 1962 {published data only}
- Retik AB, Arons MS, Ketcham AS, Mantel N. The effect of heparin on primary tumors and metastases. Journal of Surgical Research 1962;2:49-53. [Google Scholar]
Rohwedder 1977 {published data only}
- Rohwedder JJ, Sagastume E. Heparin and polychemotherapy for treatment of lung cancer. Cancer Treatment Report 1977;61(7):1399-401. [PubMed] [Google Scholar]
Sakon 2010 {published data only}
- Sakon M, Kobayashi T, Shimazui T. Efficacy and safety of enoxaparin in Japanese patients undergoing curative abdominal or pelvic cancer surgery: results from a multicenter, randomized, open-label study. Thrombosis Research 2010;125(3):e65-70. [DOI] [PubMed] [Google Scholar]
Schulman 2003 {published data only}
- Schulman S, Wahlander K, Lundstrom T, Clason SB, Eriksson H, Investigators TI, et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. New England Journal of Medicine 2003;349(18):1713-21. [DOI] [PubMed] [Google Scholar]
Schulman 2013 (RE‐MEDY) {published data only}
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. New England Journal of Medicine 2013;368(8):709-18. [DOI] [PubMed] [Google Scholar]
Schulman 2015 (RECOVER) {published data only}
- Schulman S, Goldhaber SZ, Kearon C, Kakkar AK, Schellong S, Eriksson H, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thrombosis and Haemostasis 2015;114(1):150-7. [DOI] [PubMed] [Google Scholar]
Siragusa 1999 {published data only}
- Siragusa S. Low molecular weight heparins could be important in cancer. BMJ 1999;319(7213):851. [PMC free article] [PubMed] [Google Scholar]
Song 2014 {published data only}
- Song KY, Yoo HM, Kim EY, Kim JI, Yim HW, Jeon HM, et al. Optimal prophylactic method of venous thromboembolism for gastrectomy in Korean patients: an interim analysis of prospective randomized trial.. Annals of Surgical Oncology 2014;21(13):4232-8. [DOI] [PubMed] [Google Scholar]
Spigel 2005 {published data only}
- Spigel DR. Low-molecular-weight heparin improves survival in patients with cancer. Journal of Clinical Outcomes Management 2005;12(5):241-2. [Google Scholar]
Stanford 1979 {published data only}
- Stanford CF. Anticoagulants in the treatment of small cell carcinoma of the bronchus. Thorax 1979;34:113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tethi 2011 {published data only}
- Thethi I, Hoppensteadt D, Khan H, Demir M, Adiguzel C, Litinas E, et al. Procoagulant and inflammatory mediators in small cell lung carcinoma: Potential role in thromboembolic complications. Journal of Clinical Oncology 2011;29(Suppl):2553. [Google Scholar]
Traby 2010 {published data only}
- Traby L, Kaider A, Schmid R, Kranz A, Quehenberger P, Kyrle P, et al. The effects of low-molecular-weight heparin at two different dosages on thrombin generation in cancer patients. A randomised controlled trial. Thrombosis and Haemostasis 2010;104:92-9. [DOI] [PubMed] [Google Scholar]
Vedovati 2014 {published data only}
- Becattini C, Rondelli, F, Vedovati MC, Camporese G, Giustozzi M, Boncompagni M, et al. Incidence and risk factors for venous thromboembolism after laparoscopic surgery for colorectal cancer.. Haematologica 2014;99:doi:10.3324/haematol.2014.109843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becattini C, Rondelli F, Vedovati MC, Camporese G, Giustozzi M, Boncompagni M, et al. Incidence and risk factors for venous thromboembolism after laparoscopic surgery for colorectal cancer. Haematologica 2015;100(1):e35-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becattini C, Vedovati MC, Rondelli F, Boncompagni M, Camporese G, Balzarotti R, et al. One week vs. four week heparin prophylaxis after laparoscopic surgery for colorectal cancer. The pro-laps pilot feasibility study. Journal of Thrombosis and Haemostasis 2013;11:11. [Google Scholar]
- Vedovati MC, Becattini C, Rondelli F, Boncompagni M, Camporese G, Balzarotti R, et al. A randomized study on 1 vs. 4 weeks prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer.. Journal of Thrombosis and Haemostasis 2013;11:214. [Google Scholar]
- Vedovati MC, Becattini C, Rondelli F, Boncompagni M, Camporese G, Balzarotti R, et al. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Annals of Surgery 2014;259(4):665-9. [DOI] [PubMed] [Google Scholar]
Verso 2008 {published data only}
- Agnelli G, Verso M, Bertoglio S, Ageno W, Bazzan M, Parise P, et al. A double-blind placebo-controlled randomized study on the efficacy and safety of enoxaparin for the prevention of upper limb deep vein thrombosis in cancer patients with central vein catheter. In: Journal of Clinical Oncology. Vol. 22. 2004:734S.
- Verso M, Agnelli G, Bertoglio S, Di Somma C, Paoletti F, Ageno W, et al. A double-blind placebo-controlled randomized study on the efficacy and safety of enoxaparin for the prevention of upper limb deep vein thrombosis in cancer patients with central vein catheter. 2003 Journal of Thrombosis and Haemostasis;1(Suppl 1 Jul):Abstract: no P0825. [Google Scholar]
- Verso M, Agnelli G, Bertoglio S, Di Somma FC, Paoletti F, Ageno W, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. Journal of Clinical Oncology 2005;23(18):4057-62. [DOI] [PubMed] [Google Scholar]
- Verso M, Agnelli G, Kamphuisen PW, Ageno W, Bazzan M, Lazzaro A, et al. Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Internal and Emergency Medicine 2008;3(2):117-22. [DOI] [PubMed] [Google Scholar]
Von Hugo 1981 {published data only}
- Hugo R, Hafter R, Hiller KF, Lochmuller H, Selbmann HK, Graeff H. Prevention of deep vein thrombosis in patients with gynaecologic cancer undergoing radiotherapy. A comparison of calcium-heparin and semi-synthetic heparin analogue [Thromboembolische Komplikationen waehrend der Strahlenbehandlung gynaekologischer karzinome]. Geburtshilfe und Frauenheilkunde 1981;41(3):179-83. [DOI] [PubMed] [Google Scholar]
Ward 1998 {published data only}
- Ward B, Pradhan S. Comparison of low molecular weight heparin (Fragmin) with sodium heparin for prophylaxis against postoperative thrombosis in women undergoing major gynaecological surgery. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38(1):91-2. [DOI] [PubMed] [Google Scholar]
Wester 1996 {published data only}
- Valk HW, Banga JD, Wester JW, Brouwer CB, Hessen MW, Meuwissen OJ, et al. Comparing subcutaneous danaparoid with intravenous unfractionated heparin for the treatmentof venous thromboembolism. A randomized controlled trial. Annals of Internal Medicine 1995;123(1):1-9. [DOI] [PubMed] [Google Scholar]
- Wester JPJ, Valk H, Nieuwenhuis HK, Banga JD. Risk factors for bleeding during treatment of acute venous thromboembolism. Thrombosis and Haemostasis 1996;76(5):682-8. [PubMed] [Google Scholar]
Wojtukiewicz 2003 {published data only}
- Wojtukiewicz MZ, Kozlowski L, Ostrowska K, Dmitruk A, Zacharski LR. Low molecular weight heparin treatment for malignant melanoma: a pilot clinical trial. Thrombosis and Haemostasis 2003;89(2):405-7. [PubMed] [Google Scholar]
Zacharski 2003 {published data only}
- Zacharski LR. Heparin treatment of malignancy: the case for clinical trials in colon cancer. Thrombosis Research 2003;110(4):213-4. [DOI] [PubMed] [Google Scholar]
Zheng 2014 {published data only}
- Zheng H, Gao Y, Yan X, Gao M, Gao W. Prophylactic use of low molecular weight heparin in combination with graduated compression stockings in post-operative patients with gynecologic cancer. Zhonghua zhong liu za zhi [Chinese Journal of Oncology] 2014;36(1):39-42. [PubMed] [Google Scholar]
References to ongoing studies
Borad 2011 (PGPC1) {published data only}
- A randomized phase II open-label study to assess the efficacy & safety of gemcitabine + Abraxane® with or without ODSH (2-0, 3-0 desulfated heparin) as first line treatment of metastatic pancreatic cancer. Ongoing study. November 2011. Contact author for more information.
Chibauldel 2008 (PAM07) {published data only}
- Chemotherapy with or without preventive anticoagulation for metastatic cancer of the pancreas. Ongoing study. October 2007. Contact author for more information.
Germonpre 2008 (SYRINGES) {published data only}
- Low molecular weight heparin in advanced non small cell lung cancer (NSCLC): a randomized open label phase III study evaluating the effect of enoxaparin (Clexane) on survival and symptom control in patients with stage IIIB and IV NSCLC undergoing a cisplatin based first line chemotherapy: the SYRINGES Trial. Ongoing study. June 2008. Contact author for more information.
Kakkar 2010 (GASTRANOX) {published data only}
- Kakkar AK, Doval DC, Fareed J, Kerr D, Maganji JM, Mueller I. Rationale and design of the GASTRANOX trial on a low-molecular-weight heparin [LMWH], enoxaparin, with chemotherapy vs. chemotherapy alone in inoperable gastric and gastro-oesophageal cancer. Annals of Oncology 2010;21:252-3. [Google Scholar]
Lars 2008 (RASTEN) {published data only}
- A randomized phase III study of standard treatment +/- enoxaparin in small cell lung cancer. Ongoing study. June 2008. Contact author for more information.
Meyer 2017 (PROVE) {published data only}
- Long-term Prophylaxis of Venous Thromboembolism with low-molecular-weight heparin in patients with metastatic lung cancer. Ongoing study. April 2017. Contact author for more information.
Okuno 1999 {published data only}
- Phase III double-blind trial comparing low-molecular weight heparin (LMWH) versus placebo in patients with advanced cancer. Ongoing study. December 1998. Contact author for more information.
Pandya 2002 {published data only}
- A prospective randomized controlled multicenter study of the effect of dalteparin on quality of life in unresectable pancreatic cancer. Ongoing study. October 2002. Contact author for more information.
Rosovsky 2009 {published data only}
- A randomized phase II study to evaluate the effect of two different doses of enoxaparin sodium in combination with standard chemotherapy (cisplatin plus etoposide) with respect to time to tumor progression (TTP) in patients with newly diagnosed extensive stage small cell lung cancer (SCLC) without underlying venous thromboembolism. Ongoing study. July 2008. Contact author for more information.
Additional references
Akl 2013
- Akl EA, Johnston BC, Alonso-Coello P, Neumann I, Ebrahim S, Briel M, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLOS One 2013;8(2):e57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akl 2014 (CVC)
- Akl EA, Ramly EP, Kahale LA, Yosuico VED, Barba M, Sperati F, et al. Anticoagulation for people with cancer and central venous catheters. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD006468. [DOI: 10.1002/14651858.CD006468.pub5] [DOI] [PubMed] [Google Scholar]
Akl 2014 (initial)
- Akl EA, Kahale L, Neumann I, Barba M, Sperati F, Terrenato I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD006649. [DOI: 10.1002/14651858.CD006649.pub6] [DOI] [PubMed] [Google Scholar]
Akl 2014 (long‐term)
- Akl EA, Kahale LA, Barba M, Neumann I, Labedi N, Terrenato I, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD006650. [DOI: 10.1002/14651858.CD006650.pub4] [DOI] [PubMed] [Google Scholar]
Akl 2014 (oral)
- Akl EA, Kahale L, Terrenato I, Neumann I, Yosuico VE, Barba M, et al. Oral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD006466. [DOI: 10.1002/14651858.CD006466.pub5] [DOI] [PubMed] [Google Scholar]
Akl 2016
- Akl EA, Kahale LA, Ebrahim S, Alonso-Coello P, Schünemann HJ, Guyatt GH. Three challenges described for identifying participants with missing data in trials reports, and potential solutions suggested to systematic reviewers.. Journal of Clinical Epidemiology 2016;76:147-54. [DOI] [PubMed] [Google Scholar]
Akl 2017
Alifano 2004
- Alifano M, Benedetti G, Trisolini R. Can low-molecular-weight heparin improve the outcome of patients with operable non-small cell lung cancer?: An urgent call for research. Chest 2004;126:601-7. [DOI] [PubMed] [Google Scholar]
Alshurafa 2012
- Alshurafa M, Briel M, Akl EA, Haines T, Moayyedi P, Gentles SJ, et al. Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature. PLOS One 2012;7(11):e49163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ay 2010
- Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116(24):5377-82. [DOI] [PubMed] [Google Scholar]
Ben‐Aharon 2014
- Ben-Aharon I, Stemmer SM, Leibovici L, Shpilberg O, Sulkes A, Gafter-Gvili A. Low molecular weight heparin (LMWH) for primary thrombo-prophylaxis in patients with solid malignancies–systematic review and meta-analysis. Acta Oncologica 2014;53(9):1230-7. [DOI] [PubMed] [Google Scholar]
Chazouilleres 1994
- Chazouilleres O, Poupon R, Gatineausaillant G, Roulot D, Barbare JC, Labadie H, et al. Beneficial effect of low molecular weight heparin (fraxiparin) on short term mortality in patients with unresectable hepatocellular carcinoma (HCC). A randomized study. Gastroenterology 1994;106(4):A874. [Google Scholar]
Che 2013
- Che DH, Cao JY, Shang LH, Man YC, Yu Y. The efficacy and safety of low-molecular-weight heparin use for cancer treatment: a meta-analysis. European Journal of Internal Medicine 2013;24:433-9. [DOI] [PubMed] [Google Scholar]
Chew 2007
- Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. Journal of Clinical Oncology 2007;25:70–6. [DOI] [PubMed] [Google Scholar]
Chew 2008
- Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. Journal of Thrombosis Haemostasis 2008;6:601–8. [DOI] [PubMed] [Google Scholar]
Cochrane Crowd
- Cochrane Crowd. http://crowd.cochrane.org. ..
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Available from www.cochrane-handbook.org 2009.
Connolly 2012
- Connolly GC, Dalal M, Lin J, Khorana AA. Incidence and predictors ofvenous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer 2012;78:253–8. [DOI] [PubMed] [Google Scholar]
CSR‐Web
- CSR-web. http://community.cochrane.org/tools/data-management-tools/crs. ..
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
Di Nisio 2016
- Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AWS. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD008500. [DOI: 10.1002/14651858.CD008500.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dvorak 1986
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New England Journal of Medicine 1986;315(26):1650-9. [DOI] [PubMed] [Google Scholar]
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels-Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014-21. [DOI] [PubMed] [Google Scholar]
Elliott 2017
- Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al on behalf of the Living systematic review Network. Living Systematic Reviews: 1. Introduction - the Why, What, When and How. Journal of Clinical Epidemiology (in press) 2017.
Francis 1998
- Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Seminars in Thrombosis and Hemostasis 1998;24(2):93-109. [DOI] [PubMed] [Google Scholar]
Girolami 2006
- Girolami B, Girolami A. Heparin-induced thrombocytopenia: a review. Seminars in Thrombosis and Hemostasis 2006;32(8):803-9. [DOI] [PubMed] [Google Scholar]
GRADE handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html Updated October 2013.
Guyatt 2017
Haynes 2002
- Haynes RB, Devereaux PJ, Guyatt GH. Clinical expertise in the era of evidence-based medicine and patient choice. Vox Sanguinis 2002;83(Suppl 1):383-6. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsh 1993
- Hirsh J. Low molecular weight heparin. Thrombosis and Haemostasis 1993;70(1):204-7. [PubMed] [Google Scholar]
Hirsh 2008
- Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(Suppl):141s-59s. [DOI] [PubMed] [Google Scholar]
Karnofsky 1948
- Karnofsky D. The use of nitrogen mustard in the palliative treatment of cancer. Cancer 1948;1:634-56. [Google Scholar]
Levine 2003
- Levine MN, Lee AY, Kakkar AK. From Trousseau to targeted therapy: new insights and innovations in thrombosis and cancer. Journal of Thrombosis and Haemostasis 2003;1(7):1456-63. [DOI] [PubMed] [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5(6):649-55. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
Pelzer 2009 (CONKO‐2004)
- Pelzer U, Deutschinoff G, Opitz B, Stauch M, Reitzig P, Hahnfeld S, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy - first results of the CONKO 004 trial. In: Onkologie - DGHO meeting. Vol. 580. October 2009:Abstract.
Phan 2014
- Phan M, John S, Casanegra AI, Rathbun S, Mansfield A, Stoner JA, et al. Primary venous thromboembolism prophylaxis in patients with solid tumors: a meta-analysis. Journal of Thrombosis and Thrombolysis 2014;38:241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prandoni 1992
- Prandoni P, Lensing AW, Buller HR, Carta M, Cogo A. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet 1992;339:441-5. [DOI] [PubMed] [Google Scholar]
Prandoni 2002
- Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins M, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100(10):3484-8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salat 1990
- Salat C, Breitruck H, Reinhardt B, Hiller E. Thromboprophylaxis with low molecular weight heparin (LMWH) and conventional low dose heparin in patients with malignant diseases. Blut 1990;61(2-3):142. [Google Scholar]
Simmonds 2017
Smorenburg 2001
- Smorenburg SM, Van Noorden CJ. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacological Reviews 2001;53(1):93-105. [PubMed] [Google Scholar]
Sun 2010
- Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010 Mar 30;340:c117. [DOI] [PubMed] [Google Scholar]
Synnot 2017
- Synnot A, Turner T, Elliott J with input from Elie Akl, Harriet MacLehose and the Living Systematic Review Network. Cochrane Living Systematic Reviews Interim guidance for pilots (Version 0.3, 21 April 2017). available at: http://community.cochrane.org/review-production/production-resources/living-systematic-reviews 2017.
Thodiyil 2002
- Thodiyil P, Kakkar AK. Can low-molecular-weight heparins improve outcomes in patients with cancer? Cancer Treatment Reviews 2002;28:151-5. [DOI] [PubMed] [Google Scholar]
Wallace 2017
- Wallace BC, Noel-Storr A, Marshall IJ, Cohen AM, Smalheiser NR, Thomas J. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association 2017;0(0):1-4. [DOI: 10.1093/jamia/ocx053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2009
- Young AM, Billingham LJ, Begum G, Kerr DJ, Hughes AI, Rea DW, et al. Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): an open-label randomised trial. Lancet 2009;373(9663):567-74. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang J1, Zhang YL, Ma KX, Qu JM. Efficacy and safety of adjunctive anticoagulation in patients with lung cancer without indication for anticoagulants: a systematic review and meta-analysis. Thorax 2013;68(5):442-450. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Akl 2007
- Akl EA, Doormaal FF, Barba M, Kamath G, Kim SY, Kuipers S, et al. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD006652. [DOI: 10.1002/14651858.CD006652] [DOI] [PubMed] [Google Scholar]
Akl 2011a
- Akl EA, Gunukula S, Barba M, Yosuico VED, Doormaal FF, Kuipers S, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD006652. [DOI: 10.1002/14651858.CD006652.pub2] [DOI] [PubMed] [Google Scholar]
Akl 2011b
- Akl EA, Gunukula S, Barba M, Yosuico VED, Doormaal FF, Kuipers S, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database of Systematic Reviews 2011, Issue 4. Art. No: CD006652. [DOI: 10.1002/14651858.CD006652.pub3] [DOI] [PubMed] [Google Scholar]
Akl 2014 (parenteral)
- Akl EA, Kahale LA, Ballout RA, Barba M, Yosuico VE, Doormaal FF, et al. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD006652. [DOI: 10.1002/14651858.CD006652.pub4] [DOI] [PubMed] [Google Scholar]


